Translate this page into:
Bioactive molecular family construction: Design, optimization and antifungal mechanism study of novel 2-phenylglycine derivatives
⁎Corresponding author. zbwu@gzu.edu.cn (Zhibing Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
With the widespread use of fungicides, some problems have also gradually emerged, such as resistance, toxicity and residues. Therefore, to develop novel antifungal pesticides with unique structures, safety and high efficiency, a series of 2-phenylglycine derivatives were designed and synthesized. The bioactivity results revealed good antifungal activity of compound III11 and boscalid against Nigrospora oryzae, and they both effectively inhibited spore germination with EC50 values of 17.3 and 3.1 μg/mL, respectively. An antifungal mechanism study showed that both III11 and boscalid significantly increased the content of nucleic acids, proteins and MDA, which indicated that they could destroy the integrity of the mycelial cell membrane, thus affecting the normal growth of mycelia. Molecular docking results revealed that III11 bound with some amino acid residues (SER-39, ARG-14 and ARG-43) of succinate dehydrogenase (SDH) through a unique mode involving hydrogen bonds, different from the binding mode of boscalid. Further investigation demonstrated that III11 exhibited moderate inhibitory activity against SDH (IC50 = 54.5 μg/mL), weaker than that of boscalid (IC50 = 10.7 μg/mL). The results provided sufficient support for the optimization and derivatization of 2-phenylglycine derivatives, which could be further developed and designed as potential fungicides.
Keywords
Structural derivation
Antifungal activity
Mechanism study
Molecular docking
Enzyme activity
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR
-
19F nuclear magnetic resonance
- HRMS
-
high-resolution mass spectrometry
- r.t.
-
room temperature
- EC50
-
median effective concentration
- IC50
-
median inhibition concentration
- CK
-
blank control
- SEM
-
scanning electron microscope
- FM
-
fluorescence microscope
- PBS
-
phosphate buffer saline
- PDA
-
potato dextrose agar
- PDB
-
potato dextrose broth
- TLC
-
thin layer chromatography
- EA
-
ethyl acetate
- PE
-
petroleum ether
- DCM
-
dichloromethane
- SDH
-
succinate dehydrogenase
- PI
-
propidium iodide
- MDA
-
malondialdehyde
- SD
-
standard deviation
- OD
-
optical density
- SAR
-
structure–activity relationship
Abbreviations
1 Introduction
The safety of food and economic crops is closely related to people's livelihood, but the increasing severity and scale of fungal diseases poses a serious threat to global food production and safety (Sun et al., 2022; Ray et al., 2017; Li et al., 2021; Fisher et al., 2012). At present, chemical control is still one of the most effective methods to prevent and control fungal diseases because of its low cost, high efficiency and convenience. Accompanying this, the risks of food security and fungicide resistance are also increasing (Chen et al., 2020; Bojarski and Witeska, 2020), and fungal disease control is facing considerable challenges. Therefore, there is an urgent need to continuously develop structurally unique, safe and efficient fungicides to address the growing food security problems.
1,3,4-Oxadiazole, a five-membered heterocyclic ring composed of carbon, nitrogen and oxygen atoms (Wang et al., 2021) with unique electron-rich properties, has been widely used in pharmaceuticals and pesticides and exhibits a wide range of biological activities, including antifungal (Zheng et al., 2017; Wang et al., 2019), insecticidal (Wang et al., 2019), anti-allergic (Guda et al., 2013), anti-inflammatory (Kumar et al., 2021), antibacterial (Xiang et al., 2020), and anti-convulsant activities (Fig. 1) (Harish et al., 2013). In addition, amide, widely found in natural products, is an essential structural unit and active pharmacophore skeleton in drug development (Yao et al., 2017) and has been widely used in pesticides, including fungicides (Esteve-Turrillas et al., 2017), insecticides (Li et al., 2023) and herbicides (Doležalová et al., 2020). It is worth noting that the acylhydrazone and sulfonamide structures are similar to the amide structure, which is also an efficient pharmacophore. A variety of commercialized drugs have been developed, such as flusulfamide, sulfalene, siflufenzopyr and nifursemizone (Kalli and Velmurugan, 2022; Zhang et al., 2022; Osmaniye et al., 2018; Iliev et al., 2019).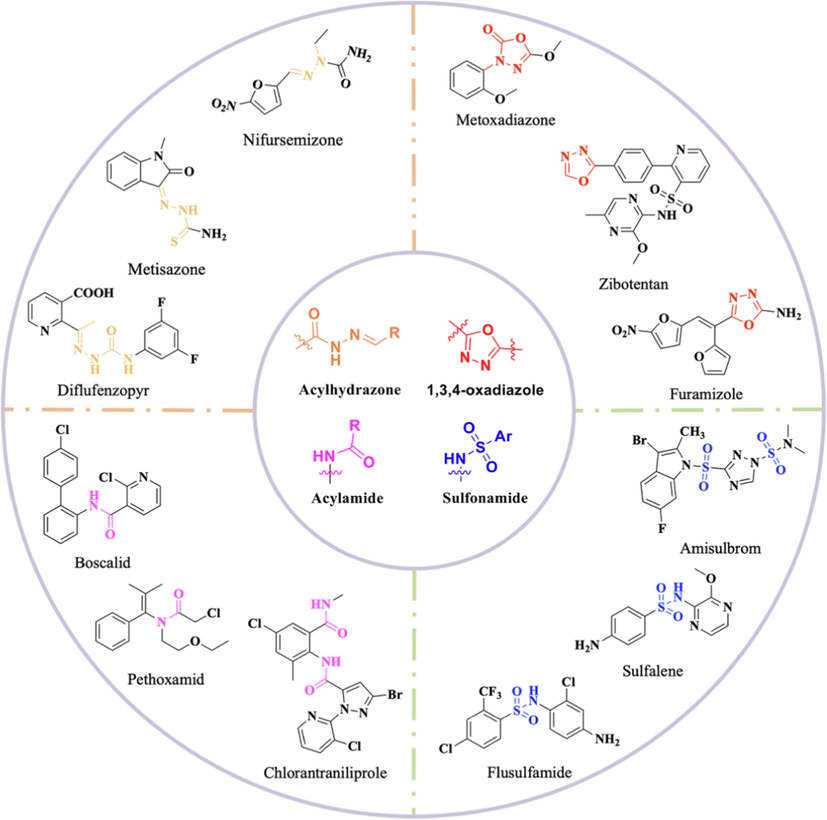
Commercialized drugs containing 1,3,4-oxadiazole, amide, acylhydrazone and sulfonamide moieties.
