Translate this page into:
Unveiling the phytochemical profile and biological potential of five Dendrobium species
⁎Corresponding author. zhangxiaodanzstu@163.com (Xiaodan Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Dendrobium, a genus of orchids celebrated for their ornamental, medicinal, and culinary uses, is the focus of this study, which examines the metabolite profiles and bioactivity of five species: Dendrobium officinale, Dendrobium nobile, Dendrobium crepidatum, Dendrobium chrysotoxum, and Dendrobium denneanum. Utilizing UPLC-Q-TOF-MSE analysis, we have identified 88 compounds from the stems and leaves of these species. Notably, extracts from D. denneanum and D. chrysotoxum exhibited the highest antioxidant activities as demonstrated by DPPH, ABTS radical scavenging, and FRAP assays. Additionally, D. officinale showed significant anti-hepatoma activity with an IC50 value of 104 μg/mL. Multivariate analysis, hierarchical cluster analysis, and metabolites correlation analysis further elucidated the bioactive potential of these species. This comprehensive approach revealed distinct metabolite profiles that correlate with the observed bioactivities. The antioxidant activities of D. denneanum and D. chrysotoxum were particularly noteworthy, suggesting their potential as natural sources of antioxidants for pharmaceutical and nutraceutical applications. The anti-hepatoma activity of D. officinale, with its notable IC50 value, indicates its promise as a therapeutic agent against liver cancer. The findings underscore the richness of bioactive compounds in Dendrobium species, highlighting their antioxidant and anti-hepatoma properties. These insights pave the way for further research into the medicinal utilization of Dendrobium leaves and contribute to the development of novel applications in healthcare and wellness industries. The potential health benefits identified in this research emphasize the importance of preserving and studying these valuable plant species for their diverse bioactivities.
Keywords
Dendrobium
Antioxidation
Anti-hepatoma
UPLC-Q-TOF-MSE
Difference analysis
1 Introduction
Dendrobium refers to a group of perennial epiphytic herbs that belong to the Orchidaceae family, which are primarily found in the tropical and subtropical regions across Asia and Oceania (Pei et al., 2017; Xiao et al., 2017). The genus is remarkably diverse, encompassing more than 1100 species, including notable ones such as D. officinale, D. nobile, D. crepidatum, D. chrysotoxum, and D. denneanum (Yu et al., 2013; Cong et al., 2017). Among these, certain species like Dendrobium officinale and Dendrobium huoshanense are particularly valued for their dual medicinal and culinary properties. These species are not only used in traditional medicine but are also incorporated into a variety of foods and beverages. They are consumed in forms such as fresh juice, wine, and a range of processed products. In particular, D. officinale and D. nobile are utilized to create popular products like Dendrobium beverages, wines, fresh-squeezed drinks, and gel candies. The growing popularity of these products reflects a rising interest in the health benefits and unique flavors offered by Dendrobium species.
Dendrobium possesses significant edible and medicinal value, containing a variety of chemical compounds. Increasingly, researchers are focusing on the study of its secondary metabolites and pharmacological effects (Hu et al., 2021a; Hu et al., 2021b; Xu et al., 2022). For decades, researchers have identified the chemical components of Dendrobium through LC-MS/MS, nuclear magnetic resonance, and other methods, and found that Dendrobium contains various types of chemical components, such as polysaccharides, alkaloids, phenols, bibenzyls, etc. Among them, polysaccharides have the pharmacological effects of anti-oxidant, anti-tumor, hypoglycemic, enhancing immunity, protecting the liver, lowering blood sugar, lowering blood pressure, anti-cancer, anti-arrhythmia, and anti-epilepsy (Chan et al., 2018). Several studies have shown that polysaccharides are one of the main active ingredients of Dendrobium. Consequently, the extractions, quantitative, and qualitative analysis of Dendrobium polysaccharides, along with their pharmacological studies, have become significant research hotspots both domestically and internationally. (Fan et al., 2018). The alkaloid components of Dendrobium exhibit neuroprotective, anti-tumor and liver injury protective effects, with the most active alkaloids primarily found in D. nobile, D. crepidatum, and D. huoshanense etc. (Liang et al., 2023). Bibenzyl compounds, characteristic components of traditional Chinese medicine Dendrobium, primarily exhibit anti-tumor, anti-cataract, antibacterial, antioxidant, and anti-angiogenesis activities (Wang et al., 2022). These active substances are predominantly found in the stems and leaves (Shen et al., 2020).
Recent reports highlight the potent proliferation inhibition of HeLa cells by nine bibenzyl compounds, including denofficisn, densiflorol A, dendrocandin B, gigantol, dendrocandin U, 3,4-dihydroxy-5,4′-dimethoxy bibenzyl, moscatilin, 4, 4′-dihydroxy-3, 5-dimethoxy bibenzyl, and (S)-3, 4, α-trihydroxy-5, 4′-dimethoxy bibenzyl (Ren et al., 2020). Concurrently, advancements in artificial cultivation techniques have led to an abundance of Dendrobium leaves as residual by-products (Fan et al., 2021). Recognized for their developmental potential, Dendrobium leaf resources are attracting increasing attention in research. This study investigates five distinct Dendrobium species such as D. officinale, D. crepidatum, D. chrysotoxum, D. nobile, and D. denneanum. Chemical analyses were conducted to assess the total contents of polysaccharides, alkaloids, and bibenzyls, alongside antioxidant and anti-hepatocarcinoma activities across these species. Utilizing UPLC-Q-TOF-MSE, the chemical constituents of stems and leaves from these Dendrobium species were identified.
In recent years, the research hotspots concerning Dendrobium alkaloids have centered on the identification of these alkaloids within various Dendrobium species and the exploration of their medicinal activities (Ding et al., 2021). Among the diverse bioactive compounds found in Dendrobium, bibenzyl compounds stand out as characteristic components. These compounds are notable for their simple structures, diverse skeleton connection methods, and abundant substituents, which contribute to their varied biological activities. Our study aimed to offer fresh perspectives on the metabolite profiles and bioactivities of Dendrobium species, hoping to spark renewed interest in these remarkable organisms. Specifically, we compared the total contents of polysaccharides, alkaloids, and bibenzyls across five different Dendrobium species, providing a comprehensive analysis of their bioactive potential.
Furthermore, the present study unveils the phytochemical characterization of both the stems and leaves of five Dendrobium species from Zhejiang and Yunnan provinces, namely D. officinale, D. nobile, D. crepidatum, D. chrysotoxum, and D. denneanum, using ultra-performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS). The biological profiles of these species were screened through in vitro antioxidant and anti-hepatoma activity tests. The results clearly demonstrate the distinct activities of the chemical substances present in these species, providing a solid experimental foundation for the development of natural antioxidant and anti-hepatoma drugs derived from Dendrobium. Additionally, this study serves as a valuable reference for the comprehensive utilization of the medicinal and edible components of Dendrobium leaves, promoting their potential in both pharmaceutical and nutritional applications.
2 Materials and methods
2.1 Plant collection
Dendrobium species such as D. officinale, D. nobile, D. denneanum, and D. chrysotoxum were collected from Jiande City, Zhejiang Province, and Wenshan City, Yunnan Province. D. crepidatum specimens were obtained from Kunming City, Yunnan Province. Collection took place in November under the supervision of Professor Liang Zongsuo from the College of Life Science and Medicine at Zhejiang Sci-Tech University. Voucher specimens were identified and authenticated by Professor Liang Zongsuo and subsequently deposited at the Key Laboratory of Plant Secondary Metabolism, Zhejiang Sci-Tech University, China (Specimen Numbers: 20211101007, 20211101008, 20211101009, 20211101010, and 20211101011).
