Translate this page into:
Thermal decomposition of foundry resins: A determination of organic products by thermogravimetry–gas chromatography–mass spectrometry (TG–GC–MS)
⁎Corresponding author. akmita@agh.edu.pl (A. Kmita),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The article presents the results of research on thermal decomposition of Ester-Cured Alkaline Phenolic No-Bake (ALPHASET) binders used in molding technology. In the ALPHASET system phenol-formaldehyde resin of resole type is cured with a liquid mixture of esters. Under the influence of the molten metal the thermal decomposition of the binder follows, resulting in the evolution of gases, often harmful, e.g. from benzene, toluene, ethylbenzene and xylenes (BTEX) or Polycyclic Aromatic Hydrocarbon (PAH) groups. The identification of gases evolved during the pyrolysis of the binders was carried out and their decomposition temperatures were determined using the Thermogravimetry–Gas Chromatography–Mass Spectrometry (TG–GC–MS) technique. The tests were subjected to two types of binders from different manufacturers. Among the products of pyrolysis there have been identified mainly benzene and its derivatives, and phenol and its derivatives. Compounds identified in pyrolytic gas are largely considered to be harmful to humans and the environment (some of the compounds are carcinogenic and mutagenic). The presented results of the TG–GC–MS measurements show that the applied analytic methods are feasible to perform a qualitative and also quantitative characterization of the binder samples.
Keywords
Resins
Decomposition
TG–GC–MS
Metal casting
Emission
1 Introduction
The metal casting industry represents an important manufacturing component that produces numerous important casting products for everyday life. With the development of the world economy, the metal casting industry has been growing rapidly in recent years. The total production of casting metals in world increased from about 92 million tons in 2010 to 110 million tons in 2015 (Modern Casting, 2015; Trinowski, 2016). The rapid expansion of metal casting industry has raised major environmental concerns associated with this industry.
The metal casting industry produces complex and irregular shaped products used in numerous industrial and consumer goods. Pouring liquid metal into a mold made of a material to withstand the heat produces a casting. For castings that require hollow interior sand cores are used. The primary production method for making a mold is the sand casting process.
Synthetic organic polymers are applied as resin binders to transform sand into hardened molds and cores. Resins commonly used by the foundry industry include phenol-formaldehyde, phenolic-urethane and furan.
Once the molten metal is poured into the sand mold and core binders will be exposed to the intense heat of metal castings and undergo thermal decomposition reactions. A variety of organic compounds and certain HAPs1 (Hazardous Air Pollutants) could be generated during this process. The thermal decomposition products condense in the molding sand and are emitted into the air during metal pouring, mold cooling, and casting shakeout. Some of these compounds are toxic and are therefore of considerable interest (Wang et al., 2007, 2011a,b; Tiede et al., 2010; Holtzer et al., 2014a,b; Shepherd, 2012; Strzemiecka et al., 2016; Dungan and Reeves, 2005; Lytle et al., 1998).
Also, from the quality point of view, castings produced with those resins are not always the best. For example, often in the surface layer of ductile iron castings made in the sands with furan resin cured with sulfonic acids the degeneration of nodular graphite into graphite flake occurs (Xiaogan et al., 1992; Riposan et al., 2013; Holtzer et al., 2014a,b; Holtzer et al., 2015a).
However, due to its advantages (high production efficiency, high durability achieved, ease of regeneration of the used sand, very good reproducibility of the shape and dimensions) sands with organic binders are still widely used in foundries. As a response to the ever more stringent environmental regulations, foundries have been seeking alternative raw materials that are more environmentally friendly than the traditional ones to diminish their pollutants and operation costs. Many new mold and core binder systems have been developed recently, predicated on the foundries’ requirement. There is a need for foundries to evaluate these new products as a part of adopting them in full-scale production.
