Translate this page into:
Characterization of the key volatile organic components of different parts of fresh and dried perilla frutescens based on headspace-gas chromatography-ion mobility spectrometry and headspace solid phase microextraction-gas chromatography-mass spectrometry
⁎Corresponding authors at: College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, No.10 Poyanghu Road, West Tuanbo New Town, Jinghai District, Tianjin 301617, PR China. hsyu@tjutcm.edu.cn (Heshui Yu), lizheng@tjutcm.edu.cn (Zheng Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Perilla frutescens was an annual herb considered as one of the “One Root of Medicine and Food”, and it was used widely for food and medical treatment. Its main active ingredients were volatile organic compounds (VOCs), but it was easily affected during drying and storage. The leaves, seeds and stems had shown differences in therapeutic effects, but the underlying reason remained unclear. In the present work, headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) and headspace solid phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC–MS) were utilized to effectively and comprehensively analyze VOCs of fresh and dried P. frutescens between the leaves, stems and seeds. Meanwhile, chemometric analysis was applied to compare and identify characteristic volatile markers. As a result, 60 VOCs were identified by HS-GC-IMS and 115 VOCs were identified by HS-SPME-GC–MS from P. frutescen. 25 potential volatile markers were selected based on a combination model of orthogonal partial least squares discriminant analysis (OPLS-DA) and random forest (RF). The models could be used to analyze the variation between fresh and dried P. frutescens and distinguish the differences in different parts effectively and comprehensively. It was the first research regarding the method development of HS-GC-IMS and HS-SPME-GC–MS that comprehensively analyzes the VOCs characterization of fresh and dried P. frutescens in different parts and the findings obtained would evaluate the quality and provide a reference for further exploration of the edible and medicinal effects of P. frutescens.
Keywords
Different parts
Headspace-gas chromatography-ion mobility spectrometry
Headspace solid phase microextraction-gas chromatography-mass spectrometry
Perilla frutescens
Volatile organic compounds

1 Introduction
Perilla frutescens (L.) Britt. was a significant traditional Chinese medicine (TCM) with dual features of both nutrition and medical treatment that belongs to the Lamiaceae family and was extensively cultivated in East Asian countries such as Japan, India and China(Bachheti et al., 2014). It was used as an ornamental plant in gardens because of their specific leaf morphology and exceptional fragrance as well as in skin care products owing to their biological activities(Ahmed 2018). It was also used as a kitchen herb in salads, sushi, and soups and as a spice, garnish, or food colorant as well(Fujiwara et al., 2018, Ahmad 2022). In addition, P. frutescens was also used as a functional food which was considered an excellent alternative to fish oil nutritional supplement and a good source of vitamins and minerals (Ahmed 2018). It could even reduce the risk of cancer, cardiovascular disease and metabolic disorders such as breast cancer, obesity, type 2 diabetes and so on (Wang et al., 2020, Ahmad 2022). In Chinese traditional medicinal system, dried parts such as P. frutescens leaves, P. frutescens stems and P. frutescens seeds were considered as different drug and have different treatment effects(Liu et al., 2013). The leaves were introduced for treating respiratory and intestinal diseases such as cough, vomiting in pregnancy, cold, fever, poisoning from fish and crabs, and so on. The stems possessed the effect of curing vomiting and belching, epigastric pain, as well as threatened miscarriage, while the seeds were used to heal cough, wheezing, and constipation(Ahmed 2018). Modern pharmacological researchers had proved that P. frutescens possessed numerous effects of anti-bacterial, anti-inflammatory, anti-depression, analgesia, anti-mutagenic, anti-carcinogenic, preventing miscarriage, and so on(Dhyani et al., 2019, Ahmad 2022).
P. frutescents had a lot of complicated volatile organic components (VOCs). However, the variety and content of VOCs in processing, stored procedures and plant growth situations would be affected to a certain extent(Song et al., 2021). There were significant differences in VOCs content due to geography, harvesting time, etc. According to the chemotypes, it could be divided into ketone type, aldehyde type, phenol type, etc.(Zhang et al., 2009). P. frutescens containing a large amount of perilla ketone was reported to have potent acute toxicity and cardio-pulmonary toxicity for experimental animals and livestock(Yu et al., 2017). Therefore, it is important to comprehensively characterize VOCs in three different parts (seeds, leaves and stems) of fresh and dried P. frutescens. Previous studies in VOCs identified mostly used two-dimensional gas chromatography (GC × GC), gas chromatography mass spectrometry (GC–MS), electronic noses (e-nose) and gas chromatography–olfactometry-mass spectrometry (GC-O-MS)(Franchina et al., 2019, Li et al., 2022). GC-O-MS could screen the important aroma-active compounds of complex samples. But it was difficult to satisfy the rapid detection of volatile compounds because of the long sample pre-processing operation time(Song and Liu 2018). Although e-nose was a relatively fast assessment detection system that possesses simple sample pretreatment and non-destructive features, the repeatability and stability were easily influenced by environmental factors and low accuracy(Zhou et al., 2017). GC × GC was a powerful precision analytical technique to study complex samples(Aspromonte et al., 2019). Its high resolution was not easy to achieve by GC–MS and GC–MS was applied more widely in practical applications. GC–MS was a detection technology applied to the separation and identification of complex component. It was provided with the advantages of safety, accuracy and high separation ability(Yin et al., 2021). Furthermore, the introduction of headspace solid-phase microextraction (HS-SPME) that integrates extraction, concentration, desorption, and sampling had greatly improved the complex procedures of GC–MS sample pretreatment (e.g., traditional essential oil extraction), and achieved efficient determination of VOCs without damaging the sample(Zanella et al., 2021). HS-GC-IMS was an emerging technique with high molecular specificity and sensitivity for detecting in the field of small molecular weight VOCs based on the difference in migration velocity of different gas phase ions in an electric field. The technology had the advantages of easy operation, affordability, and nondestructive analysis(He et al., 2020, Yin et al., 2021). HS-GC-IMS detected smaller VOCs particles than HS-SPME-GC–MS. Combining HS-SPME-GC–MS and HS-GC-IMS was able to promote detection efficiency and implement the comprehensive characterization of VOCs. To our knowledge, no research has been conducted to compare and comprehensively analyze VOCs of the different parts of fresh and dried P. frutescens combining HS-SPME-GC–MS and HS-GC-IMS.
Therefore, the aim of this study was to explore the differences in VOCs in different parts of P. frutescens and the effect of natural drying on VOCs in order to better understand the characteristic VOCs in P. frutescens. HS-GC-IMS and HS-SPME-GC–MS were used to establish the characteristic volatile fingerprints and investigate the differences of VOCs among three parts of fresh and dried P. frutescens. Multivariate statistics were used to investigate the key substances in three different parts of fresh and dried P. frutescens, which provided reference evidence for the development of functional food and phytomedicines.