2.2 Analysis of total polysaccharide, alkaloid, and bibenzyls content
2.2.1 Quantification of polysaccharide content
The phenol–sulfuric acid technique was employed to quantify the polysaccharide content in the stems and leaves of the five Dendrobium species. Initially, 9 mg of anhydrous glucose was dissolved in a 100 mL volumetric flask using distilled water to prepare a 90 μg/mL glucose standard solution. Aliquots of this standard solution were pipetted into separate 10 mL test tubes and diluted with distilled water to obtain a series of glucose standard solutions with concentrations of 18 μg/mL, 36 μg/mL, 54 μg/mL, 72 μg/mL, and 90 μg/mL. Freshly prepared 5 % phenol solution (1 mL) was added to each test tube, mixed thoroughly, and then supplemented with 5 mL of concentrated sulfuric acid. The mixture was heated in a 100 °C water bath for 20 min, cooled rapidly in an ice bath for 5 min, and the absorbance was measured at 488 nm using a multifunctional microplate reader.
To prepare the sample solution, 0.3 g of Dendrobium sample powder was refluxed with 200 mL of water for 2 h. The mixture was then transferred to a 250 mL volumetric flask, adjusted to volume with water, and filtered. Two milliliters of the filtrate was mixed with 10 mL of anhydrous ethanol and refrigerated at 4 °C for 1 h to precipitate the polysaccharides. The mixture was centrifuged at 4000 r/min for 20 min, the supernatant was discarded, and the precipitate was washed with 80 % ethanol, dissolved in hot water, and adjusted to 25 mL with water. For the determination of polysaccharide content, 1 mL of the sample solution was transferred into a test tube, and the same protocol used for preparing the polysaccharide standard curve was followed. The absorbance was measured at 488 nm, and the polysaccharide content was calculated using the standard curve.
2.2.2 Determination of alkaloid content
For the determination of alkaloid content, various reagents were prepared according to standard protocols. This included 0.01 mol/L sodium hydroxide ethanol solution, 0.2 mol/L sodium hydroxide solution, pH 4.5 buffer solution, and 0.04 % bromocresol green solution. To construct the alkaloid standard curve, 1.00 mg of dendrobine was dissolved in a 100 mL volumetric flask with chloroform to make a 10 μg/mL dendrobine standard solution. This solution was then used to prepare dendrobine standard solutions with concentrations of 1–5 μg/mL. Each solution was mixed with 5 mL of pH 4.5 buffer solution and 1 mL of 0.04 % bromocresol green solution, shaken for 3 min, and allowed to separate. Six milliliters of the lower layer was transferred to a centrifuge tube, mixed with 1 mL of 0.01 mol/L sodium hydroxide ethanol solution, and the absorbance was measured at 620 nm.
To prepare the sample solution for alkaloid content determination, 0.5 g of Dendrobium sample powder was moistened with 5 mL of ammonia water, left to sit for 30 min, then refluxed with 30 mL of chloroform for 2 h. The solution was adjusted to the original weight with chloroform, filtered, and 3 mL of the filtrate was transferred to a 10 mL volumetric flask and diluted to volume. Two milliliters of this solution was taken into a 25 mL volumetric flask and made up to volume with distilled water. For the determination, 10 mL of the sample solution was transferred to a separatory funnel, and the same protocol as the alkaloid standard curve preparation was followed. The absorbance was measured at 620 nm, and the alkaloid content was calculated using the standard curve (Yao et al., 2021; Zhou, 2021; Zhang et al., 2020).
2.2.3 Determination of bibenzyls content
The determination of bibenzyls content involved dissolving 4 mg of dendrophenol in a 5 mL volumetric flask with distilled water to obtain an 800 μg/mL standard solution. This solution was used to prepare dendrophenol standard solutions with concentrations of 0 μg/mL, 8 μg/mL, 16 μg/mL, 32 μg/mL, and 54 μg/mL. Each standard solution was mixed with 1 mL of Folin-phenol reagent and 1.7 mL of 2 mol/L Na2CO3 solution, diluted to 10 mL with water, and heated at 30 °C for 1 h. The absorbance was measured at 760 nm, and a standard curve was plotted.
For the sample solution preparation, 0.5 g of Dendrobium powder was extracted with 30 mL of 70 % ethanol using ultrasound for 1 h, filtered, mixed with 0.1 g of casein, heated at 30 °C with agitation for 1 h, filtered again, evaporated to dryness, and dissolved in water to a final volume of 100 mL. For the determination of bibenzyls content, 1 mL of the sample solution was transferred to a volumetric flask, mixed with 1 mL of Folin-phenol reagent and 1.7 mL of 2 mol/L Na2CO3 solution, and the same protocol as the bibenzyl standard curve preparation was followed. The absorbance was measured at 760 nm, and the bibenzyls content was calculated using the standard curve (Li, 2020).
2.3 Sample preparation for LC-MS analysis
The samples were dried in an oven at 50 °C until a consistent weight was achieved. Once dried, they were ground using a pulverizer, passed through a 60-mesh sieve, and sealed for preservation. For extraction, 20 mg of the powder was accurately weighed and placed in a 2 mL centrifuge tube, followed by the addition of 1 mL of 70 % methanol. The mixture underwent ultrasonic extraction at 60 °C for 45 min. After extraction, the samples were centrifuged at 8000 rpm for 10 min, and the supernatant was collected for further analysis.
2.4 UPLC-Q-TOF-MS/MS analysis
Chromatographic separation was performed using a Waters ACQUITY UPLC HSS T3 chromatographic column (2.1 mm × 100.0 mm, 1.8 μm; Waters Corporation, Milford, MA, USA). The elution was carried out with a gradient mixture of water (A) and acetonitrile (B) using the following gradient program: 0–1 min, 4 % B; 1–7 min, 4–50 % B; 7–8 min, 50–70 % B; 8–12 min, 70–95 % B; 12–15 min, 95 % B; 15–16 min, 95–4 % B; and 16–18 min, 96 % A. The column temperature was maintained at 40 °C, with a flow rate of 0.4 mL/min. A sample injection volume of 1 μL was used, and detection was conducted at 254 nm (Lou et al., 2021).
Mass spectrometry detection was conducted in electrospray modes, utilizing argon as both the collision gas and desolvation gas (Waters Corporation, Milford, MA, USA). The desolvation temperature was set to 400 °C, and the source temperature was kept at 100 °C. The full scan data range spanned from 50 to 1200 Da, with a scan frequency of 1.000 s. The spray standard concentration was locked at 400 mg/mL. For low collision energy scanning, the collision energy was set at 6.000 V, whereas for high collision energy scanning, it ranged from 30 to 70 V. The UPLC system was controlled by MassLynx 4.1 software (Waters Corporation, Milford, MA, USA).
2.5 In vitro antioxidant assays
2.5.1 Extraction
The sample was dried to a constant weight in an oven at 50 °C, then pulverized and passed through a 60-mesh sieve. A total of 10 g of the resulting powder was mixed with 500 mL of 70 % methanol and subjected to ultrasonic extraction at 60 °C for 45 min, followed by filtration. The extract was concentrated using a rotary evaporator, dried into a powder using a freeze dryer, and stored under sealed conditions at −20 °C.
