Translate this page into:
A dual-functional chemosensor based on acylhydrazone derivative for rapid detection of Zn(II) and Mg(II): Spectral properties, recognition mechanism and application studies
⁎Corresponding authors. zcx@zknu.edu.cn (Chunxiang Zhao), 20041023@zknu.edu.cn (Yahong Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
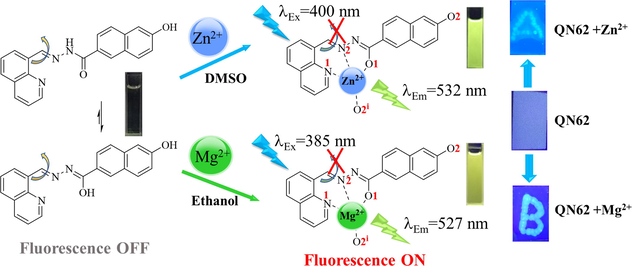
In this work, an acylhydrazone derivative (QN62) was developed via the one-step condensation of 6-hydroxy-2-naphthoic hydrazide with quinoline-8-carboxaldehyde. The structure of the QN62 compound was characterized by 1H NMR, 13C NMR, HR-MS (ESI), and X-ray crystallography. As a dual-functional turn-on fluorescence chemosensor, QN62 exhibited rapid recognition for Zn2+ in DMSO-H2O (4:1, v/v) and Mg2+ in ethanol-H2O (9:1, v/v). The enhancement in fluorescence detection was associated with the coordination reaction between QN62 and the target ions, which promoted intramolecular charge transfer and prevented the C⚌N isomerization process. Simultaneously, a rapid color change from colorless to yellowish-green or yellow under UV light (365 nm) was easily visible to the naked eye. Under optimal conditions, the limit of detection and limit of quantification were 32.3 nM and 97.8 nM for Zn2+ and 16.1 nM and 48.9 nM for Mg2+, respectively. The recognition mechanism was reasonably speculated based on analysis of the Job’s plot, HR-MS, 1H NMR, and density functional theoretical calculations. Utilizing silica-gel plates fabricated from the QN62 chemosensor, the visual and rapid identification of Zn2+ and Mg2+ was successfully achieved, which could provide a convenient approach for on-site detection in environmental fields. The QN62 chemosensor could also quantify trace amounts of Zn2+ and Mg2+ in water samples. Furthermore, the cell imaging experiments indicated that QN62 could effectively sense intracellular Zn2+ and Mg2+, providing potential applications in biological systems.
Keywords
Acylhydrazone
Dual-functional chemosensor
Zinc ion
Magnesium ion
Theoretical calculations
Application
1 Introduction
The abnormal accumulation of metal ions can cause potential hazards for the biological environment and human health (Geng, et al., 2022; Sidqi, et al., 2022; Aysha, et al., 2021). For example, Zn2+ plays a vital role in many biological processes such as signal transduction, cellular metabolism, and gene expression, but zinc homeostasis disturbances may be responsible for neurodegenerative syndromes (Maity, et al., 2019; Wang, et al., 2022; Bush, et al., 1994). In the environment, high concentrations of zinc (1300 mg/kg) can diminish soil microbial activity, resulting in phytotoxic effects (Voegelin, et al., 2005; Moreno, et al., 2009). Similarly, as a significant element in living beings and the environment, magnesium imbalances may also induce various diseases, including hypermagnesemia, diarrhea, and metabolic syndrome (Fu, et al., 2021; Matsui, et al., 2017). Thus, developing efficient and convenient methods for detecting trace metal ions has attracted widespread attention.
Fluorescence-based molecular probes have been extensively investigated for the recognition of trace metal ions due to their unique superior characteristics, such as fast response, good selectivity, high sensitivity, low detection limit, and operational simplicity (Saravana Kumar, et al., 2020; Jiang, et al., 2019). Abundant fluorescent probes have been shown to be efficient for detecting single metal ions such as Zn2+ (Bumagina, et al., 2022), Al3+ (Kolcu and Kaya, 2022), Hg2+ (Musikavanhu, et al., 2022; Mohamed, et al., 2022), Cu2+ (Ahmed, et al., 2022; Picci, et al., 2021; Khan, et al., 2022), Mg2+ (Hu, et al., 2017), Cd2+ (Mohanasundaram, et al., 2021), and Cr3+ (Khan, et al., 2022). The development of dual-functional chemosensors has also attracted great interest among researchers, as dual chemosensors can identify-two species, effectively saving time and costs compared to one-to-one detection probes (Hazra, et al., 2018; Roy, et al., 2021; Alam, et al., 2016; Anbu, et al., 2014; Gogoi, et al., 2014; Dong, et al., 2017). So far, a small number of fluorescent probes have been reported for the simultaneous detection of Zn2+ and Mg2+ ions. For example, a Schiff-base compound based on coumarin was prepared for the sensing of Zn2+ and Mg2+, showing significant enhancement of the fluorescence peaks at 558 nm for Zn2+ and 550 nm for Mg2+ (λex = 450 nm) (Maity and Bharadwaj, 2014). Alam et al. (Alam, et al., 2014) reported on a quinoline derivative as a turn-on fluorescent probe for Zn2+ and Mg2+, which exhibited emissions at 539 nm for Zn2+ and 526 nm for Mg2+ (λex = 430 nm). An acylhydrazone compound based on naphthalene and pyrazole, developed by Dhara et al. (Dhara, et al., 2016), was used as a dual sensor for Zn2+ and Mg2+ ions. In the complexation studies, the amide carbonyl O and pyrazole N atoms acted as metal binding sites to form 1:1 complexes. Wang et al. (Wang, et al., 2019) reported on isoquinoline-based acylhydrazone, which could efficiently recognize Zn2+ and Mg2+ by altering the solvent from DMF-H2O (9:1, v/v) to acetonitrile. However, some of the reported probes showed certain limitations as overlapping response signals, low sensitivity, or a multi-step synthetic route. Thus, there is scope for modulating dual chemosensor properties utilizing the selective combination of organic functional groups (Wang, et al., 2019).
Quinolines have been widely utilized as fluorophores for constructing probes due to their unique advantages of good biocompatibility, easy structural modification, and stable photophysical properties (Wang, et al., 2021; Xu, et al., 2022; Yan, et al., 2021). The nitrogen atom in the quinoline group can also be used as a binding site for coordination with metal ions, accompanied by the enhancement of fluorescence quantum yield (Wang, et al., 2021). In addition, naphthalenes are well-known, ideal fluorophores in the design of fluorescent chemosensors. An electron donating group and an electron withdrawal group have been introduced into the 2- and 6-positions of naphthalene to provide a push–pull electronic system, possessing intramolecular charge transfer (ICT) characteristics (Sun, et al., 2020; Li, et al., 2019). Moreover, acylhydrazone backbones have shown a strong coordination ability with metal ions through the N atom of imine and the O atom of carbonyl (Liao, et al., 2017). After binding with certain metal ions, acylhydrazone-based probes usually display a turn-on fluorescence response by restricting the free rotation of -C⚌N (Sun, et al., 2020).
Combining the advantages of the above functional groups, and to further continue our study on the development of dual chemosensors (Ding, et al., 2021), in this work, we rationally designed and developed a simple acylhydrazone chemosensor (QN62) through a one-step condensation reaction between 6-hydroxy-2-naphthoic hydrazide and quinoline-8-carboxaldehyde. In this work, QN62 could discriminate between Zn2+ in DMSO-H2O (4:1, v/v) medium and Mg2+ in ethanol-H2O (9:1, v/v) medium, showing significant fluorescence enhancement. The response mechanism of QN62 to Zn2+/Mg2+ was investigated by fluorescence measurements, HR-MS, 1H NMR, and theoretical calculations. The designed compound QN62 achieved visual and rapid identification of the target ions using silica-gel plates. Moreover, it was successfully used to quantify trace amounts of Zn2+ and Mg2+ in water samples and readily sensed intracellular Zn2+ and Mg2+, which could be used as a guideline for future detection in environmental and biological systems.
