Translate this page into:
In-situ crosslinking reaction of graphene oxide & waterborne epoxy resin to construct continuous phase anticorrosive coating
⁎Corresponding author at: School of Chemistry and Chemical Engineering, Yulin University, Yulin 719000, Shaanxi, PR China. chenxiaodong@yulinu.edu.cn (Xiaodong Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
GA Text: Mechanism diagram of graphene oxide (GO) & waterborne epoxy resin (WEP) and diethylenetriamine in-situ crosslinking reaction to construct anti-corrosion coating.
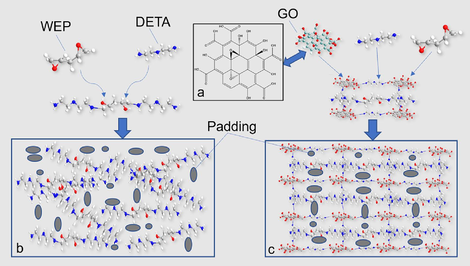
Mechanism diagram of GO&WEP and diethylenetriamine in-situ crosslinking reaction to construct anti-corrosion coating.
Abstract
The mechanism of using graphene oxide as a two-dimensional filler to increase the path length of the corrosive medium to the metal surface is limited to improving the corrosion resistance of the coating. This study propose novel dispersion and in-situ crosslinking reaction mechanism for constructing water-based heavy-duty anticorrosive coatings by using graphene oxide (GO) and waterborne epoxy resin (WEP). The results showed that GO can be uniformly dispersed directly into WEP without complex functional modifications. Oxygen-containing functional groups on moleculars structure of GO&WEP co-polymerized with diethylenetriamine (DETA) forming a continuous phase film which improved the density of the coating. Characterization techniques such as Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), thermal conductivity measurement instrument, pencil hardness tester, adhesion tester, salt spray corrosion test chamber, and electrochemical impedance spectroscopy (EIS) were used to evaluate coating structure and performance properties. The results showed that graphene characteristics enhanced physical properties and anti-corrosion performance of composite coatings. The construction mechanism of the coating is different from that of the current GO modified and dispersed in the coating as a two-dimensional filler to extend the path length of the corrosive medium to the metal surface. The coatings prepared by adding the optimum mass ratio of 0.1–0.2 % GO to WEP have the best physical properties and anti-corrosion properties. When the GO content is 0.2 wt%, the thermal conductivity increases by 86.0 %. After added 0.025 wt% GO, the coating hardness is increased by two grades directly from 2B to HB. With the addition of GO concentration increased to 0.05 wt%, the adhesion is increased from 2 to level 1.When the GO content exceeds 0.05 %, the coating will not bulge off after 4 months of salt water immersion experiment.
Keywords
Graphene oxide
Waterborne epoxy resin
Heavy corrosion prevention
Crosslinking reaction
Water-based coating
1 Introduction
Metal corrosion has caused serious waste of resources and energy loss, and brought huge economic losses to all countries in the world. The loss brought about by metal corrosion is about 4 % of GDP(J. Zou, 2019; Y.Y. Sun et al., 2021). Therefore, the development of metal corrosion and protection technology has always been the hot spot pursued by researchers. Among them, coating protection technology has become a hot spot because of its high cost performance. At present, with the enhancement of people's environmental awareness and the improvement of the world's environmental protection standards, water-based coatings have become the main development direction (Z.K. Si et al., 2021; L.Q. Meng et al., 2019).
Water-based epoxy resin (WEP), as a binder for water-based coatings, has the characteristics of low volatile organic compounds (VOC) content, easy transportation and storage, good stability, strong adhesion, good corrosion resistance and environmental friendliness, so that it has been paid more and more attention(X.Y. Han et al., 2014; N. Elhalawany et al., 2018; X.N. Menget al., 2015; Q. Pan et al., 2013). However, WEP also has the disadvantages of low hardness, easy foaming and high spatial porosity, which limits its application in the field of high performance (J. Ding,2014; J.F. Zhang et al., 2019). In order to optimize the development and utilization of WEP, many researchers have studied the modification of WEP.
Graphene oxide (GO) is an important derivative of graphene. Similar to graphene in structure, it has the advantages of good mechanical properties, compact structure, good physical barrier performance and large specific surface area. At the same time, the surface and edge of GO contain a large number of oxygen-containing functional groups such as hydroxyl, carboxyl and epoxy groups (Q.N. Zhai et al., 2020). Studies have proved that the synergistic effect of two-dimensional nanomaterials and waterborne epoxy resin can greatly improve the barrier ability of composite coating to corrosive substances. GO, as an ideal two-dimensional material, has attracted much attention in corrosion prevention because of its good barrier ability (T.C. Huang et al., 2017; H.W. Huang et al., 2020; X.B. Zhu et al., 2021; Q.S. Zhu et al., 2020; C.L. Chen et al., 2020; M. Kasaeian et al., 2018; H.K.Yu et al., 2022; K. Amirazodi et al., 2019). Therefore, researchers began to use GO to modify waterborne epoxy resin research. For example, N. Wang et al., 2019 found that adding GO to epoxy resins can effectively improve the corrosion resistance. T. Zhou et al., 2021 ameliorated the compatibility among curing agent, resin matrix and GO by end-sealing, modification and in situ grafting process, so that the cured coating had ideal anticorrosion properties. The research findings have enhanced the performance of anti-corrosion coatings. however, in terms of the coating's film-forming mechanism, it still relies on modifying and improving the uniform dispersion of graphene oxide within the coating. The anti-corrosion mechanism involves utilizing GO as a filler material in the coating to extend the path for corrosive media to reach the metal surface through its two-dimensional sheet structure, thereby increasing the longevity of the coating (Dhamodharan, D. et al., 2022; Gao, H. et al., 2023). Nevertheless, this modification process is intricate and fails to establish a continuous protective film with resin in the coating, resulting in limited enhancement in corrosion resistance. In this paper, by the comparison of molecular structures, it is found that both GO and WEP have oxygen-containing functional groups in their molecular structures. The theoretical hypothesis that GO aqueous solution can be uniformly dispersed in WEP emulsion due to their ‘similar phase solubility’ is helpful for the formation of dense anticorrosive film is put forward. The GO aqueous solution was prepared by using the improved Hummers method (W.S. Hummers Jr. et al., 1958), and the dispersion law of GO aqueous solution in WEP emulsion was revealed, which proved the reliability of the theory. Based on the acylation reaction between organic polyamine cross-linkers and carboxyl group on GO, and the addition reaction between organic polyamine cross-linkers and epoxy resin on GO, a theoretical hypothesis was proposed that GO and epoxy resin could simultaneously produce cross-linking reaction with organic polyamine to form continuous phase film, so as to increase the physical properties and anticorrosion properties of composite coatings. The anticorrosive coating was constructed by in-situ crosslinking reaction of GO&WEP complex on tinplate test plate with diethylenetriamine as crosslinker, and the formation mechanism was studied. Polarizing microscope, Infrared spectrometer (FTIR), X-ray diffraction (XRD), Scanning electron microscopy (SEM), Electrochemical impedance spectroscopy(EIS), thermal conductivity tester, pencil hardness tester, adhesion tester, salt spray corrosion test chamber and other equipment were used to test and characterize the coating structure and application performance. It was proved that GO can be uniformly dispersed in WEP by simple mechanical stirring. The mechanism of coating construction is that the oxygen-containing functional groups on the molecular structure of the two molecules are crosslinked and cross-linked with organic polyamine cross-linker to constitute a continuous phase, which improves the shielding property of the coating. Graphene characteristics of GO improve the physical properties and heavy corrosion resistance of the composite coating. This work provides a theoretical basis and a new idea for the construction of graphene oxide modified epoxy waterborne heavy corrosion coating.
