Translate this page into:
Exploration on the mechanism of “black as lacquer and sweetness as candy” based on the reactions of “crosslinking coloring and Maillard” and “oligosaccharide hydrolysis” during the processing of Radix Rehmanniae
⁎Corresponding authors at: National Key Laboratory of Chinese Medicine Modernization, State Key Laboratory of Component-based Chinese Medicine, Tianjin Key Laboratory of TCM Chemistry and Analysis, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, China; Haihe Laboratory of Modern Chinese Medicine, Tianjin, 301617, China wangyf0622@tjutcm.edu.cn (Yuefei Wang), chaix0622@tjutcm.edu.cn (Xin Chai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
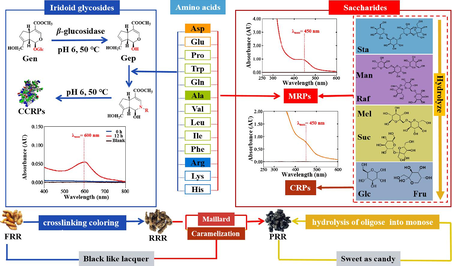
“Sweet as candy” of PRR derive from hydrolysis reaction of oligosaccharides. Crosslinking coloring and Maillard reactions contribute to “black as lacquer” of PRR. The occurrence of crosslinking coloring reaction was firstly reported in RR.
Abstract
Radix Rehmanniae (RR) has three processing forms in clinical practice, including fresh Radix Rehmanniae (FRR), raw Radix Rehmanniae (RRR), and processed Radix Rehmanniae (PRR). However, the sensory criterion, “black as lacquer and sweetness as candy”, which was traditionally used to evaluate the quality of PRR, have not been fully elucidated. In this study, the mechanism of chemical transformation based on oligosaccharide hydrolysis, crosslinking coloring, and Maillard reactions was explored to clarify the observed changes in appearance and flavor. The physicochemical properties of different processed RR products were characterized. Besides, “sweetness as candy” was explained by oligosaccharides hydrolysis, and “black as lacquer” can attribute to crosslinking coloring and Maillard reactions. Importantly, this study provided the first evidence for the existence of crosslinking coloring reaction during the processing of RR. In conclusion, our results unveiled the scientific basis of the sensory attributes “black as lacquer and sweetness as candy” in PRR, offering valuable insights for enhancing quality control of PRR.
Keywords
Radix Rehmanniae
Processing
Crosslinking reaction
Maillard reaction
Hydrolysis of oligosaccharides
- RR
-
Radix Rehmanniae
- FRR
-
fresh Radix Rehmanniae
- RRR
-
raw Radix Rehmanniae
- PRR
-
processed Radix Rehmanniae
- TCM
-
traditional Chinese medicine
- RW
-
yellow rice wine
- Cat
-
catalpol
- Leo
-
leonuride
- Fru
-
fructose
- Glc
-
glucose
- Sta
-
stachyose
- Arg
-
arginine
- Lys
-
lysine
- 5-HMF
-
5-hydroxymethyl-2-furadehyde
- 5-GMF
-
5-(α-D-glucopyranosyloxymethyl)-2-furancarboxaldehyde
- 5-GGMF
-
5-(α-D-glucopyranosyl-(1→6)-α-D-glucopyranosyloxymethyl)-2-furancarboxaldehyde
- Gep
-
genipin
- PITC
-
phenyl isothiocyanate
- Gen
-
geniposide
- Asp
-
aspartate
- Glu
-
glutamate
- His
-
histidine
- Ala
-
alanine
- Val
-
valine
- Gln
-
glutamine
- Pro
-
proline
- Leu
-
leucine
- Ile
-
isoleucine
- Trp
-
tryptophan
- Phe
-
phenylalanine
- Suc
-
sucrose
- Mel
-
melibiose
- Raf
-
raffinose
- Man
-
manninotriose
- CC
-
column chromatography
- UPLC-PDA
-
ultra-performance liquid chromatography with photo-diode array detector
- HPLC-ELSD
-
high performance liquid chromatography coupled with evaporative light scattering detector
- UV–vis
-
ultraviolet–visible
- CCRPs
-
crosslinking coloring reaction products
- MRPs
-
Maillard reaction products
- SD
-
standard deviation
- Gal
-
galactose
Abbreviations
1 Introduction
Radix Rehmanniae (RR), derived from the root of Rehmannia glutinosa Libosch., is a commonly used medicinal herb in China and other eastern countries. Three types of RR in terms of the processing methods are frequently used in clinical settings, including fresh Radix Rehmanniae (FRR), raw Radix Rehmanniae (RRR), and processed Radix Rehmanniae (PRR), which are prescribed in traditional Chinese medicine (TCM) for distinct medicinal properties (Chinese pharmacopoeia commission, 2020). RRR is characterized by its “cold” nature, which is believed to reduce the heat in blood, promote the production of body fluids, and dissipate the stasis in vessels. In contrast, PRR, possessing a “warm” character, is used to replenish blood and reinforce the body’s essence and marrow (Gong et al., 2019a). In the process of PRR stewed by yellow rice wine (RW) and steamed, its color changes from brownish-yellow to dark black, the texture becomes sticky, and the sweetness increases. As a result, the sensory qualities of being “black as lacquer and sweetness as candy” have become critical criteria for determining the endpoint of the PRR processing procedure (Wang et al., 2010; Li et al., 2022).
The obvious discrepancy in the features of processed RR products essentially reflect the inherent changes in chemical compositions and compound’s abundance. It has been reported that iridoid glycosides, phenylethanoid glycosides, saccharides, and amino acids are presented in RR, which contribute to the efficacy of RR (Jia et al., 2023). Iridoid glycosides are sensitive to the acidic environment, where they are prone to hydrolyse, resulting in the release of the glycosyl moiety (monoglycosyl or oligosaccharyl) and consequent decrease in the levels of catalpol (Cat) and leonuride (Leo) in PRR (Zhou et al., 2016a; Du et al., 2021; Yang et al., 2024). Additionally, polysaccharides could be degraded into oligosaccharides and/or monosaccharides, accounting for the great enhancement of free fructose (Fru) and glucose (Glc), while noticeable decrease of stachyose (Sta) was witnessed in PRR (Chang et al., 2011; Xue et al., 2023). Concurrently, there is a marked decline in the contents of alkaline amino acids, especially arginine (Arg) and lysine (Lys), during the processing of RR (Gao et al., 2010), accompanied by a great increase of 5-hydroxymethyl-2-furadehyde (5-HMF) in PRR (Xu et al., 2012; Zhou et al., 2017), a product of the Maillard reaction. The darken color on the surface of PRR is predominantly associated with increases in the levels of 5-HMF, Glc, and Fru, coupled with decreases in Cat and Leo contents (Meng et al., 2020). Besides, 5-(α-D-glucopyranosyloxymethyl)-2-furancarboxaldehyde (5-GMF) and 5-(α-D-glucopyranosyl-(1→6)-α-D-glucopyranosyloxymethyl)-2-furancarboxaldehyde (5-GGMF), two furfural mono/di-glucosides, were produced during the processing of PRR (Li et al., 2010; Won et al., 2014). Maillard reaction, known as a non-enzymatic browning reaction that occurs between reducing sugar’s carbonyl groups and amino groups, is primarily responsible for the dark-colored appearance of PRR (Meng et al., 2021). However, very little attention has been paid to the reaction between main saccharides (oligosaccharides and monosaccharides) and amino acids in RR, as well as their underlying mechanisms.
