Translate this page into:
Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots
⁎Corresponding authors at: National Engineering Laboratory for Tree Breeding, College of Biological Sciences and Biotechnology, Beijing Forestry University, Qinghuadonglu No. 35, Haidian District, Beijing 100083, China. lsun2013@bjfu.edu.cn (Liwei Sun), yjliubio@bjfu.edu.cn (Yujun Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Investigation of flavonoids-rich extract from Scutellaria baicalensis shoots shows that 15 of the 19 flavonoids detected with UPLC-Q-TOF-MS were identified, and it exhibited high antioxidant abilities with IC50 at 70.81, 35.34 and 33.27 μg/mL in DPPH, ABTS and CAA assays, respectively. An order of antioxidant abilities in scavenging DPPH• and ABTS+• of eight high content flavonoids in the extract was the same, and it could be well-explained by a preliminary assign-score method we created in structure-activity relationship analyses specific for flavonoids, revealing that double bonds and phenolic hydroxyls on rings A and B are augmentors, and sugar moiety is an attenuator influencing antioxidant capacity but might contribute positively in CAA assays. In addition, three of the eight flavonoids (baicalein, baicalin and scutellarin) made contributions of 58.33%, 60.36% and 51.41% to overall antioxidant activity of the flavonoids-rich extract in DPPH, ABTS and CAA assays, respectively, thus were determined as the primary or marker flavonoids of Scutellaria baicalensis shoots.
Keywords
Scutellaria baicalensis shoots
UPLC-Q-TOF-MS
Primary flavonoids
Antioxidant activity
Structure–activity relationship
A preliminary assign-score method
1 Introduction
Evidences have manifested that reactive oxygen species (ROS) is implicated in the development of many degenerative diseases such as coronary heart disease, cancer, neurodegenerative diseases, diabetes and aging (Deng et al., 2012; Melguizo et al., 2014). ROS, including superoxide free radical, hydrogen peroxide, hydroxyl free radical and single oxygen, play a pivotal role in oxidative damage caused by these diseases (Nordberg and Arner, 2001). Fortunately, antioxidants may prevent and/or relieve oxidative stress-related diseases through delaying or reducing such oxidative damage. It is well-recognized that antioxidants can be classified into two types, namely enzymatic antioxidants and non-enzymatic antioxidants (Halliwell, 2007). Enzymatic antioxidants act as the first defense line in human body such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glucose-6-phosphate dehydrogenase, while non-enzymatic antioxidants including synthetic and natural antioxidants are considered to be the second defense line. Considering the effects of potential toxicity and carcinogenicity of synthetic antioxidants (Papas, 1999), the search of natural antioxidants has been intensified in recent years.
Natural antioxidants consist of phyto-derived phenols, carotenoids and betaines as well as vitamins C and E. Phenols are a large group of plant secondary metabolites and can be classified into two sub-groups: simple phenols represented by hydroxycinnamic acids and stilbenes, and polyphenols such as flavonoids, hydrolysable tannins, and condensed tannins (Thaipong et al., 2006; Iacopini et al., 2008; Katalinić et al., 2010). Total phenols are of great significance due to their high correlation to antioxidant ability, and therefore are usually accepted as the primary parameters in evaluating antioxidant potential for a plant material (Cetkovic et al., 2007). Among total phenols, polyphenols are capable of exhibiting excellent antioxidant property due to their high radical scavenging capacity which is based on availability of specific hydroxyl groups and probability of stabilization of resulting phenolic radicals through hydrogen bonding or extended electron delocalization (Khanama et al., 2012).
Flavonoids are documented to be one of the major quantities of polyphenols ingested from the diet, especially plant-based foods such as green tea, soy bean, and onion. Flavonoids possess a chemical structure of 15-carbon skeleton constituted by two phenyl rings (rings A and B) and a heterocyclic ring C. According to their structures, flavonoids can be further categorized into three subclasses, namely flavanones (including flavones and isoflavones), flavan-3-ols (including flavonols) and anthocyanidins. Flavonoids are reported to serve as primary antioxidants to convey antioxidant ability and protection of human body against insult of harmful ROS (Xi, et al., 2014; Wu et al., 2015; Seyoum et al., 2006). Further studies demonstrated that antioxidant ability of flavonoids is largely dependent upon the number and position of hydroxyls, sugar moieties and double bonds which also contribute to their molecular polarity (Seyoum et al., 2006).
Scutellaria baicalensis, named as “Huang Qin” in Chinese, is a widely used Chinese herbal medicine taken to relieve heat dampness, purge fire detoxification, promote hemostasis, and prevent miscarriage (Han et al., 2007), and its root is the main medicinal part. In previous literatures, intensive and comprehensive analyses had been systematically conducted on compositions and biological activities of its root extract. The root has been reported to possess antiallergic, antipyretic, antibacterial, antiviral, anticancer, and anti-inflammatory properties in modern pharmacology (Long et al., 2016; Jung et al., 2012), and its active constituents are mainly flavonoids, with over 60 flavonoids being isolated (Han et al., 2007; Liu et al., 2009). On the other hand, there are few studies on the shoot of S. baicalensis. Nevertheless, recent pharmacological studies found that extract of the shoot can deliver a wide variety of therapeutic and beneficial effects, such as cardiovascular protection, hepatoprotection, neuroprotection, anti-bacterial activity, improvement of memory deficits, and anti-tumor activity (Liu et al., 2011), indicating that it might be a good candidate as a potential supplement for developing functional foods. Moreover, several reports have showed that active components in the shoot are also composed mainly by flavonoids, including isocarthamidin-7-O-glucuronide, scutellarin, apigenin-7-O-glucuronide, and baicalin (Liu et al., 2011; Seo et al., 2013; Horvath et al., 2005).
Currently, qualitative and quantitative analyses of flavonoids in plants mainly rely on high performance liquid chromatography-mass spectrometry technology (HPLC/MS), and flavonoids such as patulitrin, quercimeritrin, and quercitagetrin in whole herb of Galinsoga parviflora (Bazylko et al., 2015) and polymethoxylated flavonoids in branches of Murraya paniculata (Zhang et al., 2013) have been successfully examined through HPLC/MS. With regard to S. baicalensis, Seo et al. (2013) had identified 42 polyphenols from flowers, stems, leaves and roots of Korean S. baicalensis, and Liu et al. (2011) had determined 17 flavonoids from its stems and leaves. However, due to its low resolution, analysis speed and sensitivity, traditional HPLC/MS techniques are not an ideal approach for analyzing plant extracts which is usually consisted of complicated compositions and trace levels of compounds (Wu et al., 2016). To make up these defects, an updated technique with superior resolution and sensitivity, called ultra-performance liquid chromatography coupled with quadrupole tandem time-of-flight mass spectrometry (UPLC-Q-TOF-MS), has been employed to replace the traditional HPLC/MS in rapid analyses of phytochemicals. UPLC is a new chromatographic technique using a column with smaller packing operated under high pressure. In addition, Q-TOF-MS allows the generation of mass information with higher accuracy and precision (Abdelbary and Nebsen, 2013; Du et al., 2013). To date, a number of analyses have been successfully accomplished on identification and quantification of phytochemicals in plants by UPLC-Q-TOF-MS, e.g., anthocyanins in black rice (Hao et al., 2015) and secolignans in Peperomia dindygulensis (a folk medicinal plant) (Wang et al., 2014). However, no investigation using UPLC-Q-TOF-MS has been done on analyzing phytochemicals in roots as well as shoots of S. baicalensis.