Recognition of processes (reaction mechanisms) which occur when high temperatures are influencing materials applied for making molds and cores, requires using adequate analytical techniques. Due to a high versatility of materials applied in the foundry industry and - in consequence - their different physical and chemical properties, it is difficult to select a universal analytical technique, allowing for a complete investigation of each material. Therefore it is advisable to apply combined techniques such as Pyrolysis - Gas Chromatography/Mass Spectrometry (Py-GC/MS) (Wang et al., 2011a,b; Dungan and Reeves, 2005), Pyrolysis - Gas Chromatography/Flame Ionization Detector (Py-GC/FID) (Zhang et al., 2014; Wang et al., 2011a,b), Thermogravimetric–Fourier Transform–Infrared Spectroscopy (TGA–FT–IR), Thermogravimetric–Mass Spectrometry (TGA–MS) (Singh et al., 2012) or Thermogravimetric–Gas Chromatography–Mass Spectrometry (TGA–GC–MS) (Howell et al., 2003). Currently, there is a return to the traditional inorganic binder which is water glass, but hardened by dehydration. From the point of view of ecology this binder is very environmentally friendly. In the group of organic binders Ester-Cured Alkaline Phenolic No-Bake (ALPHASET) technology is increasingly popular (United State Patent, 1986). In numerous emission tests in which ALPHASET mold sand was used, data have shown that the Polycyclic Aromatic Hydrocarbons (PAH) and benzene, toluene, ethylbenzene and xylenes (BTEX) emissions from the sand mold binders’ pyrolysis took place, but in much smaller quantities than in the sands with other organic binders which are widely used in foundries (Table 1) (Holtzer et al., 2013; Campbell, 2011; Holtzer et al., 2015b). Ester-Cured Alkaline Phenolic No-Bake (ALPHASET) is used for small or medium batch production. It may be used for all alloy types but is of special interest for lighter alloys, because of the ease of shake out. The absence of nitrogen in the compounding reagents is a benefit for steel casting and spheroidal iron casting. TOC/DOC - Total Organic Compounds/Dissolved Organic Carbon. COD - Chemical Oxygen Demand.
Parameter
ALPHASET set
FURAN-ACID set
Electrolytic conductivity, μS/cm
1317
1483
TOC/DOC, mg/dm3
21
191
COD, mg/dm3
64
490
Phenol index, mg/dm3
<0.04
0.59
Formaldehyde, mg/dm3
0.08
0.14
Hydrocarbons, mg/dm3
0.14
0.44
The cross-linking of alkaline phenolic resole resin by adding organic esters with the advantages including low odor, virtually no smoke, easy to stripping, good finishing, low veining, minimal erosion, and very good hot strength. Since the binders are free of nitrogen and sulfur, they afford foundry engineering advantage of nodular iron, cast steel and aluminum casting (Campbell, 2011).
The curing mechanism for ALPHASET is started by the liquid ester reacting with alkali in the phenol resin, to form the alkali metal salt of the acid component of the ester (1st stage).
A secondary reaction is thought to occur when the partially polymerized resin contacts heat during the pouring of liquid metal operation, yielding an extremely rigid structure (2nd stage).
Typically, 1.5–2.0% binder based on sand and 20–25% co-reactant based on the resin are used to coat washed and dried silica sand in most core and molding operations.
The aims of the study were the thermal decomposition of binders and the determination of evolved gas composition in purpose to find information about the temperature range of degradation and the identification of gaseous degradation compounds for evaluation of their toxicity.
2 Materials and methods
The subject of the study was two binders (cured resins) used in the ALPHASET molding process from different manufacturers.
-
The sample labeled “TPA70” in which the relative proportions TPA70 resole resin and hardener/catalyst ALPHASET ACE1010 comply with the manufacturer’s recommendations – Hexion UK Limited. Resin ALPHASET TPA70 as substance classified with a health or environmental hazard contents: ⩾35–<50% mass phenol, polymer with formaldehyde and ⩾7–<25% mass potassium hydroxide. However, ALPHASET ACE1010 contained: ⩾25–<35% mass gamma-butyrolactone and ⩾25–<35% mass propylene carbonate.
-
And the sample marked “ESTROFEN” in which the relative proportions of ESTROFEN resole resin and hardener/catalyst PR5 comply with the manufacturer’s recommendations – Prec-Odlew Sp. z o. o., Poland. The resin ESTROFEN as substance classified with a health or environmental hazard contents: 35–50% mass phenol, polymer with formaldehyde, 10–12.5% mass potassium hydroxide and 0.1–0.2% mass formaldehyde. However, PR5 contained: 15–25% mass gamma-butyrolactone and 20–25% mass propylene carbonate.
For the measurement, the following apparatuses were used:
-
The Thermo-Microbalance NETZSCH TG 209 F1 Libra® with an effective resolution of 0.1 μg enables highly precise measurements under pure He atmospheres from ambient temperatures up to 1000 °C. Heating rate of sample was 10 °C/min. The weight of samples was about 1.5 mg.
