Translate this page into:
Efficient removal of Cr6+ by magnetically modified biochar from aqueous solution:Removal mechanism investigation
⁎Corresponding author. liupeng22@pzhu.edu.cn (Peng Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The magnetic biochar is prepared by one-pot method using waste wooden building formwork as feedstock in the existence of the FeCl3 for Cr6+ removal form wastewater. Characterization analysis indicates that magnetic biochar exists Fe3O4 with specific surface area of the 333.89–496.35 m2/g, contributing to Cr6+ removal. Cr6+ removal process can be well analyzed by the Pseudo-second order and Hill models with adsorption capacity of 30.41 mg/g. The adsorption process analysis indicates that Cr6+ removal mechanism includes surface complexation, electrostatic attraction, reduction and pore filling. The influence of Fe2+ on Cr6+ removal is analyzed, indicating Fe2+ participation into Cr6+ removal by reduction. The magnetic biochar has excellent reusability after three cycles. The Cr6+ removal process is also investigated using column adsorption experiment, indicating that magnetic biochar has column adsorption amount of the 56.64 mg/g. The economic feasibility of the magnetic biochar is investigated and calculated with the production cost of $2.48/1kg. The waste wooden building formwork is converted into the efficient magnetic biochar for Cr6+ removal from wastewater.
Keywords
Magnetic biochar
Cr6+
Adsorption
Reduction
Economic feasibility
1 Introduction
The heavy metals in water bodies have posed serious threat to the human health and environment (Guo et al., 2024). Cr6+ is a common heavy metals in the groundwater, which has highly toxic, soluble and mobile in the environment (Qu et al., 2022). The wastewater containing Cr6+ is mainly produced from the tanneries, metal processing, electroplating and other basic industries (Xiao et al., 2024). The toxicity of the Cr6+ is much higher than the Cr3+, which can cause the skin cancer and respiratory problems for human beings (Hong et al., 2024). Excess Cr6+ in the ecosystem is not only harmful to the humans but also inhibits the growth and development of other organisms (Mao et al., 2024). The maximum limited Cr6+ concentration is 0.05 mg/L in drinking water (Cai et al., 2023). Therefore, removal Cr6+ from above industries wastewater is necessary.
In order to control the pollution of Cr6+ wastewater, several treatment methods such as electrochemical, membrane filtration and photocatalytic degradation have been used for Cr6+ wastewater treatment (Fu and Wang, 2011). However, compared with these costly and complex methods, adsorption is the common and efficient methods for Cr6+ removal from wastewater. Many adsorbents including activated carbon, clay mineral and biochar are used for Cr6+ removal from wastewater (Fu and Wang, 2011; Zhao et al., 2021; Zou et al., 2021). For sustainably and high efficient removing Cr6+ from wastewater, it can be found that it is essential for development of the low-cost adsorbents.
Biochar is produced at the temperature of 400–600 °C in the inert atmosphere, which is also the byproducts of biomass pyrolysis. The production cost of biochar is about $448.78/t (Qin et al., 2023). Biochar can be an alternative for adsorptive elimination of Cr6+ as the result of its abundant functional groups and pore structure. Li et al. (2022) prepared the Landfill leachate sludge-based biochar for Cr6+ removal from wastewater, exhibiting adsorption capacity of 17.46 mg g−1 (Li et al., 2022). Singh et al. (2022) prepared the biochar derived from Citrobacter freundii bacterial biochar for Cr6+ removal with adsorption capacity of 19.43 mg g−1 (Singh et al., 2022). Mutabazi et al. 2024 prepared the peanut shell-derived biochar for Cr6+ wastewater purification with adsorption capacity of 16.67 mg g−1 (Mutabazi et al., 2024). Amin et al. (2019) prepared marine Chlorella sp. Residue-based biochar for Cr6+ removal with adsorption amount of 15.94 mg g−1 (Amin and Chetpattananondh, 2019). However, the above literatures only investigate the Cr6+ adsorption performance of original biochar with modest adsorption capacity, which limits uptake ability for Cr6+ removal from wastewater.
There are many methods for modification biochar to improve its pore structure, chemical surface functional groups, etc for improvement of adsorption capacity. Combining Fe-based compounds such as Fe3O4, FeC3 and zero-valent iron with biochar to prepare the Fe-based biochar is a kind of modification method to improve Cr6+ removal capacity of original biochar (Dong et al., 2021; Sun et al., 2023). The Fe-based compounds can participate in Cr6+ removal by reduction (Zhang et al., 2023; Sahu et al., 2022). Besides, the Fe-based biochar can be quickly recycled from wastewater under extra filed. Yan et al. (2023) modified biochar with chitosan for Cr6+ removal with adsorption amount of 125.34mg g−1 (Yan et al., 2023). Chu et al. (2023) modified the Fe@CSBC by loading Fe3O4 on biochar derived from corn straw, which is used for capturing Cr6+ in wastewater with adsorption amount of 138.8 mg g−1 (Chu and Nguyen, 2023). Qiu et al. (2020) prepared nano-zero-valent iron/sludge-derived biochar for Cr6+ adsorption and reduction with 64.13 mg g−1 (Qiu et al., 2020). Wang et al. (2020) used magnetic greigite/biochar composites to remove Cr6+ with removal of 93 % by adsorption and reduction (Wang et al., 2020). However, the preparation methods of the Fe-based biochar are more or less environmentally unstable and complicated to synthesize, which limits their practical application. It should be developed the simple and low-cost method for preparation of the Fe-based biochar overcome these disadvantages. FeCl3 is a kind of the chemical activation agent, which can improve the pore structure of biochar (Wang et al., 2020). FeCl3 can generate the Fe3O4 on biochar after heat.