In our previous work (Zhang et al., 2023), a series of 2-phenylglycine derivatives containing a 1,3,4-oxadiazole moiety with certain inhibitory activity against fungi, bacteria and TMV was reported. In this work, a series of novel 2-phenylglycine derivatives containing 1,3,4-oxadiazole, acylhydrazone and sulfonamide fragments were designed and synthesized to construct a new bioactive molecular family (Fig. 2). The antifungal activity against eight plant pathogenic fungi was evaluated, and the antifungal mechanism of the active compound was explored through morphological observation, quantitative analysis of mycelial growth and a cell membrane integrity study, which provided sufficient support for further development of amino acid derivatives.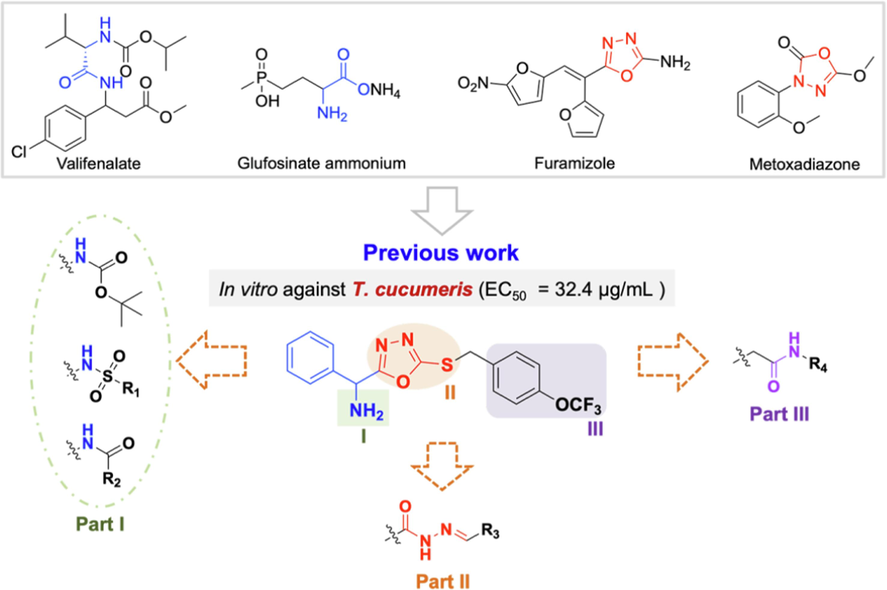
Molecular design strategy of the target compounds.
2 Materials and methods
2.1 Instruments and chemicals
1H NMR, 13C NMR and 19F NMR data were measured by a Bruker 400 NMR spectrometer (Bruker Corporation, Germany) using CDCl3 and DMSO‑d6 as solvents. HRMS data were measured by a Thermo Scientific Q Exactive high resolution mass spectrometer (Thermo Scientific, USA). The melting point was measured by an X-4B melting point meter (Nanbei Instrument Limited, China) and was uncorrected. Spore germination was observed by an Olympus-CX33 microscope (Olympus Co., Ltd., Japan). The mycelial morphology was observed using an Olympus-BX53 FM (Olympus Ltd, Japan) and a Nova Nano SEM450 (Thermo Fisher Scientific, USA). MDA and cellular contents (nucleic acids and proteins) were detected by a Cytation™5 multimode reader (BioTek Instruments, Inc. USA). All reagents and solvents were used directly without further purification or drying after purchase.
2.2 Fungi
F. prolifeatum, G. zeae, T. cucumeris, A. solani, N. oryzae, S. sclerotiorum, and F. oxysporum were purchased from Beijing Beina Chuanglian Biotechnology Institute, China; C. camelliae was provided by Guizhou Academy of Agricultural Sciences and identified at Sangon Biotech (Shanghai) Co., Ltd., China. All fungi were grown on PDA plates at 25 ± 1 °C and maintained at 4 °C.
2.3 Synthesis
Intermediates A, B, C, D, and E were synthesized according to the method reported in our previous work (Zhang et al., 2023).
2.3.1 General synthesis procedure for Fn intermediates (using F3 as an example)
Intermediate B (3.0 mmol), DMF (8.0 mL), 2-fluorobenzoic acid (3.3 mmol), DPPA (3.6 mmol) and triethylamine (6.7 mmol) were added in turn to a 50 mL round-bottom flask (Shioiri et al., 2022), stirred in an ice bath for 2 h, and then reacted at r.t. The reaction process was monitored by TLC. Then, the crude product was extracted with EA and purified by column chromatography (eluent: PE/EA = 30/1) to obtain F3, a light yellow solid, with a yield of 83% and an m.p. of 91–93 °C. The synthesis procedures for other intermediates were similar to those for F3.
2.3.2 General synthesis procedure for Gn intermediates (using G3 as an example)
A mixture of F3 (10.0 mmol), methanol (10.0 mL) and 80% hydrazine hydrate (100.0 mmol) was stirred at 100 °C for 7 h and monitored by TLC (Makane et al., 2019). Subsequently, methanol was removed by vacuum distillation. Then, the crude product was extracted with DCM and purified by column chromatography (eluent: DCM/methanol (MeOH) = 50/1) to obtain G3, a white solid, with a yield of 88% and an m.p. of 122–123 °C. The synthesis of other intermediates was similar to that of G3.
2.3.3 General synthesis procedure for Hn intermediates (using H11 as an example)
4-Aminobenzotrifluoride (10.8 mmol), tetrahydrofuran (10.0 mL), triethylamine (12.9 mmol) and bromoacetyl bromide (12.9 mmol) were added in turn to a 50 mL round-bottom flask and stirred in an ice bath (Kumar et al., 2012). The reaction process was monitored by TLC. Subsequently, tetrahydrofuran was removed by vacuum distillation. Then, the crude product was extracted with DCM and purified by column chromatography (eluent: PE/EA = 30/1) to obtain H11, a white solid, with a yield of 84% and an m.p. of 151–153 °C. The synthesis of other intermediates was to that of H11.
2.3.4 General synthesis procedure for target compounds I1–I6
Intermediate D (4.0 mmol), acetonitrile (10.0 mL), water (10.0 mL), potassium carbonate (6.0 mmol) and halogenated compounds (5.0 mmol) were added in turn to a 50 mL round-bottom flask and stirred at r.t. for 7 h (Wang et al., 2021). Then, the crude product was extracted with EA and purified by column chromatography (eluent: PE/EA = 10/1) to obtain target compounds I1–I6.
2.3.5 General synthesis procedure for target compounds I7–I14
Intermediate E (1.0 mmol), DCM (8.0 mL), substituted sulfonylchloride (2.0 mmol) and triethylamine (1.0 mol) were added in turn to a 50 mL round-bottom flask and stirred in an ice bath, and the reaction process was monitored by TLC (Wang et al., 2021). Then, the crude product was extracted with DCM and purified by column chromatography (eluent: PE/EA = 30/1) to obtain the target compounds I7–I14.
2.3.6 General synthesis procedure for target compounds I15–I18
Intermediate E (1.3 mmol), DCM (10.0 mL), EDCI (2.6 mmol), carboxylic acid (1.6 mmol) and DMAP (1.3 mmol) were added in turn to a 50 mL round bottom flask, and the reaction process was monitored by TLC (Li et al., 2016). Then, the crude product was extracted with DCM and purified by column chromatography (eluent: PE/EA = 30/1) to obtain target compounds I15–I18.
2.3.7 General synthesis procedure for target compounds II1–II8
Intermediate G (1.8 mmol), ethanol (10.0 mL) and aromatic aldehydes (2.8 mmol) were added in turn to a 50 mL round-bottomed flask and heated for 5 h (Yang et al., 2016). Subsequently, ethanol was removed by vacuum distillation. Then, the crude product was extracted with DCM and purified by column chromatography (eluent: PE/EA = 30/1) to obtain target compounds II1–II4. Target compounds II5–II8 were synthesized using a similar procedure to that used for II1–II4.
