Translate this page into:
Cell Lysis, labeling efficacy and biodistribution of intravenously administered Technetium-99 m labeled multifunctional zirconia nanoparticles in animal model
⁎Corresponding authors. saira.cssp@pu.edu.pk (Saira Riaz), shahzad.cssp@pu.edu.pk (Shahzad Naseem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Sol-gel technique was utilized to synthesized Fe3O4 stabilized ZrO2 nanoparticles with various wt. % of iron oxide (Fe3O4). XRD studies showed that synthesized nanoparticles exhibited tetragonal ZrO2 using 5––10 wt% iron oxide content. Relatively higher value of hardness (∼1114 HV – 1278 HV) as well as fracture toughness (17.65 MPam−1/2-24.48 MPam−1/2) was observed at 8–10 wt% concentration of iron oxide. High anti-oxidant activity (∼70%) as well as high cell viability against cancerous cell (∼23.4%) was observed for optimized sample. Almost 73% of encapsulation efficiency was observed for optimized sample after time period of 120 min.
Abstract
Nanoparticles, to be used in biomedical applications, have been the subject of extensive investigation in recent years. Recently, zirconia and iron have gained significant relevance to the field due to their superior mechanical and structural qualities as well as their numerous biological applications. In this research, Fe3O4-stabilized zirconia nanopowders are fabricated using a low-cost sol–gel approach. Iron oxide sol is added in 1–10 wt% increments to a 0.1 M zirconia sol. The obtained crystallite size values match well with previously reported values for the tetragonally stable (t-zirconia) phase. Hardness values of approximately 1278 HV, along with fracture toughness values of ∼ 24.48 MPa.m-1/2 are observed for stable tetragonal zirconia. Results from cell lysis experiments indicate that the synthesized nanoparticles have the potential to combat cancerous cells. An encapsulation efficacy of up to ∼ 73% is observed after a 120-minute time period. Radio labeling techniques are employed to label the as-synthesized zirconia nanoparticles using Sodium Pertechnetate (Na99mTcO4) labeled radiopharmaceuticals, allowing for biodistribution assessment. Multiple CT scans of rabbits are performed to obtain accurate results and evaluate their survival. Studies show that radiolabeled nanoparticles are highly absorbed in the animals' bladders, making them a promising choice for tumor therapy. Various physical properties of the animals are periodically examined after administration of the nanoparticle-based injection for several months, and no abnormalities are found. The results obtained through these characterizations indicate that these nanoparticles can be used as future therapeutic agents as well as drug carriers.
Keywords
Iron oxide
Zirconia
Honey
Anticancer
Drug loading
- CT scan
-
computerized tomography scan
- Wt. %
-
Weight percent
- NPS
-
nanoparticles
- PVA
-
Poly vanyl alcohol
- IOZH
-
Honey mediated Fe3O4-stabilized zirconia
- DPPH
-
2, 2, diphenyl-1-picrylhydrazyl
- MTT
-
(3-(4, 5 dimethythiazol-2-yl)-2, 5diphenyl tetrazolium bromide)
- SBF
-
Simulated body fluid
- DMEM
-
Dulbecco's Modified Eagle's Medium
- FBS
-
fetal bovine serum
Abbreviations
1 Introduction
The pharmacological properties of a therapeutic agent are inherent and cannot be altered without various fractions of drugs. Due to their small size, nanoparticles serve as an excellent choice for blood transportation. Nanoparticles can be utilized as drug carriers since their properties can be adjusted without affecting the pharmacodynamics of the medication. Research is underway to utilize nanoparticle-mediated drug delivery to mitigate the adverse effects of chemotherapy (Peer et al., 2020). Conventional chemotherapy presents challenges related to unfavorable pharmacokinetics, toxicity, and non-specific tumor targeting. To effectively treat tumors, high doses are often required. However, the therapeutic benefits are constrained by the adverse effects of such large doses (Rahman et al., 2007). In response to this situation, recent advancements in nanotechnology have been made, introducing chemotherapeutic nanoparticles that employ both active and passive targeting strategies to enhance therapeutic parameters for cancerous tissues simultaneously.
Biocompatibility is defined as the ability of a material to fulfill its intended function in medical therapies without inducing toxic, allergic, inflammatory, mutagenic, immune, or carcinogenic properties in the recipient of the therapy. Furthermore, it should elicit the most suitable beneficial tissue and cellular response in that specific context while addressing clinically relevant challenges (Harianawala et al., 2016). Radiolabeling of nanoparticles (NPs) is a valuable approach for imaging microorganisms with a high aspect ratio (Cadafalch Gazquez et al., 2016; Pijeira et al., 2022). However, after radiolabeling, a challenging task is to preserve the surface, physical, and chemical characteristics of nanomaterials to enable optimal biodistribution and cell uptake processes (Abd Elmaboud et al., 2019). This underscores the significance of nanomaterial properties and chemical manipulation (Lübbe et al., 2001; Avedisian et al., 2009). Additionally, nanomaterials possess the advantageous quality of sustainability, allowing them to retain their features even after radiolabeling.
A wide range of materials are utilized in radiolabeling and other biological applications. Doping or combining them with other substances helps enhance the material's potency and rigidity (Cadafalch Gazquez et al., 2016). Zirconia, with its remarkable mechanical qualities and exceptional strength, owes its robustness to its crystalline structures. It is biocompatible, bioinert, and non-resorbable. In vivo, zirconium-coated implants outperform untreated implants in terms of osseointegration. Osteoblast cells cultured on ZrO2 exhibit strong adhesion and growth characteristics, as well as the ability to proliferate and differentiate (Pijeira et al., 2022). Zirconia is among the metal oxide materials known for their biocompatibility and effective surface qualities necessary for biomolecule immobilization, which is crucial for improved imaging (Abd Elmaboud et al., 2019; Lübbe et al., 2001; Avedisian et al., 2009).
Zirconia exists in different crystallographic forms, including cubic (b), monoclinic (m), and tetragonal (t) structures (Bukhari et al., 2018; Sengupta et al., 2019; Quintard et al., 2002). These allotropes are stable within specific temperature ranges. The monoclinic structure is stable below ∼ 1170 °C, while the tetragonal structure is observed between ∼ 1700 °C and 2370 °C. Above 2370 °C, zirconia adopts a cubic structure (Davar et al., 2013). Zirconia finds extensive applications in various industries, including the medical field, due to its excellent mechanical properties. The phase transition, where one phase of zirconia transforms into another, occurs at specific temperature ranges. Tetragonal zirconia exhibits higher hardness and strength compared to other materials and is commonly used in applications requiring high strength and stiffness. However, when tetragonal phase undergoes a transition to the monoclinic phase it results in a 3–5 % increase in volume, formation of fissures, and a reduction in hardness (Hbaieb, 2012; Kelly and Denry, 2008). Nevertheless, doping zirconia with a small concentration of iron oxide has been shown to enhance its mechanical properties without inducing any secondary phase. To maintain the stability of the tetragonal (t) phase at lower temperatures, ions such as Fe3+, Y3+, Fe2+, are used in the treatment. It is important for the material to exhibit both compatible optical response and mechanical strength. When tetravalent atoms replace trivalent or divalent atoms, metal oxides tend to develop oxygen vacancies (Imran et al., 2019; Sundell et al., 2006).
Iron oxide is employed as a stabilizer due to its proven biocompatibility and its ability to preserve the mechanical strength of zirconia (Guo and Xiao, 2012; Jain et al., 2008). Fe3O4-doped zirconia exhibits ferromagnetic properties and low coercivity, making it suitable for biological applications (Imran et al., 2021; Mittal et al., 2022). Additionally, iron oxide enhances the development mechanism and stability process of zirconia without undergoing phase conversion (Zhukovskaya and Strakhov, 1980). Honey is a nutrient-dense, viscous, carbohydrate-rich fluid that is well-known for its disinfectant and pain-relieving properties. It has been utilized for its medicinal properties and healing purposes (El-Kased et al., 2017). Honey consists of various biomolecules and has been employed by multiple research groups for the synthesis of different nanoparticles (Philip, 2009; Balasooriya et al., 2017; Venu et al., 2011; Heshmatpour and Aghakhanpour, 2011). Various methods have been employed to produce zirconia doped with metal oxides, such as laser ablation (Han et al., 2022), RF sputtering (Smeacetto et al., 2010), and the sol–gel technique (Jodłowski et al., 2018). Among these processes, the sol–gel approach is widely preferred due to its cost-effectiveness and ability to operate at moderate thermal temperatures. The sol–gel process begins with material hydrolysis to generate a sol, followed by condensation and drying to obtain a gel. Subsequently, the gel is thermally treated under inert atmospheric conditions to yield the final product (Bokov et al., 2021).
Kao et al., (Kao et al., 2018) checked the effect of iron oxide in yttria-stabilized ZrO2 by mixing these powders using ball milling. Holz et al., (Holz et al., 2018) studied the effect of low Fe2O3 concentrations on the mechanical strength of stabilized zirconia using yttria. Zhao et al., (Zhao et al., 2010) fabricated the ZrO2 nanofibers by electro spinning process. The mean diameter of zirconia nanofibers was observed to be 80 nm after calcination at 400 °C. Tetragonal zirconia phase was observed after calcination at 400 and 600 °C. Stabilized zirconia nanofibers were prepared by reactive sol–gel electro-spinning process as reported by Xu et al., (Xu et al., 2010). YZ exhibited tetragonal phase after calcination from 500 °C to 1400 °C. Yuan et al., (Yuan et al., 2022) synthesized ZrO2 NPs using a green approach utilizing P. nirurui leaf extract. DLS analysis indicated that the average particle size of ZrO2 NPs was 121.5 nm with a negative zeta potential of 22.6 mv. Muthulakshmi et al., (Muthulakshmi et al., 2023) used Guettarda speciosa (G. speciosa) leaf extract to produce zirconia nanoparticles. Kadiyala et al., (Kanth Kadiyala et al., 2023) green synthesized zirconium oxide nanoparticles, i.e. ZrO2/rGO NCs and tested biocompatibility. In previous studies, variety of stabilizers, i.e. yttria, magnesium oxide and calcium oxide, have been used to stabilize/partly stabilize ZrO2 nanoparticles by obtaining tetragonal phase (Li et al., 2020; Cao et al., 2022; Lu et al., 2022). However, high temperatures were required to achieve stability (Peng et al., 2018).
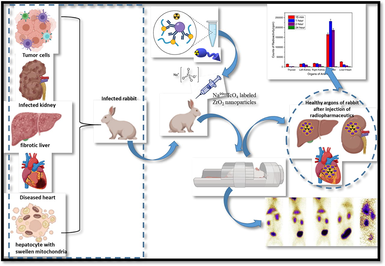
The objective of the current study is to investigate the structural, mechanical, and magnetic properties of stabilized zirconia nanoparticles with varying concentrations of Fe3O4. The concentration of Fe3O4 was increased from 1 to 10 (wt. %). XRD analysis was performed to determine the phase purity of the synthesized samples. Vibrating sample magnetometer (VSM) results were utilized to confirm the magnetic nature of the samples, demonstrating their potential for cancer diagnosis and treatment. Optimized nanoparticles were subsequently employed for biodistribution studies in rabbits. Radiolabeling was achieved by utilizing sodium pertechnetate (Na99mTcO4), eluted from the PAKGEN 99Mo/99mTc generator.
1.1 Hypothesis statement
Present research work is designed to explore the optimization of stabilized zirconia without using toxic stabilizers. Low cost sol–gel method will be used for synthesis purposes. This material will be optimized by keeping in mind its bio-activity and bio-compatibility for future biomedical application. In-vitro and in-vivo both approaches will be used to check the compatibility and efficiency level of synthesized material.
1.2 Clinical significance
It’s been predicted that the honey added Fe3O4 stabilized ZrO2 nanoparticles can act as multifunctional material. Synthesized material under optimized conditions can be used for diagnostic as well as therapeutic purposes. Optimized nanoparticles can be used for effective drug loading and releasing purposes. Effective biodistribution of these nanoparticles can help in diagnosing as well as targeting the cancer cells based on obtained in vitro and in vivo results.
2 Experimental details
2.1 Materials and method
Zirconium oxychloride octa hydrate (Sigma–Aldrich with 99.99% purity), Fe(NO3)3·9H2O (Sigma–Aldrich with 99.99% purity) were utilized without any further purification. Distilled water was doubly deionized (D.I.) before usage. Honey (natural sweetener) was collected from honeybee hive from Botanical garden of University of the Punjab, Lahore.