2.5.2 ABTS assay
For the ABTS assay, vitamin C was used as the positive control. An ABTS solution at a concentration of 7 mmol/L was mixed with a 5 mmol/L potassium persulfate solution and left to stand in darkness for 12 h to generate the ABTS+• reaction solution. This reaction solution was then diluted with ethanol to achieve an absorbance of 0.700, forming the working solution. The Dendrobium extract was dissolved in 60 % ethanol to prepare various concentration gradients: 100 μg/mL, 200 μg/mL, 400 μg/mL, 600 μg/mL, 1000 μg/mL, and 2000 μg/mL. For each concentration, 40 μL of the sample solution was mixed with 160 μL of the working solution in a 96-well plate. The absorbance (Ap) at 734 nm was measured using a microplate reader after 6 min of reaction in the dark. The absorbance of the blank (Aq) was measured using 60 % ethanol instead of the sample solution, and the absorbance of the control (Aw) was measured using distilled water instead of the diluted ABTS+• reaction solution. The scavenging rate of ABTS+• at different concentrations was calculated according to Formula (1-1) (He et al., 2023).
2.5.3 DPPH assay
A DPPH solution with a concentration of 0.1 mmol/L was prepared with anhydrous ethanol and vitamin C was used as a positive control. The Dendrobium extract was dissolved in 60 % ethanol to create various mass concentration gradients: 100 μg/mL, 200 μg/mL, 400 μg/mL, 600 μg/mL, 1000 μg/mL, and 2000 μg/mL. Following the method adjusted from references (Fan and Wang, 2022; Kedare and Singh, 2011; Lawag et al., 2021), 100 μL of each sample was mixed with 100 μL of DPPH solution in 96-well plates and allowed to react in the dark at 37 °C for 30 min. The absorbance (Ap) was measured at 517 nm using a multifunctional microplate reader. The absorbance of the blank (Aq) was measured using 60 % ethanol instead of the sample solution, and the absorbance of the control (Aw) was measured using anhydrous ethanol instead of the DPPH solution. The DPPH scavenging rate of the samples at different concentrations was calculated using formula (1-2).
2.5.4 FRAP assay
The FRAP reagent was prepared by mixing 3.6 mol/mL sodium acetate buffer (pH 3.6), 10 mmol/L TPTZ solution, and 20 mmol/L FeCl3 solution in a ratio of 10:1:1. This reagent was then dissolved with 60 % ethanol to create different mass concentration gradients ranging from 100 to 2000 μg/mL. For the assay, 20 μL of each sample solution at different concentrations was combined with 180 μL of FRAP working solution in a 96-well plate and incubated at room temperature for 5 min. Each concentration was repeated three times to obtain the absorbance value (Aj), measured at 593 nm. Vitamin C served as the positive control, and a standard curve was established using ferrous sulfate. As a blank, 180 μL of FRAP working solution was replaced with 180 μL of distilled water and mixed with 20 μL of sample solution at different concentrations, followed by a 5-minute incubation at room temperature. The absorbance value of each well at 593 nm was measured using a microplate reader. Each concentration was repeated three times to obtain the absorbance value (A0). By substituting Aj and A0 into the ferrous sulfate standard curve formula, the corresponding ferrous sulfate concentration was determined, and the difference between these values was defined as the FRAP value.
2.6 Determination of anti-hepatoma activity in vitro
Different concentration gradients of Dendrobium extract solutions were prepared using cell culture medium. Preliminary experiments determined the final concentrations for the CCK-8 assay, as the inhibitory effects on liver cancer cells varied among extracts. In a 96-well plate, 100 μL of PBS was added to the peripheral wells, and 100 μL of cell-free culture medium was added to each well in the blank control group. Huh7 cells were cultured in the 96-well plates. Cells in the exponential growth phase were harvested to form a dispersed cell suspension. One milliliter of this suspension was mixed with 14 mL of culture medium, and 100 μL of this mixture was added to each well of the experimental group. 5-Fluorouracil was used as a positive control for its known anti-tumor activity. Each experimental group had three replicates. The plates were incubated at 37 °C in a 5 % CO2 atmosphere. After 24 h, the culture medium was discarded, and 100 μL of the extract solutions with varying concentrations were added. The blank group received culture medium without extracts. The plates were incubated for another 48 h, after which 10 μL of CCK-8 was added to each well and incubated for 2 h. Absorbance (OD) was measured at 450 nm (Gao et al., 2022; Xia et al., 2023; Zhou et al., 2022). The inhibition rate of the extracts on cancer cells was calculated using Formula (1-3).
2.7 Data analysis
All data presented as means ± standard of at least three independent experiments. The mass spectrometry fragment information obtained was compared with the compound information recorded in the PubChem database(https://pubchem.ncbi.nlm.nih.gov/),Scifinder database (https://scifinder-n.cas.org/) and the literature in the MassLynx4.1 software (Waters Corporation, Milford, MA, USA)for automatic peak identification, peak matching, peak alignment, peak extraction, peak integration and normalization.After normalization, the chemometric analyses were encompassed byMetaboAnalyst 5.0 (https: //https://www.metaboanalyst.ca), where it wassubjected to, hierarchical cluster analysis, correlation analysis and PCA. Pearson’s correlation analysis was performed to determine thecorrelation amongst all variables. The resulting correlation networkswere visualized by CytoscapeVersion 3.7.2.
3 Results and discussion
3.1 Content of polysaccharide, alkaloid and bibenzyl
Understanding the polysaccharide, alkaloid, and bibenzyl content in different Dendrobium species is crucial for several reasons. Polysaccharides are known for their immunomodulatory and anti-tumor properties, making them valuable in pharmaceutical applications. Alkaloids, with their diverse biological activities, including anti-inflammatory and anti-cancer effects, are also of significant medicinal interest. Bibenzyls possess antioxidant and anti-inflammatory properties, contributing to their potential therapeutic uses. By quantifying these compounds in various Dendrobium species, this study provides valuable insights into their medicinal potential, guiding their use in traditional medicine and modern pharmacology. Moreover, the study highlights the species with the highest content of these beneficial compounds, aiding in the selection of species for specific therapeutic applications. For instance, the high polysaccharide content in D. officinale stems suggests its strong potential for use in immune-boosting and anti-tumor therapies, while the high alkaloid content in D. crepidatum positions it as a candidate for anti-inflammatory treatments.