2.3.8 General synthesis procedure for target compounds III1–III13
Intermediate D (3.0 mmol), acetone (10.0 mL), sodium bicarbonate (3.0 mmol) and Hn (3.0 mmol) were added successively to a 50 mL round bottom flask, and the reaction process was monitored by TLC after heating and refluxing for 7 h (Khanaposhtani et al., 2018). Subsequently, acetone was removed by vacuum distillation. Then, the crude product was extracted with EA and purified by column chromatography (eluent: DCM/EA = 50/1) to obtain the target compounds III1–III13.
2.3.9 General synthesis procedure for target compounds III14–III17
III4 (1.0 mmol) and DCM (8.0 mL) were added to a 50 mL round-bottom flask. After complete dissolution, trifluoroacetic acid (2.0 mL) was added, the solution was stirred at room temperature for 7 h (Liu et al., 2017), and the pH was adjusted to 8–9 by adding saturated sodium carbonate solution. Then, the crude product was extracted with DCM and purified by column chromatography (eluent: DCM/MeOH = 200/1) to obtain III14, a yellow solid, with a yield of 73% and an m.p. of 119–121 °C. The synthesis of other target compounds III15–III17 was similar to III14.
2.4 Bioassay evaluation
2.4.1 In vitro antifungal activity bioassay
According to a previously reported method (Wu et al., 2021), all target compounds were preliminarily evaluated for in vitro antifungal activity against eight plant pathogenic fungi at a concentration of 50 μg/mL. Sterile distilled water with DMSO (1%) was used as a blank control, and the commercialized fungicide boscalid was used as a positive control. When the mycelia of the blank control group grew to 6 cm, the diameter of the mycelia of the treatment group was recorded, and three replicates were performed for each treatment group. The inhibition rate was calculated according to the formula I (%) = [(C − T)/(C − 0.4)] × 100, where C is the growth diameter of fungi in the blank control group, T is the diameter of fungi in the treatment group, and I is the inhibition rate. The SD value was calculated as three repeated inhibition data points.
On the basis of in vitro antifungal activity, the EC50 values of the compounds with inhibition rates higher than 60% were further determined according to the above method (Zhang et al., 2022). The concentration gradient was 100, 50, 25, 12.5, 6.25, or 3.125 μg/mL, and the linear regression analysis and EC50 values were calculated by SPSS software (version 20.0, IBM).
2.4.2 In vivo antifungal activity evaluation
According to the method reported in the literature with some minor modifications (Wang et al., 2019). Rice was used as the testing plant, DMSO (1%) was served as a blank control, and the commercial fungicide boscalid was selected as a positive control. The in vivo protective activity of III11 against N. oryzae was further evaluated. Rice seeds (Xiangliangyou 900) were sown and grown for approximately 8 weeks at r.t. At the tillering stage of rice, 50 mL of III11 and boscalid solutions were prepared with Tween 20 and sterile distilled water at 50 μg/mL and 100 μg/mL, respectively. The solutions were sprayed evenly on the rice plants until they were was completely moist. After 4 h, the middle part of the rice leaves was ground with sandpaper, and mycelial plugs (diameter 4 mm) were placed on the wounds and fixed with wet cotton. The inoculated plants were then placed in an incubator at 25 °C and 90% relative humidity. At 6 days after inoculation, the lesion length of rice leaves was measured, and the control effect of each treatment group was calculated. The calculation formula is as follows: I (%) = (A0 − A1)/A0 × 100, where A0 is the lesion length of the blank control group, and A1 is the lesion length of the treatment group.
2.4.3 Spore germination of N. Oryzae
N. oryzae was inoculated on PDA medium and cultured at 25 °C for approximately 7 to 10 days under the conditions of a 12 h light/12 h dark cycle (Li et al., 2022). The mycelia were washed with sterile distilled water and Tween 20, fully shaken (650 rpm, 15 min), and filtered to obtain a spore suspension. Then, 200 μL of spore suspension was evenly applied to the drug-containing PDA medium and cultured in a constant-temperature incubator at 25 °C for 12 h. Finally, spore germination was observed under a microscope. The total number of spores was not less than 200 in each repeated examination. The total number of spores and spore germination were recorded, and germination was considered to have occurred when the germ tube length reached 1/2 of the spore diameter. DMSO (1%) was used as the blank control, and three replicates were performed for each treatment group. The following formula was used to calculate the spore germination rate: I = N/M × 100, where I represents the inhibition rate, N represents the spore germination number, and M represents the total spore number. The following formula was used to calculate the spore germination inhibition rate: W = (ICK − In)/ICK × 100, where W is the spore germination inhibition rate, ICK is the spore germination rate of the control group, and In is the spore germination rate of the treatment group; three replicates were performed to obtain the SD value.
2.4.4 Preparation of mycelia of N. Oryzae
N. oryzae was cultured on a PDA plate in an incubator at 25 °C for two days, and then fifteen mycelial cakes (diameter 4 mm) were cut from the edge of a normally growing colony and added to a conical flask containing PDB medium. After culturing at 25 °C for 16 h (180 rpm) in a shaker, the residual PDA was removed and transferred to another conical flask containing PDB for additional incubation for 2 days. Finally, the culture was filtered and washed three times with sterile water to obtain the mycelia.
2.5 Mechanism study of the activity of III11 against N. Oryzae
2.5.1 Morphological observation of the mycelia via SEM
N. oryzae was cultured on a PDA plate for two days, and then five mycelial cakes were cut from the edge of the colony, added to 50 mL PDB medium, and incubated in a shaker at 25 °C and 180 rpm for 36 h. DMSO (1%) was used as a blank control, and solutions of III11 and boscalid at different concentrations (25 and 50 μg/mL) were added to the culture medium, which were then incubated under the same conditions for 24 h. Then, the hyphae were washed with PBS buffer (pH = 7.2) three times, fixed with 2.5% glutaraldehyde for more than 12 h, dehydrated with ethanol (30%, 50%, 70%, 90%, and 100%) and tert-butanol for 15 min, respectively, dried in a freeze dryer, sprayed with gold, made into a sample, and observed by SEM (Yang et al., 2021).
2.5.2 Morphological observation of the mycelia via FM
Five mycelial cakes were cut from the edge of the normally growing colony and added to the PDB medium. After 36 h of culture in a shaker at 25 °C and 180 rpm, different concentrations of III11 and boscalid solutions (0, 25, and 50 μg/mL) were added, and the culture was incubated for 24 h under the same conditions. The hyphae were washed with PBS buffer solution, stained with PI, washed three times with PBS buffer solution after incubation at 37 °C for 15 min, made into samples and observed by FM (Puig et al., 2016).
2.5.3 Determination of dry weight of mycelia
According to the method described in 2.4.4, N. oryzae mycelia were cultured in PDB medium in a shaker at 25 °C and 180 rpm (Mo et al., 2021). Fresh hyphae (0.5 g) were added to 50 mL PDB medium, and then different concentrations of III11 and boscalid solutions (0, 50, and 100 μg/mL) were added. After 2 days of incubation on a shaker at 25 °C and 180 rpm, the hyphae were filtered and washed three times with equal amounts of distilled water. Ultimately, the samples were dried by vacuum freezing for 6 h, and the hyphae were weighed. Each treatment group was set up with three replications, and the average weight of dry hyphae was recorded as the dry weight of mycelia.