For preparation of zirconia sol, ZrOCl2·8H2O was utilized as precursor, while D.I. water was used as solvent to prepare 0.1 M solution (Sol I). After this, pre-synthesized sol of iron oxide (Fe3O4) at pH 9 having molarity of 0.1 M was used as a stabilizer. Detailed preparation for ZrO2 and Fe3O4 sol is reported elsewhere (Sanaullah et al., 2021; Sanaullah et al., 2021; Bashir et al., 2015; Khan et al., 2023). Various concentrations. i.e. 1 to 10 wt%, of Fe3O4 sol were added in 0.1 M solution of zirconia. Detailed study of iron oxide stabilized ZrO2 has been previously reported (Khan et al., 2023). After synthesis of iron oxide stabilized zirconia sols, 1 ml of honey was added to 100 ml of synthesized solution. Sols of all the samples prepared at various concentrations of iron oxide were dried at temperature of 80 °C to form powder. Synthetic route for stabilized nanoparticles is expressed in the form of a flow chart in Fig. 1.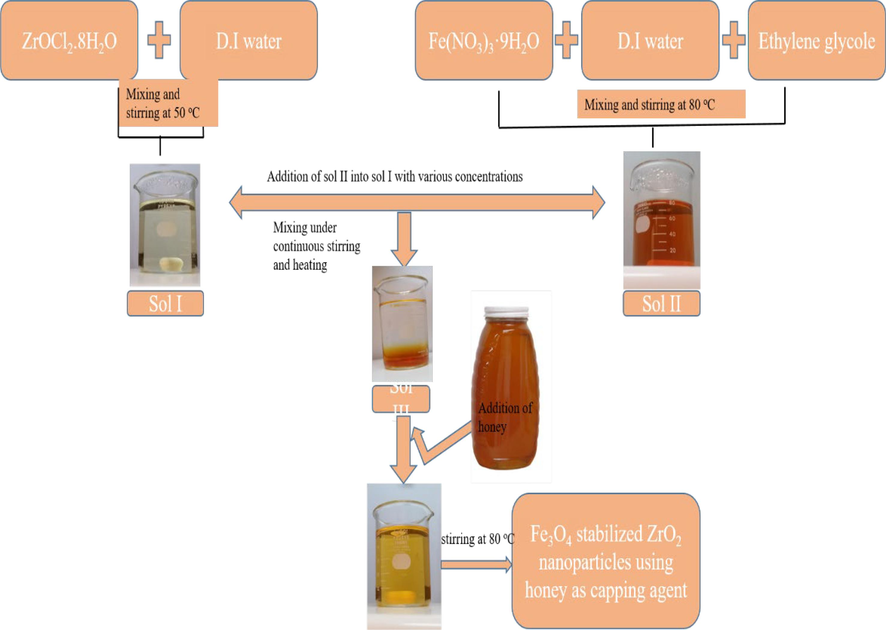
Flow chart of the synthesis of iron oxide stabilized.
2.2 Radiolabeling of zirconia nanoparticles
These synthesized zirconia nanoparticles were tested for radiolabeling and biodistribution (scintigraphy) studies. Radiolabeling was performed by using sodium pertechnetate (Na99mTcO4), eluted from PAKGEN 99Mo/99mTc generator, which is locally-produced in Pakistan. The chemicals utilized in this research work were obtained from Sigma–Aldrich. The animal/rabbit trials were accomplished after getting approval from the Animal Ethical Board of INMOL Hospital. The protocol for biodistribution of radiotracer in animal models has been developed at INMOL lab after a series of experiments on various radiopharmaceutucals, keeping in view the blood pool formation, uptake of radioconjugate in various body organs (biodistribution), its retention in those organs and excretion pattern from the body.
2.3 Synthesis of labelled zirconia nanoparticles
99mTc was used to radiolabel zirconia nanoparticles (IOZH). As an experimental procedure, the Tc-99 m metal was reduced by using 20–40 μg of SnCl2·2H2O at a pH value between 4 and 7, which was fixed using either 0.1 M NaOH or 0.1 M HCl. To get maximum labeling yield, the amount of zirconia nanoparticles varied from 0.25 mg to 0.75 mg. The optimized radiolabeling conditions were 0.5 mg of zirconia nanoparticles dissolved in 20 μL of D.I water, in presence of 40 μg of SnCl2·2H2O at pH value of 4. For radiolabeling, ∼370 MBq 99mTcO4- (equivalent to 10 mCi in saline) was added in reaction vial and incubation of vial was done for about 25–30 min at 24 ± 2 °C.
2.4 Quality control
Ascending paper chromatography was employed to evaluate radiochemical yield (% labeling efficacy) of 99mTc-zirconia nanoparticles. The content of free 99mTcO4- present in radiolabeled nano-conjugate was evaluated using Whatman chromatography paper # 3 in saline and acetone as stationary and mobile phase respectively. In this experimental setup, the amount of free 99mTcO4- moved along solvent front and 99mTc-zirconia nanoparticles reside at the base level. The content of radiocomplex and free 99mTcO4- was determined by dividing the chromatography strip into 1 cm fractions. The radioactivity was counted on gamma-counter (SCALER TIMER ST7). The amount of the colloidal content was measured by passing the radioconjugate through a 0.22 µm filter. In order to obtain the radioconjugate as a potential radiotracer, we tested its stability till 24 h at room temperature.
2.5 Stability of labelled zirconia nanoparticles
After attaining the radiolabeling parameters, the stability of labeled nanoconjugate was tested by incubating it at room temperature till 24 h. The estimation of free and colloid complex at various time intervals, e.g. 2, 4 and 24 h of incubation was assessed by using ascending paper chromatography.
2.6 Lipophilicity test and protein binding of labelled zirconia nano particles
Human blood plasma (obtained with prior approval from a healthy volunteer) was used to investigate in vitro protein binding. To initialize the procedure, 1.5 ml of human blood plasma and 500 µL of radiolabeled complex were mixed to form a slurry and then incubation of this slurry was performed at the temperature of 37 °C for a time period of 1 h. Following incubation, the mixture was separated (in blood serum and human blood cells) by centrifuging it at 3000 rpm for 10 min. The supernatant layer was combined with an equivalent amount of trichloroacetic acid before being centrifuged for 10 min at 3000 rpm. Centrifugation was performed to get the separate layers so that gamma camera could be used to measure obtained radioactivity. All studies were carried out in triplicate.
Lipophilicity is directly associated with the partition coefficient (p) measurements in-between the organic layer and aqueous layer. It was determined by performing incubation of 80 µL of the radiolabeled complex with 200 µL of phosphate buffer having pH values of 6.6, 7.0, and 7.6. Afterwards, 200 µL of n-octanol was transferred to each vial, and the three vials were shaken for around a time period of 10–15 min for the separation of two distinct layers. The radioactivity content of each layer was quantified with a gamma counter after separation.
2.7 Stability in human blood serum
To test the stability of a new radioconjugate in normal human sera, 1 ml of serum was incubated at 37 °C with 200 µL of radioconjugate. The stability was assessed by extracting an aliquot from the sample at various time periods up to 24 h during incubation. The radiolabeling effectiveness was assessed once again using paper chromatography, as previously described. Any reduction in radiolabeling yield might be due to radiolabeled conjugate degradation.
2.8 Labelled zirconia nanoparticles scintigraphy study in rabbit
To assess the practicality of a new nano-radioconjugate as a radiopharmaceutical medicine, we administered it intravenously (in the posterior ear vein) to rabbit models and acquired pictures using a single-headed gamma imaging camera. It was linked to an internet computer (carrying Macintosh operating system 7.5 and an ICONTM Workstation) through a spatial fidelity parallel-hole collimator. As part of the experiment, the animal was housed on a level hard surface with both back legs extended wide and all four legs taped using surgical tape. The local anesthesia was established by infusing 2 ml of Diazepam intramuscularly through the left thigh. The radioactive medication to be examined, for example 130 MBq (in 300 µL) of 99mTc-zirconia nanoparticles, was administered intravenously into the animal's marginal ear vein. Photos were taken at 10 min in dynamic view, followed by static photographs after 10 min, 2 h, 4 h, and 24 h after the injection to investigate the biodistribution of tracer in animals.
2.9 Characterization tools
To analyze the crystalline nature and structure of produced nanoparticles, an X-ray diffractometer with a Cu K-alpha radiation source was used. The mechanical properties of the zirconia samples were determined using a Shimadzu HMV-2 Vickers micro indenter in accordance with ASTM C1327-99 (American Society for Testing of Materials, 1999). Wayne Kerr Precision 6500B impedance analyzer was used to test the dielectric characteristics of as generated samples. To test the magnetic characteristics of the samples, a LakeShore vibrating sample magnetometer (VSM) (Series 7400 and model 7407) was used. DPPH (2, 2, diphenyl-1-picrylhydrazyl) method was applied to investigate anti-oxidant activity and to measure absorption intensity, Shimadzu UV-1800 UV–visible spectrophotometer was employed. The biodistribution of tracer in animals was studied using a single-headed Siemens optimized ORBITER gamma camera system (rabbit).
3 Results and discussion
Fig. 2 illustrates X-ray diffraction (XRD) patterns of ZrO2 nanoparticles stabilized with various concentrations of Fe3O4. The diffraction peaks i.e. ∼24.40°, ∼ 31.77°, 40.31°, ∼48.53° and ∼ 64.48° corresponding to ( −1 1 0), (1 1 1), ( −2 1 1), (0 2 2) and (0 3 2) planes (card no. 00–013-0307) indicated the formation of monoclinic entities in as synthesized nanoparticles. While, peaks appearing at angles of 30.58°, 35.56°, 42.53°, 53.84°, 62.31° & 73.28° are due to (1 1 1), (2 0 0), (1 1 2), (2 2 1), (2 2 2) and (4 0 0) planes of tetragonal phase of zirconia respectively [card no. 00–017-0923].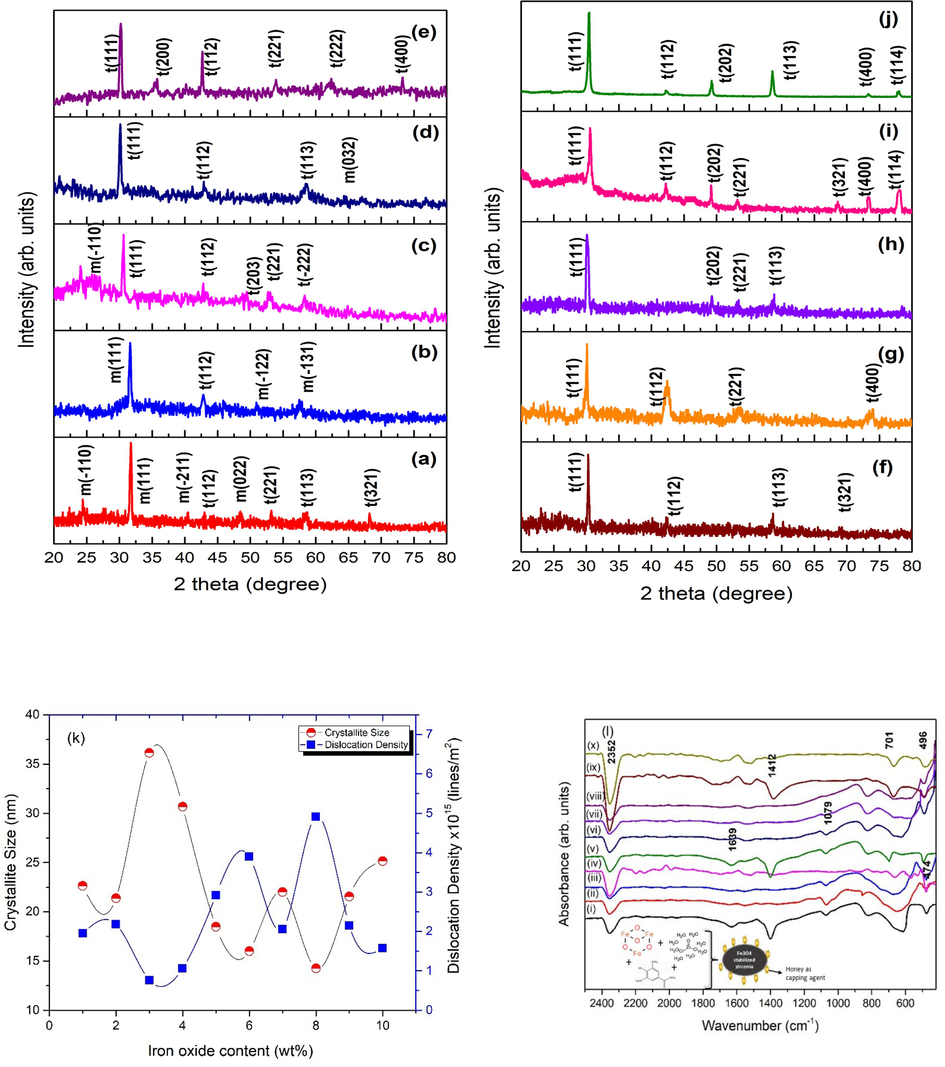
XRD patterns of nanoparticles with iron oxide content of (a-j) 1–10 wt% and (k) crystallite size and dislocation density, (l) FTIR spectra of as synthesized nanoparticles using various concentration of iron oxide as a stabilizer.
