Translate this page into:
A synthesis of thioxo[3.3.3]propellanes from acenaphthoquinone-malononitrile adduct, primary amines and CS2 in water
⁎Corresponding author. yavarisa@modares.ac.ir (Issa Yavari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Novel thioxo[3.3.3]propellanes were synthesized in moderate to good yields via reactions of aromatic or aliphatic amines and carbon disulfide with the Knoevenagel adduct resulting from acenaphthoquinone and malononitrile in water at room temperature. The merit of this reaction is highlighted by its high atom-economy, chemo-selectivity, and lack of metal promoters. The structures of the products were established by IR, NMR, and single crystal X-ray analyses.
Keywords
[3.3.3]Propellane
Dithiocarbamate
Knoevenagel condensation
Acenaphthoquinone
1 Introduction
Propellane systems are defined as tricyclic compounds containing three nonzero bridges and one zero bridge between a pair of bridgehead carbons (Ginsburg, 1975). They have significant chemical and physical properties due to their fascinating topology (Navarro and Reisman, 2012; Pihko and Koskinen, 2005; Wiberg, 1989). Due to their occurrence in several natural products and bioactive compounds, they found applications in medicinal chemistry (Qian-Cutrone et al., 1994; Dave et al., 2004; Miao et al., 2013). Since the propellanes discovery in 1965 (Nerdel et al., 1965), the commonest processes reported for their synthesis involve Diels–Alder reactions (Nicolaou et al., 2002), palladium (Trost and Shi 1991) or manganese catalyzed transformations (Asahi and Nishino 2008), rearrangement of spiro-ketones (Fitjer et al., 1994), nucleophilic substitutions of alkenes (Jamrozik et al., 1995), photochemical addition reactions (Navarro and Reisman 2012), and MCR methodologies (Rezvanian et al., 2012; Zhang and Yan 2013; Alizadeh et al., 2015).
Sulfur heterocycles have been widely explored as new materials due to their superconducting, optical, and electronic switching properties (Bendikov et al., 2004; Nielser et al., 2000; Konstantinova et al., 2004; Attanasi et al., 2009; Wang et al., 2011; Shi et al., 2011). Despite the importance of organo-sulfur compounds, there are relatively few protocols for construction of C–S bonds compared to C–N and C–O bond-forming methods. Recently, carbon disulfide was used as sulfur reagent in constructing various sulfur heterocyclic systems (Clegg et al., 2010; Maddani and Prabhu 2010; Ma et al., 2011; Özkay et al., 2016; Charitos et al., in press).
Dithiocarbamate salts, obtained from amines and CS2, have wide impacts in environmental chemistry (Kanchi et al., 2014). These salts react with different electrophiles including electron-deficient alkenes (Saidi et al., 2006; Bardajee et al., 2011), electron-rich alkenes (Ziyaei-Halimehjani et al., 2010, 2013), 2-chloro-1,3-dicarbonyl compounds (Yavari et al., 2010a), aldehydes and ketones (Ziyaei-Halimehjani et al., 2012), maleic anhydride (Ziyaei-Halimehjani and Hosseinkhany, 2015), fumaryl chloride (Alizadeh and Zohreh 2009), alkyl halides (Azizi et al., 2006), epoxides (Ziyaei-Halimjani and Saidi 2006; Azizi et al., 2007), divinyl sulfone and sulfoxides (Ziyaei-Halimehjani et al., 2016), β-nitrostyrene derivatives (Ghabraie et al., 2013), itaconic anhydride (Yavari et al., 2010b), electron-deficient chlorobenzenes (Ranjbar-Karimi et al., 2014) and 2-chloroacetamides (Yurttaş et al., 2014; Abu-Mohsen et al., 2015). To the best of our knowledge, there is no published report on the reaction between CS2 and amines in the presence of cyanochalcones.
As part of our current studies in the synthesis of heterocyclic [3.3.3]propellanes and 1,3-dithiolanes compounds (Yavari et al., 2007, 2010c; Yavari and Beheshti 2011; Diyanatizadeh and Yavari 2016), we herein report on the synthesis of a novel class of thioxo[3.3.3]propellanes by a simple and one pot three-component reaction involving aliphatic and aromatic amines, carbon disulfide, and Knoevenagel condensation product of acenaphthoquinone and malononitrile in water at room temperature.
