Translate this page into:
The pericarp of Zanthoxylum bungeanum Maxim.: An excellent source for the development of alternative drugs for improving glucose and lipid metabolism disorder related diseases
⁎Corresponding authors at: State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, No.1166 Liutai Avenue, Chengdu 610075, PR China. fansz1930@yahoo.com (Shu-Guang Hou), pengwei@cdutcm.edu.cn (Wei Peng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The pericarp of Zanthoxylum bungeanum Maxim. (ZBM) (Rutaceae), also called Sichuan pepper, is a famous condiment and herbal medicine existing in China dating back over 2000 years. In this paper, we summarized the active compounds and pharmacological effects of ZBM for regulating glucose and lipid metabolism disorders, and also discussed the challenges associated with the drug development of this herbal medicine. So far, more than 100 natural compounds have been reported from ZBM, including amides, volatile oils, flavonoids, etc., and amides and volatile oil are the predominant active components in ZBM. Current investigations suggested ZBM and its active compounds (such as Hydroxy-α-Sanshool, Hydroxy-β-Sanshool) are beneficial for ameliorating glucose and lipid metabolism disorder, such as diabetes, hyperlipidemia, obesity, as well as nonalcoholic fatty liver disease (NAFLD). Although previous research has obtained great achievements, there is still enormous gaps in drug development of this herbal medicine, and future works should be devoted to break through bottlenecks in mechanisms, safety, structure modification, clinical efficacy, modern preparation and quality control. The present review gives a comprehensive understanding of the available works of ZBM regarding its regulating effects on glucose and lipid metabolic disorders, which would be beneficial for the future drug development of ZBM for combating glucose and lipid metabolic disorders.
Keywords
Zanthoxylum bungeanum Maxim.
Sichuan pepper
Glucose and lipid metabolism disorders
Hydroxy-α-Sanshool
Hydroxy-β-Sanshool
- ACAT
-
cholesterol acyltransferase
- API
-
Active pharmaceutical ingredient
- ApoA 1
-
Apolipoprotein A1
- ApoB
-
Apolipoprotein B
- BUN
-
Blood urea nitrogen
- CB1
-
cannabinoid receptor 1
- CPT
-
Carnitine palmitoyl-transferase
- CS
-
Citrate synthase
- DM
-
Diabetes mellitus
- EAE
-
Ethanol extracts
- EEZB
-
Ethanol extracts of Z. bungeanum
- FBG
-
Fasting blood glucose
- FXR
-
Farnesoid X-activated receptor
- G-6-pase
-
Glucose-6-phosphatase
- GHb
-
Glycosylated hemoglobin
- GlcN
-
Glucosamine
- GLUT-2
-
Glucose transporter 2
- GSP
-
Glycosylated serum protein
- HAS
-
Hydroxy-α-Sanshool
- HBS
-
Hydroxy-β-Sanshool
- HBV
-
High blood viscosity
- HDL-C
-
High-density lipoprotein cholesterol
- HepG2
-
Human hepatocellular carcinomas
- HFD
-
high fat diet
- HIF
-
Hypoxia inducible factor
- HLV
-
High low viscosity
- HO-1
-
Homeobox-1
- HSD
-
High sugar diet
- IC50
-
Half maximal inhibitory concentration
- IGF1
-
Insulin-like growth factor 1
- InR
-
Insulin receptor
- LD50
-
the median lethal dose
- LDH
-
Lactic dehydrogenase
- LDL-C
-
Low-density lipoprotein cholesterol
- MDA
-
malondialdehyde
- NAFL
-
Nonalcoholic fatty liver
- NAFLD
-
Nonalcoholic fatty liver disease
- NASH
-
Nonalcoholic steatohepatitis
- NMR
-
Nuclear magnetic resonance
- NO
-
nitric oxide
- OGTT
-
Oral glucose tolerance test
- OVX
-
Ovariectomy
- PA
-
Palmitic acid
- IR
-
Insulin resistance
- PEPCK
-
Phosphoenolpyruvate caboxykinase
- PKM2
-
Pyruvate pinase M2
- SCFAs
-
short-chain fatty acids
- SCOE
-
Supercritical CO2 extracts
- SOZB
-
Seed oil of Z. bungeanum
- STZ
-
Streptozotocin
- T2DM
-
Type 2 diabetes mellitus
- TC
-
Total cholesterol
- TG
-
Triglycerides
- TP
-
Total protein
- TRPV1
-
the transient receptor potential cation channel subfamily V member 1
- ALB
-
Albumin
- TXNIP
-
Thioredoxin interacting protein gene
- UPS
-
Ubiquitin-proteasome system
- WESC
-
Water extracts of Z. bungeanum
- HFD
-
High fat diet
- ZBE
-
Zanthoxylum bungeanum Maxim extracts
- ZBM
-
Zanthoxylum bungeanum Maxim
Abbreviations
1 Introduction
Nowadays, accumulating evidence lends support to the view that functional plants used as food are precious resource for finding candidate drugs. They are rich for human health promoting substances including non-nutritive, nutritive, and bioactive compounds such as flavonoids, phenolics, anthocyanins, phenolic acids, etc., and also have perfect flavor and excellent medicinal value (Ari et al., 2022; Dawadi et al., 2022; Ozrenk et al., 2020; Sagbas et al., 2020). The dried ripe pericarp of the Zanthoxylum bungeanum Maxim. (ZBM), also called Sichuan pepper, Shu-Jiao, and Qin Jiao, is a famous condiment food and traditional herbal medicine in China (Zhang et al., 2017; National Pharmacopoeia Committee, 2020). In fact, ZBM is an ancient food existing in China over 2000 years since approximately Zhou dynasty, and firstly seen in the ancient book of Erya and named “Dajiao” (Guo, 2021; He et al., 2022; Jiang, 2019). The earliest record of the medicinal application of ZBM listed in the called Shen Nong Ben Cao Jing (a classic monograph of Chinese medicine written during the Han dynasty) as an active herbal medicine with the functions of warming spleen and kidney, relieving arthralgia and pain (Yang et al., 2023). Another famous monograph of Chinese medicine written by Li Shi-zhen named Compendium of Materia Medica recorded that ZBM possesses the effects of strengthening the teeth, darkening the hair, brightening the eyes, anti-aging, and strengthening the spirit“.
In recent years, accumulating evidence on the phytochemical investigations have revealed that ZBM possesses abundant amide alkaloids, volatile oil, flavonoids, lignans and other chemical constituents (He et al., 2022; Yang et al., 2013; Zhang et al., 2021a). Among these compounds, amides and volatile oils are the main flavor constituents corresponding to the pungent and aromatic flavor (Fig. 1), and also the active substances in ZBM (Shi et al., 2010; Liang et al., 2014). Furthermore, increasing pharmacological studies have suggested that ZBM has a wide range of bioactivities, including analgesia effects, gastrointestinal function regulating effects, lipid-lowering effect, hypoglycemic effects, anti-obesity effects, anti-aging effects and anti-senile dementia effect (Zhou et al., 2020; Zhu, 2020; Yuan et al., 2021; Wei et al., 2021; Tang et al., 2023). Interestingly, the application of ZBM for intervention of the glucose and lipid metabolism disorders was first listed in a medical monograph of the “Blending decoctions to treat sixty ailments” unearthed in Lao Guan Shan during the Qin and Han dynasties, where “Shu Jiao” was used for treating “Thirst disorder syndrome (also known as Diabetes)” (Wang et al., 2018; You, 2016). Nowadays, growing evidence lends support to the view that ZBM and its active compounds are beneficial for ameliorating glucose and lipid metabolism disorder and its related diseases; however, there is a lack of the systematic review regarding the related pharmacological effects and updating molecular mechanisms (Wang et al., 2018).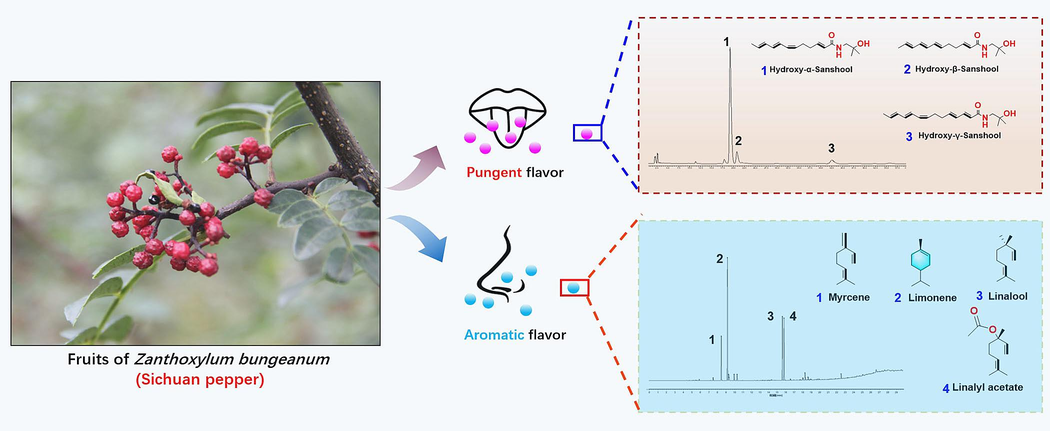
Amides and volatile oils are the main flavor components of ZBM.
In this paper, we collected all the available literatures regarding the ameliorating effects of ZBM and its active compounds on glucose and lipid metabolism disorder and its related diseases via classic books, literatures, online database, online academic search engines, and data base of the thesis of Ms. or Ph.D., etc. Then, we summarized the possible active compounds and molecular mechanisms of this herbal medicine for regulating glucose and lipid metabolism disorder, and also discussed the challenges associated with the functional food or drug development with ZBM and its active compounds for management of glucose and lipid metabolism disorder.
