Translate this page into:
Effect of microwave method on jasminaldehyde synthesis using solvent-free over Mg–Al–NO3 hydrotalcite catalyst
⁎Corresponding author. m.naciribennani@umi.ac.ma (Mohammed Naciri Bennani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Jasminaldehyde is a widely used starting material in the chemical industry. One method for synthesizing jasminaldehyde is through the aldol condensation of benzaldehyde with 1–heptanal, using a heterogeneous catalyst called hydrotalcite Mg–Al–NO3. The synthesized catalyst was characterized using various techniques, including XRD, FT–IR, TGA, DTA, SEM, EDX, and N2 adsorption–desorption isotherm (BET) to determine its structure, morphology, thermal stability, and properties. This catalyst was tested in the studied reaction under solvent-free conditions, using both microwave and conventional heating methods (in an oil bath) to evaluate the efficiency of each method in producing jasminaldehyde. The results showed that the use of microwave heating in this reaction is particularly advantageous. It allowed for the highest conversion (>99%) of 1–heptanal, with a good selectivity (80%) towards jasminaldehyde, after only 50 min of reaction time. In contrast, conventional heating required a longer reaction time of about 8 h to achieve similar conversion and selectivity values. Furthermore, Mg–Al–NO3 demonstrated nearly sustained activity in three consecutive reuse experiments, and the durability of this catalyst was characterized using several techniques.
Keywords
Microwave
Conventional Heating
Aldol–condensation
Mg–Al–NO3 Catalyst
Jasminaldehyde
1 Introduction
Industrial chemistry research aims to develop efficient heating modes to reduce reaction time and energy consumption (Bouafia et al., 2021; Henary et al., 2020; Laskar et al., 2018). Additionally, the development of new catalyst materials used in catalysis is an important area of research (Allam et al., 2020; Hebali et al., 2022) for improving conversion, enhancing product selectivity, and reducing the utilization of organic solvents, which are responsible for environmental pollution and the production of harmful waste. Jasminaldehyde plays a crucial role in the cosmetic, fragrance, alcoholic perfumes, and deodorants industries (Patil et al., 2013). However, its extraction from natural sources is an expensive process. As a result, it is commonly synthesized through aldol condensation, involving the formation of a C–C bond between benzaldehyde and 1–heptanal in liquid base media such as sodium hydroxide or potassium hydroxide under homogeneous catalysis (Sharma et al., 2008; Vashishtha et al., 2013). One major drawback of aldol condensation in a liquid base medium is the substantial solvent consumption, which is environmentally unfriendly (Sharma et al., 2008). To address this issue, researchers have explored the use and development of solid catalysts to mitigate the disadvantages of aldol condensation in liquid-base medium methods (Vogt and Weckhuysen, 2022; C. Zhu et al., 2021). Hamza and Nagaraju utilized amorphous aluminium phosphates (AlP) and metal alumino-phosphates (M−AlP) and achieved 93% conversion of 1–heptanal with 77% selectivity of jasminaldehyde under the conditions of 140 °C for 4 h (Hamza and Nagaraju, 2015). Similarly, Patil et al. reported the same conversion and selectivity values when utilizing double metal cyanide (Fe–Zn) catalyst but at a higher temperature and longer reaction time of 160 °C for 12 h (Patil et al., 2013). Kumar et al. tested the catalytic activity of mixed oxide of cerium and zirconia (CeO2–ZrO2) for jasminaldehyde production through the same reaction and achieved a yield of 89% with good selectivity of jasminaldehyde at 130 °C (Kumar et al., 2017). Sharma et al. employed a freshly activated Mg–Al hydrotalcite after calcination and reconstitution by contact with NaOH solution for 8–12 h. The results obtained were encouraging, with significant conversion of 1-heptanal and very high selectivity of jasminaldehyde at a reaction temperature of 130 °C (Sharma et al., 2010).
In general, the development of a solid catalyst with active basic sites of the Brønsted type is highly beneficial for aldol condensation reactions. Such catalysts offer an environmentally friendly catalytic process at a reasonable cost, ensuring increased durability and efficiency in various environments (Takehira, 2017). The catalyst belongs to the family of layered double hydroxides (LDHs) and is highly valuable for the production of jasminaldehyde in industrial chemistry (Takehira, 2017). It is characterized by a two-dimensional structure formed by a stack of brucite-type sheets in which trivalent cations replace some of the divalent metal cations with the chemical formula [M2+1–x M3+x (OH)2]X+ [An–x/n·mH2O]X–, this general formula accounts for the wide variety of compounds that can be synthesized by varying the nature and proportions of cations and anions (Bernard et al., 2022). These catalysts have several advantages: basic properties, possible reuse, and easy separation after reaction from the synthesized products (Morales-Serna et al., 2013).
The cross-aldol condensation of benzaldehyde (B) and 1–heptanal (H) produce two main products, jasminaldehyde (J) and (Z)-2-pentylnon-2-enal (P), via the self-condensation reaction of 1–heptanal (Scheme 1). It is important to note that the selectivity of jasminaldehyde was also influenced by the (B/H)0 M ratio, where the percentage yield of by-product (P) was reduced using a high (B/H)0 M ratio (Fan, 2020). Additionally, it should be noted that a higher concentration of 1–heptanal promotes an accelerated self-condensation reaction of 1–heptanal (Ganga et al., 2016).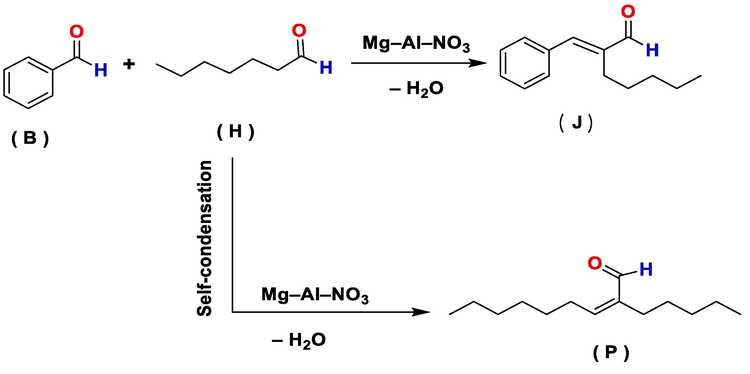
Reaction scheme for the synthesis of jasminaldehyde.
