Translate this page into:
Electrochemical determination of imatinib mesylate using TbFeO3/g-C3N4 nanocomposite modified glassy carbon electrode
⁎Corresponding authors at: Department of Biotechnology, Research Institute of Modern Biological Techniques (RIMBT), University of Zanjan, P.O. Box 45371-38791, Zanjan, Iran (A. Ramazani). aliramazani@gmail.com (Ali Ramazani), salavati@kashanu.ac.ir (Masoud Salavati-Niasari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
A new electrochemical sensor based on a glassy carbon electrode (GCE) modified by Tb (Terbium) FeO3/g-C3N4 (graphitic carbon nitride) nanocomposite has been developed. In order to characterize the nanocomposite produced, several techniques were employed, including X-ray diffraction (XRD), Fourier transform infrared, Field Emission Scanning Electron Microscope, Energy Dispersive X-ray Spectroscopy, Brunauer-Emmett-Teller, vibrating sample magnetometry, and Transmission Electron Microscopy (TEM). According to XRD data, the nanocomposite produced contained particles of about 36 ± 2 nm in size. TEM examination of the voltammetric response of the offered sensor (TbFeO3/g-C3N4 nanocomposite/glassy carbon electrode (GCE)) demonstrated a catalytic effect against imatinib. In optimized solution pH and scan rate conditions, this sensor demonstrated an excellent electrocatalytic response for detecting imatinib. Furthermore, the fabricated sensor demonstrated acceptable accuracy, reproducible behavior, and a high level of stability during all electrochemical tests. In addition, analytical parameters were determined and the results were compared with those from previous studies.
Keywords
Imatinib Mesylate
TbFeO3/g-C3N4 Nanocomposite
Electrochemical Determination
Electrochemical Sensor
Nanostructures
1 Introduction
Imatinib mesylate (IMAT) is a particular drug with the molecular formula of 4-[(4-methylpiperazin-1-yl) methyl]-N- [4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl) amino] phenyl]benzamide that is used to treat chronic myeloid leukemia (CML) and diseases such as gastrointestinal stromal tumors (Druker et al., 2001, Gibbons et al., 2008). Imatinib mesylate significantly alters the management of tumors that are resistant to cytotoxic chemotherapy by inhibiting tyrosine kinase (Hunter 2007). CML, is a malignant blood disorder in which the arms of chromosomes 9 and 22 are displaced causes abnormal proliferation of myeloid cells, leading to the production of the BCR-ABL fusion protein, which encodes a protein with high tyrosine kinase activity (Mauro and Deininger 2006, Nabi et al., 2021, Mohaghegh et al., 2022). IMAT is mostly metabolized by the enzyme CYP3A4 (Deininger and Druker 2003). Depending on the dose of this drug, patients can have very diverse circulating concentrations, which illustrates the importance of plasma IMAT concentrations for patients' clinical outcomes (Larson et al., 2008). It is therefore essential to measure the concentration of IMAT in plasma in order to control CML disease (Ajimura et al., 2011, Rahimi Kakavandi et al., 2021). Several instrumental methods, such as high-performance liquid chromatography-ultraviolet detection (HPLC/UV) have been reported in the literature. (Hasin et al., 2017), gas chromatography (Karim et al., 2012), HPLC/mass spectrometry detection (Andriamanana et al., 2013), spectrophotometric methods (Karim et al., 2014), capillary electrophoresis (Rodríguez-Flores et al., 2005) and electrochemical methods (Rodriguez et al., 2005, Hatamluyi and Es' haghi 2017, Rodríguez et al., 2018, Ghapanvari et al., 2020, Rezvani Jalal et al., 2020, Wu et al., 2021, Gondo 2022) have been used to measure IMAT drug in blood plasma and other biological samples. Although some of these techniques possess a high degree of sensitivity and selectivity, there are some limitations, including long analysis times, expensive tools, well-equipped laboratories, problematic sample preparation steps, difficult operations, and the need for skilled and trained personnel. Due to some advantages, electrochemical diagnostic methods have been considered more frequently than the methods mentioned due to the fact that they offer a high degree of sensitivity and selectivity, do not require sophisticated instruments, require a shorter analysis time, and are easier to apply (Yousefi et al., 2011, Teymourinia et al., 2020, Amiri and Mahmoudi-Moghaddam 2021, Ghalkhani et al., 2022, Sohouli et al., 2022, Sohouli et al., 2022, Bobinov et al., 2021).
In the manufacture of electrochemical sensors, the selection of appropriate nanomaterials with high selectivity, sensitivity, and stability is the primary challenge (Farvardin et al., 2020, Iranmanesh et al., 2020, Zhang et al., 2020, Moarefdoust et al., 2021, Abbasi et al., 2023, Foroughi and Jahani 2022, Jahani et al., 2022, Ghasemi et al., 2023, Taherizadeh et al., 2023). Sensors for detecting diverse analytes in diverse biological and environmental fluids can be fabricated using a variety of materials, including polymers, carbon nanomaterials, and metal oxide nanoparticles (Halali et al., 2020, Tajik et al., 2021, Terán-Alcocer et al., 2021, Abdulhusain et al., 2022, Mahde et al., 2022, Al-Alwany, 2022). Graphitic carbon nitride (g-C3N4) has recently gained more attention than other nanomaterials due to its properties such as ease of fabrication, low cost, high thermal, chemical, and mechanical stability, as well as its unique structure. A graphitic structure of g-C3N4 containing nitrogen atoms and SP2 hybridization provides more surface-active sites for adsorption of compounds (Wang et al., 2020). As a result of the graphitic layer structure of this material, a series of weak interactions between the carbon and nitrogen layers can be achieved to form very thin structural plates for use in electrochemical sensors. The thin and high-area layers enable rapid transfer of the carrier from the inside to the surface. Although g-C3N4 nanostructures have unique characteristics, they are not suitable for electrochemical analysis due to their low electrical conductivity (Hassannezhad et al., 2019). Recent research has focused on the study of ABO3-type oxide perovskites (where the A-site cation is usually occupied by an alkaline-earth or rare-earth metal, while the B-site cation is typically occupied by a transition metal cation) because they possess certain properties. The latter, such as high structural porosity, simple synthesis, thermal stability, high surface area, the ability to change the structure and form hybrids with other structures, high ionic and electronic conductivity, short electron transfer pathway, enhanced catalytic activity, special electrical properties, and significant optical potentials (Warshi et al., 2018, Salavati-Niasari et al., 2004, Teymourinia et al., 2020, Salavati-Niasari et al., 2009). For the analysis of environmental pollutants by different electrochemical methods, the use of ABO3 type perovskite-based electrode modifiers has resulted in good analytical results, including a wide linear range, good detection limits, high selectivity, accuracy and reproducibility (Warshi et