In this work, magnetic biochar is successfully prepared by pyrolysis of waste wooden building formwork using FeCl3 as chemical agent for Cr6+ wastewater purification. The magnetic biochar is characterized to analyze its physicochemical properties. Cr6+ removal capacity of magnetic biochar is also investigated. The objectives of this work are: (1) to analyze physicochemical properties of the magnetic biochar, (2) to investigate Cr6+ adsorption performance of magnetic biochar, (3) to explore possible involved Cr6+ removal mechanism, (4) to investigate Cr6+ removal capacity in column adsorption experiment.
2 Experimental section
2.1 Material
The waste wooden building formwork is collected from local construction site, which is crushed with the particle size of the 2 mm. The FeCl3 is obtained from Sinopharm Chemical Reagent Co China. Potassium dichromate (K2Cr2O7) is purchased from the Yantai Shuangshuang Chemical Co., Ltd, China.
2.2 Synthesis of the magnetic biochar
Waste wooden building formwork is mixed with 20 g FeCl3 in the aqueous solution, which is stirred for 24 h. Subsequently, mixture is dried using electric dry oven at the temperature of 80 °C for 24 h. Finally, dried mixture is heated at 400–600 °C for 30 min in the microwave furnace under nitrogen atmosphere with nitrogen flow rate of 200 mL/min. Finally, the residue in the microwave furnace is named as the MB-400 °C, MB-500 °C and MB-600 °C.
2.3 Adsorption experiment
The influence of the pH on Cr6+ adsorption is investigated at pH of 2–7. The adsorption isotherm experiments are carried out as follows. 0.1 g magnetic biochar is mixed with 100 mL Cr6+ solution with different concentration, which is stirred using the magnetic stirrer with a shaking speed of 300 r/min (pH=2). The adsorption time is 24 h to reach the adsorption equilibrium. Mixture solution is sampled to detected the residue concentration after adsorption equilibrium. After adsorption, the adsorbent and the adsorbed solution are separated by filtration using a 0.22 μm filter. Cr6+ concentration is obtained using UV–VIS spectroscopy at 540 nm. Cr6+ adsorption amounts (qe and qt) in the adsorption experiment are calculated from the following equation. Cr6+ adsorption behavior on MB-600 °C is investigated by Langmuir, Freundlich, and Hill models, which are described in the Table S1.
The experiment method of the adsorption kinetics is similar with the adsorption isotherm. Pseudo-first/second order and Intraparticle diffusion models are used to explain the Cr6+ adsorption kinetics process, which are presented in Table S2.
qt, adsorption amount of Cr6+ adsorbed over time, mg/g.
qe, Cr6+ adsorption amount, mg/g.
C0, initial Cr6+ concentration, mg/L.
Ct, Cr6+ concentration over time, mg/L.
Ce, Cr6+ equilibrium concentration, mg/L.
M, quality of the magnetic biochar, g.
V, solution volume, L.
2.4 Characterization
The specific surface area of magnetic biochar is analyzed using the Brunauer Emmett Teller (BET) method using the Autosorb instrument. The surface properties of magnetic biochar are analyzed by the X-ray photoelectron spectroscopy (XPS). The surface chemical composition is investigated using the X-ray diffractometer (XRD). Scanning electron microscopy (SEM) is used to analyze the surface morphologies of magnetic biochar. The chemical functional groups of samples are analyzed using the Fourier transform infrared spectroscopy (FTIR). Zeta potential measurements are conducted using a ZetaPALS.
3 Results and discussions
3.1 Characterization analysis
Fig. 1 shows the XRD analysis of magnetic biochar. The characteristic peaks of the Fe3O4 (2θ = 18.23°, 30.01°, 35.4°, 43.04°, 56.89°, 51.8°and 62.51°) are appeared in the magnetic biochar with good crystallinity. This result indicates that FeCl3 is converted into the Fe3O4 at temperature of the 400–600 °C. It also means that the magnetic biochar has magnetic, which can be quickly recycled from Cr6+ wastewater due to existence of the Fe3O4.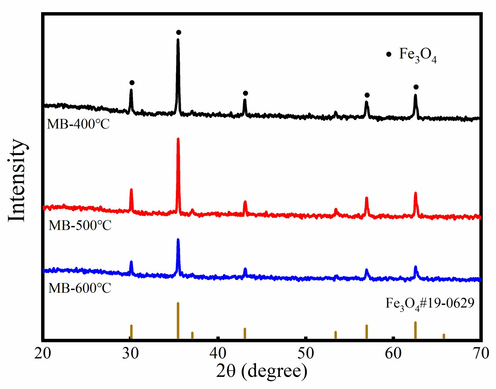
XRD pattern of the magnetic biochar.
Fig. 2 shows the N2-adsorption isotherm of the magnetic biochar. The pore structure parameter of the magnetic biochar is presented in Table 1. As Fig. 2 shown, N2 adsorption amount of the magnetic biochar significantly increases at P/P0 < 0.1, and then the curve tends to slowly increase. “Hysteresis loop” appears on magnetic biochar, indicating the mesoporous structure of magnetic biochar. As Table 1 shown, the MB-600 °C has large surface area, which is acted as the candidate of the magnetic biochar for further investigation.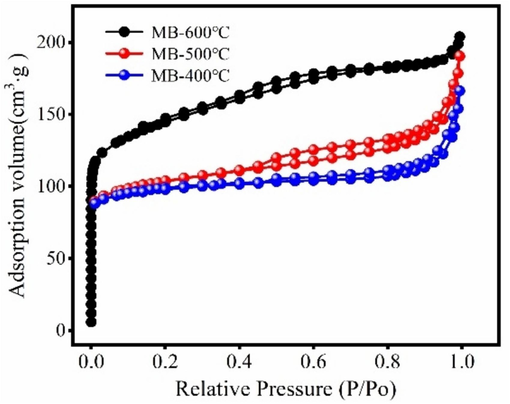
N2-adsorption/desorption isotherm of magnetic biochar.