The success of the microwave process is associated with the polarization effect of electromagnetic radiation (Tounsi et al., 2023), which transforms electromagnetic energy into thermal energy (Mudhaffar et al., 2023; Soni et al., 2020). This method has rapidly emerged as a new and particularly interesting heating method due to its ability to effectively reduce reaction time, save energy, and achieve a high conversion with good selectivity of desired products (Pratap et al., 2020; Saigal et al., 2019).
This study focuses on the synthesis and characterization of a solid Mg–Al–NO3 catalyst using various physicochemical techniques. Subsequently, the catalyst was tested for the production of jasminaldehyde through an aldol-condensation reaction between 1–heptanal and benzaldehyde, under solvent-free conditions. In addition, to compare the reaction yields obtained using two different heating methods, specifically microwave heating and conventional heating. It sought to explore the specific effects of these heating methods on the aldol-condensation reaction.
2 Experimental
2.1 Materials
To prepare the Mg–Al–NO3 hydrotalcite catalyst, the following chemical compounds were utilized: Magnesium nitrate (Mg(NO3)2·6H2O; purity = 98.9%), Aluminum nitrate (Al(NO3)3·9H2O; purity = 99.1%) and sodium hydroxide (NaOH; purity = 99.9%) were supplied by LOBA Chemie. Distilled water was used exclusively for all the experiments. The benzaldehyde (C7H6O; purity = 99.0%) and 1–heptanal (C7H14O; purity = 98.9%) employed in the reaction were purchased from Sigma-Aldrich.
2.2 Catalyst synthesis
The Mg–Al–NO3 hydrotalcite type catalyst with a molar ratio of Mg/Al equal to 3/1 was prepared by the co-precipitation method. To begin, 0.120 mol of (Mg(NO3)2·6H2O), and 0.040 mol of (Al(NO3)3·9H2O) were dissolved in 150 mL of distilled water and dropped into a flask initially containing 100 mL of distilled water. Both solutions were mixed with strong agitation at room temperature under nitrogen flow, and the pH was controlled at 10 by adding an aqueous NaOH solution (1.5 M). The suspension was then agitated at 70 °C for 17 h. The resulting solid is filtered and washed several times with warm water until the filtrate is neutral, then dried in an oven for 24 h at 70 °C.
2.3 Catalyst characterizations
The powder X–ray diffraction (P-XRD) pattern of the synthesized catalyst was recorded with a powder diffractometer (BRUKER, D8-ADVANCE A25), outfitted with a copper anticathode tube Cu-Kα filter (λ = 1.5418 Ǻ) over a 2θ range of 2–80°. The operating voltage and current were 40 kV and 20 mA, respectively. Fourier transform infrared (FT-IR) spectra for synthesized catalyst were measured using a SHIMADZU type instrument (IRAFFINIY-1S) equipped with a Tri-Glycine sulfate (TGS) detector. The sample was prepared as pellets, consisting of 5 mg of solid with 95 mg of dry KBr. The results are recorded in absorbance between (4000–400) cm−1 and 20 scans. The thermal analysis by DTA/GTA was carried out with gravity thermal analyzer type (TA60–SHIMADZU) in a temperature range between 25 and 600 °C with a heating rate of 10 °C/min. The morphologies of the catalyst were also examined using a scanning electron microscope (SEM) with energy-dispersive X–ray (EDX) analysis. The images of the catalyst were taken with a scanning electron microscope (Model: JSM–IT500 HR) at a magnification of about 4000 times. An accelerating voltage of about 10 kV was applied. The catalyst's specific surface area and average pore diameter were calculated by the standard BET (Brunauer, Emmett, and Teller) and BJH (Barrett, Joyner, and Halenda) methods, respectively. BET measurement was N2 adsorption–desorption isotherms performed at 77 K using Micromeritics ASAP-2010. The basicity of the catalysts was established by the Hammett indicator method (Cantrell et al., 2005; Devi et al., 2018), as well as the acid-base titration method (Sahu et al., 2013). The acid-base titration method determined the synthesized catalyst's basicity by dissolving 100 mg of catalyst in 10 mL of 0.1 mol. L–1 aqueous NaOH solution and stirred for 30 min. Then the resulting solution was titrated with 0.1 mol. L–1 aqueous solution of acetic acid in the presence of phenolphthalein was an indicator until the pink color became colorless.
The kinetics of the reaction was followed by using a type (SHIMADZU–2010) chromatography equipped with a capillary DB5 column (Length: 30 m, inner diameter: 0.32 mm, and film thickness: 0.22 µm) of 5% diphenyl and 95% dimethyl-siloxane, equipped with a flame ionization detector (FID). The temperature of GC oven was programmed to rise from 80° to 250 °C at a rate of 10 °C per minute. Nitrogen was used as a carrier gas, and the injection port and the FID were kept at 250 °C at all times.