2 Materials and methods
2.1 Samples and reagents
P. frutescens samples were collected from the botanical gardens of Tianjin University of Traditional Chinese Medicine where the collection district was divided into five areas. The samples were made an identification by Professor Lijuan Zhang from Tianjin University of Traditional Chinese Medicine. Then, each sample was immediately divided into two parts. One part was placed in a refrigerator (Haier Co., Ltd, Shandong, China) at − 4° C to maintain its freshness as fresh P. frutescens samples. The other part was used as dried P. frutescens sample after air-desiccated for six days in the shade, and then were sealed and stored under normal temperature. So, there were a total of 30 batches samples containing different parts (stems, leaves, seed) of fresh and dried. All samples were stored in the laboratory of Tianjin University of Traditional Chinese Medicine.
N-ketone C4-C9 standard mix for HS-GC-IMS was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). N-alkane C8-C20 standard was got from Sigma-Aldrich Chemical Co., Ltd. (Missouri USA) as external references for HS-SPME-GC–MS. Reference standards of perilla ketone (CAS: 553–84-4), caryophyllene (CAS: 87–44-5), caryophyllene oxide (CAS: 1139–30-6), linalool (CAS: 78–70-6), 1-octen-3-ol (CAS: 3391–86-4), humulene (CAS: 6753–98-6) and citral (CAS: 5392–40-5) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Seven standards were diluted with ethyl acetate to form a mixed standard (perilla keton:1.5 mg/ml, caryophyllene: 0.4 μl/mL, caryophyllene oxide:0.112 mg/ml, linalool:1μl/mL, 1-octen-3-ol: 1 μl/mL, humulene: 0.4 μl/mL and citral: 1 μl/mL).
2.2 HS-GC-IMS analysis
The experiment was analyzed on the HS-GC-IMS apparatus (Flavourspec®, G.A.S, Dortmund, Germany) with an autosampler (CTC Analytics AG, Zwingen, Switzerland) and the GC was provided with a FS-SE-54-CB-1 (CS-Chromatographie Service GmbH, Germany) capillary column (15 m × 0.53 mm ID, 0.5 μm). The fresh samples were thinly grated while the dried samples were pulverized into powder (40-mesh sieve). First, 0.3 g of sample was weighed into a 20 mL headspace glass sampling bottle (Zhejiang HAMAG technology, Ningbo, China). and incubated at 80 °C for 20 min with an incubation speed of 500 rpm. Subsequently, headspace samples (500 µl) were automatically injected into the injector (85 ℃) with splitless mode and then driven into the FS-SE-54-CB-1 capillary column of 45 °C isothermal conditions through nitrogen of 99.99% purity. And its programmed flow rate was started at 2 mL/min for 2 min, then increased to 10 mL/min within 15 min, increased to 100 mL/min over 25 min and increased to 120 mL/min over 30 min. The flow rate of purified nitrogen (99.999% purity) was at the rate of 150 mL/min as the drift gas. The analytes following GC separation and elution at 75 ℃ were driven into the IMS ionization chamber. Finally, the analytes were ionized by 3H ionization source with 300 MBq activity under the positive ion mode. The generating ions were transferred into the drift tube of 9.8 cm in length. The IMS drift tube worked at an electric-field of 5 kV and 45 °C isothermal conditions. All analyses were taken thrice. N-ketone C4-C9 standard mix was used to calculate the retention index (RI) of each compound as external references.
2.3 HS-SPME-GC–MS analysis
HS-SPME-GC–MS analysis was implemented by an Agilent 7890B instrument (Agilent, CA, USA) equipped with an Agilent 7000D mass spectrometry detector and GC autosampler system. The GC was fitted with a HP-5MS elastic quartz capillary column (30 m × 0.25 mm × 0.25 μm, 19091S-433, J&W Scientific, Folsom, CA, USA). SPME (Polydimet-hylsiloxane/Carboxen/divinylbenzene 50/30 μm phase thickness (PDMS/CAR/DVB) fiber (supelco, Bellefonte, Penn.) was installed on Multipurpose sampler (Gerstel, GER). The fresh samples were thinly grated while the dried samples were pulverized into powder (40-mesh sieve). Briefly, 0.3 g of sample was weighed into a 20 mL headspace glass sampling bottle (Zhejiang HAMAG technology, Ningbo, China) and incubated at 60 ℃ incubation temperature for 5 min. Subsequently, the SPME fiber needle inserted into the headspace glass sampling bottle for extraction for 10 min at 60 ℃. Then, the fiber needle immediately plugged into the heated injection port at desorption for 5 min (250 ℃, splitless mode). The flow rate of helium(>99.999%) as the carrier gas was 1 mL/min. The GC column temperature was programmed temperature as follows: initially programmed at 40 °C for 2 min, at 7 °C/min to 62.5 °C, at the rate of 20 ℃/min to 72.5 ℃, at 2 °C/min to 85 °C, rose to 145 °C at rate of 5 °C/min, then at 2 °C/min to 150 °C, at 5 °C/min to 155 °C, at 7 °C/min to 170 °C, eventually, at 20 °C/min to 250 °C held for 5 min. The GC total running time was 45.393 min. The MS was operated in electron ionization (EI) mode at ionizing energy of 70 eV. The injection port and ion source temperature were set at 250 ℃ and 230 ℃, respectively. The quadrupole temperature was 150 ℃. The mass spectra (MS1 full scan mode) was scanned from 50 to 600 m/z. All samples were performed in double. N-alkane C8-C20 standard was used to calculate RI of each compound as external references.
2.4 Data analysis
VOCs detected by HS-GC-IMS were identified by Laboratory Analytical Viewer (LAV) according to RI and drift time (Dt) and the fingerprint was established by Gallery Plot. VOCs detected by HS-SPME-GC–MS were tentatively identified from the standard NIST17 (matching degree greater than 700, RI) and reference compounds. Principal component analysis (PCA) and hierarchical clustering analysis (HCA) analyses were conducted by Origin 2021 software. SIMCA 14.1 was used to build the model of orthogonal partial least squares discrimination analysis (OPLS-DA). The online website MetaboAnalyst 5.0 was employed to build the Random Forest (RF) model.
