Translate this page into:
Value-added synthesized acidic polymer nanocomposite with waste chicken eggshell: A novel metal-free and heterogeneous catalyst for Mannich and hantzsch cascade reactions from alcohols
⁎Corresponding authors at: Department of Chemistry, Faculty of Sciences, University of Birjand, Birjand 97175-615, Iran. P.ghamari71@gmail.com (Pouya Ghamari kargar), P.ghamari71@birjand.ac.ir (Pouya Ghamari kargar), Bagherzadeh@birjand.ac.ir (Ghodsieh Bagherzade) gbagherzade@gmail.com (Ghodsieh Bagherzade)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
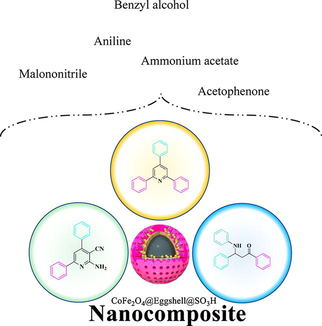
Abstract
The removal of metals from chemical reactions has raised growing concerns for conserving natural resources and environmental safety. Therefore, it is necessary to expand simple procedures that use eco-friendly materials with high elimination capacities. In this paper, we have synthesized a new nanocomposite material in which eggshell membranes act as nucleation sites for CoFe2O4 nanoparticle precipitation in the attendance of an external magnetic field. In the next step anchoring a chlorosulfonic acid on the surface of eggshell‐coated magnetic nanoparticles as solid waste was transformed into a magnetic biomaterial, green, cheap, and environmentally friendly catalyst (CoFe2O4@Eggshell@SO3H). Techniques such as FT-IR, VSM, FESEM, TEM, EDX, XRD, and TGA were used to characterize the as-synthesized catalyst. The catalytic property of the as-prepared catalyst was examined in the synthesis of 2,4,6-triarylpyridine, β-aminocarbonyl, and 2-amino-4,6-diphenylnicotinonitrile via alcohol-based oxidation. Excessive yield, quick reaction time, solvent-free condition, waste to wealth, and optimization with the layout of the experiment are the important advantages of the present work. Taken collectively, these results offer the conversion of wastage to fortune products around the world and utilization in organic metamorphosis.
Keywords
Eggshell
Cobalt ferrite
2,4,6-triarylpyridine
β-amino carbonyl
2-amino-4,6-diphenylnicotinonitrile
1 Introduction
Magnetic nanoparticles are an interesting class of nanomaterials that have been widely investigated for use in many technological applications. Nowadays, magnetic nanoparticles are one of the influential factors in the nanomaterial family, which have been extensively employed in different sciences, consisting of drug shipping, (Zhang et al., 2020) sickness recognition, (Kang et al., 2019) water desalination, (Hajizadeh et al., 2020) environment scrubbing, (Taheri-Ledari et al., 2020) and chemical catalysis (Ghamari kargar et al., 2021a; Ghamari kargar and Bagherzade, 2021a; Khashei Siuki et al., 2020). Furthermore, these magnetic nanoparticles have attracted interest within the discipline of biomedicine, because MNPs have been used to induce heating for hyperthermia treatments, to provide contrast effects for magnetic imaging, and for the remote control of targeted drug delivery (Bagherzade et al., 2021; Bakhshi et al., 2021; Karaagac et al., 2019; Sharifianjazi et al., 2020). Recently, numerous iron oxides have been known that typically refer to three types: α-Fe2O3 (hematite), γ-Fe2O3 (maghemite), and Fe3O4 (magnetite). These compounds form an outstanding category of functional materials that can be applied in various fields. Among all iron oxides, Fe3O4 has attracted more attention due to its superior magnetic properties. (Alavinia and Ghorbani-Vaghei, 2022; Zafari et al., 2021) In the last two decades, research on Fe3O4 nanoparticles has achieved significant progress not only in the synthesis of homogeneous core magnetic nanoparticles Fe3O4 nanoparticles but also in the preparation of advanced nanoarchitectures (composites, core–shell, functionalized surfaces, etc.) and the use of these nanomaterials in different fields. (Ghamari Kargar et al., 2022c; Ghamari Kargar et al., 2022d; Ghamari Kargar and Bagherzade, 2021c) Fe3O4 has attracted more attention than other iron oxides or ferrite spinel oxides (MFe2O4 with M = Co, Ni, Mn, Cu, etc.) because of its superior magnetic properties, electronic conductivity, and biocompatibility. (Xie et al., 2021; Xie and Wang, 2021; Khashei Siuki et al., 2022) In this sense, ferrite spinel oxides with superparamagnetic behavior, are presently utilized in drug transport, microwave devices, catalytic activity toward oxidative reactions, magnetic imaging, photocatalytic activity, magnetic data storage, and multi-component reactions (Radmansouri et al., 2018; Ghamari Kargar et al., 2021c). Ferrites are compounds with different and useful physical properties along with high chemical stability and low production cost is known as valuable materials. Ferrites' structure may be exclusive from garnet, hexagonal, and spinel, based on their preliminary crystal lattice (Milam-Guerrero et al., 2019; Kiani et al., 2020). Among diverse structures, spinel ferrites are the most penchant class and are divided into normal and inverse forms among these groups, cobalt ferrite NPs (CoFe2O4) have been of great fondness owing to their potential for being used in different fields (Scheme 1) (Rouhani et al., 2018; Jabbar et al., 2020; Londoño-Calderón et al., 2020; Pi et al., 2020; Ghamari Kargar and Bagherzade, 2021b; Ghamari Kargar et al., 2020b). Lately, ferrite magnetic nano-catalysts have attracted the consideration of many researchers because of their high activity, availability, selectivity, low toxicity, excellent reusability, large surface area, and easy separation (Bagherzade and Khashei Siuki; Bita Baghernejad, 2021). Ferrite magnetic nanoparticles can be functionalized with organic and inorganic compounds such as silica, (Afzalimir et al., 2020; Bakherad et al., 2018) surfactants, (Lu et al., 2008) polymers, (Ghamari kargar et al., 2021d; Ghazavi et al., 2022) cellulose, (Ghamari kargar et al., 2020c) carbon, (Safari and Javadian, 2015) chitosan, (Mohammadi et al., 2014) pectin, (Ghamari Kargar and Bagherzade, G., Ghasemi, 2021) due to their high surface-to-volume ratio. The coating layer on magnetic nanoparticles can be barricaded from aggregation or oxidation; and their stability can be enhanced.
Synthesis techniques, properties, and applications of CoFe2O4.
Another method of immobilization is using bio-derivative materials as environmentally benign supports. In current years, there has been a splendid upsurge of interest in the application of biowaste materials consisting of eggshell waste. Consistent with the literature, chemicals composition of eggshells are CaCO3 (94 %), organic matter (4 %), MgCO3 (1 %), and Ca3(PO4)2 (1 %) (Stadelman and Schmieder, 2002). Low-fee eggshell waste has been used extensively as probable support, catalyst, bone replacement, inexpensive adsorbent for removal of ionic pollutants and dyes from the aqueous medium, coating pigments for inkjet printing paper, the starting material for preparing calcium phosphate bio-ceramics and efficient bio-templates in recent years due to their high catalytic activity, reusability, ease of handling, and benign character (Fakhimi et al., 2022; Fatemeh Kamali, 2021; Ji et al., 2021; Kalantari et al., 2022). The use of waste eggshells as an aid not only provides a powerful and environmentally sound method for the production of heterogeneous catalysts but also enables the production of economically and ecologically friendly heterogeneous catalysts. Currently, waste eggshell has been used as a catalyst for the preparation of dimethyl carbonate, biodiesel synthesis, and lactose isomerization (Ghani et al., 2023; Rezayati et al., 2022). Meditation some benefits of Fe3O4-eggshell catalysts consisting of the fact that they are simple to find, their cheap supports, and the catalyst preparation method, these catalysts have been subjected to study by many researchers. Environmental compatibility, simple accessibility, and Biodegradability are three established features of these catalysts that categorize them as green catalysts (Talha and Sulaiman, 2016). The placement of acidic groups on the ferrite magnetic nanoparticles have been used as catalyst shells in different functional groups metamorphosis because catalysis has played a first-rate role in preventing pollution in our environment. One of the best Brønsted acid catalysts, which is highly active in acid‐catalyzed reactions, is sulfuric acid. Even though liquid sulfuric acid is used annually in critical chemical processes, it travails from several damages such as purification of the product, troublesome separation of sulfates, and neutralization of sulfuric acid that produces a large amount of waste which also involves significant energy and material use. Consequently, because of the economic pressures and the stringent environmental standards, tons of attention have been directed toward immobilized sulfuric acid on a solid support to simple purification of the products, effective separation of catalyst, and reduction waste. Toward this aim, many support materials inclusive of polymers, (Caetano et al., 2009) amorphous and mesoporous carbon, (Hayati et al., 2018; Zareyee et al., 2013) mesoporous silica (Morales et al., 2011; Zareyee and Serehneh, 2014) and amorphous silica (Gu et al., 2007) are regularly used for immobilization of sulfuric acid, which can be separated with the aid of conventional separation techniques along with filtration and centrifugation. They screen the benefits of easy catalyst recycling from the liquid reaction media, green chemical method, least corrosion, reusability, and increased product selectivity in multi-component reactions (Rostamnia and Doustkhah, 2015; Sobhaniaragh et al., 2021).
Multicomponent reactions (MCRs) refer to one-pot, convergent chemical reactions using more than two starting materials in which the final product retains a significant fraction of all starting materials. This state-of-the-art approach has emerged as an efficient, cost-effectiveو and environmentally friendly alternative for the sequential multi-step synthesis of diverse biologically active pharmacophores. (Sharma et al., 2020; Bakavoli et al., 2005) MCRs show a very high bond formation index (BFI) because several non-hydrogen atom bonds are formed in one synthetic transformation. Rapid chemical reactions and simple experiments are unique features of MCR, leading to diversity-oriented synthesis (DOS) and the complexity of desired end products. MCRs are particularly suitable for preparing large libraries of synthetic molecules with the aim of conducting structure–activity relationship (SAR) studies of drug-like compounds as an essential part of research in the fields related to pharmaceutical, agrochemical, and different industries. (Wang et al., 2019) Under such inspiration in the closing decades, multi-component reactions (MCRs) have been extensively utilized in organic synthesis because of their efficacy for the technology of heterocyclic compounds in a single artificial step. Those reactions constitute very effective chemical generation techniques from each economic and synthetic point of view, and they are one of the maximum influential strategies in green chemistry, business chemistry, and the contemporary drug discovery process (Gholami et al., 2020; Foroughi Kaldareh et al., 2020; Ghamari kargar et al., 2022b). Therefore, discovering of novel MCRs is an interesting topic for synthetic chemistry researchers. Generally, the MCR reactions are carried out in the presence of a homogeneous or heterogeneous catalyst. One of the most important challenges in catalysis nature is the conversion of a successful homogeneous catalyst to a heterogeneous catalyst, as homogeneous catalysts have disadvantages, including disposability, slow reaction speed, and saponification problems due to the presence of fatty acids in the starting materials. On the other hand, solid-supported heterogeneous catalysts have gained significant interest in organic synthesis because of their unparalleled properties, such as high efficiency due to more surface area, easy product/catalyst separation, reusability, low toxicity, higher reaction speed and selectivity, low cost and more stability (Ghamari Kargar et al., 2020a; Naeimi and Nazifi, 2014; Seitllari et al., 2020). Due to these facts, the development of solid-phase catalysts has received increasing attention for synthesis reactions, including MCRs. Currently, the N-heterocyclic pyridine compounds have attracted excellent attention in pharmaceutical chemistry due to possessing various activities, which include antiepileptic, vasodilator, antimalarial, anticonvulsant, aesthetic, and agrochemicals like pesticidal, fungicidal, and herbicidal (Ardeshir Khazaei et al., 2020; Nabizadeh and Jamali, 2021; Nagarapu et al., 2007). Pyridine ring systems, specifically 2,4,6-triarylpyridines represent an essential magnificence of heterocyclic compounds due to their specific function in medicinal chemistry (Kim et al., 2004; Reza et al., 2020). Further, the great thermal stabilities of these pyridines have instigated a developing interest in their use as monomeric building blocks in thin films and organometallic polymers (Ghamari kargar et al., 2022a; Hosseini and Khamesee, 2021).