al., 2018). Many studies have been conducted on the use of perovskite materials such as LaFeO3, LaCoO3, and NdFeO3 to modify electrodes for the measurement of pharmaceuticals and biological compounds such as dopamine, uric acid, ascorbic acid, catechol, and flutamide (Atta et al., 2019). Rare earth orthoferrites like RFeO3 (R ion states rare-earth ions) have recently been considered because of their innovative magnetic and electrical properties, such as controllable magnetic properties by altering the electric field and practical temperature, spin orientation capability, and photocatalytic and magneto-optical effects (Vijayaraghavan et al., 2017, Anajafi et al., 2019, Salavati-Niasari and Amiri, 2005). Research has shown that the replacement of transition elements with Fe, a metal oxide with adjustable spin and oxidation states, plays an important role in the chemical, magnetic, and electrical properties of RFeO3-based metal oxides (Zhang et al., 2017). The synthesis of nanoparticles can be classified into three categories, namely chemical, physical, and biological. By far the simplest, non-toxic, and most economical method of synthesizing nanoparticles is biological synthesis. A significant role is played by surface coatings and reducing agents during the synthesis of nanoparticles. In the physical and chemical methods of synthesis of nanoparticles, toxic and dangerous substances are used, which cause defects in nature. The cost of surface covering agents and reducing agents is high, and therefore it is not economically feasible to synthesize these materials. Accordingly, the green method is the most effective method for preparing nanoparticles. The most famous species of the genus Diospyros is Diospyros kaki. L (Oriental persimmon). D. For more than 2000 years, kaki has been cultivated in China and consumed as a food. One of the main macronutrients found in Diospyros kaki. L fruit is carbohydrates. In terms of energy, this fruit has nearly 76.60 kcal per 100 g−1, of which 75.33 kcal come from. The majority of carbohydrates in persimmons are monosaccharides and disaccharides, including glucose, fructose, and sucrose, which have a mean of 7.41, 5.96, and 1.06 g per 100 g of fresh fruitage, respectively (Matheus et al., 2022). For the first time, we synthesized TbFeO3 nanoparticles using the fruit extract Diospyros kaki. L as a natural fuel. Dual composites containing carbon-based nanomaterials (g-C3N4) and RFeO3-based novel metal oxide perovskites can overcome the low conductivity of g-C3N4 through synergistic effects. In our knowledge, there have been very few studies conducted on electrochemical analysis of the IMAT drug. The present study is the first to use a composite consisting of g-C3N4 and TbFeO3 to improve the surface of glassy carbon electrodes (GCEs). A layered or two-dimensional structure of g-C3N4 is one of the most stable forms under ambient conditions. The graphitic planes are interconnected with amino groups called tri-s-triazine units. Using these units, carbon nitride polymers can be synthesized that exhibit a variety of electronic properties. A major step in the fabrication of electrochemical sensors involves the immobilization of nanocomposite materials of carbon and perovskites on the electrode surface. Different substrate materials can be loaded to produce new electrodes with recognition capabilities. Therefore, in this study, we have developed a modified GCE based on TbFeO3/g-C3N4 nanocomposite for the determination of IMAT drugs. The electrode has been modified to demonstrate remarkable electrocatalytic activity for the detection of IMAT in biological samples with reasonable analytical results.
2 Experimental
2.1 Materials and methods
Merck provided the chemicals used in the preparation of samples, including terbium (III) nitrate pentahydrate, iron (III) nitrate nonahydrate, and melamine. We used the material as received without further purification. Crystallinity, sample composition, and phase determination were determined using Fourier transform infrared spectroscopy (FT-IR; Nicolet M. agna-550 spectrophotometer) and X-ray diffraction (XRD; Philips). Irradiation of copper with nickel (λ = 1.54 Å); scan range 10 < 2θ < 80). FE-SEM (Field Emission Scanning Electron Microscope) (Mira3 Tescan) and TEM (Transmission Electron Microscope) (HT-7700) were used to examine the surface characteristics and morphology of samples. An EDS (Energy Dispersive Spectrometry) analysis was conducted using a Philips XL30 microscope. Moreover, the magnetic properties were measured using a VSM (vibrating sample magnetometer) (Desert Precision Magnetism Company, Kashan Kavir, and Iran). N2 adsorption/desorption analysis (BET) was conducted at 195 °C using an automatic gas adsorption analyzer (Tristar 3000, Micromeritics) to determine the size and type of nanoparticle pores.
2.2 Extract preparation of Diospyros kaki.L
To extract Diospyros kaki L., about 300 g of fresh persimmon fruit were washed well and extracted using acetone: water (80:20 volume/volume). Afterwards, it is homogenized at room temperature on a mechanical stirrer for 30 min. A 10-minute centrifugation was performed on the extracted extract. Afterwards, the supernatant was collected and filtered with Whatman paper, and the extract obtained was dried in the oven and stored in the refrigerator.
2.3 Synthesis of pure TbFeO3
A green sol–gel combustion method was used in this experiment to prepare TbFeO3 nanostructures. As a first step, 1 mmol (0.1 g) of Tb(NO3)3·5H2O and 1 mmol (0.092 g) of Fe(NO3)2·9H2O are dissolved in 15 ml of distilled water. As a fuel, 0.2 g of diospyros kaki. L extract was added to the solution obtained in the previous step. We placed the solution under a temperature of 80 °C for 30 min. Upon reaching 150 °C, the viscous solution evaporated and transformed into ashes. The powder obtained after drying was placed in an oven at 80 °C for one day in order to complete the drying process. Lastly, the obtained product was calcined at 800 °C for four hours. There are carbohydrates present in Diospyros kaki. L fruit (including glucose, fructose, and sucrose) that are used as fuel. A fuel with appropriate ligand strength, metal ion protection by surface active complex, and different combustion temperatures due to carbon bulk suggest different properties of TbFeO3 nanostructures.
2.4 Fabrication of g-C3N4 and TbFeO3/g-C3N4 nanocomposites
In order to prepare g-C3N4, two grams of melamine were heated to 550 °C for four hours, and the obtained yellow powder was collected for further investigation. Afterwards, 0.45 g of g-C3N4 in 50 ml of water was subjected to sonication for 5 min and the mixture was stirred for 2 h in order to prepare the nanocomposite. After stirring for 30 min, 0.45 g of TbFeO3 nanostructure was added to this suspension. After centrifugation and washing with ethanol and water several times, the nanocomposites were dried at 75 °C for one day (Scheme 1).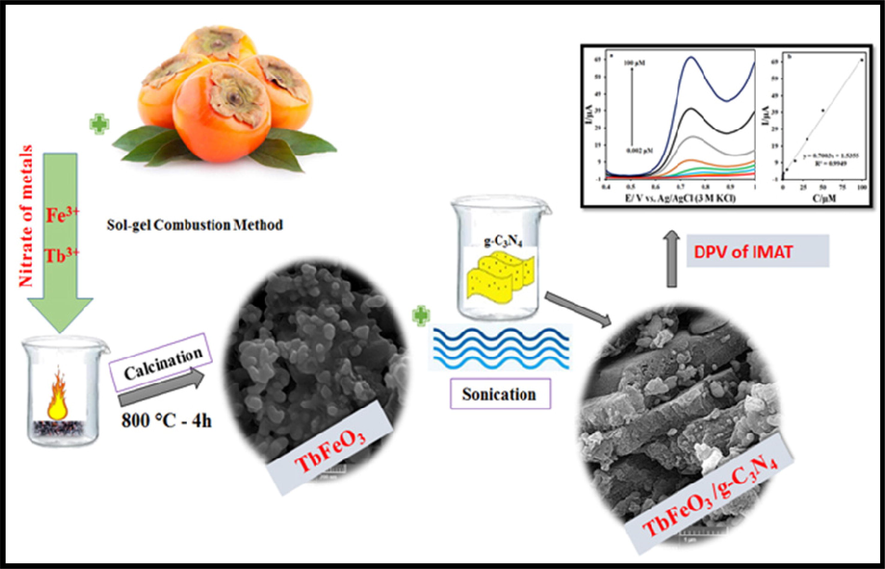
The formation process of the TbFeO3/g-C3N4 nanocomposites by sol–gel auto-combustion method using the diospyros kaki. L extract.