2 Results and discussion
Initially, the three-component reaction of methylamine, carbon disulfide and acenaphthoquinone-malononitrile adduct was investigated to establish the feasibility of the strategy and to optimize the reaction conditions. Different solvents such as H2O, MeOH, EtOH, tetrahydrofuran (THF), and CH2Cl2 were explored. The results are summarized in Table 1. When the reaction was performed in H2O in the presence of 2 equiv. of Et3N as the base for 2 h, it was found that product 6a was obtained in 71% yield (Table 1). Thus, the optimized reaction conditions used were 1 mmol of amines, 1.5 mmol of carbon disulfide, 2 mmol of Et3N, and 1 mmol of acenaphthoquinone-malononitrile adduct in H2O at room temperature.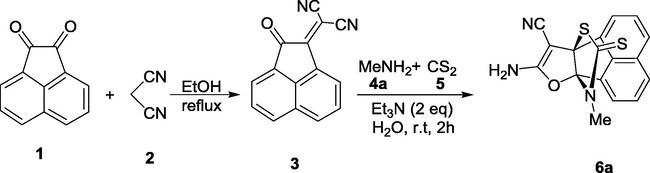
Entry
Solvent
Time (h)
Yieldb (%)
1
THF
4
60
2
MeOH
5
55
3
EtOH
5
52
4
H2O
2
71
5
CH2Cl2
4
46
Using the optimized reaction conditions for the formation of product 6a, a range of aliphatic and aromatic amines were treated with CS2 and 3 in H2O for 1–5 h at room temperature to afford thioxo[3.3.3]propellane derivatives 6a–m in moderate to good yields (Table 2).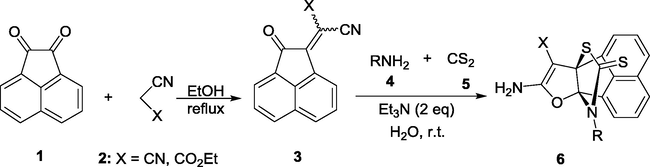
Entry
X
R
Product
Yieldb (%)
Time (h)
1
CN
Me
6a
71
2
2
CN
Et
6b
74
3
3
CN
Pr
6c
71
4
4
CN
Bu
6d
79
4
5
CN
Bn
6e
68
3
6
CN
4-Cl-C6H4-CH2
6f
80
2
7
CN
2,4-Cl2-C6H3-CH2
6g
91
1
8
CN
Ph
6h
85
5
9
CN
4-MeO-C6H4
6i
88
1
10
CN
4-Me-C6H4
6j
82
2
11
CO2Et
Et
6k
75
3
12
CO2Et
4-Cl-C6H4-CH2
6l
77
2
13
CO2Et
4-MeO-C6H4
6m
83
1
The structures of products 6a–m were deduced from their IR, 1H NMR, 13C NMR, and mass spectral data, and by single-crystal X-ray analysis of 6l. The mass spectrum of 6a displayed molecular ion peak at m/z = 337. The IR spectrum of 6a exhibited stretching bands for NH2 (3325 and 3272 cm–1), CN (2194 cm–1), and C=S (1345 cm–1) groups. The 1H NMR spectrum of 6a exhibited two sharp singlets (δ 3.54 and 7.99 ppm) for the methyl and NH2 protons. The aromatic protons appeared at δ 7.56–8.11 ppm. The 1H NMR spectra of 6b–g were similar to those of 6a except for the R groups which exhibited characteristic patterns (δ 4.09–5.43 ppm) for diastereotopic H2C-N protons. In the 13C NMR spectrum of these compounds, signals corresponding to the O–C–NH2, and C=S groups were observed at about 166 and 199 ppm, respectively.
To extend the scope of these transformations, the reaction of 1 with ethyl cyanoacetate was attempted and the results are shown in Table 2 (Entries 11–13). Compounds 6k–m was again fully characterized with their IR and NMR spectral data. Unequivocal evidence for the structure of 6l was obtained from single-crystal X-ray analysis. The ORTEP diagram of 6l is shown in Fig. 1. The structure was deduced from the crystallographic data and those of 6a–k, and 6m were assumed to be analogous on account of their similar NMR spectra.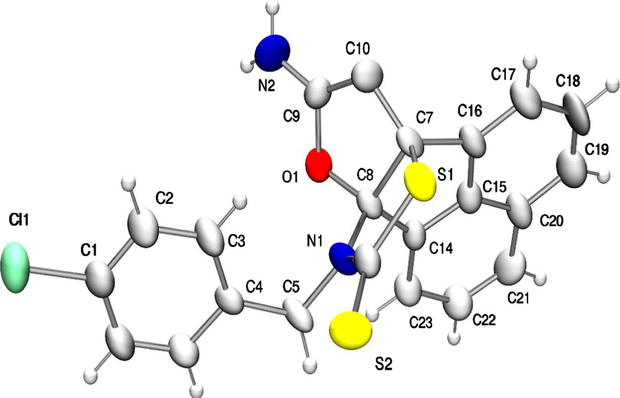
Molecular structure and numbering scheme of 6l; the thermal ellipsoids are drawn at the 40% probability level.
A plausible mechanism for the formation of products 6a–m is shown in Scheme 1. It is conceivable that the dithiocarbamate 7 undergoes S-Michael addition upon 3 to afford intermediate 8, which undergoes proton-transfer reaction to produce 9. Intermediate 9 undergoes intermolecular nucleophilic attack of nitrogen atom upon the carbonyl group to generate 10, which is convert to ketenimine intermediate 11 by deprotonation of the RXCH-CN moiety of 10. Then, O-cyclization of ketenimine 11 and subsequent imine-enamine tautomerization leads to the formation of thioxo[3.3.3]propellanes 6.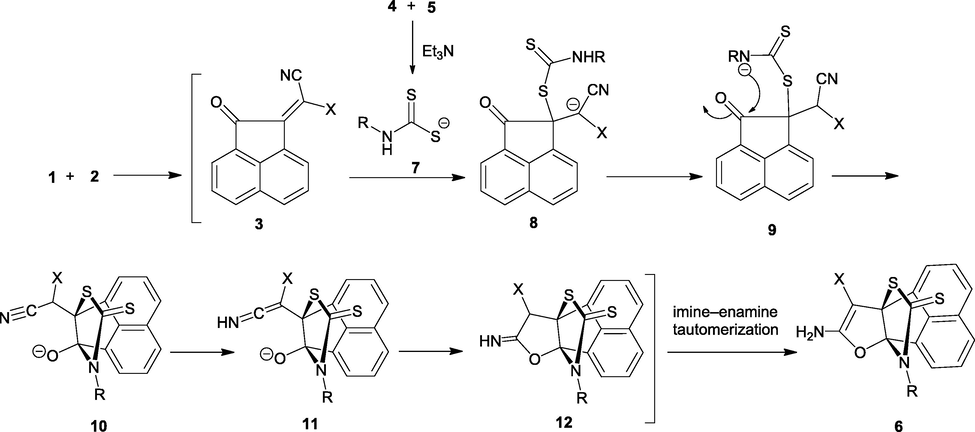
A plausible mechanism for the formation of products 6.
3 Conclusion
In summary, we have developed a simple one-pot three-component reaction involving aromatic and aliphatic amines, carbon disulfide, and the Knoevenagel condensation product of acenaphthoquinone and malononitrile or ethyl cyanoacetate for the synthesis of a new series of thioxo[3.3.3]propellanes in water at room temperature. It is noteworthy that this reaction results in the sequential C-S, C–N, and C–O bond formation in a single pot. The advantages of this method include the good yields of products, mild and simple reaction conditions (no metal catalyst or inert atmosphere, water used as a green solvent), fairly broad substrate scope, and readily available starting materials, which make it an useful protocol for the synthesis of [3.3.3]propellane systems.
