Translate this page into:
Metabolomic profiling and assessment of antimicrobial, antioxidant and genotoxic potential of Unonopsis guatterioides R.E.Fr. (Annonaceae) fruits
⁎Corresponding authors. erica.luiz@ufms.br (Érica Luiz dos Santos), nidia.yoshida@ufms.br (Nídia Cristiane Yoshida)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Unonopsis guatterioides (A.DC.) R.E.Fr., is found mainly in the Pantanal, Cerrado and Amazon biomes and, some species of this genus are used in folk medicine. The analysis by HPLC-ESI-MS revealed the presence of alkaloids previously reported for Unonopsis genus, such as asimilobine, anonaine, nornuciferine, glaucine and norglaucine. In contrast, the heliamine, norjuziphine and anomuricine alkaloids are being reported for the first time in the Unonopsis genus, while this is the first report of azafluoranthene alkaloid triclisine in the Annonaceae family. These results showed the promising application of mass spectrometric monitoring of complex extracts in the search for novel natural products for the food, pharmaceutical, and cosmetic industries, thus simplifying phytochemical analysis. Bioactive analysis based on antioxidant activity indicated that ethanolic extracts of the peels and pulps of the fruits from U. guatterioides showed low scavenging activity against the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), with IC50 values > 100 μg*mL−1, using as reference the ascorbic acid (IC50 < 50 μg*mL−1). These results are in concordance with the chemical profiles, whose major compounds proved to be O-substituted phenolic derivatives. Furthermore, ethanolic extracts of the peels (UGP-1) and pulps (UGP-2) of the fruits from U. guatterioides showed weak activity against Staphylococcus pseudintermedius, S. aureus and S. epidermidis, with MIC values above 1000 μg*mL−1. The combination of the ethanolic extract of the pulps of fruits from U. guatterioides and ampicillin resulted in an additive effect (FICI = 1.0), when tested against S. aureus and a strain of S. epidermidis. These results suggest that the pulps' ethanolic extract, when combined with the antibiotic ampicillin, can strengthen the therapy for S. aureus and S. epidermidis infection. Additionally, no genotoxic activity of the ethanolic extracts of fruits from U. guatterioides (UGP-1 and UGP-2) was detected at the tested concentrations (0.25, 1.25, 2.5 and 5.0 mg*mL−1). The genotoxic property was performed using the Somatic Mutation and Recombination assay (SMART Test), in vivo, in somatic cells of Drosophila melanogaster.
Keywords
Unonopsis guatterioides
Cerrado
HPLC ESI-MS
Antioxidant
Genotoxic and antimicrobial
1 Introduction
Tropical fruits constitute an important innovation domain for the food, pharmaceutical and cosmetic industries, due to their bioactive properties and market potential (Neri-Numa et al., 2018). Pantanal and Cerrado biomes present a great diversity of species of small native fruits, adapted to the tropical climate and resistant to several pests in the region (Alho et al., 2019; Neri-Numa et al., 2018). However, most of these fruits have not yet been inserted in the context of Brazilian agribusiness, either due to the absence of technology for scale production or even due to the scarcity of studies regarding their phytochemical, toxicological and nutritional aspects, generating a lack of knowledge of their potential use and/or application (Vieira et al., 2006).
Unonopsis guatterioides (A. DC.) R. E. Fries. (syn. U. lindmanii - Anonnaceae), popularly known as ‘pindaíva-preta’ or ‘envira-preta’, is a medium size fruit tree distributed in countries of South America (Silva et al., 2012a; Yoshida et al., 2013). In Brazil, this plant has been found mainly in the Pantanal, Cerrado and Amazon biomes (Kuhlmann, 2018; Silva et al., 2016), produces small fruits c. 1–2 cm diameter and, in the literature, there are no phytochemical studies and/or biological properties described for these fruits. Considering the current upsurge of interest in the measurement of efficacy and use of natural products for applications in food technology, cosmetic industry, therapeutic, nutraceutical and medical uses, investigations focused on the evaluation of biological and chemical profile of native fruits could expand the knowledge on their potential application in products and offer recommendations for development areas (Vieira et al., 2006). In this context, recent advances in technology have brought a revolution in the way in which secondary metabolites are viewed and consulted, since techniques such as nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC–MS), high-performance liquid chromatography combined with mass spectrometry (HPLC-MS), allowed the evaluation of large amounts of chemical information obtained from each species of plant analyzed (Alcantara et al., 2007; Moco et al., 2006; Verpoorte et al., 2007; Benamar et al., 2021; Frezza et al., 2022). For example, the method of electrospray ionization mass spectrometry (ESI-MS) has been used to screen the Unonopsis alkaloids (Silva et al., 2014), and this method, along with multivariate analysis [principal components analysis (PCA) and hierarchical cluster analysis (HCA)], has enabled the chemotaxonomic study of the Unonopsis species that occur in the Brazilian Amazon (Silva et al., 2016). These tools also allow the planning of phytochemical studies according to a specific interest (Silva et al., 2016).
Furthermore, although the medicinal properties of some plants are well known, some of them may contain toxic chemicals that can cause insidious genotoxicity and may lead to harmful effects on DNA, increasing the likelihood of mutational somatic events that lead to neoplasias. The Somatic Mutation and Recombination Test (SMART) uses Drosophila melanogaster as a test organism to detect a vast range of genetic abnormalities, such as mutations, deletions and somatic recombination caused by natural and synthetic compounds (Olimpio et al., 2021; Costa et al., 2010). Drosophila has extensive genetic homology to mammals, i.e., has 60% of orthologous genes to mammals, which makes it a suitable model organism for genotoxic investigations (Staats et al., 2018; Costa et al., 2010). Thus, in vivo tests using D. melanogaster have been vastly explored to reduce the usage of higher animals in toxicological studies (Senes-Lopes et al., 2018).
Phytochemicals, or plant secondary metabolites, are frequently investigated as antimicrobials, because they can act alone or in synergy with antibiotics, as agents that modify resistance for use against multi-drug bacteria (Duong et al., 2021). The Brazilian native fruits Euterpe oleraceae (Arecaceae) proved to be potentially active against Staphylococcus aureus, with Minimal Inhibitory Concentration (MIC) value of 7.81 µg*mL−1 and biofilm eradication concentration of 250 µg*mL−1. The methanolic extract of the fruits also showed a synergic effect with commercial antimicrobials gentamicin, chloramphenicol and ciprofloxacin against a panel of S. aureus strains (Dias-Souza et al., 2018). Hidromethanolic extracts of Terminalia hadleyana (Combretaceae) fruits showed activity against bacteria Shewanella putrefaciens, S. aureus and methicillin resistant S. aureus (MRSA) (Zhang et al., 2022). On the other hand, antioxidants are a theme of great interest to the cosmetic industry due to their tissue regenerating role and antiaging properties (Shafi et al., 2019; Masaki et al., 2010). Tropical fruits such as guava, star fruit, and papaya are widely known for their role in the human diet, and hydroalcoholic extracts of these fruits showed high antioxidant potential in 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (Krings & Berger, 2001), reinforcing the potential of the native fruits as sources of antioxidants.
Therefore, this study aimed to investigate the metabolomic profile of the ethanolic extracts from the fruits of U. guatterioides, and to evaluate the biological properties of the extracts for possible future applications. To assay the in vivo genotoxic effects, the extracts were evaluated by somatic mutation and recombination test (SMART) in the somatic cells of D. melanogaster wings, the in vitro antimicrobial activity against clinical drug-resistant S. aureus, S. pseudintermedius and S. epidermidis was evaluated by checkerboard method, and the antioxidant potential was assessed by DPPH assay.
2 Experimental
2.1 Materials
The reagents such as 2,2-diphenyl-1-picrylhidrazyl (DPPH), ascorbic acid, and formic acid were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Other reagents used, such as ethanol and methanol, were provided by Tedia Company (California, USA). All reagents were used analytical grade. Moreover, the antibacterial culture mediums and antibiotics were purchased from Sigma AldrichTM. The clinical bacterial strains used were: two human pathogens, namely Staphylococcus aureus (resistant to clindamycin, erythromycin and penicillin G) and Staphylococcus epidermidis (A: resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, linezolid, oxacillin and trimethoprim/sulfamethoxazole and B: resistant to ciprofloxacin, erythromycin, gentamicin and oxacillin), and one canine pathogen, i.e, Staphylococcus pseudintermedius (resistant to amoxicillin + clavulanic acid, gentamicin, neomycin, azithromycin, cephalexin, cephalothin, streptomycin, marbofloxacin). The veterinary strain was supplied by the Faculty of Veterinary Medicine and Animal Science of Universidade Federal de Mato Grosso do Sul, and the human clinical strains were provided by the Center of Clinical Analysis of the University Hospital, Universidade Federal de Mato Grosso do Sul (Campo Grande, Brazil).
2.2 Samples
Ripe fruits of the Unonopsis guatterioides (A.DC.) R.E.Fr (Annonaceae) were harvested in July 2021 at Jardim Aeroporto, located in Campo Grande, MS, Brazil (20°27′11.3″S 54°40′16.0″W), and identified by the botanist Dr. Flavio Macedo Alves (UFMS). After cleaning and sanitization, the fruits were kept at a temperature of 5° C until used, by a period of 24 h. The U. guatterioides samples (aerial parts) were deposited in the Herbarium of the Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande Campus, under the code 16548.
2.3 Extract preparation
The extraction procedure was carried out in accordance with Engelbrecht et al., 2021, with some modifications. The fruits were subdivided, using stainless steel knives, into three parts: peel, pulp and seeds. Separately, the pulps and peels (50 g, each) were homogenized and extracted, at room temperature, with ethanol (5 days) with the removal of the solvent at each 24 h. Subsequently, the extracts were filtered through a cotton membrane and concentrated under vacuum at 40 °C, yielding the ethanolic extract of the peels (UGP-1), 63.0% (w/w) and pulps (UGP-2), 64.2% (w/w), of the fruits from U. guatterioides. The concentrated extracts were kept at −18 °C in a light-protected environment.
