Translate this page into:
Physicochemical characterization, phytochemical analysis, and pharmacological evaluation of Sambucus wightiana
⁎Corresponding author at: Department of Pharmaceutical Sciences, University of Kashmir, 190006, India. iachashoo@gmail.com (Ishtiaq Ahmad Chashoo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sambucus wightiana (SW) is a 4–5-foot herbaceous stem with 5–9 leaflets and pinnatifid leaves (15–30 cm). It is used to treat stomach disorders, as an emetic for expelling poisonous substances, and as a laxative for controlling skin diseases. Phytochemical research based on ethnopharmacological knowledge is frequently regarded as an appropriate approach for discovering new agents from higher-altitude plants. Therefore, the present study focussed on identifying, collecting, and authenticating the S. wightiana and, isolating and characterizing the phytoconstituents using the DPPH method, reducing power, total flavonoid, phenolic content, anti-hyperglycemic and antioxidant capacity. Furthermore, the evaluation of antidiabetic studies of extracts/fraction and pure phytoconstituents of S. wightiana in alloxan-induced diabetic model and OGTT methods were carried out. The observed results revealed that the methanolic extract of Sambucus wightiana has significant anti-hyperglycemic and anti-oxidant activity. The methanolic extracts of S. wightiana, a dose of alloxan/SW-400 mg/Kg (111.55 ± 6.9 mg/dl) also decreased significantly the serum ALP level (p < 0.05). The methanol extracts of S. wightiana showed highly significant anti-hyperglycemic activity (p < 0.05). From the methanolic extracts, alloxan/SW-400 mg/Kg (89.55 ± 2.5 mg/dl) showed highly significant decrease in serum LDL level (p < 0.05) in extract-treated groups, not changing the body weight substantially and methanolic extract of S. wightiana at a dose of 400 mg/Kg exhibited substantial lipid, and blood glucose levels and liver enzymes lowering capacity compared to the diabetic control group. Consequently, the prevention of hyperglycaemia by various other drugs, S. wightiana could contribute to a new formulation with significant pharmacological effects.
Keywords
Sambucus wightiana
Flavonoids
Phenolic compounds
In vitro antioxidant properties
Anti-hyperglycaemic activity
1 Introduction
Fruits are major sources of phenolic compounds and anthocyanins (Moyer et al, 2002). These fruits include anthocyanin pigments and phenolic compounds, which have metabolic and ecological functions and offer a variety of protection against environmental stress (Khoo et al, 2017). Inclusion of these fruits in the human diet displays a broad range of therapeutic benefits and antioxidant protection which in turn reduces the risk of stroke, and coronary heart disease, improved visual acuity, and anti-carcinogenic activity, and improves cognitive behavior (Kaur and Kapoor, 2001, Prior, 2003, Zafra-Stone et al 2007). One fine source of flavonoids, phenolic compounds, and anthocyanins, with robust antioxidant capacity, are fruits of elderberries from Sambucus spp. (Koca and Karadeniz, 2009, Akbulut et al 2009). Many indigenous cultures have been using these berries as a source of medicine in earlier days. The elderberry's purple-black fruits have drawn a lot of interest because of its ability to promote health.
The literature review reveals the effects of elderberries and their derived products on specific chronic diseases (Thole et al 2006, Murkovic et al 2004, Bell and Gochenaur, 2006, Zielińska-Wasielica et al 2019). The recent research on extracts of both American elderberry (Sambucus canadensis L.) and European black or common elderberry (Sambucus nigra L.) revealed highly significant chemo-preventive potential controlling enzymes usually connected with various forms of cancer (Thole et al 2006; Khodashenas and Ghorbani, 2016). The extracts of European elderberries have also revealed anti-inflammatory, immune-stimulatory, anti-oxidative, and to a lesser degree athero-protective properties (Murkovic et al 2004, Bell and Gochenaur, 2006, Zielińska-Wasielica et al 2019). The Sambucus is a Latin word that has been derived from “sambuca” which signifies a kind of flute made from elderberry twigs. The plants included in the genus Sambucus are shrubs or herbs and small trees. The genus Sambucus belonging to the family Adoxaceae is a flowering plant. This genus's various species are commonly known as elder or elderberry. The pulp and skin of cooked berries, as well as the majority of Sambucus species, are non-toxic. This genus' uncooked berries and other plant parts are toxic.
Sambucus wightiana is a 4–5-foot herbaceous stem with 5–9 leaflets and pinnatifid leaves (15–30 cm). The flat-topped flower clusters are heavily scented, white-creamy in color, hermaphrodite in nature, and seen during the June-July season and reproduce through seeds. At an altitude of 1800–2400 m, it is commonly found on the forest floor, roadsides, heavily grazed slopes, and in gregarious clumps under semi-shade (light woodland). This species is found in the Himalayan regions of Pakistan, India, and Afghanistan. In Kashmir, it is commonly referred to as elder or dwarf elder. Other recognized local names are known in the Kashmir regions of Jammu and Kashmir's Kishtwar and Gandula. The berries, leaves, and roots are purgatives, while the inner bark and bark are diuretics, hypotensive, anti-inflammatory, diaphoretic, and expectorants against foot and mouth disease in cattle. Its fruits contain a dye that is used to color yarn (Wani et al., 2022, Khattak et al 2005). Anthocyanins, phenolics, quercetin, flavonols, chlorogenic acid, cyaniding-3-glucoside in elderberries, cyaniding-3-sambubioside, kaempferol, and other glycosylated flavonoids are all present in various parts of Sambucus species (Khattak et al 2005). Because elderberries are high in anthocyanins, they may have a higher antioxidant capacity than vitamins C and E. Elderberries are used in the cosmetics, food, and pharmaceutical industries (Ballabh et al 2008). Purgative properties are known to exist in berries, leaves, and roots. A decoction of the inner bark and root is effective and used as a diuretic, expectorant, anti-inflammatory (particularly of the bronchial tubes), diaphoretic and purgative (leaf), and hypotensive (leaf extract) (Kumar et al 2009). S. wightiana is used as a folk medicine to treat skin diseases. The berries, roots, and leaves have been used as laxatives, purgatives, and antimicrobials. Phytochemical research based on ethnopharmacological knowledge is frequently regarded as an appropriate approach for discovering new agents from higher altitude plants (Chashoo et al 2012; Ali et al., 2021).
The current study focused on identifying, collecting, and authenticating S. wightiana and, isolating and characterizing the phytoconstituents from S. wightiana using the DPPH method, reducing power, total flavonoid and phenolic content, and antioxidant capacity. Furthermore, in vitro antioxidant properties of methanolic extracts of S. wightiana and evaluation of antidiabetic studies of extracts/fraction and pure phytoconstituents of S. wightiana in alloxan-induced diabetic model and OGTT methods were performed.
2 Materials and methods
2.1 Plant material collection, authentication, and preparation of specimen
S. wightiana was collected from the upper reaches of Gulmarg, Srinagar, J & K, India at an altitude of 2800 m. The plant was recognized and authenticated by Prof. A. R. Naqshi (Ex-Taxonomist) Department of Botany, University of Kashmir, and Prof. Zulfiqar Ali Bhat, Professor, Department of Pharmaceutical Sciences, University of Kashmir, and specimen voucher no. KU/SW/13 and KU/BP/13 was assigned to S. wightiana and has been deposited in the Department of Pharmaceutical Sciences, University of Kashmir for future references.
2.2 Chemicals
The standard glibenclamide was procured from Protec Pharmaceutical Ltd. (Srinagar, India). All other reagents and chemicals employed were of analytical grade.
2.3 Macroscopic study of plant material
The herb is analogous to the other species; therefore, comprehensive analyses of the morphological characters can be useful in distinguishing them. The macroscopic identity of the plant material depends on, the size, color, shape, surface, and texture characteristics of the cut surface. During the present study, the macroscopic procedures about various parameters like color, odor, taste, size, shape, texture, and appearance of the surface were performed as per WHO (1998) guidelines.
2.4 Determination of physicochemical constants of powdered plant materials
Physicochemical constants like extractive values, ash value, swelling index, loss on drying, and pH were determined as per methods described by WHO (1998).
2.5 Extraction of plant materials
2.5.1 Aerial plant material extraction
The plant material after being dried in shadow and ultimately was powdered using ball mills. The powdered material was allowed to go through mesh no.40, subsequently weighed & employed for extraction. An amount of approximately 200 gms in the powder form was consecutively extracted in the Soxhlet apparatus with petroleum ether, chloroform, ethyl ethanoate, and methanol. The final marc was then extracted with water using the decoction method. Individually, the weighed powder was extracted with different solvents using the soxhlet and maceration methods (Tzanova et al., 2020).
