Translate this page into:
In vitro/vivo antifungal activity study of novel mandelic acid derivatives as potential fungicides against Thanatephorus cucumeris
⁎Corresponding author. zbwu@gzu.edu.cn (Zhibing Wu) wzb1171@163.com (Zhibing Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
To discover highly efficient and novel lead compounds against Thanatephorus cucumeris, a series of novel mandelic acid derivatives containing 1,3,4-thiadiazole thioether was designed and synthesized. The bioassay results revealed that target compound F10 exhibited excellent antifungal activity against T. cucumeris with EC50 value of 9.7 μg/mL. Further studies found that F10 not only significantly inhibited the growth of T. cucumeris mycelia but also effectively inhibited the formation of sclerotia, and exhibited significant in vivo protective (61.1%) and curative (67.9%) activities at 200 μg/mL. Mechanism studies demonstrated that F10 can damage the integrity of the cell membrane structure, resulting in increased permeability of the cell membrane, releasing the intracellular electrolyte and inhibiting the growth of fungi. In general, this work is helpful for managing the formation and diffusion of the infection source and provides an effective method to control rice sheath blight disease infected with sclerotia of T. cucumeris.
Keywords
Mandelic acid
1,3,4-thiadiazole thioether
Antifungal activity
Sclerotia formation, sclerotia germination
Antifungal mechanism study

- TLC
-
thin layer chromatography
- TMS
-
tetramethylsilane
- CDCl3
-
deuterated chloroform
- DMSO‑d6
-
dimethyl sulfoxide-d6
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR
-
19F nuclear magnetic resonance
- HRMS
-
high-resolution mass spectrometry
- PDA
-
potato dextrose agar
- PDB
-
potato dextrose broth
- PBS
-
phosphate buffer saline
- EC50
-
median effective concentration
- CK
-
blank control
- SM
-
stereoscopic microscopy
- SEM
-
scanning electron microscope
- FM
-
fluorescence microscope
- PI
-
propidium iodide
- MDA
-
malondialdehyde
- SD
-
standard deviation
Abbreviations
1 Introduction
Plant diseases are an important factor that can affect agricultural production (Ghorbanpour et al., 2018; Giray et al., 2020); in particular, plant diseases caused by fungi are one of the most serious threats to global crop production, food quality, and security (Chen et al., 2019b; Li et al., 2019a; Ma et al., 2019; Yang et al., 2019a; Yin et al., 2020; Dong et al., 2022). According to statistics, approximately 10% to 16% of the crop yield reduction in the world is caused by pathogenic fungi each year, and the average economic losses exceeded $220 billion (Fisher et al., 2012; Li, et al., 2019b; Yan et al., 2020; Tudi et al., 2021; Sun et al., 2022). Among the pathogenic fungi, Thanatephorus cucumeris (anamorph: Rhizoctonia solani) causes rice sheath blight, which is one of the most extensive and destructive fungal diseases in many rice-growing areas (Feng et al., 2017; Persaud et al., 2019). Its dispersal and propagation are mediated by sclerotia, aggregations of hyphae, and it can germinate a mass of hyphae under various environments (Basu et al., 2016; Lu et al., 2016; Tiwari et al., 2017). The infection process of plants with T. cucumeris is shown in Figure S1. Long-term persistence of the sclerotia and hyphae in the agricultural landscape is achieved by quiescent survival on plant debris, and T. cucumeris overwinter on straw stubble before infecting a secondary host, leading to the outbreak of disease (Fisher et al., 2012; Suwannarach et al., 2012). Sclerotia and hyphae play crucial roles in the life and infection cycle. Although many attempts have been made to treat rice sheath blight using new technology, the control of T. cucumeris still mainly relies on the utilization of chemical fungicides (Ashkani et al., 2015; Ke et al., 2017; Roese et al., 2018). It would be hugely attractive to develop a fungicide with strong inhibitory action on mycelial development, sclerotia formation or germination of T. cucumeris in one study.
1,3,4-thiadiazole is a well-known important heterocyclic compound, and its derivatives have broad-spectrum biological activities, such as antimicrobial (Mao et al., 2021), insecticidal (Chen et al., 2019a), antiviral (Gan et al., 2017), and antitumor activities (Chen et al., 2019c). Mandelic acid, as a prodrug, has been used to treat urinary infections and develop antithrombotic, antibiotic, and antitumor drugs since the early 20th century (El and Gould 2016; Saeed et al., 2017; Li et al., 2021). Mandipropamid, the only commercial mandelic acid fungicide in the field of pesticides, was put on the market in 2001. There are few reports on the antifungal activity of mandelic acid derivatives in the agricultural field, which provides an opportunity for molecular derivation.
To obtain the lead compounds as potential antifungal agents, a series of novel mandelic acid derivatives containing 1,3,4-thiadiazole thioether were designed and synthesized (Fig. 1). Bioassay evaluation found that the target compounds exhibited excellent in vitro/vivo antifungal activities against T. cucumeris, and the effect on the formation and germination of T. cucumeris sclerotia after treatment with highly active compounds was further explored. Then, the in vitro antifungal mechanism against T. cucumeris was studied.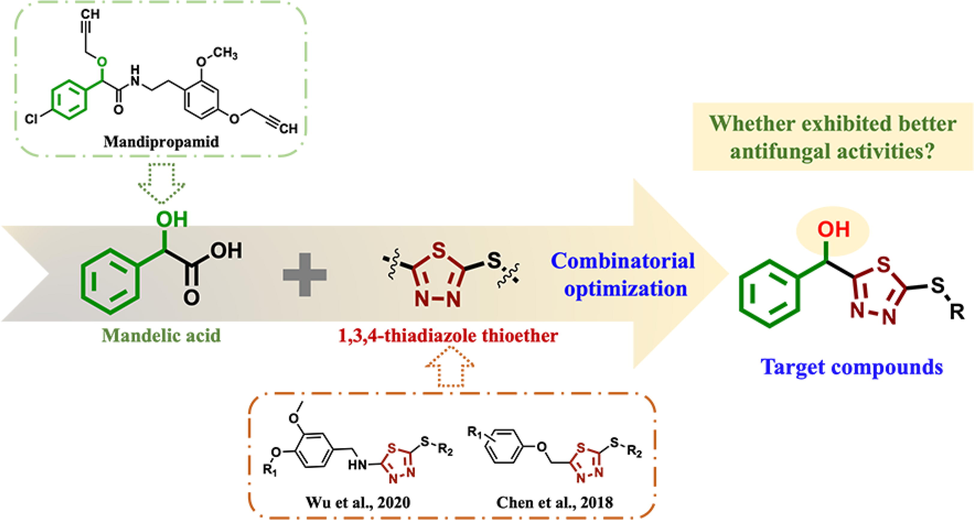
The design strategy for the target compounds.
