A DFT study of solvation effects and NBO analysis on the tautomerism of 1-substituted hydantoin
⁎Corresponding author. Tel.: +98 9173235388; fax: +98 21 88257969. m-shabanian@phd.araku.ac.ir (Meisam Shabanian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
1-Substituted hydantoins (1-SH) have been known as a benefit intermediate for producing agricultural and pharmaceuticals. The effect of solvent polarity on the tautomeric equilibria of 1-substituted hydantoin ring is studied by the density functional theory calculation (B3LYP/6–31++G(d,p)) level for predominant tautomeric forms of hydantoin derivatives (1-NO2, 1-CF3, 1-Br, 1-H, 1-CH⚌CH2, 1-OH, 1-CH3) in the gas phase and selected solvents (benzene (non-polar solvent), tetrahydrofuran (THF) (polar aprotic solvent) and water (protic solvent)). For electron withdrawing and releasing derivatives in the gas phase and solution Hy1 forms is more stable and dominant form. In addition variation of dipole moments and charges on atoms in the solvents are studied.
Keywords
1-Substituted hydantoins
DFT study
Tautomerism
Dipole moments
1 Introduction
Hydantoins, glycolylurea, consist a group of compounds that has been of considerable interest. Many of the compounds possess clinically useful activity, especially as anticonvulsants (e.g., norantoin, mepheenytoin, nirvanol, and methetoin). Many biological activities of hydantoin derivatives are known, as in their uses as herbicides and fungicides and some N-substituted derivatives of hydantoin are used as chlorinating or brominating agents in disinfectant/sanitizer or biocide products. Both the electron distribution and the stereochemistry of the hydantoins are important for their biological activity. For this reason and along with a research program to study the structure–activity relationships for this class of compounds, this paper reviews the relevant literature on the structure of hydantoin derivatives both in solution and in the solid state (Ware, 1950; Kleinpeter, 1997).
Tautomerism interconversions (Grochowski et al., 2004; Belova et al., 2008) have been investigated by chemists during last decades. Recently, study of tautomerism received renewed attention due to its importance on the determination of compounds’ properties and their area of applications. The importance of tautomerism is revealed more since in recent years the investigation about tautomerism has been the major topic in theoretical chemistry. For example, tautomerisms in keto-enol (Misra and Dalai, 2007; Zborowski and Korenova, 2004), imine–enamine (Oziminski et al., 2004; Dines and Onoh, 2006), purine (Shukla and Mishra, 2000), pirimidine (Bonacin et al., 2007) and many other systems (Ralhan and Ray, 2003) have been studied during the past decades. Thereupon, compounds containing different tautomers can be the subject of interest by theoretical chemists (Tavakol, 2010).
In this article we studied the tautomerism of seven 1-substituted hydantoins in the gas phase and solution using polarisable continuum method (PCM) at the B3LYP/6–31++G(d,p) level of theory.
2 Computational methods
All these calculations were carried out on a Pentium V personal computer by means of GAUSSIAN03 program package (Frisch et al., 2004) and for our computations. First, all the compound’s structures were drawn using Gauss View 03 (Dennington et al., 2003) and optimized in semi. To characterize all the optimized geometries the vibrational frequencies for all the conformers have been done at B3LYP levels. The stationary structures are confirmed by ascertaining that all ground states have only real frequencies. The tautomers were also optimized in solvents according to the polarisable continuum method of Tomasi and co-workers, which exploits the generating polyhedra procedure (Miertus et al., 1981; Cances et al., 1997; Cossi et al., 1998; Barone and Cossi, 1998; Barone et al., 1998) to build the cavity in the polarisable continuum medium, where the solute is accommodated. Atomic charges in all the structures were obtained using the Natural Population Analysis (NPA) method within the Natural Bond Orbital (NBO) approach (Reed et al., 1988; Najafi Chermahini et al., 2007).