2 Materials and methods
2.1 Materials
Flake graphite: Qingdao Tianhe Da Graphite Co., Ltd.. Concentrated sulfuric acid: 98 % (mass fraction), Tianjin Kemeiou Chemical Reagent Co., Ltd.. Sodium nitrate: Analytically pure, Taicang Shanghai Testing Reagent Co., Ltd.. Potassium permanganate: Analytically pure, Tianjin Kemeiou Chemical Reagent Co., Ltd.. Hydrogen peroxide: 30 % (mass fraction), Tianjin Kemeo Chemical Reagent Co., Ltd.. Concentrated hydrochloric acid: 36 % (mass fraction), Tianjin Kemeo Chemical Reagent Co., Ltd.. Waterborne epoxy resin: Hangzhou Wuhuigang Adhesive Co., Ltd.. N-butanol: Analytically pure, Tianjin Zhiyuan Chemical Reagent Co., Ltd.. Diethylenetriamine (DETA), Hangzhou Wuhuigang Adhesive Co., Ltd..
2.2 Instruments and equipment
Infrared spectrometer (FTIR), Bruker Tensor27, Bruker, Germany. X-ray diffractometer (XRD), Bruker D8 ADVANCE, Bruker, Germany. Field emission Scanning electron microscopy (SEM), Sigma 300 (σ300), Zeiss, Germany. Thermal conductivity tester, DM3615, Shanghai Dongmao Electronic Technology Co., Ltd.. Pencil hardness tester, BN703-B, Baien Company. Adhesion tester, QFZ, Cangzhou Yixuan Test Instrument Co., Ltd.. Salt spray corrosion test chamber, YFX-150, hanghai Yuejin Medical Device Co., Ltd.. Circulating water multi-purpose vacuum pump, SHB-B88, Zhengzhou Great Wall Technology & Trade Co., Ltd.. Overhead electric stirrer, OSC-10L, Qunan Experimental Instrument Co., Ltd.. Polarizing microscope, Eclipse Ci-POL, Nikon Co. Ultrasonic instrument, KQ218, Tianjin Tianma Instrument Factory. Electrochemical workstation, CS350M, Wuhan Koster Test Instrument Co., Ltd..
The microstructure of the coating was produced by field emission scanning electron microscopy σ300 (SEM). The dispersion of GO in water-based epoxy was photographed using a polarizing microscope with a 50x objective and a 10x /0.45 eyepiece. Infrared spectroscopy was used to analyze the evolution of functional groups at different stages of the coating using potassium bromide tablets in the wavelength range was 400–4000 cm-1. X-ray diffraction with Cu-Kα radiation (λ = 0.15418 nm) was used to characterize the structural changes of the coating from 5°to 80°. The thermal conductivity tester, pencil hardness tester and adhesion tester were used to test the physical properties of the composite coating. The corrosion resistance of the composite coating was tested with 3 % mass concentration sodium chloride brine immersion, and kerosene immersion and neutral salt spray test(5% sodium chloride solution, the test water temperature is 30℃, the solution PH value is 6 ∼ 7). Electrochemical impedance spectroscopy (EIS) was performed using a classical three-electrode system with graphite electrode as auxiliary electrode and calomel electrode as reference electrode. The electrolyte solution used was 3.5 % NaCl solution (mass fraction) at 25℃, and the coating film thickness was 60 μm. The frequency range of electrochemical impedance was 1.0 × 10–2 ∼ 1.0 × 105 Hz, and the measured signal was 50 mV sine wave. The test instrument was a CS350M electrochemical workstation. The experimental data were analyzed and fitted by ZVIEW software.
2.3 Specimen preparation
2.3.1 Preparation of graphene oxide aqueous solution
GO was prepared by the modified Hummers method. 1 g sodium nitrate (NaNO3) was mixed with 70 ml concentrated sulfuric acid (H2SO4) in a conical flask, followed by 2 g flake graphite, and the mixture was stirred continuously under an ice bath. After 30 min, 8 g potassium permanganate (KMnO4) was added. After 35 min of reaction, the device was placed in the water bath at 35-45℃ for another 4 h. Then 120 ml deionized water was slowly added to the reaction solution in several times. After 30 min of reaction, heat up to 95 ℃ and keep for 5 min. Add 25 ml of 30 % hydrogen peroxide (H2O2) and stir until there were no more bubbles in the solution. Finally, 5 % hydrochloric acid (HCl) and deionized water were added to centrifugally wash the reaction solution until the pH of the solid substance was neutral After adding an appropriate amount of water to the solid material, it was completely dispersed by an ultrasonic disperser to obtain an aqueous solution of GO. 10 g the aqueous solution of GO was accurately weighed and placed in an evaporation dish, and was dried under vacuum at 65℃ to constant weight. The solids content of the GO aqueous solution is then calculated.
2.3.2 Preparation of GO&WEP composite emulsion (GOEPE)
30 G water-based epoxy resin was added to 0 wt%, 0.025 wt%, 0.050 wt%, 0.075 wt%, 0.100 wt%, 0.200 wt%, and 0.740 wt% (GO mass equivalent) GO aqueous solution, respectively, and stirred in a high-speed mixer at room temperature (20-30℃) for 2 h to prepare GO&WEP composite emulsion (GOEPE) with different GO contents. The composite emulsion was coated by 30 µm coating scraper on a slide and the morphology of the wet film was observed by optical microscope. (see Table 1)
Mass ratio of GOEPE
Mass ratio of GOEPC
Sample number
EPE (g)
GO (wt%)
Sample number
EPE (g)
DETA (g)
GO (wt%)
1
30
0
1
30
15
0
2
30
0.025
2
30
15
0.1
3
30
0.05
3
30
15
0.2
4
30
0.075
4
30
15
0.74
5
30
0.1
6
30
0.2
7
30
0.74
2.3.3 In-situ construction of GO&WEP composite coating (GOEPC)
As shown in Fig. 1 and Table 1, 15 g diethylenetriamine (DETA) was added to the composite emulsion prepared in 2.3.2 with 30 g different GO contents, and an appropriate amount of deionized water was added to adjust the concentration. The mixture was stirred by the agiter for 30 min, and an appropriate amount of n-butanol was added to remove the bubbles, and the mixture was left for 20 min. The mixture was evenly coated on the tinplate plates that had been polished with 120 mesh sandpaper, cleaned and dried with anhydrous ethanol, and on glass plates that had been cleaned and dried with anhydrous ethanol, then dried at room temperature in a dust-free environment for 24 h to prepare the graphene oxide & water-based epoxy composite coating.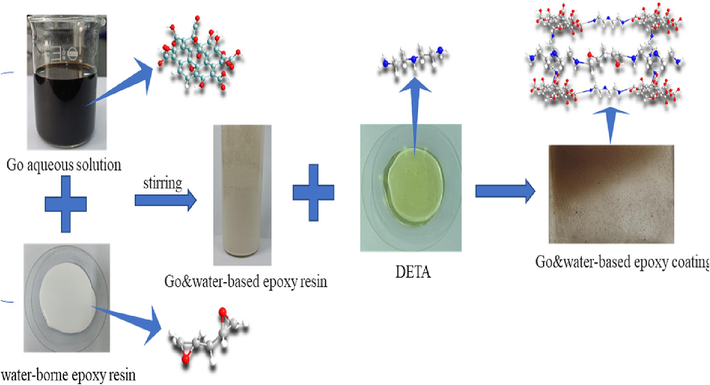
Preparation of graphene oxide & water-based epoxy composite coating.
