Translate this page into:
Unveiling the phytochemical profile and antioxidant activity of roots from six Polygala species
⁎Corresponding authors at: Key Laboratory for Research of “Qin medicine” of Shaanxi Administration of Traditional Chinese Medicine, Shaanxi Qinling Application Development and Engineering Center of Chinese Herbal Medicine, College of Pharmacy, Shaanxi University of Chinese Medicine, Xi’an 712046, China. bingyyang@126.com (Bingyue Yang), ppengliang@126.com (Liang Peng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The genus Polygala (Polygalaceae), comprising about 670 species worldwide, has more than 40 species in China. The roots of Polygala tenuifolia Willd., Polygala sibirica L., and Polygala japonica Houtt. are recorded in the Chinese Pharmacopeia. Other closely related plants of the Polygala genus are also widely used in the folk medicine of southern China, such as Polygala fallax Hemsl., Polygala arvensis Willd., and Polygala glomerata Lour. To systematically compare the chemical compositional and elucidate characteristic compounds among the Polygala genus, six Polygala species, namely P. tenuifolia, P. sibirica, P. japonica, P. fallax, P. arvensis and P. glomerata, underwent comprehensive phytochemical studies using the ultra performance liquid chromatography-quadrupole time-of-flight tandem mass Spectrometry (UPLC-Q-TOF-MS/MS) technique, resulting in the identification of 154 compounds, consisting of 62 oligosaccharide esters (40.26 %), 58 saponins (37.66 %), 29 xanthones (18.83 %), and 5 other chemicals (3.25 %). Based on the Masslynx computational tool, a comprehensive comparative phytochemical profiling was achieved and revealed differences in composition among the six Polygala species. P. sibirica exhibited higher levels of specific compounds and was more closely related to P. tenuifolia. Meanwhile, P. japonica showed greater similarity to P. fallax, P. arvensis, and P. glomerata. Oligosaccharide esters and triterpene saponins were more abundant in P. sibirica and P. tenuifolia than in other species. Furthermore, the antioxidant activities (1,1-diphenyl-2-acrylohydrazide [DPPH], 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt [ABTS], copper ion reducing antioxidant capacity [CUPRAC], and ferric ion-reducing antioxidant power [FRAP]) of the six species were evaluated. Among them, P. tenuifolia demonstrated superior antioxidant potential, with a lower half-maximal inhibitory concentration (IC50) value for scavenging DPPH radicals and excellence in CUPRAC (119.85 ± 16.28 μM/0.01 g) compared to other plants. P. japonica showed higher ABTS (98.94 ± 0.03 μM/0.01 g) and FRAP (42.58 ± 0.08 μM/0.01 g). Total flavonoids showed remarkable antioxidant capabilities among the six medicinal plants. Total saponins and phenolics also contributed to the antioxidant potential of these plants. The systematic data and results obtained may provide methodological support for further evaluating the potential use of folk Polygala plants as new effective materials in various commercial sectors, including food, cosmetic, and pharmaceutical industries.
Keywords
Polygala L
UPLC-Q-TOF-MS
Chemotaxonomy
Antioxidant
1 Introduction
Polygala L. is a prominent genus within the Polygalaceae family, comprising 673 species (World Flora Online, 2020) distributed worldwide, with the exception of Antarctica, Polynesia, and New Zealand (Marie et al., 2020). In regions such as China, Korea, and Japan, numerous species of this genus have been extensively utilized in herbal medicine for the treatment of various ailments, including insomnia, diabetes, Alzheimer's disease, neurasthenia, memory impairment, hypertension, and other conditions (Xin et al., 2020). Multiple studies have demonstrated the remarkable antioxidant, anti-inflammatory, anti-tumor, and immune-boosting properties of Polygala L. (Li et al., 2022, Zhang et al., 2020).
Traditional Chinese Medicine (TCM) and folk medicine have long employed approximately 40 different Polygala species, some with a rich historical background dating back to thousands of years (Yang et al., 2022). These medicinal plants are widely recognized and consumed as a safe and edible treatment for various neurodegenerative diseases, including Parkinson's and Alzheimer's diseases (Wang et al., 2019). P. tenuifolia is primarily distributed in China, South Korea, and Russia, serves as the original source of Polygalae Radix (known as Yuanzhi in Chinese and Onji in Japanese) (Jiang et al., 2016). Similarly, P. sibirica, another officially authorized source of Polygala radix in the Chinese Pharmacopoeia (Chinese, 2020), is widely used in both China and Japan as a TCM known for its tonic, sedative, and expectorant properties (Zhou et al., 2014). Among the genus Polygala L., P. japonica is a perennial herbaceous plant found in China, Vietnam, and Philippines, and has been historically employed in Vietnamese traditional medicine for treating amnesia and bronchitis (Li et al., 2012, Tran et al., 2018). As traditional ethno-herbals, P. fallax is commonly used in folk medicine by ethnic minorities in the Guangxi Zhuang Autonomous Region and has gained popularity as a clinical treatment for chronic diseases (Yao et al., 2020, Wu et al., 2023). P. arvensis is also one of the folk herbs traditionally used to treat jaundice, diabetes, and wound healing (Mane et al., 2022). P. glomerata, widely distributed in southern China, and has long been used to treat acute tonsillitis, stomatitis, and other inflammatory conditions, including myelitis, hepatitis pharyngitis, tuberculosis, esophageal cancer, and whooping cough (Li et al., 2014).
Over the past decades, extensive phytochemical studies have successfully identified and isolated more than 180 compounds from the roots or whole plants of Polygala species, primarily saponins, oligosaccharide esters and xanthones (Li et al., 2021, Zhao et al., 2020). Several analytical methods have been successfully applied to the analysis of the chemical composition of medicinal plants of the genus Polygala, such as UPLC/Q-TOF MS, UPLC-HRMS/MS, DI-MS/MSALL® (Li et al., 2021, Yang and Lang, 2023, Zhang et al., 2014). Among the numerous compounds found in Polygala plants, oligosaccharide esters play a crucial role in dementia prevention and brain protection (Ba et al., 2019). Pharmaceutical studies have reported the anti-inflammatory effects of saponins, along with their potential to treat anxiety, insomnia, and depression (Zhao et al., 2020). Meanwhile, xanthones represent significant components in possessing strong cytoprotective properties and potentially contributing to the protective effects on neurons in neurodegenerative diseases (Klein et al., 2012, İhsan et al., 2023). Traditional medicine remains essential for maintaining good health worldwide, especially in developing countries, accompanying a growing interest in exploring new plant materials containing bioactive compounds (Jeanette and Brijesh, 2018). However, there is limited information about the chemical composition of P. fallax, P. arvensis, and P. glomerata, and the specific substances responsible for their biological activity are unclear. Moreover, differences in the types and relative contents of chemical constituents among medicinal plants of the Polygala genus have not been extensively studied.
