Translate this page into:
Novel 1,3,4-oxadiazole thioether and sulfone derivatives bearing a flexible N-heterocyclic moiety: Synthesis, characterization, and anti-microorganism activity
⁎Corresponding authors. zhoux1534@163.com (Xiang Zhou), jhzx.msm@gmail.com (Song Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
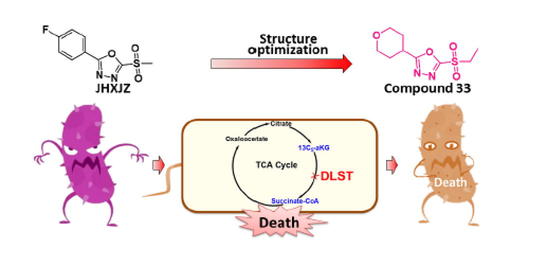
Abstract
In the search for more efficient and versatile anti-phytopathogen agents, a series of new 1,3,4-oxadiazole thioether/sulfone analogues bearing a flexible N-containing heterocyclic pattern were elaborately prepared, and their bioactivities against plant pathogenic microorganisms were systematically evaluated. Bioassay screening results demonstrated that compounds 32 and 33 significantly inhibited the growth of Xanthomonas oryzae pv. oryzae (Xoo) in vitro (32, EC50 = 5.17 mg L−1; 33, EC50 = 1.19 mg L−1), which were significantly surpass commercial bismerthiazol (BT) and thiodiazole copper (TC). Meanwhile, pot experiments confirmed the prospective applications of compound 33 in managing rice bacterial leaf blight and its good safety toward rice plants. Further studies showed that compound 33 interfered with the formation of bacterial biofilms and inhibited bacterial virulence factors. Furthermore, an in vitro antifungal bioassay showed that compound 32 possessed remarkable growth inhibitory activity against Sclerotinia sclerotiorum (S.s., EC50 = 22.16 mg L−1) and Verticillium dahlia (V.d. EC50 = 32.78 mg L−1). These results all confirmed that the designed 1,3,4-oxadiazole compounds displayed potential for managing plant microbial diseases through targeting dihydrolipoamide S-succinyltransferase (DLST).
Keywords
1, 3, 4-oxadiazole derivatives
Thioether derivatives
Sulfone derivatives
Antibacterial activity
1 Introduction
Plant diseases have attracted global attention owing to the substantial economic losses caused in agriculture (Bauske et al., 2018; Guo et al., 2021). According to statistics, about 16 %–20 % of economic crop losses globally are due to preharvest plant disease (Savary et al., 2019). Notably, rice bacterial leaf blight is an intractable plant bacterial disease that caused by Xanthomonas oryzae pv. oryzae (Xoo) and leading to reducing yields in rice-growing countries as high as 10 %–50 % per year (Lu et al., 2014). Therefore, improving the grain yield and quality has become a critical issue for agriculture worldwide. Disease prevention is an important measure for ensuring steady increases in food production, with the use of agrochemicals currently being the most important, low-cost, and effective method. However, some problems, such as environmental pollution and drug resistance have inevitably developed with the long-term use and abuse of agrochemicals, seriously restricting the production and development of modern agriculture (Fisher et al., 2018, Gould et al., 2018, Velema et al., 2013). The use/overuse of commercially bismerthiazol has already leading to the occurrence of bismerthiazol-resistant Xoo strain. Therefore, rising crisis of bactericide resistance urges the excavation of agrochemicals with novel action modes.
Nitrogen-containing heterocyclic compounds are considered potential sources of natural products for the development of new drugs (Benedik, 1998). Examples, such as oxadiazoles, triazoles, pyridines, indoles, and their derivatives, are commonly used as medicines and pesticides (Gan et al., 2017). Among them, 1,3,4-oxadiazole derivatives have gained attention not only for their significant biological abilities, including antiviral (Wang et al., 2016), antifungal, insecticidal (Formagio et al., 2008), antibacterial (Li et al., 2013, Xu et al., 2011; Wang et al., 2018), and antitumor activities (Huang et al., 1987), but also for their good selectivity, high activity, and low toxicity. And representative 1,3,4-oxadiazole derivatives were summarized and listed in Table S1. In particular, 1,3,4-oxadiazole analogues exhibit broad-spectrum bioactivities in agriculture, with some related commercial pesticides successfully developed, such as herbicide oxadiazon, herbicide oxadiargyl, and insecticide metoxadiazone (Tao et al., 2019). Therefore, the 1,3,4-oxadiazole scaffold is an important pharmacophore group in agrochemical research and development. In our previous studies, many series of 2,5-disubstituted-1,3,4-oxadiazole thioether/sulfone analogues with a rigid group at the 2-position of the 1,3,4-oxadiazole were successfully synthesized, with some target molecules showing excellent bioactivity against phytopathogens (Zhu et al., 2019, Wang et al., 2016, Chen et al., and Xiang et al., 2020). Among them, commercial candidate pesticides Jiahuangxianjunzuo and Fubianezuofeng have been developed, and valuable experience in structural modification gained (Su et al., 2016; Wu et al., 2021). For example, we found that sulfone derivatives increased adenosine triphosphate (ATP) levels and acted as a covalent inhibitor in an anti-dihydrolipoamide S-succinyltransferase (DLST) function study (Chen et al., 2019; Chen et al., 2020).
Encouraged by the aforesaid observations and as a continuation of our research efforts, three series of 1,3,4-oxadiazole thioether/sulfone analogues bearing a flexible N-containing six-membered heterocyclic moiety, such as piperazine, piperidine, and morpholine, at the 2-position of the 1,3,4-oxadiazole ring were designed (Fig. 1) and synthesized (Figs. 2-4). All obtained compounds were systematically investigated for their in vitro anti-microorganism potency against three plant pathogenic bacteria, namely, Xanthomonas oryzae pv. oryzae (Xoo), Xanthomonas axonopodis pv. citri (Xac), and Pseudomonas syringae pv. actinidiae (Psa), and six plant pathogenic fungi, namely, Gibberella zeae (G.z.), Botryosphaeria dothidea (B.d.), Fusarium oxysporum (F.o.), Thanatephorus cucumeris (T.c.), Sclerotinia sclerotiorum (S.s.), and Verticillium dahlia (V.d.). Furthermore, pot experiments were conducted under greenhouse conditions to evaluate their control efficiency of the most active target compounds toward rice bacterial blight in vivo.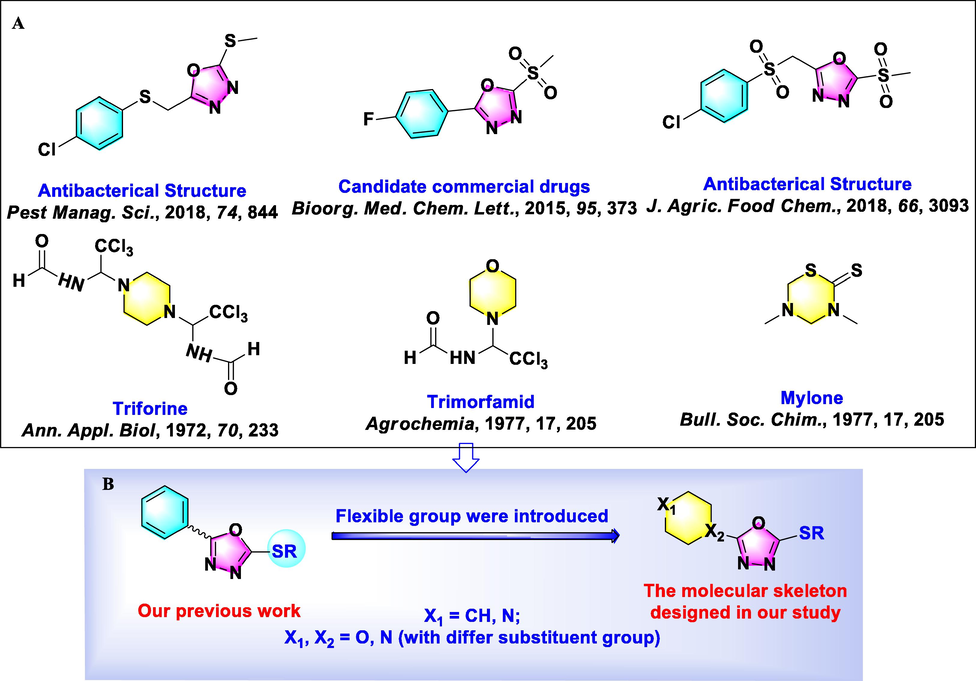
A) The structures of some bioactive 1,3,4-oxadiazoles thioether derivatives; B) Design concept for the title molecules.