3 Results and discussions
3.1 GO dispersion in WEP
Since the uniform dispersion of GO in the coating is one of the key factors to improve the corrosion resistance of the coating, this paper proposes to take advantage of the molecular structure of GO and the milky water-based epoxy resin emulsion (WEP), which both contain similar oxygen-containing functional groups and can be evenly dispersed in water, and directly add GO aqueous solution to WEP for mechanical dispersion. It should be possible to get a GOEPE mixed emulsion without modification to increase dispersibility. Experiments are used to verify the possibility of this hypothesis.
GOEPE wet film, different concentrations of GO aqueous solution were added to the milky white water-based epoxy resin emulsionwas, formed on a glass plate and photographed under a 500x polarizing microscope. In order to more clearly observe the dispersion of GO in the sample, the measurement mass ratio of GO was selected to add 0.1 %, 0.2 %, 0.74 %(a,b,c of Fig. 2). The GOEPE dry film is made of the GOEPE emulsion on the slide and then dried in 105℃oven for constant weight. photographs were taken using a 500x polarizing microscope (d,e,f of Fig. 2). By observation and analysis, it indicated that GO in GOEPE wet film were distributed in WEP evenly. With the increase of GO concentration, the color of GOEPE wet film also became darker. In GOEPE dry film, GO were tiled and dispersed in water-based epoxy organic film. With the increase of GO concentration, the area covered by GO in WEP gradually increased (d, e, f of Fig. 2). When 0.74 wt% GO was added, GO is not evenly distributed in WEP and stacked.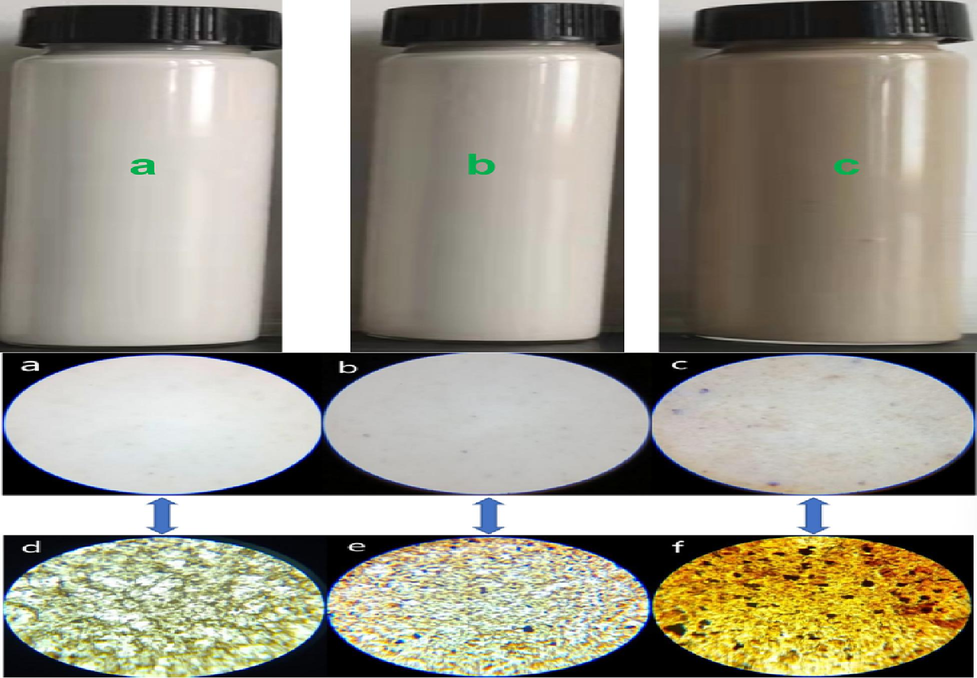
500X microscopic images of GOEPE dry and wet films with different GO contents, a. GOEPE wet films of 0.1 wt% GO. b. GOEPE wet films of 0.2 wt% GO. c. GOEPE wet films of 0.74 wt% GO. d. GOEPE dry film of 0.1 wt% GO. e. GOEPE dry film of 0.2 wt% GO. f. GOEPE dry film of 0.74 wt% GO.
This phenomenon can be well illustrates the proper concentration of GO well dispersed in the WEP. This is due to the presence of hydrophilic functional groups such as carboxyl, carbonyl, hydroxyl and epoxy groups on the edges and surfaces of GO sheets. So that it can dissolve in water and form a transparent uniform liquid. As a water-based coating, WEP also has a large number of epoxy groups, which is ‘similar phase solubility’ with GO, is conducive to the uniform dispersion of GO in WEP. The dispersion of GO in water-based epoxy resin is a key factor to improve the corrosion resistance of composite coatings. Which also proves the correctness of the previous theoretical hypothesis that a few GO can be dispersed in WEP. At the same time, the problem of graphene agglomeration in the coating system is solved and the densification of the coating is improved.
3.2 Mechanism of in-situ crosslinking reaction of GO&WEP resin to construct anticorrosive coating
In order to explore the mechanism of GO&WEP in situ crosslinking reactionto construct water-based heavy corrosion coating, polarizing microscope, XRD, FTIR and SEM were used to analyze the experiment.