2.5.2 Fluorescence analysis
Many plants fluoresce when their powder or cut surface is exposed to UV light, making it an important tool for identifying them. Using a variety of reagents, including sodium hydroxide, picric acid, acetic acid, hydrochloric acid, nitric acid, iodine, ferric chloride, and others, plant powders (mesh 40) were investigated for their fluorescence properties in daylight and UV light (254 and 366 nm) as well as after treatment (Dinesh et al 2011).
2.6 Preliminary Phytochemical investigation
Utilizing the cold extraction technique and the Soxhlet equipment, the plant material was successively extracted with the solvents using petroleum ether, chloroform, ethyl ethanoate, and water. The decoction was used to create the aqueous extract. The extracts were evaporated at a low pressure and temperature (40–50 °C). Protein, amino acids, carbohydrates, fats, steroids, glycosides, coumarins, flavonoids, alkaloids, tannins, phenolic compounds, saponins, terpenes, mucilage resins, and other compounds were discovered during the preliminary phytochemical investigation (Zhang et al 2018).
2.6.1 Determination of fat content
Anhydrous ether was applied to a known quantity of the sample (3 g) in an extraction device for six hours, followed by filtration of the extract. Utilizing a process developed by Aziz et al., (Aziz and Mitu, 2019), the solvent was evaporated and dried to a consistent weight at 105 °C.
2.6.2 Saponin determination
20 g of plant material that had been powdered and 100 ml of 20% aqueous ethanol were put to a conical flask. After 4 h of continuous stirring at 55 °C in a hot water bath, the sample was filtered and subjected to a second extraction with 200 ml of 20% ethyl alcohol. The extract was then reduced to a volume of 40 ml in a water bath that was 90 °C or thereabouts. A 250 ml separating funnel was filled with the concentrated extract, and 20 ml of diethyl ether was added while the mixture was being violently shaken. The ether layer was removed, but the aqueous layer was retained. 60 ml of n-butyl alcohol was used to repeat the purification process. Two times, 5% aqueous sodium chloride solution was used to wash the extract. The sample was oven dried to a consistent weight before the saponin concentration was determined, and the leftover solution was boiled in a water bath to remove the solvent (Obadoni and Ochuko, 2001).
2.6.3 Thin layer chromatography (TLC) profile
Prepared TLC plates were procured from the Sigma Pvt. Ltd. (Mumbai, India) and used for study. When not in use the plates were kept in a desiccator (Stahl and Sies, 2003). The sample was applied to TLC plates using glass capillaries. The spots were placed with a minimum distance of 1 cm between adjacent spots. They were also marked to determine their identity. The glass chromatogram chamber (16.5 cm × 29.5 cm) was employed for carrying out the experiments. The chamber was given 24 hrs for saturation hours before use. The different solvent combinations were tried for different extracts and the process was continued till the satisfactory resolution system was developed. The different components that were present on the TLC plates were identified using a compressed air sprayer. The detecting reagent was then diluted to a volume of about 50 ml, and the sprayer with the air compressor was put to work. Following each spray, the sprayer was cleaned separately with water, chromic acid, distilled water, and acetone. The fluorescent substances were also tested in a UV chamber. The results were obtained at two different UV wavelengths of 254 nm and 366 nm.
2.7 Microbial load determination
The microbial count of plant material was determined according WHO guidelines. 50 ml of sterile distilled water were used to dissolve 1 g of the drug's powdered form. For maximum mixing, the suspension was shaken for enough time. The suspension was then filtered using disposable sterilised filter paper. As a stock solution, the filtrate was used. This stock solution was serially diluted (1:1, 1:10, 1:1100), and 1 ml of each diluted solution was used as an inoculum (applied using the spreading method) on nutritional agar medium. The inoculum was then incubated at 37 °C for 24 h. After 24 h, the petri plates with distinctly discernible colonies were removed, and the number of colonies was calculated using a colony counter. Using the dilution factor, the dilution load/g of sample was then calculated. The medium was autoclaved at 121 °C and 15 lbs per square inch pressure (Shubha and Hiremath, 2010).
2.8 Determination of heavy metal
2.8.1 Processing of plant material for determination of lead, cadmium, and mercury
Accurately weighed 0.2 g of powdered drug was taken into a clean silica crucible. To this, 1 ml of digestion mixture containing one part by weight of concentrated nitric acid and one part by weight of concentrated perchloric acid was added. Crucible was placed in oven and the temperature raised slowly up to 100 °C. This temperature was maintained for 3 hrs. Temperature was then raised up to 120 °C and the same maintained for 2 hrs. Again, the temperature was raised very slowly up to 450 °C and maintained for 4 hrs. Then, the contents of crucible were allowed to cool. 2.5 ml of 0.5% v/v nitric acid was added to the crucible and suitably diluted with double distilled water. The contents were filtered through membrane filters. The above processed sample was subjected to atomic absorption spectrometer (AAS) analysis for determination of lead, cadmium, and mercury. Three determinations were done under each analysis to get concordant results (Rajan, 2018).
2.8.2 Processing of plants material for determination of Arsenic
50 g of powdered drug was taken into a 1 L Kjeldhal flask. 25 ml of double distilled water, 50 ml of nitric acid, and 20 ml of sulphuric acid were added to the flask. Contents of the flask were heated slowly and carefully to avoid excess foaming. Nitric acid equivalent to 1000 g/l was added drop by drop until all the organic matter got destroyed. This was identified by no darkening of the solution by further addition of nitric acid and continued heating. The flask was then allowed to cool. 75 ml of distilled water and 25 ml of ammonium oxalate (25 g/l) were added to the flask to expel nitrogen oxide from the solution. Heated again and cooled. The contents were transferred into a 250 ml volumetric flask and made-up the final volume with double distilled water. The solution was filtered through membrane filters. The processed sample was subjected to AAS analysis for determination of Arsenic. Three determinations were done under each sample to get concordant results (Rajan, 2018).
2.9 Fractionation of successive extract of S. Wightiana
The coarsely powdered material (2 kg: complete ariel parts) was extracted in a Soxhlet apparatus using several solvents, including petroleum ether (P), chloroform (C), ethyl acetate (EE), and methanol (M), at temperatures ranging from 40 to 60 °C, before being decocted with water (AQ).
2.9.1 Isolation of flavonoids from whole plant of S. Wightiana
Before being ground into powder, the complete aerial plant material was shade-dried for a week. Using a Soxhlet system and 95% ethanol, powdered material (5 kg) was extracted over the course of around 72 h. A rotating evaporator was used to filter and concentrate the extract while operating under reduced pressure and a vacuum. It was feasible to produce a 400 g (8.0% w/w) dark brown viscous mass. The extract (200 g) was subjected to lead acetate treatment, producing a yellow precipitate that was then suspended in methanol, subjected to lead removal using hydrogen sulfide gas, and finally filtered. After the filtrate was evaporated, the residue was heated in boiling water and extracted with ether. Before being acidified, the concentrated ether fraction was extracted using sodium hydrogen carbonate, sodium hydroxide, and hydrochloric acid. A reddish brown amorphous solid (500 mg), a cream-colored solid (300 mg), and a white-colored solid (200 mg) were generated by recrystallization from alcohol:water and subsequently analyzed spectroscopically (Bhujbal et al., 2009). After being isolated, the compound (C-1) was characterized.
2.9.2 Isolation of rutin from S. swightiana
500 g of plant powder and water (100 ml) were combined. The pH was changed to 6–8 using 1 M NaOH, and the mixture was then boiled at a low temperature. The mixture was filtered, and the filtrate was then treated with 1 M HCl to make it acidic (pH 5–6) in order to precipitate the rutin. The precipitate was purified by vacuum filtering, dissolved in water, and then adjusted to a pH range of 6 to 8. The solution was then heated for 30 min, filtered, and then dried at 700 °C in an oven. The compound was characterised to confirm its identity as rutin (C-2).
