Translate this page into:
Smartphone-enabled mesoporous silica nanotube chemosensors for quick and selective mercury detection in water and cosmetics
⁎Corresponding author. n_elmetwaly00@yahoo.com (Nashwa M. El-Metwaly) nmmohamed@uqu.edu.sa (Nashwa M. El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The pervasive presence of mercury in water and cosmetics poses significant health risks, necessitating the development of a method for the in-situ monitoring and extraction of mercury ions. This study introduces a novel approach utilizing Mesoporous Silica Nanotubes (MSNTs) with a unique worm-like structure, providing an expansive surface area ideal for the adsorption of a Hg2+ ion chromophore, N,N,N,N′-Tetramethyl-4,4′-diaminobenzophenone. This configuration enables rapid and visible detection of toxic mercury, with a color transition from yellow to green that is easily discernible by the naked eye. The sensitivity of the Mercury nano-sensor (MNS) is remarkably high, with a detection limit of 1.9 × 10-8 M as determined by digital image analysis, and 4.9 × 10-8 M via spectrophotometric methods—both well below the WHO guidelines for drinking water. The MNS’s low detection threshold, coupled with its reusability after simple regeneration, positions it as an effective tool for preliminary water testing. The findings suggest that the MNS, requiring only 10 mg for measurements, offers a promising solution for the real-time visualization of mercury ions, enhancing safety measures in water and cosmetic products.
Keywords
Mesoporous silica nanotube
Chemosensors
Mercury detection in water
Cosmetics
1 Introduction
Mercury, recognized as a formidable contaminant within ecosystems, is linked to numerous health conditions, including the notorious Minamata disease. Its inability to degrade within biological systems or food chains presents a profound risk to both public health and environmental stability (Zahir et al., 2005; Hylander and Goodsite, 2006; Zheng et al., 2007). Furthermore, mercury's presence can inflict harm upon the endocrine and nervous systems, as well as the brain and kidneys (Clarkson et al., 2003; Wang et al., 2010). Mercury's primary form is the water-soluble inorganic mercurous ion (Hg2+). Notable sources of mercury include cement kilns, power stations, thermometers, mercury vapor lamps, chlor-alkali plants, gold extraction processes, and barometers (Natale et al., 2006; Grandjean et al., 1998; Harada, 1995). Mercury's correspondence for thiol groups in proteins and enzymes disrupts cellular functions in humans. According to U.S. EPA standards, the maximum permissible mercury concentration is 2 ppb in water (Mercury Update, 2001). Products such as skin-whitening creams, antiseptic soaps, and eye cosmetics often contain high mercury levels. This metal can be absorbed through the skin, inhaled, or ingested, leading to accumulation within the body. Methylmercury, a particularly toxic variant, can be absorbed through skin contact with cosmetics or through the consumption of foods like fish. Notably, mercury accumulation in expectant mothers is associated with neurodevelopmental impairments in their offspring (Bose-O’Reilly et al., 2010). Symptoms of mercury exposure include psychological disturbances, skin pigmentation changes, rashes, and diminished resistance to skin infections (El-Sewify et al., 2022). While some products fail to disclose their mercury content, the exposure risk extends beyond individual users to their immediate family members. Cosmetic products can have mercury concentrations ranging from 700 to 30,000 ppm, while the acceptable limit in drinking water is only 2 ppb.
Reliable mercury quantification often employs atomic absorption and mass spectrometry techniques. Nevertheless, these methods come with high costs and the necessity for centralized laboratories. Recent advancements have seen the creation of portable X-ray fluorescence devices, which offer quick mercury assessments in various products, yet they remain financially prohibitive (Brent et al., 2017). Recent years have witnessed the emergence of analytical methods for mercury ion detection, such as electrochemical sensing (Fu et al., 2011; Leermakers et al., 2005), atomic absorption spectrometry (AAS) (Gao et al., 2010), and inductively coupled plasma mass spectrometry (ICP-MS) (Moreno et al., 2010). A shared limitation of these techniques is their reliance on costly, complex equipment. On the flip side, molecular optical sensing allows for the monitoring of a specific analyte by connecting an optical signal transducer to a selective recognition element (Chen et al., 2015). Compared to traditional methods, spectrophotometry offers several advantages, including straightforward procedures, swift operation, heightened sensitivity, ease of use, affordability of the apparatus, and the capacity for concurrent detection (You et al., 2015; Khalil et al., 2016).
Chemo-sensors have an advantage because they can convert ion concentration into signals that can be easily understood by standard devices or even by people without specialized training. A chromogenic sensor is what we call it when the signal causes a visible color change, and this is especially helpful. Essentially, a sensor is an integrated analytical device that converts the concentration of a chemical entity into a readable signal for either an observer or an instrument (McGrath and Scanaill, 2013; Radwan et al., 2020). Chemo-sensors based on mesoporous silica have garnered attention for their superior efficacy in detecting small organic compounds (Shahat et al., 2015; Radwan et al., 2022; Shahat et al., 2020; Radwan et al., 2020; Kamel et al., 2020). However, the literature is scant on the development of solid-state chemo-sensors tailored for Hg2+ ion detection (El-Sewify et al., 2023). Therefore, the creation of cost-effective, user-friendly, rapid-response, and highly selective chemo-sensors for mercury ion analysis, particularly those enabling straightforward and visual detection, remains an enticing yet formidable task.
A plethora of studies have delved into the intricate interplay between silica entities and surfactants to synthesize specific mesoporous silicas (Hudson et al., 2008; Hassan et al., 2020; Hasan et al., 2014; Hasan, 2014; Hasan and Znad, 2015; Khaleque et al., 2015). The combination of inorganic and organic elements results in the self-organization of surfactant-silica nanocomposites. The physical attributes and structures of these materials are largely influenced by the dynamics of sol–gel processes, which are governed by factors such as the pH level, water content, and temperature of the reaction milieu (Pan et al., 2018; El-Sewify et al., 2022; Ioannou-Ttofa et al., 2019; Du et al., 2021; Zhu et al., 2023; Zhang et al., 2022; El-Sewify et al., 2024; Khalil et al., 2024; Basha et al., 2024; Mohamed et al., 2024; Hosni et al., 2024; Rizk et al., 2024; Al-Hazmi et al., 2022; Awual et al., 2017; Abd El-Fattah et al., 2024; Ali et al., 2021; Shahat et al., 2022; Abd El-Fattah et al., 2024; Abd El-Fattah et al., 2024), as well as the thermodynamic properties of the surfactant-silica system (Pulicharla et al., 2017). By meticulously managing the rate of silica polymerization and the self-assembly process, one can customize the mesostructures, morphologies, and dimensions of the mesoporous silicas (Brühwiler, 2010). The burgeoning attention in mesoporous silica (MSNs) stems from their uniform mesopores, straightforward surface modification, and notable biocompatibility, making them ideal for biomedical applications. Their expansive surface area and voluminous pore spaces lay the groundwork for devising a versatile theranostic platform (Wang et al., 2016). MSNs are characterized by three distinct functional zones: the nanochannels/pores, the nanoparticle’s external surface, and the silica matrix. These nanoparticles are further distinguished by their simple surface modification, biological compatibility in living organisms, and efficient cellular internalization (Chung et al., 2007; Mazrouaa et al., 2019).