2 Experimental
2.1 Materials and instruments
All of the reagents and chemicals used in this study were of analytical grade and used without further purification. Quinoline-8-carboxaldehyde was purchased from Energy Chemical (Shanghai, China), 6-hydroxy-2-naphthoic hydrazide was purchased from J&K Scientific ltd. (Beijing, China), and all other reagents were purchased from Sinopharm Chemical (Shanghai, China). The melting point was determined by a Beijing XT4-100x microscopic melting point apparatus (Beijing Electrooptical Instrument Factory, China). The 1H NMR and 13C NMR spectra were measured by a Bruker AV-500 spectrometer (Bruker BioSpin Gmbh, Germany), the Fourier transform infrared (FT-IR) spectra were obtained on a Nicolet 6700 spectrometer (Thermo Fisher Scientific, USA), and the high-resolution mass spectra (HR-MS) spectra were obtained using a UPLC G2-XS mass spectrometer (Waters, USA). A TU-1901 UV–vis spectrophotometer (PGeneral Instrument Inc., China) and Cary Eclipse fluorescence spectrofluorometer (Agilent Technologies, USA) were used to measure absorption and fluorescent spectra, and the cell imaging experiments were observed by an Olympus FV3000 confocal laser-scanning microscope (Olympus FV3000, Japan).
A Bruker Smart CCD single-crystal diffractometer (Bruker AXS GmbH, Germany) was utilized to collect single crystal data. Measurement was performed at 173 K using a graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). SHELXS-97 program was used to solve and refine the crystal structure by full-matrix least-squares techniques on F2 (Sheldrick, 1997). The structure plots was used Diamond software (Brandenburg, 2012). Crystallographic data CCDC2192938 for the structure QN62 has been deposited with the Cambridge Crystallographic Data Centre and can be obtained free of charge via https://www.ccdc.cam.ac.uk.
2.2 Synthesis of compound QN62
The compound 6-hydroxy-N'-(quinolin-8-ylmethylene)naphthalene-2-carbohydrazide (QN62) was prepared as follows, quinoline-8-carboxaldehyde (250 mg, 1.60 mmol) was dissolved in 20 mL of 1,4-dioxane and then 6-hydroxy-2-naphthoic hydrazide (320 mg, 1.60 mmol) was added. After refluxed for 4 h, the solvent was removed under reduced pressure and the crude product was purified by column chromatography on silica gel (dichloromethane-methanol, 9:1, v/v). Crystallization from dichloromethane-methanol (4:1, v/v) gave fusiform yellow single crystals. Yield: 78 %. m.p.: 164.5–165.5 °C. IR (cm−1) (Fig. S1): 3453 (–OH), 3200 (–NH), 3043 (HC = ), 1629 (C⚌O), 1578 (C⚌N), 1288 (C—N, amide III band), 1215(C—O). 1H NMR (500 MHz, DMSO‑d6, ppm) (Fig. S2): 12.20(1H, s, OH), 10.15(1H, s, NH), 9.80(1H, s,–CH = N), 9.01(1H, dd, J = 4.1, 1.7 Hz, ArH), 8.53(1H, s, ArH), 8.47(1H, m, ArH), 8.43(1H, d, J = 7.2 Hz, ArH), 8.10(1H, d, J = 8.0 Hz, ArH), 8.01–7.91(2H, m, ArH), 7.82(1H, d,J = 8.6 Hz, ArH), 7.74(1H, t, J = 7.7 Hz, ArH), 7.64(1H, dd, J = 8.3, 4.1 Hz, ArH), 7.25–7.17(2H, m, ArH). 13C NMR (125 MHz, DMSOd6, ppm) (Fig. S3): 163.12, 157.08, 150.27, 145.27, 144.20, 136.62, 136.27, 131.25, 130.69, 129.87, 128.11, 127.98, 127.14, 126.52, 126.08, 125.53, 124.53, 121.83, 119.47, 108.59. ESI-Mass (Fig. S4): m/z calcd. for [QN62 + H]+, 342.1198; found, 342.1242.
2.3 Spectroscopic measurements
The QN62 stock solution (1.0 mM) was prepared in THF. Various solutions of metal ions (5.0 mM) were prepared by dissolving their nitrate or chloride salts in double distilled water. Test solutions were prepared with QN62 stock solution (100 μL) and a certain volume of metal ions solution, and the mixture was diluted to 5 mL by DMSO-H2O (4:1, v/v) or ethanol-H2O (9:1, v/v). Different pH of solutions was adjusted with 1.0 mM NaOH or HCl. All experiments and test procedures were performed at room temperature.
2.4 Binding constant determination
The binding constant K of complexes was determined based on the modified Benesi-Hildebrand in Eq. (1) (Zhang, et al., 2018).
2.5 Limit of detection (LOD) and limit of quantification (LOD) calculations
The limit of detection (LOD) and limit of quantification (LOQ) were calculated using the Eq. (2) and Eq. (3) (Goncalves, et al., 2022).
2.6 Quantum yield measurement
The fluorescence quantum yield was estimated using anthracene (ΦR = 0.27 in ethanol) (Rurack, 2008) as reference and using the following Eq. (4) (Immanuel David, et al., 2022).
2.7 Cell imaging experiment
The MCF-7 cells were cultured with Dulbecco's modified Eagle's medium (DMEM) medium at 37˚C in a humidified incubator with 5 % CO2. The cells were first incubated with 10 μM QN62 at 37 °C for 30 min and washed three times to remove the excess of QN62. Subsequently, the cells were exposed to 10 μM Zn2+/Mg2+ for 30 min under the same conditions. The fluorescence imaging of QN62 in MCF-7 cells was carried out on a confocal laser scanning microscope.
3 Results and discussion
3.1 Characterization of Chemosensor QN62
The chemosensor QN62 was synthesized as shown in Scheme 1. The target compound QN62 was confirmed by FT-IR, 1H NMR, 13C NMR, and HR-MS analyses, as well as by single crystal X-ray diffraction. The characteristic absorption band –CH = N- at 1578 cm−1 appeared in the FT-IR spectrum of QN62 (Fig. S1). In the 1H NMR and 13C NMR spectra of QN62, the proton and carbon signals of Schiff base CH = N resonated at their expected frequency ranges of 9.807 and 157.075 ppm, respectively (Fig. S2 and S3). In the HR-MS spectrum, the base peak was found at 342.1242 (calcd. 342.1198), corresponding to [QN62 + H]+ (Fig. S4). Moreover, the structure of compound QN62 was explicitly established by X-ray crystallography. As shown in Fig. 1a, the quinoline unit and naphthalene ring of QN62 were not in the same plane, presenting a dihedral angle of 13.87°. The lattice consisted of one QN62 unit, which incorporated one disordered dichloromethane solvent molecule (Fig. 1a), and the crystalline lattice contained intermolecular O—H⋅⋅⋅O and N—H⋅⋅⋅O hydrogen bonds (Fig. 1b). Centro-symmetrically related molecules were connected into ring R22(20) dimers by intermolecular N(3)-H(3)⋅⋅⋅O(2) hydrogen bonds and the dimers were further linked by intermolecular O(2)-H(2)⋅⋅⋅O(1) hydrogen bonds, which formed an undulating two-dimensional network (Fig. 1c). The intermolecular hydrogen bonds played a key role in the formation of crystalline solids (Shyamsivappan, et al., 2020). The QN62 compound for the crystallographic data, the selected bond lengths, and the bond angles were illustrated in Tables S1, S2, and S3, respectively.
Synthesis of compound QN62.
