Translate this page into:
A Cu (I) catalyzed large scale synthesis of an antipsychotic drug substance Clozapine with new precursor 2-chloro benzoic acid
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Development of an economic and commercial manufacturing process for an anti-psychotic drug substance clozapine with an alternative key starting material (2-chloro benzoic acid) in the place of literature reported key starting material Anthranilic acid. To avoid narcotic key starting materials usage in drug substances the author invented a commercially available raw material 2-chlorobenzoic acid, which reacts with another key starting material 4-chloro-1, 2-diamino benzene. This reaction proceeds through Ullman reaction to produce multi scale level Clozapine which quality meets the ICH requirements.
Keywords
Synthesis
Clozapine
New precursor
Commercial manufacturing
Copper (I) catalyst
1 Introduction
Clozapine chemically designated as 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b, e] [1, 4] diazepine and its chemical structure as shown as in the Fig. 1. Heritage Life Sciences Barbados, JAZZ PHARMACEUTICALS (for ODT), TASMAN PHARMA INC., (for suspension). Currently total 13 DMF’s available for Clozapine and 9 are active 4 are inactive.
Chemical structure of Clozapine.
Clozapine sold under the brand name Clozaril among others*, is an atypical antipsychotic medication. It is mainly used for schizophrenia that does not improve following the use of other antipsychotic medications. In those with schizophrenia and schizoaffective disorder it may decrease the rate of suicidal behavior. It is more effective than typical antipsychotics, particularly in those who are treatment-resistant. It is taken by mouth… Clozapine oral dosage form is marketed on various brand names like *the other brand names FAZACLO ODT, VERSACLOZ 12.5, 25, 100, 150 & 200 mg strength tablets available in the market and maximum daily dosage is 1800 mg. Clozapine approved by FDA in 1989 and marketed in various dosage forms such tablets, ODT tablets and suspension.
The first of synthesis clozapine and its analogues were reported (Hunziker et al., 1967) in 1967 (Scheme 1) and later it was disclosed as a product in journal of radiology (De Paulis et al., 1988; McLeish et al., 1993). Tomas de Paulis etal synthesized some analogues of clozapine with shorter synthesis (De Paulis et al., 1981). The authors of this present research invented a new way of synthesis with environmental friendly precursor known as 2-chloro benzoic acid instead of Anthranilic acid which synthesis involves too much of pollution. The selected route of synthesis is industrially scalable process with premium costing and all.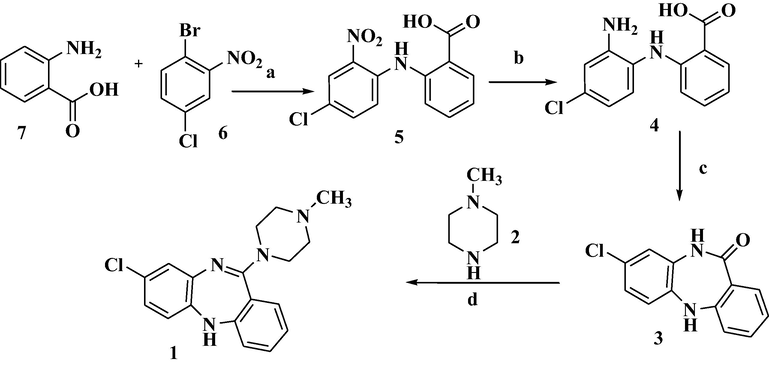
2 Experimental
2.1 Materials and methods
Most of the raw materials, Solvents and reagents were purchased from commercial manufacturing sources and very few were purchased from Aldrich and Merck Inc., Such as reagents and analytical grade raw materials. The instruments which were used for its each stage analysis purpose were such as Proton & Carbon NMR (Bruker 400 MHz), Mass (Agilent 6110AA ESI and APCI system, FT-IR (Perkin Elmer Spectrum 100 FT-IR Spectrometer).