-
For control of the measurements as well as for data acquisition, modern digital electronics and the well established NETZSCH PROTEUS 32-bit Software are employed. Combining both thermogravimetric and spectroscopic methods such as GC–MS, FTIR and/or MS, enables furthermore identification of the evolved gases.
-
The evolved gases are injected over a heated transfer line (up to 300 °C) into the heated Joint Analytical System (JAS) valve box of the Agilent 7890A Gas Chromatograph. With the help of a carrier gas He, the gases were carried over a specific sample loop and transferred to a heated column (column length 30 m). The single components of the gas mixture travel through the column and leave it in dependence of the interactions with the column material (retention time, RT) and were then detected to be the Agilent 5975 MDS (mass selective detector).
The data were obtained from thermogravimetric analysis (TGA), where the sample weight over time as the temperature increased is stored to identify the temperature at which mass was lost. This could determine approximately when an emission had occurred due to the amount of weight loss from the sample. Gas chromatography was then used to identify the compounds in released gas. Additional usage of mass spectrometry (MS) helped to observe exactly at which temperatures the compounds were coming off. It also could determine how much of the compound was produced by the intensity of the peaks (McKinley et al., 1993).
3 Results and discussion
3.1 Thermogravimetry analysis
Figs. 1a and 1b depict the TG and DTG (derivative thermogravimetry) results of TPA70 and ESTROFEN samples respectively. Both samples exhibited three overlapping mass loss steps. The mass loss (TG, %) and mass loss rate (DTG, %/min) profiles are very similar for both samples. Where in TPA70 sample in three steps the maximum rate of weight loss occurs at a lower temperature than the ESTROFEN sample, the mass loss started already at room temperature. The separation of the mass loss steps is not completely clear. Also the signal (mass loss rate) showed several minima for each sample. The residual mass was nearly equal for both samples with 45.4% (TPA70) and 45.3% (ESTROFEN) respectively.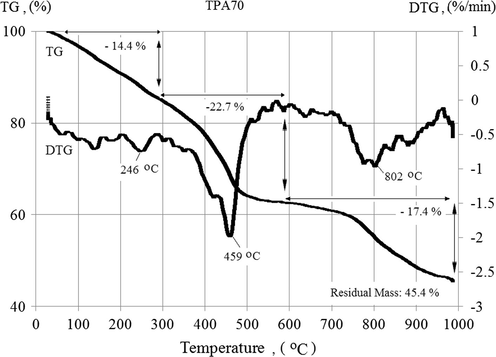
Temperature dependent mass loss (TG) and mass loss rate (DTG) of the TPA70 sample.
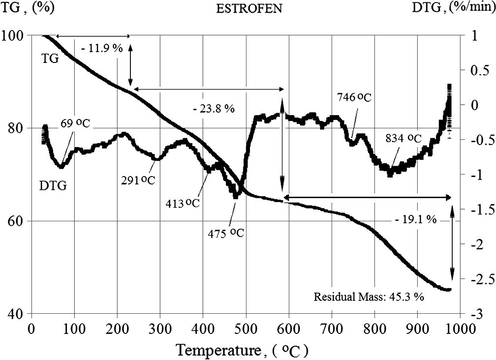
Temperature-dependent mass loss (TG) and mass loss rate (DTG) of the ESTROFEN sample.
The first significant mass loss up to almost 15% occurred from room temperature to 300 °C. Probably in this range the release of water occurred. As it was suggested by Lytle et al. (1998) the water in the novolac resin is a product of pyrolysis and originated from an elimination reaction taking place at high temperature. Because cured novolac and resole resin are structurally alike, thermal decomposition products are similar between these resins (Sobera and Hetper, 2003). A mass loss in this temperature range can be also caused by a release of CO and CO2, since one of the binder components is propylene carbonate, which is thermally decomposed at temperatures above 240 °C (Li et al., 2003). For the TPA70 binder the maximum rate of this process occurs at a temperature of 246 °C (Fig. 1a) and in case of the ESTROFEN binder at a temperature of 291 °C (Fig. 1b).
The secondary significant mass loss was noticed between 300 °C and 550 °C with a slight plateau thereafter to 650 °C. In this range the thermal decomposition of binder2 appears and several organics are released.