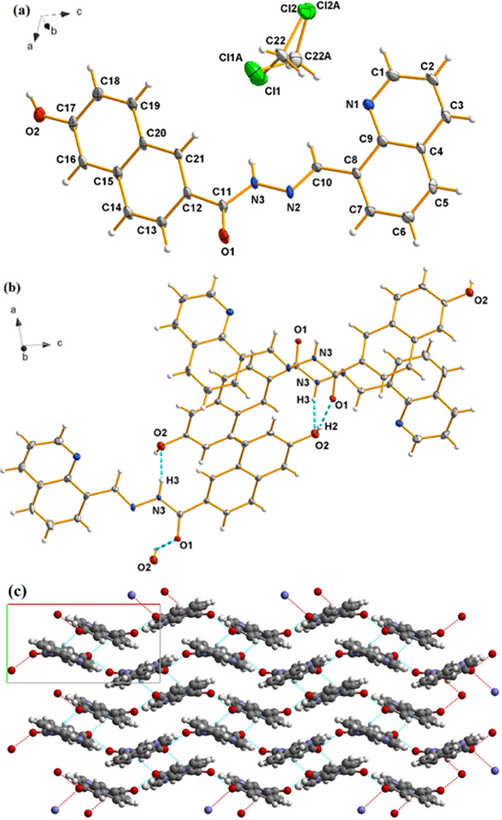
(a) Ortep view of QN62 drawn in 30 % probability thermal-displacement ellipsoids with the atom numbering scheme. (b) Part of the crystal structure of QN62 showing the intermolecular hydrogen bonds (dotted lines). (c) two-dimensional undulating structure of QN62. H-bonds are shown by dotted lines.
3.2 Spectroscopic recognition of Zn2+ and Mg2+
The fluorescent properties of chemosensor QN62 were investigated in a series of solvents, including acetonitrile, ethanol, DMF, and DMSO. In the above solvents, QN62 exhibited very weak fluorescence emissions (Fig. S5). In the designed QN62 molecule, the combination of 6-position phenolic hydroxyl and 2-position acylhydrazone of naphthalene synergistically created a push–pull system, which possessed ICT features (Kim and Kim, 2021) and could be visualized by density functional theory (DFT) calculations, as presented later. Simultaneously, the isomerization of the -C⚌N- unit in the molecule easily underwent nonradiative deactivation of the excited states (Sivakumar and Lee, 2021). Thus, weak fluorescence was possibly caused by the synergistic contribution of C⚌N isomerization and the ICT process. In the above organic solvents, the fluorescence behavior of QN62 toward common metal ions, including Ag+, Al3+, Ca2+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Fe3+, Hg2+, K+, Mg2+, Na+, Ni2+, Zn2+ and Pb2+ was measured. A dramatic enhancement in the fluorescence signals was observed only at 532 nm with the addition of Zn2+ in DMSO-H2O (4:1, v/v) media, and at 527 nm with Mg2+ in ethanol-H2O (9:1, v/v) solution (Fig. 2). With the addition of 1.0 equiv. Zn2+ and Mg2+ ions to the respective analytical systems, the enhancements in QN62 emission intensity were nearly 10-fold and 70-fold, respectively, and the fluorescence quantum yield also increased from 0.013 to 0.11 and 0.43. Other tested ions did not produce any obvious changes in the fluorescence emissions of QN62 under identical conditions. Interfering congeners Cd2+/Ca2+ exhibited minimal emission in the respective analyte system of Zn2+/Mg2+. Simultaneously, the colorless QN62 solution changed to yellowish-green/yellow in the presence of Zn2+/Mg2+ under irradiation of the 365 nm UV lamp, which was easily visible to the naked eye (Fig. 2. inset). These indicated that QN62 could serve as an excellent fluorescent probe for Zn2+ and Mg2+ by switching solvents. The enhanced fluorescence response of QN62 toward Zn2+/Mg2+ was possibly due to the interactions of the metal ions with the binding sites, which increased the electron withdrawal ability of the coordinating groups, and this was further beneficial for the ICT process (Sivakumar and Lee, 2021; Hossain, et al., 2017). The restriction of -C⚌N- isomerization also played an essential role in the fluorescence enhancement process (Wu, et al., 2018).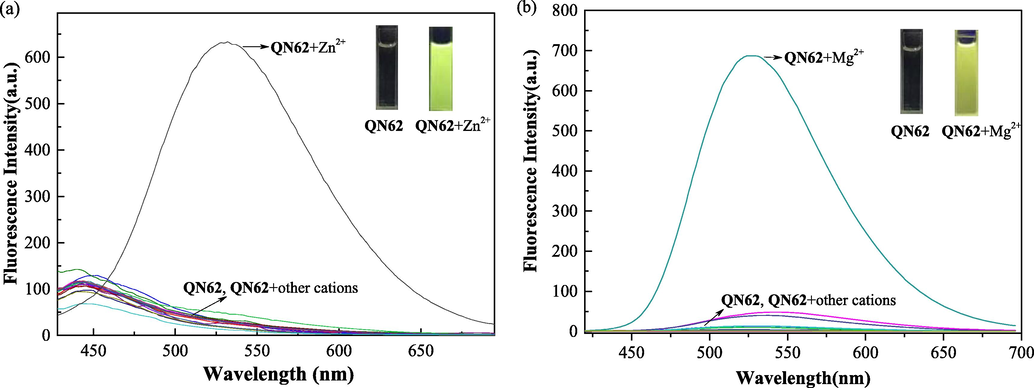
(a) Fluorescence responses of QN62 (20 μM) with different metal ions (20 μM) in DMSO-H2O (4:1, v/v) (λex = 400 nm). (b) Fluorescence responses of QN62 (20 μM) with different metal ions (20 μM) in ethanol-H2O (9:1, v/v) (λex = 385 nm). Inset: images of QN62 before and after addition of Zn2+/Mg2+ under 365 nm UV-light.
The response time of QN62 to detect Zn2+/Mg2+ was studied. As shown in Fig. S6, the fluorescence intensity of QN62 treated with Zn2+/Mg2+ rapidly reached saturation within 2 min and then remained unchanged for 30 h. This performance could potentially provide a real-time detection method for Zn2+/Mg2+ ions.
Due to the high fluorescence sensing ability of QN62 toward Zn2+/Mg2+ in DMSO/ethanol solution, the UV absorption spectral response of QN62 before and after Zn2+/Mg2+ addition was further investigated (Fig. 3). The QN62 compound displayed a broad absorption band centered at 343 nm in DMSO-H2O (4:1, v/v) and ethanol-H2O (9:1, v/v) solutions, and the longer wavelength absorption band of QN62 could be assigned to π-π* and n-π* charge transfer (CT) transitions (Djouhra, et al., 2017; Xie, et al., 2018). Upon the addition of Zn2+/Mg2+, the absorption band at 343 slightly decreased, accompanied by new bands at 400 nm for Zn2+ and 386 nm for Mg2+, indicating the coordination reaction between QN62 and Zn2+/Mg2+. After adding Zn2+/Mg2+, the red shifts of the absorption peaks of compound QN62 were due to intramolecular charge transfer (Purkait, et al., 2019).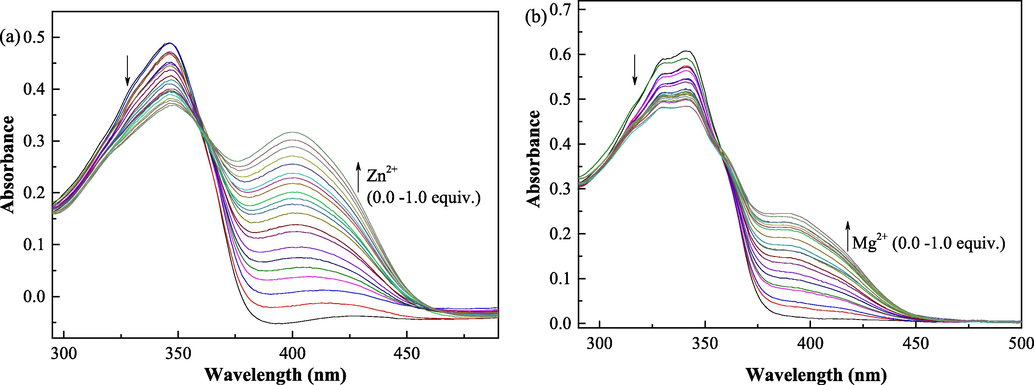
(a) Change in the absorption spectral of QN62 (20 μM) upon addition of Zn2+ (0–20 μM) in DMSO-H2O (4:1, v/v). (b) Change in the absorption spectral of QN62 (20 μM) upon addition of Mg2+ (0–20 μM) in ethanol-H2O (9:1, v/v).