2 Materials and methods
2.1 Instruments and chemicals
1H, 13C, and 19F NMR spectra were obtained by a Bruker 400 NMR spectrometer (Bruker Corporation, Germany) or JEOL-ECX 500 NMR (JEOL Corporation, Japan) using TMS as an internal standard and CDCl3 or DMSO‑d6 as the solvent. HRMS data were obtained on a Thermo Scientific Q Exactive mass spectrometer (Thermo Scientific, USA). X-ray crystallographic data were collected by a Bruker Corporation diffractometer (Bruker Corporation, Germany). Melting points were measured on an XT-4 binocular microscope melting point apparatus and were uncorrected. The morphology of mycelia was observed by a Nova Nano SEM450 scanning electron microscope (Thermo Fisher Scientific, USA) and an Olympus-BX53F fluorescence microscope (Olympus-BX53F, Olympus, Japan). Electrical conductivity was measured using a DDS-307 conductivity meter (INESA & Scientific Instrument Co., Ltd., Shanghai, China). All reagents and solvents were commercially available with chemical or analytical purity.
2.2 Fungi
Gibberella saubinetii (G. saubinetii), Alternaria solani (A. solani), Thanatephorus cucumeris (T. cucumeris), Verticillium dahlia (V. dahlia), and Gibberella zeae (Schwein.) Petch (G. zeae) were purchased from Beijing Beina Chuanglian Biotechnology Institute, China. Botryosphaeria dothidea (B. dothidea) was provided by GuiYang University and identified by Sangon Biotech (Shanghai) Co., Ltd., China. All fungi were grown on PDA plates at 25 ± 1 °C in the dark and maintained at 4 °C.
2.3 Mycelia and cultivating conditions
Five fresh mycelial dishes (4 mm) of T. cucumeris were cut from the PDA medium and added to PDB medium. They were cultivated at 25 °C and 180 rpm, and the mycelia were further treated in different ways according to the experimental requirements as follows:
(a) The hyphae were mixed and cultivated with different concentrations of target compounds (50.0, 25.0, and 10.0 μg/mL) for 96 h and filtered (Mo et al., 2021). The hyphae were washed 3 times with distilled water, dried at 65 °C for 12 h, and then used in mycelial weight experiments.
(b) After cultivation for 72 h and filtering, the hyphae were washed with distilled water 3 times, and then fresh hyphae (2.0 g) were put into PDB medium (50 mL) with different concentrations of target compounds (50.0, 25.0, and 10.0 μg/mL) and cultivated for 72 h under the same conditions and then filtered (Mo et al., 2021). The hyphae were washed 3 times with distilled water, dried at 65 °C for 12 h, and then used in mycelial loss ratio experiments.
(c) After cultivation for 48 h, different concentrations of F10 (50.0, 25.0, and 10.0 μg/mL) were added to the PDB medium, and the hyphae were incubated for 24 h under the same conditions and then filtered. Then, the hyphae were washed with PBS solution 3 times (Hou et al., 2018; Wang et al., 2019; Yang et al., 2019b) and used in the morphological study, cell membrane permeability study and MDA content determination experiments.
In all of the above experiments, the same volume of DMSO was used as the CK group, and the commercial agent triadimefon was used as a positive control.
2.4 Synthesis
2.4.1 General synthetic procedure for intermediate B
A mixture of DL-mandelic acid (A, 0.1 mol), 98% H2SO4 (1 mL), and CH3OH (30 mL) was refluxed at 100 °C, monitored reacted by TLC for 3 h. Then, the solvent was evaporated under reduced pressure to get crude product, and separated by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 30/1) to obtain intermediate B.
2.4.2 General synthetic procedure for intermediate C
B (0.1 mol) was added in the solution of 80% NH2NH2·H2O (0.4 mol), reacted for 2 h at r.t., then the mixture was filtered, and the crude product was purified by recrystallized with EtOH solution to obtain intermediate C.
2.4.3 General synthetic procedure for intermediate d
C (0.1 mol) were reacted with KOH (0.12 mol) and CS2 (0.3 mol) in C2H5OH solution for 7 h at r.t., and the solvent was concentrated under reduced pressure. The crude product was slowly added into 98% H2SO4 (15 mL), reacted for 6 h at 0 °C. Then, the mixture was dropwise poured into ice water (90 mL), filtered, and purified by silica gel column chromatography (eluent: dichloromethane/ methanol = 100/1) to obtain intermediate D.
2.4.4 General synthetic procedure for intermediates E
A mixture of D (1.0 mmol), methanol (10 mL), and NaBH4 (1.1 mmol) was stirred for 30 min at r.t. Then, the solvent was concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel (eluent: PE/EA = 10/1 – 3/1) to obtain the Intermediates E.
2.4.5 General synthetic procedure for target compounds F1–F30
To a solution of E (1.0 mmol) in CH3CN solution (8.0 mL), (C2H5)3N (0.5 mL) and different benzyl halides (1.2 mmol) were added and stirred for 24 h at r.t., then concentrated in vacuo. The residue was purified by silica gel column chromatography (eluent: PE/EA: 100/1 – 20/1) to obtain Fn (Sauer et al., 2017; Yang et al., 2021).
2.5 Crystallographic analysis of target compound F16
A single crystal of target compound F16 suitable for X-ray diffraction analysis was obtained by slow evaporation of the solution (petroleum ether/ethyl acetate) at r.t. X-ray crystallographic data were collected by a Bruker Corporation diffractometer (Bruker Corporation, Germany). The crystal was kept at 293.15 K during data collection. Using Olex2, the structure was solved with the SHELXT structure solution program using Intrinsic Phasing and refined with the SHELXL refinement package using Least Squares minimization.
2.6 Effects on in vitro mycelial growth
In vitro antifungal activities of the key intermediates and target compounds against six plant pathogenic fungi (G. saubinetii, A. solani, T. cucumeris, V. dahliae, G. zeae, and B. dothidea) were evaluated using a mycelial growth inhibition method with some modifications (Li et al., 2019b). The initial screening concentration was 100 μg/mL, 1% DMSO in sterile distilled water was used as a CK group, and the commercial fungicide triadimefon served as a positive control. Mycelia were incubated at 25 °C in the dark, and the diameters of the hyphae in the treatment groups were measured by the cross-crossing method when they reached approximately 6.0 cm in the CK groups.
The EC50 values of the target compounds that exhibited > 50% inhibitory effects at 100 μg/mL were further determined using the method described above. A gradient of concentrations of the test compounds of 200, 100, 50, 25, 12.5, 6.25, and 3.125 μg/mL were prepared.
2.7 Effect of treatment with F10 on the hyphae weight and loss ratio of T. Cucumeris
The weight of the hyphae cultivated with method (a) described above was calculated after the hyphae were dried. The loss ratio of the hyphae cultivated with method (b) described above was calculated with the formula: I (%) = [(W − R)/(W)] × 100, where W represents the dry weight of the initial hyphae and R represents the residual weight of the CK group or the treated group (Mo et al., 2021).
