Translate this page into:
A comparative study of phytochemical metabolites and antioxidant properties of Rhodiola
⁎Corresponding author. lsun2013@bjfu.edu.cn (Liwei Sun)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Rhodiola crenulata (RC) and Rhodiola fastigiata (RF) are representative species of Rhodiola with well-accepted health benefits; the roots are the medicinal part. However, prior to this study, the differences in phytochemicals between these two species and different parts of the same species remained unclear. Using LC-ESI-MS/MS, HS-SPME-GC–MS, chemical and sensory analyses, volatile compounds and non-volatile compounds, and antioxidant activities of the roots of Rhodiola crenulata and Rhodiola fastigiata and four parts (roots, leaves, flowers, and above-ground stems) of RC were investigated. The volatile compounds and non-volatile compounds of RC roots exhibited upregulation overall compared to those of RF roots, and the odorousness, phenolic content, and antioxidant activity were more pronounced in the RC roots. The phenolic content and antioxidant activity of roots and leaves, alongside the odorousness of roots and flowers, were more significant among the four parts of RC, and the RC roots and RC flowers exhibited similar odorousness. Comparison of non-volatile differential metabolites between RC roots and RC leaves showed upregulations of saccharides and phospholipids, and minor upregulations of flavonoids and phenylpropanoids in the roots; in addition, amino acids, organic acids, and vitamins were upregulated in the leaves. These results revealed the following: 1) RC roots are superior to RF roots regarding volatile compounds and non-volatile compounds, and antioxidant activity; 2) it is more favorable to select RC roots for exploiting volatile compounds compared with RC flowers in consideration of the biomass available; 3) in terms of non-volatile compounds, and antioxidant ability, RC leaves are also of great value in addition to RC roots, though these two parts show distinct characteristics.
Keywords
Rhodiola crenulata
Rhodiola fastigiata
Volatile compounds
Non-volatile compounds
Metabolomic
Antioxidant ability
1 Introduction
Rhodiola is a perennial functional plant from Crassulaceae, of which there are approximately 90 species worldwide and 73 species in China. Several Rhodiola have been used in Asian and European countries to promote human health (Tao et al., 2019; Shikov et al., 2021; Panossian et al., 2021). Modern pharmacological studies revealed that Rhodiola exhibits various benefits, such as antioxidation (Dong et al., 2021), anti-inflammation (Song et al., 2021), anti-plateau reaction (Chiu et al., 2013), anticancer (Bassa et al., 2016), antiviral (Wang et al., 2021), and cardioprotective effects, and it is used in the treatment of lung diseases (Hsu et al., 2017; Chuang et al., 2015). In China, Rhodiola crenulata is a typical functional species (Chinese Pharmacopoeia Commission, 2020), and is used for anti-hypoxia, anticancer and anti-plateau reaction treatments, for improving physical strength, and for eliminating fatigue (Ma et al., 2022; Tao et al., 2019; Hsu et al., 2017; Chiu et al., 2013). Rhodiola fastigiata has also been commonly employed (Li and Zhang, 2008; Ma et al., 2021) and, to our knowledge, is used as a main replacement for Rhodiola Crenulata in the market of China. However, research on Rhodiola fastigiata is rarely be found.
Different species of plants have different pharmacological qualities due to their differences in phytochemical compositions. This is shown in the species of Eucommia ulmoides (namely, Purple-leaf E. ulmoides and “Qinzhong”), and also in the species of chrysanthemum (namely, “Boju” and “Huaiju”) (Wu et al., 2018; Xie et al., 2012). Booker et al. (2016) identified the quality of Rhodiola by determining differences in phytochemical composition among species. Our previous study also showed that Rhodiola crenulata possessed stronger phenolic content and antioxidant activity than Rhodiola rosea (Dong et al., 2020). Nevertheless, until this study, comparative studies on the phytochemical components between Rhodiola crenulata and Rhodiola fastigiata remained undone.
Different parts of the same plant possess different pharmacological qualities due to their differences in phytochemical compositions. This is manifest in the flowers and leaves of Agastache rugosa; in the leaves, flowers, and stems of Lonicera japonica Thunb; and in the leaves and barks of Eucommia ulmoides (Park et al., 2019; Li et al., 2020a, 2020b; Wu et al., 2018). The roots is well-recognized as the medicinal parts of Rhodiola (Tao et al., 2019). Besides the roots, the leaves, above-ground stems, and flowers of Rhodiola maybe also significant, which may have substantial exploitation value. Comparative studies on the phytochemical components of the four parts of Rhodiola (roots, leaves, flowers, and above-ground stems) have been but rarely reported.
The chemical compounds of a plant can be categorized into two groups: volatile compounds and non-volatile compounds. Both volatile compounds and non-volatile compounds from plants offer a number of health benefits to humans. Plant volatile terpenoids have shown health benefits such as antibacterial, antiviral, anticancer, anti-inflammatory, and antidepressant effects (Petrović et al., 2019). Zhang et al. (2021a) reported that volatile monoterpenes, sesquiterpenes, and aromatics from herbs provide excellent antidepressant effects and minor toxic side effects. Aguiar et al. (2021) found that volatile alcohols from broccoli and spinach are antibacterial. Leelarungrayub et al. (2017) found that volatile olefins in Zingiber cassumunar Roxb are antioxidant and anti-inflammatory. Phenolic components of plant non-volatile compounds (phenolic components can be further classified mainly into three groups: flavonoids, gallic acids, and phenylpropanoids) exert effective therapeutic and preventive effects as antioxidants against a wide range of diseases, including COVID-19, cancer, cardiovascular diseases, diabetes, and neurodegenerative diseases (Stiller et al., 2021; Shen et al., 2022; Nabavi et al., 2016). Phenols are the most representative class of health-promoting Rhodiola components (Tao et al., 2019). Previous studies have shown that the content of phenolic components has a significant positive correlation with the antioxidant activity of plants (Shi et al., 2018). Dong et al. (2020) and Dong et al. (2021) also found that in Rhodiola species, the phenolic compounds content was positively related with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) antioxidant abilities. There were significant differences in antioxidant capacity between different phenolics (Velderrain-Rodríguez et al., 2018; Feduraev et al., 2022). Additionally, significant differences existed in pharmacological functions among different phenolics. Jeong et al. (2012) found that in Juniperus rigida the anti-inflammatory activity of its phenylpropanoid glycosides, lignan glycosides, and flavonoids was significantly stronger than that of its coumarins. Yoo et al. (2017) found that 4-hydroxybenzoic acid, vanillic acid, and syringic acid (which are belong to phenylmethanes, a subgroup of phenylpropanoids) inhibited the inflammatory factor TNF-α significantly more strongly than ferulic acid (which is belongs to phenylpropanes, another subgroup of phenylpropanoids). Velderrain-Rodrí et al. (2018) found that the antiproliferative activity of gallic acid on human colon cancer cells was significantly stronger than those of quercetin, catechin, rutin (which are flavonoids), syringic acid, p-coumaric acid, and ferulic acid (which are phenylpropanoids). Quercetin, as a plant-derived flavonoid, has been a research hotspot recently in the fight against lung inflammation, lung diseases induced by rhinoviruses, and lung protection (Han et al., 2020). There is a variety of quercetin-based health products marketed worldwide for protecting the lungs.
Saccharides, amino acids, and lipids are the three essential nutrients in the human body. They are involved in the whole spectrum of human physiological activities, from birth and growth to the immune response and disease fighting (Chen et al., 2015; Ryan et al., 2021; Roy and Tedeschi, 2021). Organic acids are also responsible for modulating immunity and anti-inflammatory functions while participating in energy production in the human body (Liu et al., 2020; Balta et al., 2021). B and C vitamins are widely associated with a variety of physiological activities in the human body, including antioxidation, energy metabolism, DNA synthesis, and protein synthesis (Peterson et al., 2020; Pohanka et al., 2012). It has been shown that Rhodiola contains a variety of active constituents, including volatile compounds, flavonoids, gallic acids, phenylpropanoids, amino acids, and organic acids (Lei et al., 2003; Dong et al., 2020; Dong et al., 2021).
It is well-accepted that antioxidation involves fighting against a wide variety of diseases, thereby improving human health. DPPH and ABTS antioxidant assays are two commonly used approaches for measuring plant antioxidant activity, which are valid and convenient colorimetric methods (Dong et al., 2021; Li et al., 2022).
In this study, firstly, we performed a comparison of the medicinal and functional components between the medicinal parts (namely, the roots) of Rhodiola Crenulata and Rhodiola Fastigiata, so as to find the better medicinal species of these two. Subsequently, phytochemical study was focused on the roots, leaves, flowers, and above-ground stems of the better species (namely, Rhodiola crenulata), and this was to comprehensively explore the other available parts and their characteristics compared with the roots.
In order to comprehensively evaluate the medicinal and functional components of Rhodiola, both non-volatile and volatile compounds were evaluated: 1) for the non-volatile compounds: the overall differences (namely, the contents of phenolic components and antioxidant abilities) were evaluated by chemical methods, and the differences in each category were identified by LC-ESI-MS/MS-based metabolomics; 2) regarding the volatile compounds: the overall differences (namely, the overall odorousness, and nine odor characteristic concentrations) were assessed using the sensory analyses, and the differences in each category were identified by HS-SPME-GC–MS-based metabolomics.
This study provides new information about the differences in the medicinal and functional components between the roots of Rhodiola crenulata and Rhodiola fastigiata, making for reasonable utilization of the roots of these two species. It also provides new scientific information on the exploitation of other available parts of Rhodiola crenulata in addition to the roots, which is of great value for the comprehensive development of Rhodiola crenulata.
2 Materials and methods
2.1 Plant materials
All samples of Rhodiola were collected at the same site of Balang Mountain, Wolong Town, Wenchuan County, Sichuan Province, China, on October 12, 2020. The altitude of the collection site was 4481 m; longitude: 102°53′43″ E; latitude: 30°54′25″ N. The collection site was all rocky slopes. One species was identified as Rhodiola crenulata (Hook. f. & Thomson) H. Ohba, and the other as Rhodiola fastigiata (Hook. f. & Thomson) S.H. Fu by the associate professor Shubin Dong at Beijing Forestry University. For each species, we collected 6 wild whole plants (namely, 6 biological repetitions), for a total of 12 plants. The fresh plants were freeze-dried. The same parts of 6 plants from the same species of Rhodiola were categorized and stacked into five groups, i.e., RF-R (Rhodiola fastigiata roots), RC-R (Rhodiola crenulata roots), RC-L (Rhodiola crenulata leaves), RC-F (Rhodiola crenulata flowers), and RC-S (Rhodiola crenulata above-ground stems). The samples were crushed separately as 5 groups accordingly, and samples in the same group were mixed well. All samples were stored at −40 ℃ for subsequent experiments. The specimen plants (voucher number 20201012RC for Rhodiola crenulata and number 20201012RF for Rhodiola fastigiata) were stored at the National Engineering Research Center of Tree Breeding and Ecological Restoration, Beijing Forestry University.
2.2 Extraction and determination of phenolic components and antioxidant abilities
The extraction method was adapted from Dong et al. (2021). In brief, 1.5 g of Rhodiola sample was mixed with 15 mL of 70 % methanol and extracted by ultrasonic extraction using a 300 w ultrasonic extractor (KQ-300DE Ultrasonic Extractor, Kunshan Ultrasonic Instruments Co., ltd., Kunshan, China) at room temperature for 30 min. Next, it was filtered through a 0.22 μm filter in order to collect the supernatant and obtain the residue. The extraction of the residue was repeated twice for the above-mentioned steps. The supernatants obtained from these three extractions were combined and stored at −20 °C for the measurement of total phenolic and flavonoid contents, and DPPH and ABTS antioxidant abilities.