The objective of this study was to analyze compositions as well as both chemical and cellular antioxidant abilities of the flavonoids-rich extract from S. baicalensis shoots and investigate the contribution of its main flavonoids to its overall antioxidant ability, thus providing base-line data for further development of this valuable natural resource of functional foods. Besides, a preliminary assign-score method was developed when we analyzed structure-activity relationship of the eight high content flavonoids in the flavonoids-rich extract.
2 Materials and methods
2.1 Chemicals
Macroporous resin (AB-8) and ten authentic standards (e.g. rutin, quercetin, baicalin, baicalein, scutellarin, apigenin, chrysin, apigenin-7-O-glucuronide, chrysin-7-O-glucuronide, and isocarthamidin-7-O-glucuronide) were bought from Beijing Chemical Factory and National Institute for Control of Pharmaceutical and Biological Products (Beijing, China), respectively. Purified water was from a mili-Q system (Millipore, Billerica, MA). Reagents for antioxidant assays, including 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulphonic acid (ABTS), Dulbecco’s modified Eagle’s medium (DMEM), heat-inactivated foetal bovine serum (FBS), penicillin-streptomycin solution (100X), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), 4-(2-hydroxyethyl)-1-piperazineethanesulpronic acids (HEPES), phosphate buffered saline (PBS), 2-2′-azobis (2-amidinopropane) (AAPH), and Hank’s balanced salt solution (HBSS) were purchased from Sigma-Aldrich (St. Louis, USA). Human hepatocellular carcinoma HepG2 cells were obtained from Institute of Basic Medical Science Academy (Beijing, China).
2.2 Plant materials and extraction of flavonoids
Shoots of annual Scutellaria baicalensis Georgi were collected in Great Khingan, Heilongjiang, China, washed with purified water, air-dried till equilibrium humidity, and grounded and stored at −20 °C until extraction.
Extraction of flavonoids from the shoots was conducted using a method described by Wu et al. (2015) with slight modifications. Briefly, 250 g powder prepared was refluxed for 2 h at 80 °C with purified water (plant materials:water = 1:10; w:v). The mixture was filtered through a Whatman No. 42 filter paper to obtain filtrate and the residues were subject to extraction twice more under the same condition. All the filtrates (approximately 7500 mL) were combined and then evaporated under vacuum at 80 °C to obtain 500 ml brown concentrated extract solution. The extract solution, after adjusting to pH3.1, was then added onto a chromatographic column (45 mm × 450 mm), which was packed with 100 g AB-8 resins pretreated and activated according to the manufacturer’s recommendation. After getting adsorption equilibrium, the extract was desorbed with 1500 ml of 95% ethanol at a flow rate of 2 mL/min. Next, the eluent was evaporated under vacuum to dryness, and the extract was collected and stored at −20 °C for qualitative and quantitative determinations of total flavonoids.
2.3 Quantitative determination of the flavonoids-rich extract
Total flavonoids were determined according to the method described by Sumczynski et al. (2015) with slight modifications. Briefly, 200 μL extract solution (10 mg/mL) was added to 150 μL NaNO2 solution (5%). The blend was allowed to remain for 6 and 5 min before 300 μL AlCl3 (10%) and 1000 μL NaOH (1 M) were added, respectively. Final volume of the solution was made to 2800 μL with purified water. After 10 min incubation, the mixture turned pink and the absorbance was measured at 510 nm using a UV-1700 spectrophotometer (SHIMADZU, Japan). Total flavonoid was expressed in mg rutin equivalents/g D.W.
2.4 UPLC-Q-TOF-MS analysis of flavonoid compositions
For identification of flavonoid compositions, eight standards (baicalin, baicalein, scutellarin, apigenin, chrysin, apigenin-7-O-glucuronide, isocarthamidin-7-O-glucuronide, and chrysin-7-O-glucuronide) were used based on previous reports (Liu et al., 2011; Seo et al., 2013; Horvath et al., 2005). Chromatographic separation was performed on an Acquity Ultraperformance Liquid Chromatrography (UPLC) system (Waters, USA) equipped with a Dimonsil C18 column (5 μm. 4.6 mm × 250 mm, Dikma Technologies). Extract and individual standards were dissolved in methanol at 0.5 and 0.05 mg/mL, respectively, then filtered through a 0.45-μm filter before injection. Column temperature was maintained at 23.1 °C and injection volume was 10 μL. Mobile phase consisted of methanol (A) and water with 0.05% formic acid (B). The gradient program was as follows: 0–60 min, 34–70% A; 60–70 min, 70% A; 70–75 min, 70–100% A; 75–78 min, 100–34% A. Elution was set at a flow rate of 1.0 mL/min.
The Acquity UPLC system was coupled to a Xevo-G2QTOF mass spectrometer (Waters, USA) by an ESI interface operating in negative ion resolution mode using a capillary voltage of 2.5 kv. The desolvation gas flow rate was set at 11.6 L/min, and the temperature for desolvation was 280 °C. A mass range of 50–1200 m/z was selected. UPLC-MS/MS data, including retention time, experimental and calculated m/z, molecular formula, error of the experimental m/z, DBE, and MS/MS fragments, were collected. Identification of individual compounds from the extract was based on the data in comparison with those of the eight standards and literatures.
2.5 Antioxidant capacity assays
2.5.1 DPPH assay
DPPH• scavenging activities of the flavonoids-rich extract and the eight high content flavonoids were assessed using a method described by Wang et al. (2013). Briefly, 0.1 mL methanol solution of each sample (extract or each of the eight standards) with a concentration at 40, 80, 120, 160 and 200 μg/mL was mixed with 0.25 mL freshly prepared DPPH solution (1 mM in methanol) and 2 mL ethanol. The mixture was shaken vigorously and kept standing for 20 min at room temperature in the dark, then absorbance was read at 517 nm with the UV-1700 spectrophotometer. Radical-scavenging activity was calculated by the following formula: Inhibition (%) = [(Ao − As)/Ao] × 100, where As is the absorbance of each sample and A0 is the absorbance of a control prepared without adding the sample. The inhibition was plotted against the extract concentration to determine corresponding IC50 value (the effective concentration at which DPPH radicals were scavenged by 50%).
2.5.2 ABTS assay
ABTS+• scavenging activities were assessed using a method described by Wang et al. (2013). Briefly, 62.5 mL ABTS solution (7 mM) was mixed with 1 mL potassium persulfate (140 mM), and kept standing for 16 h in the dark at room temperature to obtain ABTS stock solution. Then, 10 ml ABTS stock solution was diluted with approximately 480 mL methanol to an absorbance of 0.70 at 734 nm to obtain the working solution. An aliquot (0.15 mL) of methanol solution containing extract or each of the eight standards at 0.01, 0.02, 0.03, 0.04 or 0.05 mg/mL was mixed with 2.85 mL ABTS working solution in 10 s, and the mixture was left to stand in darkness for 10 min. Absorbance was measured at 734 nm using the UV-1700 spectrophotometer. Scavenging activity was expressed as a percentage using the same formula with that for DPPH assay. As in the DPPH assay, the inhibition was plotted against the extract concentration to determine corresponding IC50 value.