To extend the practical applicability of compound QN62, we investigated the fluorescence response of compound QN62 before and after Zn2+/Mg2+ addition at various pH values (Fig. S7). The experimental results showed that the fluorescence intensity of QN62 did not change under acidic, neutral, or alkaline conditions. However, after adding Zn2+/Mg2+, the chemosensor QN62 displayed notable emission enhancement under neutral and weakly alkaline conditions (pH 6–10 for Zn2+, pH 6–9 for Mg2+). In neutral and weakly alkaline conditions, the deprotonation process of QN62 enhanced the electron-donating ability of phenolic O−, producing a stronger binding affinity toward Zn2+/Mg2+ ions (Khan, et al., 2022). A pH value of 7.0 was selected as the experimental condition, which was within the biologically relevant pH range (5.5–7.5) (Shi, et al., 2013; Xu, et al., 2017).
To determine the sensitivity of the QN62 chemosensor for Zn2+/Mg2+, fluorometric titration was carried out (Fig. 4). The fluorescence intensity increased with a gradual increase in Zn2+/Mg2+ concentration and was saturated at 1.0 equiv. Zn2+/Mg2+. Moreover, the fluorescence intensities at 532 nm and 527 nm showed a good linear relationship with the Zn2+ and Mg2+ concentrations in the range of 0.0–1.0 equiv. (Fig. S8). These results indicated that the QN62 chemosensor could provide a potential method for the quantitative determination of Zn2+ and Mg2+. The binding constant (K) values of the complexes were calculated to be 8.29 × 104 M−1 for Zn2+ and 6.34 × 104 M−1 for Mg2+ (Fig. S9), which were comparable to previously reported probes for Zn2+ and Mg2+ (Dhara, et al., 2016; Sun, et al., 2020). The large binding constant values suggested QN62-Zn2+/QN62-Mg2+ complex formation (Jana, et al., 2016). The LOD values were calculated to be 32.3 nM for Zn2+ and 16.1 nM for Mg2+, which were low compared with the reported Zn2+/Mg2+ fluorescent probe (Table 1). Meanwhile, the LOQ values were also estimated to be 97.8 nM for Zn2+ and 48.9 nM for Mg2+. The values of LOD and LOQ were below the WHO recommended limit of Zn2+ (5 mg/L) and Mg2+ (30 mg/L) for drinking water and were lower than the total Zn2+ concentrations (200–300 μM) and Mg2+ concentrations (200–1000 μM) in human cells (Wang, et al., 2021; Kumar and Puri, 2012; Maret, 2015; Farruggia, et al., 2006).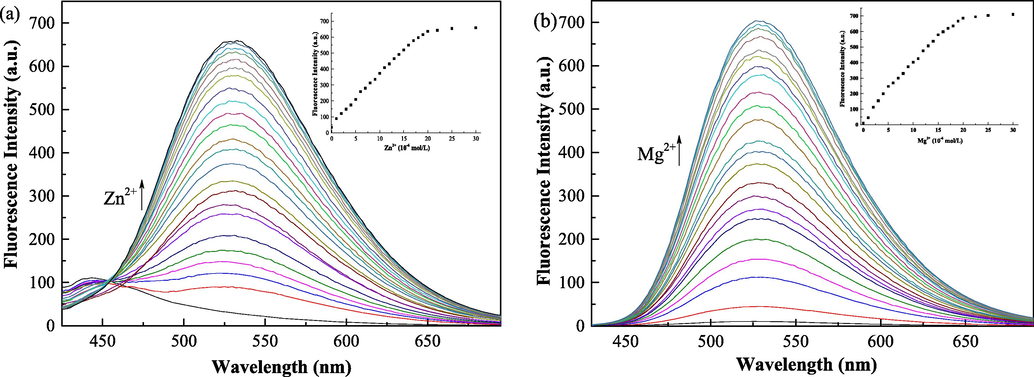
(a) Emission spectral changes of compound QN62 (20 μM) with increasing concentration of Zn2+: 0–30 μM in DMSO-H2O (4:1, v/v). (b) Emission spectral changes of compound QN62 (20 μM) with increasing concentration of Mg2+: 0–30 μM in ethanol-H2O (9:1, v/v).
Structure of chemosensor
Tested Media
Excitation/ Emission(nm)
LOD (nM)
Ref.
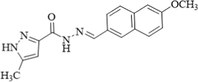
Acetonitrile/H2O tris-HCl, (7:3, v/v)
370/456 (Zn) 370/472 (Mg)
220 (Zn) 390 (Mg)
(Alam, et al., 2014)
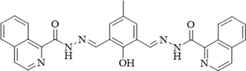
DMF/H2O(9:1, v/v, PBS buffer, pH7.4) (Zn) Acetonitrile (Mg)
460/536 (Zn) 350/560 (Mg)
307 (Zn) 297 (Mg)
(Dhara, et al., 2016)
H2O
323/478 (Zn) 323/458 (Mg)
59.4 (Zn) 89.1 (Mg)
(Patil, et al., 2018)
DMF/H2O (1:4, v/v) (Zn) DMF (Mg)
450/558 (Zn) 350/430 (Mg)
53 (Zn) 33 (Mg)
(Li, et al., 2016)
DMSO-H2O (4:1, v/v) (Zn) ethanol-H2O (9:1, v/v) (Mg)
400/538 (Zn) 385/527 (Mg)
45.1 (Zn) 14.7 (Mg)
This work
The selectivity of QN62 for Zn2+/Mg2+ mixed with other metal ions was examined by fluorescent competition experiments. As illustrated in Fig. S10a, except for Cu2+, Co2+, Fe3+, Al3+, and Cr3+, the coexistence of other competitive ions showed minimal interference on the fluorescence intensity of the QN62-Zn2+ complex in DMSO-H2O (4:1, v/v). The strong Lewis acidity of Al3+, Cr3+, and Fe3+ reduced the binding affinity of imine and phenolic hydroxyl groups towards the Zn2+ ion. In the case of Cu2+ and Co2+, the high coordination affinity and their inherent magnetic properties resulted in a decrease in fluorescence emission intensity at 532 nm (Gao, et al., 2020). Although Co2+, Fe3+, Al3+, and Cr3+ could cause interference with Zn2+ recognition, QN62 still displayed an obvious enhancement of fluorescence intensity, which could effectively sense the target ion. When QN62 acted as a magnesium ion in the fluorescent probe (Fig. S10b), the coexistence of Cu2+, Co2+, Ni2+, Al3+, Cr3+, and Fe3+ caused significant interference for the QN62-Mg2+ complex. Fluorescence quenching was possibly due to the strong complexation abilities or paramagnetic quenching properties of the interfering ions or high Lewis acidity (Wang, et al., 2021; Gao, et al., 2020; Zhou, et al., 2010; Komatsu, et al., 2007; Erdemir and Kocyigit, 2016). Additionally, we attempted to find the lowest concentration of interfering ions that would have a minimal influence on the determination of Zn2+/Mg2+. The experimental results were illustrated in Figs. S10c and Fig. S10d.
3.3 Binding studies
The sharp changes in the optical response of QN62 before and after Zn2+/Mg2+ addition revealed the formation of new species, QN62-Zn2+ and QN62-Mg2+ complexes. Because QN62 contained imine N, quinoline N, carbamoyl O, and phenolic hydroxyl O moieties, QN62 could utilize these binding sites to coordinate with Zn2+/Mg2+. To explore the binding stoichiometry of QN62 with Zn2+/Mg2+, the Job’s plot based on the emission intensity was studied (Fig. S11). When the value of [M2+]/[M2+ + QN62] was 0.5, the fluorescence intensity reached a maximum, indicating the formation of 1:1 stoichiometric complexes between QN62 and Zn2+/Mg2+. The stoichiometry of the complexes was further confirmed by the HR-MS spectral technique. The molecular ion peak m/z values of the QN62-Zn2+ and QN62-Mg2+ complexes were calculated to be 404.0377 and 364.0936, respectively, against experimental values of 404.0381 [QN62 + Zn2+ - H]+ and 364.1054 [QN62 + Mg2+ - H]+ (Fig. S12).