For total phenols, 20 μL of extract, standard (10–400 mg/L gallic acid), or blank (distilled water) was mixed with 40 μL of 25 % Folin-Ciocalteu, respectively, and then 140 μL of 700 mM Na2CO3 was added, and the solution was shaken at 250 rpm for 5 min. After incubation for 2 h at room temperature in the dark, absorbances were measured at 765 nm using a microplate reader (Tecan Infinite 200 Pro Full Wavelength Microplate Reader, Tecan, Männedorf, Switzerland). The results are expressed as mg gallic acid/100 g d.w. of Rhodiola sample.
For total flavonoids, 140 μL of extract, standard (10–100 mg/L rutin), or blank (distilled water) was mixed with 8 μL of 50 mg/mL NaNO2 for 6 min, respectively, followed by adding 8 μL of 100 mg/mL AlCl3. After a 5 min of standing time, 100 μL of 40 mg/mL NaOH was added, and the solution was incubated at room temperature for 30 min. Absorbances were measured at 410 nm with the microplate reader. The results are expressed as mg rutin/100 g d.w. of Rhodiola sample.
For DPPH antioxidant ability, 10 μL extract, standard (20–800 mg/L Trolox), or blank (distilled water) was mixed with 40 μL of 1 mM DPPH, respectively, and then 190 μL of methanol of added. After incubation for 30 min at room temperature in the dark, absorbances were measured at 517 nm using the microplate reader. The results are expressed as mg Trolox/100 g d.w. of Rhodiola sample.
For ABTS antioxidant ability, ABTS solutions were prepared by mixing an equal volume of 7 mM ABTS and 2.4 mM potassium persulfate and incubated in dark at room temperature for 12–16 h. After incubation, the ABTS solution was diluted with methanol to an absorbance of 0.7 ± 0.02 at 734 nm to obtain ABTS working solution. Subsequently, 5 μL of extract, standard (20–800 mg/L Trolox), or blank (distilled water) was mixed with 200 μL ABTS working solution, respectively. After incubation in the dark at 30 °C for 5 min, absorbance was measured at 734 nm using the microplate reader. The results are expressed as mg Trolox/100 g d.w. of Rhodiola sample.
The measurements of contents of total phenols and total flavonoids, and DPPH and ABTS antioxidant abilities were all performed in six repetitions.
2.3 Sensory analyses
Sensory analyses followed the methodology described in López-Pérez et al. (2017) with minor adaptations. In short, an expert panel was formed with six members who had>2 years of experience each in sensory analysis. The panel considered that Rhodiola samples possessed nine odor characteristics: floral, sweet, orange, grass, herbal, minty, woody, lemon, and leather. First, 2 g of each Rhodiola sample was packed into a 100 mL screw-cap glass bottle, stored at 4 °C, and transferred to room temperature for 1 h before the assay. After that, the samples were randomly given to the panel for scoring. The scoring consisted of two items: 1) overall odorousness and 2) nine odor characteristic concentrations. The scoring was on a scale of 0 to 10: 0–2 = none or not perceptible concentration, 2–4 = weak concentration, 4–6 = moderate concentration, 6–8 = high concentration, and 8–10 = extremely high concentration. The samples were scored three times by each expert panel member, with 24 h between scores.
2.4 LC-ESI-MS/MS-Based metabolomics for non-volatile compounds
The extraction of non-volatile compounds and LC-ESI-MS/MS analysis followed the method described by Dong et al. (2021). In brief, 50 mg of Rhodiola sample was mixed with 700 μL of extract solution (methanol:water = 3:1, precooled at − 40 °C). Subsequently, the mixture was homogenized at 35 Hz for 4 min and sonicated in an ice-water bath for 5 min. The homogenization and sonication cycle were repeated twice. The mixture was then further extracted overnight at 4 °C on a shaker. Following centrifugation at 13,800 × g for 15 min at 4 °C, the extract was filtrated through a 0.22 μm microporous membrane to obtain a filtrate.
The filtrates were analyzed by a LC-ESI-MS/MS system (HPLC, EXION LC system, Sciex, Framingham, MA, USA; MS, SCIEX QTrap 6500+, Sciex, Framingham, MA, USA). Extracts were separated through a Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 × 100 mm, Waters, Milford, MA, USA) as the column under following mobile phase conditions: mobile phase A was 0.1 % formic acid in water, and mobile phase B was acetonitrile. The measurements were performed using the following gradient program: 0–0.5 min: 98 % A, 2 % B; 0.5–11 min: a linear gradient to 5 % A, 95 % B; 11–13 min: 5 % A plus 95 % B was kept; a combination of 98 % A with 2 % B was set to within 0.10 min and kept running for 13.1–15 min. The column temperature was set to 40 °C. The auto-sampler temperature was set to 4 °C, and the injection volume was 2 μL. The effluent was connected to an ESI-triple quadrupole-linear ion trap (QqQ-LIT)-MS.
The separated components were detected using a triple quadrupole (QqQ)-linear ion trap (LIT) mass spectrometer equipped with an IonDrive Turbo V ESI interface (SCIEX QTrap 6500+, Sciex, Framingham, MA, USA) and operated in positive and negative ion modes. The ESI source operation parameters were as follows: ion spray voltage: +5500/−4500 V; curtain gas: 35 psi; temperature: 400 °C; ion source gas 1:60 psi; ion source gas 2:60 psi; declustering potential (DP): ±100 V. Instrument tuning and mass calibration were performed in QqQ and LIT modes with polyethylene glycol solutions of 10 and 100 μmol/L, respectively. The QqQ scan was performed by multiple reaction monitoring (MRM) with the collision gas (nitrogen) set to 5 psi. The DP and CE of single MRM transfer were achieved by further optimization of declustering potential (DP) and collision energy (CE). Based on metabolites eluted during this period, a specific set of MRM transition was monitored in each cycle. This assay was carried out in triplicate for samples described in “2.1. Plant Materials”.
Metabolite identification was based on the MetWare database (MWDB), which is a self-built database. MWDB was also reported in previous studies (Yan et al., 2019; Dong et al., 2020).
2.5 HS-SPME-GC–MS-Based metabolomics for volatile compounds
Volatile compounds extraction and HS-SPME-GC–MS analysis followed the method described in Xia et al. (2021) with minor revisions. In brief, 1 g of the sample was transferred immediately to a 20 mL head-space vial (Agilent, Palo Alto, CA, USA), containing 2 mL of NaCl saturated solution to inhibit any enzyme reaction. The vials were sealed using crimp-top caps with TFE-silicone headspace septa (Agilent, Palo Alto, CA, USA). For SPME analysis, each vial was placed in 60 ℃ for 10 min, and then a 65 µm divinylbenzene/carboxen/polydimethylsiloxane fiber (Supelco, Bellefonte, PA, USA) was exposed to the headspace of the sample for 20 min at 60℃. The identification and quantification of volatile compounds were carried out using an Agilent Model 7890B GC and a 7000D mass spectrometer (Agilent, Palo Alto, CA, USA), equipped with a 30 m × 0.25 mm × 1.0 μm DB-5MS (5 % phenyl-polymethylsiloxane) capillary column. Helium was used as the carrier gas at a linear velocity of 1.0 mL/min. The injector’s temperature was kept at 250℃, and the detector’s temperature, 280℃. The oven temperature was programmed to go from 40 ℃ (5 min), increasing at 6℃/min, to 280℃, and held for 5 min. Mass spectra were recorded in electron impact (EI) ionisation mode at 70 eV. The quadrupole mass detector, ion source, and transfer line temperatures were set, respectively, to 150, 230, and 280℃. This assay was performed in triplicate for samples described in “2.1. Plant Materials”.
Metabolites were identified using data system libraries (MWGC or NIST). MWGC and NIST were also reported in a previous study (Wang et al., 2022).
2.6 Statistical analysis
The SIMCA 16.0.2 software package (Sartorius Stedim Data Analytics AB, Umea, Sweden) was used to perform multivariate analyses, including principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), the OPLS-DA model permutation test, and plot volcano plots. The screening criteria for differential metabolites were: |log2(fold change)| > 1, p-value < 0.05 and VIP > 1, which should be satisfied simultaneously. For the determination of differential biomarker metabolite (referred to as biomarker), the high abundance of a metabolite in one Rhodiola sample versus the zero abundance of the corresponding metabolite in another Rhodiola sample was determined (for zero abundance, volatile compounds were set to 1.00 cps and 9.00 cps for non-volatile compounds). A “*” is used to indicate the biomarker in a heat map, and to distinguish it from the common differential metabolite (CDM), where CDM refers to the differential metabolite that is common between two samples. R (Bell Laboratories, Inc., Madison, WI, USA, https://www.r-project.org) was used for hierarchical cluster analysis (HCA) and heat map plots of the differential metabolites.
3 Results
3.1 The overall odorousness and odor characteristics in Rhodiola crenulata roots and Rhodiola fastigiata roots
In this experiment, the overall odorousness and nine odor characteristics of the roots of both Rhodiola crenulata and Rhodiola fastigiata were compared individually. The overall odorousness of volatile compounds was significantly stronger for RC-R than RF-R (Fig. 1A). As shown in Fig. 1B, the concentrations of four odor characteristics (floral, sweet, lemon and orange) were significantly stronger in RC-R than in RF-R (p-value < 0.05). In contrast, RF-R was significantly stronger than RC-R for the concentrations of woody and leather odor characteristics (p-value < 0.05). In addition, the concentrations of odor characteristics of “grass,” “herbal,” and “minty” did not exhibit significant differences between RC-R and RF-R. This indicates that RC-R is significantly stronger than RF-R in terms of the overall odorousness of volatile compounds; further analysis of the nine odor characteristics' concentrations revealed that the overall odorousness of RC-R is stronger than that of RF-R, which is primarily present in four odor characteristics (floral, sweet, lemon, and orange).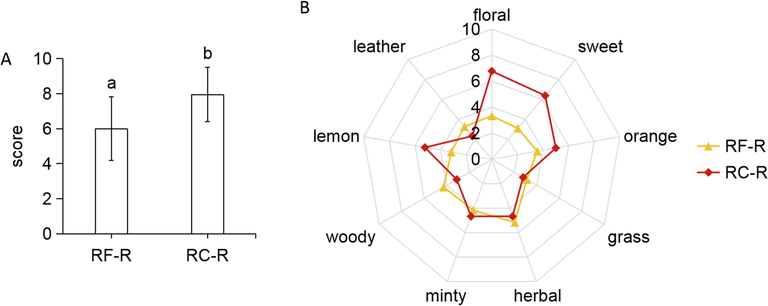
Sensory analyses of RF-R and RC-R. Overall odorousness (A); and odor characteristics’ concentrations, including floral, sweet, orange, grass, herbal, minty, woody, lemon, and leather (B).
3.2 The differences in ten categories of volatile compounds between Rhodiola crenulata roots and Rhodiola fastigiata roots
By comparing RC-R and RF-R, a total of 92 volatile compounds were identified (Table S1). Seventy-seven volatile differential metabolites were screened (Fig. 2, Table S2). Compared with RF-R, 43 biomarkers plus 25 CDMs were upregulated, and 2 biomarkers plus 7 CDMs were downregulated in RC-R, and the upregulation rate was 88.31 %. This suggests that the upregulation of volatile compounds is more pronounced in the RC-R than in the RF-R.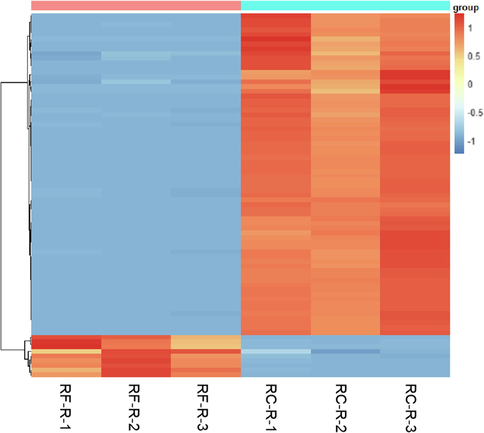
The differential metabolites of volatile compounds between RF-R and RC-R displayed by heat map. There were 45 biomarkers and 32 CDMs for volatile compounds. Three independent replicates were performed. The heat maps were generated based on cluster analysis of the relative contents of differential metabolites, including biomarkers and CDMs, in RF-R and RC-R samples. Each row represents one metabolite; each column denotes one Rhodiola sample. After normalization, the range of variation in the relative content of each metabolite was set to between −1.5 and 1.5. Upregulation indicates a significant increase in the amount of a metabolite in one Rhodiola sample compared to the amount of the corresponding metabolite in another Rhodiola sample, which is depicted in red; downregulation indicates a significant decrease in the amount of a metabolite in one Rhodiola sample compared to the amount of the corresponding metabolite in another Rhodiola sample, which is depicted in blue. The upregulation rate is the percentage of the number of upregulated differential metabolites among the number of total differential metabolites. Note that the meaning of subsequent heat maps is the same.