The crystallite size (D) was measured by mean of Williamson-Hall plot as expressed in Eq. (1) (Cullity, 1956):
Here, 2ωf is measured in radians, K is utilized to show shape factor, wavelength is expressed as λ, produced microstrains in the samples is expressed by e (=Δd/d) factor, D particularizes the size of crystallites and θ is used to demonstrate Bragg angle.
To calculate dislocation density (δ), following expression was used (Eq. (2) (Cullity, 1956).
Crystallite size and dislocation density, as estimated from the Eq. (2), are plotted as a graph with altering the concentration of iron oxide as depicted in Fig. 2(k). The Fourier transform infra-red spectra of Fe3O4 stabilized ZrO2 nanoparticles are shown in Fig. 2(l).
The morphological features of nanomaterials (size, shape, and roughness) have been observed to impact their applications, making microscopic examination a valuable tool in determining the nature of a nanomaterial. SEM micrographs, as shown in Fig. 3, show uniform large area growth of zirconia samples synthesized under different conditions, i.e. variation in iron content.
SEM images of zirconia stabilized with iron oxide content of (a-j) 1–10 wt%.
For bio-medical applications, the functional material’s adsorption on the surface of nanoparticles and hydrodynamic diameter size of particles plays a crucial role. The size of nanoparticles determined by DLS procedure helps in understating the adsorption of molecules on the surface of nanoparticles. Zetasizer (Nano ZEN3600, Malvern Instruments, UK) was used in the present study to check the hydrodynamic diameter size along with the potential values. Average zeta size and PDI of as synthesized nanoparticles are given in Table 1. Fig. 4(a-j) shows the average zeta size value of synthesized nanoparticles. The zeta potential is also an important parameter for a biomaterial surface, since it affects the protein adsorption (Cai et al., 2006), cell attachment (Cheng et al., 2005) and bacterial adhesion (Gottenbos et al., 2001) in a physiological environment. The stability of ZrO2 nanoparticles can be assessed by the zeta potential measurements (Jameel et al., 2020). Examining the zeta potential helps to better understand the comprehensive characteristics of nanoparticles for their biomedical applications. Suriyaraj et al., (Renger et al., 2004) demonstrated zeta potential of 36.5 ± 5.5 mV for ZrO2. Chau et al., (Chakravarty et al., 2010) reported zeta potential of − 32.8 mV for ZrO2 nanoparticles biosynthesized from Laurus nobilis leaf extract.
Sample name
Z average (d.nm)
PDI (polydispersityindex)
(a)
423.0
0.372
(b)
535.9
0.651
(c)
828.4
0.825
(d)
322.1
0.527
(e)
1127
0.892
(f)
291.4
0.610
(g)
420.6
0.632
(h)
372.8
0.622
(i)
398.4
0535
(j)
359.7
0.618
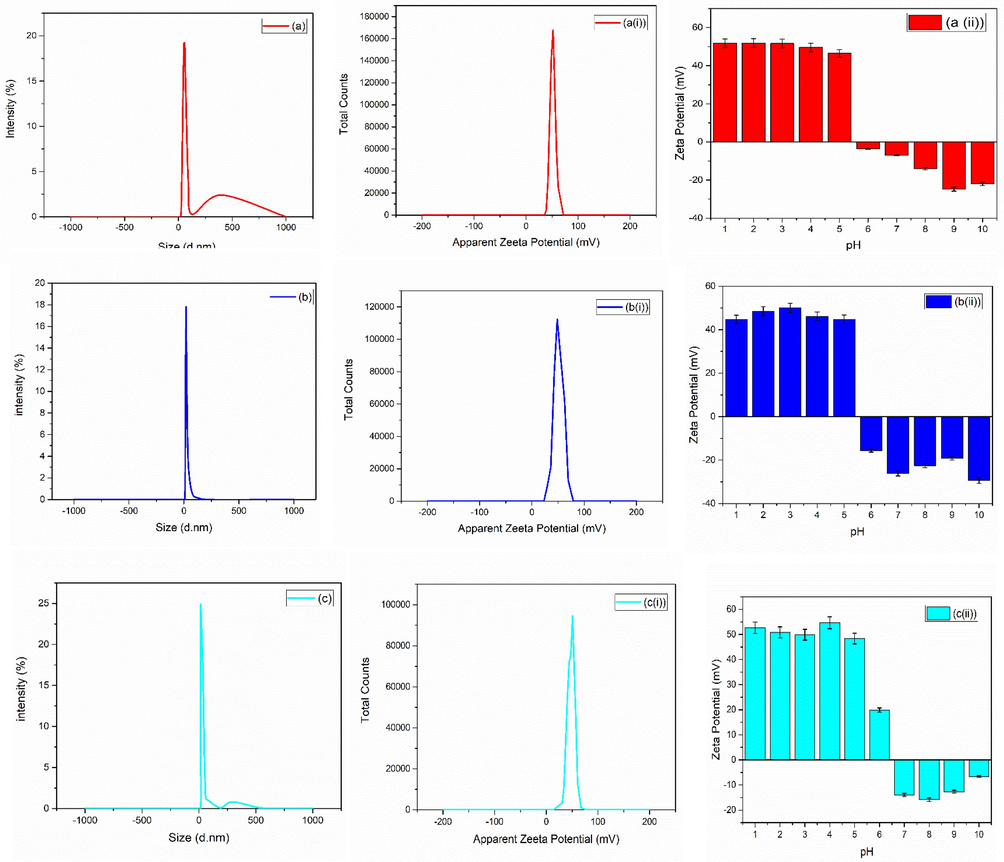
(a-j) average zeta size, (a(i)-j(i)) average zeta potential and (a(ii)-j(ii)) average zeta potential of zirconia nanoparticles at various pH of zirconia nanoparticles using 1-10 wt% of iron oxide.
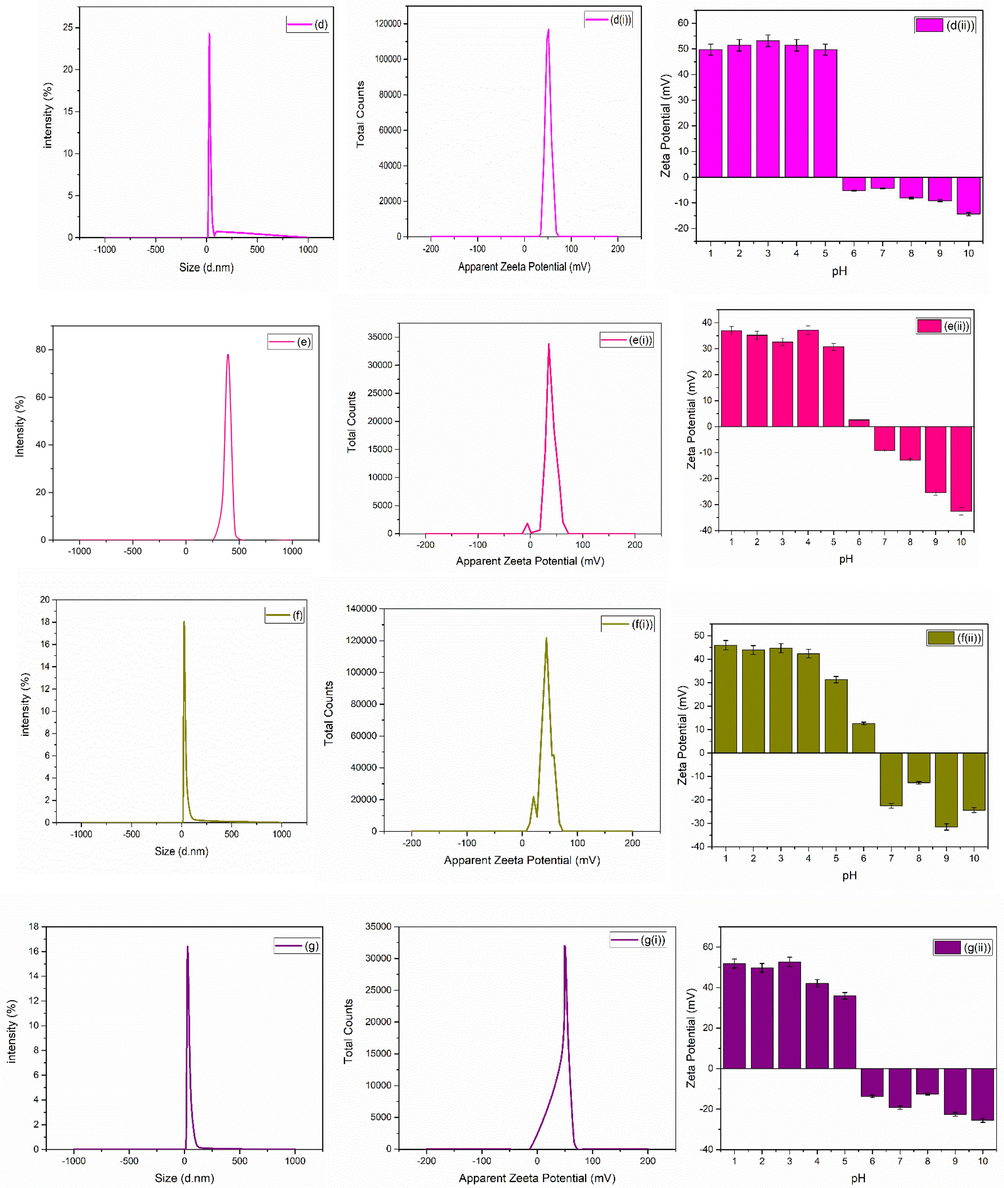
(a-j) average zeta size, (a(i)-j(i)) average zeta potential and (a(ii)-j(ii)) average zeta potential of zirconia nanoparticles at various pH of zirconia nanoparticles using 1-10 wt% of iron oxide.
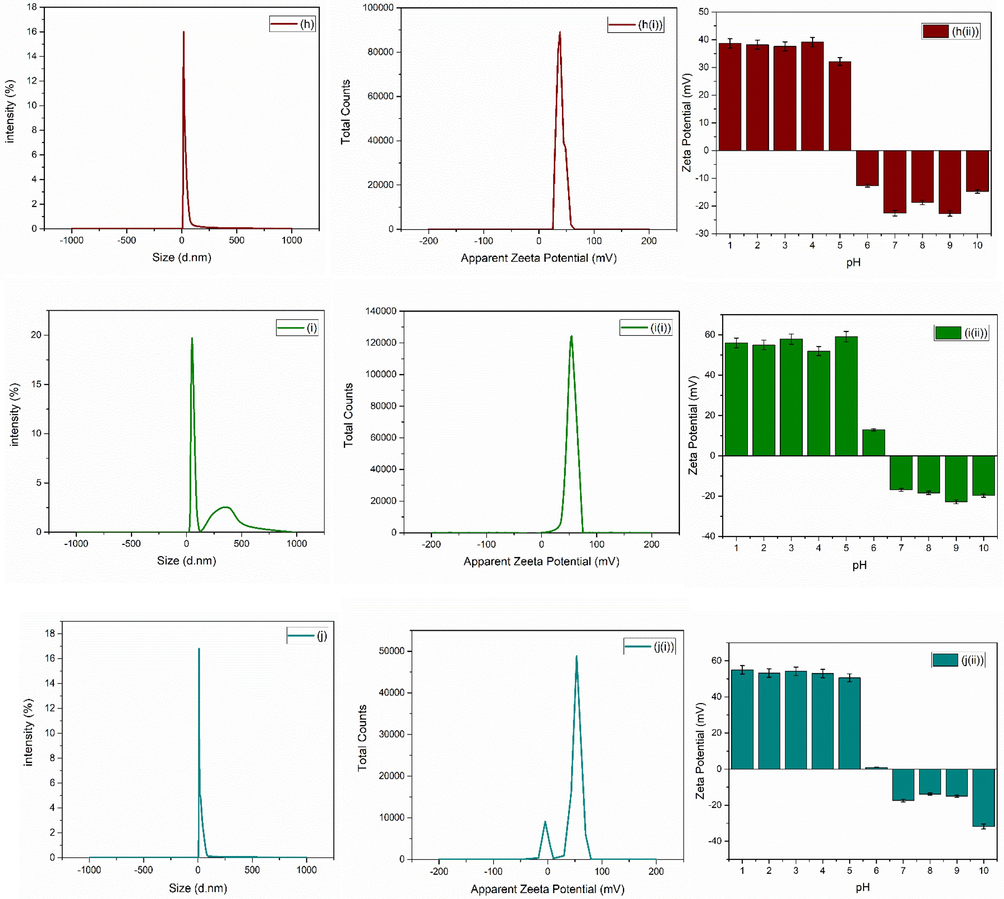
(a-j) average zeta size, (a(i)-j(i)) average zeta potential and (a(ii)-j(ii)) average zeta potential of zirconia nanoparticles at various pH of zirconia nanoparticles using 1-10 wt% of iron oxide.