In addition to pyridine, among nitrogen-containing compounds, β-amino carbonyl scaffolding is of increasing importance in organic chemistry of their affluence in a diversity of naturally occurring products and biologically active compounds (Filho et al., 2017). β‐amino carbonyl compounds are essential building blocks for the guidance of amino alcohols, peptides, lactams, and precursors to synthesize amino acids and many nitrogen‐containing biologically vital compounds (Rezayati et al., 2021; Zhang et al., 2014). Consequently, searching for powerful techniques for synthesizing β-amino carbonyls is an attractive challenge. β-Amino carbonyls have been regarded as considerable targets in organic syntheses. Some of the ways that have been noted for the preparation of β-amino carbonyls are the use of diverse catalysts (Mohammadi Ziarani et al., 2021; Lu and Cai, 2010; Goswami et al., 2013). Each of these techniques can also have its advantages, and blessings; however, it can also be afflicted by apparent drawbacks, which include extended reaction time, complicated work-up, low yield, or risky response conditions. Moreover, many of them are corrosive and risky and frequently reason environmental troubles, which limits their use in the synthesis of nitrogen-containing essential compounds. Subsequently, an environmentally benign technique for the preparation of such compounds is of wonderful problem in synthetic organic chemistry.
Therefore, to keep away from such drawbacks, the development of more simple, green protocols, non-poisonous and ‘greener’ techniques for synthesizing metal nanoparticles and pyridine and mannish reactions underneath slight situations with averting organic solvents and poisonous reagents remains in call for. Synthesis of pyridine and mannish reactions using environmentally benign materials like alcohols has received increasing attention due to a growing need to develop green technologies. So, in this work, a new and highly effective strategy is used to synthesize novel CoFe2O4@Eggshell@SO3H heterogeneous nanocomposite by using eggshell bio-ceramic and chlorosulfonic acid for coating MNPs via the co-precipitation method. To our knowledge, this is the first report on the green synthesis of CoFe2O4 supported on eggshell waste as a natural and biocompatible nanocomposite. In addition, the catalytic activity of the nanocomposite was investigated in the synthesis of 2,4,6-triphenyl pyridine, β-amino carbonyl, and 2-amino-4,6-diphenylnicotinonitrile via one pot multi-component condensation of benzyl alcohol derivatives in solvent and appropriate thermal conditions for the first time. Simple work-up procedures, ease of preparation and facile purification, improved product yields, mild reaction conditions, shorter reaction times, and recyclability of the catalyst are the main advantages of CoFe2O4@Eggshell@SO3H nanocomposite.
2 Experimental
All chemicals and device specifications used are in the supporting information file.
2.1 Preparation of CoFe2O4
The CoFe2O4 ferrite particles were prepared by the chemical co-precipitation method by digestion (Hosseinzadeh et al., 2019). The starting materials were high-purity Co(NO3)2·6H2O and Fe(NO3)3·9H2O, and NaOH was used as the precipitation agent. Firstly, The reaction is described by Co(NO3)2·6H2O and Fe(NO3)3·9H2O with Co:Fe atomic ratio of 1:2 was dissolved in deionized water by gentle heating. Then, the aqueous mixture was slowly poured into a well-stirred NaOH solution and stirred for several minutes. The digestion was performed at 80 °C for 120 min. During digestion, the particles grew and evolved into a spherical structure. After digestion, the gelatinous precipitate was filtered and washed several times using deionized water and EtOH until the pH value of the solution became neutral. Finally, the gelatinous precipitate was dried at 80 °C in the air to form a powder sample.
2.2 Preparation of eggshell powder
The eggshell was prepared according to the procedure described in the literature. (Nasrollahzadeh et al., 2016) 20 g of eggshells were collected at home and washed with warm water, and then the adhering membrane was separated manually. The eggshells were boiled in distilled water for 2 h and then washed with distilled water. After the drying of eggshells in an oven (120 °C for 2 h), the size of the eggshell was reduced using a cutting mill. Eggshells contain organic impurities that should be removed before use, so that 100 mL CH2Cl2 was added to the eggshell and sonicated for 2 h. Finally, the powder was separated using filtration and dried in an oven for 12 h.
2.3 Preparation of CoFe2O4@eggshell
2 g of the eggshell powder was added to the 100 mL water and stirred for 20 min at room temperature, and then 1 g from as‐synthesized CoFe2O4 was added to the mixture. The mixture was dispersed for 15 min using ultrasound and stirred for 2 h at 60 °C. There are two kinds of intermolecular interactions between CoFe2O4 and CaCO3, which are electrostatic, electrostatic‐electrodynamics, and electrodynamics. The intermolecular interactions are strong enough to prevent the separation of eggshells from the CoFe2O4, so experimental results confirm this. Finally, the CoFe2O4@Eggshell was filtered and dried in an oven.
2.4 Preparation of CoFe2O4@eggshell@SO3H nanocomposite
After the dispersion of CoFe2O4@Eggshell (1.5 g) in 100 mL CH2Cl2 for 15 min, 1.0 mL chlorosulfonic acid (ClSO3H; density: 1.75 g/cm3) dissolved in CH2Cl2 (20 mL) was added dropwise to the dispersed solution at 0 °C. The mixture was stirred for 24 h at room temperature. The CoFe2O4@Eggshell@SO3H product was separated by a permanent magnetic field and washed three times with absolute ethanol. The resulting nanocomposite was dried in an oven for 12 h, then characterized by FT-IR analyses.
The reaction between eggshell (CaCO3) and ClSO3H is as (Khazaei et al., 2018):
2.5 General procedure for the synthesis of 2,4,6-triarylpyridine derivatives
To a solution of benzyl alcohol (1 mmol), acetophenones (1.2 mmol), ammonium acetate (1 mmol), and 0.05 g (0.4 mol%) of nanocomposite CoFe2O4@Eggshell@SO3H was stirred at 80 °C under solvent-free condition for an appropriate time. After completion of the reaction (monitored by TLC), the reaction mixture was cooled, eluted with hot EtOH (2 mL) and then the catalyst was separated magnetically. The title compounds were obtained in their crystalline forms by recrystallization of ethanol solution. The recyclability of nanocomposite in this reaction was studied for 5 runs. After completing each run, nanocomposite was separated by external magnet, washed with EtOH, and dried overnight. All compounds were identified by melting point and in some cases, by CHN, and 1H NMR. (See in file Supporting information).
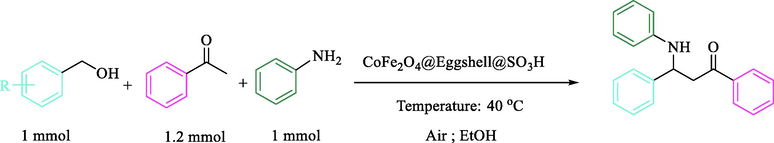
2.6 General procedure for the synthesis of β-amino carbonyl derivatives
To a solution of benzyl alcohol (1 mmol), acetophenones (1.2 mmol), aniline (1 mmol) and 0.08 g (0.5 mol%) of nanocomposite CoFe2O4@Eggshell@SO3H were stirred in 3 mL ethanol at 40 °C for an appropriate time. After completion of the reaction (monitored by TLC), water was added, and the precipitate was collected by filtration and washed with water to give the pure product. The title compounds were obtained in their crystalline forms by recrystallization of ethanol solution. The recyclability of nanocomposite in this reaction was studied for 5 runs. After completing each run, nanocomposite was separated by an external magnet, washed with EtOH, and dried overnight. All compounds were identified by melting point and also in some cases, by CHN, and 1H NMR. (See in file Supporting information).
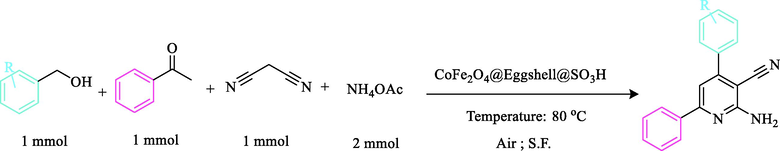
2.7 General procedure for the synthesis of 2-Amino-4,6-nicotinonitrile derivatives
To a solution of benzyl alcohol (1 mmol), malononitrile (1 mmol), acetophenones (1 mmol), ammonium acetate (2 mmol), and 0.06 g (0.45 mol%) of nanocomposite CoFe2O4@Eggshell@SO3H was stirred in solvent-free condition at 80 °C for an appropriate time. After completion of the reaction (monitored by TLC), water was added, and the precipitate was collected by filtration and washed with water to give the pure product. The title compounds were obtained in their crystalline forms by recrystallization of ethanol solution. The recyclability of nanocomposite in this reaction was studied for 5 runs. After completing each run, nanocomposite was separated by external magnet, washed with EtOH, and dried overnight. All compounds were identified by melting point and also in some cases, by CHN, and 1H NMR. (See in file Supporting information).
3 Result and discussion
Considering our research toward evaluating the catalytic activity of core/shell magnetic nanoparticles natural for the synthesis of organic compounds, (Ghamari Kargar et al., 2020b; Ghamari Kargar et al., 2021b) we structured our study to investigate the suitability of activated CoFe2O4@Eggshell@SO3H nanocomposite (Scheme 2) as a catalyst for the synthesis of 2,4,6-triarylpyridines and β-amino carbonyl and 2-amino-4,6-diphenylnicotinonitrile. The nano catalyst was characterized by techniques such as FT-IR, XRD, TGA, TEM, FESEM, VSM, DLS, and EDX.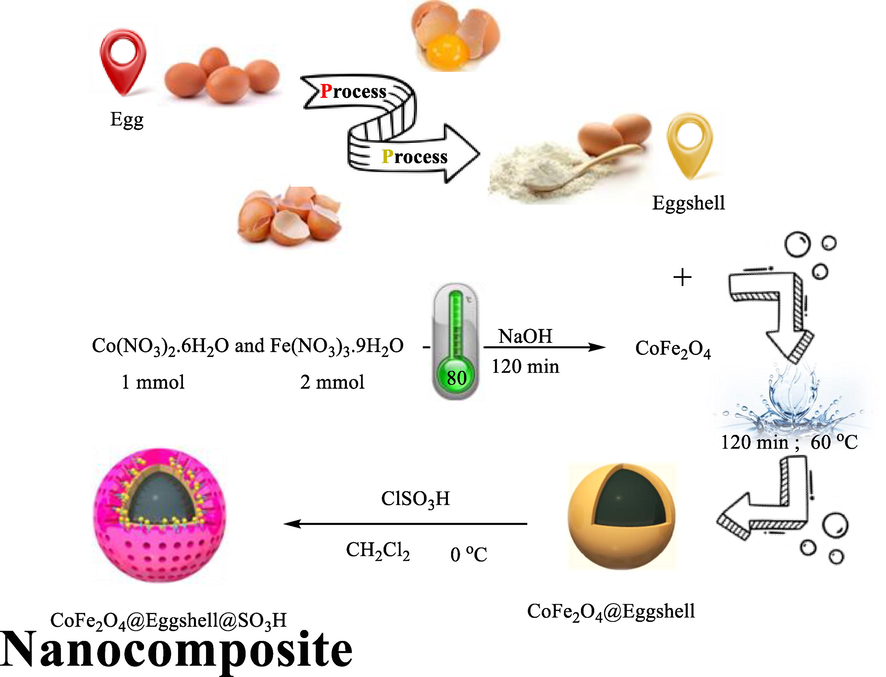
Schematic representation of synthesized CoFe2O4@Eggshell@SO3H nanocomposite.
Firstly, the FT-IR spectra of the components of the CoFe2O4@Eggshell@SO3H nanocomposite are shown in Fig. 1. In the CoFe2O4 spectrum, the absorption bands at 588 cm−1 are related to the tensile vibrations of the Fe-O bond. The weak and strong peaks observed in the eggshell are present in 713 cm−1 and 874 cm−1, which are associated with deformation inside, and outside the plate, respectively, indicating the presence of calcium carbonate (CaCO3). Also, on the other hand, the presence of carbonate, amines and amides, and hydroxyl ions in the eggshell membrane particles was found in Fig. 1, which showed significant peaks at intensities of 1403 cm−1, 1734 cm−1, 2518 cm−1, 2900 cm−1, 3401 cm−1. According to the spectral information of CoFe2O4 and Eggshell, it was observed that in the spectrum CoFe2O4@Eggshell (c), all tensile vibrations have appeared. Finally, in the nanocomposite spectrum (d), the tensile vibration of 1152 cm−1 corresponds to S⚌O in the SO3H group. In addition, it confirms the wavelength of the OH group from 3000 cm−1 to 3600 cm−1 SO3H group. These results indicate the conversion of CaCO3 to Ca (HSO4) 2 in the synthesis of nanomagnetic catalysts. The functional groups represented by vertical dotted rectangles exist in CoFe2O4, Eggshell, and CoFe2O4@Eggshell (Mahmoudiani Gilan et al., 2021).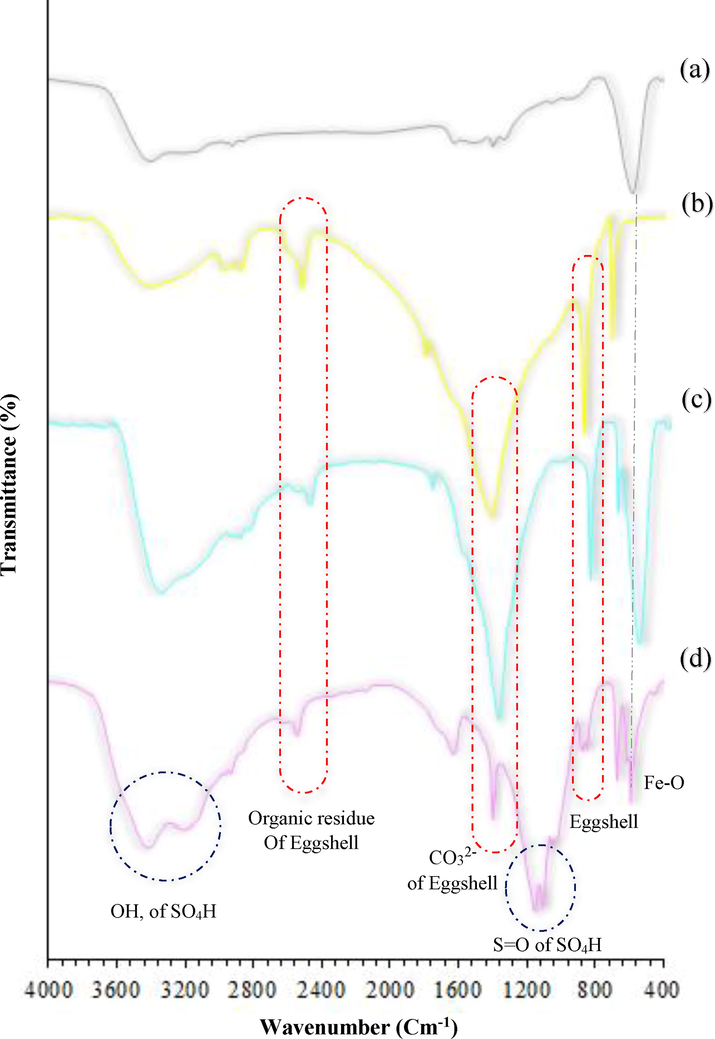
FT-IR spectra of (a) CoFe2O4, (b) Eggshell, (c) CoFe2O4@Eggshell and (d) CoFe2O4@Eggshell@SO3H nanocomposite.