The GOEPC coating film was obtained by adding diethylenetriamine to GOPEPE with different concentrations and curing at room temperature on the glass slide. The GOEPC was observed and photographed using a 500x polarizing microscope. As can be seen from Fig. 3, the surface of pure EPC without GO self-drying film at room temperature is full of bumps, which are uneven and not smooth (Fig. 3a). On the surface of GOEPC added with 0.1 wt% GO, the peripheral epoxy film with GO spreads and sinks, while the peripheral epoxy film without GO rises and floats. This is a phenomenon caused by the low concentration of GO, which is not enough to spread throughout the film (Fig. 3b). The GOEPC coating with 0.2 wt% GO has a smooth surface, and the GO sheets are laid flat in the water-based epoxy resin film, and the distribution is relatively uniform. (Fig. 3c). The surface of.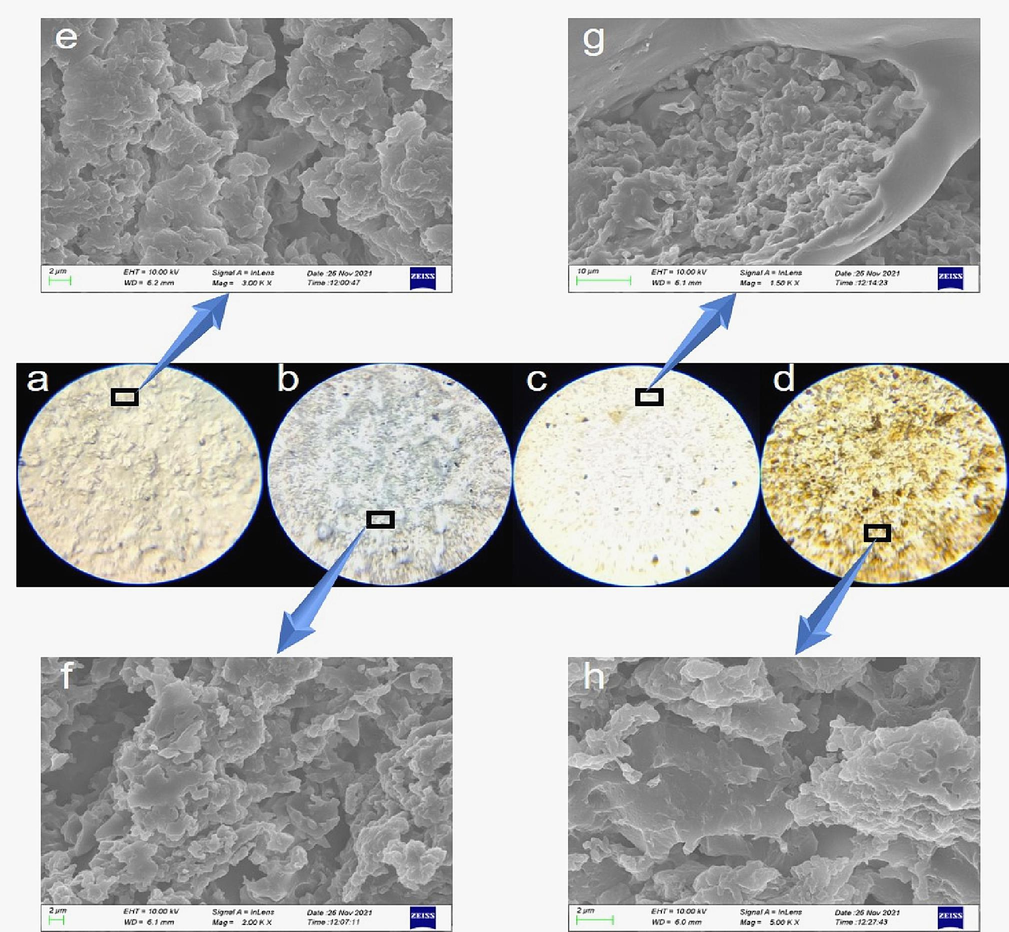
500X polarizing microscope and SEM images of GOEPC. a. Polarizing microscope view of EPC with 0% GO. b.Polarizing microscope view of GOEPC with 0.1 wt% GO. c. Polarizing microscope view of GOEPC with 0.2 wt% GO. d. Polarizing microscope view of GOEPC with 0.74 wt% GO. e. SEM image of EPC with 0%GO. f. SEM image of GOEPC with 0.1 wt% GO. g. SEM image of GOEPC with 0.2 wt% GO. h. SEM image of GOEPC with 0.74 wt% GO.
GOEPC coating with 0.74 wt% GO was tiled and stacked with GO, which was higher than that of water-based epoxy resin, resulting in rough surface of the coating, indicating that high concentration of GO was not conducive to smooth and compact coating(Fig. 3d). While e, f, g and h in Fig. 3 are microscopic morphology maps of GOEPC with different GO contents taken by SEM. After comparison, it can be found that the microscopic topography of GOEPC with 0.1 wt% GO is similar to that of pure EPC, and the particles are very loose and have obvious pores. However, when the amount of GO is added to 0.2 wt%, the microscopic morphology is obviously changed, the surrounding surface is more flat, the middle particle is more compact, and the encapsulation is good. However, when the amount of GO increased to 0.74.
wt%, it was found that the high concentration of GO would cause the phenomenon of GO stacking in WEP, resulting in rough coating. Fig. 3 proves that GO has strong surface activity in the water-based epoxy resin system, which can not only be better dispersed in WEP, but also better participate in crosslinking reaction, so as to make the coating more compact. But higher concentration of GO is not always better.
The XRD patterns of GO, EPC and GOEPC are shown in Fig. 4a. The GO obtained by Hummers reaction has a strong diffraction peak near 2θ of 12.6°, which is the characteristic diffraction peak of GO. This indicates that the crystal form of graphite has changed and a new crystal structure has been formed. According to Bragg's equation (2dsinθ = nλ), the layer spacing of GO is about 0.7 nm, more than double that of graphite. This is due to the formation of hydroxyl, carboxyl, epoxy and other oxygen-containing groups in the oxidation process, which increases the GO layer spacing. There is no obvious diffraction peak in the XRD pattern of pure EPC after curing. However, the XRD pattern of GOEPC after curing shows that a diffraction peak appears near 2θ of about 18°. However, the intensity of the diffraction peak weakens and becomes wider, and the characteristic diffraction peak of GO disappears.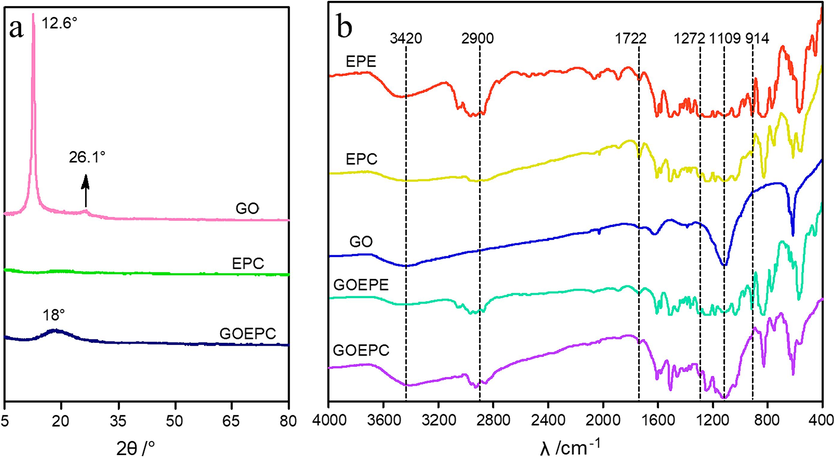
a. XRD patterns of pure water-based epoxy resin coating, graphene oxide and graphene oxide and water-based epoxy resin composite coating. b. Infrared spectrograms of water-based epoxy resin emulsion, water-based epoxy resin coating, graphene oxide, graphene oxide and water-based epoxy resin composite emulsion, and graphene oxide and water-based epoxy resin composite coating.
This indicates that the diffraction peak is a new structure of GO and WEP after the crosslinking reaction of diethylenetriamine. According to Bragg equation, the layer spacing of the new structure can be calculated to be about 0.49 nm, which is much smaller than that of GO. This is due to the acylation reaction between the carboxyl group of GO and diethylenetriamine and the addition crosslinking reaction between the epoxy group and diethylenetriamine.
Fig. 4b shows the infrared spectra of EPE, EPC, GO, GOEPE and GOEPC. From the infrared spectra of EPE, it can be observed that the absorption peak at about 2900 cm−1 is the stretching vibration peak of C–H in water-based epoxy resin. Around 914 cm−1 is the characteristic peak of the epoxy group in aqueous ring resin. As can be seen from the EPC in Fig. 4b, the characteristic peak of the epoxy group at 914 cm−1 disappears after curing of the water-based epoxy resin, which proves that the addition curing reaction between the water-based epoxy resin and diethylenetriamine leads to the ring-opening of the epoxy group in the original water-based epoxy resin (Z.J. Li et al., 2022; X.L. Liu et al., 2015). Meanwhile, the N–H absorption band corresponding to 3420 cm-1 should be the contribution of the N–H functional group that DETA participated in the crosslinking reaction. From the infrared spectrum of GO, GO has a broad absorption peak near 3430 cm−1, which is due to the stretching vibration peak of O–H in GO. The absorption peak at 1722 cm−1 is the stretching vibration peak of C = O of the carboxyl group (–COOH) on GO dry film. This peak also indicates that the GO dry film can not be completely dried after drying, but still contains water molecules, which is in line with the characteristics of GO. Moreover, the residual water molecules also have an impact on the absorption peak of 3430 cm−1. The strong absorption peak at 1272 cm−1 is the vibration absorption peak of C-O-C in GO. The peak near 1109 cm−1 is the characteristic peak of the epoxy group of GO (W. Zhu et al., 2020; J.G. Kohl et al., 2017; X.M. Zhu et al., 2019). This indicates that both water-based epoxy resin and GO have the same epoxy-group functional group and have ‘similar phase solubility’, so GO can be dispersed better in water-based epoxy emulsion. Compared with the infrared spectrum of GOEPC and GOEPE, the absorption peak of the epoxy group at 914 cm−1 disappeared, which confirmed the addition curing reaction between epoxy group ring-opening and diethylenetriamine. In addition, the absorption peak of the carboxyl group also disappeared, indicating that the carboxyl group and the amino group of diethylenetriamine underwent amidation reaction. It can be further demonstrated that GO and water-based epoxy resins can copolymerize with organic polyamines at the same time to realize in-situ construction of anticorrosive film.
