Translate this page into:
Design, synthesis and biological activity of novel chalcone derivatives containing indole
⁎Corresponding author. wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
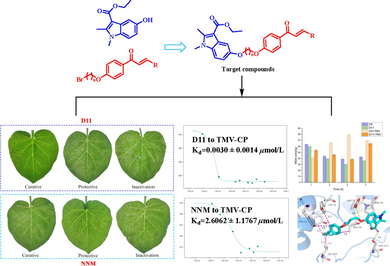
In this study, 24 chalcone derivatives containing indole were designed and synthesized. Some of the target compounds exhibited significant antiviral and antibacterial effects. The results of EC50 test against tobacco mosaic virus showed that the EC50 value of curative activity of D11 was 107.4 μg/mL, which was better than the commercial drug ningnanmycin 311.5 μg/mL, and the EC50 value of protective activity of D16 was 106.6 μg/mL, which was superior to ningnanmycin 205.4 μg/mL, the inactivated activity of D11 was comparable to that of ningnanmycin. Microscale thermophoresis experiments demonstrated D11 (Kd = 0.0030 ± 0.0014 μmol/L) had a stronger binding ability with tobacco mosaic virus capsid protein (TMV-CP), which was much higher than that of ningnanmycin (Kd = 2.6062 ± 1.1767 μmol/L). Molecular docking results showed D11 had a significantly higher affinity with TMV protein than ningnanmycin. In addition, the malondialdehyde (MDA), peroxidase (POD), succinate dehydrogenase content (SDH) assay showed D11 could improve the disease resistance of tobacco. The results of bacterial inhibition activity showed that the EC50 value of D23 against Xoo was 50.3 μg/mL, which was better than the commercial drugs thiodiazole copper (79.8 μg/mL) and bismerthiazol (106.2 μg/mL), the bacterial inhibition activity of D23 was verified by scanning electron microscopy.
Keywords
Chalcone derivatives
Indole
Antiviral activity
Bacteriostatic activity
Mechanism of action
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR
-
19F nuclear magnetic resonance
- HRMS
-
High-resolution mass spectrometry
- TMV
-
Tobacco mosaic virus
- TMV-CP
-
Tobacco mosaic virus coat protein
- MST
-
Microscale thermophoresis
- NNM
-
Ningnanmycin
- MDA
-
Malondialdehyde
- POD
-
Peroxidase
- SDH
-
Succinate dehydrogenase
- EC50
-
Median effective concentration
- SEM
-
Scanning electron microscopy
- DMSO
-
Dimethylsulfoxide
- Xoo
-
Xanthomonas oryzae pv.oryzae
- Psa
-
Pseudomonas syringae pv.actinidiae
- Xac
-
Xanthomonas axonopodis pv. citri
Abbreviations
1 Introduction
Our country is a large agricultural country with a long history, in the process of agricultural development, the agricultural economy has made remarkable achievements (Xu et al., 2021; Yan et al., 2021). In recent years, achieving high quality and high yield of crops is the key to consolidate and strengthen the agricultural economy of China (Wu et al., 2022; Chao, 2019). But as agriculture continues to develop, many problems and challenges have been gradually revealed with respect to crops (Mariana and Octávio, 2022). The increasing number of some crop diseases caused by plant viruses, phytopathogenic bacteria, etc. (Marqués et al., 2022; Liu et al., 2021), has seriously affected the quality of agricultural products and the number of varieties, so crop disease control has become a requirement of the times to supplement the shortcomings of the agricultural economy. The use of pesticides is the most effective method to prevent and control crop diseases, but problems such as enhanced drug resistance, pesticide residues and environmental pollution have also emerged subsequently (Li et al., 2021; Claudio et al., 2018; Zhao et al., 2018). Therefore, it is important to research new commercial pesticides with high efficiency, low toxicity and environmental protection (Jiang et al., 2020).
Chalcone is a precursor of flavonoid synthesis in plants with the chemical structural formula 1,3-diphenyl-2-propen-1-one, its derivatives are mainly concentrated in parts A, B and C (Fig. 1) (Li et al., 2022; Nadia et al., 2022; Chandrabose et al., 2014) and are widely found in vegetables, soybeans, fruits, spices, tea and other plants (Hiroyuki et al., 2003; Ye et al., 2016). Chalcone and its derivatives have been found to possess a variety of biological activities, such as antiviral (Fu et al., 2020), antibacterial (Chen et al., 2020; Zhou et al., 2022), anticancer (Yang et al., 2021), antioxidant (Bale et al., 2021; Zahrani et al., 2020), anthelmintic (José et al., 2018), and anti-inflammatory (Nurkenov et al., 2019; Ibrahim et al., 2021). In view of this, chalcones have also been successively used in the synthesis of biopesticides, the domestically registered isobavachalcone is a plant-derived fungicide with chalcone structure extracted from the legume tonic (Guan et al., 2014). The design and synthesis of novel pesticides with excellent activity by introducing biologically active pharmacophore into the chalcone backbone has become a hot topic in the field of pesticide chemistry. Indole (Fig. 1) is an aromatic heterocyclic alkaloid found not only in natural plants such as jasmine, oleander, citrus, croton root and orange blossom (Qin et al., 2015; Wei et al., 2018; Ding et al., 2018), but also in many animals and marine organisms (Liu et al., 2017; Lin and Tan, 2018). Both indoles and their derivatives exhibit many biological activities, such as: antibacterial (Xu et al., 2017; Kang et al., 2020), antitumor (Palassini et al., 2017), antiviral (Xie et al., 2020; Wang et al., 2021), antioxidant (Quan and Adam, 2020), and anti-inflammatory (Liu et al., 2016). As extremely important heterocyclic skeletal pharmacophore, indoles are widely used in pharmaceuticals, pesticides, fragrances, dyes, and other research fields. Therefore, the exploration of indoles has been favored by many chemists and pharmacologists.
The structures of chalcone and indole.