The XRD pattern of nanoparticles shown in Fig. 2 has seven distinct peaks for CoFe2O4 in the regions of (1 1 1) 18/17, (2 2 0) 17/30, (3 1 1) 82/35, (4 0 0) 22/43, (4 2 2) 77/53, (5 1 1) 32/57, (4 4 0) 62/72. This pattern shows that pure CoFe2O4 nanoparticles have a spinel structure (Mahhouti et al., 2019). The peak in area 29 indicates calcium carbonate and eggshell layers around CoFe2O4, which confirms the formation of the CoFe2O4@Eggshell core–shell (Mahmoudiani Gilan et al., 2021). It is worth noting that the same seven peaks have appeared for the CoFe2O4@Eggshell@SO3H magnetic nanocomposite. With the disappearance of the calcium carbonate peak and the appearance of a broad peak in the region of 18–25, it shows that the acidic group is stabilized on the eggshell and does not change CoFe2O4 crystalline phase.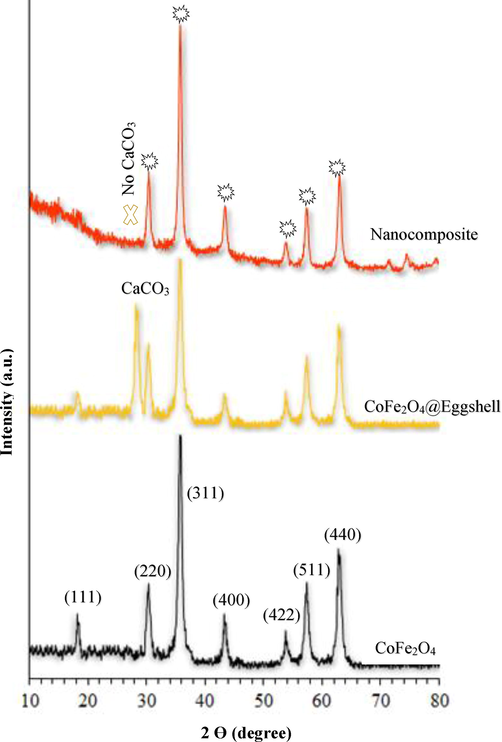
XRD pattern of (line black) CoFe2O4, (yellow line) CoFe2O4@Eggshell and (orang line) CoFe2O4@Eggshell@SO3H.
The magnetic properties of CoFe2O4 and CoFe2O4@Eggshell@SO3H were investigated by VSM analysis at room temperature (Fig. 3). According to the obtained diagram, CoFe2O4 and CoFe2O4@Eggshell@SO3H have superparamagnetic properties. The observed saturation magnetization for CoFe2O4 and CoFe2O4@Eggshell@SO3H is 63.57 and 37.63 emug−1, respectively. This decrease in magnetism for CoFe2O4@Eggshell@SO3H is due to the stabilization of the eggshell and the acidic group on the surface of the CoFe2O4.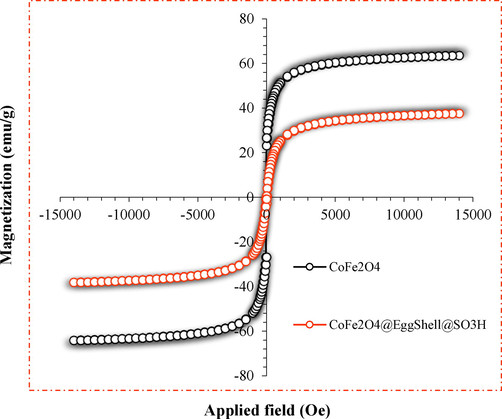
Magnetization curves of (Θ) CoFe2O4, and (Θ) CoFe2O4@Eggshell@SO3H.
TEM and FESEM analyzes were used to study the surface morphology and microstructure of CoFe2O4@Eggshell@SO3H nanocomposite. FESEM images were recorded to understand the morphological changes on the surface of the eggshell and magnetic nanocomposite. Fig. 4 show that the eggshell is a coarse porous network. According to the FESEM images, the synthesized CoFe2O4@Eggshell@SO3H are approximately spherical and well dispersed, albeit in some area’s larger structures with non-spherical morphology are perceived (Fig. 4). On the other hand, in the TEM image in Fig. 4, the presence and shapes of irregular particles coexisted with spherical particles. The presence of clear bands is known as the edges of the regular crystal lattice, which is attributed to the calcite of the eggshell powder, visible in different directions. In contrast, intertwined spherical particles show the presence of CoFe2O4.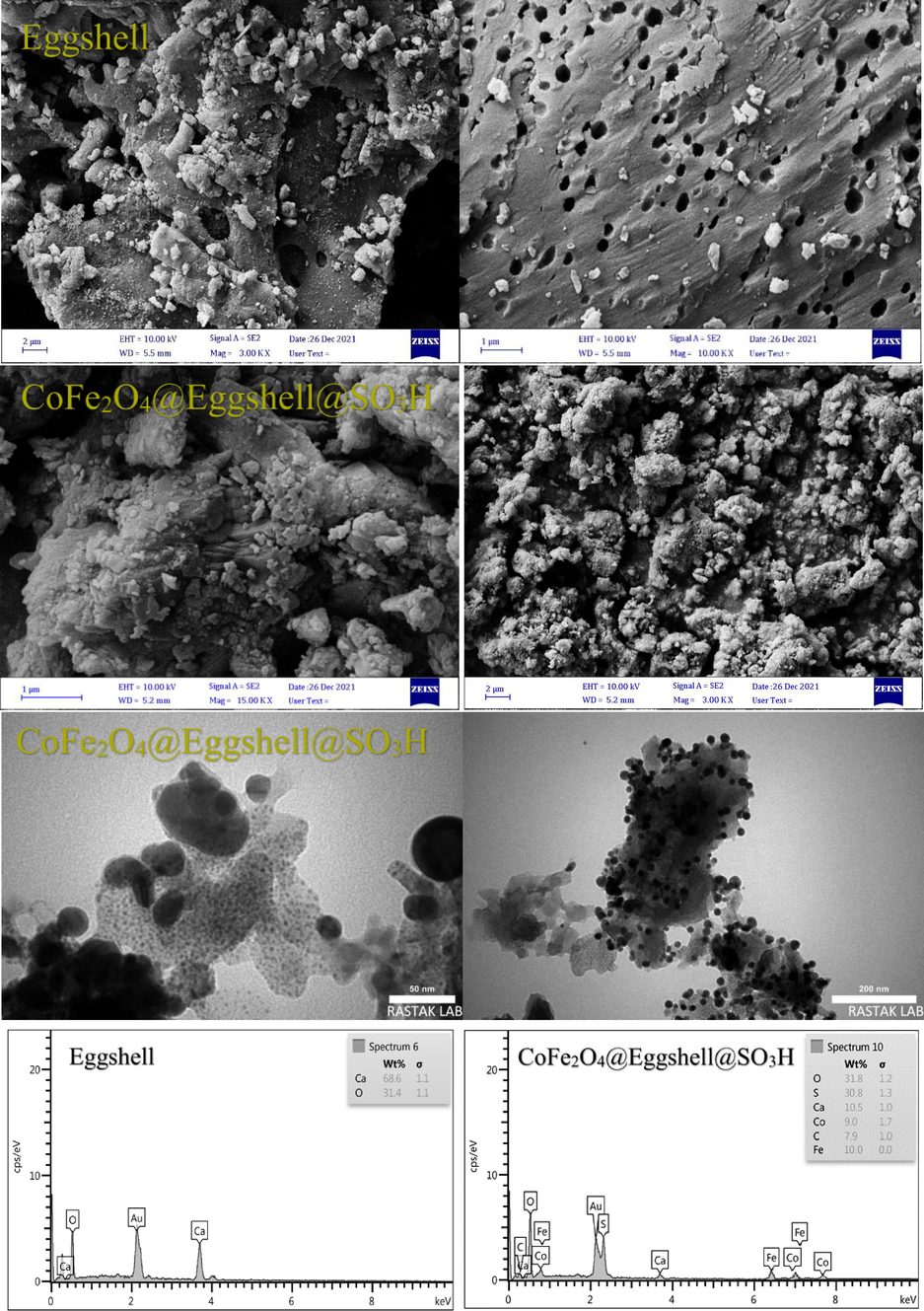
FESEM, TEM, and EDX images of eggshell, and CoFe2O4@Eggshell@SO3H nanocomposite.
The results further confirm that the eggshell has high mechanical strength, which makes it a suitable carrier in CoFe2O4 immobilization. Analysis of TEM and FESEM images shows that the average diameter of nanoparticles is about 30–47 nm. The particle size of the nanocomposite is due to the presence of the eggshell and the stabilized acidic group around the CoFe2O4 particles as the core. Also, the results of EDX analysis of CoFe2O4@Eggshell@SO3H nanocomposite are shown in Fig. 4 and confirm the presence of carbon, calcium, iron, cobalt, oxygen, and sulfur.
Also, AFM analysis was performed to evaluate the surface properties of CoFe2O4@Eggshell@SO3H nanocomposite. Fig. 5 shows 2D and 3D images of the nanocomposite. According to the 2D image, most of the particles are the same color, which indicates that the particles are about the same thickness. However, the surface porosity, texture and roughness of CoFe2O4@Eggshell@SO3H nanocomposite are shown in the 3D image. In the 3D image, there are various peaks indicate the porous and uneven surface of the magnetic nanoparticles of the eggshell. Therefore, it can be concluded that by modifying the eggshell with magnetic nanoparticles and SO3H group, the number of pores and surface roughness increases.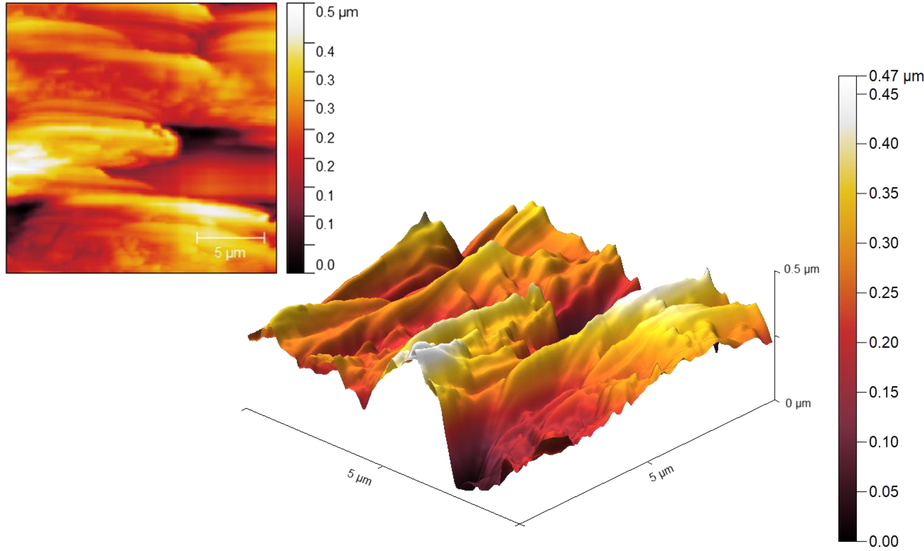
2D and 3D images of AFM analysis of CoFe2O4@Eggshell@SO3H nanocomposite.
