Translate this page into:
Phytochemical and bioactivity evaluation of secondary metabolites and essential oils of Sedum rubens growing wild in Jordan
⁎Corresponding author. hala.aljaber@bau.edu.jo (Hala I. Al-Jaber)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Sedum rubens L. (Crassulaceae family) is an interesting succulent medicinal plant that has never been investigated for its phytochemical constituents. Thus, the current study was designed to unveil its chemical constituents and bioactivity potentials. In the current study, the total phenol content (TPC), total flavonoid content (TFC) and DPPH radical scavenging properties of the hydroalcoholic (HA) and water extracts (W) were determined. Moreover, the presence of selected phenolic acids (gallic acid, chlorogenic acid, caffeic acid) and flavonoids (rutin, quercetin, hesperidin) was determined by HPLC-PDA. In addition, hydro-distilled essential oil (HDEO) composition of the plant at the pre-flowering (PF) and full-flowering (FF) stages was determined by GC/MS and GC/FID techniques. Results revealed that the FF hydroalcoholic extract had the highest TPC (136.9 mg gallic acid/g extract), TFC (234.7 mg quercetin/g extract) and DPPH• radical scavenging activity (7.10 × 10-2 ± 1.0 × 10-3 mg/mL). This extract was rich in gallic acid and caffeic acids (366, 243 mg/Kg dry plant, respectively). The study resulted in reporting four known compounds including α- & β-amyrin acetates, β-sitosterol and β-sitosterol glycoside for the first time from the plant. The HDEO at the PF and FF stages were dominated by oxygenated sesquiterpenes (21.92%) and aliphatic hydrocarbons (45.71%).
Keywords
Sedum rubens
Secondary metabolites
TPC
TFC
Antioxidant activity
HPLC-PDA
1 Introduction
Sedum genus, is considered among the largest genera belonging to the Crassulaceae family, comprising about 500 species (Hassan et al., 2021; Bensouici et al., 2016; Eid and Gonaid, 2018; Thiede and Eggli, 2007). Sedum plants, characterized by their succulent leaves and stems, are known to grow out of bare rocks and stones in their natural habitats, and thus are known as stonecrops (Diermen, 2009). These plants are widely distributed in temperate and subtropical regions of the world but mainly reported to grow wild in the Mediterranean climatic zone (Ito et al., 2017; Stephenson, 1994; Thiede and Eggli, 2007). Members of the Sedum genus have been used in traditional medicine for the treatment of many ailments including bladder infections, constipation, scurvy disease, jaundice, hepatitis and epilepsy (Romojaro et al., 2013; Liu et al., 2021; Winekenstädde et al., 2015), and many species belonging to this genus were recognized for their anti-inflammatory (Altavilla et al., 2008), antibacterial (Bensouici et al., 2016), antioxidant (Thuong et al., 2007), antinociceptive (Jung et al., 2008), hepatoprotective and anticancer activities (Kang et al., 2000; Camargo et al., 2002). Previous phytochemical screening studies revealed that Sedum plants contained different classes of secondary metabolites including terpenoids, flavonoids, and alkaloids (Al-Qudah et al., 2012; Stevens et al., 1994; Kim et al., 1996; Şöhretoğlu & Sabuncuoğlu., 2012; Şöhretoğlu et al., 2016; Yue-ling et al., 2017).
There are nine Sedum species reported to grow wild in the Flora of Jordan (Al-Eisawi, 1998) including S. hispanicum L., S. rubens L., S. pallidum Bieb., S. litoreum Guss., S. nicaeense All., S. microcarpum (Sm.) Schönl., S. caespitosum Cav., S. laconicum Boiss. and Heldr. and S. palaestinum Bioss. Despite the reputation of seveal Sedum species in in traditional medicine, literature survay revealed that S. rubens has never been investigated for its phytochemical constituents or bioactivity potentials.