2.10 NMR spectroscopy
Following the steps outlined below, NMR spectra were obtained. Briefly stated, 1 ml of D2O and methanol‑d4 (80:20) were used to dissolve the PKRE, and 600 µl of the solution was transferred to a 5 mm NMR tube. A 600 MHz Bruker AVANCE III-600 spectrometer (Bruker, Karlsruhe, Germany) with a proton NMR frequency of 600.30 MHz and a carbon NMR frequency of 150.94 MHz was used to acquire NMR spectra at 25 °C. The acquisition time for each 1H NMR spectra was 6 min and 50 s, and it consisted of 64 scans with the following parameters: 0.16 Hz/point and relaxation delay (RD) = 5 s. With low power selective irradiation at the H2O/methanol frequency, a pre-saturation sequence was used to suppress the remaining H2O/D2O/methanol‑d4 signal during the recycle delay. HMQC spectra were captured using a Q sine (SSB = 2.0) window function, a relaxation time of 1 s, and spectral widths of 64,6009.61 Hz in F2 and 36,056.78 Hz in F1. The HMBC spectra were captured with a relaxation delay of 1 s, a spectral width of 9615.38 Hz in F2 and 36230.55 Hz in F1, and several other parameters. Except for the F2 spectral width of 36,056.78 Hz, the same parameters as in the HMQC spectra were applied. The comprehensive investigation was conducted as previously mentioned (Kumar et al 2017).
2.11 In-Vitro antioxidant study
Antioxidant activity of different extracts, fractions and isolated compounds were carried out as follows:
2.11.1 Total flavonoid content
A spectrophotometric technique was used to determine the flavonoids content of the plant extract (Quettier-Deleu et al., 2000). The sample included 1 ml of a 3 to 15 mg/ml methanol extract solution as well as 1 ml of a 2% AlCl3 solution dissolved in methanol. The samples were incubated for one hour at room temperature. Using a spectrophotometer, the absorbance was measured at a wavelength of 415 nm. Triplicate samples were generated for each analysis, and the absorbance mean value was computed. The same process was carried out again using the rutin standard solution to create the calibration curve. The quantity of flavonoids in extracts was represented in terms of rutin equivalent (mg of rutin/g of extract), and the concentration of flavonoids was obtained (mg/ml) from the calibration curve based on measured absorbance.
2.11.2 Total phenolic content
The crude extracts of S. wightiana were determined using a reported method (Kupina et al 2008) The total phenolic content of extracts was calculated using the Folin-Ciocalteu technique. The extracts were oxidized with Folin-Ciocalteu reagent and then neutralized with sodium carbonate. The resulting blue color's absorbance was measured at 760 nm after 60 min using gallic acid as a reference. The amount of total phenol in each gram of extract was calculated as mg gallic acid equivalent.
2.11.3 Total antioxidant capacity
With some modifications, the phosphomolybdenum method was used to determine total antioxidant activity (Prieto et al 1999). This method's fundamental idea is based on the extract converting Mo (VI) to Mo (V), which it then does at an acidic pH to generate a green phosphate Mo (V) complex. 3 ml of the reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) were combined with 1 ml of extract in varied concentrations (between 5 and 30 mg/ml). The tubes containing the reaction solution were then sealed and incubated at 95 °C for 90 min. The samples were cooled to room temperature before measuring the solution's absorbance at 695 nm in comparison to a blank. Instead of extract, methanol was used as a blank. The samples were prepared in triplicate for each assay, and the mean absorbance value was calculated. The antioxidant activity is measured in mg of ascorbic acid equivalents (mg AAE)/g of extract.
2.11.4 DPPH radical scavenging activity
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) compound's capacity to scavenge free radicals was used to measure the antioxidant activity of plant extracts (Kedare and Singh, 2011). The methanolic extract (0.3 ml) was combined with 2.7 ml of DPPH (6 × 10-5 mol/l) at various concentrations. After being vortexed, the mixture was left in the dark for 60 min. The DPPH radical's reduction was measured by the drop in absorption at 517 nm. 1 ml of 0.1 mM DPPH solution was combined with 1 ml of methanol as a control. The values for IC50 were calculated. This formula was used to compute the % inhibitory activity: where, A1 is the extract/standard absorbance and A0 is the control absorbance. The IC50 values were computed and the inhibition curves were produced. Methanol (1 ml) and extract solution (2.5 ml) were employed as a negative control. The positive control was rutin solution.
2.11.5 Reducing power
The approach outlined in the literature by (Bhalodia et al 2013) was used to examine the extracts' reduction power. The methanolic extract (2.5 ml) was combined with 2.5 ml of 1% potassium ferricyanide and 200 mmol/l sodium phosphate buffer (pH 6.6). The mixture was incubated at 50 °C for 20 min. The mixture was centrifuged at 650 rpm for 10 min after adding 2.5 ml of 10% trichloroacetic acid (w/v). After adding the upper layer (2.5 ml) to the 2.5 ml distilled water and 0.5 ml of FeCl3 (0.1%), the absorbance at 700 nm was measured. A higher reducing power is indicated by a higher absorption. The experiments were conducted three times, and the outcomes were presented as mean values and standard deviations.
2.12 Pharmacological screening of plant extracts
Young male Wistar rats, 6–8 weeks old, 100–200 g, were procured from the Indian Institute of Integrative Medicine's animal house (Jammu and Kashmir, India). The animals were acclimated to the typical laboratory settings (12 h light/dark cycle, 50%–60% humidity, and 22 °C temperature) before the experiment. All animals were kept in polypropylene cages (n = 6) for the duration of the experiment and given pelleted food and water as needed. The Institutional Animal Ethical Committee approved the study's protocol (Approval No. 2012/07). Every experiment was conducted in accordance with the protocol that was authorized. The acute toxicity research was conducted in compliance with the OECD guidelines-425, a widely recognized methodology (OECD, 2001). S. wightiana was administered orally to overnight fasted, healthy rats (n = 6) at dosages of 800, 1600, and 3200 mg/kg, po. Additionally, isolated S. wightiana compounds were given at a dose of 100 mg/kg, po. The dose is considered hazardous, under the standards, if two or three animals die. If an animal dies, the same amount is administered once more to confirm the toxic dose. Plant extracts are regarded as harmless if no mortalities are noticed. Antidiabetic activity of different extracts, fractions and bioactive compounds were also carried out as per following protocol:
2.12.1 Gulcose tolerance test of extract/fraction/molecules
Rats that had been fasted normally were placed into ten groups of six each. As the control group, group I received saline solution as usual. Groups III and IV received a methanol extract of the aerial part of S. wightiana (SW 200 & 400 mg/kg/day), while groups V and VI received compounds isolated from S. wightiana (rutin and quercetin 5 mg/kg/day). Group II received the reference medication, glibenclamide, at a dose of 5 mg/kg body weight. The rats in all groups received 2 g after 30 min. Before and after the delivery of glucose, as well as 30, 90, and 150 min later, blood samples were obtained from the tip of the tail. The glucose oxidase method was used to assess blood glucose levels after serum had been isolated (Yashwant et al., 2011).
2.12.2 Alloxan induced antidiabetic activity of extract/fraction/molecules
Six rats from each of six groups had the following course of treatment:
Group 1: Vehicle control group, in which rats were given vehicle on a daily basis i.e. saline (normal control).
Group 2: Diabetic control group in which alloxan (150 mg/kg i.p.) was administered to the rats.
Group 3: The rats in the standard control group also received alloxan (150 mg/kg i.p.) before receiving glibenclamide (5 mg/kg/day).
Group 4 & 5: Test group in which the rats received alloxan (150 mg/kg i.p.) and a methanolic extract of the entire aerial part of S. wightiana (200 & 400 mg/kg/day).
Group 5 & 6: Alloxan (150 mg/kg i.p.) had previously been given to the rats in the test group, who also received daily doses of isolated pure components from S. wightiana (qurecetin and rutin, each at 5 mg/kg/day).
Through a feeding cannula, plant extracts, the standard drug glibenclamide (5 mg/kg), and saline were given. The normal control group (group 1), received saline for 14 days. Group 2 contains the diabetic control rats. Group 3 served as the standard control group, which received glibenclamide (5 mg/kg) for 14 days. Groups 4 to 5 (previously received alloxan) received a constant dose of SW (200 & 400 mg/kg, p.o.), were administered daily with pure isolated compounds 5 mg/kg. On the first day, a homogeneous suspension of the extracts and the standard drug glibenclamide (5 mg/kg) was freshly prepared individually using saline and alloxan, and the test compounds were administered for 14 days. Instead of drugs, the normal control group was given a vehicle.
2.12.3 Induction of diabetes in experimental rats
Rats developed diabetes after receiving a single i.p. injection of alloxan monohydrate (150 mg/kg) (Guruvayoorappan and Sudha, 2008; Rathnaker et al, 2011). Each animal's dosage of alloxan was determined individually based on body weight before being dissolved in 0.2 ml of saline (154 mM NaCl) right before injection. Two days following alloxan injection, rats having plasma glucose levels of 200 mg/dl were enrolled in the study. 48 h after the alloxan injection, the animals started receiving treatment with plant extracts.