In this study, the ultra performance liquid chromatography-quadrupole time-of-flight tandem mass Spectrometry (UPLC-Q-TOF-MS/MS) technique was utilized to identify and characterize the primary saponins, oligosaccharide esters, xanthones and other chemicals in six Polygala species. Additionally, total phenolics, total flavonoids, total saponins, as well as the in vitro antioxidant activities (1,1-diphenyl-2-picrylhydrazide [DPPH], 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt [ABTS], copper ion reducing antioxidant capacity [CUPRAC], ferric ion-reducing antioxidant power [FRAP]) of these components, were evaluated. These findings contribute to evaluating Polygala raw materials as potential novel resources for applications in diverse commercial sectors, including food, cosmetics, and pharmaceuticals.
2 Materials and methods
2.1 Plant materials
All samples were collected in June 2022 in China. Among them, P. tenuifolia was collected from the standardized cultivation in Yulin City, Shaanxi Province; P. sibirica and P. japonica were harvested in Baoji City, Shaanxi Province. P. fallax, P. arvensis, and P. glomerata were collected by local medicinal plant growers in Guilin City, Guangxi Zhuang Autonomous Region; Guiping City, Guangxi Zhuang Autonomous Region; and Shaoguan City, Guangdong Province, respectively. All six species of the genus Polygala were studied based on their roots. The formal identification of samples in this study was undertaken by Prof. Xinjie Yang (Shaanxi Museum of TCM). Voucher specimens were deposited at the Shaanxi Qinling Application Development and Engineering Center of Chinese Herbal Medicine, Shaanxi Province under voucher specimen numbers QLZCY-PT-220615, QLZCY-PS-220622, QLZCY-PJ-220622, QLZCY-PF-220625, QLZCY-PA-220610 and QLZCY-PG-220626 (Table 1).
Name
Longitude
Latitude
Altitude (m)
Collected Time
Voucher specimens numbers
P. tenuifolia
110°18′23″
38°04′09″
1109.20
15-June 2022
QLZCY-PT-220615
P. sibirica
107°17′15″
33°59′41″
1302.55
22-June 2022
QLZCY-PS-220622
P. japonica
107°32′52″
34°03′45″
910.25
22-June 2022
QLZCY-PJ-220622
P. fallax
110°49′17′′
24°25′30′′
453.30
25-June 2022
QLZCY-PF-220625
P. arvensis
109°54′12′′
23°33′17′′
352.60
10-June 2022
QLZCY-PA-220610
P. glomerata
113°40′36′′
25°03′10′′
105.50
26-June 2022
QLZCY-PG-220626
2.2 Preparation of extracts
The roots from six Polygala species were passed through a No. 5 sieve, and 200 mg was extracted with 70 % methanol (10 mL) by sonication (300 W) at room temperature for 45 min. After ultrasonic-assisted extraction, the mixture was centrifuged at 12,000 rpm for 10 min. The supernatant was filtered by a 0.22 µm microporous filter membrane, and then the filtrate was used for chromatographic analysis. A total of 30 samples from six groups of P. tenuifolia (PT1-PT5), P. sibirica (PS1-PS5), P. japonica (PJ1-PJ5), P. fallax (PF1-PF5), P. arvensis (PA1-PA5), and P. glomerata (PG1-PG5) groups were used for the experiment.
2.3 UPLC-Q-TOF-MS/MS system for analysis of six plants
Liquid chromatography analysis was performed on a Waters Acquity UPLC System equipped with a Waters ACQUITY UPLC BEH C18 column (100 mm × 4.6 mm, 1.7 μm). The volume flow rate was set at 0.3 mL/min, and the column temperature was maintained at 40 °C. The injection volume was 2 μL. A gradient elution program was employed using 0.1 % formic acid (A) and acetonitrile (B) as solvents. The elution program comprised the following time intervals: 0–20 min (92 % B), 20–40 min (70 %-68 % B), 40–45 min (68 %-50 % B), 45–50 min (50 %-0% B), 50–51 min (0 %-95 % B), and 51–55 min (95 % B).
Ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-Q-TOF-MS) was performed using an electrospray ion source (ESI) in negative ion mode. The scanning range for mass-to-charge ratio (m/z) was set from 50 to 2000. The drying gas and cone gas flow rates were maintained at 600 and 50 L·h−1. The desolvation gas and ion source temperatures were set at 250 °C and 100 °C. The capillary and cone hole voltages were set at 2.5 kV and 40 V. The collision-induced dissociation (CID) energy is 40 V. Peak areas were extracted using MassLynx software for data export.
China National Knowledge Infrastructure (CNKI), PubMed, and SciFinder databases were consulted to search for the relevant literature of the Polygala genus, and a database containing information on compound names, molecular formulae, molecular masses, and characteristic secondary fragment ions was established. The obtained raw mass spectrometry data were imported into MassLynx software, and the compounds were identified by confirming the chemical compositions of the extracted mass spectrometry information from the total ion flow diagrams with the data in the constructed database and checking the sequence of the retention time according to the polarity magnitude. Finally, a total of 154 components were identified from six medicinal plants of the Polygala genus in negative ion mode.
2.4 Antioxidant assay
2.4.1 Total phenolics, total flavonoids and total saponins
The roots (2.0000 g) of all samples were extracted three times by 60 % ethanol (v/v), each time for 30 mL, and combine the extracts for the determination. The total phenolics content was determined based on Rojas-Ocampo et al. (Rojas et al, 2021) with appropriate adjustments. The total phenolic content was determined by the Folin-Ciocalteu method. Gallic acid (98 %, Shanghai Yuanye Biological Co.) was used as the standard. The absorbance value was detected at 765 nm using a microplate reader (A-5082, Tecan Austrla Gmbh Untersbergetr), and the magnitude of the absorbance value was proportional to the content. The total phenolics content was expressed as Gallic acid equivalents (GE)/g dry weight (DW).
The total flavonoids content was determined using the aluminum nitrate colorimetric method of El Atki et al. (El Atki et al, 2019) with appropriate adjustments. The absorbance of the mixture was measured at 420 nm, and the content of total flavonoids was calculated using rutin (98 %, Shanghai Yuanye Biological Co.) as the standard, with the total flavonoids content expressed as rutin equivalents (RE)/g DW.
The roots (2.0000 g) of all samples were extracted three times by distilled water (v/v), each time for 50 mL, combined and extracted 4 times with an equal volume of water-saturated n-butanol solution. The n-butanol solution was recovered, dried and concentrated in 50 mL of methanol. Based on the determination of total saponins content by Luo et al., the sample to be tested was precisely absorbed 200 μL, and then 200 μL of 5 % vanillin-glacial acetic acid solution and 800 μL of perchloric acid were added to the sample (Luo et al., 2024). The sample was bathed in 60 °C water for 15 min, removed, cooled for 5 min, diluted with 5 mL of glacial acetic acid, shaken, stood for 15 min, and the absorbance was measured at 576 nm. The content of total saponins was calculated using tenuifolin (98 %, Chengdu Efa Biotechnology Co.) as the standard, and the total saponins content was expressed as Tenuifolin equivalents (TE)/g DW. All standard compounds were dissolved in methanol.