1H NMR titration experiments were performed in DMSO‑d6 to understand the binding mode of the QN62 compound toward Zn2+ (Fig. 5). With the gradual addition of Zn2+ ions, the amide –NH-C(=O) signal (Hb) of QN62 at 10.148 ppm slowly disappeared, while a new sharp signal at 9.372 ppm appeared, indicated that the amide converted to the enol-imine tautomeric form. With the addition of 1.0 equiv. of Zn2+, the proton signals of phenolic hydroxyl (Ha) at 12.202 ppm and enol (He) at 9.372 ppm disappeared, suggesting that deprotonation of the phenolic hydroxyl and enol in the amide groups occurred when the QN62 compound coordinated with Zn2+. The upfield signal shift of naphthalene hydrogens was possibly related to the increase in electron density caused by the deprotonation of the phenolic hydroxyl group (Dhara, et al., 2016). In addition, the peak of the imine (Hc) shifted upfield from 9.807 to 9.057 ppm, which could be possibly explained by the ICT effect (Kim and Kim, 2021). However, the signal at 9.017 ppm corresponding to the 2′-position of quinoline (Hd) shifted to 9.155 and the other protons of quinoline also underwent a different extent downfield shift, implying that quinoline N was involved in the coordination reaction between QN62 and Zn2+. These results indicated that QN62 could chelate with Zn2+ through expected binding sites. Considering that enolic O1 and phenolic hydroxyl O2 were located at the 2- and 6-positions of the naphthalene ring, deprotonated enolic O1 and phenolic hydroxyl O2 in one ligand molecule could not be coordinated to the same zinc ion center. The proposed binding mode of the QN62 probe with Zn2+ was shown in Scheme 2. Zinc ions was coordinated with two N atoms (quinoline N1, imine N2) and one O atom (deprotonated enolic O1) from one QN62 ligand molecule, and one deprotonated phenolic hydroxyl O2i of the adjacent other QN62 ligand.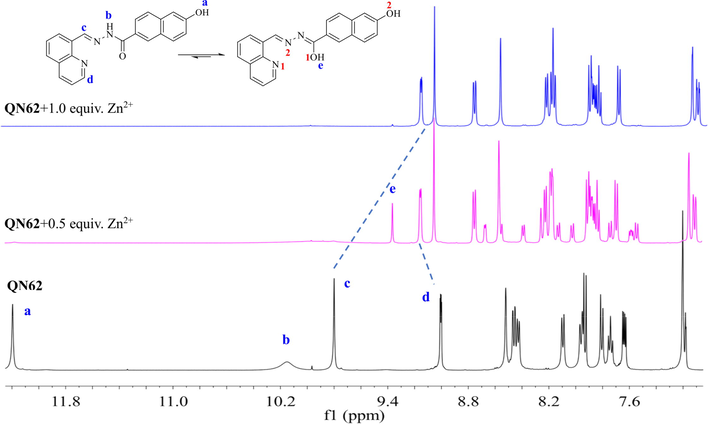
1H NMR titration of QN62 with Zn2+ in DMSO‑d6.
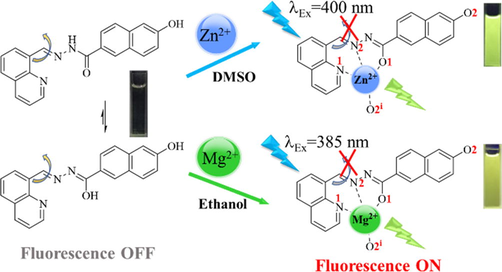
Plausible binding mode of QN62 for Zn2+ and Mg2+.
To further confirm the binding mode between QN62 and Zn2+/Mg2+, optimized structures of QN62-Zn2+ and QN62-Mg2+ were obtained by DFT calculations at the B3LYP/6-31G+ (d,p) level using the Gaussian 16 program (Becke, 1993; Frisch, et al., 2016). In the calculation process, the PCM model was adopted to consider the solvent effect of DMSO and ethanol (Tomasi, et al., 2005). As shown in Fig. 6a, the zinc or magnesium atoms in the optimized complexes coordinated with the deprotonated enol oxygen, imine nitrogen, and nitrogen of quinoline provided by one ligand molecule, and the oxygen of the deprotonated phenolic hydroxyl group of the adjacent ligand, resulting in a distorted tetrahedral coordination geometry. The distances of the coordination bond in the two complexes were in the range of 1.98–2.14 Å, which was comparable to previously reported coordination bonds (Withersby, et al., 1999; Bock, et al., 1999; Ezzayani, et al., 2022).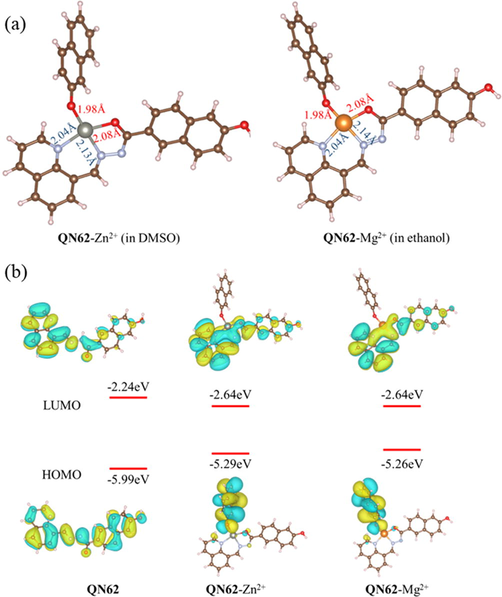
(a) Optimized structures of the complexes QN62-Zn2+ and QN62-Mg2+. (b) Frontier molecular orbitals of QN62, QN62-Zn2+ and QN62-Mg2+.
As indicated by the frontier molecular orbital diagrams shown in Fig. 6b, the enol form of QN62 had nearly a planar conformation, which could provide π-conjugation and ICT from the donor part (naphthalene ring) to the acceptor part (quinoline unit and acylhydrazone unit) (Li, et al., 2016). In compound QN62, the π-electrons of the HOMO were localized over almost the entire molecule, while the π-electrons of the LUMO were restricted to the quinoline unit and the coordination sites. Thus, QN62 possessed ICT characteristics from the naphthalene ring to the coordination sites. In the energy-optimized structures of QN62-Zn2+ and QN62-Mg2+, the distribution of π-electrons of HOMO and LUMO were very similar. In the complexes, the π-electrons of HOMO and LUMO were distributed mainly over the donor moiety and the acceptor part, respectively, indicating that the complexes possessed more obvious ICT characteristics than the free QN62 probe. These results showed that the binding of Zn2+/Mg2+ to the ligand further encouraged the ICT process by increasing the electron withdrawal ability of the acceptor part (Hossain, et al., 2017). When Zn2+/Mg2+ coordinated with QN62, the energy gaps decreased, corresponding to the red shift of the UV–vis absorption bands, and the theoretical calculations supported the originally designed concept.
Based on the experimental results and theoretical calculations, we speculated on the response mechanism of QN62 for Zn2+/Mg2+ (Scheme 2). Before the addition of Zn2+/Mg2+, the weak fluorescence of the QN62 chemosensor was due to the synergistic contribution of the C⚌N isomerization and the ICT process. With the introduction of Zn2+/Mg2+ to the QN62 system, the binding sites participated in the coordination of QN62 with Zn2+/Mg2+, which further promoted the ICT process and prevented C⚌N isomerization, resulting in a significant fluorescence enhancement.