At first, polysaccharide content was quantified using the phenol–sulfuric acid method. The calibration curve, derived from anhydrous glucose, was y = 0.0024x + 0.1034 (R2 = 0.9924). The concentration of polysaccharides in the test solutions was determined using a standard curve (Fig. 1a, d). Notably, the polysaccharide content in the stems was higher than in the leaves across all five Dendrobium species. For instance, D. nobile had 11.530 % in its stems and 2.458 % in its leaves. D. officinale exhibited the highest polysaccharide content in its stems at 49.57 %, significantly surpassing the other species, while D. crepidatum had the lowest.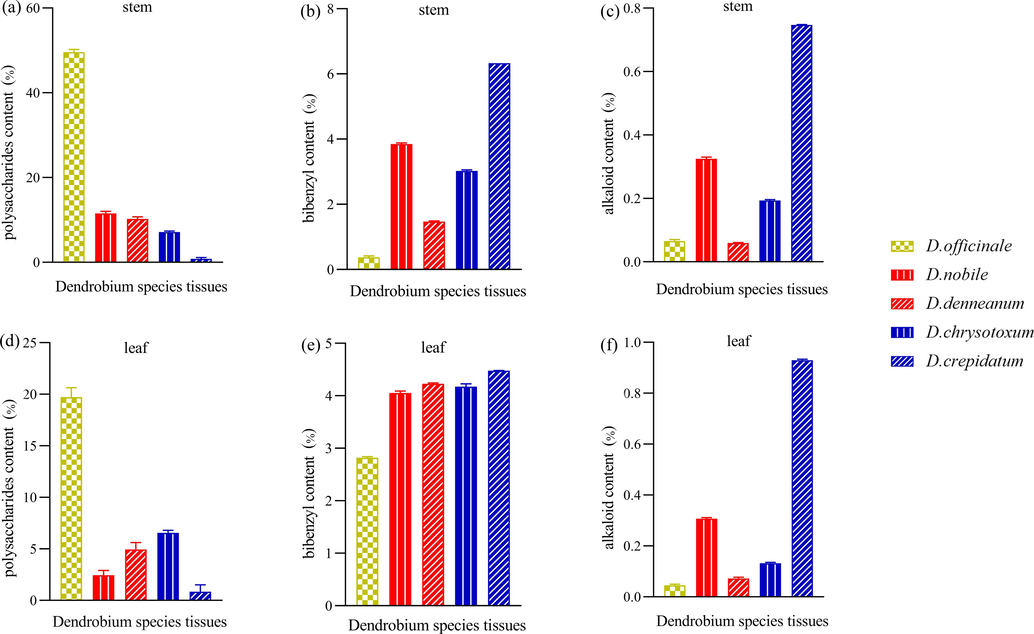
The contents of phytochemical composition in the stem and leaf of five different Dendrobium species.
Then, bibenzyl content was measured using the Folin-Ciocalteu method. The calibration curve for gigantol was y = 0.0212x + 0.1240 (R2 = 0.9924) (Fig. 1b, e). The highest bibenzyl levels were found in the stems (6.33 %) and leaves (4.475 %) of D. crepidatum. This was followed by D. nobile and D. chrysotoxum, with D. officinale showing the lowest bibenzyl content in both stems and leaves. In addition, alkaloid content was determined using a colorimetric method with acid dyes. The calibration curve for dendrobine was y = 0.0700x − 0.0526 (R2 = 0.9951). The results indicated that D. crepidatum had the highest alkaloid content in both stems and leaves, followed by D. nobile and D. chrysotoxum (Fig. 1c, f).
3.2 Metabolomic analysis
3.2.1 LC-MS/MS analysis
Methanol extracts from the roots and stems of the five Dendrobium species were subjected to detailed LC-MS/MS analysis. This metabolite profiling identified and classified 88 compounds across various phytochemical classes, including flavonoids, alkaloids, bibenzyls, phenanthrenes, terpenoids, esters, and phenolic glycosides (Table S13, Table S1-10). The following sub-sections provide a brief overview of these categories, while the Multivariate Analysis Section offers a thorough comparison of the differences among the species. Among the 33 alkaloids identified in the Dendrobium extracts, Inosine isomer (1), Inosine (4), N-methyldendrobinium (61), and Dendramine (73) were specifically found in the leaves of Dendrobium. In contrast, N-isopentenyl-dendroxinium (35), (+)-Epicatechin (37), N-trans-feruloyl tyramine (44), 2-Hydroxydendrobine (49), 6-Hydroxynobiline (55), Succinylcarnitine (58), N-isopentenyl-6-hydroxydendroxinium (76), 2′-Deoxyadenosine-5′-monophosphate (81), and Dendroxine (84) were identified in the stems. Furthermore, Dendrocrepine isomer (11), Adenosine (12), Crepidine isomer (15), Crepidatumines C (18), Dendrocrepidine C (21), N-methyldendrobinium isomer (23), Dendrocrepine (26), Dendrocrepidine B (28), Crepidamine (30), Drepidamine (32), Dendrocrepidine D (33), Homocrepidine B (34), Crepidine (40), 4-Hydroxy-dendroxine (45), Dendronobiline A (48), Dendroamine (50), Dendrine (57), Mubironine B (60), Dendrobine (64), and N-trans-p-coumaroyltyramine (77) were detected in both the leaves and stems of Dendrobium.
Various bibenzyls have been identified in the leaf and stem extracts of Dendrobium species. Pinoresinol (2) was specifically found in D. nobile, and Chrysotobibenzyl (70) was found in D. chrysotoxum. Moscatilin (74) and Chrysotoxine (86) were present in D. denneanum and D. chrysotoxum, respectively. Conversely, Crepidatin (31) was absent in D. chrysotoxum and D. officinale, while Gigantol (88) was absent in D. nobile and D. officinale. Lastly, Chrysotoxine (52) was detected in both D. officinale and D. crepidatum.
Flavonoids were the predominant category of phytochemicals, with 13 different derivatives found exclusively in the stem and leaf extracts of Dendrobium species. Isoliquiritin (41) and Apigenin (78) were identified only in D. nobile; Schaftoside (14), Isoschaftoside (16), Apigenin-6-C-α-L-arabinoside-8-C-β-D-xyloside (22), and Isoschaftoside isomer (17) were unique to D. officinale; Erigeside II (51) was specific to D. chrysotoxum; and Kaempferol (19) was found solely in D. crepidatum.
Out of the 9 Phenanthrenes identified in the Dendrobium extracts. Erianthridin(7) and Denobilone (8) were annotated only in D. nobile; Chrysotoxene(10), 2, 7-Dihydroxy-3, 4, 6-trimethoxy-phenanthrene (13) and 2,7-Dihydroxy-3,4-dimethoxyphenanthrene (85) were present onlyin D.chrysotoxum; Moscatin(62) and 9,10-Dihydro-5,9-dihydroxy-4-methoxy-2-phenanthrenyl-β-D-glucopyranoside (72) were noticed in D. denneanum.Denobilone B (27) was found in D. officinale and D. crepidatum. On the other hand, Confusarin (80) was absent in D. denneanum and D. nobile.
A total of 14 terpenoids have been identified in the leaf and stem extracts of Dendrobium species. Dendronobilin F (38), Dendroside G (42), Dendronobilin C (46), and Dendronobilin L (47) were only found in the stems of D. nobile. Dendronobilin H (6), Dendroterpenoids C (63), and Dendronobilin B (68) were specifically present in the leaf and stem extracts of D. nobile. Dendronobilin K (29), Flakinins B (65), Aduncin (67), and Dendronobiloside E (71) were found exclusively in D. crepidatum, D. chrysotoxum, D. officinale, and D. denneanum, respectively. Dendroside D isomer (83) was identified in D. officinale and D. crepidatum. Conversely, Dendronobiloside C (53) was absent in D. chrysotoxum, while Dendroside D (82) was absent in both D. chrysotoxum and D. denneanum.
The three esters identified include 3-(4-hydroxy-3-methoxy-phenyl)-acrylic acid octacosyl ester (54), Defuscin (87), and Dihydroconiferyl dihydro-p-cumarate (56), with 54 and 87 found in D. chrysotoxum and 56 in D. nobile. One fluorenone, Dengibsin (69), was observed in D. chrysotoxum. Three phenolic acids, namely Dendroflorin (3), 3,4-Dimethoxybenzoic acid (5), and 5-Hydroxy-6-methoxyphenylethanol (59), were also found in D. chrysotoxum. Two phenolic glycosides, Denneanoside A (39) and Dihydrosyringin (75), were spotted in D. denneanum. Lastly, two phenylpropanoids, p-Hydroxyphenylpropanoic acid (9) and Episyringaresinol (43), and one quinone, Emodin (79), were found in D. chrysotoxum.