2 Traditional usages
ZBM is an ancient food and herbal medicine existing in China dating back over 2000 years since approximately the Zhou dynasty, and firstly recorded in the ancient book of Erya (Guo, 2021; He et al., 2022; Jiang, 2019). In ancient China, ZBM was considered as a known symbol of goodness for flourishing population owing to its fruitful and bright red fruits. Consequently, it’s commonly prepared as ZBM wine or ZBM vinegar which were liked by young men and women, and it’s interesting that ZBM is even added to build the empress's palace, which was called “Jiao-Fang palace (椒房殿)” (Guo, 2021).
Importantly, ZBM is commonly applied to treat some diseases which was first recorded in the classic monograph of Shen Nong Ben Cao Jing (神农本草经) with the functions of warming spleen and kidney, relieving arthralgia and pain (Yang et al., 2023). Before the period of Sui & Tang dynasties, ZBM was used as a known tonifying agents for spleen and kidney, which could be used to treat infertility, hair loss, edema, jaundice, joint pain, and diseases of eye and ear, etc. Furthermore, in the period of Tang & Song dynasties, ZBM was commonly used to treat itch, toothache, embryotocia, irregular menses, and postpartum lochia, et al, instead of focusing on its function of “warming energizer and eliminating cold”. After Ming dynasty, the clinical ureses of ZBM have been extended a lot, another known TCM monograph of Compendium of Materia Medica (本草纲目) recorded that ZBM can be used to strengthen teeth, darken hair, brighten eyes, anti-aging, and strengthen spirit“. In addition, it’s also recognized that ZBM has the functions of “warming energizer and relieving pain, and destroying parasites and relieving itching”, and is beneficial for management of upset stomach, diarrhea, edema, cough, itch, toothache, and parasites, etc. Interestingly, ZBM is also used to treat the “Thirst disorder syndrome” which is also known as Diabetes in modern medicine. This application of ZBM is recorded in a newly unearthed TCM monograph of Blending decoctions to treat sixty ailments (治六十病方和齐汤法) (Wang et al., 2018; You, 2016). In Table S1, we summarized the traditional medical used ZBM in Chinese traditional medicine in the form of powders and decoctions.
3 Active components of ZBM
So far, more than 100 natural compounds have been reported from ZBM, including amide alkaloids, volatile oils, flavonoids, terpenoids, fatty acids and trace elements (Bai et al., 2023; Zhou et al., 2023; Li et al., 2023a; Tian et al., 2022; Liu et al., 2021; Gu et al., 2023; Qiao et al., 2017; Wang et al., 2023; Meng et al., 2020; Zhou et al., 2020; Liu et al., 1991; Huang et al., 1991; Meng et al., 2020; Wu et al., 2019; Hu, 2013; Yuan and Wang, 2010; Shi and Zhang, 2010; Tong, 1991; Chen and Xue, 1985), among which amides and volatile oil components are the most important active components in ZBM.
3.1 Amide alkaloids
So far, a large number of alkaloid components have been isolated from ZBM, including amides, quinolines, isoquinolines, quinolone derivatives and benzophenanthridine derivatives (Yuan et al., 2021; Wang, 2017; Chen, 2018). Importantly, it’s found that amides are the most predominant alkaloids in ZBM, most of which are natural long-chain unsaturated fatty acids, and are also considered as one of the predominant active components in this herbal medicine (Zhang et al., 2021a; Zhu et al., 2009; Yu et al., 2013; Wu et al., 2021). Since the year of 1955, the first amide isolated from the ZBM, called a-sanshool (also called echinacein or neoherculin early), phytochemical researchers have reported more than thirty amides from ZBM (Zhang et al., 2016; Yang, 2008; Tian et al., 2022; Mei, 2012; Xiong et al., 1997; Kou, 2015; Huang et al., 2012; Meng et al., 2022), mainly including Hydroxy-α-Sanshool (HAS, 1), α-Sanshool (2), Hydroxy-β-Sanshool (HBS, 3), β-Sanshool (4), Hydroxy-γ-Sanshool (5), γ-Sanshool (6), Tetrahydrobungeanool (7), Dihydrobungeanool (8), Dehydro-7-sanshool (9), Isobungeanool (10), Qinbunamide A (11), Qinbunamide B (12), Qinbunamide C (13), Hydroxy-γ-iso sanshool (14), (2E,4E,8E,10E,12E)-N-isobutyl-2,4,8,10,12-tetradecapentaenamide (15), Bungeanool (16), (2E, 6E, 8E)-N-(2-hydroxy-2-methylpropyl)-10-oxo-2,6,8-decatrienamide (17), 2′-Hydroxy-N-isobytyl-[trans-2,6,8,10] dodecatetraenamide (18), (6RS)-(2E,7E,9E)-6-hydroxy-N-(2-hydroxy-2-methylpropyl)-11-oxo-2,7,9-decatrienamide (19), N-[2-(3,4-dimethoxyphenyl)ethyl]-3-phenylacrylamide (20), Bugeanumamide A (21), (11RS)-(2E,7E,9E)-11-hydroxy-N-(2-hydroxy-2-methylpropyl)-6-oxo-2,7,9-decanetricarboxamide (22), (10RS, 11RS)-(2E,6Z,8E)-10,11-dihydroxy-N-(2- hydroxy-2-methylpropyl)-2,6,8-dodecatrienamide (23), (6RS,11RS) -(2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide (24), (2E,4E, 9E,11E)-N-(2-hydroxy-2-methylpropyl)-8-hydroxy-13-oxo-2,4,9,11-tetradecenamine (25), (2E,4E,9E,11E)-N-(hydroxy-2-methylpropyl)-8,13-dihydroxy-2,4,9,11-tetradecenamide (26), (2E)-6,6-dimethoxy-N-(2-hydroxy-2-methylpropyl)-2-hexanamide (27), hydroxy-ε-sanshool (28) (Table S2).
3.2 Volatile oils
Volatile oil compounds are the main aromatic substances affording ZBM with special fragrant odor and taste, and it’s reported that ZBM possesses the volatile oil contents ranging from 0.7% to 9.0%. nowadays, more than 50 volatile components have been isolated and identified from ZBM (Song, 2018; Zhao et al., 1992; Zhang et al., 2016; Le et al., 2014; Liu et al., 2020; Yuan and Wang, 2010; Lou, 2019; Fan et al., 2014; Zhu et al., 2022; Ding et al., 2020; Li et al., 2020a; Li et al., 2022; Long et al., 2023; Mo et al., 2009; Xiao et al., 2023; Li, 2022; Xi et al., 2021), mainly including α-Thujene (29), α-Pinene (30), Sabinene (31), β-Pinene (32), Myrcene (33), α-Phellandrene (34), α-Terpinene (35), p-Cymene (36), β-Phellandrene (37), Cineol (38), γ-Terpinene (39) 2-Carene (40), Linalool (41), α-Terpineol (42), 1-methyl-4-(1-methylethyl) (43), 3-Cyclohexene-1-ethanol (3-Cyclohexene-1-methanol) (44), Isopinene (45), Limonene (46), Geranyl acetate (47), Acetosyringone (48), (-)-Terpinen-4-ol (49), Cuminaldehyde (50), Dibutyl phthalate (51), 1-Methoxy-4-(1-propenyl)-benzene (52), Myrtenyl acetate (53) (Table S3).
3.3 Flavonoids
ZBM also contains abundant flavonoids (Song, 2018; Wu et al., 2010; Wu et al., 2011; Xiong et al., 1995; Shi et al., 2013; Guo et al., 2017; Zhang, 2013; Yuan et al., 2021; Hu et al., 2020; Li, 2018), mainly including Quercetin-3-O-α-L-Rhamnopyranosyl-(1-6)-β-D-glucopy ranoside (54), Quercetin-3-O-α-L-Rhamnopyranoside (55), Quercetin 3-O-β-glucopyranoside (56), Quercetin (57), Rutin (58), Isorhamnetin (59), Hyperoside (60), and Vitexin (61) (Table S4).
3.4 Lignans
The present study reports the isolation of lignan-like chemical components from ZBM (Zhang et al., 2016; Li et al., 2019; Yang et al., 2013; Wu et al., 2023; Yuan et al., 2021; Zhang, 2008; Li, 2021; Yang et al., 2018; Li et al., 2015; Zhu et al., 2013), including 7-methoxycoumarin (62), Methoxycoumarin (63), Neochlorogenic acid (64), Sesamin (65), Eudesmin (66), Chlorogenic acid (67) (Table S5).
3.5 Others
Other components found in ZBM include 2-phenylethyl-6-O-β-D-glucopyranoside (68), Isobutyl-O-β-D-Glucopyranoside (69), (3-methoxyphenethyl alcohol-4-O-β-D-glucopyranoside (70), (1′R, 3′R, 5′R, 8′S)-epidihydrocarboxylic acid-O-β-D-glucopyranoside (71), Benzyl-1-O- β-D-glucopyranoside (72), Benzenepropanoic acid-O-β-D-glucopyranosyloxy (73), Orcinol glucoside (74), 3-Methoxy-5-hydroxyphenol-1-O-β-D-glucopyranoside (75), 4-Hydroxyl-3,5-dimethoxyl-6-O-β-D-glucosebenzene (76), 2-(4-hydroxy-3-methoxyphenyl) ethyl-O-β-D-glucopyranoside (77), Coumaric acid-4-O-β-D Glucopyranoside (78), Hloroglucinol 1-O-β-D-glucopyranoside (79), 3,5-dihydroxy phenethyl alcohol 3-O-β- glucopyranoside (80), Betulalbuside A (81), 1-hydroxylinaloyl-6-O-β-D-glucopyranoside (82), guanosine (8 3), Arbutin (84), Syringin (85), skimmiamine (86), etc. (Table S6) (Zhang et al., 2016; Ren and Xie, 1981; Zhao, 2012; Lu et al., 2015; Liu and Wei, 1991).