2.4 HPLC ESI-MS analysis
The spectrometric analysis of the chemical composition of ethanol extracts were carried out by high-performance liquid chromatography (HPLC; Shimadzu LC-20 AD), coupled to a micrOTOF-Q III mass spectrometer (Bruker Daltonics) with electrospray ionisation source (HPLC-ESI-MS). Separately, each ethanolic extract (10 mg) was diluted in 10 mL of MeOH-H2O (1:1) and filtered through PVDF membranes with a 0.22 μm thickness (Allcrom, Brazil). Then, 4 μL of solution was injected into a C-18 column [150 mm × 2.0 mm, 5 µm, Phenomenex™ Luna PFP (2)] and diode array detector (DAD), coupled to an electrospray ionization mass spectrometer. The gradient system with a mobile phase consisting of water and methanol (both containing 0.1% formic acid), ranging from 3 to 80% (v/v), totalling 45 min of analysis, with a flow of 0.2 mL*min−1. Mass spectra in positive ion mode, in the m/z 120–1200 region, were obtained by high resolution mass spectrometry (HR-MS). The chemical profile's constituents were annotated, and molecular formula were proposed (taking only error values 5 ppm into account) through data analysis using the Compass DataAnalysis 4.2 - Bruker® software and a database built using the Scifinder™ data platform. Retention times (RT) of the compounds in the chromatographic column, [M + H]+ ions and their primary molecular ions, UV absorption spectra, m/z values, fragmentation patterns of authentic standards, and data from the literature were all examined for the purpose of data comparison.
2.5 Antioxidant activity by DPPH (2,2-diphenyl-1-picrylhydrazyl) assay
The ability of the fruits ethanolic extract to scavenge fee radicals was assessed using DPPH method, according to previous reports (Wu et al., 2005; Yao et al. 2012; Silva et al., 2017). Briefly, 30 μL of different extract concentrations (10.0–100 μg*mL−1) was mixed with -270μL of 0.1 mM DPPH solution. Both solutions were prepared in methanol. For 1.0 h, the samples were kept at room temperature in the dark. The absorbance was then determined at 517 nm with an ELISA microplate reader. Ascorbic acid was employed as a positive control at doses of 0.75-100 μg*mL−1 in methanol. As a negative control, a mixture of 270 μL of 0.1mμM DPPH methanolic solution and 30 μL of methanol was used. The tests were performed in triplicate (n = 3). The percentage of inhibition (A%) was computed to assess the radical scavenging activity using the following equation: ([A0-Aa]/A0) × 100. A0 is the absorbance of the negative control sample and Aa is the absorbance of the examined sample. By using linear regression, the IC50 was calculated, which is the antioxidant concentration that results in a 50% reduction in DPPH absorbance.
2.6 Antimicrobial assays
The antimicrobial activities of individual ethanolic extracts of the peels (UGP-1) and pulps (UGP-2) of U. guatterioides fruits, and combinations between extract UGP-1 and ampicillin (UGP-1 + AMP), extract UGP-2 and ampicillin (UGP-2 + AMP), were assayed against Staphylococcus aureus, S. pseudintermedius and S. epidermidis, following the methods described by Jesus et al., 2020.
2.7 Determination of Minimal Inhibitory concentration (MIC)
Antimicrobial activities of the extracts UGP-1, UGP-2 and ampicillin were determined by broth microdilution method (Jesus et al., 2020). To achieve a final concentration of 0.78-100 μg*mL−1 for ampicillin and 62.6 μg*mL−1-8000 μg*mL−1 for the extracts, with a final volume of (100 μL) in each well, two-fold dilutions were carried out on 96-well plates prepared with Mueller-Hinton broth (Sigma-Aldrich). The bacterial inoculum was prepared from overnight cultures of each bacterial species in Mueller-Hinton agar (Sigma-Aldrich) diluted in saline solution (0.45%) to a concentration of roughly 108CFU.mL CFU*mL-1 (0.5 in McFarland scale). Subsequently, each solution was diluted 1/10 in saline solution (0.45%) and 5 µL (104CFU.mL CFU*mL-1) were added to each well containing the test samples. All experiments were performed in triplicate and the microdilution trays were incubated at 36 °C for 18 h. After this period, 20 μL of an aqueous solution (0.5%) of triphenyl tetrazolium chloride (TTC) were added to each well and the trays were incubated for 2 h at 36 °C. In addition, in those wells where bacterial growth did occur, TTC turned from colourless to red. The MIC, which was expressed in µg*mL−1, was determined as the lowest concentration of each extract or compound at which no colour change occurred.
2.8 Synergy testing
Synergism between ampicillin and ethanolic extract of the peels (UGP-1 + AMP), ampicillin and ethanolic extract of the pulps (UGP-2 + AMP), was tested against S. aureus, S. pseudintermedius and S. epidermidis, using a standard checkerboard microtiter method (Jesus et al., 2020). In 96-well plates prepared with Mueller-Hinton broth, the extracts UGP-1 and UGP-2 were submitted to serial two-fold dilutions to achieve concentrations of 62.6 μg*mL−1 to 8000 μg*mL−1, with a final volume of 50 μL in each well. Then, each well received 50 μL of antibiotic solutions in Mueller-Hinton broth, resulting in concentrations which varied horizontally, from 100 to 0.05 μg*mL−1. The final concentration values for extracts UGP-1 and UGP-2 ranged from 31.2 μg*mL−1 to 4000 μg*mL−1. The bacterial inoculums were made as previously mentioned, and 5 μL were added to each well containing the test samples. Subsequently, the plates were incubated for 18 h at 36 °C. After TTC was added, the MIC of the combinations was accessed, and the following formulas were used to calculate the fractional inhibitory concentration (FIC) and fractional inhibitory concentration index (FICI):
The FICI values were interpreted as: synergic effect, when FICI ≤ 0.5; additivity effect, when 0.5 < FICI ≤ 1; indifferent effect as 1 < FICI ≤ 4and antagonist effect as FICI > 4 (Basri et al., 2014; Ahumada-Santos et al., 2016; Solarte et al., 2017).
2.9 Somatic mutation and recombination test (SMART Test)
This test was developed according to the methodology described by Guterres et al., 2014 and Olimpio et al., 2021. The Somatic Mutation and Recombination Test (SMART Test) was carried out through experimental crossings between the strains mwh (multiple wing hairs) and flr3 (flare 3) of Drosophila melanogaster. Two different crosses were performed with these strains: standard cross (ST - standard cross) between “mwh” males and virgin flr3 females and high bioactivation cross (HB - high bioactivation cross) between “mwh” males and virgin females “flr3”. After 8 h, eggs from each crossover were collected in culture flasks with an agar-agar basis (0.04 g*mL−1), biological yeast, and supplemented with sugar. After 72 h, the larvae that hatched were rinsed with tap water and collected using a sieve. For chronic feeding, the groups of larvae from each crossing were transferred to identified vials, containing an alternative culture medium, consisting of 1.5 g of industrialized mashed potato flakes (Yoki® Alimentos S.A., Brazil) and 5 mL of solution containing a final concentration of 0.625, 1.25, and 2.5 mg*mL−1 of extracts UGP-1 and UGP-2, respectively, from fruits of U. guatterioides. This solution was prepared with distilled water and Tween-80®. A solution containing 3% ethanol, 1% Tween-80™ and distilled water was used as negative control while, doxorubicin (DXR) at 0.125 mg*mL−1 was used as positive control. The assays were conducted in triplicate. Each cross produced 2 types of progenies, marker-heterozygous (MH) (mwh+/+flr3) and balancer-heterozygous (BH) (mwh+/+TM3, BdS) flies. These 2 genotypes' wings can be separated thanks to the dominant BdS marker. The hatched flies were collected and stored in 70% ethanol (v/v). To identify the types of mutations, the wings were removed, mounted on slides with Faure’s solution (30 g of Arabic gum, 50 g of chloral hydrate, 20 mL of glycerol and 50 mL of water) and analysed by light microscopy, with 400x magnification. The wings displayed the heterozygous marker mwh/flr3 making possible to see different types of stains on the wings. The frequency and size of spots were recorded.
2.10 Statistical analysis
For the statistical analysis of the SMART Test, i.e., to evaluate the frequencies of spots per wing, the Frei and Wrügler et al., 1988 method was used. The induced effects were distinguished by the type and size of mutant stains and analysed by a bi-caudal chi-square test for the proportions, with a significance level of α = β 0.05, where the statistical diagnosis was positive (+), negative (-) or inconclusive. The recombinogenic activity was calculated, based on the clone induction frequencies per 105 cells, as following: mutation frequencies (FM) = frequency of BH clone flies/frequency of MH clone flies; recombination frequencies (FR) = 1 - FM. Frequencies of total spots (FT) = total spots in MH flies (considering mwh and flr3 spots)/No. of flies; mutation = FT × FM; recombination = FT × FR (Olimpio et al., 2021; Guterres et al., 2014).
3 Results and discussion
Previous phytochemical studies showed that trunk peels, branches, and leaves from U. guatterioides are rich in aporphine and oxoaporphine alkaloids (Guinaudeau et al., 1988; Silva et al., 2012a), while in the bark of the xylopodium were identified triterpenes and steroids (Silva et al., 2012b). Studies investigating leishmanicidal (Silva et al. 2012c), bioherbicidal (Yoshida et al., 2019) and antimicrobial effects (Brighenti et al., 2014) of U. guatterioides have also been described. This work describes the first phytochemical study of the ethanolic extract of the fruits from U. guatterioides.