2.5.3 Cellular antioxidant activity (CAA) assay
To determine cellular antioxidant activities of the flavonoids-rich extract and the eight high content flavonoids, CAA assay reported by Liu and Huang (2014) was used with mild modifications. In brief, human hepatocellular carcinoma HepG2 cells were seeded in 100 μL growth medium (DMEM with 10% FBS and 1% penicillin-streptomycin solution) at a density of 105 cells per mL in a 96-well plate, and the plate were maintained at 37 °C and 5% CO2 in a MCO-15AC CO2 incubator (Sanyo/Panasonic, Japan). To get accurate data, the most two outside rows of wells of the plate were not used. After seeding for 24 h, the growth medium was removed and the wells were washed with PBS. The inner wells were treated for 1 h with 100 μL of each sample at concentrations of 2.5, 5.0, 10.0, 20.0 or 40.0 μg/mL plus 25 mM DCFH-DA dissolved in antioxidant treatment medium (DMEM with 2 mM L-glutamine and 10 mM HEPES). After the medium being removed, each well was washed with 100 μL PBS. Next, 100 μL of AAPH (600 μM) dissolved in oxidant treatment medium (HBSS with 10 mM Hepes) was pipetted into each well and the whole 96-well microplate was then placed in an Infinite M200 Pro Microplate Reader (Tecan, Switzerland) at 37 °C. Fluorescence reading was monitored every 5 min during 1 h at 538 and 485 nm for excitation and emission, respectively. Control wells contained the cells treated with DCFH-DA and oxidant treatment medium with AAPH while blank wells contained the cells treated with DCFH-DA and oxidant treatment medium without AAPH. Quercetin was used as a standard to confirm the accuracy of the assay. The area under the curve of fluorescence versus time was integrated to calculate the CAA value based on the following equation after subtracting the blank value (Wolfe and Liu, 2007): CAA unit = 100 − (∫SA/∫CA) × 100, where ∫SA was the integrated area under the fluorescence versus time curve of an individual sample and ∫CA was the corresponding integrated area of the control. Median effective dose (IC50) was determined from the median effect plot of log (fa/fu) versus log (dose), where fa was the fraction (CAA unit) affected and fu was the fraction (100 −CAA) unaffected by the treatment with a sample. IC50 value was converted to CAA value expressed as μg quercetin equivalents/100 μg D.W.
2.6 Statistical analysis
All experiments were conducted in triplicate, the results were expressed as mean ± SD, and the data were analyzed by SPSS software (version 17.0, Chicago, USA) and Excel 2016. Differences were considered to be significant at p < 0.05.
3 Results and discussion
3.1 Identification of flavonoids composition and quantification of eight high content flavonoids
Before phytochemical analysis by UPLC-Q-TOF-MS and determination of antioxidant ability, several extractions with different solvents were performed to select an effective extracting method. As a result, soaking extraction with water for 6 h at 80 °C was chosen. After extraction, a purification for preparation of flavonoids was proceeded with pretreated macroporous resin (AB-8) and 95% ethanol as elution solvent. Total flavonoids obtained was 765.23 mg rutin equivalents/g D.W. (Table S1).
An UPLC-Q-TOF-MS method with negative ion mode was adopted to analyze main compounds in the flavonoids-rich extract obtained above. UPLC-Q-TOF-MS base peak chromatograms of the extract were depicted in Fig. 1. Nineteen compounds (each accounted for an area larger than 0.5% of the total peak areas), which accounted for 93.25% of the total, were detected. Among these 19 compounds, the peak of compound 3 was the biggest (12.4% of the total), followed by compounds 19 (11.9%), 9 (10.1%), 13 (8.5%), 17 (8.1%), 16 (7.1%), 4 (5.0%), 6 (4.8%), 8 (4.7%), 5 (4.4%), 18 (3.5%), 11 (3.4%), 10 (1.7%), 12 (1.6%), 1 (1.5%), 7 (1.4%), 15 (1.3%), 2 (0.9%), and 14 (0.8%).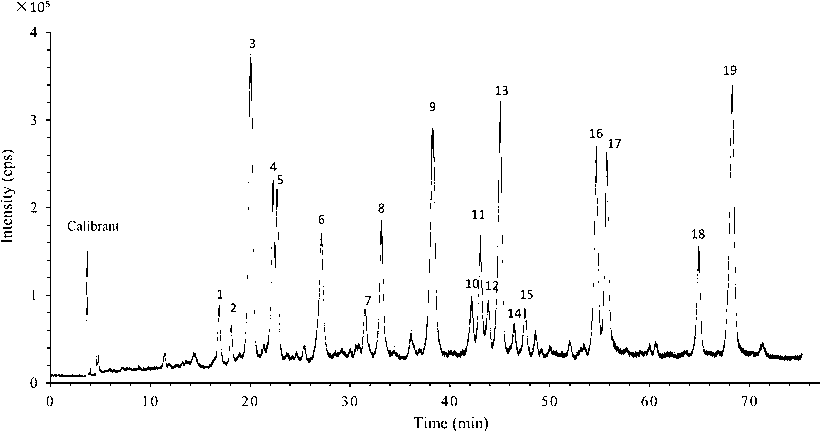
UPLC-Xevo-G2QTOF-MS base peak chromatogram of flavonoids-rich extract from S. baicalensis shoots eluted by a Dimonsil C18 column (5 μm. 4.6 × 250 mm, Dikma Technologies). The mobile phase was a gradient by two mobile phases: methanol (A) and water with 0.05% formic acid (B) (0 min, 34% A; 60 min, 70% A; 70 min, 70% A; 75 min, 100% A; 78 min, 34% A). The flow rate was 0.5 mL min-1(cps: counts per second).
Through analyzing UPLC-MS/MS data of the 19 compounds, including retention time, experimental and calculated m/z, molecular formula, error of the experimental m/z, DBE, MS/MS fragments, and proposed names of individual compounds (Table 1), it is clear that 15 of them were successfully identified as flavonoids, but four (compounds 1, 2, 4, and 5, totally 11.8% of the total peak area) were not yet ascertained. MS spectra data of compounds 1 and 2 and those of compounds 4 and 5 were completely pair-wise identical. Nonetheless, they could not be matched to the known compounds documented in literatures. Thus, identifications of these four compounds must require conducting the 1D and 2D NMR analysis. Unfortunately, quantities of these four compounds obtained were far too little to meet the requirement for NMR analysis.
