Translate this page into:
Qingqianliusus A-N, 3,4-seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus and their biological activities
⁎Corresponding author. cpujyq2010@163.com (Yuqing Jian), wangwei402@hotmail.com (Wei Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fourteen previously unreported 3,4-seco-dammarane triterpenoids named Qingqianliusus A-N (1–14), along with four known 3,4-seco-dammarane triterpenoid derivatives (15–18) were isolated from the 95 % ethanol extract of the Cyclocarya paliurus leaves. Compounds 1 and 2 possess a rare 3,11-heptacyclic lactone as natural product, and several pairs of the 3,4-seco-dammarane triterpenoid epimers with R/S configuration at C-24 were investigated and determined in detail for the first time. Compounds 8, 11, and 14 showed good α-glucosidase inhibitory effects with IC50 values of 4.97 ± 0.63, 7.08 ± 0.53, and 3.76 ± 0.77 μM, respectively. Meanwhile, compound 11 was also found potent inhibition rate of 35.83 % against COX-2, as compared with the positive control celecoxib (70.28 %). In addition, compounds 3, 7, 10, and 13 exhibited outstanding cytotoxicities against human gastric cancer cell lines (BGC-823) with IC50 values of 7.69 ± 0.21, 8.47 ± 0.41, 9.04 ± 0.61, and 8.86 ± 0.38 μM, respectively. Compounds 13 and 3 had modest activities on human colon cancer cell lines (HCT-116) with IC50 values of 8.80 ± 0.36 and 9.45 ± 0.93 μM, respectively.
Keywords
Cyclocarya paliurus
Juglandaceae
Qinqianliusu
3 4-seco-dammarane triterpenoids
Anti-hypoglycemic
Anti-inflammatory
1 Introduction
Cyclocarya paliurus (Batalin) Iljinskaja (family: Juglandaceae), also known as “Qingqianliu” in Chinese, is now widely distributed in most areas of the south of the Yangtze River such as Hunan, Jiangxi, Hubei, Jiangxi, Sichuan, Guizhou, Guangxi, and Yunnan (Xie et al., 2010; Yang et al., 1992). As a precious species, its leaves have long history of being used as food resources and health care medicines. In Hunan folk medicine, Qingqianliu were used to slake thirst as a drink, and also called Sweet Tea due to sweet taste (Kennelly et al., 1995). Moreover, according to the “Resources of Traditional Chinese Medicine”, it has the potential therapy to clear heat, reduce swelling and relieve pain. In August 2013, Qingqianliu also were approved by the National Health Commission of the People's Republic of China to become a new raw food material (Xie et al., 2015; Xie et al., 2018). Until 2017, Many enterprises exploited and developed the leaves of the title plant into health products with health care function of blood sugar, blood lipids, and blood pressure regulations, together with weight loss ( https://db.yaozh.com/baojian).
In our previous study, >210 compounds have been reported from the title plant (Chen et al., 2022). Among them, the largest number of characteristic 3,4-seco-dammarane triterpenoids could be proved as quality markers to identify the plant (Cui and Li, 2012). They can also be considered as the major active and functional constituents in Qingqianliu with anti-hyperglycemic (Li et al., 2021; Li et al., 2012; Sun et al., 2020b; Yang et al., 2018; Zhou et al., 2021), anti-inflammatory (Li et al., 2021; Liu et al., 2020), cytotoxic (Chen et al., 2018; Sun et al., 2020a; Zhou et al., 2021), anti-hyperlipidemic (Wu et al., 2017; Yang et al., 2018), and anti-microbial (Kennelly et al., 1995) activities. Obviously, our previous researches have illustrated the cytotoxic capability of 3,4-seco-dammarane triterpenoids isolated from 80 % ethanol extract of Qingqianliu (Chen et al., 2018). The aim of this study focused on further chemical constituents and pharmacological activities to provide deeper insights into Qingqianliu as a nutraceutical additive or raw food material (Ning et al., 2019; Xie et al., 2018). On this basis, fourteen undescribed 3,4-seco-dammarane triterpenoids (1–14, Fig. 1) were extracted and separated from the chloroform extract of Qingqianliu. Moreover, their structures were elucidated through X-ray crystallography and extensive spectroscopic methods. Compounds (1–14) were tested for their biological properties on α-glucosidase inhibition, COX-2 inhibition, and cytotoxic activities.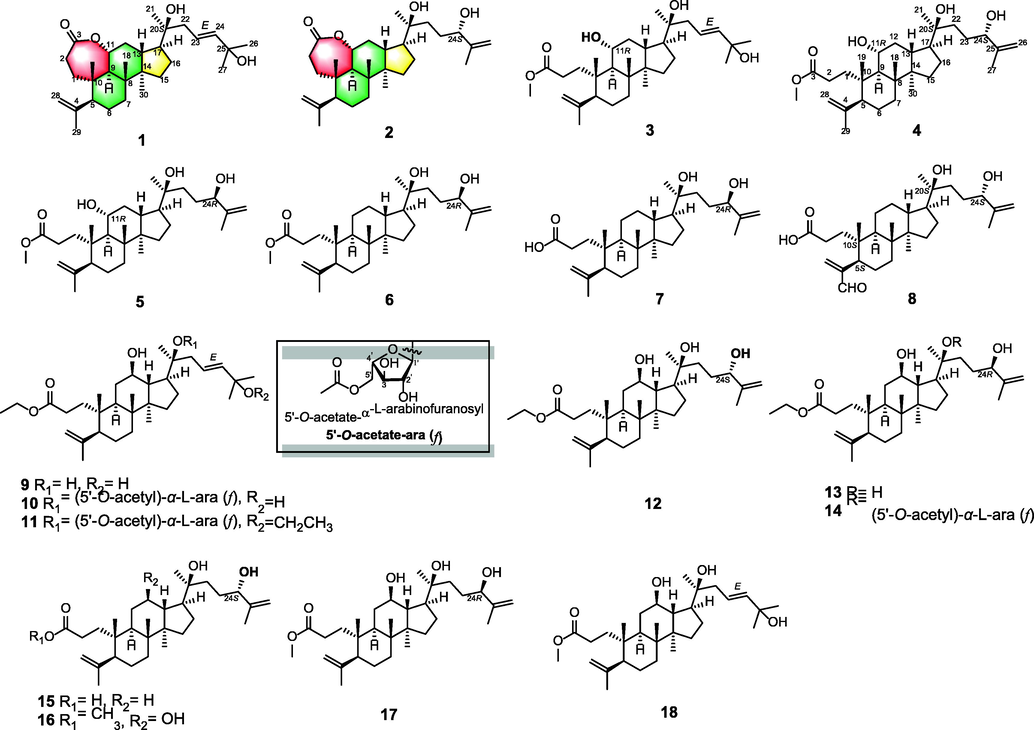
Structure of compounds 1–18 isolated from Qingqianliu.
2 Material and methods
2.1 General and solvents
UV spectra were recorded with Hewlett-Packard 8452A, UV–vis spectrophotometer (L.P., Palo Alto, Ca, USA). Using a Frontier infrared spectrometer to record IR spectra (PerkinElmer, U.S.A). HR-ESI-MS spectra were performed on Xevo G2-S QTOF mass spectrometer (Waters Co., Milford, MA, USA), 6500 series Q-TOF mass spectrometer (Agilent Co., USA) and LTQ Orbitrap Velos Pro MS (Thermo Scientific, MA, USA). The single Crystal X-ray diffraction data were obtained using a SuperNova, Dual, Cu at zero, AtlasS2 diffractometer (Rigaku Co., Japan). 1D and 2D NMR spectra were acquired on a Bruker Avance DRX-600 MHz spectrometer (Bruker Co., Billerica, MA, USA) at room temperature with CD3OD as solvent. The absolute configuration of the sugar moiety was detected by HPLC analysis at 210 nm using C18 columns (5 μm, 4.6 × 250 mm, Agilent, Palo Alto, CA, USA) on Agilent 1260 series HPLC instrument (Agilent, Palo Alto, CA, USA). Semi preparative HPLC was carried out on Agilent 1260 series semi preparative HPLC instrument (Agilent, Palo Alto, CA, USA) by using C18 columns (5 mm, 9.4 × 150 mm) with a flow rate of 2 mL/min. All analytical-grade solvents were bought from Merck KGaA (Darmstadt, Germany). Besides, chemical solvent were obtained from Shanghai Titan Scientific Co., ltd. P. R. China and silica gel (300–400 mesh) for column chromatographic were purchased from Qingdao Marine Chemical Inc. P. R. China. Similarly, Sephadex LH-20 gel were bought from GE Health care, Sweden and Lichrospher RP-18 gel (20–45 μm) from Merck KGaA (Darmstadt, Germany).
2.2 Plant material
The leaves of Cyclocarya paliurus (Batalin) Iljinskaja were purchased from Huaihua, Hunan Province, People’s Republic of China, in June 2015, and authenticated by Prof. Wei Wang of Hunan University of Chinese Medicine. The voucher specimen (No. 20150629) was preserved in the TCM and Ethnomedicine Innovation & Development Laboratory, School of Pharmacy, Hunan University of Chinese Medicine, People′s Republic of China.