This study introduces innovative solid chemo-sensors created by anchoring N,N,N,N′-Tetramethyl-4,4′-diaminobenzophenone onto the mesoporous structure of silica nanotubes (MSNTs). These sensors serve as chromogenic detectors, offering a highly selective, swift, and visual method for identifying Hg2+ ions. The resulting color change is discernible to the naked eye and can be monitored using commonplace devices like smartphones or through spectrophotometric techniques. Digital image-based colorimetry (DICA) is a novel approach that leverages these technologies for precise quantitative analysis in the field of analytical chemistry. Sample images are captured with a smartphone camera and an accompanying app, with RGB values computed on the spot. These RGB metrics are then employed to measure the concentrations of target analytes. The time required to achieve a consistent signal is remarkably brief (under 20 s). The merits of this technique include its simplicity, speed, cost-effectiveness, and accessibility to non-specialists. Digital image analysis has determined the detection threshold for Hg2+ ions to be 4.9 × 10-8 M, while the spectrophotometric approach has a limit of 1.9 × 10-8 M. MSNs have proven effective in detecting and extracting ultra-low concentrations of Hg2+ ions from water and skin-lightening cosmetic products.
2 Experimental work
2.1 Materials and instruments
Detailed descriptions of all materials and instruments used in this work are provided in the supporting information file.
2.2 Preparation of mesoporous silica nanotubes (MSNTs)
In preparing innovative mesoporous silica nanotubes, 0.25 gm of multiwall carbon nanotubes were distributed in 100 mL of Milli-Q water. Then, it was subjected to sonication for 30 min to ensure uniform suspension. Subsequently, 2 gm of cetyltrimethylammonium bromide was added, and the solution was stirred continuously for over 2 h. Following this, 1.0 gm of Brij 58, pre-dissolved in 4 mL of acetone, was added to the mixture, which was then stirred for an additional hour. To form the coating silica, 2.5 mL of TEOS was incorporated. The mixture was then stirred vigorously for another hour, after which 1.5 mL of NH4OH (25 wt%) was added. Vigorous stirring was applied to the reaction, which was allowed to proceed for 24 h at room temperature in a sealed container. The MSNTs@MWCNTs (shell@core) composite was gathered via centrifugation. The MSNTs@MWCNTs were collected and purified using isopropanol. They were then dried at 80 °C for 24 h. After that, they underwent calcination for 6 h at 500 °C. Finally, they were heated for an additional 8 h at the same temperature (Scheme 1).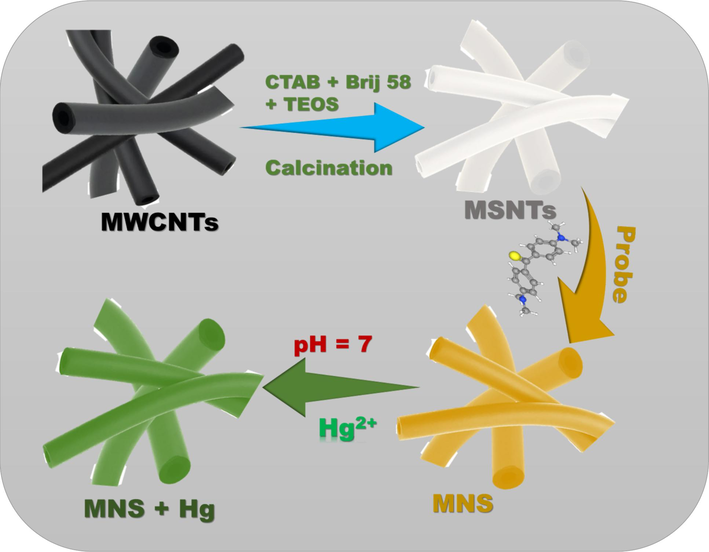
Schematic representation of MSNTs formation, probe immobilization on the MSNTs structure, and the optical signalling response of the MNS towards Hg2+ ions.
2.3 Design of mercury nano-sensor (MNS)
The MSNs were synthesized through a straightforward immobilization technique. A combination of the organic chromophore N,N,N,N′-Tetramethyl-4,4′-diaminobenzophenone (50 mg) with MSNTs (1 g) was agitated in ethanol for 4 h. After the stirring, the blend underwent filtration and was left to dry. The platform's hue visibly changed, signaling the successful bonding of the organic chromophore to the nanotube's surface. To ensure complete pore occupancy within the carrier, this process was executed multiple times. Following these steps, the resultant MSNs were purified using deionized water and desiccated at 50 °C throughout the night.
2.4 Analysis of the ultra-trace Hg2+
To prepare a Hg2 + stock solution with a concentration of 200 ppm, we added 0.0271 g of HgCl2 to a 100.0 mL volumetric flask. We also added a few droplets of 3.0 M hydrochloric acid to prevent the precipitation of Hg(OH)2. Then, we diluted the mixture with deionized water. The resulting solution was transferred to a 1 cm quartz cuvette, and a measured quantity of the Hg2+ solution was added. We promptly acquired UV–vis spectroscopic profiles without any agitation. For liquid/solid sensing assays, we used a phosphate-buffered medium to maintain the Hg2+ ion concentrations at a neutral pH of 7. In addition, 10 mg of MNS chemo-sensors were added to both the blank solution and the solution containing Hg2+. Each solution had a volume of 20 mL and was agitated at 25 °C. The mixtures were sonicated for 60 s and then analyzed using a UV–vis spectrophotometer. The colorimetric assessment of Hg2+ ions using MNS nanotubes was studied across a wide pH range. The sensing parameters included a dosage of 10 mg of MNS, a Hg2+ concentration of 2 ppm, and a total volume of 10 mL. The absorbance spectra were recorded after equilibrium was reached. In order to quantify mercury in cosmetic products, the samples were treated with nitric acid to extract Hg2 + ions from any methylmercury compounds that could be present.
3 Results and discussion
CTAB, a surfactant commonly utilized for the dispersion of nanoparticles and their transition from organic to aqueous phases (Yang et al., 2011), serves as a foundational element for the mesoporous silica coating of nanoparticles. Additionally, CTAB is frequently employed as a template in the synthesis of mesoporous silica (Zhao and Wan, 2007), underscoring the importance of establishing a straightforward, universal protocol for mesoporous silica coatings within the CTAB framework.
The MSNTs, characterized by their biocompatibility, porous structure, surface functionality, and hydrophilic design, have garnered significant interest due to their one-dimensional hollow inorganic architecture (Gao et al., 2011; Yang et al., 2011; Abou-Melha et al., 2021). The fabrication process (Scheme 1) ensured precise control over the dimensions of these novel MSNTs. The process started by suspending MWCNTs in deionized water, then forming a silica shell to make MSNTs@MWCNTs. The complete removal of MWCNTs from the composite was verified through XRD analysis, which included both low- and wide-angle diffraction patterns. The WAXRD of the MSNTs and MNS exhibited a characteristic broad peak spanning the 18–38° range, indicating the amorphous nature of the nanotube walls, as depicted in Fig. 1A. Additionally, the LAXRD patterns revealed a shoulder peak at 2θ ≈ 1.37°, confirming the presence of an ordered mesostructure, as illustrated in Fig. 1B. The LAXRD and WAXRD profiles showed that the sensor (MNS) and its precursor, the MSNTs carrier, have similar structures. This suggests that the immobilization process maintains the integrity and order of the MSNTs' mesostructure.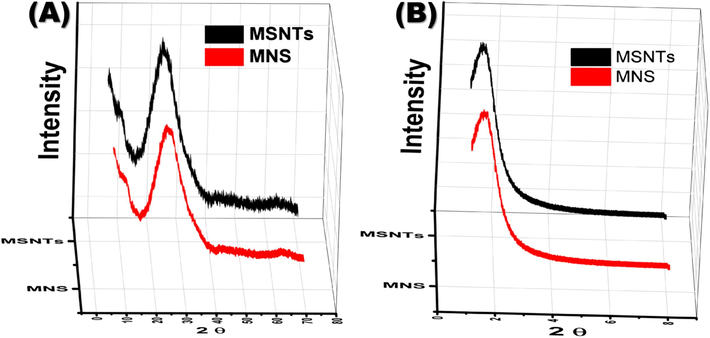
(A) WAXRD and (B) LAXRD diffraction patterns of the MSNTs and MNS.