S. rubens (Fig. 1), a Dwarf, annual, succulent, brownish herb, 3–5 cm long, recognized by its tiny pinkish flowers (9 mm) having five stamens, is reported to grow in mountainous rocky places of Jordan including Irbid, Jarash, Al-Salt and Amman (Al-Eisawi, 1998). Driven by our interest in phytochemical analysis and bioactivity evaluation of medicinal plants growing wild in Meditteranean region (Al-Jaber et al., 2020; Al-Jaber et al., 2019; Al-Jaber et al., 2018; Abu Zarga et al., 2021; Barhoumi et al., 2021; Al-Qudah et al., 2014; Al-Jaber et al., 2012), this work was designed to unveil the volatile and nonvolatile chemistry of S. rubens from Jordan and to shed more light on its bioactivity potentials. Accordingly, the hydro-alcoholic (HA) and water (W) extracts obtained from S. rubens were assayed for their total phenol content (TPC), total flavonoid content (TFC) and antioxidant activity using the DPPH• assay method. The most active extract was then investigated for the presence of certain selected bioactive phenolic acids (gallic acid, caffeic acid, chlorogenic acid) and flavonoids (catechin, rutin, hesperidin) using HPLC-PDA. Also, two terpene acetals (α- & β-amyrine acetates) and two sterols (β-sitosterol, and β-sitosterol glycoside) were isolated and are reported here for the first time from this species. The chemical composition of the hydro-distilled essential oils (HD-EO) obtained from fresh plant material at the pre-flowering (PF) and full flowering (FF) growth stages was also determined using GC/MS technique.
Sedum rubens L. from Jordan.
2 Materials and methods
2.1 Instrumentation
UV spectra were measured using the Lightwave II spectrophotometer (USA, Yarmouk University). 1H NMR spectra were recorded on Bruker NMR spectrometers (400 MHz, Hashemite University, Jordan) with TMS as an internal standard. 13C NMR spectra were recorded at 100.0 MHz. Clevenger-type apparatus (Al-Balqa Applied University) was used to extract the essential oils by hydro-distillation. IR spectra were measured on the Bruker INVENIOS spectrophotometer (Chemistry Department, Faculty of Science, Al-Balqa Applied University). Mass spectra were recorded at Bruker apex-IV (Germany, The University of Jordan). HPLC-PDA analysis was performed on Shimadzu-A20 HPLC (Japan, Chemistry Department, Faculty of Science, Al-Balqa Applied University). GC/MS analysis of essential oil constituents was performed on Varian Chrompack CP-3800 GC–MS-MS-200 (Saturn, Netherlands) equipped with DB-5 (5 % diphenyl, 95 % dimethyl polysiloxane) GC capillary column (30 m × 0.25 mm i.d., 0.25 μm film thicknesses). Quantitative analysis was conducted on a Hewlett-Packard HP-8590 gas chromatograph equipped with a split-splitless injector (split ratio 1:50) and an FID detector was used. The column was an optima-5 (5 % diphenyl, 95 % dimethyl polysiloxan) fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness).
2.2 Plant material
The plant material was collected from Shafa-badran (32°03′07″N, 35°54′03″E), 20 km to the north of the capital Amman, Jordan, during the spring season-2021 at the pre-flowering (PF, March) and full flowering (FF, April) stages. The taxonomic identity of the plant was confirmed by Prof. Dr. Hala I. Al-Jaber, Department of Chemistry, Faculty of Science, Al-Balqa Applied University, Al-Salt, Jordan. A voucher specimen (No: C/SR/2020) was deposited at the herbarium of the Faculty of Science, Al-Balqa Applied University, Al-Salt, Jordan.
2.2.1 Extracts preparation
Hydroalcoholic (Sr-HA) and water (Sr-W) extracts were prepared from S. rubens at different growth stages (PF & FF) according to the procedure described in the literature (Al-Jaber, 2016) with little modification. Briefly, a 300 g sample of the plant material at each growth stage was refluxed in 1000 mL ethanol–water (70:30; v/v). The solvent was then evaporated affording the HA extract (HA-PF: 7.2 g; HA-FF: 7.8 g). The same procedure was followed for the preparation of the W extract (W-PF: 11.7 g; W-FF: 15.3 g). These extracts were then assayed for their TPC, TFC, antioxidant activity using the DPPH• method and for their antimicrobial activity.
2.2.2 Extraction and isolation of components
Isolation of nonvolatile secondary metabolites from plant material was performed according to the procedure listed in the literature with little modification (Odeh and Al-Jaber, 2022; Al-Jaber et al., 2012). Briefly, air-dried plant material (2.4 Kg) was defatted by extraction with hexane (20 L) at room temperature for 7 days. The defatted and dried plant residue was then extracted repeatedly (5 times, 20 L, 7 days each) with a mixture of ethanol:water (70:30, EtOH:H2O; v/v). The combined extracts were then evaporated under vacuum yielding a crude gummy residue (SRE, 120 g). The dried residue (100 g) was then adsorbed on 100 g silica gel 60 and chromatographed in a column packed in CH2Cl2 (DCM) and eluted with a gradient mixture of DCM:MeOH of increasing polarity. A total of 143 fractions (500 mL each) were collected and then consolidated into 10 groups according to their TLC behavior (SRE Ι - SRE X). Further purification and isolation of secondary metabolites was achieved by a combination of CC, thin layer chromatography (TLC), and/or recrystallization.