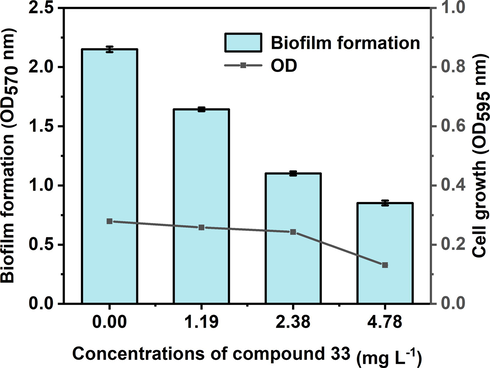
The effect of molecule 33 on bacterial biofilm by using crystal violet assay.
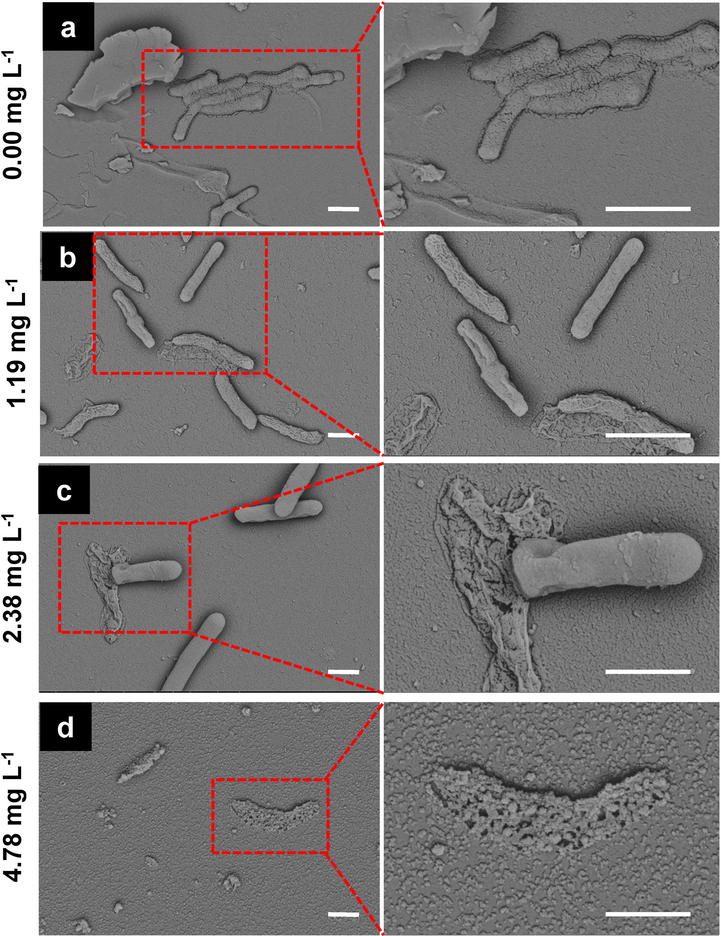
Xoo biofilms formation assay after treatment with compound 33. Scale bars are 1 μm.
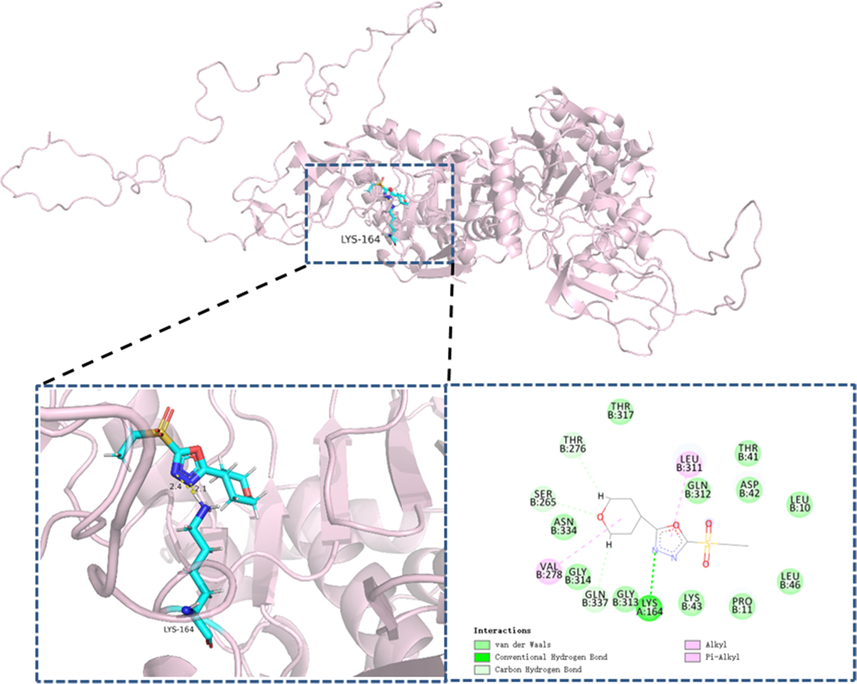
Docking pose of compound 33 in the possible domain of XooDLST.
2 Materials and methods
2.1 Instruments and chemicals
The realted NMR data of all title compounds were documented by an JEOL-ECX-500 apparatus (JEOL, Japan). Meanwhile, tetramethylsilane (TMS) served as the internal standard, and CDCl3/DMSO‑d6 used as the solvent. Parts per million (ppm) and Hz represents chemical shifts and coupling constants (J), respectively. The HRMS data were detected by Thermo Scientific Q Exactive device (UItiMate 3000, Thermo Scientific, The United States). All chemical materials were purchased from commercial market.