2.4.2 Evaluation of antioxidant capacity
The assays for DPPH, the ABTS radical scavenging rate, CUPRAC, and FRAP, were performed according to the methodology of the previous research of the subject group with appropriate adjustments (Ji et al., 2023). For half-maximal inhibitory concentration (IC50) value, extract samples were made in various concentrations. The DPPH IC50 was calculated using CurveExpert 2.0.
2.5 Statistical analysis
For each experiment, three samples were analyzed with n = 3 repetitions, and the final results are presented as mean ± standard deviation (SD) in the tables. One-way analysis of variance (ANOVA) followed by S-N-Ka test at P<0.05 was conducted. Pearson correlation coefficient and heatmap between antioxidant activity and the samples' total phenolics, total saponins, and total flavonoids levels were analyzed using SPSS 26.0 and Chiplot. The data were entered into SIMCA analysis software and subjected to multivariate statistical analyses such as principal component analysis (PCA), Partial least squares-discriminant analysis (PLS-DA), and orthogonal partial least squares-discriminant analysis (OPLS-DA).
3 Results and discussion
3.1 Identification of chemical constituents by UPLC-Q-TOF-MS/MS
UPLC-Q-TOF-MS/MS analysis was conducted in the negative ion mode to identify the chemical constituents of six medicinal plants from the Polygala genus. This mode was chosen for its better sensitivity in detecting a broad spectrum of compound classes, including oligosaccharide esters, xanthones, and saponins (Li et al., 2021). As shown in Fig. 1, base peak chromatograms of 70 % methanol extracts from the six Polygala species were obtained.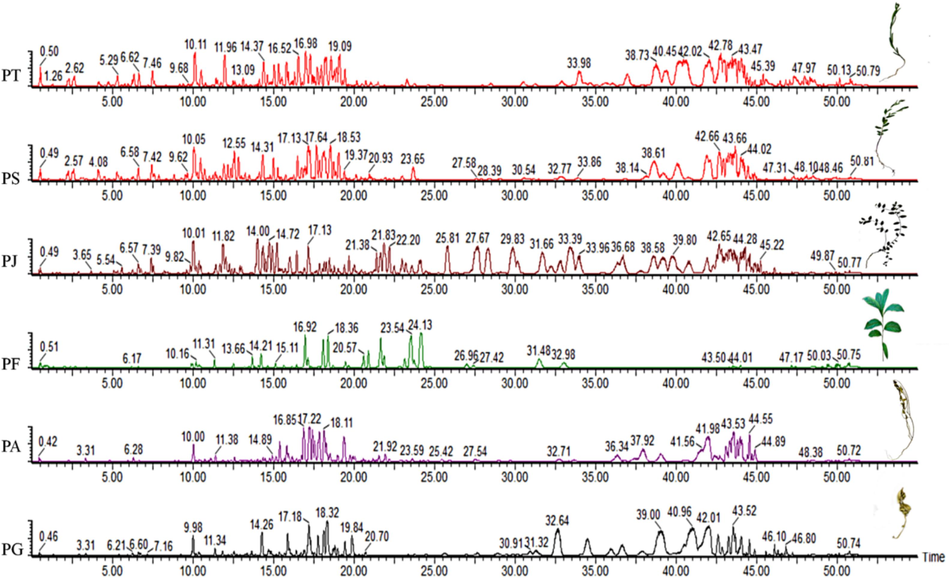
UPLC-Q-TOF-MS/MS base peak chromatograms of methanol extracts from six species in the negative ion mode. PT, P. tenuifolia; PS, P. sibirica; PJ, P. japonica; PF, P. fallax; PA, P. arvensis; PG, P. glomerata.
Oligosaccharides are widely distributed in plants, renowned for their antioxidant, anti-aging, and antibacterial properties, and hold considerable application value across cosmetics, pharmaceuticals, and the food industry. Xanthones, characteristic compounds of the Polygala genus, were found in numerous Polygala plants. Saponins emerged as the most prevalent secondary metabolites in Polygala species. As we all know, the roots of Polygala plants commonly contain numerous saponins, oligosaccharide esters, and xanthones.
The UPLC-Q-TOF-MS/MS analysis of 70 % methanol extracts from the roots of the Polygala species revealed a total of 154 compounds (Table S1, Supporting Information). These compounds comprised 62 oligosaccharide esters (40.26 %), 58 saponins (37.66 %), 29 xanthones (18.83 %), and 5 other chemicals (3.25 %). Among the species, P. tenuifolia, contained 114 compounds, including 43 oligosaccharide esters (37.72 %), 43 saponins (37.72 %), 25 xanthones (21.93 %), and 3 other chemicals (2.63 %). P. sibirica contained 103 compounds, including 43 oligosaccharide esters (41.75 %), 33 saponins (32.04 %), 23 xanthones (22.33 %), and 4 other chemicals (3.88 %). P. japonica contained 70 compounds, including 29 oligosaccharide esters (41.43 %), 21 saponins (30.00 %), 17 xanthones (24.29 %), and 3 other chemicals (4.28 %). P. fallax contained 57 compounds, including 25 oligosaccharide esters (43.86 %), 15 saponins (26.32 %), 13 xanthones (22.80 %), and 4 other chemicals (7.02 %). P. arvensis contained 70 compounds, including 27 oligosaccharide esters (45.00 %), 13 saponins (21.67 %), 18 xanthones (30.00 %), and 2 other chemicals (3.33 %). P. glomerata contained 68 compounds, including 23 oligosaccharide esters (33.82 %), 27 saponins (39.71 %), 15 xanthones (22.06 %), and 3 other chemicals (4.41 %). Oligosaccharide esters and saponins emerged as the predominant compounds across the six medicinal plants, followed by xanthones and carbohydrates. These findings, depicted in Fig. 2, were consistent with previous reports on the chemical composition of Polygala species (Feng et al, 2018, Sun et al, 2023).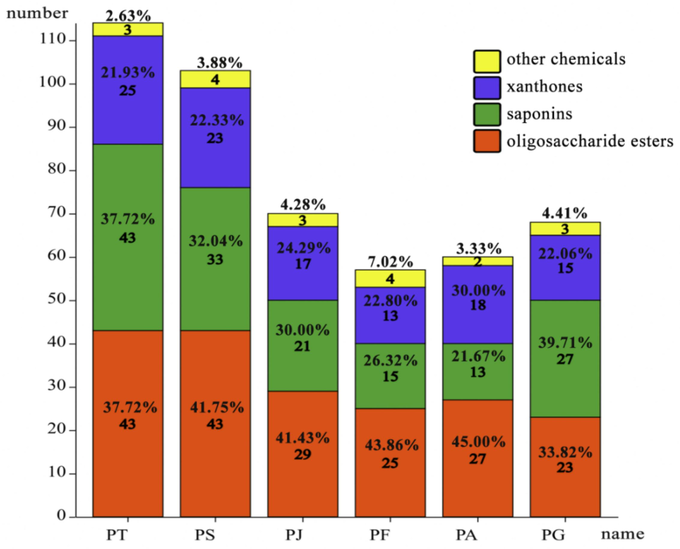
Classification of compounds of six Polygala species.