Item
Surface area (m2/g)
Pore volume (cm3 g−1)
Average pore size (nm)
MB-600 °C
496.35
0.32
2.54
MB-500 °C
350.73
0.29
3.36
MB-400 °C
333.89
0.26
3.08
The microstructure analysis of the MB-600 °C is shown in the Fig. 3a-d. As Fig. 3a-b shown, MB-600 °C has developed pore structure. Besides, the surface of the MB-600 °C has gray particulate matter. EDS mapping image analysis result indicates that C, O and Fe are found on MB-600 °C (Fig. 3c-d). The gray particulate matter on the MB-600 °C is the Fe3O4 combined with XRD analysis.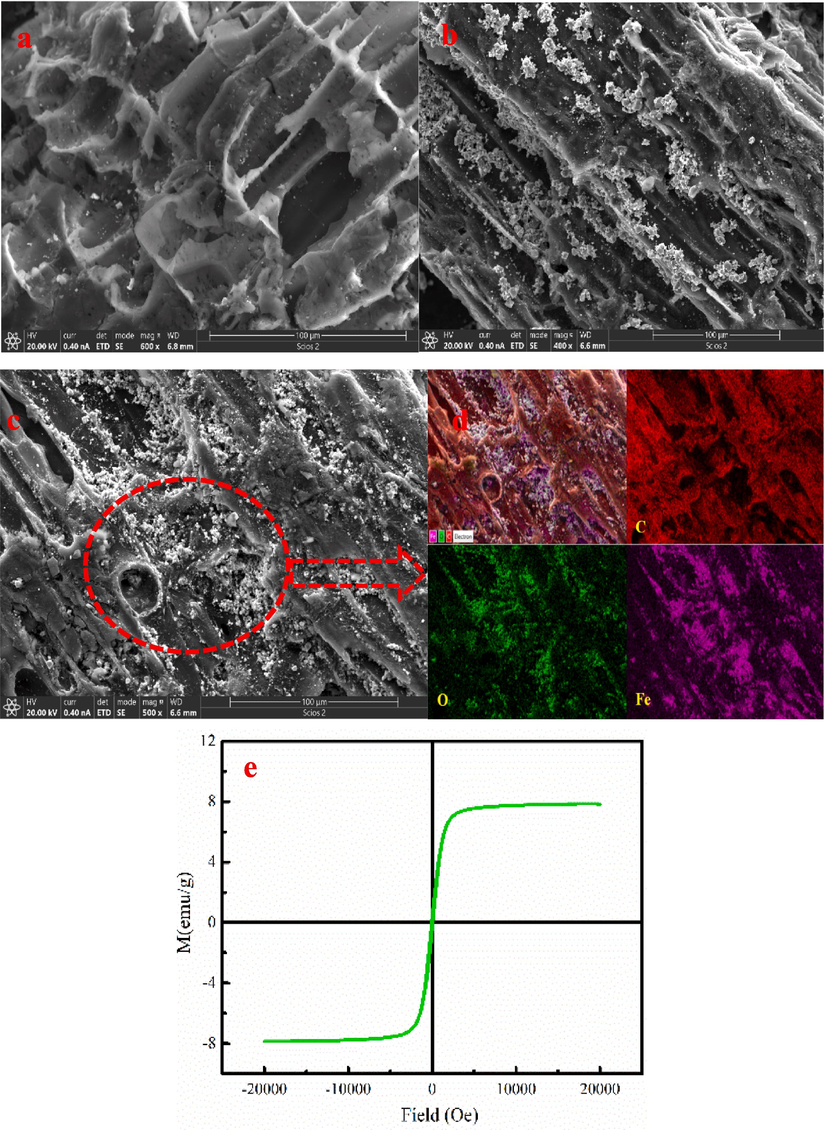
The microstructure analysis of the MB-600 °C (a-d) and the hysteresis loop of the MB-600 °C (e).
Fig. 3e shows the hysteresis loop analysis of the MB-600 °C. As Fig. 3e shown, the saturation magnetization of the MB-600 °C is 7.85 emu/g, indicating that MB-600 °C has certain of the soft magnetic property. The MB-600 °C can be quickly separated and recovered from aqueous solution under the action of applied magnetic field (Fig.S1). As Fig.S1 shown, the MB-600 °C is gathered on the wall of the bottle under the action of applied magnetic field, which is consistent with the hysteresis loop analysis.