3.1 Metabolomic analysis of the extracts
The compounds detected in the ethanolic extract of the peels of the fruits from U. guatterioides (UGP-1) and the ethanolic extract of the pulps of fruits from U. guatterioides (UGP-2) were tentatively characterized analysing various types of information such as precise molecular mass, retention time (tr), absorption wavelengths, MS/MS fragment ions generated in positive mode, fragmentation patterns of authentic standards, and comparison with spectral data from the literature. A total of 41 compounds in extract UGP-1 and in extract UGP-2 were tentatively identified as indicated in Fig. 1A, Fig. 1B and Table 1. Moreover, two classes of compounds were characterized as major in the peel and pulp of the fruits from U. guatterioides: alkaloids and flavonoids. In addition, terpene, coumarins and amines were also detected in the ethanolic extract of the pulps (Table 1). The proposals for identication of the compounds are described below.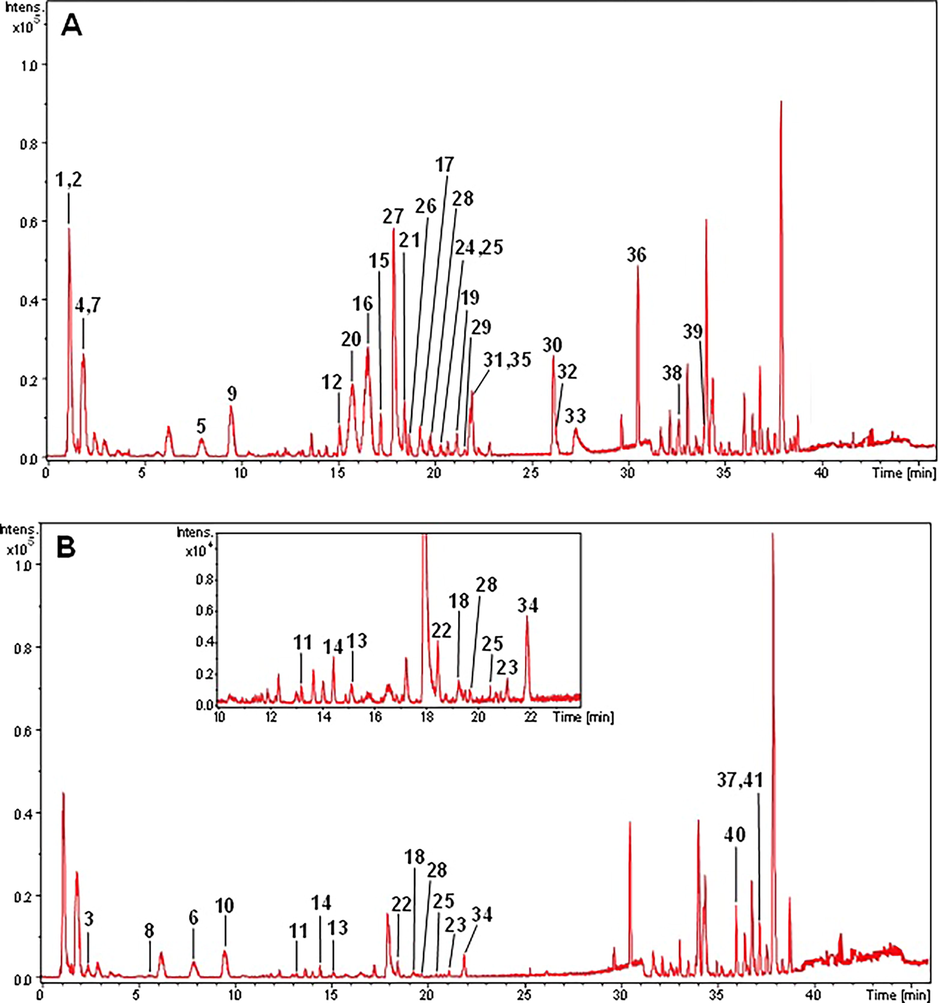
Chromatographic profile obtained by HPLC ESI-MS in positive ion mode. (A) Ethanolic extract of the peels of the fruits from U. guatterioides. (B) Ethanolic extract of the pulp of the fruits from U. guatterioides (See Table 1 for the analyte identification).
Peak
UVλmax
(nm)
Metabolite class/
Tentative assignment
Molecular mass
(m/z)
Molecular formula
MS/MS[a] (m/z) fragments
Reference
Extract
Peels
Pulp
1
281
Alkaloid
399.0881 [M + H]+
C12H19N2O13
219.0272
–
+
–
2
273
Alkaloid
381.0788
[M + H]+
C12H17N2O12
203.0487
–
+
–
3
260
Alkaloid
268.1037
[M + H]+
C10H14N5O4
n.d.
–
–
+
4
254
Alkaloid
268.1030
[M + H]+
C9H18NO8
n.d.
–
+
–
5
n.d.
Alkaloid
317.0845
[M + Na]+
C12H14N4O5
n.d.
–
+
–
6
n.d.
Alkaloid
317.0830
[M + H]+
C8H17N2O11
n.d.
–
–
+
7
n.d.
Disaccharide
325.1129
[M + H]+
C12H21O10
n.d.
–
+
–
8
n.d.
Alkaloid/
Heliamine/
194.1158
[M + H]+
C11H16NO2
n.d.
Pummangura et al., 1982
–
+
9
n.d.
Alkaloid
188.0701
[M + Na]+
C9H11NO2
n.d.
–
+
–
10
n.d.
Alkaloid
188.0714
[M + H]+
C11H10NO2
n.d.
–
–
+
11
n.d.
Coumarin/
Esculetin-O-acetylglucoside
383.0970
[M + H]+
C17H19O10
n.d.
–
–
+
12
n.d
Alkaloid
314.1379
[M + Na]+
C16H21NO4
n.d.
–
+
–
13
n.d.
Amide/
Moupinamide
314.1386
[M + H]+
C18H20NO4
n.d.
Moreira and Leitão, 2001
–
+
14
n.d.
Terpene/
Abscisic acid
265.1452
[M + H]+
C15H21O4
n.d.
Sousa et al., 2022
–
+
15
280
Alkaloid/
Pallidine
328.1533
[M + H]+
C19H22NO4
n.d.Leboeuf et al., 1982
+
–
16
280; 517
Anthocyanin/
Cyanidin-O-rutinoside595.1648
[M+]C27H31O15
287.0549
Ling et al., 2009;
Ivanova et al., 2010+
+
17
n.d.
Alkaloid
342.1694
[M + Na]+
C18H25NO4
n.d.
–
+
–
18
n.d.
Alkaloid/
Norglaucine
342.1726
[M + H]+
C20H24NO4
n.d.
Lúcio et al., 2015
–
+
19
n.d.
Alkaloid/
Glaucine
356.1825
[M + H]+
C21H26NO4
n.d.
Lúcio et al., 2015
+
–
20
280;
517
Anthocyanin/
Cyanidin 3-O-glucoside or Ideain
449.1056
[M+]C21H21O11
305.0641, 287.0536
Ling et al., 2009;
Ivanova et al., 2010;
Barman et al., 2021
+
+
21
n.d.
Alkaloid
330.1684
[M + Na]+
C17H25NO4
192.1031
–
+
–
22
n.d.
Alkaloid/
Anomuricine
330.1703
[M + H]+
C19H24NO4
n.d.
Lúcio et al., 2015
–
+
23
n.d.
Flavonoid/
Quercetin-3-O-β-D-apiofuranosyl-(1 → 2)-galactopyranoside
619.1301
[M + Na]+
C27H24N4O12
n.d.
Silva et al., 2019
+
–
24
n.d.
Alkaloid/
Asimilobineb
268.1328
[M + H]+
C17H18NO2
251.1082; 219.0839; 190.9968
Lúcio et al., 2015
+
–
25
n.d.
Alkaloid/
Norjuziphine
286.1431
[M + H]+
C17H20NO3
n.d.
Lúcio et al., 2015
+
+
26
n.d.
Alkaloid
314.1749
[M + Na]+
C17H25NO3
269.1155; 237.0910; 175.0656
–
+
–
27
n.d.
Alkaloid
342.1699
[M + Na]+
C18H25NO4
297.1109; 282.0879; 265.0856
–
+
–
28
n.d.
Flavonoid /
Quercitrin449.1070
[M + H]+
C21H21O11
303.0505 285.0432
Andriamadio et al., 2015
+
+
29
n.d.
Flavonoid/
Rutin633.1394
[M + Na]+
C27H30O16
465.1048; 303.0493
Novaes et al., 2018
+
–
30
n.d.
Alkaloid/
Anonaineb
266.1177
[M + H]+
C17H16NO2
249.0916; 219.0799; 249.0906; 191.0857
Silva et al., 2012a
+
–
31
n.d.
Alkaloid/
Triclisine
264.1022
[M + H]+
C17H14NO2
n.d.
Guinaudeau et al., 1988
+
–
32
n.d.
Alkaloid/
Nornuciferine282.1501
[M + H]+
C18H20NO2
265.1218
Silva et al., 2012a
+
–
33
n.d.
Alkaloid
276.0645
[M + Na]+
C15H11NO3
n.d.
–
+
–
34
350
Flavonoid/
Quercetinb
303.0493
[M + H]+
C15H11O7
285.0475
Novaes et al., 2018
+
+
35
260; 355
Flavonoid/
Isoquercitrin465.1035
[M + H]+
C21H21O12
303.0499
Novaes et al., 2018
+
–
36
n.d.
Alkaloid
375.1430
[M + Na]+
C19H20N4O3
228.0662; 198.0975; 159.0750
–
+
–
37
n.d.
Unknown
431.2057
[M + H]+
C24H31O7
177.0084
–
–
+
38
n.d.
Alkaloid
389.1560
[M + H]+
C16H25N2O9
n.d.
–
+
–
39
n.d.
Alkaloid
403.1718
[M + H]+
C17H27N2O9
n.d.
–
+
–
40
n.d.