2.2 General synthetic process for intermediates and target compounds
2.2.1 Synthesis of the compound 1
Raw material 1-methylpiperazine (0.90 g, 8.6 mmol), and TEA (1.75 g, 17.2 mmol), were charged into a 50 mL one-neck round-bottom flask containing 30 mL CH2Cl2. After that, this mixture was further stirred for 10 min under ice bath. Phenyl carbonochloridate (1.52 g, 9.4 mmol) was then added dropwise to react at room temperature for 5 h. After the reaction was complete, the mixture was diluted with CH2Cl2 and washed with water. The organic layer was further separated and concentrated by vacuum distillation. Finally, desired compound 1 was purified by silica gel chromatography using eluent (CH2Cl2: MeOH = 70:1–50:1, V/V).
2.2.2 Synthesis of the compound 2
The synthesis of compound 2 was prepared referring with previous literature (Tripathi et al., 2019). In a 25 mL one-neck round-bottom flask, prepared compound 1 (0.50 g, 2.3 mmol) and 3 mL of hydrazine hydrate were added followed by heating at 100 °C for 12 h. Upon completion of the reaction, the mixture was diluted with CH2Cl2 and washed with water. The organic layer was separated and concentrated by vacuum distillation. The residue was purified by silica gel chromatography using CH2Cl2 and MeOH (50:1–20:1, V/V) as eluent to afford the compound 2.
2.2.3 Synthesis of the compound 3
Compound 3 was synthesized according to previous work (Wang et al., 2019). To a solution of compound 2 (0.37 g, 2.3 mmol), and KOH (0.24 g, 4.4 mmol) in 20 mL of EtOH were added. The resulting mixture was stirred for 10 min, added CS2 (0.19 g, 2.4 mmol) at 85 °C for another 24 h till almost finished conversion of reation. After reaction completion, the mixture was concentrated, diluted with CH2Cl2 and washed with water. The organic layer was separated, concentrated by vacuum distillation, and further purified by silica gel chromatography using CH2Cl2 and MeOH (30:1, V/V) as eluent to yield the compound 3.
2.2.4 Synthesis of the compound 11
A 50 mL three-neck round-bottom flask was charged with piperidine-4-carboxylic acid (1.10 g, 8.1 mmol), 2-(isopropylthio)acetohydrazide (1.36 g, 8.9 mmol) and phosphorus oxychloride (10 mL) were added successively followed by heating at 78 °C for 2.5 h. The excess phosphorus trichloride was removed in vacuo and the residue was diluted with ethyl acetate and washed with water. The organic layer was further separated and concentrated by vacuum distillation. The residue was purified by silica gel column chromatography (CH2Cl2: MeOH = 10: 1, V/V) to afford compound 11 as a light brown oil 0.69 g, yield 33.66 %.
2.2.5 Synthesis of the compound 21
A reaction mixture consisted of tetrahydro-2H-pyran-4-carboxylic acid (6.00 g, 44.0 mmol) and 2.5 mL H2SO4 in 50 mL CH3OH. Furthermore, this misture was reacted at 80 °C for 2.5 h. After reaction completion, this mixture was concentrated to removed excess CH3OH, resulting in yielding desirable residue. Moreover, this residue was further separated by ethyl acetate, washed with water, and purified by silica gel chromatography using eluent (CH2Cl2: MeOH = 30:1, V/V) to abtain compound 21 with a white oily liquid.
2.2.6 Synthesis of the compound 22
A solution of compound 21 (2.00 g, 14.0 mmol), 3 mL hydrazine hydrate in a 2 mL of CH3OH was reacted for 20 h, the excess CH3OH was removed by vacuum distillation and the corresponding residue was extract by ethyl acetate. The organic layer was washed with water, separated and concentrated by vacuum distillation. Finally, the compound 22 was purified by silica gel chromatography using CH2Cl2 and MeOH (30:1, V/V) as eluent to achieve white oily liquid.
2.2.7 Synthesis of the compound 23
In a 50 mL three-neck round-bottom flask, the compound 22 (0.50 g, 3.30 mmol) and KOH (0.42 g, 6.6 mmol) were added and dissolved in 30 mL EtOH. After the mixture was stirred for 10 min, CS2 (0.63 mL, 8.2 mmol) was added and then reacted at 85 °C for 1 d. Then, the reaction was quenched by water and extracted by CH2Cl2. The organic layer was separated and concentrated by vacuum distillation. The target compound 23 was purified by silica gel chromatography with eluent (CH2Cl2: MeOH = 30:1, V/V) to afford an well yield of 49.18 %.
2.2.8 Synthesis of the title products 4–10
To achieve desirable target products 4–10, typical reaction process was carried out. Briefly, appropriate intermediate 3 (0.37 g, 1.8 mmol) and KOH (0.12 g, 2.1 mmol) were added in succession and dissolved in 5 mL DMF, and this mixture was stirred for 10 min. After that, the CH3I (0.28 g, 1.9 mmol) was added dropwise into this reation and subsequently reacted for 6 h at room temperature. After reaction was complete, this mixture was extracted with ethyl acetate. Finally, desirable target compounds 4–10 were purified by silica gel chromatography using CH2Cl2 and MeOH (70:1–40:1, V/V) as eluent to afford the target compounds 4–10.
2.2.9 Synthesis of the target compounds 12–20
A mixture of K2CO3 (0.23 g, 1.6 mmol) and compound 11 (0.35 g, 1.4 mmol) in 3 mL of DMF was stirred within 10 min under ice bath, 3-bromoprop-1-yne (0.18 g, 1.5 mmol) was added dropwise and then reacted at 60 °C for 2 d. After the mixture was completed, this mixture poured into water and extracted with ethyl acetate. Furthermore, achieved organic layer was separated, concentrated by vacuum distillation, and further purified by silica gel chromatography using CH2Cl2 and MeOH (100:1–50:1, V/V) as eluent to afford corresponding target compounds 12–20.
2.2.10 Synthesis of the target compounds 24–31
In a solution of compound 23 (0.31 g, 16.0 mmol), and K2CO3 (0.24 g, 17.6 mmol) in 10 mL DMF was stirred under ice bath within 10 min. Then, various halogenated hydrocarbon (17.6 mmol) was added dropwise to this mixture for reaction with 6 h. After the completion of the reaction, the mixture was quenched by water and extracted 3 times for ethyl acetate. Finally, the organic layer was combined, concentrated by vacuum distillation, and further purified by silica gel chromatography using a mixture of CH2Cl2 and MeOH (70:1–40:1, V/V) to give target compounds 24–31.
2.2.11 Synthesis of the target compounds 32–34
The synthesis of target compounds containing sulfone moiety was prepared referring with previous literature (Chen et al., 2019; Li et al., 2018). Briefly, In a mixture of ammonium molybdate (0.12 g, 0.19 mmol) in 30 % hydrogen peroxide was stirred at ice bath for 10 min. Then, the compound 24–26 (1.1 mmol) in ethanol (2 mL) were added dropwise and reacted at room temperature for 8 h. After that, the mixture was poured into water and extracted by ethyl acetate. After that, the organic layer was concentrated by vacuum distillation. Moreover, achieved residue was further purified by silica gel chromatography using a mixture of CH2Cl2 and MeOH (70:1–40:1, V/V) to successfully yield the target compounds 32–34.