3 Results and discussion
3.1 Gas phase
Structures of hydantoin derivatives are depicted in Fig. 1 and the results of energy comparisons of five tautomers in the gas phase and different solvents are given in Table 1. In agreement with previous results, in the gas phase all Hy1 forms are more stable than the other forms. The major difference between Hy1 and the other forms in the gas phase was found for 1-methyle hydantoin with 24.07 kcal mol−1. The order of stability of Hy1 tautomer over the other tautomers in the gas phase is CH3 > H > CH⚌CH2 > OH > Br > NO2 > CF3.
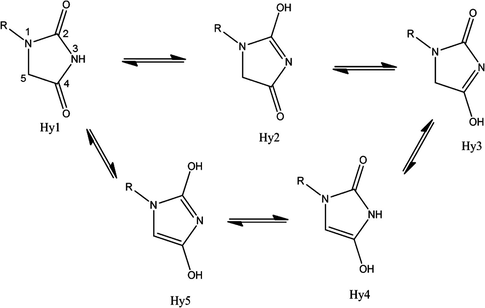
- Tautomeric forms of hydantoin derivatives and numbering of hydantoin ring.
| R | Tautomer | Gas (1.0) | Benzene (2.2) | THF (7.6) | Water (78.4) |
|---|---|---|---|---|---|
| NO2 | Hy1 | −581.2074691 | −581.2221109 | −581.2320934 | −581.2367362 |
| Hy2 | −581.1814368 | −581.1932298 | −581.2015427 | −581.2060233 | |
| Hy3 | −581.1834681 | −581.2002005 | −581.2120739 | −581.217642 | |
| Hy4 | −581.1711189 | −581.1904285 | −581.2039496 | −581.2107403 | |
| Hy5 | −581.1591994 | −581.1753041 | −581.186004 | −581.190941 | |
| CF3 | Hy1 | −713.7845745 | −713.7963805 | −713.8042665 | −713.8078671 |
| Hy2 | −713.7488251 | −713.7589838 | −713.7683018 | −713.7743826 | |
| Hy3 | −713.7610168 | −713.7750443 | −713.7850663 | −713.7897254 | |
| Hy4 | −713.7479111 | −713.7641756 | −713.7753535 | −713.7810888 | |
| Hy5 | −713.7376467 | −713.7510511 | −713.7597643 | −713.7637658 | |
| Br | Hy1 | −2947.821781 | −2947.8339021 | −2947.8420475 | −2947.8455984 |
| Hy2 | −2947.7831188 | −2947.7974126 | −2947.8156584 | −2947.8145562 | |
| Hy3 | −2947.7956906 | −2947.8101048 | −2947.8203488 | −2947.8249864 | |
| Hy4 | −2947.7824059 | −2947.7991082 | −2947.8105747 | −2947.8161127 | |
| Hy5 | −2947.7746538 | −2947.7883321 | −2947.7971482 | −2947.800963 | |
| H | Hy1 | −376.7405171 | −376.7556339 | −376.7659129 | −376.7703092 |
| Hy2 | −376.6983782 | −376.7196312 | −376.7361504 | −376.7441062 | |
| Hy3 | −376.7021526 | −376.7221592 | −376.7370945 | −376.7443175 | |
| Hy4 | −376.7013924 | −376.7208266 | −376.7341045 | −376.7402239 | |
| Hy5 | −376.6932919 | −376.7100632 | −376.7209578 | −376.7255222 | |
| CH3 | Hy1 | −416.0542389 | −416.066013 | −416.0739606 | −416.0774713 |
| Hy2 | −416.0082409 | −416.0245805 | −416.0369986 | −416.0439921 | |
| Hy3 | −416.0158605 | −416.0327035 | −416.0454155 | −416.0517596 | |
| Hy4 | −416.0156317 | −416.0313783 | −416.0421626 | −416.0474911 | |
| Hy5 | −416.0059184 | −416.0194233 | −416.0281578 | −416.0320566 | |
| CH⚌CH2 | Hy1 | −454.1438696 | −454.155888 | −454.1638078 | −454.1672786 |