2.4 Reaction procedure for the synthesis of jasminaldehyde
To optimize the reaction parameters, the synthesis of jasminaldehyde was conducted using both conventional oil bath heating and microwave heating methods. The reaction was performed using both procedures to compare their effectiveness and determine the optimal conditions for jasminaldehyde production.
2.4.1 Conventional heating method
The reaction was carried out in 50 mL two-necked flask submerged in an oil bath, equipped with a reflux set-up, and fitted with a bar magnet. Benzaldehyde (79 mmol) and 1–heptanal (15.8 mmol) were previously distilled with a molar ratio (B/H)0 = 5 was added, and 100 mg of Mg–Al–NO3 hydrotalcite catalyst was added to the flask at 120 °C with stirring and under nitrogen flow for 8 h. The fractions were taken progressively with a syringe and filtered by centrifugation. The catalyst fractions were progressively taken with a syringe and filtered by centrifugation.
2.4.2 Microwave method
The reaction was microwave-assisted using the microwave ovens and the same procedure as mentioned above in the conventional heating method (Sec.2.4.1). The reactions were carried out at a power of 100 W at a temperature of 90 °C at different reaction times (10; 20; 30; 40; and 50 min). The catalyst fractions were progressively taken with a syringe and filtered by centrifugation.
2.5 Method of analysis
Reaction kinetics were monitored by analyzing reaction mixture samples versus time using gas chromatography (GC), under the same conditions. Prior to injection, fractions of the reaction mixture were centrifuged, and diluted in decane used as an internal standard, at a concentration of 0.1 mol. L–1, diluted in methanol. By injecting 0.1 µl of each diluted fraction of compound into the GC column, the components of the reaction mixture were identified by determining their retention times. To identify the chromatographic peaks of the reactants and the secondary product 2-pentylnon-2-enal, obtained by self-condensation of 1–heptanal (carried out under the same conditions as those employed in the reaction studied), they were analyzed individually under the same conditions as those employed in the reaction studied), they were analyzed individually under the same conditions.
The progress of the reaction was monitored as a function of the consumption of 1–heptanal. The conversion of 1–heptanal and selectivity of jasminaldehyde were calculated through the following formulas:
3 Results and discussion
3.1 XRD analysis
The diffragtogram of (XRD) for the prepared catalyst is presented in Fig. 1. The resulting diffraction pattern revealed all lines (00 L), principally the lines (0 0 3), (0 0 6), and (0 1 2), well resolved and intense, characteristic of pure and well-crystallized LDHs phases, which indicate a hexagonal lattice with the rhombohedral space group R3m in agreement with the literature data (Arhzaf et al., 2020; Houssaini et al., 2021). With the knowledge of the reticular planes (hkl) and the inter-reticular distances (dhkl), calculated from Bragg's law, to determine the parameters of the mesh, we used the following relationship:
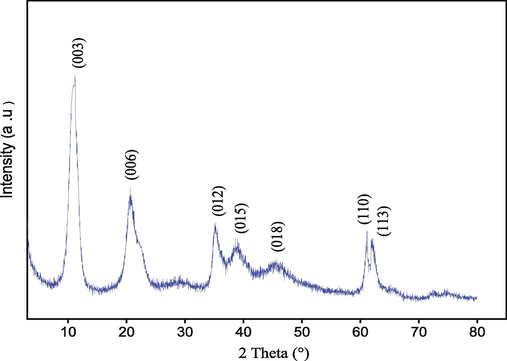
Powder XRD pattern of Mg–Al–NO3 catalyst.
The crystallographic parameter (a) is half of the cation-cation distance and determined from the position of the line (1 1 0) at 2θ = 61.12° and calculated according to the relation: (a = 2d110). Similarly, from the position of the line (0 0 3) at 2θ = 11.2°, the inter-reticular distance (d003) used to determine the crystallographic parameter (c) and calculated by the relation: (c = 3d003), which corresponds to the thickness of a layer of the sheet. Also, X–ray diffraction is a convenient method for determining the average crystallite size. From the Debye-Scherrer relationship between the crystallite diameter (L) and the half-value width (β) using the X–ray peak (0 0 3) of the catalyst, we have determined the parameter (L) by the following formula (Abdelsadek et al., 2022; Houssaini et al., 2021):
Where (k) is a constant related to the crystallite shape, normally taken to be equal to 0.89. X–ray wavelength is described by (λ) and measured in (Å). The width of the diffraction peak (β) is measured at half maximum in radians, and (θ) is the value of the theta angle relative to a line (0 0 3). The main crystallographic parameters of the mesh and the average size of the crystallites obtained for the synthesized catalyst are collected in Table 1. The crystallographic parameters present the same values generally obtained for the double lamellar hydroxide compounds (An et al., 2020). Also, they indicate good purity and crystallinity for the Mg–Al–NO3 synthesized.