2.8 Effect on sclerotia formation and germination
The hyphae were cultivated on PDA medium containing various concentrations (25.0 and 50.0 μg/mL) of F10 in the dark at 25 °C for 14 continuous days and photographed and recorded by SM.
The sclerotia germination inhibitory experiment was performed using the method described previously with some modifications. First, T. cucumeris were incubated in the dark for 14 d to obtain sclerotia; then, PDA plates containing different concentrations of F10 (25.0 and 50.0 μg/mL) were prepared; finally, 15 sclerotia were picked and placed on each PDA plate. DMSO and triadimefon were used as the CK and the positive control, respectively. All treatment groups were cultivated in the dark at 25 °C for 72 h and then observed, photographed and recorded by SM. The inhibitory effect of F10 on sclerotia germination was calculated by the formula: I (%) = [(G − F)/(G − d)] × 100, where G represents the diameter of sclerotia germination of the CK group, F represents the diameter of sclerotia germination after treatment with F10, and d represents the average diameter of sclerotia (Hou et al., 2018; Zhang et al., 2018).
2.9 In vivo activity against rice sheath blight
The rice plants were cultivated and used to evaluate the protective and curative activities against rice sheath blight infected with T. cucumeris. For the protective activity, the rice plants were treated with target compound F10 by spraying with 200 μg/mL and then inoculated with one T. cucumeris sclerotia (2 mm) 24 h later on a leaf sheaf of each plant. However, for the curative activity, the plants were first inoculated with T. cucumeris sclerotia for 24 h and then sprayed with F10 (200 μg/mL). All of the treatments were replicated for the same batch plants and maintained at 25 ± 1 °C and 90% RH with a 12 h light/12 h dark photoperiod, the same volume of DMSO was used as the CK group, with triadimefon as a positive control. The control efficacies were calculated as follows after 7 d of inoculation: control efficacy (%) = (D0-D1)/D0 × 100%, where D0 and D1 are the diameters of the CK and the treatment group, respectively (Zhang et al., 2018).
2.10 Morphological study of mycelia from T. cucumeris by SEM and FM
After fixation with 2.5% glutaraldehyde at 4 °C for 24 h, the hyphae were washed with PBS 3 times before being dehydrated with a series of ethanol solutions (30%, 50%, 70%, 90% anhydrous ethanol and 100% tertiary butanol). Then, the hyphae were observed by SEM after freeze drying and gold-spraying (Yang et al., 2019b).
The PBS solution with hyphae in a 2 mL centrifuge tube was removed by centrifuging at 4 °C and 6000 rpm for 5 min. A PI solution of 1/10 PBS volume (10.0 μg/mL) was added to the centrifuge tube for staining after the supernatant was removed. The centrifuge tube was wrapped in tin foil and incubated in a thermostatic mixer at 37 °C and 1000 rpm in the dark for 15 min. After coloring, the hyphae were washed with PBS 3 times, observed and photographed by FM (Yang et al., 2019b).
2.11 Study on cell membrane permeability
The relative permeability of the cell membrane of T. cucumeris was evaluated according to a previous method with some modifications. The hyphae were washed with distilled water three times and filtered. Hyphae (2.0 g) were added to a beaker containing 50 mL distilled water and shaken well, and the electrical conductivity of mycelia was measured at 0, 1, 2, 4, 6, 8, 10, and 12 h (electrical conductivity at each time point). After 12 h, the beaker was placed in a boiling water bath for 5 min, and the absolute electrical conductivity was measured and calculated with the formula: relative conductivity (%) = electrical conductivity at each time/absolute electrical conductivity × 100% (Wang et al., 2017; Wang et al., 2019; Elsherbiny et al., 2021).
2.12 MDA content determination of mycelia
The hyphae were washed with distilled water 3 times and filtered under vacuum for 10 min. Then, 0.1 g hyphae were added to 1.0 mL MDA extract solution (produced by Beijing Solaibao Technology Co., Ltd., Beijing, China), homogenized in a tissue morcellator, and centrifuged at 8000 g and 4 °C for 15 min. The supernatant was removed, and MDA content was determined using an MDA detection kit (Beijing Solaibao Technology Co., Ltd., China) (Yang et al., 2020; Mo et al., 2021).
2.13 Data analysis
The EC50 values were calculated with Origin 2021 software by regression equation and R2. All treatments were performed with three replicates by conventional methods, and data in the same groups were evaluated by the deviation value test. The results are presented as the means ± SDs. To determine the effects of the treatments, all the data in the study were analyzed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA) for statistical variances (ANOVA) between repeated experiments to determine whether there were significant differences among the biological characteristics. A Duncan's multiple range test was used for mean separations when the treatment effects were statistically significant (P < 0.05).
3 Results and discussion
3.1 Synthesis
The synthetic route of the target compounds is shown in Fig. 2. Intermediate B was obtained by esterification of the raw material DL-mandelic acid (A) and then hydrazinolysis was performed to obtain intermediate C. CS2 and KOH were added to form the transition state hydrazine salt and then cyclized in the presence of 98% H2SO4 at 0 °C to obtain intermediate D, reducing D obtained intermediate E, which was electrophilically substituted to form E obtained the target compounds Fn. The intermediates and target compounds were characterized by 1H, 13C, 19F NMR and HRMS. The physical data and copies are provided in the Supporting Information.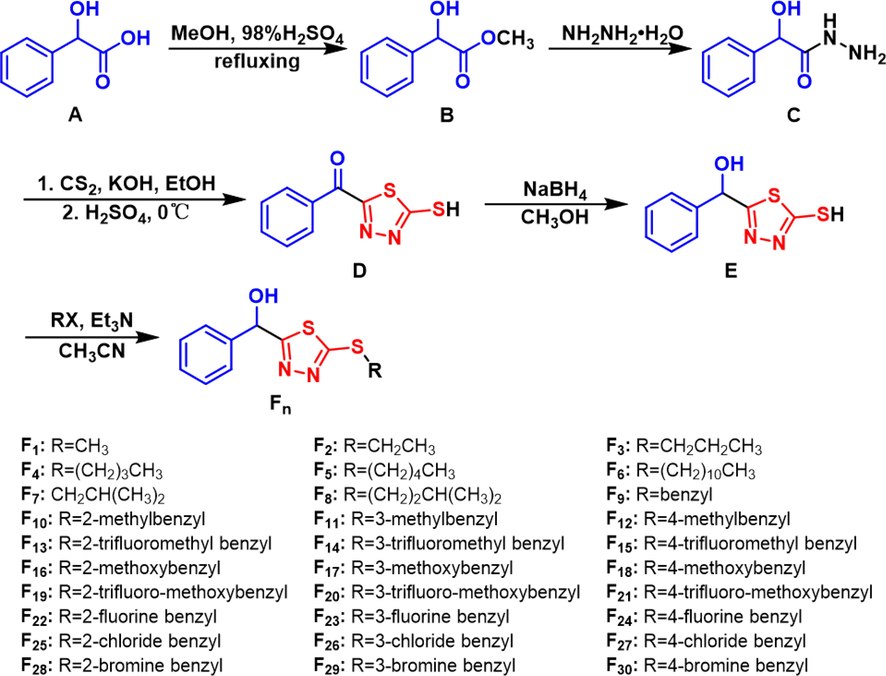
Synthetic route of the target compounds F1–F30.