| Hy2 | −454.098425 | −454.1151325 | −454.1276585 | −454.1351203 | |
| Hy3 | −454.1170664 | −454.1311998 | −454.1411281 | −454.1456436 | |
| Hy4 | −454.1021849 | −454.1183108 | −454.1295859 | −454.134804 | |
| Hy5 | −454.0907753 | −454.1046771 | −454.113678 | −454.1175365 | |
| OH | Hy1 | −451.9012817 | −451.9178215 | −451.9290471 | −451.9339936 |
| Hy2 | −451.86771 | −451.890121 | −451.9033081 | −451.9092211 | |
| Hy3 | −451.8748703 | −451.8769226 | −451.906554 | −451.912549 | |
| Hy4 | −451.860528 | −451.8769226 | −451.8959356 | −451.9012212 | |
| Hy5 | −451.8507417 | −451.8698025 | −451.8823162 | −451.8878452 |
This indicates that the stability of Hy1 form decreases with the increase withdrawing effect of substituents. The calculated dipole moments for the hydantoins are presented in Table 2. In the Hy2 tautomers, the electron withdrawing derivatives have smaller dipole moments than the electron releasing ones; but in Hy4 forms electron donating derivatives have lower dipole moments values than electron withdrawing substituents. This maybe explained by the consideration of charge values on the atoms of hydantoin ring. It is well known that in haydantoin N1 and N3 atoms carry the most negative charge. The Hy2 isomer of 1-NO2 derivative has the least charge density on N1 and N3. It is noticeable that the differences between the dipole moments of 1-H and 2-H forms are related to nature substituents.
| R | Tautomer | Gas (1.0) | Benzene (2.2) | THF (7.6) | Water (78.4) |
|---|---|---|---|---|---|
| NO2 | Hy1 | 2.6038 | 3.2247 | 3.7160 | 3.9700 |
| Hy2 | 3.0670 | 3.8083 | 4.4560 | 4.8578 | |
| Hy3 | 6.2865 | 7.8248 | 9.0553 | 9.6786 | |
| Hy4 | 6.9441 | 8.5545 | 9.8821 | 10.5669 | |
| Hy5 | 3.6635 | 4.6294 | 5.4782 | 5.9043 | |
| CF3 | Hy1 | 1.3475 | 1.5638 | 1.7249 | 1.7905 |
| Hy2 | 5.0520 | 6.3704 | 7.2312 | 8.0778 | |
| Hy3 | 5.5358 | 6.8991 | 8.0413 | 8.6616 | |
| Hy4 | 5.9082 | 7.1693 | 8.1428 | 8.6173 | |
| Hy5 | 1.7713 | 2.1689 | 2.4807 | 2.5991 | |
| Br | Hy1 | 1.9298 | 2.2431 | 2.4729 | 2.5882 |
| Hy2 | 7.0953 | 8.8554 | 6.7323 | 11.0407 | |
| Hy3 | 5.0617 | 6.3101 | 7.3319 | 7.8312 | |
| Hy4 | 5.1598 | 6.3006 | 7.2013 | 7.6384 | |
| Hy5 | 1.3389 | 1.5940 | 1.7887 | 1.8825 | |
| H | Hy1 | 2.7821 | 3.2723 | 3.6437 | 3.8137 |
| Hy2 | 7.6712 | 9.8882 | 11.8194 | 12.5808 | |
| Hy3 | 7.8371 | 9.6297 | 11.0666 | 11.7583 | |
| Hy4 | 5.2094 | 6.3199 | 7.1838 | 7.5898 | |
| Hy5 | 1.8784 | 2.2479 | 2.5263 | 2.6560 | |
| CH3 | Hy1 | 3.0149 | 3.4165 | 3.7002 | 3.8320 |
| Hy2 | 8.8193 | 10.6037 | 12.0025 | 12.7320 | |
| Hy3 | 7.7192 | 9.4854 | 10.9136 | 11.6196 | |
| Hy4 | 4.9890 | 6.0886 | 6.9381 | 7.3840 | |