2.3 Experimental section
The methods for antibacterial bioassay in vitro and in vivo were reported in our previously works (Zhou et al., 2017). The typical mycelium growth rate technique was used for in vitro antifungal bioassay from our reported method (Zhou et al., and Zheng et al., 2017, Zeng et al., 2020).
2.4 Plant toxicity test of compound 33 in rice
Rice plant (variety: Xiangliangyou) growth about eight weeks were used in this experiment. Rice leaves were sprayed evenly with 200 mg/L of compound 33 solution or the equivalent DMSO was used as blank control. Then, all the treated rice plants were grown in a plant growth incubator (Light: 28 ℃ for 16 h; Dark:25 ℃ for 8 h) with 90 % RH. Finally, the results were assessed at 14 days after spraying.
2.5 Biofilm inhibition assay
According to literature report (Yang et al., 2016; Zhao et al., 2021), Xoo cells were cultured to reach OD595 = 0.2 in nutrient broth medium, and further treated with compound 33 to afford final concentrations of 0, 1.19, 2.38 and 4.78 mg/L. The bacteria-containing/without compound solution was added to sterile 96-well plate and cultured at 28 °C for 72 h in a digital biochemical incubator (Bazargani et al., 2016; Kang et al., 2020). After incubation, the 96-well plates were cleaned with sterile water, and the residual bacterial solution was removed and dried at 37 °C. Fixed with Carnot's fixative solution for 30 min, then washed with sterile H2O. The dried samples were treated with 1 % crystal violet The dried samples were stained with 1 % crystal violet for 30 min. After that, these samples were further cleaned with H2O. Finally, 95 % ethanol was used to dissolve the previously stained sample. OD570 was determined with a microplate reader to evaluate the quantification of biofilm formation.
2.6 Biofilm imaging assay
Biofilm study was carried out according to previous work (Milling et al., 2011, Zhou et al., 2019; Kakkar et al., 2015). Xoo cells were cultivated to OD595 = 0.1, transferred to six-well polystyrene plates and then covered with round glass. After 5 days of treatment with compound 33 at different dosages (0, 1.19 mg/L, 2.38 mg/L, or 4.78 mg/L), Xoo cells were rinsed gently with phosphate-buffered saline media, and these samples were further post-fixed in situ in 2.5 % glutaraldehyde solution. After that, these samples were then gradually dehydrated with increasingly concentrated ethanol. At last, these samples were observed with scanning electron microscopy (SEM) after undergoing steps such as freeze-drying and gold plating.
2.7 Molecule docking
For the docking study, according to previous work (Zhu et al., 2022), the three-dimensional model of XooDLST was obtained by using Swiss-Model: the protein sequence of XooDLST was A0A0K0GL90, which achieved in Uniprot, and the template of DLST (PDB code:7b9k.1.A) was used. Then, the theoretical model of XooDLST was further assessed, and result was provided in Support Information. Finally, docking outcomes were achieved by Sybyl X 2.0, and further displayed by Discovery Studio 2020 and PyMol software. Docking accuracy was certified as root-mean-square deviation (RMSD) by Sybyl X 2.0 software.
3 Results and discussion
3.1 Synthesis of target compounds 4–10, 12–20 and 24–34
The synthetic routes of target compounds 4–10, 12–20, and 24–34 were displayed in Schemes 1–3, respectively. Three series of 1,3,4-oxadiazole thioether derivatives with a thioether group at the 5-position and a piperazine/piperidine/morpholine moiety at the 2-position were synthesized, and three 1,3,4-oxadiazole sulfone derivatives were successfully constructed with a morpholine ring at the 2-position. Analysis of the structure–activity relationships in these compounds was limited owing to the long synthetic route, complex raw materials, and different synthetic conditions. First, using 1-methylpiperazine as the starting material, compound 3 was prepared in a three-step sequence, comprising acylation, hydrazinolysis, and cyclization, then derivatized with differently substituted halogenated compounds to obtain target compounds 4–10 in moderate yields. Second, 1-methylpiperidine-4-carboxylic acid and 2-(isopropylthio)acetic acid were reacted using phosphorus oxychloride as solvent at 78 °C to obtain compound 11, and a subsequent substitution reaction in DMF proceeded promptly to afford target compounds 12–20 in higher yields. Finally, tetrahydro-2H-pyran-4-carboxylic acid was used as raw material to yield compound 22 by undergoing substitution and acylation steps, then ring-closed with CS2 and derivatized with differently substituted halogenated compounds to obtain target compounds 24–31. Sulfone compounds 32–34 were then prepared by oxidation of 24–26, respectively, using H2O2 as oxidant and ammonium molybdate as catalyst (referenced by Li et al., 2018; Chen et al., 2019). These title compounds were confirmed by NMR and HRMS (Corresponding data could be shown in the supporting information Figure S1-Figure S91).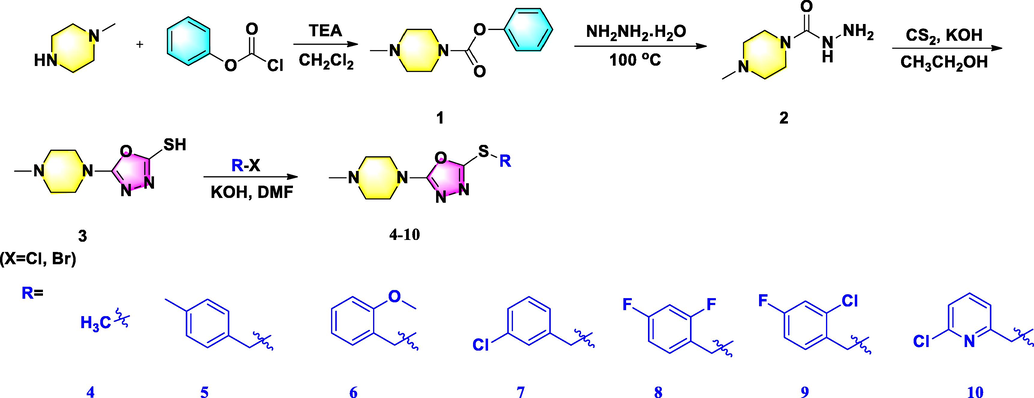
Synthetic route for the title molecules 4–10.
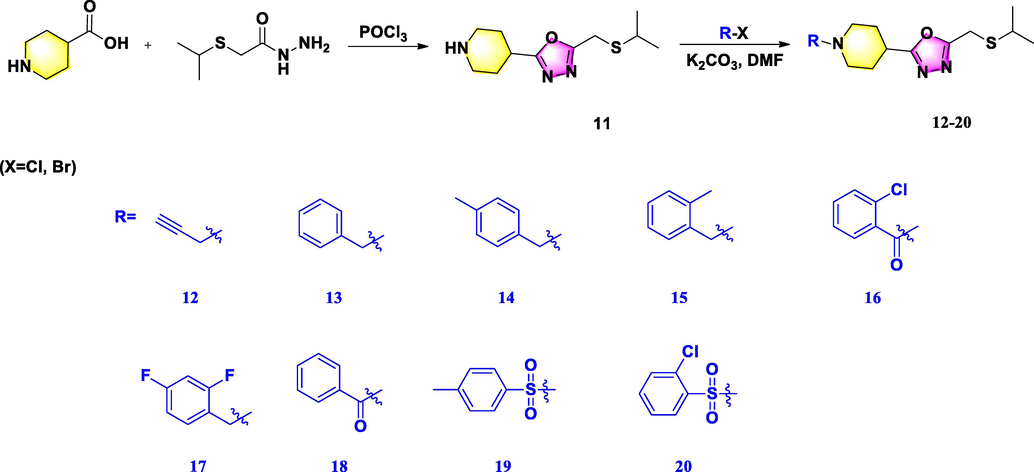
Synthetic route for the title molecules 12–20.