2.12.4 Blood sample collection for the measurement of glucose
Up to the end of the study, blood samples were taken from the rat's tip of the tail on a weekly basis (i.e., 2 weeks). On the first, seventh, and fourteenth study days, measures of body weight and fasting blood glucose were taken. Using a one-touch electronic glucometer and glucose test strips, blood sugar levels were tested using a procedure based on the glucose dehydrogenase method. Days 0, 7, and 14 after treatments were used to measure blood glucose levels. Under light ether anaesthesia, blood was taken from the retro-orbital plexus of overnight fasting rats on day 14. A blood sample without anticoagulant was spun at 3,000 × g for 5 min to extract serum for biochemical parameter measurement. Until biochemical parameters were assessed, serum was stored at −20 °C. Utilizing the A15 Analyzer Automatic Clinical Chemistry (Biosystems S.A., Spain), the serum was examined. Glucose, total cholesterol, triglycerides, creatinine, serum HDL, LDL, urea, and alkaline phosphatase were the biochemical markers that were assessed.
2.13 Statistical analysis
Results were displayed as mean ± standard error mean (SEM). The one-way ANOVA and Dunnett's test were used to analyze the deviations using GraphPad Instat software (San Diego, CA, USA). P was regarded as statistically significant at 0.05.
3 Results
3.1 Fluorescence analysis of S. Wightiana
Under both daylight and UV light, S. wightiana (#40) whole plant powder was studied. The findings are displayed in Table S1. S. wightiana showed different fluorescence characteristics with different treatments.
3.2 Phytochemical screening
3.2.1 Phytochemical analysis of multiple extracts
The results for different fractions of S. wightiana fractions, such as PE, chloroform, EA, methanol, and aqueous fractions are summarized in Table S2. Different group of compounds was detected in different fractions of S. wightiana.
3.2.2 Phytochemical screening of methanol extract of S. wightiana
The whole plant of S. wightiana was extracted with methanol in a Soxhlet system and examined for phytochemicals. Table S3 provides a summary of the findings.
3.2.3 Fat and volatile content of S. wightiana
S. wightiana has been found to have fat and volatile levels of 2.05% and 0.5% (flowers), respectively.
3.2.4 Saponin content of S. wightiana
The saponin content of S. wightiana was determined as 48.4 mg/g.
3.2.5 Thin layer chromatography profile
The results for TLC profile of different fractions of S. wightiana are summarized in Table S4. Different phytoconstituents were separated with different Rf values.
3.3 Determination microbial load in S. wightiana
The results for microbial load of dilutions of S. wightiana are summarized in Table S5. The microbial load was negligible in control. In addition, no microbial colonies were countable in different dilutions of S. wightiana.
3.4 Heavy metal and mineral contents
The results for heavy metal contents of S. wightiana are summarized in Table 1. All the heavy metals such, as Pb, Cd, Hg, As, Cr, and Ni were detected in very low amount and acceptable limits.
Heavy metal
Amount (ppm)
Limit (ppm)
Lead (Pb)
0.005
NMT 10.0
Cadmium (Cd)
0.051
NMT 0.3
Mercury (Hg)
0.2
NMT 1.0
Arsenic (As)
0.61
NMT 10.0
Chromium (Cr)
0.127
NMT 2.0
Nickle (Ni)
0.016
NMT 1.63
The results for mineral contents of S. wightiana are summarized in Table 2. All the minerals such, as Co, Mn, Fe, Zn, and Cu were also detected in very low amount and acceptable limits.
Mineral
Amount (ppm)
Permissible limit of mineral
Cobalt (Co)
0.057
No regulatory limits by WHO
Manganese (Mn)
0.085
No regulatory limits by WHO
Magnesium (Mg)
3.408
No regulatory limits by WHO
Iron (Fe)
0.095
NMT 20.0 ppm
Zinc (Zn)
0.087
NMT 27.4 ppm
Copper (Cu)
0.054
NMT 3.00 ppm
3.5 In-Vitro antioxidant study
Antioxidant capacity of methanol extract of S. wightiana was carried out using different methods (Ajileyea et al 2015, Izuta et al 2009).
3.5.1 Total flavonoid content
A spectrophotometric technique was used to measure the flavonoid content using an AlCl3 solution. The quantity of flavonoids in extracts was then quantified in terms of rutin equivalent (mg rutin/g of extract), depending on the concentration of flavonoid that was read (mg/ml) on the calibration line based on the observed absorbance. Rutin made up 113.74 mg of the total flavonoid content per gram of extract.
3.5.2 Total phenolic content
The total phenolic content was ascertained using the Folin-Ciocalteu colorimetric technique. As mg GAE/g, the total phenolic content was measured. 249.15 mg GAE/g of phenols were discovered to be present overall.
3.5.3 Total antioxidant capacity
Using AA as a reference, the total antioxidant capacity (TAC) was calculated. TAC mg of AA/g was used to calculate the TAC. The TAC for AA was determined to be 195.54 mg/g.
3.5.4 DPPH radical scavenging activity
As a recognized benchmark for free radical scavenging activity, rutin was chosen. Table 3 lists the findings for DPPH scavenging activity. Standard rutin and S. wightiana extract both had IC50 values of 269.46 and 549.36 g/ml, respectively. *The values present the mean of three measurements ± SD.
Plant extract
Conc. (µg/ml)
Absorbance
Inhibition (%)
IC50 (µg/ml)
Rutin (200 µg/ml)
100
0.916
24.48 ± 0.018
269.46
200
0.684
43.61 ± 0.016
300
0.504
58.45 ± 0.013
400
0.356
70.65 ± 0.025
500
0.292
75.92 ± 0.026
600
0.220
81.86 ± 0.024
200
0.937
26.45 ± 0.016
300
0.572
55.102 ± 0.023
400
0.392
69.230 ± 0.041
500
0.252
80.219 ± 0.011
600
0.182
85.714 ± 0.017
S. wightiana SWM (1000 µg/ml)
100
0.956
10.904 ± 0.013
539.36
200
0.851
20.689 ± 0.028
300
0.737
31.314 ± 0.020
400
0.677
36.905 ± 0.070
500
0.581
45.852 ± 0.021
600
0.476
55.638 ± 0.026
3.5.5 Reducing power
BHT was chosen as a standard for reducing power assessment. The results for reducing power assessment are summarized in Table 4. Standard showed excellent reducing power. Good reducing power potential was also seen in S. wightiana extract.
Plant extract
Conc. (µg/ml)
Absorbance
Standard/BHT (1000 µg/ml)
100
0.796
200
1.508
300
2.444
400
3.120
500
3.219
600
3.285
SWM (1000 µg/ml)
100
0.381
200
0.473
300
0.682
400
0.846
500
1.009
600
1.115
3.6 Isolation of flavonoids from methanol extract of whole aerial part of Sambucus wightiana
Compounds C-1 and C-2 were isolated from the whole plant of S. wightiana.
3.6.1 Compound 1: Quercetin 3-O-β-D-glucopyranoside (C-1)
Quercetin 3-O-β-D-glucopyranoside (C-1) was obtained as creamish powder. The compound also produced color reactions for a hydroxyl flavone with a variety of reagents. The following physicochemical properties were found in compound C-1.
Chemical formula: C21H20O12.
Melting point = 190–260 °C.
UV–Vis: (ethanol) λmax; 245, 345 nm.
HR Mass: Molecular weight: 464.09, M+1 (465.24) (Figure S1).
NMR: 1H NMR (chemical shift δ in ppm, coupling constant J in Hz) 6.20 (1H, d, J = 2 Hz, C6-H), 6.42 (1H, d, J = 2 Hz, C8-H),7.69 (1H, d, J = 2.2 Hz, C2′-H), 6.90 (1H, d, J = 8.5 Hz,C5′-H),7.56 (1H, dd, J = 8.5,2.2 Hz, C6′-H),9.41(1H, s, C4′-OH), 9.35 (1H, s, C3′OH), 9.63 (1H, s, C3-OH), 12.52 (1H, s, C5-OH), 10.82 (1H, s, C7-OH) (Figure S2).
13C NMR (chemical shift δ in ppm) 148.40 (C-2),134.84 (C-3), 178.26 (C-4), 157.13 (C-5), 98.40 (C-6), 164.48 (C-7), 93.30 (C-8), 161.83 (C-9), 104.5 (C-10), 121.57 (C-1′), 115.53 (C-2′), 145.02 (C-3′), 148.40 (C-4′), 114.9 (C-5′), 121.4 (C-6′) (Figure S3). The final molecular structure of compound C-1 is presented in Figure S4. And these data were matched with references (Mir et al., 2017).