3.4 Reversibility of the chemosensor QN62
The reversibility of the detection process was further evaluated by alternating the addition of Zn2+/Mg2+ and ethylenediaminetetraacetic acid (EDTA) in the QN62 solutions (Fig. 7). Upon the addition of EDTA to the QN62-Zn2+/QN62-Mg2+ sensing solutions, the fluorescence intensity displayed a significant decrease and approached its original value, suggesting that EDTA could complex Zn2+/Mg2+ from the complexes and release free QN62. With the re-addition of Zn2+/Mg2+, the emission signals recovered, and this reversible cycle was repeated five times with a small emission signal decay. The results indicated that QN62 served as a reversible chemosensor for Zn2+/Mg2+ detection.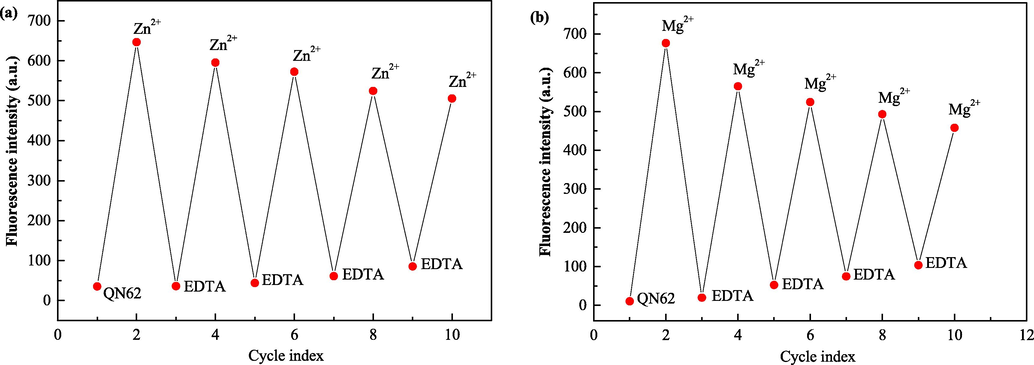
(a) Reversible changes in fluorescence intensity of QN62- (20 μM) at 532 nm upon alternate addition of Zn2+ and EDTA in DMSO-H2O (4:1, v/v). (b) Reversible changes in fluorescence intensity of QN62 (20 μM) at 527 nm upon alternate addition of Mg2+ and EDTA in ethanol-H2O (9:1, v/v).
3.5 Application of chemosensor QN62 in test strip, water samples and cell imaging
To expand the potential practicability of QN62, we attempted to recognize Zn2+ and Mg2+ using silica-gel plates. Silica-gel plates were prepared by immersion in QN62 solution for a few seconds and then air-dried. Subsequently, the plates were successively treated with Zn2+ or Mg2+ by using the micro-capillary. We found that silica-gel plates immersed with QN62 displayed no fluorescence under UV light (Fig. 8). After micro-capillary treatment with Zn2+ or Mg2+, the silica-gel plates showed yellowish-green or yellow fluorescence. These findings suggested that the silica-gel plates made from QN62 could provide a convenient device for the on-site monitoring of Zn2+ and Mg2+.
Photograph of the silica-gel plates containing QN62 for the detection of Zn2+ and Mg2+ under UV light (365 nm).
To evaluate the applicability of the QN62 compound in the samples, we carried out recovery tests using water samples from the campus area. Different concentrations of Zn2+/Mg2+ were added to water samples containing QN62. The experimental results showed that all the samples had acceptable recoveries with low standard deviation (SD) values, which illustrated the practical application of the QN62 chemosensor for detecting Zn2+ and Mg2+ in water samples (Table 2).
Source of samples
Zn2+
Mg2+
Added (μM)
Found (μM)
Recovery (%) ± SD
Added (μM)
Found (μM)
Recovery (%) ± SD
Tap water
5.00
4.84
96.80 ± 4.54
5.00
5.29
105.80 ± 3.30
10.00
9.88
98.80 ± 2.94
10.00
10.16
101.60 ± 2.71
15.00
15.14
100.93 ± 2.50
15.00
15.10
100.67 ± 1.88
Bottled water
5.00
4.94
98.80 ± 3.26
5.00
5.35
107.00 ± 3.19
10.00
9.85
98.50 ± 2.16
10.00
10.31
103.10 ± 2.38
15.00
14.88
99.20 ± 2.05
15.00
15.33
102.20 ± 1.26
Cell imaging experiments were carried out to investigate whether QN62 could detect intracellular Zn2+ and Mg2+, and MCF-7 cells were selected. As shown in Fig. 9, the cells treated with free QN62 (10 μM) showed negligible fluorescence. After incubation with Zn2+/Mg2+ (10 μM), green fluorescence was observed, and the merged images showed that the fluorescence signal was located in the intracellular region. The cell imaging results suggested that the QN62 chemosensor could sense intracellular Zn2+ and Mg2+.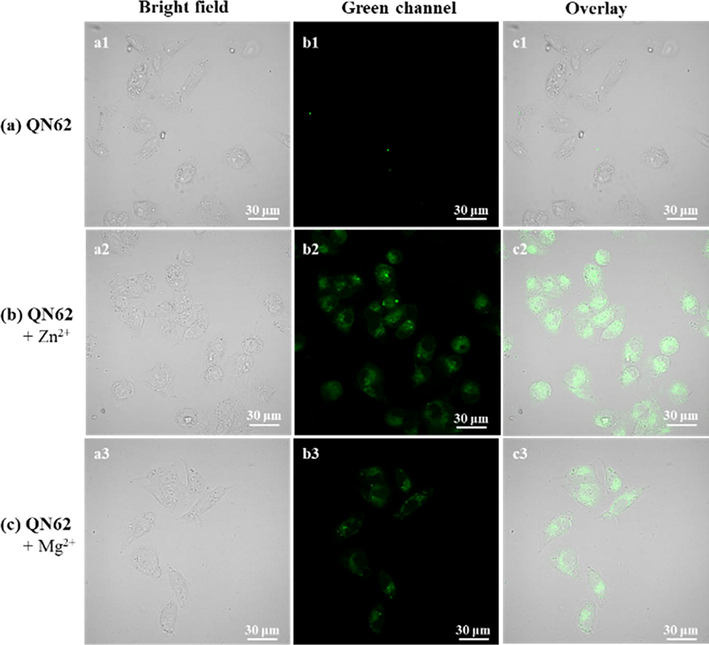
Bioimaging of exogenous Zn2+ and Mg2+ in MCF-7 cells with QN62 (10 μM). (a) Cells stained with QN62 for 30 min. (b) Cells treated with QN62 and then with 10 μM Zn2+ for 30 min. (c) Cells treated with QN62 and then with 10 μM Mg2+ for 30 min. a1-a3: Bright field; b1-b3: Green channel; c1-c3: Merged images. Green channel: λex = 405 nm and collection: 500–550 nm.
4 Conclusions
In this study, a new dual-functional chemosensor QN62 based on 6-hydroxy-2-naphthoic hydrazide moieties was successfully designed, synthesized, and characterized by standard techniques and single-crystal X-ray crystallography. QN62 exhibited simultaneous turn-on fluorescence for Zn2+ in DMSO-H2O (4:1, v/v) solution and Mg2+ in ethanol-H2O (9:1, v/v) solution. Moreover, the recognition progress induced a rapid color change from colorless to yellowish-green or yellow under UV light (365 nm), which was easily visible to the naked eye. After the addition of Zn2+ or Mg2+ to the corresponding detection system, the fluorescence enhancement response was observed, which could be associated with the coordination of target ions, promoting intramolecular charge transfer (ICT) and preventing C⚌N isomerization. The limits of detection of QN62 for Zn2+ (32.3 nM) and Mg2+ (16.1 nM) were sufficiently low compared to recent literature reports. The detection mechanism was reasonably speculated based on analysis of the Job’s plot, HR-MS, 1H NMR, and theoretical calculations. The reversibility experiments indicated that QN62 could be recycled simply through EDTA treatment. Visual and rapid identification of Zn2+ and Mg2+ was successfully achieved utilizing silica-gel plates made from the QN62 chemosensor. The practical applicability of the QN62 chemosensor in real sample analysis was validated by the addition of Zn2+/Mg2+ in water samples. Furthermore, the QN62 chemosensor could be effectively used for the bioimaging of Zn2+ and Mg2+ in cells. Based on these results, the QN62 chemosensor could be used as an addendum in the field of multi-analyte solvent-dependent sensing.