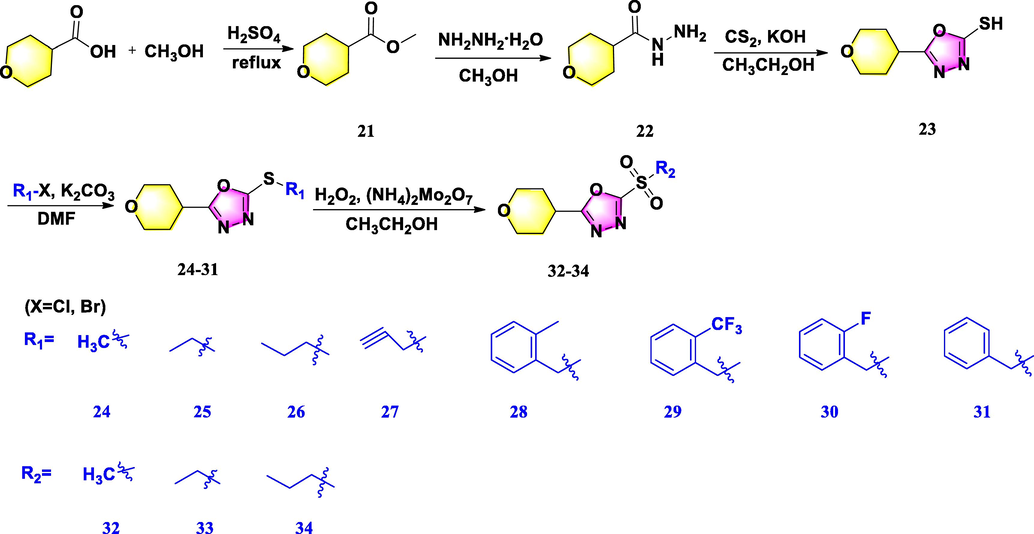
Synthetic route for the title molecules 24–34.
3.2 In vitro bioassay results of compounds 4–10, 12–20, and 24–34
The in vitro bioassay results of the synthesized title compounds towards Xoo, Xac, and Psa were assayed by introducing turbidimetric method, with positive controls pesticides [commercialized bismerthiazol (BT) and thiodiazole copper (TC)]. Notably, the preliminary screening results (Table 1) suggested that most of the designed 1,3,4-oxadiazole thioether derivatives possessed weak antibacterial ability, with only 5, 14, 15, and 16 exhibiting an inhibition activity of>50 % at 100 mg L−1. In addition, thioether compounds substituted with a piperidine structure at 100 mg L−1, such as 14, 15, and 16, showed good anti-Xac ability, with inhibition rates of 56.46 %, 55.62 %, and 86.96 %, respectively. Pleasingly, the anti-Xoo ability was greatly improved when thioether derivatives (24–26) were oxidized to sulfone derivatives (32–34). The designed sulfone derivatives showed distinct selectivity for the tested bacterial strain and excellent bioactivity against Xoo, with inhibition rates of > 95 % at both 100 and 50 mg L−1. However, they possessed poor antibacterial ability against Xac and Psa, with inhibition rates of < 50 % at 100 mg L−1. Furthermore, EC50 values of the partially active compounds against Xoo were further evaluated, with the results (Table 2) showing that compounds 32–34 displayed excellent anti-Xoo activity, with corresponding EC50 values of 5.18, 1.20, and 8.26 mg L−1, respectively, which were much surpass to commercialized BT (35.04 mg L−1) and TC (>100 mg L−1). These outcomes displayed that the integration of two bioactive components can facilitate the discovery of highly effective, biologically active alternatives.
Compounds
Xoo
Xac
Psa
100 mg/L
50 mg/L
100 mg/L
50 mg/L
100 mg/L
50 mg/L
4
0
0
0
0
0
0
5
18.53 ± 1.51
0
73.83 ± 1.03
40.74 ± 8.25
48.35 ± 6.23
34.88 ± 5.68
6
0
0
36.22 ± 4.11
25.79 ± 6.78
34.80 ± 2.98
28.91 ± 2.17
7
55.24 ± 0.99
32.11 ± 0.57
31.11 ± 9.90
0
59.70 ± 1.46
33.74 ± 0.66
8
58.58 ± 1.06
15.78 ± 1.44
0
0
0
0
9
34.75 ± 1.08
0
0
0
37.83 ± 2.72
19.41 ± 2.20
10
44.89 ± 0.69
28.79 ± 3.65
0
0
40.05 ± 2.31
10.88 ± 1.79
12
33.83 ± 1.05
27.03 ± 1.49
35.24 ± 5.05
22.74 ± 5.52
14.34 ± 1.78
0
13
16.42 ± 2.29
10.85 ± 1.29
43.83 ± 3.11
33.15 ± 6.26
0
0
14
41.47 ± 2.48
25.91 ± 3.69
56.46 ± 1.75
41.02 ± 3.63
0
0
15
16.37 ± 7.48
0
55.62 ± 5.74
39.19 ± 5.97
0
0
16
48.74 ± 2.40
0
86.96 ± 0.67
68.38 ± 1.72
16.29 ± 1.69
0
17
21.80 ± 1.54
15.23 ± 1.63
18.20 ± 4.61
0
0
0
18
0
0
16.04 ± 1.76
0
13.29 ± 2.1
0
19
0
0
19.09 ± 1.57
0
0
0
20
32.08 ± 4.20
23.38 ± 3.56
37.91 ± 6.79
0
0
0
23
100
96.72 ± 4.47
35.43 ± 5.64
0
28.59 ± 0.64
23.28 ± 1.83
24
40.31 ± 4.42
16.71 ± 0.55
15.57 ± 3.02
0
16.72 ± 3.37
10.96 ± 2.57
25
36.68 ± 4.19
19.29 ± 0.39
0
0
0
0
26
39.25 ± 1.03
0
0
0
0
0
27
49.47 ± 5.24
15.67 ± 4.78
41.24 ± 1.09
16.02 ± 2.69
39.44 ± 0.76
26.27 ± 3.55
28
31.67 ± 3.62
12.23 ± 1.72
32.05 ± 2.56
22.57 ± 2.44
24.24 ± 6.75
15.82 ± 1.13
29
24.66 ± 3.49
0
0
0
0
0
30
15.82 ± 1.82
10.62 ± 0.47
0
0
0
0
31
20.97 ± 1.31
10.26 ± 1.92
0
0
0
0
32
100
100
49.52 ± 2.01
37.20 ± 1.94
29.98 ± 1.69
0
33
100
99.19 ± 0.70
41.36 ± 3.01
0
0
0
34
BT
TC
100
100
40.1 ± 0.1997.24 ± 2.13
53.90 ± 0.39
26.30 ± 0.3845.78 ± 1.98
42.8 ± 0.58
34.8 ± 0.3916.59 ± 1.38
24.70 ± 0.58
18.90 ± 0.7831.98 ± 2.01
27.90 ± 0.17
15.20 ± 0.1511.09 ± 1.79
10.50 ± 0.48
0
compounds
Xoo
Regression equation
R2
EC50 (mg L-1)
23
32
33
34
BT
TC
y = 6.544x-2.467
y = 4.158x + 2.030
y = 3.892x + 4.698
y = 3.873x + 1.447
y = 4.182x-1.459
y = 3.405x-1.8210.985
0.992
0.999
0.975
0.919
0.99413.84 ± 1.17
5.18 ± 0.30
1.20 ± 0.09
8.26 ± 0.68
35.04 ± 0.77
100.6 ± 0.89
3.3 Antifungal activity
Furthermore, the most recognized mycelium growth rate method was carried out to achieve their antifungal properties toward G. zeae, F. oxysporum, B. dothidea, S. sclerotiorum, V. dahlia, and T. cucumeris. Commercial agents fluopyram (FP) and boscalid (BS) served as positive controls pesticides. As shown in Table 3, all thioether derivatives displayed no activity against the tested fungi, with only sulfone derivative 32 showing relatively good fungicidal activity, with inhibition rates of 53.83 %, 45.64 %, 51.32 %, 98.86 %, 99.42 %, and 52.13 %, respectively. Furthermore, the EC50 values of compound 32 against S. sclerotiorum and V. dahlia were measured as 22.16 and 32.78 mg L−1, respectively. SAR analysis showed that the fungicidal ability was not present when a piperazine/piperidine/morpholine