3.6.2 Compound 2: Rutin
Rutin was obtained in the form of a yellowish needle/amorphous solid. The compound also produced colour reactions for a hydroxyl flavone with a variety of reagents. The TLC of rutin in solvent system i.e. ethyl acetate: glacial acetic acid: formic acid: water (100:11:11:25) showed single spot at Rf = 0.42. The following physicochemical properties were recorded in compound C-2.
Chemical formula: C27H30O16.
Melting point: 190–195 °C.
UV (ethanol) ƛmax = 252, 358 nm.
FT-IR (KBR, cm−1): 3434.56, 2938.92, 2087.17, 1647.38, 1504.88, 1458, 1363, 1235, 1204, 1132, 1063, 969, 826, 728, 656, 631, 596, 453 (Figure S5).
HR Mass: Molecular weight: M/Z 610.52 (M + ) (Figure S6).
1H NMR: (CD3OD; Chemical shift δ in ppm, coupling constant J in Hz) 6.21 (1H, d, J = 2, C6-H), 6.40 (1H, d, J = 2, C8-H),7.63 (1H, d, J = 2.1, C2′-H),6.86 (1H, d J = 9, C5′-H),7.66 (1H, dd, J = 9,2.1, C6′-H),8.05 (1H, s, C4′-OH), 8.07 (1H, s, C3′-OH), 12.34 (1H, s, C5-OH), 10.86 (1H, s, C7-OH), 5.23– (1H, d, J = 7.4, H1-G), 5.11 (1H, d, J = 6.45, H1-R), 1.11 (3H, d, J = 6.74, CH3-R) (Figure S7).
13C NMR: (chemical shift δ in ppm) 157.94 (C-2), 134.22 (C-3), 178.03 (C-4), 157.9 (C-5), 98.54 (C-6), 164.64 (C-7), 93.45 (C-8), 161.6 (C-9), 104.21 (C-10), 122.14 (C-1′), 114.65 (C-2′), 144.45 (C-3′), 148.41 (C-4′), 116.28 (C-5′), 121.7 (C-6′),103.30 (C1-G), 74.33 (C2-G), 76.79 (C3-G), 72.54(C4-G), 75.84 (C5-G), 67.15 (C6-G), 101.02 (C1-R), 68.31 (C2-R), 70.84 (C3-R), 70.7 (C4-R), 70.0 (C5-R), 16.47 (C6-R) [R and G represent signals from rhamnose and glucose moieties, respectively] (Figure S8). The final molecular structure of compound C-2 is presented in Figure S9. And these data were matched with references (Mir et al., 2017).
3.7 Acute toxicity studies
The extracts/fractions/compounds were observed to be safe and based on safe dose. The experimental doses were selected for S. wightiana for animal studies. The acute oral toxicity study of S. wightiana showed no mortality up to 3000 mg/kg for whole plant extract while successive extracts were also found safe up to 1600 mg/kg. The pure compounds flavonoids such as qurecetin and rutin were found safe up to 100 mg/kg.
3.7.1 Effect on total cholesterol
The effect of methanol extract and isolated compounds of S. wightiana on total cholesterol levels in alloxan induced diabetic albino rats after 14 days of treatment is shown in (Fig. 1). The result exhibits that alloxan induced diabetic control (DC) group albino rats show increase in total cholesterol levels (271.16 ± 10.5 mg/dl) compared to the normal control (NC) group (145.36 ± 3.2 mg/dl) given only vehicle. However, the standard glibenclamide (67.33 ± 1.856 mg/dl) significantly decreased the serum total cholesterol levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). The methanol extract of S. wightiana also decreased the serum total cholesterol levels compared to the DC group in the ascending order as SW-200 mg/Kg (158.46 ± 5.6 mg/dl) and SW-400 mg/Kg (138.46 ± 4.6 mg/dl) (p < 0.05). In case of isolated compounds, the order follows as rutin (5 mg/Kg) (148.32 ± 5.3 mg/dl) < qurecetin (5 mg/Kg) (144.38 ± 4.1 mg/dl) (p < 0.05).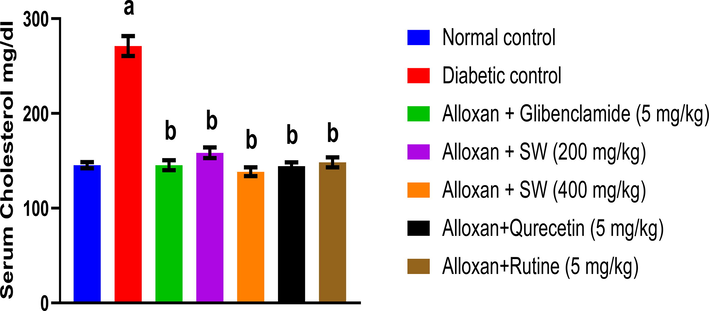
Effect of methanolic extract and isolated compound on total cholesterol level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.001 compared' to NC and bp < 0.001 compared to DC.
3.7.2 Effect on high density lipoprotein (HDL) cholesterol
The effect of alloxan (150 mg/kg, i.p.) administration on HDL levels in DC produced significantly lower levels of HDL cholesterol in serum when compared to the normal control group (p < 0.05). The effect of methanol extract and isolated compounds of S. wightiana on HDL levels in alloxan induced diabetic albino rats is shown in (Fig. 2). The result reveals that alloxan induced DC group rats shows decrease in HDL level (30.0 ± 1.9 mg/dl) compared to NC group (36.83 ± 2.5 mg/dl) given only vehicle. However, the standard glibenclamide (36.73 ± 1.5 mg/dl) significantly increased the HDL levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). From both the methanol extracts of S. wightiana, highly significant activity was observed in SW-200 mg/Kg (36.63 ± 2.1 mg/dl) (p < 0.05), while in case of isolated compounds, highly significant increase in serum HDL level was observed by qurecetin (5 mg/Kg) (39.71 ± 1.8 mg/dl) (p < 0.05).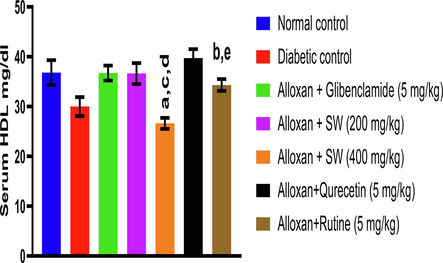
Effect of methanolic extract and isolated compound on HDL level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.05 compared to NC, bp < 0.05 compared to DC, cp < 0.05′ compared to alloxan + glibenclamide (5 mg/kg), dp < 0.05 compared' to alloxan + SW (200 mg/kg), ep < 0.05 compared to alloxan + SW (400 mg/kg).
3.7.3 Effect on low density lipoprotein (LDL) cholesterol
After 14 days of treatment, the effect of methanol extract and isolated compounds of S. wightiana on LDL levels in alloxan-induced diabetic albino rats is shown in Fig. 3. The result showed that alloxan induced DC group in rats shows increase in LDL levels (189 ± 12.4 mg/dl) compared to the NC (91.32 ± 1.2 mg/dl) given only vehicle. However, the standard glibenclamide (92.35 ± 3.1 mg/dl) significantly decreased LDL levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). The methanol extracts of S. wightiana showed highly significant anti-hyperglycemic activity (p < 0.05). From the methanolic extracts, alloxan/SW-400 mg/Kg (89.55 ± 2.5 mg/dl) showed highly significant decrease in serum LDL level (p < 0.05). Thus, authenticating the traditional claim of the studied crude drug as potent anti-hyperglycemic agent.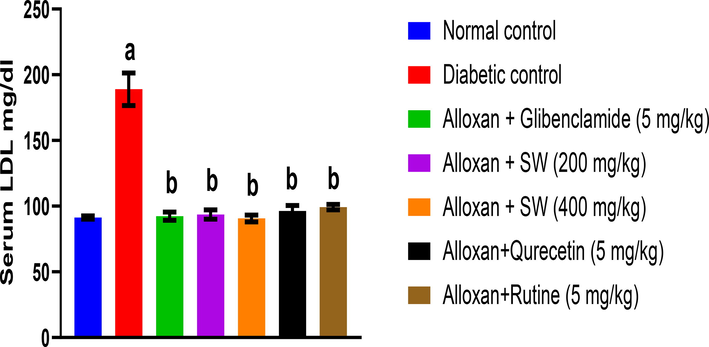
Effect of methanolic extract and isolated compound on LDL level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.001 compared to NC, bp < 0.001 compared to DC, cp < 0.001 compared to alloxan + glibenclamide (5 mg/kg), dp < 0.001 compared to alloxan + SW (200 mg/kg), ep < 0.001 compared to alloxan + SW (400 mg/kg), fp < 0.001 compared to alloxan + qurecetin (5 mg/Kg).
