A facile green approach to the synthesis of Bi2WO6@V2O5 heterostructure and their photocatalytic activity evaluation under visible light irradiation for RhB dye removal
⁎Corresponding authors. muhammad.iftikhar@phys.uol.edu.pk (M.I. Khan), m.aziz@qu.edu.sa (Mahvish Fatima), nmalwadai@pnu.edu.sa (Nourah Alwadai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bismuth tungsten oxide and vanadium pentoxide (Bi2WO6/V2O5) heterostructures are produced by a green synthesis approach using Azadirachta indica extract for photocatalytic performance. The hydrothermal method at temperatures between 120 °C and 140 °C is used to synthesize Bi2WO6. Bi2WO6 and V2O5 phases are formed in pure orthorhombic wells according to the XRD pattern. The SEM displays V2O5 nanorods, Bi2WO6 hierarchical microspheres that resemble flowers at 120 °C, and particles with a particle-like character at 140 °C. In V2O5, the asymmetric stretching vibrations of the triplely coordinated oxygen (chain oxygen) bonds and the vibration of the doubly coordinated oxygen (bridge oxygen) bonds are responsible for a peak at 611 cm−1. In FTIR spectra between 600 and 1600 cm−1, the major absorption bands in Bi2WO6 are attributed to the W-O stretching, Bi-O stretching, and W-O-W bridging stretching modes. Bi2WO6@V2O5 at 120 °C has the lowest bandgap energy (2.32 eV) and optical electronegativity (0.62), as well as the highest refractive index (2.57), extinction coefficient (2.21), and dielectric constant (εr = 0.72 and εi = 11.4) among all samples, making it a suitable material for photocatalysis. Rhodamine blue (RhB) dye degradation is used to measure the photocatalytic activity (PCA) of certain materials. The results showed that heterostructure V2O5@Bi2WO6 synthesized at 120 °C is more attractive among all samples due to high degradation of RhB dye under sunlight irradiation in 90 min.
Keywords
V2O5
Bi2WO6
Dye degradation
Optical properties
Fourier transform infrared spectroscopy
Scanning Electrons Microscopy
1 Introduction
With the economy's rapid expansion, many water pollution problems, especially those involving organic toxins, have gotten worse. Dye is commonly used in the printing, textile, and paper industries. They evolved over time into organic pollutants that must be avoided. There are large differences in the chemistry, consistency, toxicity, and anti-degradation of dye stuffs. If waste water is not sufficiently treated to eliminate them, they will also quickly cause considerable environmental harm. Many biological (aerobic and anaerobic degradation), physical (filters with coagulation, ozonation, adsorption, and precipitation), and chemical approaches (such as reverse osmosis, ion exchange, and advanced oxidation processes) have been employed to address the problem of organic contaminants (Piaskowski et al., 2018; Bal and Thakur, 2022; DUBROW and BOARDMAN, , 1996). Among other methods, photocatalytic destruction of organic compounds is one that is particularly well suited. In the areas of energy and reducing environmental contaminants, photocatalysis has received a lot of attention. Sunlight is converted into chemical energy using this environmentally beneficial energy source. The overview of photocatalysis states that electrons in the valence band (VB) are radiated and moved into the conduction band (CB), whereas the holes are present in VB, generating an electrons-holes pairs when the energy of the sunlight is greater than or equal to the bandgap of the semiconductor. The hole has enough oxygen to oxidize different organic pollutants and create the hydroxyl radical, •OH, in an H2O system. The hydroxyl radical (•OH) in water is generated by the CB's electron interacting with the oxygen in H2O to generate a superoxide radical, which in turn reacts with the OH ion. The hole has the ability to produce an •OH radical in water as well as oxidize a variety of organic pollutants (Liu, 2015). Since this •OH is a potent oxidant, it oxidizes the pollutant's organic molecules to render them inert. The most common substances used as photo catalysts are TiO2 (Lettieri, 2021), g-C3N4 (Wen, 2017), Bi2O3 (Li, 2020), BiVO4 (Sharifi, 2021), and V2O5 (Zhang, 2021). The high melting (681 °C) and boiling points (1,750 °C), yellow color, and band gap energy (Eg) of 2.3 eV make vanadium pentoxide (V2O5), an oxyanion of the transition metal vanadium (Group-5, block-d), an excellent chemical for the degradation of dyes. Lithium-ion assault and battery (Cheah et al., 2012), gas detectors (Schneider et al., 2016), oxidation accelerators (Kim, 2010), electro-chemical capacitances (Dong et al., 2000), and electrochromic devices (Iida et al., 2008) are only a few of the numerous applications for V2O5. V2O5 is being promoted as a potential candidate for the degradation of acetone, Methyl Blue dye hydrocarbon, Methly Orange, and Rhodamine B, among other intriguing practical uses, due to its photocatalytic activity in powder form. Its method is based on the charge of recombination per electron-hole unit. However, the good sensitivity of pure V2O5 nanostructures in the visible light area is limited the quick remixing of photogenerated electrons and holes, which restrict their practical applicability. Researchers have used various tactics to increase its photocatalytic activity, including element doping, heterostructures, morphological modulation, and surface modification (Xie, 2021; Xiang, 2019). The heterostructure approach is considered promising since it minimizes recombination. Proper valence and conduction band edge potentials in hetero-junction devices are crucial to aid the redox reaction in photocatalytic processes. The main factor in the production of reactive species is heterostructure interfaces, which also slow down the rate at which charge carriers recombine (Salari and Kohantorabi, 2021). In the three basic types of heterostructures, i.e., I, II, and III types, band alignment depends on semiconductors' bandgaps and electronic affinities. Due to the greater position of the CB and VB potentials in type 2, type 2 is the most effective at slowing down the rate of recombination (CB). Furthermore, extending photoabsorption demands a narrow band gap, which invariably results in poor redox ability, whereas improving redox ability necessitates a wide band gap, which compromises the light-harvesting capability. Numerous researchers have employed Z-scheme and S-scheme photocatalytic systems to address this problem (Salari and Kohantorabi, 2021; Salari, 2020; Salari and Zahiri, 2022). Inspired by natural photosynthesis, a Z-scheme photocatalytic device with two-step photoexcitation has been presented (see Fig. 1). Z-scheme photocatalysts have stronger redox abilities than typical semiconductor photocatalysts due to the photogenerated electrons' (holes') tendency to remain at the semiconductor layer at a lower VBM (higher CBM) energy level. This allows for a wider spectrum of solar light harvesting. The photocatalytic effectiveness is thereby noticeably increased.
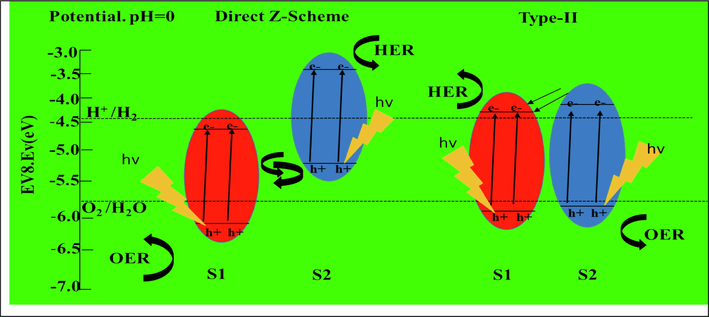
- Direct Z-scheme and type II photocatalysts are shown schematically.