ring was introduced at the 2-position and different sulfur moieties were introduced at the 5-position of the 1,3,4-oxadiazole ring. Furthermore, the potency was significantly improved when thioether derivatives were oxidized to sulfone derivatives. For example, the fungicidal activities of sulfone derivatives 32–34 were much better than those of corresponding thioether derivatives 24–26, respectively. Furthermore, introducing a methyl group (compound 32) onto the sulfone moiety resulted in more broad fungicidal potency compared with introducing a multi-carbon group (compounds 33 and 34). This outcome indicated that an increasing chain length hampered the antifungal ability of the designed sulfone compounds (see Tables 3 and 4).
Compounds
G.z.
F.o.
B.d.
S.s.
V.d.
T.c.
100 mg/L
100 mg/L
100 mg/L
100 mg/L
100 mg/L
100 mg/L
4
0
0
0
0
0
0
5
0
0
0
0
0
0
6
0
0
0
0
0
0
7
0
0
0
0
0
0
8
0
0
0
0
0
0
9
0
0
0
0
0
0
10
0
0
0
0
0
0
12
0
0
0
0
0
0
13
0
0
0
0
0
0
14
0
0
0
0
0
0
15
0
0
0
0
0
0
16
0
0
0
0
0
0
17
0
0
0
0
0
0
18
0
0
0
0
0
0
19
0
0
0
0
0
0
20
0
0
0
0
0
0
23
0
0
0
0
0
0
24
0
0
0
0
0
0
25
0
0
0
0
0
0
26
0
0
0
0
0
0
27
0
0
0
0
0
0
28
0
0
15.73 ± 1.65
0
15.42 ± 1.94
0
29
0
0
0
0
0
0
30
0
0
0
0
0
0
31
0
0
0
0
0
0
32
53.83 ± 1.94
45.64 ± 1.46
51.32 ± 1.41
98.86 ± 1.08
99.42 ± 1.17
52.13 ± 1.94
33
11.33 ± 1.88
0
0
49.04 ± 1.87
16.03 ± 1.15
0
34
35.87 ± 1.99
30.15 ± 1.17
54.32 ± 1.73
24.85 ± 1.07
29.43 ± 2.64
60.13 ± 3.05
BS
98.23 ± 1.95
88.44 ± 2.27
91.59 ± 2.17
60.01 ± 1.17
FP
100
90.38 ± 1.1
57.59 ± 11.19
85.01 ± 2.06
Compounds
Pathogens
Regression equation
EC50 (mg L-1)
32
S.s.
y = 2.289x + 1.919
22.17 ± 0.73
32
BS
FP
V.d.
S.s.
S.s.
y = 4.659x-2.062
y = 8.725x-11.712
y = 2.357x + 1.28732.78 ± 0.70
82.28 ± 2.14
37.56 ± 1.57
3.4 Effects of various dosage of compound 33 on biofilm
Bacterial biofilm is a crucial virulence factor for phytopathogenic bacteria, and provides crucial physical barriers to delay of plant defense responses as well as reduce bactericidal potency. Therefore, to further confirm the inhibitory potency of compound 33 on biofilm, biofilm experiments were carried out, and evaluated using crystal violet staining. The measured optical density value (OD570) of compound 33 on biofilms is displayed in Fig. 2. In the absence of compound 33 (control sample), the amount of biofilm formation was largest (2.15), and decreased with an increasing concentration of 33. Biofilm formation was clearly decreased by 12.7 %, 21.3 %, and 30.4 % after cotreatment with compound 33 at various concentrations (1.19, 2.38, and 4.78 mg L−1, respectively).
3.5 Biofilm formation imaging assay through using scanning electron microscopy (SEM)
To further verify the biofilm disruption effects of compound 33, SEM was conducted to visualize the bacterial biofilm formation of Xoo. As shown in Fig. 3, unbroken intact biofilms and Xoo were observed in control samples. Meanwhile, in the absence of compound 33, Xoo cells were tightly covered in the extracellular matrix. However, after treatment of Xoo cells with compound 33, a broken extracellular matrix was observed, and numerous Xoo cells emerged on the biofilm surface. At a compound dosage of 2.38 mg L−1, part of the cell biofilm was completely destroyed and some cells had died. More interestingly, when the dosage was upgraded to 4.78 mg L−1, all cells were dead. Above-mentioned results showed that treatment with compound 33 (1.19–4.78 mg L−1) inhibited not only the Xoo cell membrane, but also the formation of a large number of biofilms, and thereby further inhibiting bacterial virulence factors.
3.6 Molecular docking
To account for the most profiling compound 33 against phytopathogen, molecular docking was conducted to investigate their possible interactions with the XooDLST. The theoretical model of XooDLST from section 2.6 was utilized in all docking simulations. As displayed in Fig. 4, the core scaffold of compound 33 was engaged in the binding pocket of XooDLST through interaction with Lys 164 residue. In particular, we found that N atom on 1,3,4-oxadiazole ring of compound 33 could interact with amino group of Lys 164 residue via hydrogen bonding, the O atom on 1,3,4-oxadiazole ring of compound 33 interacts with other residues through alkyl, and van der Waals interaction. More interestingly, lysine was found, and certified as the crucial amino acid for succinylation modification. Suggesting that compound 33 might inhibit the succinylation modification of Lys 164 residue, and thereby resulting in disrupting the function of DLST.