The MSNTs and MNS show Type IV classification in their nitrogen adsorption–desorption isotherms, as depicted in Fig. 2. These isotherms display dual hysteresis loops within the MSNTs' isotherm, indicating the presence of mesoporous structures enabled by capillary condensation. The mesopores within the SiO2 layer produce a minor hysteresis loop in the P/P0 range of 0.3 to 0.5 after CTAB extraction. Conversely, the pronounced hysteresis loop at P/P0 values exceeding 0.8 is ascribed to the intrinsic mesopores of the MSNTs themselves (Vila et al., 2009). Post-immobilization of the organic chromophore, a reduction in the MSNTs’ surface area is evident from the N2 isotherms (Fig. 2A). The Brunauer-Emmett-Teller (BET) surface area of the MSNTs is quantified at 1120 m2/g, surpassing that of the MNS, which is 922 m2/g. Similarly, the pore volume of the MSNTs, measured at 1.56 cm3/g, exceeds the MNS’s 1.33 cm3/g (Fig. 2B). The preservation of chromophores both on the exterior and interior of the nanotubes accounts for the diminished pore volumes and surface areas observed in the MNS. These findings elucidate the systematic process of infilling and ornamenting the microporous voids within the MSNTs with organic receptors.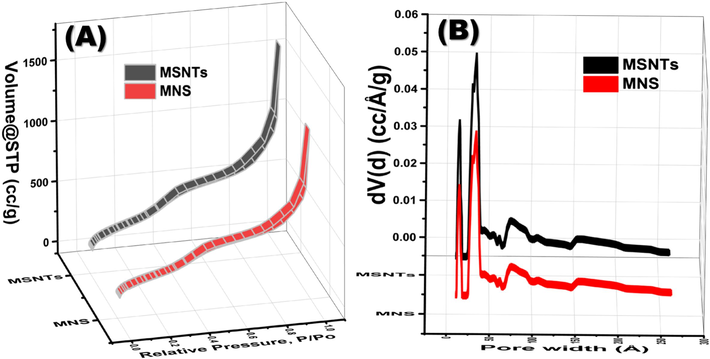
Nitrogen adsorption–desorption analysis of the MSNTs and MNS and pore size distributions of the MSNTs and MNS (B).
The morphological characterization of MSNTs was conducted using SEM and TEM techniques, with the findings illustrated in Fig. 3. Initially, the pristine MWCNTs exhibited an average diameter ranging from 15-30 nm. Post-coating with mesoporous SiO2, a notable increase in diameter to approximately 55–85 nm was observed, indicative of a 40–50 nm thick SiO2 mesoporous layer enveloping the MWCNTs. TEM analysis corroborated the uniform coating of the mesoporous SiO2 layer across all samples, with minimal free SiO2 particles detected. Intriguingly, most of the pores were aligned perpendicular to the MWCNTs’ surface, a feature that is anticipated to enhance mass transfer across the silica coating’s inner and outer surfaces, thereby rendering this structure advantageous for nanocarrier applications (Ding et al., 2009).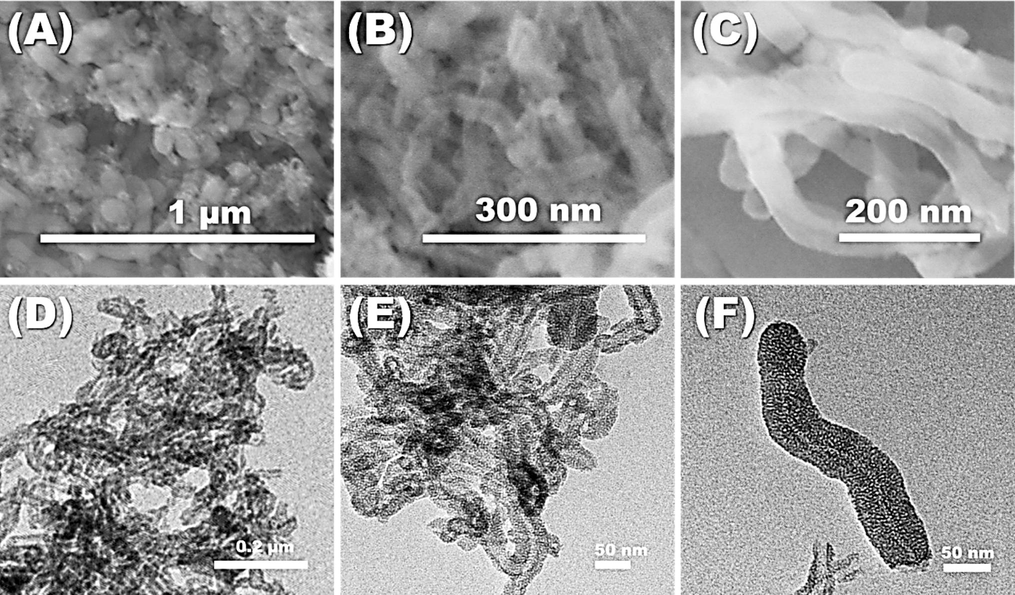
Representative FESEM images of (A) the MSNTs, (B) the MNS chemosensor, and (C) the MNS chemosensor after many times of reusability, the TEM images of (D) the MSNTs, (E) the MNS chemosensor, and (F) the MNS chemosensor after many times of reusability.
SEM imagery (Fig. 3A-C) revealed the engineered morphology of the nanotube structures, which were dispersed and interconnected randomly, forming the MSNTs’ cavities. These carriers were synthesized through reproducible methods that ensured control over the structural morphology. The emergence of a worm-like structure is attributed to the Si-O-Si interactions, leading to the spontaneous assembly and clustering of silicon dioxide around the MWCNTs. The dispersion of the carrier in the solution regulated the self-assembly of MSNTs and the formation of significant openings. TEM images (Fig. 3D–F) displayed the MSNTs arranged asymmetrically to create significant openings. The findings demonstrate that cylindrical MSNTs, with diameters around 65 nm, were randomly clustered and layered around the cavities, encapsulating the highly sensitive receptors. A uniform coating of dense, thin organic receptor layers was applied to the MSNTs’ cavities, culminating in the MNS. The direct immobilization of organic chromophore onto the silica nanotubes facilitated ongoing monitoring, rapid detection (within seconds), and the capture of Hg2+ contaminants in both water and cosmetic products. The chromophores were incorporated without affecting the structural morphology of the MSNTs. This was confirmed by the FESEM image (Fig. 3 B) and the TEM image (Fig. 3 E). In addition, the FESEM image (Fig. 3 C) and the TEM image (Fig. 3 C) confirm the stability of the sensor even after reusability many times.