Peak
RT (min)
m/z measured
Molecular formula
m/z calculated
Error (ppm)
DBE
MS/MS Fragments
Proposed compound
1
16.90
563.1397
C26H27O14
563.1401
−0.7
13.5
503.0976, 473.2211, 443.1250, 383.1451, 353.4324
Unidentified
2
18.08
563.1401
C26H27O14
563.1401
0
13.5
503.2180, 473.0555, 443.2177, 383.1113, 353.1528
Unidentified
3
20.01
463.8077
C21H19O12
463.0877
0
12.5
287.0553, 181.0446, 167.0586
Isocarthamidin-7-O-Glu acid
4
22.29
463.0847
C21H19O12
463.0877
−0.6
12.5
287.0553, 269.0446, 175.0586, 131.3212
Unidentified
5
22.68
463.0875
C21H19O12
463.0877
−0.4
12.5
287.0552, 269.0441, 175.0786, 131.1745
Unidentified
6
27.12
461.0719
C21H17O12
461.0720
−0.2
13.5
285.0395
Scutellarin
7
31.46
461.0715
C21H17O12
461.0720
−1.1
13.5
285.0393, 267.1287, 257.4673, 241.3984, 239.1703, 213.5543
Isoscutellarein-7-O-Glu acid
8
33.11
445.0771
C21H17O11
445.0771
0
13.5
269.0450, 251.1109, 241.8732, 223.1253
Bacailin
9
38.24
445.0764
C21H17O12
445.0771
−1.6
13.5
269.0444, 225.0908, 117.4377
Apigenin-7-O-Glu acid
10
42.19
475.0877
C22H19O12
477.0877
0
13.5
299.0552, 284.0586
5,7,8-Trihydroxy-6-methoxyflavone-7-O-Glu acid
11
43.06
461.0713
C21H17O12
461.0720
−0.7
13.5
285.0393, 267.1287, 257.4673, 241.3984, 239.1703, 213.5543
Isoscutellarein-8-O-Glu acid
12
43.82
445.0765
C21H17O11
445.0771
−1.3
13.5
269.0443, 251.0649, 241.9603, 223.6092
Nowogonin-7-O-Glu acid
13
45.08
429.0828
C21H17O10
429.0822
0.6
13.5
253.0500, 209.1276, 143.6528
Chrysin-7-O-Glua cid
14
46.41
459.0922
C22H19O11
459.0927
−0.5
13.5
283.0602, 268.0654
OroxylinA-7-O-Glu acid
15
47.54
459.0925
C21H17O12
459.0927
−0.4
13.5
283.0604, 268.0807
Wogonoside
16
54.67
269.0452
C15H9O5
269.0450
0.2
11.5
225.1632, 117.5361
Apigenin
17
55.65
269.0449
C15H9O5
269.0450
0.1
11.5
251.2309, 241.6732, 223.0081
Bacailein
18
64.93
283.0599
C15H11O5
283.0606
−0.7
11.5
268.0286
Wogonin
19
68.22
253.0497
C15H9O4
253.0501
−0.4
11.5
209.1342, 143.7623
Chrysin
Among the 15 identified flavonoids, eight, namely, compounds 3, 6, 8, 9, 13, 16, 17 and 19, were rigorously identified by comparing their retention times and MS/MS spectra with those of corresponding standards (Table 1), and all these compounds have been documented in S. baicalensis shoots (Liu et al., 2011; Seo et al., 2013; Horvath et al., 2005). Mass spectrum of compound 3 (tR = 20.01 min) showed a [M−H]− base peak with a m/z at 463 and an intense fragment anion with a m/z at 287 (aglycone anion), which was generated by loss of a glucuronic acid moiety (176 Da), and MS2 of the anion at 287 further yielded two products at 181 and 167. By comparing the retention time and mass spectra with those of standards, compound 3 was identified as isocarthamidin-7-O-glucuronide. Compound 8 (tR = 33.11 min) showed a [M−H]− at 445 and an aglycone anion at 269 (also by loss of glucuronic acid moiety). In MS2 spectra, the fragment at 269 produced three anions at 251, 241, and 223, which may result from loss of a H2O, a CO, and successive of a H2O and a CO, respectively. Based on these information, compound 8 was determined as baicalin. Compound 9 (tR = 38.24 min) produced a [M−H]− at 445 along with an aglycone anion at 269 (a loss of glucuronic acid moiety). MS2 of the anion at 269 further yielded three fragments at 225, 151 and 117. Based on these information, compound 9 was identified as apigenin-7-O-glucuronide. Compounds 6 and 13 (tR = 27.12 and 45.08 min) were determined as scutellarin and chrysin-7-O-glucuronide, which exhibited [M−H]− base peaks at 461 and 429, and aglycone anions at 285 and 253 (each also produced by a loss of glucuronic acid moiety), respectively. There were no further fragment anions in its mass spectrum for compound 6, and MS2 of the anion at 253 of compound 13 yielded two anions at 209 and 143. Compounds 16, 17, and 19 (tR = 54.67, 55.65, and 68.22 min) yielded three [M−H]− base peaks at 269, 269, and 253, and MS2 fragments of the three anions were shown to be identical to those of compounds 9 (apigenin-7-O-glucuronide), 8 (baicalin), and 13 (chrysin-7-O-glucuronide), indicating that apigenin-7-O-glucuronide, baicalin, and chrysin-7-O-glucuronide were glucuronides of compounds 16, 17, and 19, thus, they were identified as apigenin, baicalein, and chrysin, respectively.
Authentic standards of compounds 7, 10, 11, 12, 14, 15, and 18 (totally 13.7% of the total peak area) were not available, thus they were identified by comparing their retention times and MS spectra data solely with literatures (Table 1). Compounds 7 and 11 (tR = 31.46 and 43.06 min, respectively) both yielded a [M−H]− at 461 and an aglycone anion at 285. MS2 spectra of the anions at 285 of the two compounds yielded five completely identical fragments at 267, 257, 241, 239, and 213 by loss of a H2O, a CO, a CO2, successive of a H2O and a CO, and four H2O, respectively. Based on such information and their retention times reported (Liu et al., 2011), compounds 7 and 11 were determined as flavones, and were identified as isoscutellarein-7-O-glucuronide and isoscutellarein-8-O-glucuronide, respectively. Compound 10 (tR = 42.19 min) exhibited a [M−H]− at 475 and an aglycone anion at 299. MS2 of the anion at 299 yielded a fragment at 284 (loss of a radical CH3 fragment), which revealed the presence of a methoxyl and three hydroxyls on the aglycone of this glucuronide. Based on comparison with known flavonoids in S. baicalensis documented by Liu et al. (2009), compound 10 was determined as 5,7,8-trihydroxy-6-methoxyflavone-7-O-glucuronide. Compound 12 (tR = 43.82 min) exhibited a [M−H]− at 445 and an aglycone anion at 269 (loss of a glucuronic acid moiety). MS2 of the anion at 269 produced three fragments at 251, 241, and 223 by loss of a H2O, a CO, and successive of a H2O and a CO, respectively. Based on these results and comparison with the literature (Liu et al., 2011), compound 12 was determined as nowogonin-7-O-glucuronide. Compounds 14 and 15 (tR = 46.41 and 47.54 min) were a pair of isomers, and both of them generated a significant [M−H]− at 459. The anion at 459 further yielded an anion at 283 (−176 Da by loss of a glucuronide acid) and the anion at 283 further yielded an anion at 268 (−15 Da by loss of a —CH3 group). By comparison of retention times of these two isomers with those reported by Han et al. (2007), compounds 14 and 15 were determined as oroxylin-A-7-O-glucuronide and wogonoside, respectively. Compound 18 (tR = 64.93 min) released a [M−H]− at 283, and its MS2 yielded a fragment at 268 by loss of a CH3. Based on such data and literature reported by Zhang et al. (2007), compound 18 was determined as wogonin.
Collectively, 15 of the 19 flavonoids were successfully identified, and peak areas of the eight flavonoids with authentic standards accounted for 67.6% of the total, thus they were defined as high content flavonoids in S. baicalensis shoots for further antioxidant analysis. Authentic standards of compounds 11 and 18 (totally 6.9% of the total peak area) were not available, and compounds 7, 10, 12, 14 and 15 (totally 6.8% of the total peak area) were too low to be further analyzed, therefore, these seven compounds were not chosen. Quantitative determination by UPLC showed that the eight flavonoids accounted for 57.39% of the flavonoids-rich extract and 75.00% of the total flavonoids, and their order of contents from the highest to the lowest was: baicalein > baicalin > scutellarin > apigenin-7-O-glucuronide > chrysin-7-O-glucuronide > isocarthamidin-7-O-glucuronide > apigenin > chrysin (Table S1).
3.2 Antioxidant capacities of flavonoids-rich extract and its eight high content flavonoids
It is well-recognized that single antioxidant assay presents certain limitation on reflecting actual antioxidant ability. In order to reflect the actual situation as far as possible, in vitro chemical and cell-based assays were conducted in the present study to evaluate antioxidant capacities of the flavonoids-rich extract from S. baicalensis shoots and its eight high content flavonoids.