Importantly, band bending at the junction interface is caused by the chemical potential’s difference between 1 and 2 semiconductors. The built-in field created by the band bending causes the photogenerated holes and electrons to move in opposition to one another, causing them to be spatially separated on different sides of heterojunctions (Phuruangrat, 2014). Additionally, it has been discovered that the heterostructures junction area crystal structure plays an important role in improving the quantum efficiency of the photocatalyst. Lattice mismatch most likely results from two semiconductors with different interlayer spacings. Lattice mismatching induced defects at the interfaces could trap photogenerated charges and block the migration of both electrons and holes. However, the electrical structure is also changed by interface strain brought on by the lattice mismatch. The lattice strain might create an electric field, which would increase how well electrons and holes are separated (Najafi-Ashtiani, 2018). Improved photocatalytic activity and water splitting efficiency are achieved by using type-II heterostructures successfully to increase charge separation efficiency. To create heterostructures with V2O5, various semiconductors like P-g-C3N4 (Zhang, 2021), ZnO composites (Aliaga, 2018), MoO3 (Chuai, 2015), and Bi2WO6 have been employed. Bi2WO6 is an excellent material for type 2 heterostructures, with V2O5 being the best of all these. One kind of Aurivillius oxide, Bi2WO6, has a perovskite-like slab of WO6 and (Bi2O2)2 in a layer-like structure. Due to its narrow band gap, it is a severe competitor to the inherent visible light-sensitive photocatalytic system (almost 2.75 eV). It was initially developed for the photodegradation of organic compounds and then used for the evolution of oxygen via water splitting when exposed to visible light (Chung, 2019). Bi2WO6 nanoparticles, nest-like nanoplates, nanocage superstructures, and flower-like structures have been developed and used so far for photocatalytic applications to protect the environment. Hadi Salar developed a heterostructure of Bi2WO6 /Bi2MoO6 and noticed that the degradation of the methyl orange dye under visible light was complete in 20 min (Salari, 2020). By synthesising a heterostructure of Bi2WO6 and MnO2, Hadi Salari and Hadis Yaghmaei were able to degrade the methylene blue dye when exposed to visible light. (Salari and Yaghmaei, 2020).
Numerous techniques, such as sol–gel, co-precipitation, molten salt, solvothermal, and hydrothermal processes, can be used to manufacture V2O5 and Bi2WO6 (Chen, 2021; Liu, 2018). The nanoparticles are made using bio-inspired materials since they are non-toxic. This synthesis technique is environmentally benign because no hazardous substances are used in the process. Plants are viewed as “natural chemical factories,” producing a vast array of metabolites. These are used to stabilize NPs and act as redox mediators. Applying plant components to manufacture nanoparticles is easier and more stable than traditional techniques. This method also avoids toxic substances and is inexpensive, easy to use, and beneficial to the environment (Ahmad, 2022).
The heterostructure of Bi2WO6 and V2O5 is created in the current work using a green way to increase the effectiveness of dye degradation. Although V2O5 has an excellent ability to remove polluting colours from wastewater, a high recombination rate lowers the efficacy of the process. Bi2WO6 is a suitable material for photocatalysis because it has a low recombination rate compared to V2O5 and high electrical characteristics. Due to its low recombination rate, the heterostructure of Bi2WO6 and V2O5 will be very effective against the RhB dye. As diverse morphologies of Bi2WO6 impact the effectiveness of dye degradation, two types of morphologies have been generated here, and heterostructures of these morphologies have been created using V2O5. The literature needs to examine this work. These heterostructures, which are 2D materials, can also be utilised in supercapacitors and batteries.
2 Experimentation
2.1 Synthesis of bismuth tungsten oxide (Bi2WO6)
Bismuth tungsten oxide nanoparticles were synthesized using the green method using Bismuth Nitrate, Azadirachta indica, and Sodium tungsten oxide. 20 g of delicately chopped Azadirachta indica leaves were added to 100 ml of water, boiled for 2 h at 100 °C, and filtered with the help of filter paper. A finely filtered solution has been obtained. 2.91 g of Bi(NO3)3·5H2O and 0.99 g of Na2WO4·2H2O were dissolved in 30 ml of green extract and stirred at room temperature for 20 min. A dark, homogeneous solution has been obtained. Finally, 30 ml of the homogenous solution was transferred into a Teflon-lined 50-mL autoclave and kept at 120 °C for 8 h. The processed samples were collected by centrifugation after the autoclave had cooled to room temperature, rinsed twice with ethanol and distilled water, and then dried for 12 h at 80 °C in a drying oven. Similarly, the same procedure has repeated afterward for the preparation of Bi2WO6 at 140 °C. The reaction equation is given below. 4Bi(NO3)3·5H2O + 2Na2WO4·2H2O + 2H2O Bi2WO6 + 4NaOH + 12NO2 + 3O2
The schematic diagram is shown in Fig. 2.
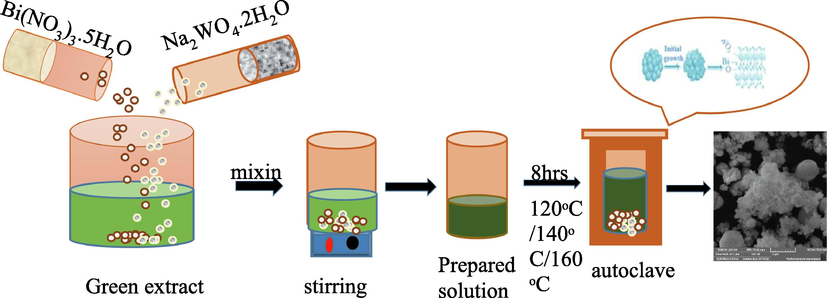
- Schematic diagram of synthesis of Bi2WO6.
2.2 Synthesis of vanadium pentoxide (V2O5)
Vanadium pentaoxide (V2O5) was made from ammonium metavanadate using Triton X-100 as a precursor (NH4VO3). 19.9 g of NH4VO3 (BDH chemicals, England) was dissolved in 999.9 ml of Azadirachta indica leaf solution, then 0.2 g of Triton X-100 (scintillation grade) was added, and the emulsification was completed by heating at 90 °C for 60 min. To acidify the VO3 solution, concentrated HNO3 (35 percent, Sigma–Aldrich) was added drop by drop. The brownish-black precipitates had aged overnight. The pH of the solution was adjusted through repeated washings with water, and the last remnants on the surface were removed through washing with ethanol. The filtrates were dried for the full night at 100 °C in a vacuum chamber. Yellowish-brown V2O5 was created by calcining the material at 500 °C for 5 h in a furnace at a temperature with a cooling and heating rate of 25 °C/min.