In the comparison between RC-R and RF-R, 77 volatile differential metabolites were categorized into 10 categories, i.e., volatile terpenoids, aromatics, alcohols, olefins, esters, aldehydes, ketones, acids, alkanes, and heterocyclic compounds. Taking RF-R as a reference, 31 volatile terpenoids were all upregulated in RC-R (Fig. 3), including 23 biomarkers and 8 CDMs. Regarding the 17 aromatics, 4 biomarkers plus 7 CDMs were upregulated, and 1 biomarker plus 5 CDMs were downregulated in the RC-R compared to the RF-R. Regarding the 8 alcohols (5 biomarkers plus 3 CDMs) and 4 olefins (3 biomarkers plus 1 CDM), all were upregulated in the RC-R. For 7 esters, 3 biomarkers plus 3 CDMs were upregulated, and 1 biomarker was downregulated, in the RC-R (Fig. 4). In the five above-mentioned categories of volatile compounds, the number of upregulated differential metabolites (60) represented 77.92 % of the total number of volatile differential metabolites (77). This indicates that the majority of metabolites in these 10 volatile categories were more prevalent in RC-R than in RF-R, in particular, terpenoids, aromatics, alcohols, olefins, and esters.
The differences in differential metabolites of terpenoids between RF-R and RC-R displayed by heat map. There were 23 biomarkers and 8 CDMs for terpenoids. Three independent replicates were performed.
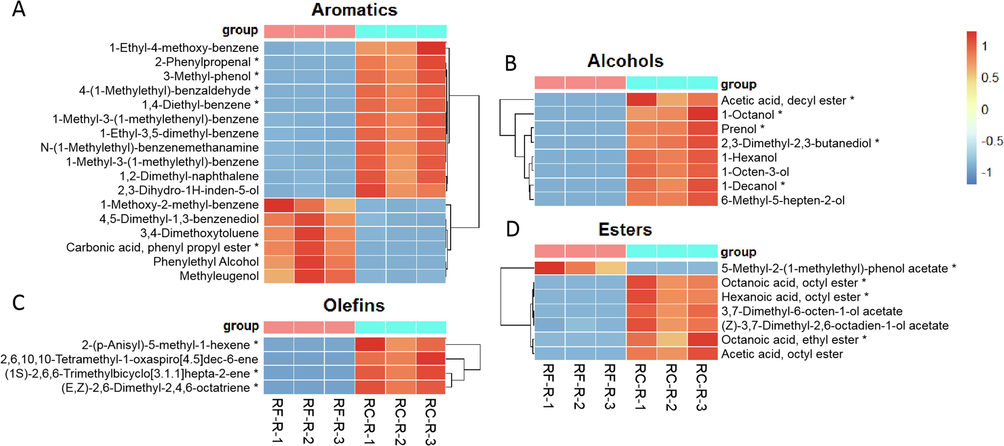
The differences in differential metabolites of aromatics, alcohols, olefins, and esters between RF-R and RC-R displayed by heat map. There were 5 biomarkers and 12 CDMs for aromatics (A), 5 biomarkers and 3 CDMs for alcohols (B), 3 biomarkers and 1 CDM for olefins (C), and 4 biomarkers and 3 CDMs for esters (D). Three independent replicates were performed.
3.3 The contents of phenolic components and antioxidant activity in Rhodiola crenulata roots and Rhodiola fastigiata roots
As shown in Fig. 5, the contents of non-volatile compounds, total phenols, and total flavonoids, and DPPH and ABTS antioxidant abilities, were significantly higher in RC-R than RF-R.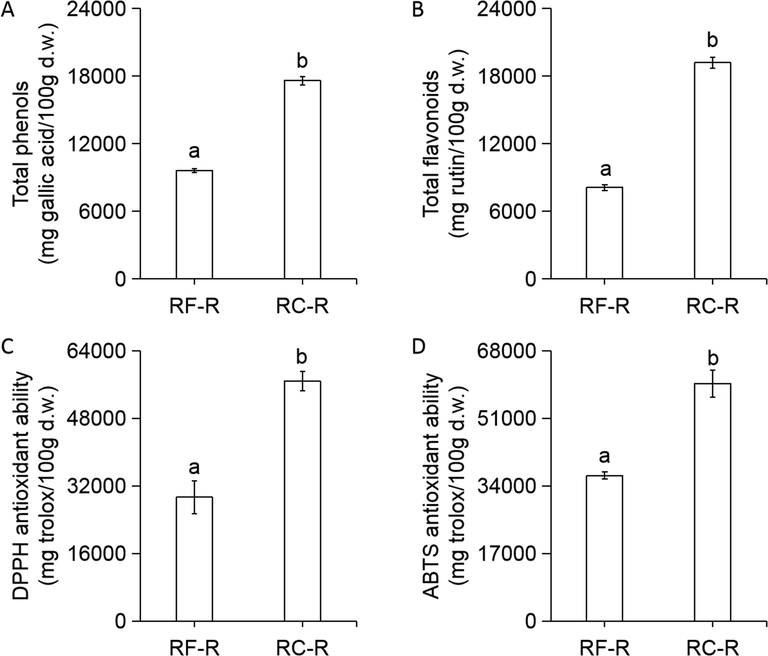
Contents of phenolic components and antioxidant abilities of RF-R and RC-R. Total phenols (A); total flavonoids (B); DPPH antioxidant ability (DPPH is an abbreviation of 2,2-diphenyl-1-picrylhydrazyl) (C); and ABTS antioxidant ability (ABTS is an abbreviation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) (D). Gallic acid and rutin were used as standards for total phenols and total flavonoids, respectively. Trolox was used as a standard for DPPH and ABTS antioxidant ability. Results are presented as the mean ± SD of six independent experiments (n = 6) and are expressed as mg standard per 100 g d.w. of plant materials. In each column, different letters (a and b) mean significant differences between two groups (p < 0.05) found via a t-test.
3.4 The variations in eight categories of non-volatile compounds between Rhodiola crenulata roots and Rhodiola fastigiata roots
In total, 680 non-volatile compounds were identified in the comparison between RC-R and RF-R (Table S3), and 491 differential metabolites were selected (Table S4). Compared with RF-R, 134 biomarkers plus 246 CDMs were upregulated and 19 biomarkers plus 92 CDMs were downregulated in RC-R, and the upregulation rate was 77.39 %. This indicates that the upregulation for non-volatile differential metabolites was more evident in the roots of Rhodiola crenulata compared to roots of Rhodiola fastigiata.
For the purpose of discovery of the specific differences, non-volatile compounds were further classified into eight categories, plus others. These eight categories were flavonoids, gallic acids, phenylpropanoids, amino acids, saccharides, organic acids, phospholipids, and free fatty acids and glycerides.
3.4.1 Flavonoids
For flavonoid metabolites, a total of 101 differential metabolites were identified in the comparison of RC-R and RF-R. In terms of the differential metabolites of flavonoids, taking RF-R as a relative reference, 93 upregulated differential metabolites and 8 downregulated differential metabolites were identified in RC-R, and the upregulation rate was found to be 92.08 %.
Based on the corresponding aglycones and reports, major flavonoids in Rhodiola were further classified into four subclasses, namely, quercetin and derivatives, kaempferol and derivatives, luteolin and derivatives, and catechins and derivatives (Dong et al. 2021; Tao et al. 2019). Therefore, the differences in these four flavonoid classes between the roots of these two species were tested. This was also applicable for subsequent comparison of flavonoids between RC-R and RC-L.
Analysis revealed that the upregulated flavonoids in RC-R were involved in all these four subclasses: 1) quercetin and derivatives (22 biomarkers plus 6 CDMs up-regulated and 1 CDM downregulated in RC-R), 2) kaempferol and derivatives (11 biomarkers plus 8 CDMs were all upregulated in RC-R), 3) luteolin and derivatives (4 biomarkers plus 2 CDMs were all upregulated in RC-R), and 4) catechins and derivatives (7 biomarkers plus 4 CDMs were upregulated and 5 CDMs were downregulated in the RC-R) (Fig. 6). The number of upregulated differential metabolites in these four groups (64) accounted for 68.82 % of the total number of upregulated differential metabolites in flavonoids (93). This suggests that the majority of flavonoid differential metabolites, notably the above-mentioned four groups, were more upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata.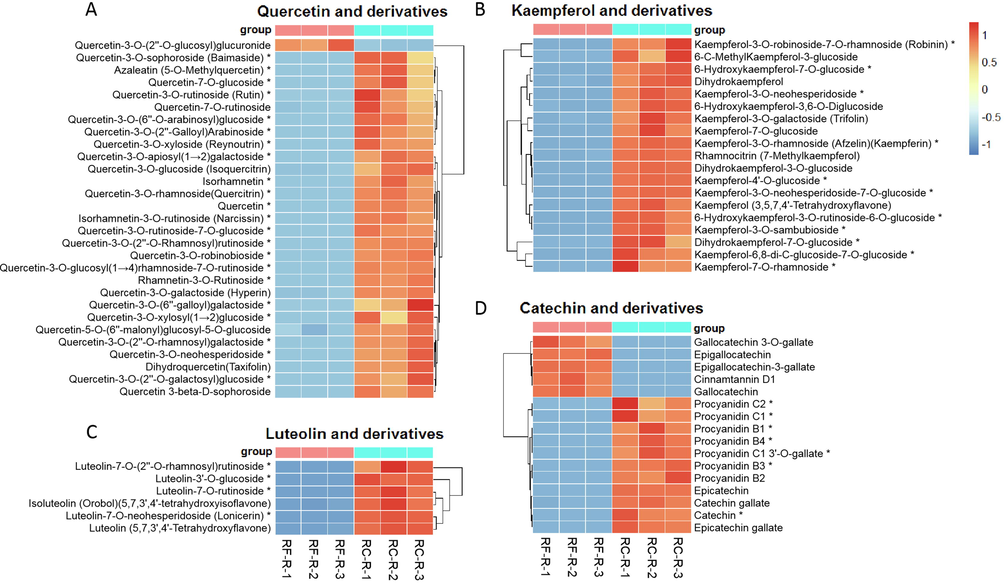
The differential metabolites of flavonoids between RF-R and RC-R displayed by heat map. There were 22 biomarkers and 7 CDMs for quercetin and derivatives (A), 11 biomarkers and 8 CDMs for kaempferol and derivatives (B), 4 biomarkers and 2 CDMs for luteolin and derivatives (C), and 7 biomarkers and 9 CDMs for catechin and derivatives (D). Three independent replicates were performed.