| Hy5 | 2.3506 | 2.5999 | 2.7825 | 2.8707 | |
| CH⚌CH2 | Hy1 | 1.8262 | 2.0860 | 2.2985 | 2.3802 |
| Hy2 | 7.4423 | 9.2216 | 10.6757 | 11.4296 | |
| Hy3 | 4.6670 | 5.8457 | 6.8213 | 7.3045 | |
| Hy4 | 3.7335 | 4.5451 | 5.2161 | 5.5522 | |
| Hy5 | 1.4103 | 1.5789 | 1.7212 | 1.7831 | |
| OH | Hy1 | 2.0019 | 2.5576 | 3.0234 | 3.2656 |
| Hy2 | 7.2315 | 6.0234 | 7.0601 | 7.5663 | |
| Hy3 | 4.5590 | 5.9384 | 7.1507 | 7.8244 | |
| Hy4 | 4.4355 | 5.5754 | 6.9989 | 5.4223 | |
| Hy5 | 2.0633 | 2.3916 | 2.6936 | 2.8601 |
The calculated values of NBO charges using the Natural Population Analysis (NPA) of optimized structures of hydantoin derivatives are listed in Table 3. As it was noticed previously, the hydantoin’s nitrogen atoms at position 1 or 3 carry the largest negative charge and these positions will most effectively interact with electrophiles. There is no uniform trend for the variation of charges to relate to the different substituents of hydantoins in the gas phase, Table 3.
| R | ∊= | 1.0 | 2.2 | 7.6 | 78.4 | 1.0 | 2.2 | 7.6 | 78.4 | 1.0 | 2.2 | 7.6 | 78.4 | 1.0 | 2.2 | 7.6 | 78.4 | 1.0 | 2.2 | 7.6 | 78.4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atom | Hyl | Hy2 | Hy3 | Hy4 | Hy5 | ||||||||||||||||
| NO2 | Nl | −0.035 | −0.332 | −0.318 | 3.347 | −0.319 | −0.304 | −0.294 | 3.358 | −0.357 | −0.336 | −0.323 | −0.315 | −0.310 | −0.293 | −0.280 | 3.366 | −0.285 | −0.275 | −0.267 | 3.372 |
| C2 | 0.821 | 0.834 | 0.842 | 3.420 | 0.769 | 0.788 | 0.802 | 3.401 | 0.799 | 0.811 | 0.817 | 0.819 | 0.798 | 0.806 | 0.811 | 3.404 | 0.719 | 0.731 | 0.740 | 3.371 | |
| N3 | −0.680 | −0.674 | −0.670 | 3.166 | −0.545 | −0.573 | −0.595 | 3.198 | −0.562 | −0.583 | −0.598 | −0.604 | −0.657 | −0.654 | −0.652 | 3.175 | −0.598 | −0.606 | −0.609 | 3.197 | |
| C4 | 0.685 | 0.697 | 0.705 | 3.352 | 0.656 | 0.668 | 0.674 | 3.335 | 0.617 | 0.638 | 0.654 | 0.662 | 0.479 | 0.490 | 0.500 | 3.253 | 0.457 | 0.461 | 0.465 | 3.233 | |
| C5 | −0.334 | −0.336 | −0.339 | 2.830 | −0.345 | −0.345 | −0.345 | 2.827 | −0.320 | −0.323 | −0.326 | −0.327 | −0.215 | −0.220 | −0.223 | 2.888 | −0.196 | −0.205 | −0.212 | 2.892 | |
| CF3 | Nl | −0.593 | −0.587 | −0.583 | −0.581 | −0.589 | −0.577 | −0.569 | −0.563 | −0.604 | −0.598 | −0.593 | −0.590 | −0.555 | −0.552 | −0.549 | −0.547 | −0.509 | −0.510 | −0.512 | 3.244 |
| C2 | 0.853 | 0.845 | 0.852 | 0.855 | 0.780 | 0.801 | 0.810 | 0.820 | 0.812 | 0.820 | 0.825 | 0.827 | 0.805 | 0.807 | 0.808 | 0.807 | 0.722 | 0.729 | 0.733 | 3.367 | |
| N3 | −0.681 | −0.676 | −0.673 | −0.671 | −0.557 | −0.599 | −0.614 | −0.636 | −0.562 | −0.585 | −0.603 | −0.612 | −0.653 | −0.651 | −0.649 | −0.648 | −0.606 | −0.618 | −0.625 | 3.187 | |