3.1.1 Saponins
The basic parent nucleus of the triterpene saponins in Polygala species was the oleanolane-type pentacyclic triterpenoid. The cleavage of glycosidic bonds resulted in the sequential neutral loss of glucose (Glc), fucose (Fuc), rhamnose (Rha), xylose (Xyl), apigenin (Api), galactose (Gal), and H2O and CO2, ultimately yielding fragmentation ions m/z 455. Further loss of CH2O produced fragment ions at m/z 425. The diagnostic fragment ions of m/z 455 and 425 were used to screen out saponins, with a tolerated offset of 10 mDa. Secondly, the precursor ion was confirmed. The neutral loss was applied to pursue those components bearing featured substitution.
As one of the diagnostic compounds, compound 46 (Rt = 2.732 min) was identified as tenuifolin. In detail, m/z 679 was assigned as the deprotonated molecular ion [M−H]−, and the molecular formula was calculated as C36H56O12. When the ion population within the m/z 679–680 mass window, actually m/z 679 ion cluster, entered the collision chamber, the primary fragment ions were observed at m/z 547, 503, 461, 277, and 161, respectively. The structure of tenuifolin as well as the proposed mass cracking pathways in response to those primary fragment ion species are shown in Fig. 3A.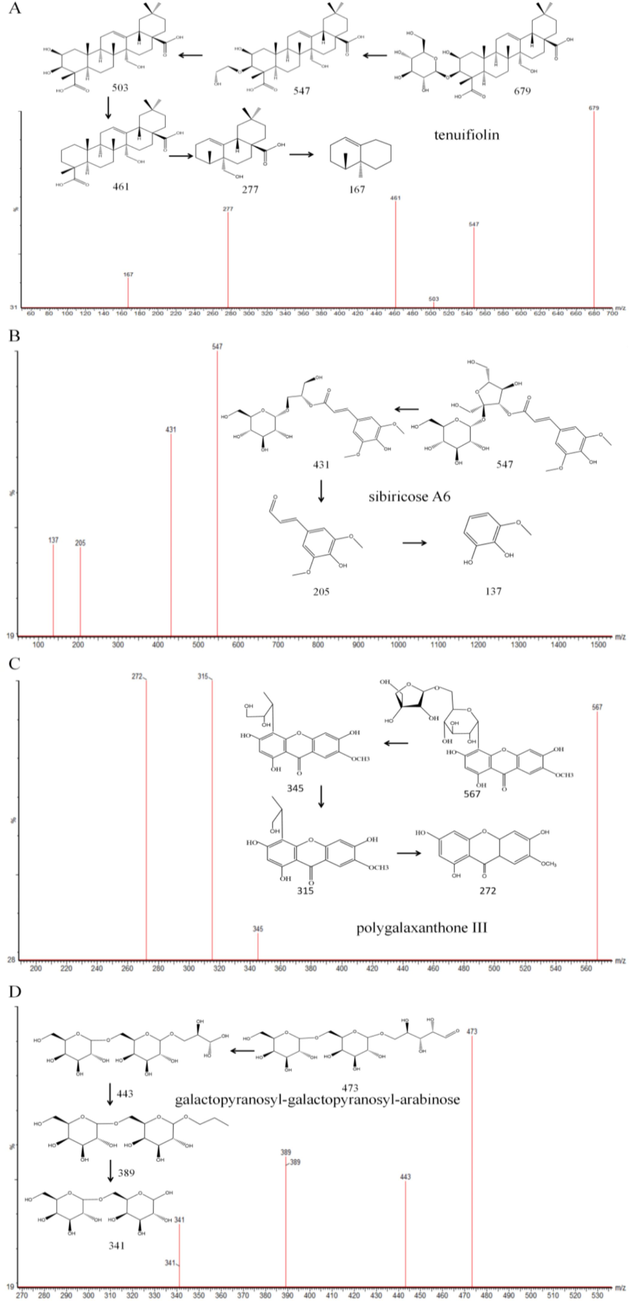
Possible cleavage pathways of four typicla compounds: (A) tenuifiolin, (B) sibiricose A6, (C) polygalaxanthone III, (D)galactopyranosyl-galactopyranosyl-arabinose.
3.1.2 Oligosaccharide esters
Oligosaccharide esters are a class of biologically active compounds in Polygala species. Greater structural diversity occurs for oligosaccharide esters, although most of them are derived from the sucrose backbone. Additional substituents such as glucopyranosyl and rhamnopyranosyl groups are linked via glycosidic bonds, and some acyl groups, such as benzoyl, cinnamoyl, feruloyl, and sinapoyl moieties, serve as the usual substitutes via ester bonds. Finally, 43 oligosaccharide esters were identified in the samples by comparison with the literature.
Compound 31 (Rt = 2.566 min) was characterized as a representative oligosaccharide ester. The deprotonated molecular ion [M−H]− was observed at m/z 547, and the molecular formula was calculated as C23H32O15. When the m/z 547 ion cluster enters the collision chamber, the main fragment ions were observed at m/z 431, 205 and 175 respectively. After retrieving the library, compound 31 was assigned as sibiricose A6. The inferred mass cleavage pathway of these main fragment ions is shown in Fig. 3B.
3.1.3 Xanthones
Xanthones in Polygalae species mostly have similar chemical skeletons. Substitutions usually occur at C-1, C-2, C-3, C-6, and C-7 sites of the aglycone, and sometimes C-4 and C-8 substitutions are observed. The substituents include methoxy, hydroxyl, methylene dioxy, monosaccharides, and disaccharides. When fission occurs for the linkage between glucosyl and xanthone skeleton, the neutral loss of 162 Da (Glc), 132 Da (Api), and 146 Da (Rha) is generated.
Compound 34 was assigned as polygalaxanthone Ⅲ (Rt = 5.179 min), with a deprotonated molecular ion [M−H]− at m/z 567, and the molecular formula calculated as C25H28O15. When the ion population within the m/z 567–568 mass window, actually m/z 567 ion cluster, entered the collision chamber, the primary fragment ion species were observed at m/z 345, 315, and 272, respectively. The structure of polygalaxanthone Ⅲ as well as the proposed mass cracking pathways in response to those primary fragment ion species are shown in Fig. 3C.
3.1.4 Other chemicals
Galactopyranosyl-galactopyranosyl-arabinose, assigned to compound 24 (Rt = 0.444 min), is one of the particular constituents in PT. The m/z 473 peak was identified as a deprotonated molecular ion peak [M−H]−, with the molecular formula observed as C17H30O15. When the ion group within the mass window of m/z 473–474, which is actually the m/z 473 ion cluster, enters the collision chamber, the main fragment ion species are observed at m/z 443, 389, and 341, respectively. The structure of galactopyranosyl-galactopyranosyl-arabinose and the inferred mass cleavage pathway of these main fragment ions are shown in Fig. 3D.