3.4.2 Gallic acids
For comparison between RC-R and RF-R, 28 differential metabolites were grouped into this category, including gallic acid derivatives and hydrolyzed tannins formed from gallic acid and sugars, respectively (Fig. 7). In terms of the differential metabolites of gallic acids, taking RF-R as a relative reference, 10 biomarkers and 16 CDMs identified in RC-R showed upregulation, and 1 biomarker and 1 CDM showed downregulation; the upregulation rate was 92.86 %. Therefore, gallic acids were significantly upregulated in Rhodiola crenulata roots compared to those of Rhodiola fastigiata.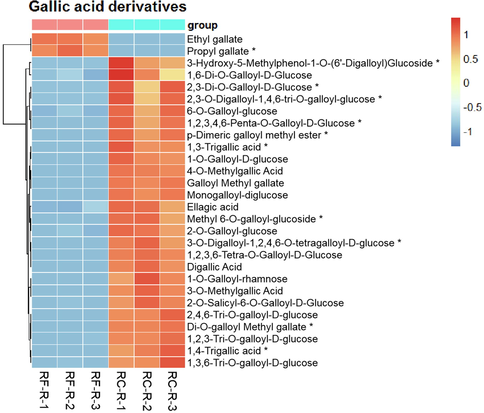
The differences in differential metabolites of gallic acid derivatives between RF-R and RC-R displayed by heat map. There were 11 biomarkers and 17 CDMs for gallic acid derivatives. Three independent replicates were performed.
3.4.3 Phenylpropanoids
For phenylpropanoids metabolites, a total of 94 differential metabolites were identified in the comparison between RC-R and RF-R. Sixty-three upregulated differential metabolites and thirty-one downregulated differential metabolites were identified in RC-R when RF-R was used as a relative reference, with an up-regulation rate of 67.02 %.
On the basis of their basic structures and biosynthetic pathways, phenylpropanoids were further grouped into seven subclasses, namely, cinnamic acid-coumaroyl and derivatives, phenylmethanes, phenylpropanes, phenylethanes, shikimic acids, lignans, and lignins. Therefore, the differences in these seven phenylphanoids between the roots of these two species will be further focused on. We also compare the phenylphanoids between RC-R and RC-L.
Analysis revealed that the upregulated phenylpropanoids in RC-R were mainly concentrated in the following six groups: 1) cinnamic acid-coumaroyl and derivatives (6 biomarkers plus 9 CDMs were up-regulated and 3 CDMs were down-regulated in RC-R), 2) phenylmethanes (3 biomarkers plus 9 CDMs up-regulated and 2 biomarkers plus 7 CDMs down-regulated in RC-R), 3) phenylpropanes (10 biomarkers plus 8 CDMs upregulated and 6 biomarkers plus 9 CDMs downregulated in RC-R), 4) shikimic acids (4 biomarkers plus 4 CDMs were all up-regulated in RC-R), 5) lignans (1 biomarker plus 2 CDMs were all upregulated in RC-R), and 6) lignins (4 CDMs were all upregulated in the RC-R) (Fig. 8A, B, C, and E, Fig. S4A and C). For the above six groups, up to 95.24 % (60) of the total number of upregulated differential metabolites (63) in phenylpropanoids were upregulated. This indicates that the above six groups of phenylpropanoids are upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata, particularly in the four groups of cinnamic acid-coumaroyl and derivatives, shikimic acids, lignans, and lignins.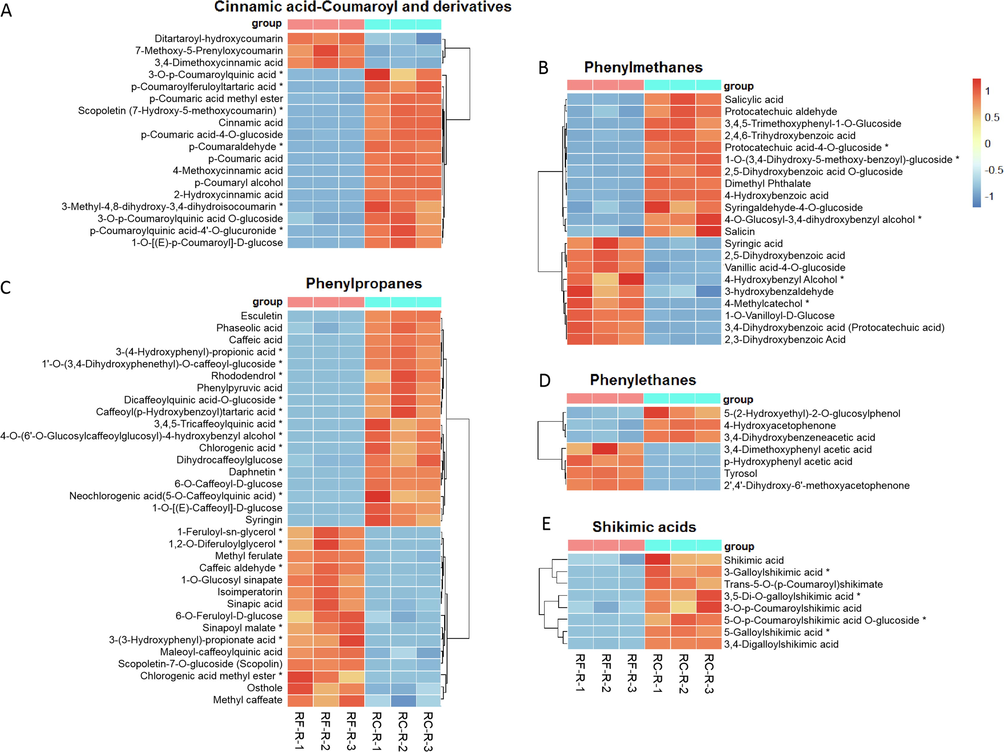
The differences in differential metabolites of phenylpropanoids between RF-R and RC-R displayed by heat map. There were 6 biomarkers and 12 CDMs for cinnamic acid-coumaroyl and derivatives (A), 5 biomarkers and 16 CDMs for phenylmethanes (B), 16 biomarkers and 17 CDMs for phenylpropanes (C), 0 biomarker and 7 CDMs for phenylethanes (D), and 4 biomarkers and 4 CDMs for shikimic acids (E). Three independent replicates were performed.
For phenylethanes, the number of downregulated compounds in the RC-R was slightly more than the number of upregulated ones (Fig. 8D).
3.4.4 Amino acids
For amino acid metabolites, 59 differential metabolites were identified in the comparison of RC-R and RF-R (Fig. S5). There were 46 upregulated differential metabolites and 13 downregulated differential metabolites identified in RC-R when taking RF-R as a relative reference, and the upregulation rate was 77.97 %. Of these, the amino acids upregulated in Rhodiola crenulata roots (i.e., RC-R) covered the six amino acid families, i.e., 1) aromatic amino acids, 2) glutamic acids, 3) serines, 4) aspartic acids, 5) histidine, and 6) alanines. This suggests that the above-mentioned amino acid families were upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata.
3.4.5 Saccharides and organic acids
For saccharide metabolites, 18 differential metabolites were identified in the comparison between RC-R and RF-R (Fig. S6A). Four biomarkers and eight CDMs were upregulated, and six CDMs were downregulated, in RC-R compared with RF-R; the upregulation rate was 66.67 %. For organic acid metabolites, 38 differential metabolites were identified in the comparison of RC-R and RF-R (Fig. S6B). Five biomarkers and nineteen CDMs were upregulated, and fourteen CDMs were downregulated, in RC-R compared to RF-R, and the upregulation rate was 63.16 %. The above results indicate that saccharides and organic acids were upregulated in the roots of Rhodiola crenulata compared to those of Rhodiola fastigiata.
3.4.6 Phospholipids, and free fatty acids and glycerides
For lipid metabolites, 67 differential metabolites were identified in the comparison of RC-R and RF-R, including 38 phospholipids (7 biomarkers plus 28 CDMs upregulated and 3 CDMs downregulated in RC-R), and 29 free fatty acids and glycerides (4 biomarkers plus 22 CDMs upregulated and 3 CDMs downregulated in RC-R). The upregulation rate was 91.05 % (Fig. S7). This shows that phospholipids with free fatty acids and glycerides were significantly upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata.
3.5 The overall odorousness and odor characteristics in the four parts of Rhodiola crenulata
In this experiment, the overall odorousness and nine odor characteristics’ concentrations of four parts (roots, leaves, flowers, and above-ground stems) from Rhodiola crenulata were compared. In terms of overall odorousness, RC-F and RC-R were significantly greater than RC-L and RC-S, but no significant differences were found between RC-F and RC-R (Fig. 9A). As shown in Fig. 9B, none of the concentrations of the nine odor characteristics showed significant differences between RC-F and RC-R. With respect to the concentrations of odor characteristics of floral, sweet, orange, minty, and lemon, RC-F and RC-R were both substantially stronger than RC-L and RC-S (p-value < 0.01). For the odor characteristic of grass, RC-L was significantly stronger than RC-S, RC-R, and RC-F (p-value < 0.01). The overall odorousness and characteristic odor concentrations of Rhodiola crenulata roots and flowers were very similar and were evaluated to be better than those of the leaves and above-ground stems. Since the biomass of Rhodiola crenulata roots is significantly greater than that of flowers, it is considered more favorable to choose the roots as the site for the development of volatile compounds in Rhodiola crenulata.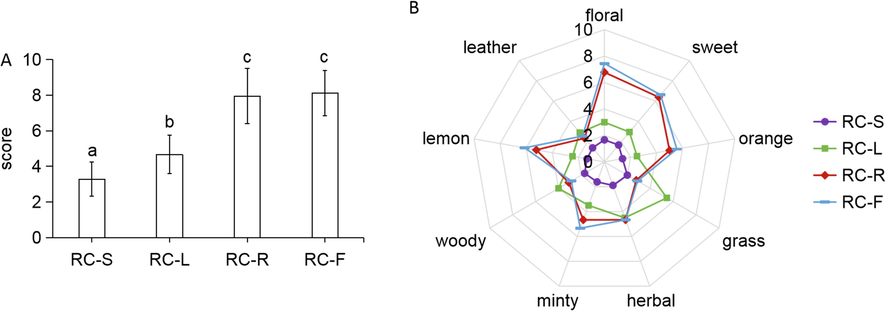
Sensory analyses of RC-S, RC-L, RC-R, and RC-F. Overall odorousness (A); odor characteristics’ concentrations, including floral, sweet, orange, grass, herbal, minty, woody, lemon, and leather (B).
3.6 The contents of phenolic components and antioxidant activity in the four parts of Rhodiola crenulata: roots, leaves, flowers, and above-ground stems
As shown in Fig. 10A, C and D, the total phenolic contents, and DPPH and ABTS antioxidant abilities of the four parts of Rhodiola crenulata in descending order were RC-R > RC-L > RC-F > RC-S. Fig. 10B shows that the total flavonoid contents of the four parts of Rhodiola crenulata were RC-F > RC-R > RC-L > RC-S in descending order, and there were significant differences among the four parts.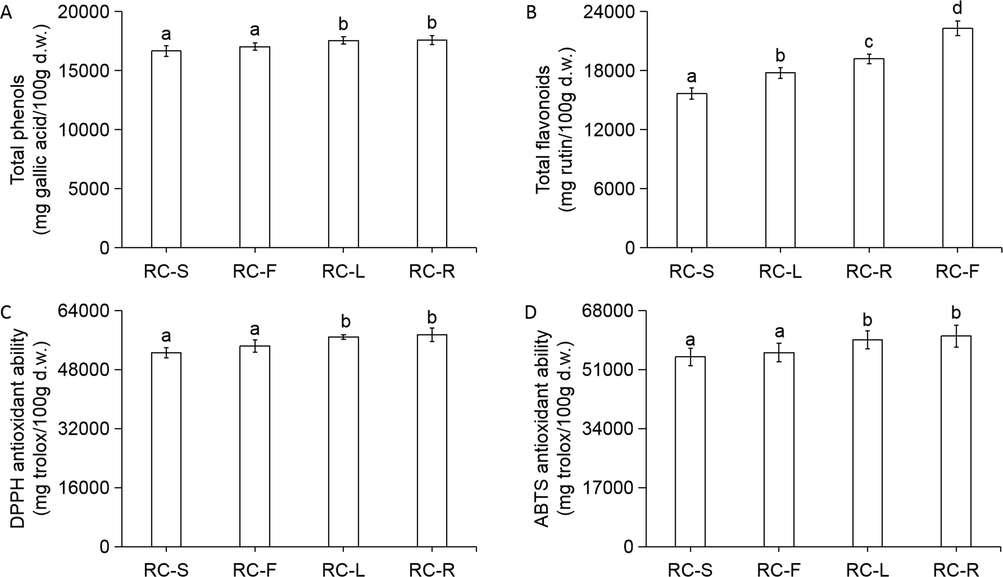
Contents of phenolic components and antioxidant abilities of RC-S, RC-F, RC-L, and RC-R. Total phenols (A); total flavonoids (B); DPPH antioxidant ability (C); ABTS antioxidant ability (D). Gallic acid and rutin were used as standards for total phenols and total flavonoids, respectively. Trolox was used as a standard for DPPH and ABTS antioxidant ability. Results are presented as mean ± SD of six independent experiments (n = 6) and are expressed as mg standard per 100 g d.w. of plant materials. In each column, different letters (a, b, c and d) mean significant differences between two groups (p < 0.05) found via a t-test.