3 Results and discussions
3.1 The characteristic VOCs of fresh and dried P. frutescens
3.1.1 Differences of VOCs between fresh and dried P. frutescens by HS-GC-IMS
HS-GC-IMS analysis was the latest non-targeted and rapid analytical technique which had great potential for detecting small-molecule(Chen et al., 2021). For exploring the differences of small-molecule VOCs among fresh and dried P. frutescens, the small-molecule VOCs were detected by HS-GC-IMS. In the present study, 60 small-molecule VOCs were identified according to RI and Dt in all P. frutescens samples by HS-GC-IMS (Supplementary Information, Table A.1 and Fig.S1). Due to the adduct formation between partial molecules and reactant ions, a single VOCs with high concentration or higher proton affinity than that of water could form multiple signals presenting as dimer or polymer(Zhang et al., 2021). Therefore, the identified components included both dimers and monomers. However, the differences among fresh and dried P. frutescens could not be quickly identified by table. Therefore, the fingerprints were established between fresh samples and dried samples in the same part (Fig. 1A&B&C). The color represented the peak intensity. The peak intensity increased from blue to white and then to red. The compositional differences were evident between fresh and dried samples in fingerprints. 22 small-molecule VOCs (heptan-2-ol, vanillin, benzyl alcohol, etc.) only were identified in fresh stem (Fig. 1A, a), Conversely, 10 small-molecule VOCs (e.g., 4-methylguaiacol, coumarin, 4-ethylphenol, etc.) were recognized only in dried stems (Fig. 1A, b). Compounds “Unknown” were unidentified compound. After comparing fresh and dried P. frutescens stems, it was found that fresh stems had many special components that dried stems do not have. For example, benzothiazole of fresh stems had anti-cancer, anti-inflammatory, analgesic antidiabetic, etc.(Das et al., 2022), which suggested that future research could develop related products for fresh P. frutescens stems. 18 small-molecule VOCs (p-cymen-7-ol, nerol, eugenol, etc.) were only identified in fresh leaves (Fig. 1B, c). Nerol and eugenol with antibacterial and antioxidant properties had long been used in food and cosmetology(Wang et al., 2019, Ulanowska and Olas 2021). 12 small-molecule VOCs were only identified in dried leaves, such as neryl acetate, ethyl 3-phenylpropanoate, and so on (Fig. 2B, d). Vanilla was detected both in fresh and dried leaves, which was the world’s most popular flavor in food, cosmetics, and medicine(Arya et al., 2021). 5 small-molecule VOCs (α-terpineol, 2-acetylthiazole, etc.) were identified exclusively in dried seeds (Fig. 1C, e), while geraniol and phenyl ethyl alcohol were just recognized in fresh seeds (Fig. 1C, f). Geraniol were regarded as good natural preservatives on account of antibacterial and antioxidant(Lin et al., 2021). After drying, the components existing in the fresh samples disappeared in the dried sample, and many new components were produced in the dried samples (Fig. 1A&B&C), especially in the stems and leaves samples, which demonstrated those compounds were sensitive to drying process and were decomposed or degraded. Besides, these components may contribute to the corresponding aroma between fresh and dried P. frutescens. The signal strength represented the concentration of flavor molecules (Fig. 1A&B&C) of HS-GC-IMS, the white color indicated the lower concentration while the red color box showed higher and the darker the color gets, the higher the concentration of fingerprint plots(Chen et al., 2021). Compared to dried samples (seeds, leaves and stems), fresh samples (seeds, leaves and stems) seemed to have a stronger signal intensity. The changes of signal intensities of small-molecule VOCs were due to the chemical reactions (Maillard reaction, interaction between different molecules and degradation of macromolecules) during drying(Guo et al., 2018). Fresh P. frutescens was more suitable as flavorings or vegetables to use because it had a strong flavor concentration. Besides, the small-molecule VOCs that had been identified were classified into six ingredient categories (Fig. 1D). Alcohols (11 components) and others (7 components) were most found in fresh P. frutescens leaves. Esters (9 components) were most found in dried P. frutescens leaves. Ketones (6 components) mainly focused on dried P. frutescens seeds. Aldehydes (3 components) were relatively abundant in fresh P. frutescens seeds. Thus, the aroma of P. frutescens were more derived from alcohols and Esters. In the current study, most of the small molecules contained in P. frutescens had strong oxidative and antibacterial bioactivities, making it a potential ingredient for functional food development. Unfortunately, there were still many small-molecule VOCs of P. frutescens that had not yet been identified. Therefore, the IMS database needed to be further supplemented in future researches.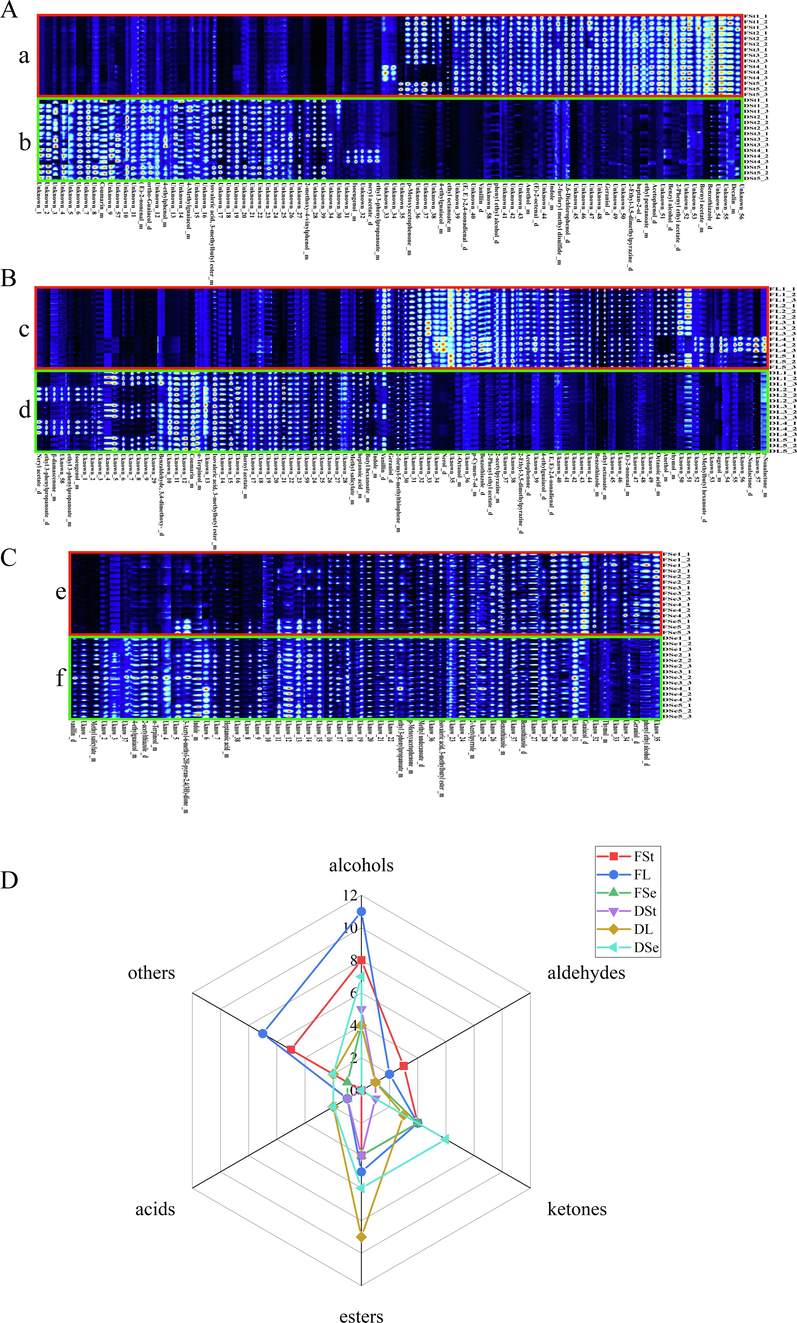
Fingerprints and radar chart of the fresh and dried P. frutescens from HS-GC-IMS. (Each row stands for a selected signal peak in one sample and each column stands for a signal peak of a compound in a different sample. Fst: fresh stems (a), FL: fresh leaves (b), FSe: fresh seeds (c), DSt: dried stems (d), DL: dried leaves (e), DSe: dried seeds (f). (A) stems, (B) leaves, (C) seeds. (D) Component classification identified by HS-GC-IMS.