3.2 Crystal structure of target compound F16
As shown in Fig. 3, the crystal structure of F16 was confirmed via X-ray diffraction analysis. Crystallographic data of compound F16: C17H16N2O2S2 (M = 344.44 g/mol): monoclinic, space group P21/c (no. 14), a = 9.5745(12) Å, b = 7.6138(10) Å, c = 23.623(3) Å, β = 92.271(5)°, V = 1720.7(4) Å3, Z = 4, T = 293.15 K, μ(CuKα) = 2.890 mm−1, and Dcalc = 1.330 g/cm3; 11,899 reflections were measured (7.49° ≤ 2Θ ≤ 144.152°), and 3279 reflections were unique (Rint = 0.1979, Rsigma = 0.1657) and were used in all calculations. The final R1 was 0.1532 (I > 2σ(I)), and wR2 was 0.5971 (all data). The crystallographic data of target compound F16 were deposited in the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2159799. The detailed crystallographic data are provided in Table S1 in the Supporting Information.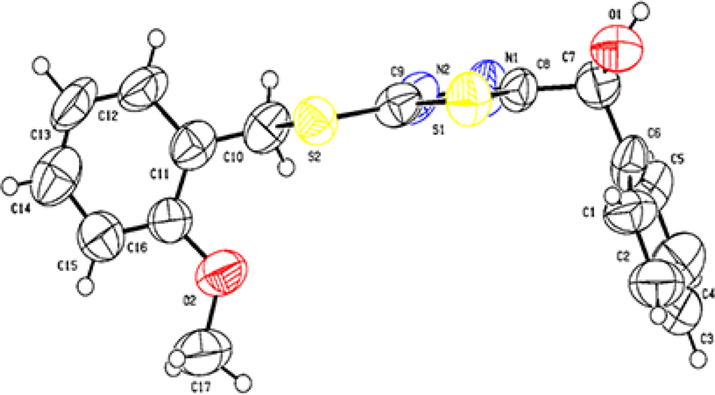
Single crystal structure of F16.
3.3 Effects of mycelial growth treated with target compounds
Preliminary in vitro antifungal activity results of target compounds against six pathogenic fungi are provided in Table S2 in the Supporting Information, and revealed that most target compounds exhibited better antifungal bioactivities against G. saubinetii and T. cucumeris. As shown in Table 1, the EC50 value of F15 against G. saubinetii was 11.4 μg/mL, similar to that of triadimefon (14.8 μg/mL); the EC50 values of F4, F9, F10, and F11 against T. cucumeris were 15.3, 11.8, 9.7, and 18.7 μg/mL, respectively, similar to that of triadimefon (11.0 μg/mL). Structure-activity relationship (SAR) analysis indicated that compounds exhibited better antifungal activities when substituted group were introduced to 1,3,4-thiadizazole-2-thiol part. Compared the antifungal activities against six pathogenic fungi (A. solani, G. saubinetii, V. dahlia, G. zeae, T. cucumeris and B. dothidea) between E and Fn in Table S2, we found that most compounds with substituted group exhibited better antifungal activities than that with thioether group, especially when benzyl and substituted-benzyls were introduced to 1,3,4-thiadizazole-2-thiol part. In addition, as shown in Table S2 to S4, among ‘R’ groups, most compounds with benzyl and substituted-benzyls group exhibited better antifungal activities than that with alkyl group, especially when benzyl, 2-methylbenzyl, and 4-trifluoromethyl benzyl were introduced to 1,3,4-thiadizazole-2-thiol part. Moreover, the antifungal activity of the most title compounds with the meta-substituted were generally better than that of the compounds with para-substituent, such as F11, F23, and F26 (the EC50 values were 28.9, 37.1, and 31.4 μg/mL, respectively), which were better than that of F12, F24, and F27 against G. saubinetii; F11, F14, F17, F20, and F26 (the EC50 values were 18.7, 27.7, 25.7, 20.0, and 32.3 μg/mL, respectively) were better than that of F12, F15, F18, F21, and F27 against T. cucumeris. When R = methylbenzyl, the activity of title compounds with the ortho- and meta-substituted were mostly better than that of the compound with para-substituent, such as the EC50 values of F10 (9.7, 50.0, and 23.3 μg/mL), and F11 (18.7, 45.9, and 28.9 μg/mL) against T. cucumeris, A. solani, and G. saubineti both better than the para-substituent F12. When R = trifluoro-methoxybenzyl, the activity of title compound with the para-substituent was mostly better than that of the compound with ortho-substituted, such as F21 (24.3, 33.7, 41.2, and 23.6 μg/mL) was better than that of F19 (51.2, 55.3, 56.4, and 47.6 μg/mL) against T. cucumeris, A. solani, G. zeae, and G. saubinetii. The regression equations and R2 are provided in Tables S3 and S4 in the Supporting Information.
No.