In general, binding modes was called “well-docked”, that the RMSD value was < 2.0 Å with respect to the minimized reference structure. Both the “turned” and “nonturned” compound 33’s poses were used for evaluating the RMSD value. The parameters (Similarity: 9.461; RMSD: 0.427) were provided by Sybyl X 2.0 software. Thus, the results declared that the predicted poses seem reasonable due to the RMSD of 0.427 was lower than 2.0.
Above-mentioned outcomes indicated that the morpholine ring was favor to target the XooDLST to a certain extent.
3.7 The control efficiency of compound 33 toward rice bacterial leaf blight
To achieve a potential control efficiency for managing plant bacterial diseases, compound 33 was selected based on its superior bioactivity (anti-Xoo potency, EC50 = 1.19 mg L−1) to evaluate the control efficiency toward rice bacterial leaf blight. As displayed in Table 5, compound 33 exhibited good activity toward rice bacterial leaf blight, providing corresponding acceptable curative (53.63 %) and good protective activities (50.00 %) at 200 mg L−1. These were slightly superior than commercial BT (curative activity: 39.09 %; protective activity: 38.18 %) and TC (curative activity: 39.54 %; protective activity: 37.27 %), respectively. Furthermore, 0.1 % of orange peel essential oil (OPO) and organic silicon (SI) were used as auxiliaries, which are expected to enhance the physical and chemical properties of the compound, to obtain better potency. Therefore, pot experiments were conducted concurrently, with the addition of auxiliaries shown to significantly enhance the control efficacy of compound 33 (200 mg L−1) toward rice bacterial blight. Improved curative and protective activities of 60.66 % and 53.03 % (organic silicon), and 61.36 % and 55.45 % (orange oil), respectively, were obtained, which were superior to these of compound 33 alone.
Treatment
Protective activity (14 days after spraying)
Curative activity (14 days after spraying)
Morbidity (%)
Disease index (%)
Control efficiency (%)
Morbidity (%)
Disease index (%)
Control efficiency (%)
33
100
40.74
50.00
100
37.77
53.63
33-SI
100
38.27
53.03
100
29.91
60.66
33-OPO
100
36.29
55.45
100
31.48
61.36
BT
100
50.37
38.18
100
49.62
39.09
TC
100
51.11
37.27
100
49.25
39.54
CK
100
81.48
100
81.48
3.8 Phytotoxicity of compound 33 at 200 mg L−1 toward rice plants after seven days under greenhouse conditions
To further verify the safety of our designed compounds toward plants, the most bioactive compound against Xoo, 33, was selected and assessed for its phytotoxicity toward rice plants, and providing an equivalent amount of DMSO used as the blank control. The results showed no adverse impact on the rice leaf and haulm at 7 days after treatment with compound 33 at 200 mg L−1 (Figure S92), which proved that compound 33 was safe toward rice. All experiments suggested that the designed molecular skeleton showed potential application prospects as an antibacterial agent in agriculture.
4 Conclusions
An array of 1,3,4-oxadiazole thioether/sulfone derivatives bearing a piperazine/piperidine/morpholine group were elaborately prepared, and their antibacterial potency toward three phytopathogenic bacteria and six phytopathogenic fungi were systematically evaluated. The preliminary antibacterial screening outcomes showed that these compounds possessed broad-spectrum bioactivities toward the tested bacterial strains. Compounds 32 and 33 showed significant antibacterial potency toward Xoo, with EC50 values of 5.17 and 1.19 mg L–1, respectively, which were surpass to commercialized BT and TC. Pot experiments indicated that 200 mg L−1 of compound 33 exhibited good efficiency toward rice bacterial blight, with acceptable curative efficiency (53.63 %) and protective efficiency (42.72 %), which were slightly better than commercialized BT (39.09 % and 38.18 %) and TC (39.54 % and 37.27 %). Interestingly, adding organic silicon or orange peel essential oil as 0.1 % auxiliaries significantly enhanced the control efficacy of compound 33 toward rice bacterial blight, resulting in improved curative and protective activities of 53.03 % and 63.28 % (organic silicon), and 55.45 % and 61.36 % (orange peel essential oil), respectively. Meanwhile, compound 33 showed low phytotoxicity toward rice plants at 200 mg L−1. Furthermore, antifungal bioassay outcomes demonstrated that newly designed 1,3,4-oxadiazole sulfone derivative 32 exhibited broad-spectrum activity against the tested fungi, especially S. sclerotiorum and V. dahlia, providing EC50 values of 22.16 and 32.78 mg L−1, respectively. Furthermore, a hypothetical model (Fig. 5) of compound C11 targeting DLST was proposed by using SEM, biofilm assay, and molecular docking study. Considering these merits of title compounds (e.g., simple molecular frameworks and significant bioactivities), these types of sulfone compound can be served as promising antimicrobial alternatives through targeting DLST, and further structural modification is underway in our laboratory.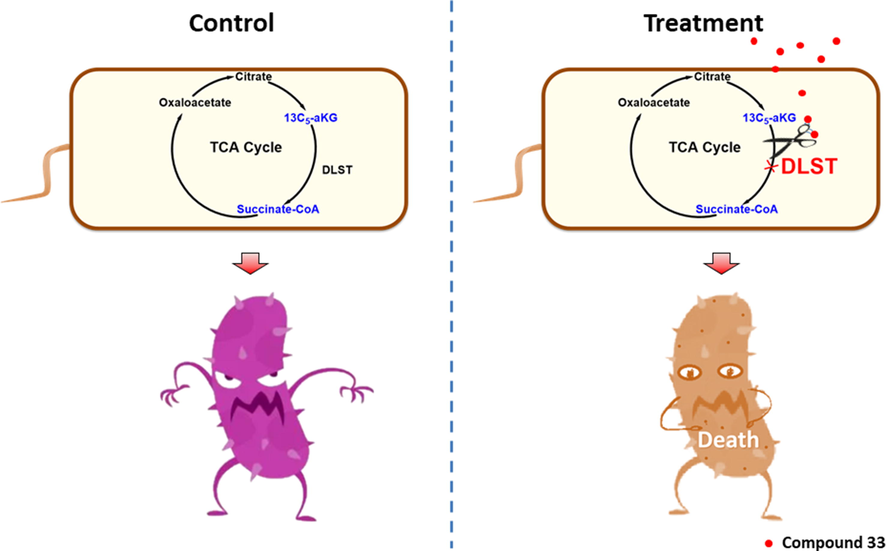
Hypothetical model of compound 33 inhibiting DLST function of Xoo and resulting in disruption of Xoo’s invasion process.
CRediT authorship contribution statement
Fang Wang: Writing – original draft, Data curation. Bin-Xin Yang: Data curation. Tai-Hong Zhang: Formal analysis. Qing-Qing Tao: Data curation. Xiang Zhou: Data curation, Resources, Writing – review & editing. Pei-Yi Wang: Methodology. Song Yang: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
Acknowledgments
We acknowledge the supports from National Natural Science Foundation of China (21877021, 32160661), the Guizhou Provincial S&T Project (2018[4007]), the Guizhou Province [Qianjiaohe KY number (2020)004]), Program of Introducing Talents of Discipline to Universities of China (D20023, 111 Program) and GZU (Guizhou University) Found for Newly Enrolled Talent (No. 202229). The authors thank Simon Partridge (PhD) for supervising the draft of this manuscript.