2.3 Extraction and isolation
The crude extract (4 Kg) was extracted from Qingqianliu (20.0 Kg) using 95 % ethanol percolation for three times at room temperature. Afterwards, the ethanol extract was suspended in water and then extracted with petroleum ether (PE), chloroform (CHCl3), and n-butanol (n-BuOH) respectively. The chloroform layer (980.0 g) was subjected to silica gel column (60 × 15.5 cm) chromatography eluted with CH2Cl2-CH3OH (0:1–1:1) to gain nineteen fractions (Fr. E1-E19). Fraction E7 (5.2 g) was purified using silica gel column (60 × 15.5 cm) chromatography eluted with CYH-EA (0:1–1:1) and reverse-phase HPLC (2.0 mL/min, MeCN-H2O, 7.0:3.0) afforded 10 (4.0 mg) and 14 (4.0 mg). Combined fractions E8 and E9 named fractions E8-9 (33.9 g), which through silica gel column with PE-EA (0:1–1:1) to give thirteen fractions (Fr. F1-F13). Sub fraction F9 (2.5 g) through silica gel column chromatography yield 1 (12.5 mg), 2 (7.0 mg), 3 (15.2 mg), 4 (5.8 mg), 5 (4.1 mg), 11 (4.4 mg), 12 (27.4 mg), and 13 (32.4 mg). Similarly, fraction F10 (4.6 g) was subjected using Sephadex LH-20 column (4 × 77 cm) chromatography eluted with CH3OH and reverse-phase HPLC (2.0 mL/min, MeCN-0.01 % F3CCOOH-H2O, 6.5:3.5) to produce 6 (9.1 mg), 7 (4.4 mg), 8 (3.8 mg), 9 (17.9 mg), 15 (4.0 mg), and 18 (8.2 mg). Compounds 16 (5.2 mg) and 17 (4.2 mg) were separated from fraction E13 (10.0 g) via silica gel column (60 × 15.5 cm) chromatography eluted with CYH-EA (0:1–1:1) and purified by reverse-phase HPLC (2.0 mL/min, MeCN-H2O, 7.0:3.0).
2.3.1 Qingqianliusu A (1)
Colorless cubic crystal; mp 169 ∼ 171℃; [α]23 D = + 67.0 (c 0.19, MeOH); UV (MeOH) λmax (log ε): 204 (3.93) nm; IR (vmax): 3369, 2950, 2959, 2930, 2907, 1728 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 495.3450 [M + Na]+ (calcd. for C30H48O4Na, 495.3450).
No.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
1a
1.78 (m)
1.78 (m)
2.35 (m)
2.35 (m)
2.35 (m)
1.61 (m)
1.61 (m)
1.49 (m)
1.62 (m, 2H)
1.63 (m, 2H)
1.63 (m, 2H)
1.63 (m, 2H)
1.63 (m, 2H)
1.63 (m, 2H)
1b
1.33 (t-like, 13.5)
1.32 (t-like, 13.5)
1.54 (m)
1.56 (m)
1.55 (m)
1.59 (m)
1.59 (m)
0.86 (m)
2a
2.81 (dd, 15.4, 12.7)
2.81 (dd, 15.7, 12.4)
2.64 (m)
2.64 (m)
2.63 (m)
2.39 (m)
2.34 (m)
2.59 (ddd, 16.0, 12.1, 4.0)
2.38 (m)
2.37 (m)
2.38 (m)
2.38 (m)
2.38 (m)
2.38 (m)
2b
2.30 (dd, 15.4, 8.1)
2.30 (dd, 15.4, 8.1)
2.33 (m)
2.34 (m)
2.34 (m)
2.21 (m)
2.15 (m)
2.12 (ddd, 16.0, 12.1, 5.5)
2.21 (m)
2.20 (m)
2.21 (m)
2.20 (m)
2.20 (m)
2.21 (m)
5
1.87 (m)
1.87 (m)
2.07 (dd, 12.9, 3.4)
2.07 (dd, 12.1, 4.1)
2.07 (dd, 12.8, 3.4)
2.05 (dd, 12.8, 3.2)
2.05 (dd, 12.7, 3.1)
2.74 (dd, 13.2, 3.2)
2.05 (m)
2.05 (m)
2.06 (m)
2.06 (m)
2.06 (m)
2.05 (m)
6a
2.02 (qd, 13.2, 4.0)
2.00 (qd, 13.3, 3.9)
1.88 (m)
1.88 (m)
1.87 (m)
1.90 (m)
1.88 (m)
1.98 (dd, 13.2, 3.2)
1.91 (m)
1.91 (m)
1.92 (m)
1.91 (m)
1.91 (m)
1.93 (m)
6b
1.44 (m)
1.43 (m)
1.33 (m)
1.32 (m)
1.32 (m)
1.36 (m)
1.35 (m)
1.23 (m)
1.39 (m)
1.40 (m)
1.40 (m)
1.39 (m)
1.39 (m)
1.40 (m)
7a
1.65 (m)
1.66 (m)
1.57 (m)
1.57 (m)
1.56 (m)
1.61 (m)
1.62 (m)
1.62 (m)
1.60 (m)
1.61 (m)
1.61 (m)
1.59 (m)
1.60 (m)
1.61 (m)
7b
1.23 (m)
1.23 (m)
1.20 (m)
1.19 (m)
1.19 (m)
1.25 (m)
1.24 (m)
1.24 (m)
1.28 (m)
1.27 (m)
1.26 (m)
1.27 (m)
1.27 (m)
1.27 (m)
9
1.84 (d, 9.6)
1.84 (d, 9.7)
1.73 (m)
1.73 (m)
1.74 (m)
1.60 (m)
1.61 (m)
1.70 (m)
1.68 (dd, 13.4, 2.9)
1.66 (dd, 13.5, 3.1)
1.66 (dd, 13.6, 3.1)
1.67 (dd, 13.2, 3.2)
1.66 (d, 13.3, 3.1)
1.66 (m)
11a
4.78 (q-like, 8.8)
4.78 (q-like, 8.8)
3.92 (td, 10.8, 5.0)
3.91 (td, 10.9, 5.0)
3.91 (td, 10.8, 5.0)
1.44 (m)
1.46 (m)
1.44 (m)
1.77 (m)
1.74 (m)
1.75 (m)
1.76 (m)
1.75 (m)
1.76 (m)
11b
1.36 (m)
1.33 (m)
1.32 (m)
1.33 (m)
1.31 (m)
1.29 (m)
1.31 (m)
1.30 (m)
1.32 (m)
12a
2.47 (ddd, 13.8, 8.0, 4.5)
2.46 (ddd, 13.8, 7.9, 4.5)
2.13 (m)
2.12 (m)
2.12 (m)
1.89 (m)
1.88 (m)
1.87 (m)
3.58 (td, 10.6, 5.1)
3.69 (td, 10.4, 5.3)
3.71 (td, 10.5, 5.3)
3.56 (td, 10.6, 5.1)
3.57 (td, 10.6, 5.1)
3.70 (td, 10.4, 5.3)
12b
1.68 (m)
1.68 (m)
1.45 (m)
1.46 (m)
1.46 (m)
1.30 (m)
1.31 (m)
1.30 (m)
–
–
–
–
–
–
13
1.89 (m)
1.87 (m)
1.88 (m)
1.87 (m)
1.85 (m)
1.74 (m)
1.72 (m)
1.72 (m)
1.80 (m)
1.80 (m)
1.77 (m)
1.79 (m)
1.79 (m)
1.78 (m)
15a
1.54 (m)
1.52 (m)
1.41 (m)
1.41 (m)
1.41 (m)
1.49 (m)
1.49 (m)
1.47 (m)
1.56 (m)
1.59 (m)
1.58 (m)
1.55 (m)
1.53 (m)
1.59 (m)
15b
1.10 (m)
1.11 (m)
1.06 (m)
1.08 (m)
1.08 (m)
1.09 (m)
1.08 (m)
1.07 (m)
1.07 (m)
1.06 (m)
1.06 (m)
1.09 (m)
1.09 (m)
1.09 (m)
16a
1.80 (m)
1.79 (m)
1.75 (m)
1.74 (m)
1.75 (m)
1.75 (m)
1.74 (m)
1.73 (m)
1.88 (m)
1.87 (m)
1.90 (m)
1.88 (m)
1.88 (m)
1.91 (m)
16b
1.66 (m)
1.62 (m)
1.63 (m)
1.61 (m)
1.60 (m)
1.60 (m)
1.58 (m)
1.59 (m)
1.40 (m)
1.41 (m)
1.39 (m)
1.32 (m)
1.35 (m)
1.35 (m)
17
1.79 (m)
1.78 (m)
1.79 (m)
1.80 (m)
1.78 (m)
1.75 (m)
1.75 (m)
1.74 (m)
2.08 (m)
2.24 (m)
2.27 (m)
2.07 (m)
2.06 (m)
2.27 (m)
18
1.00 (s)
1.00 (s)
1.06 (s)
1.05 (s)
1.05 (s)
1.06 (s)
1.06 (s)
1.09 (s)
1.08 (s)
1.06 (s)
1.07 (s)
1.06 (s)
1.06 (s)
1.06 (s)
19
1.20 (s)
1.20 (s)
1.07 (s)
1.07 (s)
1.07 (s)
0.88 (s)
0.88 (s)
0.84 (s)
0.91 (s)
0.91 (s)
0.91 (s)
0.91 (s)
0.91 (s)
0.91 (s)
21
1.14 (s)
1.14 (s)
1.16 (s)
1.14 (s)
1.14 (s)
1.13 (s)
1.12 (s)
1.13 (s)
1.15 (s)
1.33 (s)
1.32 (s)
1.15 (s)
1.14 (s)
1.33 (s)
22a
2.20 (m)
1.50 (m)
2.21 (m)
1.54 (m)
1.58 (m)
1.59 (m)
1.58 (m)
1.50 (m)
2.32 (m)
2.54 (dd, 14.3, 5.6)
2.59 (dd, 14.2, 6.2)
1.53 (m)
1.54 (m)
1.75 (m)
22b
2.18 (m)
1.40 (m)
2.19 (m)
1.41 (m)
1.39 (m)
1.40 (m)
1.39 (m)
1.38 (m)
2.20 (m)
2.37 (m)
2.32 (m)
1.31 (m)
1.30 (m)
1.63 (m)
23a
5.67 (ddd, 15.6, 7.5, 6.2)
1.65 (m)
5.68 (ddd, 15.7, 7.5, 6.3)
1.64 (m)
1.65 (m)
