Translate this page into:
A facile synthesis of 1,3,4-oxadiazole-based carbamothioate molecules: Antiseizure potential, EEG evaluation and in-silico docking studies
⁎Corresponding authors. samreengul@gcuf.edu.pk (Samreen Gul Khan), Imran.ch@bzu.edu.pk (Imran Imran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In present work, a series of novel structural hybrids of 1,3,4-oxadiazole and carbamothioate was designed by chemical modification of 2-(4-isobutylphenyl)propanoic acid. Target compounds (7a-f) were synthesized in significant yields (84–88 %) by coupling compound (4) with different electrophiles under different reaction conditions. The structures of oxadiazole based carbamothionate derivatives were confirmed by spectroscopic (FTIR, 1H NMR, 13C NMR) and physiochemical methods. During in-vivo experimentation, all synthesized compounds were tested through 6 Hz (32 mA) and PTZ (80 mg/kg) mouse seizure models. The 7b and 7c showed significant outcomes (P < 0.05) in terms of seizure severity, protection and mortality. The behavioural outcomes of PTZ tests were further strengthened with video-electroencephalogram (vEEG) findings in which EEGs were analyzed for epileptic spikes to understand the impact of 7b and 7c treatment on these ictal activities. The 7b was found most efficient in reducing the seizure spiking activity in brains of PTZ-treated mice while both 7b and 7c significantly reduced overall PTZ-induced seizure severity. The molecular docking studies also predicted the BBB permeability, reduced binding energies and good compound interaction with GABAA receptors and SV2A protein. Therefore, the observed pharmacological outcomes might be attributed to the GABAA agonistic and SV2A modulating potential of these oxadiazole-carbamothioate hybrid compounds.
Keywords
Epilepsy
6 Hz
PTZ
1,3,4-oxadiazole
Electroencephalogram
Seizure
1 Introduction
More than 70 million of world’s population is suffering from epilepsy (Löscher et al., 2020). Uncontrolled epilepsy or failure to attain seizure control with standard anticonvulsant medications is intricately associated with pharmacoresistant epilepsy (Sharma et al., 2015). Drug resistance epilepsy (DRE) is not only responsible for the intractable seizures but also causes neurobiochemical changes in the brain leading to neuropsychiatric dysfunctions, life-long dependency and disease-induced socioeconomic burden (Kwan and Brodie, 2002). A systematic review has recently reported that the overall prevalence and incidence of DRE have elevated from 0.11 to 0.58 % and 0.06 to 0.51 %, respectively (Fattorusso et al., 2021).
The quality of life for epileptic patients is improved by prescribing conventional therapeutic choices. However, their utilization is associated with idiosyncratic dose-related toxicity and addictive potential (Zaccara et al., 2007). Additionally, pharmacoresistant epilepsy necessitates the development of appropriate novel therapeutic options. Currently available antiseizure drugs are effective in preventing epileptogenesis and convulsions in<80 % of epileptic patients (Perucca, 2021). Furthermore, the extended use of antiepileptic drugs is related to unwanted adverse effects that might prove life-threatening (Obniska et al., 2012). Due to the aforementioned reasons, scientists ate continuously attempting to synthesize new chemical agents of antiepileptic potential with better safety profiles.
The screening of novel compounds for anticonvulsant potential relies profoundly on the established preclinical animal models of epileptic seizures. Generally, experimental seizure induction involves electrical and/or chemical stimulation of the rodent brain (Löscher, 1999). The 6-Hertz (6 Hz) and pentylenetetrazole (PTZ)-induced seizure models are broadly used in the initial screening of antiepileptic compounds (Löscher, 2011). The 6 Hz model utilizes the corneal application of different current intensities (22, 32 or 44 mA) to identify the new compounds with antiseizure potential. However, at high stimulation intensities i.e. 44 mA, most of the AEDs lose their efficacy so it can be used to identify new compounds that can be employed in the treatment of refractory partial epilepsy (Barton et al., 2001).
Molecular hybridization is a simple and effective tool to covalently combine multiple drug pharmacophores. Our ongoing research focuses on design and synthesis of pharmacologically active diverse polyvalent scaffolds as anticonvulsant agent. 1,3,4-oxadiazole is reported to have versatile nature and great importance in medicinal chemistry such as anticonvulsant agents (Almasirad et al., 2014; Zarghi and Arfaei, 2011), anticancer (Yadav et al., 2017), antifungal (Karaburun et al., 2019), antioxidant (Abd-Elzaher et al., 2016), anti-inflammatory (Chawla et al., 2018, Gulnaz et al., 2019), antibacterial (Zheng et al., 2018), anti-HIV (Akhtar et al., 2007; Khan et al., 2016) antimicrobial ((Bala et al., 2014)), antitumor (Mansouri et al., 2021), antipyretic (Xu et al., 2018) as well as pyrophosphatases and phosphodiesterases (Akhtar et al., 2007). Carboxamide is reported to be significantly important pharmacophore of antiepileptic drugs like levetiracetam, carbamazepine, and brivaracetam (Ahmad et al., 2019). On this basis, we have designed a hybrid of oxadiazole and carbamothioate pharmacophore together in one molecule to prevent the progression of epilepsy. We selected 2-(4-isobutylphenyl) propanoic acid core for chemical derivatization. Our designed molecular framework contains hydrophobic aryl rings on each side of oxadiazole and carbamothioate. In the current study, the synthesized oxadiazole-carbamothioate derivatives 7a, 7b, 7c, 7d, 7e and 7f were preliminarily tested for antiseizure potential through 6 Hz (32 mA) and PTZ (80 mg/kg) mouse models. This study aimed to further authenticate the antiseizure potential of potent compounds by examining their effects on electroencephalogram (EEG) activity in the PTZ model. The effects of compounds were evaluated for epileptic spikes analysis to examine whether these ictal activities in EEG are affected by test compounds. For further understanding of observed outcomes, the binding energies and interactions of compounds were predicted with target molecular receptors.