2.2 Results and discussions
Most of the pharmaceutical companies producing Clozapine active pharmaceutical ingredient as per innovator mentioned route of synthesis only, which involves 2-Aminobenzoic acid (anthranilic acid) and 4-chloro-2-aminobromobenzene were as main starting materials. The entire reported process supposed to be not an environment friendly and which generates lot of effluent during its synthesis.
To avoid generation of un-necessary pollutants by using narcotic, bromo substituted starting materials; the authors invented a new precursor to produce Clozapine in commercial scale with less costing. The developed process is promising towards ecofriendly production atmosphere and to deliver a good quality material to concern schizoaffective disorder patients.
The entire process scale up was taken at factory level after assessing the risk of intermediates, byproducts and reagents genotoxicity by functional structural alert.
The main drawback of the above invention is the cost of starting material and also the narcotic effect of Anthranilic acid. The use of Anthranilic acid in the preparation of Clozapine may not be suggested due to its narcotic properties. Anthranilic acid and its derivatives are widely recognized to show narcotic effects. Hence, it needs to develop the process for the preparation of Clozapine using other than Anthranilic acid as a raw material or starting material.
The present inventors has developed a novel and an improved process (Scheme 2) for the preparation of Clozapine which results in greater efficiency, with better product purity than the prior art processes (Scheme 1).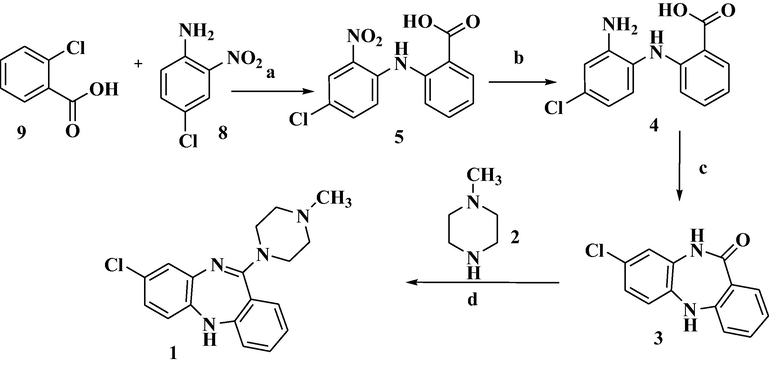
Proposed route of synthesis.
2.3 Experimental procedures
2.3.1 Synthesis of 2-(4-chloro-2-nitrophenylamino) benzoic acid (5)
In a clean & dry Round bottom flask fitted with reflux condenser, 250gm of 4-chloro-2-nitroaniline (8), 150gm of 2-chlorobenzoic acid (9) was taken in 2000 mL of N, N-Dimethylformamide stirred for 10–15 min until it get dissolve and then added 200gm of anhydrous potassium carbonate (K2CO3) followed by addition of 25 gm of Copper (I) iodide at room temperature under gentle stirring. Slowly heated the reaction mass temperature to reflux and maintained for 8–9 h. After completion of the starting materials over the HPLC, the reaction mass was cool to room temperature and quenched in to Water (6000 mL). The obtained reaction mixture pH was adjusted to 2–3.5 with Conc. Hydrochloric acid. The resulting precipitated solid material was isolated through Buckner filtration and dried to afford 330 gm (Yield: 77%) of 2-(4-chloro-2-nitrophenylamino) benzoic acid (5) with purity NLT 99.0% and any other impurity NMT 1.0%.
2.3.2 Synthesis of 2-(2-amino-4-chlorophenylamino) benzoic acid (4)
In a clean and dry Round bottom flask 150.0 gm of 2-(4-chloro-2-nitrophenylamino) benzoic acid was dissolved in 112.5 gm of 5% aqueous solution of sodium hydroxide under gentle stirring at room temperature (RT). After 10 min of stirring it was added a mixture of 300.0gm of Sodium dithionate with 1000 mL of water over a period of 15 min and temperature is exothermic to 50 °C and the reaction mixture temperature was maintained for 60–70 min until its starting material get complies over HPLC monitoring (starting material NMT 2.0%). The reaction mixture was cooled to 25–35 °C (RT) and the resulting solution pH was adjusted to 2–3 with Conc. Hydrochloric acid. The precipitated light brown colored solid was filtered and dried, Reported the yield 120.0 gm (89.1%) with purity NLT 98.0% and any other impurity content NMT 1.0%.