Iridoid glycosides, serving as reactants in crosslinking coloring reaction, can undergo reactions with amino acids in the presence of β-glucosidase to yield gardenia red or blue pigments (Liu et al., 2008; Hobbs et al., 2018). Genipin (Gep), a colorless iridoid as monoterpene, can react with primary amines from amino acids or proteins to produce water-soluble bluish-violet pigments (Touyama et al., 1994). The increasing interest in such natural polymer pigments has led to their application across various fields, including food, cosmetics, pharmaceuticals, and material science (Neri-Numa et al., 2017). Even being rich in iridoid glycosides and amino acids, RR has been rarely reported in the crosslinking coloring reaction, which might be crucial for pigment formation during the process from FRR to PRR. Therefore, it is imperative to intensify research on chemical transformation mechanisms to elucidate how and why processing alters the chemical components.
In this study, the physicochemical properties of different processed products from RR along with the dynamic variations on the contents of amino acids and saccharides were identified and examined during the processing of RR. The crosslinking coloring reaction initiated by iridoid glycosides and amino acids, Maillard reaction formed between saccharides and amino acids, and caramelization reaction of saccharides were performed to illustrate the scientific basis of “color black as lacquer”. Additionally, the hydrolysis of saccharides provided a scientific explanation for the characteristic of “sweetness as candy”. In conclusion, our study not only deepens the understanding of the scientific basis underpinning RR processing, but also offers recommendations for optimizing processing method and establishing quality control standards for PRR.
2 Material and methods
2.1 Reagents and chemicals
Hydrochloric acid was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China). Glacial acetic acid, sodium bicarbonate, and sodium acetate anhydrous were bought from Tianjin Fengchuan Chemical Reagent Co., Ltd. (Tianjin, China). Phenyl isothiocyanate (PITC), triethylamine, and formic acid were supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). n-Hexane was bought from Tianjin Concord Technology Co., Ltd. (Tianjin, China). Reference standards including geniposide (Gen), Gep, Cat, aspartate (Asp), glutamate (Glu), Lys, Arg, histidine (His), alanine (Ala), valine (Val), glutamine (Gln), proline (Pro), leucine (Leu), isoleucine (Ile), tryptophan (Trp), phenylalanine (Phe), Fru, Glc, sucrose (Suc), melibiose (Mel), raffinose (Raf), manninotriose (Man), and Sta were obtained from Shanghai Yuanye Biotech Co., Ltd. (Shanghai, China). Leo was provided by Chengdu Ruifen Si Biotech Co., Ltd. (Chengdu, China). The purity of all chemicals was greater than 98 %. β-Glucosidase, acetonitrile (HPLC grade), and methanol (HPLC grade) were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Column chromatography (CC) was carried out using MCI Gel (CHP20/P120, Mitsubishi Chemical Corporation, Tokyo, Japan). Water used in the experiment was purified by a Milli-Q system (Millipore, Milford, MA, USA).
The sodium acetate buffer solutions at pH 4.8 and 6.0 were prepared according to the guidelines released by Chinese Pharmacopoeia, respectively.
FRR samples, collected in Jiaozuo city (Henan, China) in November 2020, were authenticated by Prof. Tianxiang Li of Tianjin University of Traditional Chinese Medicine (Tianjin, China) based on original, characteristic, microscopical, and chemical identifications specified by Chinese Pharmacopoeia.
2.2 Preparation of RRR and PRR
To acquire RRR, three batches (2500 g/batch) of FRR with a uniform size (weight of 110 ± 15 g, diameter of 58 ± 10 mm) were selected after removing their fibrous roots. The FRR samples were then dried at 50 °C for 10 days (10 h per day) until the moisture was less than 15 %.
PRR samples were obtained through the following procedure: Three batches of RRR samples were mixed with RW at a weight ratio of 10:4, soaked for 24 h, and then steamed for 12 h until softened. The softened samples were taken out, dried, blended with resulting steamed liquid, and continued to be steamed for 6 h. Afterwards, they were dried at 60 °C in the oven until the texture became viscous and dark-black colored, and then cut into thick slices (2-4 mm).
2.3 Preparation of standard and sample solutions
2.3.1 Preparation of standard and sample solutions for quantitative analysis of amino acids in different processed products of RR and RW
Thirteen reference compounds were accurately weighed, dissolved, and diluted with 0.02 M hydrochloric acid to obtain individual stock solutions. Each stock solution was accurately measured and employed to obtain a mixed standard solution, leading to accomplishment of the final concentrations of Pro at 10.79 µg/mL, Leu at 3.67 µg/mL, Asp at 30.66 µg/mL, Arg at 185.04 µg/mL, Trp at 6.37 µg/mL, Lys at 3.98 µg/mL, Ile at 5.57 µg/mL, Phe at 4.02 µg/mL, Glu at 37.31 µg/mL, Gln at 304.2 µg/mL, Ala at 7.52 µg/mL, His at 12.36 µg/mL, and Val at 3.88 µg/mL. A series of the diluted solutions of mixed standards were prepared to construct calibration curves. Subsequently, 100 μL of 0.4 M PITC and 100 μL of 0.1 M triethylamine acetonitrile solution were mixed with 200 μL of the mixed standard solution, and then placed at room temperature for 1 h. Afterwards, 400 μL of n-hexane was added, with shaking for 1 min and standing for 10 min. Finally, the lower solution was filtered through a 0.22 μm membrane filter and then diluted with ultrapure water (v:v, 1:2) to obtain the standard solutions for ultra-performance liquid chromatography with photo-diode array detector (UPLC-PDA) analysis.