Unknown
417.1881
[M + Na]+
C21H30O7
389.1612
–
–
+
41
n.d.
Alkaloid
385.1631
[M + Na]+
C21H22N4O2
n.d.
–
–
+
3.2 Identification of alkaloids
In the mass spectra of the extract of the peels and pulps, respectively, of fruits from U. guatterioides, diagnostic fragmentations related to aporphine alkaloids were observed. Previous research has demonstrated that the initial losses of [M + H – 17]+ or [M + H – 31]+ are a key fragmentation of aporphine alkaloids, supporting the substitution pattern of the heterocyclic nitrogen (–NH3 or –CH3NH2) (Stévigny et al., 2004; Silva et al., 2014). Furthermore, subsequent losses of 32 and 28 Da corresponding to CH3OH and CO, respectively, are observed if hydroxy and methoxy groups are in adjacent positions on the aromatic ring. Otherwise, fragment ions arising from the loss of CH3 and OCH3 radicals are observed in the spectrum. On the other hand, formaldehyde (CH2O) and CO losses are observed when a methylenedioxy group is present. In summary, these are the main diagnostic fragmentations for aporphine alkaloids identification (Stévigny et al., 2004). Therefore, the peak 24, with [M + H]+ at m/z 268.1328 and molecular formula C17H18NO2, was proposed as aporphine alkaloid asimilobine (Table 1 and Scheme 1). The fragmentation of the molecular ion at m/z 268.1328 indicated the presence of an aporphine alkaloid containing an amino group by the initial loss of NH3 (17 Da). This process resulted in fragment ions at m/z 251.1082 [M + H-17]+ (Scheme 1A). The subsequent losses of CH3OH (32 Da) and CO (28 Da) corresponding to fragment ions at m/z 219.0839 and 190.9968, respectively, confirmed the presence of both hydroxyl and methoxyl groups in vicinal locations on the aromatic ring (Scheme 1A) (Stévigny et al., 2004). These results are consistent with the aporphine alkaloid asimilobine, which in the genus Unonopsis was identified in U. guatterioides (Yoshida et al., 2013, Silva et al., 2012a) and U. duckei (Silva et al., 2014). Additionally, the peak 30, with [M + H]+ at m/z 266.1177 was identified as anonaine (Table 1). In the MS spectrum (Fig. 2A), the fragmentation at m/z 266.1177 produced a fragment ion at m/z 249.0916 [M + H-17]+, which represents a neutral loss of NH3 (17 Da). The MS2 spectrum (Fig. 2B) showed successive losses of CH2O (30 Da) and CO (28 Da) which resulted in fragment ions at m/z 219.0799 from fragment at m/z 249.0906 and fragment ions at m/z 191.0857 from fragment at m/z 219.0799, respectively, confirming the fragmentation pattern of aporphine alkaloids containing a methylenedioxy group (Scheme 1B) (Stévigny et al., 2004). The aporphine alkaloid anonaine has already been isolated from U. guatterioides (Guinaudeau et al., 1988; Silva et al., 2012a, Yoshida et al., 2013). On the other hand, the loss of NH3 (17 Da) was also observed for nornuciferine, peak 32, with a molecular ion [M + H]+ at m/z 282.1501 (Table 1), whose fragment ion at m/z 265.1218 correspond to [M + H - NH3]+. In the genus Unonopsis this compound was previously isolated from U. guatterioides and U. duckei (Silva et al., 2012a; Silva et al., 2014). Glaucine, peak 19, was tentatively identified as an aporphine alkaloid, with [M + H]+ at m/z 356.1825 (Table 1). Thus, the aporphine alkaloids asimilobine, anonaine, nornuciferine and glaucine, were found in the ethanolic extract of the peels of fruits from U. guatterioides (Table 1), while the aporphine alkaloid norglaucine was tentatively identified as the compound detected at m/z 342.1726 [M + H]+ and was observed in the ethanolic extract of the pulps of fruits from U. guatterioides (Table 1). In the genus Unonopsis glaucine and norglaucine were described for the first time in U. duckei (Silva et al., 2014).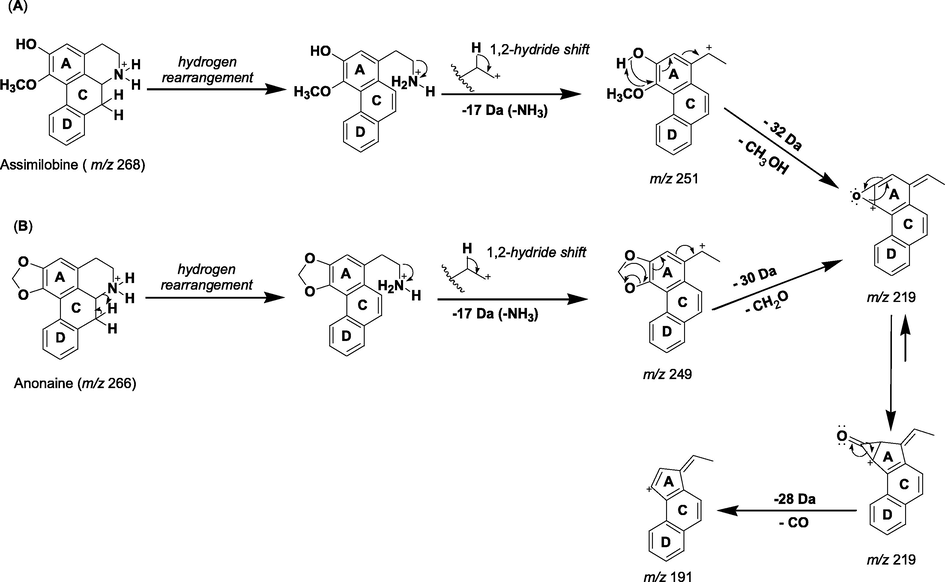
Proposed fragmentation pathway of aporphine alkaloids. (A) Asimilobine: presence of vicinal hydroxyl and methoxyl groups at ring A. (B) Anonaine: methylenedioxy bridge at ring A.
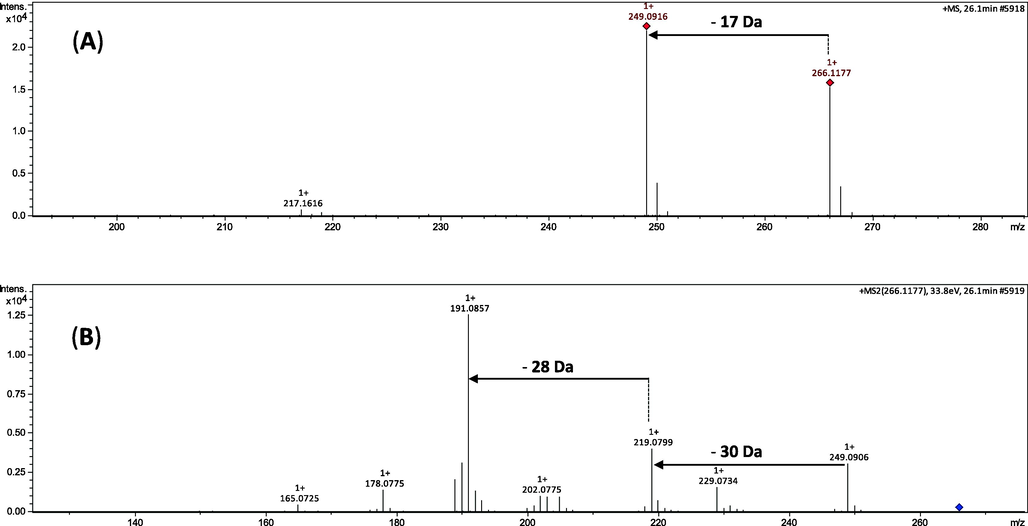
Anonaine tentative identification ESI-MS/MS. (A) Fragmentation spectrum MS of the ion at m/z 266. (B) Fragmentation spectrum MS2 of the ion at m/z 249.
Other subclasses of alkaloids such as the morphinadienone alkaloid pallidine ([M + H]+ at m/z 328.1533, peak 15) (Silva et al., 2018), and azafluoranthene alkaloid triclisine ([M + H]+ at m/z 264.1022, peak 31) (Khunnawutmanotham et al., 2015), were tentatively identified in the ethanolic extract of the peels of fruits from U. guatterioides. Morphinadienone alkaloids are rare in Annonaceae family and pallidine is the main representative in this family. In the genus Unonopsis pallidine was previously described in U. floribunda (Silva et al., 2018). On the other hand, azafluoranthene or indeno[1,2,3-ij]-isoquinoline belonging to a small group of alkaloids that are rarely found in nature but has been found in species of Menispermaceae family (Ponnala et al., 2013; Khunnawutmanotham et al., 2015). However, in this study we report for the first time the occurrence of triclisine, an azafluoranthene alkaloid, in the Annonaceae family. In addition, the compound 25 (peak 25) was proposed as norjuziphine, a benzylisoquinoline alkaloid (Chen et al., 2001; Lúcio et al., 2015). Based on the main ion [M + H]+ at m/z 286.1431, the molecular formula was established as C17H20NO3 (Table 1). This compound was found in both ethanolic extract of the peels and pulps of fruits from U. guatterioides (Table 1). Norjuziphine have been identified in Polyalthia acuminata, Porcelia macrocarpa and Onychopetalum amazonicum species of the Annonaceae family (Lúcio et al., 2015; Lima et al., 2020). The tetrahydroisoquinoline alkaloid heliamine (peak 8) and tetrahydrobenzylisoquinoline alkaloid anomuricine (peak 22) were tentatively identified as the compounds detected at [M + H]+ at m/z 194.1158 and [M + H]+ at m/z 330.1703, respectively. Both were found in the ethanolic extract of the peels of fruits from U. guatterioides. In the Annonaceae family, heliamine has been identified in Duguetia surinamensis (Paz et al., 2019) while anomuricine has been found in Annona muricata (Lúcio et al., 2015). Thus, this is the first report of the occurrence of heliamine, norjuziphine and anomuricine in the genus Unonopsis.