2.3.3 Synthesis of 8-Chloro-5, 10-dihydrodibenzo [b, e][1, 4]diazepin-11-one(3)
100.0gm of compound 4 was dissolved in a mixture of 800 mL of N, N-Dimethylformamide, 10 mL of Conc. Sulfuric acid In a clean and dry Round bottom flask at RT under vigorous stirring. The entire reaction mass temperature was slowly heated to 150–155 °C and maintained for 7 h till its starting material gets complies over HPLC. Once reaction completed reduce the reaction mass temperature to 100 °C and removed the solvent under reduced pressure to recover the solvent about 80%. The resulting syrupy material was diluted with 200 mL of Water and its pH adjusted to 9.5 to 10 with aqueous Sodium hydroxide and the precipitated solid material was filtered and dried under vacuum afforded 80.0 gm (Yield: 86.02%)
2.3.4 Synthesis of 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b, e] [1, 4] diazepine Clozapine (1)
In a Clean & dry round bottom flask 1000 mL Toluene and 100.0gm of 8-chloro-11-oxo-10, 11-dihydro-5H-dibenzo-l, 4-diazepine was taken at room temperature(RT). The resulting mass was stirred for 30 min, the reaction mass was cooled to 10–15 °C and a mixture solution of 97.0 mL (164.0gm) of Titanium (IV) chloride in 200.0 mL of Toluene was added drop wise to the above cooled solution over a period of 30 min with constant stirring. The reaction mixture was heated to RT and then to reflux, maintained for 3 h. The reaction mass was then cooled to 10 °C. 200.0gm of 1-Methylpiperizine was added to the reaction mass during a period of 30 min, and further it was maintained for 30–45 min at 10 °C. The reaction mixture then further heated to reflux for 4 h. Check TLC with 10% Methanol in chloroform (1:9) mobile phase. After completion of the reaction mass the excess solvent was distilled off to get the crude residue, it was partitioned between 2 M aqueous Hydrochloric acid (500.0 mL) and Ethyl acetate (500.0 mL). The separated aqueous layer was adjusted pH with Sodium hydroxide (pH ∼ 14) and extracted with Ethyl acetate (3X200.0 mL), the combined organics were washed with Water (2 × 100.0 mL), and followed by saturated Brine solution. The dried organic layer was distilled under reduced pressure, the optioned solid Clozapine was crystalized in Acetone and Isopropyl Ether afforded 85.0 gm (Yield: of 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b, e] [1, 4] diazepine Clozapine (1) with Purity NLT 99.0%, any other Individual impurity NMT 0.10%.
3 Conclusion
As explained in the above experimental section, we have developed an optimized new route of synthesis for clozapine. It is feasible process for commercial scale manufacturing with eco-friendly low cost raw materials. The entire synthesis opted in four simple steps by keeping all the industrial requirements such as safety, time cycle and operational easiness. Finally landed up with quality active pharmaceutical ingredient with cost effective route of synthesis with purity not less than 99.87%, Any other individual impurity NMT 0.10% with 60% overall yield.
Acknowledgement
The author thankful to SMS pharmaceuticals Ltd. Management team for supporting the finance and research infrastructure to get this novel research output and special thanks to AR&D team for their continuous analytical support.
References
- 11-amino-5h-dibenzo [B, E]-1, 4-diazepine. 10. Mitteilung uber siebengliedrige heterocyclic. Helv. Chim. Acta. 1967;50(6):1588-1599.
- [CrossRef] [Google Scholar]
- J. Labelled Compd. Radiopharm.. 1988;25(9):1027-1033.
- [CrossRef]
- J. Anal. Prof. Drug Substances Excipients. 1993;22:145-184.
- [CrossRef]
- Synthesis of clozapine analogues and their affinity for clozapine and spiroperidol binding sites in rat brain. J. Med. Chem.. 1981;24(9):1021-1026.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.05.003.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