3.3 Antioxidant activity
According to the theory of oxygen free radicals, biological oxidation can negatively impact human health and contribute to diseases. The balance of free radicals within the body is crucial for human well-being, and antioxidants play a key role in maintaining this equilibrium (Wang et al., 2017). Antioxidant compounds are increasingly valued in the pharmaceutical and nutraceutical industries (Trifan et al., 2022). Consuming plants rich in antioxidants can help keep the human body healthy and mitigate the damage caused by reactive oxygen species. Given this context, efforts were made to determine if the tested Dendrobium species could serve as a source of natural antioxidants. To explore the in vitro antioxidant potential of Dendrobium extracts, various chemical assays were conducted, including radical quenching (ABTS and DPPH) and reducing power (FRAP). According to Zhou and Lv, the DPPH free radical scavenging activity of D. officinale leaves was higher than that of the stems (Zhou and Lv, 2012), which aligns with our experimental results.
The results are presented in Table 1 further, non-biological radicals like DPPH and ABTS are commonly used in vitro experiments to evaluate the radical-scavenging abilities of plant extracts. From Table 1, the results show varying degrees of antioxidant efficacy in the extracts of stems and leaves from different Dendrobium species. The highest radical scavenging ability was observed in D. denneanum leaves (ABTS: 79.94 μg/mL, DPPH: 93.56 μg/mL), followed by D. chrysotoxum leaves (ABTS: 122.30 μg/mL, DPPH: 78.34 μg/mL) and D. nobile stems (ABTS: 194.07 μg/mL, DPPH: 110.93 μg/mL). The weakest radical scavenging ability was recorded in D. officinale stems (ABTS: 1002.47 μg/mL, DPPH: 390.67 μg/mL). The term “reducing power” refers to the ability of antioxidant compounds to donate electrons, as measured by the FRAP assay, which involves the conversion of Fe3+to Fe2+. D. chrysotoxum leaves (0.99 μg/mL) and D. denneanum leaves (1.13 μg/mL) demonstrated the highest levels of capability in the FRAP assay. These empirical results revealed the potent antioxidant potential of certain Dendrobium leaves, such as those from D. denneanum and D. chrysotoxum, indicating their potential as natural antioxidant agents. Given that Dendrobium stems are the primary raw material in the market; this study provides a scientific basis for improving the resource utilization efficiency of Dendrobium. Data are presented as mean ± standard deviation (SD) of three determinations; different superscript letters withincolumns indicate significant differences in the tested extracts for the same parts (p <0.05). ABTS: 2,2′-azino-bis(3- ethylbenzothiazoline) 6-sulfonic acid; DPPH: 1,1-diphenyl-2-picrylhydrazyl; FRAP: ferric ion reducing antioxidant power. IC50: the concentration at which 50 % inhibition of liver cancer cell proliferation occurs.
specimen
ABTS
IC50(μg/mL)DPPH
IC50(μg/mL)FRAP
IC50(μg/mL)CCK-8
IC50(μg/mL)
D. officinale-stem
1002.47±58.20a
390.67±5.48b
412.82±152.86a
1166±0.207d
D.officinale-leaf
729.23±57.57b
236.37±3.70f
16.13±1.27b
104±0.008h
D.nobile-stem
194.07±7.50f
110.93±1.86h
2.36±0.32b
1316±0.038c
D.nobile-leaf
220.30±6.25e
131.53±2.44g
2.88±0.38b
815±0.071e
D.denneanum-stem
282.07±12.08d
269.30±5.30e
4.13±0.27b
4151±0.082a
D.denneanum-leaf
79.94±9.00h
93.56±5.26i
1.13±0.044b
450±0.087b
D.chrysotoxum-stem
496.73±25.17c
335.83±4.87c
3.38±0.22b
1052±0.048d
D.chrysotoxum-leaf
122.30±0.60g
78.34±3.09j
0.99±0.01b
494±0.029g
D.crepidatum-stem
321.57±21.04d
279.73±3.80d
7.88±0.45b
935±0.139e
D.crepidatum-leaf
538.93±11.88c
474.63±5.32a
25.87±3.13b
638±0.028f
VC
9.97±0.15i
1.48±0.04k
0.01±0.00b
−
5-Fluorouracil
−
−
−
30±0.001i
3.4 Anti-hepatoma activity
Dendrobium, as a natural botanical resource, harbors medicinal attributes that extend to shielding the liver and curbing the proliferation of liver cancer cells (Zhao et al., 2021). In this study, in vitro assays were performed to assess the activity of an ethanolic extract of D. chrysotoxum leaves. The results showed weak in vitro inhibitory activity against HeLa human cervical cancer cells with a measured IC50 value of 450 μg/mL (Prasad et al., 2017). This is consistent with the IC50 of 494 μg/mL for D. chrysotoxum leaves in our experiment. The current study utilized the Huh7 human liver cancer cell line to investigate the anti-hepatoma potential of Dendrobium extracts. Extracts from the stems and leaves of five different Dendrobium species were introduced at various concentrations during the exponential growth phase of the liver cancer cells. After 24 h, the proliferation rate of the liver cancer cells was measured using the CCK-8 method. The extracts from the Dendrobium stems and leaves exhibited varying degrees of inhibitory effects on the growth of human liver cancer cells. The inhibitory impact on liver cancer cell proliferation showed a concentration-dependent pattern, with higher concentrations of Dendrobium extracts leading to greater suppression. According to Table S3, the most effective anti-hepatoma activity was found in D. officinale leaves (IC50: 104 μg/mL), followed by D. chrysotoxum leaves (IC50: 494 μg/mL) and D. crepidatum leaves (IC50: 638 μg/mL). The weakest anti-hepatoma activity was observed in the stems (IC50: 4151 μg/mL) and leaves (IC50: 3450 μg/mL) of D. denneanum. Additionally, all five leaf extracts demonstrated a higher rate of inhibiting liver cancer cell proliferation compared to their corresponding stem extracts.
These findings are particularly significant considering the current utilization practices of Dendrobium. The Chinese Pharmacopoeia predominantly recognizes the stem of Dendrobium as the medicinal part, while the leaves are typically discarded (Commission, 2020). However, our study suggests that the leaves, which are often overlooked and discarded, possess substantial anti-cancer properties. This revelation could lead to more comprehensive use of Dendrobium plants, reducing waste and enhancing the sustainability of medicinal plant utilization. In conclusion, the study highlights the untapped potential of Dendrobium leaves in anti-cancer applications. By demonstrating the significant anti-hepatoma activity of leaf extracts, particularly from D. officinale, this research advocates for the reconsideration of current practices regarding Dendrobium usage. The integration of leaves in medicinal applications could enhance the therapeutic value derived from these plants, promoting a more sustainable approach to their cultivation and utilization. Future studies should focus on isolating and characterizing the specific bioactive compounds responsible for these effects, paving the way for the development of novel anti-cancer therapies based on Dendrobium extracts.