Acknowledgements
The authors greatly acknowledge the National Natural Science Foundation of China (Grant No. 81803388, No. 31902419, No. 21701203) and the Key Scientific and Technological Project of Zhoukou City (2021GG01011) for the financial support of our work. We thank LetPub (http://www.letpub.com) for linguistic assistance during the preparation of this manuscript, and thank Dr. Yali Cui for the assistance in cell imaging.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Weiwu Song, Zengchen Liu, GuangLu Liu reports financial support was provided by the National Natural Science Foundation of China. Chunxiang Zhao reports administrative support was provided by the Key Scientific and Technological Project of Zhoukou City.
References
- Irreversible coumarin based fluorescent probe for selective detection of Cu2+ in living cells. Spectrochim. Acta, Part A. 2022;264:120313
- [CrossRef] [Google Scholar]
- A novel chromo- and fluorogenic dual sensor for Mg2+ and Zn2+ with cell imaging possibilities and DFT studies. Analyst. 2014;139:4022-4030.
- [CrossRef] [Google Scholar]
- ESIPT blocked CHEF based differential dual sensor for Zn2+ and Al3+ in a pseudo-aqueous medium with intracellular bio-imaging applications and computational studies. RSC Adv.. 2016;6:1268-1278.
- [CrossRef] [Google Scholar]
- Differentially selective chemosensor with fluorescence off-on responses on Cu2+ and Zn2+ ions in aqueous media and applications in pyrophosphate sensing, live cell imaging, and cytotoxicity. Inorg. Chem.. 2014;53:6655-6664.
- [CrossRef] [Google Scholar]
- Multi-functional colorimetric chemosensor for naked eye recognition of Cu2+, Zn2+ and Co2+ using new hybrid azo-pyrazole/pyrrolinone ester hydrazone dye. Dyes Pigm.. 2021;196
- [CrossRef] [Google Scholar]
- Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys.. 1993;98:5648-5652.
- [CrossRef] [Google Scholar]
- Manganese as a replacement for magnesium and zinc functional comparison of the divalent ions. J. Am. Chem. Soc.. 1999;121:7360-7372.
- [CrossRef] [Google Scholar]
- Brandenburg, K, 2012. DIAMOND. Visual crystal structure information System. Version 3.2i; Crystal impact. Bonn, Germany. http://www.crystalimpact.com.
- Dipyrromethene chromo-fluorogenic chemosensors for quantitative detection and express analysis of Zn2+ ions. J. Mol. Liq.. 2022;345
- [CrossRef] [Google Scholar]
- Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464-1467.
- [CrossRef] [Google Scholar]
- A novel pyrazole based single molecular probe for multi-analyte (Zn2+ and Mg2+) detection in human gastric adenocarcinoma cells. RSC Adv.. 2016;6:105930-105939.
- [CrossRef] [Google Scholar]
- A novel quinoline derivative as dual chemosensor for selective sensing of Al3+ by fluorescent and Fe2+ by colorimetric methods. J. Mol. Struct.. 2021;1231
- [CrossRef] [Google Scholar]
- A selective naked-eye chemosensor derived from 2-methoxybenzylamine and 2,3-dihydroxybenzaldehyde - synthesis, spectral characterization and electrochemistry of its bis-bidentates Schiff bases metal complexes. Spectrochim. Acta, Part A. 2017;184:299-307.
- [CrossRef] [Google Scholar]
- A simple quinolone Schiff-base containing CHEF based fluorescence 'turn-on' chemosensor for distinguishing Zn2+ and Hg2+ with high sensitivity, selectivity and reversibility. Dalton Trans.. 2017;46:6769-6775.
- [CrossRef] [Google Scholar]
- Anthracene excimer-based “turn on” fluorescent sensor for Cr3+ and Fe3+ ions: Its application to living cells. Talanta. 2016;158:63-69.
- [CrossRef] [Google Scholar]
- Application of a new synthesized magnesium porphyrin complex in the degradation of methylene blue dye. J. Mol. Struct.. 2022;1258
- [CrossRef] [Google Scholar]
- 8-Hydroxyquinoline derivatives as fluorescent sensors for magnesium in living cells. J. Am. Chem. Soc.. 2006;128:344-350.
- [CrossRef] [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.V.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J.A., Jr.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.J.; Heyd, J.J.; Brothers, E.N.; Kudin, K.N.; Staroverov, V.N.; Keith, T.A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.P.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Fox, D.J., 2016. Gaussian 16, Revision C.01, Gaussian, Inc.: Wallingford CT.
- A quinoline-based chromogenic and ratiometric fluorescent probe for selective detection of Mg2+ ion: Design, synthesis and its application in salt lake brines and bioimaging. Dyes Pigm.. 2021;185
- [CrossRef] [Google Scholar]
- Quinoline-based hydrazone for colorimetric detection of Co2+ and fluorescence turn-on response of Zn2+. Spectrochim. Acta, Part A. 2020;230:118025
- [CrossRef] [Google Scholar]
- Geng, R., Tang, H., Ma, Q., Liu, L., Feng, W., Zhang, Z., 2022. Bimetallic Ag/Zn-ZIF-8: An efficient and sensitive probe for Fe3+ and Cu2+ detection. Colloids. Surf., A 632. https://doi.org/10.1016/j.colsurfa.2021.127755.
- A benzothiazole containing CHEF based fluorescence turn-ON sensor for Zn2+ and Cd2+ and subsequent sensing of H2PO4− and P4O74− in physiological pH. Sens. Actuators, B. 2014;202:788-794.
- [CrossRef] [Google Scholar]
- Turn-on, photostable, nontoxic and specific, iron(II) sensor. Spectrochim. Acta, Part A. 2022;265
- [CrossRef] [Google Scholar]
- Remarkable difference in Al3+ and Zn2+ sensing properties of quinoline based isomers. Dalton Trans.. 2018;47:13972-13989.
- [CrossRef] [Google Scholar]
- A schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al3+. Sens. Actuators, B. 2017;239:1109-1117.
- [CrossRef] [Google Scholar]
- A turn-on fluorescent chemosensor based on acylhydrazone for sensing of Mg2+ with a low detection limit. RSC Adv.. 2017;7:29697-29701.
- [CrossRef] [Google Scholar]
- A photoswitchable “turn-on” fluorescent chemosensor: Quinoline-naphthalene duo for nanomolar detection of aluminum and bisulfite ions and its multifarious applications. Food Chem.. 2022;371:131130
- [CrossRef] [Google Scholar]
- Deciphering the CHEF-PET-ESIPT liaison mechanism in a Zn2+ chemosensor and its applications in cell imaging study. New J. Chem.. 2016;40:5976-5984.
- [CrossRef] [Google Scholar]
- A functionalized fluorochrome based on quinoline-benzimidazole conjugate: from facile design to highly sensitive and selective sensing for picric acid. Dyes Pigm.. 2019;162:367-376.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and applications of schiff base chemosensor for determination of Cu2+ ions. J. Saudi Chem. Soc.. 2022;26
- [CrossRef] [Google Scholar]
- Synthesis, Characterization and Applications of Schiff Base Chemosensor for Determination of Cr(III) Ions. J. Fluoresc.. 2022;32:1889-1898.
- [CrossRef] [Google Scholar]
- Color-tunable indolizine-based fluorophores and fluorescent pH sensor. Molecules. 2021;27
- [CrossRef] [Google Scholar]
- Carbazole-based Schiff base: A sensitive fluorescent ‘turn-on’ chemosensor for recognition of Al(III) ions in aqueous-alcohol media. Arabian J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Development of an iminocoumarin-based zinc sensor suitable for ratiometric fluorescence imaging of neuronal zinc. J. Am. Chem. Soc. 2007
- [CrossRef] [Google Scholar]
- A review of permissible limits of drinking water. Indian J. Occup. Environ. Med.. 2012;16:40-44.