The maximum rate of decomposition of the sample TPA70 takes place at a temperature of 459 °C, and ESTROFEN samples at 475 °C. At temperatures up to 500 °C both samples lost most of its mass (65–68% of the total mass loss).
The third significant loss of mass (nearly - 20%) occurred from 700 °C to 1000 °C, with a maximum speed at a temperature of 802 °C and 834 °C, respectively for TPA70 and ESTROFEN samples. After heating to 1000 °C the final weight of both samples represents about 45% of the initial weight.
3.2 Gas chromatography – mass spectrometry
During the TG run the evolved gases were condensed in the cryotrap at −50 °C and separated via the GC column and detected by MS after completion of the TG run. The total ion chromatograms (TIC) after heating the cryotrap are shown in Figs. 2 and 3. The comparison of the measured mass spectra to the National Institute of Standards Technology database (NIST MS, 2000) gave possible candidates for the released compounds. For some peaks structural similar compounds (e.g. structural isomers) were found with high hit quality. The results are shown in Table 2 (TPA70) and Table 3 (ESTROFEN). Also the comparison of the TIC for both samples did not give significant difference, probably only in concentration of released compound. CAS - number of chemical substance in Chemical Abstracts Service Registry.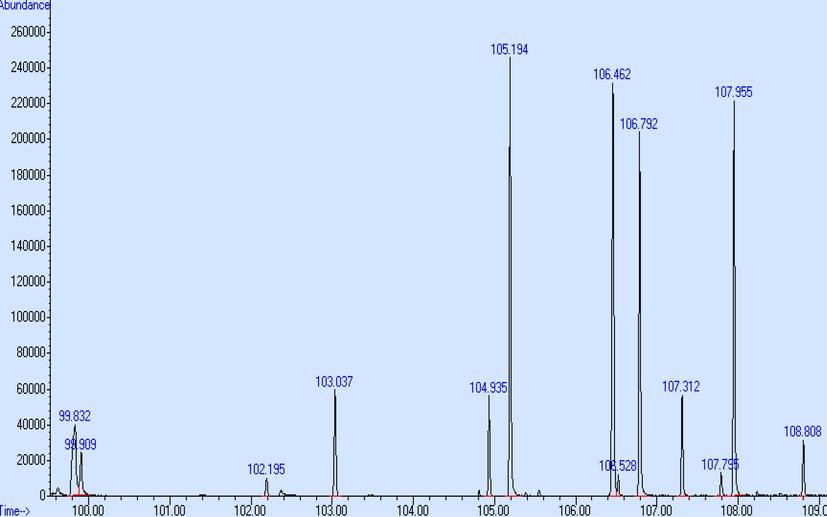
Total ion chromatogram (TIC) of sample TPA70 after heating the cryotrap.
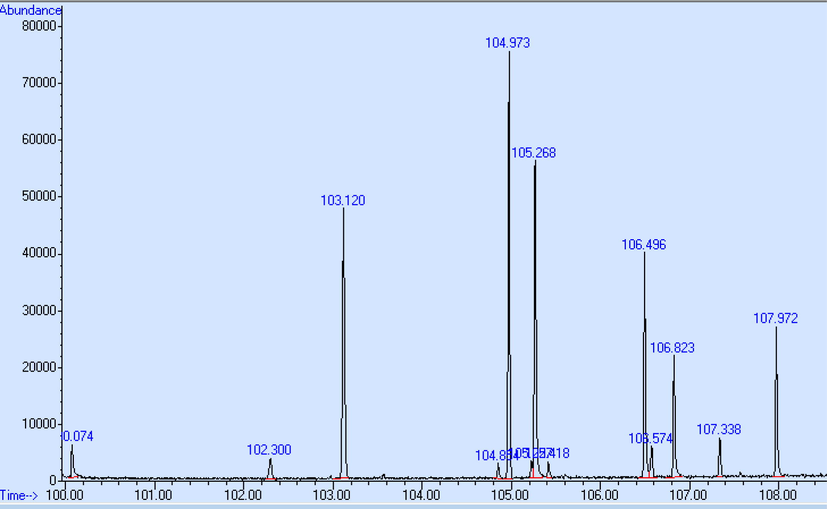
Total ion chromatograms (TIC) of sample ESTROFEN after heating the cryotrap.