Fig. 5 shows SEM images of pure water-based epoxy coating (EPC-X(X = 1,2)), GO dry film, and GO and water-based epoxy composite coating (GOEPC-X(X = 1,2,3,4)). From Fig. 5a, fracture cracks of EPC coating are parallel and relatively regular (as showed by the yellow line in the Fig. 5a), which are typical epoxy resin uneven polymerization process left wide gaps between the polymer aggregates. From the observation of the micro-surface of EPC coating in Fig. 5b, it is found that there are pores on the surface. This is caused by the evaporation of solvent water in the process of curing and film formation of EPC coating, which makes pores appear in the coating and reduces its ability to hinder corrosive substances. Fig. 5d shows SEM morphology of GOEPC composite coating constructed by in-situ crosslinking reaction of 0.06 g graphene oxide, 30 g water-based epoxy resin and 15 g diethylenetriamine under the same conditions. In the GOEPC composite coating, GO is basically evenly sandwiched between the epoxy resin polymer, and the GO lamellae is adhered to each other by the reaction with organic polyamine crosslinking agent to form a continuous phase (as showed in the yellow line in Fig. 5d). At the same time, it can be seen from the local magnification of the composite coating (Fig. 5f yellow circle) that GO and epoxy resin are connected together by crosslinking reaction with DETA at the same time, which makes up for the pore defects caused by the volatilization of WEP water and makes the composite coating more dense. As shown in Fig. 5e, the GO modified water-based epoxy composite coating has a morphology similar to that of the GO dry film shown in Fig. 5c, that is, the GO thin as a wing. This shows that GO does play a supporting role in the composite coating, and it also shows that GO has a good dispersion in WEP, forming a continuous film and significantly improving the compactness (Fig. 5g). The advantages of GO and epoxy resin are superposition, which makes up for the disadvantages of pure epoxy resin film forming alone, thus improving the heavy corrosion resistance of composite coating and making the composite coating more ductile. The GO was mixed evenly in water-based epoxy resin, and the diethylenetriamine crosslinking agent was added to cross-link and cure. The GO&WEP resin was crosslinked in-situ on tinplate plate and glass plate to construct an anti-corrosion coating. The construction mechanism of the anticorrosive coating can be revealed by the above polarizing microscope, XRD, FTIR and SEM characterization methods as follows mechanism diagram of GO&WEP and diethylenetriamine in-situ crosslinking reaction to construct anti-corrosion coating (Fig. 6). As an important derivative of graphene, GO has different amounts of hydroxyl, carboxyl and epoxy functional groups at different sites (as shown in Fig. 6a). In construction of the heavy anticorrosion coating, diethylenetriamine acts as an organic polyamine crosslinking agent to acylate the carboxyl group on GO. At the same time, diethylenetriamine will make the epoxy group of GO and water-based epoxy resin ring open, and further addition reaction occurs(Z.N. Yu, 1996; J. Zhou et al., 2019). In this way, GO can be crosslinked with water-based epoxy resin at the same time to form a continuous phase, which improves the shading of the coating, and thus improves its physical properties and heavy corrosion resistance. Fig. 6b shows the schematic diagram of the addition and curing of pure water-based epoxy resin molecules and diethylenetriamine molecules. The distribution of the solidified molecules is disorderly, resulting in pores on the surface of the solidified film, which is not dense enough. Fig. 6c is a diagram of the GO/epoxy in-situ crosslinked water-based resin heavy anticorrosive coating. In this coating construction process, the acylation reaction, its chemical reaction formula is shown in Fig. 7a, and addition reaction between GO and diethylenetriamine (shown in Fig. 7b) formed a larger molecular weight and continuous phase anticorrosive film. The corrosion resistance of the coating was further improved.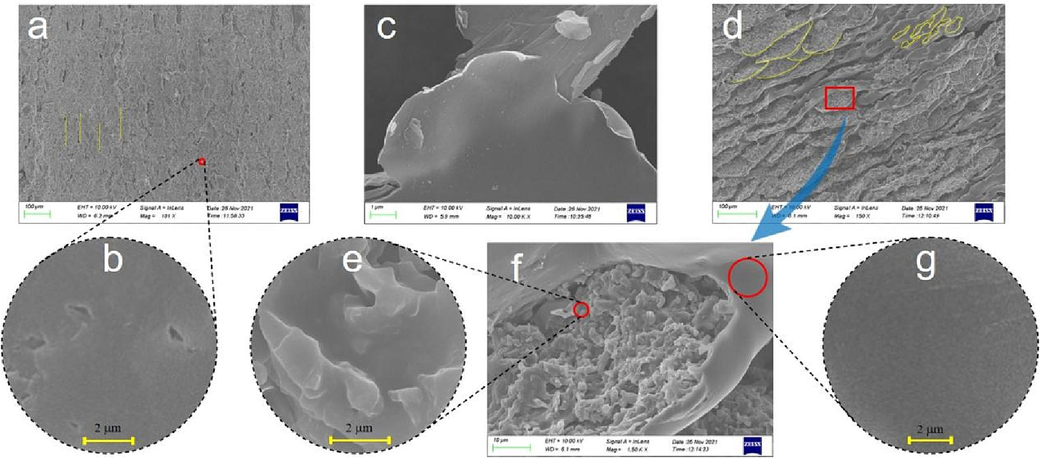
SEM images of pure water-based epoxy resin coating, GO dry film, and GO and water-based epoxy composite coating, a. EPC-1, b. EPC-2, c. GO, d. GOEPC-1, e. GOEPC-2, f. GOEPC-3, g. GOEPC-4.