3.7 The differences in flavonoids, phenylpropanoids, and gallic acids between the roots and leaves of Rhodiola crenulata
A total of 685 non-volatile compounds were identified in the roots and leaves of Rhodiola crenulata, and 450 differential metabolites were selected (Tables S5 and S6). Taking RC-L as a relative reference, 36 biomarkers plus 202 CDMs were found upregulated and 27 biomarkers plus 185 CDMs downregulated in RC-R, giving an upregulation rate of 52.89 %.
For uncovering of the specific differences, non-volatile compounds were further classified into eight categories plus others. These eight categories included flavonoids, gallic acids, phenylpropanoids, saccharides, phospholipids, amino acids, organic acids, and vitamins.
For flavonoid metabolites, 87 differential metabolites were identified in the comparison of RC-R and RC-L. Of these, 54 upregulated differential metabolites and 33 downregulated differential metabolites were identified in RC-R when using RC-L as a relative reference, making an upregulation rate of 62.07 %. Further analysis revealed that the upregulated flavonoids in the roots were notably manifested in catechins (Fig. 11D). In contrast, the other three flavonoid groups showed less significant differences in their up- and downregulation in the roots versus leaves (Fig. 11A, B and C). This suggests that catechins accumulated more in the roots of Rhodiola crenulata than the leaves.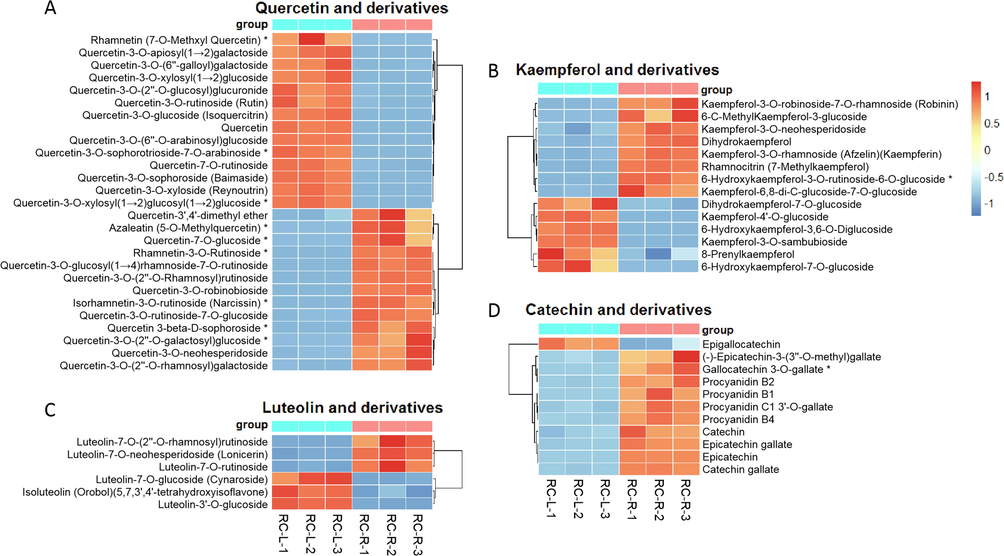
The differences in differential metabolites of flavonoids between RC-L and RC-R displayed by heat map. There were 9 biomarkers and 18 CDMs for quercetin and derivatives (A), 1 biomarker and 13 CDMs for kaempferol and derivatives (B), 0 biomarker and 6 CDMs for luteolin and derivatives (C), and 1 biomarker and 10 CDMs for catechin and derivatives (D). Three independent replicates were performed.
For the 17 differential gallic acid metabolites, taken RC-L as a relative reference, 1 biomarker plus 8 CDMs were found upregulated in RC-R, and 8 CDMs were downregulated (Fig. 12); the upregulation rate was 52.94 %. This suggests that the relative differences in gallic acids were not significant between the roots and leaves of Rhodiola crenulata.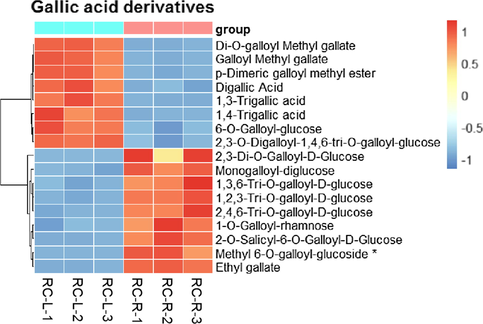
The differences in differential metabolites of gallic acid derivatives between RC-L and RC-R displayed by heat map. There were 1 biomarker and 16 CDMs for gallic acid derivatives. Three independent replicates were performed.
For the phenylpropanoids, a total of 100 differential metabolites were identified in the comparison between RC-R and RC-L. Fifty-six upregulated differential metabolites and forty-four downregulated differential metabolites were identified in RC-R when using RC-L as a relative reference, making an upregulation rate of 56 %. Of these, there were four groups of phenylpropanoids upregulated in the roots, i.e., 1) phenylmethanes (1 biomarker plus 12 CDMs upregulated and 2 biomarkers plus 8 CDMs downregulated in RC-R), 2) shikimic acids (3 CDMs upregulated and 2 CDMs downregulated in RC-R), 3) lignans (1 biomarker plus 5 CDMs upregulated and 2 CDMs downregulated in RC-R), and 4) lignins (4 were all up-regulated in RC-R) (Fig. 13B, E and Fig. S4B, D). Cinnamic acid-coumaroyl and derivatives, phenylpropanes, and phenylethanes were not significant in their up-/downregulation when comparing roots with leaves (Fig. 13A, C and D). These results suggest that, compared to the leaves of Rhodiola crenulata, the above-mentioned four groups of phenylpropanoids facilitate more accumulation in the roots.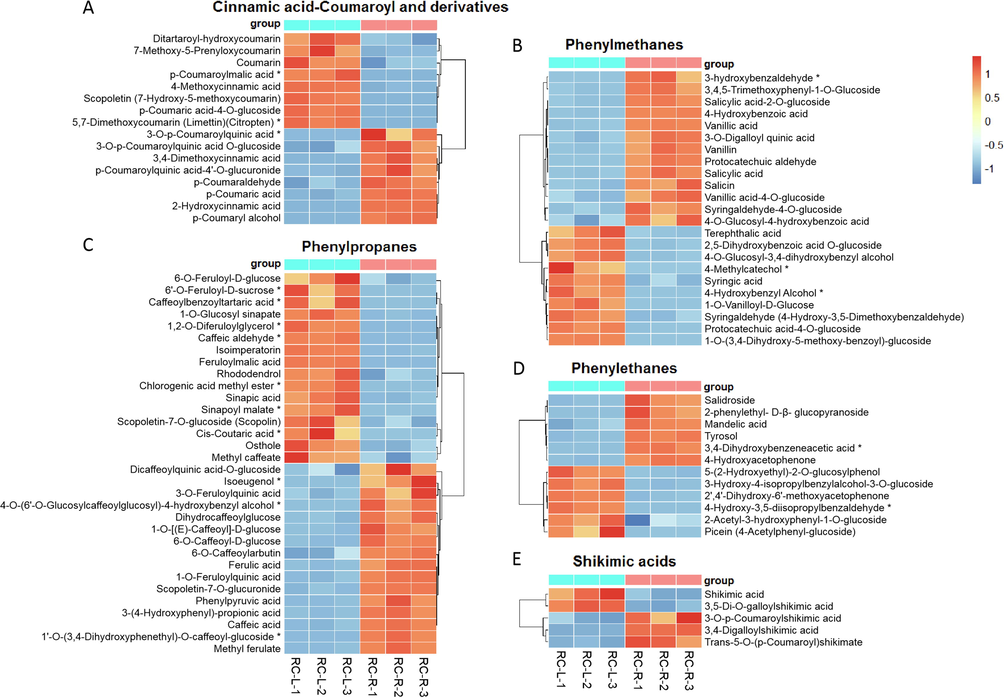
The differences in differential metabolites of phenylpropanoids between RC-L and RC-R displayed by heat map. There were 3 biomarkers and 13 CDMs for cinnamic acid-coumaroyl and derivatives (A), 3 biomarkers and 20 CDMs for phenylmethanes (B), 10 biomarkers and 22 CDMs for phenylpropanes (C), 2 biomarkers and 10 CDMs for phenylethanes (D), and 0 biomarker and 5 CDMs for shikimic acids. Three independent replicates were performed.
3.8 The differences in saccharides and phospholipids between the roots and leaves of Rhodiola crenulata
For saccharide metabolites, 16 differential metabolites were identified in the comparison between RC-R and RC-L (Fig. 14A). Three biomarkers plus 11 CDMs were upregulated, and 2 CDMs were downregulated, in RC-R compared to RC-L, making an upregulation rate of 87.5 %. This shows that saccharides accumulated more significantly in the roots of Rhodiola crenulata than the leaves.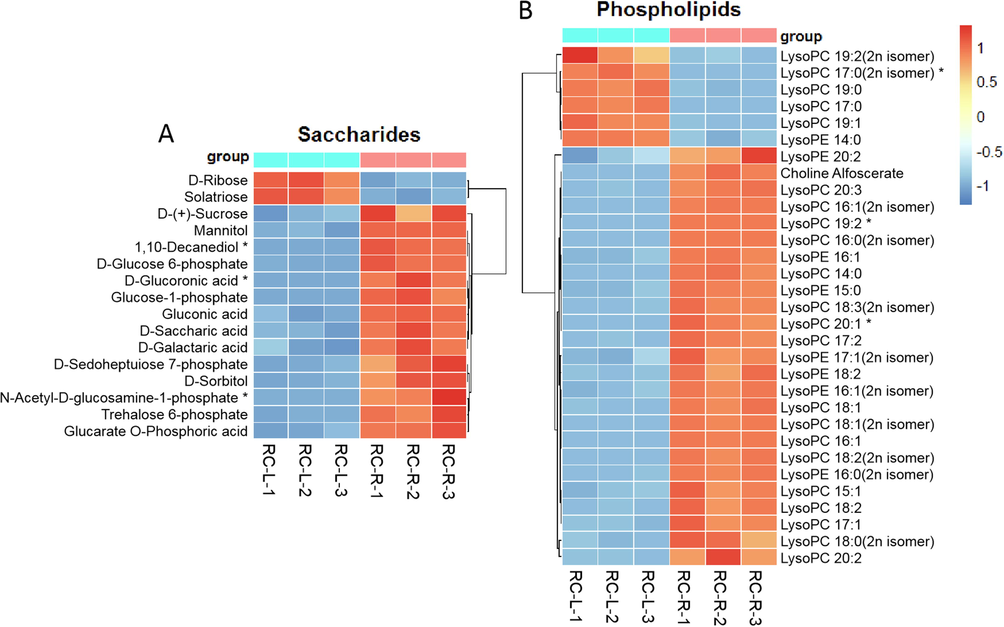
The differences in differential metabolites of saccharides and phospholipids between RC-L and RC-R displayed by heat map. There were 3 biomarkers and 13 CDMs for saccharides (A), and 3 biomarkers and 28 CDMs for phospholipids (B). Three independent replicates were performed.