4 Pharmacological activities of ZBM
4.1 Hypoglycemic effects
Diabetes mellitus (DM), a known endocrine disease, is a typical glycol-metabolic disorder caused by complex and multipath factors which result in high glucose levels in blood, and can further lead to the damages in kidney, cardiovascular organs, and nervous tissue, etc (American Diabetes Association, 2013). Among all DM patients, the type 2 diabetes mellitus (T2DM) accounts for 90% of the total population (Zimmet et al., 2001). A meta-analysis reports that T2DM can exacerbate the disease progression of nonalcoholic steatohepatitis (NASH) and NAFLD, and their prevalence is closely linked, which adds to the economic burden (Younossi et al., 2019). Currently, besides insulin injection, oral hypoglycemic drug is another predominant way for treating DM, especially for the T2DM (Tan et al., 2019). Nowadays, development of more effective and safer oral drugs for DM treatment are still a hot spot for the pharmaceutical researchers (Li et al., 2019; Wong, 2018).
In the past decades, hypoglycemic effects of ZBM and its components have been comprehensively investigated with preclinical animal experiments in vivo and in vitro (Table 1). High concentrations of palmitic acid (PA) can cause lipid deposition in liver cells, leading to insulin resistance (IR) which is a key reason for the development of T2DM, and PA induced HepG2 is a commonly used cell model for evaluating the candidate hypoglycemic drugs for improving IR. Based on the in vitro model of HepG2 cells stimulated by PA (200 μmol/L), Zhang et al. reported that water extracts of ZBM (WESC) (20, 40, 60 μg/mL) can notably suppress the PA-induced lipid formation, ameliorate PA-induced oxidative stress in HepG2 cells via increasing anti-oxidative enzymes’ activities. Integration of network pharmacology and experimental validation analysis, Zhang et al. explored the related molecular mechanisms, and found that the hypoglycemic effects of WESC is closely involved in activation of AMPK/PI3K/Akt pathway (Zhang et al., 2022a). Similarly, Huang et al verified by network pharmacology, molecular docking and in vitro experiments that 40 μmol/L of the ZBM extracts (ZBE) could down-regulate the expression of AKT1, IL6, HSP90AA1 and FOS, which supported the results of network pharmacology and slow down the disease process of DM (Huang et al., 2023). In the year of 2021, Lu et al. evaluated the effects of total amides in ZBM (0.4 and 0.8 mg/kg) on DM mice induced by high fat diet (HFD) & high sugar diet (HSD) and streptozotocin (STZ) injection (60 mg/kg). The authors reported that total amides treatment reduced food and water intake in DM mice, whereas increased body weight of DM mice. In addition, the Lu et al. also found total amides can improve sugar tolerance, decrease triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and malondialdehyde (MDA) in serum, as well as alleviated pathological changes in liver of DM mice (Lu et al., 2021). Furthermore, another investigation by You et al. studied the hypoglycemic effects of total amides in ZBM. Based on HFD feeding combined STZ injection, You et al. successfully prepared the DM rats, and they found that treatment with total amides (3, 6, 9 mg/kg) for 4 weeks can increase body weight, improve glucose tolerance, increase hepatic glycogen, myo-glycogen and insulin levels, as well as reduced pathological changes in pancreatic tissues. Importantly, results of You et al. also revealed total amides treatment decreased 2 key enzymes for the gluconeogenesis including phosphoenolpyruvate caboxykinase (PEPCK) and glucose-6-phosphatase (G-6-pase) in the liver, whereas increased the glycolysis enzyme of glucokinase) in the liver, as well as increased the pancreatic duodenal homeobox-1 (HO-1), glucokinase, and glucose transporter 2 (GLUT-2) in the pancreas. Furthermore, the possible molecular mechanisms are involved in upregulation of the transient receptor potential cation channel subfamily V member 1 (TRPV1) and down-regulation of cannabinoid receptor 1 (CB1) (You, 2016; You et al., 2015). Later in 2017, based on STZ injection induced DM rats, Ren et al. reported treatment with total amides from ZBM (2, 4, 8 mg/kg) for 4 weeks can reduce liver weight, food intake, blood urea nitrogen (BUN). In addition, total amides could also increase skeletal muscle weight, insulin, insulin-like growth factor 1 (IGF1), total protein (TP), albumin (ALB), globular proteins and ALB proteins/globulin protein levels in serum, and increase TP, RNA, RNA/DNA in skeletal muscle and mRNA expression of insulin receptor (InR) in liver and skeletal muscle. Further in-depth research revealed the total amides could ameliorate protein metabolism disorder in DM mice via activating PI3K/PKB/mTOR signaling and regulating ubiquitin–proteasome system (UPS) (Ren et al., 2017). As a key enzyme in glucose metabolism, α-glucosidase plays an important role in increasing blood glucose. Therefore, inhibiting the activity of α-glucosidase has become one of the means to treat diabetes (Song et al., 2019). One study showed that alkaloids extracted from ZBM with different polar solvents had significant inhibitory effects on α-glucosidase, which was consistent with the glucose-lowering mechanism of acarbose, suggesting that it has great potential as α-glucosidase inhibitors (Li et al., 2020b). ACAT, cholesterol acyltransferase; BUN, blood urea nitrogen; DM, diabetes mellitus; FBG, fasting blood glucose; G-6-pase, glucose-6-phosphatase; GHb, glycosylated hemoglobin; GlcN, glucosamine; GLUT-2, glucose transporter 2; GSP, glycosylated serum protein; HAS, hydroxy-α-sanshool; HBS, Hydroxy-β- sanshool; HFD, high fat diet; HSD, high-sugar diet; HO-1, homeobox-1; IGF1, insulin-like growth factor 1; InR, insulin receptor; IR, insulin resistance; ITT, intraperitoneal injection of insulin tolerance test; OGTT, Oral glucose tolerance test; OVX, bilateral ovariectomy; PA, palmitic acid; PEPCK, phosphoenolpyruvate carboxykinase; SCFAs, short-chain fatty acids; SCOE, supercritical CO2 extract; STZ, streptozotocin; TP, total protein; TC, total cholesterol; TG, triglycerides; UPS, ubiquitin–proteasome system.
Compounds/extracts
Models
Dosage
Detail pharmacological effects
Related mechanisms
Ref.
Hypoglycemic effects
Water extracts
PA induced HepG2 cells
20,40, 60 μg/mL
Inhibiting PA-induced lipid formation;
Ameliorating PA-induced oxidative stress.Activating AMPK/PI3K/Akt signaling.
Zhang et al., 2022a
Total amides
HFD feeding + HSD feeding + STZ induced mice
0.4, 0.8 mg/kg, p.o., for 4 weeks
Reducing food and water intake, and increasing body weight;
Improving sugar tolerance;
Decreasing TG, TC, LDL-C and MDA in serum;
Alleviating pathological changes in liver.
Lu et al., 2021
Total amides
HFD feeding + STZ induced rats
3, 6, 9 mg/kg, p.o., for 4 weeks
Increasing body weight of DM model rats;
Improving glucose tolerance;
Increasing hepatic glycogen, myoglycogen and insulin;
Reducing pathological changes in pancreatic;
Reducing gluconeogenesis and increasing glycolysis.Decreasing PEPCK and G-6-pase, and increasing glucokinase;
Increasing HO-1, GLUT-2;
Up-regulating TRPV1 and down-regulating CB1.
You, 2016; You er al., 2015
Total amides
STZ induced in rats
2,4,8 mg/kg, p.o., for 4 weeks
Reducing liver weight;
Increasing skeletal muscle weight and reducing BUN;
Increasing insulin, IGF1, TP, albumin, globulin and albumin/globulin;
Increasing TP, RNA, RNA/DNA in skeletal muscle;
Increasing InR in liver and skeletal muscle.Increasing mRNA of IGF1 and IGF1 receptor;
Increasing mRNA of PI3K, PKB and mTOR in skeletal muscle, and decreasing Atrogin-1, MuRF1 and FOXO;
Promoting protein synthesis via activating PI3K/PKB/mTOR signaling;
Inhibiting protein catabolism via UPS.Ren et al., 2017
HAS
HFD feeding + STZ-induced mice
1.25, 2.5, 5 mg/kg, p.o., for 4 weeks
Improving type 2 diabetes;
Protecting liver and pancreas;
Increasing liver glycogen.Activating PI3K/Akt/GSK-3β/GS signaling.
Zhang et al., 2022b
HAS
GlcN induced HepG2 cells
2.5, 5, 10 μg/mL
HAS
HFD feeding + HSD feeding + STZ induced mice
8 mg/kg, p.o., for 4 weeks
Decreasing GSP, GHb, TG, and TC;
Alleviating IR.Regulating gut microbiota and metabolites;
Regulating lipid and amino acid metabolism pathways.Xu et al., 2022
Hyperoside
HFD feeding + alloxan induced in KM mice
100, 200,400 mg/kg, p.o., for 4 weeks
Improving FBG and OGTT;
Restoring insulin secretion and mitochondrial function;
Reducing intracellular Ca2+ & oxidative stress.Inhibiting TXNIP;
Inhibiting ROS-induced apoptosis of pancreatic β-cells.Zhang et al. (2021b)
H2O2 induced MIN6 cells
10, 20, 40 μg/mL
Lipid-lowering effects
SCOE
OVX rats
15 mg/kg, p.o., 4 weeks
Increasing the intestinal function and peristalsis of OVX rats;
Reducing fat and TG in liver via decreasing emulsification and absorption of fat;
Promoting bile acid and neutral steroid excretion;
Accelerating TC decomposition while prevent its synthesis.Upregulating ileal FXR and liver CYP7A1 expression;
Reducing ileal FXR, ASBT & IBABP and liver HMG-CoA expression.Ren, 2014
Volatile oils
OVX rats
9 mg/kg, p.o., 4 weeks
Increasing the intestinal function and peristalsis of OVX rats;
Reducing fat and TG in liver via decreasing emulsification and absorption of fat.