4 Experimental
Compound 3 was prepared from acenaphthoquinone and malononitrile (or ethyl cynoacetate) according to the literature (Mhaidat et al., 2007; Chen et al., 2014). Other materials were obtained from Merck and used without further purification. Elemental analyses for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer. FT-IR spectra were recorded on a Shimadzu IR-460 instrument using the KBr self-supported pellet technique. 1H and 13C NMR spectra were recorded on a Bruker DRX-500 Avance spectrometer at 500 and 125 MHz. NMR spectra were obtained in solution of DMSO-d6 using tetramethylsilane (TMS) as internal standard. Mass spectra were obtained on a Finnigan-MAT-8430EI-MS apparatus at ionization potential of 70 eV. The melting points of the products were determined in open capillary tubes by using Electrothermal-9100 apparatus. Column chromatography was performed using silica (Merck #60). Silica plates (Merck) were used for TLC analysis.
4.1 Synthesis of thioxo[3.3.3]propellane derivatives (6a–m)
Compound 3 (1 mmol, 0.230 g) was added to a stirred solution of amine (1 mmol), CS2 (1.5 mmol, 0.114 g), and Et3N (2 mmol, 0.202 g) in H2O (5 mL) at room temperature. After completion of the reaction [about 1–5 h, TLC (n-hexane/EtOAc, 1:1) monitoring], the mixture filtered and the precipitate purified by flash column chromatography on silica gel using EtOAc/n-hexane (1:1) as eluent (for compound 6a-6e and 6k) or recrystallization from EtOAc (for compounds 6f–6j, 6l, and 6m) to afford the pure product 6.
4.2 8-Amino-12-methyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6a
Violet solid (0.24 g, 71%). mp: 254–258 °C. 1H NMR (500 MHz, DMSO-d6): δH 3.54 (3 H, s, Me), 7.57 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.69 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.76 (1 H, t, 3J = 8.0 Hz, Ar-H), 7.93 (1 H, d, 3J = 8.0 Hz, Ar-H), 7.99 (2 H, s, NH2), 8.02 (1 H, d, 3J = 7.0 Hz, Ar-H), 8.09 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 34.3 (Me), 58.9 (C-S), 73.4 (CCN), 117.7 (CN), 119.2 (OCN), 119.8 (CH), 122.3 (CH), 126.0 (CH), 129.0 (CH), 129.9 (CH), 130.5 (CH), 132.5 (C), 134.6 (C), 135.5 (C), 143.0 (C), 166.5 (CNH2), 196.6 (C=S). IR (KBr) (νmax, cm−1): 3325 and 3272 (NH2), 2194 (CN), 1656 (OC=C), 1593, 1429 (C=CAr), 1345 (C=S). EI-MS: m/z (%) = 337 (M+, 30), 322 (100), 280 (20), 256 (56), 229 (40), 178 (10), 154 (15), 127 (9). Anal. Calc. for C17H11N3OS2 (337.42): C, 60.52; H, 3.29; N, 12.45. Found: C, 60.82; H, 3.32; N, 12.53%.
4.3 8-Amino-12-ethyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6b
Cream color solid (0.26 g, 74%). mp: 257–260 °C. 1H NMR (500 MHz, DMSO-d6): δH 1.28 (3 H, t, 3J = 7.0 Hz, Me), 4.12–4.26 (2 H, AB-m, ΔυAB = 42.0 Hz, CH2) 7.55 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.68 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.76 (1 H, t, 3J = 7.7 Hz, Ar-H), 7.93 (1 H, d, 3J = 8.3 Hz, Ar-H), 7.99 (2 H, s, NH2), 8.09 (2 H, t, 3J = 7.5 Hz, 2 Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 13.5 (Me), 42.3 (CH2), 58.4 (C-S), 73.2 (CCN), 117.1 (CN), 119.2 (OCN), 119.8 (CH), 121.7 (CH), 125.8 (CH), 128.3 (CH), 129.4 (CH), 130.5 (CH), 131.8 (C), 134.1 (C), 135.5 (C), 142.5 (C), 165.8 (CNH2), 196.2 (C=S). IR (KBr) (νmax, cm−1): 3275 and 3196 (NH2), 2197 (CN), 1657 (OC=C), 1596, 1434 (C=CAr), 1384 (C=S). EI-MS: m/z (%) = 351 (M+, 28), 323 (100), 291 (23), 267 (60), 240 (23), 189 (15), 165 (13). Anal. Calc. for C18H13N3OS2 (351.44): C, 61.52; H, 3.73; N, 11.96. Found: C, 61.80; H, 3.81; N, 12.00%.
4.4 8-Amino-12-propyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6c
Colorless solid (0.26 g, 71%). mp: 250–252 °C. 1H NMR (500 MHz, DMSO-d6): δH 0.95 (3 H, t, 3J = 7.2 Hz, Me), 1.66–1.91 (2 H, AB-m, ΔυAB = 114.5 Hz, CH2), 3.93–4.16 (2H, AB-m, ΔυAB = 93.9 Hz, CH2-N), 7.55 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.67 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.75 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.91 (1 H, d, 3J = 7.5 Hz, Ar-H), 7.98 (2 H, s, NH2), 8.04 (1 H, d, 3J = 7.0 Hz, Ar-H), 8.08 (1 H, d, 3J = 7.5 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 12.5 (Me), 21.4 (CH2), 48.5 (CH2), 58.4 (C-S), 73.1 (CCN), 117.1 (CN), 118.9 (OCN), 119.1 (CH), 121.6 (CH), 125.3 (CH), 128.3 (CH), 129.3 (CH), 129.8 (CH), 131.9 (C), 134.1 (C), 135.2 (C), 142.5 (C), 165.8 (CNH2), 196.5 (C=S). IR (KBr) (νmax, cm−1): 3314 and 3265 (NH2), 2197 (CN), 1659 (OC=C), 1594, 1436 (C=CAr), 1385 (C=S). EI-MS: m/z (%) = 365 (M+, 25), 321 (100), 289 (31), 265 (61), 238 (44), 187 (10), 163 (24), 136 (9). Anal. Calc. for C19H15N3OS2 (365.47): C, 62.44; H, 4.14; N, 11.50. Found: C, 62.70; H, 4.22; N, 11.56%.