For phospholipid metabolites, 31 differential metabolites were identified in the comparison between RC-R and RC-L (Fig. 14B). The upregulation rate of phospholipids (2 biomarkers plus 23 CDMs upregulated and 1 biomarker plus 5 CDMs downregulated) was 80.65 % in RC-R compared to RC-L. This indicates that phospholipids accumulated more significantly in the roots of Rhodiola crenulata than the leaves.
3.9 The differences in amino acids, organic acids, and vitamins between the roots and leaves of Rhodiola crenulata
For amino acid metabolites, 40 differential metabolites were identified in the comparison between RC-R and RC-L (Fig. 15). Twenty-seven upregulated differential metabolites and thirteen downregulated differential metabolites were identified in RC-L if RC-R was used as a relative reference, for an upregulation rate of 67.5 %. The amino acids upregulated in leaves covered the six amino acid families, i.e. 1) glutamic acids, 2) aspartic acids, 3) serines, 4) aromatic amino acids, 5) alanines, and 6) histidines. These results suggest that the above-mentioned amino acid families facilitate more accumulation in the leaves of Rhodiola crenulata compared to the roots.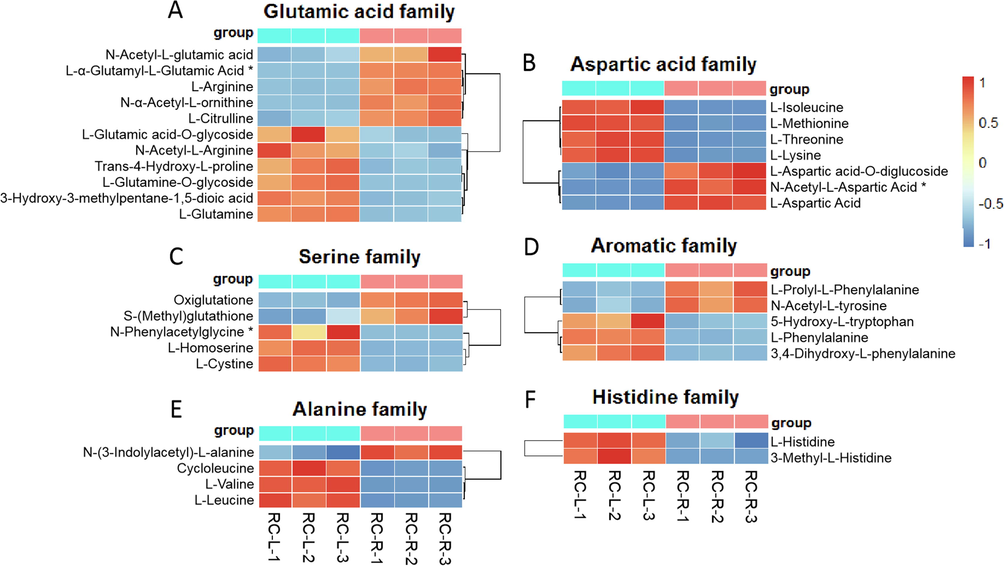
The differences in differential metabolites of amino acids between RC-L and RC-R displayed by heat map. There were 1 biomarker and 10 CDMs for the glutamic acid family (A), 1 biomarker and 6 CDMs for the aspartic acid family (B), 1 biomarker and 4 CDMs for the serine family (C), 0 biomarker and 5 CDMs for the aromatic family (D), 0 biomarker and 4 CDMs for the alanine family (E), and 0 biomarker and 2 CDMs for the histidine family (F). Three independent replicates were performed.
For organic acid metabolites, 38 differential metabolites were identified in the comparison between RC-R and RC-L (Fig. 16A). Twenty-five CDMs were upregulated and one biomarker plus twelve CDMs were downregulated in RC-L compared to RC-R, for an upregulation rate of 65.79 %. This indicates that more organic acids accumulated in the leaves of Rhodiola crenulata compared to the roots.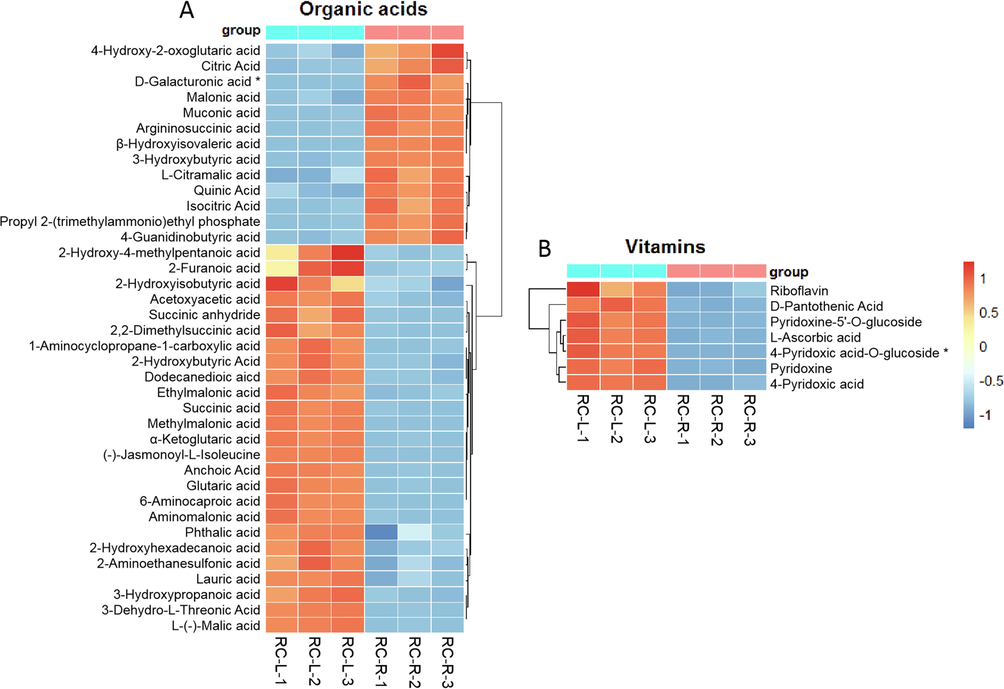
The differences in differential metabolites of organic acids and vitamins between RC-L and RC-R displayed by heat map. There were 1 biomarker and 37 CDMs for organic acids (A), and 1 biomarker and 6 CDMs for vitamins (B). Three independent replicates were performed.
For vitamin metabolites, seven differential metabolites were identified in the comparison between RC-R and RC-L (Fig. 16B). One was vitamin C (l-ascorbic acid), and the other six were all B vitamins. Compared to RC-R, all seven differential metabolites of vitamins exhibited upregulation in RC-L (1 biomarker plus 6 CDMs). This indicates that vitamins, especially vitamins C and B, accumulated in the leaves of Rhodiola crenulata compared to the roots.
4 Discussion
4.1 Comparison between Rhodiola crenulata roots and Rhodiola fastigiata roots
4.1.1 The volatile compounds of Rhodiola crenulata roots are more valuable for exploitation than those of Rhodiola fastigiata roots
The overall odorousness of volatile compounds of Rhodiola crenulata roots was significantly stronger than that of Rhodiola fastigiata roots (see Fig. 1A). It was primarily presented in four odor characteristics (floral, sweet, lemon, and orange), which were significantly stronger than those of Rhodiola fastigiata roots (see Fig. 1B). Compared with the roots of Rhodiola fastigiata, the upregulation of volatile differential metabolites in the roots of Rhodiola crenulata was evident (see Fig. 2), particularly the upregulation of five categories of volatile compounds, i.e., terpenoids, aromatics, alcohols, olefins, and esters, which accounted for 77.92 % of the total volatile differential metabolites (see Figs. 3 and 4). We argue that the upregulation of these five categories of volatile compounds in roots of Rhodiola crenulata resulted in a higher overall odorousness than the roots of Rhodiola fastigiata.
The odors of some volatile differential metabolites coincided with floral, sweet, lemon, and orange. For example, the odor of 3-carene is lemony and sweet, the odor of citronellol is floral, the odor of linalool is floral and sweet, and the odor of limonene is lemony and orangey (Xiao et al., 2017; Guo et al., 2021). In this study, we discovered that all of these volatile terpenoids mentioned above were upregulated in the roots of Rhodiola crenulata (see Fig. 3). These findings help explain why the odor characteristics of floral, sweet, lemon and orange are stronger in the roots of Rhodiola crenulata.
Plant-volatile-compound-based treatment protocols may improve the subhealth condition, which is a popular, economical, and safe treatment modality available today with few side effects. Han et al. (2017) found that bergamot essential oil aromatherapy is an effective adjunctive treatment protocol that improves the mental health and well-being of individuals. Pandur et al. (2021) found that lavender essential oil inhibited the production of four pro-inflammatory factors, IL-6, IL-8, IL-β, and TNFα, in THP-1 cells, thereby exerting an anti-inflammatory therapeutic effect on human respiratory infections. Kennedy et al. (2018) discovered that plant volatile terpenoids enhance cognitive performance and reduce neurological fatigue in humans. Thus, from the perspective of human health, Rhodiola crenulata roots possess higher exploitation value of volatile compounds than Rhodiola fastigiata roots.
4.1.2 The non-volatile compounds in Rhodiola crenulata roots showed better qualities than those in Rhodiola fastigiata roots regarding human health benefits
Regarding non-volatile phenols, our results indicate that Rhodiola crenulata root is significantly superior to Rhodiola fastigiata root in terms of total phenolic content, total flavonoid content, and antioxidant abilities (see Fig. 5). This result is consistent with the significant upregulation of flavonoids, gallic acids, and phenylpropanoids, the three well-known major components of phenols, in Rhodiola crenulata root (see Figs. 6, 7 and 8). First, in comparison with Rhodiola fastigiata roots, we identified upregulation of differential flavonoid metabolites in Rhodiola crenulata roots, predominantly of the four subcategories quercetins, kaempferols, luteolins, and catechins, which are the major flavonoids in Rhodiola (Tao et al., 2019). We also found that the vast majority of differential gallic acid metabolites were upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata. Furthermore, differential phenylpropanoid metabolites were upregulated in Rhodiola crenulata roots, particularly cinnamic acid-coumaroyl and derivatives, shikimic acids, lignans, and lignins. Therefore, it can be clearly seen that phenols exhibited more pronounced upregulation and accumulation in the roots of Rhodiola crenulata compared to those in the Rhodiola fastigiata roots.
Phenols are the main antioxidants of plants (Amarowicz and Pegg, 2019). DPPH and ABTS antioxidant tests are commonly used assays for antioxidant activity (Wołosiak et al., 2022). Zhou et al. (2020) found that increased phenolic and flavonoid contents in kiwifruit pulp were the main reason for the increased scavenging capacity of DPPH and ABTS free radicals. Khatri and Chhetri (2020) reported that seven medicinal plants showed a significant positive correlation between total phenolic content and antioxidant activity. These reports are consistent with our findings (see Figs. 5-8), which indicates that the accumulation of phenols in Rhodiola crenulata roots may be the main reason for their significantly higher scavenging capacity of DPPH and ABTS free radicals than Rhodiola fastigiata roots.