Catalyst
θ (0 0 3) (°)
θ (1 1 0) (°)
d (0 0 3) (Å)
d (1 1 0) (Å)
a (Å)
c (Å)
β (0 0 3) (°)
L (0 0 3) (nm)
Mg–Al–NO3
5.60
30.56
7.89
1.52
3.04
23.69
1.59
5.00
3.2 FTIR analysis
The synthesized catalyst was further analyzed using Fourier transform infrared spectroscopy to identify the main molecular groups present and the types of vibrations occurring between the atoms. The infrared spectrum of the synthesized catalyst is depicted in Fig. 2. The FT–IR analysis allows for the identification of the principal characteristic molecular groups of the hydrotalcite phases (Ziyat et al., 2021). In the spectrum, a broad band observed between 3445 and 3500 cm−1 corresponds to the vibration of hydroxide groups bound to various divalent and trivalent cations, as well as water molecules in the lamellar space (Trujillano et al., 2018). Another weak band at 1635 cm−1 is attributed to the deformation vibrations of interlayer water molecules (Ramlee et al., 2022). Additionally, the nitrate anions present in the catalyst are characterized by an intense band at 1383 cm−1 (An et al., 2020). At lower frequencies bands can be observed in the range of 830–425 cm –1, which correspond to M−O and M−O−M type lattice vibrations (Ziyat et al., 2021).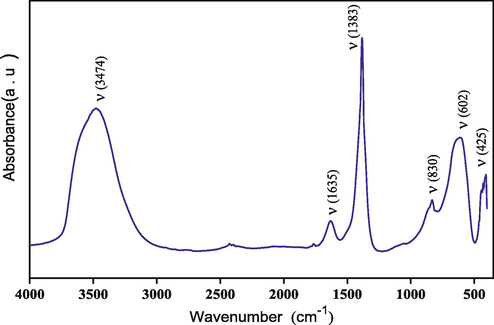
FT–IR spectra of Mg–Al–NO3 catalyst.
3.3 TGA and DTA analysis
TGA Thermogravimetric analysis (TGA) detects the mass change of a sample as a function of temperature and is useful for monitoring the evolution of the chemical composition of solids. TGA profile for Mg–Al–NO3 demonstrated a two-step weight loss (Fig. 3), accompanied by endothermic transformations characteristic of LDHs phases (Prentice et al., 2020). The first step occurred between 25 and 225 °C, resulted in a weight loss of 14.5% and was attributed to the removal of physisorbed water molecules from the surface and water molecules located in the interlamellar domain, which is represented by a first DTA endothermic peak at 158 °C. The second step, which took place in the temperature range of 225–600 °C, resulted in a 30.5% loss of mass and was indicated by a second endothermic peak in the DTA at 450 °C, attributed to the beginning of the decomposition of the nitrate compensating anions and the dehydroxylation of the layers. The results were consistent with typical findings in the literature on the thermal decomposition of hydrotalcite materials, according to Arhzaf et al. (Arhzaf et al., 2021).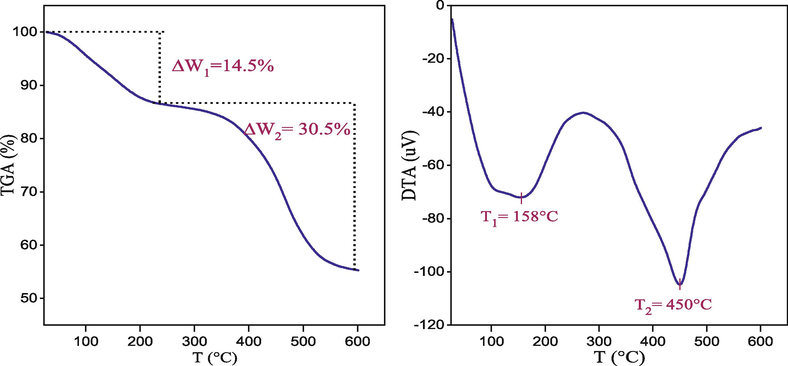
TG–DTA curves of Mg–Al–NO3 catalyst.
3.4 SEM–EDX analysis
The texture of the synthesized Mg–Al–NO3 based catalyst shows well-dispersed particles in the form of platelets of small crystallite size, in agreement with the value obtained by the Debye-Scherrer method (Table 1). The morphology of the resulting catalyst is shown in Fig. 4. It is similar to the LDHs found by Jinxia Xu, and al. (Xu et al., 2017), in synthesizing Mg–Al–NO3 hydrotalcite by the co-precipitation method. An energy-dispersive X-ray was carried out to investigate the chemical element analysis for the Mg–Al–NO3 catalyst. The molar ratio of Mg/Al is very close to that initially introduced during the synthesis process, which indicates that the two cations, Mg2+ and Al3+, are completely precipitated. The elemental analysis results of Mg–Al–NO3 catalyst are summarized in Table 2.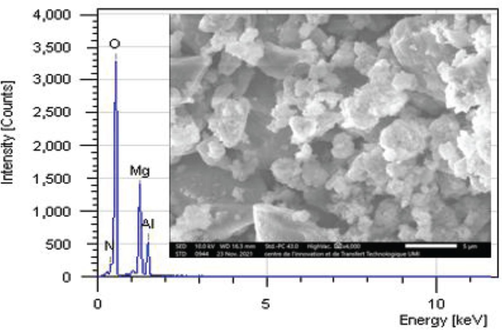
SEM image at 5 µm and EDX analysis of Mg–Al–NO3 catalyst.