1.65 (m)
1.64 (m)
1.61 (m)
5.74 (ddd, 15.7, 8.6, 6.0)
5.73 (ddd, 15.7, 8.0, 5.6)
5.74 (ddd, 15.8, 8.5, 6.1)
1.74 (m)
1.76 (m)
1.80 (m)
23b
1.57 (m)
1.55 (m)
1.55 (m)
1.57 (m)
1.56 (m)
1.58 (m)
1.57 (m)
1.54 (m)
1.53 (m)
24
5.62 (d, 15.6)
3.97 (t, 6.5)
5.63 (d, 15.7)
3.96 (t, 6.7)
3.98 (t, 6.1)
3.96 (t, 6.2)
3.96 (t, 6.2)
3.96 (t, 6.5)
5.63 (d, 15.7)
5.63 (d, 15.7)
5.55 (d, 15.8)
4.00 (t, 6.4)
3.98 (t, 5.9)
3.98 (t, 6.4)
26a
1.27 (s)
4.91 (br s)
1.28 (s)
4.91 (br s)
4.92 (br s)
4.92 (br s)
4.92 (br s)
4.91 (br s)
1.29 (s)
1.29 (s)
1.28 (s)
4.93 (br s)
4.93 (br s)
4.74 (br s)
26b
4.81 (br s)
4.82 (br s)
4.81 (br s)
4.82 (br s)
4.81 (br s)
4.81 (br s)
4.82 (br s)
4.82 (br s)
4.71 (br s)
27
1.26 (s)
1.72 (s)
1.26 (s)
1.75 (s)
1.73 (s)
1.73 (s)
1.72 (s)
1.72 (s)
1.28 (s)
1.28 (s)
1.27 (s)
1.73 (s)
1.73 (s)
1.74 (s)
28a
4.86 (br s)
4.85 (br s)
4.85 (br s)
4.85 (br s)
4.85 (br s)
4.86 (br s)
4.86 (br s)
6.42 (br s)
4.88 (br s)
4.88 (br s)
4.88 (br s)
4.88 (br s)
4.88 (br s)
4.88 (br s)
28b
4.74 (br s)
4.74 (br s)
4.69 (br s)
4.69 (br s)
4.69 (br s)
4.69 (br s)
4.69 (br s)
6.22 (br s)
4.71 (br s)
4.71 (br s)
4.71 (br s)
4.71 (br s)
4.71 (br s)
4.71 (br s)
29
1.75 (s)
1.75 (s)
1.75 (s)
1.76 (s)
1.75 (s)
1.75 (s)
1.75 (s)
9.44 (s)
1.76 (s)
1.77 (s)
1.76 (s)
1.76 (s)
1.77 (s)
1.76 (s)
30
0.97 (s)
0.97 (s)
0.97 (s)
0.97 (s)
0.96 (s)
0.94 (s)
0.95 (s)
0.98 (s)
0.95 (s)
0.95 (s)
0.96 (s)
0.95 (s)
0.96 (s)
0.97 (s)
3-COOCH3
\
\
3.63 (s)
3.62 (s)
3.62 (s)
3.66 (s)
3-COOCH2 CH3
4.11 (q, 7.1)
4.11 (q, 7.1)
4.12 (q, 7.1)
4.11 (q, 7.1)
4.11 (q, 7.2)
4.11 (q, 7.1)
3-COOCH2CH3
1.24 (t, 7.1)
1.25 (t, 7.1)
1.25 (t, 7.1)
1.25 (t, 7.1)
1.25 (t, 7.2)
1.25 (t, 7.1)
1′
5.27 (d, 2.9)
5.27 (d, 2.9)
5.27 (d, 2.8)
2′
3.94 (dd, 4.9, 2.9)
3.95 (dd, 4.8, 2.9)
3.96 (dd, 4.8, 2.8)
3′
3.82 (dd, 6.4, 4.9)
3.82 (dd, 6.3, 4.8)
3.82 (dd, 6.6, 4.8)
4′
4.11 (m)
4.10 (m)
4.07 (m)
5′a
4.26 (m)
4.26 (m)
4.26 (m)
5′b
4.13 (m)
4.11 (m)
4.13 (m)
5′-OCOCH3
2.06 (s)
2.06 (s)
2.06 (s)
25-OCH2CH3
3.39 (q, 7.0)
25-OCH2CH3
1.12 (t, 7.0)
No.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
1
40.6t
40.6,t
31.7t
37.7t
37.7t
35.9t
36.0t
36.2t
35.8t
35.8t
35.8t
35.8t
35.8t
35.7t
2
30.5t
30.5t
30.2t
30.3t
30.2t
29.4t
29.4t
28.8t
29.6t
29.7t
29.7t
29.6t
29.6t
29.6t
3
179.6s
179.6s
177.3s
177.3s
177.3s
176.4s
178.2s
178.0s
175.8s
175.7s
175.8s
175.7s
175.7s
175.7s
4
148.3s
148.3s
149.1s
149.1s
149.1s
148.9s
149.0s
154.2s
148.7s
148.7s
148.7s
148.6s
148.6s
148.7s
5
58.7 d
58.7 d
52.8 d
52.8 d
52.8 d
52.0 d
52.0 d
40.2 d
51.7 d
51.7 d
51.7 d
51.7 d
51.7 d
51.7 d
6
26.3t
26.3t
26.1t
26.1t
26.1t
25.9t
25.9t
26.3t
25.8t
25.8t
25.8t
25.8t
25.8t
25.8t
7
34.7t
34.7t
35.8t
35.8t
35.8t
35.1t
35.1t
35.2t
34.6t
34.5t
34.5t
34.6t
34.6t
34.5t
8
41.8s
41.8s
41.7s
41.6s
41.6s
41.3s
41.3s
41.3s
40.6s
40.7s
40.7s
40.6s
40.6s
40.6s
9
53.5 d
53.5 d
46.4 d
46.4 d
46.4 d
42.3 d
42.3 d
42.3 d
41.7 d
41.3 d
41.3 d
41.7 d
41.7 d
41.3 d
10
40.7s
40.7s
40.6s
40.6s
40.6s
40.2s
40.2s
39.5s
40.2s
40.2s
40.2s
40.2s
40.2s
40.2s
11
78.5 d
78.5 d
71.6 d
71.6 d
71.6 d
23.2t
23.2t
23.4t
32.5t
31.3t
31.3t
32.6t
32.6t
31.3t
12
37.0t
37.0t
40.1t
40.0t
40.0t
28.6t
28.6t
28.5t
71.8 d
71.5 d
71.4 d
71.7 d
71.7 d
71.3 d
13
39.9 d
40.0 d
42.0 d
42.0 d
42.0 d
43.6 d
43.6 d
43.6 d
49.3 d
50.0 d
50.0 d
49.0 d
49.0 d
49.6 d
14
51.0s
51.0s
51.6s
51.6s
51.6s
51.9s
51.9s
51.9s
53.1s
53.0s
53.0s
53.1s
53.1s
52.8s
15
31.6t
31.7t
31.8t
31.9t
31.9t
32.3t
32.3t
32.3t
32.0t
31.4t
31.4t
32.1t
32.1t
31.5t
16
25.8t
26.0t
25.7t
25.9t
25.9t
25.8t
25.8t
25.8t
27.2t
26.8t
26.9t
27.4t
27.4t
27.0t
17
51.9 d
52.4 d
50.3 d
50.5 d
50.8 d
51.0 d
51.1 d
50.8 d
54.5 d
52.7 d
52.6 d
55.0 d
55.1 d
52.8 d
18
17.5 q
17.4 q
16.9 q
16.8 q
16.8 q
15.8 q
15.8 q
15.8 q
16.1 q
16.2 q
16.2 q
15.9 q
16.0 q
16.1 q
19
19.3 q
19.4 q
21.3 q
21.1 q
21.1 q
20.8 q
20.8 q
19.6 q
20.7 q
20.6 q
20.6 q
20.8 q
20.8 q
20.6 q
20
75.7s
75.4s
75.9s
75.6s
75.6s
75.9s
75.9s
75.8s
74.5s
83.8s
83.7s
74.1s
74.3s
84.0s
21
26.1 q
25.5 q
26.0 q
25.5 q
25.4 q
25.2 q
25.2 q
25.4 q
27.1 q
23.3 q
23.3 q
26.7 q
26.7 q
22.7 q
22
45.2t
37.9t
45.4t
38.3t
38.3t
38.2t
38.2t
38.2t
40.3t
39.9t
39.8t
32.2t
32.3t
32.6t
23
123.7 d
30.1t
123.8 d
30.2t
30.3t
30.2t
30.2t
30.1t
124.1 d
123.5 d
126.9 d
30.0t
30.1t
30.4t
24
142.2 d
77.4 d
142.1 d
77.4 d
77.3 d
77.3 d
77.3 d
77.5 d
141.6 d
142.0 d
139.6 d
77.4 d
77.4 d
77.2 d
25
71.2s
148.8s
71.2s
148.8s
149.0s
149.0s
149.0s
148.8s
71.2s
71.2s
76.3s
148.8s
149.2s
149.3s
26
29.9 q
111.6t
29.9 q
111.6t
111.3t
111.3t
111.3t
111.6t
29.8 q
29.9 q
26.9 q
111.4t
111.1t
111.2t
27
30.0 q
17.5 q
30.0 q
17.5 q
17.8 q
17.8 q
17.8 q
17.5 q
30.0 q
30.0 q
26.9 q
17.7 q
17.9 q
18.0 q
28
114.6t
114.6t
114.2t
114.2t
114.2t
114.0t
113.9t
138.3t
114.6t
114.2t
114.2t
114.2t
114.2t
114.2t
29
23.9 q
23.8 q
23.8 q
23.8 q
23.8 q
23.7 q
23.7 q
196.6 d
23.7 q
23.7 q
23.7 q
23.7 q
23.7 q
23.7 q
30
16.1 q
16.1 q
16.6 q
16.7 q
16.7 q
16.9 q
16.9 q
16.9 q
17.0 q
17.2 q
17.2 q
17.1 q
17.1 q
17.3 q
3-COOCH3
51.9 q
51.9 q
51.9 q
52.1 q
3-COOCH2CH3
61.6t
61.6t
61.6t
61.6t
61.6t
61.6t
3-COOCH2CH3
14.5 q
14.5 q
14.5 q
14.5 q
14.5 q
14.5 q
1′-OCH-
103.5 d
103.6 d
103.4 d
2′-CH-
83.7 d
83.6 d
83.5 d
3′-CH-
77.9 d
78.0 d
78.1 d
4′-CH-
82.1 d
82.2 d
82 d
5′-CH2-O
65.2t
65.3t
65.3t
5′-OCO-
172.6s
172.6s
172.6s
5′-OCOCH3
20.7 q
20.7 q
20.7 q
25-OCH2CH3
59.0t
25-OCH2CH3
16.4 q
2.3.2 Qingqianliusu B (2)
Colorless amorphous powder; [α]23 D = + 76.0 (c 0.25, MeOH); UV (MeOH) λmax (log ε): 203 (3.71) nm; IR (vmax): 3407, 2944, 2873, 1709 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 495.3451 [M + Na]+ (calcd. for C30H48O4Na, 495.3450).