2.5.4 Determination of release cellular contents
0.2 g fresh hyphae (collected in 2.4.4) was suspended in 25 mL sterile water. Then, compound III11 or boscalid was added to a final concentration of 25 μg/mL (Dou et al., 2022). The obtained samples were cultured in a shaker at 25 °C at a speed of 180 rpm. The absorbance at 260 and 280 nm was measured at 0, 12, 24, 36, 48, 60, and 72 h, respectively.
2.5.5 Determination of MDA content
According to the method described in 2.4.4, the mycelia of N. oryzae were cultured on a shaker at 25 °C and 180 rpm for 2 days (Wu et al., 2016). Then, different concentrations of III11 solutions (0, 25, 50, and 100 μg/mL) were added, and the culture was incubated for 3 days under the same conditions. The hyphae were filtered, washed with an equal amount of distilled water, and then dried by vacuum freezing for 6 h. Finally, the MDA content was determined by an MDA detection kit (Solarbio Technology Co., Ltd., China) according to the instructions, and each treatment group consisted of three replicates.
2.5.6 Molecular docking
Docking analysis was performed using AutoDock Vina 1.1.2 (Du et al., 2021). The protein structure of SDH was downloaded from the PDB database (PDB code: 2FBW). PyMOL 2.3.0 was used to remove the protein-bound water and the original ligand, and the protein structure was imported into Mgtools 1.5.6 for hydrogenation, calculation of charge and merging of nonpolar hydrogen. In addition, the three-dimensional structure of the compound was downloaded from PubChem and then imported into Chemdraw 3D, and the energy was minimized by MM2 to obtain the lowest energy dominant conformation. The two-dimensional binding modes were obtained by Discovery Studio 3.5.
2.5.7 SDH enzyme assay
A succinate dehydrogenase assay kit (Suzhou Comin Biotechnology Co., Ltd.) was used to determine the effects of III11 and boscalid on SDH enzyme activity (Sun et al., 2022). N. oryzae was grown in PDB medium for 2 days, and then different concentrations of III11 and boscalid solutions (0, 6.25, 12.5, 25, 50, 100 and 200 μg/mL) were added, and the culture was incubated at 25 °C for an additional 48 h. Ultimately, the mycelia were filtered and dried by vacuum freezing, and SDH inhibitory activity was detected according to the kit instructions.
Data processing was mainly done by IBM SPSS Statistics 20. And the significant difference analysis using Duncan’s test method.
3 Results and discussion
3.1 Chemistry
As shown in Scheme 1, intermediate C was obtained by esterification, amino protection and hydrazinolysis, according to our previous work (Zhang et al., 2023). Key intermediate D was formed by the reaction of intermediate C and carbon disulfide under alkaline conditions. Target compounds I1 − I6 were obtained from intermediate D, which reacted with different halogenated compounds by a simple nucleophilic substitution, with a yield range of 69–82%. After removing the protective group of In intermediates in the presence of trifluoroacetic acid, En intermediates were obtained. Then, target compounds I7 − I14 were synthesized by a substitution reaction of En and different sulfuryl chlorides, with a yield range of 62–78%; target compounds I15−Ⅰ18 were synthesized by a condensation reaction of En and different acids, with a yield range of 75–82%.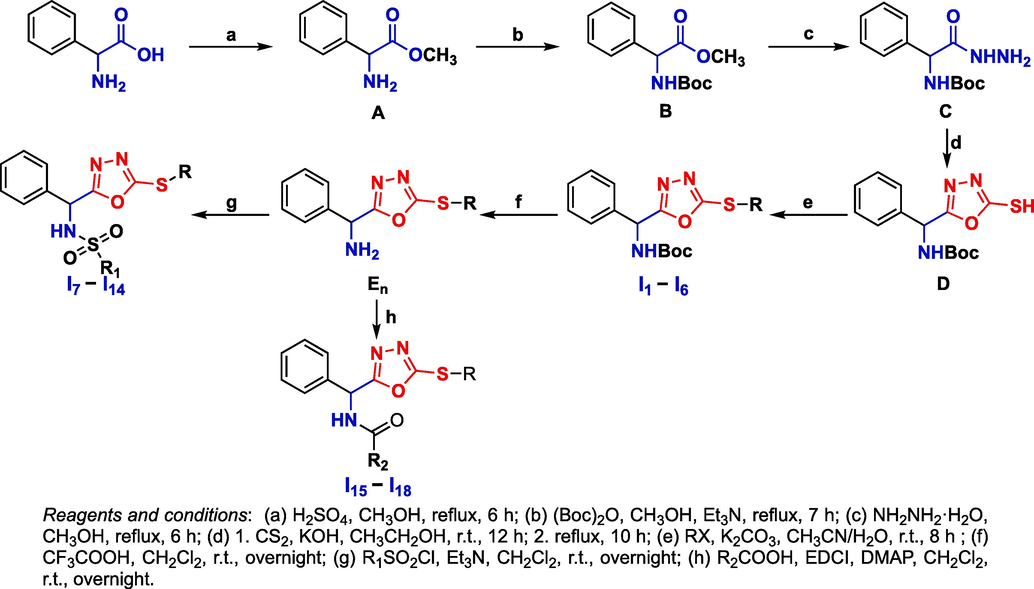
Synthetic route of target compounds I1 − I18.
Fn intermediates were obtained by a condensation reaction of A and different carboxylic acids in the presence of DPPA at room temperature, with a yield range of 74–83%. Gn intermediates were obtained by hydrazinolysis of Fn intermediates. Target compounds II1 − II8 were obtained through a reaction of Gn intermediates and C with aromatic aldehydes, with a yield range of 73–88% (Scheme 2). Target compounds III1 − III13 were obtained by a substitution reaction of D and H, and then III14 − III17 were obtained by removing the protective group (Scheme 3). All target compounds were identified by 1H NMR, 13C NMR, 19F NMR and HRMS. The spectral data of target compounds Ⅰ1 − I18, II1 − II8, and III1 − III17 are provided in the Supporting information.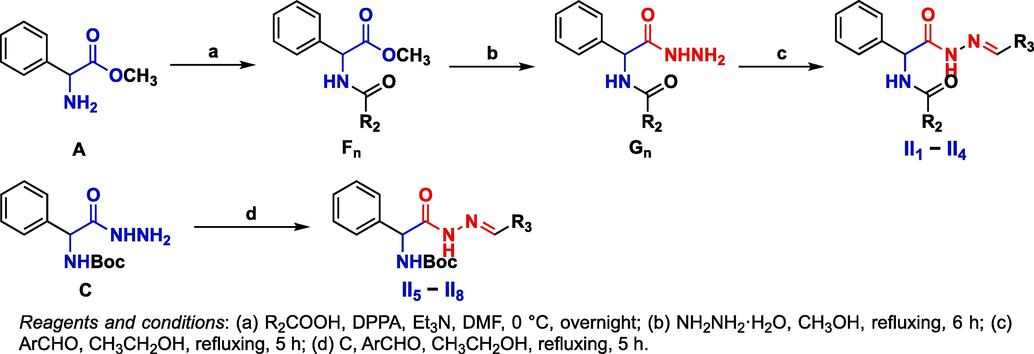
Synthetic route of target compounds II1 − II8.