2.5 Preparation of sensor
An electrochemical sensor was initially prepared by cleaning the GCE with alumina powder and washing it with water and acetone. After synthesizing the nanocomposite, it was dispersed in 1 mg of ethanol and 2 µL of it was placed on the electrode surface and dried at 50 °C. This electrode was referred to as TbFeO3/g-C3N4/GCE. The same procedure was followed for the preparation of g-C3N4/GCE.
3 Result and discussions
3.1 X-ray diffraction analysis
Fig.S1 illustrates the XRD patterns of (a) graphite carbon nitride and (b) TbFeO3 nanoparticles prepared with diospyros kaki.L extract at 800 °C for four hours. Based on the XRD pattern of g-C3N4 and the significant diffraction peak at 2θ = 27.2°, this can be attributed to the (0 0 2) facet of Graphite Carbon Nitride. Several diffraction peaks of pure TbFeO3 have been observed at 21.1°, 23.01°, 26.2°, 32.18°, 33.06°, 34°, 40.03°, and 59.4°, which correspond to (1 1 2), (2 0 2), (1 2 3), (2 0 0), (3 0 1), (1 1 5) and (0 0 4) crystal planes of TbFeO3 perovskite nanostructures with reference code (47–0068). Therefore, it confirms that TbFeO3 nanostructures produced by the combustion method have a high degree of crystallinity. A heterojunction TbFeO3/g-C3N4 nanocomposites sample shown in Fig. 1 retained the characteristics of pure TbFeO3 and g-C3N4 in the diffraction peaks. Also, clear peaks in the range of 27.2° were observed, which confirms the presence of g-C3N4. The crystal size was calculated using Scherer's equation as follows: D = Kλ/βcosθ where λ is the wavelength (Cu-K), β is the broadening of the diffraction line calculated with half maximum intensity, θ is the angle Bragg for a certain diffraction, and Dp is the crystallite size. Comparing TFCN nanocomposite (36.30 nm) with pure TbFeO3 (Dp = 34.5 nm), the size of the TFCN nanocomposite was reduced from the sharp peak severity of the (1 1 2) plane. It is reasonable to assume that the TbFeO3 particles accumulated on the surface of the g-C3N4 nanosheets are widely distributed. As a result of the above findings, it is possible to confirm the interaction between TbFeO3 and g-C3N4.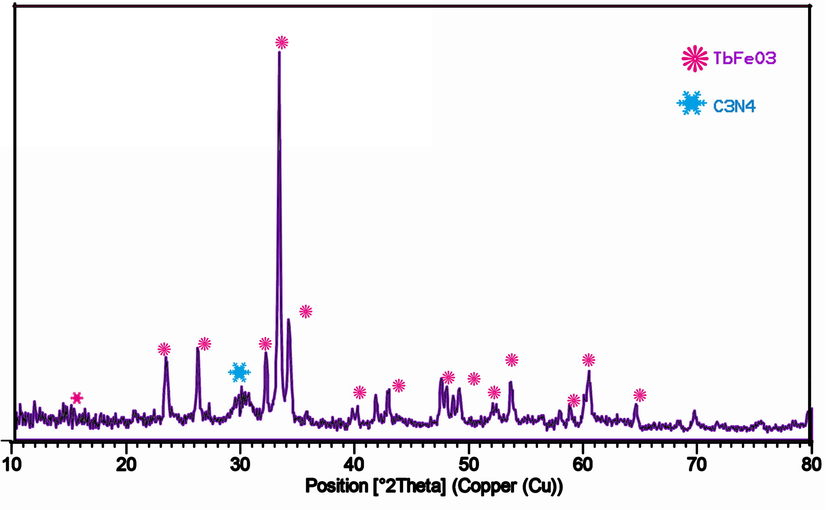
XRD patterns of the TbFeO3/g-C3N4 (1–1) nanocomposites.
3.2 FT-IR analysis
Fig. S2 (a-c) shows the Fourier transform infrared spectra of TbFeO3, g-C3N4 and TbFeO3/g-C3N4 nanocomposites. There are wide peaks at 3443 cm−1 and 1630 cm−1 in the spectra of TFO nanoparticles and TFO/CN nanocomposites. This may be a result of the O–H stretching vibrational mode. There is also a possibility that the peaks at 439 and 565 cm−1 are due to Fe-O stretching vibrations (Yang et al., 2013). The calcination of the TbFeO3 nanoparticles at 800 °C resulted in a weak peak at 1112 cm−1, which could be caused by atmospheric carbon dioxide. It is possible to relate the absorption peak appearing at about 3200 cm−1 in the CN and TFO/CN spectra to the N–H bending and stretching vibrations caused by uncondensed terminal amine groups. Accordingly, direct-heated melamine products still possess amine functionalities. The typical aromatic heterocycle Graphite Carbon Nitride was found in the region between 1200 and 1650 cm−1, where a number of strong peaks were observed. (Wang et al., 2017, Tian et al., 2018). As for CN, the defined peaks at 1242, 1436, and 1566 cm−1 corresponded to the stretching forms of the g-C3N4 framework structure of the conjugated aromatic ring. Graphite Carbon Nitride also exhibits a defined absorption peak at 804.94 cm−1 as a result of the s-triazine breathing vibration (Liu et al., 2018).
3.3 FESEM and TEM analysis
The SEM was used to examine the microscopic surface morphology of g-C3N4, TbFeO3 and TFCN samples. Fig. S3 (a,b) shows SEM images of TbFeO3 nanoparticles treated at 800 °C for 4 h with extract of Diospyros kaki. L as fuel. The results indicate that pure TbFeO3 consists of large numbers of accumulated spherical nanoparticles. In Fig. S4 (a,b), SEM images are shown of well dispersed TbFeO3 particles on graphite carbon nitride. Furthermore, its surface area is relatively larger. It is therefore possible to use the surface as a suitable substrate for nanoparticles.