Nanoparticles consisting of magnetic oxides, like magnetic iron oxide (Fe3O4), are very useful for the purpose of imaging as well as separation techniques. Due to their biological and chemical inertness, they are frequently used as a coating material to increase functionality of antibodies or enzymes as separation agents. To utilize zirconia nanoparticles stabilized using Fe3O4, it is very crucial to find out their magnetic behavior as for biomedical applications magnetic properties play an important role. MH loops of synthesized nanoparticles exhibited soft ferromagnetic behavior as shown in Fig. 5.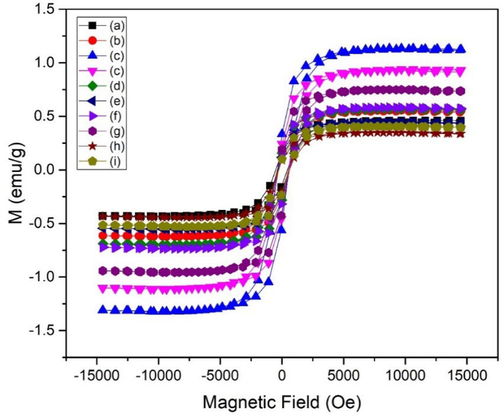
MH curves of as synthesized nanoparticles using various concentration of iron oxide (a-j:1-10 wt%) as a stabilizer.
It is essential to look into the impedance capabilities of materials that is to be used as regrow/repair bone tissue and biological cells. These qualities may be greatly influenced by the applied or externally present field, influencing the behavior of nanoparticles. The suitable dielectric constant values are in the range of ∼ 18–68 for biomedical applications at high values of frequency (Yentekakis and Chu, 2020; SanaUllah et al., 2022). The impedance response of IOZH nanoparticles was thoroughly investigated. The room temperature behavior of all samples generated with varying iron oxide concentrations was investigated. Capacitance and resistance were obtained by means of impedance analyzer. Frequency dependent response of dielectric constant (ε) and tangent loss (tanδ) were obtained from Eqs. (3) and (4) (Macdonald and Barsoukov, 2005).
C = capacitance by mean of parallel plate configuration; d = thickness of the prepared sample; A = area ; ɛo = free space permittivity; ρ = resistivity; f = frequency.
Fig. 6 depicts a decrease in the value of the dielectric constant along with tangent loss with increment in frequency, displaying typical dispersion behavior.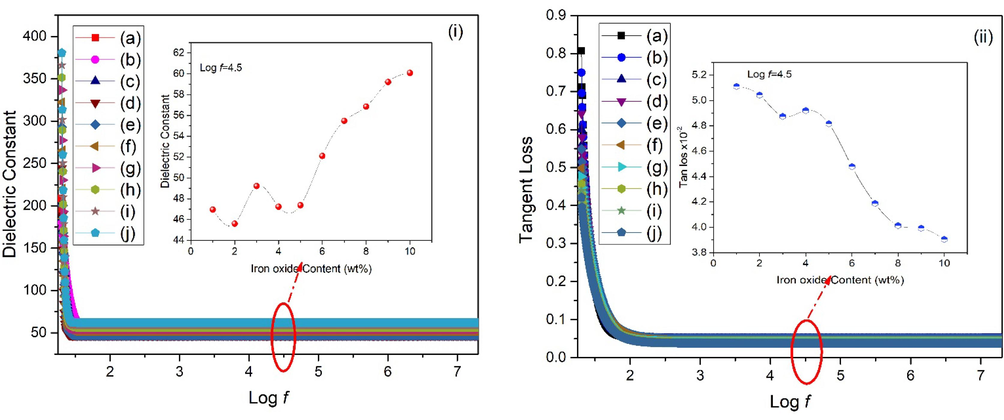
(i) Dielectric constant and (ii) tangent loss of as synthesized nanoparticles using various concentration of iron oxide (a-j) 1-10 wt%. (insets show variation for all samples at fixed frequency).
The hardness of material is a key component that describes its potential use under various atmospheres.
Fig. 7 (a-f) displays the hardness value at a total of 2500 μm2 of microstructural sites. The hardness maps were created by combining image data from a Shimadzu HMV-2 Vickers micro indenter and Origin software (Origin (Pro), 2016, OriginLab Company, Northampton, USA). Indentation displays random values in each position due to the varied and random orientation of each grain hardness value. That’s the main reason that each region of sample prepared using nanoparticles displays a dissimilar/distinct mechanical property as also shown by mean of color filled contour plot even after using same dwell time and force, i.e. 4.903 N load for 15 sec according to ASTM C-1327.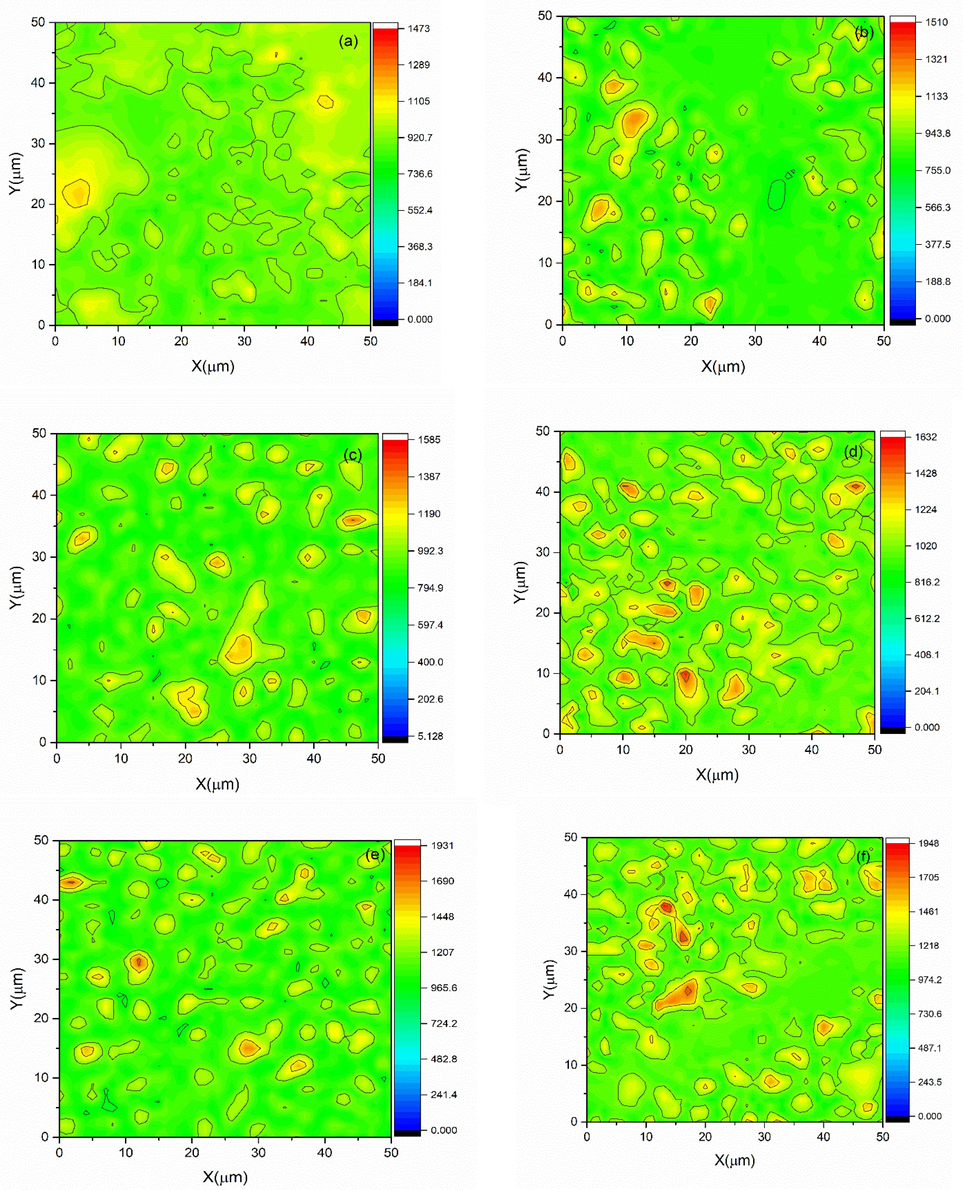
Hardness mapping of samples with iron oxide concentration of (a-f) 5–10 wt%.
Simulated body fluid (SBF) based test was utilized to investigate colloidal stability as well as biodegradability of as synthesized samples as used previously (Pattnaik et al., 2011). Nanoparticle based compressed round shape discs were submerged in SBF for a time period of 1, 2, 4, 8, 13, 20, and 26 weeks. The area (mm2), diameter (mm), and thickness (mm) of the compacted disc were measured, before starting the submersion. Biodegradation results of samples are shown in Fig. 8(a).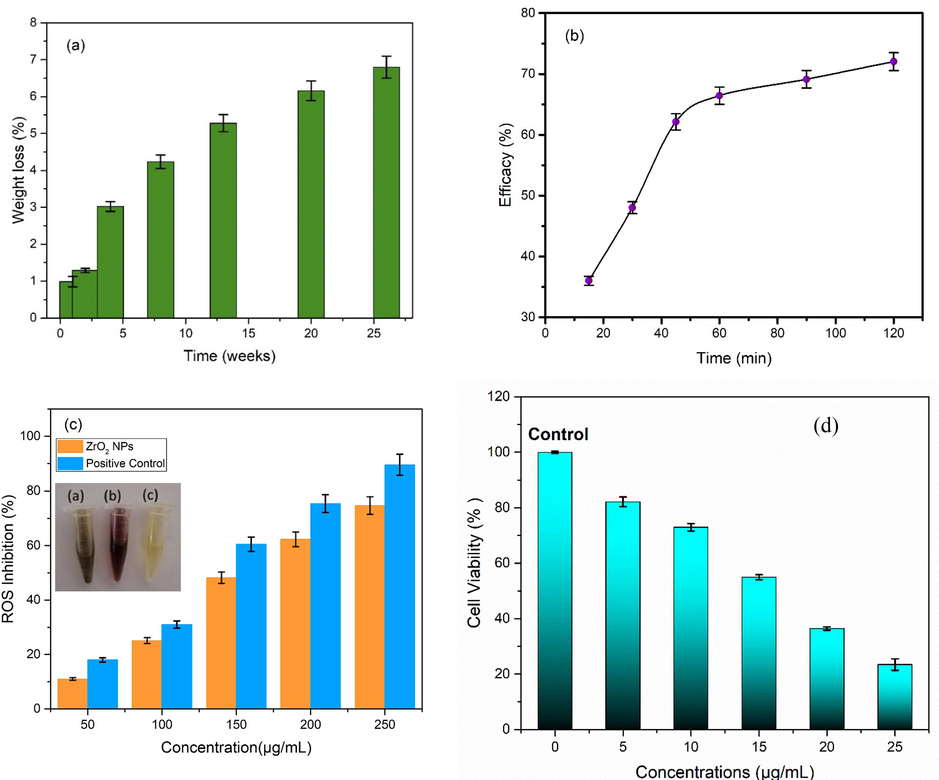
(a) Biodegradation assay, (b) drug encapsulation efficacy, (c) ROS inhibition (inset shows color changing capacity of nanoparticles) and (d) cell viability capability of iron oxide stabilized zirconia nanoparticles.
Fig. 8(b) shows the labelling efficacy of curcumin. An ethanolic solution containing curcumin (200 μg/mL having pH 7) was set up to evaluate the drug (curcumin) loading efficacy of IOZH. The curcumin solution was formerly incorporated with IOZH nanoparticles, and the slurry was agitated for 2 h at room temperature in the dark (25 °C) (Janes et al., 2001; Farahani et al., 2020; Mohanty et al., 2010). Centrifugation was used to dry drug-load nanoparticles after stirring. The IOZH nanoparticles were further treated at least 3–4 times with ethanol to neutralize the unbound medicine i.e. curcumin. After washing, the curcumin-loaded stabilized IOZH nanoparticles were heated for 5 h at 50°C.