3.5 Multivariate analysis
A Pearson correlation analysis was performed to assess the relationship between bioactive compounds and biological activities. Fig. 2 shows the correlation heatmap, revealing a strong correlation (R > 0.7) between total polysaccharide content and scavenging and reducing abilities. However, the anti-hepatoma ability assays correlated moderately with total polysaccharide levels. In recent years, studies have shown that the physiological activity of D. officinale is closely related to the polysaccharide contents. In Fig. 2, the individual compounds exhibited different correlations with the biological activities. Crepidamine (30), Aduncin (67) and Apigenin (78) were strongly correlated with free radical scavenging, reducing power and anti-hepatoma ability, while Schaftoside (14), Isoschaftoside (16), Rutin (24), Quercetin (25) and Dendroside D (82) were moderately correlated with free radical scavenging, reducing power and anti-hepatoma ability.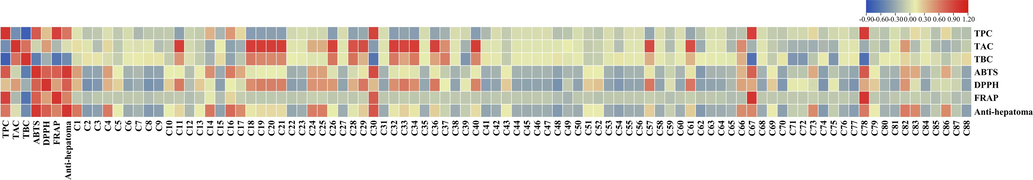
Correlation analysis of the phytochemical composition and biological activities. ABTS: 2,20 -azino-bis (3-ethylbenzothiazoline) 6-sulfonic acid; DPPH: 1,1-diphenyl-2-picrylhydrazyl; FRAP: ferric ion reducing antioxidant power; TPC: total polysaccharide content; TAC: total alkaloid content; TBC: total bibenzil content. Compounds are numbered as in Table S13.
The PCA results illustrating the overall distribution trend of Dendrobium species are depicted in Fig. S11. Upon examining the intra-group relationships, it is visually apparent that the stems and leaves of D. crepidatum and D. nobile exhibit complete separation. In contrast, the stems and leaves of the remaining three Dendrobium species overlap to a significant extent, suggesting that the disparity between the stems and leaves of D. crepidatum and D. nobile is more pronounced than that of the other species. Moreover, on a broader group level, D. crepidatum and D. nobile form distinct clusters apart from the other three Dendrobium groups, whereas D. chrysotoxum, D. officinale, and D. denneanum demonstrate considerable overlap, indicating a high degree of similarity among these species.
3.6 Hierarchical cluster analysis
In Hierarchical cluster analysis, we have conducted a comprehensive examination of metabolite distribution across the stems and leaves of five Dendrobium species. Fig. 3a presents a dendrogram from hierarchical cluster analysis (HCA), revealing distinct clusters formed by samples from these species' leaves and stems. Notably, D. officinale, D. chrysotoxum, and D. denneanum clustered closely together, suggesting similarities in their metabolic ingredient compositions. Additionally, we employed Ward's hierarchical clustering algorithm to analyze the distribution of 88 different metabolites, depicted in the clustering heatmap (Fig. 3b). This heatmap highlights significant differences in compound distribution among the species. Specifically, D. officinale predominantly contains alkaloids and flavonoids, D. nobile is rich in alkaloids and terpenoids, D. denneanum exhibits bibenzyl, phenanthrene, and terpenoids, D. chrysotoxum contains phenanthrene, bibenzyl, and phenolic acids, while D. crepidatum is characterized by alkaloids and flavonoids. These findings underscore the substantial variation in compound richness among the five Dendrobium species, providing valuable insights into their metabolic diversity and potential bioactivity profiles.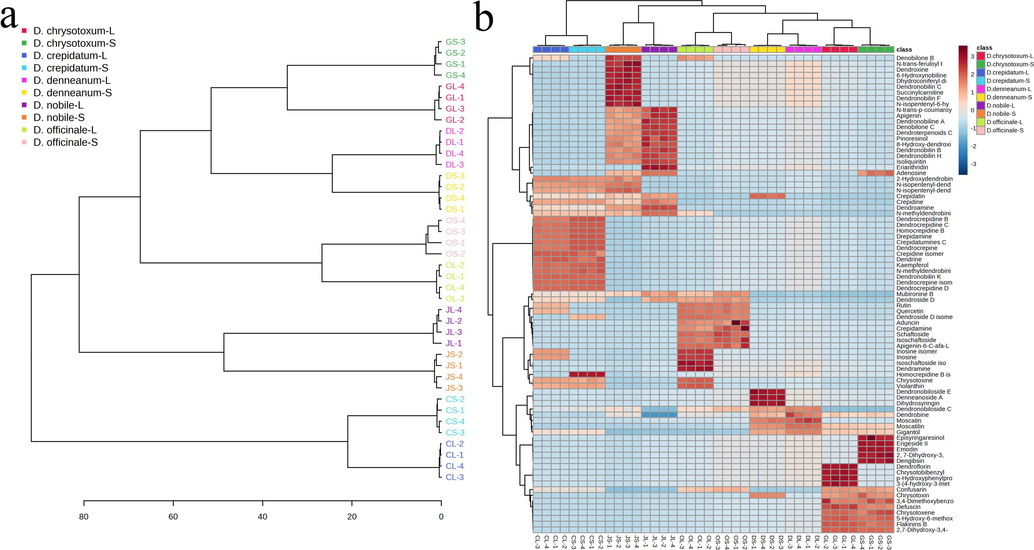
Hierarchical cluster analysis. a. HCA of 40 Dendrobium samples. b. Heat map visualization for deferent tissues of Dendrobium by 88 screened potential biomarkers. Abscissa indicates the samples information, the ordinate indicates the differential metabolites which are presented as the metabolite names. The color squares changed from blue to red indicate the increasing amount of the metabolites. Data are presented five replicates. Clustered image map (red color: high content; blue color: low content) based on the chemical composition dataset. GS and GL is the "Stem" and "Leaf" of D.chrysanthum, DS and DL is the "Stem" and "Leaf" of D.denneanum, OS and OL is the "Stem" and "Leaf" of D. officinale, JS and JL is the "Stem" and "Leaf" of D. nobile, CS and CL is the "Stem" and "Leaf" of D. crepidatum.