2 Chemistry and experimental details
2.1 Chemistry
Melting points of all derivatives were checked using open capillary tube methods and an electrical melting point (Stuart SMP10 melting point apparatus). 2-(4-isobutylphenyl) propanoic acid was used as a starting material. Infrared spectra were obtained using potassium bromide discs on an FT-IR spectrophotometer (BRUKER) with a wavelength range of 4000–400 cm-1 and expressed in wave number (cm-1) at GC University's Hi Tech Lab. 1H NMR and 13C NMR spectra were carried out in deutrated solvent i.e. the exchangeable protons were exchanged by D2O. DMSOd6 on Avance Bruker using 500 MHz spectrophotometer (1H NMR) and 75 MHz to 100 MHz (13C NMR) NMR spectrophotometer at Department of Chemistry, University of Copenhagen, Denmark. The chemical shift was expressed in δ ppm. Reaction progress and completion was monitored by TLC.
2.1.1 Synthesis of methyl 2-(4-isobutylphenyl)propanoate (2)
Dry methanol (30–35 ml) was added into ibuprofen (1) (6.g, 0.02 mol) in a round bottom flask. Conc. H2SO4 (2.5 ml) was added into the flask containing methanol and ibuprofen. The reaction was set to reflux for 6 to 7 h. The reaction completion was checked using thin layer chromatographic technique. After successful completion, the solvent was removed by evaporation. Distilled water was added into the crude product and ester was extracted using solvent extraction technique. Diethyl ether was used for extraction of ester. Sodium carbonate solution was then added into ester containing layer. The ester was washed and dried over anhydrous sodium carbonate. The product was then concentrated. The compound (2) was obtained as pale-yellow oily liquid; molecular formula C14H20O2; Mol. Wt. 220.15; Yield: (90 %); b.p. 263–265 ∘C; IR (KBr) cm−1: 1736.34 (C⚌O stretch (ester), 1203.37 (C—C—O stretch), 1162.81 (O—C—C stretch); Anal. C, 76.33; H, 9.15; O, 14.52 (supplementary data, S1).
2.1.2 Synthesis of 2-(4-isobutylphenyl)propanehydrazide (3)
2-(4-isobutylphenyl)propanehydrazide was synthesized by dissolving methyl 2-(4-isobutylphenyl)propanoate (2 g, 0.02 mol) in 15 ml methanol (MeOH) was taken in oven dried RBF, then added hydrazine hydrate (2 ml). Then the solution was set to reflux for 10–12 h at to 90–100O C. After the completion, chilled water was added in reaction to get product. Ibuprofen hydrazide was separated as a white crystalline solid. The spectroscopic and physical date of the 2-(4-isobutylphenyl) propanehydrazide is given as Rf value (n-hexane: ethyl acetate 7:3) = 0.83; molecular formula C13H20N2O; Mol. Wt. 220.16; Yield: (88 %); m.p. 77–78 ∘C; IR (KBr) cm−1: 3272.75, 2963.11, 1640.11, 1604.85, 1466.29, 1366.60, 906.66, 686.81. Mol. Wt. 220.16. Anal. C, 70.87; H, 9.15; N, 12.72; O, 7.26 (supplementary data, S2).
2.1.3 Synthesis of 5-(1-(4- isobutylphenyl)ethyl)-1,3,4-oxadiazole-2-thiol (4)
In an oven dried round bottom flask, 2-(4-isobutylphenyl)propanehydrazide (0.02 mol) was dissolved in methanol (30 ml). KOH (0.02 mol) and CS2 (0.03 mol) were also added in reaction flask. The mixture was refluxed at 95 ͦ C for 10–11 h. Reaction progress as well as completion was continuously monitored by TLC. After the completion, chilled water was added in flask to get precipitates of product the product. Mixture was acidified with HCl to pH-4.-3. Precipitates were filtered and washed with water. The product was also recrystallize using absolute ethanol. 5-(1-(4- isobutylphenyl)ethyl)-1,3,4-oxadiazole-2-thiol was separated as off white crystalline solid having molecular formula C14H18N2O3S; Mol. Wt. 482.26; Yield: (84 %); m. p. 115–118 °C;IR (KBr) cm−1: 2448.56, 1507.89, 1454.29, 1294.98, 1174.54, 1069.78, 989.93, 736.98. Anal. C, 69.67; H, 9 = 7.84; N, 5.80; O, 9.94; S, 6.64 (supplementary data, S3-S5).
2.1.4 Synthesis of N-substituted aryl/alkyl 2-bromocarbamides (6a-f)
In an oven dried round bottom flask, the N-substituted aryl/alkyl amines (5a-5f,12.0 mol) were dissolved in 10.0 ml of 5 % Na2CO3 solution. Carbonic dibromide (12.0 mmoles) was introduced gradually into the above reaction mixture. The mixture containing round bottom flask was shacked gently till precipitation. Reaction progress as well as completion was continuously checked by TLC. After the completion, chilled water was added in flask to get precipitates of product the product. Precipitates of the product (6a-f) were filtered and dried. The product were also recrystallized using absolute ethanol.