3.2 Influence of pH
Cr6+ has various of forms at different pH (Asoubar et al., 2023). Fig. 4 presents Cr6+ adsorption behavior on MB-600 °C at pH of 2–7. Fig. 4 shows that MB-600 °C has large Cr6+ adsorption amount at pH of 2–4. There exists lots of H+ in the aqueous solution, which makes MB-600 °C have positive charge. Therefore, MB-600 °C can adsorb Cr6+ by electrostatic adsorption. The H+ concentration is general decrease as pH increases. Therefore, Cr6+ adsorbing amount is generally decrease with increasing in pH. The zeta potential value of the MB-600 °C is about 5.2 (Fig.S2). This result indicates that MB-600 °C has positive potential at pH<5.2, and becomes negative potential at pH>5.2. The MB-600 °C has large Cr6+ adsorption amount at pH=2. Therefore, the desire pH for MB-600 °C adsorption Cr6+ is 2.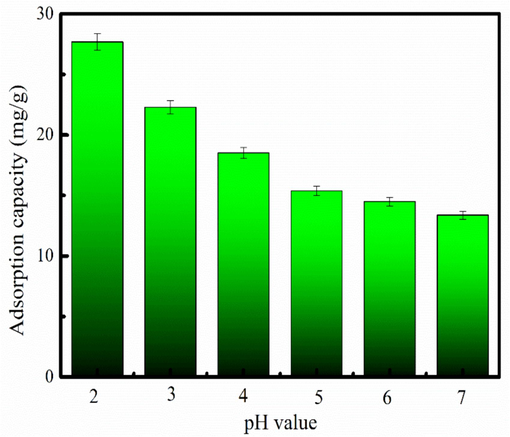
Influence of the solution of the pH on Cr6+ adsorption.
3.3 Adsorption isotherms investigation
The Cr6+ adsorption behavior on MB-600 °C is investigated by Langmuir, Freundlich, and Hill models. Fig. 5a shows the Cr6+ adsorption data fitting adsorption isotherm equations, and fitting parameters are listed in Table.2. As Table 2 shown, compared with Freundlich model, the R2 value of Cr6+ adsorption data fitting the Langmuir/Hill equation has large R2. Cr6+ adsorption amount obtained from Hill model is 30.41 mg/g. The parameter (KL) of Langmuir model is used to calculate the dimensionless factor RL that can investigate feasible of Cr6+ adsorption on MB-600 °C (Cheng et al., 2021). Calculated method is in the following equation.
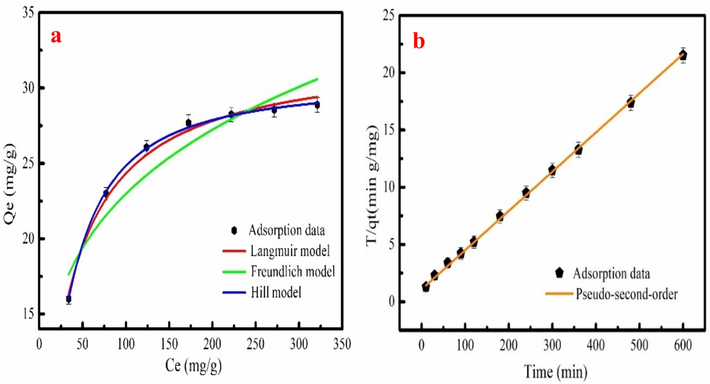
The Cr6+ adsorption data fitting the adsorption isotherm models (a) and The Cr6 + adsorption data fitting Pseudo-second-order equation (b).
Isotherm models
Models Parameter
Fitting result
Cr6+
Langmuir
qm (mg/g)
32.47
KL (L/mg)
0.0299
R2
0.9927
Freundlich
1/n
0.2453
KF ((mg/g).(L/mg)1/n)
7.42
R2
0.90083
Hill
Qm
30.41
n
1.28
K
31.49
R2
0.9990
If the RL belongs to 0–1, Cr6+ adsorption process is feasible. RL values of Cr6+ adsorption on MB-600 °C is 0.0872–0.4009, indicating that MB-600 °C is feasible for Cr6+ adsorption. Besides, the values of the heterogeneity (1/n) calculated from Freundlich that can represent the bond distribution (Tang et al., 2018). The 1/n value of Cr6+ adsorption on MB-600 °C is 0.2453, which is less than 1. This result confirms that Cr6+ adsorption process is feasible and favorable (Xiang et al., 2019).
The Cr6+ adsorption amounts of other adsorbents are also compared with MB-600 °C (Table S3). Cr6+ adsorption amount of MB-600 °C is larger than that reported adsorbents. These results prove that the MB-600 °C is the promising adsorbent for Cr6+ wastewater purification.
3.4 Adsorption kinetics investigation
The Cr6+ adsorption data is analyzed by the Pseudo-first/second order and Intraparticle diffusion equations. The calculated results are listed in Table 3. Correlation coefficient (R2) value of Cr6+ adsorption process fitting Pseudo-second order is 0.9989, which is higher than other adsorption kinetics models (Table 3). Thus, Pseudo-second order is better to describe Cr6+ adsorption kinetic process, indicating that chemical adsorption influences Cr6+ adsorption (Cheng et al., 2022). Fig. 5b shows Cr6+ adsorption data fitting Pseudo-second-order equation. The Intraparticle diffusion fitting result has two stages (Fig.S3). The C1 and C2 value of Cr6+ calculated from Intraparticle diffusion model are 1.32 and 19.91, respectively. The calculated C value isn’t zero, indicating that Cr6+ adsorption on MB-600 °C isn’t alone controlled by Intraparticle diffusion.