3.1 Quantitative determination of mercury(II) ions using UV–Vis spectrophotometry
A major challenge in chemical sensors is creating optical chemosensors that are highly stable. The long-lasting stability of the carefully crafted optical chemosensors is demonstrated by minor fluctuations in sensor performance over time, especially during storage. Spectral analyses of these sensors have consistently yielded uniform results, affirming the absence of sensitivity degradation post-storage in an opaque container.
The optimal sensing parameters greatly affect the fidelity and intensity of color distribution, which is crucial for detecting ultratrace levels of mercury ions. To ascertain the efficacy of MNS optical chemosensors, various factors were scrutinized, including the limitations of Hg2+ ion detection, pH levels, response duration, and the quantity of chemosensors employed (measured in milligrams). The absorbance spectra in Fig. 4A were carefully examined to find the optimal pH for detecting Hg2+ ions. A gamut of standard Hg2+ ion solutions was fine-tuned with diverse buffer solutions. At a neutral pH of 7, a pronounced absorbance spectrum was observed, designating it as the optimal condition for Hg2+ ion sensing.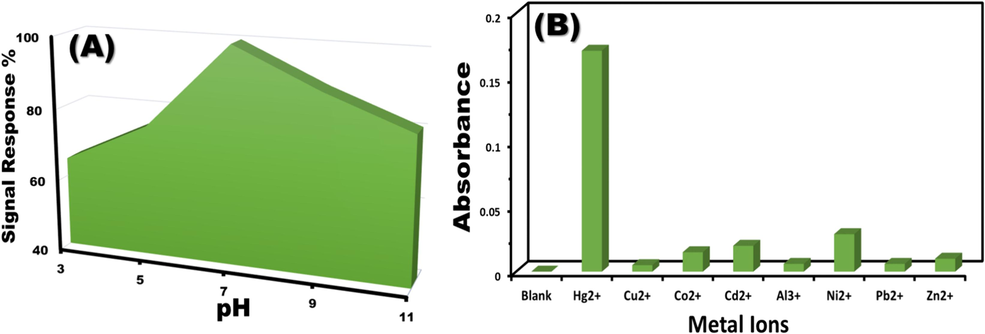
(A) pH-dependent response of MNS optical chemosensors to 1 ppm Hg2+ ions. (B) Interference studies: Effect of common interfering cations on the absorbance spectra of MNS chemosensors.
The test samples were exposed to standard solutions containing common interfering cations to investigate the selectivity of MNS chemosensors for Hg2+ detection. The spectrophotometric measurements were carried out at a wavelength of λ = 560 nm, following the optimal conditions (pH 7, ambient temperature, 10 mL sample volume, and 10 mg sensor dosage). Fig. 4B illustrates that the introduction of interfering ions did not significantly alter the absorption spectra. However, the addition of Hg2+ ions resulted in the emergence of a new absorbance peak at λ = 560 nm. The absorbance of common ions was documented, revealing a marked enhancement in the absorption spectra upon Hg2+ ion interaction. The established tolerance levels for various interfering ions are as follows: Cu2+ (20 ppm), Zn2+ (100 ppm), Pb2+ (100 ppm), Co2+ (100 ppm), Al3+ (100 ppm), Ni2+ (100 ppm), and Cd2+ (100 ppm). These results affirm the MNS chemosensors’ exceptional specificity for Hg2+ ions.
Under the designated optimal sensing conditions, the absorbance of the MNS chemosensor was meticulously examined (refer to Fig. 5A, B). At the peak wavelength (λmax) of 560 nm, a notable intensification in the MNS absorption spectra was observed in tandem with the escalating concentrations of Hg2+ ions. This amplification in absorbance intensity is ascribed to the charge transfer occurring during the formation of the complex. The calibration curve for the MNS demonstrated a direct linear relationship with the Hg2+ ion concentrations at lower levels, as depicted in Fig. 5C. This linear correlation (illustrated in Fig. 5D) facilitates the tracking of Hg2+ ions in ultra-trace quantities, within the highly sensitive detection range of 0.002 – 0.65 µM. The color transition upon the creation of the MNS-Hg2+ complex was discernible to the naked eye, negating the need for intricate methodologies. The calculated limit of detection (LOD) for Hg2+ ions operating the MNS chemosensors was determined to be 1.9 × 10-8M, comfortably below the permissible threshold for water (as outlined in Table 1).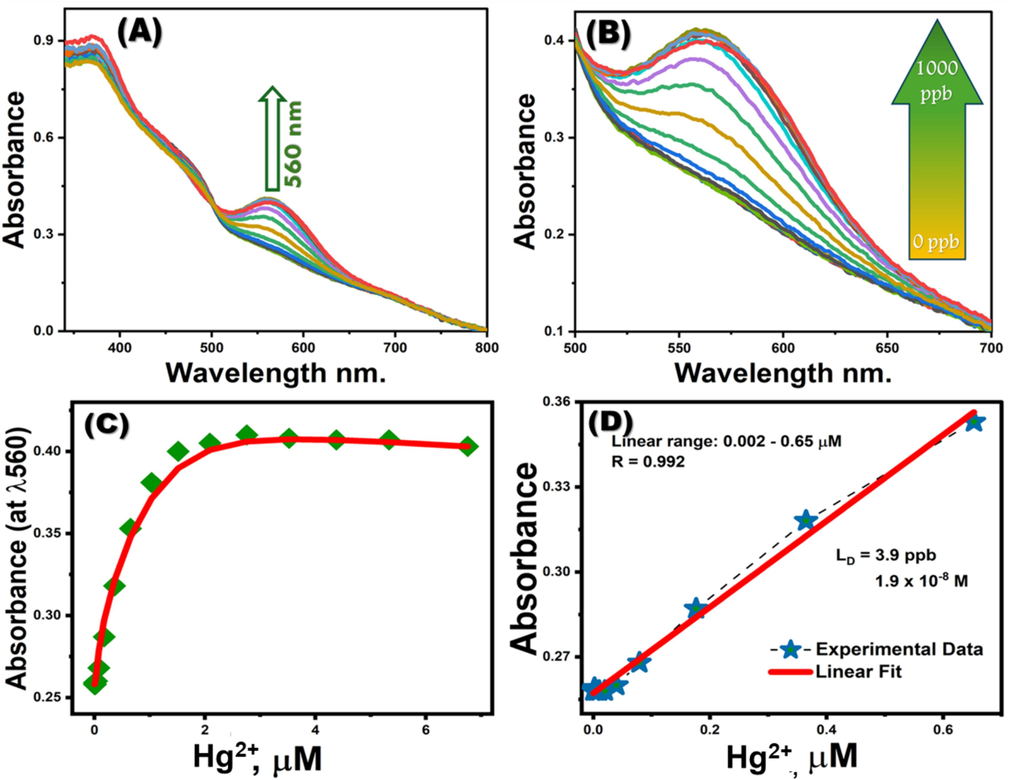
(A, B) Absorption spectra of MNS optical chemosensors titrated with Hg2+ ions. (C) Calibration curves for MNS optical chemosensors, measured at λ560nm. (D) Linear-fit line within the linear concentration range of the calibration plots for MNS optical chemosensors at λ560nm with varying Hg2+ concentrations.