Fraction SRE I (1.0 g) afforded α-amyrine acetate (80 mg) and β-amyrine acetate (90 mg). Fraction SRE III (2.0 g) afforded β-Sitosterol (21 mg). Fraction IX afforded β-sitosterol glycoside (40 mg). All compounds were identified based on their spectral data (1D, 2D NMR, HREIMS, IR).
2.3 Determination of total phenol (TP) and total flavonoid (TF) contents
The TPC and TFC were performeusing the Folin- Ciocalteu and the Aluminum chloride methods, respctively and according to the procedure descibed in the literature (Al-Jaber 2017; Al-Jaber et al., 2020).
2.4 DPPH• free radical scavenging activity
The DPPH• radical scavenging capacity of the Sr-HA and Sr-W extracts obtained from the fresh S. rubens at the different flowering stages was determined according to the procedure listed in the literature (López-Alarcón & Denicola, 2013; Al-Qudah et al., 2014).
2.5 HPLC-PDA analysis parameters
Analysis of some selected phenolic acids and flavonoids was performed on Shimadzu-A20 HPLC (Japan) equipped with a PDA detector (at 290 nm), utilizing C-18 column (Phenomenex Luna, 250 × 4.6 mm, 5 µm, ambient temperature). The analyzed samples were freshly prepared in HPLC grade methanol, and then filtered through a 0.45 μm nylon filtration disk prior to analysis. An isocratic elution mode was employed using a mobile phase mixture consisting of 70% A: (water: formic acid (99:1; v/v)) and 30% B: (methanol). The flow rate was 1 mL/min and the injection volume was 20 µL.
A stock solution of the screened phenolic acids and flavonoids (caffeic acid, catechin, chlorogenic acid, gallic acid (100 ppm each), rutin (40 ppm) and hesperidin (20 ppm)) was prepared in HPLC-grade methanol. Sample solution obtained from SRE (4000 ppm) was prepared and then assayed immediately for its phenolic acids and flavonoids compounds using HPLC-PDA. The concentration of detected compounds was done based on calibration curves for authentic compounds.
2.6 Antimicrobial activity
In-vitro antibacterial activity of the Sr-HA and Sr-W extracts at the PF and FF stages were examined against three gram-positive bacteria (Staphylococcus aureus (ATCC 29213), Staphylococcus epidermidis (ATCC12228), and Enterococcus faecalis (ATCC 19433)), and two gram-negative bacteria: (Salmonella (ATCC 104799) and Escherichia coli (ATCC 2452)) using the agar well diffusion method according to the procedure described in the literature (Al-Qudah et al., 2012; Al-Jaber 2016). Positive controls used were trimethoprim (5 μg/mL, gram positive) and tobramycin (10 μg/mL, gram negative).
2.7 Extraction of essential oils, GC/MS and GC/FID analysis
Essential oils (EOs) were extracted from fresh plant material according to the procedure described in the literature (Al-Jaber et al., 2018, Al-Jaber 2017). Briefly, 300 g sample of fresh plant material collected at each flowering stage, was coarsely powdered and then hydro-distilled for 3 hours in a Clevenger type apparatus. The essential oil was then extracted (twice) with GC-grade n-hexane. The obtained HD-EOs were dried using anhydrous MgSO4 and then stored in amber glass vials at 4 °C until analysis was performed.
Analysis of the chemical constituents of the HD-EOs was performed according to the procedure described in the literature, using the same instruments, columns with the same temperature program described in our previous work (Al-Jaber et al., 2018; Al-Jaber 2017; Adams, 2017). Identification of the HD-EO components was achieved based on their experimentally determined GC/MS retention indices with reference to a homologous series of C8-C20 n-alkanes values measured with columns of identical polarity, and by comparing their mass spectra to those in the built libraries (Nist Co and Wiley Co, USA) and/or authentic samples. The relative peak areas were measured and used to calculate the concentration of the detected compounds.