The RR sample powder (1.0 g) was accurately weighed and ultrasonically extracted (144 W, 35 kHz) with 30 mL of 0.02 M hydrochloric acid solution for 1 h, followed by centrifugation at 13,322 g for 10 min to collect the supernatant. Weighed RW (0.4 g) was mixed with 30 mL of 0.02 M hydrochloric acid solution, followed by centrifugation at 13,322 g for 10 min to collect the supernatant. The derivatization procedure of amino acids derived from the supernatant of RR samples and RW was consistent with that of the standard solution. All solutions were stored at 4 °C until analyzed by UPLC-PDA.
2.3.2 Preparation of standard and sample solutions for quantifying oligosaccharides and monosaccharides in different processed products of RR
Seven reference compounds were accurately weighed and dissolved with ultrapure water to obtain stock solutions separately, which were then used to prepare a mixed standard solution with the final concentrations at 0.2400 mg/mL Fru, 0.3986 mg/mL Glc, 0.4000 mg/mL Suc, 0.3981 mg/mL Mel, 0.1993 mg/mL Raf, 0.2581 mg/mL Man, and 0.8761 mg/mL Sta, respectively. Subsequently, the working solutions at the different concentrations were diluted for constructing calibration curves.
Accurately weighed sample powder (0.25 g) was ultrasonically extracted (144 W, 35 kHz) with 25 mL of 60 % methanol aqueous solution for 40 min. After centrifugation at 13,322 g for 10 min, the supernatant was collected and filtered through a 0.22 µm membrane filter and then diluted with ultrapure water (v:v, 1:3) to obtain the sample solution. All solutions were stored at 4 °C until analyzed by high performance liquid chromatography coupled with evaporative light scattering detector (HPLC-ELSD).
2.3.3 Preparation of sample solution for exploration on hydrolysis reaction of oligosaccharide
The saccharide standards were accurately weighed and dissolved in sodium acetate buffer solution (pH 4.8) to obtain respective stock solutions at the concentrations of 25.02 mg/mL for Suc, 25.07 mg/mL for Mel, 25.02 mg/mL for Raf, 25.02 mg/mL for Man, and 25.06 mg/mL for Sta. Afterwards, 1.5 mL of each saccharide solution was mixed with 8.5 mL of sodium acetate buffer solution (pH 4.8) and then hydrolyzed for 18 h at 100 °C. The supernatant was filtered, diluted with ultrapure water (v:v, 1:3), and stored at 4 °C before HPLC-ELSD analysis.
2.3.4 Preparation of sample solution for illumination on the crosslinking coloring reaction
β-Glucosidase (1.96 mg) was weighed precisely, mixed with sodium acetate buffer solution at pH 6.0 (400 µL), and stored at 4 °C. The enzyme activity was 110.74 U/mL.
Standards, including Gen, Gep, Cat, Leo, Arg, Ala, and Glu, were accurately weighed and dissolved in sodium acetate buffer solution (pH 6.0) to obtain respective stock solutions at the concentrations of 2.247 mM for Gen, 2.279 mM for Gep, 2.247 mM for Arg, 2.270 mM for Ala, 2.265 mM for Glu, 2.247 mM for Leo, and 2.257 mM for Cat. The stock solutions were stored at 4 °C before analysis. Afterwards, 1 mL Gen, 1 mL Arg, and 10 µL β-glucosidase stock solutions were mixed with sodium acetate buffer solution (pH 6.0) to give final volume of 15 mL (β-glucosidase 0.074 U/mL), and then the mixed solution reacted at 50 °C for 12 h. Ultraviolet–visible (UV–vis) spectrophotometry was used to measure the spectra of the reacted solution, and the concentrations of Gen and Gep in the reacted solution were detected by UPLC-PDA every 2 h. According to the same procedure, Gen and β-glucosidase were reacted with Ala and Glu, respectively, while Cat and β-glucosidase, as well as Leo and β-glucosidase, were reacted with Arg, Ala, and Glu, respectively.
2.3.5 Preparation of sample solution for clarification on the Maillard and caramelization reactions
Standards were accurately weighed and dissolved with sodium acetate buffer solution (pH 4.8) to obtain respective stock solutions at the concentrations of 600.011 mM for Fru, 600.061 mM for Glc, 600.088 mM for Suc, 600.042 mM for Raf, 600.020 mM for Sta, 59.963 mM for Arg, 59.906 mM for Asp, and 60.051 mM for Ala. The stock solutions were stored at 4 °C before analysis. Afterwards, 1 mL each of Fru and Arg stock solutions were mixed with sodium acetate buffer solution (pH 4.8) to give final volume at 15 mL. The mixture then reacted at 100 °C for 18 h, followed by UV–vis analysis to explore the Maillard reaction. According to the same procedure, Glc (Suc, Raf, or Sta) was reacted with Ala, Arg, and Asp, respectively.
Fru stock solution (1 mL) was mixed with sodium acetate buffer solution (pH 4.8) to give final volume at 15 mL, and then reacted at 100 °C for 18 h for UV–vis analysis. According to the same procedure, Glc, Suc, Raf, and Sta underwent self-reactions respectively to explore the caramelization reaction.
2.4 The enrichment of crosslinking coloring reaction products (CCRPs) and Maillard reaction products (MRPs) from processed products of RR
RRR sample powder (2.5 g) was ultrasonically extracted (144 W, 35 kHz) with 75 mL ultrapure water for 30 min. After centrifugation at 13,322 g for 10 min, the supernatant was collected and concentrated to 30 mL. The concentrated solution was subjected to MCI Gel CC by elution with MeOH-H2O (0:100, 50:50, 75:25, v/v) and 150 mL of each gradient elution was collected to afford fractions with CCRPs. Each fraction was then concentrated and analyzed by UV–vis. The enriched CCRPs from PRR samples were analyzed by the established method.
RRR sample powder (0.25 g) was ultrasonically extracted (144 W, 35 kHz) with 25 mL 75 % methanol aqueous solution for 30 min. After centrifugation at 13,322 g for 10 min, the supernatant was collected. Then, 10 mL of the supernatants was taken into the sealed dialysis bag (molecular weight cutoff 3500 Da) and immersed in ultrapure water for 24 h. During this period, the ultrapure water was refreshed three times. The enriched MRPs were analyzed via a UV–vis system. The successful method was undertaken to enrich MRPs from PRR samples.
2.5 UPLC-PDA analysis
The amino acids contents of different processed products of RR and RW were analyzed by an ACQUITY UPLC system (Waters, Milford, MA, USA) equipped with an ACQUITY UPLC® BEH C18 column (2.1 × 100 mm, 1.7 µm, Waters) maintained at 52 °C, using the method outlined by Zhang et al. (2016) with slight modifications. The mobile phase system consisted of pH 6.5 sodium acetate buffer solution (A) and acetonitrile (B), using the following gradient program: 0-2 min, 1 %-4% B; 2-4 min, 4 %-15 % B; 4-5 min, 15 %-17 % B; 5-6 min, 17 %-20 % B; 6-10 min, 20 %- 40 % B.