Parameters
Spectrophotometric method
(DICA) method
LD (M)
1.9 x 10-8
4.9 x 10-8
DR (µM)
0.002 to 0.65
0.024 to 0.5
LQ (M)
6.5 x 10-8
1.3 x 10-7
R2
0.992
0.986
The interaction between Hg2+ ions and the MNS material resulted in the formation of Hg2-MNS complexes. This 1:2 stoichiometric ratio between MNS and Hg2+ ions was further set through Job’s plot analysis (Fig. 6.). The visual manifestation of these complexes is clearly evident in the images presented in Fig. 6.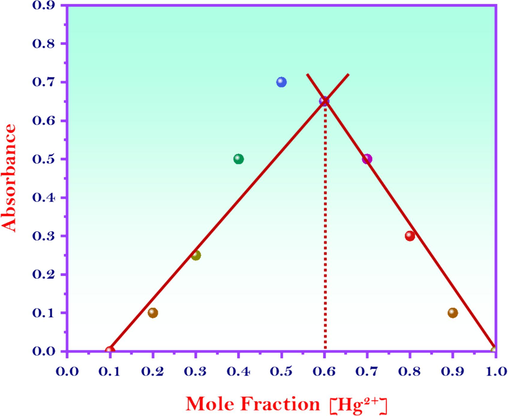
Job’s plot analysis for the interaction between the MNS and the Hg2+ ions.
Limit of detection (LD), detection range (DR), Limit of quantitation (LQ).
3.2 Hg2+ ion sensing with digital image-based colorimetry
The histogram tool, pivotal in digital image-based colorimetry analysis (DICA), scans each pixel within the selected color regions to deduce the mean integer values of Red, Green, and Blue (RGB) intensities. These intensities (IR, IG, and IB) correlate with the concentration of Hg2+ ions, as illustrated in Fig. 7A. Notably, the intensities of the green (IG) and red (IR) components diminish as the Hg2+ ion concentration increases, while the blue intensity (IB) remains relatively unchanged. The predominance of the red component in the sensor's color response is consistent with the spectrophotometric method, which shows high absorption at 560 nm. By associating the color intensity with the absorbance at each concentration level, a calibration curve can be constructed that mirrors the one obtained from the spectrophotometric method (shown in Fig. 7B and C and Fig. 8). Fig. 7D corroborates that Hg2+ ions can be sensitively detected at extremely low concentrations, specifically 4.9 × 10-8M. Table 1 discusses the DICA analytical parameters, highlighting a performance that rivals that of the spectrophotometric method. The analytical parameters gleaned from the use of a mobile camera device substantiate the efficacy of DICA for the rapid sensing of toxic Hg2+ ions within seconds. Thus, DICA emerges as a competitive alternative for the quantification of mercury ion concentrations.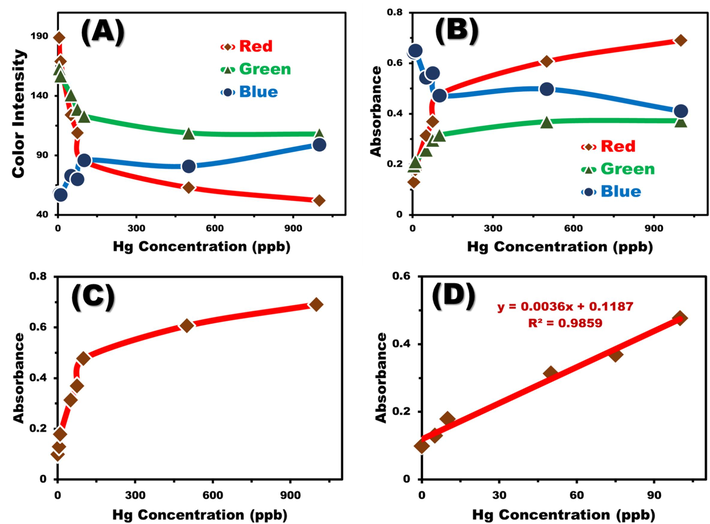
(A) Correlation between Hg2+ ion concentrations and RGB intensities derived from digital images analyzed using the Histogram tool. (B) Relationship between Hg2+ ion concentrations and calculated RGB absorbances for mobile camera images. (C) Correlation between Hg2+ ion concentrations and the calculated absorbance of red color, demonstrating an increase in red color absorbance with rising Hg2+ ion concentrations. (D) Linear correlation between red color absorbance and Hg2+ ion concentration. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
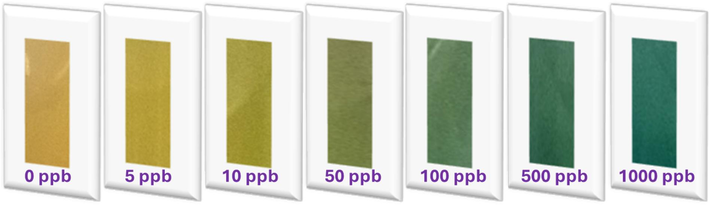
Captured input pics for the DICA, showcasing the complexation between Hg2+ and the MNS probe across various concentrations.
3.3 Computational analysis of MNS probe and its interaction with Hg2+
Using density functional theory (DFT) and the Gaussian 09 program (Ambroise et al., 2021; Sarangi et al., 2020; Fouda and Besley, 2018; Besley, 2021), a theoretical analysis was performed to understand the binding mechanism between the MNS probe and Hg2 + ions, including energy level assessments. The electronic structure of the MNS probe and [(MNS)2-Hg(II)] complexes was characterized, focusing on their HOMO and LUMO using different functional basis sets. The optimized geometrical structures of the MNS probe and the MNS-Hg(II) complex are displayed in Fig. 9A.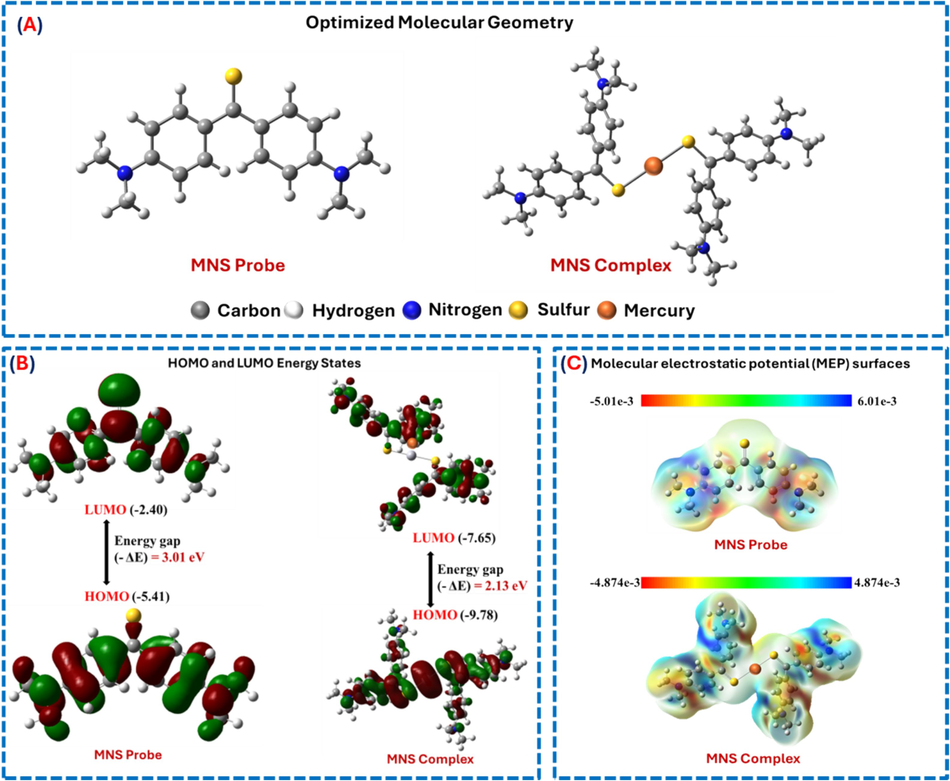
(A) Optimized molecular geometry of the MNS probe and MNS-Hg(II) complex. (B) Frontier molecular orbital (FMO) analysis of the MNS probe and MNS-Hg(II) complex. (C) Molecular electrostatic potential (MESP) mapping of the MNS probe and MNS-Hg(II) complexation.