2.1.5 Synthesis of N-substituted 5-(1-(4-isobutylphenyl) ethyl)-1,3,4-oxadiazole-2-yl-2-sulfanyl carbamide derivatives (7a-f)
Different N-substituted 5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazole-2-yl-2-sulfanyl carbamide derivatives were synthesized in good yield by the stirring 4 with different substituted aralkyl/ alkyl/aryl 2-bromocarbamides (6a-f) using DMF and NaH at room temperature. Reaction progress as well as completion was continuously checked by TLC. After the completion, chilled water was added in flask to get precipitates of product. Precipitates of the product were filtered and dried. The product was also recrystallized using absolute ethanol.
2.1.6 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl)(2,3 dimethylphenyl) carbamothioate (7a)
Off white amorphous solid; (83 %); C23H27N3O2S; Mol. Wt. 409.55; m.p 157–159 °C; IR (KBr, cm−1) 2906.21, 2363.96, 2313.72, 2164.19, 1680.73, 1515.73, 698.36, 637.52; 1H NMR (500 MHz, DMSO) δ 9.29 (s, 1H, N—H), 7.44–7.42 (d, 1H, J = 10, H-6′), 7.20–7.19 (d, 2H, J = 10, aromatic-H-9 & aromatic-H-13), 7.15–7.13 (d, 2H, J = 5, aromatic-H-10 & aromatic-H-12), 7.06–7.03 (t, 1H, J = 5 & 10, aromatic-H-5′), 6.94–6.92 (d, 1H, J = 10, aromatic -H-4′), 4.32–4.28 (q, 1H, aromatic-H-6), 2.43–2.41 (d, 1H, J = 5 CH2-14), 2.23 (s, 3H, CH3-2′) 2.07 (s, 3H, CH3-3′), 1.84–1.79 (m, 1H, H-15) 1.59–1.58 (d, J = 5, 3H, CH3-7), 0.86–0.85 (d, J = 5 Hz, 6H, H-16,H-17).13C NMR (126 MHz, DMSO) 162.15(C, C⚌O),161.37(C, C5), 139.98(C, C-11), 138.41 (2C, C-3′& C-8), 137.06(C-3′), 136.63(C-1′), 129.26(C-10 &C-12), 128.09(C-2′), 126.85(C-9 & C-13), 125.51(C-4′ & C-5′), 119.40(C-6), 44.18(CH2, C-14), 36.06(C-6), 29.40(C-15), 22.13(2CH3, C-16 & C-17), 20.19(CH3, C-7), 19.38(CH3, C-3′), 13.75(CH3, C-2′); EIMS m/z: 409.18 (100.0 %), 410.19 (24.9 %), 411.18 (4.5 %), 411.19 (2.7 %), 412.18 (1.1 %), 410.18 (1.1 %); Elemental Analysis: C, 67.45; H, 6.65; N, 10.26; O, 7.81; S, 7.83 (supplementary data, S6-S9).
2.1.7 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl) (4methoxyphenyl)carbamothioate (7b)
Off white amorphous solid; (80 %); C22H25N3O3S; Mol. Wt. 411.52; m.p 145–147 °C; IR (KBr, cm−1) 2906.21 (C—H), 2363.96 (C—O), 2313.72 (C⚌C), 2164.19 (C⚌C), 1515.73 (C⚌O ring), 698.36 (C—H), 679.66 (C—H), 666.02 (C—O—C), 651.67 (C⚌C), 637.52 (C—H). 1H NMR(500 MHz, DMSO) δ 10.10 (s, 1H, N—H), 7.43–7.41, (d, 2H, J = 10 Ar H-2′ & H-6′), 7.21–7.19 (d, 2H, J = 10 aromatic-H-9 & aromatic-H-13), 7.15–7.13 (d, 2H, J = 10, aromatic-H-10 & aromatic-H-12), 6.91–6.89 (d, 2H, J = 10, aromatic-H-3′ & aromatic-H-5′), 4.34–4.29 (q, 1H, H-6), 3.71 (s, 3H, OCH3-4′), 2.43–2.41 (d, 2H, J = 10 CH2-14), 1.82–1.78 (m, 1H, H-15) 1.60 (d, J = 5, 3H, CH3-7), 0.86–0.84 (d, J = 10 Hz, 6H, H-16 & H-17). 13C NMR (126 MHz, DMSO) 161.39, (C⚌O),159.98.17(C5), 153.83(C-4′), 140.15(C11), 138.24(C-8), 131.80(C-10 & C-12), 129.53(C-1), 126.53(C-9 & C-13) 117.82(C-2′ & C-6′), 113.93(C-3′ & C-5′), 55.31(CH3, OCH3-4′), 43.19(CH2, C-14), 36.06(1C, C-6), 29.67(1CH,C-15) 21.86 (2C, CH3-1 & CH3-17), 19.38(1C, CH3-7). EIMS m/z: 411.16 (100.0 %), 412.17 (23.8 %), 413.16 (4.5 %), 413.17 (2.7 %), 412.16 (1.1 %), 414.16 (1.1 %); Elemental Analysis: C, 64.21; H, 6.12; N, 10.21; O, 11.66; S, 7.79 (supplementary data, S10-S13).