3.1.2 Differences of VOCs between fresh and dried P. frutescens by HS-SPME-GC–MS
HS-SPME-GC–MS was used widely and effectively for analyzing VOCs. In order to explore comprehensively VOCs between fresh and dried P. frutescens, HS-SPME-GC–MS was performed. A total of 115 VOCs were identified by HS-SPME-GC–MS (Supplementary Information, Table A.2). Seven compounds had passed standard validation (Fig. 2A, a), and the remaining components had been identified through literatures, database, and RI. The components detected by HS-SPME-GC–MS were more than HS-GC-IMS. The total ion chromatograms (TIC) of all P. frutescens samples were shown in Supplementary Information (Supplementary Information, Fig. S2). Elsholtzia ketone, cuminaldehyde, perilla ketone, caryophyllene, and linalool with relatively high content(Fig. 2A, b) possessed pharmacological activities for cardiovascular, respiratory systems and tumor cells(Ahmed 2018, Chen et al., 2020). After natural shade drying, these VOCs displayed change to some extent. Compared to the fresh P. frutescens stems, a significant increase in the content of elsholtzia ketone, perilla ketone, caryophyllene, and linalool was found in the dried P. frutescens stems. Compared with the fresh P. frutescens leaves, the contents of perilla ketone, caryophyllene, and linalool were increased in all samples of dried P. frutescens leaves, but elsholtzia ketone and cuminaldehyde were decreased in dried P. frutescens leaves excluding sample 4 which had a slight increase. Elsholtzia ketone and cuminaldehyde were decreased in sample 5 and increased in the remaining dried P. frutescens seeds compared with the fresh P. frutescens seeds, whereas caryophyllene was decreased except for sample 5. Two other compounds (perilla ketone and linalool) were both increased and decreased between different batches of dried P. frutescens seeds. Interestingly, elsholtzia ketone and cuminaldehyde content seemed to exhibit synergistic effects after shade drying whether leaves and stems or seeds of P. frutescens. The differences may be a factor of dried P. frutescens as medicine. Some VOCs such as (R)-l-perillaldehyde, α-farnesene and trans-2-hexenal were only detected in dried stems. Conversely, some VOCs such as (R)-cuparene, beta-elemene and citral were only identified in fresh stems. Cis-linalool oxide, (R)-l-perillaldehyde and α-cubebene were just detected from dried leaves while butylated hydroxytoluene, α-longipinene, and 2-methylbutyraldehyde were only tested from fresh leaves. 1-(6,6-dimethylbicyclo[3.1.0]hex-2-en-2-yl)-ethanone and ylangenol were only found in the dried seeds. Conversely, some ingredients (e.g. 3-carene, β-guaiene, egomaketone, etc.) were only detected in the fresh seeds. The VOCs formed after drying may be attributed to precursors in fresh samples being degraded, isomeric species or oxidation reactions during the drying process(Song et al., 2021). Furthermore, small-molecule VOCs detected by HS-GC-IMS may be involved in synthesis and conversion processes of the aforementioned compounds detected by HS-SPME-GC–MS. For example, limonene could produce α-terpineol by biotransformation(Molina et al., 2019). Terpenes were relatively abundant in fresh and dried P. frutescens (Fig. 2D). Terpenes were a vital member of the VOCs safeguarding plants against pests, diseases, and weeds, and also offered a wide range of prospects in the pharmaceutical, food, cosmetic, and flavoring industries(Ninkuu et al., 2021).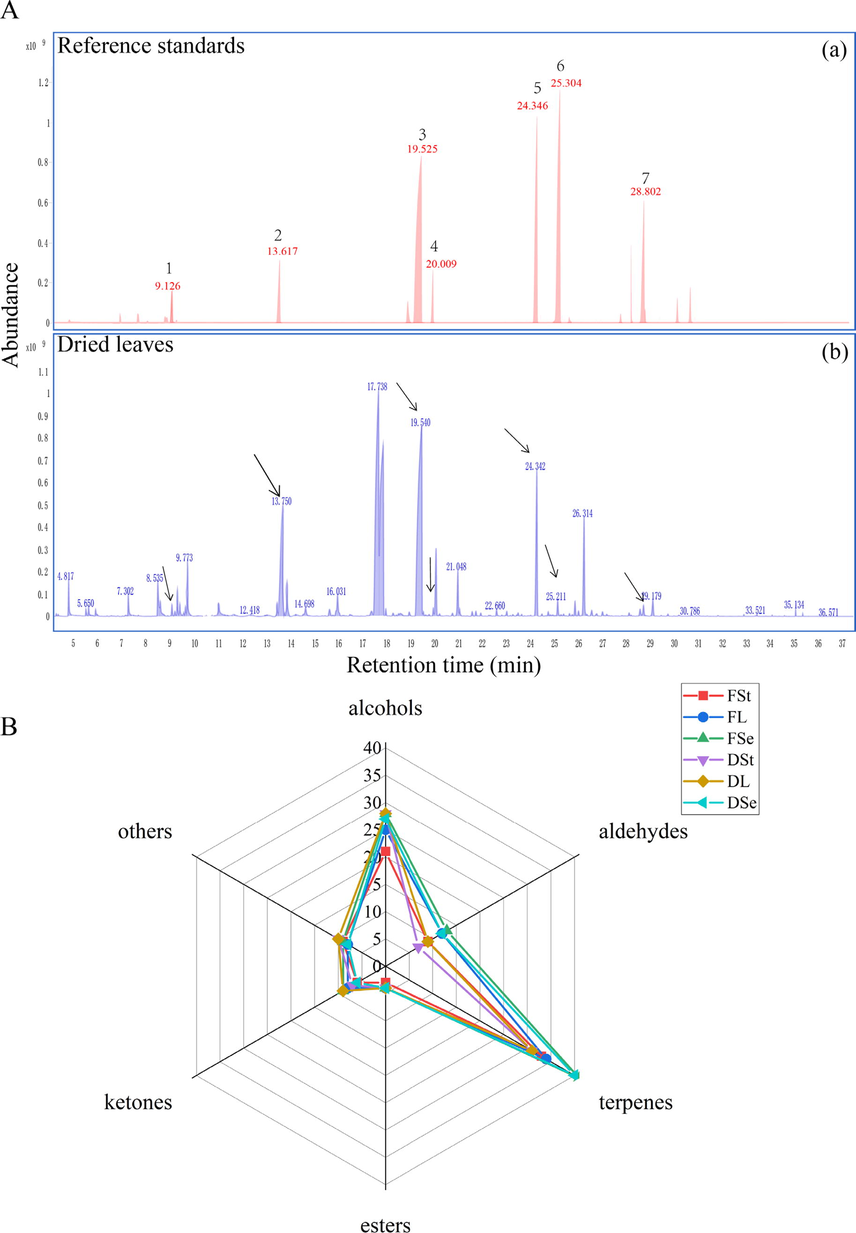
The total ion chromatograms (TIC) (A) and radar chart (B) of the fresh and dried P. frutescens from HS-SPME-GC–MS. (A): The total ion chromatograms of mixed standard chemicals (a) and dried P. frutescens leaves samples (b). 1: 1-Octen-3-ol; 2: Linalool; 3: Perilla ketone; 4: Citral; 5: Caryophyllene; 6: Humulene, 7: Caryophyllene oxide. (B): Component classification identified by HS-GC-IMS HS-SPME-GC–MS detected. Fst: fresh stems, FL: fresh leaves, FSe: fresh seeds, DSt: dried stems, DL: dried leaves, DSe: dried seeds.