Catalyst
(%) Mass of chemical elements
R Exp
R The
Mg–Al–NO3
Mg
O
Mg
Al
N
Mg/Al
Mg/Al
59.58
26.93
10.03
1.49
2.97
3.00
3.5 BET measurement
The experimental measurement of the specific surface area is based on nitrogen adsorption according to the principle of the Brunauer, Emmet, and Teller methods (Yukselen and Kaya, 2006). The specific surface area is easily deduced by knowing the surface area occupied by a nitrogen molecule at 77 K (16.2 Å2) and the volume adsorbed on the catalyst monolayer. The N2 adsorption–desorption isotherms at low-temperature (77 K) performed on the synthesized catalyst are shown in Fig. 5. The obtained isotherms are classified as type IV according to the IUPAC classification, which is characteristic of mesoporous materials. Moreover, the hysteresis loop is of type H3, indicating the formation of the catalyst in platelets, typical of hydrotalcite materials (Zhang et al., 2019). The pore diameter distribution curves obtained from the BJH adsorption dV/dD pore volume are shown in Fig. 5. The mean pore diameter was determined by BJH method involves analyzing the desorption branch of the isotherm that corresponds to a thermodynamically stable equilibrium. The value of the specific surface area, the average pore diameter, and the pore volume for the synthesized catalyst are presented in Table 3.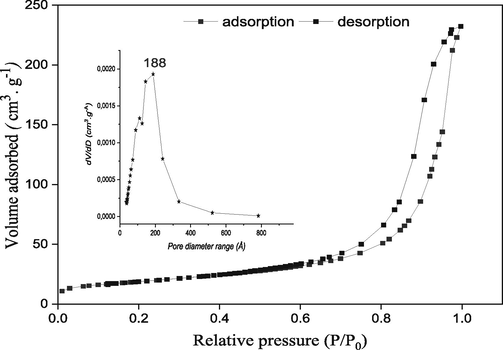
N2 adsorption/desorption isotherms and pore diameter for Mg–Al–NO3.
Catalyst
S BET (m2. g−1)
V P (cm3. g−1)
D P (Å)
Mg–Al–NO3
95.25
0.353
188
3.6 Basicity measurement
Basicity is defined as the number of basic sites in a solid per unit weight or surface area. The total basicity of the synthesized catalyst Mg–Al–NO3 was determined using the acid-base titration method. The amount of base in the catalyst was then calculated using the following equation:
Where CAA is the concentration of the acetic acid standard solution, ΔVAA is the difference in volume between the acetic acid solution consumed at equivalence by the solution containing the solid and the blank (NaOH solution), and ms represents the mass of catalyst used in the test. Based on the assay, the total basicity of Mg–Al–NO3 was determined to be 1.32 mol. g−1. However, when considering the specific surface area, the calculated basicity becomes (1.32/95.25 = 0.0138 mol. m−2), which indicates a good basicity of Bronsted-type basic sites. This basicity corresponds to the hydroxide groups (OH–) located on the catalyst surface (Winter et al., 2006). These basic sites are generally efficient and favorable for the aldol condensation reaction.
4 Kinetics of the reaction
4.1 Effect of the heating method on the conversion and the selectivity of jasminaldehyde
In this experiment, a comparative analysis was conducted to evaluate the heating efficiency of the conventional and microwave methods by examining the conversion (%) and total reaction time in the synthesis of jasminaldehyde. Fig. 6 summarizes the results of the conversion of 1-heptanal (%), selectivity of jasminaldehyde, and reaction time in the aldol condensation reaction between 1–heptanal and benzaldehyde using the Mg–Al–NO3 catalyst. The application of microwave irradiation has a significant impact on the yields and reaction time. With microwave heating at a power of 100 W (T = 90 °C), the reaction was completed within 50 min, resulting in a 99.5% conversion of 1–heptanal, and high selectivity of selectivity of jasminaldehyde (80%). In contrast, under the conditions of the conventional heating method at a temperature of 120 °C, a similar yield and selectivity of jasminaldehyde (79%) were achieved, but after 8 h of reaction time. The microwave method for heating organic syntheses has garnered significant interest due to its advantages over conventional processes. The conventional methods often require longer heating times, higher energy consumption, and more complex procedures, resulting in increased costs (Rana and Rana, 2014).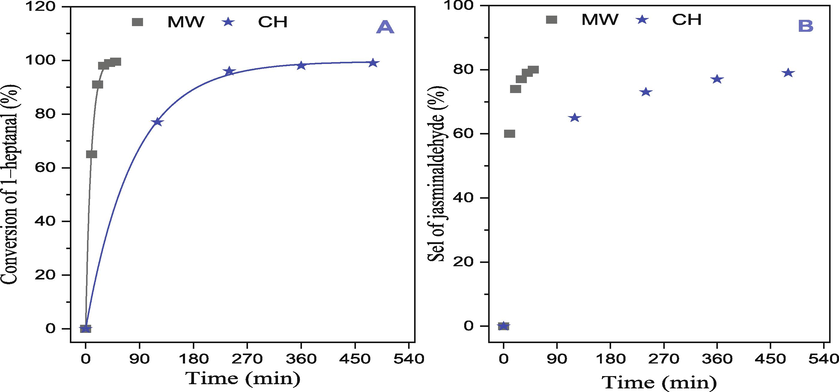
Conversion of 1–heptanal and selectivity of jasminaldehyde as a function of reaction time over Mg–Al–NO3 catalyst by two heating methods: (MW = Microwave and CH = Conventional heating).
To evaluate the impact of catalyst site type, it is crucial to conduct the reaction under identical conditions using the catalyst calcined at 450 °C. The study revealed that the calcined catalyst demonstrated a comparable conversion of 1–heptanal to the non-calcined catalyst. However, the selectivity for jasminaldehyde decreased from 80% to 69% after calcination. This suggests that the catalyst with Bronsted (OH–) group basic sites exhibited higher selectivity for jasminaldehyde compared to those with Lewis (O2–) basic sites. The study also speculated that the presence of Lewis-type basic sites, potentially formed due to the formation of a mixed oxide during calcination, could have facilitated the self-condensation of two 1–heptanal molecules. This process might have contributed to the decline in selectivity towards jasminaldehyde in the reaction.