The prepared nanocomposite was also analyzed using TEM to determine its microstructure and size. Fig. 2. For the TbFeO3/g-C3N4 nanocomposites shown in TEM images in Fig. 2, we can clearly distinguish spherical aggregates of TbFeO3 with an average diameter of about 30 nm, which is very similar to the crystallite size (Dp = 36 nm) calculated through XRD correspondence data.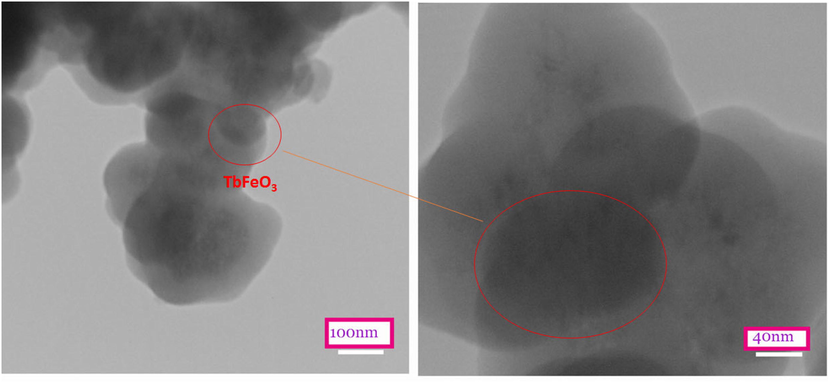
TEM images of TbFeO3/g-C3N4 (1–1) nanocomposites.
3.4 EDX analysis
The Fig. S5(a,b) illustrates the elemental analysis (EDX) of synthesized TFO nanostructures and TFO/CN nanocomposites. The specific peaks of Fe, Tb, N, and O elements in Fig. S5 (b) indicate the presence of g-C3N4 as a substrate for TbFeO3 nanostructures. EDX images confirm the stoichiometric ratio of the ingredients and the balanced dispensation of the ingredients. Furthermore, in TbFeO3/g-C3N4 nanocomposites, the peaks clearly show the presence of C, N, Fe, and Tb elements.
3.5 VSM analysis
VSM analysis (vibrating sample magnetometer) with a topmost applied field of 1000 Oe at ambient temperature was used for studying and evaluating the magnetized properties of pure TbFeO3 nanoparticles and TbFeO3/g-C3N4 nanocomposites. In Fig. S6(a,b), we can observe the ferromagnetic behavior of both samples, with the difference that the maximum saturation magnetization (Ms) of TbFeO3/g-C3N4 nanocomposites (0.53 emu/g) is lower than that of pure TbFeO3 nanoparticles (1.51 emu/g). In contrast, the superior coercive force can maintain a substantial portion of the saturation field when the driving force is removed. In addition, a thin hysteresis loop implies a minimal amount of wasted energy. As a result, the saturated magnetic value of TbFeO3 is approximately 1.51 emu/g. accordingly, the higher MS in these products is primarily attributable to the smaller particle size, as well as the greater cationic difference between the two surfaces. Due to this, we observed lower coercivity values and a special magnetic response that would probably be easily shifted and collected by an external magnetic field.
3.6 BET analysis
A survey was conducted to determine the specific surface areas of the samples and their porous structures. This was done based on nitrogen adsorption–desorption experiments at 77 K. The corresponding curves and data are shown in Fig. S7(a–d). These isotherms were classified as type III by the International Union of Pure and Applied Chemistry (IUPAC). The hysteresis type H3 is associated with mesoporous and microporous materials, both of which were graded. Table.S1 shows the average diameter, total volume, and average pore diameter of nanomaterials.
3.7 Performance of sensors
Using the cyclic voltammetry (CV) method, the GCE, g-C3N4/GCE, and TbFeO3/g-C3N4/GCE were used to investigate the electrochemical behavior of IMAT in phosphate buffer solution with pH = 7. The voltammograms obtained from the IMAT solution are shown in Fig. 3. According to the results, the oxidation current of IMAT at the surface of the unmodified electrode is 2.35 µA, while in the g-C3N4/GCE and TbFeO3/g-C3N4/GCE electrodes it is 7.46 µA and 15.77 µA, respectively. Moreover, the oxidation potential of IMAT on the surfaces of GCE, g-C3N4/GCE, and TbFeO3/C3N4/GCE is 0.82, 0.81, and 0.78 V, respectively, with an oxidation current starting at 0.65 V. Due to the high surface area of the electrode and the effects of carbon nitride and ferrous materials, the oxidative current of TbFeO3/g-C3N4/GCE increases the electrode surface. In addition, these points improve the conductivity of carbon nitride and TbFeO3, which results in a higher oxidation current on the surface of this electrode than on other electrodes. This may be a result of the electrostatic attraction of the analyte to the electrode surface and the existence of a large surface area for the oxidation of IMAT. Using the Randles-Sevcik equation, the surface areas of GCE, g-C3N4/GCE, and TbFeO3/g-C3N4/GCE are 0.028, 0.037, and 0.044 cm2, respectively.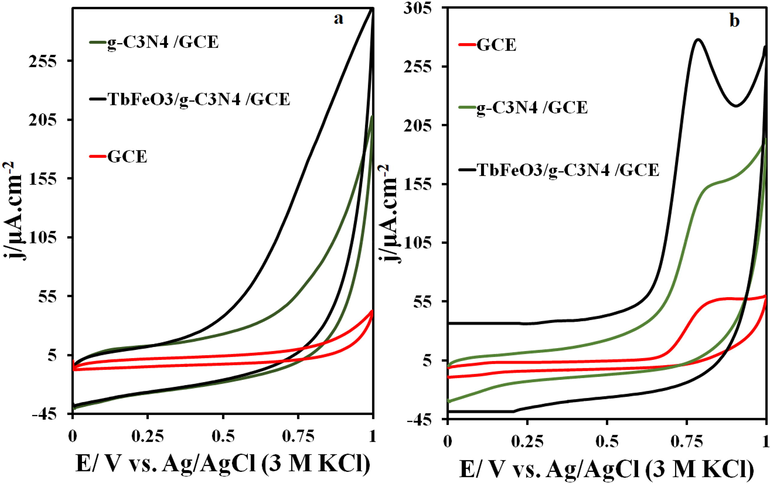
CVs of GCE, g-C3N4/GCE, and TbFeO3/g-C3N4/GCE surface in the presence of 5 μM IMAT and (b) the CVs on the GCE, g-C3N4/GCE, and TbFeO3/g-C3N4/GCE surface in the 0.1 M phosphate buffer solution (pH = 7.0), at the scan rate of 50 mV s−1, T = 25 °C.