| C4 | 0.685 | 0.696 | 0.703 | 0.707 | 0.657 | 0.665 | 0.672 | 0.670 | 0.612 | 0.632 | 0.647 | 0.654 | 0.462 | 0.467 | 0.471 | 0.473 | 0.442 | 0.440 | 0.439 | 3.219 | |
| C5 | −0.324 | −0.328 | −0.330 | −0.331 | −0.338 | −0.330 | −0.341 | −0.332 | −0.307 | −0.311 | −0.313 | −0.312 | −0.201 | 0.205 | −0.207 | −0.207 | −0.186 | −0.194 | −0.200 | 2.899 | |
| Br | Nl | −0.602 | −0.595 | −0.589 | 3.207 | −0.586 | −0.575 | −0.558 | −0.561 | −0.612 | −0.603 | −0.595 | −0.591 | −0.559 | −0.553 | −0.549 | −0.547 | −0.507 | −0.509 | −0.510 | 3.244 |
| C2 | 0.812 | 0.819 | 0.823 | 3.410 | 0.767 | 0.781 | 0.790 | 0.797 | 0.790 | 0.795 | 0.707 | 0.707 | 0.777 | 0.777 | 0.774 | 0.772 | 0.690 | 0.695 | 0.698 | 3.349 | |
| N3 | −0.682 | −0.678 | −0.676 | 3.163 | −0.583 | −0.621 | −0.670 | −0.664 | −0.557 | −0.584 | −0.603 | −0.613 | −0.653 | −0.652 | −0.651 | −0.651 | −0.613 | −0.629 | −0.639 | 3.180 | |
| C4 | 0.686 | 0.696 | 0.702 | 3.350 | 0.657 | 0.662 | 0.665 | 0.663 | 0.605 | 0.624 | 0.638 | 0.645 | 0.456 | 0.460 | 0.463 | 0.465 | 0.440 | 0.436 | 0.434 | 3.215 | |
| C5 | −0.317 | −0.321 | −0.324 | 2.838 | −0.330 | −0.332 | −0.332 | −0.334 | −0.305 | −0.310 | −0.313 | −0.315 | −0.204 | −0.208 | −0.210 | −0.210 | −0.194 | −0.202 | −0.209 | 2.894 | |
| H | Nl | −0.694 | −0.687 | −0.681 | −0.678 | −0.696 | −0.681 | −0.660 | −0.653 | −0.707 | −0.697 | −0.687 | −0.683 | −0.644 | −0.641 | −0.638 | −0.637 | −0.597 | −0.600 | −0.603 | −0.604 |
| C2 | 01.810 | 0.814 | 0.817 | 0.818 | 0.754 | 0.773 | 0.784 | 0.789 | 0.786 | 0.789 | 0.788 | 0.788 | 0.773 | −0.769 | 0.764 | 0.761 | 0.683 | 0.685 | 0.686 | 0.687 | |
| N3 | −0.689 | −0.686 | −0.684 | −0.683 | −0.583 | −0.635 | −0.678 | −0.693 | −0.526 | −0.569 | −0.604 | −0.622 | −0.660 | −0.661 | −0.661 | −0.661 | −0.629 | −0.651 | −0.666 | −0.673 | |
| C4 | 0.682 | 0.691 | 0.696 | 0.698 | 0.653 | 0.658 | 0.658 | 0.658 | 0.586 | 0.606 | 0.622 | 0.630 | 0.447 | 0.446 | 0.446 | 0.447 | 0.431 | 0.424 | 0.418 | 0.415 | |
| C5 | −0.330 | −0.333 | −0.335 | −0.335 | −0.351 | −0.348 | −0.345 | −0.345 | −0.331 | −0.333 | −0.334 | −0.335 | −0.215 | −0.217 | −0.217 | −0.216 | −0.208 | −0.215 | −0.220 | −0.222 | |
| CH3 | Nl | −0.519 | −0.509 | −0.501 | −0.497 | −0.514 | −0.476 | −0.215 | −0.469 | −0.533 | −0.519 | −0.507 | 3.251 | −0.474 | −0.467 | −0.461 | 3.272 | −0.428 | −0.426 | −0.425 | −0.425 |
| C2 | 0.821 | 0.826 | 0.830 | 0.831 | 0.774 | 0.798 | 0.626 | 0.803 | 0.798 | 0.801 | 0.802 | 3.398 | 0.785 | 0.783 | 0.780 | 3.386 | 0.693 | 0.698 | 0.701 | 0.702 | |
| N3 | −0.685 | −0.681 | −0.697 | −0.678 | −0.602 | −0.671 | −0.553 | −0.685 | −0.523 | −0.565 | −0.599 | 3.193 | −0.656 | −0.658 | −0.658 | 3.171 | −0.631 | −0.651 | −0.664 | −0.670 | |