3 Results and discussion
In the current study, Sr-HA and Sr-W extracts obtained from fresh S. rubens at the PF and FF stages were assayed for their TPC, TFC and DDPH• radical scavenging capacity (Table 1). The results clearly revealed that the HA extract at the FF stage had the highest TPC and TFC (136.90 ± 0.02 mg gallic acid/g dry extract; 234.68 ± 0.01 mg quercetin/g dry extract, respectively) when compared to W extract at the FF stage or to those obtained during the PF stage. The results also indicated that this extract (FF-HA) had also the highest DPPH• radical scavenging activity (IC50 7.1 × 10-2 ± 1.0 × 10-3 mg/mL).
Extracts
TPC
TFC
DPPH•
IC50 (mg/mL)
PF
HA
107.70 ± 0.02
116.78 ± 0.01
8.7 × 10-2 ± 3.0 × 10-3
W
23.90 ± 0.01
20.54 ± 0.01
0.134 ± 5.0 × 10-3
FF
HA
136.90 ± 0.02
234.68 ± 0.01
7.10 × 10-2 ± 1.0 × 10-3
W
22.30 ± 0.01
45.12 ± 0.01
0.194 ± 1.8 × 10-2
Ascorbic acid
–
–
4.20 × 10-3 ± 1.0 × 10-4
The HA extract was subjected to HPLC-PDA analysis to determine the presence of six selected compounds that included three phenolic acids (gallic, caffeic and chlorogenic) and three flavonoids (rutin, quercetin and hesperidin). Of these six compounds, chlorogenic acid and hesperidin were not detected at all. The other compounds were detected at appreciable concentration levels (Table 2, Fig. S2) that accounted for the high TPC, TFC and DPPH• radical scavenging activity. The extract was especially rich in both gallic acid and caffeic acid (366, 243 mg/Kg dry plant, respectively). It is worth mentioning that all four compounds detected by HPLC-PDA method in the current study are reported here for the first time from S. rubens.
Compound
Structure
Formula
Rt (min)
Concentration
(mg compound/Kg dry plant)
Gallic acid
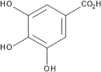
C7H6O5
3.658
366
Caffeic acid
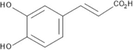
C9H8O4
8.717
243
Rutin

C27H30O16
26.102
199
Hesperidin

C28H34O15
32.613
152
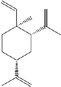
Moreover, two triterpenes and two sterols (Fig. 2) were isolated and characterized using spectroscopic techniques like NMR (1D & 2D), FT-IR and HR-ESIMS (Fig. 3). These compounds included α-amyrin acetate (1), β-amyrin acetate (2), β-sitosterol (3) (Okoye et al., 2014), and β-sitosterol glycoside (4) (Peshin & Kar, 2017), all of which are also reported for the first time from S. rubens.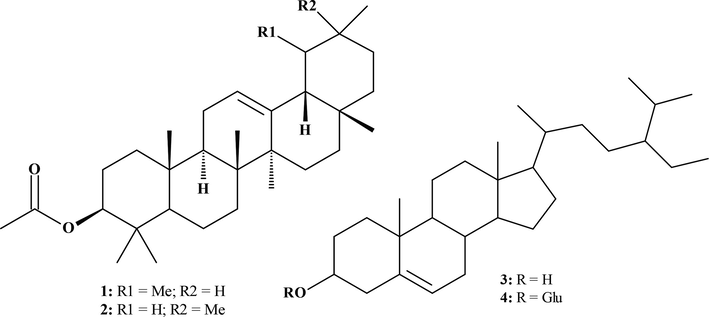
Compounds identified in the hydroalcoholic extract of S. rubens from Jordan.
Hydro-distillation of the fresh aerial parts of S. rubens at the PF stage afforded a pale yellow oil (yield 0.05%, w/w). GC/MS analysis of the PF-HD-EO (Table 3) resulted in the identification of 28 compounds amounting to 98.28 % of the total oil content. Two main classes dominated the composition, namely oxygenated sesquiterpenes (21.92%) and rose ketones (20.91%). These two classes were mainly represented by 7-epi-α-eudesmol (7.81%) and (E)-β-damascenone (16.34%), respectively. Other classes of compounds were also detected including oxygenated monoterpenes (17.66%), sesquiterpene hydrocarbons (18.11%), aliphatic hydrocarbons and their derivatives (15.85%) in addition to aromatic compounds and their derivatives (3.82%). *Identified by Co-injection of authentic samples, a: Literature Kovats (Retention) index; b: Experimentally calculated Kovats index using C8 – C20 n-alkanes on HP-5MS capillary column. MS: Identification by mass spectrum (NIST, Wiely and local generated library), Co-ln: Co-Injection with an authentic compound.