To evaluate the scavenging capability of as synthesised IOZH nanoparticles against reactive oxygen species, DPPH assay was utilized (Das et al., 2013), (Serpen et al., 2007; Shin et al., 2017). Methanolic stock solution of IOZH nanoparticles were prepared to made working solutions having concentrations from 50 to 250 µg/mL (with an interval of 50 µg/mL). Further this working solution was added into 1 ml of methanolic DPPH solution (1 mM). The same amount of ascorbic acid was utilized as a positive test control. Scavenging capability of samples is shown in Fig. 8(c).
To evaluate the in-vitro anticancer capability of as synthesized IOZH nanoparticles through MTT assay, HCT-116 cell lines (colon cancer cells) were cultivated in DMEM (Dulbecco's Modified Eagle's Medium) along with 10% heat-inactivated FBS (fetal bovine serum) and antibiotic (penicillin) (Balaji et al., 2017 and Rajapriya et al., 2020). The incubation took place at 37 °C with 5% CO2 vapor present. To assess the vitality of optimized samples, the MTT test was utilized. Fig. 8 (d) depicts the proclivity of customized nanoparticles to diminish the cell viability (%) of colon cancer cell lines (HCT-116). When the concentration of ideal sample of as generated nanoparticles grew, the percentage of cell viability declined continuously. A concentration of 5 to 25 μg/mL (with 5 μg/mL interval) was utilized as working solution.
The antibacterial activity of the nanoparticles (prepared with 5-10wt%) was performed using standard agar well diffusion. The in vitro antibacterial activity studies was performed against 24-h culture of bacterial strains Bacillus subtilis (taken from Institute of MicroBiology & Molecular Genetics, University of the Punjab, New campus, Lahore, Pakistan). Petri plates containing 20 ml nutrient agar were prepared and wells were formed. Bacterial strains were swabbed into petri dishes. Varying concentrations (5-25 µg/ml) of nanoparticles were added to check the antibacterial response (Manjunatha et al., 2021). Zone of inhibition was measured in mm and recorded after 24 hours of incubarion at 37°C as shown in Fig. 9 (a-f). Measured zone of inhibition (mm) are listed in Table 2.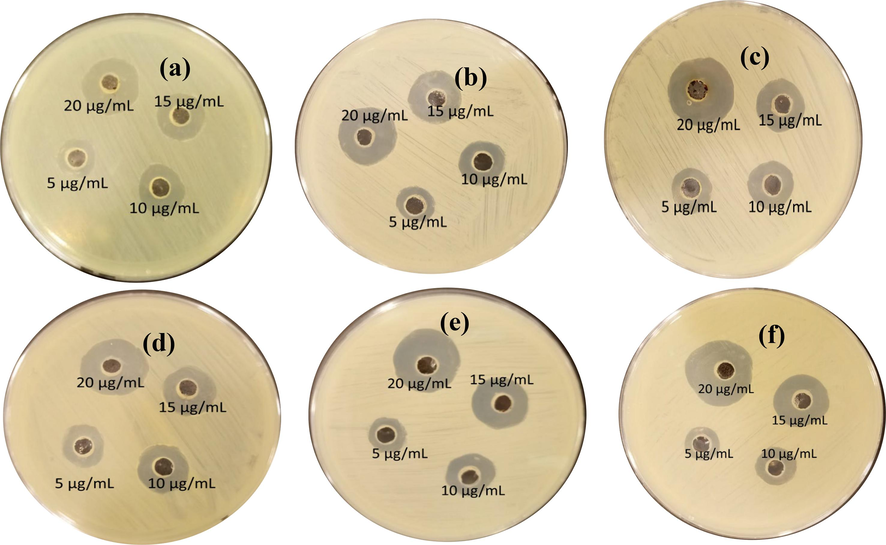
Antibacterial activity of nanoparticles using iron oxide concentration of (a) 5 wt%, (b) 6 wt%, (c) 7 wt%, (d) 8 wt%, (e) 9 wt% and (f) 10 wt%.
Nanoparticles concentrations
Sample name (zone of inhibition in mm)
(a)
(b)
(c)
(d)
(e)
(f)
0
–
–
–
–
–
–
5 μg/mL
9
10
10.5
11
11
11
10 μg/mL
12
12
13
14
14
15
15 μg/mL
13
15
14
15
16
17
20 μg/mL
19
20
20
21
21
22
Fig. 10 shows the results obtained from gram staining. Fig. 10(a) shows staining results for untreated bacterial strains. While Fig. 10(b-g) represents gram staining of nanoparticles treated bacteria. For nanoparticle treated bacteria, plates shown in Fig. 10 were utilized. Smeared bacterial culture was taken from extreme edge of 20 μg/mL zone of inhibition for each plates. In Fig. 10(a) it can be observed that cells present different sporulating stages i.e. unipolar and bipolar. The bacteria can be seen in the form of chains and stained positive. As the stress of nanoparticles increased the cells staining is affected and towards the negative behaviour. The mature spores were also observed at higher IOZH i.e. 10 wt%. The cells also tends to arrange either singly or in pairs at progression towards higher wt%.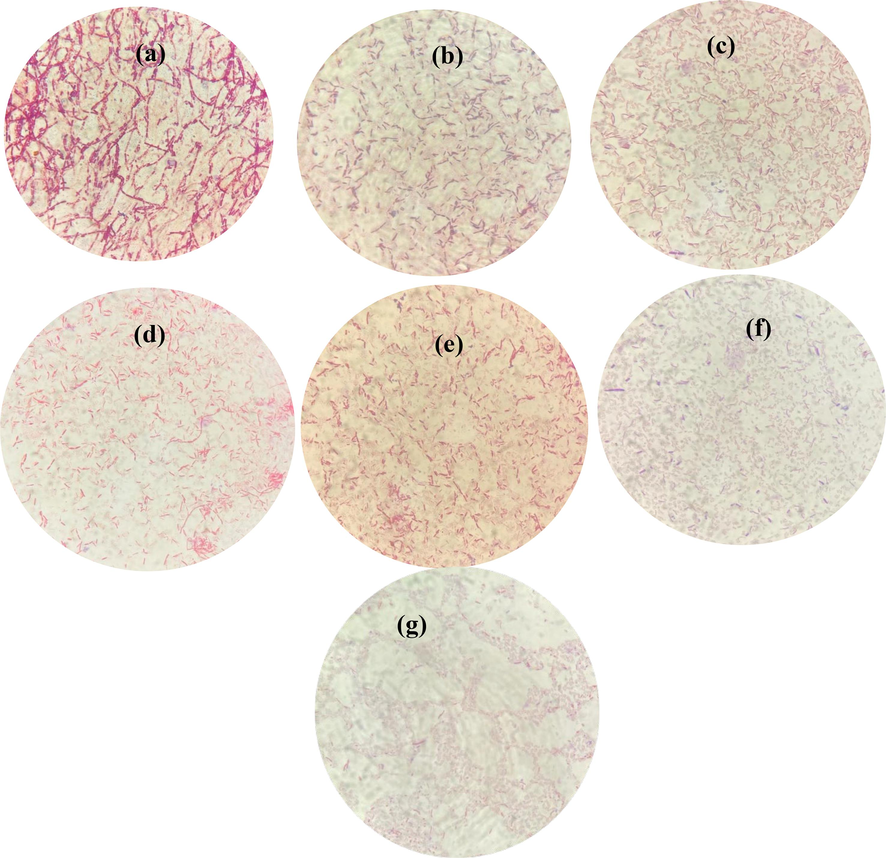
Gram staining results of (a) untreated, IOZH using iron oxide concentration of (b) 5 wt%, (c) 6 wt%, (d) 7 wt%, (e) 8 wt%, (f) 9 wt% and (g) 10 wt%.
Radiolabeling of zirconia nanoparticles includes an estimation of the radiolabeling yield, radiochemical purity and the stability of the radio-product. All of above said parameters were estimated through paper chromatography (in acetone as a mobile phase). In this chromatography system, free 99mTcO4- moved along the solvent (with Rf = 1) and the radiolabeled conjugate remained at the base line. To estimate the amount of colloid, the radioactive complex was filtered through a 0.22 µm filter and the content of radioactivity in filtrate and filter was measured in gamma counter which showed a negligible amount of colloid in final radio-formulation. Fig. 11 (a) shows the effect of pH on the radiolabeling yield.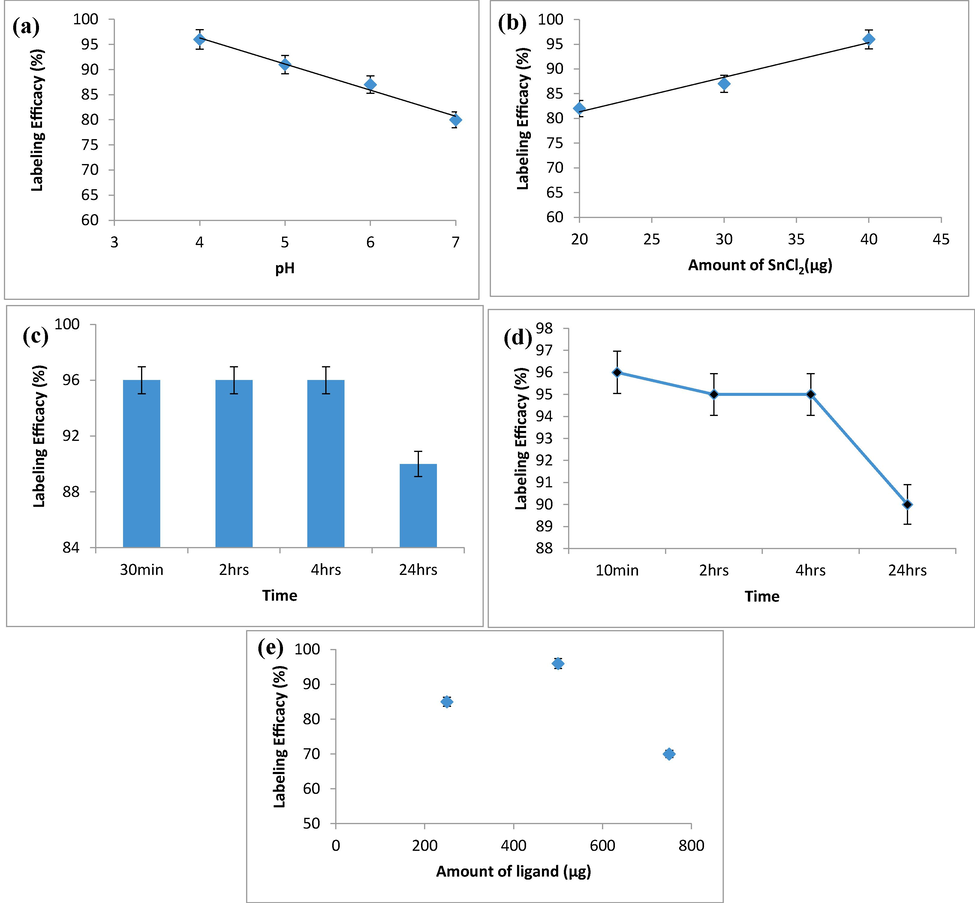
(a) Effect of pH on radiolabeling efficacy, (b) Effect of amount of reducing agent on radiolabeling efficacy, (c) Stability of radiolabeled complex at room temperature, (d) Stability of radiolabeled complex in human blood Serum, (e) Effect of amount of the ligand on radiolabeling yield (%).
Since the amount of reducing agent, e.g., SnCl2·2H2O plays a pivotal role in radiolabeling with Tc-99 m, so we studied the correlation between the amount of SnCl2·2H2O and the radiolabeling efficacy. Data are shown in Fig. 11 (b). The stability of 99mTc-zirconia nanoconjugate that reflects the rate of complexation was evaluated at room temperature. Results are shown in Fig. 11 (c). The stability of 99mTc-zirconia nanoconjugate was also evaluated in human blood serum as shown in Fig. 11 (d).