Declaration of Competing Interest
The authors declare no competing financial interest.
References
- Spatial and temporal distribution of mutations conferring QoI and SDHI Resistance in Alternaria solani across the united states. Plant Dis.. 2018;102(2):349-358.
- [CrossRef] [Google Scholar]
- Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control.. 2016;61:156-164.
- [CrossRef] [Google Scholar]
- Microbial denitrogenation of fossil fuels. Trends Biotechnol.. 1998;16(9):390-395.
- [CrossRef] [Google Scholar]
- Sulfone-based probes unraveled dihydrolipoamide S-succinyltransferase as an unprecedented target in phytopathogens. J. Agr. Food Chem.. 2019;67(25):6962-6969.
- [CrossRef] [Google Scholar]
- Target discovery in Ralstonia solanacearum through an activity-based protein profiling technique based on bioactive oxadiazole sulfones. J. Agr. Food Chem.. 2020;68(8):2340-2346.
- [CrossRef] [Google Scholar]
- Novel amide derivatives containing 1,3,4-thiadiazole moiety: Design, synthesis, nematocidal and antibacterial activities. Bioorg. Med. Chem. Lett.. 2019;29(10):1203-1210.
- [CrossRef] [Google Scholar]
- Novel sulfone derivatives containing a 1,3,4-oxadiazole moiety: design and synthesis based on the 3D-QSAR model as potential antibacterial agent. Pest Manag. Sci.. 2020;76(9):3188-3198.
- [CrossRef] [Google Scholar]
- Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360(6390):739-742.
- [CrossRef] [Google Scholar]
- Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Bioorg. Med. Chem.. 2008;16(22):9660-9667.
- [CrossRef] [Google Scholar]
- Synthesis and antiviral evaluation of novel 1,3,4-oxadiazole/thiadiazole-chalcone conjugates. Bioorg. Med. Chem. Lett.. 2017;27(18):4298-4301.
- [CrossRef] [Google Scholar]
- “Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance. Science. 2018;360(6390):728-732.
- [CrossRef] [Google Scholar]
- Future Direction of agrochemical development for plant disease in china. Food Energy Secur.. 2021;10(4):e293.
- [Google Scholar]
- Novel 1,3,4-oxadiazol-2(3H)-ones as potential pest control agents. J. Agr. Food Chem.. 1987;35(3):368-373.
- [CrossRef] [Google Scholar]
- Xanthomonas campestris cell-cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan. J. Exp. Bot.. 2015;66(21):6697-6714.
- [CrossRef] [Google Scholar]
- Ferulic acid inactivates Shigella flexneri through cell membrane destructieon, biofilm retardation, and altered gene expression. J. Agr. Food Chem.. 2020;68(27):7121-7131.
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial activities, and 3D-QSAR of sulfone derivatives containing 1,3,4-oxadiazole moiety. Chem. Biol. Drug Des.. 2013;82(5):546-556.
- [CrossRef] [Google Scholar]
- Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agri. Food Chem.. 2018;66(12):3093-3100.
- [CrossRef] [Google Scholar]
- Molecular detection of Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and Burkholderia glumae in infected rice seeds and leaves. Crop. J.. 2014;2(6):398-406.
- [CrossRef] [Google Scholar]
- Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One. 2011;6(1):e15853.
- [Google Scholar]
- The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol.. 2019;3(3):430-4439.
- [CrossRef] [Google Scholar]
- Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules. 2016;22(1):64.
- [CrossRef] [Google Scholar]
- Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agr. Food Chem.. 2019;67(27):7626-7639.
- [CrossRef] [Google Scholar]
- Design and development of molecular hybrids of 2-pyridylpiperazine and 5-phenyl-1,3,4-oxadiazoles as potential multifunctional agents to treat Alzheimer's disease. Eur. J. Med. Chem.. 2019;183:111707
- [CrossRef] [Google Scholar]
- Optical control of antibacterial activity. Nat. Chem.. 2013;5(11):924-928.
- [CrossRef] [Google Scholar]
- Synthesis of thiazolium-labeled 1,3,4-oxadiazole thioethers as prospective antimicrobials. In Vitro and in vivo bioactivity and mechanism of action. J. Agr. Food Chem.. 2019;67(46):12696-12708.
- [CrossRef] [Google Scholar]
- Synthesis andlarvicidal activity of 1,3,4-oxadiazole derivatives containing a 3-chloropyridin-2-yl-1H-pyrazole scaffold. Monatsh. Chem.. 2018;149(3):61l-l.
- [CrossRef] [Google Scholar]
- Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfoxide/sulfone derivatives. Bioorg. Med. Chem. Lett.. 2016;26(4):1214-1217.
- [CrossRef] [Google Scholar]
- Synthesis and antibacterial activity of pyridinium-tailored 2,5- substituted-1,3,4- oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg. Med. Chem. Lett.. 2016;26(4):1214-1217.
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial activity, and mechanisms of novel 6-sulfonyl-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole derivatives. J. Agr. Food Chem.. 2021;69(16):4645-4654.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel 1,3,4-oxadiazole sulfone compounds containing3,4-dichloroisothiazolylamide moiety and evaluation of rice bacterial activity. Pestic. Biochem. Physiol.. 2020;170:104695
- [CrossRef] [Google Scholar]
- Synthesis and bioactivity of novel sulfone derivatives containing 2,4-dichlorophenyl substituted 1,3,4-oxadiazole/thiadiazole moiety as chitinase inhibitors. Pestic. Biochem. Physiol.. 2011;101(1):6-15.
- [CrossRef] [Google Scholar]
- New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016;21(4):468.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antimicrobial behavior of novel oxadiazoles containing various N-containing heterocyclic pendants. Pest Manag. Sci.. 2020;76(8):2681-2692.
- [CrossRef] [Google Scholar]
- (+)-Terpinen-4-ol inhibits Bacillus cereus biofilms formation by upregulating the interspecies quorum sensing signals diketopiperazines and diffusing signaling factors. J. Agr. Food Chem.. 2021;69(11):3496-3510.
- [CrossRef] [Google Scholar]
- Synthesis and bioactivities of novel 2-(thioether/sulfone)-5-pyrazolyl-1,3,4-oxadiazole derivatives. Chin. Chem. Lett.. 2017;28(2):253-256.
- [CrossRef] [Google Scholar]
- Inhibition of quorum sensing and virulence in Serratia marcescens by hordenine. J. Agr. Food Chem.. 2019;67(3):784-795.
- [CrossRef] [Google Scholar]
- Antimicrobial activities of pyridinium-tailored pyrazoles bearing 1,3,4-oxadiazole scaffolds. J. Saudi Chem. Soc.. 2017;21(7):852-860.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological profiles of Novel 1,3,4-oxadiazole-2-carbohydrazides with molecular diversity. J. Agr. Food Chem.. 2022;70(9):2825-2838.
- [CrossRef] [Google Scholar]
- Integration of naturally bioactive thiazolium and 1,3,4-oxadiazole fragments in a single molecular architecture as prospective antimicrobial surrogates. J. Saudi Chem. Soc.. 2019;24(1):127-138.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supporting information including 1H NMR, 13C NMR, 19F NMR and HRMS spectra associated with this article can be found.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104479.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