Ren, 2014
Total amide
OVX rats
45 mg/kg, p.o., 4 weeks
Reducing bile acid reabsorption;
Reducing TG absorption and TG content in liver;
Accelerating cholesterol decomposition;
Reducing cholesterol synthesisIncreasing ileal FXR and reducing ileal ASBT & IBABP expression;
Down-regulating hepatic FXR and up-regulating CYP7A1 expression; Downregulating hepatic HMG-CoA expression.Ren, 2014
Total amides
HFD feeding rats
3, 6, 9 mg/kg, p.o., for 6 weeks
Decreasing TC, TG in liver and blood;
Alleviating fatty changes in the live;
Increasing TC excretion.Downregulating liver SREBP-2 & HMG-CoA and ileal ASBT & IBABP;
Upregulating TRPV1 in liver and ileal.You, 2016
Total amide
HFD feeding mice
5, 10, 20 mg/kg, p.o. for 12 weeks
Reducing body weight and fat accumulation;
Alleviating liver pathological changes;
Improving oxidative stress, OGTT and ITT;
Reducing TG, TC, LDL-C and increased HDL-C;Decreasing ratio of Firmicutes/Bacteroidota;
Increasing the abundance of Allobaculum, Bacteroides and Dubosiella;
Increasing the production of SCFAs;
Activating AMPK/Nrf2 signaling.Peng et al., 2023
Total amide
HFD feeding rats
7 mg/kg, p.o., for 4 weeks
Reducing the body weight of obese rats;
Lowering blood and liver lipid levels;
Improving liver pathology;
Increasing cholesterol and TG excretion;
Increasing the total bile acid in small intestine and feces.
Chen et al., 2017
Powders of ZBM
HFD feeding mice
1.5 g/kg, crude herbs mass equal, p.o. for 12 weeks
Alleviation effects on HFD-induced NAFLD;
Regulating fatty acid and TC metabolism.Rebalance gut microbiota dysbiosis and regulate metabolic profiles
Huang et al., 2023
β-Sanshool
Hi5 cell microsomes of hACAT-1/hACAT-2
IC50 = 39.0 μM (ACAT-1)
IC50 = 79.7 μM (ACAT-2)ACAT-1 and ACAT-2 inhibitors;
Regulating TC metabolism.
Park et al., 2007
γ-Sanshool
IC50 = 12.0 μM (ACAT-1).IC50 = 82.6 μM (ACAT-2)
HBS
IC50 = 154.2 μM (ACAT-1)IC50 = 263.0 μM (ACAT-2)
HAS
HFD feeding mice
9, 18, 36 mg/kg, p.o., for 4 weeks
Reducing abdominal fat and liver lipid content by reducing hepatic oxidative stress.
Wang et al., 2019
Ethanol extracts
3 T3-L1 cells
100, 200, 300 μg/mL
Inducing apoptosis of adipocytes.
Upregulating PARP, Bax;
Downregulating Bcl-2, Mcl-1.Wang et al., 2020
HFD feeding mice
4 g/kg, p.o., for 10 weeks
HAS
HFD feeding mice
9, 18, 36 mg/kg, p.o., for 4 weeks
Reducing liver oxidative stress;
Lowering TC, TG, LDL-C and increasing HDL-C.
Wang et al., 2019
HAS
HFD feeding mice
2, 4, 8 mg/kg, p.o., for 8 weeks
Reducing body weight, TC, TG, LDL-C, HIF-1, ATP, LDH, CPT and CS in liver;
Increasing activity of SOD and CAT;
Improving lipid and energy metabolism disorder.Activating AMPK/HIF1/PKM2 signaling.
Ren et al., 2023
Besides crude extracts, some monomers from ZBM were also reported to be promising agents with hypoglycemic activities. Recently, based on T2DM animal model induced by HFD feeding combined with STZ injection, Zhang et al. investigated the hypoglycemic potentials of HAS which is considered as the most predominant amide compound in ZBM, and found that orally administration of HAS (1.25, 2.5, 5 mg/kg) for 4 weeks can obviously improve T2DM symptoms, fasting blood glucose, as well as alleviate pathological changes in liver and pancreas. Combined with the in vitro cell model induced by glucosamine (GlcN), further experiments showed HAS can increase the glucose uptake and glycogen synthesis in liver tissues and GlcN stimulated HepG2 cells, and in-depth study uncovered these effects of HAS were closely correlated in activating PI3K/Akt/GSK-3β/GS signaling (Zhang et al., 2022b). Another literature in 2022 also reported the hypoglycemic potentials of HAS on in vivo DM animal model induced by HFD and HSD feeding combined with STZ injection. It’s reported that treatment with HAS (8 mg/kg) orally for 4 weeks can decrease the triglycerides (TG), total cholesterol (TC), glycosylated serum protein (GSP) and glycosylated hemoglobin (GHb) in serum. Interestingly, this paper reported the possible mechanisms for hypoglycemic effect of HAS are depended on regulation of gut microbiota and metabolites and lipid and amino acid metabolism pathway (Xu et al., 2022). In another study, Zhang et al. identified 26 phenolic and alkylamide components of HPRH, and principal component analysis was used to verify the inhibitory effects of these key components on α-glucosidase in vitro, and nuclear magnetic resonance (NMR) and molecular docking techniques were used to identify the possible binding sites of α-glucosidase inhibition (Luo et al., 2023). Oxidative stress would result in various disorders in glucose and lipid metabolism. Interestingly, another known flavonoid glycoside compound named Hyperoside from ZBM was reported to have notable hypoglycemic effect. In 2021, Zhang et al. found hyperoside (100, 200, 400 mg/kg) oral treatment can improve fasting blood glucose (FBG) and oral glucose tolerance test (OGTT) in HFD-fed and alloxan-induced DM mice. Using H2O2 induced MIN6 cells as model, mechanistic studies found that hyperoside can restore insulin secretion and mitochondrial function by inhibition of ROS-induced apoptosis of pancreatic β-cells via downregulating TXNIP, reducing intracellular Ca2+ and reducing oxidative stress (Zhang et al., 2021c).
Collectively, all these representations mentioned above suggest ZBM and its active compounds have significant hypoglycemic activity and can be developed as alternative or therapeutic drugs for the treatment of glucose metabolism disorders related diseases, such as diabetes. However, there is not enough systemic results about the pharmacological mechanisms corresponding to the hypoglycemic effects of this herbal medicine. From previous works, we can find that activating AMPK/PI3K/Akt signaling is an important pathway for the hypoglycemic effects of ZBM, which could regulate the down-stream signals, including /PKB/mTOR, GSK-3β/GS, etc. Importantly, there are some potential up-stream drug receptors for ZBM to activate the AMPK/PI3K/Akt signaling, such as TRPV1, GLUT-2, CB1 and IGF1. Besides, ZBM could affect some enzymes related to glycometabolism, such as PEPCK, G-6-pase, etc. Lastly, regulating ROS and gut microbiota was also mentioned in previous literatures, and considered as the possible pharmacological mechanisms regarding the hypoglycemic effects of ZBM.
4.2 Lipid-lowering effects
In various clinical diseases, such as T2DM, glucose metabolism disorders are commonly accompanied by lipid metabolism disorders. Consequently, it would be a very ideal way for treating these diseases via regulation of the sugar and lipid metabolism simultaneously (Agbu and Carthew, 2021). At present, there are also some clinical drugs to reduce blood lipids, such as statins and fibrates, but these drugs have more or less adverse reactions, which is not conducive to their clinical development (Dugani et al., 2016; Pagidipati et al., 2016). Interestingly, besides hypoglycemic activity, increasing evidence also lends support to the view that ZBM and its active components possess notable lipid-lowering effects (Wang et al., 2019). In the year of 2014, Ren et al. investigated the lipid-lowering effect of supercritical CO2 extracts of ZBM (SCOE, mainly containing volatile oils and total amides) using bilateral ovariectomy (OVX) rat model. Ren et al. found that oral administration of SCOE (15 mg/kg) for 4 weeks can increase the intestinal function and peristalsis of OVX rats, reduce fat and TG in liver via decreasing emulsification and absorption of fat, promote bile acid and neutral steroid excretion, as well as accelerate TC decomposition while prevent its synthesis. In-depth molecular mechanisms study revealed SCOE can upregulate ileal Farnesoid X-Activated Receptor (FXR) and liver CYP7A1 expression, reduce ileal FXR, ASBT & IBABP and liver HMG-CoA expression. Ren et al. also investigated the volatile oils of ZBM, and found that the volatile oil (9 mg/kg) oral treatment for 4 weeks could increase the intestinal function and peristalsis of OVX rats, and reduce fat and TG in liver via decreasing emulsification and absorption of fat. However, the volatile oil cannot obviously promote the TC decomposition and cannot inhibit the HMG-CoA expression, so TC content in liver of the volatile oil treated OVX rats is also increased significantly. Cholesterol acyltransferase (ACAT) can link free cholesterol with long-chain fatty acids to form cholesterol esters, which can promote the development of cardiovascular diseases. A study showed that four amide components extracted from ZBM can reduce the activity of ACAT and reduce the synthesis of cholesterol lipids (Park et al., 2007). Next, Ren et al. further investigated the lipid-lowering effect of total amides in ZBM, similar to the SCOE, total amides (4 mg/kg) oral treatment for 4 weeks showed a promising effect for lowering the TG and TC in liver tissues, and the molecular mechanisms are related to increase of the intestinal function and peristalsis, acceleration of cholesterol decomposition via downregulating hepatic FXR and up-regulating CYP7A1 expression, and reduction of cholesterol synthesis via downregulating hepatic HMG-CoA expression (Ren, 2014). Another 2 studies found total amides in ZBM (3, 6, 9 mg/kg) oral treatment for 6 weeks significantly improved lipid metabolism disorders in HFD feeding rats. Total amides can decrease TC, TG in liver and blood, alleviate fatty changes in the live, accelerate TC decomposition while prevent its synthesis via downregulating liver SREBP-2 & HMG-CoA and ileal ASBT & IBABP, and increase TC excretion via upregulating TRPV1 in liver and ileal (Lv, 2014). Recently, Peng et al. studied effects of the total amides in ZBM on HFD-feeding induced nonalcoholic fatty liver (NAFL), the results revealed total amides (5, 10, 20 mg/kg) oral treatment for 12 weeks can notably ameliorate the NAFL. Total amides treatment can reduce body weight and fat accumulation in NAFL mice, alleviate liver pathological changes, reduce TG, TC, low-density lipoprotein cholesterol (LDL-C) and increased the high-density lipoprotein cholesterol (HDL-C), improve oxidative stress, OGTT and ITT. The alteration of gut microbiota can affect the development of metabolic diseases and help to treat metabolic disorders (Aron et al., 2021). The potential molecular mechanisms are closely related to decreasing ratio of Firmicutes/Bacteroidota and increasing the abundance of Allobaculum, Bacteroides and Dubosiella, increasing the production of the short-chain fatty acids (SCFAs), and activating AMPK/Nrf2 signaling (Peng et al., 2023). Chen et al. reported that after oral treatment with total amides (7 mg/kg) or total amides (7 mg/kg) combined capsaicin (9 mg/kg) for 4 weeks, the body weight of the HFD feeding rats was significantly decreased, lipid profiles in blood and liver were improved, fatty changes in the liver was alleviated, and the TG, TC and total bile acid in feces were increased, etc. (Chen et al., 2017). Furthermore, another study in 2023 also reported that ZBM is promising herbal medicine for treating NAFLD. It’s reported that the powders of ZBM (1.5 g/kg) oral treatment for 12 weeks can alleviate the HFD feeding induced NAFLD in mice via regulating fatty acid and TC metabolism, as well as rebalancing gut microbiota dysbiosis (Huang et al., 2023). Interestingly, two other studies have shown that seed oil of Z. bungeanum (SOZB) can reduce TG, TC, high-density lipoprotein (HDL-C) in the blood of hyperlipemia rats. Low-density-lipoprotein cholesterol (LDL-C) levels and also reduce high blood viscosity (HBV), high low viscosity (HLV) (Heng et al., 2005; Liu et al., 2007). However, another study demonstrated that SOZB could reduce the levels of TG, TC, MDA and nitric oxide (NO) in the blood of hyperlipidemic hamsters through the activation of PPAR-γ, suggesting that SOZB is a promising new natural antilipemic drug (Chen et al., 2014). For the different extraction parts of ZBM, for example, the n-butanol fraction isolated from the ethanol extracts of Z. bungeanum (EEZB) was shown to reduce the hyperlipidemic effects in apoE-ko mice, and at the cellular level. It was demonstrated that the extract fraction could significantly reduce the levels of TC, TG, free cholesterol and apolipoprotein B (ApoB) secretion in Hepg2 cells, while apolipoprotein A1 (ApoA 1) secretion was increased (Wu et al., 2014).