With these encouraging in vitro results in mind, the potential of 99mTc-zirconia nanoparticles was investigated in vivo by injecting it intravenously into a rabbit and generating its scintigraphy profiling on a Gamma Camera. After administering radiopharmaceutical into rabbits, we analyzed the imaging in dynamic mode for 10 min (pictures at 1 min, 3 min, 4 min, 7 min, and 10 min) after injection (in anterior mode). Static photos were taken at 10 min, 1 h, 2 h, 4 h, and 24 h (p.i.) to clearly evaluate radiotracer accumulation in the animal; data are displayed in Fig. 12 (ii). Additionally, the rabbits were maintained under careful surveillance for 72 h following the trial to identify any abnormalities. Fig. 12 (ii) depicts the absorption of 99mTc-zirconia nanoparticles in various animal tissues at several discrete time intervals.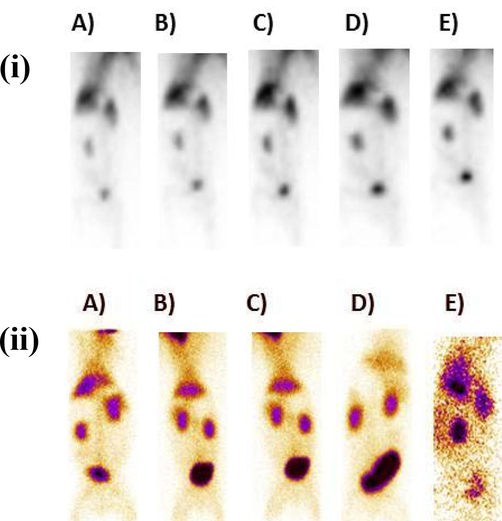
(i) Scintigraphy profile of 99mTc-zirconia nanoparticles in dynamic view (anterior mode) in a rabbit model for 1–10 min, after injection. Images at A) 1 min, B) 3 min, C) 5 min, D) 7 min, E) 10 min and (ii) whole-body static images of rabbit in anterior view indicating the biodistribution of 99mTc-zirconia nanoparticles at various time points after injection, images at A) 15 min B) 1 h, C) 2 h, D) 4 h, and E) 24 h.
The findings of scintigraphy data are supportive of the argument that uptake of a radioconjugate depends on several factors, e.g. nature of compound, pH value, lipophilicity and blood plasma protein binding level, etc. The high uptake value of 99mTc-zirconia nanoconjugate in the vital organs e.g. heart, liver, kidney and bladder [Fig. 13] indicates the potential of the novel radiocomplex to be further investigated, thus making these nanoparticles a potential nano-radiolabeled diagnostic agent.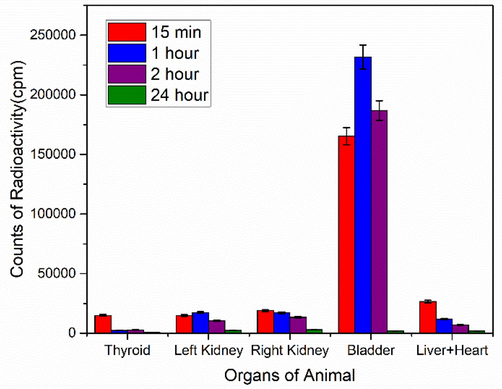
Uptake of Radio-nanoconjugate in various organs of rabbit at discrete time points.
4 Discussion
X-ray diffraction (XRD) results, presented in Fig. 2, showed formation of stabilized ZrO2 nanoparticles under optimized conditions, i.e. the content of iron oxide. Monoclinic peaks of zirconia disappeared after increasing concentration of iron oxide from 5 to 10 wt%. Elimination of monoclinic phase is due to nucleation process which results after surpassing of super-saturation limit of stabilizer ions in the solution. A solid solution is formed in zirconium cation (Zr4+) and is replaced with ions of iron (Fe3+ or Fe2+). Because of the smaller sized atom replacement, oxygen vacancies are produced, which plays a crucial role in the stability of t-zirconia even at low temperatures, which was previously unattainable. (Sanaullah et al., 2021; Tyagi et al., 2006; Cong et al., 2009). Honey was utilized during synthesis that may act as 1) capping agent, 2) reducing and 3) stabilizing agent (Bahari et al., 2023). Fructose and glucose are major moieties of honey. According to previous reports, fructose and glucose plays an important role for the reduction of OH– ion (hydroxyl ions) as they have reducing nature (Cong et al., 2009; Sanaullah et al., 2020). It can be seen from Fig. 2(k) that nanoparticles had a lower crystallite size that was below 30 nm, particularly for 5 to 10 wt%. of iron oxide content. It has formerly been shown that biologic implants as well as coatings exhibit little or no aggregation when the crystallite size is < 30 nm (Garvie et al., 1975). It has also previously been found that nucleation rates are inversely linked to crystallite size (Liu et al., 1995; Marsh, 1998). Due to existence of monomers in less quantity, crystallite size also reduces associated with rapidly occurring nucleation. As a result, nucleation rate plays a crucial role in controlling and restricting the Ostwald ripening process for controlled t-zirconia synthesis (Kelly and Denry, 2008; Sajjadi and Brooks, 2000; Sajjadi, 2007). Honey can be employed as a capping agent, which aids in the prevention of aggregation of ZrO2 nanocrystals and has contributed significantly to the development of t-zirconia.
Fig. 2(l), i.e. FTIR spectra, shows that Zr-O-Zr bonds are prevalent in the 470–545 cm−1 range. For 1–4 wt% of iron oxide, mixed bands (tetragonal and monoclinic) are visible. The bands at 470 cm−1 are likely due to a monoclinic phase fraction, but the band at 496 cm−1 is likely due to a tetragonal phase of zirconia. (Batool et al., 2020; Asha et al., 2019; Khalili and Chenari, 2020). For samples treated with high concentrations of iron oxide (5–––10 wt%), the band (470 cm−1) shifts towards higher wavenumber, which corresponds to tetragonal zirconia (496 cm−1) as revealed by X-ray diffraction findings [Fig. 2(a-b)]. Many researches have previously documented the existence of t-ZrO2 phase at this wavenumber (Sanaullah et al., 2021; Fang et al., 2013; Lopez et al., 2007). The conspicuous band at 2352 cm−1 is caused by O–H stretching of as-yielded nanoparticles. The band observed at 1639 cm−1is because of the O–H stretching (Natalia et al., 2018). The peak at 1412 cm−1 corresponds to O–H bonding. While vibration bands present at 827 cm−1 are assigned to C = CH (Sahoo et al., 2011).
SEM images [Fig. 3] showed formation of hard agglomerated grains (Fig. 3(a–d)) for the samples with mixed structural phases. These hard agglomerated structures were observed because of the presence of multiple large numbers of tightly packed grains (Singh et al., 2017; Adeyemi et al., 2022) due the presence of two competing crystal phases. Large area growth with uniform surface morphology was observed with the increase in iron content from 5 to 10 wt% [Fig. 3 (e-j)]. Further, hydrodynamic diameter was also calculated and shown in Fig. 4. Various dilutions of zirconia nanoparticles were prepared in the present research work to investigate various parameters by adjusting pH using HCl and NH3. Ultrasonication time of 20 min was used and process was repeated twice after cooling the solution. Analysis was performed in triplicate. Zeta potential of as synthesized nanoparticles are shown in Fig. 4(ai-ji). Variation in zeta potential with the change in pH values is shown in Fig. 4 (aii-jii). Fig. 4 shows a clear indication of pH dependent response of zirconia nanoparticles. Positive values of zeta potential were observed for acidic pH values (1–6). Whereas iso-electric point was observed with further increase in pH. Negative values of zeta potential were observed with the further increase in pH values to basic conditions. When the pH was 2, the zeta potential reached + 53.2 ± 230 mV, which gradually decreased to + 52.97 ± 2.29 mV and + 0.95 ± 0.04 mV with the increase in pH to 4–6. Further, 0 mV of zeta potential was observed at ∼ 6 pH, and is known as the isoelectric point of the material under investigation. The stability of the nanoparticles in the form of solution fluid strongly depends on its pH nature (Nishakavya et al., 2022). For relatively higher pH vales, i.e. 8 and 10, the value of zeta potential was increased to − 13.83 ± 0.60 mV and − 31.8 ± 137 mV, respectively. These values might have been observed because of the charge value of oxide along with the dissociation of the hydroxyl groups present at the surface (Hiremath et al., 2021; Ordóñez et al., 2020).
Magnetization response of the samples were examined and plotted in Fig. 5. The observed soft ferromagnetic behavior can be attributed to the induced spin polarization by means of interaction due to attraction forces between host and doped electrons. The local electrons demonstrate similar direction of spin after the exchange interactions with polarized electrons of Fe2+ and Fe3+ ions (Xia et al., 2011). Therefore, because of these exchange interactions, ferromagnetic behavior was exhibited in the as synthesized nanoparticles. Honey was also added during synthesis, as a capping agent, and according to previous reports organic based molecules may exhibit magnetic ordering (Miller and Epstein, 1994; Miller, 2011). The presence of oxygen vacancies, as previously noted, results in the formation of t-zirconia. As the crystallographic phase changes from pure t-ZrO2 to mixed zirconia, coercivity and saturation magnetization might change (Lin et al., 2018).
Dispersion phenomenon, as shown in Fig. 6, occurs after the introduction of an external electric field. It takes some time for these carriers to align in the direction of the externally applied field. As a result, these carriers have enough time to align at lower frequencies. At higher frequencies, however, space charge carriers do not have enough time to respond, leading them to lag behind applied field and provide a lower dielectric constant value. Variation in dielectric constant with increment of stabilizer concentration may be related to particle size (SanaUllah et al., 2022; Gabriel et al., 1996). The increase in grain size with increasing iron oxide content promotes simpler domain wall motion, which results in an increase in the value of r. Furthermore, a larger concentration of iron oxide promotes the creation of a stronger intervening layer (insulator) near the grains. When compared to the resistance and capacitance of grain, these grain boundaries have a large resistance and capacitance (Ji and Brace, 2011; Jha, 2013). Because of the formation of mixed phases, a low dielectric constant value is achieved at lower stabilizer concentrations, as seen in Fig. 2. Meanwhile, increasing the concentration of stabilizer resulted in an increase in dielectric constant [Fig. 6(i)]. Relatively higher dielectric constant values are correlated with the stabilized zirconia samples [Fig. 2]. Figure S1 shows variation in real and imaginary impedance along with Cole-Cole plots. One semi-circle observed in Cole-Cole plots corresponded to a high frequency response of grains (Bukhari et al., 2018; Batool et al., 2020). At high concentrations of stabilizer, the area under the curve grows, indicating a higher value of impedance, whereas small semi circles suggest a lower value of impedance. Materials with small grain size have a high amount of grain boundaries and consequently have a comparatively high hardness / mechanical strength.
Mechanical properties of synthesized material, shown in Fig. 7, is significant to switch/swap absorption routes while maintaining therapeutic effectiveness. Commonly, not only leakage is prevented due to the attracting force between liposomes and sample, but this force is also responsible for the modulation of pathway efficacy to penetrate. Samples with higher value of hardness can easily enter the cell wall by mean of endocytosis route. Because of shape distortion and non-uniform ligand distribution, soft NP might be hindered. (Sangtani et al., 2017; Dasgupta et al., 2017). Hardness is the property of a substance that resists the indent created by the applied force. Grain size has a significant impact on the hardness of the polycrystalline material. Materials with smaller grain sizes have bigger grain boundaries, resulting in better mechanical strength. A Vickers hardness micro-indenter was operated to assess the material's hardness along with the fracture toughness. An array of indentations in a 50 μm by 50 μm matrix was built to measure the homogeneity and uniformity of samples. A contour map was employed to assess the mechanical behaviour of this matrix with an array of indents in terms of visual response.
The size of crystallites has a strong effect on the hardness of substance. The Hall-Patch equation, which is provided below as Eq. (5) (Niihara et al., 1982), can be used to correlate crystallite/grain size with hardness.
Eq. (6) (Niihara et al., 1982) is used to calculate fracture toughness.
H is for Hardness in HV, E stands for Young's modulus, d stands for mean diagonal length of the indent, and c stands for mean length of the Palmqvist fracture. Increased value of fracture toughness, i.e. 20–24 MPam-1/2, was observed for the samples prepared with 5–10 wt% of iron oxide content. Because of the presence of the dominating monoclinic phase and high grain size values, samples prepared with 1-4 wt% Fe3O4 concentration had comparatively low hardness values [Fig. S2 (a-d)]. Whereas, cracks may form as a result of the structural shift from tetragonal to monoclinic (Batool et al., 2020).
Biodegradation test of as synthesized optimized sample, as shown in Fig. 8(a) was measured by means of Eq. (7) (Öchsner and Merkel, 2013).
Where Vs and Sa are the volume of SBF (mL) and disc's area (mm2) respectively. Samples were dipped into a little quantity of SBF before being heated to 36 °C, which is the average human body temperature. The disks were washed, and drying was performed at room temperature without any further heat treatment. Using Eq. (8), the possible weight reduction (%) was calculated (Öchsner and Merkel, 2013).
Where w1 and w2 are the weight of the compact disc before and after being soaked, respectively. The weight loss data is shown in Fig. 9(a).