In order to find novel pesticide compounds with excellent antiviral and bacterial inhibition, 24 chalcone derivatives containing indole were synthesized according to the principle of active splicing in drug synthesis (Fig. 2 and Scheme 1). All target compounds were tested for antiviral and bacterial inhibition activities. The results of antiviral tests showed that the D11 had excellent curative, protective and inactivated activities against tobacco mosaic virus. The Molecular docking, MST, MDA, POD and SDH assays were performed on D11, molecular docking and MST results showed D11 had a strong binding ability with TMV-CP, the experiments of MDA, POD, SDH showed D11 could improve the disease resistance of tobacco. In addition, the results of bacterial inhibition tests showed D23 has excellent inhibitory effect on Xoo. SEM experiments were performed on D23 to observe the effect of D23 on Xoo cells. This study provides a useful guiding basis for the development of new pesticides.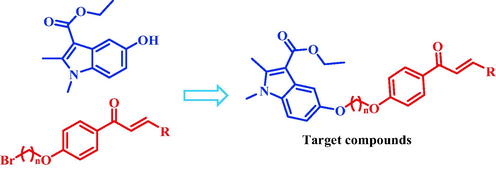
Design of target compounds.
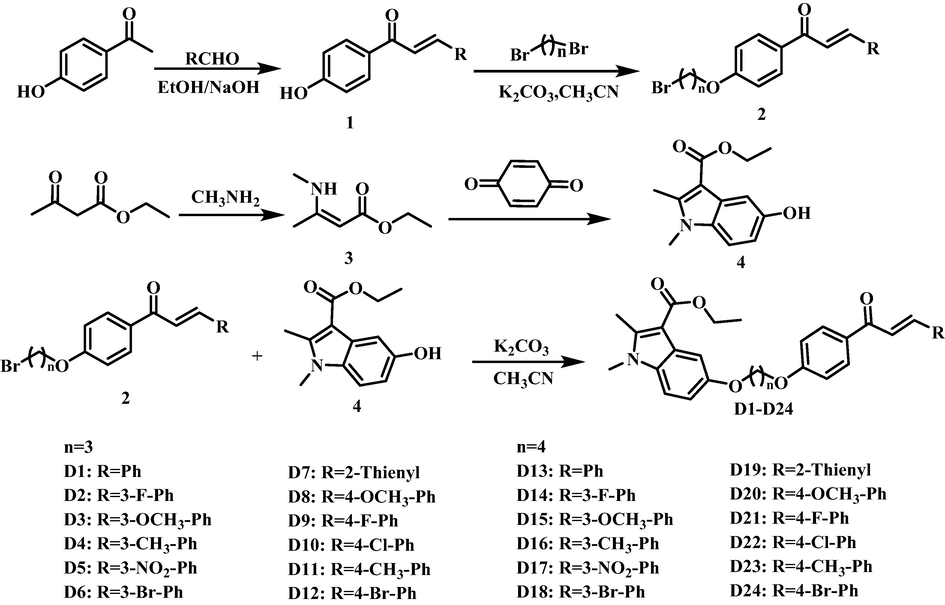
Synthetic route of compounds D1-D24.
2 Materials and methods
2.1 Instruments and chemicals
2.1.1 Instruments
The melting points were determined by using an XT-4 binocular microscope (Beijing, China) and uncorrected. 1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra were obtained using JEOL-ECX500 (Tokyo, Japan) and Bruker 400 NMR spectrometer (Bruker Corporation, Germany). High-resolution mass spectrometry (HRMS) was conducted by using a Thermo Scientific Q Exactive (Thermo Scientific, Missouri, USA). The Kd values of compounds to TMV-CP were determined by NanoTemper Monolith NT.115 microscale thermophoresis instrument (München, Germany). Enzyme activity data were determined by MultiskanFC Microplate reader (Beijing, China).
2.1.2 Chemicals
Various reaction materials were purchased from Shanghai Titan Technology Co., Ltd (Shanghai, China). Bismerthiazol, Thiodiazole copper and ningnanmycin were purchased from Shanghai Tansoole Platform (Shanghai, China). All chemical reagents and solvents are analytically pure. The enzyme activity kit was purchased from Beijing Solarbio Technology Co., Ltd (Beijing, China), Other chemical materials were purchased from Bositai Technology Co., Ltd (Chongqing, China).
2.2 Synthesis
2.2.1 Synthesis of intermediates 1–4
Intermediates 1 and 2 were synthesized according to the method of the literature (Chen et al., 2020; Zhou et al., 2022). Intermediates 3 and 4 were synthesized according to the literature (Huang et al., 2019). The methylamine solution was slowly added to a round bottom flask containing ethyl acetoacetate, the reaction was carried out at room temperature for 3 h. After completion of the reaction, the organic phase was washed with water, and the organic phase was dried to obtain the pale-yellow liquid intermediate 3. The P-benzoquinone, acetone and intermediate 3 were added to the reaction flask in sequence and reacted at 30 °C for 2 h. After concentrating under reduced pressure, acetone was precrystallized to obtain intermediate 4.
2.2.2 Synthesis of target compounds D1-D24
Intermediate 4 (3.60 mmol), K2CO3 (9.00 mmol) and 30 mL CH3CN were added to 100 mL round bottom flask, condense and reflux at 80 °C for 60 min. Intermediate 2 (3.0 mmol) was added to the reaction system, continue to heat and reflux the reaction for 8 h. The reaction system was concentrated under reduced pressure, and then pour into 500 mL of ice water, a white solid was precipitated. After standing for 60 min, the reaction system was filtered under reduced pressure and dried, the crude product was recrystallized with anhydrous ethanol. Finally, the target compounds D1-D24 were purified by column chromatography (petroleum ether: ethyl acetate = 2:1, v/v).
2.3 Anti-TMV activity assay
According to the previously reported method in the literature (Peng et al., 2021). The antiviral activity of the compounds against TMV in vivo was tested at an agent concentration of 500 μg/mL. In addition, EC50 values for against TMV were tested at concentrations of 500, 250, 125, 62.5 and 31.25 μg/mL. The commercial drug NNM was used as the control agent. Each measurement was repeated three times.
2.4 Microscale thermophoresis experiment(MST)
The interaction of the target compounds to TMV-CP was investigated by previously reported approach in the literature (Liu et al., 2022).
2.5 Molecular docking
The molecular docking of compound D11 to TMV-CP was carried out according to the method reported in the literature (Ding et al., 2022). AutoDockTools and PyMOLWin software were used to simulate and validate the binding ability of target compounds to TMV proteins.
2.6 Defensive enzyme activity measurement
The defense enzyme activity of D11 was tested according to the methods in the literature (Liu et al., 2022; Gan et al., 2021). Nicotiana Tabacum K326 was induced whole leaves in a greenhouse with D11 and a solvent (DMSO + 1% Tween water), where the solvent was used as a negative control (CK). After 12 h of induction, TMV was inoculated on the tobacco leaves and the leaves were collected on 1, 3, 5 and 7 d. Then added to liquid nitrogen to obtain CK, CK + TMV, D11 and D11 + TMV groups, which were ground into powder and measuring the enzyme activity content in the leaves according to the method in the kit instructions.