The thermogravimetric analysis (TGA) of CoFe2O4@Eggshell@SO3H was used to determine the thermal stability and content of organic functional groups on the surface of magnetic nano particles (Fig. 6). Fig. 6 shows the TGA curve for the CoFe2O4@Eggshell@SO3H nanocomposite in the temperature range of 25–800 °C. A weight loss of 5 % at temperature below about 200 °C is related to the desorption of water molecules from the nanocomposite surface. In addition, the nanocomposite is stable up to 450 °C, and the weight loss from 450 to 650 °C is related to the decomposition of organic matter. The eggshell stabilized on the surface of CoFe2O4. Fig. 4 shows that the eggshell is stable up to 450 °C and is suitable for reactions that are stable at this temperature.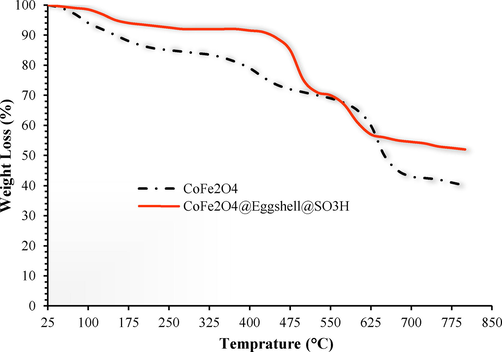
TGA diagram of (black line) CoFe2O4, and (orange line) CoFe2O4@Eggshell@SO3H.
The specific surface area and pore size distributions of the nanocomposite were obtained from BET multi-point and single-point methods using N2 adsorption–desorption isotherm data (according to the Barrett–Joyner–Halenda method). All the parameters are summarized in Table 1.
Nanocomposite
Pore volume (cc/g)
Pore radius (Å)
BET Surface area (m2/g)
CoFe2O4@Eggshell@SO3H
0.06
13.01
17.95
After catalyst characterization, we structured our study to check the adaptability of CoFe2O4@Eggshell@SO3H nanocomposite as a heterogeneous catalyst for the synthesis of 2,4,6-triarylpyridines and β-amino carbonyl, and 2-amino-4,6-diphenylnicotinonitrile (Scheme 3). The oxidative synthesis of 2,4,6-triarylpyridines, and β-amino carbonyl, and 2-amino-4,6-diphenylnicotinonitrile derivatives using alcohols need both oxidation and acid catalysts. The dual properties of CoFe2O4@Eggshell@SO3H nanocomposite, oxidation capability and acidic property made these catalysts capable of being used in both oxidation, and synthetic sections of the reactions. Finally, the effect of the solvent on time, catalyst loading, and temperature for all three of the model reaction was assessed (Figs. 7-9).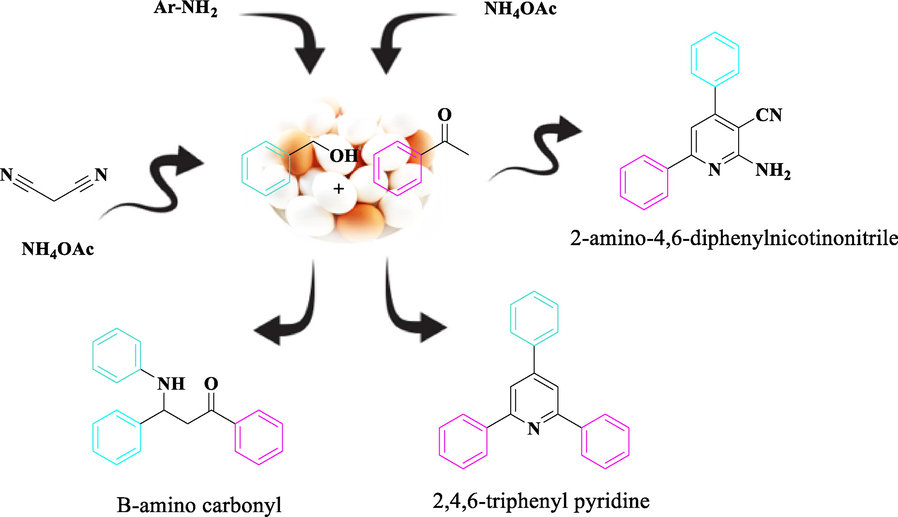
Hantzsh pyridine and Mannich as model reactions.
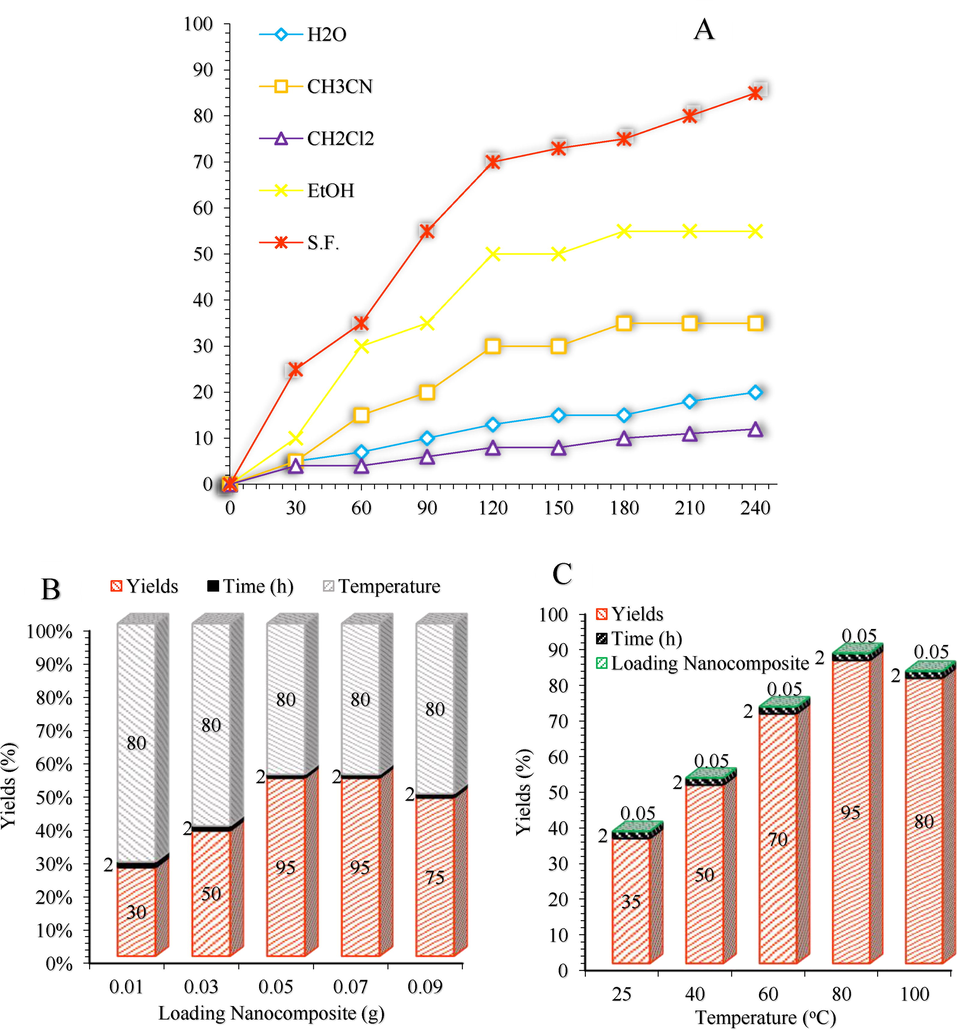
The screening effect of the solvent, temperature, and catalyst on the 2,4,6-triarylpyridine reaction. Reaction conditions: Benzyl alcohol (1 mmol), Acetophenone (2 mmol), Ammonium acetate (1 mmol), Solvent, Temperature, Catalyst, and Air as the oxidant.
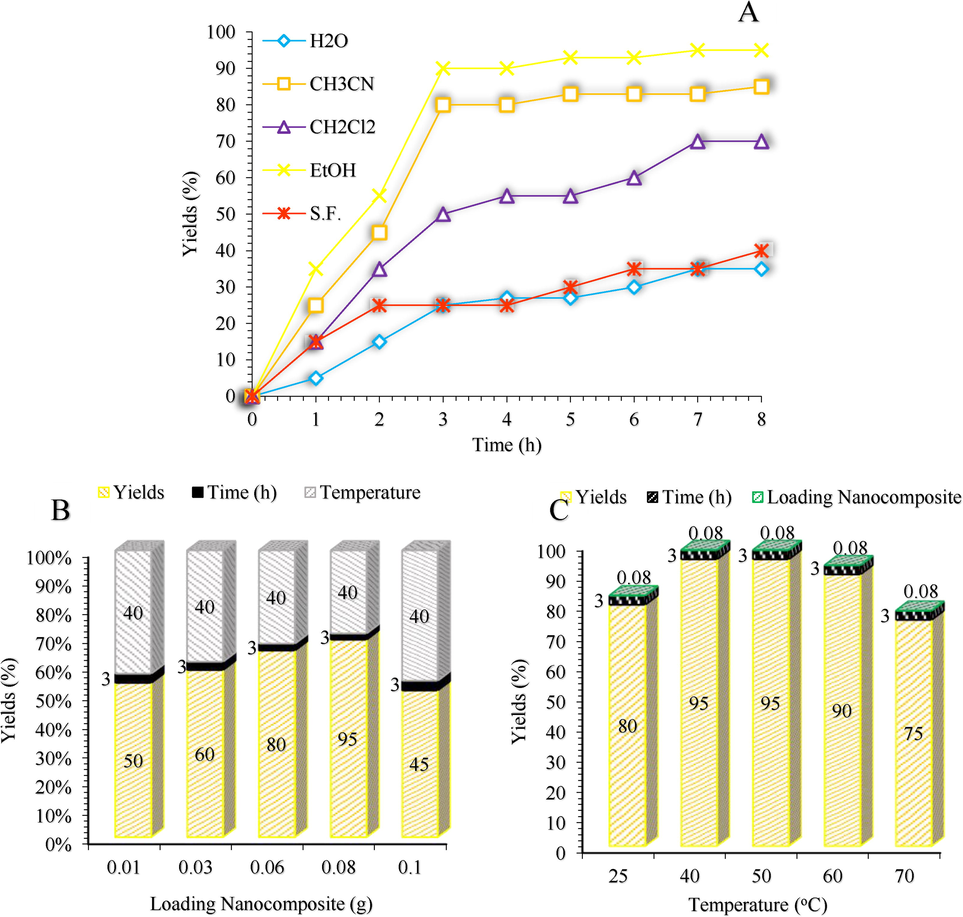
The screening effect of the Solvent, temperature and catalyst on the β-amino carbonyl reaction. Reaction conditions: Benzyl alcohol (1 mmol), Acetophenone (1.2 mmol), Aniline (1 mmol), Solvent, Temperature, Catalyst, and Air as the oxidant.
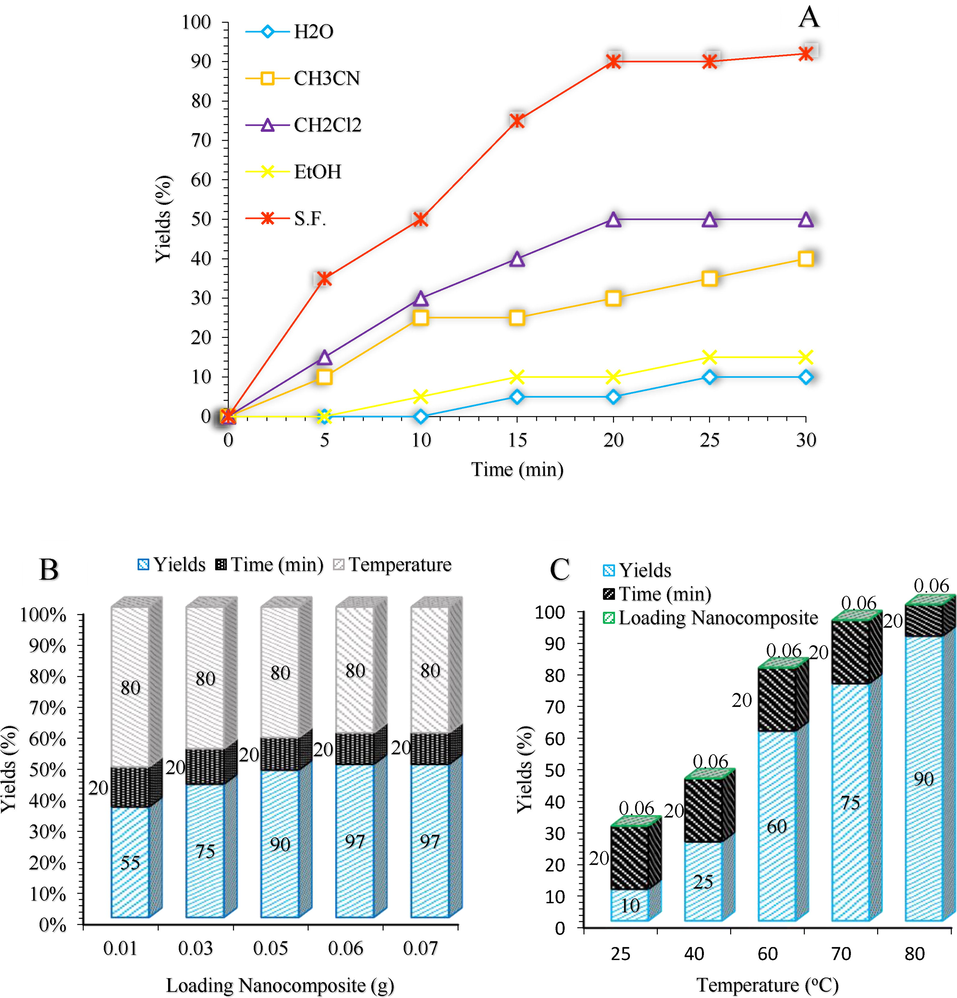
The screening effect of the Solvent, temperature and catalyst on the 2-amino-4,6-diphenylnicotinonitrile reaction.