2.1.8 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl) (4-ethoxyphenyl)carbamothioate (7c)
Off white amorphous solid; (85 %); C23H27N3O3S; Mol. Wt. 425.55; m.p 137–139 °C; IR (KBr, cm−1) 3734.81, 2363.60, 2312.63, 2164.57, 2121.33, 2034.35, 1981.56, 1965.41, 1949.75, 1938.69, 1695.15, 1516.80, 641.28; 1H NMR (400 MHz, CDCl3) 7.27–7.25 (d, 2H, J = 8, aromatic-H-2′ & aromatic-H-6′), 7.18–7.16 (d, 2H, J = 8 aromatic-H-9 & aromatic-H-13), 7.11–7.09 (d, 2H, J = 8 aromatic-H-10 & aromatic-H-12), 6.83–6.81 (d, 2H, J = 8, aromatic-H-3′ & aromatic-H-5′) 4.23–4.17 (q, 1H, H-6), 3.98–3.97 (d, 2H, OCH2-4′), 2.44–2.42(d, 2H, J = 8 CH2-14), 1.86–1.77 (m, 1H, H-15) 1.69 (d, J = 8, 3H, CH3-7), 1.39–1.36 (t, 3H, OCH2CH3-4′), 0.88–0.86 (d, J = 8 Hz, 6H, H-16 & H-17) (supplementary data, S14-S16). 13C NMR (126 MHz, DMSO) 161.85,(C, C⚌O),160.01(C, C5), 153.49(C, C-4′), 140.13(C, C11), 138.45(C, C-8), 131.85(C-10 & C-12), 129.26(C, C-1), 126.84(C-9 & C-13) 118.13(C-2′ & C-6′), 114.76(C-3′ & C-5′), 63.15(1CH2, OCH2-4′), 44.19(CH2, C-14), 36.01(1C, C-6), 29.74(1CH,C-15) 22.23(2C, CH3-1 & CH3-17), 19.38(1C, CH3-7), 14.82(OC2H5, CH3-4′); EIMS m/z: 425.18 (100.0 %), 426.18 (24.9 %), 427.17 (4.5 %), 427.18 (3.0 %), 428.18 (1.1 %), 426.17 (1.1 %); Elemental Analysis: C, 64.92; H, 6.40; N, 9.87; O, 11.28; S, 7.53 (Fig. 1).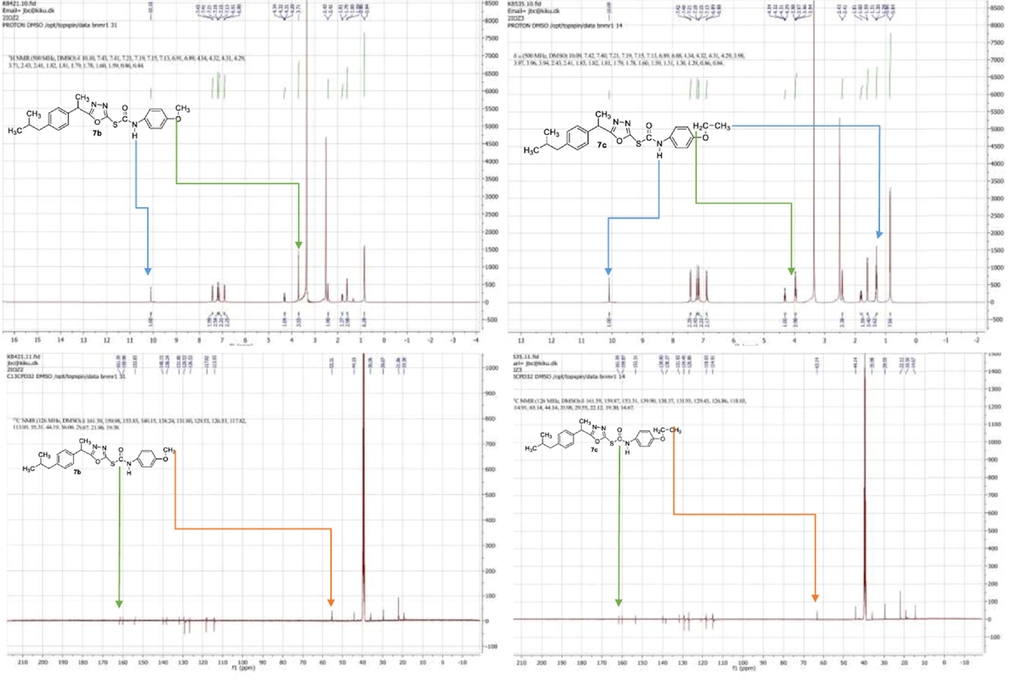
1H NMR and 13C NMR spectra of 7b and 7c.
2.1.9 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl) (2-methylyphenyl)carbamothioate (7d)
Off white amorphous solid; (86 %); C22H25N3O2S; Mol. Wt. 395.52; m.p 191–193 °C; IR (KBr, cm−1) 2906.21, 2363.96, 2313.72, 2164.19, 1690.15, 1515.73, 698.36; 1H NMR (500 MHz, DMSO) δ 9.32 (s, 1H, N—H), 7.74–7.72 (d, 1H, J = 10, H-3′), 7.21–7.20 (d, 2H, J = 5, aromatic-H-9 & aromatic-H-13), 7.17–7.15 (d, 2H, J = 10, aromatic-H-10 & aromatic-H-12), 7.15 (d, 1H, J = 5 & 10, aromatic-H-6′), 7.14 (t, 1H, aromatic-H-5′), 6.99–6.97 (t, 1H, J = 5, aromatic-H-4′), 4.34–4.30 (q, 1H, H-6), 2.43–2.42 (d, 1H, J = 5 CH2-14), 2.22 (s, 3H, CH3-2′) 1.84–1.77 (m, 1H, H-15) 1.61–1.59 (d, J = 5, 3H, CH3-7), 0.86–0.85 (d, J = 5 Hz, 6H, H-16,H-17). 13C NMR (126 MHz, DMSO) 162.21(C⚌O),160.92(C5), 139.90(C-11), 138.51 (C-8), 136.80(C-1′), 130.49(C-2′), 129.11(C-3′), 128.24(C-10 & C-12), 126.85(C-4′), 126.35(C-9 & C-13), 123.29(C-5′), 120.17(C-6′), 43.89(CH2, C-14), 36.63(C-6), 29.41(C-15), 22.44(2CH3, C-16 & C-17), 19.41(CH3, C-7), 17.99(CH3, C-2′); EIMS m/z: 395.17 (100.0 %), 396.17 (23.8 %), 397.16 (4.5 %), 397.17 (2.7 %), 396.16 (1.1 %), 398.17 (1.1 %); Elemental Analysis: C, 66.81; H, 6.37; N, 10.62; O, 8.09; S, 8.11 (supplementary data, S17-S21).