3.8 Optimizing modifier value
As part of the preparation of electrochemical sensors, different quantities of nanocomposite A were investigated and IMAT was measured by cyclic voltammetry at pH = 7.0. A maximum oxidation current of IMAT was obtained using 2 μL of the synthesized nanocomposite. The oxidation current of IMAT was equal to and less than the value of 2 μL, when the amount of modifier was more than 2 μL on the surface of the electrode. In this case, the electrode surface is blocked by a higher value of the modifier, preventing IMAT penetration and reducing oxidation current. Therefore, the amount of 2 μL was selected as the optimum content (Table S2).
3.9 pH effect
An electrolyte solution with pH 2.0–9.0 was used to measure IMAT on the surface of TbFeO3/C3N4/GCE.
At different pHs, no oxidation–reduction peaks related to TbFeO3/g-C3N4/GCE are observed in the electrolyte alone (Fig. 4 a). In addition, only one oxidation peak appears in the potential window of 0–1.25 V in the presence of the drug, which is related to the IMAT. CVs in Fig. 4b show the results of IMAT oxidation at pH 2.0 to 9.0 within a potential window of 0.0 to 1.25 V. The results also show that the change in pH of the electrolyte solution causes a shift in the IMAT oxidation potential, which indicates the involvement of the electrons and protons in IMAT electro-oxidation. As shown in Fig. 4c, the maximum oxidation current of IMAT at TbFeO3/C3N4/GCE was obtained at pH = 7.0, which was used as the optimal pH for other tests. The dependence of the oxidation peak potential of IMAT on pH is also shown in Fig. 4d. The slope of the line obtained for this relation was 58 mV/pH, which is close to the slope of the Nernst equation. Accordingly, the number of electrons and protons participating in the oxidation of IMAT is the same.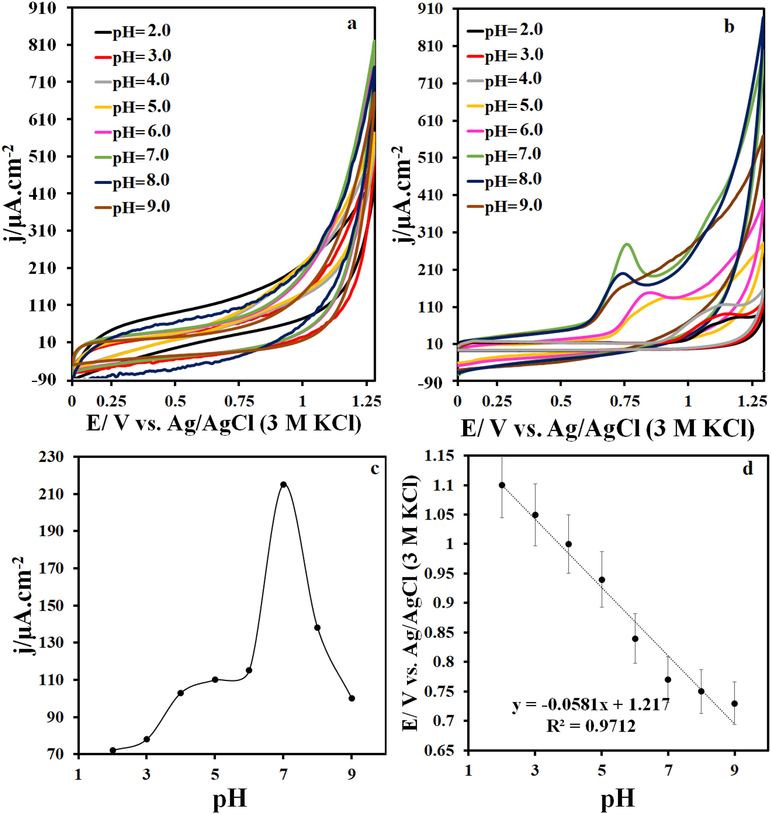
(a) CVs on the TbFeO3/g-C3N4/GCE at various pH, (b) CVs on the TbFeO3/g-C3N4/GCE at various pH in the presence of 5 μM IMAT, (c) plots of the I vs. pH value and (d) Ip vs. pH value at the scan rate of 50 mV s−1.
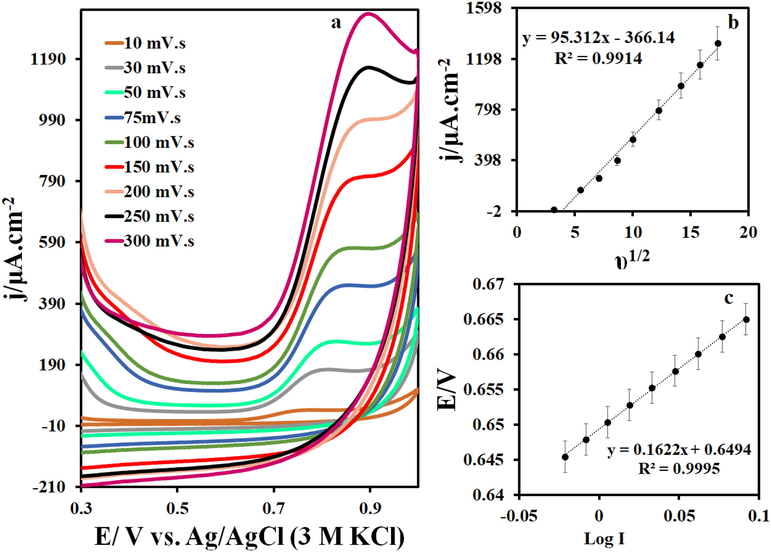
(a) CVs in 0.1 M phosphate buffer solution (pH = 7.0) containing 5 μM IMAT at different scan rates (10–300 mVs−1), (b) the plot of I vs. υ1/2 and (c) E vs. log I.
3.10 Scan rate
On a TbFeO3/C3N4/GCE surface, cyclic voltammetry was used to determine scan rate during IMAT oxidation at pH = 7. The IMAT cyclic voltammograms for scan rates ranging from 10 to 300 mV/s are shown in Fig. 5a. As the scanning rate increases, oxidative current increases as well. IMAT electro-oxidation was found to be a diffusion-controlled process based on the linear increase in peak currents with the square root of the scan rate, as illustrated in Fig. 5b. Also, the potential diagram based on the logarithm of IMAT current at 10 scan rate was used in order to calculate the electron transfer coefficient. According to Equation (Slope = 2.303 RT/ (α) nF) and Fig. 5c, the electron transfer coefficient is approximately 0.61.