2.7 Determination of MDA content
The MDA content of TMV infected tobacco leaves treated with D11 was tested according to the method described in the literature (Zhang et al., 2017). Tobacco leaves on 1, 3, 5 and 7 d were collected as samples and divided into CK, CK + TMV, D11 and D11 + TMV groups, subsequently the absorbance of each sample was measured at 532 nm and 600 nm according to the method described in the kit instructions, the MDA content was calculated by referring to the kit instructions.
2.8 Measurement of antibacterial activity
According to the literature (Tang et al., 2022), D1-D24 were determined by a modified 96-well plate method against Xoo, Xac, Psa for their antibacterial activity. The corresponding concentration of DMSO solution was used as a negative control, the commercial drugs thiodiazole copper and bismerthiazol were used as positive controls. Three parallel experiments were performed for each sample.
2.9 Scanning electron microscopy
SEM experiments were performed on the bacterial inhibition of D23, all methods were referred to previous literature (Peng et al., 2021).
3 Results and discussion
3.1 Chemistry
The D1-D24 were synthesized according to the designed route of Scheme 1. All the target compounds were characterized by NMR and HRMS, the detailed data are in the Supporting Material.
3.2 Antiviral activity of D1-D24 against TMV in vivo
The against TMV activity of the synthesized target compounds was determined at concentration of 500 μg/mL with the half-leaf blight spot methods, the results are shown in Table 1, most of the compounds showed excellent against TMV activity, the curative activities of compounds D11, D12, D15, D16, D18 and D22 were 74.7, 65.8, 70.2, 65.9, 71.9 and 73.6%, which were superior to NNM 57.1%. The protective activities of compounds D2, D4, D10, D11, D12, D16 and D18 were 72.2, 71.3, 72.4, 73.6, 75.5, 77.0 and 74.1%, which were better than NNM 68.2%. The inhibition rate of inactivated activity of D11 was 86.1%, which was comparable to that of NNM. Among them, the tobacco leaf morphology of D11 against TMV in vivo is shown in Fig. 3. D11 showed significant inhibition of TMV in both curative and protective activities, which was superior to the commercial drug NNM.
Compounds
Curative activity (%) a
Protective activity (%) a
Inactivated activity (%) a
D1
63.6 ± 5.4
68.3 ± 1.8
71.3 ± 5.2
D2
50.9 ± 5.3
72.2 ± 2.6
72.5 ± 3.3
D3
56.4 ± 0.9
59.9 ± 1.3
70.9 ± 6.1
D4
41.1 ± 4.3
71.3 ± 3.6
74.8 ± 2.7
D5
43.3 ± 3.6
66.2 ± 0.8
62.9 ± 2.4
D6
50.6 ± 4.7
59.6 ± 1.7
66.9 ± 4.7
D7
52.9 ± 4.7
66.0 ± 3.0
61.6 ± 2.9
D8
31.3 ± 2.2
55.7 ± 1.6
55.5 ± 1.8
D9
62.6 ± 1.8
63.0 ± 6.4
72.5 ± 5.1
D10
59.0 ± 1.6
72.4 ± 1.7
78.2 ± 1.5
D11
74.7 ± 2.8
73.6 ± 2.1
86.1 ± 1.0
D12
65.8 ± 4.3
75.5 ± 0.7
58.7 ± 0.9
D13
47.4 ± 5.5
51.6 ± 5.4
41.4 ± 2.0
D14
38.3 ± 0.4
60.6 ± 4.5
56.7 ± 2.7
D15
70.2 ± 4.5
56.6 ± 2.5
80.9 ± 0.9
D16
65.9 ± 3.6
77.0 ± 3.2
84.5 ± 4.3
D17
43.9 ± 5.7
54.6 ± 1.5
60.3 ± 4.7
D18
71.9 ± 0.5
74.1 ± 1.7
72.9 ± 1.2
D19
45.2 ± 2.7
38.0 ± 3.3
55.4 ± 3.6
D20
65.4 ± 4.6
56.3 ± 3.1
57.3 ± 2.1
D21
62.0 ± 3.2
70.9 ± 0.3
57.4 ± 6.5
D22
73.6 ± 3.4
61.5 ± 2.2
54.6 ± 5.2
D23
58.2 ± 2.1
68.1 ± 4.6
55.0 ± 2.5
D24
58.7 ± 2.9
68.8 ± 3.5
64.4 ± 2.5
NNM b
57.1 ± 1.9
68.2 ± 1.6
91.8 ± 0.6
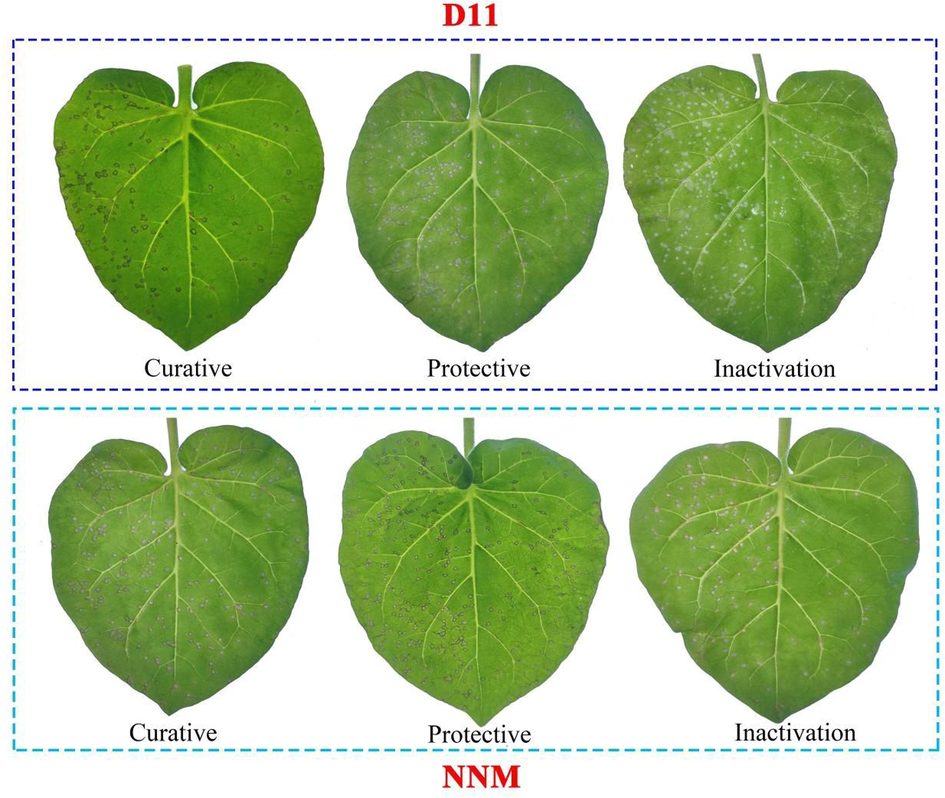
Against TMV activities of D11 and NNM in vivo.