2.1.10 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl) (phenyl)carbamothioate (7e)
Off white amorphous solid; (84 %) C21H23N3O2S; Mol. Wt. 381.49; m.p 133–135 °C; IR (KBr, cm−1) 3666.46, 2346.03, 2313.66, 2160.86, 2120.94, 2023.86, 2008.29, 1695.15, 1618.61, 1562.64, 1540.63 1445.89; 1H NMR (500 MHz, DMSO) δ 9.32 (s, 1H, N—H), 7.52–7.50 (d, 2H, J = 10, aromatic-H-2′ & aromatic-H-6′), 7.32–7.30 (t, 2H, J = 5, aromatic-H-3′ & aromatic-H-5′), 7.21–7.20 (d, 2H, J = 5, aromatic-H-9 & aromatic-H-13), 7.15–7.13 (d, 2H, J = 10, aromatic-H-10 & aromatic-H-12), 6.98–6.95(t, 1H, J = 5, aromatic-H-4′), 4.36–4.33 (q, 1H, H-6), 2.42–2.41 (d, 1H, J = 5 CH2-14), 1.83–1.79 (m, 1H, H-15) 1.61–1.60 (d, J = 5, 3H, CH3-7), 0.86–0.85 (d, J = 5 Hz, 6H, H-16,H-17). 13C NMR (126 MHz, DMSO) 161.96(C⚌O), 159.98(C5), 140.10(C-11), 138.71 (C-8), 138.32(C-1′), 129.28(C-3′ & C-5′) 128.95(C-10 &C-12), 126.86(C-4′), 126.86(C-9 & C-13), 121.44(C-4′), 116.70(C-2 & C-6′), 43.89(CH2, C-14), 36.08(C-6), 29.40(C-15), 22.16(2CH3, C-16 & C-17), 19.36(CH3, C-7); EIMS m/z: 381.15 (100.0 %), 382.15 (22.7 %), 383.15 (4.5 %), 383.16 (2.5 %), 382.15 (1.1 %), 384.15 (1.0 %); Elemental Analysis: C, 66.12; H, 6.08; N, 11.01; O, 8.39; S, 8.40 (supplementary data, S22-S23).
2.1.11 S-(5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-yl) (2-chlorophenyl)carbamothioate (7f)
Off white amorphous solid; (88 %); C21H22ClN3O2S Mol. Wt. 415.94; m.p 121–123 °C; IR (KBr, cm−1) 2906.21, 2363.96, 2313.72, 2164.19, 1680.15, 1515.73, 698.36, 679.66; 1H NMR (500 MHz, DMSO) δ 9.32 (s, 1H, N—H), 7.88 (d, 1H, J = 10, aromatic-H-6′), 7.64 (d, 1H, J = 10, aromatic-H-3′), 7.40–7.37 (t, 1H, J = 5 & 10, aromatic-H-5′), 7.22–7.20 (d, 2H, J = 10, aromatic-H-9 & aromatic-H-13), 7.15–7.13 (d, 2H, J = 10, aromatic-H-10 & aromatic-H-12), 7.06–7.03 (t, 1H, J = 5 & 10, aromatic-H-4′), 4.35–4.32 (q, 1H, H-6), 2.43–2.41 (d, 1H, J = 10 CH2-14), 1.85–1.78 (m, 1H, H-15) 1.60–1.59 (d, J = 5, 3H, CH3-7), 0.86–0.84 (d, J = 10 Hz, 6H, H-16,H-17).13C NMR (126 MHz, DMSO) 162.72(C, C⚌O),160.11(C5), 139.91(C-11), 138.32 (C-8), 137.00(C-1′), 133.11(C-3′), 129.27(C-10 &C-12), 128.23(C-4′), 127.27(C-9 & C-13), 125.36(C-5′), 123.03(C-6′), 114.89(C-2′), 44.39(CH2, C-14), 36.29(C-6), 29.77(C-15), 22.30(2CH3, C-16 & C-17), 19.42(CH3, C-7); EIMS m/z: 415.11 (100.0 %), 417.11 (32.0 %), 416.12 (22.7 %), 418.11 (7.3 %), 417.11 (4.5 %), 417.12 (2.5 %), 419.10 (1.4 %), 416.11 (1.1 %), 418.11 (1.0 %); Elemental Analysis: C, 60.64; H, 5.33; Cl, 8.52; N, 10.10; O, 7.69; S, 7.71 (supplementary data, S24-S28).
2.2 Screening for antiseizure potential
2.2.1 Animals
The 6–8 weeks old Balb/c mice of both sexes weighing 25–40 g were used in this study. The animals used in this study were obtained from the animal house of the Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The housing conditions were maintained with 25 °C and 12 h day/night cycle. The animals were given free access to standard rodent food and water. Before experimentation, the animals were habituated to the test environment and experimenter’s handling to maximally avoid handling-induced stress. All experimental studies on animals were under the ARRIVE guidelines keeping in view the minimal number of animal usage and permitted by the departmental Ethical Committee vide # 29/SC/2022.