Besides crude extracts, some monomers from ZBM were also reported to be promising agents with lipid-lowering effects. A study by Park et al. reported that β-Sanshool, γ-Sanshool, and HBS from ZBM are promising cholesterol acyltransferase (ACAT)-1 and ACAT-2 inhibitors with significant modulating ability on TC metabolism (Yong et al., 2007). Using the Hi5 cell microsomes of hACAT-1/hACAT-2, Park et al determined the half maximal inhibitory concentration (IC50) value of these compound mentioned above for the ACAT-1 and ACAT-2, and found the IC50 values of β-Sanshool, γ-Sanshool, and HBS for ACAT-1 and ACAT-2 are 39.0 μM (ACAT-1) & 79.7 μM (ACAT-2), 12.0 μM (ACAT-1) & 82.6 μM (ACAT-2), and 154.2 μM (ACAT-1) & 263.0 μM (ACAT-2), respectively (Yong et al., 2007). In 2019, Wang et al. found that HAS (9, 18, 36 mg/kg) oral treatment could improve lipid metabolism in HFD feeding mice with reduced TG, TC and LDL by reducing abdominal fat and liver lipid content by reducing hepatic oxidative stress (Wang et al., 2019).
Owing to the continuously improving standard of living and changing dietary habit, obesity, also a lipid metabolism disorders related disease, has become an epidemic problem, which has become a predominant health concern for public health worldwide (Dragano et al., 2020). It is worth noting that obesity is the important pathological factor for various serious diseases, for instance, diabetes, coronary heart disease, fatty liver, and even cancers, etc (Piché et al., 2020). However, the available drugs in clinical for treating obesity are limited, so it’s urgent for pharmaceutical researchers to discovery more alternative drugs for combating obesity as well (Gadde et al., 2018; Ryan, 2022). In 2019, Wang found the ethanol extracts of ZBM (EAE, 4 g/kg) oral treatment for 10 weeks could reduce the body weight of HFD feeding mice. Furthermore, based on network pharmacology analysis and experimental in vivo and in vitro, it’s found that EAE promotes apoptosis of adipocytes to exert slimming effects (Wang et al., 2020). In 2019, Wang et al. investigated the effects of HAS (9, 18, 36 mg/kg) on HFD feeding mice. Besides lipid-lowering effect of HAS mentioned above, it’s also found that oral treatment of HAS significantly reduced body weight and abdominal fat accumulation in HFD feeding rats, regulated blood and liver lipid metabolism, improved liver tissue lesions, reduced liver lipid deposition, improved liver oxidative stress, and the mechanisms was related to regulation of ApoE and PPARγ (Wang, 2020; Li et al., 2023). Recently, another literature by Ren et al. also investigated the anti-obesity effects of HAS. Ren et al. found that oral administration of HAS (2, 4, 8 mg/kg) for 8 weeks can notably reduce body weight, TC, TG, LDL-C, hypoxia Inducible factor (HIF)-1, ATP, lactic dehydrogenase (LDH), carnitine palmitoyl-transferase (CPT) and citrate synthase (CS) in liver of HFD feeding mice, and increase the activity of SOD and CAT. The molecular mechanisms are involved in improving lipid and energy metabolism disorder via activating AMPK/HIF1/pyruvate kinase M2 (PKM2) signaling in HFD feeding mice (Ren et al., 2023). In addition, another study found that after giving dried and ripe peppercorns powder to HFD fed C57/BL8 weeks, serum TG, TC and LDL-C levels were decreased, and body weight was also significantly decreased, while the degree of liver steatosis was also improved (Huang et al., 2023).
In summary of above describe, it’s suggested ZBM and its active compounds have significant lipid-lowering effect and can be developed as alternative or therapeutic drugs for the treatment of lipid metabolism disorders related diseases, such as hyperlipidemia, NAFLD, overweight or obesity, etc. Previous research has uncovered lots of possible molecular mechanism for the lipid-lowering effects of ZBM. Importantly, upregulation of FXR might be an important mechanism for lipid-lowering effects of ZBM, which would be beneficial for reducing fat and TG in liver via decreasing emulsification and absorption of fat, promoting bile acid and neutral steroid excretion, and accelerating TC decomposition, etc. Additionally, it’s also indicated ZBM can downregulate liver SREBP-2 & HMG-CoA and ileal ASBT & IBABP, which would be helpful decreasing TC, TG in liver and blood, alleviating fatty changes in the live, and increasing TC excretion. Furthermore, regulation of gut microbiota to product SCFAs is also a potential mechanism for the lipid-lowering effects of ZBM. Besides, the lipid-lowering effects of ZBM might be also related to activating AMPK/Nrf2, AMPK/HIF1/PKM2, etc. However, more in-depth investigations are also needed to uncover the molecular mechanisms of the lipid-lowering effects of ZBM in the future.
5 Safety and pharmacokinetics
As a traditional condiment food and herbal medicine in China, ZBM is generally considered to be completely safe under commonly used dosage (Fu et al., 2022). However, historical Materia Medica books record ZBM has mild toxicities due to that ZBM has strong odor & pungent taste and can be used to kill insects and relieve itching. Moreover, it’s recorded that overdosing on ZBM might result in respiratory distress. The “Valuable Prescriptions for Emergencies (Qianjin Yao fang)”, which written by the known medical scientist of Sun Si-miao in Tang dynasty, recorded that “long term consumption would result in dyspnea”. In addition, another classic monograph of Chinese medicine named “Chinese medical records” written by Zhang Xi-chun (early modern of China) also recorded in the “Chinese Medical Records” that “I tried to chew 30 ZBM due to stomach cold, and after swallowing it, I felt dyspnea…”. In the year of 2022, Zhang et al. reported the median lethal dose (LD50) of HAS, the predominant active component in ZBM, is 73 mg/kg in mice (Zhang et al., 2022b); xanthotoxin (XAT, 80 μg/mL) contained in ZBM has certain photoactivation toxicity to Caenorhabditis elegans, and can cause in the overall RNA changes of C. elegans (Zhang, 2020).
Nowadays, pharmacokinetic studies showed that after 30 min of oral administration of total amide in healthy subjects, the maximum plasma concentration of HAS can reach 0.76–2.66 μmoL/L with a median half-life of 1.6–1.7 h, and its pharmacokinetic profile is similar to that of HBS and HAS. What’s more, after oral administration of the total amide, the HAS and HBS could be detected in both plasma and urine, and HAS may be rapidly absorbed in the gastrointestinal tract, and formed glycosidic acid conjugates in plasma, subsequently transported to whole body, and finally excreted through kidneys. After subcutaneous injection of total amide of ZBM, HAS, HBS and hydroxy-γ-sanshool can be rapidly absorbed by the body and widely distributed in the plasma with the absolute bioavailability of 100. 2%, 76. 2% and 90. 3%, respectively (Munekage et al., 2011; Li et al., 2019).