3.2 The characteristic VOCs of three parts of fresh and dried P. frutescens
3.2.1 Differences of VOCs in three parts of fresh or dried P. frutescens by HS-GC-IMS
Dried parts such as leaves, stems and seed were regarded as different drugs from ancient times to the present, but the underlying reason remains unclear. The fingerprint profiles form by HS-GC-IMS (Fig. 3) indicated there was distinct variability among different parts of P. frutescens. Among the different parts of fresh P. frutescens, the part with the largest amount and content of components was the fresh leaves (Fig. 3A, b), and the least was the fresh seeds (Fig. 3A, c). The flavor concentration of P. frutescens leaves was significantly higher, making them one of the favorite vegetables. The researches on different parts of fresh P. frutescens were very meaningful and could promote the development from multiple perspectives in the food field. Among the different parts of dried P. frutescens, the part with the largest number of components was the dried seeds (Fig. 3B, f), and the least was the dried stems (Fig. 3B, d). The differences in the amount and content of components in different parts of dried P. frutescens may be one of the reasons why different parts (seeds, leaves and stems) of P. frutescens as different drugs from the results (Fig. 3B). After the dataset was reduced dimensionality, principal component analysis and hierarchical cluster analysis (HCA: Fig. 4A&B) and (PCA: Fig. 4C&D) were carried out for chemometric analysis. Linkage method of HCA was average-link. Correlation was selected as type of distance. As displayed by the results of HCA (Fig. 4A&B), all fresh samples could be divided into three groups (I, II and IV) which correspond to the seeds, leaves and stems except for two of the samples (stem 5 and leaves 4) was misassigned (Fig. 4A). Meanwhile, other dried samples were clustered into three major clusters (I, II and IV) according to three medicinal parts (seeds, leaves and stems) although three samples of stem 4, leaves 4 and leaves 5 were not clustered into corresponding clusters (Fig. 4B). The result of PCA (Fig. 5C: PC1:50.9%, PC2:20.3%, PC3:9.6%; Fig. 4d: PC1:40 %, PC2:25.9%, PC3:16.5%) was similar to HCA, but HCA relatively achieved better results. Overall, fingerprint profiles, PCA and HCA indicated that VOCs of small molecules in P. frutescens from different parts (leaves, stems and seeds) showed apparent differences. At present, some small-molecule VOCs had been reported to contribute to anti-cancer, such as geraniol, benzothiazole, geranyl acetate, and so on(e Silva et al., 2022). These small-molecule VOCs differences may be associated with the treatment of different kinds of diseases. Thus, the small-molecule VOCs of P. frutescens in pharmacological studies deserved further investigation. Interestingly, although all samples came from the botanical gardens, there were certain differences between batches indicating that using HS-GC-IMS was able to detect subtle differences, which the instrument provided a reference for quality control of P. frutescens. Meanwhile, P. frutescens had genetic instability and easily mated with closely related species to form similar morphology, but there were obvious differences in chemical composition(Hu et al., 2010). Thus, it also suggested more samples were necessary to explore the cultivation conditions, harvesting time, and processing conditions of high-quality P. frutescens to guarantee stable quality.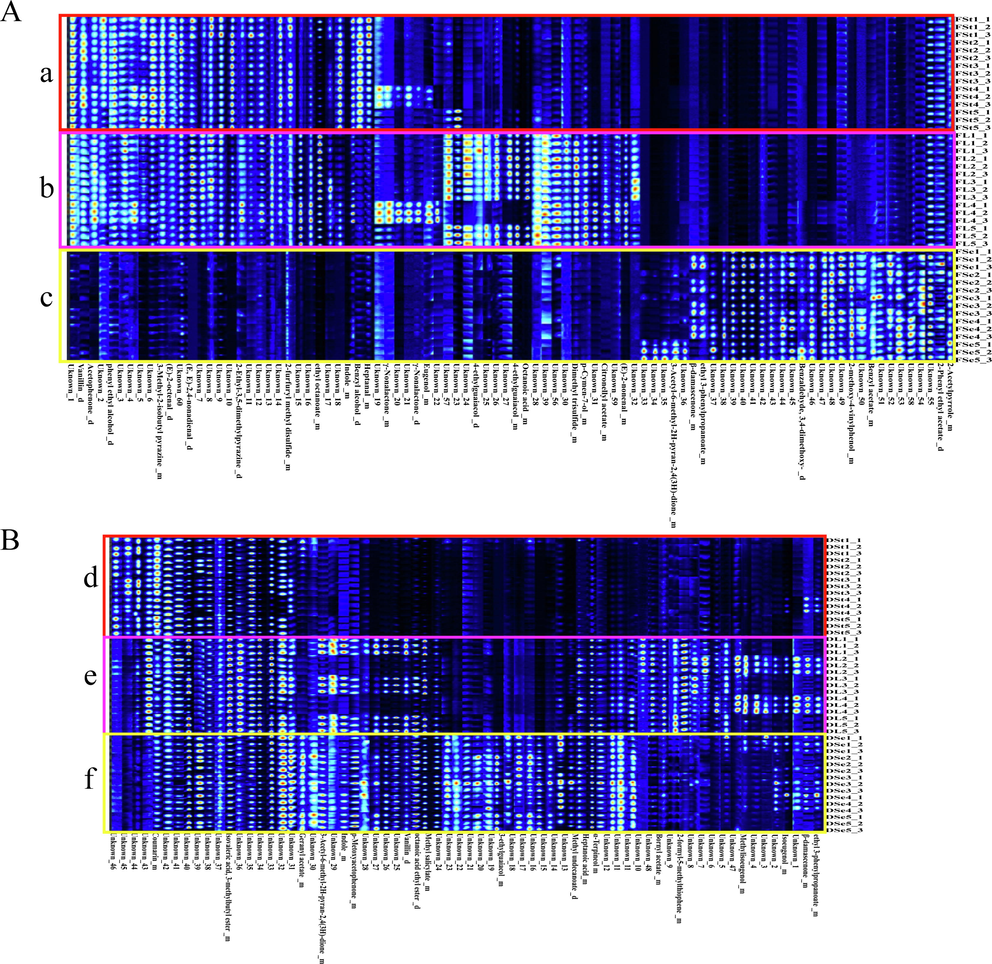
Fingerprints among three different parts of P. frutescens from HS-GC-IMS. (Each row stands for a selected signal peak in one sample and each column stands for a signal peak of a compound in a different sample). Fst: fresh stems (a), FL: fresh leaves (b), FSe: fresh seeds (c), DSt: dried stems (d), DL: dried leaves (e), DSe: dried seeds (f). (A) Different parts of fresh P. frutescens, (B) Different parts of dried P. frutescens.