Furthermore, the binding energy and charge transfer mechanism of the MNS probe and the MNS-Hg(II) complex were assessed using Frontier Molecular Orbital (FMO) analysis, as shown in Fig. 8B. Surprisingly, our research indicates that the MNS probe, which includes ligating groups like (=S), had a more positive interaction with Hg(II) at a binding ratio of 2:1 (Marczenko and Balcerzak, 2000). The energy transition differences (EHOMO-ELUMO) indicate that the MNS probe has an energy band gap value of 3.01 eV, while the MNS-Hg(II) complex has a value of 2.13 eV, as shown in Fig. 9B. The observed decrease in the band gap suggests that the MNS probe has effectively interacted with Hg(II), as indicated by the hyperchromic shift in absorption spectra.
The presence of sulfur atoms in the MNS probe significantly contributed to the stability of the Hg-S bond. Additionally, a MESP 3D mapping of both the MNS probe and the [(MNS)2-Hg(II)] complex was performed to identify active coordination sites and the electrostatic potential distribution across the molecule, shown in Fig. 9C. Electrophilic, neutral, and nucleophilic potentials were identified through visual inspection, each represented by distinct colors—blue, green, and red, respectively. Regions showing a red stain, which indicates negative potential due to active functional groups (=S and −N-CH3), improved the chances of strong binding interaction with Hg2+ when forming complexes using the MNS probe. Complex formation led to a significant enhancement in negative potential, primarily attributed to the stable coordination of the = S—Hg bond. Thus, the presence of functional groups such as = S in MNS molecules was found to be essential in promoting the formation of charge transfer transition complexes with Hg(II), as demonstrated by MESP analysis.
3.4 Reusability and long-term stability analytical assessment for the MNS
The MNS sensor's performance was evaluated in terms of long-term stability and reusability. To assess reusability, the sensor was subjected to multiple cycles of Hg2+ ion detection and regeneration. Following Hg2+ ion recognition, the MNS material was regenerated by decomplexing the bound Hg2+ ions using 2 mL of 0.05 M thiourea (Fig. 10A). The regenerated MNS exhibited minimal changes in absorbance compared to a blank sample, indicating the absence of probe molecule leaching. The sensor demonstrated excellent reusability, maintaining its sensing efficiency for up to six cycles. Beyond this point, a slight decline in performance was observed, potentially due to minor probe detachment from the MSNT carrier. Fig. 3 C&F confirm the stability of the morphology of the MNS chemecalsensor even after many times reusability.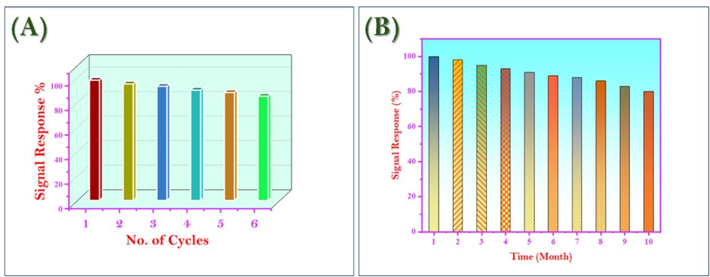
(A) Reuse study of MNS material (B) Long-term performance and stability study for the MNS material.
To evaluate the sensor's long-term stability, its color and spectral response were monitored over a period of 10 months (Fig. 10B). The results demonstrated the chemical stability of the MNS material toward Hg2+ ion detection, indicating its suitability for prolonged use without significant performance degradation.
3.5 Analytical assessment of the presence of competing during Hg2+ detection with MNS
To evaluate the MNS’s selectivity for Hg2+ ions amidst potential interfering species, rigorous selectivity and competitive experiments were conducted. The sensor’s performance was assessed under optimized conditions. The tolerance limit, specified as the maximum interfering ion concentration causing less than a five percent error in absorbance measurements, was determined. As comprehensively detailed in Fig. 11, the MNS exhibited exceptional selectivity for Hg2+ ions. While the MNS demonstrated high performance for most tested ions, the influence of Au3+, Ag+, and Pd2+ was mitigated through the addition of 1 mL of 2 mM sodium thiosulfate solution as a masking agent. The MNS’s consistent detection of Hg2+ ions in the existence of significant concentrations of other ions underscores its potential for practical applications in complex matrices.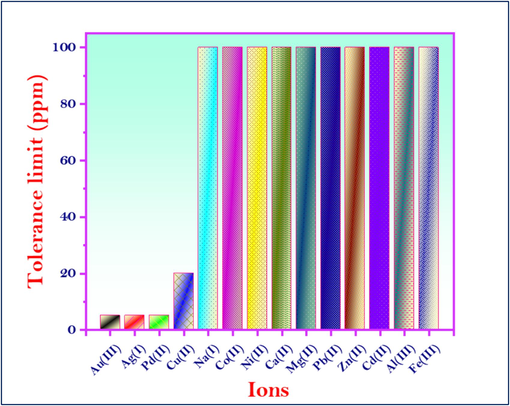
Effect of interfering ions on the detection of Hg2+ ions using MNS optical chemosensors.
3.6 Applicability of MNS nanotubes
In the practical application of MNS chemosensors, various water samples and skin-whitening creams were subjected to mercury ion detection. Under the established optimal sensing parameters, the water samples collected from different locations were spiked with a consistent concentration of Hg2+ ions. The water samples underwent a triplicate analysis for mercury ion detection, which was later confirmed by ICP-MS testing. The congruence between the ICP-MS data and our findings underscores the efficacy and competitiveness of the MNS chemosensors in mercury detection, as detailed in Table 2. For the skin-whitening cream samples (containing methylmercury as a source of mercury), a pre-treatment involving digestion of 1 g of sample with 10 mL concentrated nitric acid, followed by filtration then dilute the solution to 100 mL for further analysis. Subsequently, 10 mg of MNS optical chemosensors were introduced to the treated samples under optimal conditions. The data pertaining to these samples, as compiled in Table 2, demonstrate the chemosensors’ exceptional selectivity and sensitivity in quantifying Hg2+ ion concentrations. This reinforces the potential of MNS chemosensors as a reliable tool for mercury detection in real-world scenarios.