Based on the results of the initial screening, the EC50 values of some compounds against TMV were further tested and the results are shown in Table 2. The EC50 values of curative activity of D11, D15, and D22 were 107.4, 108.4, and 104.0 μg/mL, which were better than that of NNM 311.5 μg/mL. The EC50 values of protective activity of D2, D12, and D16 were 106.3, 114.8, and 106.6 μg/mL, which were better than NNM 205.4 μg/mL.
Compounds
Toxic regression equation
r
EC50 (µg/mL)a
Curative
D11
y = 1.0913x + 2.7836
0.9773
107.4
D12
y = 0.8599x + 3.0692
0.9958
175.9
D15
y = 0.8985x + 3.1714
0.9671
108.4
D16
y = 0.8241x + 3.1336
0.9975
184.0
D18
y = 0.8439x + 3.2733
0.9889
111.2
D22
y = 0.8564x + 3.2726
0.9543
104.0
NNMb
y = 0.9414x + 2.6527
0.9830
311.5
Protective
D2
y = 0.9063x + 3.1633
0.9346
106.3
D4
y = 0.8776x + 3.1610
0.9614
124.6
D10
y = 1.0705x + 2.5815
0.9929
181.6
D11
y = 0.9735x + 2.7799
0.9485
190.8
D12
y = 0.8974x + 3.1514
0.9568
114.8
D16
y = 0.9464x + 3.0810
0.9633
106.6
D18
y = 0.8208x + 3.1481
0.9727
180.4
NNMb
y = 0.9289x + 2.8519
0.9589
205.4
The structure–activity relationship (SAR) was analyzed according to the anti-TMV activities shown in Table 1. The target compounds showed better protective, curative and inactivated activities against TMV. when the number of C-atoms of brominated alkanes was n = 3 and R was an electron-giving substituted phenyl group. For example, the curative activity was D11 (R = 4-CH3-Ph, 74.7%) > D12 (R = 4-Br-Ph, 65.8%), the protective activity was D11 (R = 4-CH3-Ph, 73.6%) > D10 (R = 4-Cl-Ph, 72.4%) > D9 (R = 4-F-Ph, 62.6%), the inactivated activity was D11 (R = 4-CH3-Ph, 86.1%) > D10 (R = 4-Cl-Ph, 78.2%). When the number of brominated alkane C atoms n = 4, R was halogen substituted phenyl, the target compounds showed higher protection and curative against TMV, such as curative activity D22 (R = 4-Cl-Ph, 73.6%) > D16 (R = 3-CH3-Ph, 65.9%), protective activity D18 (R = 3-Br-Ph, 74.1%) > D15 (R = 3-OCH3-Ph, 56.6%).
In summary, D11 (n = 3, R = 4-CH3-Ph) showed significant inhibitory activity against TMV in terms of curative, protective and inactivated activities, when n = 4, R was halogen-substituted phenyl, the protective and curative activities of the target compound against TMV were enhanced.
3.3 Binding ability of D11, D13, D15 and NNM to TMV-CP
The results of MST study of D11, D13, D15 and NNM to TMV-CP are shown in Fig. 4. The dissociation constants Kd values of D11, D13, D15 and NNM for TMV-CP were 0.0030 ± 0.0014 μmol/L, 8.0268 ± 3.3977 μmol/L, 0.1371 ± 0.0310 μmol/L and 2.6062 ± 1.1767 μmol/L. It is thus clear that D11, D15 have better affinity for TMV-CP than NNM and much surpassed than D13. In summary, the magnitude of binding capacity with TMV-CP was D11 > D15 > NNM > D13. which was consistent with the results of the initial against TMV screening.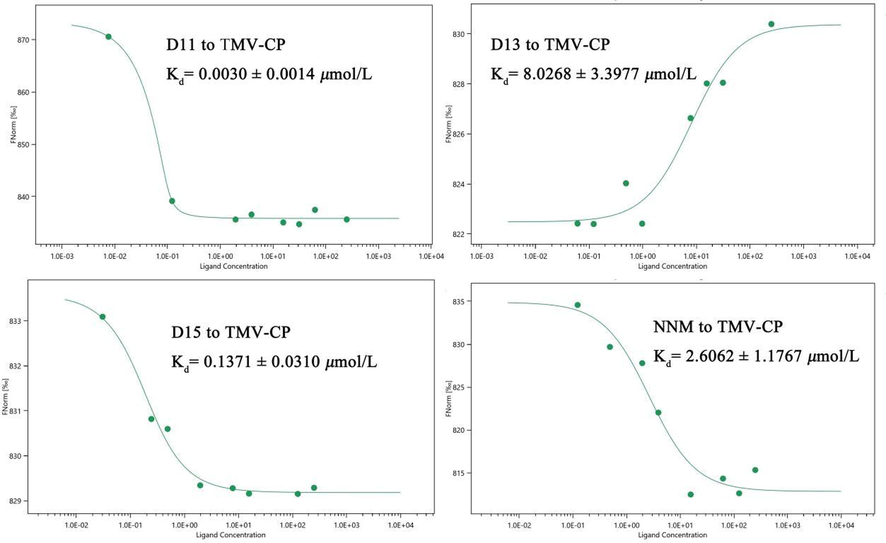
MST results of D11, D13, D15 and NNM.