3.2.1 DPPH• and ABTS+• antioxidant abilities
DPPH and ABTS assays were performed to determine the free radical scavenging abilities of the flavonoids-rich extract and its eight high content flavonoids (Fig. 2). As showed in Fig. 2A, trolox, the positive control, exhibited the highest DPPH• scavenging ability at all concentrations, and the antioxidant ability rose in a concentration-dependent manner, reaching an elimination of almost all DPPH• (98.9%) at 0.20 mg/mL. At concentrations up to 0.04 mg/mL, the activity increased abruptly, then its increasing speed slowed down between 0.04 and 0.12 mg/mL, followed by a much more gradual increase thereafter. In contrast to trolox, the radical-scavenging ability of the extract was less than half of trolox at all concentrations, and it initiated in a rapid increase up to 0.04 mg/ml and changed into a gradual increase from 0.04 to 0.2 mg/ml, reaching a final elimination of rarely 42.78% DPPH•, which was in agreement with those reported by Seo et al. (2013).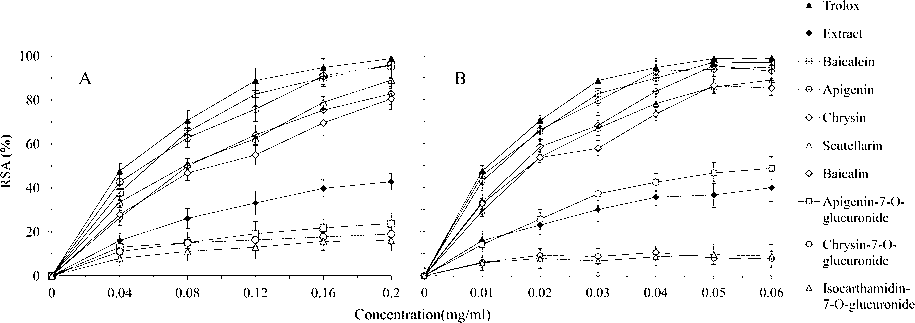
Antioxidant capacity of the S. baicalensis shoot flavonoids-rich extract and eight high content flavonoids by DPPH and ABTS assays. Six and seven concentrations of the samples were chosen to be subjected to DPPH, and ABTS respectively, to examine their corresponding DPPH (A) and ABTS (B) radical scavenging activity. The absorbance was determined at 517 nm for DPPH, and at 734 nm for ABTS. Trolox was used as the positive control to ensure that the results were reliable. The results were presented as mean ± SD of three independent experiments (n = 3) and expressed as the concentrations of the samples versus DPPH or ABTS radical scavenging activity (RSA) (%).
For the eight high content flavonoids, five of them exhibited much higher radical-scavenging abilities than that of the extract but slightly lower than that of trolox at all concentrations. Their scavenging ability order was baicalein > apigenin > scutellarin > chrysin > baicalin, with the highest two scavenging rates being 96.1% and 95.6%, respectively. Trends of scavenging abilities of the other three (apigenin-7-O-glucuronide, chrysin-7-O-glucuronide and isocarthamidin-7-O-glucuronide) were similar to and their activities at all concentrations were much less than that of the extract, showing much weaker antioxidant abilities in contrast to those of the former five flavonoids.
Scavenging activities of the eight high content flavonoids in the extract by the ABTS assay (Fig. 2B) exhibited similar increasing patterns comparing to both the positive trolox control and the extract and they showed roughly the same order with that in the DPPH assay (Fig. 2A), namely, trolox > baicalein > apigenin > scutellarin > chrysin > baicalin > apigenin-7-O-glucuronide > extract > chrysin-7-O-glucuronide > isocarthamidin-7-O-glucuronide, with an exception of apigenin-7-O-glucuronide being little higher in ABTS scavenging ability but much lower in DPPH scavenging ability than that of the extract. The highest two scavenging rates of baicalein and apigenin were 97.03% and 94.88%, respectively, slightly lower than that of the trolox (98.9%). However, the lowest two scavenging rates of chrysin-7-O-glucuronide and isocarthamidin-7-O-glucuronide were only 7.88% and 9.01%, respectively, and they showed no increase or even certain extend of decrease (as indicated by somehow negative slopes of the two curves) of scavenging abilities at concentrations between 0.02 and 0.06 mg/mL. These differences between ABTS and DPPH assays might be due to their different reaction mechanism.
IC50 values of the extract for DPPH• and ABTS+• scavenging abilities were calculated to be 70.81 and 35.34 μg/mL, respectively, which were significantly lower than those of Etlingera fimbriobracteata (518.29 μg/mL for DPPH), Ipomoea batatas (298.55 for DPPH) and Lawsonia Inermis (97.4 for DPPH and 101.35 for ABTS) leaves. These three leaves are widely accepted as having potential for developing functional foods (Daula et al., 2016; Zhang et al., 2016; Dhaouadi et al., 2015), suggesting that S. baicalensis shoots extract possessed noticeable antioxidant ability, and this might be largely attributed to the strong antioxidant abilities delivered by five of the eight high content flavonoids (i.e., baicalein, apigenin, scutellarin, chrysin, and baicalin; Fig. 2).
3.2.2 Cellular antioxidant ability (CAA)
To further validate the chemically determined DPPH• and ABTS+• scavenging abilities, CAA assay were adopted by using human hepatocellular carcinoma HepG2 cells, and quercetin was used as a positive control. Concentrations of the extract and eight high content flavonoids were under the non-cytotoxic level to HepG2 cells. Protective effects of the extract at concentrations up to 40.0 μg/mL against AAPH-induced oxidation in HepG2 cells were determined, and low fluorescence value manifested the strong antioxidant ability (Liu and Huang, 2014). Fluorescence values of the extract showed time-dependent increases at all concentrations, the increment rates till 20 min were lower than those from 20 to 50 min, and the fluorescence at each time point decreased as the concentration increased (Fig. S1A). IC50 value of the extract was calculated to be 33.27 ± 1.086 μg/mL based on the linear regression of the median effect curves as described in Material and Methods (Fig. S1B). In addition, IC50 values of the eight high content flavonoids were then calculated based on the same linear regression (Table 2).
Flavonoids
IC50 (μg/ml)a
Numbersb
Total scores
DPPH
ABTS
CAA
A
B
D
S
Baicalein (1)
37.18 ± 0.35
17.53 ± 0.39
19.53 ± 0.62
3
0
7
0
51
Apigenin (2)
37.94 ± 0.21
18.22 ± 0.31
63.65 ± 1.34
2
1
7
0
50
Scutellarin (3)
41.25 ± 0.28
19.52 ± 0.43
22.03 ± 0.85
2
1
7
1
49
Chrysin (4)
42.67 ± 0.37
20.16 ± 0.45
67.60 ± 1.29
2
0
7
0
48
Baicalin (5)
43.28 ± 0.41
21.98 ± 0.28
23.62 ± 0.96
2
0
7
1
47
Apigenin-7-O-glucuronide (6)
111.58 ± 4.21
32.67 ± 0.38
112.99 ± 3.23
1
1
7
1
46
Chrysin-7-O-glucuronide (7)
132.13 ± 3.34
79.34 ± 1.34
139.80 ± 5.23
1
0
7
1
44
Isocarthamidin-7-O-glucuronide (8)
149.78 ± 4.25
84.25 ± 1.12
147.65 ± 5.31
2
1
6
1
43
The above IC50 values, including those of the flavonoids-rich extract and each of the eight high content flavonoids, were then converted to CAA values expressed as μg quercetin equivalents. As shown in Fig. 3,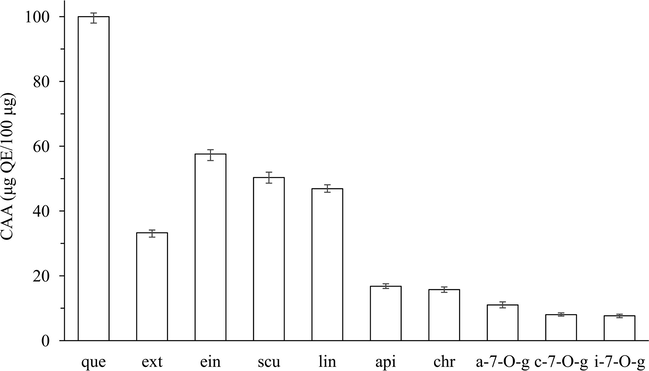
Cellular antioxidant activity of extract and eight high content flavonoids. (que: quercetin, ext: extract, ein: baicalein, scu: scutellain, lin: baicalin, api: apigenin, chr: chrysin, a-7-O-g: apigenin-7-O-glucuronide, c-7-O-g: chrysin-7-O-glucuronide, i-7-O-g: isocarthamidin-7-O-glucuronide). Six concentrations (2.50–40.0 μg/ml) of each sample were used against the oxidation from dichlorofluorescein (DCFH) to 2′, 7′-dichlorofluorescein (DCF) induced by peroxyl radicals in HepG2 cells. Quercetin was used as the positive control to ensure that the results were reliable, and the results were expressed as μg Quercetin Equivalents/100 μg. Data are the mean ± SD of three different experiments.