4.5 8-Amino-12-butyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6d
Colorless solid (0.30 g, 79%). mp: 248–251 °C. 1H NMR (500 MHz, DMSO-d6): δH 0.92 (3 H, t, 3J = 7.3 Hz, Me), 1.38 (2 H, six, 3J = 7.2 Hz, CH2), 1.63–1.87 (2 H, AB-m, ΔυAB = 104.7 Hz, CH2), 3.95–4.20 (2 H, AB-m, ΔυAB = 96.0 Hz, CH2-N), 7.55 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.66 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.74 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.89 (1 H, d, 3J = 8.0 Hz, Ar-H), 7.98 (2 H, s, NH2), 8.02 (1 H, d, 3J = 7.0 Hz, Ar-H), 8.06 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 14.0 (Me), 19.7 (CH2), 28.7 (CH2), 46.8 (CH2), 58.4 (C-S), 73.1 (CCN), 117.7 (CN), 119.1 (OCN), 119.2 (CH), 121.6 (CH), 125.3 (CH), 128.1 (CH), 129.1 (CH), 129.2 (CH), 131.9 (C), 134.1 (C), 135.2 (C), 142.4 (C), 165.8 (CNH2), 196.5 (C=S). IR (KBr) (νmax, cm−1): 3316 and 3262 (NH2), 2194 (CN), 1658 (OC=C), 1594, 1437 (C=CAr), 1383 (C=S). EI-MS: m/z (%) = 379 (M+, 20), 335 (100), 303 (14), 279 (47), 252 (36), 220 (16), 177 (20), 150 (10), 44 (9). Anal. Calc. for C20H17N3OS2 (379.50): C, 63.30; H, 4.52; N, 11.07. Found: C, 63.61; H, 4.60; N, 11.15%.
4.6 8-Amino-12-benzyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6e
Pink solid (0.28 g, 68%). mp: 249–252 °C. 1H NMR (500 MHz, DMSO-d6): δH 5.43 (2 H, AB-q, 2J = 16.5 Hz, ΔυAB = 43.8 Hz, CH2-N), 7.07 (2 H, d, 3J = 7.2 Hz, 2 Ar-H), 7.20 (3 H, m, 3 Ar-H), 7.56 (1 H, t, 3J = 7.6 Hz, Ar-H), 7.60 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.69 (1 H, t, 3J = 7.6 Hz, Ar-H), 7.84 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.92 (1 H, d, 3J = 8.00 Hz, Ar-H), 7.96 (2 H, s, NH2), 8.02 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 51.3 (CH2), 59.1 (C-S), 73.1 (CCN), 117.6 (CN), 119.5 (OCN), 119.9 (CH), 122.6 (CH), 126.1 (CH), 127.6 (CH), 128.0 (2 CH), 128.5 (2 CH), 128.9 (CH), 129.6 (CH), 129.7 (CH), 130.3 (C), 132.5 (C), 134.6 (C), 135.7 (C), 143.1 (C), 166.5 (CNH2), 197.9 (C=S). IR (KBr) (νmax, cm−1): 3295 and 3230 (NH2), 2190 (CN), 1650 (OC=C), 1595, 1433 (C=CAr), 1378 (C=S). EI-MS: m/z (%) = 413 (M+, 30), 369 (100), 337 (20), 261 (15), 247 (40), 220 (16), 105 (22), 77 (8). Anal. Calc. for C23H15N3OS2 (413.51): C, 66.81; H, 3.66; N, 10.16. Found: C, 67.12; H, 3.73; N, 10.20%.
4.7 8-Amino-12-(4-chlorobenzyl)-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6f
Colorless solid (0.36 g, 80%). mp: 241–246 °C. 1H NMR (500 MHz, DMSO-d6): δH 5.43 (2 H, t, 2J = 17.0 Hz, ΔυAB = 17.9 Hz, CH2-N), 7.11 (2 H, d, 3J = 8.3 Hz, 2 Ar-H), 7.29 (2 H, d, 3J = 8.3 Hz, 2 Ar-H), 7.60 (2 H, m, 2 Ar-H), 7.69 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.89 (1 H, d, 3J = 7.2 Hz, Ar-H), 7.92 (1 H, d, 3J = 8.0 Hz, Ar-H), 7.96 (2 H, s, NH2), 8.03 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 49.2 (CH2), 58.5 (C-S), 73.6 (CCN), 117.1 (CN), 118.9 (OCN), 119.2 (CH), 121.9 (CH), 126.1 (CH), 128.4 (2 CH), 128.5 (3 CH), 128.8 (CH), 129.0 (CH), 130.4 (C), 131.9 (C), 134.0 (C), 134.4 (C), 135.0 (C), 142.5 (C), 165.8 (CNH2), 197.5 (C=S). IR (KBr) (νmax, cm−1): 3315 and 3265 (NH2), 2195 (CN), 1654 (OC=C), 1592, 1432 (C=CAr), 1374 (C=S). EI-MS: m/z (%) = 447 (M+, 40), 403 (100), 371 (37), 336 (14), 260 (15), 246 (9), 219 (42), 195 (54), 90 (12). Anal. Calc. for C23H14ClN3OS2 (447.96): C, 61.67; H, 3.15; N, 9.38. Found: C, 61.95; H, 3.22; N, 9.45%.