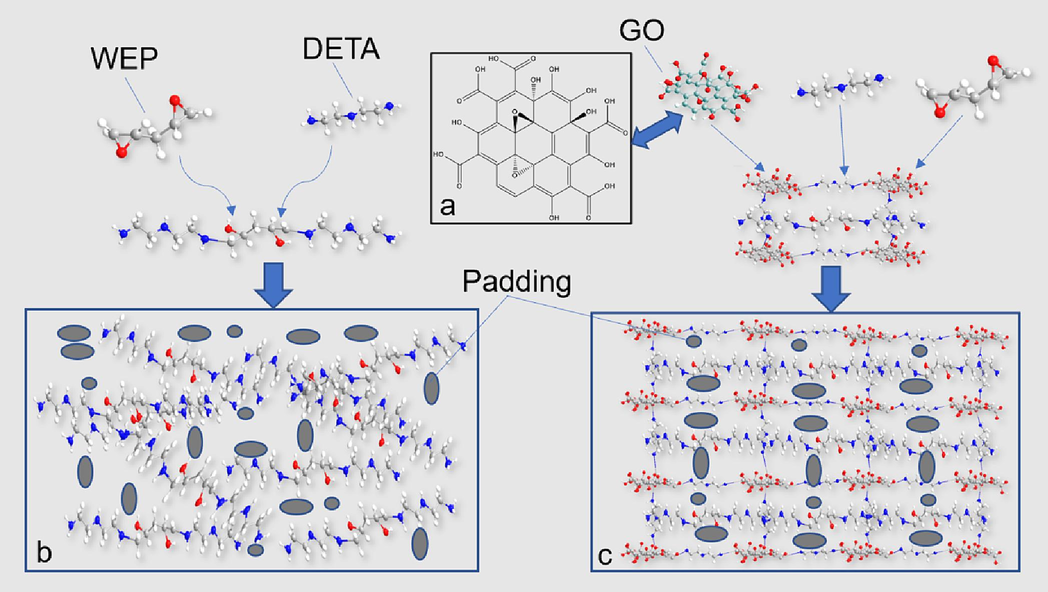
Mechanism diagram of GO&WEP and diethylenetriamine in-situ crosslinking reaction to construct anti-corrosion coating.
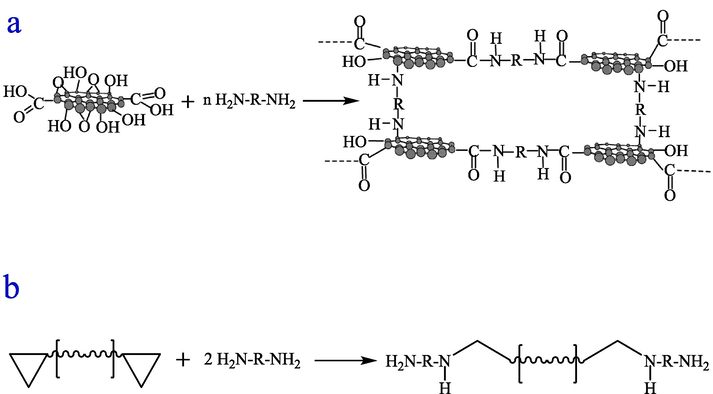
A. Cross-linking reaction between GO and organic polyamine cross-linking agent, b. Reaction equation of addition curing reaction between waterborne epoxy resin and organic polyamine cross-linking agent.
3.3 Effect of GO content on physical properties of WEP anticorrosive coating
3.3.1 Thermal conducting property
Fig. 8 shows the thermal conductivity of GOEPC composite coatings with different GO contents. The thermal conductivity of pure waterborne epoxy resin coating is 0.086 W·m−1·K−1. With the increase of GO content, on the whole, the thermal conductivity of GOEPC is getting larger and better. This is made possible by the fact that GO itself has very good thermal conductivity. When GO content is 0.025 wt%, the thermal conductivity is 0.108 W/(m·K), which increases by 25.6 % compared with pure water-based epoxy resin coating. When the GO content is 0.2 wt%, the thermal conductivity is 0.160 W/(m·K), the thermal conductivity increases by 86.0 %. Continue to increase the content of GO thermal conductivity is still increasing, but the growth rate becomes slow. This indicates that the thermal conductivity of the composite coating is better under the crosslinking reaction of GO and water-based epoxy resin with diethylenetriamine within a certain range. However, with the increasing GO content in the composite coating, the crosslinking reaction in the composite coating have reached saturation, so the thermal conductivity increases slowly. At the same time, too much GO is easy to cause agglomeration, which will affect the dispersion degree of GO in WEP.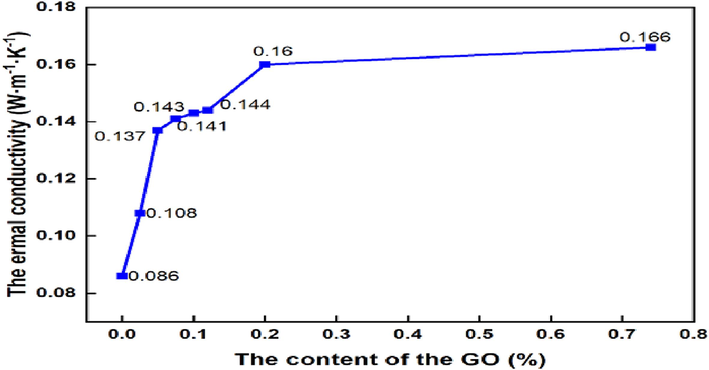
Thermal conductivity of GOEPC with different GO contents.
3.3.2 Hardness properties of coating film
Fig. 9 shows the pencil hardness grades of GOEPC composite coatings with different GO contents. The hardness of pure WEP coatings is 2B, but after adding 0.025 wt% GO, the hardness is increased by two grades directly to HB. Thus, GO plays a role in the composite coating, which also proves that GO and WEP cross-link with diethylenetriamine, resulting in higher hardness of the composite coating. More GO is added, the stiffness still increases, but the degree of increase decreases. This phenomenon is because the organic polyamine cross-linking agent added each time is quantitative, so the added GO cannot be all cross-link reaction with diethylenetriamine. Therefore, it can be proved that the cross-linking effect of GO and diethylenetriamine has a great influence on the hardness of the composite coating.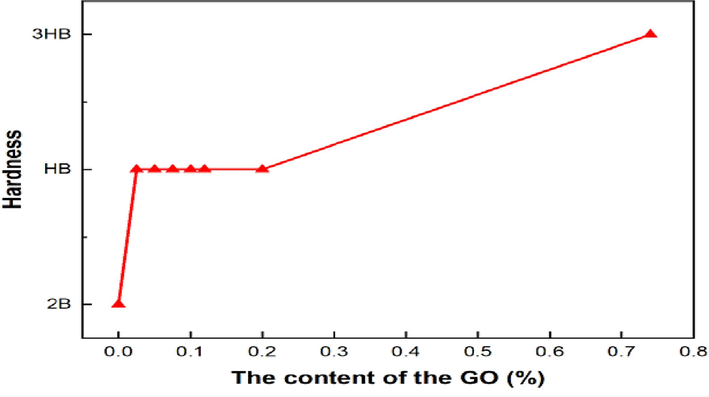
Pencil hardness of GOEPC with different GO contents.
3.3.3 Coating adhesive force properties
It can be seen from Fig. 10 the bar chart that the adhesive force of pure water-based epoxy resin is level 2, but the adhesive force is also level 2 when the amount of GO added is 0.025 wt%. This happens because the amount of GO added is small, and it is difficult to form a complete film after the reaction with diethylenetriamine. With the addition of GO concentration increased to 0.05 wt%, the hydroxyl group on its molecular structure and the nitrogen atom on the membrane molecular structure will adsorb to the metal in the form of chemical bonds, thus improving the adhesion of the anti-corrosion film, so the adhesion rises to level 1. The adhesion of the coating formed by GO cross-linking with diethylenetriamine is obviously better than that of the pure waterborne epoxy resin coating.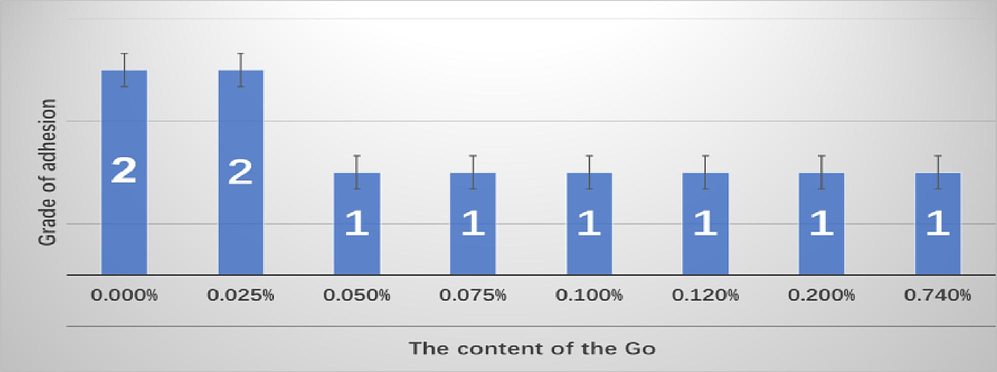
Coating adhesive force properties of GOEPC with different GO contents.