2.2.2 Chemicals and drugs
All synthetic compounds named 7a, 7b, 7c, 7d, 7e and 7f were injected through the intraperitoneal route at a dose of 100 mg/kg. The dilution of each compound was made by dissolving 10 mg of the compound in 1 ml of 1 % tween 80 just before administration. The levetiracetam (50 mg/kg) (Nieoczym et al., 2013) and diazepam (5 mg/kg) (Ahmad et al., 2019) were dissolved in distilled water and injected intraperitoneally.
2.2.3 6 Hz seizure model
For the induction of psychomotor seizures, the mice were given corneal stimulation (6 Hz, 32 mA, 0.2 ms pulse duration, 3 s duration) (Barton et al., 2001). The corneas were treated with 1–2 drops of 0.5 % tetracaine hydrochloride about 15–20 min before electrical stimulation. The corneal electrodes were made wet with normal saline to increase the electrical conductivity to the corneas of manually held mice. The mice were immediately released after corneal stimulation (UGO BASILE rodent shocker 7801) and monitored for behavioral changes. The 6 Hz seizures are presented as animal’s stunned posture with rearing, Straubing of tail, twitching of vibrissae and forelimb clonus for 60–120 s. The animals were considered protected if resumed their normal behavior within 10 s after stimulation. In the current study, each synthetic compound (100 mg/kg; i.p.) was injected 45–60 min before 6 Hz corneal stimulation. The outcomes were compared with vehicle-treated mice injected with 1 % Tween 80 (1 ml/kg; i.p.) using levetiracetam (50 mg/kg; i.p.) as standard treatment.
2.2.4 Acute PTZ test
The protection from PTZ-induced seizures is one of the primary protocols relied on to claim the antiepileptic potential of test compounds. The acute PTZ model is characterized by full body tonic/clonic seizures in rodents after the administration of the lethal dose of PTZ. The initial signs of clonic convulsions include facial and forelimb movements followed by full-body tonic-clonic seizures that eventually lead to the front and hind limb extension which is followed by the animal’s death (Koutroumanidou et al., 2013).
The randomly chosen mice were divided into nine groups (n = 4). The animals of all groups were pre-treated with test compounds (100 mg/kg) and after 45–60 min of treatment, the animals were exposed to PTZ (80 mg/kg) (Herrera-Calderon et al., 2018) and the response was monitored for 30 min. The animals were monitored behaviorally for latency to first myoclonic twitch, latency to the episode of generalized tonic-clonic seizures, latency to seizure with hind limb extension and latency to death (Liu et al., 2019) and outcomes were compared with PTZ control animals.
2.2.5 Recording of video/EEG (vEEG) in acute PTZ test
The intraperitoneally injected ketamine/xylazine cocktail was used to anesthetize the mice (Bielefeld et al., 2017) and animals were fixed onto the stereotaxic apparatus equipped with a heating pad to circumvent the risk of hypothermia. After shaving the fur from the required area, the skin of the head was swabbed with 70 % ethanol to prepare it for aseptic surgery. The cortical screw electrodes were stereotaxically implanted at AP + 3 mm; LL ± 1.5 mm. Additionally, one screw served as the indifferent reference electrode (AP − 2 mm; L − 1.5 mm). The screw electrodes were fixed in the skull using an additional skull screw for anchoring (Bröer et al., 2016). All mounted screws and the headset were covered with dental cement. Animals were continuously monitored during the surgical procedure and were housed individually in clear polycarbonate until they recover and move freely in the cage.
After providing the 5–7 days of recovery, the individually caged mice were connected to the 8-channels bioamplifiers (ADInstruments ltd., Sydney, Australia) and an analog–digital converter (PowerLab 8/30 ML870, ADInstruments) for EEG recording. The vEEG was recorded using a laptop equipped with a Logitech camera for video recording.
After introducing into the EEG-dedicated cages, the animals were allowed to acclimatize for 30 min. The baseline was recorded for 15–20 min and the synthetic compounds 7b and 7c (100 mg/kg; i.p.) were injected to separate groups of animals (n = 4). After an hour, the PTZ (80 mg/kg; i.p.) was administered and vEEG was recorded for 30 min using a signal sampling rate of 200 Hz and bandpass filtered between 0.1 and 60 Hz (Anjum et al., 2018). The EEGs were analyzed for electrographic alterations and spikes which were the electroencephalogram changes of at least twice the amplitude of the EEG baseline. A previously reported spike analysis algorithm by Muneeb et al. was used to analyze the spiking activity. The outcomes were expressed as the latency (sec) to the first post-PTZ spike and the number of spikes in 90 sec followed by the first spike (Anjum et al., 2018).