No
aRI Lit.
bRI Exp.
Compound
% PF
% FF
Identification mode
1
1000
1000
n-Decane
–
7.24
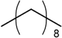
MS, RI
2
1040
1044
Santolina alcohol
–
1.42
MS, RI
3
1042
1048
Benzene acetaldehyde
–
0.92
MS, RI
4
1069
1073
m-Tolualdehyde
–
2.42
MS, RI
5
1096
1103
Linalool*
2.61
1.92
MS, RI, Co-In
6
1106
1108
trans-Vertocitral C
3.75
3.32
MS, RI
7
1209
1210
2E-Octenol acetate
1.54
3.32
MS, RI
8
1205
1205
Verbenone
–
1.22
MS, RI
9
1225
1225
Citronellol
–
0.85
MS, RI
10
1257
1256
Linalool acetate*
9.32
1.05
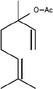
MS, RI, Co-In
11
1256
1263
trans-Sabinene hydrate acetate
1.13
–
MS, RI
12
1290
1298
Thymol*
0.89
–
MS, RI, Co-In
13
1300
1300
n-Tridecane
–
0.99
MS, RI
14
1299
1307
Carvacrol
1.19
–
MS, RI
15
1306
1313
Undecanal
13.33
29.23
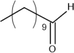
MS, RI
16
1321
1317
(2E,4E)-Decadienol
–
0.58
MS, RI
17
1321
1324
neo-Verbanol acetate
0.85
1.22
MS, RI
18
1382
1377
Ethyl-(4E)-decenoate
0.98
–
MS, RI
19
1384
1383
(E)-β-Damascenone
16.34
7.43
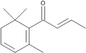
MS, RI
20
1400
1403
β-Elemene
5.23
2.61
MS, RI
22
1400
1413
β-Longipinene
8.62
–
MS, RI
23
1419
1423
E-Caryophyllene*
–
1.90
MS, RI, Co-In
24
1433
1433
β-Gurjunene
0.91
–
MS, RI
25
1446
1445
Seychellene
3.35
0.97
MS, RI
26
1466
1460
2E-Dodecenal
–
4.36
MS, RI
27
1484
1486
(E)-β-Ionone
3.71
3.34
MS, RI
28
1481
1487
methyl-γ-Ionone
0.87
–
MS, RI
29
1513
1515
Modhephen-8-β-ol
0.98
–
MS, RI
30
1522
1521
Eugenol acetate
1.74
2.21
MS, RI
31
1522
1523
Isobornyl isovalerate
1.32
–
MS, RI
32
1541
1541
10-epi-cis-Dracunculifoliol
–
0.76
MS, RI
33
1619
1617
epi-Cedrol
–
0.91
MS, RI
34
1622
1620
β-Cedrene epoxide
1.21
–
MS, RI
35
1625
1624
Silphiperfol-6-en-5-one
0.99
–
MS, RI
36
1645
1648
Cubenol
4.41
1.71
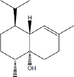
MS, RI
37
1646
1653
Torreyol
1.02
–
MS, RI
38
1663
1662
7-epi-α-Eudesmol
9.81
2.23
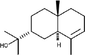
MS, RI
39
1667
1667
14-hydroxy-(Z)-Caryophyllene
–
0.87
MS, RI
40
1677
1672
Z-Nerolidyl acetate
1.02
–
MS, RI
41
1688
1690
8-Cedren-13-ol
–
2.02
MS, RI
42
1843
1844
α-Vetivone
1.17
10.21
MS, RI
43
1908
1908
Rimuene
–
1.31
MS, RI
44
1969
1970
Sandaracopimara-8(14),15-diene
–
0.80
MS, RI
Oxygenated Monoterpenes
17.66
11.00
Sesquiterepene hydrocarbons
18.11
5.48
Oxygenated sesquiterpenes
21.92
18.70
Rose ketones
20.91
10.77
Aliphatic hydrocarbons and their derivatives
15.85
45.71
Diterpene hydrocarbons
–
2.12
Aromatic compounds and their derivatives
3.82
5.54
Total Identified
98.28
99.32