Isotherm models
Models Parameter
Fitting result
Cr6+
Pseudo-first order
qe.cal (mg/g)
25.21
K1 (1/min)
0.028
R2
0.9448
Pseudo-second order
qe.cal (mg/L)
29.17
K2 (g/mg min)
1.077
R2
0.9989
Intraparticle diffusion
C1
1.32
K31 (mg/g min1/2)
2.07
R2
0.9944
C2
19.91
K32 (mg/g min1/2)
0.35
R2
0.8976
3.5 Removal mechanism
The zeta potential of the MB-600 °C is about 5.2. The surface potential of the MB-600 °C is positive charge at pH of 2. Therefore, MB-600 °C can adsorb Cr6+ by electrostatic attraction. As Fig. 6 shown, MB-600 °C has the OH, C=O, C-O and Fe-O group, demonstrating that MB-600 °C has abundant functional group. Besides, the oxygen-containing functional groups can easily obtain the protons at pH of 2 (Liang et al., 2023). The oxygen-containing functional groups can be used as the electron donor, contributing to Cr6+ removal by adsorption/reduction (Singh et al., 2022; Zhao et al., 2024). After Cr6+ adsorption, the peak intensity and peak location of oxygen containing functional groups have changed, indicating that these groups involve in Cr6+ removal. Fe2+ comes from the dissolution of Fe3O4, which might be an important reaction pathway for Cr6+ removal by reduction (Zhao et al., 2024).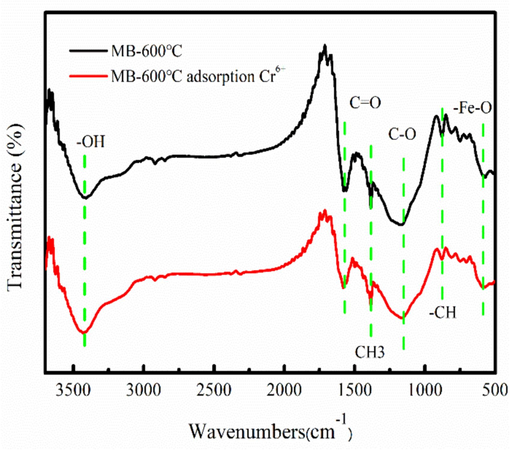
FT-IR spectra of MB-600 °C before and after Cr6+ adsorption.
Fig. 7a-b shows the C 1 s peaks of MB-600 °C before and after Cr6+ adsorption. The peaks at 288.58, 285.21 and 284.60 eV are C=O, C-O and C–C group, respectively (Tang et al., 2021; Wang et al., 2020). For MB-600 °C, upon reaction with Cr6+, the C-O percentage decreases from 35.29 % to 28.27 %, which indicates that –CHO is an electron donor that is oxidized into –COOH by Cr6+ (Zhang et al., 2020). Generally, the C-O group is the adsorption and reduction site of the MB-600 °C, and adsorption and reduction occur simultaneously (Liu et al., 2023) (Eq.4). However, C=O proportion decreases, which maybe C=O involves in Cr6+removal(Eq.5) (Mutabazi et al., 2024).
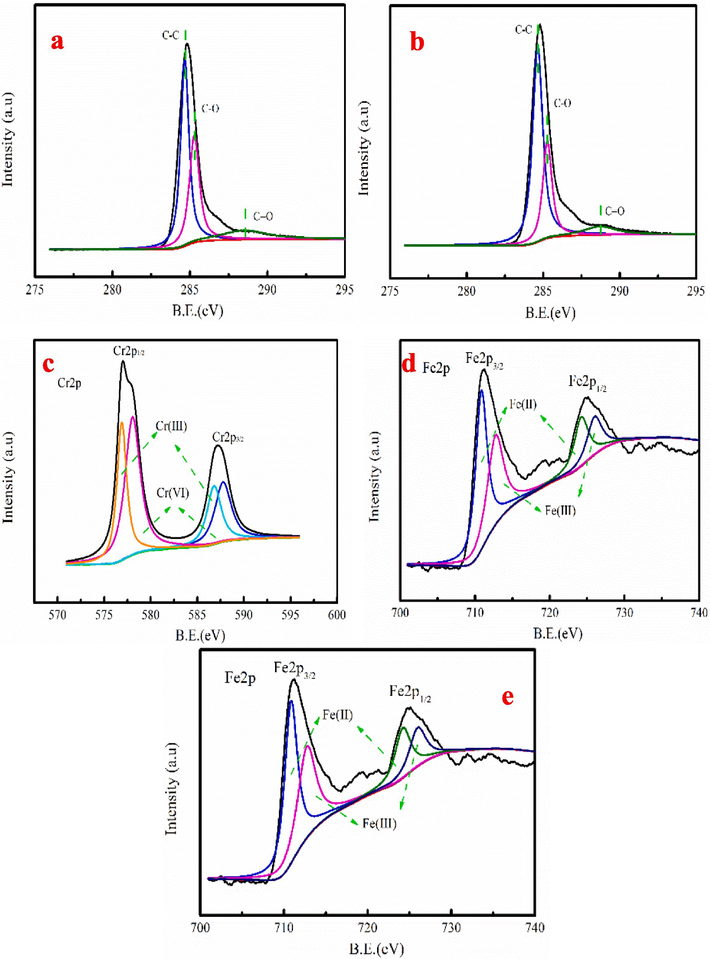
The detail survey of the C1s before (a) and after Cr6+ adsorption (b), detail survey of the Cr2p after adsorption Cr6+ (c) and detail survey of the Fe2p after adsorption Cr6+ (d-e).
As Fig. 7c shown, the two peaks of Cr 2p1/2 and Cr 2p3/2 correspond to the 587.24 eV and 577.04 eV, respectively. The peaks at 576.89 eV and 586.80 eV indicate the presence of Cr3+, suggesting that Cr6+ is partially reduced into Cr3+ (Luo et al., 2021). Fig. 7c-d shows the Fe 2p spectra of the MB-600 °C before and after Cr6+ adsorption, which can be divided in four peaks. The peaks at 710.85 eV and 724.21 eV correspond to Fe2+, while Fe3+ appears at peaks of 712.74 eV and 725.98 eV. Peak area ratio of Fe2+/Fe3+ decreases by 0.37 after Cr6+ adsorption, implying that the dissolved Fe2+ involves in Cr6+ reduction process. The analysis result is consistent with Cr2p spectrum analysis. The Fe2+ is reducing substances, which can reduce Cr2O72- into Cr3+ (Eq.6) (Li et al., 2019).