2.2.6 Molecular docking
The AutoDock Tools v1.5.6 and AutoDock v4.2 were used to carry out the molecular docking of all target compounds (Morris et al., 2009). RCSB protein data bank (https://www.rcsb.org/) was used for downloading the crystal structure of GABAA-receptors (PDB ID 6HUP, co-crystallized with Diazepam (DZP)) (Berman et al., 2007). The synaptic vesicle protein 2A (SV2A) sequence was retrieved from the National Center for Biotechnology Information (NP_476558.2). Then I-Tasser server was used to convert the protein sequence (Roy et al., 2010) into 3D model of the protein. The structures of compounds were geometry/energy minimized with Chem 3D pro 12.0 and saved as a PDB file format. In the case of GABAA receptors, the grid dimensions were calculated from an active site with the co-crystallized ligand of protein structure using Discovery Studio Visualizer version 4.0 (Waltham, MA, USA). The active site for the docking study was selected by defining a grid of dimensions 60 × 60 × 60 size with 0.375 Å over the co-crystallized ligand. For SV2A protein, the grid box was confirmed with the CB-Dock (blind docking) online tool (https://clab.labshare.cn/cb-dock/php/). The ligands were docked onto the SV2A protein using a 70 Å × 70 Å × 70 Å grid box which covered all putative residues involved in racetam recognition according to the literature, and a Lamarckian genetic algorithm was used for docking (Correa-Basurto et al., 2015; Shi et al., 2011). A hundred confirmations were generated and poses of the ligands were sorted based on the lowest free binding energy (Stroganov et al., 2008).
where, (a) final intermolecular energy = vdW + dissolve energy and electrostatic energy + H-bond, (b) total internal energy, (c) torsional free energy, and (d) unbound system’s energy. The best active binding mode with the lowest energy was designated and visualized through Discovery Studio Visualizer v 4.0.
2.2.7 Data analysis
Every animal was independently observed for the parameters i.e. latency of mycolic twitch, full body generalized seizures and death which had normal distribution and evaluated by one-way ANOVA followed by Dunnet’s multiple comparison test using GraphPad Prism version 8. The EEGs were analyzed with LabChart version 8 and the inter-group comparison for latency to first spike and spike counts in electroencephalogram was made by the non-parametric Mann-Whitney test. All data were expressed as Mean ± SEM considering P < 0.05 significant.
3 Results and discussion
3.1 Chemistry
Present work is a synthetic approach for the synthesis of N-substituted 5-aryl-1,3,4-oxadiazole-2-carbamothioate derivatives (7a-f) which is based upon chemical modification of 2-(4-isobutylphenyl)propanoic acid for improving their safety profile. Previously we have synthesized different oxadiazole based derivatives containing acetamide functionalities and it is proved from literature that oxadiazole possess good anti-convulsant activity So in continuation of our previous work on heterocyclic compounds (Gul et al., 2017, Khan et al., 2020, Khan et al., 2021), we have cyclized –COOH group of 2-(4-isobutylphenyl)propanoic acid into five membered heterocycle e.goxadiazole nucleus 4. Oxadiazole was modified at SH by replacing its H-group with different electrophiles yielding final compounds (7a-7f).
The synthetic route of synthesized compounds (7a-7f) is in Scheme 1. Compound (2) was obtained by refluxing the 2-(4-isobutylphenyl)propanoic (1) with absolute methanol for six hour using Fischer esterification method (Gulnaz et al., 2019). The ester of 2-(4-isobutylphenyl)propanoic was gently refluxed with 80 % hydrazine hydrate (Iqbal et al., 2017) in methanol to get 2-(4-isobutylphenyl) propanehydrazide (3). In third step, the compound 3 was cyclized into 5-(1-(4-isobutylphenyl) ethyl)-1,3,4-oxadiazole-2-thiol (4) by gently refluxing the compound 3 in methanol with carbon disulfide and KOH. After completion, the reaction mixture in flask was acidified by conc. HCl and product 4 was isolated by filtration. Compound 4 was reacted with different substituted aralkyl/alkyl/aryl 2-bromocarbamides (6a-f) using DMF and NaH at room temperature. Reaction progress was continuously checked by TLC. After successful completion, the solvent in flask was evaporated. Chilled water was introduced in reaction mixture to get product. Product were filtered and dried. The product was also recrystallized using absolute ethanol. The structures of oxadiazole based carbamothionate derivatives were confirmed by spectroscopic (FTIR, 1H NMR, 13C NMR) and physiochemical methods.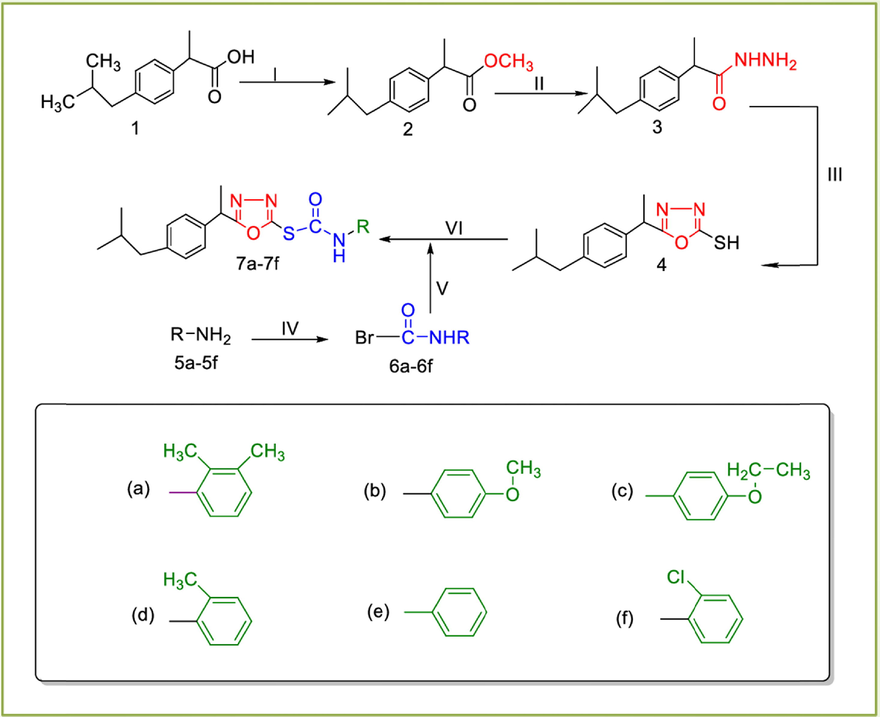
Synthesis of target compounds (7a-7f): (I) H2SO4/EtOH/refluxing for 3–4 h (II) N2H4/MeOH/stirring for 5–6 h (III) CS2/KOH/EtOH/refluxing for 3–6 h (IV) carbonicdibromide/H2O/5% Na2CO3 soln./stirring for 1 h (V) DMF/NaH/stirring for 2–3 h.