3.11 Analytical performance
In this study, different concentrations of IMAT were evaluated using differential pulse voltammetry (DPV) on a surface composed of TbFeO3/g-C3N4/GCE at pH = 7. An example of a differential pulse voltammogram for IMAT is shown in Fig. 6a. The oxidation current of IMAT also increases with an increase in IMAT concentration. Fig. 6b shows the IMAT calibration curve. For this drug, the slope of the line obtained is 0.7 μA/μM, which indicates the appropriate sensitivity of the TbFeO3/g-C3N4/GCE for IMAT measurements. Furthermore, the detection limit and the quantitative limit for IMAT at the TbFeO3/g-C3N4/GCE surface are 0.6 and 2 nM, respectively. All validation parameters were included in the calibration analysis results. There is a standard error of intercept and slope reported in the bracket in the calibration equation below (j = 5.92 (±0.004) C + 14.32 (±0.018)). Three concentrations of calibration range were examined with three replicates for intraday and interday RSD (Table S3).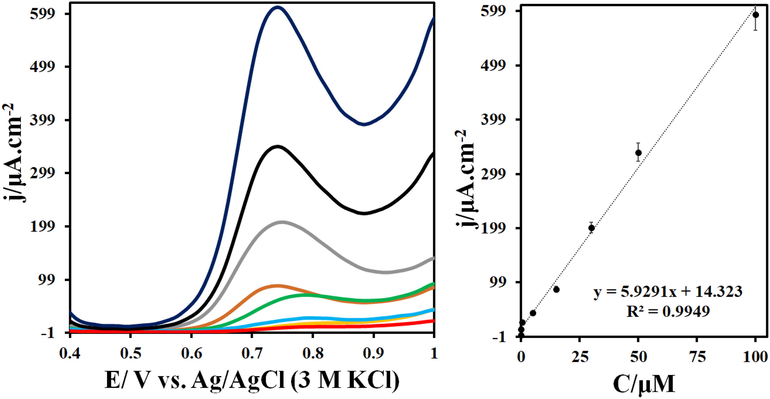
(a) DPVs of TbFeO3/g-C3N4/GCE in the presence of 0.002–100 μM IMAT in 0.1 M phosphate buffer (pH = 7.0). (b)The related linear relationships between the I and IMAT concentrations (calibration curve) in the range of 0.002 to 100 µM. (Conditions: step: 0.005 V, modulation amplitude: 0.025 V, modulation time: 0.05 s, interval time: 0.05 s and scan rate: 0.05 V/s).
The reproducibility of the GCE was determined by modifying four electrodes using TbFeO3/g-C3N4/GCE and then measuring IMAT at pH = 7.0. According to the results, the standard deviation of the oxidation current of IMAT at a potential of 0.78 V is 4.63%, which indicates excellent reproducibility. IMAT measurements were conducted sequentially four times at optimum pH to investigate TbFeO3/g-C3N4/GCE repeatability. The relative standard deviation of applied currents was found to be 3.96 percent. Based on these data, it appears that the TbFeO3/g-C3N4/GCE has an appropriate repeatability. Five measurements were taken during a one-month period at pH = 7.0 to determine the stability of TbFeO3/g-C3N4/GCE for IMAT measurement. In various measurements conducted over a period of one month, the oxidation current changes for IMAT were 8.38%, which indicates that the electrode is stable. We also studied the stability of the TbFeO3/g-C3N4/GCE in 0.1 M KCl and 1 mM K3Fe(CN)6/K4Fe(CN)6 solution for 100 consecutive cycles, and the results are shown in Fig. S9. The results indicated that only 1.25% of the current of oxidation/reduction peaks decreased over 100 consecutive cycles, indicating the stability of the sensor.
In order to examine the selectivity of a drug, it must be measured concurrently with compounds present in the body or compounds that are capable of being administered simultaneously with the desired drug. There are many minerals in the body that contain Na, Ca, and Mg, or nerves that contain receptors such as dopamine (DA) and uric acid (UA); therefore, measuring the desired drug in the presence of inorganic compounds such as NaCl is essential. It has also been checked based on the sources of simultaneous prescription of IMAT and acetaminophen drugs (AC), which demonstrates the importance of simultaneous measurement. The IMAT measurements were conducted on TbFeO3/g-C3N4/GCE surfaces containing various compounds listed in Table 1. In the presence of compounds such as dopamine, uric acid, and acetaminophen with a concentration of 150 mM, the oxidation current change for 5 mM IMAT is equal to 1.8%. Likewise, in the presence of 1 mM sodium chloride, sodium sulfate, iron chloride, copper nitrate, and calcium nitrate, the oxidation current change for IMAT is equal to <1%. Among the oxidation current of IMAT, hydrogen peroxide and caffeine at a concentration of 500 mM caused the greatest change, which is equal to 1.5%. The measurement of IMAT on the surface of the electrode prepared with TbFeO3/g-C3N4 is therefore appropriate (Fig. S10).
Compound
Concertation (μM)
% Changes in IMAT current
Dopamine, Uric acid and Acetaminophen
150
< 1.8 %
Sodium chloride, Sodium sulfate, Iron (II) chloride, Copper nitrate and Calcium nitrate
1000
< 1 %
Hydrogen peroxide and Caffeine
500
< 1.5%
Human blood samples were measured using the standard addition method at pH = 7 on TbFeO3/g-C3N4/GCE surfaces. In Table 2, the concentrations of drugs added to blood samples are presented along with their standard deviations and recovery percentages. According to the results, the optimum recovery percentage in real samples is approximately 96–98, suggesting that the modified electrode is capable of measuring IMAT accurately.
Sample
Added (µM)
Found (µM)
Recovery (%) (n = 3)
RSD%
Plasma
0
–
–
–
0.50
0.48
96.00
5.33
5.00
4.86
97.20
4.83
15.00
14.76
98.40
4.70
Table 3 compares the performance of TbFeO3/g-C3N4/GCE in IMAT measurement with that of other methods. Based on the results of this study, the modified electrode appears to be comparable to other methods in terms of achieving a detection limit and a wide range of concentrations. The modified electrode was prepared by a simple and cost-effective procedure that is more readily available than materials used in other electrochemical methods. As a result, the modified electrode used in this study is desirable in terms of its detection limit and wide range of concentrations.
Sensor
Method
Linear range (µM)
LOD (nM)
Synthesis method
Ref.
Dendrimer/PEG
DPV
0.01–200
7.3
expensive
(Hatamluyi and Es' haghi 2017)
MWCNT/SPCE
SWV
0.005–0.912
7.0
Time-consuming, expensive
(Rodríguez et al., 2018)
MWCNT/NiO-ZnO/GCE
DPV
0.015–2
2.4
expensive, Fairly hard
(Chen et al., 2019)
Cu-BTC/RGO/GCE
DPV
0.04–80
6.0
Time-consuming, expensive
(Rezvani Jalal et al., 2020)
Fe3O4@MWCNTs@PANNFs/CPE
DPV
0.001–0.85
0.4
Time-consuming, expensive and Fairly hard
(Ghapanvari et al., 2020)
RGO/AgNPs/GCE
DPV
0.001–280
1.1
Time-consuming, expensive
(Wu et al., 2021)
Cu-BTC/MWCNT/GCE
DPV
0.01–220
4.1
Fairly hard, expensive
(Pour et al., 2021)
TbFeO3/g-C3N4/GCE
DPV
0.002–100
0.6
Very Simple, affordable
This work
4 Conclusion
In this study, TbFeO3/g-C3N4 was placed on the surface of a GCE to measure IMAT using differential and cyclic pulse voltammetry. By combining TbFeO3/g-C3N4, an excellent nanocomposite was created with a high surface area and good conductivity, which led to satisfactory results in the IMAT tests. Due to the increased electron transfer rate at the surface of the TbFeO3/g-C3N4/GCE electrode at pH = 7.0, the oxidation current of IMAT was higher than the oxidation current of the other electrodes. The excellent results are attributed to the large electrode surface area provided by g-C3N4 and TbFeO3, as well as their excellent catalytic properties. Thus, these electrodes can be used as electrochemical sensors based on their performance. IMAT showed a linear detection limit of 0.002–100 μM equal to 0.6 nM. One of the most important outcomes of the electrochemical sensor prepared for IMAT is its excellent detection limit in comparison to other methods. Besides its special selectivity, repeatability, and stability, this sensor showed beneficial results in measuring IMAT in real samples.