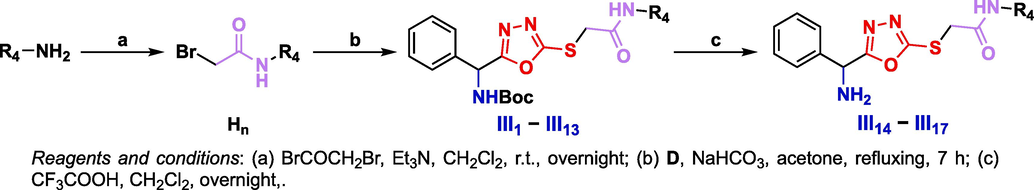
Synthetic route of the target compounds III1 − III17.
3.2 Antifungal activity analysis
3.2.1 In vitro antifungal activity of target compounds
The antifungal activity of intermediate D and all target compounds against eight plant pathogenic fungi (Fusarium prolifeatum, Gibberella zeae, Thanatephorus cucumeris, Alternaria solani, Nigrospora oryzae, Sclerotinia sclerotiorum, Fusarium oxysporum, and Colletotrichum camelliae) were evaluated at a concentration of 50 μg/mL. The antifungal results revealed that most target compounds exhibited better activity than that of intermediate D. Notably, compound III11 (81.9%) showed the best activity against N. oryzae, which was better than that of intermediate D (40.0%) and boscalid (76.3%). Compound III15 (82.1%) displayed excellent activity against S. sclerotiorum, which was far better than that of intermediate D (0%) and comparable to that of the commercialized fungicide boscalid (79.4%). Detailed antifungal data are provided in Tables S1–S3 in the Supporting information.
The EC50 values of target compounds with inhibition rates more than 60% were further screened. As shown in Table 1, some target compounds exhibited high antifungal activity by optimizing 2-phenylglycine derivatives. The EC50 values of I3 and III11 against N. oryzae were 25.1 and 17.3 μg/mL, respectively. The EC50 values of III5 and III15 against S. sclerotiorum were 21.3 and 25.2 μg/mL, respectively, which were significantly improved from intermediate D.
No
N. oryzae
No
S. sclerotiorum
EC50 (μg/ml)
regression equation
R2
EC50 (μg/ml)
regression equation
R2
I3
25.1 ± 0.7
y = 1.6342x + 2.712
0.9881
I6
107.4 ± 5.1
y = 1.4706x + 2.013
0.8359
I4
33.5 ± 1.5
y = 1.3131x + 2.9982
0.9897
I7
29.3 ± 3.5
y = 1.5817x + 2.6794
0.9420
III2
55.3 ± 3.9
y = 1.4609x + 2.4539
0.9744
I10
61.7 ± 3.6
y = 1.8087x + 1.7617
0.9464
III4
42.4 ± 2.4
y = 1.1574x + 3.1159
0.9372
I13
34.4 ± 4.2
y = 1.7531x + 2.3055
0.9781
III7
27.6 ± 1.9
y = 1.0169x + 3.5353
0.9782
III5
21.3 ± 1.1
y = 1.5766x + 2.9047
0.9806
III9
40.7 ± 1.0
y = 2.2536x + 1.3728
0.9879
III15
25.2 ± 1.4
y = 1.9103x + 2.3215
0.9986
III11
17.3 ± 0.1
y = 1.5863x + 3.0343
0.9879
BA
2.8 ± 0.1
y = 1.0289x + 4.5455
0.9109
BA
3.1 ± 0.2
y = 0.7420 × + 4.6360
0.9524
Preliminary structure–activity relationship (SAR) analysis demonstrated that the antifungal activity of the target compounds was obviously reduced when 1,3,4-oxadiazole-thioether was replaced by acylhydrazone, as was the case for II1 to II8; however, when 1,3,4-oxadiazole-thioether was retained and the amino and sulfhydryl groups were replaced by organic acids and halogenated hydrocarbons, respectively, the target compounds exhibited better antifungal activity, as was the case for I3 and III11. Therefore, 1,3,4-oxadiazole-thioether may be a key functional moiety for the antifungal activity of 2-phenylglycine derivatives.
3.2.2 In vivo antifungal activity of III11 against N. Oryzae on rice leaves
The in vivo protective activities of III11 and boscalid against N. oryzae on rice leaves were 44.3% and 71.0%, respectively, at a concentration of 50 μg/mL. However, the in vivo protective activity of III11 (65.8%) was increased by approximately 21% at 100 μg/mL, similar to that of boscalid (76.3%) (Fig. 3). The detailed protective activity data are included in the Supporting information.
In vivo protective activity against N. oryzae on rice leaves. (A) Blank control; (B) and (C) treated with III11; (D) and (E) treated with boscalid.
3.2.3 Effect of III11 treatment on N. oryzae spore germination
The germination rates of spores after treatment with III11 were 34.9 (25 μg/mL) and 24.6% (50 μg/mL), respectively, which were significantly lower than those of the blank control group (49.0%) (Fig. 4A). Moreover, the inhibition rates of spore germination treated with III11 were 28.7% (25 μg/mL) and 49.8% (50 μg/mL), lower than those of boscalid (43.0% and 56.1%) (Fig. 4B). As shown in Fig. 4C to 4E, the spores of N. oryzae were treated with different concentrations of III11 (25 and 50 μg/mL), and III11 significantly reduced the number and length of germ tubes, thereby affecting the spore germination rate. The results demonstrated that III11 could inhibit the spore germination of N. oryzae, and the inhibition effect was more significant with increasing concentration.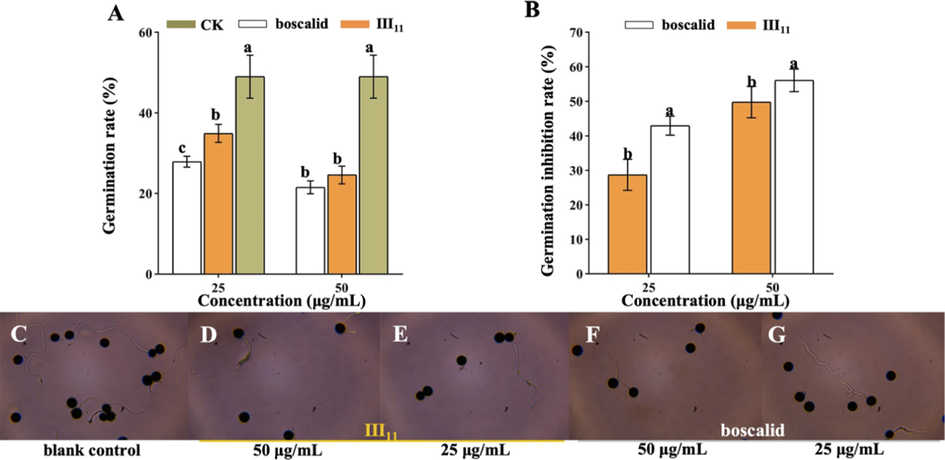
Effects on spore germination after treatment with III11 and boscalid. (A) Spore germination rate; (B) spore germination inhibition rate; from (C) to (E), spore germination after treatment with different compounds for 12 h. Error bars denote the standard error of the mean for three independent experiments. Different lowercase letters in a column indicate significant differences between mean values evaluated by Duncan’s multiple range test (P less than 0.05).