A Venn diagram analysis was performed to visualize the shared and specific compounds among the six Polygala species. The diagram showed 38 compounds shared by two species, 27 compounds shared by three species, 26 compounds shared by four species, 14 compounds shared by five species, and 19 compounds shared by all six species. Furthermore, 10 specific compounds were detected in P. tenuifolia, 11 in P. sibirica, 6 in P. japonica, 2 in P. arvensis, and 1 each in P. fallax and P. glomerata. Particularly, P. fallax had the fewest specific compounds, amounting to half of those found in P. tenuifolia (Fig. 4).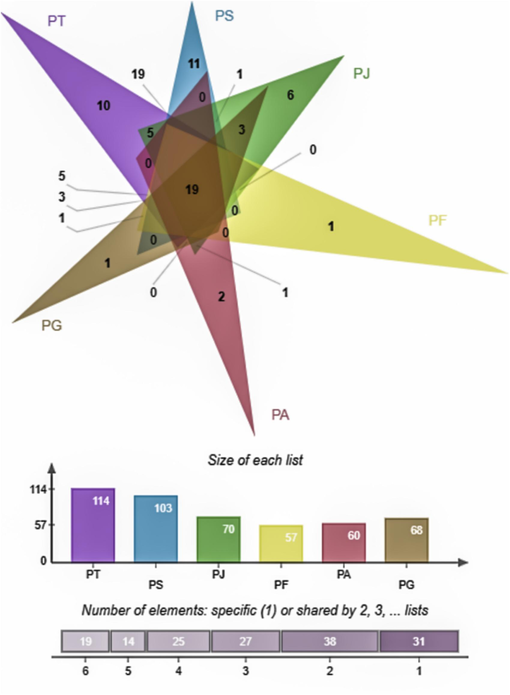
Venn diagram of compounds shared among six Polygala species.
3.2 Differential compound analysis
Differential compound analysis was conducted to identify compounds with distinct accumulation patterns among the six Polygala species. A total of 67 up-regulated compounds and 124 down-regulated compounds were identified, with 46 compounds displaying both up- and down-regulation across five comparisons (the criteria was set to satisfy VIP values ≥ 1, Fold change ≥ 2, p ≤ 0.05). Metabolite profiling revealed unique composition profiles for PS vs. PT, PJ vs. PT, PF vs. PT, FG vs. PT, and PA vs. PT, identifying 66 (27 up-regulated, 39 down-regulated), 104 (26 up-regulated, 78 down-regulated), 94 (13 up-regulated, 81 down-regulated), 84 (20 up-regulated, 64 down-regulated), and 87 (14 up-regulated, 73 down-regulated) differential metabolites, respectively. These differential metabolites may serve as chemical markers for distinguishing the six Polygala species and could have potential applications (Figure S1, Supporting Information).
Hierarchical cluster analysis (HCA) revealed distinct clustering patterns among the samples, indicating metabolite variations across species. Samples PT, PS, PJ, PF, PA, and PG showed separation, suggesting changes in metabolite composition. Furthermore, P. arvensis and P. glomerata formed a cluster, while P. fallax clustered with these two species. The six species were divided into two major branches, with P. tenuifolia and P. sibirica forming one group and P. japonica clustered with the remaining species.
Additionally, heatmap analysis was employed to visualize the content profiles of 154 compounds across these species (Fig. 5). The results revealed substantial differences in compound types and contents among the six species, underscoring clear interspecies disparities. Notably, PG, PA, and PF had the highest number and content of compounds, with 79 (onjisaponin MF), 1 (gentitein), and 91 (polygalasaponin ⅩⅩⅩⅦ) compounds, respectively. Intraspecies differences were also observed, potentially due to interspecific variation. Specific compounds such as onjisaponin MF, gentitein, and polygalasaponin ⅩⅩⅩⅦ were identified as trace components between PG, PA, and PF samples. PT displayed higher content levels of 140 (tenuifoside A), 120 (tenuifoliose L), and 139 (tenuifoside A). PS exhibited elevated content levels of 85 (tenuifoliose C2), 84 (senegose K), and 98 (senegose A). PJ showed higher content levels of 77 (tenuifoliose T), 67 (polygalasaponin VI), and 60 (3-acetyl-3′,6-O-disinapoylsucrose), while other compounds demonstrated distinct accumulation patterns. These compounds can be used as marker constituents for species identification in PG, PA, and PF samples.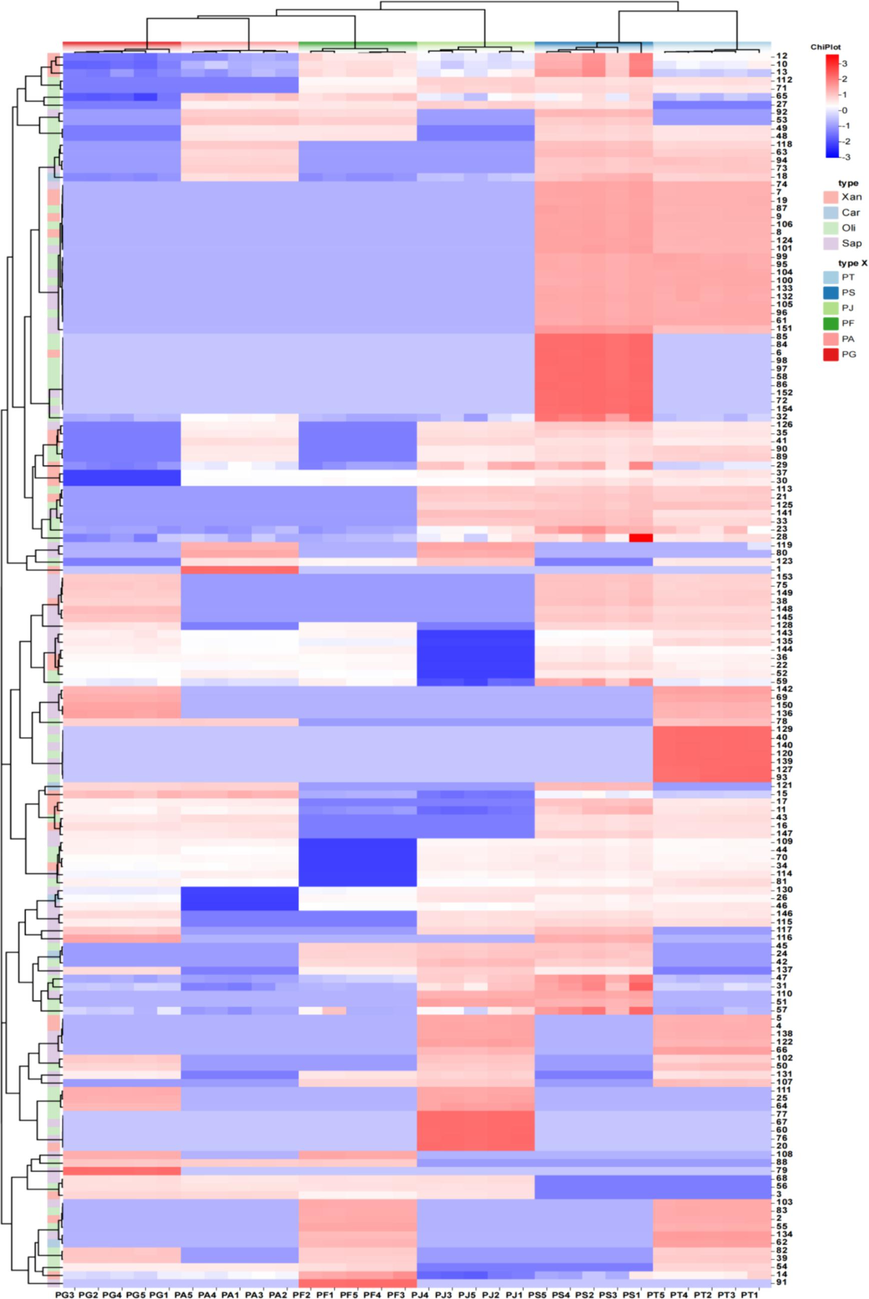
Heat map of euclidean distance correlation coefficient between metabolites and different samples. The abscissa repented different samples, and the ordinate represented metabolites.