Furthermore, MB-600 °C can also realize Cr6+ removal by the pore filling (Su et al., 2022; Yang et al., 2020). The MB-600 °C shows flourishing pore structure that plays a vital role in Cr6+ removal process. The summary of Cr6+ adsorption mechanism is shown in the Fig. 8.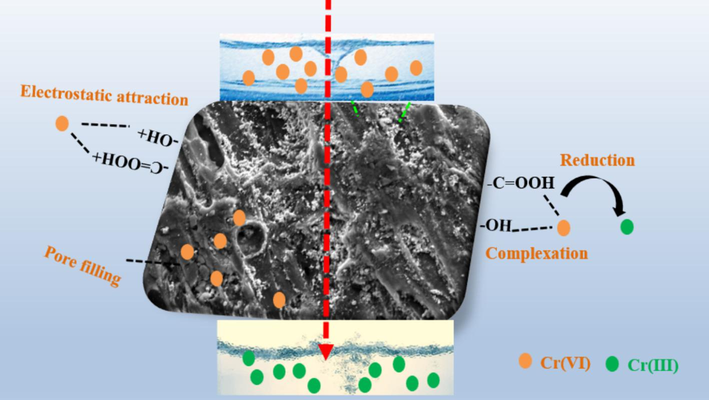
The summary of Cr6+ adsorption mechanism.
3.6 Reusability investigation
Fig.S4 shows reusability results of MB-600 °C. As Fig.S4 shown, the Cr6+ adsorption capability of MB-600 °C decreases as cycle number increases. The Cr6+ adsorption amount is dropped to 19.84 mg/g after three cycles, which decreases by 28.32 %. The reason is that part of active sites of MB-600 °C loses adsorption capacity after regeneration. MB-600 °C still shows large Cr6+ adsorption capacity after regeneration, which has practical application potential in Cr6+ wastewater purification.
3.7 Effect of Fe2+
The Fe 2p spectra analysis of the MB-600 °C before and after Cr6+ adsorption indicates that Fe2+ involves in Cr6+ removal. Besides, the electron transfer occurs between Fe2+ and Cr6+. Fe3O4 can dissolve to generate Fe2+ because the pH of Cr6+ adsorption solution is 2. This result indicates that the Fe2+ exists adsorption solution and participates in Cr6+ reduction. Fe2+ is an important reductant with a low standard electrode potential of 0.77 V/SHE and can transform Cr6+ to Cr3+ (E0(HCrO4-/Cr3+) = 1.35 V/SHE) (Jiang et al., 2019). Therefore, the influence of Fe2+ on Cr6+ removal is analyzed in existence of 1,10-phenanthroline that make Fe2+ cannot participate in Cr6+ removal (Liu et al., 2019). As Fig. 9a shown, the Cr6+ removal is decrease in existence of the 1,10-phenanthroline compared to without adding 1,10-phenanthroline. The analysis result demonstrates that Fe2+ participates in Cr6+ removal.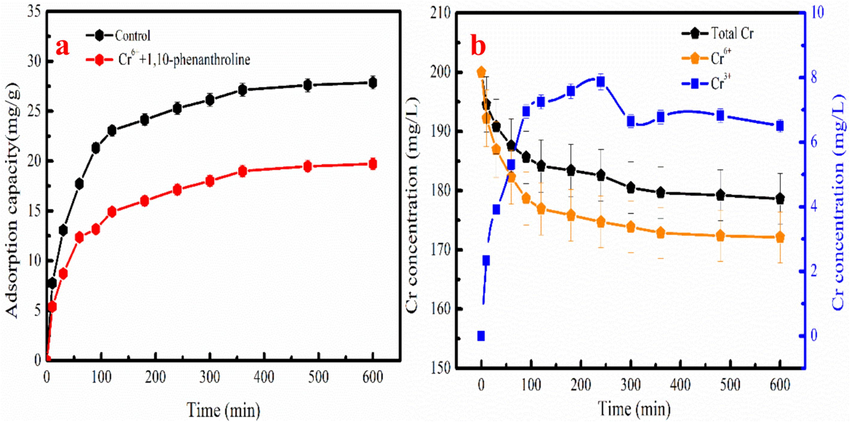
The influence of 1,10-phenanthroline on Cr6+ removal(a), the total Cr, Cr6+, and Cr3 +concentration in the Cr removal process(b).
As Fig. 9b shown, the total Cr and Cr6+ concentration rapidly decrease with increasing in adsorption time without adding 1,10-phenanthroline. The total Cr concentration is gradual decrease and Cr3+ concentration exhibits an increasing trend, indicating that Cr6+ adsorption and reduction simultaneously occur. While, Cr3+ concentration increases as time continues to increase. However, Cr3+ concentration gradually decreases as adsorption time continues to increase, indicating that part of Cr3+ is adsorbed on MB-600 °C. The equation of Fe2+ involves in Cr6+ removal is listed in Eq. (6). Cr6+ removal by MB-600 °C is restrained in existence of 1,10-phenanthroline, demonstrating that Fe2+ plays an important in Cr6+ removal.