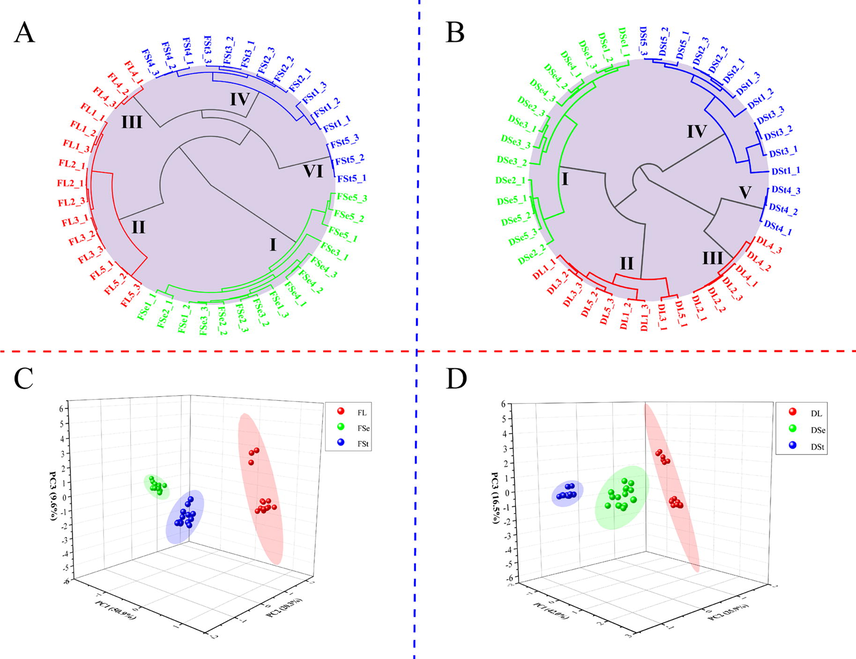
(A)&(B): Dendrograms of different parts resulting from HCA. (C)&(D): Classification of different parts analyzed samples by PCA analysis. FSt: fresh stems; FSe: fresh seeds; FL: fresh leaves; DSt: dried stems; DSe: dried seeds; DL: dried leaves.
![OPLS-DA and RF of different parts of P. frutescens (blue: seeds, green: stems, red: leaves). FSt: fresh stems; FSe: fresh seeds; FL: fresh leaves; DSt: dried stems; DSe: dried seeds; DL: dried leaves. (A)&(B) Discriminatory classification map. (C)&(D) The variable importance for the projection (VIP) predictive of the VOCs (a&b were VIP value greater than 1). (E) significant features of different parts of the fresh. (F) significant features of different parts of the dried. Additionally, (1R,4R,5S): (1R,4R,5S)-1,8-dimethyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene. (1S,4S,4aS): (1S,4S,4aS)-1-isopropyl-4,7-dimethyl-1,2,3,4,4a,5-hexahydronaphthalene. (2R,3S,5S,6R): (2R,3S,5S,6R)-2,5-bis(4-Methoxyphenyl)-3,6-dimethyl-1,4-dioxane-rel- 1,1,4,7-Tetramethyl: 1,1,4,7-Tetramethyl-1a,2,3,4,6,7,7a,7b-octahydro-1H-cyclopropa[e]azulene.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104867-fig6.png)
OPLS-DA and RF of different parts of P. frutescens (blue: seeds, green: stems, red: leaves). FSt: fresh stems; FSe: fresh seeds; FL: fresh leaves; DSt: dried stems; DSe: dried seeds; DL: dried leaves. (A)&(B) Discriminatory classification map. (C)&(D) The variable importance for the projection (VIP) predictive of the VOCs (a&b were VIP value greater than 1). (E) significant features of different parts of the fresh. (F) significant features of different parts of the dried. Additionally, (1R,4R,5S): (1R,4R,5S)-1,8-dimethyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene. (1S,4S,4aS): (1S,4S,4aS)-1-isopropyl-4,7-dimethyl-1,2,3,4,4a,5-hexahydronaphthalene. (2R,3S,5S,6R): (2R,3S,5S,6R)-2,5-bis(4-Methoxyphenyl)-3,6-dimethyl-1,4-dioxane-rel- 1,1,4,7-Tetramethyl: 1,1,4,7-Tetramethyl-1a,2,3,4,6,7,7a,7b-octahydro-1H-cyclopropa[e]azulene.
3.2.2 Differences of VOCs of three parts in fresh or dried P. frutescens by HS-SPME-GC–MS
Although there were no significant differences in the types of VOCs identified by HS-SPME-GC–MS, differences in content of components of different parts were observed (Supplementary Information, Fig.S2 and TableA2). Since there was genetic diversification and varied the content of components in VOCs, previous studies based on the main component content of essential oil divided into the following chemotypes: perillaketone (PK), perillaldehyde (PA), elsholtziaketone (EK), citral (C), phenylpropanoids (myristicin, dillapiole, elemicin) (PP), D-limonene and piperitone (DLP), β-caryophyllene myristicine (MT), perillene (PL) and piperitenone types (PT)(Kimura and Ito 2020). In this study, cuminaldehyde, elsholtzia ketone, perilla ketone and caryophyllene were mainly detected. The content of these components was ranked in the top five of the total VOCs in most of the samples either fresh or dried P. frutescens. Thus, there were two chemotypes identified, including PK-type and EK-type according to the main VOCs in the HS-SPME-GC–MS data. Besides, cuminaldehyde made up the largest content of component in some samples, which had been reported that according to a new classification of five essential oils based on cluster analysis of Euclidean distance, it was divided into fifth categories (EK chemotypes)(Wei et al., 2007). Therefore, the results of the current study supported with classification finding of Euclidean distance analysis and provided a scientific reference for EK chemotypes. Chemotypes of P. frutescens were related to the harvest period, sowing period and growing environment(Ghimire et al., 2017). Therefore, the result appeared variations of these VOCs between batches and had two chemotypes in the collection place.