2.3 Synthesis of heterostructure (Bi2WO6 @V2O5)
Synthesized vanadium pentoxide, bismuth tungsten oxide, and Azadirachta indica extract were used to synthesise heterostructures. 0.55 g of V2O5 were added to 30 ml of green extract in a beaker and stirred continuously at 26 °C; then, 2.09 g of Bi2WO6 nanoparticles were added to it. The dark, greenish solution has been obtained. Two samples of heterostructures (V2O5@Bi2WO6) have been prepared using Bi2WO6 (synthesized at 120 °C and 140 °C) and V2O5. After that, fill another beaker of 500 ml up to 100 ml with water and place on a hot plate at 80 °C. A beaker of the prepared solution was kept in the beaker of 500 ml for 3–4 h, and a very thick solution was obtained. The solution was dried at 80 °C in the oven for 4–5 h. Finally, heterostructures have been obtained. At the end of the experiments, five samples of Bi2WO6 were prepared at 120 °C and 140 °C. At 120 °C and 140 °C, V2O5 and their heterostructures Bi2WO6@V2O5 were obtained.
2.4 Characterization
An X-ray diffractometer (Rigaku Diffractometer) with X-ray wavelength (=0.154 nm) is used to look at the structural characteristics of NPs. Nicolet Instrument Corp., Madison, Wisconsin, USA, provided the Nicolet Nexus 870 FT-IR spectrometer having a detector of deuterated triglycine sulphate for FTIR investigation. An FTIR spectrophotometer simultaneously gathers high-resolution spectral information over a broad spectrum of wavelengths. The material’s morphology was revealed by scanning electron microscopy (SEM, Nova-400). A UV2102PC spectrophotometer was used to examine the optical characteristics.
2.5 Photocatalytic test
The target pollutant compounds were the pollutant dye molecules Rhodamine B (RhB). The photocatalytic experiment was performed in a beaker using a 300 W Xe light with a 400 nm cutoff filter as the visible light source. 50 ml of RhB dye solution was mixed with 20 mg of photocatalysts produced. 2 gL-1 of catalyst load and a RhB concentration of 10 mgL-1 were selected. The mixture was subjected to 30 min of magnetic stirring in the dark to reach an adsorption–desorption equilibrium. A transparent dye solution was created by centrifuging 4 ml of the suspensions, after which the initial concentration, Co, was measured using a UV2102PC spectrophotometer. The Xe bulb was turned on after that. When light photons reached the suspension's surface, the photocatalytic process was started. The solution was constantly agitated with a magnetic stirrer throughout this operation. To quantify the concentration of RhB dye following light degradation, samples were taken from the slurry mixture (generally labelled as C). (Co-C)/Co was used to compute the deterioration efficiency. Where Co denotes zero time and C represents experiment time (Wang, 2014).
3 Result and discussion
3.1 XRD analysis
XRD examines the crystalline properties of V2O5, Bi2WO6, and Bi2WO6@V2O5 nanoparticles. The peaks of V2O5 at 2θ = 15.3°, 20.3°, 21.7°, 26.1°, 31°, and 32.4° with the structural plane (2 0 0), (0 1 0), (1 1 0), (4 0 0), (0 1 1), and (1 1 1) indicate the orthorhombic structure of V2O5, according to ICDD No. 96–101-1292, space group Pmn21 (Zhang, 2021). The characteristic diffraction peaks at 2θ = 19.04° (0 2 1), 28.08° (1 3 1), 32.21° (0 6 0), 33.89° (6 0 0), 38.7° (2 2 1), 50.75° (4 2–1), 48.74° (2 2 2), and 59.46° (12 0–5), etc., indicate the orthorhombic structure of Bi2WO6 according to (PDF card no. 79–2381). The peaks mentioned above are present in the heterojunctions' XRD patterns, but there is no trace of any secondary impurity phases. In heterostructures, peaks of Bi2WO6 and V2O5 are observed. No peaks related to BiO, BiV, etc., indicate the formation of a pure heterostructure of Bi2WO6@V2O5. The peak intensity of heterostructured Bi2WO6@V2O5 is increased, which shows that V2O5 and Bi2WO6 interact with each other, confirming that the heterostructure's crystallinity is improved. The high peak intensity of the heterostructure might be because the crystal nucleation center’s preferred V2O5 owing to the aromatic system, and then a heterostructure with a larger particle size than Bi2WO6 was formed (Fig. 3). Because the peak power of pure Bi2WO6 at 140 °C is low, the heterostructure synthesized at 140 °C had a low peak intensity.
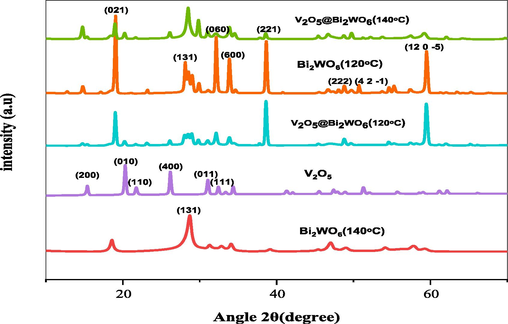
- XRD pattern of V2O5, Bi2WO6 and V2O5 @Bi2WO6 at 120 °C and 140 °C.
3.2 FTIr
The FTIR spectra of synthesized nanostructures are shown in Fig. 4. The FTIR peaks of Bi2WO6 at 627.67 cm−1 and 902.18 cm−1 are responsible for the vibrations of bending and stretching for W–O–W and O–W–O in WO3. For particular, the location of the W-O stretching vibrations is 644 cm−1. W–O extending, Bi–O stretching, and W–O–W bridging stretching modes related to the Bi − O band positioned at 844.8 cm−1 (Phuruangrat, 2014). The W = O stretching vibration is related to the peak at 950.98 cm−1 (Najafi-Ashtiani, 2018). FTIR peaks of V2O5 are observed at 825 cm−1 correspond to V–O–V stretching mode (Surya Bhaskaram et al., 2016) and at 724 cm−1 and 742 cm−1 show the symmetric and asymmetric stretching vibrations of V–O (Geng et al., 2014). According to research, the triple coordinated oxygen bonds (chain oxygen) asymmetric stretching vibrations and double coordinated oxygen bonds (bridge oxygen) vibration are responsible for the peak at 611 cm−1 (Wu et al., 2015). The bridge V-O-V stretching is attributed to bands ranging from 700 to 900 cm−1. The bands appearing at 957 cm−1 are attributed to vanadly stretching modes (δ V-O) (Farahmandjou and N.J.J.o.N.R. Abaeiyan, , 2017). For heterostructures of V2O5@Bi2WO6, the peaks are observed at 627 cm−1, 617 cm−1, and 950.98 cm−1 attributed to the vibrations of bending and stretching for O–W–O in WO3 and W = O stretching vibration, respectively, which confirmed the presence of Bi2WO6. The other peaks observed at 825 cm−1, 742 cm−1, and 957 cm−1 are attributed to the corresponding V–O–V stretching mode, the symmetric and asymmetric stretching vibrations of V–O and a vanadly stretching methods (δ V-O), respectively, which confirmed the presence of V2O5. There are no other impurity peaks observed. The peak intensities of heterostructured V2O5@Bi2WO6 at 120 °C are high, which shows that V2O5 and Bi2WO6 interact with each other, confirming the increased crystallinity of heterostructure.