Retention time, min
Database hit
CAS no
Probability, %
99.832
Acetic acid
64-19-7
90
102.195
Column material
–
–
103.037
Benzene, 1,3-dimethyl
108-38-3
97
p-Xylene
106-42-3
95
104.813
Benzene, 1-ethyl-3-methyl
620-14-4
83
104.935
Benzene, 1,2,3-trimethyl
526-73-8
97
105.194
Phenol
108-95-2
95
106.462
Phenol, 2-methyl
95-48-7
97
106.528
Benzene, 4-ethyl-1,2-dimethyl
934-80-5
90
Benzene, 1,2,4,5-tetramethyl
95-93-2
90
Benzene, 1-ethyl-2,3-dimethyl
933-98-2
90
106.792
Phenol, 4-methyl
874-41-0
96
107.312
Phenol, 2,6-dimethyl
576-26-1
97
107.795
No clear identification
–
–
107.955
Phenol, 3,5-dimethyl
108-68-9
97
108.808
Phenol, 2,4,6-trimethyl
527-60-6
95
Retention time, min
Database hit
CAS no
Probability/%
100.074
Acetic acid
64-19-7
83
102.300
Column material
–
–
103.120
p-Xylene
106-42-3
97
Benzene, 1,3-dimethyl-
108-38-3
95
104.854
Benzene, 1-ethyl-3-methyl
620-14-4
90
Benzene, 1-ethyl-2-methyl
611-14-3
90
104.973
Benzene, 1,3,5-trimethyl-
108-67-8
97
105.268
Phenol
108-95-2
95
106.496
Phenol, 2-methyl
95-48-7
95
106.574
Benzene, 1-ethyl-2,4-dimethyl
874-41-9
90
Benzene, 1-ethyl-2,3-dimethyl-
933-98-2
90
Benzene, 1-methyl-2- (1-methylethyl)
527-84-4
90
106.823
Phenol, 4-methyl
874-41-0
91
107.338
Phenol, 2,3-dimethyl
526-75-0
91
107.972
Phenol, 2,4-dimethyl
105-67-9
95
Sample TPA70 was additionally investigated in the quasi-continuous mode, which means injection of evolved gas every 4 min into the GC at a constant column temperature of 150 °C.
The results are shown in Fig. 4 and Table 4. Here, the temperature dependent release could be followed.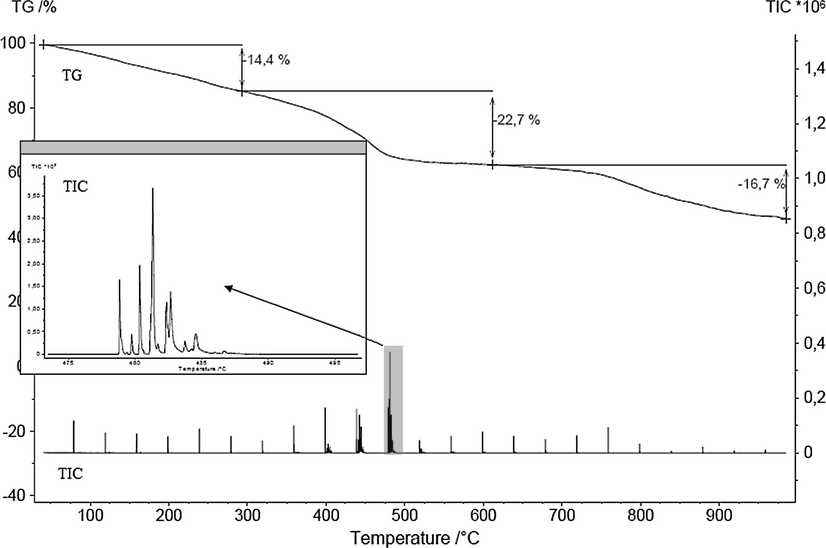
Temperature dependent mass loss (TG) and total ion current (TIC) of sample TPA70.
Retention time, min
Database hit
CAS no
Probability/%
45.072
Toluene
108-88-3
91
45.134
p-Xylene
106-42-3
97
Benzene, 1,3-dimethyl-
108-38-3
97
45.229
Benzene, 1,3,5-trimethyl
108-67-8
95
45.330
Phenol, 2-methyl
95-48-7
94
45.364
Phenol, 4-methyl
874-41-0
93
Benzene, 1-ethyl-3,5-dimethyl
934-74-7
74
45.470
Phenol, 2,3-dimethyl
526-75-0
95
45.549
Phenol, 2,4-dimethyl
105-67-9
93
The analysis of the MS data gave the release of water up to 300 °C, the release of several organic compounds during the second mass loss step and the release of CO and CO2 during the third mass loss step. The released organic compounds were again identified by database comparison (NIST MS, 2000). Table 4 shows the database hits with high probability. This measurement mode gives less candidates as concentration and separation in weaker with this method.