| C4 | 0.684 | 0.693 | 0.699 | 0.701 | 0.653 | 0.658 | −0.407 | 0.658 | 0.588 | 0.608 | 0.624 | 3.314 | 0.458 | 0.455 | 0.456 | 3.226 | 0.434 | 0.428 | 0.423 | 0.421 | |
| C5 | −0.315 | −0.319 | −0.322 | −0.323 | −0.330 | −0.332 | −0.236 | −0.333 | −0.315 | −0.318 | −0.321 | 2.839 | −0.206 | −0.209 | −0.210 | 2.895 | −0.196 | −0.203 | −0.208 | −0.210 | |
| CH⚌CH2 | Nl | −0.502 | −0.496 | −0.491 | −0.489 | −0.490 | −0.478 | −0.469 | −0.464 | −0.512 | −0.505 | −0.499 | −0.496 | −0.467 | −0.463 | −0.460 | −0.459 | −0.431 | −0.432 | −0.433 | 3.287 |
| C2 | 0.825 | 0.833 | 0.837 | 0.839 | 0.772 | 0.789 | 0.800 | 0.807 | 0.803 | 0.808 | 0.810 | 0.811 | 0.795 | 0.795 | 0.793 | 0.793 | 0.707 | 0.711 | 0.714 | 3.356 | |
| N3 | −0.681 | −0.678 | −0.675 | −0.673 | −0.580 | −0.620 | −0.650 | −0.665 | −0.563 | −0.590 | −0.610 | −0.619 | −0.668 | −0.666 | −0.662 | −0.661 | 0.619 | −0.636 | −0.647 | 3.175 | |
| C4 | 0.686 | 0.696 | 0.702 | 0.705 | 0.655 | 0.661 | 0.664 | 0.664 | 0.607 | 0.625 | 0.639 | 0.646 | 0.435 | 0.447 | 0.458 | 0.464 | 0.440 | 0.435 | 0.431 | 3.214 | |
| C5 | −0.323 | −0.327 | −0.330 | −0.331 | −0.335 | −0.337 | −0.339 | −0.339 | −0.308 | −0.313 | −0.316 | −0.318 | −0.140 | −0.158 | −0.176 | −0.187 | −0.190 | −0.198 | −0.203 | 2.897 | |
| OH | Nl | −0.243 | −0.237 | −0.228 | 3.390 | −0.244 | −0.214 | −0.203 | −0.196 | −0.259 | −0.249 | −0.238 | −0.231 | −0.206 | −0.196 | −0.177 | −0.165 | 0.136 | −0.131 | −0.127 | −0.125 |
| C2 | 0.807 | 0.814 | 0.817 | 3.406 | 0.755 | 0.770 | 0.781 | 0.789 | 0.785 | 0.790 | 0.791 | 0.791 | 0.782 | 0.779 | 0.771 | 0.764 | 0.678 | 0.682 | 0.684 | 0.685 | |
| N3 | −0.684 | −0.680 | −0.677 | 3.162 | −0.566 | −0.643 | −0.664 | −0.674 | −0.569 | −0.594 | −0.614 | −0.626 | −0.661 | −0.659 | −0.659 | −0.663 | −0.626 | −0.646 | −0.659 | −0.664 | |
| C4 | 0.683 | 0.694 | 0.699 | 3.349 | 0.656 | 0.663 | 0.666 | 0.666 | 0.608 | 0.626 | 0.639 | 0.646 | 0.456 | 0.459 | 0.460 | 0.446 | 0.437 | 0.432 | 0.428 | 0.426 | |
| C5 | −0.334 | −0.336 | −0.338 | 2.830 | −0.351 | −0.348 | −0.348 | −0.348 | −0.319 | −0.323 | −0.326 | −0.327 | −0.212 | −0.218 | −0.224 | −0.189 | −0.207 | −0.217 | −0.223 | −0.225 | |
3.2 Solvent effects
Solvent effects are relevant in the tautomers stability phenomena, since polarity differences among the tautomers can induce significant changes in their relative energies in solution. PCM/B3LYP calculations were used to analyze the solvent effects on tautomerism of hydantoin derivatives. It is important to stress that the PCM model does not consider the presence of explicit solvent molecules; hence specific solute–solvent