2.3.3 Qingqianliusu C (3)
Colorless powder; [α]23 D = + 38.5 (c 0.29, MeOH); UV (MeOH) λmax (log ε): 204 (3.91) nm; IR (vmax): 3408, 2963, 2871, 1717 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 527.3712 [M + Na]+ (calcd. for C31H52O5Na, 527.3712).
2.3.4 Qingqianliusu D (4)
Colorless amorphous powder; [α]23 D = + 36.0 (c 0.25, MeOH); UV (MeOH) λmax (log ε): 203 (3.84) nm; IR (vmax): 3358, 2944, 2878, 1732 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 527.3719 [M + Na]+ (calcd. for C31H52O5Na, 527.3712).
2.3.5 Qingqianliusu E (5)
White amorphous powder; [α]23 D = + 39.4 (c 0.33, MeOH); UV (MeOH) λmax (log ε): 203 (3.81) nm; IR (vmax): 3269, 2940, 2868, 1734 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 527.3717 [M + Na]+ (calcd. for C31H52O5Na, 527.3712).
2.3.6 Qingqianliusu F (6)
White amorphous powder; [α]23 D = + 39.9 (c 0.28, MeOH). UV (MeOH) λmax (log ε): 204 (4.00) nm; IR (vmax): 3364, 2950, 2943, 2868, 1732 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 511.3764 [M + Na]+ (calcd. for C31H52O4Na, 511.3763).
2.3.7 Qingqianliusu G (7)
White amorphous powder; [α]23 D = + 36.0 (c 0.22, MeOH); UV (MeOH) λmax (log ε): 203 (3.69) nm; IR (vmax): 3388, 2949, 2869, 1712 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 497.3589 [M + Na]+ (calcd. for C30H50O4Na, 497.3607).
2.3.8 Qingqianliusu H (8)
White amorphous powder; [α]23 D = + 14.3 (c 0.21, MeOH); UV (MeOH) λmax (log ε): 204 (3.76) nm; IR (vmax): 3432, 2937, 2868, 1708, 1692 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 511.3382 [M + Na]+ (calcd. for C30H48O5Na, 511.3399).
2.3.9 Qingqianliusu I (9)
Colorless amorphous powder; [α]23 D = + 33.2 (c 0.21, MeOH); UV (MeOH) λmax (log ε): 204 (3.89) nm; IR (vmax): 3259, 2949, 2875, 1735 cm−1. 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 541.3869 [M + Na]+ (calcd. for C32H54O5Na, 541.3868).
2.3.10 Qingqianliusu J (10)
White amorphous powder; [α]23 D = + 7.2 (c 0.29, MeOH); UV (MeOH) λmax (log ε): 204 (3.95) nm; IR (vmax): 3351, 2927, 1734 cm−1; 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 715.4400 [M + Na]+ (calcd. for C39H64O10Na, 715.4397).
2.3.11 Qingqianliusu K (11)
White amorphous powder; [α]23 D = + 12.0 (c 0.25, MeOH); UV (MeOH) λmax (log ε): 204 (3.82) nm; IR (vmax): 3401, 2973, 1734 cm−1. 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 743.4718 [M + Na]+ (calcd. for C41H68O10Na, 743.4710).
2.3.12 Qingqianliusu L (12)
White amorphous powder; [α]23 D = + 24.2 (c 0.41, MeOH); UV (MeOH) λmax (log ε): 204 (3.94) nm; IR (vmax): 3292, 2942, 2872, 1733 cm−1. 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 541.3863 [M + Na]+(calcd. for C32H54O5Na, 541.3869).
2.3.13 Qingqianliusu M (13)
White amorphous powder; [α]23 D = + 40.8 (c 0.59, MeOH); UV (MeOH) λmax (log ε): 204 (3.77) nm; IR (vmax): 3265, 2941, 2875, 1732 cm−1. 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 541.3859 [M + Na]+(calcd. for C32H54O5Na, 541.3869).
2.3.14 Qingqianliusu N (14)
White amorphous powder; [α]23 D = + 7.2 (c 0.28, MeOH); UV (MeOH) λmax (log ε): 203 (3.77) nm; IR (vmax): 3287, 2916, 2849, 1728 cm−1. 1H and 13C NMR (CD3OD, Tables 1 and 2); HR-ESI-MS m/z 715.4382 [M + Na]+ (calcd. for C39H64O10Na, 715.4397).
2.4 Determination of the absolute configurations of the Sugars
The acid hydrolysis was adopted from reported method (Liu et al., 2020; Shen et al., 2020). After the comparison of the retention times (tR) of monosaccharide derivatives with those of standard products: l-Arabinose (19.75 min) and d-Arabinose (23.96 min), compounds 10, 11, and 14 were determined to be l-Arabinose. Detailed description of the procedure can be seen in the Supporting Information (Text S1.).
2.5 X-ray crystallographic Analysis
All data were obtained using a SuperNova, AtlasS2 diffractometer with graphite monochromated Cu Ka radiation (λ = 1.54184 Å). The structure was solved by direct method with the SHELXTL software package and was refined by full-matrix least-squares techniques. All the nonhydrogen atoms were refined anisotropically and H atoms were located in geometrical calculations. Crystal data for compounds 1 and 18 were listed below.
2.5.1 Crystal data for 1
CCDC: 2133487, C30H48O4, M = 472.68 g/mol, monoclinic space group P21, a = 14.0820(8) Å, b = 8.2422(3) Å, c = 24.0159(11) Å, β = 100.684(5)°, V = 2739.1(2) Å3, Z = 4, T = 149.99(10) K, μ(Cu Kα) = 0.576 mm−1, Dcalc = 1.146 g/cm3, 21,646 reflections measured (6.388 ≤ 2Θ ≤ 150.052), 9167 unique (Rint = 0.0538, Rsigma = 0.0530) which were used in all calculations. The final R1 was 0.0781 (I > 2σ(I)) and wR2 was 0.2170. Flack parameter = − 0.3(2).
2.5.2 Crystal data for 18
CCDC: 2133486, C31H52O5, M = 504.72 g/mol, tetragonal space group P41212, a = 19.3567(2) Å, c = 16.0224(3) Å, V = 6003.30(17) Å3, Z = 8, T = 149.99(10) K, μ(Cu Kα) = 0.578 mm−1, Dcalc = 1.117 g/cm3, 32,912 reflections measured (6.458 ≤ 2Θ ≤ 133.17), 5302 unique (Rint = 0.0326, Rsigma = 0.0186) which were used in all calculations. The final R1 was 0.0626 (I > 2σ(I)) and wR2 was 0.1679. Flack parameter = -0.05(6).
2.6 α-Glucosidase inhibitory activity Assay
The α-glucosidase inhibition assay was adopted from reported method (Perez et al., 2019; Smirnova et al., 2020; Zhou et al., 2021). The detailed protocol was described in the Supporting Information (Text 2).
2.7 Anti-inflammatory activity against COX-2
The COX-2 inhibition assay was detected with previous report (Chai et al., 2008; Shataer et al., 2021). The detailed procedures for this assay were discussed in the Supporting Information (Text 3).
2.8 Cytotoxic Bioassays
Using the previously described MTT method (Chen et al., 2018; Gao et al., 2016; Peng et al., 2019; Sun et al., 2021; Yan et al., 2014; Zhou et al., 2021), the cytotoxicities of new compounds against the human cancer cell lines (BGC-823, MCF-7, HCT-116, and HepG-2) were measured. Paclitaxel was used as positive control. The detailed process can be seen in the Supporting Information (Text 4).
3 Results and discussion
3.1 Structure Determination
The chloroform extract of Qingqianliu was isolated and fractionated over Sephadex LH-20, silica gel, RP-18 (ODS), and semi-preparative HPLC to obtain fourteen undiscovered compounds named Qingqianliusus A-N (1–14), as well as four reported compounds, 20S,24S-dihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid (15) (Aoki et al., 1988), cyclocariol B (16) (Chen et al., 2018), cyclocariol A (17) (Chen et al., 2018), and cyclocariol E (18) (Chen et al., 2018). In addition, compound 15 was reported from Qingqianliu for the first time.