4.2 Effect of the heating method on the rate constant
To examine the effect of the jasminaldehyde synthesis methods reaction rate, by calculating the reaction rate constants (k) for each method and reaction rate versus time can be expressed as follows:
The integrated form of this equation is:
Where α and β represent the partial orders corresponding to each reactant and kapp = K.[benzaldehyde]β is the apparent rate constant because benzaldehyde is present in excess in the reaction medium. As shown in Fig. 7A, the curves obtained show that the disappearance of 1–heptanal decreases according to an exponential law, identical to first-order kinetics. According to the integrated equation, obtained by plotting – ln(
/
against t, lead to the straight lines as shown in Fig. 7B, which confirm that the aldol condensation reaction follows a pseudo-first-order law. The values of the apparent rate constants (kapp = K.
), determined from the slopes of the previous lines, are given in Table 4.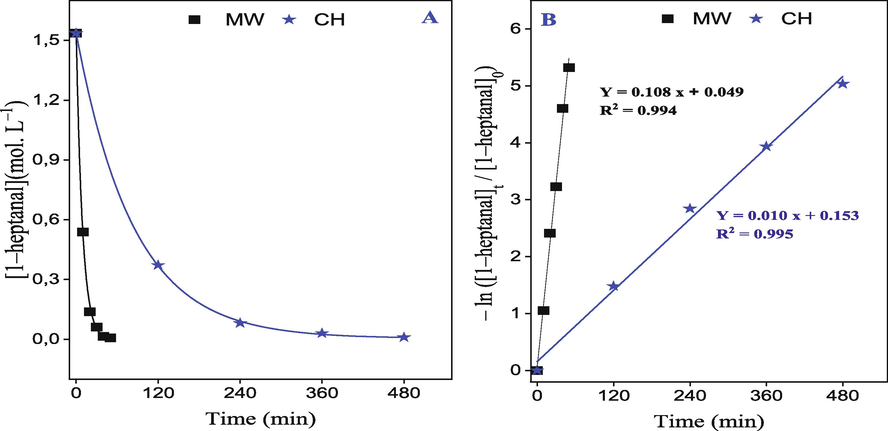
Kinetic of 1–heptanal consumption versus the time of reaction for two heating methods: (MW = Microwave and CH = Conventional heating).
Methods
kapp (min−1)
Microwave
Conventional Heating0.108
0.010
The findings in Table 4 indicate that using microwave heating results in a reaction rate constant 10 times greater than that achieved through conventional heating under identical circumstances. These results suggest that the microwave method is more effective and economical for synthesizing jasminaldehyde. Compared to traditional heating methods, using a microwave to heat products can save time, produce more products, and selectively heat the desired compound. This is because the microwave generates heat inside the product, preventing overcooking on the surface. Additionally, using a microwave greatly, reduces between 25% and 34% of energy consumption, which has economic and environmental benefits (Z. Zhu et al., 2021), Based on the findings, it appears that utilizing the microwave technique shows potential in creating jasminaldehyde and could potentially be utilized in other areas of chemical synthesis.
5 Recycle study
To study the reusability and the effect of microwave irradiation on the stability of the Mg–Al–NO3 catalyst in the investigated reaction, the Mg–Al–NO3 catalyst was tested through four recycling runs under the previous optimal reaction conditions. After each catalytic reaction, the catalyst was recovered from the reaction solution by centrifugation, washed multiple times with acetone to remove reactants/products from its surface, and then dried at a temperature of 60 °C for 5 h. The results of 1–heptanal conversion (%) and jasminaldehyde selectivity are presented in Table 5.
Runs of catalyst
Conv of 1–heptanal (%)
Sel of jasminaldehyde (%)
Ratio (Sel/Conv)
Fresh
1
2
399.5
91
86
7880
65
49
29
0.80
0.71
0.57
0.37
Successive cycles of the experiment revealed a decrease in the conversion of 1–heptanal from 99.5% to 78% and a significant decrease in the selectivity of jasminaldehyde from 80% to 29% when compared to the fresh catalyst. This decline in conversion and selectivity can be primarily attributed to a reduction in the number of active basic sites on the catalyst surface. The loss of initial catalytic activity of the Mg–Al–NO3 catalyst after each cycle is due to a decrease in the number of active basic sites involved in the aldol condensation reaction. This reduction is caused by the presence of products or reagents that block the catalyst pores and are not completely desorbed during the drying process at a temperature of 60 °C.