We choose the reaction of benzyl alcohol, and acetophenone, and ammonium acetate as the model 2,4,6-triarylpyridines reaction (Scheme 4). The study of the influence of the solvent, as well as its absence, illustrated that solvent free conditions as green and sustainable reaction conditions rendered the best results (Fig. 7a). To optimize the reaction catalyst, an amount of 0.05 g was found to minimize the 2,4,6-triarylpyridine formation (Fig. 7b). Screening the amount of the catalyst illustrated that decreasing the catalyst amount from 0.07 g to 0.05 g led not to a decrease in the yield of the product. But weaker activity was obtained by further reducing the amount of catalyst (Fig. 7b). Also, a further increase in the amount of catalyst did not have any significant effects on the product yield. Finally, the temperature dependency of the model reaction was investigated (Fig. 7c) and found that the reaction proceeded efficiently under 80 °C.
Schematic illustration of the 2,4,6-triarylpyridines as the model reaction by CoFe2O4@Eggshell@SO3H.
With an optimized catalytic system in hand (Fig. 7), to develop the scope of the reaction, a wide range of alcohols, acetophenone and ammonium acetate was subjected to nanocomposite reaction under the optimized conditions (Table 2). In all cases, aromatic alcohols with substituents carrying either electron-withdrawing or electron-donating groups reacted effectively and gave the goods correctly yields (Table 2). It was found that aromatic aldehydes with electron-withdrawing groups reacted faster than those with electron-donating groups, as expected. These results justified one more time the efficiency of the nanocomposite.
Entry
Alcohols
Time (h)
Yield (%)
m.p. (oC)
Obtained
Reported [ref.]
1
H
120
95
135–137
135–138 (Tabrizian et al., 2015)
2
para-F
120
95
178–180
New
3
para-Cl
90
95
122–124
123–124 (Tabrizian et al., 2015)
4
para-Br
75
97
166–168
165–166 (Tabrizian et al., 2015)
5
para-OH
90
92
197–198
196–198 (Tabrizian et al., 2015)
6
para-MeO
150
96
100–102
100–101 (Tabrizian et al., 2015)
7
para-Me
130
93
125–128
127–129 (Tabrizian et al., 2015)
8
para-NO2
100
97
194–196
194–195 (Tabrizian et al., 2015)
9
orto-NO2
120
95
190–192
New
10
orto-Cl
100
95
115–118
115–117 (Tabrizian et al., 2015)
In subsequent examination, optimization of reaction conditions was carried out in the β-amino carbonyl three-component reaction of benzyl alcohol, acetophenone, and aniline in various solvents, and the presence of temperatures and different amounts of catalyst (Scheme 5). Based on the literature survey, as indicated in Fig. 8a, the efficiency and the yield of the model reaction in EtOH were higher than those obtained in other solvents. To extend the optimization of the prepared catalyst, the best results related to the amount of nanocomposite, and the temperature are shown in Fig. 8b and Fig. 8c.
Schematic Illustration of the β-amino carbonyl as the model reaction by CoFe2O4@Eggshell@SO3H.
To research the generality of this protocol and based on the above-optimized endings, we explored the scope and limitations of this favorable reaction by varying the structure of the benzyl alcohol component. In this attempt, diverse benzyl alcohol derivatives were successfully produced using acetophenone, and aniline in the presence of a catalytic amount of CoFe2O4@Eggshell@SO3H in EtOH at 40 °C in excellent yields (Table 3). The process was found to be excellent in terms of yields and times using benzyl alcohol substituted with electron-donating as well as electron-withdrawing moieties.
Entry
Alcohols
Time (h)
Yield (%)
m.p. (oC)
Obtained
Reported [ref.]
1
H
180
95
168–170
168–169 (Rahmatpour et al., 2019)
2
para-F
180
90
103–105
102–104 (Rahmatpour et al., 2019)
3
para-Cl
150
95
116–118
117–118 (Kundu and Nayak, 2012)
4
para-Br
120
97
128–130
128–129 (Kundu and Nayak, 2012)
5
para-OH
150
96
182–184
181–182 (V.N.K. et al., 2008)
6
para-MeO
210
93
150–152
149–150 (Rahmatpour et al., 2019)
7
para-Me
180
95
131–133
130–132 (Rahmatpour et al., 2019)
8
para-NO2
150
97
108–110
108–110 (Rahmatpour et al., 2019)
9
orto-NO2
210
92
123–125
New
10
orto-Cl
180
95
119–121
New
After the successful synthesis of 2,4,6-triarylpyridine and β-amino carbonyl three-component reactions, we have used our efforts to synthesize the 2-amino-4,6-diphenylnicotinonitrile reaction (Scheme 6). Based on the literature survey, as indicated in Fig. 9, the efficiency and the yield of the model reaction in solvent free condition was higher than those obtained in other solvents (Fig. 9a). To extend the optimization of the prepared catalyst, the best results related to the amount of nanocomposite, and the temperature are shown in Fig. 9b and 9c.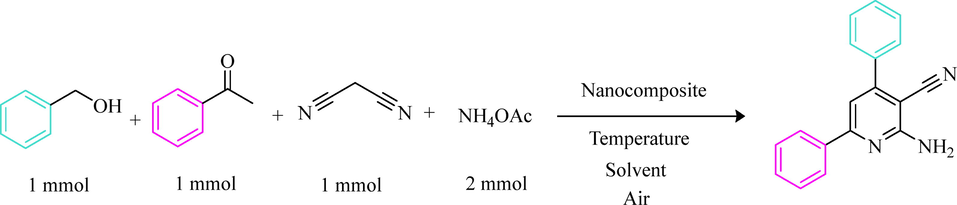
Schematic illustration of the 2-amino-4,6-diphenylnicotinonitriles as the model reaction by CoFe2O4@Eggshell@SO3H.
Reaction conditions: Benzyl alcohol (1 mmol), Malononitrile (1 mmol), Ammonium acetate (2 mmol), Acetophenone (1.2 mmol), Solvent, Temperature, Catalyst, and Air as the oxidant.
To research the generality of this protocol and based on the above-optimized endings similar to previous reactions, we explored the scope and limitations of this favorable reaction by varying the structure of the benzyl alcohol component. In this attempt, diverse benzyl alcohol derivatives were successfully produced using acetophenone, and aniline in the presence of a catalytic amount of CoFe2O4@Eggshell@SO3H in solvent-free condition at 80 °C in excellent yields (Table 4). The process was found to be excellent in terms of yields and times using benzyl alcohol substituted with electron-donating as well as electron-withdrawing moieties.
Entry
Alcohols
Time (min)
Yield (%)
m.p. (oC)
Obtained
Reported [ref.]
1
H
20
97
185–187
186–187 (Heravi et al., 2015)
2
para-F
20
95
211–214
210–213 (Zolfigol et al., 2016)
3
para-Cl
20
97
170–172
169–171 (Zolfigol et al., 2016)
4
para-Br
15
98
213–216
215–218 (Safari et al., 2012)
5
para-OH
20
95
223–225
225–227 (Erşatır and Yıldırım, 2021)
6
para-MeO
25
90
179–181
181–182 (Heravi et al., 2015)
7
para-Me
20
92
220–223
224–227 (Zolfigol et al., 2016)
8
para-NO2
15
95
210–212
212–213 (Heravi et al., 2015)
9
orto-NO2
15
95
139–141
140–142 (Rekunge et al., 2021)
10
orto-Cl
15
96
190–192
193–196 (Khaksar and Yaghoobi, 2012)
To illustrate the need for CoFe2O4@Eggshell@SO3H nanocomposite for these reactions, an experiment was conducted in the absence of CoFe2O4@Eggshell@SO3H nanocomposite, and nanocomposite components CoFe2O4 alone and CoFe2O4@Eggshell as analogs of the nanocomposite (Table 5). A simple comparison shows that a desirable activation of CoFe2O4 occurred by eggshell and SO3H in the nanocomposite, and the best yield was obtained in the presence of CoFe2O4@Eggshell@SO3H nanocomposite (Table 5; entry 4, 8, 12). In Scheme 7, the catalytic mechanism for CoFe2O4@Eggshell@SO3H-catalyzed formylation is demonstrated on the basis of the findings of previous research. (Taheri et al., 2017; Hosseinzadeh-Khanmiri et al., 2018) At first, the alcohol oxidation is carried out with CoFe2O4@Eggshell@SO3H nanocomposite acts as an efficient Brønsted acid catalyst lively active sites especially. CoFe2O4@Eggshell@SO3H protonates the carbonyl group and causes it to be more reactive under the nucleophilic attack of oxygen groups. With the release of protons, a mixture reaction is formed to regenerate the catalyst structure through a proton transfer. The synthesized aldehyde enters the synthesis cycle of multicomponent reactions as a starting material. For this purpose, the reactions mechanism of 4,6-triarylpyridine, and β-amino carbonyl, and 2-amino-4,6-diphenylnicotinonitrile in the presence of CoFe2O4@ Eggshell@SO3H was investigated (Scheme 7).
Reactions
Entry
Catalyst
Time (h)
Yields (%)
2,4,6-triarylpyridine a
1
–
24
Trace
2
CoFe2O4 (0.05 g)
4
35
3
CoFe2O4@Eggshell (0.05 g)
2
75
4
CoFe2O4@Eggshell@SO3H (0.05 g)
2
95
β-amino carbonyl b
5
–
24
Trace
6
CoFe2O4 (0.08 g)
6
40
7
CoFe2O4@Eggshell (0.08 g)
3
80
8
CoFe2O4@Eggshell@SO3H (0.08 g)
3
95
2-amino-4,6-diphenylnicotinonitrile c
9
–
24
Trace
10
CoFe2O4 (0.06 g)
1
45
11
CoFe2O4@Eggshell (0.06 g)
0.3
85
12
CoFe2O4@Eggshell@SO3H (0.06 g)
0.3
97
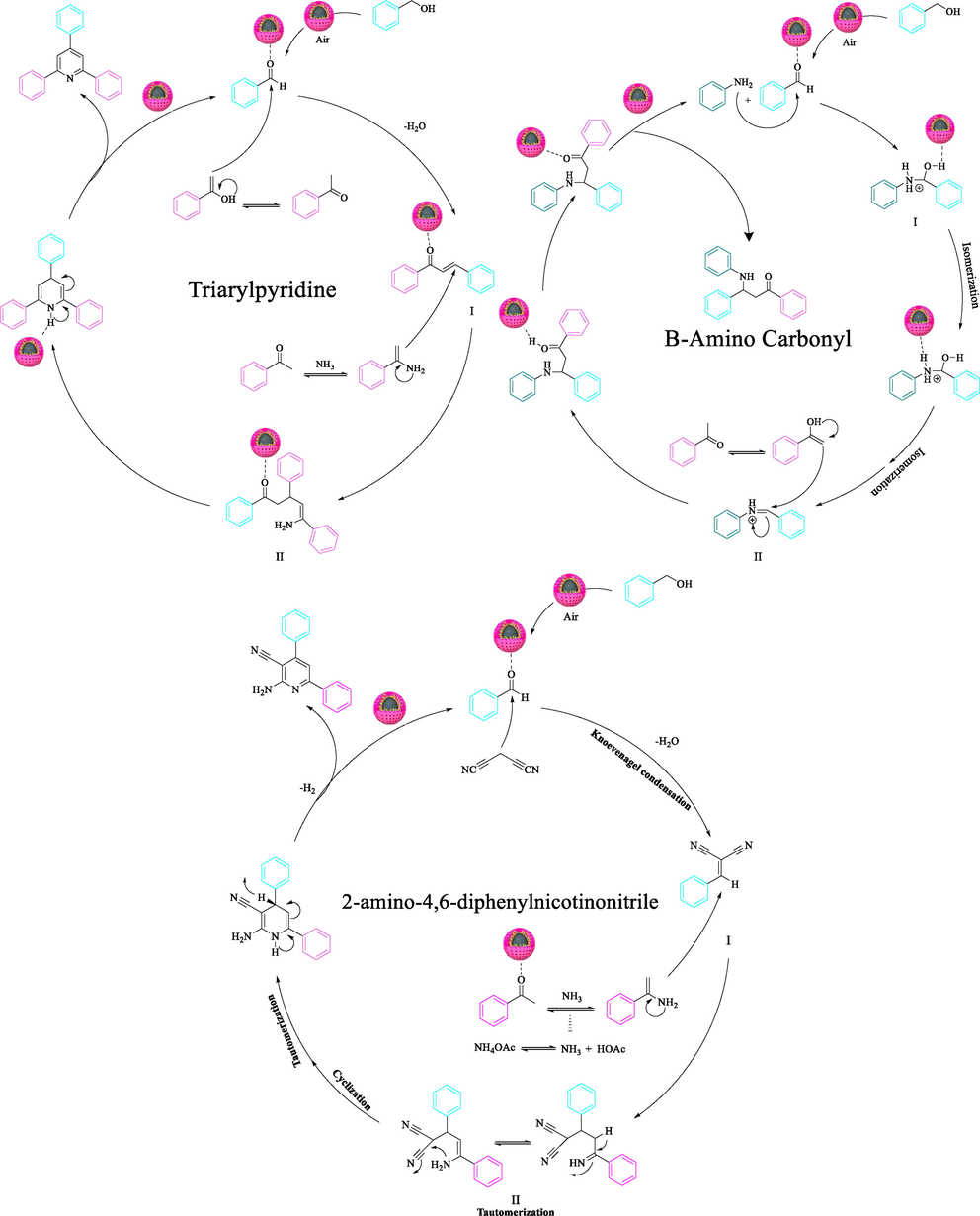
A plausible mechanism for the preparation of 2,4,6-triarylpyridine, and β-amino carbonyl, and 2-amino-4,6-diphenylnicotinonitrile using the CoFe2O4@Eggshell@SO3H nanocomposite.