Analysis of the HD-EO obtained from the plant material at the FF stage (yield 0.08 %, w/w) lead to the identification of 31 components accounting for 99.32 % of the total composition (Table 3). The oil at this stage had a quite different composition when compared to the EO at the PF stage. Aliphatic hydrocarbons and their derivatives were detected at significantly higher concentration levels as compared to their content in the PF stage (45.71%, 15.85%., respectively) with undecanal being the main component detected in this class (29.23%). Also, it was noticed that all different terpenoid classes were detected at lower concentration levels compared to their content in the previous growth stage including oxygenated sesquiterpenes (18.70%), oxygenated monoterpenes (11.00%), sesquiterpene hydrocarbons (5.48%) and diterpene hydrocarbons (2.12%). Also, rose ketones content decreased to 10.77% of the total content. Fig. 3 shows the variation in the chemical composition of the different classes of volatiles organic compounds detected in the HD-EO of S. rubens at the main two growth stages.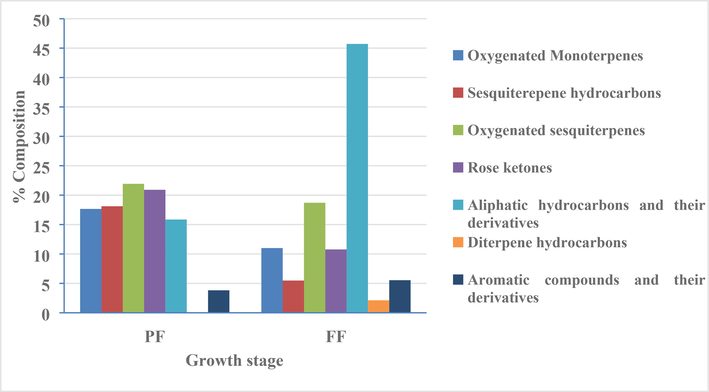
Variation in the chemical composition of S. rubens at PF and FF stages.
Despite that several Sedum species were subjected to phytochemical investigation of their volatile and nonvolatile constituents and evaluation of their possible bioactivity effects, S. rubens was never investigated. Stanković et al., (2012) examined the TPC, TFC and DPPH radical scavenging activity for the methanol extract of S. acre, the results obtained (18.25 mg gallic acid/g extract; 8.42 mg rutin equivalent/g extract; 987.16 mg/mL., respectively) were all lower than the resutls obtained in the current study. Antimicrobial screening studies of S. acre against 13 strains of bacteria and four species of fungi using microdilution method showed considerable antibacterial activity, the antifungal activity was weak (Stanković et al., (2012). Ertaş et al (2014) reported that the methanol extract of S. sediforme (Jacq.) Pau from Turkey had the highest TP and TF content (335.71 ± 4.81 and 26.66 ± 0.75 μg/mg extract, pyrocatechol equivalents and quercetin equivalents., respectively). The butanol fraction of S. caeruleum from Algeria had higher antioxidant activity (28.35 ± 1.22 μg/mL) as compared to our findings (Bensouici et al., 2016). Del Carmen Beltrán-Orozco et al, (2013) evaluated the TPC in the ethanolic extract of S. praealtum flowers (688.42 ± 21.4 GAE/100 mg extract) and reported the isolation of kampferol and quercetin from this extract. Rutin, caffeic acid and chlorogenic acid (138.62 ± 8.17; 151.25 ± 8.93; 23.30 ± 2.18 μg analyte/g extract, respectively) were quantified in S. sediforme from Istanbul-Turkey (Ertaş et al., 2014). Investigation of the antimicrobial activity of the methanolic extract revealed weak antimicrobial activity against Gram-positive and –negative bacteria (Ertaş et al., 2014). LC-MS/MS of S. sediforme (Jacq.) Pau crude extract from Greece revealed the presence of gallic acid, caffeic acid, quercetin and luteolin but lacked chlorogenic acid (Winekenstädde et al., 2015).