In order to explore the rule of crosslinking coloring reaction, UPLC analysis was performed on an ACQUITY UPLC® HSS T3 column (2.1 × 100 mm, 1.8 μm, Waters) maintained at 35 °C. The mobile phase consisted of 0.1 % formic acid solution (A) and acetonitrile (B), using a following gradient program: 0-6 min, 1 %-40 % B. Both mobile phase systems were maintained at flow rate of 0.3 mL/min. The sample injection was 2 µL, and the monitoring wavelengths were set at 254 and 203 nm.
2.6 HPLC-ELSD analysis
Using the method described in a previous study (Hu et al., 2013), the analysis of oligosaccharides and monosaccharides in different processed RR products and the exploration of oligosaccharides hydrolysis were performed on a Waters 2695 HPLC system coupled with ELSD. The separation was achieved on a Prevail Carbohydrate ES column (4.6 mm × 250 mm, 5 μm, Shanghai Chenqiao Biotech Co., Ltd., China) operated at 30 °C. The mobile phase was composed of acetonitrile (A) and water (B), using following elution condition: 0-20 min, 28 %-36 % B. The sample injection was 5 µL. The evaporator temperature for the ELSD was set at 100 °C with a nitrogen gas flow at 1.0 L/min.
2.7 UV–vis analysis
UV–vis analysis was performed on a UV-2600 system (SHIMADZU, Tokyo, Japan) equipped with quartz cells with 1 mm path length. The absorbance spectra were recorded ranging from 200 to 800 nm.
2.8 Statistical analysis
The tested values were represented as mean ± standard deviation (SD). Excel 2016 and SPSS Statistics 24.0 were used for data analysis. Unpaired t-test was used for multiple comparisons (P<0.05 for significant difference) and plotted with Origin 2019b.
3 Results and discussion
3.1 Influence of processing on the physicochemical properties of RR
By taking the weight, water loss rate, buffering capacity, and pH into account, the variations of key physicochemical properties were comprehensively and systematically investigated during the processing of RR. As shown in Fig. 1(A-D), a notable transition from FRR to PRR was observed, characterized by darkening color, sweetening flavor, and stickier texture, which indicated the change of internal physicochemical environment of RR. The lab values about color changes from FRR to PRR are provided in Fig. S1. Obvious decrease in the weight of RR was recorded, with moisture reducing by 71.5 % after extended heating. To evaluate buffering capacity, different volumes of 0.5 % HCl (0 ∼ 1000 µL) and 0.5 % NaOH (0 ∼ 1000 µL) were added into 50 mL of ultrapure water and FRR juice, respectively. Contrary to water, whose pH value fluctuated between 2.49 and 11.40, the pH value of FRR juice consistently stabilized around 6.5, demonstrating its incredible buffering ability. Besides, the pH value showed slight decrease from FRR to RRR, followed by a distinct drop from RRR to PRR, with pH value falling from 6.5 to 4.8. This decline could be ascribed to the decomposition of the proteins into acidic peptides or free amino acids released under high temperature and long-term heating during the processing of PRR (Ni et al., 1989).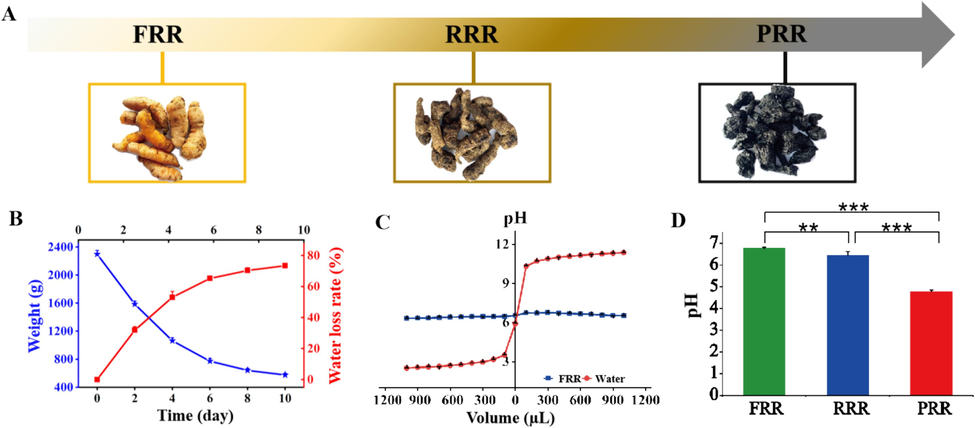
(A) Variations in the appearance during the processing from FRR to PRR. (B) Variations of the weight and water loss rate during the processing of RRR. (C) The buffering capacity of FRR. (D) The pH values in different processed RR products. *P<0.05; **P<0.01; ***P<0.001.
3.2 Unveiling the transformation mechanism of saccharides hydrolysis for “sweetness as candy”
Five oligosaccharides (Suc, Mel, Raf, Man, and Sta) and two free monosaccharides (Glc and Fru) were determined in different processed RR products, whose content variations are illustrated in Fig. S2. It was observed that Sta was the most abundant oligosaccharide, reaching up to 562.4 mg/g in FRR, yet dramatically decreased to 42.93 mg/g in PRR induced by processing. On the contrary, Man, Mel, Glc, and Fru were barely undetectable in FRR, yet witnessed to climb in content especially from RRR to PRR, with concentrations reaching 329.3 mg/g, 51.39 mg/g, 101.2 mg/g, and 163.2 mg/g in PRR, respectively. The levels of Suc and Raf were highest in RRR, followed by FRR, and were lowest in PRR, suggesting the hydrolysis of oligosaccharides during processing.