Furthermore, the stability of the Mg–Al–NO3 catalyst was confirmed through thorough characterization. As illustrated in Fig. 8A, X-ray diffraction analysis did not reveal any noticeable changes in the pattern of the used catalyst compared to the fresh catalyst. This suggests that the structure of the Mg–Al–NO3 remained unchanged after the reaction. Image obtained through scanning electron microscopy as well as analyses using energy-dispersive X-ray spectroscopy were conducted on the spent catalyst used in the experiment. The SEM images did not show any visible differences between the fresh and spent catalysts (Fig. 8B). However, the EDX analysis highlighted a low proportion of carbon (9%) in the spent catalyst, indicating the presence of reaction products trapped within the catalyst pores that were not entirely removed during the drying step. This convincingly demonstrates the high stability of the Mg–Al–NO3 catalyst throughout the reaction.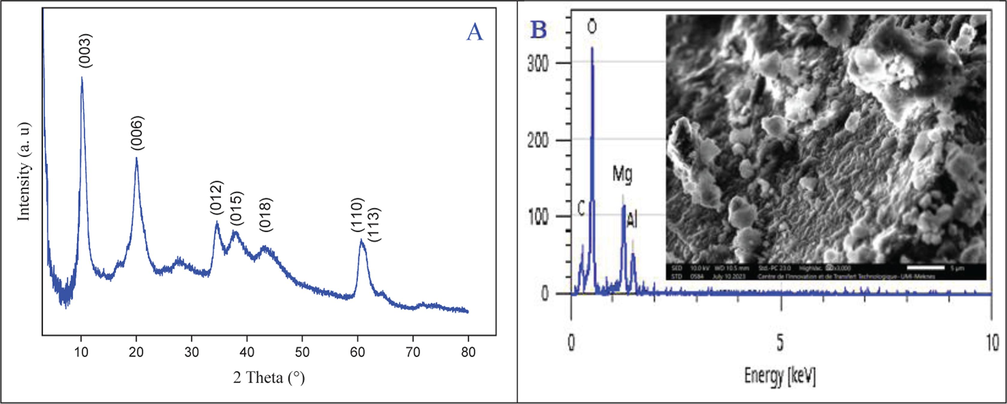
(A) Powder XRD pattern of used Mg–Al–NO3; (B) SEM image and EDX analysis of used Mg–Al–NO3 catalyst.
6 Conclusions
In summary, the bi-metallic Mg–Al–NO3 catalyst was successfully synthesized using the co-precipitation method under a nitrogen atmosphere, employing sodium hydroxide as the precipitating agent. Characterization techniques such as XRD, FT–IR, and SEM confirmed that the catalyst exhibited a well-formed structure without impurities, and its particles were small and evenly distributed. The catalyst was found to possess a substantial amount of Brønsted-type basic sites, approximately 1.32 mol. g−1, which is sufficient for catalyzing the aldol condensation reaction between benzaldehyde and 1–heptanal. The catalytic activity of the synthesized catalyst was evaluated in the solvent-free synthesis of jasminaldehyde, with a focus on comparing the heating efficiency of microwave and conventional heating methods. The results demonstrated that microwave irradiation significantly enhance reaction kinetics and reduce the energy consumption. The reaction is achieved after 50 min at only 90 °C under microwave irradiation. In contrast, the conventional heating required a longer reaction time of about 8 h, and a temperature of 120 °C for product the jasminaldehyde. Furthermore, the durability of the Mg–Al–NO3 catalyst was confirmed through examination of the used catalyst using XRD, SEM, and EDX techniques. The catalyst exhibited excellent stability, maintaining its structure and activity, even after three reaction cycles.
CRediT authorship contribution statement
Jamal Houssaini: Conceptualization. Mohammed Naciri Bennani: Conceptualization, Supervision. Said Arhzaf: Validation. Hamid Ziyat: Validation. Marwa Alaqarbeh: .
Acknowledgments
We are grateful to thank the Laboratory of Chemistry and Biology Applied to the Environment, affiliate at the CNRST, the Research Team of Materials and Applied Catalysis at Moulay Ismail University in Morocco.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdelsadek, Z., Gonzalez-Cortes, S., Bali, F., OuizaCherifi, Halliche, D., Masset, P.J., 2022. Evaluation of the reactivity, selectivity and lifetime of hydrotalcite-based catalysts using isopropanol as probe molecule. Res. Chem. Intermed. https://doi.org/10.1007/s11164-021-04640-2.
- A generalized 4-unknown refined theory for bending and free vibration analysis of laminated composite and sandwich plates and shells. Comput. Concr.. 2020;26:185-201.
- [CrossRef] [Google Scholar]
- Effect of the solvent on the basic properties of mg–al hydrotalcite catalysts for glucose isomerization. Catalysts. 2020;10:1-13.
- [CrossRef] [Google Scholar]
- Effect of Mg/Al molar ratio on the basicity of Mg-Al mixed oxide derived from Mg-Al hydrotalcite. Mediterr. J. Chem.. 2020;10:625.
- [CrossRef] [Google Scholar]
- Solvent-free aldol condensation of furfural and acetone on calcined Mg-Al hydrotalcites. Moroccan J. Chem.. 2021;9:614-627.
- [CrossRef] [Google Scholar]
- Stability of hydrotalcite (Mg-Al layered double hydroxide) in presence of different anions. Cem. Concr. Res.. 2022;152
- [CrossRef] [Google Scholar]
- Bending and free vibration characteristics of various compositions of FG plates on elastic foundation via quasi 3D HSDT model. Steel Compos. Struct.. 2021;41:487-503.
- [CrossRef] [Google Scholar]
- Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen.. 2005;287:183-190.
- [CrossRef] [Google Scholar]
- Comparative study of potassium salt-loaded MgAl hydrotalcites for the knoevenagel condensation reaction. ACS Omega. 2018;3:7086-7095.
- [CrossRef] [Google Scholar]
- Acid–base bifunctional magnesium oxide catalyst prepared from a simple hydrogen peroxide treatment for highly selective synthesis of jasminaldehyde. Energy Sources Part A Recover. Util. Environ. Eff.. 2020;42:2501-2515.
- [CrossRef] [Google Scholar]
- Bifunctional organocatalysts for the synthesis of jasminaldehyde and their derivatives. Indian J. Chem. - Sect. A Inorg. Phys. Theor. Anal. Chem.. 2016;55A:950-955.
- [Google Scholar]
- Amorphous metal-aluminophosphate catalysts for aldol condensation of n-heptanal and benzaldehyde to jasminaldehyde. Cuihua Xuebao/Chinese J. Catal.. 2015;36:209-215.