There have been few reports concerning the investigation of essential oil composition of Sedum plants. Yaylı et al (2010) reported the essential oil composition of two Sedum species from Turkey, S. pallidum var. bithynicum and S. spurium. Investigation revealed that both plants were dominated by oxygenated sequiterpenes (30.7%, 27.7%., respectively) and identified each of caryophyllene oxide (12.8%) and hexahydrofarnesyl acetone (15.7%) as the main constituents. The essential oil of S. palladium from Iran revealed hexadecanoic acid (33.5%, (Dahpour et al., 2012) as the main component. On the other hand, Al-Qudah et al (2012) investigated the essential oil composition of S. microcarpum (Sm.) schönl from Jordan. In this study, 55 compounds amounting to 97.18 % of the total content were identified; of which 36.39% were attributed to oxygenated monoterpenoids (36.39%). Its worth mentioning that myrtenol (21.7%), the main component detected in Al-Qudah et al (2014) study was not detected at all in our current results. In the study conducted by Ertaş et al (2014), a total of 24 compounds amounting to 91.6% of the total content were identified in the essential oil of S. sediforme from Turkey, that were dominated by the sesquiterpene hydrocarbon α-selinene (20.4%).
4 Conclusion
Sedum genus is considered as the largest genera of the Crassulaceae family in terms of the number of species. Literature survey revealed that only 15 species out of 500 Sedum species were investigated. The current study is the first report to explore the volatile and nonvolatile chemical constituents of S. rubens and its bioactivity potentials. The chemical diversity of S. rubens was indicated by the detection of terpenoids, sterols, phenolic acids and flavonoids that was reflected by the high TPC, TFC and DPPH radical scavenging activity of the HA extract obtained at the full flowering stage. Our results led to the isolation of four known compounds including α- & β-amyrin acetates (1, 2), β-sitosterol & β-sitosterol glycoside (3, 4), all are reported here for the first time from this species. Additionally, HPLC-PDA profiling of the HA extract revealed the presence of caffeic acid, catechin, gallic acid and rutin, all are reported for the first time from this species. The essential oil composition varied depending on the growth stage of the plant material, the PF stage was found to be rich in oxygenated sesquiterpenes and rose ketones, while the FF stage contained mainly aliphatic hydrocarbons and their derivatives.
Acknowledgments
This work was funded by the Deanship of Scientific Research and Innovation at Al-Balqa Applied University, Al-Salt, Jordan (grant No 66/2019/2020) to whom the authors are especially grateful.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A new cyclic polyketide and other constituents from Ononis spinosa growing wildly in Jordan and their antioxidant activity. J. Asian Nat. Prod. Res.. 2021;24(3):290-295.
- [Google Scholar]
- Identification of essential oil components by Gas Chromatography/Mass spectrometry (4.1 ed.). Corp, Illinois, USA: Carol Stream, IL, Allured Publ; 2017.
- Field guide to wild flowers of Jordan and neighboring countries. Amman, Jordan: The National Library; 1998.
- Variation in essential oil composition of Iris nigricans Dinsm. (Iridaceae) endemic to Jordan at different flowering stages. Arab. J. Chem.. 2016;9:S1190-S1196.
- [CrossRef] [Google Scholar]
- Essential oil composition, total phenolic and total flavonoid content and in-vitro antioxidant properties of hydro-alcoholic and water extracts of Salvia deserti growing wild in Jordan. JJC.. 2017;12(1):11-19.
- [Google Scholar]
- New terpenes from Salvia palaestina Benth. and Salvia syriaca L. growing wild in Jordan. J. Asian Nat. Prod. Res.. 2012;14(7):618-625.
- [Google Scholar]
- Essential oil composition and anticholinesterase activity evaluation of Achillea fragrantissima growing wild in Jordan. J. Herbs Spices Med. Plants.. 2018;24(3):272-281.
- [Google Scholar]
- Two new 28-noroleanane type triterpenoids and other constituents from Gladiolus atroviolaceus growing wild in Jordan. JJC.. 2019;14(1):11-16.
- [Google Scholar]
- HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ.. 2020;8:e9769.
- [Google Scholar]
- Investigating the chemical composition and the antimicrobial activity of the essential oil and crude extracts of Sedum microcarpum (Sm.) schönl. growing wild in Jordan. Pharmacogn. J.. 2012;4(33):1-6.
- [Google Scholar]
- Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry.. 2014;99:115-120.
- [Google Scholar]
- Anti-inflammatory effects of the methanol extract of Sedum telephium ssp. maximum in lipopolysaccharide-stimulated rat peritoneal macrophages. Pharmacology. 2008;82:250-256.
- [CrossRef] [Google Scholar]
- A new diterpene and other constituents of Salvia multicaulis from Jordan. Nat. Prod. Res.. 2021;36(19):4921-4928.