4.8 8-Amino-12-(2,4-dichlorobenzyl)-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6g
Colorless solid (0.44 g, 91%). mp: 252–257 °C. 1H NMR (500 MHz, DMSO-d6): δH 5.39 (2 H, AB-q, 2J = 17.4 Hz, ΔυAB = 40.1 Hz, CH2-N), 6.45 (1 H, d, 3J = 8.5 Hz, Ar-H), 7.13 (1 H, d, 3J = 8.5 Hz, Ar-H), 7.57 (1 H, t, 3J = 7.50 Hz, Ar-H), 7.63 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.66 (1 H, s, Ar-H), 7.71 (2 H, t, 3J = 7.50, 7.0 Hz, 2 Ar-H), 7.93 (1 H, d, 3J = 8.0 Hz, Ar-H), 8.01 (2 H, s, NH2), 8.03 (2 H, d, 3J = 8.0 Hz, 2 Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 47.9 (CH2), 59.1 (C-S), 74.5 (CCN), 117.3 (CN), 118.9 (OCN), 119.8 (CH), 121.9 (CH), 126.1 (CH), 127.9 (CH), 128.8 (CH), 129.0 (CH), 129.5 (C), 129.8 (CH), 130.4 (C), 131.7 (CH), 132.4 (C), 133.2 (C), 133.3 (CH), 134.4 (C), 135.1 (C), 142.8 (C), 166.3 (CNH2), 198.3 (C=S). IR (KBr) (νmax, cm−1): 3310 and 3266 (NH2), 2198 (CN), 1657 (OC=C), 1594, 1435 (C=CAr), 1375 (C=S). EI-MS: m/z (%) = 481 (M+, 55), 436 (100), 404 (34), 369 (12), 334 (53), 258 (45), 244 (19), 218 (23), 191 (8), 86 (17). Anal. Calc. for C23H13Cl2N3OS2 (482.40): C, 57.27; H, 2.72; N, 8.71. Found: C, 57.56; H, 2.80; N, 8.79%.
4.9 8-Amino-12-phenyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6h
Cream color solid (0.34 g, 85%). mp: 248–251 °C. 1H NMR (500 MHz, DMSO-d6): δH 6.36 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.29 (2 H, br s, 2 Ar-H), 7.48 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.60 (3 H, br s, 3 Ar-H), 7.64 (1 H, d, 3J = 7.5 Hz, Ar-H), 7.72 (1 H, t, 3J = 8.2 Hz, Ar-H), 7.92 (1 H, d, 3J = 8.0 Hz, Ar-H), 8.01 (1 H, d, 3J = 8.0 Hz, Ar-H), 8.06 (2 H, s, NH2). 13C NMR (125 MHz, DMSO-d6): δC 59.4 (C-S), 75.0 (CCN), 117.7 (CN), 119.1 (OCN), 119.9 (CH), 121.9 (CH), 122.0 (CH), 126.2 (2 CH), 128.8 (CH), 129.4 (C), 130.4 (CH), 130.7 (CH), 130.9 (CH), 132.5 (C), 134.8 (C), 135.9 (C), 137.4 (2 CH), 143.1 (C), 166.6 (CNH2), 199.4 (C=S). IR (KBr) (νmax, cm−1): 3316 and 3266 (NH2), 2191 (CN), 1652 (OC=C), 1583, 1426 (C=CAr), 1335 (C=S). EI-MS: m/z (%) = 399 (M+, 29), 355 (100), 323 (24), 311 (54), 279. (14), 202 (10), 165 (34), 150 (64), 108 (54), 76 (9). Anal. Calc. for C22H13N3OS2 (399.49): C, 66.15; H, 3.28; N, 10.52. Found: C, 66.48; H, 3.34; N, 10.60%.
4.10 8-Amino-12-(4-methoxyphenyl)-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6i
Light blue solid, 0.38 g, 88%. mp: 255–258 °C. 1H NMR (500 MHz, DMSO-d6): δH 3.85 (3 H, s, OMe), 6.46 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.11 (2 H, d, 3J = 7.0 Hz, 2 Ar-H), 7.20 (2 H, br s, 2 Ar-H), 7.51 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.62 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.70 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.92 (1 H, d, 3J = 8.2 Hz, Ar-H), 8.01 (1 H, d, 3J = 8.0 Hz, Ar-H), 8.06 (2 H, s, NH2). 13C NMR (125 MHz, DMSO-d6): δC 55.4 (OMe), 58.7 (C-S), 73.9 (CCN), 115.1 (2CH), 117.1 (CN), 117.8 (2CH), 119.8 (OCN), 121.3 (CH), 125.4 (CH), 128.1 (CH), 128.7 (CH), 128.8 (C), 129.3 (CH), 131.6 (CH), 131.9 (C), 134.2 (C), 135.5 (C), 142.5 (C), 160.3 (C), 166.0 (CNH2), 198.9 (C=S). IR (KBr) (νmax, cm−1): 3316 and 3280 (NH2), 2194 (CN), 1658 (OC=C), 1591, 1429 (C=CAr), 1349 (C=S). EI-MS: m/z (%) = 429 (M+, 28), 385 (100), 353 (77), 341 (81), 309 (83), 266 (85), 230 (97), 202 (79), 165 (90), 150 (84), 108 (75), 76 (80), 44 (18). Anal. Calc. for C23H15N3O2S2 (429.51): C, 64.32; H, 3.52; N, 9.78. Found: C, 64.63; H, 3.61; N, 9.85%.
4.11 8-Amino-11-thioxo-12-(p-tolyl)-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carbonitrile 6j
Violet solid (0.34 g, 82%). mp: 102–106 °C. 1H NMR (500 MHz, DMSO-d6): δH 2.42 (3 H, s, Me), 6.42 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.17 (2 H, br s, 2 Ar-H), 7.38 (2 H, d, 3J = 7.0 Hz, 2 Ar-H), 7.49 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.62 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.70 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.91 (1 H, d, 3J = 8.2 Hz, Ar-H), 7.99 (1 H, d, 3J = 8.0 Hz, Ar-H), 8.05 (2 H, s, NH2). 13C NMR (125 MHz, DMSO-d6): δC 21.9 (Me), 59.3 (C-S), 74.8 (CCN), 117.7 (CN), 119.1 (CH), 119.8 (OCN), 122.0 (CH), 126.1 (2CH), 128.7 (CH), 129.4 (C), 130.0 (CH), 130.7 (C), 131.1 (C), 132.5 (2 CH), 134.7 (CH), 134.8 (C), 136.0 (C), 140.6 (CH), 143.1 (C), 166.6 (CNH2), 199.3 (C=S). IR (KBr) (νmax, cm−1): 3294 and 3151 (NH2), 2194 (CN), 1659 (OC=C), 1588, 1430 (C=CAr), 1337 (C=S). EI-MS: m/z (%) = 413 (M+, 27), 369 (100), 337 (31), 325 (12), 293 (70), 278 (63), 242 (42), 214 (27), 177 (19), 162 (53), 120 (41), 88 (8), 74 (74). Anal. Calc. for C23H15N3OS2 (413.51): C, 66.81; H, 3.66; N, 10.16. Found: C, 67.13; H, 3.74; N, 10.23%.