It has been reported that flavonoids, gallic acids, and phenylpropanoids all exhibit strong antioxidant activity and play important roles in the prevention and treatment of cancer, inflammation, and bacterial infections (Shen et al., 2022; Kim, 2007; Liu et al., 2017). We therefore believe that Rhodiola crenulata roots possesses better qualities than Rhodiola fastigiata roots in terms of health benefits. Rhodiola crenulata is the only Rhodiola species listed in the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2020), which supports the findings of this study that Rhodiola crenulata is more abundant in phenols and offers higher antioxidant capacity.
In addition, figs. S5-S7 show that amino acids, saccharides, organic acids, phospholipids, and free fatty acids and glycerides were upregulated in the roots of Rhodiola crenulata compared to the roots of Rhodiola fastigiata. Plant-derived amino acids are essential for human growth, development, reproduction, and health (Hou et al., 2019); saccharides and organic acids are involved in human energy metabolism and provide energy for the body (Qi and Tester, 2019;Liu et al., 2020); lipids are indispensable sources of reserve energy and nutrition for the body and provide essential fatty acids for the body to prevent and treat chronic diseases (Zhang et al., 2021b).
Thus, we are convinced that the health benefits of Rhodiola crenulata roots are superior to those of Rhodiola fastigiata roots in terms of flavonoids, gallic acids, phenylpropanoids, amino acids, saccharides, organic acids, phospholipids, and free fatty acids and glycerides.
4.2 Comparison among the four parts of Rhodiola crenulata: roots, leaves, flowers, and above-ground stems
4.2.1 The root is more suitable for exploiting Rhodiola crenulata’s volatile compounds
Our results revealed that the overall odorousness of both root and flower parts was significantly stronger than the odorousness of leaves and above-ground stems. The overall odorousness difference between the root and flower parts was insignificant, and the odor characteristics’ concentrations were very comparable (see Fig. 9). Since the root biomass is greater than that of the flower, we believe that it is more appropriate to use root for extracting Rhodiola crenulata’s volatile compounds.
4.2.2 The roots and leaves are more appropriate for the extraction of phenolic components and antioxidants of Rhodiola crenulata
The contents of total phenols and flavonoids, and the DPPH and ABTS antioxidant abilities of Rhodiola crenulata roots, leaves, flowers, and above-ground stems were generally high. Total phenolic and flavonoid contents in the above-ground stems, though the lowest concentrations in the four parts, were as high as 16663.18 ± 475.20 mg gallic acid/100 g d.w. and 15680.49 ± 585.28 mg rutin/100 g d.w., respectively, which are significantly higher values than those of the well-known and phenolic-rich blueberry (total phenols: 944 ± 22 mg gallic acid/100 g d.w.; total flavonoids: 3608 ± 56 mg rutin/100 g d.w.) (Huang et al., 2012). Therefore, we concluded that the roots, leaves, flowers, and above-ground stems of Rhodiola crenulata are of high exploitation value (see Fig. 10). By comparison, we further found that roots and leaves had significantly higher total phenolic content and DPPH and ABTS antioxidant abilities than flowers and above-ground stems, and there were no significant differences between roots and leaves (see Fig. 10A, C and D); in terms of total flavonoid content, the order from most to least was flowers, roots, leaves, and above-ground stems (see Fig. 10B). Consider the following: The biomass of roots is the largest among the four parts, though the biomass of leaves is also large and can be reproduced every year. Flowers have high flavonoid content but less biomass. Therefore, we believe that roots and leaves are the most valuable for phenolic components and antioxidants among the four parts of Rhodiola crenulata. Accordingly, we subsequently compared the non-volatile compounds of Rhodiola crenulata roots and leaves, including flavonoids, gallic acids, phenylpropanoids, saccharides, phospholipids, amino acids, organic acids, and vitamins.
4.2.3 Both the roots and leaves of Rhodiola crenulata possess excellent non-volatile compounds, and both parts show distinct characteristics
In the comparison between roots and leaves, regarding differential phenol metabolites, when the leaves were used as a reference, the upregulation rate of flavonoids in roots was 62.07 %, that of gallic acids was 52.94 % and that of phenylpropanoids was 56 %. It can be seen that the up- and downregulation of phenols, mainly made up of flavonoids, gallic acids, and phenylpropanoids, did not vary significantly between roots and leaves. This finding is consistent with the results that there were no significant differences between roots and leaves in terms of total phenolic content, and DPPH and ABTS antioxidant abilities. Specific differences in differential metabolites of flavonoids, gallic acids, and phenylpropanoids between roots and leaves revealed that catechins, a group of flavonoids, were significantly upregulated in roots (see Fig. 11D); phenylmethanes, shikimic acids, lignans, and lignins, four groups of phenylpropanoids, were significantly upregulated in roots (see Fig. 13B and E, Fig. S4B and D). In a study of Drimia maritima (L.), Stearn, Zhang, et al. (2022) found that it accumulated more lignans in the below-ground parts (23.05 ± 0.42 mg/g) compared to the above-ground ones (2.02 ± 0.04 mg/g). Wang et al. (2015) found in a study of Ardisia quinquegona that lignins were accumulated three more times in the roots than the leaves. These findings are in agreement with our results, i.e., the upregulation of lignans and lignins in the roots of Rhodiola crenulata compared with the leaves.
Catechins inhibit the proliferation of influenza viruses and treat muscle diseases due to aging (Chang et al., 2020; Li et al., 2020a, 2020b); phenylmethanes have anti-inflammatory and therapeutic effects against parasitic diseases (Chen et al., 2022); shikimic acids is anti-influenza (Zhang et al., 2020); lignans interact with gastrointestinal microorganisms and exert anti-inflammatory and anti-apoptotic effects (Senizza et al., 2020); and lignins exert anti-tumor and anti-viral activities (Shu et al., 2021). Thus, the flavonoids (referring to catechins) and phenylpropanoids (referring to phenylmethanes, shikimic acids, lignans, and lignins) upregulated in the roots might offer better health benefits than leaves.
It should be noted that the contents of total phenols and flavonoids in roots and leaves are similar. Therefore, the leaves of Rhodiola crenulata are also valuable in the development of phenols. At the same time, we found a significant number of quercetins (See Table S5, S6) present in both roots and leaves (35 in total, of which 27 were differential metabolites), and the numbers of up- and downregulated compounds were almost equal in these two parts (6 biomarkers plus 7 CDMs upregulated and 3 biomarkers plus 11 CDMs downregulated in roots compared to leaves). This indicates that for the quercetins, the quality of the roots is similar to that of the leaves. Note that quercetin is a potent inhibitor of lung inflammation and lung cancer (Sul and Ra, 2021; Huang et al., 2021), on the basis of which, several lung-protective health products have been developed, such as Quercetin Capsules (SorLife, USA), Quercetin with Bromelain (Now, USA), and Quercetin Complex (Solgar, USA). Therefore, both the roots and leaves of Rhodiola crenulata are of great use from the perspective of developing quercetin. Meanwhile, the total number of non-differential phenol metabolites between these two parts was 74, including 25 flavonoids, 12 gallic acids, and 37 phenylpropanoids. Together with the aforementioned result that the total phenolic and total flavonoid contents of roots and leaves were similar, we speculate that there should be some substitutions between these two parts.
In addition, by comparing roots with leaves, we also found that saccharides and phospholipids were upregulated in roots. Six amino acid families, namely, glutamic acids, aspartic acids, serines, aromatic amino acids, alanines, and histidines; and organic acids and vitamins were upregulated in leaves (see Figs. 14-16). Given that studies have shown that saccharides synthesized in leaves by photosynthesis are transported to the roots to participate in energy metabolism and root development (Kim et al., 2021), our results suggest that the roots of Rhodiola crenulata are the site of sugar storage. Phospholipids are important components of plant cell membranes and are involved in the regulation of root hair development (Champeyroux et al., 2020). Together with the literature, it has been shown that the upregulation of phospholipids in the roots facilitates the growth and development of Rhodiola crenulata root hairs. Amino acids and organic acids play important roles in biosynthesis (Tegeder and Hammes, 2018; Igamberdiev and Bykova, 2018), and the upregulation of several amino acids and organic acids in leaves suggests that leaf biosynthesis of Rhodiola crenulata should be more active than that in the root. Vitamin C and B vitamins play vital roles in human health (Pohanka et al., 2012; Peterson et al., 2020). We believe that the upregulation of vitamin C and various B vitamins in the leaves implies that the leaves of Rhodiola crenulata are more advantageous than the roots for replenishing the body with vitamins.
5 Conclusion
This comparative study on the volatile and non-volatile compounds, and antioxidant activities of the medicinal parts (namely the roots) of both Rhodiola crenulata and Rhodiola fastigiata indicate that the overall odorousness, concentrations of different odor characteristics, total phenolic and flavonoid contents, and DPPH and ABTS antioxidant abilities of Rhodiola crenulata roots were significantly higher than those of Rhodiola fastigiata roots. Volatile compounds (in total, ten categories, including terpenoids, aromatics, alcohols, olefins, esters, etc.) and non-volatile compounds (in total, eight categories, including flavonoids, gallic acids, phenylpropanoids, amino acids, saccharides, organic acids, phospholipids, and free fatty acids and glycerides) in the Rhodiola crenulata roots were significantly higher than those in the Rhodiola fastigiata roots. Thus, we strongly believe that the health benefits of Rhodiola crenulata are superior to those of Rhodiola fastigiata due to the significant enrichment of various active phytochemicals in Rhodiola crenulata roots.
In light of this, Rhodiola crenulata was selected to further investigate the differences in its four parts: roots, leaves, flowers, and above-ground stems. First, overall odorousness, odor characteristics, contents of phenolic components, and antioxidant abilities were compared. Second, we focused on comparing the differences in non-volatile compounds in roots and leaves, including flavonoids, gallic acids, phenylpropanoids, saccharides, phospholipids, amino acids, organic acids, and vitamins. Based on the results, we concluded the following: 1) from the perspective of exploiting volatiles, it is more beneficial to select the roots; 2) in terms of non-volatile compounds, and antioxidant ability, the leaves of Rhodiola crenulata are also of great value, in addition to the roots in the traditional sense; 3) saccharides and phospholipids are upregulated in the Rhodiola crenulata roots, and flavonoids and phenylpropanoids exhibit minor upregulations in the Rhodiola crenulata roots, whereas amino acids, organic acids, and vitamins are upregulated in the Rhodiola crenulata leaves. Accordingly, we are confident that both the roots and the leaves of Rhodiola crenulata possess excellent health benefits. The benefits of these two parts are to a certain extent present in both with regard to phenols, but there are distinct characteristics regarding saccharides, phospholipids, amino acids, organic acids, and vitamins.
Acknowledgement
This work was supported by the Fundamental Research Funds for the Central Universities (grant number 2018ZY29), Beijing Forestry University, China. This work was also supported by the National Natural Science Foundation of China (grant number 31800270). We sincerely thank the associate professor Shubin Dong in Beijing Forestry University for authentication of Rhodiola crenulata (Hook. f. & Thomson) H. Ohba and Rhodiola fastigiata (Hook. f. & Thomson) S.H. Fu.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules. 2021;26:3653.
- [CrossRef] [Google Scholar]
- Natural antioxidants of plant origin. Adv. Food Nutr. Res.. 2019;90:1-81.
- [CrossRef] [Google Scholar]
- The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci. Rep.. 2021;11(1):16202.
- [CrossRef] [Google Scholar]
- Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23(1):87-94.
- [CrossRef] [Google Scholar]
- From Traditional Resource to Global Commodities:—A Comparison of Rhodiola Species Using NMR Spectroscopy—Metabolomics and HPTLC. Front. Pharmacol.. 2016;7:254.
- [CrossRef] [Google Scholar]
- Signaling phospholipids in plant development: small couriers determining cell fate. Curr. Opin. Plant Biol.. 2020;57:61-71.
- [CrossRef] [Google Scholar]
- Catechin inhibiting the H1N1 influenza virus associated with the regulation of autophagy. J. Chin. Med. Assoc.. 2020;83(4):386-393.