The 2,4,6-triphenylpyridine reaction involves four steps aldol condensation, Michael addition, cyclization, and finally air oxidation. Condensation of an aldehyde and acetophenone forms and aldol product (I); on the other hand, a molecule of acetophenone with ammonia forms an enamine adduct. The addition of enamine to the aldol product, followed by cyclization, gives dihydropyridine (II). Finally, air oxidation afforded the final product (Scheme 7). Also, a plausible mechanism for synthesizing β-amino carbonyl using the CoFe2O4@Eggshell@SO3H nanocomposite is presented in Scheme 7. Initially, we assumed that the reaction occurs via a condensation, between aldehyde and aniline to form intermediate I on the active sites of the CoFe2O4@Eggshell@SO3H nanocomposite after dehydration. Next, the imine intermediate activated by as CoFe2O4@Eggshell@SO3H nanocomposite Brønsted acid undergoes a nucleophilic attack by enol which in turn converts to the final product and simultaneously releases the catalyst for the next catalytic cycle. This mechanism has been supported by the literature. Finally, a proposed mechanism for the synthesis of 2-amino-4,6-diphenylnicotinonitrile based on scheme 7 is presented. In the first step, the carbonyl aldehyde synthesized with alcohol under aerobic conditions is activated by the nanocomposite CoFe2O4@Eggshell@SO3H and reacts. Immediately, malononitrile reacts with activated arylaldehyde via Knoevenagel condensation followed by dehydration to generate intermediate I. On the other hand, ammonia produced from ammonium acetate reacts with activated acetophenone and forms an enamine mediator. Then enamine reacts with mediator I and forms mediator II. Here, the presence of CoFe2O4@Eggshell@SO3H nanocomposite as a catalyst helps to prepare the desired 2-amino-4,6-diphenylnicotinonitrile products after intramolecular cycle, automerization, and aromatization.
For a better assessment of the catalytic performance of CoFe2O4@Eggshell@SO3H, a fixed of person catalytic experiments were performed with the employing homogeneous, and heterogeneous Brønsted acid catalysts beneath the equal reaction condition with aromatic aldehydes (Fig. 10). It should be noted that CoFe2O4@Eggshell@SO3H nanocomposite, for the first time with very suitable conditions and more activity, has prepared Pyridine and Mannich reactions from alcohols (Fig. 10A-B).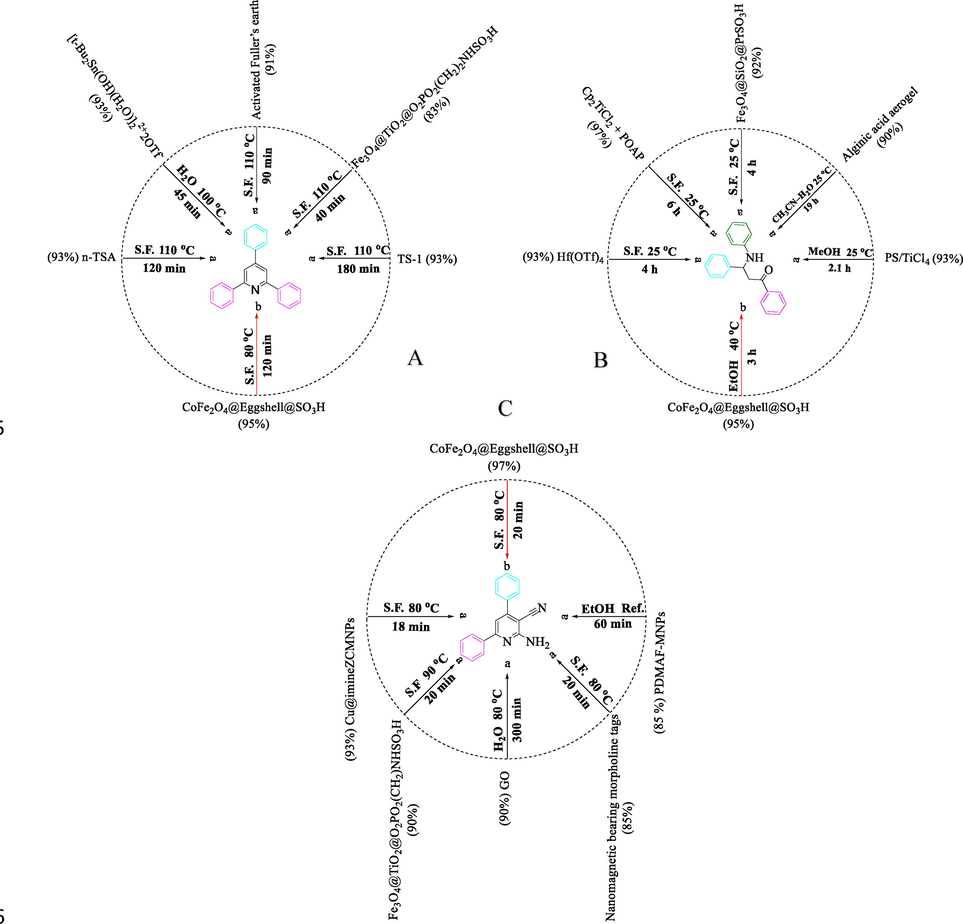
A: a Comparison of the activity of some catalysts in 2,4,6-triarylpyridine reaction of benzaldehyde, ammonium acetate and acetophenone. (Tabrizian et al., 2015; Rekunge et al., 2018; Wang et al., 2019; Zolfigol et al., 2018; Gadekar and Lande, 2018) b (Benzyl alcohol, ammonium acetate, and acetophenone). B: a Comparison of activity of some catalysts in β-amino carbonyl reaction of benzaldehyde, aniline and acetophenone. (Rahmatpour et al., 2019; Zhu et al., 2015; Yeszhanov et al., 2018; Han et al., 2020; Pettignano et al., 2015) b (Benzyl alcohol, aniline, and acetophenone). C: a Comparison of activity of some catalysts in 2-amino-4,6-diphenylnicotinonitrile reaction of benzaldehyde, aniline and acetophenone. (Yahyazadeh et al., 2018; Zolfigol and Yarie, 2017; Khalili, 2016; Kalhor et al., 2019; Bodaghifard et al., 2017) b (Benzyl alcohol; malononitrile, ammonium acetate, and acetophenone).
CoFe2O4@Eggshell@SO3H nanocomposite can recycle greater without problems from the reaction mixture with an external magnet and reuse numerous times without a decrease in catalytic activity. The recyclability of CoFe2O4@Eggshell@SO3H nanocomposite was investigated in model reactions under optimized reaction conditions. After finish reaction, EtOAc was added to the reaction mixture, and the catalyst was isolated by an external magnet, washed with EtOH and EtOAc (2 × 5 mL), and dried under vacuum. The catalyst was successfully recycled five times and the turnover number (TON) in all these five periods is specified. The results showed that this CoFe2O4@Eggshell@SO3H nanocomposite could be easily recovered and reused for several times without significant loss of activity or performance (Fig. 11).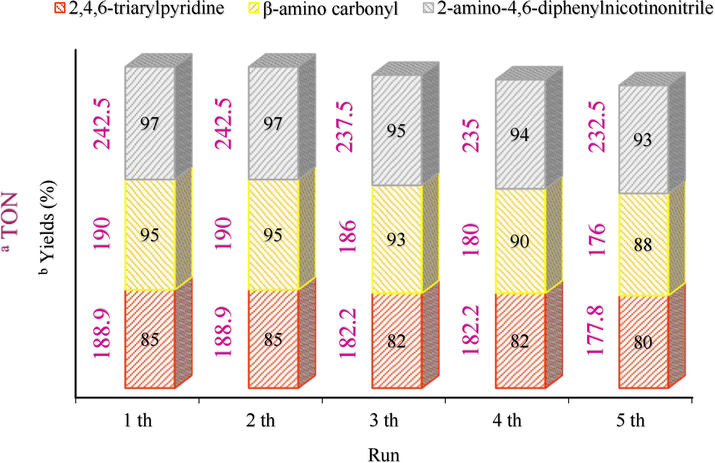
Recovery of nanocomposite in 5 runs for all three model reactions; 2,4,6-triarylpyridine (red), β-amino carbonyl (yellow), and 2-amino-4,6-diphenylnicotinonitrile (blue) a Turnover number; b Isolated yield.
The recyclability of CoFe2O4@Eggshell@SO3H in the model 2,4,6-triarylpyridine reaction was studied. After each run, CoFe2O4@Eggshell@SO3H was separated by a magnet, and washed with EtOH, and finally, dried overnight. Figure S1 shows the satisfactory reusability of the nanocomposite in this reaction (Figure S1 seen in SI file). CoFe2O4@Eggshell@SO3H was proved to be very robust. No significant deactivation was observed during the analogous five reaction cycles. The FESEM image, XRD and FT-IR spectrum of the reused catalyst after 5 cycles are shown in Figure S1a-c. The results were observed to be exactly the same as the pre-reaction spectrum, which indicates the high stability of the nanocomposite in the reaction path (Fig. S1: see in SI file). In order to investigate the heterogeneity of CoFe2O4@Eggshell@SO3H nanocomposite during the reaction, leaching test for model reactions such as 6,4,4-triphenylpyridine in the presence of EtOH (benzyl alcohol/ acetophenone/ammonium acetate), β-aminocarbonyl in the presence of EtOH (benzyl alcohol/acetophenone/aniline) and 2-amino-4,6-diphenylnicotinonitrile in the presence of CH2Cl2 (benzyl alcohol/acetophenone/ ammonium acetate/malononitrile) were performed. The nanocomposite was magnetically removed in situ after half of the reaction time (50 % conversion) from model reactions and the reactants were allowed to undergo further reaction (for 2,4,6-triarylpyridine after 50 % conversion: 20 % yields; for β-amino carbonyl after 50 % conversion: 45 % yields; for 2-amino-4,6-diphenylnicotinonitrile after 50 % conversion: 30 % yields). These results indicated that after the removal of the heterogeneous catalyst, no progress was observed in any of the reactions, indicating the absence of the nanocomposite in the reaction medium and confirming that the synthesized acid nanocomposite acts heterogeneously in the reaction (Table 6). a Reaction conditions: Benzyl alcohol (1 mmol), Acetophenone (2 mmol), Ammonium acetate (1 mmol), EtOH., 80 °C, Catalyst (0.05 g), air as the oxidant. b Reaction conditions: Benzyl alcohol (1 mmol), Acetophenone (1.2 mmol), Aniline (1 mmol), EtOH., 40 °C, Catalyst (0.08 g), air as the oxidant. c Reaction conditions: Benzyl alcohol (1 mmol), Acetophenone (2 mmol), Ammonium acetate (1 mmol), malononitrile (1 mmol), CH2Cl2., 80 °C, Catalyst (0.06 g), air as the oxidant.