Alkaloids are compounds widely distributed in the plant kingdom and occur in plants belonging to Annonaceae, Apocynaceae, Asteraceae, Berberidaceae, Boraginaceae, Buxaceae, Celastraceae, Fabaceae, Lauraceae, Liliaceae, Loganiaceae, Menispermaceae, Papaveraceae, Piperaceae, Poaceae, Ranunculaceae, Rubiaceae, Rutaceae, Amaryllidaceae, Erythroxylaceae, and Solanaceae families (Mondal et al., 2019). Numerous alkaloids are well-known as effective chemotherapy drugs, to treat neurological problems, metabolic disorders and infectious diseases (Faisal et al., 2023).
3.3 Identification of flavonoids
The HPLC-ESI-MS analysis of the ethanolic extract from the peels of the U. guatterioides fruits allowed the identification of seven flavonoids derivatives including rutin, quercitrin, quercetin, isoquercitrin, quercetin-3-O-β-D-apiofuranosyl-(1 → 2)-galactopyranoside and two anthocyanins. Additionally, the analysis of the ethanolic extract of the pulps favoured the identification of the flavonoids quercitrin, quercetin and quercetin-3-O-β-D-apiofuranosyl-(1 → 2)-galactopyranoside (Table 1).
The proposed fragmentations for rutin, quercitrin and isoquercitrin are shown in Scheme 2. The peak 29 (Table 1) was identified as rutin or quercetin-3-O-rutinoside. The molecular formula of this compound was determined to be C27H30O16, through of the analysis of its pseudomolecular ion at m/z 633.1394 [M + Na]+. Additionally, the loss of a terminal rhamnose unit (146 Da) yielded an ion at m/z 465.1048 which was formed due to cleavage at the glycosidic O-linkage and a concurrent H-rearrangement. The extra loss of glucose (162 Da) or the direct loss of the rutinose residue produced the aglycone ion, quercetin, at m/z 303.0493 (Scheme 2) (Cuyckens and Claeys, 2004). Furthermore, the quercitrin, peak 28 (Fig. 1B and Scheme 2), with molecular ion at m/z 449.1070 [M + H]+, showed molecular formula C21H21O11 (Table 1). Additionally, in mass spectrum was observed the loss of a terminal rhamnose (146 Da) unit which yielded the fragment ion at m/z 303.0505, indicating ion aglycone quercetin in structure. This fragment was also confirmed by the presence of fragment ion in m/z 285.0432 resulting from loss of a water molecule (Scheme 2) (Wen-Zhi et al., 2012).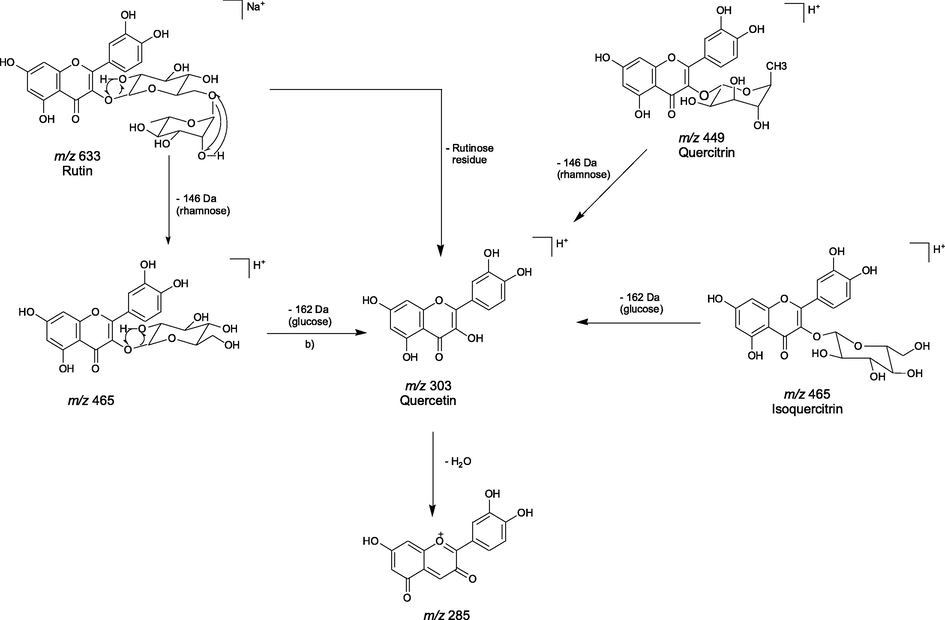
Proposed fragmentation mechanism for flavonoids derivatives: rutin, quercitrin, quercetin and isoquercitrin.
The peak 35 (Fig. 1A) produced molecular ion at m/z 465.1035 [M + H]+ and a fragment ion at m/z 303.0499 indicating to the loss of glucose residue. Consequently, the peak 35 was concluded to be isoquercitrin (Scheme 2). Quercetin was observed in peels and pulps of the fruits from U. guatterioides (peak 34) and showed molecular formula C15H11O7 (Table 1). The occurrence of this substance was also confirmed by presence of the fragment ion at m/z 285.0475 (Scheme 2). The peak 23, with pseudomolecular ion [M + Na]+ at m/z 619.1301, was assigned as quercetin-3-O-β-D-apiofuranosyl-(1 → 2)-galactopyranoside, based on its absorption, mass spectra and bibliographic reference (Silva et al., 2019).
Moreover, the compounds 16 and 20, both correponding to peaks with relative higher intensities in U. guatterioides peel′s chromatogram than pulp′s (Table 1), had its structures tentatively assigned to anthocyanins based on a molecular ion fragmentation of 287 m/z, possibly related to a cyanidin aglycone (Ling et al. 2009), and due its maximum absorbance above 500 nm (Ivanova et al. 2011; Barman et al., 2021) (Fig. 2). According to literature, anthocyanins of Annonaceae plants had their pharmacological potential mainly investigated and attributed for sickle cell disease treatment, being only qualitatively reported from Annona, Melodorum, Uvariopsis and Uvariodendron specimens (Mpiana et al. 2012, Ngbolua et al. 2016, Ngbolua et al. 2017, Konczak & Sakulnarmrata 2022). The only chemical characterization described for Annonaceae anthocyanins resulted on cyanidin 3-O-glucoside report on Uvaria hamiltonii flowers extract (Barman et al. 2021).
These flavonoids have been found in Annonaceae species. Thus, rutin, quercitrin and isoquercitrin were identified in Annona muricata L. (Souza et al., 2018; Ramos et al., 2022), quercetin in Annona crassiflora (Prado et al., 2020; Ramos et al., 2022) and quercetin-3-O-β-D-apiofuranosyl-(1 → 2)-galactopyranoside in Annona nutans (Silva et al., 2019). However, this is the first report of these compounds from the Unonopsis genus.
Flavonoids are widely distributed in the plant kingdom and are part of the most relevant and diversified group of phenolic compounds. Due to their numerous health-promoting qualities, they stick out for having high economic appeal (Ramos et al., 2022).
3.4 Other compounds
Other compounds were also identified in ethanolic extract from pulp of the fruits from U. guatterioides (Table 1), such as the coumarin esculetin-O-acetylglucoside (peak 11), the amide moupinamide (peak 13) and the terpene abscisic acid (peak 14), which were tentatively identified as the compounds detected with [M + H]+ at m/z 383.0970, [M + H]+ at m/z 314.1386 and [M + H]+ at m/z 265.1452, respectively (Moreira and Leitão, 2001; Sousa et al., 2022). These compounds are being reported for the first time in the Annonaceae family.
The total ion chromatogram of the ethanolic extract from the peels has a different chemical profile when compared to that from pulps (Fig. 1A and Fig. 1B). The ethanolic extract of the peels of fruits showed greater overall quantities of flavonoids (Table 1), which is in agreement with the expected results, since it is common for a greater accumulation of these compounds on the surface of the fruits and leaves, as they act as a protection against UV rays and/or as a defence against insects (Simmonds et al., 2003). Moreover, the ethanolic extract from the peels and pulps presented alkaloids as the major compounds. These results are also in agreement with the substances found in species of the Annonaceae family which present a wide variety of chemical constituents (Ramos et al., 2022), but alkaloids are the major chemical constituents (Lúcio et al., 2015).
3.5 Antimicrobial assays
In this study, the extracts UGP-1 and UGP-2 were tested against clinical resistant strains of S. pseudintermedius, S. aureus and S. epidermidis.
S. pseudintermedius is an opportunistic pathogen associated with skin and wound infections in dogs, cats and horses, rarely causing infection in humans (Robb et al., 2017; Bhooshan et al., 2020). However, in addition to skin infections, there have been an increasing number of cases reports of S. pseudintermedius infections in humans, most of which have been related to the intense contact of dogs and tutors (Robb et al., 2017). On the other hand, S. aureus is an opportunistic pathogen that colonizes asymptomatically the skin and mucosa of mammals and birds, which is also frequently observed in hospital settings (Mehraj et al., 2016). Additionally, S. aureus also has a considerable impact on agriculture and public health as a major cause of infection in a plethora of animal hosts (Haag et al., 2019; Szczuka et al., 2022). In this context, one of the most common bacterial species that is universally present on human skin and mucous membranes is S. epidermidis, which is typically recognized as a commensal bacterium (Huttenhower et al., 2012). In instance, intravascular devices, cerebrospinal fluid shunts, intraocular lenses, prosthetic joints, and heart valve replacements are among the medical implants that are easily colonized by many strains of S. epidermidis (Kleinschmidt et al., 2015).