To further explore the impact of processing on the changes of saccharides contents in RR, the mechanisms were discussed under simulated processing condition of PRR (100 °C, pH 4.8) for 18h. As shown in Fig. 2A, Suc, composed of Glc and Fru, was hydrolyzed into the corresponding monosaccharides after 18 h of heating, revealing the increase of Glc and Fru and the decrease in Suc levels in PRR. Similarly, Raf, which is formed by Fru and Mel, broke down into corresponding Fru and Mel, leading to a decline in Raf and an increase in Mel levels. Furthermore, the increase of Man and Fru can be attributed to the release of fructofuranosyl in Sta. However, Mel and Man exhibited no significant change in content after 18 h of heating, suggesting that the glycosidic bond between Fru and galactose (Gal) is stable and may require more severe conditions for hydrolysis. The processing-induced chemical transformation pathways of saccharides in RR are presented in Fig. 2B, from which the tasteless saccharides such as Sta and Raf are gradually converted into sweeter monosaccharides such as Fru, contributing to a “sweetness as candy” flavor in PRR.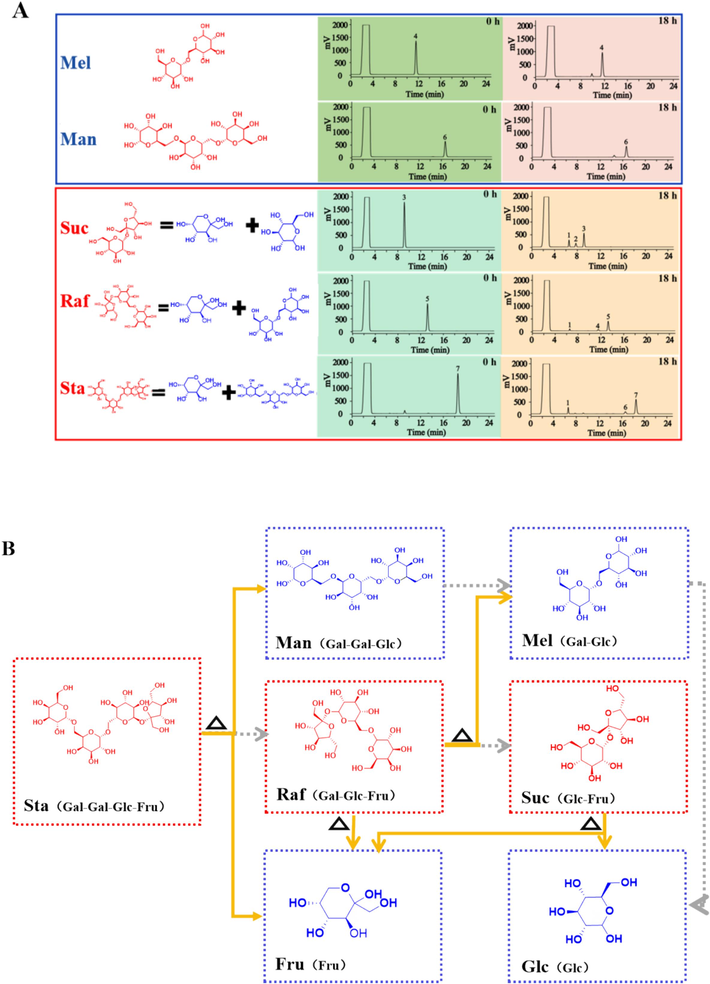
(A) Representative HPLC-ELSD chromatograms of reacted solutions of saccharides hydrolysis at 0 h and 18 h (1. Fru; 2. Glc; 3. Suc; 4. Mel; 5. Raf; 6. Man; 7. Sta). (B) The processing-induced chemical transformation pathways of saccharides in RR (Solid arrows: validated degradation pathways; dotted arrows: possible degradation pathways).
3.3 Exploration on the CCRPs in different processed RR products to explain the mechanism of crosslinking reaction for“black as lacquer”
Gen, driven by β-glucosidase secreted by medicinal plants, is transformed into Gep and subsequently reacts with methylamine to produce blue pigment (Bentes et al., 2015). Researchers have documented the presence of β-glucosidase in FRR (Zhao et al., 2006). Our previous study has demonstrated that iridoid glycosides, especially Cat, Leo, and Gen, presented declining tendency from RRR to PRR (Yang et al., 2024). In this study, the dynamic variations in amino acids contents in different processed RR products were investigated using UPLC-PDA analysis. As shown in Fig. S3, the contents of alkaline amino acids (Lys, His, and Arg), acidic amino acids (Asp and Glu), and neutral amino acids (Trp and Gln) decreased by more than 78 % during the process from FRR to PRR. Noteworthily, RW also contains abundant amino acids, significantly influencing the contents of amino acids in PRR. Therefore, it is speculated that iridoid glycosides, catalyzed by β-glucosidase, can react with various amino acids to form CCRPs in PRR.
To substantiate our hypothesis, an MCI CC method was performed to enrich the CCRPs in different processed RR products. The ratio of material/solvent (1:10, 1:20, 1:30, and 1:40) and extraction time (20, 30, and 40 min) were optimized to maximize extraction efficiency and satisfactorily yield CCRPs. As shown in Fig. 3, the absorbance measurements of enriched fractions from FRR, RRR, and PRR revealed a characteristic absorption peak at 540 nm in RRR and PRR sample solutions, with the peak being particularly noticeable in the 50 % MeOH fraction of PRR. However, there was no absorption at 540 nm in FRR. This phenomenon offered stronger evidence that crosslinking coloring reaction does occur during the processing of both RRR and PRR, with more obvious effects observed in PRR.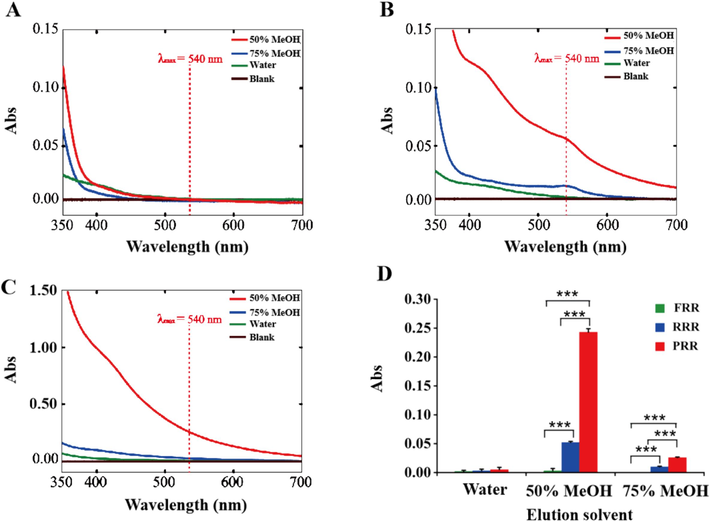
UV–vis absorption curves of CCRPs in (A) FRR, (B) RRR, and (C) PRR. (D) The absorbance of different enriched fractions from FRR, RRR, and PRR at 540 nm (n = 3). *P<0.05; **P<0.01; ***P<0.001.