3.4 Molecular docking of compounds D11, D13 and NNM with TMV-CP
The molecular docking results of D11, D13 and NNM are shown in Table 3 and Fig. 5. D11 formed two hydrogen bonds with TMV-CP, in which the indole carbonyl oxygen atom of D11 formed a hydrogen bond with residue SER-215 (3.0 Å) of TMV-CP, the indole hydroxyl oxygen atom formed a hydrogen bond with residue ASP-70 (3.8 Å), the total binding free energy was −7.44 kcal/mol. Obviously, the more hydrogen bonds and the lower the binding free energy, the more stable the docking result. In supplement to this, D11 also formed seven hydrophobic bonds with other residues, which provided a certain intermolecular force for D11 and showed a significant inhibitory activity against TMV-CP. Although the commercial drug NNM formed two hydrogen bonds with amino acid residue GLU-306 (3.0 Å), and the total binding free energy was −1.05 kcal/ mol, which shows that the affinity with TMV protein was D11 > NNM. In contrast, D13 formed only one hydrogen bond with residue ASN-127 (3.0 Å), the total binding free energy was −5.29 kcal/ mol, forming three hydrophobic bonds, the effect of hydrogen bonding in molecular docking was most obvious, so the affinity with TMV protein was D13 < NNM. In conclusion, the order of binding ability with TMV protein was D11 > NNM > D13, which is consistent with the results of the preliminary screening data.
Compounds
AA-Residues
Binding-Energy(kcal/ mol)
Distance
D11(A)
ASP-70
−7.44 kcal/ mol
3.8 Å
SER-215
3.0 Å
D13(B)
ASN-127
−5.29 kcal/ mol
3.0 Å
NNM(C)
GLU-306
−1.05 kcal/ mol
3.0 Å
GLU-306
3.0 Å
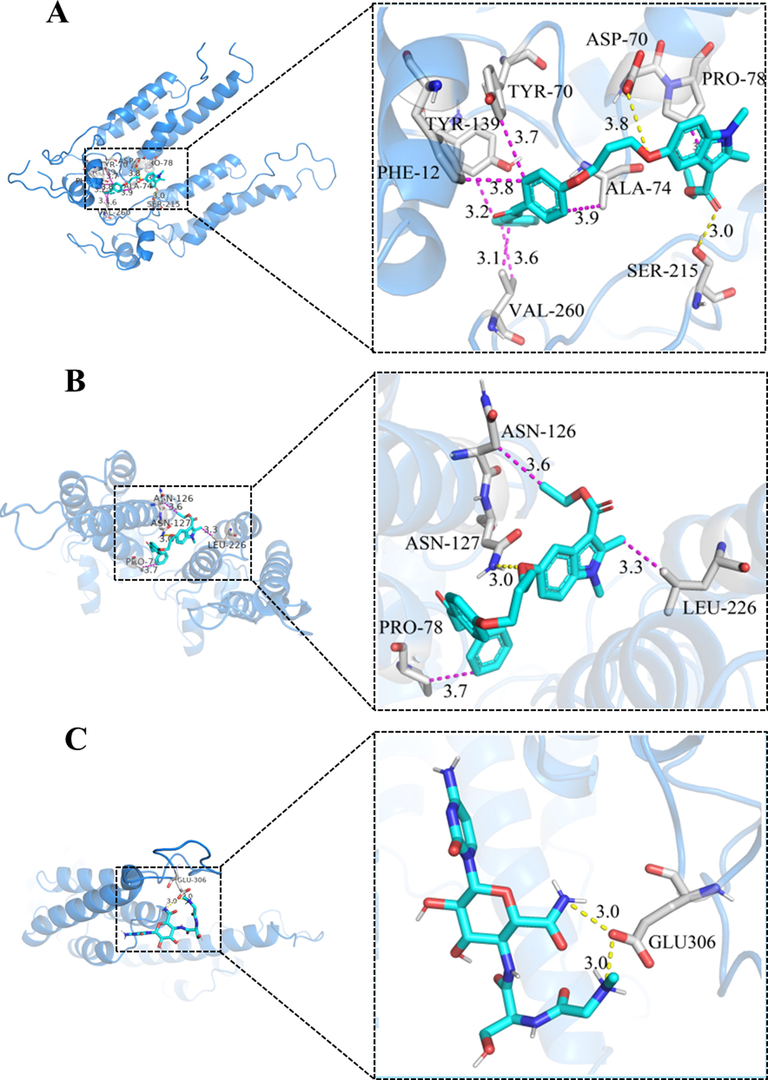
Molecular docking results of D11 (A), D13 (B) and NNM (C).
3.5 Effects of D11 on defensive enzyme activities
Peroxidase (POD) has the dual effect of eliminating the toxicity of peroxyphenols and amines in plants. The changes of POD content in tobacco after treatment with D11 are shown in Fig. 6. The POD content gradually increased in all groups during 1–7 d of TMV inoculation, the POD content in the D11 + TMV group reached the highest value at 7 d, which was 110% higher than that in the CK + TMV group during the corresponding period. In addition, the POD content of the D11 + TMV group was higher than that of the CK + TMV group from 1 to 7 d. The POD content of the D11 group was higher than that of the CK group during 2–7 d. The above experimental results showed that D11 could increase POD to a certain extent and improve the disease resistance of plants, which in turn inhibited the virus infestation.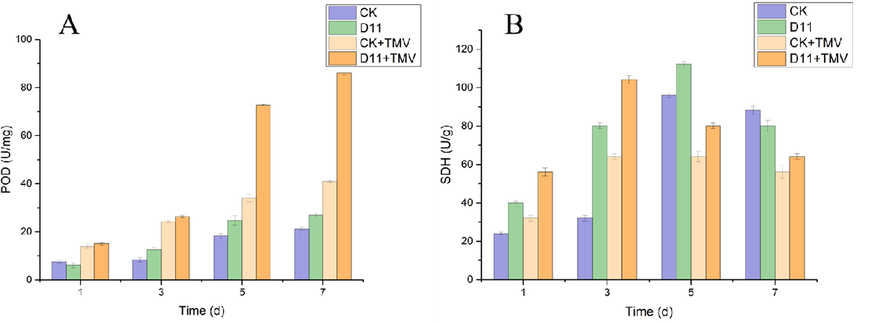
Effects of D11 on POD(A) and SDH(B) activity in tobacco leaves. Vertical bars refer to mean Standard Deviation (n = 3).