CRediT authorship contribution statement
Mahin Baladi: Investigation, Formal analysis, Methodology, Software, Writing – review & editing, Writing – original draft. Hakimeh Teymourinia: Investigation, Writing – review & editing, Software, Formal analysis, Methodology, Writing – original draft, Software. Elmuez A. Dawi: Software, Writing – review & editing, Visualization. Mahnaz Amiri: Supervision, Software, Data curation, Validation, Resources. Ali Ramazani: Software, Data curation, Validation, Resources. Masoud Salavati-Niasari: Software, Formal analysis, Methodology, Writing – review & editing, Writing – original draft, Conceptualization, Supervision, Project administration, Investigation, Data curation, Validation, Resources, Visualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of the DNA-based voltammetric biosensor for detection of vincristine as anticancer drug. Front. Chem.. 2023;2023(10):1060706.
- [CrossRef] [Google Scholar]
- Anabasis articulata exerts an anti-arthritic effect on adjuvant-induced arthritis in rats. J. Adv. Pharm. Technol. Res.. 2022;13(4):276-280.
- [CrossRef] [Google Scholar]
- Capillary electrophoresis method for plasmatic determination of imatinib mesylate in chronic myeloid leukemia patients. Electrophoresis. 2011;32:1885-1892.
- [Google Scholar]
- Arrhythmia related to hypertensive left ventricular hypertrophy in Iraqi patients: frequency and outcome. J. Med. Life. 2022;15(9):1115.
- [CrossRef] [Google Scholar]
- Green synthesis of ZnO/ZnCo2O4 and its application for electrochemical determination of bisphenol A. Microchem. J.. 2021;160:105663
- [Google Scholar]
- NdFeO3 as a new electrocatalytic material for the electrochemical monitoring of dopamine. Anal. Bioanal. Chem.. 2019;411:7681-7688.
- [Google Scholar]
- Simultaneous analysis of anticancer agents bortezomib, imatinib, nilotinib, dasatinib, erlotinib, lapatinib, sorafenib, sunitinib and vandetanib in human plasma using LC/MS/MS. J. Chromatogr. B. 2013;926:83-91.
- [Google Scholar]
- Electrochemical sensing platform based on nano-perovskite/glycine/carbon composite for amlodipine and ascorbic acid drugs. Electroanalysis. 2019;31:448-460.
- [Google Scholar]
- Historical aspects of microsurgical treatment of brain aneurysms. Hist. Med.. 2021;7(2):179-188.
- [CrossRef] [Google Scholar]
- A facile electrochemical sensor based on NiO-ZnO/MWCNT-COOH modified GCE for simultaneous quantification of imatinib and itraconazole. J. Electrochem. Soc.. 2019;166:B697.
- [Google Scholar]
- Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev.. 2003;55:401-423.
- [Google Scholar]
- Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med.. 2001;344:1038-1042.
- [Google Scholar]
- The synthesis and characterization of 3D mesoporous CeO 2 hollow spheres as a modifier for the simultaneous determination of amlodipine, hydrochlorothiazide and valsartan. Anal. Methods. 2020;12:1767-1778.
- [Google Scholar]
- Investigation of a high-sensitive electrochemical DNA biosensor for determination of Idarubicin and studies of DNA-binding properties. Microchem. J.. 2022;179:107546
- [Google Scholar]
- Preparation of an electrochemical sensor based on a HKUST-1/CoFe2O4/SiO2-modified carbon paste electrode for determination of Azaperone. Microchem. J.. 2022;107199
- [Google Scholar]
- A modified carbon paste electrode based on Fe 3 O 4@ multi-walled carbon nanotubes@ polyacrylonitrile nanofibers for determination of imatinib anticancer drug. J. Appl. Electrochem.. 2020;50:281-294.
- [Google Scholar]
- Simultaneous determination of amitriptyline and venlafaxine using a novel voltammetric sensor of carbon paste electrode modified with octahedral Pd2+-doped Co3O4 composite. Mater. Chem. Phys.. 2023;296:127176
- [Google Scholar]
- Phase I and pharmacokinetic study of imatinib mesylate in patients with advanced malignancies and varying degrees of renal dysfunction: a study by the National Cancer Institute Organ Dysfunction Working Group. J. Clin. Oncol.. 2008;26:570-576.
- [Google Scholar]
- Effect of Isotiocyanate Therapy on trophoblast cell culture hyperglycemia atmosphere in apoptosis, caspase-3, NO, VEGF. Hist. Med.. 2022;8(1):62-67.
- [CrossRef] [Google Scholar]
- Perovskite nanomaterials as optical and electrochemical sensors. Inorg. Chem. Front.. 2020;7:2702-2725.
- [Google Scholar]
- Validation of assay method for the estimation of imatinib mesylate in tablet dosage form by HPLC. Eur. J. Biomed. Pharm. Sci. 2017
- [Google Scholar]
- A graphitic carbon nitride (gC 3 N 4/Fe 3 O 4) nanocomposite: an efficient electrode material for the electrochemical determination of tramadol in human biological fluids. Anal. Methods. 2019;11:2064-2071.
- [Google Scholar]
- A layer-by-layer sensing architecture based on dendrimer and ionic liquid supported reduced graphene oxide for simultaneous hollow-fiber solid phase microextraction and electrochemical determination of anti-cancer drug imatinib in biological samples. J. Electroanal. Chem.. 2017;801:439-449.
- [Google Scholar]
- Treatment for chronic myelogenous leukemia: the long road to imatinib. J. Clin. Invest.. 2007;117:2036-2043.
- [Google Scholar]
- Synthesis of La 2 O 3/MWCNT nanocomposite as the sensing element for electrochemical determination of theophylline. Anal. Methods. 2020;12:4319-4326.
- [Google Scholar]
- Development and characterization of La2O3 nanoparticles@ snowflake-like Cu2S nanostructure composite modified electrode and application for simultaneous detection of catechol, hydroquinone and resorcinol as an electrochemical sensor. Electrochim. Acta. 2022;416:140261
- [Google Scholar]
- Potentiometric determination of imatinib under batch and flow injection analysis conditions. Int. J. Electrochem. Sci.. 2012;7:9668-9681.