EC50 value ± SDs (μg/mL) a
G. saubinetii
A. solani
V. dahlia
G. zeae
T. cucumeris
F3
102.8 ± 3.5
91.9 ± 2.0
–
–
31.3 ± 0.6
F4
84.2 ± 0.6
119.0 ± 4.7
–
–
15.3 ± 0.8
F9
86.8 ± 0.8
62.5 ± 1.6
–
87.3 ± 1.7
11.8 ± 0.1
F10
23.3 ± 0.1
50.0 ± 1.4
88.4 ± 0.8
63.0 ± 1.8
9.7 ± 0.1
F11
28.0 ± 1.1
45.9 ± 1.9
89.2 ± 0.7
54.9 ± 1.3
18.7 ± 0.8
F13
43.3 ± 0.5
42.3 ± 3.1
58.7 ± 1.3
104.0 ± 1.2
35.4 ± 1.1
F14
52.2 ± 2.4
37.1 ± 0.3
73.4 ± 0.8
112.7 ± 4.1
27.7 ± 0.4
F15
11.4 ± 0.2
36.6 ± 1.5
69.6 ± 2.4
167.5 ± 10.9
46.1 ± 0.7
F16
92.7 ± 1.0
37.0 ± 1.8
–
–
–
F17
90.1 ± 1.4
63.8 ± 0.2
–
108.1 ± 1.4
25.7 ± 1.7
F19
47.6 ± 2.3
56.4 ± 1.1
66.2 ± 1.1
55.3 ± 2.0
51.2 ± 1.2
F20
37.6 ± 1.6
42.1 ± 0.9
72.1 ± 0.8
49.1 ± 0.5
20.0 ± 0.4
F21
23.6 ± 1.1
41.2 ± 1.8
72.9 ± 1.5
33.7 ± 1.3
24.3 ± 0.3
F22
69.2 ± 1.0
70.5 ± 0.5
–
–
–
F23
37.1 ± 1.3
49.6 ± 1.3
110.6 ± 4.6
81.4 ± 2.6
34.9 ± 1.0
F24
47.7 ± 0.6
76.4 ± 1.4
102.9 ± 1.1
119.9 ± 2.5
32.7 ± 1.1
F25
43.9 ± 0.3
35.8 ± 1.6
–
93.8 ± 2.4
60.6 ± 3.4
F26
31.4 ± 0.8
47.1 ± 1.3
95.8 ± 0.8
86.3 ± 3.9
32.3 ± 1.0
F28
49.2 ± 2.6
53.2 ± 2.1
175.8 ± 4.7
92.3 ± 4.0
116.4 ± 3.8
F29
91.0 ± 3.8
48.2 ± 1.8
–
122.9 ± 7.0
53.9 ± 0.1
triadimefon
14.8 ± 0.5
45.3 ± 0.6
2.9 ± 0.2
16.9 ± 0.1
11.0 ± 0.7
As shown in Fig. 4A, compared with the dry weight of the hyphae in the CK group (310.7 mg), the dry weights of the hyphae of T. cucumeris treated with different concentrations of F10 (50.0, 25.0, and 10.0 μg/mL) were 5.7, 13.3, and 56.7 mg, respectively, which were less than those treated with triadimefon (47.7, 82.7, and 100.7 mg, respectively). The weight of hyphae was significantly decreased after treatment with different concentrations of F10 (50.0, 25.0, and 10.0 μg/mL), and the loss rates were 82.8%, 79.7% and 72.9%, respectively, which were all higher than those of triadimefon (74.1%, 68.1% and 60.5%, respectively) (Fig. 4B).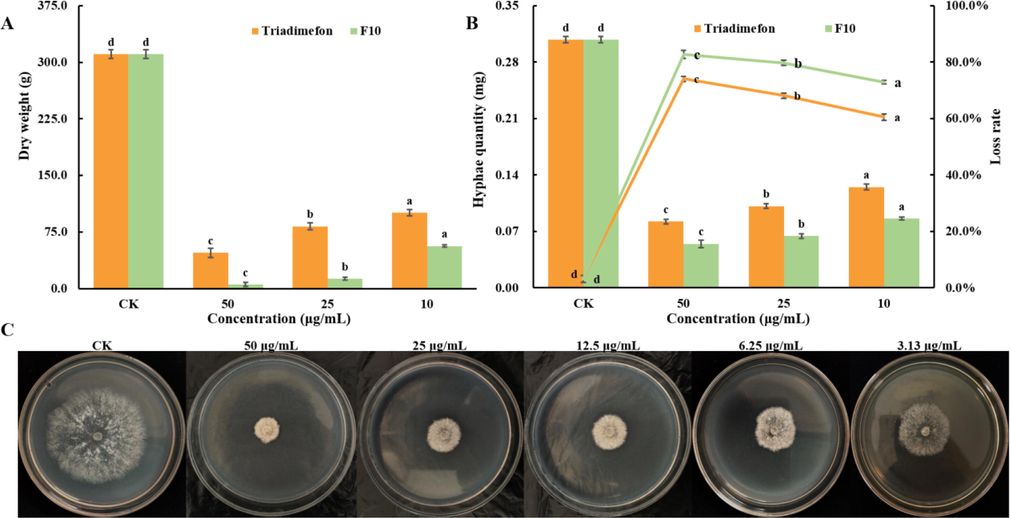
A: The dry weight of hyphae treated with F10 and triadimefon; B: The loss rate of hyphae treated with F10 and triadimefon; C: Effect of treatment with F10 at different concentrations on the mycelial growth process. Error bars denote the standard error of the mean for three independent experiments. Different lowercase letters denote statistically significant differences at P < 0.05.
The dry weight of the hyphae of T. cucumeris gradually decreased with increasing F10 concentration, which was consistent with the result shown in Fig. 4C, revealing that the target compound F10 can effectively inhibit the growth of T. cucumeris hyphae. The loss rates of T. cucumeris reflected the weight loss of hyphae, and we speculated that target compound F10 may disrupt the structure of mycelium, leading to the leakage of substances in the hyphae, such as soluble sugars and proteins. Experiments for further verification were as follows.
3.4 Sclerotia formation and germination of T. cucumeris treated with F10
The sclerotia of T. cucumeris are an important primary infection source that can cause the disease of rice sheath blight, and the formation of sclerotia can be divided into three stages: sclerotia initial (SIs), sclerotia developing (SDs), and sclerotia mature (SMs) (Georgiou et al., 2006; Dong et al., 2018). As shown in Fig. 5, a small amount of sclerotia was formed after treatment with F10 at a concentration of 25 μg/mL from 7 d to 14 d, but the sclerotia were hardly formed after treatment with F10 at 50 μg/mL. In contrast, sclerotia can be formed under triadimefon treatment at concentrations of both 25 μg/mL and 50 μg/mL.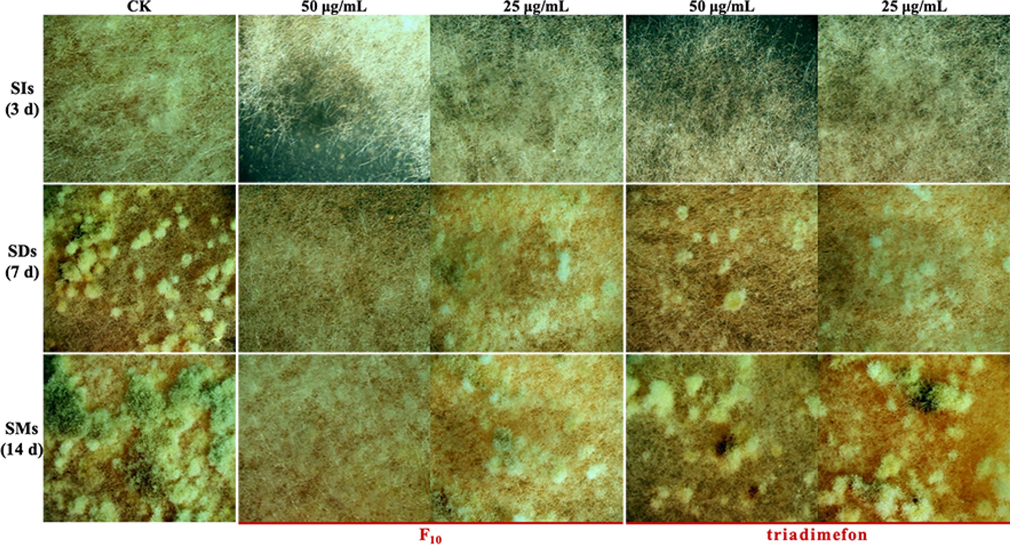
Sclerotia formation of T. cucumeris treated with F10 and triadimefon.