interactions are not described and the calculated solvation effects arise only from mutual solute–solvent electrostatic polarization. The data presented in Table 1 show that the polar solvents increase the stability of all hydantoins in comparison to gas phase. The difference between the total energies of Hy1 and the other forms do not show a regular trend when changing from gas phase to most polar solvents (water).
The solvent interactions have not pronounced an effect on the order of stability of the tautomers in the gas phase. The solvent represented by a polarizable continuum is found to show significant effect on the dipole moments of the individual solute conformers. The dipole moments (l) increase by changing the gas phase to the solution as well as by increasing the solvent polarity. The most significant variations being obtained in hydantoin in Hy2 form with 4.9096 D, Table 2. We have examined the charge distribution of tautomers in the solvent as well as gas phase by using calculated NBO charges. The charge distribution in solvents with the increase of polarity differently varies for any atoms.
4 Conclusion
-
In the gas phase and solution all Hy1 forms were more stable than the other tautomers. On the other hand, interestingly enough, the highly elaborate B3LYP/6–31++G(d,p) type DFT calculations yield results in estimating the stability order of these tautomers as Hy1 > Hy2 > Hy2 > Hy4 > Hy5 (Table 1). In the solution and with increase of polarity; Hy1 isomers were more stable. With increase of polarity, total energies of all compounds were more negative.
-
The dipole moments of all compounds are affected by solvent. With increase of the polarity of solvents the dipole moments of the tautomers were increased.
-
The charges on all five positions were affected by substituents and solvents.
References
- J. Phys. Chem. A.. 1998;102:1995.
- J. Comp. Chem.. 1998;19:404.
- J. Phys. Chem. A.. 2008;112:3209.
- Vib. Spectrosc.. 2007;44:133.
- J. Chem. Phys.. 1997;107:3032.
- Chem. Phys. Lett.. 1998;286:253.
- Dennington, R., Keith, T., Millam, J., Eppinnett, K., Hovell, W.L., Gilliland, R., 2003. GaussView, Version 309. Semichem, Inc., Shawnee Mission, KS.
- Spectrochim. Acta A.. 2006;64:891.
- Frisch, M.J. et al. 2004. Gaussian 03, Revision CO2, Gaussian, Inc., Wallingford CT.
- J. Mol. Struct.. 2004;689:43.
- Struct. Chem.. 1997;8:161.
- J. Chem. Phys.. 1981;55:117.
- THEOCHEM. 2007;807:33.
- THEOCHEM. 2007;820:7.
- THEOCHEM. 2004;680:107.
- THEOCHEM. 2003;634:83.
- Chem. Rev.. 1988;88:899.
- Comput. J. Chem.. 2000;21:826.
- THEOCHEM. 2010;956:97.
- Chem. Rev.. 1950;46:403.
- THEOCHEM. 2004;683:15.