CAA values of the eight flavonoids are presented from left to right in a decreasing order. Among them, baicalein exhibited the strongest value (57.89 ± 0.258 μg quercetin equivalents/100 μg), followed by scutellarin (51.34 ± 0.364) and baicalin (47.88 ± 0.276), while the other five displayed low values ranging from 7.66 ± 0.134 to 16.73 ± 0.185. CAA value of the extract (33.99 ± 0.213) was significantly lower than those of the top three and higher than those of the other five, which is also significantly higher than those of numerous vegetables (from 0.28 to 1.69) and adlays (from 0.16 to 2.98) (Liu and Huang, 2014), indicating that S. baicalensis shoots extract can convey a considerable antioxidant effect by protection from oxidant insult to cell membrane, thus stabilized oxidant homeostasis in human liver cells. It was worth noting that, apigenin and chrysin, which exhibited low antioxidant abilities in the CAA assay, showed a relatively high level of antioxidant activity in DPPH and ABTS assays. This discrepancy may be caused by several factors such as solubility in the incubation medium and the growth situation of HepG2 cells (Wolfe et al., 2008). Moreover, the lack of correlation (Table S2) between chemical and CAA assays suggests that it is essential to adopt the multiple approaches to achieve a comprehensive understanding of antioxidant abilities for flavonoids. Nevertheless, the first three of the eight high content flavonoids from the extract, namely, baicalein, scutellarin, baicalin, possessed the highest antioxidant abilities both in the chemical DPPH and ABTS assays as well as the CAA assay.
3.3 Structure-antioxidant activity relationship of the eight high content flavonoids
Oxidative stress has been implicated in the onset of human diseases such as cardiovascular diseases, cancer, neurodegenerative diseases, diabetes, and ageing (Deng et al., 2012). Evidences indicate that scavenging properties of antioxidant compounds are essential for attenuating oxidative stress, thus preventing development of pathologies (Majo et al., 2005). Flavonoids have been recognized as effective antioxidants, and their roles are directly associated with their specific structural features, such as the position and number of hydroxyls and the number of double bonds on aromatic rings A and B as well as the heterocyclic ring C (Seyoum et al., 2006; Amaral et al., 2009; Modak et al., 2005).
Fig. 4 shows chemical structures of the eight high content flavonoids in a decreased order of antioxidant ability. In order to analyze the structure-activity relationship more intuitively, we arbitrarily assigned different scores to the four structurally important features, i.e., double bonds (each 6 score), hydroxyls on rings A (each 3 score) and B (each 2 score), and sugar moieties (each −1 score), which exert influences at different degree to the antioxidant ability of individual flavonoids (Heijnen et al., 2001; Seyoum et al., 2006). The minus mark indicates negative influence, suggesting that the sugar moiety might be an attenuator to the antioxidant ability. A total score was calculated for each flavonoid, and a big score represents a high antioxidant ability.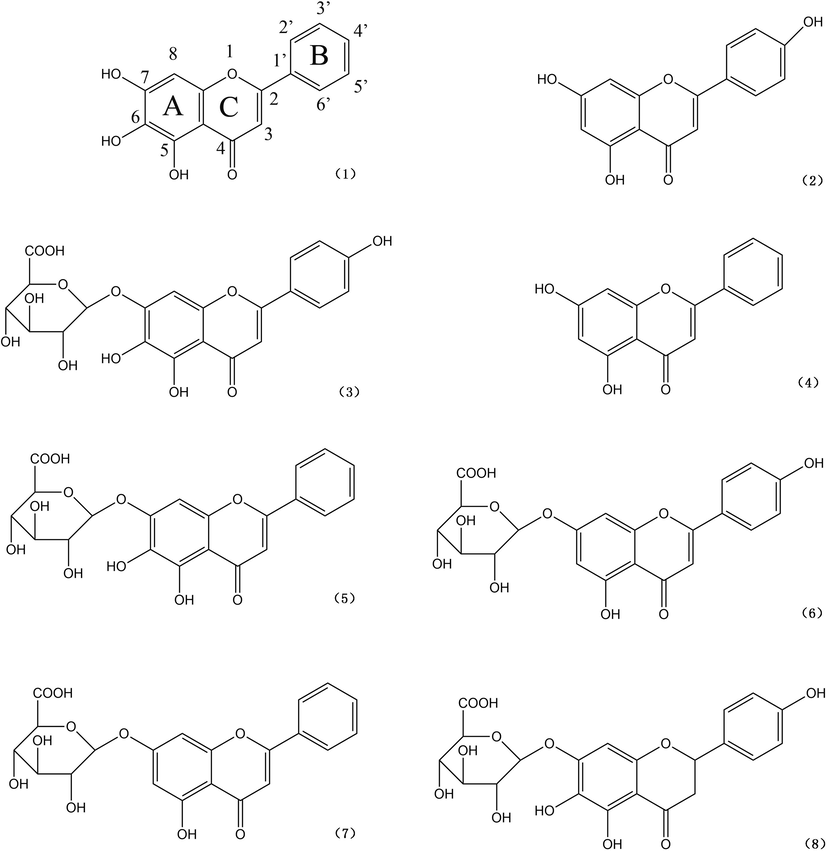
Chemical structures of the eight high content flavonoids. (1) baicalein; (2) apigenin; (3) scutellarin; (4) chrysin; (5) baicalin; (6) apigenin-7-O-glucuronide; (7) chrysin-7-O-glucuronide; (8) isocarthamidin-7-O-glucuronide.
As shown in Table 2, baicalein possessed the strongest antioxidant ability with a total score of 51, and this was attributed to seven double bonds in the two aromatic rings (7 × 6 = 42; a feature holding by all the high content flavonoids except the last one, isocarthamidin-7-O-glucuronide) and three hydroxyls existed on ring A (3 × 3 = 9). The relatively lower ability of apigenin (50 score) was due to the absent of one hydroxyl on ring A (−3 score) and the present of one hydroxyl on ring B (+2 score) comparing to baicalein, and this can be explained perfectly by a hypothesis that a hydroxyl on ring A is more active than that on ring B (Seyoum et al., 2006). As to the third strongest antioxidant scutellarin (49 score), it has the same structure with apigenin except for a sugar moiety at C-7 (-1 score), causing the decrease of its antioxidant ability in comparison with apigenin (Heijnen et al., 2001).