4.12 Ethyl 8-amino-12-ethyl-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carboxylate 6k
Light yellow solid (0.30 g, 75%). mp: 192–195 °C. 1H NMR (500 MHz, DMSO-d6): δH 1.31 (6 H, t, 3J = 7.0 Hz, 2 Me), 4.12–4.21 (2 H, AB-m, CH2-N), 4.19 (2 H, br s, NH2), 4.25 (2 H, q, 3J = 7.0 Hz, O-CH2), 7.63 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.71 (1 H, t, 3J = 7.0 Hz, Ar-H), 7.73 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.86 (1 H, d, 3J = 8.3 Hz, Ar-H), 8.04 (1 H, d, 3J = 7.0 Hz, Ar-H), 8.06 (1 H, d, 3J = 7.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 12.6 (Me), 15.7 (Me), 42.1 (CH2), 58.1 (CH2), 59.3 (C-S), 79.7 (CCO2Et), 120.6 (CH), 121.2 (CH), 121.4 (OCN), 124.9 (CH), 128.1 (CH), 128.9 (CH), 129.6 (CH), 131.8 (C), 134.5 (C), 135.7 (C), 143.7 (C), 164.6 (C=O), 171.5 (CNH2), 197.3 (C=S). IR (KBr) (νmax, cm−1): 3388 and 3215 (NH2), 1681 (C=O), 1626 (OC=C), 1531, 1442 (C=CAr), 1379 (C=S). EI-MS: m/z (%) = 398 (M+, 30), 353 (100), 309 (17), 277 (48), 249 (42), 223 (21), 172 (35), 148 (53), 121 (12), 15 (11). Anal. Calc. for C20H18N2O3S2 (398.50): C, 60.28; H, 4.55; N, 7.03. Found: C, 60.55; H, 4.62; N, 7.10%.
4.13 Ethyl 8-amino-12-(4-chlorobenzyl)-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carboxylate 6l
Cream color solid (0.38 g, 77%). mp: 163–165 °C. 1H NMR (500 MHz, DMSO-d6): δH 1.05 (3 H, t, 3J = 7.0 Hz, Me), 4.21 (2 H, q, 3J = 7.0 Hz, OCH2), 4.22 (2 H, br s, NH2), 5.42 (2 H, AB-q, 2J = 17.0 Hz, ΔυAB = 20.0 Hz, N-CH2), 7.16 (2 H, d, 3J = 7.5 Hz, 2 Ar-H), 7.30 (2 H, d, 3J = 7.5 Hz, 2 Ar-H), 7.58 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.65 (1 H, t, 3J = 7.5 Hz, Ar-H), 7.74 (1 H, d, 3J = 7.0 Hz, Ar-H), 7.87 (1 H, d, 3J = 8.0 Hz, Ar-H), 7.89 (1 H, d, 3J = 7.5 Hz, Ar-H), 8.01 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 15.8 (Me), 50.2 (CH2), 60.4 (CH2), 60.9 (C-S), 80.6 (CCO2Et), 118.5 (OCN), 121.3 (CH), 122.2 (CH), 125.7 (CH), 128.7 (CH), 129.1 (2 CH), 129.3 (C), 129.6 (CH), 129.7 (C), 130.3 (CH), 132.4 (CH), 132.6 (C), 135.1 (C), 135.3 (CH), 136.1 (C), 144.3 (C), 165.8 (C=O), 171.4 (CNH2), 199.2 (C=S). IR (KBr) (νmax, cm−1): 3388 and 3305 (NH2), 1680 (C=O, OC=C), 1547, 1436 (C=CAr), 1327 (C=S). EI-MS: m/z (%) = 494 (M+, 28), 449 (100), 405 (17), 373 (38), 338 (74), 262 (49), 248 (57), 221 (28), 189 (26), 174 (42), 132 (15), 100 (31). Anal. Calc. for C25H19ClN2O3S2 (495.01): C, 60.66; H, 3.87; N, 5.66. Found: C, 60.97; H, 3.95; N, 5.73%.