As shown in Fig. 6, the sclerotia germination rates all reached 100% after treatment with F10 and triadimefon at concentrations of 50 and 25 μg/mL. However, treatment with 50 and 25 μg/mL F10 slowed the speed of sclerotia germination, and the rates of slowdown were 38.5% and 24.2%, respectively, which were greater than those of triadimefon (28.8% and 22.5%, respectively).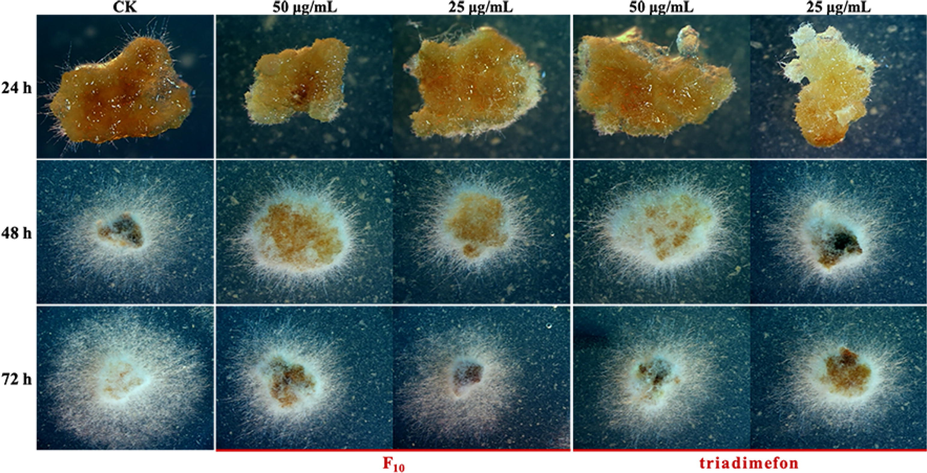
Sclerotia germination of T. cucumeris treated with F10 and triadimefon.
After the target compound F10 treated the T. cucumeris, the number of formed sclerotia decreased or hardly formed. Compound F10 displayed excellent inhibitory activity. These results revealed that compound F10 can effectively inhibit the sclerotia formation of T. cucumeris but had no obvious inhibitory activity against sclerotia germination. Combining the antifungal activity results, we found that F10 effectively inhibited the growth of hyphae and the formation of sclerotia, and hyphae were selected for further mechanism studies of F10 against T. cucumeris.
3.5 In vivo protective and curative activities treated with F10
As shown in Table 2 and Fig. 7, compound F10 exhibited significant in vivo curative (67.9%) and protective (61.1%) activities on rice plants leaf after inoculating with T. cucumeris sclerotia 7 d, which better than triadimefon (61.9% and 46.8%). And the results indicated that F10 exhibited excellent in vivo protective and curative activities against T. cucumeris, which could be used to control rice sheath blight.
Curative activity
Protective activity
Compound
Lesion lengtha (cm)
Control efficacy (%)
Lesion lengtha (cm)
Control efficacy (%)
F10
0.43 ± 0.10
67.9 ± 7.3
0.49 ± 0.13
61.1 ± 7.9
triadimefon
0.51 ± 0.14
61.9 ± 6.7
0.67 ± 0.13
46.8 ± 4.1
CK
1.34 ± 0.35
–
1.26 ± 0.20
–

In vivo curative and protective activities of F10 and triadimefon against T. cucumeris at 200 μg/mL.
3.6 Morphology study of the mycelia of T. cucumeris treated with F10
As shown in Fig. 8A and 8B, the mycelia of the CK group were regular in shape, uniform in thickness and complete in structure. However, the mycelia treated with F10 and triadimefon at different concentrations (50.0, 25.0, and 10.0 μg/mL) appeared uneven, wrinkled, irregularly shrunken, collapsed, and destroyed in a dose-dependent manner. Comparing the morphological changes of the hyphae under the treatment at the same concentration, we found that compound F10 had a more obvious effect than triadimefon.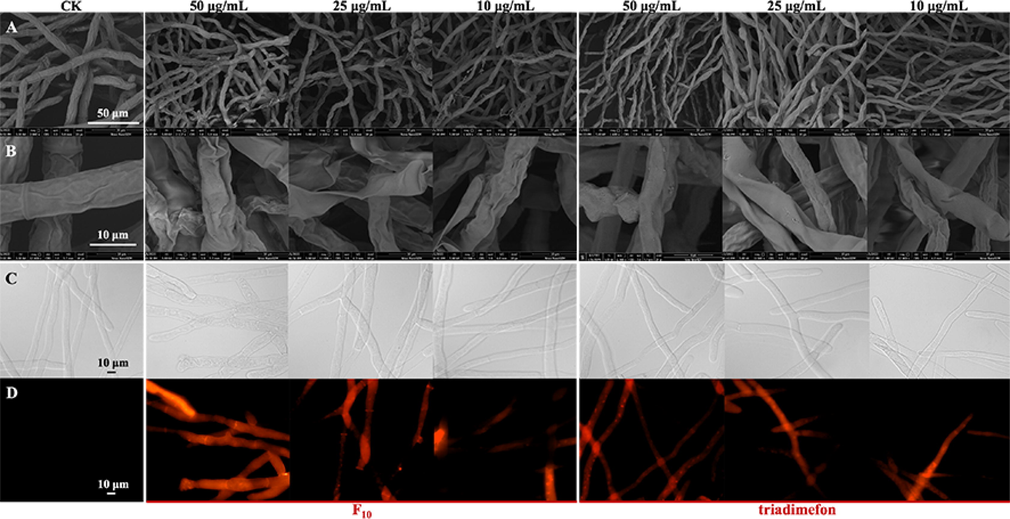
Morphological observation of T. cucumeris treated with F10 and triadimefon. A and B: Observed by SEM; C and D: observed by FM; C: bright field; D: fluorescence field.
PI is a fluorescent dye used to identify the membrane integrity of cells, and it can enter cells across damaged cellular membranes and emit red fluorescence (Yang et al., 2019b; Yang et al., 2020; Mo et al., 2021). Rather than passing through normal and intact cell membranes, the nucleus can be stained by a fluorescent dye that passes through damaged cell membranes (Mo et al., 2021; Xiao et al., 2021). As shown in Fig. 8C and 8D, no fluorescence was observed in the CK group, which indicated that the hyphae were intact and unharmed. The hyphae showed different degrees of fluorescence after treatment with F10 and triadimefon for 24 h at different concentrations. A small amount of red fluorescence was observed in the hyphae after the treatment at a low concentration (10 μg/mL), while greater fluorescence was observed at a high concentration (50 μg/mL), which indicated that as the concentration of the compounds increased, the hyphal breakage became more severe, consistent with the results observed by SEM.