6 Conclusion and perspectives
As early as in the Qin and Han dynasties, the known classic monograph of Chinese medicine named “Huang di's Canon of Medicine” have discussed the insights of traditional Chinese medicine on the etiology of obesity and diabetes, and recorded that obesity is induced by excessive consumption of fatty foods, and subsequently would result in “Thirst disorder syndrome (also known as diabetes)” (Wang et al., 2022; Tong and Zhou, 2003). Since pre-Qin dynasty, ZBM has been used to treat diabetes and its related diseases (Ling et al., 2022). Furthermore, current investigations support to the view that ZBM and its active components are beneficial for amelioration of the glucose and lipid metabolism disorder and its related diseases (Table 7, Fig. 2), such as diabetes, hyperlipidemia, obesity, as well as NAFLD, etc.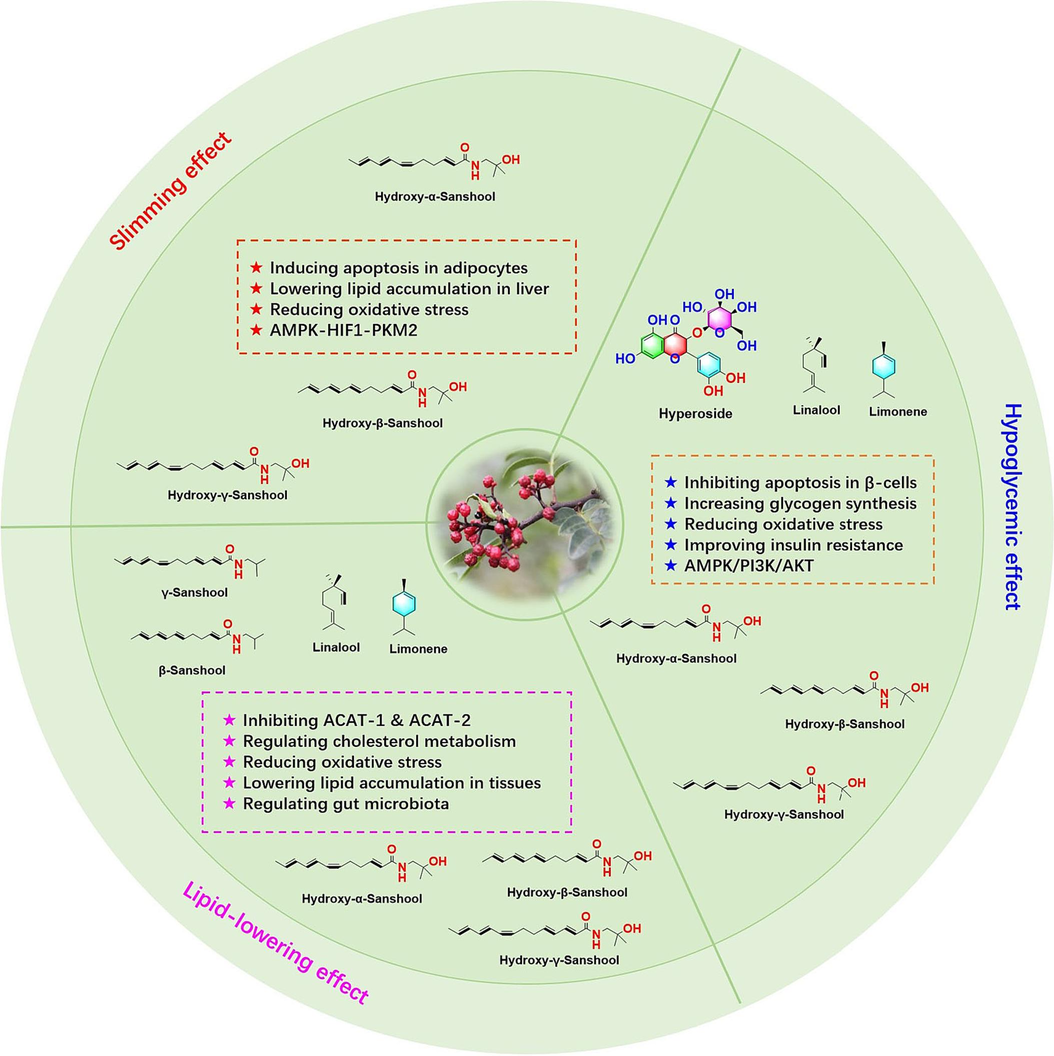
Pharmacological effects of ZBM and its active components.
However, there are also several challenges associated with the development of ZBM and its active components in the management of glucose and lipid metabolic disorders. Firstly, the existing studies have reported that ZBM and its active ingredients have a wide variety of pharmacological activities against glucose and lipid metabolic disorders, but most of them are still in vitro or in the laboratory stage, and clinical studies have not yet been conducted. For example, HAS, the most important amide component in ZBM, has been shown to have therapeutic effects on diabetes, hyperlipidemia and obesity, but clinical trials have not been carried out to confirm its clinical value. Therefore, more multicenter-based clinical studies, which are recognized as the gold standard for assessing clinical efficacy, are urgently needed to devoted to explore systematically the clinical therapeutic effects of HAS and other active ingredients in management of glucose and lipid metabolic disorders and its related diseases (Li et al., 2023b).
Secondly, ZBM pericarp is commonly used as a condiment; however, as a drug for treatment of diseases, too much consumption of ZBM is harmful to the body. Currently, most of the available studies mainly focus on the pharmacological activity of ZBM, but there are fewer studies on its toxicity and side effects, as well as the related toxicity mechanisms and toxic target organs. Safety evaluation is the key step of drug development, therefore, more new technologies, animal experiments, and clinical experiments should be used to explore the possible toxicity, toxic target organs, as well as toxic mechanisms. In addition, more works should be also devoted to explore the feasible detoxification ways towards to its possible toxicities, including administration method requirements, maximum dosage, detoxifying substances, etc.
Thirdly, amides in ZBM are unstable due to its characteristics of chemical structures, and druggability of these components are needed to be improved. For instance, HAS is the predominant active amides in ZBM with promising potentials in regulating glucose and lipid metabolism; however, HAS is unstable due to its specific unsaturated double bond structure, which is easily damaged by exposing to air, oxygen, acidic solution, and UV light, and is also more prone to isomeric transformation, hydrolysis, and oxidation under normal storage conditions. Besides, although sanshools show potential bio-activities in previous investigations, the pharmacological activities of these compounds have not met the requirement as clinical drugs. Therefore, it needs to adopt modern drug design strategies to modify the structures of HAS to ensure its activity. In addition, the way of structural modifications is also beneficial for enhancing active constituents in ZBM.
Fourth, almost all the current research on ZBM and active components are still at the experimental stage of the active pharmaceutical ingredient (API), and the related preparation investigation is lacking. Therefore, lots of efforts should be devoted to the development of modern pharmaceutical dosage forms for extracts of monomers form ZBM, which would be beneficial for enhancing their safety, clinical efficacy, and patient compliance.
Fifth, the current available quality control methods for ZBM are relatively backward via determination of total volatile oil and total alkaloids using UV analysis. Currently, the instrumental analysis techniques have been greatly improved, and the HPLC/HPLC-MS or GC of GC–MS have been widely used to control the herbal medicines’ quality. Furthermore, the quality markers (Q-Markers) strategy has been aroused increasing concern in establishment of quality control system of herbal medicines. Therefore, the modern HPLC/GC combined Q-Markers determination strategy would be focused on establishment the quality control systems of ZBM.
Sixth, most of studies regarding ZBM focus on the pericarp due to the traditional use of this herbal medicine. ZBM seeds also contain oil and abundant compounds, however their pharmacological activities have not been thoroughly uncovered. Therefore, a great deal of research should be devoted to investigate the phytochemistry and pharmacology of the seeds of this herbal medicine in the future.
Collectively, although previous research on ZBM has obtained great achievements, there is still enormous gaps in the development of modern pharmaceutical preparations, and future works should be devoted to break through the bottlenecks in mechanisms exploration, safety evaluation, chemical structure modification, clinical efficacy evaluation, modern preparation technology and quality controlling. The present review gives a comprehensive understanding of the available research works of ZBM regarding its regulating effects on glucose and lipid metabolic disorders, which would be beneficial for the future drug development of extracts or monomers from ZBM in clinical for combating glucose and lipid metabolic disorders, especially for the diabetes, hyperlipidemia, obesity, as well as NAFLD, etc.
7 Authorship contribution statement
All authors made a significant contribution to the work. Wei Peng and Shuguang Hou: conceived the manuscript. Juan Guo and Chengxun He: collected the data, drafted the manuscript. Qing Zhang, Wenwen Chen and Die Qian: drew tables and figures. Wei Peng and Chunjie Wu: revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the Natural Science Foundation of Sichuan Province of China (No. 2022NSFSC0720), State Administration of Traditional Chinese Medicine of Sichuan Province of China (No.2020HJZX001), and Health Commission of Sichuan Province (No. 21PJ130).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Bio.. 2021;22(6):425-438.
- [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67-S74.
- [Google Scholar]
- Morphological and agronomic characterization of Turkish Vaccaria hispanica (Mill.) Rauschert populations. Turk. J. Agric. For. 2022;46(6):933-946.
- [Google Scholar]
- Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 2021;160(2):573-599.
- [Google Scholar]
- Analysis of aroma components of volatile oils obtained from Zanthoxylum bungeanum Maxim. by headspace solid-phase extraction and microwave-assisted method based on GC-MS. China Spice.. 2023;48(03):187-190.
- [Google Scholar]
- Research and Application of Molecular Imprinting Purification Technique for Pepper Numbing Substances. Chongqing: Southwest University; 2018.
- Extract of Zanthoxylum bungeanum maxim. seed oil reduces hyperlipidemia in hamsters fed high-fat diet via activation of peroxisome proliferator-activated receptor γ. Trop. J. Pharm. Res. 2014;13:1837-1843.
- [Google Scholar]
- Regulatory effects of Zanthoxylum bungeanum hemp and capsaicin on lipids in rats. Jiangsu Agri. Sci.. 2017;45(21):168-172.
- [Google Scholar]
- Dawadi, P., Shrestha, R., Mishra, S., Bista, S., Raut, R.K., Joshi, T.P., Bhatt, L.R., 2022. Nutritional value and antioxidant properties of Viburnum mullaha Buch. Ham. Ex D. Don fruit from central Nepal. Turk. J. Agric. For., 46 (5), 781-789.
- GC-MS compositional analysis of volatile oil of pepper from different origins and study of antibacterial test. Lishizhen Med. Mater. Med. Res.. 2020;31(12):2993-2996.