To elucidate the formation mechanisms of CCRPs in RRR, Cat, Leo, and Gen, respectively represented by the epoxy ether[c]pyran, cyclopentano[c]pyran, and cyclopentenyl[c]pyran ring systems, were employed for reaction with Ala, Arg, and Glu (neutral, alkaline, and acidic amino acids) seperately under simulated processing conditions of RRR (50 °C, pH 6.0) with the presence of β-glucosidase. As depicted in Fig. 4, in 12 h, the content of Gen consistently decreased, whereas the content of its aglycone, Gep, initially increased within the first 6 h and then approached an equilibrium state, indicating that Gen is metabolized into Gep by β-glucosidase. Concurrently, the absorption values of CCRPs formed from reactions between Gen and different amino acids gradually raised in the presence of β-glucosidase, manifesting that Gep can react with amino acids to produce CCRPs. Notably, characteristic absorption peaks at 590-600 nm were detected in the UV–vis spectra at 12 h. In contrast, Cat and Leo fail to form CCRPs (Figs. S4 and S5) due to the inability of β-glucosidase to transform these compounds into their corresponding aglycones.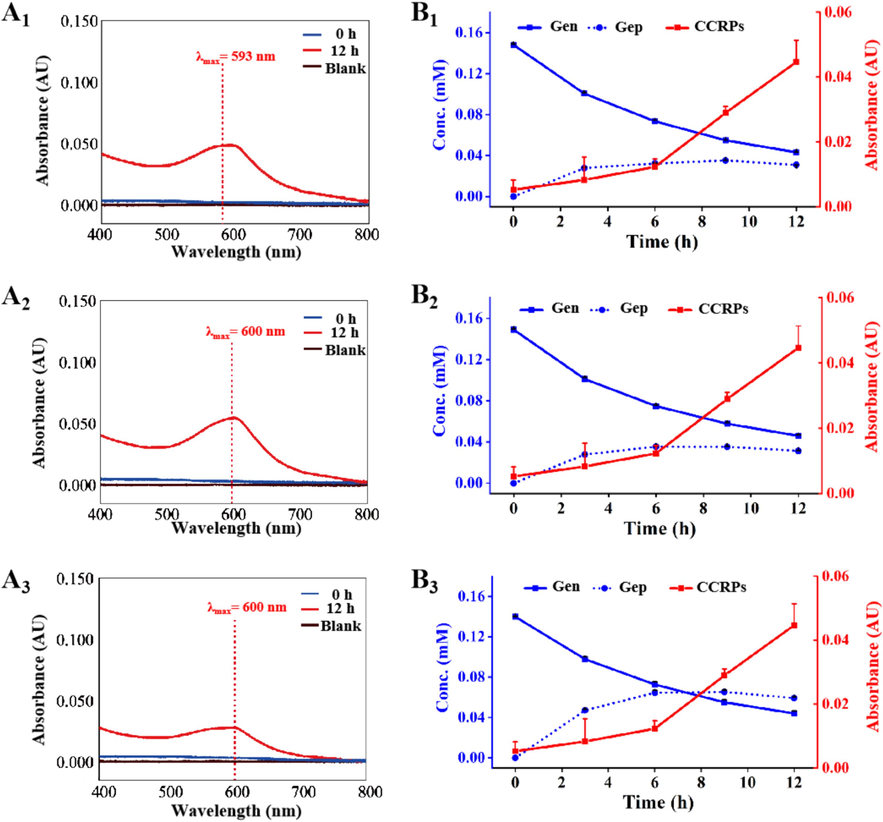
UV–vis spectra of CCRPs from reactions between Gen and (A1) Ala, (A2) Arg, or (A3) Glu in the presence of β-glucosidase. Dynamic variations on the contents of Gen and Gep, and the absorbance of CCRPs from reactions between Gen and (B1) Ala, (B2) Arg, or (B3) Glu in the presence of β-glucosidase in 12 h.
The formation of CCRPs from Gep is happened through nucleophilic attack by primary amine groups on the C-3 atom of Gep, subsequently embedding tertiary nitrogen in the six-member ring in place of an oxygen atom (Mu et al., 2013). Gardenia blue, the final product of a crosslinking coloring reaction, is supramolecular assemblies, which is formed via the covalent polymerization of basic units into dimers, followed by non-covalent self-assembly. The development of color is influenced by various factors, such as pH, temperature, type of amino acid, and genipin-to-amino acid ratio (Brauch, 2016; Zhang et al., 2023). Crosslinking coloring reaction derived from reactions between Gep and amino acids provides a plausible basis for the darkening color of PRR.
3.4 Exploration on the MRPs in different processed RR products to explain the mechanism of Maillard and caramelization reactions for“black as lacquer”
Maillard reaction not only generates volatile small molecules and brown macromolecules that are crucial for the food and nutrition industries, but also plays an essential role in the processing of TCMs (Cao et al., 2009; Liu et al., 2020). MRPs are formed in the early, intermediate, and final stages. As significant Maillard reaction intermediates, Amadori rearrangement products are key flavor and browning precursors (Chen et al., 2022), which can be monitored through absorbance at 294 nm. The increase in absorbance at 420 nm serves as an indicator for browning development in the final stage of the reaction (Zhou et al., 2016b; Sun et al., 2023). The MRPs formation may be significantly affected by the type and concentration of precursors, pH, time, and temperature (Gong et al., 2019b). Along with the Maillard reaction, caramelization of saccharides also occurs, which is a group of reactions that take place when carbohydrates are exposed to high temperatures in the absence of amino groups (Henning and Glomb, 2016; Wang et al., 2022). The two reactions take place simultaneously, influencing each other and enhancing chemodiversity in the processing (Eliodório et al., 2023). Therefore, MRPs are likely presented in PRR which is evidenced by the gradually deepened color during the processing of RR.
The preparation method of MRPs was optimized by investigating extraction solvent (H2O, 25 %, 50 %, and 75 % methanol aqueous solution), the ratio of material/solvent (1:50, 1:100, and 1:200), and extraction time (20, 30, and 40 min) to achieve the highest extracting efficiency. To minimize the impact of extraneous components, dialysis was employed to further enrich MRPs and remove interfering substances. The dialysis results confirmed that MRPs exist in different processed products of RR. As shown in Fig. 5, distinct absorption peaks at 440 nm were observed in FRR, RRR, and PRR, showing the highest absorption in PRR, which indicates that the processing conditions for PRR are more favourable of producing MRPs. However, the formation mechanisms of the MRPs in PRR remain uncertain and deserve further exploration.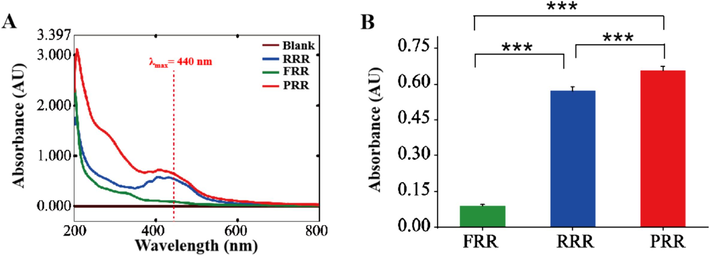
(A) UV–vis absorption curves of MRPs in different processed RR products. (B) The absorbance value of MRPs at 440 nm (n = 3). *P<0.05; **P<0.01; ***P<0.001.