The antimicrobial activities of individual ethanolic extracts of the peels (UGP-1) and the pulps (UGP-2) of fruits of U. guatterioides were assessed against clinical S. aureus (resistant to clindamycin, erythromycin and penicillin G), S. pseudintermedius (resistent to amoxicillin, clavulanic acid, gentamicin, neomycin, azithromycin, cefalexin, cephalothin, streptomycin and marbofloxacin) and two strains of S. epidermidis (A: resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, linezolid, oxacillin and trimethoprim/sulfamethoxazole and B: resistant to ciprofloxacin, erythromycin, gentamicin and oxacillin). For plant extracts, Wamba et al. 2018 ranked the antimicrobial activity as: significant activity if MIC values are below 100 μg*mL−1, moderate activity if 100 ≤ MICs ≤ 625 μg*mL−1 and weak activity if MICs > 625 μg*mL−1. Using these criteria, the ethanolic extract of fruits from U. guatterioides UGP-1 and UGP-2 showed weak activity for all staphylococcal species evaluated, with MIC values ≥ 1000 μg*mL−1 (Table 2). AMP: ampicillin; (UGP-1): ethanolic extract of the peels of fruits from U. guatterioides; (UGP-2): ethanolic extract of the pulps of fruits from U. guatterioides. Synergic effect (FICI ≤ 0.5); additive effect (FICI > 0.5–1); indifferent effect (FICI > 1–4) and antagonist effect (FICI > 4).
Pathogens
Sample/combination
Individual MIC
Combined MIC
FIC
FICI
Effect
Staphylococcus pseudintermedius
UGP-1
≥2000
1000
0.5
–
Indifferent
AMP
25
25
1
UGP-1 + AMP
–
–
–
1.5
UGP-2
≥2000
1000
0.5
–
Indifferent
AMP
25
25
1
UGP-2 + AMP
–
–
–
1.5
Staphylococcus
aureus
UGP-1
2000
1000
0.5
–
Indifferent
AMP
1.56
1.56
1
UGP-1 + AMP
–
–
–
1.5
UGP-2
≥4000
2000
0.5
–
Additive
AMP
1.56
0.78
0.5
UGP-2 + AMP
–
–
–
1
Staphylococcus epidermidis
(A)
UGP-1
1000
500
0.5
–
Indifferent
AMP
3.12
3.12
1
–
UGP-1 + AMP
–
–
–
1.5
UGP-2
≥2000
1000
0.5
–
Additive
AMP
3.12
1.56
0.5
–
UGP-2 + AMP
–
–
–
1
Staphylococcus epidermidis
(B)
UGP-1
1000
125
0.0125
–
Indifferent
AMP
1.56
1.56
1
–
UGP-1 + AMP
–
–
–
1.12
UGP-2
≥2000
31.25
0.016
–
Indifferent
AMP
1.56
1.56
1
–
UGP-2 + AMP
–
–
–
1.16
On the other hand, the synergism, a positive interaction between compounds, is a successful approach to combat antimicrobial resistance (Xu et al., 2018; Silva et al., 2019). Substances present in plant extracts can work in synergism with antibiotics potentiating their impact and assisting the host in fighting against drug-resistant bacteria (Silva et al., 2019a, 2019b; Chassagne et al., 2021). Thus, in this study, combinations between UGP-1 and ampicillin (UGP-1 + AMP), UGP-2 and ampicillin (UGP-2 + AMP) were also evaluated for their antibacterial activities (Table 2). According to the fractional inhibitory concentration index (FICI), two combinations (UGP-1 + AMP) and (UGP-2 + AMP) were indifferent (no interaction) against S. pseudintermedius with FICI values of 1.5 each (Table 2). The combination of the ethanolic extract of the pulps of fruits from U. guatterioides (UGP-2) and ampicillin resulted in an additive effect (FICI = 1.0), when tested against S. aureus, reducing the antibiotic MIC from 1.56 to 0.78 µg*mL−1 (Table 2). This combination also showed an additive effect when tested against S. epidermidis (A), reducing the antibiotic MIC from 3.12 to 1.56 µg*mL−1 (Table 2). According to Simões et al. (2009), natural products can influence in a variety of bacterial cell biochemical targets. However, the precise mechanism of action and the causes of phytochemical antibacterial specificity are still mostly understood.
In general, a positive synergistic combination reduces the minimum dose necessary to obtain effective antimicrobial effects. Moreover, it can decrease both the risk of side effects, toxicity and the costs of treatment (Silva et al., 2019a, 2019b). The ethanolic extract of the peels of fruits from U. guatterioides (UGP-1) displayed an indifferent interaction with ampicillin, with a FICI value of 1.5 (Table 2). There were no antagonistic interactions found (Table 2).
Synergistic antimicrobial combinations have great promise for lowering prospective bacterial resistance, overcoming existing antibiotic resistance, preventing host toxicity, and boosting antimicrobial effectiveness (Duong et al., 2021).
It is interesting to notice that the differential chemical profile impacts in the activity of the fruits, once the peels extract (UGP-2) showed higher activity that the pulps (UGP-1). An additive effect resulting from the combination of pulp extract (UGP-2) with the antibiotic ampicillin was observed. It is important to note that in the combination, both extract and AMP showed a 2-fold reduction in MIC, revealing the potential use of UGP-2 as an adjuvant in combating bacterial resistance. Promising results against multi-drug resistant bacteria were observed in the methanolic extracts of Curcuma longa and Moringa olifera, that was found to contain alkaloids, flavonoids terpenoids, carbohydrates as main constituents, which may be responsible for therapeutic activity against gram-positive bacteria Streptococcus aureus, Bacillus subtulis, and gram-negative Escherichia coli and Proteus vulgari (Thakur et al., 2022).
3.6 Somatic mutation and recombination test (SMART)
In this study, the genotoxicity of the U. guatterioides fruit extracts UGP-1 and UGP-2 was evaluated by wing spot test of D. melanogaster in the descendants from standard (ST) and high bioactivation (HB) crosses. Data were analysed using wings with the heterozygous mwh/flr3 marker and the frequencies of spots observed were classified as: twin stains (both subclones mwh and flr3), simple large (two or more stains) and simple small (one to two stains). All extracts were tested at concentrations of 1.25, 2.5 and 5.0 mg*mL−1 (Table 3). Only marker-trans-heterozygous flies (mwh/flr3) were evaluated. aStatistical diagnosis according to Frei and Würgler (1988): +, positive; w+, weakly positive; -, negative; i, inconclusive. Multiplication factor for the assessment of significantly negative results (m). Significance levels: α = β = 0.05 when compared with respective control. b Including rare flr3 single spots. c Considering mwh clones from mwh single and twin spots.
Genotypeand
concentration
(mg.mL−1)Number of flies
(N)Spots per fly (number of spots) statistical diagnosisa
Total spots with mwh clonesc
(n)
Small single spots
(1–2 cells)b
Large single spots
(>2 cells)b
Twin spots
Total spots
ST cross
(m = 2)
(m = 5)
(m = 5)
(m = 2)
Negative control
20
0.20
(04)
0.10
(02)
0.05
(01)
0.35
(07)
7
DXR (0.25)
20
0.95
(19)+
1.90
(38)+
1.70
(34)+
4.55
(91)+
89
1 (1.25)
20
0.20
(04)-
0.00
(00)-
0.00
(00)-
0.20
(04)-
4
1 (2.5)
0.15
(03)-
0.05
(01)-
0.05
(01)-
0.25
(05)-
5
1 (5.0)
20
0.05
(01)-
0.05
(01)-
0.05
(01)-
0.15
(03)-
3
2 (1.25)
20
0.15
(03)-
0.10
(02)-
0.05
(01)-
0.30
(06)-
6
2 (2.5)
20
0.25
(05)-
0.00
(00)-
0.00
(00)-
0.25
(05)-
5
2 (5.0)
20
0.25
(05)-
0.10
(02)-
0.05
(01)-
0.40
(08)-
8
HB cross
Negative control
20
0.30
(06)
0.15
(03)
0.05
(01)
0.50
(10)
10
DXR (0.25)
20
1.30
(26)+
2.70
(54)+
2.35
(47)+
6.35
(1 2 7)+
124
1 (1.25)
20
0.35
(07)-
0.00
(00)
0.00
(00)
0.35
(07)
7
1 (2.5)
20
0.25
(05)-
0.00
(00)
0.10
(02)
0.35
(07)
7
1 (5.0)
20
0.30
(06)-
0.00
(00)
0.00
(00)
0.30
(06)
6
2 (1.25)
20
0.15
(03)-
0.05
(01)-
0.00
(00)-
0.20
(04)-
4
2 (2.5)
20
0.35
(07)-
0.05
(01)-
0.00
(00)-
0.40
(08)-
8
2 (5.0)
20
0.30
(06)-
0.15
(03)-
0.05
(01)-
0.50
(10)-
10
With the positive control, doxorubicin (0.25 mg*mL−1), the frequency in the total of mutant stains was of 4.55 in the descendants of the ST crossing and 6.35 in the descendants of the HB crossing, while the negative control showed a frequency in the total of mutant stains of 0.35 and 0.50 in ST and HB descendants, respectively (Table 3). The results obtained from the treatment UGP-1 and UGP-2 were compared to the negative control. The frequency of stains between the doses of UGP-1 and UGP-2 ranged from 0.15 to 0.40 in the standard (ST) crossing, while the negative control showed a frequency in the total spots of the mutant of 0.35 in the descendants of the ST crossing (Table 3). Similarly, the negative control showed a frequency in the total of mutant stains of 0.50 in the descendants from high bioactivation (HB) crossing, while the frequency of stains between the doses of the ethanolic extracts UGP-1 and UGP-2 ranged from 0.20 to 0.50 in the HB crossing (Table 3). Nevertheless, in all groups treated with extracts UGP-1 and UGP-2 of fruits from U. guatterioides, the occurrence of mutant stains in individuals originating from ST and HB crosses did not statistically vary from the negative control (p ≤ 0.05), indicating which the extracts showed no genotoxicity at the tested concentrations (Table 3).