4.13.1 X-ray Crystal-structure determination of compound 6l
The X-ray diffraction measurement was carried out on STOE IPDS 2T diffractometer with graphite-monochromated MoKα radiation. The single crystal suitable for X-ray analysis was obtained from DMSO/EtOAc solution and mounted on a glass fiber and used for data collection. Cell constants a = 15.837(3) Å, b = 14.091(3) Å, c = 11.496(2) Å, Alpha = 90°, Beta = 92.48° (3), Gamma = 90°, cell volume = 2563.0(9) Å3 and orientation matrixes for data collection were obtained by least-square refinement of the diffraction data from 3217 for compound 6l. Diffraction data were collected in a series of ω scans in 1° oscillations and integrated using the Stoe X-AREA software package (Stoe and Cie, X–AREA Program, 2005). Numerical absorption correction was applied using X-Red32 software. The structure was solved by direct methods and subsequent difference Fourier maps and then refined on F2 by a full-matrix least-squares procedure using anisotropic displacement parameters. Atomic factors are from the International Tables for X-ray Crystallography. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were placed in ideal positions and refined as riding atoms with relative isotropic displacement parameters. All refinements were performed using the X-STEP32, SHELXL-2014 and WinGX-2013.3 programs (Farrugia, 1999; Coppens et al., 1965; Burnett and Johnson, 1996; Macrae et al., 2006; Sheldrick, 2008). CCDC-1502615 contains the supplementary crystallographic data for this compound 6l. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4.14 Ethyl 8-amino-12-(4-methoxyphenyl)-11-thioxo-9a,6b-(epithiomethanoimino)acenaphtho[1,2-b]furan-9-carboxylate 6m
Yellow solid (0.40 g, 83%). mp: 194–196 °C. 1H NMR (500 MHz, DMSO-d6): δH 1.33 (3 H, br-m, Me), 3.85 (3 H, br-s, MeO), 4.01 (2 H, br-m, CH2O), 4.21 (2 H, br-s, NH2), 6.47 (1 H, br-m, Ar-H), 7.13 (2 H, br-m, 2 Ar-H), 7.22 (2 H, br-m, 2 Ar-H), 7.47 (1 H, br-m, Ar-H), 7.65 (1 H, br-m, Ar-H), 7.84 (1 H, br-m, Ar-H), 7.94 (1 H, d, 3J = 8.2 Hz, Ar-H), 8.1 (1 H, d, 3J = 8.0 Hz, Ar-H). 13C NMR (125 MHz, DMSO-d6): δC 15.8 (Me), 56.5 (OMe), 60.0 (CH2O), 64.0 (C-S), 80.7 (CCO2Et), 115.6 (OCN), 115.7 (C), 121.3 (CH), 121.7 (C), 121.8 (C), 125.6 (CH), 128.5 (CH), 129.1 (C), 129.9 (2 CH), 130.2 (C), 132.3 (3 CH), 135.2 (CH), 136.4 (CH), 144.3 (C), 160.8 (C=O), 165.3 (CNH2), 200.6 (C=S). IR (KBr) (νmax, cm−1): 3382 and 3278 (NH2), 1686 (C=O), 1628 (C=C), 1530, 1428 (C=CAr), 1324 (C=S). EI-MS: m/z (%) = 476 (M+, 34), 431 (100), 387 (68), 355 (52), 343 (63), 311 (12), 268 (36), 232 (41), 204 (75), 167 (27), 152 (12), 110 (45), 78 (15). Anal. Calc. for C25H20N2O4S2 (476.57): C, 63.01; H, 4.23; N, 5.88. Found: C, 63.33; H, 4.31; N, 5.95%.
References
- Synthesis and evaluation of anti-acetylcholinesterase activity of some benzothiazole based new piperazine-dithiocarbamate derivatives. Drug Res.. 2015;65:176-183.
- [Google Scholar]
- Regioselective multicomponent sequential synthesis of oxa-aza[3.3.3]propellanes. Lett. Org. Chem.. 2015;12:153-158.
- [Google Scholar]
- A novel multicomponent method for the synthesis of 2-thioxo-1,3-thiazolidin-4-ones. Synlett 2009:2146-2148.
- [Google Scholar]
- Manganese(III)-based dioxapropellane synthesis using tricarbonyl compounds. Tetrahedron. 2008;64:1620-1634.
- [Google Scholar]
- An efficient one-pot, three-component synthesis of 5-hydrazinoalkylidene rhodanines from 1,2-diaza-1,3-dienes. Org. Lett.. 2009;11:2265-2268.
- [Google Scholar]
- Straightforward and highly efficient catalyst-free one-pot synthesis of dithiocarbamates under solvent-free conditions. Org. Lett.. 2006;8:5275-5277.
- [Google Scholar]
- Waste-free and environment-friendly uncatalyzed synthesis of dithiocarbamates under solvent-free conditions. Synlett 2007:2797-2800.
- [Google Scholar]
- One-pot synthesis of dithiocarbamates undser solvent-free conditions in the presence of KF/Al2O3. Asian J. Biochem. Pharm. Res.. 2011;1:178-184.
- [Google Scholar]
- Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives. The brick and mortar of organic electronic. Chem. Rev.. 2004;104:4891-4946.
- [Google Scholar]
- ORTEP-III Report ORNL-6895. Tennessee, USA: Oak Ridge National Laboratory; 1996.
- Synthesis and anticancer activity of novel 3,6-disubstituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives. Arab. J. Chem. 2016
- [CrossRef] [Google Scholar]
- Design, synthesis and insecticidal activity of spiro heterocycle containing neonicotinoid analogs. Chin. Chem. Lett.. 2014;25:197-200.
- [Google Scholar]
- Bimetallic aluminum (salen) complex for the synthesis of 1,3-oxathiolane-2-thiones and 1,3-dithiolane-2-thiones. J. Org. Chem.. 2010;75:6201-6207.
- [Google Scholar]
- Calculation of absorption corrections for camera and diffractometer data. Acta Crystallogr.. 1965;18:1035-1038.
- [Google Scholar]
- Preparation of ‘cage molecule’ based polyazido core units for dendrimer synthesis. Tetrahedron Lett.. 2004;45:2159-2162.
- [Google Scholar]
- A synthesis of functionalized spiro isobenzofuran-1,6′-[1,3] thiazines from phthalic anhydride-malononitrile adduct and ammonium carbamodithioate. J. Sulfur Chem.. 2016;37:54-60.
- [Google Scholar]
- WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst.. 1999;32:837-838.
- [Google Scholar]
- Synthesis and rearrangement of dispiro[3.1.3.2]-, dispiro[3.0.3.3]- and dispiro[3.0.4.2]undecanes- new entries to [3.3.3]propellanes. Tetrahedron. 1994;50:10867-10878.
- [Google Scholar]
- Efficient synthesis of functionalized dithiocarbamate derivatives through one-pot three-component reaction and evaluation of their antimicrobial activities. J. Iran. Chem. Soc.. 2013;4:725-732.
- [Google Scholar]
- Propellanes: Structure and Reactions. Weinheim: Verlag Chemie; 1975.
- Propellane, 11. Synthese von dioxa[4.3.3]propellanen aus vicinalen tetrakis(hydroxymethyl)cycloalkanen. Monatsh. Chem.. 1995;126:587-591.