3.3 Preliminary antifungal mechanism against N. oryzae
3.3.1 Morphological analysis of N. Oryzae via SEM
As shown in Fig. 5A and 5B, the shape of mycelia in the blank control group was normal, full and regular. However, the surface of mycelia became rough and ruptured after treatment with III11 at a concentration of 25 μg/mL (Fig. 5C and 5D). With increasing concentration, the surface was greatly shrunk and the structure was seriously deformed (Fig. 5E and 5F). In addition, more severe shrinkage and damage to the mycelia were observed after treatment with boscalid than after treatment with III11 (Fig. 5G, 5H, 5I, and 5 J). Boscalid was reported to destroy the structure of mycelia, thus inhibiting the growth of mycelia (Wang et al., 2021). The results demonstrated that III11 destroyed the structure of mycelia, and the damage was more obvious with increasing concentration.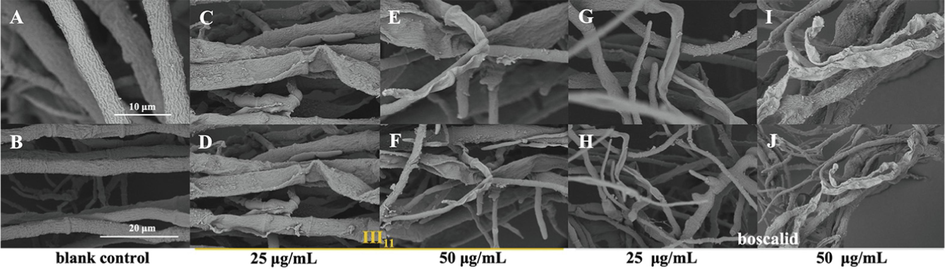
Morphological observation of N. oryzae by SEM. (A) and (B) CK group; (C), (D), (E) and (F) treated with III11; (G), (H), (I) and (J) treated with boscalid.
3.3.2 Morphological analysis of N. oryzae via FM
PI, a nuclear staining reagent, can insert into double-stranded DNA molecules to produce red fluorescence (Zhang et al., 2018). PI cannot pass through the intact cell membrane, but can pass through the damaged cell membrane to stain the nucleus. As shown in Fig. 6A, no red fluorescence was observed in the blank control group, but there was obvious red fluorescence in the mycelia after treatment with III11 and boscalid (Fig. 6A, 6B, 6C, and 6D). Moreover, the higher the drug concentration, the more severe the cell membrane damage and the more pronounced the fluorescence. The results revealed that III11 destroyed the cell membrane and altered its integrity.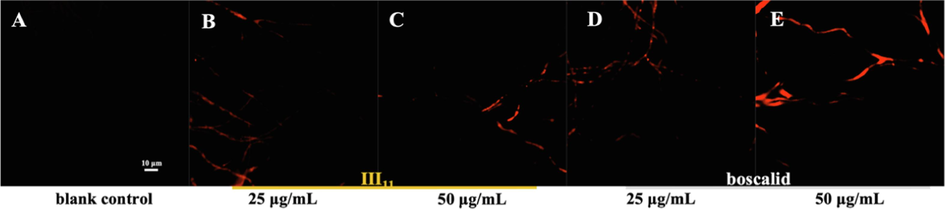
Morphological observation of N. oryzae by FM. (A) CK group; (B) and (C) treated with III11; (D) and (E) treated with boscalid.
3.3.3 Release of cellular contents of N. oryzae treated with III11
The absorbances at 260 and 280 nm were measured by a microplate reader to evaluate the concentrations of nucleic acids and proteins in the mycelial suspensions (Yang et al., 2021). As shown in Fig. 7, the absorbance value of the III11 treatment group was significantly higher than that of the blank control but slightly lower than that of boscalid treatment group, which indicated significant release of the nucleic acids and proteins of mycelia cells after treatment with III11 at 25 μg/mL. We speculated that active molecules may disrupt the cell membrane structure of the mycelia, resulting in the release of cellular contents.
Released cellular contents of mycelia treated with III11 and boscalid at 25 μg/mL. (A) Nucleic acid concentration; (B) protein concentration.
3.3.4 MDA content of N. oryzae treated with III11
MDA is a product of lipid peroxidation and one of the important indicators to evaluate the integrity of cell membrane structure and function (Yang et al., 2022; Aksakal, 2020). As shown in Fig. 8, following treatment with III11 at 25, 50, and 100 μg/mL, the MDA contents were 25.8, 42.3, and 54.8 nmol/g, respectively, which were higher than those of the blank control (18.0 nmol/g). This indicates that III11 induces lipid peroxidation in N. oryzae, causing membrane damage and thereby altering the integrity of cell membrane structure and function, which supports the findings reported in 2.3.3. The effect of boscalid was better than that of III11. Combining the results of SEM and FM revealed that active molecules can destroy the cell membrane structure of the mycelia and release the cellular contents, resulting in inhibition of mycelia growth.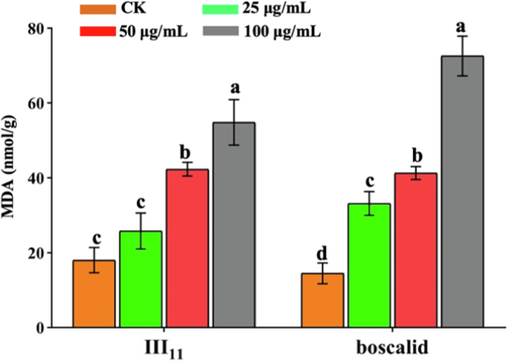
MDA content of N. oryzae treated with III11 and boscalid.
3.3.5 Effect on mycelial growth of N. oryzae treated with III11
The dry weight of mycelia directly reflects the effect of drugs on mycelial growth (Hou et al., 2018). As shown in Fig. 9, the dry weights of mycelia after treatment with III11 and boscalid at 50 μg/mL were 308.0 and 48.6 mg, respectively, which were both lower than those of the blank control (354.8 mg). Moreover, with increasing concentrations of III11 and boscalid, the dry weights of mycelia decreased to 285.5 and 34.2 mg, respectively. The results showed that III11 inhibited the growth of N. oryzae and reduced the dry weight of mycelia, and the inhibitory effect of boscalid was better than that of compound III11.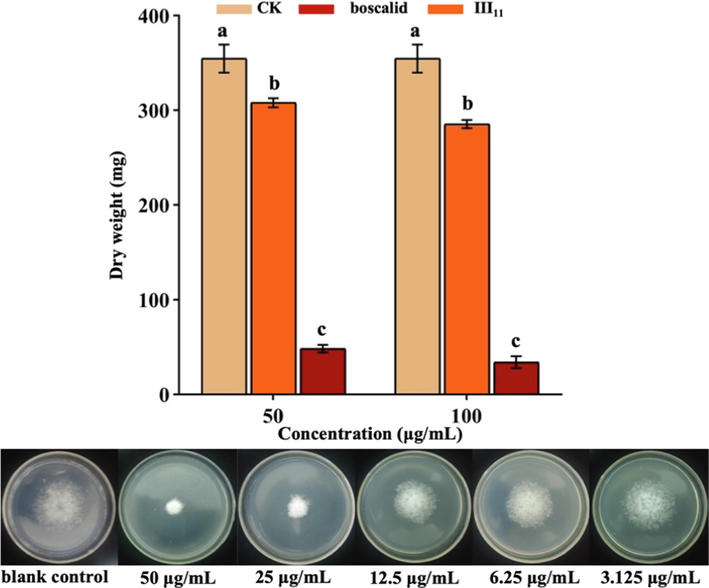
The dry weight of mycelia treated with III11 and boscalid.