3.8 Explore using DFT calculations
Combined with the results of XPS and FTIR, it could be found that the oxygen-containing functional group on the MB-600 °C mainly included hydroxyl, COOH, –OH and Fe-O groups. Thus, the MB-600 °C model with various oxygen-containing functional groups is constructed (Cheng et al., 2021). Binding energies of the COOH, –OH and Fe-O groups interactions with Cr6+ are calculated using DFT. Fig. S5 shows the calculated result. According to the calculation result in the Fig.S5, it exists energy barrier in Cr6+ adsorption process. Compared with the binding energies of Cr6+ interactions with –OH and –COOH group, the −Fe-O group is more negative. The analysis data demonstrates that-Fe-O group plays a critical role in Cr6+ removal by surface complexation.
3.9 Column adsorption
Thomas and Yoon-Nelson equation are employed for Cr6+ adsorption process using fixed-bed adsorption (Table S4). The fitting analysis results are presented in Table S5. As Table S5 shown, Cr6+ breakthrough curves can be analyzed using the Thomas and Yoon-Nelson equation with high R2. Yoon-Nelson equation is better to describe Cr6+ adsorption process because of large R2. According to Thomas equation calculation, Cr6+ adsorption amount of the MB-600 °C is 56.64 mg/g. It demonstrates that MB-600 °C has enormous potential in governing environmental concerns of wastewater. Fig.S6 presents breakthrough curve of Cr6+ adsorption on MB-600 °C.
3.10 Economic feasibility
Preparation cost of the MB-600 °C is very important parameter for actual application on an industrial scale. According to previous literature, the total production cost of MB-600 °C is presented in Table S6 (Jiang et al., 2019). As Table S6 shown, the approximate preparation cost is about $2.48 for 1 kg MB-600 °C. However, production cost of 100 mesh activated carbon is estimated as $ 40/1kg. Compared with commercial activated carbon, the production cost of MB-600 °C is cheap. Cr6+ adsorption amount of activated carbon and nZVI/sewage sludge co-pyrolyzed magnetic biochar are 23.35 and 13.27 mg/g, respectively (Mutabazi et al., 2024; Liu et al., 2020). Estimated treatment cost of 1 Kg Cr6+ is $ 81.55 for MB-600 °C. Compared with the above adsorbent, the estimated treatment cost of the 1 Kg Cr6+ is cheap. Therefore, MB-600 °C can be acted as low-cost adsorbent for Cr6+ removal.
4 Conclusions
The magnetic biochar is successfully prepared using the simple one-step pyrolysis method for Cr6+ removal from wastewater. The Cr6+ adsorption capacity is 30.41 mg/g at pH=2. The electrostatic attraction, surface complexation, reduction and pore filling are responsible for Cr6+ removal, based on mechanism analysis. Existence of Fe2+ contributes to Cr6+ removal by reduction in aqueous solution. The −Fe-O group is important in Cr6+ removal process compared to –COOH and –OH groups based on DFT calculation. The magnetic biochar has large column adsorption amount of 56.64 mg/g. The production cost of the magnetic biochar is about $2.48/1kg, based on preliminary economic analysis.
CRediT authorship contribution statement
Chao Lv: Resources, Methodology, Investigation, Formal analysis, Conceptualization. Peng Liu: Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition.
Acknowledgments
The authors would like to express their gratitude to the Ph.D. Research Start-up Funding Project of Panzhihua University (XJ2022001301), and Panzhihua Directed Science and Technology Planning Projects (2022ZD-G-4) for financial support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biochar from extracted marine Chlorella sp. residue for high efficiency adsorption with ultrasonication to remove Cr(VI), Zn(II) and Ni(II) Bioresour. Technol.. 2019;289:121578
- [Google Scholar]
- Hexavalent chromium reduction and Rhodamine B degradation by visible-light-driven photocatalyst of stannum indium sulfide-samarium vanadate. npj Clean Water. 2023;6:213411
- [Google Scholar]
- Efficient removal of Cr(VI) and As(V) from an aquatic system using iron oxide supported typha biochar. Environ. Res.. 2023;225:115588
- [Google Scholar]
- Efficient and selective removal of Pb(II) from aqueous solution by modification crofton weed: Experiment and density functional theory calculation. J. Clean. Prod.. 2021;280
- [Google Scholar]
- Efficient and selective removal of Pb(II) from aqueous solution by modification crofton weed: Experiment and density functional theory calculation. J. Clean. Prod.. 2021;280:124407
- [Google Scholar]
- High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour. Technol.. 2022;343:126081
- [Google Scholar]
- Improved Cr (VI) adsorption performance in wastewater and groundwater by synthesized magnetic adsorbent derived from Fe3O4 loaded corn straw biochar. Environ. Res.. 2023;216:114764
- [Google Scholar]
- Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater.. 2021;416:125930
- [Google Scholar]
- Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage.. 2011;92:407-418.
- [Google Scholar]
- Preparation of three kinds of efficient sludge-derived adsorbents for metal ions and organic wastewater purification. Arab. J. Chem.. 2024;17:105671
- [Google Scholar]
- Easy separation dual-function Cu2O@LDH@Fe3O4 adsorbent for the removal of Cr(VI) under dark conditions: Experimental and mechanistic study. Sep. Purif. Technol.. 2024;332:125734
- [Google Scholar]
- The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: State-of-the-art and perspectives. J. Hazard. Mater.. 2019;365:205-226.
- [Google Scholar]
- Characteristics and adsorption of Cr(VI) of biochar pyrolyzed from landfill leachate sludge. J. Anal. Appl. Pyrol.. 2022;162:105449
- [Google Scholar]
- Intercalation of nanosized Fe3C in iron/carbon to construct multifunctional interface with reduction, catalysis, corrosion resistance, and immobilization capabilities. ACS Appl. Mater. Interfaces. 2019;11:15709-15717.