Qingqianliusu A (1), an optical rotation value of [α]23 D = + 67.0 (c 0.19, MeOH), was obtained as cubic crystals (CH3CN-CH3OH) with mp 169 ∼ 171℃. The molecular formula C30H48O4 was established by [M + Na]+ ion at m/z 495.3450 (calcd. for C30H48O4Na, 495.3450) in HR-ESI-MS (Fig. S6). Its IR spectrum (Fig. S8) revealed characteristic absorption peaks for hydroxyl (3369 cm−1), methyl (2950, 2930, 1457, 1387 cm−1), double bond (1644 cm−1), and carbonyl groups (1728 cm−1). The 1H NMR data (Table 1) exhibited resonances for four olefinic proton singlets at δH 5.67 (ddd, J = 15.6, 7.5, 6.2 Hz, H-23), 5.62 (d, J = 15.6 Hz, H-24), 4.86 (br s, H-28a), and 4.74 (br s, H-28b); one oxygenated methine at δH 4.78 (q-like, J = 8.8 Hz, H-11); as well as seven methyl singlets at δH 1.75 (s, H3-29), 1.26 (s, H3-27), 1.27 (s, H3-26), 1.20 (s, H3-19), 1.14 (s, H3-21), 1.00 (s, H3-18), and 0.97 (s, H3-30). According to the 13C NMR (Table 2), DEPT-135°, and HSQC spectra (Fig. S12), 30 carbon signals including one carbonyl at δC 179.6 (C-3); four olefinic carbons at δC 148.3 (C-4), 142.2 (C-24), 123.7 (C-23), and 114.6 (C-28); one oxygenated sp3 methine at δC 78.5 (C-11); five sp3 quaternary carbons at δC 75.7 (C-20), 71.2 (C-25), 51.0 (C-14), 41.8 (C-8), and 40.7 (C-10); seven methyls at δC 29.9 (C-26), 30.0 (C-27), 26.1 (C-21), 23.9 (C-29), 19.3 (C-19), 17.4 (C-18), and 16.1 (C-30); as well as eight sp3 methylenes at δC 45.2 (C-22), 40.6 (C-1), 37.0 (C-12), 34.7 (C-7), 31.6 (C-15), 30.5 (C-2), 26.3 (C-6), and 25.8 (C-16) were determined. All the above data indicated that 1 possessed a 3,4-seco-dammarane triterpenoid skeleton.
The 1H–1H COSY correlations (Fig. 2) of 1 demonstrated the presence of four spin-coupling systems (C-1 to C-2, C-5 to C-7, C-9 to C-15, C-22 to C-24). The HMBC spectrum (Fig. S13) showed the correlations from δH 4.78 (H-11) to δC 179.6 (C-3)/40.7 (C-10), δH 2.81 (H-2a)/2.30 (H-2b) to δC 40.7 (C-10), as well as δH 1.78 (H-1a)/1.33 (H-1b) to δC 179.6 (C-3), suggesting the existence of a 3,11-heptacyclic lactone unit, which was supported by the down-field shift of the resonance with δH 4.78 (q-like, J = 8.8 Hz, H-11). The correlations from δH 5.67 (H-23) to δC 75.7 (C-20)/71.2 (C-25), δH 1.26 (H-26)/1.27 (H-27) to δC 142.2 (C-24), δH 1.14 (H-21) to δC 51.9 (C-17)/45.2 (C-22) and δH 1.89 (H-13)/1.80 (H-16a) to δC 75.7 (C-20) showed that an eight-carbon side chain containing a double bond was attached to C-17. The configuration of the Δ23 double bond was E geometry due to the large coupling constant (3JH-23/H-24 = 15.6 Hz). In addition, the correlations of δH 1.75 (H-29) to δC 114.6 (C-28)/58.7 (C-5), δH 4.86 (H-28a)/4.74 (H-28b) to δC 58.7 (C-5), δH 1.00 (H3-18) to δC 34.7 (C-7)/53.5 (C-9) /51.0 (C-14), δH 1.00 (H3-30) to δC 41.8 (C-8)/39.9 (C-13) /31.6 (C-15), implied the planar structure of 1 (Fig. 2). The relative configuration of 1 were determined by ROESY spectrum (Fig. S14), in which the correlations of δH 1.87 (H-5)/1.84 (H-9)/1.79 (H-17)/0.97 (H3-30) revealed that H-5, H-9, H-17, and H3-30 were cofacial and randomly allocated to be α-oriented. Consequently, the β-orientations of H-11, H-13, H3-18, and H3-19 were defined by the ROESY cross-peaks of δH 4.78 (H-11)/1.89 (H-13)/1.00 (H3-18)/1.20 (H3-19). Furthermore, a single crystal X-ray diffraction analysis was thus successfully performed to expressly assign the stereochemistry of 1 (Fig. 3). The Flack parameter of −0.3 (2) could only allow the relative configuration of 1. However, by analyzing several 3,4-seco-dammarane analogues (Aoki et al., 1988; Aoki et al., 1990; Chen et al., 2018; Phan et al., 2011; Takayuki and Toshifumi, 1979), as well as X-ray data (Fig. S4) of known analogue cyclocariol E (18) with a small Flack parameter of 0.05(6), it was found that the skeleton configurations of these compounds were stable. Therefore, based on the above evidence, the absolute structure of 1 was established as 23(E),11R,20S-11,20,25-trihydroxy-3,4-seco- dammara-4(28),23-dien-3 oic acid-3,11-heptacyclic lactone.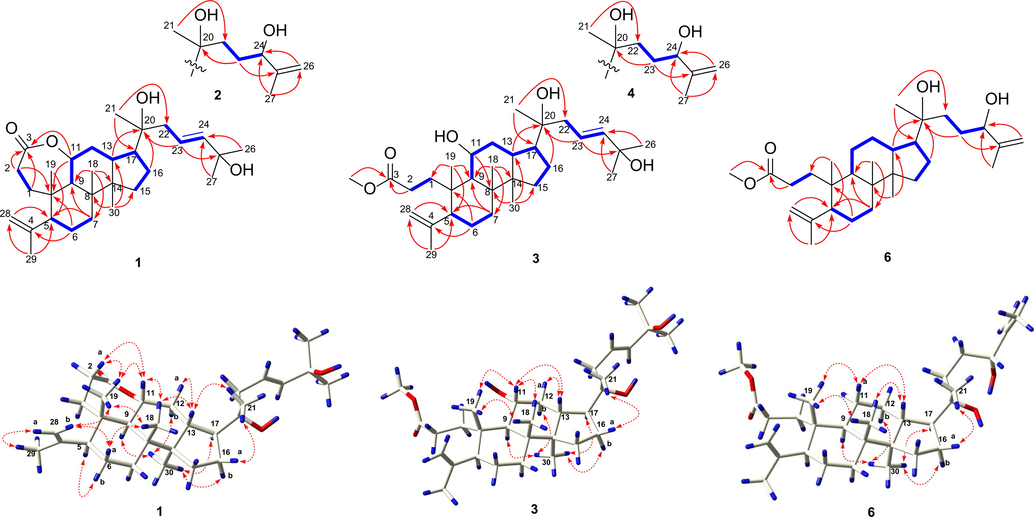
Key 1H–1H COSY (blue thick bonds) and HMBC (red solid arrows) correlations of compounds 1–4, and 6, together with NOESY (red dash arrows) correlations of compounds 1, 3, and 6.
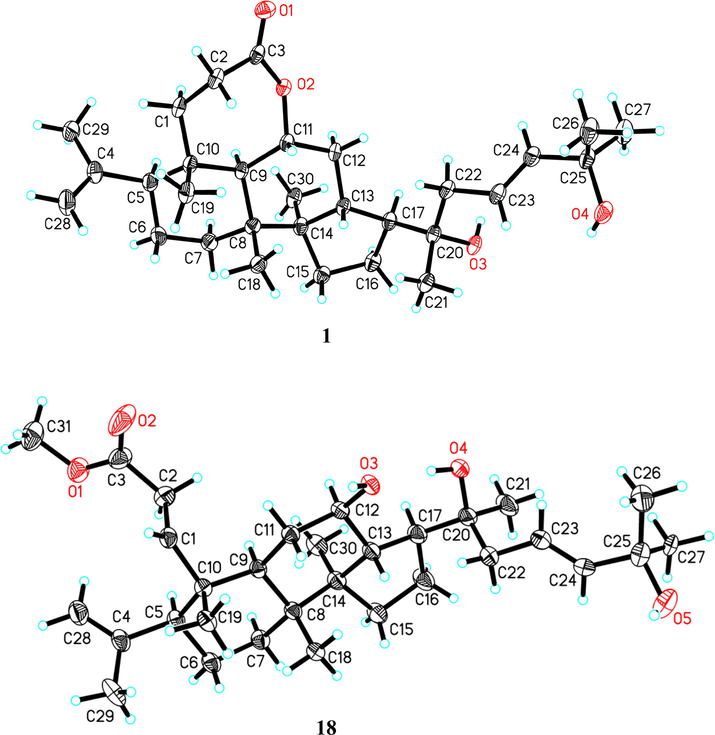
Crystal structures of compounds 1, and 18.
Qingqianliusu B (2) was obtained as a colorless amorphous powder with [α]23 D = + 76.0 (c 0.25, MeOH). The molecular formula, C30H48O4, was based on its sodium adduct molecular ion [M + Na]+ at m/z 495.3451 (calcd. for C30H48O4Na, 495.3450) in HR-ESI-MS spectrum (Fig. S15). Comparison of the NMR data (Tables 1 and 2) of 2 with compound 1 suggested that they share a similar skeleton with the same 3,11-heptacyclic lactone unit. The only difference between 1 and 2 were at the eight-carbon side chain. Terminal double bond with chemical shift at δH 4.91 (H-26a)/4.81 (H-26b), δC 148.8 (C-25) and 111.6 (C-26) in 2 replaced E-type disubstituted double bond in 1. Compound 2 also included one quaternary oxygen-bearing carbon at δC 75.4 (C-20), together with one oxygenated methine at δC 77.4 (C-24). The key HMBC correlations (Fig. S22) from δH 4.91 (H-26a)/4.81 (H-26b)/1.72 (H3-27) to δC 77.4 (C-24), δH 1.65 (H-23a)/1.57 (H-23b) to δC 148.8 (C-25)/75.4 (C-20), and δH 1.14 (H-21) to δC 52.4 (C-17)/37.9 (C-22) revealed eight carbon side chain structure of 2. Notably, the absolute configuration of chiral center (C-24) on the branch chain was determined by summarizing NMR data characteristics of epimers with R/S configuration at C-24 and single crystal data of corresponding compound cyclocariol B (16) (Chen et al., 2018). Please see “3.3. Determination of the Absolute Configurations of Epimers” of this article for more details. Thus, the structure of 2 was assigned as 23(E),11R,20S,24S-11,20,24-trihydroxy-3,4-seco-dammara-4(28),25-dien-3 oic acid-3,11-heptacyclic lactone.