3.7 Antioxidant activity
Natural sources with high antioxidant capacity represent a vast potential to prevent or minimize the oxidative stress that causes many chronic diseases (Carvalho et al., 2021; Becker et al., 2019). Any substance, even at low concentrations, that substantially slows down or prevents oxidation processes in living things is considered an antioxidant (Becker et al., 2019). The antioxidant capacity of ethanolic extracts of the peels (UGP-1) and pulps (UGP-2) of fruits of U. guatterioides were investigated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. The results in IC50 are shown in Table 4. UGP-1: ethanolic extract of the peels of fruits from U. guatterioides; UGP-2: ethanolic extract of the pulps of fruits from U. guatterioides. Positive controla.
Sample
IC50 value (μg*mL−1)
UGP-1
> 100
UGP-2
> 100
Ascorbic acida
<50
For DPPH assay, Phongpaichit et al., 2007 classifies the antioxidant activity of extracts as: strong activity to IC50 values of 10–50 µg*mL−1, moderate activity to IC50 values ranging from 50 to 100 μg*mL−1, and weak activity for values above ˃ 100 μg*mL−1. According to Table 4, the ethanolic extracts of the fruits of U. guatterioides showed weak antioxidant activity, with IC50 > 100 μg*mL−1 when compared to reference antioxidant (ascorbic acid) with IC50 < 50 μg*mL−1. These results are in agreement with the chemical profile of the ethanolic extract of the fruits from U. guatterioides obtained by HPLC ESI-MS. The ESI-MS fingerprint UGP-1 and UGP-2 showed the predominance of alkaloids and flavonoids with O-substituents, which possibly explain the low antioxidant activity of the extracts by the in vitro model DPPH. The antioxidant capacity of substances is related to their ability to single electron transfer and/or a radical hydrogen transfer to one molecule to eliminate the unpaired condition of the other molecule bearing free radical (Dos Santos et al., 2018; Becker et al., 2019). For this model test, characteristics such as free hydroxyl groups and an extended conjugation system enhance the antioxidant potential of compounds. Therefore, O-methylation and possibly other O-modifications of hydroxyl group in compounds such as the flavonoids rutin, quercetrin, isoquercetrin found in the fruits of U. guatterioides (Table 1), inactive/decreases its own antioxidant activity and of the extracts (Basile et al., 2005; Dos Santos et al., 2018; Xiao et al., 2019).
Some typical Brazilian fruits, when testing fresh fruits by the DPPH method, pointed out promising results for puçá-preto (Mouriri pusa – Memecylaceae) with EC50 values of 414 g*g-1 DPPH, camu-camu (Myrciaria dubia - Myrtaceae) with EC50 values of 478 g*g-1 DPPH and acerola (Malpighia emarginata – Malpighiaceae) with EC50 values of 670 g*g-1 DPPH, indicating an association between antioxidant capacity and phenol contents, corresponding to 868 mg GAE/100g, 1,176 mg GAE/100g, and 1,063 mg GAE/100g, respectively (Rufino et al., 2010).
4 Conclusions
A total of 41 compounds were tentatively identified in ethanolic extracts of the peels and pulps of the fruits from U. guatterioides. The HPLC-ESI-MS analysis revealed the presence of alkaloids previously reported for Unonopsis genus, such as asimilobine, anonaine, nornuciferine, glaucine and norglaucine. In contrast, the heliamine, norjuziphine and anomuricine alkaloids are being reported for the first time in the Unonopsis genus, while this is the first report of azafluoranthene alkaloid triclisine in the Annonaceae family. These data demonstrated the potential of monitoring complex extracts by HPLC-ESI-MS in the search for new natural products that can be used in the food, pharmaceutical and cosmetic industries, simplifying the phytochemical analysis. Furthermore, combination of the ethanolic extract of the pulps of fruits from U. guatterioides and ampicillin resulted in an additive effect, when tested against S. aureus and S. epidermidis (A). These results suggest that the pulps' ethanolic extract, when combined with the antibiotic ampicillin, can strengthen the therapy for S. aureus and S. epidermidis (A) infection. Bioactive analysis based on antioxidant activity indicated that ethanolic extracts of the peels and pulps of the fruits from U. guatterioides showed low scavenging activity against the free radical (DPPH). These findings are in agreement with the chemical profiles, whose major compounds, phenolic, do not proved free hydroxyls to act as antioxidants. Additionally, no genotoxic activity of the ethanolic extracts of the peels and pulps of the fruits from U. guatterioides was detected at the tested concentrations.
Funding
This research was finantially supported by Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul - FUNDECT, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, and CPq-PROPP-UFMS.
Acknowledgements
The authors are grateful to Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul - FUNDECT-MS (Project N°71/038.838/2022, Grant number 342/2022), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES (Finance Code 001), and CPq-PROPP-UFMS for their financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibacterial synergism of Echeveria subrigida (B. L. Rob & Seaton) and commercial antibiotics against multidrug resistant Escherichia coli and Staphylococcus aureus. Eur. J. Integrative Med.. 2016;8(5)
- [CrossRef] [Google Scholar]
- Chemometric analysis applied in 1H HR-MAS NMR and FT-IR data for chemotaxonomic distinction of intact lichen samples. Anal. Chim. Acta. 2007;595
- [CrossRef] [Google Scholar]
- Threats to the biodiversity of the Brazilian pantanal due to land use and occupation. Ambiente e Sociedade. 2019;22
- [CrossRef] [Google Scholar]
- HPLC/MS analysis of polyphenols, antioxidant and antimicrobial activities of Artabotrys hildebrandtii O. Hffm. extracts. Nat. Product Res.. 2015;29(23)
- [CrossRef] [Google Scholar]
- Specialized metabolites contributing to colour and scent volatiles in Uvaria hamiltonii flowers. Nat. Product Res.. 2021;35
- [CrossRef] [Google Scholar]
- Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol.. 2005;102(1)
- [CrossRef] [Google Scholar]
- Bacteriostatic antimicrobial combination: Antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. Biomed Res. Int.. 2014;2014
- [CrossRef] [Google Scholar]
- Determination of the antioxidant capacity of red fruits by miniaturized spectrophotometry assays. J. Braz. Chem. Soc.. 2019;30(5)
- [CrossRef] [Google Scholar]
- Pyrrolizidine alkaloids from Pardoglossum cheirifolium. Chem. Nat. Compd.. 2021;57
- [CrossRef] [Google Scholar]
- Staphylococcus pseudintermedius: an undocumented, emerging pathogen in humans. GMS Hygiene and Infection Control. 2020;15
- [CrossRef] [Google Scholar]
- Systematic screening of plant extracts from the brazilian pantanal with antimicrobial activity against bacteria with cariogenic relevance. Caries Res.. 2014;48(5)
- [CrossRef] [Google Scholar]
- Health benefits of phytochemicals from Brazilian native foods and plants: Antioxidant, antimicrobial, anti-cancer, and risk factors of metabolic/endocrine disorders control. In Trends Food Sci. Technol.. 2021;111
- [CrossRef] [Google Scholar]
- A systematic review of plants with antibacterial activities: a taxonomic and phylogenetic perspective. Front. Pharmacol.. 2021;11
- [CrossRef] [Google Scholar]
- A new tetrahydroprotoberberine n-oxide alkaloid and anti-platelet aggregation constituents of Corydalis tashiroi. Planta Med.. 2001;67(5)
- [CrossRef] [Google Scholar]
- Genotoxicity of lapachol evaluated by wing spot test of Drosophila melanogaster. Genet. Mol. Biol.. 2010;33(3)
- [CrossRef] [Google Scholar]
- Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom.. 2004;39(1)
- [CrossRef] [Google Scholar]
- Euterpe oleracea pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb. Pathog.. 2018;114
- [CrossRef] [Google Scholar]
- Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three Myrcia Species. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Developing antimicrobial synergy with AMPs. Front. Medical Technol.. 2021;3
- [CrossRef] [Google Scholar]
- Chemical characterization, antioxidant and cytotoxic activities of the edible fruits of Brosimun gaudichaudii Trécul, a native plant of the Cerrado Biome. Chem. Biodiversity. 2021;18(7)
- [CrossRef] [Google Scholar]
- Alkaloids as potential antivirals. A comprehensive review. Nat. Products Bioprospecting. 2023;13(4)
- [CrossRef] [Google Scholar]
- Phytochemical analysis on the seeds of a new Iranian Plantago ovata Forssk. population specimen. Nat. Prod. Res.. 2022;36
- [CrossRef] [Google Scholar]
- Evaluation of the genotoxic activity of ethanol extract and secondary metabolites isolated from Aiouea trinervis Meisn. (Lauraceae) Genet. Mol. Res.. 2014;13(1)
- [CrossRef] [Google Scholar]
- Structure, function and diversity of the healthy human microbiome. Nature 2012:486.
- [CrossRef] [Google Scholar]
- Rapid MALDI-TOF-MS detection of anthocyanins in wine and grape using different matrices. Food Anal. Methods. 2011;4
- [CrossRef] [Google Scholar]
- Antimicrobial potential of Pectis substriata essential oil (Asteraceae) against drug-resistant Staphylococcus strains. An. Acad. Bras. Cienc.. 2020;92(4)
- [CrossRef] [Google Scholar]
- Divergent total syntheses to azafluoranthene and dehydroaporphine alkaloids. Eur. J. Organic Chem.. 2015;28
- [CrossRef] [Google Scholar]
- Staphylococcus epidermidis as a cause of bacteremia. Future Microbiol.. 2015;10
- [CrossRef] [Google Scholar]
- Encapsulation of Melodorum fruticosum Lour. anthocyanin-rich extract and its incorporation into model food. LWT. Food Sci. Technol.. 2022;153
- [CrossRef] [Google Scholar]
- Kuhlmann M. 2018. Frutos e sementes do Cerrado: espécies atrativas para a fauna. Vol. 2, first ed. Ipsis gráfica e editora, Brasília.