- [CrossRef] [Google Scholar]
- Benzaldehyde Attenuates the Fifth Stage Larval Excretory-Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress. Biomolecules. 2022;12:177.
- [CrossRef] [Google Scholar]
- Chinese Pharmacopoeia Commission, 2020. Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing, pp. 161.
- Rhodiola crenulata extract for prevention of acute mountain sickness: a randomized, double-blind, placebo-controlled, crossover trial. BMC Complementary Altern. Med.. 2013;13:298.
- [CrossRef] [Google Scholar]
- Adjunctive Treatment with Rhodiola crenulata in Patients with Chronic Obstructive Pulmonary Disease – A Randomized Placebo Controlled Double Blind Clinical Trial. PLoS One. 2015;10(6):e0128142.
- [Google Scholar]
- Evaluation of Two Major Rhodiola Species and the Systemic Changing Characteristics of Metabolites of Rhodiola crenulata in Different Altitudes by Chemical Methods Combined with UPLC-QqQ-MS-Based Metabolomics. Molecules. 2020;25:4062.
- [CrossRef] [Google Scholar]
- Altitudinal Variation of Metabolites, Mineral Elements and Antioxidant Activities of Rhodiola crenulata (Hook.f. & Thomson) H.Ohba. Molecules. 2021;26:7383.
- [CrossRef] [Google Scholar]
- Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae) Antioxidants. 2022;11:311.
- [CrossRef] [Google Scholar]
- Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem.. 2021;341:128230
- [CrossRef] [Google Scholar]
- Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res.. 2020;7(1):e000392.
- [Google Scholar]
- Bergamot (Citrus bergamia) essential oil inhalation improves positive feelings in the waiting room of a mental health treatment center: a pilot study. Phytother. Res.. 2017;31(5):812-816.
- [CrossRef] [Google Scholar]
- Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids. 2019;51(8):1153-1165.
- [CrossRef] [Google Scholar]
- Rhodiola crenulata extract counteracts the effect of hypobaric hypoxia in rat heart via redirection of the nitric oxide and arginase 1 pathway. BMC Complementary Altern. Med.. 2017;17(1):29.
- [CrossRef] [Google Scholar]
- Growth suppression in lung cancer cells harboring EGFR-C797S mutation by quercetin. Biomolecules. 2021;11(9):1271.
- [CrossRef] [Google Scholar]
- Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ., Sci., B. 2012;13(2):94-102.
- [CrossRef] [Google Scholar]
- Role of organic acids in the integration of cellular redox metabolism and mediation of redox signalling in photosynthetic tissues of higher plants. Free Radic. Biol. Med.. 2018;122:74-85.
- [CrossRef] [Google Scholar]
- Anti-inflammatory phenolics isolated from Juniperus rigida leaves and twigs in lipopolysaccharide-stimulated RAW264.7 macrophage cells. J. Enzyme Inhib. Med. Chem.. 2012;27(6):875-879.
- [CrossRef] [Google Scholar]
- Volatile terpenes and brain function: investigation of the cognitive and mood effects of Mentha × Piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients. 2018;10(8):1029.
- [CrossRef] [Google Scholar]
- Reducing sugar, total phenolic content, and antioxidant potential of nepalese plants. BioMed Res. Int.. 2020;2020:7296859.
- [CrossRef] [Google Scholar]
- Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull.. 2007;30(6):1052-1055.
- [CrossRef] [Google Scholar]
- Cellular export of sugars and amino acids: role in feeding other cells and organisms. Plant Physiol.. 2021;187(4):1893-1914.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of niosomes entrapped with Plai oil (Zingiber cassumunar Roxb.) by therapeutic ultrasound in a rat model. Int. J. Nanomed.. 2017;12:2469-2476.
- [CrossRef] [Google Scholar]
- Chemical composition of the essential oils of two Rhodiola species from Tibet. Z. Naturforsch., C: J. Biosci.. 2003;58(3–4):161-164.
- [CrossRef] [Google Scholar]
- Characterization of chemical compositions by a GC–MS/MS approach and evaluation of antioxidant activities of essential oils from Cinnamomum reticulatum Hay, Leptospermum petersonii Bailey, and Juniperus formosana Hayata. Arab. J. Chem.. 2022;15:103609
- [CrossRef] [Google Scholar]
- Comparison of chemical constitution and bioactivity among different parts of Lonicera japonica Thunb. J. Sci. Food Agric.. 2020;100(2):614-622.
- [CrossRef] [Google Scholar]
- Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr.. 2020;60(3):515-528.
- [CrossRef] [Google Scholar]
- Application of microscopy in authentication of traditional Tibetan medicinal plants of five Rhodiola (Crassulaceae) alpine species by comparative anatomy and micromorphology. Microsc. Res. Tech.. 2008;71(6):448-458.
- [CrossRef] [Google Scholar]
- Metabolomics analysis to evaluate the anti-inflammatory effects of polyphenols: glabridin reversed metabolism change caused by LPS in RAW 264.7 Cells. J. Agric. Food Chem.. 2017;65(29):6070-6079.
- [CrossRef] [Google Scholar]
- Glucose challenge metabolomics implicates the change of organic acid profiles in hyperlipidemic subjects. Biomed. Chromatogr.. 2020;34(6):e4815.
- [Google Scholar]
- Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res. Int.. 2017;99:1002-1010.
- [CrossRef] [Google Scholar]
- Application of UHPLC fingerprints combined with chemical pattern recognition analysis in the differentiation of six Rhodiola species. Molecules. 2021;26(22):6855.
- [CrossRef] [Google Scholar]
- Chemical characteristics of Rhodiola Crenulata and its mechanism in acute mountain sickness using UHPLC-Q-TOF-MS/MS combined with network pharmacology analysis. J. Ethnopharmacol.. 2022;294:115345
- [CrossRef] [Google Scholar]
- Post-Stroke depression modulation and in Vivo antioxidant activity of gallic acid and its synthetic derivatives in a Murine model system. Nutrients. 2016;8(5):248.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of lavender (Lavandula angustifolia Mill.) essential oil prepared during different plant phenophases on THP-1 macrophages. BMC Complement. Med. Ther.. 2021;21(1):287.
- [CrossRef] [Google Scholar]
- Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev.. 2021;41(1):630-703.
- [CrossRef] [Google Scholar]
- In vitro antioxidant and antimicrobial properties of flower, leaf, and stem extracts of Korean Mint. Antioxidants. 2019;8(3):75.
- [CrossRef] [Google Scholar]
- B Vitamins and their role in immune regulation and cancer. Nutrients. 2020;12(11):3380.
- [CrossRef] [Google Scholar]
- Terpene core in selected aromatic and edible plants: Natural health improving agents. Adv. Food Nutr. Res.. 2019;90:423-451.
- [CrossRef] [Google Scholar]
- Ascorbic acid: an old player with a broad impact on body physiology including oxidative stress suppression and immunomodulation: a review. Mini-Rev. Med. Chem.. 2012;12(1):35-43.
- [CrossRef] [Google Scholar]
- Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN. 2019;33:18-28.
- [CrossRef] [Google Scholar]
- The role of lipids, lipid metabolism and ectopic lipid accumulation in axon growth, regeneration and repair after CNS injury and disease. Cells. 2021;10(5):1078.
- [CrossRef] [Google Scholar]
- Interorgan metabolism of amino acids in human health and disease. Adv. Exp. Med. Biol.. 2021;1332:129-149.
- [CrossRef] [Google Scholar]
- Lignans and gut microbiota: an interplay revealing potential health implications. Molecules. 2020;25(23):5709.
- [CrossRef] [Google Scholar]
- Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem.. 2022;383:132531
- [CrossRef] [Google Scholar]
- Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr.. 2018;7(1):148-154.
- [CrossRef] [Google Scholar]
- Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol.. 2021;268:113685.
- [CrossRef] [Google Scholar]
- Biological activities and emerging roles of lignin and lignin-based products─A review. Biomacromolecules. 2021;22(12):4905-4918.
- [CrossRef] [Google Scholar]
- Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed. Pharmacother.. 2021;142:111949
- [CrossRef] [Google Scholar]
- From fighting critters to saving lives: polyphenols in plant defense and human health. Int. J. Mol. Sci.. 2021;22(16):8995.
- [CrossRef] [Google Scholar]
- Quercetin prevents LPS-Induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26(22):6949.
- [CrossRef] [Google Scholar]
- Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev.. 2019;39(5):1779-1850.
- [CrossRef] [Google Scholar]
- The way out and in: phloem loading and unloading of amino acids. Curr. Opin. Plant Biol.. 2018;43:16-21.
- [CrossRef] [Google Scholar]
- Gallic acid content and an antioxidant mechanism are responsible for the Antiproliferative Activity of 'Ataulfo' Mango Peel on LS180 cells. Molecules. 2018;23(3):695.
- [CrossRef] [Google Scholar]
- Transcriptomic and metabolomic analyses provide insights into the formation of the peach-like aroma of Fragaria nilgerrensis Schlecht. Fruits. Genes. 2022;13(7):1285.
- [CrossRef] [Google Scholar]
- Phenolic profile within the fine-root branching orders of an evergreen species highlights a disconnect in root tissue quality predicted by elemental- and molecular-level carbon composition. New Phytol.. 2015;206(4):1261-1273.
- [CrossRef] [Google Scholar]
- Network pharmacology and molecular docking analysis on mechanisms of Tibetan Hongjingtian (Rhodiola crenulata) in the treatment of COVID-19. J. Med. Microbiol.. 2021;70(7):001374
- [CrossRef] [Google Scholar]
- Verification of the conditions for determination of antioxidant activity by ABTS and DPPH Assays-A practical approach. Molecules. 2022;27(1):50.
- [CrossRef] [Google Scholar]
- Metabolite profiles, bioactivity, and HPLC fingerprint of different varieties of Eucommia ulmoides Oliv.: Towards the utilization of medicinal and commercial chinese endemic tree. Molecules. 2018;23(8):1898.
- [CrossRef] [Google Scholar]
- Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims) Hortic. Res.. 2021;8(1):14.
- [CrossRef] [Google Scholar]
- Verification of key odorants in rose oil by gas chromatography-olfactometry/aroma extract dilution analysis, odour activity value and aroma recombination. Nat. Prod. Res.. 2017;31(19):2294-2302.
- [CrossRef] [Google Scholar]
- Comparative evaluation of cultivars of Chrysanthemum morifolium flowers by HPLC-DAD-ESI/MS analysis and antiallergic assay. J. Agric. Food Chem.. 2012;60(51):12574-12583.
- [CrossRef] [Google Scholar]
- A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chem.. 2019;275:618-627.
- [CrossRef] [Google Scholar]
- Simultaneous determination and anti-inflammatory effects of four phenolic compounds in Dendrobii Herba. Nat. Prod. Res.. 2017;31(24):2923-2926.
- [CrossRef] [Google Scholar]
- Natural volatile oils derived from herbal medicines: a promising therapy way for treating depressive disorder. Pharmacol. Res.. 2021;164:105376
- [CrossRef] [Google Scholar]
- Development of anti-influenza agents from natural products. Med. Res. Rev.. 2020;40(6):2290-2338.
- [CrossRef] [Google Scholar]
- Untargeted phenolic profiling and functional insights of the aerial parts and bulbs of Drimia maritima (L.) Stearn. Plants. 2022;11(5):600.
- [CrossRef] [Google Scholar]
- Dietary bioactive lipids: a review on absorption, metabolism, and health properties. J. Agric. Food Chem.. 2021;69(32):8929-8943.
- [CrossRef] [Google Scholar]
- Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric.. 2020;100(8):3283-3290.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104420.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2
Supplementary data 3
Supplementary data 3
Supplementary data 4
Supplementary data 4
Supplementary data 5
Supplementary data 5
Supplementary data 6
Supplementary data 6
Supplementary data 7
Supplementary data 7