Fig. 8(b) demonstrated that the encapsulation efficiency of IOZH nanoparticles was high as compared to the other samples. Optimized samples rapidly encapsulated within first half hour, tailed by gradual encapsulation. After 120 min, the encapsulation efficiency was determined to be ∼ 73% [Fig. 9(b)]. The interaction of hydrogen ions (positively charged and found in curcumin) with iron and oxygen ions (negatively charged) might be the reason of this rapid encapsulation.
Eq. (9) (Gooneh-Farahani et al., 2020) was utilized to test the efficacy of encapsulated drug using stabilized nanoparticles.
Tc shows total amount of curcumin and Sc shows curcumin present in the supernatant.
Fig. 8 (c) shows nanoparticles and ascorbic acid in order to compare the scavenging % of as synthesized samples. To evaluate the absorbance value, UV–visible spectrophotometer was employed. The free radical scavenging capacity of DPPH was examined by using the subsequent Eq. (10) (Lee et al., 2014):
The symbols Ao and As reflect the values of DPPH absorption obtained in the absence as well as presence of IOZH nanoparticles tested for antioxidant activity, respectively.
The observed percentage of scavenging inhibition capability with the optimized sample was ∼ 70%. This enhanced antioxidant activity is associated with the smaller particles size as well as electron cloud, carried by IOZH nanoparticles. above described findings suggested that these as synthesized IOZH nanoparticles are not only safe to be administrated for imaging purpose but, also, capable of reducing excess amount of ROS.
The effect of nanoparticles on the cell viability of cancer cells is shown in Fig. 8(d). Cells were subjected to varied nanoparticle concentrations to investigate the influence of IOZH nanoparticles on the viability of cancer cells (Gooneh-Farahani et al., 2020). The MTT (3-(4, 5 dimethythiazol-2-yl)-2, 5diphenyl tetrazolium bromide) test assay was used to measure the percentage viability of the as synthesized IOZH sample. Cells were cultured for a time period of 24 h at a temperature of 37 °C prior to exposure of optimized IOZH nanoparticles in this assay. During a time period of 24 h incubation, different IOZH nanoparticle concentrate ions were applied to the well plates at the temperature of 37 °C with CO2 (5%) and air (95%). This procedure was performed in triplicate with untreated (HCT-116) cells acting as a control treatment. Under the same settings, MTT was added to the microplates and incubated for a time period of 4 h. A micro pipit was subsequently utilized to eject the media. Dimethyl sulfoxide (DMSO) was employed to decompose MTT-generated formazan crystals, and the plates were well agitated. At 570 nm, absorbance was measured using a microplate reader. Eq. (11) (Rajapriya et al., 2020) was used to determine the percentage of cell inhibition.
Here As and Ao represents sample and control absorbance, i.e. untreated cancer cells, respectively.
The outcomes of this assay suggested that cell viability is concentration dependent i.e. it decreases as concentration of working solution increases. These health hazard cancerous cells demonstrated around ∼ 23.4% of cell viability after a time period of 24 h after treatment with working solution having 25 μg/mL concentration. The percentage of cell viability in HCT-116 cells, as shown in Fig. 8 (d) showed that iron oxide stabilized nanoparticles are toxic to malignant cell lines and might be used in cancer therapies.
Gram Stained microbial images [Fig. 10] were observed by making smeared of bacterial culture from extreme edge of inhibition zone for each plates shown in Fig. 9. Fig. 10(b) shows the effect of nanoparticle (having 6 wt% of iron content) treatment. It was observed that sporulating stages were increased as compared to the mature spores and the cells are arranged in the form of pairs. The formation of spores is mainly due to the stress provided. It can be seen that the spores retained some of the stains in this case but originally can be observed in colorless form. In Fig. 10(c, d) it can be observed that increasing concentration of NPs affects gram staining as well as result in early sporulation under stress conditions. The staining color shift from gran positive to gram negative/ gram variablecan also be observed from Fig. 10. The cell size reduced and chains of bacteria were reduced to single or double cells. The cell size and resolution has also been affected greatly. The staining results in Fig. 10 (f, g) shows the effect of highest concentration of NPs against the Bacillus subtilis strain. The cells completely went to the endospore stage and the cell size was reduced. It can be seen that the resolution of bacteria has also been affected and vegetative cells have been reduced and sporulation increased to deal with the stress provided.
To assess an optimum value of pH for doing radiolabeling, the zirconia nanoparticles were labeled with Tc-99 m at various pH (4–7) values. In all experiments, a constant amount of SnCl2·2H2O, e.g., 40 µg, was used to reduce the Tc-99 m metal. The data from these investigations show that at pH 4, the radiolabeling yield was 96 ± 2% which reduced to 91 ± 2% at pH 5, dramatically lowered to 87 ± 2% at pH 6 and further dropped to 81% at pH 7 that was not in acceptable range. So, pH 4 was selected as optimum value for radiolabeling because it produced the highest labeling efficacy [Fig. 11 (a)].
Results presented in Fig. 11(b) indicate that the radiolabeling efficacy (%) by using reducing agent in the range 20–40 µg was 82%, 87% and 96%, respectively. However, the maximum radiolabeling yield, e.g., greater than 95 ± 2% was observed by using 40 µg of reducing agent.
The radiolabeling yield (%), shown in Fig. 11 (c), was 96 ± 2 % after 30 min of incubation that was equally stable up to 4 h and further decreased to 90 ± 2 % after 24 h.
The data, shown in Fig. 11 (d), clearly indicate that the radiolabeled nanoparticles were stable more than 90 ± 2% up to 24 h. The effect of amount of ligand, e.g. (zirconia nanoconjugate) on radiolabeling yield (%) was evaluated by varying the amount of the ligand required in radioconjugate formulation. The data indicate that at low value of ligand, e.g. 250 µg, the radiolabeling yield was very low, e.g. 85 ± 2%, which increased to maximum value of 96 ± 2% at 500 µg of the ligand, and significantly dropped to 70 ± 1% by further enhancing the amount of ligand to 750 µg. The data are graphically shown in Fig. 11 (e).
Lipophilicity study revealed that the radiolabeled zirconia particles are hydrophilic in nature. Additionally, the nanoparticle-based radio-conjugates bind to a diversity of protein molecules abundant in the plasma, leading to very little drug in the blood. The amount of free or unbound medicine in the human body might just have significant adverse consequences or pharmacological activity. The recent study showed that 82.3 ± 1.5% of 99mTc-zirconia nanoconjugate displayed binding with proteins in blood, which is much higher than expected for strong binding with proteins.
The scintigraphy results [Figs. 12 and 13] show that the radiotracer (after injection) is initially taken up by the heart, then by the liver, which is removed slowly, and lastly stored in the bladder, where a gradual increase in activity level is noted with time. That might be because the 99mTc-zirconia nanoparticles attach to bladder cells quickly and specifically, indicating the prospect of practical application in the identification of bladder malignancies. Previously Sung et al., (2015), Blasiak et al., (2013) and Büyükok et al (2019) used nanopartcles for diagnosis and therapy.
As it can be clearly anticipated from the pre-clinical data that the most probable pathway for the excretion of radio-nanoconjugate and its associated metabolites was through the kidney which is considered as the normal excretory route in nuclear medicine procedures so it should be considered as an additional diagnostic feature of the novel nano-tracer under investigation.
5 Conclusions
To fulfil the increased mandate of nanoparticles to be used in biomedical applications, the Fe3O4 stabilized zirconia nanostructures were synthesized by using sol–gel route. Iron oxide sol was added to zirconia sol at a concentration of 1–10 wt% (with a 1 wt% interval), and honey was used as a capping agent. The findings of X-ray diffractions revealed a reduction in volume and crystallite size of as synthesized samples when 5 wt- 10 wt% Fe3O4 concentrations were added. In the instance of phase pure tetragonal zirconia, reducing the volume of nanoparticles resulted in a better value of hardness and fracture toughness. Tetragonally stabilized samples exhibit dielectric constant values that are suitable for biological applications. High anti-oxidant activity (∼70%) as well as highest cell viability against cancerous cell (∼23.4%) was observed in optimized sample. Almost 70% of encapsulation efficiency was observed for optimized sample after time period of 120 min. Biodistribution of Sodium Pertechnetate (Na99mTcO4) labelled zirconia nanoparticles showed high uptake by the bladder and negligible uptake in heart and kidney indicating the in-vivo stability of labeled conjugates. Results obtained using these characterizations shows that these nanoparticles could be used for future therapeutical as well as drug carrying agents.
Acknowledgement
Authors are thankful to Higher Education Commission (HEC), Pakistan, for providing research grant to Centre of Excellence in Solid State Physics. The authors extend their appreciation to Researchers Supporting Project number (RSP2023R165), King Saud University, Riyadh, Saudia Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Numerical examination of gold nanoparticles as a drug carrier on peristaltic blood flow through physiological vessels: cancer therapy treatment. BioNanoScience. 2019;9(4):952-965.
- [Google Scholar]
- Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties. Molecules. 2022;27(10):3206.
- [Google Scholar]
- American Society for Testing of Materials 1999. Designation C1327-99 Standard test method for Vickers indentation hardness of advanced ceramics Annual Book of ASTM Standards 15.01. ASTM, Philadelphia.
- Synthesis and Characterization of ZrO 2 Nanoparticles using Microwave Assisted Method and Its Antimicrobial Activity. Journal of Nanoscience and Technology 2019:642-644.
- [Google Scholar]
- Nanoparticles for cancer treatment: role of heat transfer. Ann. N. Y. Acad. Sci.. 2009;1161(1):62-73.
- [Google Scholar]
- Role of Honey as a Bifunctional Reducing and Capping/Stabilizing Agent: Application for Silver and Zinc Oxide Nanoparticles. Nanomaterials. 2023;13(7):1244.
- [Google Scholar]
- Balasooriya, E. R., Jayasinghe, C. D., Jayawardena, U. A., Ruwanthika, R. W. D., Mendis de Silva, R., & Udagama, P. V. (2017). Honey mediated green synthesis of nanoparticles: new era of safe nanotechnology. Journal of Nanomaterials, 2017.
- Nano-zirconia–evaluation of its antioxidant and anticancer activity. Journal of Photochemistry and Photobiology B: Biology. 2017;170:125-133.
- [Google Scholar]
- Fe 3 O 4 stabilized zirconia: structural, mechanical and optical properties. J. Sol-Gel Sci. Technol.. 2015;74(2):281-289.
- [Google Scholar]
- Microwave assisted sol-gel synthesis of bioactive zirconia nanoparticles–Correlation of strength and structure. J. Mech. Behav. Biomed. Mater.. 2020;112:104012
- [Google Scholar]
- Blasiak, B., van Veggel, F.C., & Tomanek, B. (2013). Applications of nanoparticles for MRI cancer diagnosis and therapy. Journal of Nanomaterials, 2013, 12-12.
- Nanomaterial by sol-gel method: synthesis and application. Adv. Mater. Sci. Eng.. 2021;2021:1-21.
- [Google Scholar]
- Room temperature stabilized TiO2 doped ZrO2 thin films for teeth coatings–A sol-gel approach. J. Alloy. Compd.. 2018;767:1238-1252.
- [Google Scholar]
- Bioevaluation of 99mTc (I) carbonyl-radiolabeled amino acid coated magnetic nanoparticles in vivo. Materials Chemistry and Physics. 2019;235:121751.
- [Google Scholar]
- Flexible yttrium-stabilized zirconia nanofibers offer bioactive cues for osteogenic differentiation of human mesenchymal stromal cells. ACS Nano. 2016;10(6):5789-5799.
- [Google Scholar]
- Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces. 2006;49(2):136-144.
- [Google Scholar]
- Effect of thermal treatment at high temperature on phase stability and transformation of Yb2O3 and Y2O3 co-doped ZrO2 ceramics. Sci. Rep.. 2022;12(1):9955.
- [Google Scholar]
- Practicality of tetragonal nano-zirconia as a prospective sorbent in the preparation of 99 Mo/99m Tc generator for biomedical applications. Chromatographia. 2010;72:875-884.
- [Google Scholar]
- In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials. 2005;26(32):6288-6295.
- [Google Scholar]
- Effect of oxygen vacancy on phase transition and photoluminescence properties of nanocrystalline zirconia synthesized by the one-pot reaction. J. Phys. Chem. C. 2009;113(31):13974-13978.
- [Google Scholar]
- Elements of X-Ray Diffraction. New York, NY, USA: Addison-Wesley; 1956.
- Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces. 2013;111:556-560.
- [Google Scholar]
- Nano-and microparticles at fluid and biological interfaces. J. Phys. Condens. Matter. 2017;29(37):373003
- [Google Scholar]
- Controllable synthesis of metastable tetragonal zirconia nanocrystals using citric acid assisted sol–gel method. Ceram. Int.. 2013;39(3):2933-2941.