3.3 Chemometric analysis
PCA was used to visualize the distribution of samples within a mathematical model space, providing insights into sample aggregation and dispersion. In our analysis (Fig. 6A), all samples fell within the 95 % confidence interval, confirming the reliability of the results. The PCA model demonstrated strong predictive ability and discrimination, with R2X=0.654 and Q2 = 0.564, both exceeding the threshold of 0.5. The clustering patterns depicted in the PCA score plot revealed distinct separation among the six Polygala species (Fig. 6B), with each species forming discrete clusters, indicating significant dissimilarities between species. The clusters of P. tenuifolia and P. sibirica were relatively closer compared to the other four species, suggesting a smaller distinction between these two species. Conversely, clusters of P. japonica and P. fallax showed greater proximity. These clustering patterns may be attributed to various factors. The smaller distance between the clusters of P. tenuifolia and P. sibirica could be attributed to limited literature on P. arvensis and P. glomerata, resulting in less chemical information available for these species. On the other hand, P. japonica has been extensively studied and included in the Chinese Pharmacopoeia, while P. fallax possesses ornamental and medicinal value with diverse applications, contributing to the closer proximity between their clusters.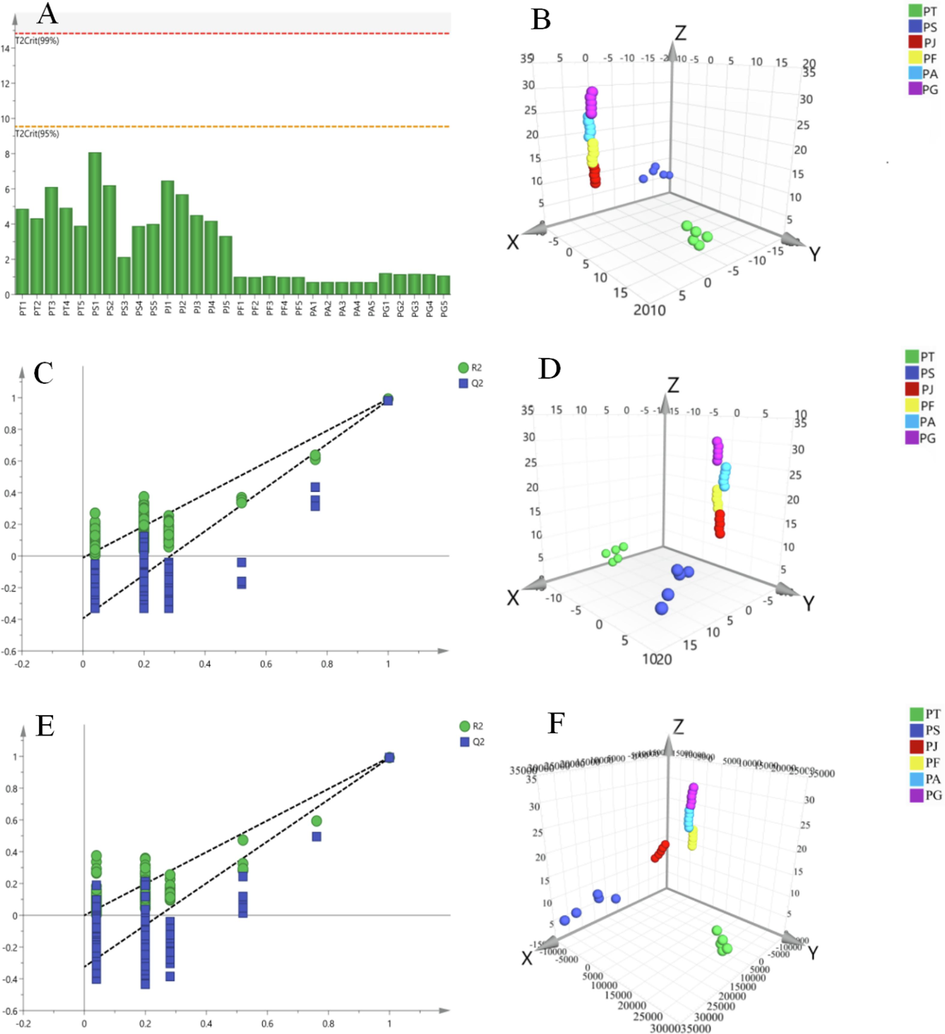
Chemometric analysis of six Polygala species: (A) Hotelling's T2 Range, (B) Scores plot of PCA-X, (C) Permutation Plot of PLS-DA, (D) Scores plot of PLS-DA, (E) Permutation Plot of OPLS-DA, (F) Scores plot of OPLS-DA.
PCA should consider intra-group errors and unrelated random errors that may obscure the study's focus. Overemphasis on details at the expense of overall patterns can hinder the identification of inter-group differences and differential compounds. To address this issue, it is essential to incorporate a priori knowledge of the samples and employ supervised pattern recognition methods. In this study, PLS-DA was utilized. Fig. 6C displays blue Q2 points lower than the original blue Q2 points on the far right, indicating the reliability and validity of the results. The PLS-DA model (Fig. 6D) demonstrated favorable outcomes with Q2 = 0.504, R2X=0.829, and R2Y=0.588, exceeding the threshold of 0.5, consistent with PCA results.
In comparison to PLS-DA, OPLS-DA reduces model complexity while enhancing explanatory power without compromising predictability. The OPLS-DA model (Fig. 6E) demonstrated strong predictive ability and a good fit, with Q2 = 0.546, R2X=0.891, and R2Y=0.546, surpassing the threshold of 0.5. The clear cluster separation observed in the score matrix (Fig. 6F) highlighted distinct clustering among the six groups of Polygala species, providing valuable insights into their differentiation. Replacement tests further confirmed the robust predictive capability of the OPLS-DA model, reinforcing its validity and reliability.
3.4 Antioxidant correlation assay
The total saponins (TS) content varied among the examined six Polygala species, ranging from 34.463 to 66.034 mg/g (Table 2), with PS exhibiting the highest concentration (66.034 mg/g) and PA displaying the lowest value(34.463 mg/g). Similar trends were observed for total phenolics content (TP) (0.834 to 4.488 mg/g) and total flavonoids content (TF) (11.256 to 45.636 mg/g), with PS demonstrating the highest values and PF recording the lowest. These results indicate that PS extract had the highest TS, TP, and TF compared to other extracts. Factors such as solvent, pH, extraction method, temperature, and extraction time can influence the content of these bioactive compounds (Ayele et al., 2022, Kavita et al., 2015, Matos et al., 2023). Notes: Values (mean ± standard deviation, n = 3) within the column are significantly different (p < 0.05) from each other represented by the superscript letters (a-g).