The rules of Maillard reaction occurring between saccharides and representative amino acids were explored under simulated preparation condition of PRR (100 °C, pH 4.8), whose results are presented in Fig. 6. The UV–vis absorbance at 450 nm was applied to assess changes on the MRPs from the final stage in Fru, Fru-Ala, Fru-Arg, and Fru-Asp reaction systems. From the UV–vis absorption spectra, obvious characteristic absorption peak at 450 nm can be witnessed in Fru, Fru-Ala, Fru-Arg, and Fru-Asp reaction systems. Similarly, the Sta, Sta-Ala, Sta-Arg, and Sta-Asp reaction systems exhibited strong absorption peak at the focused wavelength. In contrast, Glc, Suc, Raf, and their combinations with Ala, Arg, and Asp showed no significant absorbance at 450 nm. This suggests that Fru and Sta are primary saccharides contributing to the black color of PRR, thus explaining the mechanism behind the “black as lacquer” appearance of PRR.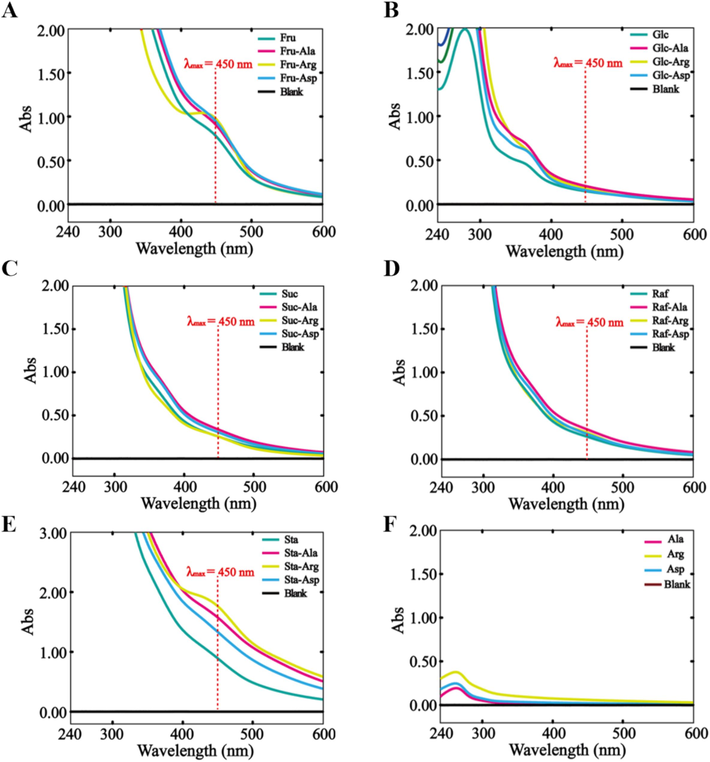
UV–vis spectra of MRPs from reactions between (A) Fru and different amino acids (Ala, Arg, and Asp), (B) Glc and different amino acids (Ala, Arg, and Asp), (C) Suc and different amino acids (Ala, Arg, and Asp), (D) Raf and different amino acids (Ala, Arg, and Asp), and (E) Sta and different amino acids (Ala, Arg, and Asp), respectively. (F) UV–vis spectra obtained by reactions of amino acids themselves.
The products from Maillard, crosslinking coloring, caramelization, and saccharides hydrolysis reactions are closely related to processing conditions. With the extended steaming time and increased steaming cycles, the PRR became darker in surface color and sweeter in taste. This is due to the fact that the extended steaming time and increased steaming cycles promote the formation of black materials and degradation of saccharides during the processing of PRR. Shennong’s Classic of Materia Medica also have documented that RR must undergo multiple cycles of steaming and sun-drying, even to the extent of being “nine times steamed and nine times sun-dried,” to ensure it achieves a quality “black as lacquer and sweetness as candy”. Therefore, the steaming time and temperature should be strictly controlled to obtain PRR with high quality and efficacy. Furthermore, based on a deeper understanding of the scientific principles underlying the processing of PRR, our study suggested that the level of monosaccharides and black materials are the important quality indicators for PRR.
4 Conclusions
Based on the sensory description as “black as lacquer and sweetness as candy” in PRR, the dynamic variations in components and the characteristics of chemical reactions during the processing of RR were systematically investigated. Illumination on the transformation mechanism of saccharides hydrolysis is meaningful to clarify the feature of “sweetness as candy”. Additionally, the production of CCRPs and MRPs and the exploration of their formation mechanism are beneficial to clarify “black as lacquer” attribute, primarily through the pathways of crosslinking coloring, Maillard, and caramelization reactions. In conclusion, our study will play a pivotal role in elucidating the chemical reactions induced by processing of RR, enabling enhanced control over the color and flavor of PRR, and ensuring that PRR maintains consistent and desirable therapeutic effects for clinical applications.
CRediT authorship contribution statement
Jingjing Yang: Visualization, Writing – original draft. Cheng Xue: Conceptualization, Data curation, Methodology, Visualization. Lihua Zhang: Data curation, Methodology, Writing – review & editing. Ning Meng: Writing – review & editing. Jing Yang: Data curation, Visualization. Ying Cui: Writing – review & editing. Yuefei Wang: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing. Xin Chai: Data curation, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing.
Acknowledgments
This research was funded by the Science and Technology Program of Tianjin (23ZYJDSS00030 and 22ZYJDSS00100), the Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine (22HHZYSS00007 and 22HHZYJC00007), and Shandong Provincial Natural Science Foundation (ZR2021LZY035).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Influence of the composition of unripe genipap (Genipa americana L.) fruit on the formation of blue pigment. J Food Sci Technol.. 2015;52(6):3919-3924.
- [CrossRef] [Google Scholar]
- Underutilized fruits and vegetables as potential novel pigments sources. In: Carle R., Schweiggert R., eds. Handbook on natural pigments in food and Beverages: Industrial applications for improving food color (pp.305e327). Cambrige: Woodhead Publishing; 2016.
- [Google Scholar]
- Effective components of Chinese herbal compound decoction and Maillard reaction. Chin J Integr Med.. 2009;15(3):224-228.
- [CrossRef] [Google Scholar]
- Traditional processing strongly affects metabolite composition by hydrolysis in Rehmannia glutinosa roots. Chem Pharm Bull (tokyo).. 2011;59(5):546-552.