Succinate dehydrogenase (SDH), a marker enzyme of mitochondria, is one of the hubs linking oxidative phosphorylation and electron transfer, its activity can generally be used as an indicator to evaluate the degree of operation of the tricarboxylic acid cycle. The changes of SDH content in tobacco after D11 treatment are shown in Fig. 6. After inoculation with TMV, the SDH content in tobacco treated with D11 gradually increased during the 1–3 d and reached the highest value in the 3 d, then decreased during the 3–7 d. In the 3 d, the SDH content of the D11 + TMV group was much higher than that of the CK + TMV group by 63%, and the SDH content of the D11 + TMV group was higher than that of the CK + TMV group in the 1–7 d. The SDH content of the D11 group gradually increased during 1–5 d and reached the highest value at 5 d, decreasing during 5–7 d. At 5 d, the SDH content of the D11 group was 17% higher than that of CK group. The SDH content of D11 was higher than that of the CK group from 1 to 5 d. It was shown that D11 could improve the disease resistance of plants against virus particles, thus reducing the symptoms of tobacco plants.
3.6 Malondialdehyde content analysis
Malondialdehyde (MDA) content reflects the degree of membrane lipid peroxidation caused by tobacco infestation. As shown in Fig. 7, after TMV inoculation, MDA content in tobacco treated with D11 decreased gradually from the 1–5 d, reached the lowest value at the 5 d, and increased from the 5 to 7 d. Especially at the 5 d, the MDA content in the D11 + TMV group was much lower than that in the CK + TMV group by about 60%. In addition, the MDA content in the D11 group was less than that in the CK group during the period 1–7 d. Thus, it can be concluded that D11 can reduce the MDA content in tobacco plants, effectively prevent TMV infestation and reinfestation, improve the disease resistance of tobacco.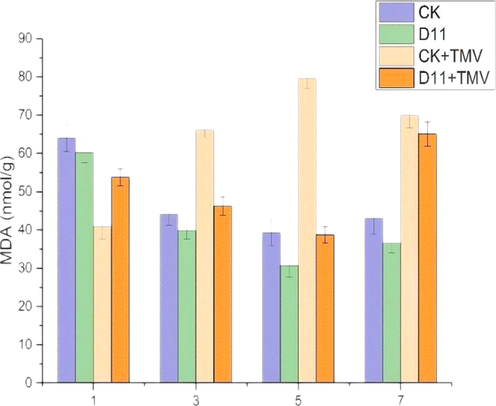
Effects of D11 on MDA content in tobacco. Vertical bars refer to mean Standard Deviation(n = 3).
3.7 Antibacterial activity of D1-D24
The inhibitory activities of the synthesized target compounds against Xoo, Xac, and Psa were determined at the concentration of 100 μg/mL and 50 μg/mL. The results of the tests are shown in Table 4. Some compounds showed inhibitory activity against both Xac and Xoo. At a concentration of 100 μg/mL, D18 showed 71.2% inhibition of Xac, which was better than the commercial drugs thiodiazole copper (61.5%) and bismerthiazol (41.2%), D23 showed 68.2% inhibition of Xoo, which was higher than the commercial drugs thiodiazole copper (60.6%) and bismerthiazol (49.3%). At 50 μg/mL, D18 inhibited Xac by 60.0%, which was superior to thiodiazole copper (42.1%) and bismerthiazol (33.2%), D23 inhibited Xoo by 52.8%, which was superior to thiodiazole copper (32.7%) and bismerthiazol (26.0%).
Compounds
Inhibition rate (%)a
Xac
Psa
Xoo
100 μg/mL
50 μg/mL
100 μg/mL
50 μg/mL
100 μg/mL
50 μg/mL
D1
7.8 ± 0.7
8.0 ± 2.9
36.8 ± 2.8
30.8 ± 4.5
17.4 ± 4.1
17.6 ± 4.0
D2
5.7 ± 2.0
7.0 ± 1.2
46.0 ± 2.2
43.7 ± 0.5
20.9 ± 1.9
10.9 ± 1.8
D3
13.4 ± 2.0
14.3 ± 1.3
44.1 ± 4.3
40.8 ± 1.7
19.3 ± 3.8
19.4 ± 3.7
D4
12.9 ± 0.2
8.2 ± 0.9
28.4 ± 3.0
33.9 ± 2.5
4.6 ± 2.6
2.5 ± 0.7
D5
16.0 ± 4.6
7.9 ± 4.5
42.6 ± 2.9
35.0 ± 0.2
13.4 ± 2.5
19.6 ± 2.5
D6
11.0 ± 0.8
8.7 ± 1.6
34.6 ± 3.8
29.8 ± 3.5
4.7 ± 2.2
24.6 ± 2.9
D7
1.9 ± 1.4
4.4 ± 1.2
36.5 ± 2.3
40.1 ± 3.0
22.5 ± 0.5
24.2 ± 0.5
D8
10.1 ± 0.6
10.1 ± 3.5
5.7 ± 2.2
33.2 ± 1.6
28.3 ± 0.7
26.6 ± 0.7
D9
16.6 ± 1.6
13.3 ± 2.1
19.2 ± 3.4
13.2 ± 1.1
18.7 ± 0.1
9.1 ± 1.7
D10
14.5 ± 1.3
15.9 ± 2.1
23.9 ± 1.6
17.6 ± 2.9
13.5 ± 2.1
17.2 ± 2.2
D11
14.1 ± 0.5
13.2 ± 2.7
34.3 ± 0.6
22.5 ± 3.4
9.6 ± 0.9
23.8 ± 1.7
D12
18.9 ± 1.6
14.5 ± 2.4
26.9 ± 4.8
29.9 ± 2.5
6.9 ± 2.1
7.5 ± 4.0
D13
13.5 ± 2.8
10.7 ± 2.0
27.1 ± 1.5
27.9 ± 3.0
14.8 ± 0.2
24.5 ± 0.6
D14
13.9 ± 1.1
16.4 ± 3.7
26.4 ± 1.9
13.9 ± 3.3
15.8 ± 1.2
13.7 ± 1.7
D15
14.0 ± 0.8
13.8 ± 0.9
35.4 ± 2.4
20.6 ± 4.0
16.4 ± 0.5
15.6 ± 2.4
D16
14.9 ± 0.4
13.6 ± 0.2
15.4 ± 1.2
15.3 ± 2.0
31.5 ± 1.4
21.1 ± 2.2
D17
22.2 ± 0.4
3.4 ± 2.6
34.2 ± 4.0
11.1 ± 1.3
40.3 ± 0.9
29.5 ± 2.1
D18
71.2 ± 1.4
60.0 ± 0.4
33.1 ± 2.7
20.6 ± 2.1
40.0 ± 3.8
30.9 ± 2.0
D19
64.2 ± 0.4
14.7 ± 1.3
23.5 ± 2.5
29.4 ± 0.7
33.8 ± 3.5
30.4 ± 2.1
D20
53.2 ± 3.1
22.7 ± 3.9
28.3 ± 1.7
18.9 ± 2.5
21.1 ± 0.8
24.1 ± 0.2
D21
41.9 ± 2.7
24.9 ± 0.5
31.3 ± 2.0
10.6 ± 1.4
7.8 ± 0.8
21.5 ± 1.3
D22
23.9 ± 1.9
0.9 ± 1.8
40.5 ± 0.8
13.2 ± 1.6
29.4 ± 0.2
28.2 ± 2.7
D23
5.7 ± 0.9
8.1 ± 2.9
25.1 ± 4.5
27.1 ± 2.6
68.2 ± 1.7
52.8 ± 0.2
D24
6.0 ± 4.5
15.7 ± 0.7
17.1 ± 2.5
5.5 ± 1.0
16.9 ± 0.7
14.6 ± 1.7
Bismerthiazolb
41.2 ± 1.9
33.2 ± 3.4
49.8 ± 2.4
38.4 ± 3.1
49.3 ± 3.2
26.0 ± 2.7
Thiodiazole copperb
61.5 ± 0.2
42.1 ± 1.6
61.5 ± 0.5
47.2 ± 3.8
60.6 ± 0.8
32.7 ± 0.4
To further confirm the results obtained from the initial screening, the EC50 values of some compounds for bacterial inhibition were tested and the results are shown in Table 5, D18 gave an EC50 value of 40.2 μg/mL for Xac, which was superior to the commercial drugs thiodiazole copper (60.8 μg/mL) and bismerthiazol (101.9 μg/mL). The EC50 value of D23 for Xoo was 50.3 μg/mL, which was better than that of thiodiazole copper (79.8 μg/mL) and bismerthiazol (106.2 μg/mL).