Comprehensive analysis of the results of morphological studies demonstrated that F10 can obviously damage the mycelial shape of T. cucumeris and structural integrity. We speculated that F10 may affect the permeability of the cell membrane, and experiments for further verification were carried out.
3.7 Cell membrane permeability of T. cucumeris treated with F10
The conductivity changes of hyphae can reflect alterations in cell membrane permeability (Zhang et al., 2018). As shown in Fig. 9A, compared with the CK group, after treatment with F10 at different concentrations (50.0, 25.0, and 10.0 μg/mL), the relative electric conductivities of the hyphae were rapidly raised in a dose-dependent manner in the first 4 h, from 42.4% to 70.4%, from 41.6% to 65.9%, and from 38.7% to 58.9%, respectively. Then, the electrical conductivity increased slowly in each group from 4 h to 8 h, and the increases were 3.1, 6.0, and 11.5%, respectively. The increase tended to be mild from 8 to 12 h, at<2.0%. The relative electrical conductivity data indicated that F10 may cause the leakage of substances (soluble sugars and proteins) in the hyphae and increase the ionic concentration. The results revealed that after F10 treatment, cell membrane permeability was obviously affected (Xin et al., 2020; Elsherbiny et al., 2021; Yang et al., 2021).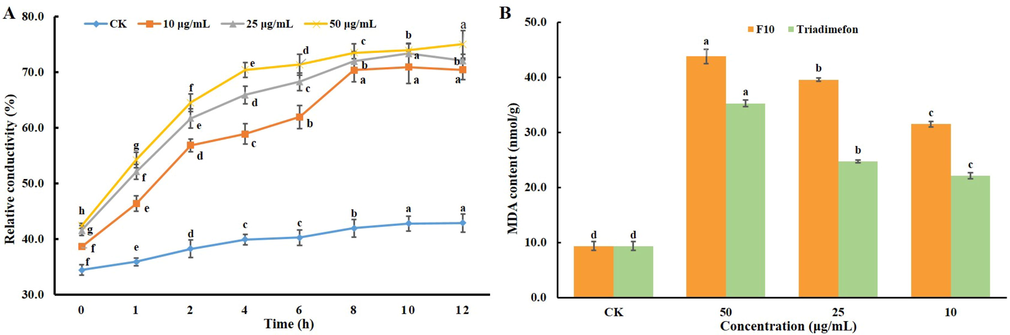
A: Cell membrane permeability of the hyphae of T. cucumeris treated with F10 at different concentrations and times; B: MDA content of hyphae treated with F10 and triadimefon at different concentrations (50.0, 25.0, and 10.0 μg/mL). Error bars denote the standard error of the mean for three independent experiments. Different lowercase letters denote statistically significant differences at P < 0.05.
3.8 MDA content for mycelia of T. cucumeris treated with F10
The MDA assay is an evaluation of membrane structure and one of the important indicators of integrity. The higher the MDA content is, the more serious the damage is to the cell membrane (Yang et al., 2019b; Yang et al., 2021). As shown in Fig. 9B, the MDA content of hyphae after F10 treatment for 24 h was obviously higher than that of the CK group, and the MDA content was increased by 368.1%, 322.9% and 236.8% after treatment with different concentrations of F10 (50.0, 25.0, and 10.0 μg/mL, respectively), which were all greater increases than those with triadimefon treatment. Combining the results of morphology, cell membrane permeability and MDA content studies, we found that target compound F10 can obviously damage the cell membranes of mycelia from T. cucumeris at lower concentrations (10 μg/mL).
The sclerotia and mycelia are both important infection sources for plant diseases. In this work, target compound F10 with high antifungal activity against T. cucumeris was further studied, and the results revealed that F10 can inhibit the sclerotia formation of T. cucumeris and break the cell membranes of mycelia, which affects the growth of mycelia, and exhibited excellent in vivo protective and curative activities. All results of this study demonstrated that this series of compounds with high activity can be used to effectively control rice sheath blight which infected with T. cucumeris.
4 Conclusion
In summary, thirty novel mandelic acid derivatives containing 1,3,4-thiadiazole thioether were designed and synthesized. Bioassay evaluation revealed that most target compounds exhibited excellent antifungal activities against T. cucumeris; among them, the EC50 value of F10 was 9.7 μg/mL. Further studies found that F10 not only significantly inhibited the growth of T. cucumeris mycelia but also effectively inhibited the formation of sclerotia in T. cucumeris. Morphology studies of the mycelium showed that F10 can obviously affect the mycelium shape and structural integrity of T. cucumeris. Further mechanism studies demonstrated that this series of target compounds can damage the integrity of the cell membrane structure, resulting in increased permeability of the cell membrane, release of intracellular electrolytes and inhibition of fungal growth. In general, target compounds can significantly inhibit the growth of T. cucumeris hyphae and the formation of sclerotia in vitro and effectively control the diseases caused by T. cucumeris in vivo, which is helpful for managing the formation and diffusion of the infection sources and provides an effective method to control rice sheath blight disease.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 32160655, 21662008), and Breeding Program of Guizhou University (No. 201931).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front. Plant Sci.. 2015;6
- [CrossRef] [Google Scholar]
- Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol.. 2016;65:1333-1346.
- [CrossRef] [Google Scholar]
- Novel 1,3,4-selenadiazole-containing kidney-type glutaminase inhibitors showed improved cellular uptake and antitumor activity. J. Med. Chem.. 2019;62(2):589-603.
- [CrossRef] [Google Scholar]
- Novel amide derivatives containing 1,3,4-thiadiazole moiety: Design, synthesis, nematocidal and antibacterial activities. Bioorg. Med. Chem. Lett.. 2019;29(10):1203-1210.
- [CrossRef] [Google Scholar]
- Discovery of novel isothiazole, 1,2,3-thiadiazole, and thiazole-based cinnamamides as fungicidal candidates. J. Agric. Food Chem.. 2019;67(45):12357-12365.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of novel psoralen-based 1,3,4-oxadiazoles as potent fungicide candidates targeting Pyruvate kinase. J. Agric. Food Chem.. 2022;70(11):3435-3446.
- [CrossRef] [Google Scholar]
- Anti-Rhizoctonia solani activity by polymeric quaternary ammonium salt and its mechanism of action. React. Funct. Polym.. 2018;125:1-10.
- [CrossRef] [Google Scholar]
- Role of old antimicrobial agents in the management of urinary tract infection. Expert Rev. Clin. Pharmacol.. 2016;9(8):1047-1056.