- [CrossRef] [Google Scholar]
- Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activitie. Pharm Biol.. 2016;54(1):174-179.
- [Google Scholar]
- Study of the anti-inflammatory effect of Sedum praealtum (Siempreviva) in the rat: dose-dependent response. Proc. West Pharmacol. Soc.. 2002;45:129-130.
- [Google Scholar]
- Chemical composition of essential oil, antibacterial activity and brine shrimp lethality of ethanol extracts from Sedum pallidum. J. Med. Plant Res.. 2012;6(16):3105-3109.
- [CrossRef] [Google Scholar]
- Phytochemical investigation of two Crassulaceae species: Rhodiola rosea L., the New “Herbal Stress Buster”, and Sedum dasyphyllum L.. Thèse e doctorat Univ. Genève Sc; 2009. p. :4122.
- Crassulaceae (chemistry and pharmacology)-A review. Futu. J. Pharmac. Sci.. 2018;4:234-240.
- [Google Scholar]
- Chemical compositions by Using LC-MS/MS and GC-MS and biological activities of Sedum sediforme (Jacq.) Pau. J. Agric. Food Chem.. 2014;62(20):4601-4609.
- [Google Scholar]
- Phytochemical constituents and biological activity of selected genera of family Crassulaceae: a review. S. Afr. J. Bot.. 2021;141:383-404.
- [Google Scholar]
- Sedum danjoense (Crassulaceae), a new species of succulent plants from the Danjo Islands in Japan. Phytotaxa.. 2017;309:23-34.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Sedum sarmentosum extract. J. Ethnopharmacol.. 2008;116:138-143.
- [CrossRef] [Google Scholar]
- Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J. Ethnopharmacol.. 2000;70:177-182.
- [CrossRef] [Google Scholar]
- Alkaloids of some Asian Sedum species. Phytochemistry.. 1996;41(5):1319-1324.
- [CrossRef] [Google Scholar]
- Improvement of adventitious root formation in Sedum aizoon L. and the production of flavonoids. S. Afr. J. Bot.. 2021;137:483-491.
- [Google Scholar]
- Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal. Chim. Acta.. 2013;763(6):1-10.
- [CrossRef] [Google Scholar]
- Phytochemical evaluation of the ethanol extract and volatile oils of Sedum rubens from Jordan and its antioxidant activity. MSc. Thesis. Jordan: Al-Balqa Applied University, Al-Salt; 2022.
- beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol.. 2014;52(11):1478-1486.
- [Google Scholar]
- Isolation and characterization of β-sitosterol-3-O-βD-glucoside from the extract of the flowers of Viola odorata. Br. J. Pharm. Res.. 2017;16(4):1-8.
- [Google Scholar]
- Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr.. 2013;64(8):944-952.
- [CrossRef] [Google Scholar]
- Secondary metabolites, cytotoxic response by neutral red retention and protective effect against H2O2 induced cytotoxicity of Sedum caespitosum. Phytochemistry.. 2012;41(5):1319-1324.
- [Google Scholar]
- Phytochemical content, antioxidant and cytotoxic Activities of Sedum spurium. Nat. Prod. Commun.. 2016;11(11):1693-1696.
- [Google Scholar]
- Evaluation of biological activities of goldmoss stonecrop (Sedum acre L.) Turkish J. Biol.. 2012;36:580-588.
- [Google Scholar]
- Stephenson, R., 1994. Sedum: Cultivated Stonecrops. Timber Press Inc.
- Epicuticular wax composition of some European Sedum species. Phytochemistry.. 1994;35(2):389-399.
- [CrossRef] [Google Scholar]
- Crassulaceae, Flowering Plants. Eudicots. Springer; 2007. p. :83-118.
- Anti-oxidant constituents from Sedum takesimense. Phytochemistry. 2007;68:2432-2438.
- [Google Scholar]
- Phytochemical profile of the aerial parts of Sedum sediforme and anti-inflammatory activity of myricitrin. Nat. Prod. Commun.. 2015;10(1):83-88.
- [Google Scholar]
- Chemical constituents and antimicrobial activities of the essential oils from Sedum allidum var. bithynicum and S. spurium grown in Turkey. Pharm. Biol.. 2010;48(2):191-194.
- [CrossRef] [Google Scholar]
- HPLC determination of quercetin in three plant drugs from genus Sedum and conjecture of the best harvest time. Pharmacognosy J.. 2017;9(6):725-728.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104712.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