Compounds
Toxic regression equation
r
EC50 (μg/mL)a
Xac
D18
y = 0.9279x + 3.5119
0.9396
40.2
Bismerthiazlb
y = 0.7952x + 3.4030
0.9745
101.9
Thiodiazole copperb
y = 0.6306x + 3.8749
0.9376
60.8
Xoo
D23
y = 1.0131x + 3.2763
0.9962
50.3
Bismerthiazlb
y = 0.8603x + 3.2570
0.9863
106.2
Thiodiazole copperb
y = 0.6147x + 3.8307
0.9498
79.8
According to the bacterial inhibitory activities shown in Table 4, The structure–activity relationship (SAR) analysis showed that the inhibitory activities of D18 (n = 4, R = 3-Br-Ph) and D6 (n = 3, R = 3-Br-Ph) against Xac were 71.2 and 11.0%, at a concentration of 100 μg/mL. Indicating that the growth of Xac could be inhibited when the number of C atoms of brominated alkanes was 4. In addition, when R was electron-absorbing group can enhance the inhibition rate of the compounds against Xac, such as D18 (n = 4, R = 3-Br-Ph) showed significantly higher inhibition rate than D16 (n = 4, R = 3-CH3-Ph), the effect of introducing heterocyclic groups was analyzed, such as D19 (n = 4, R = 2-Thienyl) showed higher inhibition rate than D20 (n = 4, R = 4-OCH3-Ph), indicating that the introduction of heterocyclic groups contributes to the inhibition of Xac activity. Similarly, the structure activity relationship of the compounds on Xoo inhibition was analyzed at 100 μg/mL concentration, the inhibition rate of D23 (n = 4, R = 4-CH3-Ph) was 68.2%, significantly higher than that of D16 (n = 4, R = 3-CH3-Ph), where R of D23 was para-substituted phenyl and R of D16 was m-substituted phenyl, thus, it is clear that when R is a para-electron giving substituent group, it is beneficial to improve the Xoo inhibiting activity. In addition, the inhibition rate of D23 and D11 was D23 (n = 4, R = 4-CH3-Ph) > D11(n = 3, R = 4-CH3-Ph), suggesting that when the number of C-atoms of brominated alkanes was 4, the inhibition of Xoo was also enhanced.
3.8 Effect of D23 on the hyphae morphology of Xoo
The mechanism of action of D23 on Xoo was further investigated by SEM. As shown in the Fig. 8, the cells in the CK group without drug treatment were uniformly full and showed a complete cylindrical shape. After treatment with D23, at 50 μg/mL, some of the cells were flattened and the cell membrane was ruptured, at 100 μg/mL, cell rupture increased significantly, exhibiting contraction, collapse and deformation. SEM analysis showed that the greater the drug concentration, the greater the degree of cell membrane damage, which further suggests that D23 can disrupt cell membranes and has potential antibacterial effects.
SEM of the hyphae of Xoo with or without compound D23 treatment. (A) CK groups (DMSO); (B) D23 at 50 µg/ mL and (C) D23 at 100 µg/ mL.
4 Conclusions
In this study, a series of chalcone derivatives containing indole were synthesized and their structures were characterized. The results of biological assays showed that some of the target compounds exhibited excellent antiviral and antibacterial activities. Among them, D11 surpassed the commercial drug NNM in terms of both Curative and protective activities. The Molecular docking, MST, MDA, POD and SDH assays were performed on D11, the results of mechanism of action were consistent with the preliminary screening datas. The inhibitory activity of the D23 against Xoo was tested by 96-well plate assay, and the test results showed that D23 had excellent inhibitory effect on Xoo, and scanning electron microscopy experiments were performed. This study lays the foundation for the application of chalcone derivatives in pesticides.
Acknowledgement
The authors gratefully acknowledge the National Nature Science Foundation of China (No. 32072446), the Science Foundation of Guizhou Province (No. 20192452).
The Supporting Information includes the characterization data, HNMR and HRMS spectrogram for the target compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chalcones and bischalcones analogs as DPPH and ABTS radical scavengers. Lett. Drug. Des. Discov. 2021;18:249-257.
- [Google Scholar]
- Advances in chalcones with anticancer activities. Recent. Pat. Anti-canc.. 2014;10:97-115.
- [Google Scholar]
- The orientation and policy suggestions for the agricultural high-quality development. J. Manage.. 2019;32:28-35.
- [Google Scholar]
- Synthesis and antibacterial activity of chalcone derivatives containing thioether triazole. J. Heterocyclic. Chem.. 2020;57:983-990.
- [Google Scholar]
- Industrial prune processing and its effect on pesticide residue concentrations. Food. Chem.. 2018;268:264-270.