- [Google Scholar]
- Recent updates on obesity treatments: available drugs and future directions. Neuroscience. 2020;437:215-239.
- [Google Scholar]
- Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol.. 2016;1(2):136-145.
- [Google Scholar]
- Comparative study on the volatile oil content and composition of Zanthoxylum bungeanum from different origins. Chin. Med. Clin.. 2014;5(2):16-19.
- [Google Scholar]
- Toxicity of pepper based on ancient Chinese herbal texts. Chin. Pharmacovigil.. 2022;19(4)
- [Google Scholar]
- Correlation between colorimetric analysis of flavonoid content and color values of Zanthoxylum bungeanum. Chin. J. Exp. Formul.. 2017;23(6):91-97.
- [Google Scholar]
- Chemical composition, pharmacological effects of Zanthoxylum bungeanum and prediction of quality markers. Shandong J. Trad. Chin. Med.. 2022;41(12):1351-1358.
- [Google Scholar]
- Study on therapeutic effects of seeds oil of Zanthoxylum bungeanum Maxim on experimental hyperlipidemia in rat. Med. J. Chin. PLA. 2005;30:1012-1013.
- [Google Scholar]
- Hu, H.S., 2013. GC/MS analysis of the chemical composition of volatile oil from pepper. Guangdong Chem. Ind. 22, 126–127, 104.
- Investigation on the mechanisms of Zanthoxylum bungeanum for treating diabetes mellitus Based on network pharmacology, molecular docking, and experiment verification. Biomed. Res. Int.. 2023;2023:9298728.
- [Google Scholar]
- Integrative multi-omics unravels the amelioration effects of Zanthoxylum bungeanum Maxim. on non-alcoholic fatty liver disease. Phytomedicine. 2023;109(154576)
- [Google Scholar]
- Studies on the chemical composition of Phyllostachys nigra (II) Wuhan Botan. Res.. 1991;01:89-93.
- [Google Scholar]
- New alkylamides from pericarps of Zanthoxylum bungeanum. Chin. Chem. Lett.. 2012;23(11):1247-1250.
- [Google Scholar]
- Jiang, L., 2019. Research on the cultivation and use of pepper in Gansu since Ming and Qing dynasties 2019 Northwest Normal University, Xi’an.
- Analysis of Zanthoxylum armatum Components and Study of Insecticidal Activity. Wuhan: Wuhan Light Industry University; 2015. Master's thesis
- Extraction of volatile oil and gas chromatography-mass spectrometry analysis of the chemical composition of Dahongpao Zanthoxylum bungeanum. Food Sci.. 2014;2:261-265.
- [Google Scholar]
- Extraction and Antioxidant and Antibacterial Activity of Flavonoid Polyphenols from Pepper Peel. Xi’an: Northwest Agriculture and Forestry University of Science and Technology; 2018.
- Study on the Chemical Constituents and Their Biological Activities in Pepper. Xi’an: Northwest University for Nationalities; 2021.
- Analysis of Chemical Composition Differences of Pepper in Different Provinces and its Origin Discrimination. Hefei: Anhui Agricultural University; 2022.
- Amides from Zanthoxylum bungeanum Maxim. (Rutaceae) are promising natural agents with neuroprotective activities. Arab. J. Chem.. 2023;16(7):104817
- [Google Scholar]
- Advances in pharmacological research on sanguinarine. Chin. Pharmacol. Bull.. 2019;35(2):172-175.
- [Google Scholar]
- Gas chromatography-mass spectrometry study on volatile oils of wild pepper in Hubei. Lishizhen Med. Mater. Med. Res.. 2022;33(8):1878-1881.
- [Google Scholar]
- A new lignan dimer from wild pepper (In English) Chinese J. Trad. Chin. Med.. 2015;14:2843-2848.
- [Google Scholar]
- Study on the extraction process and in vitro antioxidant activity of Zanthoxylum bungeanum flavonoids. China Spice.. 2019;44(9):94-100.
- [Google Scholar]
- Chemical profiles and screening of potential α-glucosidase inhibitors from Sichuan pepper using ultra-filtration combined with UHPLC-Q-TOF-Science Direct. Ind. Crop. Prod.. 2020;143
- [Google Scholar]
- GC-MS analysis of volatile components in different concoctions of pepper. Nat. Prod. Res. Devel.. 2020;32(9):1470-1476.
- [Google Scholar]
- A vortex-enhanced magnetic solid phase extraction for the selective enrichment of four quaternary ammonium alkaloids from Zanthoxyli Radix. J. Chromatogr B. 2023;1217:123617
- [Google Scholar]
- Research progress of chemical composition and pharmacological effects of pepper. West Chin. J. Pharm.. 2014;01:91-94.
- [Google Scholar]
- Preventive and therapeutical effect of the kernel of Zanthoxylum bungeanum seed oil on experimental hyperlipidemia in rat. J. Fourth Mil. Med. Univ.. 2007;28:411-413.
- [Google Scholar]
- Study on the chemical composition of Zanthoxylum schinifolium Sieb. et Zucc. J. Pharmaceut. Sci.. 1991;11:836-840.
- [Google Scholar]
- Determination of the chemical composition of medicinal pepper. Chin. Herb. Med.. 1991;01:16-18.
- [Google Scholar]
- Process optimization and GC-MS analysis of supercritical CO2 extraction of Zanthoxylum bungeanum volatile oil. Modern Food Sci. Technol.. 2020;36(05):73-80.
- [Google Scholar]
- Comparative analysis of volatile oil components of four species of pepper from Guizhou. Chin. Food Ind.. 2023;9:80-83.
- [Google Scholar]
- Study on Chemical Composition and Pharmacological Activity of Zanthoxylum bungeanum. Guangzhou: Jinan University; 2019. Master's thesis
- Improvement of glucolipid metabolism disorder in mice with type 2 diabetes by Zanthoxylum bungeanum hemp. Modern Food Sci. Technol.. 2021;37(03):37-45.
- [Google Scholar]
- GC-MS-AMDIS combined with retention index analysis to compare the composition of volatile oils of green Zanthoxylum bungeanum and Zanthoxylum armatum. Chin. Med. Clin.. 2015;6(05):18-21.
- [Google Scholar]
- Radical oxygen species: an important breakthrough point for botanical drugs to regulate oxidative stress and treat the disorder of glycolipid metabolism. Front. Pharmacol.. 2023;14:1166178.
- [Google Scholar]
- Lv, J., 2014. Functional evaluation of the hypolipidemic effect of peppermint hemp and study of the mechanism of action. M.S., Southwestern University, Chongqing.
- Study on the Material Basis of Numbness of Zanthoxylum armatum. Chengdu: Southwest Jiaotong University; 2012. Master's thesis
- Phenolic chemical composition and its antioxidant activity in green pepper. Chin. Herb. Med.. 2020;08:2095-2101.
- [Google Scholar]
- Current status of research on the active components of pepper alkaloids. China Food Ind.. 2022;348(10):110-113.
- [Google Scholar]
- Chemical composition analysis and aroma comparison of the volatile oils of nine-leaf green pepper and big red robe pepper by supercritical CO2 extraction. China Spice.. 2009;34(3):102-105.
- [Google Scholar]
- Pharmacokinetics of daikenchuto, a traditional Japanese medicine (kampo) after single oral administration to healthy Japanese volunteers. Drug Metab. Dispos.. 2011;39(10):1784-1788.
- [Google Scholar]
- National Pharmacopoeia Committee, 2020. Pharmacopoeia of the People's Republic of China. Part I. Beijing: China Pharmaceutical Science and Technology Press. 159-160.
- Characterization of European cranberrybush (Viburnum opulus L.) genetic resources in Turkey. Sci. Hortic.. 2020;273:109611
- [Google Scholar]
- Human acyl-CoA: cholesterol acyltransferase inhibitory activities of aliphatic acid amides from Zanthoxylum piperitum DC. Biol. Pharm. Bull.. 2007;30(1):205-207.
- [Google Scholar]
- Human acyl-CoA, cholesterol acyltransferase inhibitory activities of aliphatic acid amides from Zanthoxylum piperitum DC. Biol. Pharm. Bull.. 2007;30(1):205-207.
- [Google Scholar]
- Zanthoxylum bungeanum amides ameliorates nonalcoholic fatty liver via regulating gut microbiota and activating AMPK/Nrf2 signaling. J. Ethnopharmacol.. 2023;2023
- [CrossRef] [Google Scholar]
- Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res.. 2020;126(11):1477-1500.
- [Google Scholar]
- Qiao, M.F., Liu, Y., Yuan, X.J., Yi, Y.W., Peng, Y.Q., Deng, J., 2017. Analysis of chemical composition and antibacterial activity of Maoxian pepper China Spices 04, 59–63, 73.
- Study on the mechanism of regulation of lipid metabolism in vivo by Zanthoxylum bungeanum essence. Chongqing: Southwest University; 2014.
- Studies on the alkaloids of Zanthoxylum bungeanum roots. J. Pharm. Sci.. 1981;6(09):672-677.
- [Google Scholar]
- Zanthoxylum alkylamides ameliorate protein metabolism disorder in STZ-induced diabetic rats. J. Mol. Endocrinol.. 2017;58(3):113-125.
- [Google Scholar]
- The mechanisms of hydroxy-α-sanshool from Zanthoxyum bungeanum Maxim activates AMPK-HIF1-PKM2 pathway to fix the obesity. J. Func. Foods.. 2023;107:105599
- [Google Scholar]
- Morphological and biochemical character-ization of diverse strawberry tree (Arbutus unedo L.) genotypes from Northern Turkey. Agronomy. 2020;10(10):1581.
- [Google Scholar]
- Shi, X.P., Zhang, W.M., 2010. A comparative study on the volatile chemical composition of red and green pepper China Spices 02 2010 102–105, 112.
- Progress in the study of Zanthoxylum alkaloids. Chin. Wild Plant Resour.. 2010;29(04):1-7.