- [CrossRef] [Google Scholar]
- Quantitative determination of Nε-(carboxymethyl)lysine in sterilized milk by isotope dilution UPLC-MS/MS method without derivatization and ion pair reagents. Food Chem.. 2022;385:132697
- [CrossRef] [Google Scholar]
- Chinese Pharmacopoeia Commission. 2020. Chinese Medical Science and Technology Press. vol. 4.
- Research progress on effect of processing on properties and efficacy of traditional Chinese medicine containing iridoid terpenoids based on stability of compounds. Chin Tradit Herb Drugs.. 2021;52(16):5039-5051.
- [CrossRef] [Google Scholar]
- Effects of caramelization and Maillard reaction products on the physiology of Saccharomyces cerevisiae. Fungal Biol.. 2023;127(12):1534-1543.
- [CrossRef] [Google Scholar]
- Analysis of amino acids in Rehmannia glutinosa Libosch. during heating process. Amino Acids & Biotic. Resources.. 2010;32(3):52-54.
- [CrossRef] [Google Scholar]
- Comparisons of antithrombosis, hematopoietic effects and chemical profiles of dried and rice wine-processed Rehmanniae Radix extracts. J Ethnopharmacol.. 2019;231:394-402.
- [CrossRef] [Google Scholar]
- Advances in effects and regulation of Maillard reaction on quality of Chinese materia medica. Chin Tradit Herb Drugs.. 2019;50(1):243-251.
- [CrossRef] [Google Scholar]
- Pathways of the Maillard reaction under physiological conditions. Glycoconj J.. 2016;33(4):499-512.
- [CrossRef] [Google Scholar]
- Genotoxicity evaluation of the naturally-derived food colorant, gardenia blue, and its precursor, genipin. Food Chem Toxicol.. 2018;118:695-708.
- [CrossRef] [Google Scholar]
- Determination of oligosaccharide contents in different processed Radix Rehmannia Praeparata by HPLC-ELSD. Shizhen Guo Yi Guo Yao.. 2013;24(4):877-879.
- [CrossRef] [Google Scholar]
- Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix. Biomed Pharmacother.. 2023;168:115809
- [CrossRef] [Google Scholar]
- A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J Ethnopharmacol.. 2022;285:114820
- [CrossRef] [Google Scholar]
- A novel strategy to rapidly explore potential chemical markers for the discrimination between raw and processed Radix Rehmanniae by UHPLC-TOFMS with multivariate statistical analysis. J Pharm Biomed Anal.. 2010;51(4):812-823.
- [CrossRef] [Google Scholar]
- Kinetics of color development, pH decreasing, and anti-oxidative activity reduction of Maillard reaction in galactose/glycine model systems. Food Chem.. 2008;108(2):533-541.
- [CrossRef] [Google Scholar]
- Maillard conjugates and their potential in food and nutritional industries: A review. Food Front.. 2020;1(4):382-397.
- [CrossRef] [Google Scholar]
- Exploration of the relationship between the processing of Rehmannia glutinosa and Maillard Reaction based on HPLC-MS and network pharmacology. Zhongyaocai.. 2020;43(1):61-70.
- [CrossRef] [Google Scholar]
- The dynamic changes and mechanisms of Rehmanniae Radix processing based on Maillard reaction. Tradit Med Res.. 2021;6(1):1-10.
- [CrossRef] [Google Scholar]
- Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J Biomed Mater Res a.. 2013;101(2):385-393.
- [CrossRef] [Google Scholar]
- Genipin: A natural blue pigment for food and health purposes. Trends Food Sci Technol.. 2017;67:271-279.
- [CrossRef] [Google Scholar]
- Comparative analysis of free amino acids in Radix Rehmanniae and its processed products. China J Chin Mater Med.. 1989;14(3):21-22.
- [Google Scholar]
- Structure and flavor characteristics of Maillard reaction products derived from soybean meal hydrolysates-reducing sugars. LWT–Food Science and Technology.. 2023;185:115097
- [CrossRef] [Google Scholar]
- Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem Pharm Bull.. 1994;42(3):668-673.
- [CrossRef] [Google Scholar]
- Formation of DDMP, HMF and furfural in caramelization and Maillard reaction. Sci Technol Food Ind.. 2022;43(12):100-107.
- [CrossRef] [Google Scholar]
- Determination of endpoint of procedure for Radix Rehmanniae steamed based on ultraviolet spectrophotometry combination with continuous wavelet transform and kernel independent component analysis. Anal Chim Acta.. 2010;679(1–2):43-48.
- [CrossRef] [Google Scholar]
- Simultaneous analysis of furfural metabolites from Rehmanniae Radix Preparata by HPLC-DAD-ESI-MS. Food Chem.. 2014;142:107-113.
- [CrossRef] [Google Scholar]
- Simultaneous determination of iridoid glycosides, phenethylalcohol glycosides and furfural derivatives in Rehmanniae Radix by high performance liquid chromatography coupled with triple-quadrupole mass spectrometry. Food Chem.. 2012;135(4):2277-2286.
- [CrossRef] [Google Scholar]
- Simultaneous determination of eight saccharides contents in Radix Rehmanniae and its different processed products by HPLC-ELSD. Chin J Pharm Anal.. 2023;43(6):939-949.
- [CrossRef] [Google Scholar]
- Exploration of the dynamic variations of the characteristic constituents and the degradation products of catalpol during the process of Radix Rehmanniae. Molecules.. 2024;29(3):705.
- [CrossRef] [Google Scholar]
- Determination of amino acid in fermented Cordyceps preparation by HPLC with precolumn derivatization method. Chin J Pharm Anal.. 2016;36(8):1338-1348.
- [CrossRef] [Google Scholar]
- Unraveling the mechanism of the supramolecular self-assembly during the in vivo metabolism of geniposide from Chinese medicine. Materials & Design.. 2023;225:111546
- [CrossRef] [Google Scholar]
- Extraction and salting out purification of α-galactosidase and β-glucosidase from the fresh roots of Rehmannia glutinosa. Zhongyaocai.. 2006;29(2):137-139.
- [CrossRef] [Google Scholar]
- Integrating targeted glycomics and untargeted metabolomics to investigate the processing chemistry of herbal medicines, a case study on Rehmanniae Radix. J Chromatogr A.. 2016;1472:74-87.
- [CrossRef] [Google Scholar]
- Chemomics-based marker compounds mining and mimetic processing for exploring chemical mechanisms in traditional processing of herbal medicines, a continuous study on Rehmanniae Radix. J Chromatogr A.. 2017;1530:232-240.
- [CrossRef] [Google Scholar]
- The effects of reactants ratios, reaction temperatures and times on Maillard reaction products of the L-ascorbic acid/L-glutamic acid system. Food Sci Technol.. 2016;36(2):268-274.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105917.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