The heating of the mold or core sands by the heat from the hot metal leads to the thermal decomposition of the binder. The decomposition of the binders (TPA70 and ESTROFEN) released mainly to benzene and its derivatives and phenol and its derivatives, the low–volatile compounds. The compounds identified here are typical products of thermal decomposition of resole resin as described in the literature (Sobera and Hetper, 2003; Blazso, 1991) and phenol and its methyl derivatives formed as a result of breaking the bonds between the aromatic ring and methylene group along with hydrogenation of the latter to methyl groups.
According to Dungan and Reeves (2005) the pyrolysis of phenolic resole resin (minimum pyrolysis temperature was 650 °C) is obtained with a certain amount of low-volatile compounds with two condensed rings. The creation of considerable amounts of xanthane and its methyl derivatives is characteristic for resole cured resin (Sobera and Hetper, 2003).
4 Conclusions
The presented results of the TG–GC–MS measurements show that the applied analytic methods are feasible to perform a qualitative and also quantitative characterization of the binder samples. The technique TG–GC–MS allowed us to determine the scope of temperature and composition of the emitted substances.
Evolved compounds were investigated by GC–MS, which had enabled the identification of each released compound by a previous separation of the gases before the MS detection.
Two techniques of measuring the released gases were applied:
-
During the TG run the evolved gases every 1 min were condensed in the cryotrap at −50 °C and separated via the GC column and detected by MS after completion of the TG run (TPA70, ESTROFEN).
-
The quasi-continuous mode, which means injection of evolved gas every 4 min into the GC at a constant column temperature of 150 °C (TPA70).
The TG–GC–MS measuring technique showed a high similarity of the mass loss and the composition of the gases released during the heating of ESTROFEN and TPA70 binder samples in the range of temperatures up to 1000 °C which indicates that the output of both binders is similar. Thus, in the range of 300 °C the weight loss was mainly due to the loss of water but also may be with CO and CO2 emission (one of the components of the binder is propylene carbonate (a mixture of esters), which is thermally decomposed at a temperature > 240 °C) (Li et al., 2003). The second mass loss step is related to the release of a number of organic compounds. The decomposition of the binders (TPA70 and ESTROFEN) released mainly to benzene and its derivatives and phenol and its derivatives, the low–volatile compounds.
For the TPA70 binder the maximum rate of this process is at a temperature of 459 °C (Fig. 1a) and for the ESTROFEN binder this process is at 475 °C (Fig. 1b). This is the effect of the complete decomposition of these binders. The third step of mass loss was associated with CO and CO2 emission. Secreted compounds are mainly volatile aromatics including benzene and derivatives thereof, methyl and ethyl, p-xylene, phenol and derivatives thereof, methyl and ethyl. Some of the released gases (phenol and some of its derivatives and certain derivatives of benzene) resulting from thermal decomposition of binders used in the ALPHASET molding technology pose a threat to humans and the environment. The measurement by quasi-continuous mode gives less compounds than condense in the cryotrap at – 50 °C as concentration and separation in weaker with the first method.
In order to reduce the amount of harmful substances, generated during a decomposition of the ALPHASET binder, efforts should be undertaken to decrease amounts of the molding sand reaching temperatures above 400 °C. Thus, the contact time of a casting with a molding sand should be limited, which means that a casting should be knocked out (removed from a mold) as fast as possible after liquid metal pouring into a mold and solidification. Since the highest concentration of hazardous gases occurs during the mold pouring with liquid metal and the casting knocking out, these operations should be done when the exhaust installation is efficiently operating. Exhausted gases, before being introduced into the atmosphere, should be purified e.g. by adsorption on active carbon.
Acknowledgments
The study was performed within the Project from Ministry of Science and Higher Education No. 11.11.170.318/7 realized in 2016 at AGH—University of Science and Technology.
References
- Sequence of phenolic units in phenol-formaldehyde polycondensates studied by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis. 1991;19:251-263.
- [Google Scholar]
- Complete Casting Handbook. Oxford, UK: Published by Elsevier Ltd.; 2011.