3.7.4 Effect on creatinine levels
After 14 days of treatment, the effect of methanol extract and isolated compounds of S. wightiana on creatinine levels in alloxan-induced diabetic rats is shown in Fig. 4. The result showed that alloxan induced DC group shows increase in creatinine levels (2.4 ± 0.1 mg/dl) compared to the NC group (0.54 ± 0.3 mg/dl) given only vehicle. However, the standard glibenclamide (0.58 ± 0.1 mg/dl) significantly decreased creatinine levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). The methanol extracts of S. wightiana showed highly significant anti-hyperglycemic activity (p < 0.05). Thus, authenticating the traditional claim of the studied crude drug as potent anti-hyperglycemic agents.
Effect of methanolic extract and isolated compound on creatinine level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.001 compared to NC and bp < 0.001 compared to DC.
3.7.5 Effect on urea levels
After 14 days of treatment, the effect of methanol extract and isolated compounds of S. wightiana on urea levels in alloxan-induced diabetic rats is shown in Fig. 5. The result showed that alloxan induced DC group shows increase in urea levels (62.6 ± 1.8 mg/dl) compared to the NC group (31.83 ± 2.2 mg/dl) given only vehicle. However, the standard glibenclamide (31.24 ± 4.0 mg/dl) significantly decreased urea levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). S. wightiana methanol extracts demonstrated highly significant anti-hyperglycemic activity (p < 0.05). From the methanolic extracts of S. wightiana at a dose of alloxan/SW-400 mg/Kg (30.33 ± 1.2 mg/dl) showed highly significant decrease in serum urea level (p < 0.05). Thus, authenticating the traditional claim of the studied crude drug as potent anti-hyperglycemic agent.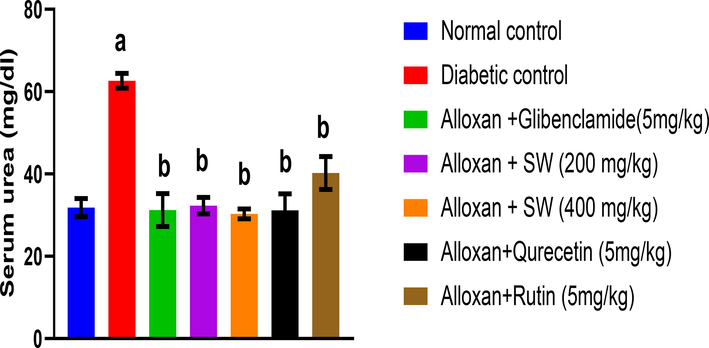
Effect of methanolic extract and isolated compound on urea level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.001 compared to NC, and bp < 0.001 compared to DC.
3.7.6 Effect on ALP levels
After 14 days of treatment, the effect of methanol extract and isolated compounds of S. wightiana on serum ALP levels in alloxan-induced diabetic rats is shown in Fig. 6. The result showed that alloxan induced DC rats shows increase in serum ALP levels (276 ± 3.6 mg/dl) compared to the NC group (120 ± 3.2 mg/dl) which were given only vehicle. However, the standard glibenclamide (130.75 ± 2.9 mg/dl) significantly decreased ALP levels compared to DC indicating the anti-hyperglycemic activity (p < 0.05). The methanol extracts of S. wightiana showed highly significant anti-hyperglycemic activity (p < 0.05). From the methanolic extracts of S. wightiana, a dose of alloxan/SW-400 mg/Kg (111.55 ± 6.9 mg/dl) also decreased significantly the serum ALP level (p < 0.05). Thus, authenticating the traditional claim of the studied crude drug as potent anti-hyperglycemic agent.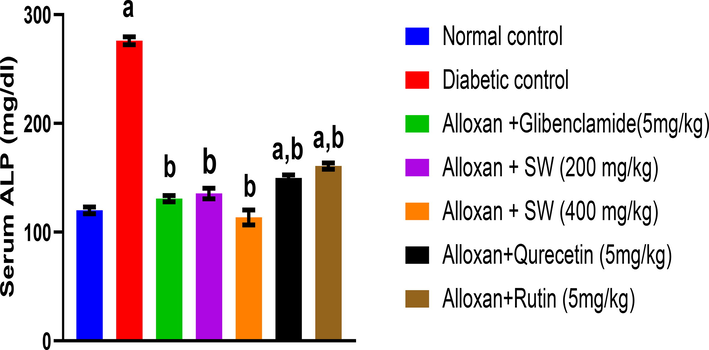
Effect of methanolic extract and isolated compound on ALP level in alloxan-induced diabetic rats. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA followed by Tukey’s multiple comparisons' test; ap < 0.001 compared to NC and bp < 0.05 compared to DC.
3.7.7 Anti-Hyperglycemic activity
The purpose of this study was to investigate the efficacy of a methanolic extract of S. wightiana and its isolated compounds against alloxan-induced diabetes, as well as their effect in a glucose tolerance test (GTT). The activity was found dose dependent in the extracts of both the plants while isolated compound at dose level of 5 mg/kg showed the protection and antidiabetic potential as compared to the standard glibenclamide (5 mg/kg). Therefore, both the plants have potential antidiabetic activity.
3.7.8 Effect of glucose tolerance test
The data shown in (Fig. 7 A-D) indicates that S. wightiana and isolated compounds remained persistently euglycemic throughout the experimental period. The initial plasma glucose level in DC group was greater compared to methanolic extract of S. wightiana, and isolated compounds treated groups. During the experimental period in DC group, the glucose level was elevated from 67 to 98 mg/dl. Whereas, in the methanolic extract of S. wightiana (glucose + SW 400 mg/kg, p.o) group, the elevated glucose level was gradually decreased from 67 to 60 mg/dl. Whereas, in the DC group treated with S. wightiana, a significant antihyperglycemic effect was evident from the 7th day, and the decrease in glucose was maximum and reached near normal levels by the 14th day of treatment (p < 0.05).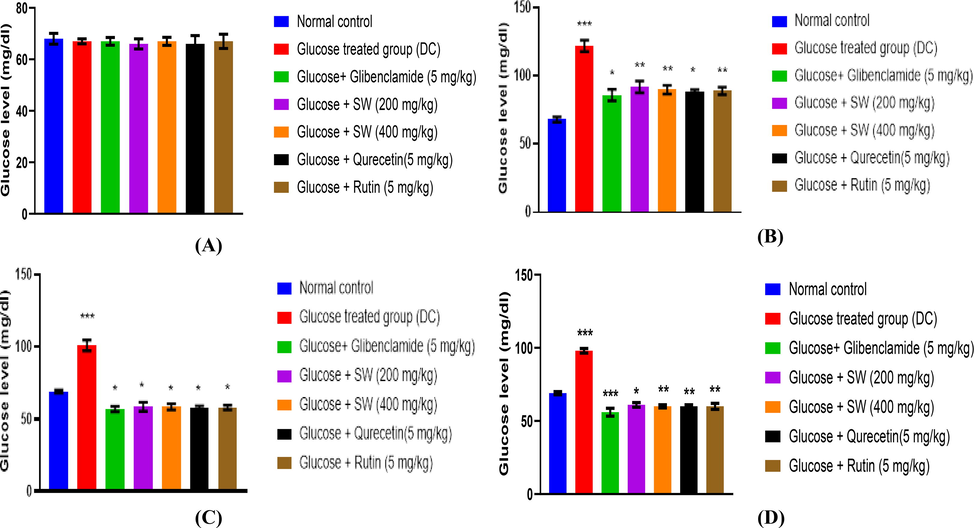
Graphical representation of effect of methanolic extract of S. wightiana on glucose level at (A): 0 min; (B): 30 mins; (C): 90 mins; (D): 150 mins in alloxan induced diabetic rats after 14 days of treatment. All values are presented as mean ± SEM, n = 3. Data was analysed by ANOVA; all groups were compared with the NC group followed by Tukey’s test. No significance difference was found (A); *p < 0.05; **p < 0.01; ***p < 0.001 (B); *p < 0.05; ***p < 0.001 (C); *p < 0.05; ***p < 0.001 (D).