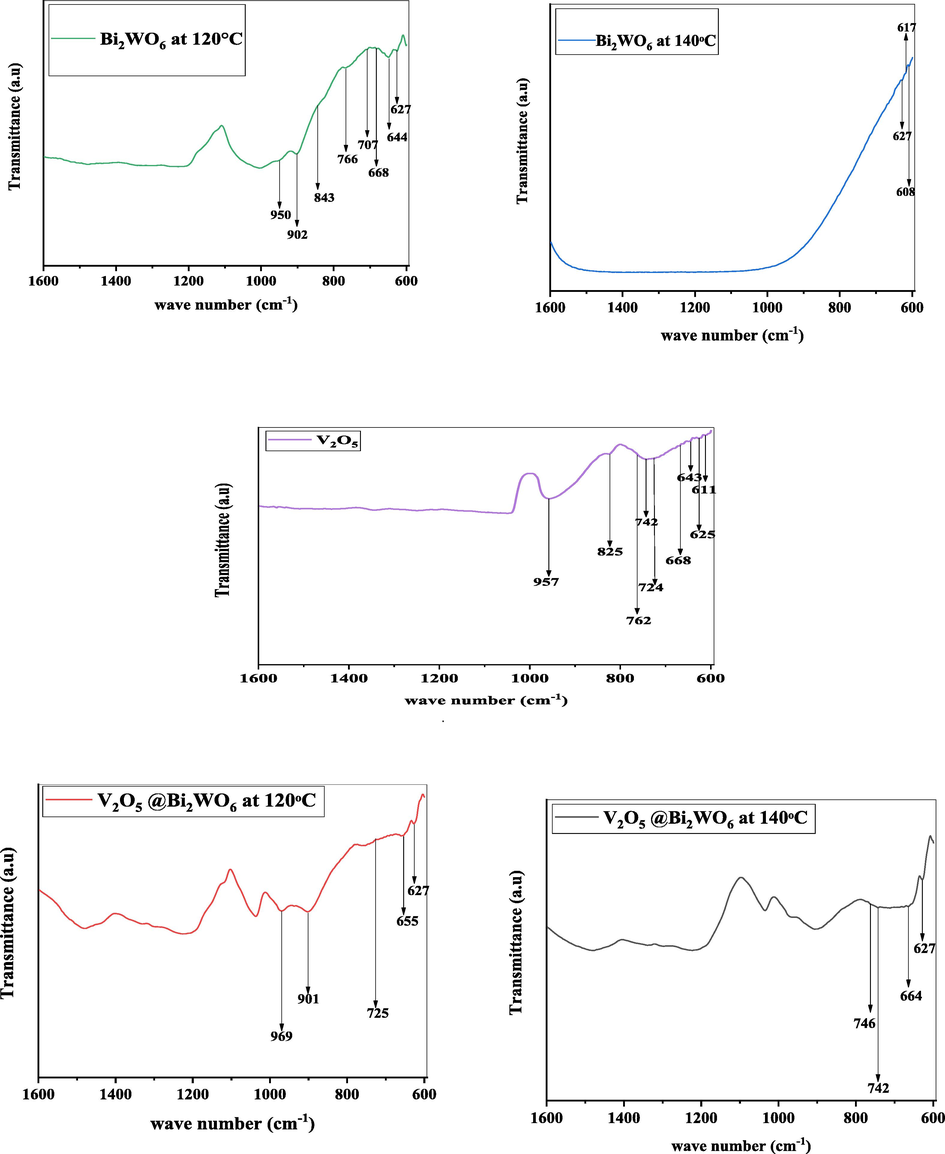
- FTIR analysis of V2O5, Bi2WO6 at 120 °C and 140 °C,V2O5 @Bi2WO6 at 120 °C and 140 °C.
3.3 Scanning electron microscopy (SEM)
Like crystal structure, morphology is an essential part of nanoparticles that affects the dye's degradation efficiency. The morphological features of hydrothermally synthesized V2O5, Bi2WO6 at 120 °C, Bi2WO6 at 140 °C, V2O5@Bi2WO6 at 120 °C, and V2O5@Bi2WO6 at 140 °C nanostructures are presented in Fig. 4.
Fig. 5 represents the morphology of V2O5, which is composed of rod-like structures. These structures are produced by the supersaturated reactant solution process, in which tiny crystallised nuclei develop in the solution and expand until they take on the final rod-like shape. Due to improved photo-generated electron transport and collection, this shape is very effective at dye degradation (Lu, 2019). Fig. 5 shows the morphological pictures of Bi2WO6 samples obtained at 120 °C and 140 °C, respectively. Microspheres that resemble flowers are ready when the reaction temperature reaches 120 °C. The hierarchical self-assembly method is used to create these microspheres from thin nanosheets. A three-step process is suggested for the creation mechanism of the flower-like Bi2WO6 superstructures in Fig. 6 in light of the facts previously described. According to studies, even if the formation of smaller crystallites is kinetically favourable, giant crystallites are thermodynamically favourable during the first accumulation. Consequently, many nanoparticles self-aggregate into microparticles that resemble spheres during the first stage of the hydrothermal process (step 1). In order to help the transition from solid spherical microparticles to superstructure, nanoparticles continue to aggregate into spherical microparticles as hydrothermal time (2–4 h) extends (step 2 in Scheme 1). The following scenarios could account for the origin of morphological evolution. In step 2, layers that resemble perovskite (Bi2O2)n2n+ and (WO4)n2n– are alternated to form the orthorhombic Bi2WO6 crystal. The smaller crystallites within the solid spherical microparticles eventually dissolve due to the difference in concentration between the surface and solution, and following hydrothermal treatment, Bi2O22+ & WO42- ions are generated in the acidic aqueous solution. Numerous tiny protuberances can be seen on the surface of the solid microsphere, providing a wealth of high-energy locations for the growth of nanocrystalline structures. Due to the sphere shape of the microparticles, the Bi2O22+ and WO42- ions in the solution preferentially transfer to their faces and spontaneously form on the microscopic protuberances. Ostwald ripening, a dissolution–recrystallization phase, is a frequent occurrence in the crystal formation process (step 2).

- SEM images of V2O5, Bi2WO6 & V2O5 @Bi2WO6 at 120 °C & 140 °C.
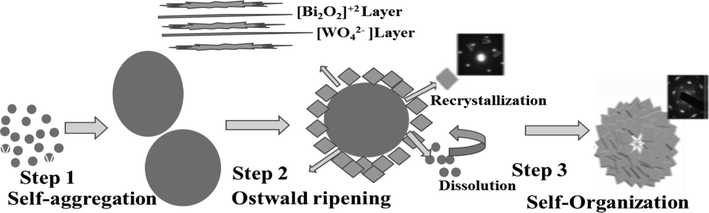
- The formation mechanism of flower-like structure (Zhang, 2007).