3.7 Metabolites correlation analysis
Correlation analysis is a strong approach for characterizing the metabolic affinities of metabolites with significant differences, and it may also be used to find inter-regulatory interactions between metabolites during changes in biological states. In the present study, Pearson correlation analysis was employed to uncover the interrelationships between the 88 differential components. The Pearson correlation coefficient-based correlation analysis results were then visualized with a correlation heat map (Fig. 4). In all groups, a total of 7744 correlations were analyzed, amongst which 1271 metabolic pairs resulted in significant correlation (| r |>0.5, p <0.001. Amongst the 1271 correlating pairs, 1081 positive correlations (r>0.5, p <0.001) and 190 negative correlations (r <-0.5, p <0.001) were observed (Table S11). As revealed by Fig. 4, these metabolites showed four distinctive components groups indicated by a dotted box. Out of these groups, there were a large number of alkaloids, terpenoids molecules in group 1, and fluorenones, phenanthrene, phenolic acids, bibenzyls compounds in group 2, and flavonoids, terpenoids, alkaloidscompounds in group 3, while group 4 mainly contained alkaloids, flavonoids, bibenzyl molecules.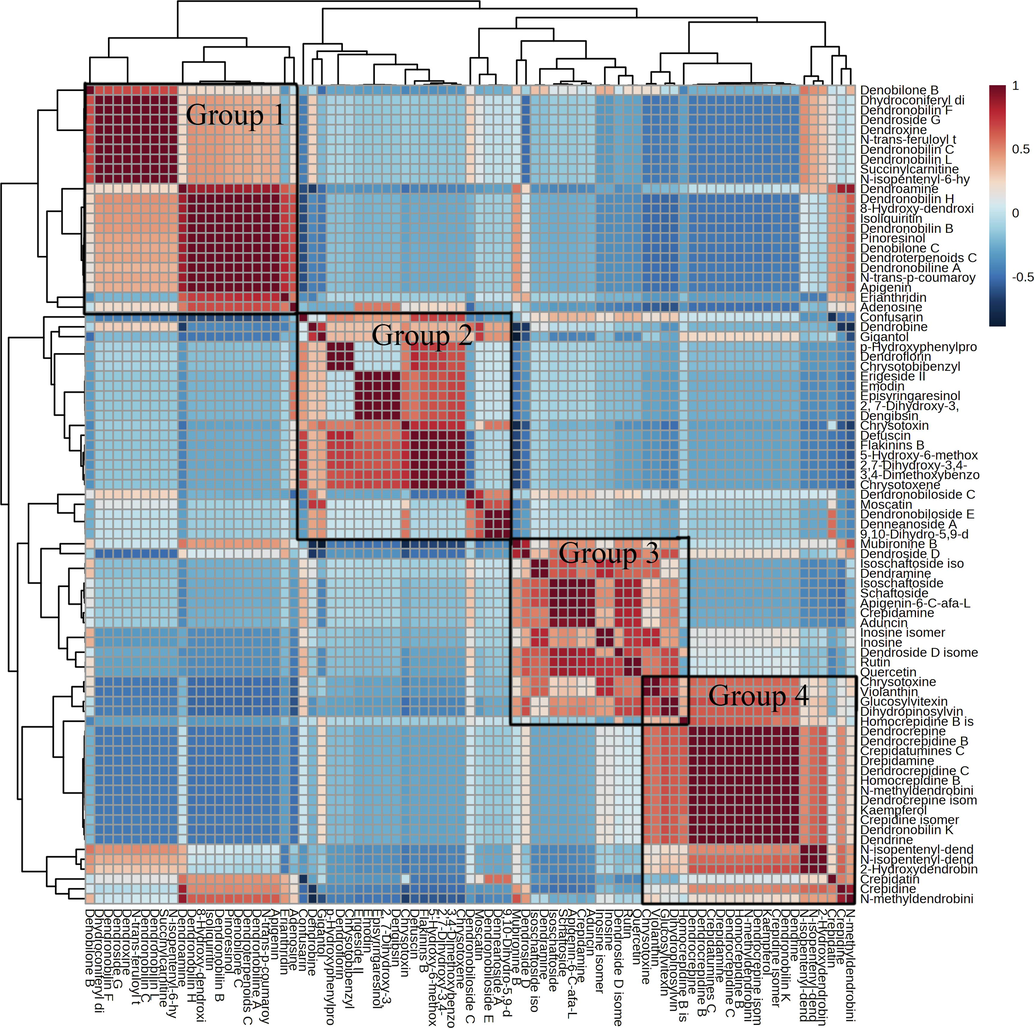
Analysis of metabolite-metabolite association. The Pearson correlation coefficient for a pair of metabolites is represented by a square. A positive correlation is indicated by red, a negative correlation by blue, and a non-significant correlation by white. The darker the color, the stronger the positive or negative correlation.
To further visualize the synergistic regulatory relationships among various metabolites, we constructed a correlation network of metabolite pairs with correlation coefficients | r | > 0.5 and p <0.001 (Fig. 5.). The network contained 85 nodes, with 1231 edges. Examining at Fig. 5, it is apparent that both Dendronobiloside C, Gigantol and Dendrobine had significant negative correlations with other metabolites, and they were terpenoids, gigantol and alkaloids compounds, which indicated the three metabolites may be decomposed for the synthesis of other metabolites. Moreover, the correlation coefficients between these compounds are known. From the data in the table S12, it can be seen visually that 6-Hydroxynobiline, Dihydroconiferyl dihydro-p-cumarate, Dendronobilin F, Dendroside G, Dendroxine, N-trans-feruloyl tyramine, Dendronobilin C, Succinylcarnitine, N-isopentenyl-6-hydroxydendroxinium are highly correlated with the other metabolites. The high percentage of alkaloids, terpenoids and esters among these compounds may indicate that they play an important role in the anabolism of other compounds. The association of these metabolites revealed not just the relationship between anabolism and catabolism, but also the functional correlation of variable substances.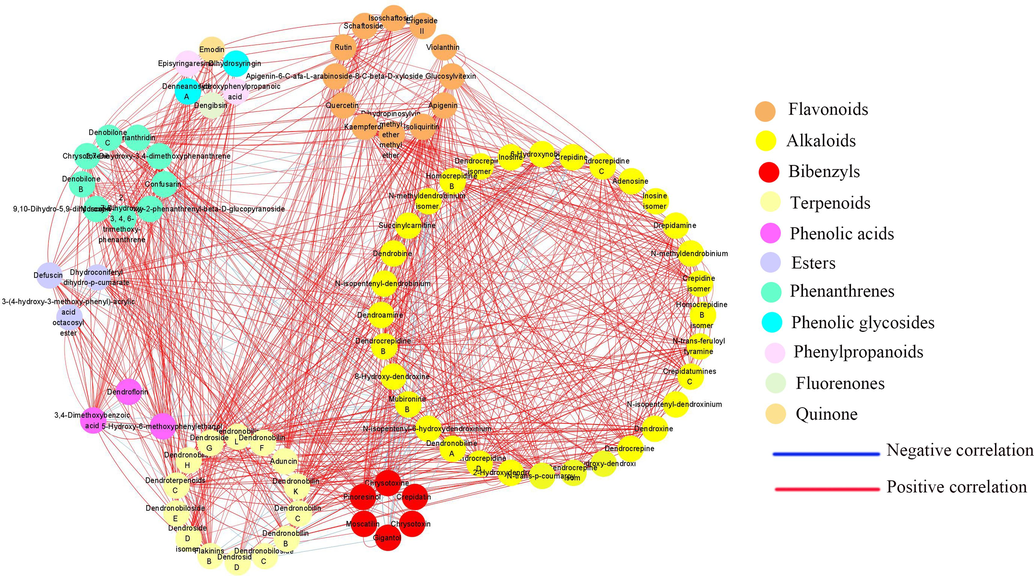
The significant correlation network for five Dendrobium species. Nodes represent metabolites and the size of nodes are related to the connectivity degree. The larger the degree is, the larger the node is. And the color of the edge represents correlation, which means red indicates positive correlation, and blue indicates negative correlation. The width of the edge represents the absolute value of the correlation coefficient. If the line is thick, the correlation is great.
4 Conclusions
This study comprehensively explored the pharmacological potential and chemical composition of various Dendrobium species, focusing on their anti-hepatoma activity, antioxidant properties, and metabolite profiles. The investigation revealed significant variability in bioactive compounds among Dendrobium stems and leaves, highlighting D. officinale as particularly rich in polysaccharides and exhibiting potent anti-hepatoma effects. Conversely, D. denneanum displayed comparatively lower activity. The correlation analysis demonstrated strong associations between polysaccharide content and antioxidant capabilities, underscoring the physiological relevance of these compounds in Dendrobium species. Hierarchical cluster analysis further differentiated species based on metabolite profiles, emphasizing distinct chemical compositions between stems and leaves within Dendrobium species. These findings not only contribute to understanding the medicinal potential of Dendrobium but also advocate for the sustainable utilization of its leaf components, traditionally overlooked in favor of the stems. Future research should focus on elucidating the specific mechanisms underlying these biological activities and optimizing extraction techniques to maximize the therapeutic benefits of Dendrobium extracts. Overall, our research could pave the way for the extensive utilization of Dendrobium species as valuable reservoirs of bioactive metabolites, offering promising antioxidant and anti-hepatoma properties. Additionally, it provides a theoretical foundation for maximizing the utilization of Dendrobium leaves.