Chronic treatment of diabetic rats with methanolic extract of S. wightiana for 14 days reduced plasma glucose levels to near normal levels (Fig. 8). The plasma glucose levels in the NC and DC groups during the experiment clearly show that studied crude drug had antihyperglycemic activity.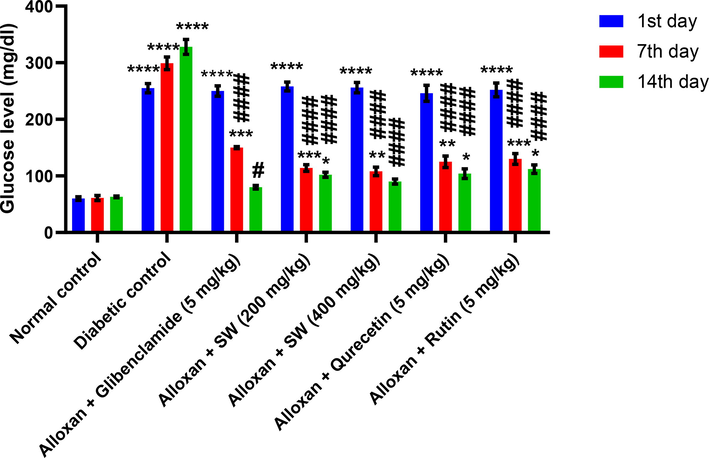
Graphical representation of effect of methanolic extract/isolated compounds of S. wightiana on glucose level on 1st, 7th, and 14th day in alloxan induced diabetic rats after 14 days of treatment.
3.7.9 Effect of glucose on body weight
The data given in Fig. 9 revealed a gradual increase in the body weight of DC rats at 1, 7 and 14 days of experimental period when compared to corresponding initial weights. Thus, the results revealed the beneficial effect of methanolic extract of S. wightiana and isolated compounds treatment against the weight loss observed under diabetic conditions.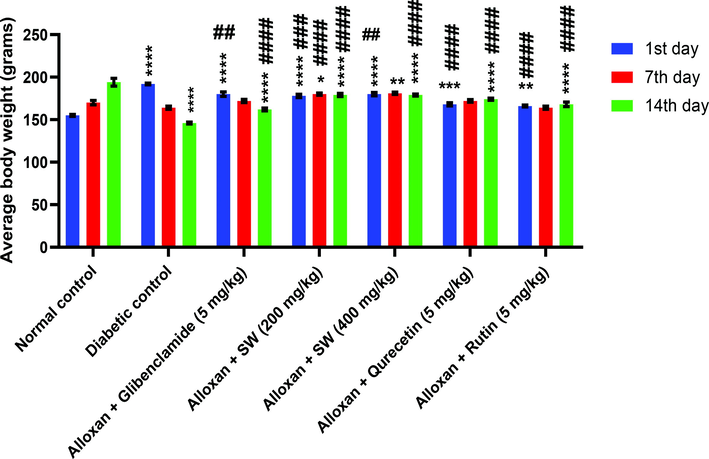
Graphical representation of effect of methanolic extract/isolated compounds of S. wightiana on glucose level on 1st, 7th and 14th day in alloxan induced diabetic rats after 14 days of treatment.
4 Discussion
The present work demonstrates the physicochemical, preliminary phytochemical investigation, and pharmacological evaluation of S. wightiana, which will help in the appropriate identification of these plants for future investigation as the pharmacognostic study is a primary and reliable criterion in the identification and determination of quality and purity of crude drugs (Bhattacharya and Zaman, 2009). The plant material should be entirely free from innocuous foreign matter. The percentage of foreign matter was found to be negligible in S. wightiana indicating their quality. The higher water content in medicinal plants encourages the growth of various microbes, the presence of insects, or any fungal growth and deterioration ultimately leading to hydrolysis (Shoaib et al 2020, Nabi et al 2022). Thus, there should be a limit set for the water content of every drug. The limit of detection of the dry powder of S. wightiana was 3.20%, which was within the prescribed limits. The pH value determination at different concentrations gives an idea about the presence of basic and acidic types of phytoconstituents present in the plant materials. The pH value of 1% and 10% solution of the aerial part of S. wightiana were determined to be 6.54 and 6.24, respectively, suggesting the presence of acidic compounds in the plant (Shoaib et al 2020). In most fruits and vegetables, flavonoids and phenolics are the main anti-oxidative compounds (Le et al., 2014), which have considerably high free radical scavenging activities. In this regard, the methanolic extract of S. wightiana was screened for flavonoid content and was investigated using the AlCl3 method (Quettier-Deleu et al., 2000), and phenolic content was determined using Prussian blue assay (Kupina et al 2018). Rutin equivalents per gram of dry weight (mg RU Eq/g) and gallic acid equivalents per gram of dry weight (mg GA-Eq/g) were used to express the total flavonoid content and total phenolic content, respectively. According to the findings of this study, methanol extracts of S. wightiana plants have high antioxidant activity. The antioxidant potential of S. wightiana may be attributed to the number of compounds discovered during extract phytochemical screening. S. wightiana's high concentration of phytochemicals and secondary metabolites, including alkaloids, phenolics, flavonoids, terpenoids, and tannins, is what gives it its antioxidant capabilities (Mohiuddin et al 2022).
Diabetics develop vascular diseases prematurely and with increased frequency when compared to nondiabetics (Galicia-Garcia et al 2020, Asmat et al 2016). It has been suggested that the hyperlipidemia (Prabhavathi et al 2014, Sarfraz et al 2016) (Shoaib et al., 2022; Shoaib et al., 2020) observed in non-ketotic diabetes may be related to the increased incidence of vascular diseases. Diabetics with vascular complications have higher serum cholesterol (Poznyak et al 2020) than diabetics without complications. Diabetes has also been affiliated with an inflated risk for developing premature atherosclerosis due to increase in triacylglycerides and LDL levels and decrease in HDL levels (Katakami, 2018). Possible modifications of lipoproteins, such as LDL, include non-enzymatic glycation and oxidation. Lipoproteins undergo non-enzymatic glycation in the presence of glucose, just as other serum proteins (Ghaffari and Mojab, 2007). The enhanced serum LDL and plasma glucose concentration observed under diabetic conditions are favorable for increased non-enzymic glycation of LDL. There is evidence that after chemical modification, such as glycation, LDL gains or increases its atherogenic potential (Rabbani et al 2011). This explains how LDL contributes to the early onset of atherosclerosis in diabetes patients (Aronson and Rayfield, 2002). The results showed that S. wightiana methanolic extract and isolated compounds significantly reduced blood sugar levels in alloxan-induced hyperglycemic rats while having no discernible effects on body weight. According to measurements including the lipid profile, serum creatinine, serum urea, and serum ALP, they can also help diabetics feel better. Our research indicates that methanolic extract at a high dose (400 mg/kg) is more effective than at a low dose (200 mg/kg) after 14 days of treatment. As a result of the discussion above, the plant's methanolic extract has a similar impact to the industry standard, glibenclamide (5 mg/kg). This could be because the extracts act on some β-cells to exert their insulin-releasing effect. Similarly, isolated compounds demonstrated antihyperglycemic activity at 5 mg/kg. p.o. The current diabetes treatments are associated with undesirable side effects and have limited efficacy. The findings of this study suggest that plant extracts and isolated compounds can effectively control serum lipid and blood glucose levels while being less toxic. The extract of S. wightiana and isolated compounds were the most effective in lowering lipid levels, blood glucose levels, and liver enzymes.
Long-term treatment is typically acknowledged for herbal medications. Therefore, any development in this area will be very significant to people everywhere, especially in nations where a sizable segment of the populace relies on alternative medicine to meet their healthcare needs. Furthermore, given its remarkable antioxidant activity against a wide range of diseases, a formulation that can be subjected to similar studies and commercialized in the future could be developed (Shoaib et al 2022).
5 Conclusion
Despite major strides in understanding and managing diabetes, still managing the complications arising out of the disease is an unabated challenge. Although an extensive range of antidiabetics in the market are available, medicines derived from herbs have been successfully used to treat this disease with minimal side effects. The observed results from this study revealed that the methanolic extract of S. wightiana has highly significant anti-hyperglycemic and antioxidant activity. The methanolic extract of S. wightiana at a dose of 400 mg/Kg exhibited substantial lipid, blood glucose levels, and liver enzyme lowering capacity compared to the diabetic control group. Also, S. wightiana exhibited the effect of decreasing the elevated urea, and creatinine levels and maintaining the total weight within the body. Studies on humans are essential to establish the role of S. wightiana as an antihyperglycemic agent. Once the safety and efficacy of these extracts will be established in humans, the formulation can be developed from the same. These formulations should be expected to be very economical and easily available antihyperglycemic agents.