TS (mg/g)
TP (mg/g)
TF (mg/g)
ABTS (μM/0.01 g)
CUPRAC (µM/0.01 g)
FRAP (µM/0.01 g)
DPPHIC50(mg/mL)
PT
48.957 ± 0.540a
2.250 ± 0.002e
30.531 ± 0.164b
96.16 ± 0.06d
119.85 ± 16.28c
30.85 ± 0.16e
3.51 ± 0.09f
PA
34.463 ± 0.292a
0.834 ± 0.001f
12.078 ± 0.220b
93.62 ± 0.32c
74.92 ± 1.05d
13.34 ± 0.23e
7.73 ± 0.19f
PS
66.034 ± 1.240a
4.488 ± 0.005d
45.636 ± 0.246b
97.43 ± 0.09c
98.58 ± 0.25c
23.37 ± 0.19e
5.66 ± 0.02f
PJ
58.349 ± 0.377a
2.994 ± 0.001f
28.689 ± 1.222b
98.94 ± 0.03c
65.06 ± 0.11d
42.58 ± 0.08e
7.44 ± 0.04g
PF
48.442 ± 0.318a
2.753 ± 0.036d
11.256 ± 0.038b
61.89 ± 0.38c
6.21 ± 0.07f
5.79 ± 0.20f
8.86 ± 0.85e
PG
41.913 ± 0.371a
4.215 ± 0.025d
16.731 ± 1.578b
89.04 ± 0.07c
43.87 ± 0.10d
11.30 ± 1.03e
5.80 ± 0.06e
Antioxidants are widely present in fruits (Huang et al., 2023), vegetables (Parada et al., 2023) and TCMs. Polygala species are recognized for their abundant totalphenolics, total flavonoids, and total saponins, which possess remarkable antioxidant effects. In this study, extracts obtained from the dry roots of six Polygala species were assessed for TP, TF, and antioxidant capacity indicators (DPPH, ABTS, CUPRAC, and FRAP, Table 1). The PJ, PT, and PF extracts exhibited the higher antioxidant capacities, while PA showed the lowest. The superior antioxidant activity of extracts from both extraction modes was attributed to the presence of flavonoid compounds. Previous studies have also reported the high antioxidant activity of these three plant species due to their flavonoid contents (Ali et al., 2022). Correlation analysis revealed a positive relationship between ABTS and TP, TS, and TF (0.503, 0.852, and 0.606, respectively) (Fig. 7), consistent with the study of P. tenuifolia (Li et al., 2022). These findings suggest that higher concentrations of polyphenols are necessary to achieve stronger antioxidant activity. The positive correlations between total phenolics, total saponins, and total flavonoids and antioxidant values further support the association between higher polyphenol concentrations and enhanced antioxidant activity (Behnaz et al., 2017).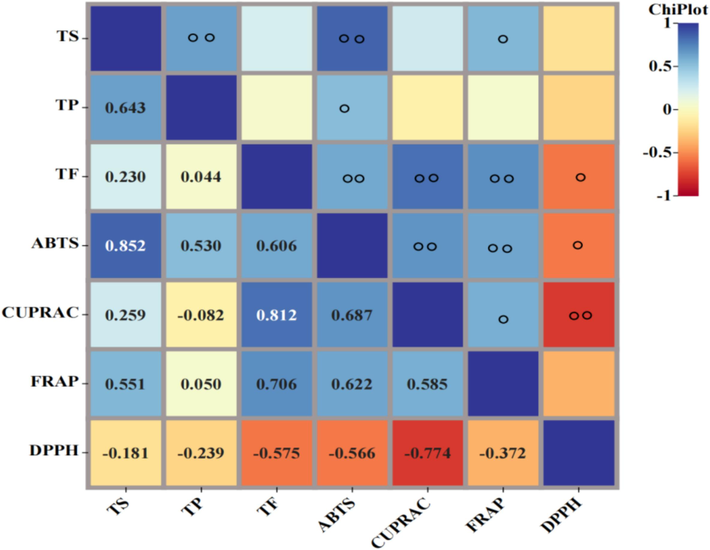
Correlation analysis of the TS, TP, TF and antioxidant activities ○ At the 0.05 level (two-tailed), the correlation is significant ○○ At the 0.01 level (two-tailed), the correlation is significant.
Overall, the observed differences may be attributed to genus variations (Rojas et al, 2021). Several species from the Polygala family have been reported to have different antioxidant capacities (DPPH, ABTS, CUPRAC, and FRAP) due to the influence of the species differential. In addition, the ability to scavenge free radicals is likely influenced by the type and concentration of radicals and the chemical structure of the polyphenols involved (Parada et al., 2023).
The content of total flavonoids was strongly associated with ABTS, CUPRAC, and FRAP but negatively correlated with DPPH IC50. Additionally, the content of total phenolics was in a positive correlation with ABTS, and the content of total saponins was also in a positive correlation with ABTS and FRAP (Fig. 7).
4 Conclusion
In this study, UPLC-Q-TOF-MS was used to characterize the chemome of six medicinal plants belonging to the Polygala genus, namely P. tenuifolia, P. japonica, P. sibirica, P. fallax, P. arvensis, and P. glomerata. Through this method, a total of 154 components were tentatively identified, including 62 oligosaccharide esters (40.26 %), 58 triterpene saponins (37.66 %), 29 xanthones (18.83 %), and 5 other chemicals (3.25 %). Differential compound analysis revealed 67 up-regulated compounds and 124 down-regulated compounds among the six Polygala species. Multivariate statistical analysis highlighted the presence of specific compounds in each of the six different Polygala species. P. sibirica and P. tenuifolia exhibited higher levels of specific compounds, possibly attributable to their longer growth period and wild plant collection practices. Conversely, P. japonica demonstrated closer similarities to P. fallax, P. arvensis, and P. glomerata. Furthermore, oligosaccharide esters and saponins were more abundant in P. sibirica and P. tenuifolia compared to other species, considered as the primary pharmacologically active ingredients in Polygalae radix. Additionally, an antioxidant assay revealed significant levels of total flavonoids and total saponins in samples from the Polygala genus, displaying varied antioxidant activity levels. The characterization of differentially identified compounds in the Polygala genus holds meaningful implications, providing valuable phytochemical information that could serve as a foundation for genetic diversity analysis, clinical applications, and the identification and conservation of medicinal plant resources within the Polygala genus.
CRediT authorship contribution statement
Yiyao Jing: Conceptualization, Data curation, Writing – original draft. Benxiang Hu: Resources. Haiyue Ji: Software, Validation. Fan Zhao: Supervision, Validation. Bo Li: Visualization. Yao Luo: Software, Validation, Visualization. Han Zhang: Writing – review & editing. Gang Zhang: Writing – review & editing. Yonggang Yan: Resources, Supervision. Xiaolin Dang: Formal analysis, Investigation. Bingyue Yang: Validation, Writing – review & editing. Liang Peng: Funding acquisition, Writing – review & editing.