CRediT authorship contribution statement
Lingxia Peng: Writing – original draft, Formal analysis, Data curation, Conceptualization. Jiani Yu: Writing – original draft, Formal analysis, Data curation, Conceptualization. Jiahao Fang: Software, Resources, Project administration, Methodology, Investigation. Feng Yin: Software, Resources, Project administration, Methodology, Investigation. Gurusamy Abirami: Investigation, Software, Supervision. Jianxiong Wu: Resources, Software. Ganggui Lou: Software, Resources, Project administration. Hongju Li: Project administration, Methodology, Investigation. Lijun Yang: Formal analysis, Project administration. Jie Xia: Software, Resources. Dongfeng Yang: Supervision, Software, Resources. Zongsuo Liang: Writing – review & editing, Software, Resources. Xiaodan Zhang: Writing – review & editing, Validation, Supervision.
Acknowledgments
This work was supported by Zhejiang Province Basic Public Welfare Research Program Project [LGN22H280004]; The ability establishment of sustainable use for valuable Chinese medicine resources [2060302]; The Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province [No. 2021C02074]. Zhejiang Sci-Tech University Excellent Postgraduate Dissertation Cultivation Fund [LW-YP2024069].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidation and melanogenesis inhibition of various dendrobium tosaense extracts. Molecules.. 2018;23(7)
- [CrossRef] [Google Scholar]
- Commission, C. P., 2020. Pharmacopoeia Committee of the People's Republic of China. Beijing: China Medical Science and Technology Press.
- Dendrocrepidamine, a novel octahydroindolizine alkaloid from the roots of Dendrobium crepidatum. J. Asian. Nat. Prod. Res.. 2021;23(11):1085-1092.
- [CrossRef] [Google Scholar]
- Analysis of nutrients in flowers of Dendrobium officinale. J. Food Safety Quality.. 2021;12(21):8334-8341.
- [CrossRef] [Google Scholar]
- Partial characterization and antioxidant activities of polysaccharides sequentially extracted from Dendrobium officinale. J. Food Measur. Character.. 2018;12(2):1054-1064.
- [CrossRef] [Google Scholar]
- Study on crude protein extraction and DPPH free radical scavenging activity of Semen Astragali Complanati by different methods. Liaoning Chem. Indus.. 2022;51(11):1512-1515.
- [CrossRef] [Google Scholar]
- Marine collagen peptides: A novel biomaterial for the healing of oral mucosal ulcers. Dent Mater J.. 2022;41(6):850-859.
- [CrossRef] [Google Scholar]
- Optimization of extraction process of flavonoids from aronia melanocarpas' leaves and analysis of their antioxidant and bile salt binding capacity. Sci. Technol. Food Industry.. 2023;44(02):253-260.
- [CrossRef] [Google Scholar]
- Molecular cloning and structural analysis of key enzymes in Tetrastigma hemsleyanum for resveratrol biosynthesis. Int. J. Biol. Macromol.. 2021;190:19-32.
- [CrossRef] [Google Scholar]
- The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: A valuable Chinese herbal medicine. J. Ethnopharmacol.. 2021;271:113836
- [CrossRef] [Google Scholar]
- Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.. 2011;48(4):412-422.
- [CrossRef] [Google Scholar]
- Optimisation of bee pollen extraction to maximise extractable antioxidant constituents. Antioxidants (Basel). 2021;10(7)
- [CrossRef] [Google Scholar]
- The study of the best harvest time and the comparation of slice and segment of dendrobium denneanum. Chengdu University of Traditional Chinese Med. 2020
- [CrossRef] [Google Scholar]
- Research progress on pharmacological activity and mechanism of action of alkaloids in Dendrobium. Food Nutrition China. 2023;29(04):40-47.
- [CrossRef] [Google Scholar]
- Differences in the chemical composition of Dendrobium officinale Kimura et Migo and Dendrobium crepidatum Lindl based on UPLC-Q-TOF-MS/MS and metabolomics. Acta Pharmaceutica Sinica.. 2021;56(12):3331-3344.
- [CrossRef] [Google Scholar]
- Diversity of endophytic bacteria of Dendrobium officinale based on culture-dependent and culture-independent methods. Biotechnol. Biotechnol. Equipment.. 2017;31(1):112-119.
- [CrossRef] [Google Scholar]
- Dendrobium chrysanthum ethanolic extract induces apoptosis via p53 up-regulation in HeLa cells and inhibits tumor progression in mice. J. Compl. Integr. Med.. 2017;14:20160070.
- [Google Scholar]
- Bibenzyl derivatives from leaves of dendrobium officinale. Nat. Product Commun.. 2020;15(2)
- [CrossRef] [Google Scholar]
- Antagonistic activity of combined bacteria strains against southern blight pathogen of Dendrobium officinale. Biological Control.. 2020;151
- [CrossRef] [Google Scholar]
- Unveiling the phytochemical profile and biological potential of five artemisia species. Antioxidants.. 2022;11(5)
- [CrossRef] [Google Scholar]
- Research progress on chemical constituents and pharmacological effects of Dendrobium officinale. West China J. Pharma. Sci.. 2022;37(04):472-476.
- [CrossRef] [Google Scholar]
- Total free radical species and oxidation equivalent in polluted air. Sci. Total Environ.. 2017;609:1103-1113.
- [CrossRef] [Google Scholar]
- Screening out biomarkers of tetrastigma hemsleyanum for anti-cancer and anti-inflammatory based on spectrum-effect relationship coupled with UPLC-Q-TOF-MS. Molecules.. 2023;28(7)
- [CrossRef] [Google Scholar]
- Black spot on the medicinal orchid Dendrobium officinale caused by Cladosporium oxysporum in China. Can. J. Plant Pathol.. 2017;40(1):100-104.
- [CrossRef] [Google Scholar]
- Insights into the plateau adaptation of Salvia castanea by comparative genomic and WGCNA analyses. J. Adv. Res.. 2022;42:221-235.
- [CrossRef] [Google Scholar]
- Comparison of polysaccharide and total alkaloid contents in Dendrobium officinale in different harvest periods. J. Traditi. Chin. Veterinary.. 2021;40(03)
- [CrossRef] [Google Scholar]
- Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in Dendrobium officinale using nested PCR-DGGE. Appl. Microbiol. Biotechnol.. 2013;97(22):9825-9836.
- [CrossRef] [Google Scholar]
- Physiological Responses of Dendrobium officinale under exposure to Cold Stress with Two Cultivars. Phyton-Int. J. Experiment. Botany.. 2020;89(3):599-617.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles from Dendrobium officinale and its anticancer effect on liver cancer. Drug Deliv.. 2021;28(1):985-994.
- [CrossRef] [Google Scholar]
- Study on Functional Components and Alkaloid Content of Dendrobium crepidatum. Chinese Academy Forestry 2021
- [CrossRef] [Google Scholar]
- Metabolic disorders sensitise endometrial carcinoma through endoplasmic reticulum stress. Cell Mol. Biol. Lett.. 2022;27(1):110.
- [CrossRef] [Google Scholar]
- Comparative studies on scavenging DPPH free radicals activity of flavone C-glycosides from different parts of Dendrobium officinale. China J. Chin. Mater. Med.. 2012;37:1536-1540.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105922.
Appendix A
Supplementary material
The following are the Supplementary material to this article:Supplementary Data 1
Supplementary Data 1