Qingqianliusu C (3), a colorless powder with [α]23 D = + 38.5 (c 0.29, MeOH), had a molecular formula C31H52O5 as determined by HR-ESI-MS on its sodium adduct molecular ion [M + Na]+ at m/z 527.3712 (calcd. for C31H52O5Na, 527.3712) (Fig. S24). In the 13C NMR spectrum (Fig. S28), singlets were exhibited for four oxygenated carbon singlets at δ 75.9 (C-20), 71.6 (C-11), 71.2 (C-25), and 51.9 (3-COOCH3). The 1H and 13C NMR data (Tables 1 and 2) of 3 were analogous to those of cyclocariol E (18) (Chen et al., 2018), illustrating that 3 had similar 3,4-seco-dammarane skeleton with cyclocariol E. Moreover, in the HMBC spectrum (Fig. 2 and Fig. S31), key correlations from δH 3.63 (s, 3-COOCH3) to δC 177.3 (C-3) showed that a methoxy group was connected to the carbonyl. The correlations from δH 3.92 (td, J = 10.8, 5.0 Hz, H-11) to δC 41.7 (C-8) and 40.6 (C-10) verified the hydroxyl was located at C-11 in 3, while one hydroxyl located at C-12 in cyclocariol E (18). The α-orientations of H-5, H-9, H-17, and H3-30 were further to confirm with the ROESY correlations (Fig. 2 and Fig. S32) of δH 2.07 (H-5)/1.73 (H-9)/1.79 (H3-17)/0.97 (H3-30). Then, key correlations of δH 3.92 (H-11)/1.87 (H-13) /1.00 (H3-18)/1.20 (H3-19) suggested that H-11, H-13, H3-18, and H3-19 are β-oriented. Thus, the configuration of C-11 in 3 was proved as S by comparing with the structure and ROESY correlation (Fig. 2) of 1. Accordingly, the compound 3 was determined as 23(E),11R,20S-11,20,25-trihydroxy-3,4-seco-dammara-4(28),23-dien-3-oic acid methyl ester.
Qingqianliusu D (4), colorless amorphous powder with [α]23 D = + 36.0 (c 0.25, MeOH). The HR-ESI-MS spectrum (Fig. S33) of 4 showed a quasi-molecular ion peak at m/z [M + Na]+ at m/z 527.3719 (calcd. for C31H52O5Na, 527.3712), which illustrated a molecular formula C31H52O5. The 1H and 13C NMR data (Tables 1 and 2) of 4 were closely identical to 3 except for the side chain at C-17. A pair of terminal disubstitutd double bond carbon singlets at δC 148.8 (C-25) and δC 111.6 (C-26), one quaternary oxygen-bearing carbon at δC 75.6 (C-20), together with one oxygenated methine at δC 77.4 (C-24) suggested that compound 4 and 2 have the same side chain connected at C-17 (Table 2). Consequently, compound 4 was deduced as 11R,20S,24S-trihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid methyl ester.
Qingqianliusu E (5) was isolated as a white amorphous powder with [α]23 D = + 39.4 (c 0.33, MeOH), which has the same molecular formula of C31H52O5 as 4, as evidenced by the 13C spectrum (Fig. S46) and its sodium adduct molecular ion [M + Na]+ at m/z 527.3717 (calcd. for C31H52O5Na, 527.3712) in the HR-ESI-MS spectrum (Fig. S42). The 1H and 13C NMR (Tables 1 and 2) of 5 showed almost identical with 4 except for a slight difference in the chemical shifts [ΔδC = δC (5) - δC (4)] at C-25 (ΔδC + 0.35), C-26 (ΔδC −0.30), and C-27 (ΔδC + 0.31), which could be attributed to the difference configuration of the hydroxyl group (−OH) at C-24. Therefore, compound 5 was found to be epimer of 4 at C-24 according to spectroscopic data and characterized as 11R,20S,24R-trihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid methyl ester.
Qingqianliusu F (6) was also obtained as white amorphous powder with [α]23 D = + 39.9 (c 0.28, MeOH). The molecular formula C31H52O4 was confirmed by the HR-ESI-MS m/z 511.3764 [M + Na]+ (calcd. for C31H52O4Na, 511.3763) (Fig. S51) and 13C NMR data (Table 2). Comprehensive analysis of the NMR data (Tables 1 and 2) of 5 and 6 revealed that compound 6 has one more methylene (δH 1.44/1.36, δC 23) while one less oxymethine (δH 3.91, δC 71.6). Moreover, the diagnostic 1H–1H COSY spectrum (Fig. S56) of δH 1.60 (m, H-9) and δH 1.44/1.36 (m, H2-11) indicated that there was no hydroxyl group substitutied at C-11. Therefore, the structure of 6 was determined as 20S,24R-dihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid methyl ester.
Qingqianliusu G (7) was separated as white amorphous powder with [α]23 D = + 36.0 (c 0.22, MeOH), which had a molecular formula of C30H50O4 determined by its quasi-molecular ion peak [M + Na]+ at m/z 497.3589 (calcd. for C30H50O4Na, 497.3607) in the HR-ESI-MS (Fig. S60), 14 mass units less than those of 6. The 1H and 13C NMR spectral data (Tables 1 and 2) of 7 were also very similar to those of 6, except for the disappearance of one methoxyl group. As a result, compound 7 was deduced as 20S,24R-dihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid.
Qingqianliusu H (8), a white amorphous powder with [α]23 D = + 14.3 (c 0.21, MeOH), had the molecular formula C30H48O5 by the 13C NMR data (Fig. S73) and quasi-molecular ion peak [M + Na]+ at m/z 511.3382 (calcd. for C30H48O5Na, 511.3399) in the HR-ESI-MS (Fig. S69). The IR absorption (Fig. S71) at 3432, 2868, 1708 and 1692 cm−1 represented the presence of hydroxyl (—OH), aldehyde (—CHO) and carbonyl groups (—COOH), respectively. Analysis of the 1D and 2D NMR data (Tables 1 and 2) showed 8 was the high similarity with those of the know compound 20S,24S-dihydroxy-3,4-seco-dammara-4(28),25-dien, 3-oic acid (15). The only slight difference in the group observed was the methyl group (—CH3) in 15 replaced by the aldehyde group (—CHO) in 8 at C-4. The correlations from δH 6.42 (H-28a)/6.22 (H-28b) to δC 196.6 (C-29)/40.2 (C-5) in the HMBC spectrum (Fig. S1 and Fig. S76) proved the linkage of aldehyde group located at C-4. These data generated the assignment of 8 as 20S,24S-dihydroxy-3,4-seco-dammara-4(28),25-dien,4-aldehyde group,3-oic acid.
Qingqianliusu I (9) was isolated as a colorless amorphous powder with [α]23 D = + 33.2 (c 0.21, MeOH). The molecular formula of 9 was determined to be C32H54O5 by 13C NMR spectrum (Fig. S82) and positive-ion peak [M + Na]+ at m/z 541.3869 (calcd. for C32H54O5Na, 541.3868) in the HR-ESI-MS (Fig. S78). The 1D and 2D NMR data (Tables 1 and 2) were nearly identical with cyclocariol E (18) (Chen et al., 2018), except for the replacement of the methoxy moiety (—OCH3) by ethoxy unit (—OCH2CH3) at C-3 in 9. Furthermore, the crucial 1H–1H COSY (Fig. S83) correlation of 9 illustrated the presence of ethyl fragment δC 4.11 (H-3-COOCH2CH3) and 1.24 (H-3-COOCH2CH3). In its key HMBC correlation (Fig. S85) from δH 4.11 (H-3-COOCH2CH3) to δC 175.8 (C-3) suggested that ester ethyl unit (—OCH2CH3) at C-3. Therefore, the structure of 9 was determined as 23(E),12R,20S-11,20,25-trihydroxy-3,4-seco-dammara-4(28),23-dien-3-oic acid ethyl ester.
Qingqianliusu J (10), a white amorphous powder with [α]23 D = + 7.2 (c 0.29, MeOH), was assigned as molecular formula of C39H64O10 determined on the basis of a positive-ion peak [M + Na]+ m/z 715.4400 (calcd. for C39H64O10Na, 715.4397) in the HR-ESI-MS (Fig. S87). A detailed analysis of 1D NMR data (Table 1 and Table 2), compound 10 were almost similar to 9 except for the additional glycosyl derivatives unit, which was proven by presence seven carbon singlets including one carbonyl signal at δH 172.6 (C-5′–OCOCH3); four oxygenated methines at δH 103.5 (C-1′), 83.7 (C-2′), 82.1 (C-4′), and 77.9 (C-3′); one oxygenated methylene at 65.2 (C-5′); and one methyl signal at δH 20.7 (C-5′–OCOCH3). The glycosyl unit can be identified as α-arabinose by comparing the 13C NMR data with known compound cyclocarioside O (Liu et al., 2020). In addition, an acetoxy group (—OCOCH3) attached to the C-5′ of α-arabinose based on the HMBC correlations (Fig. S1 and Fig. S94) of δH 4.26 (H-5′a)/4.13 (H-5′b) with δC 172.6 (C-5′–OCOCH3). Then, the key HMBC correlations from anomeric hydrogen δH 5.27 (H-1′) with δC 83.8 (C-20) determined the position of glycosyl unit was located at C-20. Besides, the sugar was recognized with acid hydrolysis and HPLC analysis (Fig. S5) of l-arabinose standard. Therefore, the structure of 10 was deduced as 23(E),12R,20S-12,20,25-trihydroxy-3,4-seco-dammara-4(28),23-dien,3-oic acid ethyl ester-20-O-α-L-(5′-O-acetyl)-arabinofuranoside.