- [Google Scholar]
- Antibacterial indole alkaloids with complex heterocycles from voacanga africana. Org. Lett.. 2018;20:2702-2706.
- [Google Scholar]
- Marine sesquiterpenes for plant protection: discovery of laurene sesquiterpenes and their derivatives as novel antiviral and antiphytopathogenic fungal agents. J. Agric. Food Chem.. 2022;70:6006-6014.
- [Google Scholar]
- New chalcone derivatives: synthesis, antiviral activity and mechanism of action. RSC Advances.. 2020;10:24483-24490.
- [Google Scholar]
- Synthesis of novel antiviral ferulic acid–eugenol and isoeugenol hybrids using various link reactions. J. Agric. Food Chem.. 2021;69:13724-13733.
- [Google Scholar]
- The influence of isobavachalcone to cellular structure and mycelial morphology of valsa mali miygabe et yamada. Agrochemicals.. 2014;53:290-292.
- [Google Scholar]
- Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem.. 2003;51:571-581.
- [Google Scholar]
- Synthesis and biological evaluation of indole-3-carboxamide derivatives as antioxidant agents. Chinese. Chem. Lett.. 2019;30:2157-2159.
- [Google Scholar]
- Novel chalcone/aryl carboximidamide hybrids as potent anti-inflammatory via inhibition of prostaglandin E2 and inducible NO synthase activities: design, synthesis, molecular docking studies and ADMET prediction. J. Enzym. Inhib. Med. Chem.. 2021;36:1067-1078.
- [Google Scholar]
- Design, synthesis and antibacterial activities against Xanthomonas oryzae pv. oryzae, Xanthomonas axonopo-dis pv. Citri and Ralstonia solanacearum of novel myricetin derivatives containing sulfonamide moiety. Pest Manag. Sci.. 2020;76:853-860.
- [Google Scholar]
- Synthesis of leading chalcones with high antiparasitic, against hymenolepis nana, and antioxidant activities. Braz. J. Pharm. Sci.. 2018;54:2175-9790.
- [Google Scholar]
- Streptindole and its derivatives as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem.. 2020;68:7839-7849.
- [Google Scholar]
- Increasing structural diversity of prenylated chalcones by two fungal prenyl transferases. J. Agric. Food Chem.. 2022;70:1610-1617.
- [Google Scholar]
- The present situation of pesticide residues in china and their removal and transformation during food processing. Food. Chem.. 2021;354:129552
- [Google Scholar]
- Bioactive alkaloids from indole-3-car-binol exposed culture of daldiniaeschscholzii. Chinese. J. Chem.. 2018;36:749-753.
- [Google Scholar]
- Tulongicin, an antibacterial tri-indole alkaloid from a deep-water topsentia sp. sponge. J. Nat. Prod.. 2017;80:2556-2560.
- [Google Scholar]
- Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega.. 2021;6:30826-30833.
- [Google Scholar]
- Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone. Arab. J. Chem.. 2022;15:104019
- [Google Scholar]
- Design, synthesis, and structure–activity relationship study of novel indole-2-carboxamide derivatives as anti-inflammatory agents for the treatment of sepsis. J. Med. Chem.. 2016;59:4637-4650.
- [Google Scholar]
- CRISPR/Cas: The new frontier in plant improvement. ACS Agric. Sci. Technol.. 2022;2:202-214.
- [Google Scholar]
- Diagnostics of infections produced by the plant viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13. ACS Synth. Biol.. 2022;11:2384-2393.
- [Google Scholar]
- Synthesis of chalcones derivatives and their biological activities: A Review. ACS Omega.. 2022;7:27769-27786.
- [Google Scholar]
- Synthesis, structure, and anti-inflammatory activity of functionally substituted chalcones and their derivatives. Russ. J. Gen. Chem.. 2019;89:1360-1367.
- [Google Scholar]
- Long-term efficacy of methotrexate plus vinblastine / vinorelbine in a large series of patients affected by desmoid-type fibromatosis. Cancer J.. 2017;23:86-91.
- [Google Scholar]
- Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives. J. Agric. Food. Chem.. 2021;69:11085-11094.
- [Google Scholar]
- Indole alkaloids with antibacterial activity from aqueous fraction of alstonia scholaris. Tetrahedron.. 2015;71:4372-4378.
- [Google Scholar]
- In silico study of the radical scavenging activities of naturalindole-3-carbinols. J. Chem. Inf. Model.. 2020;60:316-321.
- [Google Scholar]
- Synthesis of novel antibacterial and antifungal quinoxaline derivatives. RSC Advances.. 2022;12:2399-2407.
- [Google Scholar]
- Studies on the biological activity of gem-difluorinated 3, 3′-spirocyclic indole derivatives. Chinese. Chem. Lett.. 2021;8:1-4.
- [Google Scholar]
- Antimicrobial indole alkaloids with adductive C9 aromatic unit from gelsemium elegans. Tetrahedron. Lett.. 2018;59:2066-2070.
- [Google Scholar]
- Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: a meta-analysis. Peer J.. 2022;10:12861.
- [Google Scholar]
- Synthesis and antiviral/ fungicidal/ insecticidal activities study of novel chiral indole diketopiperazine derivatives containing acylhydrazone moiety. J. Agric. Food Chem.. 2020;68:5555-5571.
- [Google Scholar]
- Design, synthesis and invitro anti-mycobacterial evaluation of gatifloxacin-1H-1,2,3-triazole-isatin hybrids. Bioorg. Med. Chem. Lett.. 2017;227:3643-3646.
- [Google Scholar]
- The ratoon rice system with high yield and high efficiency in china: progress, trend of theory and technology. Field. Crop. Res.. 2021;272:108282
- [Google Scholar]
- Research progress on the effect of drought on root system of summer maize. IOP Conf. Ser.: Earth Environ. Sci.. 2021;705:012003.
- [Google Scholar]
- Changes in gastrodia tuber ethanol extracts during grifola frondosa fermentation. Chem. Nat. Compd.. 2016;52:1-4.
- [Google Scholar]
- Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules.. 2020;25:4566-4581.
- [Google Scholar]
- Use of lentinan to control sharp eyespot of wheat, and the mechanism involved. J. Agric. Food Chem.. 2017;65:10891-10898.
- [Google Scholar]
- Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem.. 2018;66:6504-6512.
- [Google Scholar]
- Design, synthesis, and antifungal activity of novel chalcone derivatives containing a piperazine fragment. J. Agric. Food Chem.. 2022;70:1029-1036.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104776.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