- [CrossRef] [Google Scholar]
- Antifungal action and induction of resistance by β-aminobutyric acid against Penicillium digitatum to control green mold in orange fruit. Pestic. Biochem. Physiol.. 2021;171:104721
- [CrossRef] [Google Scholar]
- Survival of Rhizoctonia solani AG1 IA, the causal agent of rice sheath blight, under different environmental conditions. J. Phytopathol.. 2017;165:44-52.
- [CrossRef] [Google Scholar]
- Emerging fungal threats to animal, plant and ecosystem health. Nature.. 2012;484(7393):186-194.
- [CrossRef] [Google Scholar]
- Synthesis and antiviral evaluation of novel 1,3,4-oxadiazole/thiadiazole-chalcone conjugates. Bioorg. Med. Chem. Lett.. 2017;27(18):4298-4301.
- [CrossRef] [Google Scholar]
- Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr. Comp. Biol.. 2006;46(6):691-712.
- [CrossRef] [Google Scholar]
- Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control.. 2018;117:147-157.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel cylopentapyrazoles bearing 1,2,3-thiadiazole moiety as potent antifungal agents. Bioorg. Chem.. 2020;95:103509
- [CrossRef] [Google Scholar]
- Molecular and biological characterization of Sclerotinia sclerotiorum resistant to the anilinopyrimidine fungicide cyprodinil. Pestic. Biochem. Physiol.. 2018;146:80-89.
- [CrossRef] [Google Scholar]
- Advances in understanding broad-spectrum resistance to pathogens in rice. The Plant J.. 2017;90(4):738-748.
- [CrossRef] [Google Scholar]
- Antifungal activity of pregnane glycosides isolated from periploca sepium root barks against various phytopathogenic fungi. Ind. Crop. Prod.. 2019;132:150-155.
- [CrossRef] [Google Scholar]
- Combing multiple site-directed mutagenesis of penicillin G acylase from Achromobacter xylosoxidans PX02 with improved catalytic properties for cefamandole synthesis. Int. J. Biol. Macromol.. 2021;175:322-329.
- [CrossRef] [Google Scholar]
- Design, synthesis and antifungal activity evaluation of isocryptolepine derivatives. Bioorg. Chem.. 2019;92:103266
- [CrossRef] [Google Scholar]
- The impacts of natural antioxidants on sclerotial differentiation and development in Rhizoctonia solani AG-1 IA. Eur. J. Plant Pathol.. 2016;146(4):729-740.
- [CrossRef] [Google Scholar]
- (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem.. 2019;67(4):1138-1145.
- [CrossRef] [Google Scholar]
- Pine rosin as a valuable natural resource in the synthesis of fungicide candidates for controlling Fusarium oxysporumn cucumber. J. Agric. Food Chem.. 2021;69(23):6475-6484.
- [CrossRef] [Google Scholar]
- Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Physiol.. 2021;178:104942
- [CrossRef] [Google Scholar]
- Plant extracts, bioagents and new generation fungicides in the control of rice sheath blight in Guyana. Crop. Prot.. 2019;119:30-37.
- [CrossRef] [Google Scholar]
- Agricultural diversification reduces the survival period of Sclerotinia sclerotiorum sclerotia. Eur. J. Plant Pathol.. 2018;151(3):713-722.
- [CrossRef] [Google Scholar]
- Developments in the synthesis of the antiplatelet and antithrombotic drug (S)-clopidogrel. Chirality.. 2017;29(11):684-707.
- [CrossRef] [Google Scholar]
- Synthesis and antioxidant properties of organosulfur and organoselenium compounds derived from 5-substituted-1,3,4-oxadiazole/thiadiazole-2-thiols. Tetrahedron Lett.. 2017;58(1):87-91.
- [CrossRef] [Google Scholar]
- Discovery of novel pyrazole amides as potent fungicide candidates and evaluation of their mode of action. J. Agric. Food Chem.. 2022;70(11):3447-3457.
- [CrossRef] [Google Scholar]
- Biocontrol of Rhizoctonia solani AG-2, the causal agent of damping-off by Muscodor cinnamomi CMU-Cib 461. World J. Microb. Biot.. 2012;28(11):3171-3177.
- [CrossRef] [Google Scholar]
- Host Delivered RNAi, an efficient approach to increase rice resistance to sheath blight pathogen (Rhizoctonia solani) Sci. REP-UK. 2017;7:(1).
- [CrossRef] [Google Scholar]
- Agriculture development, pesticide application and its impact on the environment. Int. J. Env. Res. Pub. He.. 2021;18(3):1112.
- [CrossRef] [Google Scholar]
- Antifungal activity of zedoary turmeric oil against Phytophthora capsici through damaging cell membrane. Pestic. Biochem. Physiol.. 2019;159:59-67.
- [CrossRef] [Google Scholar]
- Sensitivity and biochemical characteristics of Sclerotinia sclerotiorum to propamidine. Pestic. Biochem. Physiol.. 2017;135:82-88.
- [CrossRef] [Google Scholar]
- Streptomyces sp. FX13 inhibits fungicide-resistant Botrytis cinerea in vitro and in vivo by producing oligomycina. Pestic. Biochem. Physiol.. 2021;175:104834
- [CrossRef] [Google Scholar]
- In vitro fungicidal activity and in planta control efficacy of coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol.. 2020;162:78-85.
- [CrossRef] [Google Scholar]
- Bioassay-guided isolation of two antifungal compounds from magnolia officinalis, and the mechanism of action of honokiol. Pestic. Biochem. Physiol.. 2020;170:104705
- [CrossRef] [Google Scholar]
- Development of novel (+)-nootkatone thioethers containing 1,3,4-oxadiazole/thiadiazole moieties as insecticide candidates against three species of insect pests. J. Agric. Food Chem.. 2021;69(51):15544-15553.
- [CrossRef] [Google Scholar]
- In vitro and in vivo antifungal activity and preliminary mechanism of cembratrien-diols against Botrytis cinerea. Ind. Crop. Prod.. 2020;154:112745
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of novel succinate dehydrogenase inhibitor derivatives as potent fungicide candidates. J. Agric. Food Chem.. 2019;67(47):13185-13194.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antifungal evaluation of novel quinoline derivatives inspired from natural quinine alkaloids. J. Agric. Food Chem.. 2019;67(41):11340-11353.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antifungal evaluation of 8-Hydroxyquinoline metal complexes against phytopathogenic fungi. J. Agric. Food Chem.. 2020;68(40):11096-11104.
- [CrossRef] [Google Scholar]
- Discovery of β-Carboline oxadiazole derivatives as fungicidal agents against rice sheath blight. J. Agric. Food Chem.. 2018;66(37):9598-9607.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online. They including 1H NMR, 13C NMR, 19F NMR and HRMS spectra data and pictures for intermediates and target compounds; crystallographic data of target compound F16; in vitro antifungal activity results of target compounds: the regression equations and R2 of the target compounds.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104884.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1