3.3.4 Test for resistance to neutral salt spray, salt water and kerosene
According to salt spray test(5% sodium chloride solution, the test water temperature is 30℃, the solution pH value is 6 ∼ 7), GOEPC composite coatings with different GO contents are shown in Fig. 11 after 7 days in the salt spray chamber. In the neutral salt spray resistance test, it was found that the pure EPC coating changed from colorless and transparent to white (Table 1), which was caused by the re-absorption of water in the dry film of the coating. But the addition of GO greatly prevented this the re-absorption of water phenomenon. Although the rust in Fig. 11 is due to the spread of corrosion products caused by the absence of coating on the back and side edges of the steel plate. The results of salt spray resistance test show that the composite coating formed by the crosslinking of GO, water-based epoxy resin and diethylenetriamine has good physical barrier effect, which greatly improves the corrosion resistance. Therefore, the composite coating formed by the crosslinking reaction of GO, water-based epoxy resin and diethylenetriamine is more suitable for the direction of heavy corrosion protection.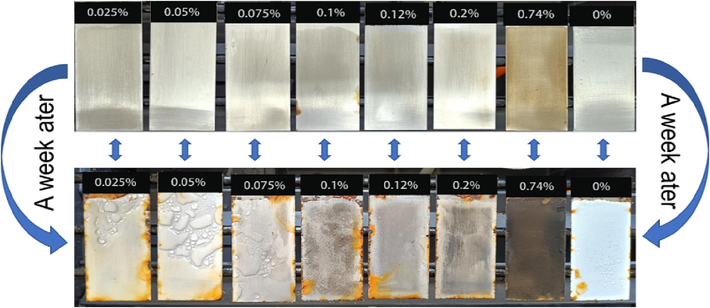
Variation of GOEPC neutral salt spray with different GO contents after 7 days.
The Table 2 also showed bulging and shedding phenomena of samples with different GO concentrations were immersed in 3 % saline and kerosene after four month. it was found that part of samples with 0.025 wt% and 0.050 wt% GO in saline did not bubble and fall off, the part exposed to saline began to bubble and shed. However, parts of the pure waterborne epoxy coating in the salt water began to bubble and peel off, and rust spots were seen forming on the tinplate under the coating. This indicates that the composite coating constructed by the crosslinking reaction of GO and waterborne epoxy resin with diethylenetriamine is more compact and has better shielding effect. Each group of samples in kerosene, has been soaked for more than four months, are intact. This indicates that the amount of GO added below 0.74 % has no effect on the kerosene resistance of the coating.
GO addition concentration
0.000
wt%0.025
wt%0.050
wt%0.075
wt%0.100
wt%0.120
wt%0.200
wt%0.740
wt%
Salt spray resistance (7 days)
No bulging, color from colorless to white
No bulging, slightly white color
No bulging, slightly white color
No bulging
No bulging
No bulging
No bulging
No bulging, dark color
Saline resistance (four months)
bulging
Small bulging
Small bulging
No bulging
No bulging
No bulging
No bulging
bulging
Kerosene resistance (four months)
No bulging
No bulging
No bulging
No bulging
No bulging
No bulging
No bulging
No bulging
3.3.5 Effect of GO content on electrochemical impedance of coating
For coatings containing 0 %, 0.1 %, 0.2 %, 0.4 % (mass concentration) GO, their electrochemical impedance spectra were tested using the three-electrode method, as shown in Fig. 12.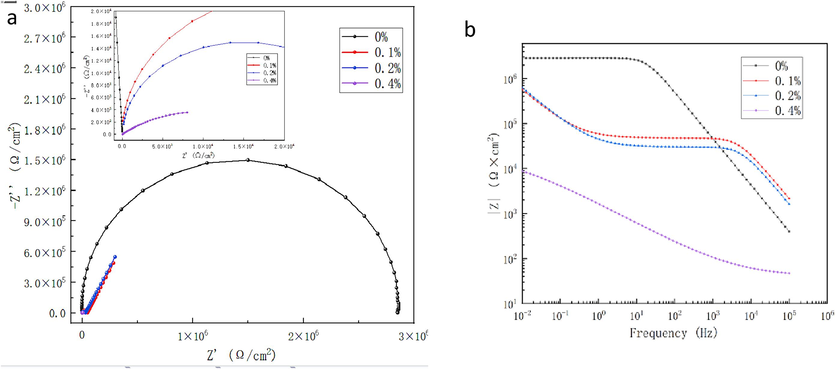
Electrochemical impedance spectra of aqueous epoxy coatings with different GO mass concentrations. (a) Nyquist diagram, (b) bode diagram.
It can be seen from Fig. 12a that the Nyquist diagram is obviously a capacitive reactance arc when GO is not added (0 %), and that 0.1 %, 0.2 %, and 0.4 % of GO are all diffusion lines, and the real part impedance of 0.4 % coating is lower than that of other coatings. As can be seen from the bode figure in Fig. 12b, the low-frequency starting impedances |Z|0.01 of the coatings with 0 %, 0.1 %, 0.2 %, and 0.4 %GO added are 2.85 × 106Ω·cm2, 5.65 × 105Ω·cm2, 6.25 × 105Ω·cm2, and 8.77 × 103Ω·cm2, respectively. The bode diagrams shows that the impedance modulus in the low frequency region of the coating with 0 % GO is significantly higher than that of the coatings with GO, while the impedance modulus of coatings with 0.1 % and 0.2 % GO is higher than coating with 0 % GO in the high frequency region. In addition, the |Z|0.01 of the coating with 0.4 % GO is 102 orders of magnitude lower than that of other coatings with GO content. This is because the impedance in the low frequency region is mainly determined by the resistance of the coating, the impedance in the high frequency region is determined by the capacitive reactance of the coating, and the high conductivity of GO reduces the resistance value of the coating. However, when the coating with 0.1 %-0.2 % GO is added, the composite coating after the cross-linking reaction of GO and water-based epoxy resin with organic polyamine crosslinker is more dense, the shielding effect is better, and the blocking ability of salt water erosion is improved than that without GO coating. When GO is added in excess, the excess GO is stacked and aggregated in the coating, which will reduce the density of the coating. This result is consistent with the results of other coating application performance tests.