Qingqianliusu K (11) was deduced as a white amorphous powder with [α]23 D = + 12.0 (c 0.25, MeOH). The molecular formula of 11, C41H68O10, yielded a positive sodium ion HR-ESI-MS peak (Fig. S96) at m/z 743.4718 [M + Na]+ (calcd. for C41H68O10Na, 743.4710), which is 28 Da more than that of 10. Detailed comparison of the 1H and 13C NMR data (Tables 1 and 2) showed that 11 was almost similar to 10, except for the hydroxyl group (–OH) at C-25 was replaced by the ethoxy (–OCH2CH3), which was proven by the key HMBC correlations (Fig. S103) from δH 3.39 (H-25-OCH2CH3) to δC 76.3 (C-25). In addition, the glycosyl unit was assigned as α-l-arabinofuranoside based on acid hydrolysis and HPLC analysis (Fig. S5). Therefore, compound 11 was a characteristic 3,4-seco-dammarane triterpenoid glycoside named as 23(E),12R,20S-12,20,25-trihydroxy-3,4-seco-dammara-4(28),23-dien,3-oic acid ethyl ester-25-O-ethyl,20-O-α-L-(5′-O-acetyl)-arabinofuranoside.
Qingqianliusu L (12) was obtained as a white amorphous powder and had a molecular formula of C32H54O5, which was established by HR-ESI-MS (Fig. S105) from a positive sodium ion at m/z 541.3863 [M + Na]+ (calcd. for C32H54O5Na, 541.3869). Comparing the 1H and 13C NMR data (Tables 1 and 2) of 12 and cyclocariol B (16)(Chen et al., 2018), which demonstrated a considerable degree of similarity except for the replacement of the ester methoxy moiety (—OCH3) by ethoxy unit (—OCH2CH3) at C-3 in 12. Accordingly, the structure of 12 was deduced as 12R,20S,24S-trihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid ethyl ester.
Qingqianliusu M (13), a white amorphous powder with [α]23 D = + 40.8 (c 0.59, MeOH), had a molecular formula of C32H54O5, the same as that 12, as determined by the [M + Na]+ ion peaks at m/z 517.3859 (calcd. for C32H54O5Na, 541.3869) in the HR-ESI-MS (Fig. S114). Detailed analysis of 1D NMR spectroscopic (Tables 1 and 2), compound 13 contained exactly a 3,4-seco-dammarane triterpenoids skeleton as 12, except for a slight difference in the chemical shifts [ΔδC = δC (13) - δC (12)] at C-25 (ΔδC + 0.39), C-26 (ΔδC −0.34), and C-27 (ΔδC + 0.27), which suggested that 13 was a epimer at C-24 of 12. Therefore, compound 13 was elucidated as 12R,20S,24R-trihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid ethyl ester.
Qingqianliusu N (14) was obtained as a white amorphous powder with [α]23 D = + 7.2 (c 0.28, MeOH). It provided the molecular formula C39H64O10, which was determined by HRESI-MS ion [M + Na]+ at m/z 715.4382 (calcd. for C39H64O10Na, 715.4397) (Fig. S123). The 1D NMR data (Tables 1 and 2) of 14 were highly analogous to those of 13 except for the additional glycosyl derivatives unit, which indicated that 14 also possesses a 3,4-seco-dammarane triterpenoids skeleton. The glycosyl unit of 14 was identified as α-(5′-O-acetyl)-arabinofuranoside by comparing the 13C data (Table 2) of compounds 10 and 11. Moreover, the acid hydrolysis results were also in agreement with the presence of l-arabinofuranoside standard (Fig. S5). Thus, 14 was identified as 12R,20S,24R-trihydroxy-3,4-seco-dammara-4(28),25-dien,3-oic acid methyl ester-20-O-α-L-(5′-O-acetyl)-arabinofuranoside.
3.2 Determination of the absolute configurations of isomers
To further clarify the absolute configuration of the hydroxyl group (—OH) substituted at the C-24, according to the 13C NMR date (Table 2) of C-25 to C-27 in the four pairs of epimers 24R/24S (17/16, 13/12, 7/15, and 5/4) were compared and analyzed. One pair of known isomers cyclocariol B (16) and cyclocariol A (17) has been isolated by our group. The X-ray crystallographic analysis of 17 demonstrated that the absolute configuration at C-24 was R. As shown in Table 3 and Fig. 4, the rules demonstrated that the slight variations between the epimers were the chemical shifts (24R-24S) at C-25 (ΔδC + 0.20 to + 0.41), C-26 (ΔδC −0.34 to −0.30), and C-27 (ΔδC + 0.25 to + 0.32). Apparently, it could be basically inferred that compounds 5, 7, and 13 were found to be R configuration at C-24, while its corresponding epimers 4, 15, and 12 were in the S configuration.
No.
Epimer (1)
Epimer (2)
Epimer (3)
Epimer (4)
17 (24R)
16 (24S)
ΔδC
(24R-24S)
13 (24R)
12 (24S)
ΔδC
(24R-24S)
7 (24R)
15 (24S)
ΔδC
(24R-24S)
5 (24R)
4 (24S)
ΔδC
(24R-24S)
25
149.26
148.85
0.41
149.24
148.85
0.39
148.99
148.79
0.20
149.14
148.79
0.35
26
111.10
111.44
−0.34
111.10
111.44
−0.34
111.27
111.61
−0.34
111.30
111.60
−0.30
27
17.92
17.67
0.25
17.94
17.67
0.27
17.81
17.49
0.32
17.83
17.52
0.31
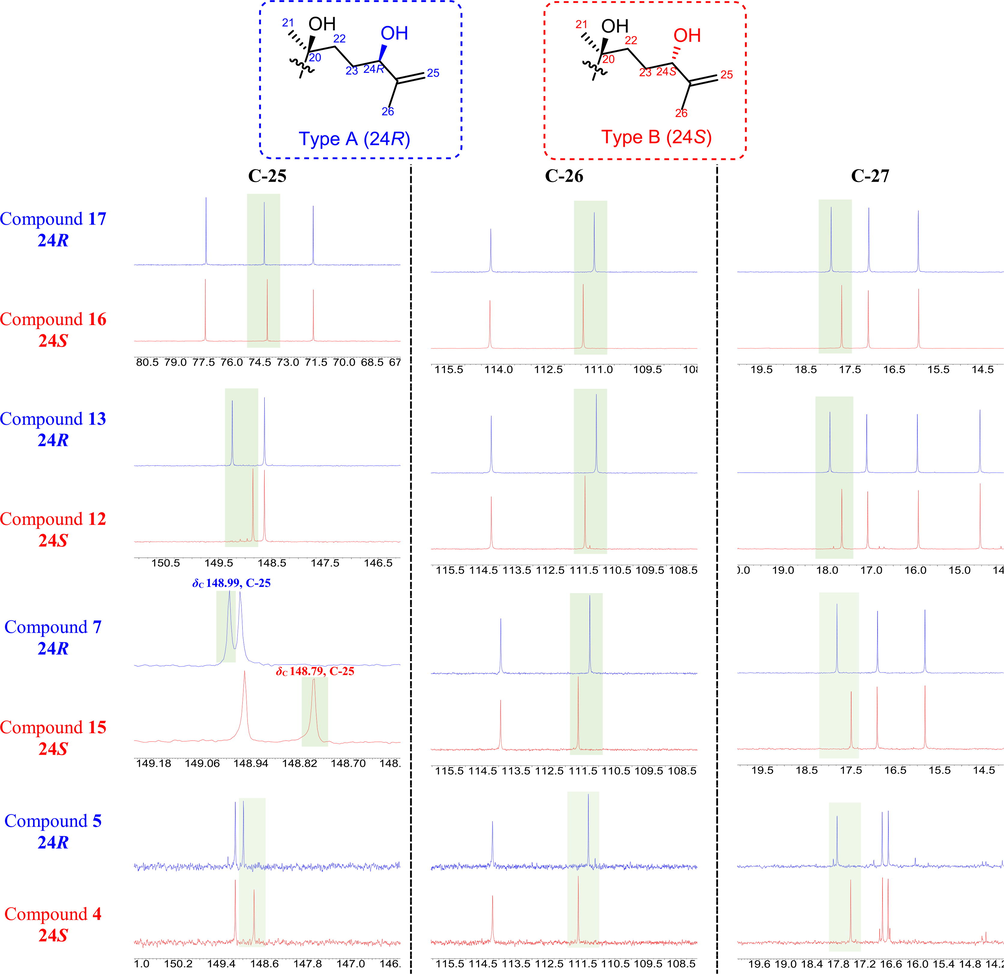
Comparison of key 13C NMR data differences for the isomers 24R (represented in blue) and 24S (represented in red) (CD3OD, 150 MHz).
3.3 α-Glucosidase inhibitory activity assay
Diabetes mellitus is a complex chronic metabolic disorder with an increasing prevalence every year (Xiao et al., 2017; Zhu et al., 2015). The inhibitors of α-glucosidase had become one of the clinically effective drugs for the prevention and treatment of diabetes (Chen et al., 2021; Li et al., 2012). On this basis, in order to further explore the potential hypoglycemic effect of the active components in Qingqianliu, α-glucosidase inhibitory activity was tested on fourteen 3,4-seco-dammarane triterpenoids (1–14). The results (Table 4) showed that only three compounds 8, 11, and 14 exhibited strong α-glucosidase inhibitory activities with the IC50 ranges from 3.76 ± 0.77 to 7.08 ± 0.53 μM, while compound 10 showed weak activity with IC50 value of 19.62 ± 0.70 μM, as compared with positive control acarbose (IC50 1.07 ± 0.31 μM). The structure–activity relationship analysis indicated that the presences of the aldehyde group at C-4 and arabinose unit at C-20 could improve the α-glucosidase inhibitory activity.
compoundsa
IC 50 (μM)b
8
4.97 ± 0.63
10
19.62 ± 0.70
11
7.08 ± 0.53
14
3.76 ± 0.77
Acarbosec
1.07 ± 0.31
3.4 Anti-inflammatory activity against COX-2
COX-2, known as Prostaglandin-endoperoxide synthase (PTGS), keeps closely related to the occurrence of inflammation and tumors (Jiang et al., 2022). The inhibitors of COX-2, which become a hot spot in drug research, could prevented inflammation and the formation of malignant tumors by blocking prostaglandin synthesis (Liu et al., 2020; Shataer et al., 2021). With celecoxib as positive control, the COX-2 inhibitory activity of compounds (1–14) were evaluated. The results were demonstrated in the Table 5. Only six compounds 1, 2, 4, 6, 10, and 11 showed COX-2 inhibitory activity with inhibition rates from 10.23 % to 35.83 % at the concentration of 25 µM. Among them, compound 11 had the highest inhibition rate and reached half of the inhibition rate of celecoxib. The results indicated that an additional sugar moiety at C-20 plays an important role in COX-2 inhibitory activity.