Bi2WO6 nanocrystals also prefer to form single crystals under hydrothermal conditions and develop into two-dimensional, plate-like structures because of their strong intrinsic anisotropy. Mass diffusion and Ostwald ripening cause solid spherical microparticles to become exhausted as the hydrothermal time increases, which causes a substantial amount of Bi2WO6 nanoplates to be produced. Contrary to published theories, which rely on hydrophobic attraction, emulsified soft or complicated templates, or both, geometric constraints of building blocks should have been a major factor in the formation of a spherical shape. Additionally, the orientated nanoplates serve as the foundation for the flower-like Bi2WO6 superstructures. A spherical superstructure would naturally result from the lateral contact of simple arrays of the nanoplates, which can quickly develop a curvature. Consequently, in situ-generated nanoplates finally self-organize to form Bi2WO6 superstructures that resemble flowers (Step 3) (Zhang, 2007). The mechanism of flower like structure is explained in Fig. 6.
Bi2WO6 has an altered, well-defined, and more aggregated particle size when the synthesis temperature is raised to 140 °C. In order to reduce structural flaws, the flower-like structure is converted into high-grain-size particles (Cui, 2016). SEM was used to analyse the morphology of V2O5 @Bi2WO6 at 120 °C and 140 °C, respectively. The synthesis of heterostructure (V2O5@Bi2WO6 at 120 °C) is confirmed by an enlarged SEM image of V2O5@Bi2WO6 giving microflowers that are haphazardly obsessed with nanorods. Rod-like nanostructures emerge within larger grains in the heterostructure at 140 °C. The creation of a heterostructure (V2O5@Bi2WO6 at 140 °C) was confirmed by the existence of nanorods and bigger grains. Additionally, both structures are effective at reducing dye degradation (Li, 2017).
3.4 UV visible analysis
The UV–visible spectroscopy is used to measure the bandgap of samples, as shown in Fig. 7.
- Band gap of V2O5, Bi2WO6 and V2O5 @Bi2WO6 at 120 °C and 140 °C.
The minimum amount of energy required to liberate an electron trapped in a bound state and enable conduction is known as the band gap of a semiconducting material. It is essential to study the transition of an electron. Tauc proposed the following relation for calculating band gap energy (Eg) as (Khan, 2017):
Where
The relation between a material's optical constants and how light affects a material's properties is explained. The most significant optical constants that may be computed using UV–vis spectroscopy are the dielectric constant (
3.4.1 Refractive index
The fundamental property of optical materials known as the refractive index (n) describes how light waves bend as they pass through different media (Khan, 2022). The “n” is one of the most important factors in determining a sample's optical qualities. The examination of dyes or substances with narrow absorption ranges is typically done using refractive index detection. It can be given as
Where c is the vacuum-bound speed of light and v is the phase velocity. The study of light scattering is essential. Hervé and Vandamme developed the relationship for “n” as a result of vibration theory as (Bahadur and Mishra, 2013):
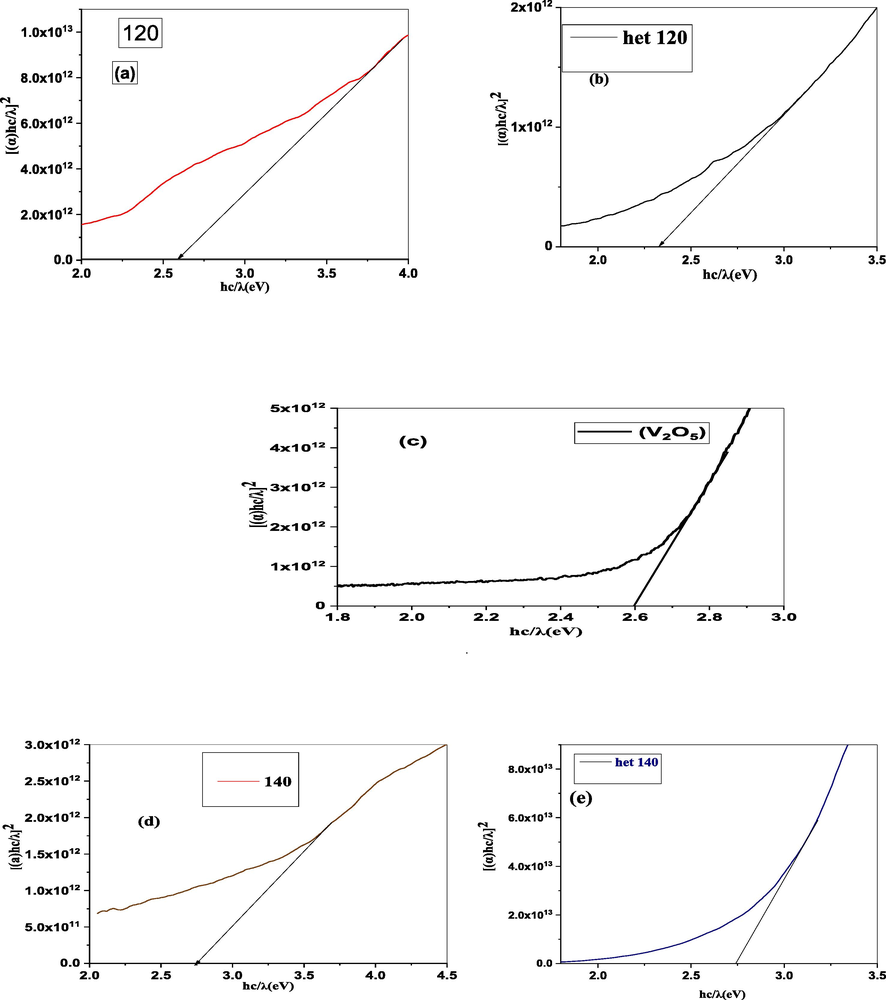
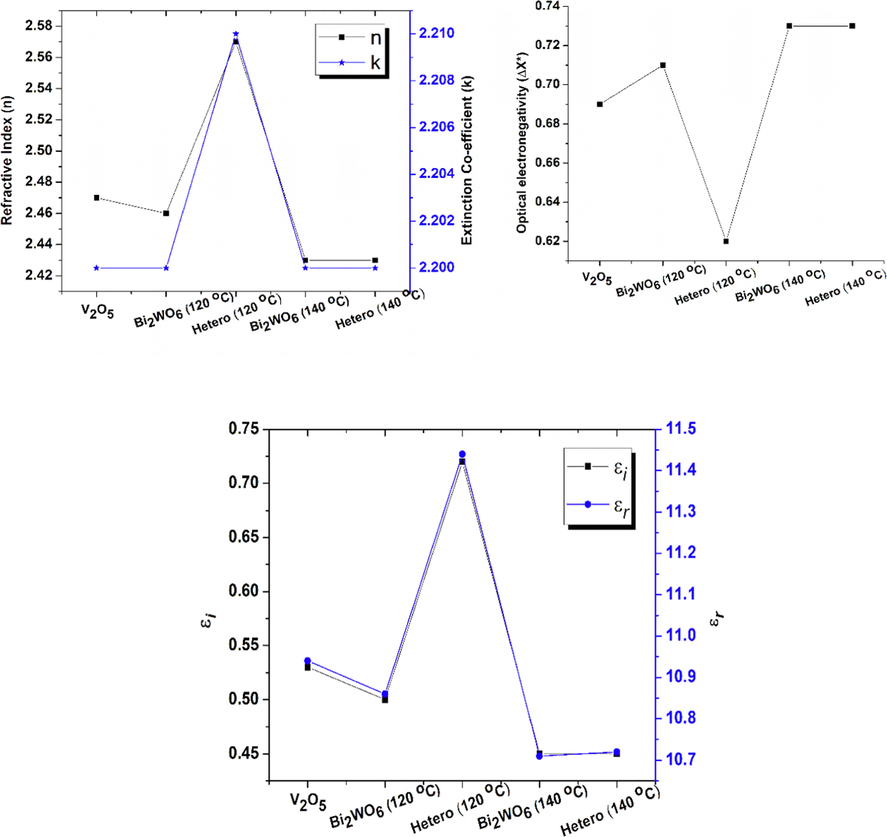
Where A = 13.6 eV and B = 3.4 eV are constants. Calculated ‘n’ for V2O5, Bi2WO6 at 120 °C and 140 °C, V2O5 @ Bi2WO6 at 120 °C and 140 °C are 2.47, 2.46, 2.43, 2.57, and 2.43, respectively. In heterostructures, the refractive index rises, causing light to deflect more toward normal. Light moves through materials more slowly the higher their refractive indices, which causes a greater change in light direction. Different bonding of Bi2WO6 and V2O5 caused higher ‘n’ which is due to oxygen vacancies (Amir-Al Zumahi, 2020).