Chrysin (48 score), baicalein (47 score) and apigenin-7-O-glucuronide (46 score) are all found to have two hydroxyls. Although baicalin and apigenin-7-O-glucuronide both carry a sugar moiety at C-7 (-1 score), the two hydroxyls on ring A of baicalin (2 × 3 = 6 score) make it possessing higher antioxidant ability than that of apigenin-7-O-glucuronide, which carries one hydroxyl on ring A and the other on ring B (3 + 2 = 5 score).
Lastly, chrysin-7-O-glucuronide (44 score) and isocarthamidin-7-O-glucuronide (43 score) shows much weaker antioxidant abilities. Although there are three hydroxyls and a sugar moiety (3 × 2 + 2 − 1 = 7 score) presented in isocarthamidin-7-O-glucuronide versus only one hydroxyl and a sugar moiety (3 − 1 = 2 score) in chrysin-7-O-glucuronide, the former showed still lower antioxidant activity than that of the latter. This was attributed to lack of a double bond (-6 score) between C-2 and C-3 in the heterocyclic ring C of isocarthamidin-7-O-glucuronide.
Collectively, our findings demonstrated that antioxidant capacities of the eight flavonoids were highly tied to their structural features including number of double bonds, number and position of phenolic hydroxyls and attachment of sugar moiety. Furthermore, the assign-score method we established was commendably suitable for evaluation of antioxidant abilities of the eight flavonoids in the DPPH and ABTS assays. Although differences of the score between each two adjacent flavonoids were calculated the same or similar due to all the hydroxyls on either ring A or ring B were assigned an exactly same score, they might not be the same because a hydroxyl (and/or a sugar moiety) on different position of an aromatic ring must exert different influence on the antioxidant ability of an individual flavonoid. On the other hand, our preliminary assign-score method could not be matched well with the order of antioxidant abilities of the eight flavonoids in the CAA assay. As shown in Table 2, CAA assay showed an order of antioxidant abilities from the highest to the lowest as follows: (1), (3), (5), (2), (4), (6), (7) and (8). The reverse of compounds (2) and (4) by compounds (3) and (5) might be caused by many factors such as solubility in the incubation medium, the growth situation of HepG2 cells, and the permeability of cell membrane to individual flavonoids (Wolfe et al., 2008). One more possibility was that an additional sugar moiety attached to ring A of (3) or (5) comparing to that of (2) or (4) might be decomposed by certain hydrolytic enzymes on the HepG2 cell membrane when they entered into the cells, thus increasing their antioxidant abilities to some extent in CAA assay. Nevertheless, the preliminary assign-score method could be regarded as a tentative innovation in the structure-activity relationship analysis, and it is to be refined when dealing with far more than eight flavonoids as did in the current work.
3.4 Contribution of individual eight flavonoids to overall antioxidant ability
To determine the contribution of individual eight high content flavonoids to overall antioxidant ability of the flavonoids-rich extract from S. baicalensis shoots, a calculation formula was developed as follows: Contribution (%) = [Ei/E0] × C, where Ei is the trolox (DPPH and ABTS assays) or quercetin equivalent (CAA assay) antioxidant activity of an individual flavonoid, E0 is the trolox (DPPH and ABTS assays) or quercetin (CAA assay) equivalent antioxidant activity of the flavonoids-rich extract, and C is the content of an individual flavonoid in the extract. Table 3 highlighted that the eight flavonoids made strong contributions to the overall antioxidant abilities of the flavonoids-rich extract in both the DPPH, ABTS and CAA assays (81.73%, 86.18% and 59.64%, respectively). Even more important, although they are not consistent with their corresponding antioxidant ability orders in either DPPH, ABTS or CAA assays (Table 3) due to differences in contents of individual flavonoids (Table S1), the three orders of antioxidant activity contributions of individual eight flavonoids are exactly the same, i.e., baicalein > baicalin > scutellarin > chrysin > apigenin > apigenin-7-O-glucuronide > chrysin-7-O-glucuronide > isocarthamidin-7-O-glucuronide (Table 3). Furthermore, baicalein, baicalin and scutellarin remained the superiors in both the two set criterions, namely, the antioxidant ability orders (Table 2) and contribution orders (Table 3), confirming further that these three flavonoids were more than just the predominant flavonoids in contents (32.83%), they were also the main contributors (56.7% in average of the three assays) to the overall antioxidant abilities in the flavonoids-rich extract as well. Thus they can be defined as the primary flavonoids in the flavonoids-rich extract from S. baicalensis shoots. Data are the mean ± SD of three repeated tests.
Flavonoids
Antioxidant ability
Contribution
DPPHa
ABTSa
CAAb
DPPH
ABTS
CAA
Baicalein
96.66 ± 3.58
90.87 ± 3.23
57.89 ± 1.53
29.23 ± 1.03
30.95 ± 0.98
26.14 ± 1.56
Baicalin
83.04 ± 2.76
72.47 ± 2.95
47.88 ± 1.23
17.89 ± 0.56
17.59 ± 0.87
15.41 ± 1.12
Scutellarin
87.13 ± 2.99
81.61 ± 2.19
51.34 ± 1.65
11.21 ± 0.27
11.82 ± 0.66
9.86 ± 1.06
Chrysin
84.13 ± 1.87
79.02 ± 2.84
16.73 ± 0.84
7.47 ± 0.08
7.89 ± 0.21
2.22 ± 0.03
Apigenin
94.72 ± 3.03
87.43 ± 3.01
17.77 ± 0.77
6.53 ± 0.12
6.79 ± 0.13
1.83 ± 0.11
Apigenin-7-O-glucuronide
32.21 ± 1.03
48.76 ± 2.09
10.01 ± 0.89
3.94 ± 0.05
6.73 ± 0.09
1.82 ± 0.24
Chrysin-7-O-glucuronide
27.20 ± 0.93
20.07 ± 1.03
8.09 ± 0.56
3.10 ± 0.03
2.31 ± 0.07
1.23 ± 0.06
Isocarthamidin-7-O-glucuronide
23.99 ± 0.87
18.91 ± 0.98
7.66 ± 0.58
2.36 ± 0.11
2.10 ± 0.01
1.13 ± 0.05
Total of the eight
529.08 ± 17.06
499.14 ± 18.32
217.37 ± 8.05
81.73 ± 2.25
86.18 ± 3.02
59.64 ± 4.24
Total of the top three
266.83 ± 9.33
244.95 ± 8.37
157.11 ± 4.41
58.33 ± 1.86
60.36 ± 2.51
51.41 ± 3.88
Extract
50.76 ± 2.87
45.07 ± 1.96
33.99 ± 1.34
100
100
100
4 Conclusions
In the present study, nineteen flavonoids were clearly detected and 15 were identified successfully in the flavonoids-rich extract from S. baicalensis. The extract showed high antioxidant capacities in DPPH, ABTS and CAA assays. Eight high content flavonoids were selected for analysis of individual antioxidant activities. The order of their antioxidant abilities in both DPPH and ABTS assays was baicalein > apigenin > scutellarin > chrysin > baicalin > apigenin-7-O-glucuronide > chrysin-7-O-glucuronide > isocarthamidin-7-O-glucuronide. Structure-activity relationship analyzed by a preliminary assign-score method revealed that phenolic hydroxyls on rings A and B and double bonds are augmentors, and sugar moiety is an attenuator influencing antioxidant capacity. Furthermore, we found that the eight flavonoids made great antioxidant contributions to overall antioxidant activities in all the three assays. Among the eight flavonoids, baicalein, baicalin and scutellarin were not only the predominant flavonoids but the superior contributors, thus they were defined as the primary flavonoids of the flavonoids-rich extract from S. baicalensis shoots. Our findings would provide useful information for further development of S. baicalensis shoots as potential supplements for various functional foods.