Compoundsa
Sample concentration (µM)
Inhibition rate (%)
1
25
12.11
2
25
15.73
4
25
10.23
6
25
20.62
10
25
17.44
11
25
35.83
Celecoxibb
1
70.28
3.5 Cytotoxic bioassays
All unreported 3,4-seco-dammarane triterpenoids (1–14) were evaluated for their cytotoxicities against for human cancer cell lines (BGC-823, MCF-7, HCT-116, and HepG-2). As shown in Table 6, compounds 3, 7, 10, and 13 displayed moderate activities against BGC-823 with IC50 values from 7.69 ± 0.21 to 9.04 ± 0.61. Moreover, compound 13 showed modest activity against MCF-7 and HCT-116 with IC50 values of 9.19 ± 0.78 and 8.80 ± 0.36. respectively. Structurally, compounds 13 and 12 as a pair of isomers, which 13 exhibited stronger activities than 12. It can be inferred that the hydroxyl group at the C-24 was R configuration in the same type of 3,4-seco-dammarane triterpenoids skeleton possess potential cytotoxicity effect.
Compoundsb
Cell lines (IC50 values, μMa)
BGC-823
MCF-7
HCT-116
HepG-2
3
7.69 ± 0.21
>20
9.45 ± 0.93
>20
7
8.47 ± 0.41
>20
>20
>20
10
9.04 ± 0.61
>20
>20
>20
12
>20
>20
11.24 ± 0.22
>20
13
8.86 ± 0.38
9.19 ± 0.78
8.80 ± 0.36
13.41 ± 0.90
Paclitaxelc (nM)
8.93 ± 0.75
11.53 ± 0.26
14.71 ± 0.85
13.27 ± 1.18
4 Conclusion
In conclusion, as a new raw food material with the edible and medicinal values, the investigations of chemical constituents with bioactive effects in Qingqianliu keeps urgently needed. In this study, fourteen new 3,4-seco-dammarane triterpenoids (1–14) and four known 3,4-seco-dammarane triterpenoids (15–18) were isolated and identified. Notably, two 3,4-seco-dammarane triterpenoids (1 and 2) with the rare structural features of heptacyclic lactone linked by oxygen between C-3 and C-11 in parent were obtained. Furthermore, three compounds (10, 11, and 14) with their sugar units were attached to the C-20 while most at C-11 and C-12 were also found. Four compounds (6–8 and 15) were rare 3,4-seco-dammarane triterpenoids without hydroxyl at position C-11 and C-12. In addition, the NMR data rules of determining the absolute configuration of four pairs of epimers at C-24 were discovered and summarized for the first time.
All undescribed compounds were calculated for anti-hypoglycemic, anti-inflammatory, and cytotoxic activities in vitro. Compounds 8, 11, and 14 exhibited good α-glucosidase inhibitory activity compared with the positive control acarbose. Compound 10 showed a weak inhibitory effect on α-glucosidase and COX-2, together with strong inhibitory activity on BGC-823, respectively. These studies provided a preliminary biological activity basis for the anti-hyperglycemia, anti-inflammatory, and cytoxicity latent impacts of Qingqianliu, and furnished references for its development and utilization as functional foods or medicines.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 82174078, 81803708, 81874369, and 82074122); Changjiang Scholars Program in Ministry Education, People’s Republic of China (T2019133); and Hunan Administration of Tradition Chinese Medicine (Xiangcai2021-105).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Six novel secodammarane-type triterpenes from male Flowers of Alnus japonica. Phytochemistry. 1988;27(9):2915-2920.
- [Google Scholar]
- Triterpenoids, diarylheptanoids and their glycosides in the flowers of Alnus species. Phytochemistry. 1990;29(11):3611-3614.
- [Google Scholar]
- Itosides J-N from Itoa orientalis and structure - anti-COX-2 activity relationship of phenolic glycosides. J. Nat. Prod.. 2008;71(5):814-819.
- [Google Scholar]
- Confining copper nanoclusters on exfoliation-free 2D boehmite nanosheets: fabrication of ultra-sensitive sensing platform for α-glucosidase activity monitoring and natural anti-diabetes drug screening. Biosens. Bioelectron.. 2021;182:113198.
- [CrossRef] [Google Scholar]
- Cyclocarya paliurus (Batalin) Iljinskaja: Botany, Ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol.. 2022;285:114912
- [Google Scholar]
- Seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus. Phytochemistry. 2018;145:85-92.
- [Google Scholar]
- Chemical constituents from leaves of Cyclocarya paliurus. Chin. Tradit. Herb. Drug. 2012;43(11):2132-2136.
- [Google Scholar]
- Bioassay Guided Fractionation Identified Hederagenin as a Major Cytotoxic Agent from Cyclocarya paliurus Leaves. Planta Med.. 2016;82(1–2):171-179.
- [Google Scholar]
- PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res.. 2022;10(1):27.
- [Google Scholar]
- Novel Highly Sweet Secodammarane Glycosides from Pterocarya paliurus. J. Agric. Food Chem.. 1995;43(10):2602-2607.
- [Google Scholar]
- New Triterpenoids from the Leaves of Cyclocarya paliurus. Planta Med.. 2012;78(03):290-296.
- [Google Scholar]
- α-Glucosidase inhibitory and anti-inflammatory activities of dammarane triterpenoids from the leaves of Cyclocarya paliurus. Bioorg. Chem.. 2021;111:104847
- [Google Scholar]
- 3,4-seco-Dammarane Triterpenoid Saponins with Anti-Inflammatory Activity Isolated from the Leaves of Cyclocarya paliurus. J. Agric. Food Chem.. 2020;68(7):2041-2053.
- [Google Scholar]
- Identification of alpha-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct.. 2019;10(4):1893-1902.
- [Google Scholar]
- Cyclopalitins A and B, nortriterpenoids from aerial parts of Cyclocarya paliurus. Phytochem. Lett.. 2019;31:114-117.
- [Google Scholar]
- Metabolite profiling and in vitro biological activities of two commercial bitter melon (Momordica charantia Linn.) cultivars. Food Chem.. 2019;288:178-186.
- [CrossRef] [Google Scholar]
- Three new dammarane glycosides from Betula alnoides. Phytochem. Lett.. 2011;4(2):179-182.
- [Google Scholar]
- Chemical Composition of the Hazelnut Kernel (Corylus avellana L.) and Its Anti-inflammatory, Antimicrobial, and Antioxidant Activities. J. Agric. Food Chem.. 2021;69(14):4111-4119.
- [Google Scholar]
- Flavonoid glycosides from the rhizomes of Pronephrium penangianum. Phytochemistry. 2020;179:112500.
- [CrossRef] [Google Scholar]
- Synthesis of dammarenolic acid derivatives with a potent α-glucosidase inhibitory activity. Med. Chem. Res.. 2020;29(4):1-9.
- [Google Scholar]
- Hypoglycemic triterpenoid glycosides from Cyclocarya paliurus (Sweet Tea Tree) Bioorg. Chem.. 2020;95:103493.
- [CrossRef] [Google Scholar]
- Cytotoxic triterpenoid glycosides from leaves of Cyclocarya paliurus. Nat. Prod. Res.. 2020;35(21):4018-4024.
- [Google Scholar]
- Two new triterpenoid glycosides from leaves of Cyclocarya paliurus. Nat. Prod. Res. 2021:1-6.
- [Google Scholar]
- New C31 Secodammarane-type Triterpenoids, Alnuseric Acid and Alniselide, in the Male Flowers of Alnus serrulatoides. Bull. Chem. Soc. Jpn.. 1979;52(4):1153-1155.
- [Google Scholar]
- Antihyperlipidaemic effect of triterpenic acid-enriched fraction from Cyclocarya paliurus leaves in hyperlipidaemic rats. Pharm. Biol.. 2017;55(1):712-721.
- [Google Scholar]
- Cyclocarya paliurus tea leaves enhances pancreatic beta cell preservation through inhibition of apoptosis. Sci. Rep.. 2017;7(1):9155.
- [Google Scholar]
- Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem.. 2010;119(4):1626-1632.
- [Google Scholar]
- Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem.. 2015;186:97-105.
- [Google Scholar]
- Protective effect of flavonoids from Cyclocarya paliurus leaves against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol.. 2018;119:392-399.
- [Google Scholar]
- Cytotoxic dammarane-type triterpenoids from the stem bark of Dysoxylum binecteriferum. J. Nat. Prod.. 2014;77(2):234-242.
- [Google Scholar]
- Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohyd. Polym.. 2018;183:11-20.
- [Google Scholar]
- Studies on the sweet principles from the leaves of Cyclocarya paliurus(Batal.) Iljinsk. Acta Pharm. Sin.. 1992;27(11):841-844.
- [Google Scholar]
- Bioactive dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. Phytochemistry. 2021;183:112618
- [Google Scholar]
- Two triterpeniods from Cyclocarya paliurus (Batal) Iljinsk (Juglandaceae) promote glucose uptake in 3T3-L1 adipocytes: The relationship to AMPK activation. Phytomedicine. 2015;22(9):837-846.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104441.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