Acknowledgments
This research was funded by the Researchers Supporting Project (number RSP2023R146) at King Saud University, Riyadh, Saudi Arabia. "The authors are also thankful to AlMaarefa University for their generous support".
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of University of Kashmir (Approval No. 2012/07).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation and characterization of antioxidant and antimicrobial compounds from Anacardium occidentale L. (Anacardiaceae) leaf extract. J. King Saud Uni. Sci.. 2015;27:244-252.
- [Google Scholar]
- Some physicomechanical and nutritional properties of berberry (Berberis vulgaris L.) fruits. J. Food Process. Eng.. 2009;32:497-511.
- [Google Scholar]
- Protective role of herbal formulation-divine noni againstcisplatin-induced cytotoxicity in healthy cells by activating Nrf2 expression: an in-vivo and in-vitro approach. Phytomed. Plus. 2021;1:100009
- [CrossRef] [Google Scholar]
- How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc. Diabetol.. 2002;1:E1.
- [Google Scholar]
- Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J.. 2016;24:547-553.
- [Google Scholar]
- Analysis of fatty acid and determination of total protein and phytochemical content of Cassia sophera Linn leaf, stem, flower, and seed. Beni-Suef Univ. J. Bas. Appl. Sci.. 2019;8:E3.
- [Google Scholar]
- Traditional medicinal plants of cold desert Ladakh—Used against kidney and urinary disorders. J. Ethnopharmacol.. 2008;118:331-339.
- [Google Scholar]
- Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol.. 2006;100:1164-1170.
- [Google Scholar]
- In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu.. 2013;34:209-214.
- [Google Scholar]
- Pharmacognostical evaluation of Zanthoxylum nitidum bark. Int. J. PharmTech. Res.. 2009;1:292-298.
- [Google Scholar]
- Antioxidant effects of roots of Clerodendrum serratum Linn. Phcoh. Res.. 2009;1:294-298.
- [Google Scholar]
- Antimicrobial studies of Sambucus wightiana Wall. ex. Wight & Arn. J. Pharm. Res.. 2012;5:2467-2468.
- [Google Scholar]
- Pharmacognostical and phytochemical evaluation of Angelica archangelica Linn. Int. J. Drug Dev. Res.. 2011;3:173-188.
- [Google Scholar]
- Influence of flavonols as in vitro on low density lipoprotein glycation. Iran Biomed. J.. 2007;11:185-191.
- [Google Scholar]
- Phytopharmacological evaluation of byesukar for hypoglycaemic activity and its effect on lipid pro-file and hepatic enzymes of glucose metabolism in diabetic rats. Ann. Hepatol.. 2008;7:358-363.
- [Google Scholar]
- 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of bee products and their constituents determined by ESR. Biol. Pharm. Bull.. 2009;32:1947-1951.
- [Google Scholar]
- Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscl. Thromb.. 2018;25:27-39.
- [Google Scholar]
- Antioxidants in fruits and vegetables-the millennium′s health. Int. J. Food. Sci. Technol.. 2001;36:703-725.
- [Google Scholar]
- Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.. 2011;48:412-422.
- [Google Scholar]
- In vitro enzyme inhibition activities of crude ethanolic extracts derived from medicinal plants of Pakistan. Nat. Prod. Res.. 2005;19:567-1557.
- [Google Scholar]
- Optimisation of nitrate reductase enzyme activity to synthesise silver nanoparticles. IET nanobiotechnology. 2016;10:158-161.
- [CrossRef] [Google Scholar]
- Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res.. 2017;61:E1361779.
- [Google Scholar]
- Antioxidant properties of blackberry and blueberry fruits grown in the black sea region of Turkey. Sci. Hort.. 2009;121:447-450.
- [Google Scholar]
- Picrorhiza kurroa enhances β-cell mass proliferation and insulin secretion in streptozotocin evoked β-cell damage in rats. Front. Pharmacol.. 2017;8:E537.
- [Google Scholar]
- An ethnobotanical study of medicinal plants used by the locals in Kishtwar, Jammu and Kashmir, India. Ethnobotanical Leaflets. 2009;13:1240-1256.
- [Google Scholar]
- Determination of total phenolic content using the Folin-C assay: Single-laboratory validation, first action. J. AOAC Int.. 2018;101:1466-1472.
- [Google Scholar]
- Processed vietnamese ginseng: Preliminary results in chemistry and biological activity. J. Ginseng Res.. 2014;38:154-159.
- [Google Scholar]
- Isolation, Characterization and Bioactivities of Sambucus wightiana derived Dotriacontanoic acid. Int. J. Trend Sci. Res. Dev.. 2017;1:1323-1332.
- [CrossRef] [Google Scholar]
- GC-MS analysis, phytochemical screening, and antibacterial activity of Cerana indica propolis from Kashmir region. Separations. 2022;9:E363.
- [Google Scholar]
- Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem.. 2002;50:519-525.
- [Google Scholar]
- Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr.. 2004;58:244-249.
- [Google Scholar]
- Phytochemical profiling and antibacterial activity of methanol leaf extract of Skimmia anquetilia. Plants. 2022;11:E1667.
- [Google Scholar]
- Phytochemical studies and comparative efficacy of crude extracts of some homeostatic plants in Edo and Delta states of Nigeria. Global J. Pure Appl. Sci.. 2001;8:203-208.
- [Google Scholar]
- The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci.. 2020;21:E1835.
- [Google Scholar]
- Glycosylated haemoglobin (HbA1c)- A marker of circulating lipids in type 2 diabetic patients. J. Clin. Diag. Res.. 2014;8:20-23.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: Specific application for the determination of Vit E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr.. 2003;78:570-578.
- [Google Scholar]
- Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol.. 2000;72:35-42.
- [Google Scholar]
- Glycation of LDL by methylglyoxal increases arterial atherogenicity: a possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes. 2011;60:1973-1980.
- [Google Scholar]
- Analytical techniques in biochemistry and molecular biology. New York, XVIII: Springer; 2018. p. :441.
- Hypoglycemic activity of a polyherbal product in alloxan induced diabetic rats. Drug Invent. Today. 2011;3:1-2.
- [Google Scholar]
- Prevalence and pattern of dyslipidemia in hyperglycemic patients and its associated factors among Pakistani population. Saudi J. Biol. Sci.. 2016;23:761-766.
- [Google Scholar]
- Antidiabetic activity of standardized dried tubers extract of Aconitum napellus in streptozotocin-induced diabetic rats. 3 Biotech. 2020;10:E56.
- [Google Scholar]
- Integrating nanotechnology with naturally occurring phytochemicals in neuropathy induced by diabetes. J. Mol. Liq.. 2022;350:E118189.
- [Google Scholar]
- A comparative evaluation of the anticancer properties of European and American elderberry fruits. J. Med. Food. 2006;9:498-504.
- [Google Scholar]
- Selectivity of current extraction techniques for flavonoids from plant materials. Processes. 2020;8:E1222.
- [Google Scholar]
- Scientific appraisal and therapeutic properties of plants utilized for veterinary care in Poonch district of Jammu and Kashmir. India. Biology. 2022;11:E1415.
- [Google Scholar]
- Hypoglycaemic and anti-diabetic activity of stem bark extracts Erythrina indica in normal and alloxan-induced diabetic rats. Saudi Pharm. J.. 2011;19:35-42.
- [Google Scholar]
- Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res.. 2007;51:675-683.
- [Google Scholar]
- Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med.. 2018;13:E20.
- [Google Scholar]
- Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods. 2019;8:E326.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105170.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
: Mass spectra (TOFMS ES+) of compound C-1; : 1H NMR spectra of compound C-1; : 13CNMR spectra of compound C-1; : Molecular structure of compound C-1: Quercetin glycoside/Quercetin 3-O-β-D-glucopyranoside; : FTIR spectra of compound C-2 (rutin); : TOF MS ES + 2.19 e3 of rutin; : 1H NMR spectra of rutin; : 13C NMR spectra of rutin; : Molecular structure of rutin (C-2); : Results of fluorescence analysis; : Phytochemical analysis of successive Sambucus wightiana extracts; : Phytochemical screening of total methanol extract; : TLC of extracts of Sambucus wightiana with Rf values of various phytoconstituents; : Colony forming units on nutrient agar medium.