Different parts of P. frutescens had been used in different medicine clinically. In order to further explore the different VOCs among different parts and screen out suitable volatile markers, the study combined orthogonal partial least squares discriminant analysis (OPLS-DA) and the Random Forest (RF) for analysis. The RF was a supervised method to handle high-dimensional data. The model displayed the best overall performance on volatile markers-selected (Chen et al., 2013). OPLS-DA was commonly used for visualizing high dimensional data and discriminant analysis of latent metabolites related to metabolic changes(Qi et al., 2020), which proved to be powerful tools in qualitative data analysis. Moreover, the variable importance of the projection (VIP) in OPLS-DA was formed by measuring variables with scores. VIP ≥ 1 was used to identify the variables important to the model and were generally considered to be important variables. The larger the VIP value, the more important the variable to the divergence was between different parts. Meanwhile, VIP ≥ 1 was considered to be a significant factor for samples classification(Galindo‐Prieto et al., 2014). In the study, two HS-SPME-GC–MS datasets (different parts of fresh P. frutescens and different parts of dried P. frutescens) were calculated by SIMCA 14.1 software based on the OPLS-DA model respectively (Fig. 5). All P. frutescens samples could be divided into three groups according to the different medicinal parts (seeds, leaves and stems) whether fresh (Fig. 5A) or dried P. frutescens (Fig. 5B), which indicated that differences existed among three parts. The explanatory and predictive abilities of the models were evaluated from R2 and Q2. And the closer R2 and Q2 were to 1, the better fitness of the model was (Wang et al., 2019). The R2 and Q2 of the two OPLS-DA models were greater than 0.9 (R2 = 0.978, Q2 = 0.915 and R2 = 0.965, Q2 = 0.92), which indicated excellent explanatory and predictive effects of the OPLS-DA model for the classification of different parts of P. frutescens. Based on the criteria of VIP ≥ 1, 56 (a) and 60 (b) important variables were selected in different parts of fresh and dried P. frutescens respectively from the VIP plot of OPLS-DA (Fig. 5C&D). To further explore favorable volatile markers from these essential variables, a RF algorithm was used to build RF model. The classification trees of RF model were set as 1000. During trees building, one-third of the samples were used as training data and the remaining samples were used as test data to get a judicial assessment of the out-of-bag (OOB) error. Theoretically, the cross-verification was conducted internally in the RF algorithm between the operation by the OOB dataset. The lower OOB error was a criterion of classifier quality(Bulgarevich et al., 2018). After several trees, the cumulative OOB error rates decreased to 0.0667 in different parts of fresh P. frutescens and to 0 in different parts of dried P. frutescens. Fig. 5E&F showed the first fifteen significant features in the ranking through the RF model. γ-muurolene, α-cubebene, △-cadinene, copaene, β-guaiene and germacrene D were more abundant in fresh seeds (Fig. 5E) with antibacterial and antioxidant, which were suitable for exploitation in various skin-care products(Bahadori et al., 2017, Falleh et al., 2020). (R)-cuparene, γ-terpinene, dibutyl phthalate, p-cymene, 2-nonen-1-ol, 1,2-epoxy-5-methylhexane, butylated hydroxytoluene and toluene were identified as significant volatile markers in fresh stems (Fig. 5E), in which γ-terpinene and p-cymene with anti-inflammatory and antioxidant effects had synergistic antibacterial effect(Sousa et al., 2022). Only 2-methoxyethanol was screened as volatile marker of the fresh leaves (Fig. 5E). Most of the volatile markers of fresh P. frutescens had antibacterial effects, which may be beneficial for the development of functional food related to oral cavity. Meanwhile, dried leaves had volatile markers of 3-hexen-1-ol, benzaldehyde and trans-2-hexenal, dried seeds had β-copaene, copaene, eudesma-4(14),11-diene and (1S,4S,4aS)-1-isopropyl-4,7-dimethyl-1,2,3,4,4a,5-hexahydronaphthalene, and dried stems had 2-nonen-1-ol, cis-4-thujanol, (1R,4R,5S)-1,8-dimethyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene, p-cymene, 1,2-epoxy-5-methylhexane, tetradecane, 3-octanol and 2-methoxyethano(Fig. 5F). P-cymene exhibited remarkable antiproliferative potential against several cancer cell lines such as human cervical cancer, human non-small cell lung cancer (NSCLC) A549 and A427 cell lines(Balahbib et al., 2021). These potential volatile markers provided the reference for studying differences in therapeutic effect.
4 Conclusion
In the present study, the experiment analyzed qualitatively and relatively quantitatively VOCs of fresh and dried P. frutescent accessing to HS-GC-IMS and HS-SPME-GC–MS, as well as using multivariate statistics screened for related volatile markers in different parts. The results demonstrated that the differences of VOCs in the stems, the leaves and the seeds of fresh and dried P. frutescens were obviously observed according to HS-GC-IMS and HS-SPME-GC–MS. Thus, attention should be paid to distinguishing different parts of P. frutescens, which provided a scientific reference for the different applications of different parts of P. frutescens in clinical treatment. In small-molecule flavor, there were fewer shared VOCs between dried and fresh P. frutescens and the content of VOCs was reduced in dried samples. Many characteristic VOCs were detected in fresh and dried P. frutescens. These results were helpful for the development of unique functional food. In VOCs detected by HS-SPME-GC–MS, the content of most of the components was increased during the process of drying. Comprehensive analysis of fresh and dried P. frutescens could acquire more information which was beneficial for future development and application. In addition, the RF model screened out 25 important volatile markers such as trans-2-hexenal, p-cymene and α-cubebene, etc. Therefore, the current study could provide a reference for further exploration in the field of the food and TCM of P. frutescens.
Acknowledgments
This work was supported by the financial support of National Natural Science Foundation of China (Grant No. 82074276) and Science and Technology Program of Tianjin(No.22ZYJDSS00100). The authors would like to thank the support from Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine. (No. ZYYCXTD-D-202002).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A systematic review on multi-nutritional and phytopharmacological importance of Perilla frutescens. International Journal of Green Pharmacy.. 2022;16
- [CrossRef] [Google Scholar]
- Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules. 2018;24:102.
- [CrossRef] [Google Scholar]
- Vanillin: a review on the therapeutic prospects of a popular flavouring molecule. Advances in traditional medicine.. 2021;21:1-17.