Entry
Model reactions
Yields (%) Before of leaching test
Yields (%) After of leaching test
1 a
2,4,6-triarylpyridine
50
20
2b
β-amino carbonyl
95
45
3c
2-amino-4,6-diphenylnicotinonitrile
65
30
4 Conclusion
In total, the current study found a path to the alteration of eggshell waste to a worthy solid catalyst. The functionalization of chlorosulfuric acid on core‐shell CoFe2O3@eggshell gives a heterogeneous, green and recyclable solid acid catalyst for the synthesis of Hantzsh pyridine, and Mannich compounds of various aromatic alcohol are reported for the first time. Furthermore, the prevailing synthesis technique is a type of green, powerful, easy, economical, and environmental strategy for the preparation of other composites with eggshells, and other biomaterials as supports. The highlights of this work were simple work-up procedures, ease of preparation and facile purification, improved product yields, mild reaction conditions, shorter reaction times and recyclability of the catalyst at least 5th times are the main advantages of CoFe2O4@Eggshell@SO3H nano-composite. I hope this work motivates researchers to prepare catalysts from the available waste international. So, research and experimentation into waste-to-wealth conversion are strongly encouraged.
Acknowledgments
We gratefully acknowledge the support of this work by the University of Birjand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of CTOD resistance curves in clamped SE(T) specimens with weld centerline cracks. Eng. Fract. Mech.. 2020;240:107326.
- [Google Scholar]
- Magnetic Fe3O4 nanoparticles in melamine-based ternary deep eutectic solvent as a novel eco-compatible system for green synthesis of pyrido[2,3-d]pyrimidine derivatives. J. Mol. Struct.. 2022;1270:133860
- [CrossRef] [Google Scholar]
- Fe3O4 bonded Pyridinium-3-carboxylic acid-N-sulfonic Acid Chloride as an efficient catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Chem. Methodol.. 2020;4:543-553.
- [CrossRef] [Google Scholar]
- Biosynthesis of organic nanocomposite using Pistacia Vera L. hull: An efficient antimicrobial agent. Medbiotech J. 2021;5:41-48.
- [Google Scholar]
- Use of pectin as a suitable substrate for catalyst synthesis Fe3O4@Pectin@Ni (II) and its application in oxidation reaction. Medbiotech J.. Ghamari kargar, P., 2021;05:1-8.
- [CrossRef] [Google Scholar]
- Synthesis of optically active imidazo[1,2-a]pyrimidin-3(2H)-ones. Mendeleev Commun.. 2005;15:145-146.
- [CrossRef] [Google Scholar]
- Silica-anchored Cu(I) aminothiophenol complex: An efficient heterogeneous catalyst for synthesis of 1,4-disubstituted 1,2,3-triazoles in water. Iran. J. Catal.. 2018;8(3):179-187.
- [Google Scholar]
- Biosynthesis of organic nanocomposite using Pistacia vera L. Hull: an efficient antimicrobial agent. Bioinorg. Chem. Appl.. 2021;2021:1-18.
- [CrossRef] [Google Scholar]
- Nano-cerium oxide/aluminum oxide: an efficient and useful catalyst for the synthesis of Tetrahydro[a]xanthenes-11-one derivatives. Chem. Methodol.. 2021;5:90-95.
- [CrossRef] [Google Scholar]
- (Triazinediyl)bis sulfamic acid-functionalized silica-coated magnetite nanoparticles: preparation, characterization and application as an efficient catalyst for synthesis of mono-, bis-, tris- and spiro-perimidines. J. Iran. Chem. Soc.. 2017;14:365-376.
- [CrossRef] [Google Scholar]
- Esterification of fatty acids to biodiesel over polymers with sulfonic acid groups. Appl. Catal. A Gen.. 2009;359:41-46.
- [CrossRef] [Google Scholar]
- Green synthesis of 2-amino-3-cyanopyridines via a cooperative vinylogous anomeric-based oxidation and their antiproliferative effects on liver, breast, and prostate cancer studies. Synth. Commun. 2021:1-14.
- [CrossRef] [Google Scholar]
- Discrete multi-load truss sizing optimization: model analysis and computational experiments. Optim. Eng.. 2022;23:1559-1585.
- [Google Scholar]
- Effective and convenient synthesis of 2-amino-4H-chromenes promoted by melamine as a recyclable organocatalyst. Eurasian Chem. Commun.. 2021;3:278-290.
- [CrossRef] [Google Scholar]
- Multicomponent Mannich reactions: general aspects, methodologies and applications. Tetrahedron. 2017;73:6977-7004.
- [CrossRef] [Google Scholar]
- Nicotinic acid-supported cobalt ferrite-catalyzed one-pot synthesis of substituted chromeno[3,4-b]quinolines. Appl. Organomet. Chem.. 2020;34
- [CrossRef] [Google Scholar]
- Solid acid catalyst TS-1 zeolite-assisted solvent-free one-pot synthesis of poly-substituted 2,4,6-triaryl-pyridines. Res. Chem. Intermed.. 2018;44:3267-3278.
- [CrossRef] [Google Scholar]
- The anchoring of a Cu(ii)–salophen complex on magnetic mesoporous cellulose nanofibers: green synthesis and an investigation of its catalytic role in tetrazole reactions through a facile one-pot route. RSC Adv. 2021;11:19203-19220.
- [Google Scholar]
- Robust, highly active, and stable supported Co(ii) nanoparticles on magnetic cellulose nanofiber-functionalized for the multi-component reactions of piperidines and alcohol oxidation. RSC Adv.. 2021;11:23192-23206.
- [CrossRef] [Google Scholar]
- A green synthesis strategy of binuclear catalyst for the C-C cross-coupling reactions in the aqueous medium: Hiyama and Suzuki-Miyaura reactions as case studies. Front. Chem.. 2021;9
- [CrossRef] [Google Scholar]
- Simple synthesis of the novel Cu-MOF catalysts for the selective alcohol oxidation and the oxidative cross-coupling of amines and alcohols. Appl. Organomet. Chem.. 2020;34
- [CrossRef] [Google Scholar]
- Novel biocompatible core/shell Fe3O4@NFC@Co(ii) as a new catalyst in a multicomponent reaction: an efficient and sustainable methodology and novel reusable material for one-pot synthesis of 4H-pyran and pyranopyrazole in aqueous media. RSC Adv.. 2020;10:37086-37097.
- [CrossRef] [Google Scholar]
- Design and synthesis of magnetic Fe3O4@NFC-ImSalophCu nanocatalyst based on cellulose nanofibers as a new and highly efficient, reusable, stable and green catalyst for the synthesis of 1,2,3-triazoles. RSC Adv. 2020;10:32927-32937.
- [Google Scholar]
- A porous metal-organic framework (Ni-MOF): An efficient and recyclable catalyst for cascade oxidative amidation of alcohols by amines under ultrasound-irradiations. Mol. Catal.. 2022;526:112372.
- [Google Scholar]
- BioMOF-Mn: An Antimicrobial Agent and an Efficient Nanocatalyst for Domino One-Pot Preparation of Xanthene Derivatives. Inorg. Chem.. 2022;61(28):10678-10693.
- [Google Scholar]
- Nickel nanoparticles adorned on magnetized cellulose nanofibers: ultrasound-facilitated cross coupling reactions. Cellulose. 2022;29(17):9183-9198.
- [Google Scholar]
- Cu/cellulose-modified magnetite nanocomposites as a highly active and selective catalyst for ultrasound-promoted aqueous O-arylation Ullmann and sp-sp2 Sonogashira cross-coupling reactions. Sustain. Chem. Pharm.. 2022;27:100672
- [CrossRef] [Google Scholar]
- Introduction of a trinuclear manganese(iii) catalyst on the surface of magnetic cellulose as an eco-benign, efficient and reusable novel heterogeneous catalyst for the multi-component synthesis of new derivatives of xanthene. RSC Adv. 2021;11:4339-4355.
- [Google Scholar]
- A novel water‐dispersible and magnetically recyclable nickel nanoparticles for the one‐pot reduction‐Schiff base condensation of nitroarenes in pure water. J. Chinese Chem. Soc.. 2021;68:1916-1933.
- [Google Scholar]
- Synthesis, characterization and cytotoxicity evaluation of a novel magnetic nanocomposite with iron oxide deposited on cellulose nanofibers with nickel (Fe3O4@NFC@ONSM-Ni) RSC Adv.. 2021;11:17413-17430.
- [CrossRef] [Google Scholar]
- Magnetic nanoparticles embedded in pectin‐based as an environmentally friendly recyclable nanocatalyst. Medbiotech J. 2021;5:9-14.
- [Google Scholar]
- Copper (II), supported on a post-modified magnetic Pectin Fe3O4@Pectin∼Imidazole∼SO3H-Cu(II): an efficient biopolymer-based catalyst for selective oxidation of alcohols with aqueous TBHP. Sci. Iran 2021
- [CrossRef] [Google Scholar]
- Synthesis of cellulose nanofibers-based ImSalophen@Fe3O4 as a green sorbent for magnetic solid-phase extraction of chlorophenols followed by quantification via high-performance liquid chromatography-ultraviolet detection. Microchem. J.. 2023;187:108368.
- [Google Scholar]
- Implementation of NCHRP 9-44A Fatigue Endurance Limit Prediction Model in Mechanistic-Empirical Asphalt Pavement Analysis Web Application. Transp. Res. Rec. J. Transp. Res. Board. 2022;2676:696-706.
- [Google Scholar]
- Glycolic acid-supported cobalt ferrite-catalyzed one-pot synthesis of pyrimido[4,5-b]quinoline and indenopyrido[2,3-d]pyrimidine derivatives. Appl. Organomet. Chem.. 2020;34
- [CrossRef] [Google Scholar]
- S. V. Goswami, P.B. Thorat, A. V. Chakrawar, S.R. Bhusare, A mild and efficient one-pot synthesis of $$\upbeta $$ -amino carbonyl compounds via Mannich reaction under ambient temperature condition, Mol. Divers. 17 (2013) 33–40. https://doi.org/10.1007/s11030-012-9414-x.
- Selectivity enhancement of silica-supported sulfonic acid catalysts in water by coating of ionic liquid. Org. Lett.. 2007;9:3145-3148.
- [CrossRef] [Google Scholar]
- Convenient Cr(VI) removal from aqueous samples: executed by a promising clay-based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid. ChemistrySelect. 2020;5:2441-2448.
- [CrossRef] [Google Scholar]
- Hf(OTf)4 as a highly potent catalyst for the synthesis of mannich bases under solvent-free conditions. Molecules. 2020;25:388.
- [CrossRef] [Google Scholar]
- Using magnetic nanoparticles Fe3O4 as a reusable catalyst for the synthesis of pyran and pyridine derivatives via one-pot multicomponent reaction. J. Iran. Chem. Soc.. 2015;12:2075-2081.
- [Google Scholar]
- How deep can forest vegetation cover extend their hydrological reinforcing contribution? Hydrol. Process.. 2018;32:2570-2583.
- [Google Scholar]
- Modeling And Simulation And Imaging Of Blood Flow In The Human Body. NVEO-NATURAL VOLATILES Essent. OILS. 2021;8:13235-13244.
- [Google Scholar]
- Sulfonic acid-functionalized silica-coated magnetic nanoparticles as a reusable catalyst for the preparation of pyrrolidinone derivatives under eco-friendly conditions. Silicon. 2019;11:2933-2943.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of a Ni nanoparticle stabilized on Ionic liquid-functionalized magnetic Silica nanoparticles for tandem oxidative reaction of primary alcohols. Appl. Organomet. Chem.. 2018;32:e4452.
- [Google Scholar]
- Structural, dielectric and magnetic properties of Mn+2 doped cobalt ferrite nanoparticles. J. Magn. Magn. Mater.. 2020;494:165726
- [CrossRef] [Google Scholar]
- A New Microfluidic Device Integrated With Quartz Crystal Microbalance to Measure Colloidal Particle Adhesion. In: Biomedical and Biotechnology. Vol 5. American Society of Mechanical Engineers; 2021.
- [Google Scholar]
- Proline-Cu complex based 1,3,5-Triazine coated on Fe3O4 magnetic nanoparticles: a nanocatalyst for the knoevenagel condensation of aldehyde with malononitrile. ACS Appl. Nano Mater.. 2022;5:1783-1797.
- [CrossRef] [Google Scholar]
- Novel magnetic nanoparticles with morpholine tags as multirole catalyst for synthesis of hexahydroquinolines and 2-amino-4,6-diphenylnicotinonitriles through vinylogous anomeric-based oxidation. Res. Chem. Intermed.. 2019;45:3453-3480.
- [CrossRef] [Google Scholar]
- Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater. Sci. Eng. C.. 2019;98:545-549.
- [CrossRef] [Google Scholar]
- The influence of synthesis parameters on one-step synthesized superparamagnetic cobalt ferrite nanoparticles with high saturation magnetization. J. Magn. Magn. Mater.. 2019;473:262-267.