- [CrossRef] [Google Scholar]
- Sensing mechanism for a fluorescent off-on chemosensor for cyanide anion. J. Lumin.. 2016;179:203-210.
- [CrossRef] [Google Scholar]
- A dual-function probe based on naphthalene for fluorescent turn-on recognition of Cu2+ and colorimetric detection of Fe3+ in neat H2O. Spectrochim. Acta, Part A. 2019;210:266-274.
- [CrossRef] [Google Scholar]
- A single chemosensor for multiple analytes: Fluorogenic and ratiometric absorbance detection of Zn2+, Mg2+ and F−, and its cell imaging. Sens. Actuators, B. 2016;226:279-288.
- [CrossRef] [Google Scholar]
- A novel acylhydrazone-based derivative as dual-mode chemosensor for Al3+, Zn2+ and Fe3+ and its applications in cell imaging. Sens. Actuators, B. 2017;244:914-921.
- [CrossRef] [Google Scholar]
- A molecular dual fluorescence-ON probe for Mg2+ and Zn2+: higher selectivity towards Mg2+ over Zn2+ in a mixture. J. Lumin.. 2014;155:21-26.
- [CrossRef] [Google Scholar]
- Modulation of fluorescence sensing properties of quinoline-based chemosensor for Zn2+: application in cell imaging studies. J. Lumin.. 2019;210:508-518.
- [CrossRef] [Google Scholar]
- Analyzing free zinc(Ⅱ) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics. 2015;7:202-211.
- [CrossRef] [Google Scholar]
- Highly selective tridentate fluorescent probes for visualizing intracellular Mg2+ dynamics without interference from Ca2+ fluctuation. Chem. Commun.. 2017;53:10644-10647.
- [CrossRef] [Google Scholar]
- New stilbene-biscarbothioamide based colorimetric chemosensor and turn on fluorescent probe for recognition of Hg2+ cation. J Photochem. Photobiol.. 2022;A 433
- [CrossRef] [Google Scholar]
- Mohanasundaram, D., Bhaskar, R., Gangatharan Vinoth Kumar, G., Rajesh, J., Rajagopal, G., 2021. A quinoline based Schiff base as a turn-on fluorescence chemosensor for selective and robust detection of Cd2+ ion in semi-aqueous medium. Microchem. J. 164. https://doi.org/10.1016/j.microc.2021.106030
- Soil organic carbon buffers heavy metal contamination on semiarid soils: effects of different metal threshold levels on soil microbial activity. Eur. J. Soil Biol.. 2009;45:220-228.
- [CrossRef] [Google Scholar]
- A simple quinoline-thiophene Schiff base turn-off chemosensor for Hg2+ detection: spectroscopy, sensing properties and applications. Spectrochim. Acta, Part A. 2022;264:120338
- [CrossRef] [Google Scholar]
- Monoterpenoid derivative based ratiometric fluorescent chemosensor for bioimaging and intracellular detection of Zn2+ and Mg2+ ions. J. Photochem. Photobiol. A. 2018;364:758-763.
- [CrossRef] [Google Scholar]
- Switching-on fluorescence by copper(Ⅱ) and basic anions: a case study with a pyrene-functionalized squaramide. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- An azine-based carbothioamide chemosensor for selective and sensitive turn-on-off sequential detection of Zn(II) and H2PO4-, live cell imaging and INHIBIT logic gate. Spectrochim. Acta, Part A. 2019;207:164-172.
- [CrossRef] [Google Scholar]
- Dual chemosensors for metal ions: a comprehensive review. Trends Anal. Chem.. 2021;138
- [CrossRef] [Google Scholar]
- Fluorescence quantum yields methods of determination and standards. Springer Ser. Fluoresc.. 2008;5:101-145.
- [CrossRef] [Google Scholar]
- An “Off-On-Off” type fluorescent chemosensor for the relay detection of Zn2+ and H2PO4- in aqueous environment. Inorg. Chim. Acta. 2020;502
- [CrossRef] [Google Scholar]
- SHELXL-97. Program for Crystal Structure Refinement: University of Göttingen, Germany; 1997.
- A highly selective fluorescence “turn-on” probe for Cu(II) based on reaction and its imaging in living cells. Inorg. Chem.. 2013;52:12668-12673.
- [CrossRef] [Google Scholar]
- Novel quinoline-based thiazole derivatives for selective detection of Fe3+, Fe2+, and Cu2+ ions. ACS Omega. 2020;5:27245-27253.
- [CrossRef] [Google Scholar]
- Photochemical processing potential of a novel Schiff base as a fluorescent probe for selective monitoring of Al3+ ions and bioimaging in human cervical cancer HeLa cells. J. Photochem. Photobiol.. 2022;A:424.
- [CrossRef] [Google Scholar]
- Paper-based fluorescence chemosensors for metal ion detection in biological and environmental samples. BioChip J.. 2021;15:216-232.
- [CrossRef] [Google Scholar]
- A novel dual-function probe for recognition of Zn2+ and Al3+ and its application in real samples. Spectrochim. Acta, Part A. 2020;228:117786
- [CrossRef] [Google Scholar]
- Quantum mechanical continuum solvation models. Chem. Rev.. 2005;105:2999-3093.
- [CrossRef] [Google Scholar]
- Changes in zinc speciation in field soil after contamination with zinc oxide. Environ. Sci. Technol.. 2005;39:6616-6623.
- [CrossRef] [Google Scholar]
- A fast-response turn-on quinoline-based fluorescent probe for selective and sensitive detection of zinc(II) and its application. Microchem. J.. 2021;160
- [CrossRef] [Google Scholar]
- A dual functional turn-on non-toxic chemosensor for highly selective and sensitive visual detection of Mg2+ and Zn2+: the solvent-controlled recognition effect and bio-imaging application. Analyst. 2019;144:4024-4032.
- [CrossRef] [Google Scholar]
- Recent studies focusing on the development of fluorescence probes for zinc ion. Coord. Chem. Rev.. 2021;429
- [CrossRef] [Google Scholar]
- A fluorescent sensor for Zn2+ and Cd2+ based on a diarylethene derivative with an indole-2-methylhydrazone moiety. J. Photochem. Photobiol.. 2022;A:424.
- [CrossRef] [Google Scholar]
- Solvent control in the synthesis of 3,6-bis(pyridin-3-yl)-1,2,4,5-tetrazine-bridged cadmium(II) and zinc(II) coordination polymers. Inorg. Chem.. 1999;38:2259-2266.
- [CrossRef] [Google Scholar]
- Quinoline containing acetyl hydrazone: An easily accessible switch-on optical chemosensor for Zn2+. Spectrochim. Acta, Part A. 2018;188:324-331.
- [CrossRef] [Google Scholar]
- A novel highly selective probe with both aggregation-induced emission enhancement and intramolecular charge transfer characteristics for CN- detection. Sens. Actuators, B. 2018;257:154-165.
- [CrossRef] [Google Scholar]
- A fluorescent sensor for selective recognition of Al3+ based on naphthalimide Schiff-base in aqueous media. J. Lumin.. 2017;192:56-63.
- [CrossRef] [Google Scholar]
- Naphthalimide appended isoquinoline fluorescent probe for specific detection of Al3+ ions and its application in living cell imaging. Spectrochim. Acta, Part A. 2022;265:120364
- [CrossRef] [Google Scholar]
- A novel double target fluorescence probe for Al3+/Mg2+ detection with distinctively different responses and its applications in cell imaging. Spectrochim. Acta, Part A. 2021;261:120067
- [CrossRef] [Google Scholar]
- A dual-responsive colorimetric and fluorescent chemosensor based on diketopyrrolopyrrole derivative for naked-eye detection of Fe3+ and its practical application. Spectrochim. Acta, Part A. 2018;189:594-600.
- [CrossRef] [Google Scholar]
- Both visual and fluorescent sensor for Zn2+ based on quinoline platform. Inorg. Chem.. 2010;49:4002-4007.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104603.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