- [CrossRef] [Google Scholar]
- Current application and potential use of GC× GC in the pharmaceutical and biomedical field. J. Pharm. Biomed. Anal.. 2019;176:112817
- [CrossRef] [Google Scholar]
- A phytopharmacological overview on Perilla frutescens. Int. J. Pharm. Sci. Rev. Res.. 2014;26:55-61.
- [Google Scholar]
- Bahadori, M. B., G. Zengin, S. Bahadori, et al., 2017. Chemical composition of essential oil, antioxidant, antidiabetic, anti-obesity, and neuroprotective properties of Prangos gaubae. Natural Product Communications. 12, 1934578X1701201233. https://doi.org/10.1177/1934578X1701201233.
- Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol.. 2021;153:112259
- [CrossRef] [Google Scholar]
- Pattern recognition with machine learning on optical microscopy images of typical metallurgical microstructures. Sci Rep.. 2018;8:2078.
- [CrossRef] [Google Scholar]
- Random forest in clinical metabolomics for phenotypic discrimination and biomarker selection. Evid. Based Complement. Alternat. Med.. 2013;2013:298183
- [CrossRef] [Google Scholar]
- Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crop. Prod.. 2020;143:111908
- [CrossRef] [Google Scholar]
- The Aroma Fingerprints and Discrimination Analysis of Shiitake Mushrooms from Three Different Drying Conditions by GC-IMS. GC-MS and DSA. Foods.. 2021;10:2991.
- [CrossRef] [Google Scholar]
- Benzothiazole based fluorescent probes for the detection of biomolecules, physiological conditions, and ions responsible for diseases. Dyes Pigm.. 2022;199:110074
- [CrossRef] [Google Scholar]
- A review on nutritional value, functional properties and pharmacological application of perilla (Perilla frutescens L.) Biomedical and Pharmacology Journal.. 2019;12:649-660.
- [CrossRef] [Google Scholar]
- e Silva, G. d. S., J. N. de Jesus Marques, E. P. M. Linhares, et al., 2022. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chemico-Biological Interactions. 362, 109994. https://doi.org/10.1016/j.cbi.2022.109994.
- Essential oils: A promising eco-friendly food preservative. Food Chem.. 2020;330:127268
- [CrossRef] [Google Scholar]
- Evaluation of different adsorbent materials for the untargeted and targeted bacterial VOC analysis using GC× GC-MS. Anal. Chim. Acta. 2019;1066:146-153.
- [CrossRef] [Google Scholar]
- Anthocyanins in perilla plants and dried leaves. Phytochemistry. 2018;147:158-166.
- [CrossRef] [Google Scholar]
- Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS) J. Chemom.. 2014;28:623-632.
- [CrossRef] [Google Scholar]
- GC–MS analysis of volatile compounds of Perilla frutescens Britton var. Japonica accessions: Morphological and seasonal variability. Asian Pac. J. Trop. Med.. 2017;10:643-651.
- [CrossRef] [Google Scholar]
- Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC–MS. J. Chromatogr. B. 2018;1099:46-55.
- [CrossRef] [Google Scholar]
- Application of GC-IMS in Detection of Food Flavor Substances. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2020.
- [Google Scholar]
- Primary identifications and palynological observations of Perilla in China. J. Syst. Evol.. 2010;48:133-145.
- [CrossRef] [Google Scholar]
- Bioconversion of essential oil components of Perilla frutescens by Saccharomyces cerevisiae. J Nat Med.. 2020;74:189-199.
- [CrossRef] [Google Scholar]
- Assessment of the ‘taro-like’aroma of pumpkin fruit (Cucurbita moschata D.) via E-nose, GC–MS and GC-O analysis. Food Chem.. 2022;X. 15:100435
- [CrossRef] [Google Scholar]
- The Inhibitory Efficiencies of Geraniol as an Anti-Inflammatory, Antioxidant, and Antibacterial, Natural Agent Against Methicillin-Resistant Staphylococcus aureus Infection in vivo. Infection and Drug Resistance.. 2021;14:2991-3000.
- [CrossRef] [Google Scholar]
- Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J.. 2013;7:1-11.
- [CrossRef] [Google Scholar]
- Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresour. Technol.. 2019;294:122180
- [CrossRef] [Google Scholar]
- Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci.. 2021;22:5710.
- [CrossRef] [Google Scholar]
- Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules. 2020;25:6027.
- [CrossRef] [Google Scholar]
- Song, C. F., Ma, X. T. Wang, J. L. et al., 2021. Effects of ultrasound and blanching pretreatments on mass transfer and qualities of hot-air drying of perilla (Perilla frutescens L.) leaves. Journal of Food Processing and Preservation. 45, e15947. 10.1111/jfpp.15947.
- GC-O-MS technique and its applications in food flavor analysis. Food Res. Int.. 2018;114:187-198.
- [CrossRef] [Google Scholar]
- Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep.. 2022;12:1-15.
- [CrossRef] [Google Scholar]
- Biological Properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci.. 2021;22:3671.
- [CrossRef] [Google Scholar]
- Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop.. 2019;22:229-237.
- [CrossRef] [Google Scholar]
- Perilla oil regulates intestinal microbiota and alleviates insulin resistance through the PI3K/AKT signaling pathway in type-2 diabetic KKAy mice. Food Chem. Toxicol.. 2020;135:110965
- [CrossRef] [Google Scholar]
- Untargeted and targeted discrimination of honey collected by Apis cerana and Apis mellifera based on volatiles using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem.. 2019;67:12144-12152.
- [CrossRef] [Google Scholar]
- Analysis and Evaluation of Aromatic Constituents of Perilla. Food Sci. 2007:301-305.
- [Google Scholar]
- Strategy for the multi-component characterization and quality evaluation of volatile organic components in Kaixin San by correlating the analysis by headspace gas chromatography/ion mobility spectrometry and headspace gas chromatography/mass spectrometry. Rapid communications in mass spectrometry : RCM.. 2021;35:e9174.
- [Google Scholar]
- Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J.. 2021;168:106527.
- [CrossRef] [Google Scholar]
- Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol.. 2017;108:375-391.
- [CrossRef] [Google Scholar]
- Comparison of headspace solid-phase microextraction high capacity fiber coatings based on dual mass spectrometric and broadband vacuum ultraviolet absorption detection for untargeted analysis of beer volatiles using gas chromatography. Anal Chim Acta.. 2021;1141:91-99.
- [CrossRef] [Google Scholar]
- Essential oil variations in different Perilla L. accessions: chemotaxonomic implications. Plant Syst. Evol.. 2009;281:1-10.
- [CrossRef] [Google Scholar]
- Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS-GC-IMS. Ultrason. Sonochem.. 2021;80:105807
- [CrossRef] [Google Scholar]
- Identification of chinese herbal medicines with electronic nose technology: Applications and challenges. Sensors. 2017;17:1073.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104867.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1