- [CrossRef] [Google Scholar]
- A concise and versatile synthesis of 2-amino-3-cyanopyridine derivatives in 2,2,2-trifluoroethanol. J. Fluor. Chem.. 2012;142:41-44.
- [CrossRef] [Google Scholar]
- Graphene oxide: a reusable and metal-free carbocatalyst for the one-pot synthesis of 2-amino-3-cyanopyridines in water. Tetrahedron Lett.. 2016;57:1721-1723.
- [CrossRef] [Google Scholar]
- A green method for synthesizing nickel nanoparticles supported by magnetized pectin: applied as a catalyst for aldehyde synthesis as a precursor in Xanthan synthesis. ChemistrySelect. 2020;5:13537-13544.
- [CrossRef] [Google Scholar]
- New Acetamidine Cu(II) Schiff base complex supported on magnetic nanoparticles pectin for the synthesis of triazoles using click chemistry. Sci. Rep.. 2022;12:3771.
- [CrossRef] [Google Scholar]
- Waste to wealth: conversion of nano-magnetic eggshell (Fe3O4@Eggshell) to Fe3O4@Ca(HSO4)2: cheap, green and environment-friendly solid acid catalyst. Appl. Organomet. Chem.. 2018;32:e4308.
- [Google Scholar]
- Promising new catalytic properties of a Co (II)-carboxamide complex and its derived Co3O4 nanoparticles for the Mizoroki-Heck and the Epoxidation reactions. Appl. Organomet. Chem.. 2020;34
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione. Eur. J. Med. Chem.. 2004;39:433-447.
- [CrossRef] [Google Scholar]
- (±)-Camphor-10-sulfonic acid catalyzed direct one-pot three-component Mannich type reaction of alkyl (hetero)aryl ketones under solvent-free conditions: application to the synthesis of aminochromans. RSC Adv.. 2012;2:480-486.
- [CrossRef] [Google Scholar]
- Magnetic properties of cobalt ferrite octahedrons obtained from calcination of granular nanotubes growing on bacterial nanocellulose. J. Magn. Magn. Mater.. 2020;495:165899
- [CrossRef] [Google Scholar]
- Mannich reactions catalyzed by perchloric acid in Triton X10 aqueous micelles. Catal. Commun.. 2010;11:745-748.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of magnetic Co nanoparticles: a comparison study of three different capping surfactants. J. Solid State Chem.. 2008;181:1530-1538.
- [CrossRef] [Google Scholar]
- Chemical synthesis and magnetic properties of monodisperse cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron.. 2019;30:14913-14922.
- [CrossRef] [Google Scholar]
- Utilization of eggshell waste as green catalyst for application in the synthesis of 1,2,4,5-tetra-substituted imidazole derivatives. Res. Chem. Intermed.. 2021;47:2173-2188.
- [CrossRef] [Google Scholar]
- Crystal chemistry and competing magnetic exchange interactions in oxide garnets and spinels. J. Solid State Chem.. 2019;274:1-9.
- [CrossRef] [Google Scholar]
- Chitosan synergistically enhanced by successive Fe3O4 and silver nanoparticles as a novel green catalyst in one-pot, three-component synthesis of tetrahydrobenzo[α]xanthene-11-ones. J. Mol. Catal. A Chem.. 2014;393:309-316.
- [CrossRef] [Google Scholar]
- Magnetically recoverable catalysts for the preparation of pyridine derivatives: an overview. RSC Adv.. 2021;11:17456-17477.
- [CrossRef] [Google Scholar]
- Sulfonic acid-functionalized catalysts for the valorization of glycerol via transesterification with methyl acetate. Ind. Eng. Chem. Res.. 2011;50:5898-5906.
- [CrossRef] [Google Scholar]
- Life and death of colloidal bonds control the rate-dependent rheology of gels. Nat. Commun.. 2021;12:4274.
- [Google Scholar]
- Sulfonated diatomite as heterogeneous acidic nanoporous catalyst for synthesis of 14-aryl-14-H-dibenzo[a, j]xanthenes under green conditions. Appl. Catal. A Gen.. 2014;477:132-140.
- [CrossRef] [Google Scholar]
- Apuri, HClO4–SiO2 as a novel and recyclable catalyst for the synthesis of 2,4,6-triarylpyridines under solvent-free conditions. Catal. Commun.. 2007;8:1973-1976.
- [CrossRef] [Google Scholar]
- Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites. Appl. Catal. B Environ.. 2016;191:209-227.
- [CrossRef] [Google Scholar]
- Alginic acid aerogel: a heterogeneous Brønsted acid promoter for the direct Mannich reaction. New J. Chem.. 2015;39:4222-4226.
- [CrossRef] [Google Scholar]
- Cobalt ferrite supported on carbon nitride matrix prepared using waste battery materials as a peroxymonosulfate activator for the degradation of levofloxacin hydrochloride. Chem. Eng. J.. 2020;379:122377
- [CrossRef] [Google Scholar]
- Doxorubicin hydrochloride - loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol.. 2018;116:378-384.
- [CrossRef] [Google Scholar]
- Titanium tetrachloride incorporated crosslinked polystyrene copolymer as an efficient and recyclable polymeric Lewis acid catalyst for the synthesis of Β -amino carbonyl compounds at room temperature. Synth. Commun. 2019:1-16.
- [CrossRef] [Google Scholar]
- An efficient, green solvent-free protocol for the synthesis of 2,4,6-triarylpyridines using reusable heterogeneous activated Fuller’s earth catalyst. J. Iran. Chem. Soc.. 2018;15:2455-2462.
- [CrossRef] [Google Scholar]
- One-pot expeditious synthesis of 2-Amino-4,6-(disubstituted)nicotinonitriles using activated fuller’s Earth as catalyst. Org. Prep. Proced. Int.. 2021;53:112-119.
- [CrossRef] [Google Scholar]
- A. reza M. Jalal Albadi, Heshmat allah Samimi, Alumina-Supported Cobalt Nanoparticles Efficiently Catalyzed the Synthesis of Chromene Derivatives under Solvent-Free Condition, Chem. Methodol. 4 (2020) 565–571. https://doi.org/https://doi.org/10.22034/chemm.2020.107071.
- Design of a Schiff Base complex of copper coated on epoxy-modified core-shell MNPs as an environmentally friendly and novel catalyst for the one-pot synthesis of various chromene-annulated heterocycles. ACS Omega. 2021;6:25608-25622.
- [CrossRef] [Google Scholar]
- Magnetic silica-coated picolylamine copper complex [Fe3O4@SiO2 @GP/Picolylamine-Cu(II)]-catalyzed Biginelli Annulation reaction. Inorg. Chem.. 2022;61:992-1010.
- [CrossRef] [Google Scholar]
- Increased SBA-15-SO3H catalytic activity through hydrophilic/hydrophobic fluoroalkyl-chained alcohols (RFOH/SBA-15–Pr-SO3H) Synlett. 2015;26:1345-1347.
- [CrossRef] [Google Scholar]
- The effect of agarose content on the morphology, phase evolution, and magnetic properties of CoFe2O4nanoparticles prepared by sol-gel autocombustion method. Int. J. Appl. Ceram. Technol.. 2018;15:758-765.
- [CrossRef] [Google Scholar]
- Ultrasound assisted the green synthesis of 2-amino-4H-chromene derivatives catalyzed by Fe3O4-functionalized nanoparticles with chitosan as a novel and reusable magnetic catalyst. Ultrason. Sonochem.. 2015;22:341-348.
- [CrossRef] [Google Scholar]
- Ultrasound-promoted an efficient method for one-pot synthesis of 2-amino-4,6-diphenylnicotinonitriles in water: a rapid procedure without catalyst. Ultrason. Sonochem.. 2012;19:1061-1069.
- [CrossRef] [Google Scholar]
- Magnetic CoFe2O4 nanoparticles doped with metal ions: a review. Ceram. Int.. 2020;46:18391-18412.
- [CrossRef] [Google Scholar]
- Sequential and direct multicomponent reaction (MCR)-based dearomatization strategies. Chem. Soc. Rev.. 2020;49:8721-8748.
- [CrossRef] [Google Scholar]
- H. Stadelman, W. J., & Schmieder, Functional uses of eggs: An overview. Eggs and health promotion, (2002) 3–8.
- Towards the prediction of hydrogen–induced crack growth in high–graded strength steels. Thin-Walled Struct. 2021;159:107245.
- [Google Scholar]
- One-pot, solvent-free and efficient synthesis of 2,4,6-triarylpyridines catalyzed by nano-titania-supported sulfonic acid as a novel heterogeneous nanocatalyst. Chinese Chem. Lett.. 2015;26:1278-1282.
- [CrossRef] [Google Scholar]
- Application of polydopamine sulfamic acid-functionalized magnetic Fe3O4 nanoparticles (Fe3O4@PDA-SO3H) as a heterogeneous and recyclable nanocatalyst for the formylation of alcohols and amines under solvent-free conditions. New J. Chem.. 2017;41:5075-5081.
- [CrossRef] [Google Scholar]
- Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small. 2020;16:2002733.
- [CrossRef] [Google Scholar]
- S. Talha, N. S., & Sulaiman, Overview of catalysts in biodiesel production., ARPN J. Eng. Appl. Sci. 11 (2016) 439–442.
- V.N.K. and B.R. J. Venu Madhav, P. Someshwar, Dipyridine copper chloride-catalysed one-pot synthesis of β-amino carbonyl compounds via Mannich reaction, J. Chem. Res. (2008) 201–202.
- Constructing robust covalent organic frameworks via multicomponent reactions. J. Am. Chem. Soc.. 2019;141:18004-18008.
- [CrossRef] [Google Scholar]
- Cationic organotin cluster [t -Bu2Sn(OH)(H2O)]22+ 2OTf − -catalyzed one-pot three-component syntheses of 5-substituted 1 H -tetrazoles and 2,4,6-triarylpyridines in water. Appl. Organomet. Chem.. 2019;33
- [CrossRef] [Google Scholar]
- Sustainable biodiesel production from low-quantity oils utilizing H6PV3MoW8O40 supported on magnetic Fe3O4/ZIF-8 composites. Renew. Energ.. 2021;168:927-937.
- [CrossRef] [Google Scholar]
- Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: a magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel. 2021;283:118893
- [CrossRef] [Google Scholar]
- Four-component synthesis of 2-Amino-3-Cyanopyridine derivatives catalyzed by Cu@imineZCMNPs as a novel, efficient and simple nanocatalyst under solvent-free conditions. Catal. Lett.. 2018;148:1254-1262.
- [CrossRef] [Google Scholar]
- Copper nanotube composite membrane as a catalyst in Mannich reaction. Chem. Pap.. 2018;72:3189-3194.
- [CrossRef] [Google Scholar]
- Poly(sulfonamide-thiourea) grafted γ-Fe2O3@talc as a green catalyst for the synthesis of substituted tetrahydropyridines. Mater. Chem. Phys.. 2021;270:124840
- [CrossRef] [Google Scholar]
- Chemoselective synthesis of geminal diacetates (acylals) using eco-friendly reusable propylsulfonic acid based nanosilica (SBA-15-Ph-PrSO3H) under solvent-free conditions. J. Mol. Catal. A Chem.. 2013;378:227-231.
- [CrossRef] [Google Scholar]
- Recyclable CMK-5 supported sulfonic acid as an environmentally benign catalyst for solvent-free one-pot construction of coumarin through Pechmann condensation. J. Mol. Catal. A Chem.. 2014;391:88-91.
- [CrossRef] [Google Scholar]
- Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale. 2020;12:3855-3870.
- [CrossRef] [Google Scholar]
- Development of β-amino-carbonyl compounds as androgen receptor antagonists. Acta Pharmacol. Sin.. 2014;35:664-673.
- [CrossRef] [Google Scholar]
- Titanocene dichloride and poly(o-aminophenol) as a new heterogeneous cooperative catalysis system for three-component Mannich reaction, Catal. Sci. Technol.. 2015;5:4346-4349.
- [CrossRef] [Google Scholar]
- Application of biological-based nano and nano magnetic catalysts in the preparation of arylbispyranylmethanes. RSC Adv.. 2016;6:92862-92868.
- [CrossRef] [Google Scholar]
- Catalytic application of sulfonic acid-functionalized titana-coated magnetic nanoparticles for the preparation of 1,8-dioxodecahydroacridines and 2,4,6-triarylpyridines via anomeric-based oxidation. Appl. Organomet. Chem.. 2018;32
- [CrossRef] [Google Scholar]
- Fe3O4@TiO2@O2PO2 (CH2)NHSO3 H as a novel nanomagnetic catalyst: Application to the preparation of 2-amino-4,6-diphenylnicotinonitriles via anomeric-based oxidation. Appl. Organomet. Chem.. 2017;31:e3598.
- [Google Scholar]
Appendix A
Supplementary material
Support information is available for free. All chemicals and device specifications, CHN and 1H NMR spectral information is located in the supporting information. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104564.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