Sample
ICP-MS Analysis
(ppm)Amount spiked of Hg(II)
(ppb)Proposed method
Spectrophotometer
DICA
Recovery
E%
Error%
Recovery
E%
Error%
Tap Water Sample 1
521 ppm Ca2+, 1056 ppm Mg2+, 1.8 ppm Fe2+, 1.6 ppm Fe3+
20
18.9
94.5
5.5
17.8
89
11
Tap Water Sample 2
619 ppm Ca2+, 305 ppm Mg2+, 1.1 ppm Fe2+, 1.8 ppm Fe3+
50
48.5
97
3
47.9
95.6
4.4
*Cosmetic Sample 1
0.1 ppm
(100 ppb)−
98.2
98.2
0.8
97.4
97.4
2.6
4 Conclusion
The culmination of this research has yielded MNS optical chemosensors that stand out for their water stability and enduring structural integrity, maintaining uniform morphology over extended periods. The MSNTs carriers, functionalized with chromophores, facilitate ease of use in both solution and solid states, enabling spectrophotometric analysis. These chemosensors are adept at detecting Hg2+ ions in ultra-trace amounts in aqueous environments and skin-whitening products, boasting a detection threshold a hundredfold more sensitive than chromophore applications alone, and surpassing the sensitivity of several previously reported Hg2+ optical sensors. The rapid response time of these sensors, clocking in at under 20 s to reach signal stability, coupled with the visually discernible color shift, enhances their user-friendliness. This color change can be conveniently monitored via smartphones or spectrophotometric methods. The high reversibility and reproducibility of the MNS chemosensors, along with their multiple-use capability, underscore their practicality. At an optimal pH of 7 and a temperature of 25 °C, the MNS chemosensors exhibit unparalleled selectivity for Hg2+ ions, making them highly suitable for water quality analysis. The long-term stability of these chemosensors is remarkable, ensuring sustained efficiency over numerous applications. The UV–Vis spectrophotometer has determined a detection limit of 1.9 × 10-8 M for the MNS nanomonitors. Furthermore, the integration of mobile phone cameras and computer software has demonstrated a comparable sensitivity, with a detection limit as low as 4.9 × 10-8 M. The findings from this study confirm the MNS chemosensors’ potential for effective monitoring and sequestration of Hg2+ ions, positioning them as an asset in environmental and health safety applications.
CRediT authorship contribution statement
Albandary Almahri: Writing – original draft, Software, Resources, Methodology, Funding acquisition, Formal analysis. Nashwa M. El-Metwaly: Writing – review & editing, Visualization, Supervision, Project administration, Data curation.
Acknowledgments
The authors extended their appreciations to Princes Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2024/01/28923).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A highly sensitive chemosensor based on a metal-organic framework for determining zinc ions in cosmetics creams and wastewater. Appl. Organomet. Chem.. 2024;38(5):e7407.
- [Google Scholar]
- Extraction and detection of mercury in electronic waste using efficient modified MOF. Colloids Surf. A Physicochem. Eng. Asp.. 2024;686:133256
- [Google Scholar]
- Novel composite based on cellulose nanomaterial for detection and selective removal of cadmium (II) ions from wastewater. Microchem. J.. 2024;198:110175
- [Google Scholar]
- Functionalized silica nanotubes with azo-chromophore for enhanced Pd2+ and Co+ ions monitoring in E-wastes. J. Mol. Liq.. 2021;329:115585
- [Google Scholar]
- Adsorption of organic dye onto mesoporous aluminosilica monoliths: Equilibrium, kinetic and thermodynamic studies. Chemistryselect. 2022;7(30):e202201995
- [Google Scholar]
- Effective removal of Methyl blue and Crystal Violet dyes using improved polysulfone/ZIF-8 nanocomposite ultrafiltration membrane. Biointerface Res. Appl. Chem.. 2021;12:7942-7956.
- [Google Scholar]
- Probing basis set requirements for calculating core ionization and core excitation spectra using correlated wave function methods. J. Chem. Theory Comput.. 2021;17(5):2832-2842.
- [Google Scholar]
- Awual, M. R., Hasan, M. M., & Shahat, A. (2017). Erratum to:“Functionalized novel mesoporous adsorbent for selective lead (II) ions monitoring and removal from wastewater”[Sens. Actuators B Chem. 203 (2014) 854–863]. Sensors and Actuators B: Chemical, 100(242), 777.
- Tailoring the structure of Fe-based NH2-MIL-88 via 2-hydroxyacetophenone for arsenic removal from aqueous solutions: Kinetics, adsorption isotherms studies, and optimization through Box-Behnken design. Sens. Actuators, A: Phys.. 2024;373:115398
- [Google Scholar]
- Modeling of the spectroscopy of core electrons with density functional theory. Wiley Interdiscip. Rev.: Comput. Mol. Sci.. 2021;11(6):e1527.
- [Google Scholar]
- Bose-O’Reilly, S.; McCarty, K. M.; Steckling, N.; Lettmeier, B.; Mercury Exposure and Children’s Health, Curr Probl Pediatr Adolesc Health Care. 2010, 186–215.
- Validation of handheld X-ray fluorescence for in situ measurement of mercury in soils. J. Environ. Chem. Eng.. 2017;5(1):768-776.
- [Google Scholar]
- Label-free colorimetric detection of mercury via Hg2+ ions-accelerated structural transformation of nanoscale metal-oxo clusters. Sci. Rep.. 2015;5:16316.
- [Google Scholar]
- The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959-2966.
- [Google Scholar]
- The toxicology of mercury–current exposures and clinical manifestations. N. Engl. J. Med.. 2003;349:1731-1737.
- [CrossRef] [Google Scholar]
- A simple route to coat mesoporous SiO2 layer on carbon nanotubes. J. Mater. Chem.. 2009;19:3725-3731.
- [Google Scholar]
- Plasmonic gold nanoparticles stain hydrogels for the portable and high-throughput monitoring of mercury ions. Environ. Sci. Tech.. 2021;56(2):1041-1052.
- [Google Scholar]
- Optical chemosensors for environmental monitoring of toxic metals related to Alzheimer's disease. RSC Adv.. 2022;12(50):32744-32755.
- [Google Scholar]
- Superior adsorption and removal of aquaculture and bio-staining dye from industrial wastewater using microporous nanocubic Zn-MOFs. Micropor. Mesopor. Mater.. 2022;329:111506
- [Google Scholar]
- Multi-responsive paper chemosensors based on mesoporous silica nanospheres for quantitative sensing of heavy metals in water. RSC Adv.. 2023;13(10):6433-6441.
- [Google Scholar]
- Fluorescent sensor/tracker for biocompatible and real-time monitoring of ultra-trace arsenic toxicants in living cells. J. Hazard. Mater. 2024135429
- [Google Scholar]
- Assessment of basis sets for density functional theory-based calculations of core-electron spectroscopies. Theor. Chem. Acc.. 2018;137(1):6.
- [Google Scholar]
- Stripping voltammetric detection of mercury (II) based on a surface ion imprinting strategy in electropolymerized microporous poly(2-mercaptobenzothiazole) films modified glassy carbon electrode. Anal. Chim. Acta.. 2011;685:21-28.
- [Google Scholar]
- Mercury speciation in hair by headspace injection–gas chromatography–atomic fluorescence spectrometry (methylmercury) and combustion-atomic absorption spectrometry (total Hg) Talanta. 2010;82:1919-1923.
- [Google Scholar]
- C. Gao, Q. Zhang, Z. Lu, Y. Yin, Templated synthesis of metal nanorods in silica nanotubes, J. Am. Chem. Soc. 133 (2011) 19706e19709.