4 Conclusion
In this paper, the dispersion of GO aqueous solution in WEP and the changes of structure and physical properties of in-situ coatings constructed by adding different contents of GO were studied and reveal the reaction mechanism of crosslinking reaction of GO&water-based epoxy resin and diethylenetriamine to construct anti-corrosion coatings. Comparing the molecular structure of the GO and WEP by polarizing microscope, SEM, FTIR, XRD. Proved GO has the same oxygen-containing functional groups with waterborne epoxy resin, which is‘similar miscibility’. Therefore, a few GO in waterborne epoxy resin with good dispersion. Characterization test of the coating application performance were by the thermal conductivity tester, pencil hardness tester, adhesion tester, salt spray corrosion test chamber, salt water resistance test, kerosene resistance test and EIS. The results showed that dilute concentration of GO aqueous solution could be uniformly dispersed within WEP emulsion. Oxygen functional groups on moleculars structure of GO&WEP crosslinked with diethylenetriamine forming a continuous phase film which improved the density of the coating. The coatings prepared by adding the optimum mass ratio of 0.1–0.2 % GO to WEP have the best physical properties and anti-corrosion properties. When the GO content is 0.2 wt%, the thermal conductivity increases by 86.0 %. After added 0.025 wt% GO, the coating hardness is increased by two grades directly from 2B to HB. With the addition of GO concentration increased to 0.05 wt%, the adhesion rises from 2 to level 1. The composite coating formed by the crosslinking of GO, water-based epoxy resin and diethylenetriamine has good physical barrier effect, which greatly prevented the re-absorption of water phenomenon and improves the corrosion resistance. When the GO content exceeds 0.05 %, the coating will not bubble off after 4 months of salt water immersion experiment. The amount of GO added below 0.74 % has no effect on the kerosene resistance of the coating.The addition of GO will improve the conductivity of the WEP coating and the shielding against corrosive media. This study provide a new method and theoretical basis for the preparation of graphene anti-corrosion coating.
Research funding
This project was supported by National Natural Science Foundation of China (Grant 22268046), Science and Technology Project of Yulin City (Grant CXY-2021–106-01).
CRediT authorship contribution statement
Xiaodong Chen: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Zhan Qu: Writing – original draft, Investigation, Formal analysis. Mihui Xie: Investigation. Meiju Zhang: Writing – original draft, Investigation. Jiannan Ai: Investigation. Guoyu Ren: Investigation. Yanli Gao: Investigation. Yonglin Yang: Writing – review & editing.
Acknowledgments
The authors express their gratitude to the National Natural Science Foundation of China (Grant 22268046), Science and Technology Project of Yulin City (Grant CXY-2021-106-01).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Polypyrrole doped graphene oxide reinforced epoxy nanocomposite with advanced properties for coatings of mild steel. J. J. Polym. Res.. 2019;26:244.
- [Google Scholar]
- Bio-inspired superior barrier self-healing coating: Self-assemble of graphene oxide and polydopamine-coated halloysite nanotubes for enhancing corrosion resistance of waterborne epoxy coating. J. Prog. Org. Coat.. 2020;139:105402
- [Google Scholar]
- Dhamodharan, D. , Dhinakaran, V. , Nagavaram, R. , Ghoderao, P. P. , H.-S., B. , Wu, L. . 2022. Experimental and numerical study on smectic aligned zirconium phosphate decorated graphene oxide hybrids effects over waterborne epoxy multi-functional properties enhancement. J. Ind. Eng. Chem. (107-), 107.
- Properties of TDE-85 epoxy resin modified with carboxyl-terminated liquid nitrile rubber. J. China Synthetic Rubber Ind.. 2014;37:11-13.
- [Google Scholar]
- Novel anticorrosive emulsion-type paints containing organic/inorganic nanohybrid particles. J. Prog. Org. Coat.. 2018;77:548-556.
- [Google Scholar]
- Amorphous cellulose edge-functionalized graphene oxide for anticorrosive reinforcement of waterborne epoxy coatings. Appl. Surf. Sci.. 2023;616:156576-.
- [Google Scholar]
- Heavy-duty coatings in China and its development trend. J. China Coat.. 2014;29:13-18.
- [Google Scholar]
- Advanced anti-corrosion coatings prepared from α-zirconium phosphate/polyurethane nanocomposites. J. RSC Adv.. 2017;7:9908-9913.
- [Google Scholar]
- Exfoliation and functionalization of α-zirconium phosphate in one pot for waterborne epoxy coatings with enhanced anticorrosion performance. J. Prog. Org. Coat.. 2020;138:105390
- [Google Scholar]
- Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-Benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM) J. Corros. Sci.. 2018;145:119-134.
- [Google Scholar]
- Adhesion of Epoxy (Pseudobarnacles) to Glass that has been Treated with Hydrophobic Carbosilane-Based Coatings. J. Prog. Org. Coat.. 2017;107:1-4.
- [Google Scholar]
- A novel silk fibroin-graphene oxide hybrid for reinforcing corrosion protection performance of waterborne epoxy coating. J. Colloids Surf. A: Physicochem. Eng. Asp.. 2022;634:127959
- [Google Scholar]
- Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating – I. High-temperature ball milling treatment. J. Corros. Sci.. 2015;90:463-471.
- [Google Scholar]
- Research progress and development trend of environment-friendly waterborne coatings. J. China Adhes.. 2019;28:5-60.
- [Google Scholar]
- Preparation and properties of chromium-free insulation coating for grain-oriented silicon steel. J. Electroplat. Finishing. 2015;34:1275-1281.
- [Google Scholar]
- Chromium-free inorganic/organic composite passivation film on surface of galvanized steel. J. Electroplat. Finish.. 2013;32:37-42.
- [Google Scholar]
- Research status and development trend of environmental friendly waterborne coatings. J. Coat. Protect.. 2021;42 24–29+46
- [Google Scholar]
- Research progress of graphene anticorrosive coating. J. Inorg. Chem. Ind.. 2021;53:30-35.
- [Google Scholar]
- Phytic Acid Intercalated Graphene Oxide for Anticorrosive Reinforcement of Waterborne Epoxy Resin Coating. J. Polymers. 2019;11:1950.
- [Google Scholar]
- Coating Technology (in Chinese). Chemical Industry Press; 1996.
- Electrostatic self-assembly of Zn 3 (PO 4) 2 /GO composite with improved anticorrosive properties of water-borne epoxy coating. J. Inorg. Chem. Commun.. 2022;119:108015
- [Google Scholar]
- Preparation, structure control and application of graphene oxide. J. Chem. Ind. Eng. Prog.. 2020;39:4061-4072.
- [Google Scholar]
- Graphene oxide modification and its application in waterborne epoxy resin. J. China Adhes.. 2019;28:30-33.
- [Google Scholar]
- Silk fibroin-graphene oxide functionalized melamine sponge for efficient oil absorption and oil/water separation. J. Appl. Surf. Sci.. 2019;497(C):143762.1-143762.8.
- [Google Scholar]
- In-situ grafted graphene oxide-based waterborne epoxy curing agent for reinforcement corrosion protection of waterborne epoxy coating. J. Surf. Coat. Technol.. 2021;412:127043
- [Google Scholar]
- Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance. J. Prog. Org. Coat.. 2020;140:105488
- [Google Scholar]
- Effect of TZVCC drying temperature on the adhesion performance of the epoxy coating on AA6063. J. Adhes.. 2020;96:565-579.
- [Google Scholar]
- In-situ modulation of interactions between polyaniline and graphene oxide films to develop waterborne epoxy anticorrosion coatings. J. Prog. Org. Coat.. 2019;26:244.
- [Google Scholar]
- Orientation of Ultrathin alpha-ZrP Nanosheets in Aqueous Epoxy Resin for Anticorrosive Coatings. J. ACS Appl. Nanomater.. 2021;4:5413-5424.
- [Google Scholar]
- Preparation and properties of graphene oxide modified waterborne epoxy anticorrosive coatings. J. Chem. Eng.. 2019;33:1-4.
- [Google Scholar]