Acknowledgement
This work was financially supported by the special funds for Forestry Public Welfare Scientific Research Projects (No. 201404718), China.
Conflict of interest
The authors declared that there were no conflicts of interest.
References
- Application of a novel UPLC–MS/MS method for the pharmacokinetic/bioequivalence determination of atorvastatin and ezetimibe in human plasma. J. Pharm. Res.. 2013;7(1):24-32.
- [Google Scholar]
- Plant extracts with anti-inflammatory properties—a new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Bioorg. Med. Chem.. 2009;17(5):1876-1883.
- [Google Scholar]
- Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsoga ciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochem. Lett.. 2015;11:394-398.
- [Google Scholar]
- Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii extracts. Int. J. Mol. Sci.. 2007;8(10):1013-1027.
- [Google Scholar]
- Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K.Schum.) R.M.Sm. Ind. Crops Prod.. 2016;84:189-198.
- [Google Scholar]
- Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods. 2012;4:906-914.
- [Google Scholar]
- Commercial Lawsonia inermis L. dried leaves and processed powder: phytochemical composition, antioxidant, antibacterial, and allelopathic activities. Ind. Crops Prod.. 2015;77:544-552.
- [Google Scholar]
- Determination of deltonin in rat plasma by using HPLC–MS/MS and the application of this method in pharmacokinetic studies. J. Chromatogr. B. 2013;931(15):1-5.
- [Google Scholar]
- Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci.. 2015;64:92-99.
- [Google Scholar]
- Biochemistry of oxidative stress. BiochemicalSociety Transactions. 2007;35:1147-1150.
- [Google Scholar]
- Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B. 2007;848(2):355-362.
- [Google Scholar]
- Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol. In Vitro. 2001;15:3-6.
- [Google Scholar]
- Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant Huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A. 2005;1062(2):199-207.
- [Google Scholar]
- Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal.. 2008;8:589-598.
- [Google Scholar]
- Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J. Ethnopharmacol.. 2012;141(1):345-349.
- [Google Scholar]
- Polyphenolic profile, antioxidant propertiesand antimicrobial activity of grape skin extracts of 14 Vitisvinifera varieties grown in Dalmatia (Croatia) Food Chem.. 2010;119(2):715-723.
- [Google Scholar]
- Phenolic acids, flavonoids and total antioxidant capacityof selected leafy vegetables. J. Funct. Foods. 2012;4:979-987.
- [Google Scholar]
- Investigation of flavonoid profile of Scutellaria bacalensis Georgi by high performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Chromatogr. A. 2009;1216(23):4809-4814.
- [Google Scholar]
- Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J. Chromatogr. B. 2011;879:1023-1028.
- [Google Scholar]
- Assessments of antioxidant effect of black tea extract and its rationals by erythrocytehaemolysis assay, plasma oxidation assay and cellular antioxidant activity (CAA) assay. J. Funct. Foods. 2014;18:1095-1105.
- [Google Scholar]
- Three new lignan glucosides from the roots of Scutellaria baicalensis. Acta Pharmaceut. Sin. B. 2016;6(3):229-233.
- [Google Scholar]
- Flavanones in citrus fruit: structure–antioxidant activity relationships. Food Res. Int.. 2005;38(10):1161-1166.
- [Google Scholar]
- The potential of Artemisia vulgaris leaves as asource of antioxidant phenolic compounds. J. Funct. Foods. 2014;10:192-200.
- [Google Scholar]
- Structure–antioxidant activity relationships of flavonoids isolated from the resinous exudate of Heliotropium sinuatum. Bioorg. Med. Chem. Lett.. 2005;15(2):309-312.
- [Google Scholar]
- Reactive oxygen species, antioxidants and the mammalian thioredoxin system. FreeRadical Biol. Med.. 2001;31:1287-1312.
- [Google Scholar]
- Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67:2058-2070.
- [Google Scholar]
- Determination of polyphenol components of Korean Scutellaria baicalensis Georgi using liquidchromatography–tandem mass spectrometry: Contribution to overall antioxidant activity. J. Funct. Foods. 2013;5:1741-1750.
- [Google Scholar]
- Total phenolics, flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem.. 2015;174:319-325.
- [Google Scholar]
- Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal.. 2006;19:669-675.
- [Google Scholar]
- Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crops Prod.. 2013;42:587-594.
- [Google Scholar]
- Qualitative and quantitative analyses of bioactive secolignans from folk medicinal plant Peperomia dindygulensis using UHPLC-UV/Q-TOF-MS. J. Pharmaceut. Biomed. Anal.. 2014;94:1-11.
- [Google Scholar]
- Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem.. 2007;55:8896-8907.
- [Google Scholar]
- Cellular antioxidant activity of common fruits. J. Agr. Food Chem.. 2008;56:8418-8426.
- [Google Scholar]
- Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa(Ait.) Hassk. Food Chem.. 2015;173:194-202.
- [Google Scholar]
- Lipidomics study of plasma phospholipid metabolism in early type 2 diabetes rats with ancient prescription Huang-Qi-San intervention by UPLC/Q-TOF-MS and correlation coefficient. Chem.-Biol. Interact.. 2016;256:71-84.
- [Google Scholar]
- Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem.. 2014;161:230-238.
- [Google Scholar]
- Development of the fingerprints for the quality evaluation of scutellariae radix by HPLC-DAD and LC-MS-MS. Chromatographia. 2007;66:13-20.
- [Google Scholar]
- Characterization of thirty-nine polymethoxylated flavonoids (PMFs) in the branches of Murraya paniculata by HPLC-DAD-ESI-MS/MS. Chinese J. Nat. Med.. 2013;11(1):63-70.
- [Google Scholar]
- Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem.. 2016;208:61-66.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2017.08.002.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1
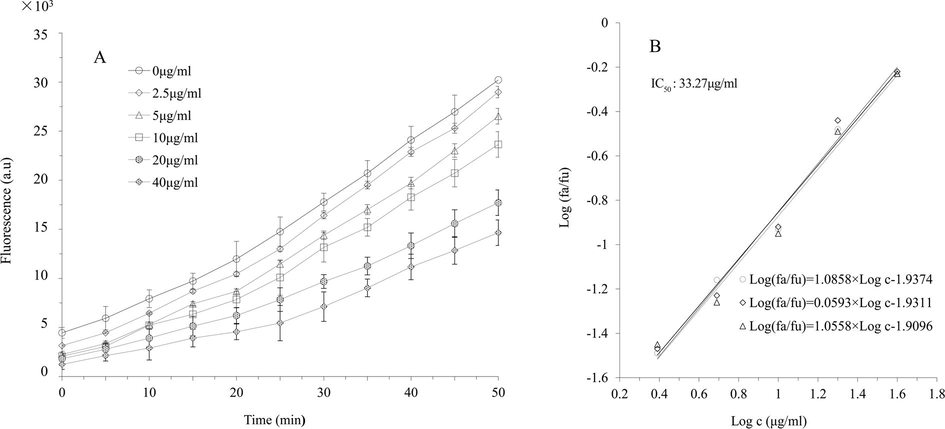
Protective effect of S. baicalensis extract at different concentrations (2.5, 5.0, 10.0, 20.0 or 40.0 μg/mL) against the oxidation from dichlorofluorescein (DCFH) to 2′,7′-dichlorofluorescein (DCF) induced by peroxyl radicals in HepG2 cells (A) and the median effect plots for the inhibition effect on the peroxyl radical-induced DCFH oxidation (B) (a.u: arbitrary unite; c: concentration).