3.3.6 Molecular docking analysis
In the SAR analysis, both compound III11 and boscalid (SDHI) contained amide structures. To explore the potential binding mechanism, molecular docking studies of the ligands III11 and boscalid with SDH (PDB code: 2FBW) were performed, and both ligands were found to be effectively embedded in the binding pocket of SDH (Fig. 10). Analysis of the binding mode found that III11 formed hydrogen bonds with amino acid residues SER-39, ARG-14 and ARG-43, and the distances of the hydrogen bonds were 3.1, 3.8, and 3.3 Å, respectively. These hydrogen bonds greatly enhance the interaction between the ligands and SDH. Interestingly, as a traditional commercialized SDHI, boscalid only formed a hydrogen bond with HIS-42, and the distance was 3.2 Å. Therefore, it could be speculated that the binding mode of III11 was inconsistent with that of boscalid. These results suggest that compound III11 is a potential SDHI and deserves further structural optimization and mechanism exploration.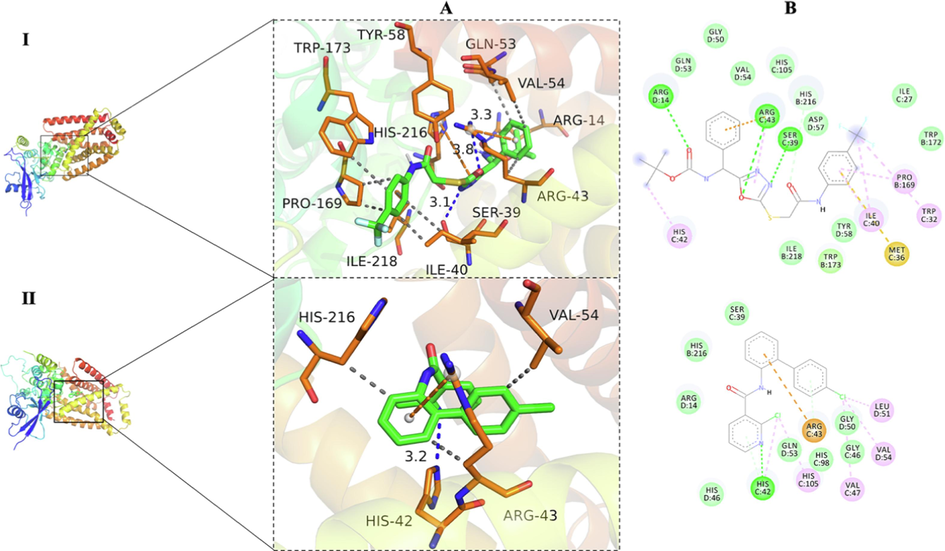
Molecular docking and binding sites of compound III11 (I) and boscalid (II) with the SDH protein (PDB code: 2FBW). A: 3D map; B: 2D map.
3.3.7 Effects of III11 treatment on SDH enzymatic activity
As shown in Table 2, compound III11 exhibited moderate SDH inhibitory activity (IC50 = 54.5 μg/mL), lower than that of boscalid (IC50 = 10.7 μg/mL), which was consistent with the in vitro antifungal results.
Although boscalid was reported to destroy the structure of mycelia (Wang et al., 2021), a systematic in vitro mechanism study of boscalid and compound III11 against N. oryzae in this work first revealed that both induced lipid peroxidation of cell membranes. This resulted in an increase in MDA content, thereby changing the permeability of cell membranes and causing leakage of intracellular nucleic acids and proteins, thus inhibiting the normal growth of mycelia.
4 Conclusions
In summary, a series of novel 2-phenylglycine derivatives containing 1,3,4-oxadiazole, acylhydrazone and sulfonamide fragments were designed and synthesized to construct a new active molecular family. The bioassay results revealed good antifungal activities of some compounds against N. oryzae and S. sclerotium. The EC50 values of I3, III7, III11, and boscalid against N. oryzae were 25.1, 27.6, 17.3, and 3.1 μg/mL, respectively. An antifungal mechanism study demonstrated that both III11 and boscalid promoted the peroxide reaction of lipids in the cell membrane and increased the MDA content by acting on mycelial cells. This destroyed the integrity of the cell membrane, resulting in the release of the cellular contents and the inhibition of mycelia growth. In addition, the SDH enzyme activity study showed an inhibitory effect of III11 on SDH and that III11 has a unique binding mode with SDH, different from that of boscalid. This is the first systematic report of the inhibitory mechanism of boscalid against N. oryzae. These results provided good academic and experimental foundation for the optimization and derivatization of amino acid derivatives and indicate that novel 2-phenylglycine derivatives containing 1,3,4-oxadiazole and amide structures are worthy of further development.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 32160655, 21662008), and Breeding Program of Guizhou University (No. 201931).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of boscalid toxicity on daphnia magna by using antioxidant enzyme activities, the expression of genes related to antioxidant and detoxification systems, and life-history parameters. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2020;237:108830
- [CrossRef] [Google Scholar]
- Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish-a review. Environ. Sci. Pollut. R.. 2020;27(16):19236-19250.
- [CrossRef] [Google Scholar]
- Novel carboxylated pyrroline-2-one derivatives bearing a phenylhydrazine moiety: Design, synthesis, antifungal evaluation and 3D-QSAR analysis. Bioorg. Med. Chem. Lett... 2020;30(21):127519
- [CrossRef] [Google Scholar]
- Selectivity and efficacy of herbicides dimethachlor and pethoxamid in rocket crop. Plant Protect. Sci.. 2020;56(4):305-316.
- [Google Scholar]
- Design, synthesis and antifungal mechanism of novel acetophenone derivatives containing 1,3,4-thiadiazole-2-thioethers. New J. Chem.. 2022;46(19):9017-9023.
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of novel antifungal leads: 3,5-dichlorobenzyl ester derivatives. J. Agric. Food Chem.. 2021;69(51):15521-15529.
- [CrossRef] [Google Scholar]
- Highly sensitive monoclonal antibody-based immunoassays for boscalid analysis in strawberries. Food Chem.. 2017;267:2-9.
- [CrossRef] [Google Scholar]
- Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186-194.
- [CrossRef] [Google Scholar]
- Syntheses and anti-allergic activity of 2-((bis(trimethylsilyl)methylthio/methylsulfonyl)methyl)-5-aryl-1,3,4-oxadiazoles. Eur. J. Med. Chem.. 2013;62:84-88.
- [CrossRef] [Google Scholar]
- Synthesis of novel 1-[5-(4-methoxy-phenyl)-[1,3,4]oxadiazol-2-yl]-piperazine derivatives and evaluation of their in vivo anticonvulsant activity. Eur. J. Med. Chem.. 2013;65:276-283.
- [CrossRef] [Google Scholar]
- Molecular and biological characterization of Sclerotinia sclerotiorum resistant to the anilinopyrimidine fungicide cyprodinil. Pestic. Biochem. Phys.. 2018;146:80-89.
- [CrossRef] [Google Scholar]
- Cancer cell growth inhibition by aroylhydrazone derivatives. Biotechnol. Biotec. Eq.. 2019;33(1):756-763.