- [Google Scholar]
- New insights into co-adsorption of Cr6+ and chlortetracycline by a new fruit peel based biochar composite from water: Behavior and mechanism. Colloids Surf. A Physicochem. Eng. Asp.. 2023;672:131764
- [Google Scholar]
- Removal and reduction of Cr(Ⅵ) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge. J. Clean. Prod.. 2020;257:120562
- [Google Scholar]
- Highly efficient immobilization of NZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere. 2019;220:1003-1013.
- [Google Scholar]
- The adsorption and reduction of anionic Cr(VI) in groundwater by novel iron carbide loaded on N-doped carbon nanotubes: Effects of Fe-confinement. Chem. Eng. J.. 2023;452:139357
- [Google Scholar]
- Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI) Chem. Eng. J.. 2021;407:127050
- [Google Scholar]
- Interfacial engineering of 0D/2D Cd0.5Zn0.5S/Ti3C2 for efficient photocatalytic synergistic removal of antibiotics and Cr(VI) J. Environ. Chem. Eng.. 2024;12:111649
- [Google Scholar]
- Cr(VI) adsorption on activated carbon, sludge derived biochar, and peanut shells derived biochar: Performance, mechanisms during the reuse process and site energy distribution analysis. J. Water Process Eng.. 2024;57:104679
- [Google Scholar]
- Preparation of pyrolysis products by catalytic pyrolysis of poplar: Application of biochar in antibiotic wastewater treatment. Chemosphere. 2023;338:139519
- [Google Scholar]
- Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation. Environ. Pollut.. 2020;265:115018
- [Google Scholar]
- Two-step ball milling-assisted synthesis of N-doped biochar loaded with ferrous sulfide for enhanced adsorptive removal of Cr(Ⅵ) and tetracycline from water. Environ. Pollut.. 2022;306:119398
- [Google Scholar]
- Mechanism enhanced active biochar support magnetic nano zero-valent iron for efficient removal of Cr(VI) from simulated polluted water. J. Environ. Chem. Eng.. 2022;10:107077
- [Google Scholar]
- S. Singh, A.G. Anil, T.S.S.K. Naik, B. U, S. Khasnabis, B. Nath, V. Kumar, S. Subramanian, J. Singh, P.C. Ramamurthy, Mechanism and kinetics of Cr(VI) adsorption on biochar derived from Citrobacter freundii under different pyrolysis temperatures, Journal of Water Process Engineering, 47 (2022) 102723.
- One-step synthesis of nitrogen-doped porous biochar based on N-doping Co-activation method and its application in water pollutants control. Int. J. Mol. Sci.. 2022;23
- [Google Scholar]
- Biochar-anchored low-cost natural iron-based composites for durable hexavalent chromium removal. Chem. Eng. J.. 2023;476:146604
- [Google Scholar]
- Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J.. 2018;336:160-169.
- [Google Scholar]
- Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms. J. Hazard. Mater.. 2021;413:125415
- [Google Scholar]
- A novel g-C3N4 modified biosynthetic Fe(III)-hydroxysulfate for efficient photoreduction of Cr(VI) in wastewater treatment under visible light irradiation. Chem. Eng. J.. 2020;398:125632
- [Google Scholar]
- Mechanism of Cr(VI) removal by magnetic greigite/biochar composites. Sci. Total Environ.. 2020;700:134414
- [Google Scholar]
- Adsorption behavior of Cr (VI) by magnetically modified Enteromorpha prolifera based biochar and the toxicity analysis. J. Hazard. Mater.. 2020;395:122658
- [Google Scholar]
- A sustainable ferromanganese biochar adsorbent for effective levofloxacin removal from aqueous medium. Chemosphere. 2019;237:124464
- [Google Scholar]
- Can Cr(VI) and tetracycline be simultaneously removed by stabilized FeS nanoparticles via aeration at circumneutral conditions? J. Water Process Eng.. 2024;60:105146
- [Google Scholar]
- Efficient removal of Cr(VI) by the modified biochar with chitosan schiff base and MnFe2O4 nanoparticles: Adsorption and mechanism analysis. J. Environ. Chem. Eng.. 2023;11:109432
- [Google Scholar]
- Batch interaction of emerging tetracycline contaminant with novel phosphoric acid activated corn straw porous carbon: Adsorption rate and nature of mechanism. Environ. Res.. 2020;181:108899
- [Google Scholar]
- Hydrodynamic analysis of carbon nanotube clusters in distributor-less conical fluidized beds with step-by-step scaling. Chin. J. Chem. Eng. 2023
- [Google Scholar]
- Novel carbothermal synthesis of Fe, N co-doped oak wood biochar (Fe/N-OB) for fast and effective Cr(VI) removal. Colloids Surf. A Physicochem. Eng. Asp.. 2020;600:124926
- [Google Scholar]
- Effects and mechanisms on Cr(VI) and methylene blue adsorption by acid (NH4)2S2O8 modified sludge biochar. Sep. Purif. Technol.. 2024;345:127100
- [Google Scholar]
- Microscopic mechanism about the selective adsorption of Cr(VI) from salt solution on O-rich and N-rich biochars. J. Hazard. Mater.. 2021;404:124162
- [Google Scholar]
- Ball milling biochar iron oxide composites for the removal of chromium (Cr(VI)) from water: Performance and mechanisms. J. Hazard. Mater.. 2021;413:125252
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105943.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