- Pyrolysis of foundry sand resins: a determination of organic products by mass spectrometry. J. Environ. Sci. Heal.. 2005;40:1557-1567.
- [Google Scholar]
- Investigations of properties of moulding sands with resins applied in the ALPHASET technology. Arch. Foundry Eng.. 2013;13(1):31-37.
- [Google Scholar]
- The influence of reclaim addition on the emission of PAHs and BTEX from moulding sands with furfuryl resin with the average amount of furfuryl alcohol. Arch. Foundry Eng.. 2014;14(1):37-42.
- [Google Scholar]
- Analysis of the sulphur content in moulding sands with furan resins. Trans. Foundry Res. Inst.. 2015;55(2):19-28.
- [Google Scholar]
- Influence of the reclaim addition to the moulding sand matrix obtained in the ALPHASET technology on the emission of gases - comparison with moulding sand with furfuryl resin. Arch. Foundry Eng.. 2015;15(1):121-125.
- [Google Scholar]
- Influence of the reclaim addition to the moulding sand with furan resin on the emission of toxic gases at high temperature. In: 71th World Foundry Congress: Advanced Sustainable Foundry, Bilbao, 19-21 May. 2014.
- [Google Scholar]
- Assessment of the thermal degradation characteristics of isomeric poly(styrene)s using TG, TG/MS and TG/GC/MS. Thermochim. Acta. 2003;396(1–2):167-177.
- [Google Scholar]
- Thermal decomposition characteristics of poly(propylene carbonate) using TG/IR and Py-GC/MS techniques. Polym. Degrad. Stabil.. 2003;81(1):157-165.
- [Google Scholar]
- Determination of novolac resin thermal decomposition products by pyrolysis-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis. 1998;45:121-131.
- [Google Scholar]
- Air emissions from foundries: a current survey of literature suppliers and foundrymen. AFS Trans. 1993:979-990.
- [Google Scholar]
- Modest growth in worldwide casting market. 49th Census of World Casting Production 2015:26-31.
- [Google Scholar]
- NIST MS Search 2, 2000. 0 Libera, Chemm. SW, Version 2,0, Fairfield, CA.
- Control of surface graphite degeneration in ductile iron for windmill applications. Int. J. Metalcast.. 2013;7(1):9-20.
- [Google Scholar]
- Curing and thermal tegradation of phenol-formaldehyde binders. Russ. Cast Prod.. 1974;2:55-56.
- [Google Scholar]
- Understanding emission characteristics of a foundry sand binder. In: Proceedings of the National Conference on Undergraduate Research (NCUR) 2012. Ogden, UT: Weber State University; 2012. March 29-31
- [Google Scholar]
- Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrolysis. 2012;94:99-107.
- [Google Scholar]
- Pyrolysis-gas chromatography-mass spectrometry of cured phenolic resins. J. Chromatog. A. 2003;993(131–135):217.
- [Google Scholar]
- Examination of the chemical changes in cured phenolo-formaldehyde resins during storage. J. Chromatogr. A. 2016;1441(106):115.
- [Google Scholar]
- Emission of organic compounds from mould and core binders used for casting iron, aluminum and bronze in sand moulds. J. Environ. Sci. Heal. A. 2010;45(14):1866-1876.
- [Google Scholar]
- Comparing moulding and core trends in the U.S. and EU casting industries. Huttenes- Albertus Chemische Werke GmbH 2016:1-13.
- [Google Scholar]
- United States Patent, 1986. Foundry moulds and cores. Patent No. Re.32,720, Borden (UK) Limited England.
- Characterization of hydrocarbon emissions from green sand foundry core binders by analytical pyrolysis. Environ. Sci. Technol.. 2007;41:7922-7927.
- [Google Scholar]
- Hazardous air pollutant formation from pyrolysis of typical Chinese casting materials. Environ. Sci. Technol.. 2011;45:6539-6544.
- [Google Scholar]
- Comparative analysis of hazardous air pollutants emissions of casting materials measured in analytical pyrolysis and conventional metal pouring emission tests. Environ. Sci. Technol.. 2011;45:8529-8535.
- [Google Scholar]
- Nodular iron surface deterioration due to PTSA in resin. AFS Trans.. 1992;100:9-15.
- [Google Scholar]
- Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as a binder catalyst. J. Therm. Anal. Calorim.. 2014;116:373-381.
- [Google Scholar]