- [Google Scholar]
- Dithiocarbamates as hazardous remediation agent: a critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem.. 2014;7:11-25.
- [Google Scholar]
- Domino condensation/S-arylation/heterocyclization reactions: copper-catalyzed three-component synthesis of 2-N-substituted benzothiazoles. Angew. Chem. Int. Ed.. 2011;50:1118-1121.
- [Google Scholar]
- Mercury: visualization and analysis of crystal structures. J. Appl. Cryst.. 2006;39:453-457.
- [Google Scholar]
- A concise synthesis of substituted thiourea derivatives in aqueous medium. J. Org. Chem.. 2010;75:2327-2332.
- [Google Scholar]
- Structure and photoconductive behavior of (E-) and (Z-)-1-cyano-1-carbethoxymethylene acenaphthen-2-one taken by stereoselective synthesis. Mat. Lett.. 2007;61:321-325.
- [Google Scholar]
- Cu(I)-catalyzed domino reaction of 3-cyclopropylideneprop-2-en-1-ones. J. Org. Chem.. 2013;78:2687-2692.
- [Google Scholar]
- Rapid construction of the aza-propellane core of acutumine via a photochemical [2 + 2] cycloaddition reaction. Org. Lett.. 2012;14:4354-4357.
- [Google Scholar]
- The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed.. 2002;41:1668-1698.
- [Google Scholar]
- Tetrathiafulvalenes as building blocks in supramolecular chemistry II. Chem. Soc. Rev.. 2000;29:153-164.
- [Google Scholar]
- Design, synthesis, and AChE inhibitory activity of new benzothiazole-piperazines. Bioorg. Med. Chem. Lett.. 2016;26:5387-5394.
- [Google Scholar]
- Arthrinone, a novel fungal metabolite from Arthrinium sp. FA 1744. J. Nat. Prod.. 1994;57:1656-1660.
- [Google Scholar]
- Three-component reaction between amines, carbon disulfide and electron-deficient derivatives of chloropyridine or chlorobenzene: synthesis of 3,6-diazaspiro[4.5]deca-2,7,9-trien-6-ium chloride and dithioylcarbamates derivatives. J. Iran. Chem. Soc.. 2014;11:289-295.
- [Google Scholar]
- Chemo- and regioselective 4CR synthesis of oxathiaaza[3.3.3]propellanes via sequential C-S, C-N and C-O bond formation in a single pot. Synlett. 2012;23:2526-2530.
- [Google Scholar]
- One-pot synthesis of dithiocarbamates accelerated in water. J. Org. Chem.. 2006;71:3634-3635.
- [Google Scholar]
- Synthesis of 2-thio-substituted benzothiazoles via a domino condensation/S-arylation/heterocyclization process. J. Org. Chem.. 2011;76:4200-4204.
- [Google Scholar]
- Stoe & Cie, X-AREA: Program for the acquisition and analysis of data, 2005, Version 1.30; Stoe & Cie GmbH: Darmatadt, Germany.
- Low-temperature, palladium (II)-catalyzed, solution-phase oxidation of methane to methanol derivative. J. Am. Chem. Soc.. 1991;113:700-701.
- [Google Scholar]
- Mercaptobenzothiazoles via DBU-promoted tandem reaction of o-haloanilines and carbon disulfide. Org. Lett.. 2011;13:3202-3205.
- [Google Scholar]
- A facile synthesis of 2-imino-4-methylene-1,3-dithiolanes. Helv. Chim. Acta. 2011;49:831-834.
- [Google Scholar]
- A one-pot synthesis of N-alkylthiazoline-2-thiones from CS2, primary amines, and 2-chloro-1,3-dicarbonyl compounds in water. Monatsh. Chem.. 2010;141:49-52.
- [Google Scholar]
- One-pot synthesis of functionalized 4-oxo-2-thioxo-1,3-thiazinanes from primary amines, CS2, and itaconic anhydride. Mol. Divers.. 2010;14:611-615.
- [Google Scholar]
- Reaction of N-heterocycles with acetylenedicarboxylates in the presence of N-alkylisatins or ninhydrin. efficient synthesis of spiro compounds. Monatsh. Chem.. 2007;138:677-681.
- [Google Scholar]
- Diastereoselective synthesis of spiro-functionalized tetraalkyl benzoisoquinopyrrolonaphthyridine-tetracarboxylates from isoquinoline, dialkyl acetylenedicarboxylates, and indane-1,3-dion. Tetrahedron Lett.. 2010;51:396-398.
- [Google Scholar]
- Synthesis, anticandidal activity, and cytotoxicity of some thiazole derivatives with dithiocarbamate side chains. Turk. J. Chem.. 2014;38:815-824.
- [Google Scholar]
- One-pot domino reactions for synthesis of heterocyclic[3.3.3]propellanes and spiro[cyclopenta[b]pyridine-4,2'-indenes. Tetrahedron. 2013;69:4915-4921.
- [Google Scholar]
- Investigation of the reaction of dithiocarbamic acid salts with aromatic aldehydes. Org. Lett.. 2012;14:3838-3841.
- [Google Scholar]
- One-pot three-component Route for the synthesis of rhodanine derivatives in water. Synthesis. 2015;47:3147-3152.
- [Google Scholar]
- Synthesis of dithiocarbamate by Markovnikov addition reaction in aqueous medium. Green Chem.. 2010;12:1306-1310.
- [Google Scholar]
- Multicomponent synthesis of dithiocarbamates starting from vinyl sulfones/sulfoxides and their use in polymerization reactions. RSC Adv.. 2016;6:75223-75226.
- [Google Scholar]
- Regiospecific synthesis of dithiocarbamates via a Markovnikov addition reaction. Synthesis. 2013;45:1483-1488.
- [Google Scholar]
- Synthesis of β–hydroxy dithiocarbamate derivatives via regioselsctive addition of dithiocarbamate anion to epoxide in water. Can. J. Chem.. 2006;84:1515-1519.
- [Google Scholar]
Appendix A
Supplementary material
The 1H and 13C NMR spectra of the products. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2017.01.010.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