- [CrossRef] [Google Scholar]
- Design, synthesis and anti-diabetic activity of piperazine sulphonamide derivatives as dipeptidyl peptidase-4 inhibitors. Pharmacia. 2022;69(4):987-993.
- [CrossRef] [Google Scholar]
- Design, synthesis, docking study, alpha-glucosidase inhibition, and cytotoxic activities of acridine linked to thioacetamides as novel agents in treatment of type 2 diabetes. Bioorg. Chem.. 2018;80:288-295.
- [CrossRef] [Google Scholar]
- Discovery of novel glitazones incorporated with phenylalanine and tyrosine: Synthesis, antidiabetic activity and structure–activity relationships. Bioorg. Chem.. 2012;45:12-28.
- [CrossRef] [Google Scholar]
- Investigation of indole functionalized pyrazoles and oxadiazoles as anti-inflammatory agents: Synthesis, in-vivo, in-vitro and in-silico analysis. Bioorg. Chem.. 2021;114:105068
- [CrossRef] [Google Scholar]
- Design, synthesis, fungicidal activity, and unexpected docking model of the first chiral boscalid analogues containing oxazolines. J. Agric. Food Chem.. 2016;64(46):8927-8934.
- [CrossRef] [Google Scholar]
- The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorgan. Med. Chem.. 2021;50:116476
- [CrossRef] [Google Scholar]
- Chlorantraniliprole in foods: Determination, dissipation and decontamination. Food Chem.. 2023;406:135030
- [CrossRef] [Google Scholar]
- Baseline sensitivity and control efficacy of a new QiI fungicide, florylpicoxamid, against Botrytis cinerea. Pest Manag. Sci.. 2022;78(12):5184-5190.
- [CrossRef] [Google Scholar]
- Novel alpha-amino acid-derived phase-transfer catalyst application to a highly enantio- and diastereoselective nitro-Mannich reaction. Org. Biomol. Chem.. 2017;15(43):9234-9242.
- [CrossRef] [Google Scholar]
- Novel 1,3,4-oxadiazoles as antitubercular agents with limited activity against drug-resistant tuberculosis. Future Med. Chem.. 2019;11(6):499-510.
- [CrossRef] [Google Scholar]
- Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Phys.. 2021;178:104942
- [CrossRef] [Google Scholar]
- Synthesis of new benzothiazole acylhydrazones as anticancer agents. Molecules. 2018;23(5):1054.
- [CrossRef] [Google Scholar]
- Interaction of antifungal peptide BP15 with stemphylium vesicarium, the causal agent of brown spot of pear. Fungal Biol-UK. 2016;120(1):61-71.
- [CrossRef] [Google Scholar]
- Fungal disease detection in plants: Traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron.. 2017;87:708-723.
- [CrossRef] [Google Scholar]
- Cutting edge of diphenyl phosphorazidate (DPPA) as a synthetic reagent–A fifty-year odyssey. Org. Chem. Front.. 2022;9(12):3360-3391.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological activity of novel laccase inhibitors as fungicides against rice blast. J. Agric. Food Chem.. 2022;70(45):14367-14376.
- [CrossRef] [Google Scholar]
- Design, synthesis, and fungicidal evaluation of novel 1,3-benzodioxole-pyrimidine derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2022;70(24):7360-7374.
- [CrossRef] [Google Scholar]
- Unique para-aminobenzenesulfonyl oxadiazoles as novel structural potential membrane active antibacterial agents towards drug-resistant methicillin resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett.. 2021;41:127995
- [CrossRef] [Google Scholar]
- Novel cinnamic acid derivatives containing the 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial activities, and mechanisms. J. Agric. Food Chem.. 2021;69(40):11804-11815.
- [CrossRef] [Google Scholar]
- Novel quinazolin-4(3H)-one derivatives containing a 1,3,4-oxadiazole thioether moiety as potential bactericides and fungicides: Design, synthesis, characterization and 3D-QSAR analysis. Journal of Saudi Chemical Society.. 2019;23:1144-1156.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N'-phenylacethydrazide derivatives as potential fungicides. Mol. Divers.. 2019;23(3):573-583.
- [CrossRef] [Google Scholar]
- Bioactivity-guided synthesis accelerates the discovery of 3-(Iso)quinolinyl-4-chromenones as potent fungicide candidates. J. Agric. Food Chem.. 2021;69:491-500.
- [CrossRef] [Google Scholar]
- Molecular design and preparation of 2-aminothiazole sulfanilamide oximes as membrane active antibacterial agents for drug resistant Acinetobacter baumannii. Bioorg. Chem.. 2021;113:105039
- [CrossRef] [Google Scholar]
- Synthesis and insecticidal evaluation of novel N-pyridylpyrazole derivatives containing diacylhydrazine/1,3,4-oxadiazole moieties. J. Heterocycl. Chem... 2019;56(4):1330-1336.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation, and 3D-QSAR studies of N-(substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2021;69(4):1214-1223.
- [CrossRef] [Google Scholar]
- Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol.. 2016;178:72-79.
- [CrossRef] [Google Scholar]
- Design and synthesis of novel 1,3,4-oxadiazole sulfone compounds containing 3,4-dichloroisothiazolylamide moiety and evaluation of rice bacterial activity. Pestic. Biochem. Phys.. 2020;170:104695
- [CrossRef] [Google Scholar]
- Design and discovery of novel antifungal quinoline derivatives with acylhydrazide as a promising pharmacophore. J. Agric. Food Chem.. 2021;69(30):8347-8357.
- [CrossRef] [Google Scholar]
- Novel hydrazone derivatives containing pyridine amide moiety: Design, synthesis, and insecticidal activity. Bioorg. Med. Chem. Lett.. 2016;26(4):1161-1164.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antifungal activity of novel thiophene/furan-1,3,4-oxadiazole carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2021;69(45):13373-13385.
- [CrossRef] [Google Scholar]
- Synthesis, antifungal activity and in vitro mechanism of novel 1-substituted-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives. Arab. J. Chem.. 2022;15(8):103987
- [CrossRef] [Google Scholar]
- Design, synthesis, and fungicidal evaluation of novel pyrazole-furan and pyrazole-pyrrole carboxamide as succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2017;65:5397-5403.
- [CrossRef] [Google Scholar]
- Cell permeability and nuclear DNA staining by propidium iodide in basidiomycetous yeasts. Appl. Microbiol. Biot.. 2018;102(9):4183-4191.
- [CrossRef] [Google Scholar]
- Novel coumarin 7-carboxamide/sulfonamide derivatives as potential fungicidal agents: Design, synthesis, and biological evaluation. Molecules. 2022;27(20):6904.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological activity of novel fungicides containing a 1,2,3,4-tetrahydroquinoline scaffold and acting as laccase inhibitors. J. Agric. Food Chem.. 2022;70(6):1776-1787.
- [CrossRef] [Google Scholar]
- Design, synthesis and bioassay of 2-phenylglycine derivatives as potential pesticide candidates. Chem. Biodivers.. 2023;20(1):e202200957
- [CrossRef] [Google Scholar]
- Synthesis and bioactivities of novel 2-(thioether/sulfone)-5-pyrazolyl-1,3,4-oxadiazole derivatives. Chinese Chem. Lett.. 2017;28(2):253-256.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105347.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1