- [Google Scholar]
- Spectrophotometric determination of imatinib mesylate using charge transfer complexs in pure form and pharmaceutical formulation. Chem. Rapid Commun.. 2014;2:55-63.
- [Google Scholar]
- Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood, J. Am. Soc. Hematol.. 2008;111:4022-4028.
- [Google Scholar]
- Facile strategy to fabricate Ni2P/g-C3N4 heterojunction with excellent photocatalytic hydrogen evolution activity. Int. J. Hydrogen Energy. 2018;43:21355-21364.
- [Google Scholar]
- Kinetic adsorption and release study of sulfadiazine hydrochloride drug from aqueous solutions on GO/P(AA-AM-MCC) composite. Int. J. Drug Del. Technol.. 2022;12(4):1583-1589.
- [CrossRef] [Google Scholar]
- Persimmon (Diospyros kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products–A review. Food Rev. Intl.. 2022;38:384-401.
- [Google Scholar]
- An electrochemical sensor based on hierarchical nickel oxide nanostructures doped with indium ions for voltammetric simultaneous determination of sunset yellow and tartrazine colorants in soft drink powders. Anal. Methods. 2021;13:2396-2404.
- [Google Scholar]
- Multifunctional magnetic nanoparticles for MRI-guided co-delivery of erlotinib and L-asparaginase to ovarian cancer. J. Microencapsul.. 2022;39:394-408.
- [Google Scholar]
- Mucin-1 conjugated polyamidoamine-based nanoparticles for image-guided delivery of gefitinib to breast cancer. Int. J. Biol. Macromol.. 2021;174:185-197.
- [Google Scholar]
- High sensitive electrochemical sensor for imatinib based on metal-organic frameworks and multiwall carbon nanotubes nanocomposite. Microchem. J.. 2021;165:106147
- [Google Scholar]
- Method development for determination of imatinib and its major metabolite, N-desmethyl imatinib, in biological and environmental samples by SA–SHS–LPME and HPLC. Biomed. Chromatogr.. 2021;35:e5088.
- [Google Scholar]
- In situ growth of metal–organic framework HKUST-1 on graphene oxide nanoribbons with high electrochemical sensing performance in imatinib determination. ACS Appl. Mater. Interfaces. 2020;12:4859-4869.
- [Google Scholar]
- Voltammetric determination of Imatinib (Gleevec) and its main metabolite using square-wave and adsorptive stripping square-wave techniques in urine samples. Talanta. 2005;66:202-209.
- [Google Scholar]
- Electrochemical sensor for leukemia drug imatinib determination in urine by adsorptive striping square wave voltammetry using modified screen-printed electrodes. Electrochim. Acta. 2018;269:668-675.
- [Google Scholar]
- Nonaqueous capillary electrophoresis method for the analysis of gleevec and its main metabolite in human urine. J. Chromatogr. A. 2005;1068:175-182.
- [Google Scholar]
- Synthesis and characterization of alumina-supported Mn(II), Co(II), Ni(II) and Cu(II) complexes of bis (salicylaldiminato) hydrazone as catalysts for oxidation of. Appl. Catal. A. 2005;290(1–2):46-53.
- [CrossRef] [Google Scholar]
- Alumina-supported FeCl3, MnCl2, CoCl2, NiCl2, CuCl2, and ZnCl2 as catalysts for the benzylation of benzene by benzyl chloride. J. Mol. Catal. A Chem.. 2004;209(1–2):209-214.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of ZnO nanocrystals from thermolysis of new precursor. Chem. Eng. J.. 2009;146(3):498-502.
- [CrossRef] [Google Scholar]
- Preparation of a supercapacitor electrode based on carbon nano-onions/manganese dioxide/iron oxide nanocomposite. J. Storage Mater.. 2022;52:104987
- [Google Scholar]
- Manganese dioxide/cobalt tungstate/nitrogen-doped carbon nano-onions nanocomposite as new supercapacitor electrode. Ceram. Int.. 2022;48:295-303.
- [Google Scholar]
- Synthesis of a dual-functional terbium doped copper oxide nanoflowers for high-efficiently electrochemical sensing of ofloxacin, pefloxacin and gatifloxacin. Talanta. 2023;255:124216
- [Google Scholar]
- Nanomaterials modified electrodes for electrochemical detection of Sudan I in food. J. Food Meas. Charact.. 2021;15:3837-3852.
- [Google Scholar]
- Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials. 2021;11:252.
- [Google Scholar]
- GQDs/Sb2S3/TiO2 as a co-sensitized in DSSs: improve the power conversion efficiency of DSSs through increasing light harvesting by using as-synthesized nanocomposite and mirror. Appl. Surf. Sci.. 2020;512:145638
- [Google Scholar]
- Remarkably enhanced H2 evolution activity of oxidized graphitic carbon nitride by an extremely facile K2CO3-activation approach. Appl. Catal. B. 2018;232:322-329.
- [Google Scholar]
- A facile synthesis of LaFeO3-based perovskites and their application towards sensing of neurotransmitters. ChemistrySelect. 2017;2:5570-5577.
- [Google Scholar]
- Earth-abundant Ni2P/g-C3N4 lamellar nanohydrids for enhanced photocatalytic hydrogen evolution and bacterial inactivation under visible light irradiation. Appl. Catal. B. 2017;217:570-580.
- [Google Scholar]
- Mesoporous g-C3N4/β-CD nanocomposites modified glassy carbon electrode for electrochemical determination of 2, 4, 6-trinitrotoluene. Talanta. 2020;208:120410
- [Google Scholar]
- Structural, optical and electronic properties of RFeO3. Ceram. Int.. 2018;44:8344-8349.
- [Google Scholar]
- Detection of imatinib based on electrochemical sensor constructed using biosynthesized graphene-silver nanocomposite. Front. Chem.. 2021;9:670074
- [Google Scholar]
- Preparation, characterization and photocatalytic properties of terbium orthoferrite nanopowder. Adv. Powder Technol.. 2013;24:242-245.
- [Google Scholar]
- Polymeric nanocomposite materials: preparation and characterization of star-shaped PbS nanocrystals and their influence on the thermal stability of acrylonitrile–butadiene. Polyhedron. 2011;30(6):1055-1060.
- [CrossRef] [Google Scholar]
- Electrochemical sensor based on an electrode modified with porous graphitic carbon nitride nanosheets (C3N4) embedded in graphene oxide for simultaneous determination of ascorbic acid, dopamine and uric acid. Microchim. Acta. 2020;187:1-10.
- [Google Scholar]
- Fast response, highly sensitive and selective mixed-potential H2 sensor based on (La, Sr)(Cr, Fe) O3-δ perovskite sensing electrode. ACS Appl. Mater. Interfaces. 2017;9:17218-17225.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104963.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