3.4.2 Extinction coefficient
The extinction coefficients of pure V2O5, Bi2WO6 at 120 °C and 140 °C, and V2O5@Bi2WO6 at 120 °C and 140 °C) are determined by the subsequent formula (Khan, 2022).
It reveals a relationship between electronegativity difference ΔX* and refractive index n.
3.4.3 Optical electronegativity
The optical electronegativity (
Calculated values of V2O5, Bi2WO6, and V2O5@Bi2WO6 at 120 °C and 140 °C are 0.69, 0.71, 0.73, 0.62, and 0.73, respectively. The concept of chemical bonding is directly affected by the presence of optical electronegativity. The size of (
3.4.4 Dielectric constant
The dielectric constant (
The light’s speed is explained by its real component, which details how much it slows down in a matter. A material's dielectric properties, or the electric field via which energy is absorbed as a result of dipole motion, are described in the imaginary part of the equation. The loss factor is determined by the ratio of εi and εr. Both the imaginary (εi) and real (εr) components of the dielectric constant are significantly influenced by photon energy (hv). The calculated εr for V2O5 and Bi2WO6 at 120 °C and 140 °C are 0.53, 0.50, 0.45, 0.72, and 0.45, respectively, and the calculated εi are 10.94, 10.86, 10.71, 11.44, and 10.72, respectively, as shown in Fig. 8. The term “decreased real part” in this context refers to a reduction in the speed of light in a medium. As a result, there is significant light scattering. The imaginary component is also an increase, which refers to a rise in light absorption. Among all samples, V2O5@Bi2WO6 at 120 °C has the highest value of εr, which refers to the generation of a large number of electron and hole pairs due to the high absorption of light in the material. The values of εr are greater than εi in all samples, despite this, suggesting that the values of εr and εi are changed by raising the concentrations of the various materials. (Khan, 2022). Table 1 gives the calculated optical properties.
- Refractive index, extinction coefficient, optical electronegativity and dielectric constant (real & imaginary) of V2O5, Bi2WO6 and V2O5 @Bi2WO6 at 120 °C and 140 °C.
| Samples | Eg | n | k | ΔX* | εr | εi |
|---|---|---|---|---|---|---|
| V2O5 | 2.6 | 2.47 | 2.20 | 0.69 | 0.53 | 10.94 |
| Bi2WO6 (120 °C) | 2.65 | 2.46 | 2.20 | 0.71 | 0.50 | 10.86 |
| V2O5 @Bi2WO6 (120 °C) | 2.32 | 2.57 | 2.21 | 0.62 | 0.72 | 11.44 |
| Bi2WO6 (140 °C) | 2.74 | 2.43 | 2.20 | 0.73 | 0.45 | 10.71 |
| V2O5 @Bi2WO6 (140 °C) | 2.73 | 2.43 | 2.20 | 0.73 | 0.45 | 10.72 |
3.5 Photocatalytic performance
Fig. 9 shows the comparison of RhB dye molecule clearance against the irradiation period. Electrons from the VB are released from V2O5 and travel towards the CB to form electron-hole pairs when exposed to sunlight with an energy level equivalent to its Eg. Oxygen and electrons combine to form hydroxyl-free radicals. For oxidation, one can rely on cavitation's capacity to carry out a direct oxidation process or mix with water to form hydroxyl-free radicals. The relationship below explains this mechanism (Liu, 2015). (See Fig. 10).
- Photocatalytic activity procedure.
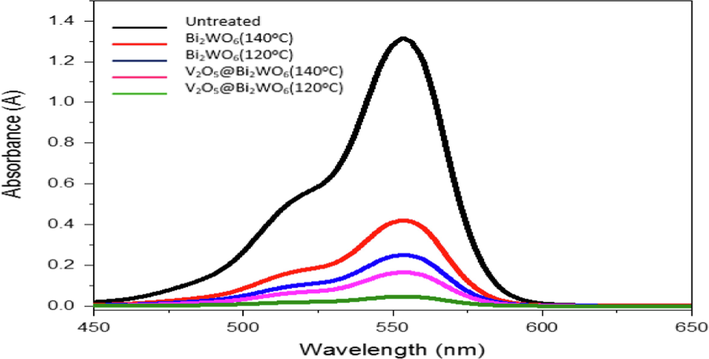
- Photocatalytic degradation of rhb by using v2O5, Bi2WO6 and V2O5 @Bi2WO6 at 120 °C and 140 °C.