- [Google Scholar]
- Separation of flavonoid compounds in Zanthoxylum bungeanum by high-speed counter-current chromatography. Food Sci.. 2013;12:6-10.
- [Google Scholar]
- Study on the chemical composition of Zanthoxylum bungeanum and Zanthoxylum armatum. Lanzhou: Lanzhou University of Technology; 2018. Master's thesis,
- Bamboo leaf pepper pair α- study on the inhibitory effect and mechanism of glucosidase. J. Food Biotechnol.. 2019;38(1):58-62.
- [Google Scholar]
- Type 1 and 2 diabetes mellitus: a review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Synd.. 2019;13(1):364-372.
- [Google Scholar]
- Network pharmacology and GC-MS to explore the mechanism of action of volatile oil of Piper betony in intervention of hyperlipidemia. J Chongqing Univ. Commerce Indu. 2023:1-12.
- [Google Scholar]
- Low temperature affects fatty acids profiling and key synthesis genes expression patterns in Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci.. 2022;23(4):2319.
- [Google Scholar]
- Study on chemical composition and biological activity of pepper. Xi’an: Northwest Agriculture and Forestry University; 2017.
- Study on the lipid-regulating and anti-atherosclerotic effects of Zanthoxylum bungeanum hydroxy-α- sanshool and its mechanism. Chengdu: Chengdu University of Traditional Chinese Medicine; 2020.
- Antiobesity, regulation of lipid metabolism, and attenuation of liver oxidative stress effects of hydroxy- α -sanshool isolated from Zanthoxylum bungeanum on high-fat diet-induced hyperlipidemic rats. Oxid Med Cell Longev.. 2019;2019:1-13.
- [Google Scholar]
- An analysis of the characteristics of the medicine used in the grouping of prescriptions for the treatment of thirst in the Sixty Diseases Formula of Lao Guan Shan Medical Brief. Chin. J. Trad. Chin. Med.. 2018;05:1785-1787.
- [Google Scholar]
- Research on the structure of the theoretical framework of obesity diagnosis and treatment in Chinese medicine. J. Liaoning Univ. Chin. Med.. 2022;24(8):119-122.
- [Google Scholar]
- Study on the chemical composition of n-butanol fraction of pepper pericarp. Chin. Herb. Med.. 2023;05:1353-1361.
- [Google Scholar]
- Network pharmacology-based strategy for the investigation of the anti-obesity effects of an ethanolic extract of Zanthoxylum bungeanum Maxim. Front Pharmacol.. 2020;11:572387
- [Google Scholar]
- A review on classification and biological activities of alkaloids from the genus Zanthoxylum species. Mini-Rev Med Chem.. 2021;21(3):336.
- [Google Scholar]
- Network pharmacology-based analysis of the underlying mechanism of Huajiao for pain relief. Evid. Based Compl. Alt. Med.. 2021;2021:5526132.
- [Google Scholar]
- Determination of three flavonoid glycosides of Zanthoxylum bungeanum by reversed-phase high performance liquid chromatography. Phys. Chem. Exam.. 2010;46(12):1441-1443.
- [Google Scholar]
- Study on the extraction technology of total flavonoids from Zanthoxylum bungeanum and analysis of flavonoid composition. Food Res. Devel.. 2011;32(02):16-20.
- [Google Scholar]
- Study on the chemical composition and biological activity of alkaloids of Cymbopogon japonicus. Chin. Herb. Med.. 2019;06:1305-1309.
- [Google Scholar]
- Analysis of the whole components of red pepper from different origins. Nat. Prod. Res. Devel.. 2023;05:837-851.
- [Google Scholar]
- The effects of Zanthoxylum bungeanum extract on lipid metabolism induced by sterols. J. Pharmacol. Sci. 2014;127:251-259.
- [Google Scholar]
- Research progress of chemical composition and pharmacological effects of pepper. West Chin. J. Pharm.. 2021;06:717-722.
- [Google Scholar]
- GC-MS analysis of volatile oil of Jiu Ye Qing pepper. Yunnan Chem. Indu.. 2023;50(6):78-81.
- [Google Scholar]
- Flavonol glucosides in pericarps of Zanthoxylum bungeanum. Phytochemistry. 1995;39(3):723-725.
- [Google Scholar]
- Alkylamides from pericarps of Zanthoxylum bungeanum. Phytochemistry. 1997;46(6):1123-1126.
- [Google Scholar]
- Effects of hydroxy-alpha-sanshool on intestinal metabolism in insulin-resistant mice. Foods. 2022;11(14):2040.
- [Google Scholar]
- Aroma constituents and alkylamides of red and green Zanthoxylum bungeanum (Zanthoxylum bungeanum and Zanthoxylum schinifolium) J. Agri. Food Chem.. 2008;56(5):1689-1696.
- [Google Scholar]
- Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem.. 2013;61:1772-1778.
- [Google Scholar]
- Advances in the study of active ingredients of Zanthoxylum bungeanum. Food Sci.. 2018;19:303-312.
- [Google Scholar]
- Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J Agric Food Chem.. 2013;61(8):1772-1778.
- [Google Scholar]
- Analysis of the efficacy of pepper and the principle of eliminating stasis and generating new energy based on the theory of “looking and smelling” in Chinese medicine. J. Jiangxi Univ. Trad. Chin. Med.. 2023;01:16-19.
- [Google Scholar]
- Study on the effect of Zanthoxylum bungeanum numbing substances on glycolipid metabolism in rats and its molecular mechanism. Chongqing: Southwest University; 2016.
- Hypoglycemic effects of Zanthoxylum alkylamides by enhancing glucose metabolism and ameliorating pancreatic dysfunction in streptozotocin-induced diabetic rats. Food Functi.. 2015;6(9):3144-3154.
- [Google Scholar]
- The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol.. 2019;71(4):793-801.
- [Google Scholar]
- Progress in anti-inflammatory activity of Zanthoxylum bungeanum. J. Chengdu Univ.. 2021;40(4):340-350.
- [Google Scholar]
- Research progress on repellent and insecticidal activity of Piper spp. Chin. Health Insect.. 2021;06:569-577.
- [Google Scholar]
- Advances in the study of phenylpropanoid components and their pharmacological activities in Zanthoxylum spponigans. Chin. J. Trad. Chin. Med.. 2021;46(22):5760.
- [Google Scholar]
- Chemical composition of Zanthoxylum bungeanum and its pharmacodynamic study. Modern. Biomed. Progr.. 2010;10(03):552-554.
- [Google Scholar]
- Isolation and composition analysis of numbing substances of pepper. Chongqing: Southwest University; 2008.
- Study on flavonoid and polyphenol contents and antioxidant activity of Zanthoxylum bungeanum. Xi’an: Northwest Agriculture and Forestry University; 2013. M.S. Thesis
- Photoactivation toxicity study of Zanthoxylum bungeanum toxin against Cryptobacterium hidradenum. Haikou: Hainan University; 2020. Master's thesis
- Chemical composition of essential oils from Zanthoxylum bungeanum Maxim. and their bioactivities against Lasioderma serricorne. J. Oleo. Sci.. 2016;65(10):871-879.
- [Google Scholar]
- Hydroxy-α-sanshool isolated from Zanthoxylum bungeanum Maxim. has antidiabetic effects on high-fat-fed and streptozotocin-treated mice via increasing glycogen synthesis by regulation of PI3K/Akt/GSK-3β/ GS signaling. Front. Pharmacol.. 2022;13:1089558.
- [Google Scholar]
- Interactions between Phenols and Alkylamides of Sichuan peper (Zanthoxylum Genus) in α-glucosidase inhibition: a structural mechanism analysis. J. Agric. Food Chem.. 2021;69(20):5583-5598.
- [Google Scholar]
- Zanthoxylum bungeanum Maxim. (Rutaceae): a systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci.. 2017;18(10):2172.
- [Google Scholar]
- Research progress on the chemical composition of Chinese pepper and its prevention and treatment of neuropsychiatric diseases. Nat. Prod. Res. Devel.. 2021;33(11):1969-1981.
- [Google Scholar]
- Hyperoside from Z. Bungeanum leaves restores insulin secretion and mitochondrial function by regulating pancreatic cellular redox status in diabetic mice. Free Radical Biol. Med.. 2021;162:412-422.
- [Google Scholar]
- Fructus Zanthoxyli extract improves glycolipid metabolism disorder of type 2 diabetes mellitus via activation of AMPK/PI3K/Akt pathway: Network pharmacology and experimental validation. J. Integr Med.. 2022;20(6):543-560.
- [Google Scholar]
- Study on the chemical composition in Zanthoxylum bungeanum peel. West Chin. J. Pharm.. 2016;31(02):109-112.
- [Google Scholar]
- Research progress on chemical composition, pharmacological effects and resource development of Zanthoxylum bungeanum. China Spice.. 2012;37(03):1-5.
- [Google Scholar]
- Study on the chemical composition of volatile oil of Zanthoxylum bungeanum. J. Lanzhou Univ.. 1992;4:74-77.
- [Google Scholar]
- M.J. Zhou F.F. Shi K. Chen M. Kang X.F. Liang Research progress on the medicinal value of Zanthoxylum bungeanum Agric. Prod. Process. 01 2020(1). 65–67, 72.
- Analysis of flavonoid components in the peel of Zanthoxylum armatum DC. based on UPLC-Q-TOF-MS technique. Food Ind.. 2023;44(02):308-311.
- [Google Scholar]
- Research status and development prospects of active ingredients of Zanthoxylum bungeanum. Grain Oil.. 2020;33(04):4-6.
- [Google Scholar]
- Analysis of the components of volatile oils of two types of pepper by GC-MS. Asia-Pac. Trad. Med.. 2022;18(9):68-73.
- [Google Scholar]
- Research on chemical composition of wild pepper. J. Central China Norm. Univ. (Natural Sci. Ed.). 2009;03:424-427.
- [Google Scholar]
- Study of two new phenylpropanoid components in wild pepper. Organ. Chem.. 2013;06:1345-1348.
- [Google Scholar]
- Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782-787.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105594.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