- [Google Scholar]
- Honey-based hydrogel: in vitro and comparative in vivo evaluation for burn wound healing. Sci. Rep.. 2017;7(1):1-11.
- [Google Scholar]
- Photoluminescence properties and photocatalytic activities of zirconia nanotube arrays fabricated by anodization. Opt. Mater.. 2013;35(7):1461-1466.
- [Google Scholar]
- A Novel and Inexpensive Method Based on Modified Ionic Gelation for pH-responsive Controlled Drug Release of Homogeneously Distributed Chitosan Nanoparticles with a High Encapsulation Efficiency. Fibers and Polymers. 2020;21(9):1917-1926.
- [Google Scholar]
- The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol.. 1996;41(11):2251.
- [Google Scholar]
- Garvie, R. C., Hannink, R. H., & Pascoe, R. T. 1975. Ceramic Steel, 258 (703). Nature, London.
- A novel and inexpensive method based on modified ionic gelation for pH-responsive controlled drug release of homogeneously distributed chitosan nanoparticles with a high encapsulation efficiency. Fibers Polym.. 2020;21:1917-1926.
- [Google Scholar]
- Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother.. 2001;48(1):7-13.
- [Google Scholar]
- Effect of Fe 2 O 3 doping on sintering of yttria-stabilized zirconia. J. Eur. Ceram. Soc.. 2012;32(16):4157-4164.
- [Google Scholar]
- Ultrashort pulsed laser ablation of zirconia-alumina composites for implant applications. J. Mater. Process. Technol.. 2022;299:117335
- [Google Scholar]
- Biocompatibility of zirconia. Journal of Advanced Medical and Dental Sciences Research. 2016;4(3):35.
- [Google Scholar]
- Reducing sintering temperature of yttria stabilized zirconia through addition of lithium nitrate and alumina. Ceram. Int.. 2012;38(5):4159-4164.
- [Google Scholar]
- Synthesis and characterization of nanocrystalline zirconia powder by simple sol–gel method with glucose and fructose as organic additives. Powder Technol.. 2011;205(1–3):193-200.
- [Google Scholar]
- Hiremath, P.G., Rajashekhara, S., Binnal, P., & Theodore, T. 2021. Chapter 9—Fluoride Contamination in Underground Water and Its Treatment. Management of Contaminants of Emerging Concern (CEC) in Environment; Singh, P., Hussain, CM, Rajkhowa, S., Eds, 249-280.
- Effect of Fe2O3 doping on colour and mechanical properties of Y-TZP ceramics. Ceram. Int.. 2018;44(15):17962-17971.
- [Google Scholar]
- Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles. Ceram. Int.. 2019;45(8):10106-10113.
- [Google Scholar]
- Biodistribution of iron-oxide-stabilized 99m Tc-ZrO 2 nanoparticles in rabbit using honey as a capping agent—microwave-assisted sol–gel approach. J. Sol-Gel Sci. Technol.. 2021;98:95-112.
- [Google Scholar]
- Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm.. 2008;5(2):316-327.
- [Google Scholar]
- Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Structures & Nano-Objects. 2020;23:100484
- [Google Scholar]
- Janes, K.A., Fresneau, M.P., Marazuela, A., Fabra, A., Alonso, M.J. 2001. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control Release. 15;73(2-3), 255-67.
- Electrical characterization of zirconium substituted barium titanate using complex impedance spectroscopy. Bull. Mater. Sci.. 2013;36(1):135-141.
- [Google Scholar]
- Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys. Med. Biol.. 2011;56(16):5249.
- [Google Scholar]
- Characterisation of well-adhered ZrO2 layers produced on structured reactors using the sonochemical sol–gel method. Appl. Surf. Sci.. 2018;427:563-574.
- [Google Scholar]
- Efficient One-Pot Solvothermal Synthesis and Characterization of Zirconia Nanoparticle-Decorated Reduced Graphene Oxide Nanocomposites: Evaluation of Their Enhanced Anticancer Activity toward Human Cancer Cell Lines. ACS Omega. 2023;8(2):2406-2420.
- [Google Scholar]
- Effect of iron oxide coloring agent on the sintering behavior of dental yttria-stabilized zirconia. Ceram. Int.. 2018;44(5):4689-4693.
- [Google Scholar]
- Stabilized zirconia as a structural ceramic: an overview. Dent. Mater.. 2008;24(3):289-298.
- [Google Scholar]
- Successful electrospinning fabrication of ZrO2 nanofibers: A detailed physical–chemical characterization study. J. Alloy. Compd.. 2020;828:154414
- [Google Scholar]
- In vivo biodistribution, antioxidant and hemolysis tendency of superparamagnetic iron oxide nanoparticles–Potential anticancer agents. Arab. J. Chem.. 2023;16(4):104602
- [Google Scholar]
- Antioxidant and [alpha]-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. Int. Food Res. J.. 2014;21(1):379.
- [Google Scholar]
- One-step preparation of CaO-doped partially stabilized zirconia from fused zirconia. Ceram. Int.. 2020;46(5):6484-6490.
- [Google Scholar]
- A comparative study on the direct and pulsed current electrodeposition of cobalt-substituted hydroxyapatite for magnetic resonance imaging application. Materials. 2018;12(1):116.
- [Google Scholar]
- Effects of Surface Conditions on Diamond Nucleation. Diam. Chem. Vap. Depos 1995:92-130.
- [Google Scholar]
- Esterification and transesterification on tungstated zirconia: Effect of calcination temperature. J. Catal.. 2007;247(1):43-50.
- [Google Scholar]
- Fabrication and reaction mechanism of MgO-stabilized ZrO2 powders by combustion synthesis. Ceram. Int.. 2022;48(5):7261-7264.
- [Google Scholar]
- Clinical applications of magnetic drug targeting. J. Surg. Res.. 2001;95(2):200-206.
- [Google Scholar]
- Impedance spectroscopy: theory, experiment, and applications. History. 2005;1(8):1-583.
- [Google Scholar]
- Antimicrobial and nonlinear optical studies of copper oxide nanoparticles. J. Electron. Mater.. 2021;50:3415-3421.
- [Google Scholar]
- On the interpretation of crystal size distributions in magmatic systems. J. Petrol.. 1998;39(4):553-599.
- [Google Scholar]
- Magnetically ordered molecule-based materials. Chem. Soc. Rev.. 2011;40(6):3266-3296.
- [Google Scholar]
- Organic and organometallic molecular magnetic materials—designer magnets. Angew. Chem. Int. Ed. Eng.. 1994;33(4):385-415.
- [Google Scholar]
- Magnetic nanoparticles: An overview for biomedical applications. Magnetochemistry. 2022;8(9):107.
- [Google Scholar]
- The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597-6611.
- [Google Scholar]
- Biofabrication of zirconia nanoparticles: synthesis spectral characterization and biological activity evaluation against pathogenic bacteria. Biointerface Res Appl Chem. 2023;13:190.
- [Google Scholar]
- Crystal structure analysis of lithium zirconate prepared from local sand at a various ratio of Li2CO3 to ZrO2. Space. 2018;12:c1
- [Google Scholar]
- Evaluation of K Ic of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett.. 1982;1(1):13-16.
- [Google Scholar]
- Size attenuated copper doped zirconia nanoparticles enhances in vitro antimicrobial properties. Appl. Biochem. Biotechnol.. 2022;194(8):3435-3452.
- [Google Scholar]
- Bending Element. In One-Dimensional Finite Elements. Berlin, Heidelberg: Springer; 2013. p. :57-110.
- Synthesis of ZrO2 nanoparticles and effect of surfactant on dispersion and stability. Ceram. Int.. 2020;46(8):11970-11977.
- [Google Scholar]
- Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. International journal of biological macromolecules. 2011;49(5):1167-1172.
- [Google Scholar]
- Nanocarriers as an emerging platform for cancer therapy. Nano-Enabled Medical Applications 2020:61-91.
- [Google Scholar]
- Robocasting of dense yttria-stabilized zirconia structures. J. Mater. Sci.. 2018;53:247-273.
- [Google Scholar]
- Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2009;73(4):650-653.
- [Google Scholar]
- Radiolabeled nanomaterials for biomedical applications: radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm Chem.. 2022;7(1):8.
- [Google Scholar]
- Comparative lattice-dynamical study of the Raman spectra of monoclinic and tetragonal phases of zirconia and hafnia. J. Am. Ceram. Soc.. 2002;85(7):1745-1749.
- [Google Scholar]
- Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int. J. Nanomed.. 2007;2(4):567.
- [Google Scholar]
- Synthesis and characterization of zinc oxide nanoparticles using Cynara scolymus leaves: enhanced hemolytic, antimicrobial, antiproliferative, and photocatalytic activity. J. Clust. Sci.. 2020;31(4):791-801.
- [Google Scholar]
- Colloid probe investigation of the stabilization mechanism in aqueous 1, 2-propanediol nano-zirconia dispersions. PCCP. 2004;6(7):1467-1474.
- [Google Scholar]
- Qualitative analysis of controlled release ciprofloxacin/carbopol 934 mucoadhesive suspension. J. Adv. Pharm. Technol. Res.. 2011;2(3):195.
- [Google Scholar]
- Nanoparticle formation by monomer-starved semibatch emulsion polymerization. Langmuir. 2007;23(3):1018-1024.
- [Google Scholar]
- Unseeded semibatch emulsion polymerization of butyl acrylate: bimodal particle size distribution. J. Polym. Sci. A Polym. Chem.. 2000;38(3):528-545.
- [Google Scholar]
- Antibacterial performance of glucose-fructose added MW based zirconia coatings–Possible treatment for bone infection. J. Mech. Behav. Biomed. Mater.. 2020;104:103621
- [Google Scholar]
- Tangerine mediated synthesis of zirconia as potential protective dental coatings. Mater. Sci. Eng. C. 2021;120:111653
- [Google Scholar]
- Microwave assisted synthesis of Fe3O4 stabilized ZrO2 nanoparticles–Free radical scavenging, radiolabeling and biodistribution in rabbits. Life Sci.. 2021;271:119070
- [Google Scholar]
- Free radical scavenging and antimicrobial activities of MW assisted sol-gel synthesized honey mediated zirconia. J. Sol-Gel Sci. Technol.. 2022;103(2):457-475.
- [Google Scholar]
- Multifunctional nanoparticle composites: progress in the use of soft and hard nanoparticles for drug delivery and imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.. 2017;9(6):e1466.
- [Google Scholar]
- Sengupta, P., Bhattacharje, A., Maiti, H.S. 2019. Zirconia: A unique multifunctional ceramic material 72, 1981-1998.
- A New Procedure To Measure the Antioxidant Activity of Insoluble Food Components. J. Agric. Food Chem.. 2007;55:7676-7681.
- [Google Scholar]
- Evaluation of antioxidant activity of the residues generated from ethanol concentration of lignocellulosic biomass using pervaporation. Journal of Industrial and Engineering Chemistry. 2017;52:51-58.
- [Google Scholar]
- Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sens. Actuators B. 2017;241:1170-1178.
- [Google Scholar]
- Yttria-stabilized zirconia thin film electrolyte produced by RF sputtering for solid oxide fuel cell applications. Mater. Lett.. 2010;64(22):2450-2453.
- [Google Scholar]
- Thermodynamics of doping and vacancy formation in BaZrO 3 perovskite oxide from density functional calculations. Phys. Rev. B. 2006;73(10):104112
- [Google Scholar]
- Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Scientific reports. 2015;5(1):12135.
- [Google Scholar]
- Synthesis and characterization of nanocrystalline mesoporous zirconia using supercritical drying. J. Nanosci. Nanotechnol.. 2006;6(6):1584-1593.
- [Google Scholar]
- Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf A Physicochem Eng Asp. 2011;384(1–3):733-738.
- [Google Scholar]
- Room-temperature ferromagnetic properties of Fe-doped ZnO rod arrays. Solid State Sci.. 2011;13(2):388-393.
- [Google Scholar]
- Fabrication and characterization of dense zirconia and zirconia-silica ceramic nanofibers. J. Nanosci. Nanotechnol.. 2010;10(9):5672-5679.
- [Google Scholar]
- Yentekakis, I. V., & Chu, W. 2020. Advances in Heterocatalysis by Nanomaterials.
- Biosynthesis of zirconium nanoparticles (ZrO2 NPs) by Phyllanthus niruri extract: Characterization and its photocatalytic dye degradation activity. Food Chem. Toxicol.. 2022;168:113340
- [Google Scholar]
- Studies of electrospinning process of zirconia nanofibers. Fibers Polym.. 2010;11(8):1119-1122.
- [Google Scholar]
- Effect of iron oxide on the phase composition of zirconia refractories. Refractories. 1980;21(1–2):48-52.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105267.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