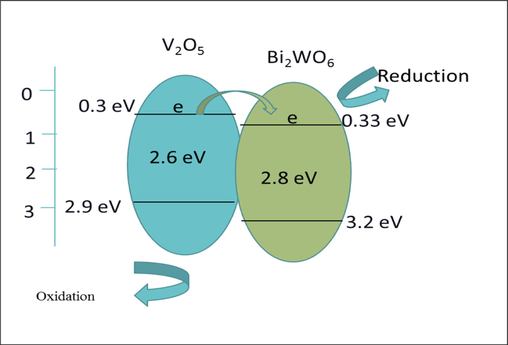
Bi2WO6 is a superior photocatalytic substance, much like V2O5. The band gaps of the synthesised Bi2WO6 at 120 °C and 140 °C were 2.65 eV and 2.74 eV, respectively. These Bi2WO6 decompose RhB dye very effectively and exhibit excellent light sensitivity in the visible region. RhB dye degradation efficiency was observed to be 81 % and 68 %, respectively, for materials produced at 120 °C and 140 °C. Given that this sample has strong crystallinity and shape, the efficiency at 120 °C is high. Its morphology consists of microspheres that resemble flowers, and these microspheres are made from thin nanosheets via a hierarchical self-assembly method that produces sharp edges. Flower-like microspheres are unusual in aquatic photocatalytic activity due to their fundamental characteristics, such as shorter photo-generated diffusion route distances, more efficient incidental light absorption due to excessive dispersion, simple settling, and a wide surface area. Due to the lower recombination rate, these materials' efficiency can be increased by exploiting their heterostructures (Tonda, 2018). Type II heterostructures are produced by Bi2WO6 and V2O5. Both materials' band gaps are in the visible range and span a wide range of light. Additionally, because interfaces and heterojunctions between V2O5 and Bi2WO6 occur in the heterostructures, the recombination rate is decreased. As seen in Fig. 9, the heterojunction between V2O5 and Bi2WO6 is essential for separating electron-hole pairs during photocatalysislight. Additionally, because interfaces and heterojunctions between V2O5 and Bi2WO6 occur in the heterostructures, the recombination rate is decreased. As seen in Fig. 9, the heterojunction between V2O5 and Bi2WO6 is essential for separating electron-hole pairs during photocatalysis. The number of heterojunctions is correlated with higher photocatalytic activity.
However, as shown in Fig. 9, the conduction and valence band boundaries of V2O5@Bi2WO6 and these two materials are close to one another in heterostructures. Light that has been exposed to the electrons in V2O5@Bi2WO6 jumps from VB to CB, leaving holes behind in VB. From the CB of V2O5 to the CB of Bi2WO6, electrons move downward and are caught by oxygen reduction, which produces superoxide radicals, which in turn produce oxygen and hydrogen peroxide (H2O2). Hydrogen peroxide is produced when the residual holes in VB undergo oxidation in the presence of water (H2O2). In addition, H2O2 breaks into two highly reactive hydroxyl radicals (OH•). OH• oxidizes organic contaminants. This substance is reduced, hence its strong photocatalytic activity.
At 120 °C, V2O5@Bi2WO6 exhibits the maximum degrading efficiency of 96.5 %, while at 140 °C, it is only 87 %. Because Bi2WO6 has flower-like structures and V2O5 has particle-like structures, the two materials combine at 120 °C to form V2O5@Bi2WO6. As previously mentioned, flower-like structures have strong photocatalytic activity. This material exhibits significant photocatalytic activity because the recombination rate is decreased in heterostructure.
To evaluate the effect of individual species (such as holes (h+), electrons (e-), hydroxyl radicals (•OH)) in the degradation of dye during the advanced oxidation process, the scavenging studies were performed. For scavenging studies, EDTA, 2-propanol, and AgNO3, which scavenge the h+, •OH, and e- species, respectively. This study is useful to appraise the effect of these oxidizing and reducing agents because these are the major species, which are product during the advanced oxidation process for the redox reactions. The findings revealed that the dye removal was different for scavengers as well as for the blank (without the scavenger) (Fig. 11). In case of AgNO3, EDTA and 2-propanol, the dye removal was observed to be 85.5, 44.5 and 23 (%), respectively. In case of blank, the dye removal was 96.5 % and in case of scavenging the h+, •OH, and e- species, the dye degradation rate of reduced, which indicates that these species have imperative role in the degradation of dye. Based on scavenging analysis, it was observed the •OH was the main specie, which is responsible for the oxidative removal of dyes followed by the h+ and e-.
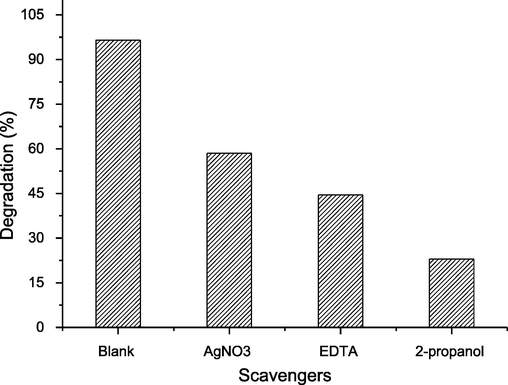
- Dye removal in the presence of different scavengers.
4 Conclusions
The green technique uses a hydrothermal pathway to create V2O5 and Bi2WO6 at 120 °C, Bi2WO6 at 140 °C, 120 °C, and V2O5@Bi2WO6 at 120 °C and 140 °C, respectively. According to FTIR data, V2O5, Bi2WO6, and V2O5@Bi2WO6 were formed at 120 and 140°Celsius, respectively. The heterostructure's improved crystallinity is confirmed by the increased peak intensity of heterostructured V2O5@Bi2WO6 at 120 °C. The morphology of V2O5 is a rod-like structure with tiny crystalline nuclei generated in a supersaturated reactant solution, according to SEM data. The nuclei gradually grow until the final rod-like structure is formed. Bi2WO6 at 120 °C has flower-like microspheres. These microspheres are constructed from thin nanosheets via the hierarchical self-assembly process. Bi2WO6 at 140 °C showed well-defined and more aggregated particles. In V2O5@Bi2WO6 at 120 °C presence of microflowers and randomly oriented nanorods confirmed its formation. From UV–vis spectroscopy, V2O5@ Bi2WO6 at 120 °C has the lowest Eg 2.3 eV. Among all samples of heterostructure, V2O5@Bi2WO6 at 120 °C shows efficient degradation of dye due to the smallest band gap and higher refractive index.
5 Research funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R124), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R124), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Large Groups Project under grant number L.R.G.P2/9/44.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R124), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Large Groups Project under grant number L.R.G.P2/9/44.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis of NiO nanoparticles using Aloe vera gel extract and evaluation of antimicrobial activity. Mater. Chem. Phys. 2022126363
- [Google Scholar]
- Enhancement photocatalytic activity of the heterojunction of two-dimensional hybrid semiconductors ZnO/V2O5. Catalysts. 2018;8(9):374.
- [Google Scholar]
- Amir-Al Zumahi, S., et al., Extraction, optical properties, and aging studies of natural pigments of various flower plants. 2020. 6(9): p. e05104
- Photovoltaic properties of ZnO films co-doped with Mn and La to enhance solar cell efficiency. Nanomaterials. 2022;12(7):1057.
- [Google Scholar]
- Bahadur, A. and M.J.A.P.P.A. Mishra, Correlation between refractive index and electronegativity difference for ANB8-N type binary semiconductors. 2013. 123(4): p. 737-740
- Correlation between refractive index and electronegativity difference for ANB8-N type binary semiconductors. Acta Phys. Pol. A. 2013;123(4):737-740.
- [Google Scholar]
- Distinct approaches of removal of dyes from wastewater: a review. Mater. Today:. Proc.. 2022;50:1575-1579.
- [Google Scholar]
- Chaves, A., et al., Bandgap engineering of two-dimensional semiconductor materials. 2020. 4(1): p. 1-21
- Electrochemical lithium insertion behavior of combustion synthesized V2O5 cathodes for lithium-ion batteries. J. Electrochem. Soc.. 2012;159(3):A273.