Acknowledgments
This research was financially supported by (1) the National Natural Science Foundation of China, China (Grant No. 82003899). (2) General Project of Social Development of Shaanxi Provincial Department of Science and Technology (2024SF-YBXM-457). (3) Youth Project of General Project of Shaanxi Provincial Science and Technology Department, China (2024JC-YBQN-0989). (4) Yulin Science and Technology Plan Project (YF-2021-74). (5) Subject Innovation Team of Quality Control and Resources Development of “Qin drug” of Shaanxi University of Chinese Medicine, China (2019-QN01).
Declaration of interest statement
This manuscript has not been published previously by any of the authors and is not under the consideration for publication in another journal. And the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- LC-MS/MS characterization of phenolic metabolites and their antioxidant activities from australian native plants. Metabolites. 2022;12:1016.
- [CrossRef] [Google Scholar]
- Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chemistry.. 2022;16:30.
- [CrossRef] [Google Scholar]
- Intestinal Absorption Profile of Three polygala oligosaccharide esters in polygalae radix and the effects of other components in polygalae radix on their absorption. Evid. Based Complement. Alternat. Med.. 2019;2019:1379531.
- [CrossRef] [Google Scholar]
- Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem.. 2017;220:153-161.
- [CrossRef] [Google Scholar]
- Chinese, P. C., (2020). Pharmacopoeia of the People's Republic of China. China, PP. 315.
- Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater. Today:. Proc.. 2019;13:777-783.
- [CrossRef] [Google Scholar]
- Studies on the chemical and intestinal metabolic profiles of Polygalae Radix by using UHPLC-IT-MS n and UHPLC-Q-TOF-MS method coupled with intestinal bacteria incubation model in vitro. J. Pharm. Biomed. Anal.. 2018;298–306
- [CrossRef] [Google Scholar]
- Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Scientia Horticulturae.. 2023;321
- [CrossRef] [Google Scholar]
- Jeanette, M., Brijesh, S., (2018). Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomedicine pharmacotherapy Biomedecine pharmacotherapie. 104, 343-365. https://doi.org / 10.1016/j.biopha.2018.05.044.
- Trehalose and brassinolide enhance the signature ingredient accumulation and antioxidant activity in the hairy root cultures of Polygala tenuifolia Willd. Ind. Crop. Prod.. 2023;196
- [CrossRef] [Google Scholar]
- Predicting the potential distribution of polygala tenuifolia willd. under climate change in China. PloS One.. 2016;9:e0163718
- [CrossRef] [Google Scholar]
- Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal.. 2015;2:243-252.
- [CrossRef] [Google Scholar]
- A pharmacognostic approach to the Polygala genus: phytochemical and pharmacological aspects. Chemistry Biodiversity.. 2012;2:181-209.
- [CrossRef] [Google Scholar]
- A review on the phytopharmacological studies of the genus Polygala. J. Ethnopharmacology.. 2020;249:112417
- [CrossRef] [Google Scholar]
- Optimization of ultrasonic-assisted extraction of active components and antioxidant activity from polygala tenuifolia: a comparative study of the response surface methodology and least squares support vector machine. Molecules. 2022;10:3069.
- [CrossRef] [Google Scholar]
- Three triterpenoid saponins from the roots of Polygala japonica Houtt. Fitoterapia.. 2012;7:1184-1190.
- [CrossRef] [Google Scholar]
- Glomexanthones A-C, three xanthonolignoid C-glycosides from Polygala glomerata Lour. Fitoterapia.. 2014;93:175-181.
- [CrossRef] [Google Scholar]
- Direct infusion-tandem mass spectrometry combining with data mining strategies enables rapid chemome characterization of medicinal plants: a case study of Polygala tenuifolia. J. Pharm. Biomed. Anal.. 2021;204:114281
- [CrossRef] [Google Scholar]
- Comprehensive evaluation of chemical constituents and antioxidant activity between crude and processed Polygalae radix based on UPLC-Q-TOF-MS/MS combined with multivariate statistical analysis. Heliyon.. 2024;6:e27622
- [CrossRef] [Google Scholar]
- Chemo-profiling by UPLC-QTOF MS analysis and in vitro assessment of Anti-inflammatory activity of Field Milkwort (Polygala arvensis Willd.) S. Afr. J. Bot.. 2022;149:49-59.
- [CrossRef] [Google Scholar]
- Chemical prospection and antioxidant activity of Humiria balsamifera (Aubl.) A. St. Hil. AND Hymenaea courbaril L. Nat. Prod. Res.. 2023;38:1-5.
- [CrossRef] [Google Scholar]
- Improved antioxidant capacity of three Brassica vegetables by two-step controlled fermentation using isolated autochthone strains of the genus Leuconostoc spp. and Lactiplantibacillus spp. Food Chem: Molecular Sci.. 2023;6:100163
- [CrossRef] [Google Scholar]
- Antioxidant capacity, total phenolic content and phenolic compounds of pulp and bagasse of four Peruvian berries. Heliyon.. 2021;8:e07787.
- [CrossRef] [Google Scholar]
- Triterpenoid saponins and phenylpropanoid glycosides from the roots of Polygala japonica Houtt. with anti-inflammatory activity. Phytochemistry.. 2018;60–66
- [CrossRef] [Google Scholar]
- Protective effects of tenuifolin isolated from Polygala tenuifolia Willd roots on neuronal apoptosis and learning and memory deficits in mice with Alzheimer’s disease. Food & Function. 2019;10(11):7453-7460.
- [CrossRef] [Google Scholar]
- World Flora Online, 2020. WFO Plant List. https://wfoplantlist.org/taxon/wfo-4000030736-2023-12? page=1/(accessed 2013).
- Research progress on the medicinal and edible polygala FALLAX Hemsl. (Polygalaceae) Plant. Horticulturae.. 2023;9:7.
- [CrossRef] [Google Scholar]
- Polygalaxanthone III, an active ingredient in polygala japonica Houtt., repaired malassezia-stimulated skin injury via STAT3 phosphorylated activation. Molecules. 2022;21:7520.
- [CrossRef] [Google Scholar]
- Research on anti-hepatocellular carcinoma activity and mechanism of Polygala fallax Hemsl. Journal of Ethnopharmacology.. 2020;260:113062.
- [CrossRef] [Google Scholar]
- Cloning, yeast expression, and characterization of a β-Amyrin C-28 oxidase (CYP716A249) Involved in triterpenoid biosynthesis in Polygala tenuifolia: regular articles. Biol. Pharm. Bull.. 2020;12:1839-1846.
- [CrossRef] [Google Scholar]
- Polygalae Radix: a review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia.. 2020;147:104759
- [CrossRef] [Google Scholar]
- Chemical investigation of the roots of Polygala sibirica L. Chin. J. Nat. Med.. 2014;3:225-228.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105915.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