- Alkaloids of Annonaceae. XXXV. Alkaloids of Desmos tiebaghiensis. J. Nat. Prod.. 1982;45(5)
- [CrossRef] [Google Scholar]
- Integrative approach based on leaf spray Mass Spectrometry, HPLC–DAD–MS/MS, and NMR for comprehensive characterization of isoquinoline-derived alkaloids in leaves of Onychopetalum amazonicum R E. Fr. J. Brazilian Chem. Soc.. 2020;31(1)
- [CrossRef] [Google Scholar]
- A rapid and sensitive LC–MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J. Chromatogr. B. 2009;877
- [CrossRef] [Google Scholar]
- Alkaloids of the Annonaceae: occurrence and a compilation of their biological activities. Alkaloids: Chem. Biol.. 2015;74
- [CrossRef] [Google Scholar]
- Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci.. 2010;58(2)
- [CrossRef] [Google Scholar]
- Epidemiology of Staphylococcus aureus nasal carriage patterns in the community. Curr. Top. Microbiol. Immunol.. 2016;398
- [CrossRef] [Google Scholar]
- A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol.. 2006;141
- [CrossRef] [Google Scholar]
- Alkaloids for cancer prevention and therapy: current progress and future perspectives. Eur. J. Pharmacol.. 2019;858(5)
- [CrossRef] [Google Scholar]
- Quantitative determination of liriodenine and moupinamide in five species of Mollinedia by high performance liquid chromatography. Phytochem. Anal. 2001;12(4)
- [CrossRef] [Google Scholar]
- Antisickling properties, thermal and photochemical degradations of anthocyanin extracts from Annona senegalensis (Annonaceae) Int. J. Biol. Chem. Sci.. 2012;6
- [CrossRef] [Google Scholar]
- Small Brazilian wild fruits: nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res. Int.. 2018;103
- [CrossRef] [Google Scholar]
- Antisickling and antibacterial activities of Uvariopsis congensis. Discovery Phytomed.. 2016;3
- [CrossRef] [Google Scholar]
- Anti-Sickle cell anemia and bacterial inhibitory effects of uvariodendron molundense (diels) R.E.Fr. (Annonaceae) from Ubangi River Basin, DR Congo. J. Biosci. Med.. 2017;5
- [CrossRef] [Google Scholar]
- Flavonols from Annona coriacea Mart. (Annonaceae) Biochem. Syst. Ecol.. 2018;78
- [CrossRef] [Google Scholar]
- Evaluation of cytotoxicity, acute toxicity, genotoxicity, mutagenic and antimutagenic potential of Elaeocarpus Serratus L. fruit extract. J. Clin. Toxicol.. 2021;11(5)
- [Google Scholar]
- Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol. Med. Microbiol.. 2007;51(3)
- [CrossRef] [Google Scholar]
- A New Route to azafluoranthene natural products via direct arylation. Eur. J. Organic Chem.. 2013;3013(6)
- [CrossRef] [Google Scholar]
- Antioxidant, antiproliferative and healing properties of araticum (Annona crassiflora Mart.) peel and seed. Food Res. Int.. 2020;133
- [CrossRef] [Google Scholar]
- Cactus alkaloids. XLIX. New trace alkaloids (dehydrosalsolidine and heliamine) from the saguaro, Carnegiea gigantea, and confirmation by mikes (MS/MS) J. Nat. Prod.. 1982;45(3)
- [CrossRef] [Google Scholar]
- An integrative approach to the flavonoid profile in some plants' parts of the Annona genus. Plants. 2022;11(21)
- [CrossRef] [Google Scholar]
- Skin infection caused by a novel strain of Staphylococcus pseudintermedius in a Siberian husky dog owner. JMM Case Rep.. 2017;4(3)
- [CrossRef] [Google Scholar]
- Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chemistry. 2010;121(4)
- [CrossRef] [Google Scholar]
- Genotoxicity of Turnera subulata and Spondias mombin × Spondias tuberosa extracts from Brazilian Caatinga Biome. J. Med. Food. 2018;21(4)
- [CrossRef] [Google Scholar]
- The impact of natural antioxidants on the regenerative potential of vascular cells. Front. Cardiovasc. Med.. 2019;6
- [CrossRef] [Google Scholar]
- Dereplication of aporphine and oxoaporphine alkaloids from Unonopsis guatterioides by ESI-IT-MS. Quim. Nova. 2012;35(5)
- [CrossRef] [Google Scholar]
- Steroids and triterpene from the bark of Unonopsis guatterioides R. E. FR. (Annonaceae). International. J. Pharm. Pharm. Sci.. 2012;4(2)
- [Google Scholar]
- Leishmanicidal activity of fractions rich in aporphine alkaloids from Amazonian Unonopsis species. Revista Brasileira de Farmacognosia. 2012;22(6)
- [CrossRef] [Google Scholar]
- Phytochemical study of the alkaloidal fractions of Unonopsis duckei R. E. Fr. guided by electrospray ionisation ion-trap tandem mass spectrometry. Phytochem. Anal. 2014;25(1):45-49.
- [CrossRef] [Google Scholar]
- Chemotaxonomy of the Amazonian Unonopsis species based on leaf alkaloid fingerprint direct infusion ESI-MS and chemometric analysis. J. Braz. Chem. Soc.. 2016;27(3)
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant activities of selected plants used by populations from Juruena Valley, Legal Amazon, Brazil. Int. J. Pharm. Pharm. Sci.. 2017;9(5)
- [CrossRef] [Google Scholar]
- Morphinadienone and other isoquinoline-derived alkaloids from the trunk bark of Unonopsis floribunda Diels (Annonaceae) Biochem. Syst. Ecol.. 2018;79
- [CrossRef] [Google Scholar]
- Plant extracts display synergism with different classes of antibiotics. An. Acad. Bras. Cienc.. 2019;91(2)
- [CrossRef] [Google Scholar]
- Anti-inflammatory, antinociceptive and antioxidant activities of the hydromethanolic fraction from Annona nutans leaves. Biosci. J.. 2019;35(5)
- [CrossRef] [Google Scholar]
- Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64(1)
- [CrossRef] [Google Scholar]
- Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep.. 2009;26
- [CrossRef] [Google Scholar]
- Combination of antimicrobials and essential oils as an alternative for the control of Salmonella enterica multiresistant strains related to foodborne disease. Foodborne Pathog. Dis.. 2017;14(10)
- [CrossRef] [Google Scholar]
- Plant growth regulators induce differential responses on primary and specialized metabolism of Annona emarginata (Annonaceae) Ind. Crop. Prod.. 2022;189
- [CrossRef] [Google Scholar]
- Phytochemical analysis and central effects of Annona muricata Linnaeus: possible involvement of the gabaergic and monoaminergic Systems. Iranian J. Pharm. Res.. 2018;17
- [Google Scholar]
- Drosophila melanogaster as a versatile model organism in food and nutrition Research. J. Agric. Food Chem.. 2018;66(15)
- [CrossRef] [Google Scholar]
- Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectrometry. Rapid Commun. Mass Spectrom.. 2004;18(5)
- [CrossRef] [Google Scholar]
- Occurrence and characteristics of Staphylococcus aureus isolated from dairy products. Molecules. 2022;27(14)
- [CrossRef] [Google Scholar]
- Investigating synergistic activity of methanolic extract of curcuma longa and Moringa olifera for in vitro antioxidant and antibacterial activities. Bull. Pharm. Res.. 2022;12(1–3)
- [CrossRef] [Google Scholar]
- NMR-based metabolomics at work in phytochemistry. Phytochem. Rev.. 2007;6
- [CrossRef] [Google Scholar]
- Frutas nativas da região Centro-Oeste. Brasília: Embrapa Recursos Genéticos e Biotecnologia; 2006. p. :320.
- Syzygium jambos displayed antibacterial and antibiotic-modulating activities against resistant phenotypes. Evid. Based Complement. Alternat. Med.. 2018;2018
- [CrossRef] [Google Scholar]
- Collision-induced dissociation of 40 flavonoid aglycones and differentiation of the common flavonoid subtypes using electrospray ionization ion-trap tandem mass spectrometry and quadrupole timeof-flight mass spectrometry. Eur. J. Mass Spectrom.. 2012;18(6)
- [CrossRef] [Google Scholar]
- Phenolic antioxidants from the heartwood of Acacia confusa. Journal of Agricultural and Food Chemistry. 2005;15
- [CrossRef] [Google Scholar]
- Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutation Res./Environ. Mutagenesis Related Subjects. 1988;203(4)
- [CrossRef] [Google Scholar]
- Structure-antioxidant capacity relationship of dihydrochalcone compounds in Malus. Food Chem.. 2019;275
- [CrossRef] [Google Scholar]
- Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep.. 2018;8(1)
- [CrossRef] [Google Scholar]
- Screening and quantitative analysis of antioxidants in the fruits of Livistona chinensis R. Brusing HPLC-DAD–ESI/MS coupled with pre-column DPPH assay. Food Chemistry. 2012;135(4)
- [CrossRef] [Google Scholar]
- An azafluorenone alkaloid and a megastigmane from Unonopsis lindmanii (Annonaceae) J. Braz. Chem. Soc.. 2013;24(4)
- [CrossRef] [Google Scholar]
- Chemical characterization and bioherbicidal potential of the essential oil from the leaves of Unonopsis guatterioides (A.DC.) R.E.Fr. (Annonaceae). Natural Product Research. 2019;33(22)
- [CrossRef] [Google Scholar]
- Proximate composition, functional and antimicrobial properties of wild harvest Terminalia carpentariae fruit. J. Food Meas. Charact.. 2022;16(1)
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105133.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