- [Google Scholar]
- Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal.. 2021;42(9):1413-1438.
- [Google Scholar]
- Characterization of V2O5/MoO3 composite photocatalysts prepared via electrospinning and their photodegradation activity for dimethyl phthalate. Chin. J. Catal.. 2015;36(12):2194-2202.
- [Google Scholar]
- Development of efficient bismuth tungstate for photocatalytic and photoelectrochemical reactions. UNSW Sydney; 2019.
- Cui, Z., et al., Effect of experimental parameters on the hydrothermal synthesis of Bi2WO6 nanostructures. 2016. 11(1): p. 1-9
- Electrochemical properties of high surface area vanadium oxide aerogels. Electrochem. Solid St.. 2000;3(10):457.
- [Google Scholar]
- DUBROW, S.F. and G.D. BOARDMAN, AEROBIC-ANAEROBIC DEGRADATION OF TEXTILE DYE WASTEWATER. Environmental chemistry of dyes and pigments, 1996: p. 75.
- Farahmandjou, M. and N.J.J.o.N.R. Abaeiyan, Chemical synthesis of vanadium oxide (V2O5) nanoparticles prepared by sodium metavanadate. 2017. 5(1): p. 00103.
- Geng, Y., P. Zhang, and S.J.R.A. Kuang, Fabrication and enhanced visible-light photocatalytic activities of BiVO 4/Bi 2 WO 6 composites. 2014. 4(86): p. 46054-46059
- Fabrication of pulsed-laser deposited V2O5 thin films for electrochromic devices. J. Mater. Process. Technol.. 2008;197(1–3):261-267.
- [Google Scholar]
- Structural, electrical and optical properties of multilayer TiO2 thin films deposited by sol–gel spin coating. Results Phys.. 2017;7:1437-1439.
- [Google Scholar]
- Improving the structural, optical and photovoltaic properties of Sb-and Bi-Co-doped MAPbBr 3 perovskite solar cell. Coatings. 2022;12(3):386.
- [Google Scholar]
- Improving the structural, optical and photovoltaic properties of Sb-and Bi-Co-doped MAPbBr 3 perovskite solar. Cell. 2022;12(3):386.
- [Google Scholar]
- Enhanced performance as a lithium-ion battery cathode of electrodeposited V2O5 thin films by e-beam irradiation. J. Solid State Electrochem.. 2010;14(10):1801-1805.
- [Google Scholar]
- Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials. 2021;14(7):1645.
- [Google Scholar]
- Acid etching followed by hydrothermal preparation of nanosized Bi2O4/Bi2O3 pn junction as highly efficient visible-light photocatalyst for organic pollutants removal. J. Colloid Interface Sci.. 2020;576:291-301.
- [Google Scholar]
- Li, S., et al., Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. 2017. 501: p. 156-163
- Low temperature synthesis of Bi2WO6 and its photocatalytic activities. Mater. Res. Bull.. 2015;66:96-100.
- [Google Scholar]
- V 2 O 5-based nanomaterials: synthesis and their applications. RSC Adv.. 2018;8(8):4014-4031.
- [Google Scholar]
- Hydroxyapatite nanomaterials: synthesis, properties, and functional applications. In: Nanomaterials From Clay Minerals. Elsevier; 2019. p. :485-536.
- [Google Scholar]
- Structural, optical and electrical properties of WO3–Ag nanocomposites for the electro-optical devices. Appl. Phys. A. 2018;124(1):1-9.
- [Google Scholar]
- Najafi-Ashtiani, H., et al., Structural, optical and electrical properties of WO3–Ag nanocomposites for the electro-optical devices. 2018. 124(1): p. 1-9
- Hydrothermal synthesis, characterization, and visible light-driven photocatalytic properties of Bi2WO6 nanoplates. J. Nanomater.. 2014;2014
- [Google Scholar]
- Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int.. 2018;101(5):1371-1384.
- [Google Scholar]
- Correlation between optical electronegativity and refractive index of ternary chalcopyrites, semiconductors, insulators, oxides and alkali halides. Opt. Mater.. 2008;31(2):209-212.
- [Google Scholar]
- Facile synthesis of new Z-scheme Bi2WO6/Bi2MoO6 p–n junction photocatalysts with high photocatalytic activity: structure, kinetics and mechanism approach. Mater. Res. Bull.. 2020;131:110979
- [Google Scholar]
- Efficient photocatalytic degradation of environmental pollutant with enhanced photocarrier separation in novel Z-scheme a-MnO2 nanorod/a-MoO3 nanocomposites. J. Photochem. Photobiol. A Chem.. 2020;401:112787
- [Google Scholar]
- Heterogeneous photocatalytic degradation of organic pollutant in aqueous solutions by S-scheme heterojunction in nickel molybdate nanocomposites. J. Environ. Chem. Eng.. 2021;9(5):105903
- [Google Scholar]
- Z-scheme 3D Bi2WO6/MnO2 heterojunction for increased photoinduced charge separation and enhanced photocatalytic activity. Appl. Surf. Sci.. 2020;532:147413
- [Google Scholar]
- Design of S-scheme 3D nickel molybdate/AgBr nanocomposites: tuning of the electronic band structure towards efficient interfacial photoinduced charge separation and remarkable photocatalytic activity. J. Photochem. Photobiol. A Chem.. 2022;426:113751
- [Google Scholar]
- Tailored BiVO4 for enhanced visible-light photocatalytic performance. J. Environ. Chem. Eng.. 2021;9(5):106025
- [Google Scholar]
- Surya Bhaskaram, D., R. Cheruku, and G.J.J.o.M.S.M.i.E. Govindaraj, Reduced graphene oxide wrapped V2O5 nanoparticles: green synthesis and electrical properties. 2016. 27(10): p. 10855-10863.
- g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Interfaces. 2018;10(3):2667-2678.
- [Google Scholar]
- Tsymbal, E.Y., et al., Multifunctional oxide heterostructures. 2012: OUP Oxford.
- Wang, H., et al., Preparation, characterization and photocatalytic performance of g-C3N4/Bi2WO6 composites for methyl orange degradation. 2014. 40(7): p. 9077-9086
- Wu, Y., G. Gao, and G.J.J.o.M.C.A. Wu, Self-assembled three-dimensional hierarchical porous V 2 O 5/graphene hybrid aerogels for supercapacitors with high energy density and long cycle life. 2015. 3(5): p. 1828-1832.
- Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci.. 2019;495:143520
- [Google Scholar]
- Facile construction for new core-shell Z-scheme photocatalyst GO/AgI/Bi2O3 with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci.. 2021;581:148-158.
- [Google Scholar]
- Fabrication of flower-like Bi 2 WO 6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem.. 2007;17(24):2526-2532.
- [Google Scholar]
- V2O5/Pg-C3N4 Z-scheme enhanced heterogeneous photocatalytic removal of methyl orange from water under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp.. 2021;608:125580
- [Google Scholar]