- [CrossRef] [Google Scholar]
- Effect of the variable visco-Pasternak foundations on the bending and dynamic behaviors of FG plates using integral HSDT model. Geomech. Eng.. 2022;28:49-64.
- [CrossRef] [Google Scholar]
- Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv.. 2020;10:14170-14197.
- [CrossRef] [Google Scholar]
- Study of the catalytic activity of the compounds hydrotalcite type treated by microwave in the self-condensation of acetone. Int. J. Anal. Chem.. 2021;2021
- [CrossRef] [Google Scholar]
- Simple and efficient synthesis of jasminaldehyde over modified forms of zirconia: Acid-base bifunctional catalysis. Indian J. Chem. Technol.. 2017;24:548-554.
- [CrossRef] [Google Scholar]
- Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv.. 2018;8:20131-20142.
- [CrossRef] [Google Scholar]
- Efficient activity of magnesium-aluminium hydrotalcite in the synthesis of amides. RSC Adv.. 2013;3:23046-23050.
- [CrossRef] [Google Scholar]
- Impact of viscoelastic foundation on bending behavior of FG plate subjected to hygro-thermo-mechanical loads. Struct. Eng. Mech.. 2023;86:167-180.
- [CrossRef] [Google Scholar]
- Solvent-free synthesis of jasminaldehyde using double metal cyanide based solid acid catalysts. Indian J. Chem. - Sect. A Inorg. Phys. Theor. Anal. Chem.. 2013;52:1564-1569.
- [Google Scholar]
- Microwave assisted synthesis of propyl esters over modified versions of zirconia: Kinetic study. Chem. Data Collect.. 2020;30:100579
- [CrossRef] [Google Scholar]
- The effects of (di-, tri-valent)-cation partitioning and intercalant anion-type on the solubility of hydrotalcites. J. Am. Ceram. Soc.. 2020;103:6025-6039.
- [CrossRef] [Google Scholar]
- Biochemical and physical characterization of immobilized Candida rugosa lipase on metal oxide hybrid support. Catal. Artic.. 2022;12:854.
- [CrossRef] [Google Scholar]
- Microwave reactors: A brief review on its fundamental aspects and applications. OALib. 2014;01:1-20.
- [CrossRef] [Google Scholar]
- Role of calcinations and basicity of hydrotalcite as catalyst for environmental benign novel synthesis of 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives of curcumin. Catal. Sci. Technol.. 2013;3:1520-1530.
- [CrossRef] [Google Scholar]
- Saigal, Khan, S., Rahman, H., Shafiullah, Khan, M.M., 2019. Nitroketene N, S -acetals: Synergistic building blocks for the synthesis of heterocycles. RSC Adv. 9, 14477–14502. https://doi.org/10.1039/c9ra00630c.
- Synthesis of jasminaldehyde using magnesium organo silicate as a solid base catalyst. J. Mol. Catal. A Chem.. 2008;280:61-67.
- [CrossRef] [Google Scholar]
- Applied Catalysis A : General Reconstructed Mg / Al hydrotalcite as a solid base catalyst for synthesis of jasminaldehyde“. Appl. Catal. A, Gen.. 2010;386:34-42.
- [CrossRef] [Google Scholar]
- Trends in Food Science & Technology Microwave-induced thermal sterilization- A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol.. 2020;97:433-442.
- [CrossRef] [Google Scholar]
- Recent development of layered double hydroxide-derived catalysts − Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci.. 2017;136:112-141.
- [CrossRef] [Google Scholar]
- Free vibration investigation of functionally graded plates with temperature-dependent properties resting on a viscoelastic foundation. Struct. Eng. Mech.. 2023;86:1-16.
- [CrossRef] [Google Scholar]
- Controlling the synthesis conditions for tuning the properties of hydrotalcite-like materials at the nano scale. Chem. Eng.. 2018;2:1-15.
- [CrossRef] [Google Scholar]
- A novel approach for selective cross aldol condensation using reusable NaOH-cationic micellar systems. Appl. Catal. A Gen.. 2013;466:38-44.
- [CrossRef] [Google Scholar]
- The concept of active site in heterogeneous catalysis. Nat. Rev. Chem.. 2022;6:89-111.
- [CrossRef] [Google Scholar]
- On the nature and accessibility of the Brønsted-base sites in activated hydrotalcite catalysts. J. Phys. Chem. B. 2006;110:9211-9218.
- [CrossRef] [Google Scholar]
- Chloride absorption by nitrate, nitrite and aminobenzoate intercalated layered double hydroxides. J. Mater. Sci.. 2017;52:5908-5916.
- [CrossRef] [Google Scholar]
- Comparison of methods for determining specific surface area of soils. J. Geotech. Geoenviron. Eng.. 2006;132:931-936.
- [CrossRef] [Google Scholar]
- Biodiesel production by esterification reaction on k+ modified mgal-hydrotalcites catalysts. Catalysts. 2019;9
- [CrossRef] [Google Scholar]
- Organic electrochemistry: Molecular syntheses with potential. ACS Cent. Sci.. 2021;7:415-431.
- [CrossRef] [Google Scholar]
- Thermochemical pretreatments of maize stem for sugar recovery: Comparative evaluation of microwave and conventional heating. Ind. Crops Prod.. 2021;160
- [CrossRef] [Google Scholar]
- Kinetic, isotherm, and mechanism investigations of the removal of nitrate and nitrite from water by the synthesized hydrotalcite Mg–Al. Res. Chem. Intermed.. 2021;47:2605-2627.
- [CrossRef] [Google Scholar]