- Cognitive performance of children prenatally exposed to “Safe” levels of methylmercury. Environ. Res.. 1998;77:165-172.
- [Google Scholar]
- Minamata disease: methylmercury poisoning in Jaorganic chromophore caused by environmental pollution. Crit. Rev. Toxicol.. 1995;25:1-24.
- [Google Scholar]
- A novel fine-tuning mesoporous adsorbent for simultaneous lead(II) detection and removal from wastewater. Sens. Actuators, B. 2014;202:395-403.
- [Google Scholar]
- Mesoporous silica based novel conjugate adsorbent for efficient selenium(IV) detection and removal from water. Micropor. Mesopor. Mater.. 2014;197:331-338.
- [Google Scholar]
- Organic-inorganic based nano-conjugate adsorbent for selective palladium(II) detection, separation and recovery. Chem. Eng. J.. 2015;259:611-619.
- [Google Scholar]
- A novel and potential chemical sensor for effective monitoring of Fe(II) ion in corrosion systems of water samples. Microchem. J.. 2020;154:104578
- [Google Scholar]
- Novel multi-function chromoionophoric probe-based nano-conjugate material for highly efficient detection, removal, and recovery of Pd (II) and Co (II) ions from electronic wastes and electroplating wastewater. ChemistrySelect. 2024;9(28):e202400557
- [Google Scholar]
- Environmental costs of mercury pollution. Sci. Total Environ.. 2006;368:352-370.
- [CrossRef] [Google Scholar]
- Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated waste-water effluents. Chem. Eng. J.. 2019;355:91-102.
- [Google Scholar]
- Efficient dual sensor of alternate nanomaterials for sensitive and rapid monitoring of ultra-trace phenols in sea water. J. Mol. Liq.. 2020;297:111798
- [Google Scholar]
- Simultaneous ultra-trace palladium(II) detection and recovery from wastewater using new class meso-adsorbent. J. Ind. Eng. Chem.. 2015;21:405-413.
- [Google Scholar]
- A ratiometric and selective fluorescent nanosensor for determining Hg2+ ions based on a novel functionalized metal–organic framework. Appl. Organomet. Chem.. 2024;38(8):e7571.
- [Google Scholar]
- Colorimetric determination of Cu (II) ions in biological samples using metal-organic framework as scaffold. Sens. Actuators B: Chem.. 2016;233:272-280.
- [Google Scholar]
- Mercury in environmental samples: Speciation, artifacts and validation. TrAC Tren. in Anal. Chem.. 2005;24:383-393.
- [Google Scholar]
- Marczenko, Z., & Balcerzak, M. (2000). Separation, preconcentration and spectrophotometry in inorganic analysis (Vol. 10). Elsevier.
- Nano-composite multi-wall carbon nanotubes using poly(pphenylene terephthalamide) for enhanced electric conductivity. J. Environ. Chem. Eng.. 2019;7:103002
- [Google Scholar]
- M.J.S. McGrath, C.N. Scanaill, Sensing and sensor fundamentals, in: Sensor Technologies: Healthcare, Wellness and Environmental Applications, Apress, New York, 2013, pp. 15e50.
- Mercury Update: Impact on Fish Advisories, EPA Off. Water Mercur. Updat. Impact Fish Advis. EPA Fact Sheet EPA-823-F-01-011, Washingt. DC. 2001.
- Efficient and low-cost mesoporous magnetic carbon composites derived from date palm stones for environmental remediation of hexavalent chromium. J. Porous Mater. 2024:1-15.
- [Google Scholar]
- Simultaneous analysis of mercury and selenium species including chiral forms of selenomethionine in human urine and serum by HPLC column-switching coupled to ICP-MS. Analyst. 2010;135:2700-2705.
- [Google Scholar]
- Capture of mercury ions by natural and industrial materials. J. Hazard. Mater.. 2006;132:220.
- [Google Scholar]
- Synergistic degradation of antibiotic sulfamethazine by novel pre-magnetized Fe0/PS process enhanced by ultrasound. Chem. Eng. J.. 2018;354:777-789.
- [Google Scholar]
- Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut., Ser. B. 2017;221:1-14.
- [Google Scholar]
- Multiuse Al-MOF chemosensors for visual detection and removal of mercury ions in water and skin-whitening cosmetics. ACS Sustain. Chem. Eng.. 2020;8(40):15097-15107.
- [Google Scholar]
- Decorated nanosphere mesoporous silica chemosensors for rapid screening and removal of toxic cadmium ions in well water samples. Microchem. J.. 2020;156:104806
- [Google Scholar]
- Monitoring of cobalt and cadmium in daily cosmetics using powder and paper optical chemosensors. ACS Omega. 2022;7(18):15739-15750.
- [Google Scholar]
- Carboxymethyl-imidazolium O-vanillin Schiff base grafted into NH2-tagged MIL-101 (Cr) for effective removal of cupric ions from aqueous effluents. Environ. Sci. Pollut. Res. 2024:1-16.
- [Google Scholar]
- On the basis set selection for calculations of core-level states: Different strategies to balance cost and accuracy. Mol. Phys.. 2020;118(19–20):e1769872.
- [Google Scholar]
- Novel and potential chemical sensors for Au(III) ion detection and recovery in electric waste samples. Microchem. J.. 2020;158:105312
- [Google Scholar]
- Azo-chromophore based on functionalized silica nanotubes for enhanced identification of Pd (II) ions in e-residues. J. Mater. Res. Technol.. 2022;17:2550-2559.
- [Google Scholar]
- A. Shahat, E.A. Ali Mohammad, M.F. El Shahat, Colorimetric determination of some toxic metal ions in post-mortem biological samples, Sensor. Actuator. B Chem. 221 (2015) 1027e1034.
- Carbon nanotubes-mesoporous sil-ica composites as controllable biomaterials. J. Mater. Chem.. 2009;19:7745-7752.
- [Google Scholar]
- Highly stable Zr (IV)-based metal−organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc.. 2016;138:6204-6216.
- [Google Scholar]
- Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron.. 2010;25:1994-1998.
- [Google Scholar]
- X.F. Yang, H. Tang, K.S. Cao, H.J. Song, W.C. Sheng, Q. Wu, Templated-assisted one-dimensional silica nanotubes: synthesis and applications, J. Mater. Chem. 21 (2011) 6122e6135.
- Phase transfer and its applications in nanotechnology. Chem. Soc. Rev.. 2011;40:1672-1696.
- [Google Scholar]
- Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev.. 2015;115:7840-7892.
- [Google Scholar]
- Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol.. 2005;20:351-360.
- [CrossRef] [Google Scholar]
- Hydrogels allow the precise growth tracking of plasmonic gold nanoparticles for mercury analysis. J. Mater. Chem. C. 2022;10(39):14508-14516.
- [Google Scholar]
- On the controllable softtemplating approach to mesoporous silicates. Chem. Rev.. 2007;107:2821-2860.
- [Google Scholar]
- Population health risk due to dietary intake of heavy metals in the industrial area of Huludao City, China. Sci. Total Environ.. 2007;387:96-104.
- [Google Scholar]
- Mercury-mediated epitaxial accumulation of Au atoms for stained hydrogel-improved on-site mercury monitoring. Anal. Chem.. 2023;95(51):18859-18870.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105984.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