In 1H NMR spectrum, chemical shift value for –NH proton in all derivatives was observed in between 10.10 and 9.29. The aliphatic and aromatic protons resonate between 4.35 and 0.85 ppm and 7.88–6.81 ppm respectively. Analysis of 1H NMR spectra for compounds (7a-7f) exhibited the segregation of peaks in four distinct regions. The protons of the aromatic ring system were the most deshielded and observed in between 7.88 and 6.81 ppm. Chemical shift value for aromatic protons of ibuprofen were observed as doublet with double integration in between 7.21 and 7.01 ppm. The aliphatic –CH3 protons of ibuprofen were the most shielded one and observed around 0.86–0.84. Key evidence of formation of carbamide moiety was appearance of NH peak around 10.10–9.29 ppm. 13C NMR spectroscopy also support the formation of carbamide by showing peaks at 162.15 ppm that correspond to C⚌O group of carbamide, and peaks at 161.15 ppm corrosponds to carbon nucleus of triazole ring. Characteristics signals at 22 ppm are due to two –CH3 carbon nuceus of ibuprofen. Segregation of signals between 44.18 and 20.19 ppm correspond to CH2 groups of ibuprofen. We have reported structure activity relationship by introducing some electron withdrawing substitution and compared them with electron donating group substitution on phenyl group. On the basis of medicinal importance, anticonvulsant activities of all compounds were checked.
3.2 6 Hz
In the 6-Hz test, only two compounds out of seven compounds showed protection. The 7b and 7e protected 2/4 while 7f protected 1/4 of the animals from psychomotor seizures at pretreatment time 1 h. However, the most prominent protection was observed in animals treated with 7c as 4/4 of the tested animals resumed the normal exploratory behaviour within 10 sec of shock application. The other two compounds were also tested at the same dose but no animals were protected. The outcomes were compared with levetiracetam (50 mg/kg), which protected 4/4 of animals (Table 1).
Group
Dose (mg/kg)
Animals tested
Animals protected
% Protection
Control
–
4
0
0 %
Levetiracetam
50 mg/kg
4
4
100 %
7a
100 mg/kg
4
0
0 %
7b
100 mg/kg
4
2
50 %
7c
100 mg/kg
4
4
100 %
7d
100 mg/kg
4
0
0 %
7e
100 mg/kg
4
2
50 %
7f
100 mg/kg
4
1
25 %
The 6 Hz model utilized the rectangular pulses of low frequency (6-Hz) of 0.2 ms with a current intensity of 32 mA delivered via corneal electrodes for 2–3 sec to induce seizures that mimicked psychomotor seizures occurring in human limbic epilepsy (Metcalf et al., 2017). At this low current intensity, the 6 Hz test worked as a discriminating screening tool to identify the antiseizure potential of currently synthesized compounds.
3.3 Acute PTZ test
The animals were visually monitored for behavioral changes for 30 min after PTZ injection. The one-way ANOVA revealed significant differences among all groups for all observed parameters i.e. in onset of first myoclonic twitch [F (7,24) = 16.08, P < 0.0001], tonic-clonic seizures [F (7,24) = 59.17, P < 0.0001], tonic hind limb extension [F (7,24) = 18.20, P < 0.0001], and death [F (7,24) = 16.42, P < 0.0001.
The PTZ control animals showed the onset of the first myoclonic twitch within 58.00 ± 5.61 sec which was significantly delayed to 311.25 ± 42.67 (P < 0.01) sec in animals pre-treated by 7b. The onset of seizures remained continuous and progressed into full body tonic-clonic seizures after 87.50 ± 12.5 sec after PTZ administration. But, the administration of 7b showed an increased latency of 518.00 ± 117.37 sec (P < 0.01) in the onset of tonic-conic seizures. Unfortunately, the onset of the first myoclonic twitch and full body tonic-clonic seizures were not significantly affected by the remaining five compounds.
As the literature reports that PTZ-induced generalized tonic-clonic seizures are followed by tonic hind limb extension which causes respiratory arrest and death in rodents. In the current study, the latency to onset of tonic hind limb extension was also noted and compared among all groups. The 75 % of animals treated with 7b did not progress to seizures with hind limb extension and remained protected from death. It was also interesting to note the significant latency of 1044.50 ± 437.76 sec to seizures with hind limb extension in 7c treated mice. Moreover, the two out of four animals did not show the PTZ-induced hindlimb extension and death. These outcomes were significantly prominent (P < 0.01) as compared to PTZ control animals where all four animals presented the seizures with hind limb extension within 203.00 ± 28.40 sec followed by death within 234.59 ± 29.07 sec (Table 2).
Group
Latency to first myoclonus twitch
(sec)
Latency to tonic clonic seizures
(sec)
Latency to tonic hind limb extension
(sec)
Latency to death
(sec)
% Protection from tonic hind limb extension
% Survial
PTZ control
58.00 ± 5.61
87.50 ± 12.5
203.00 ± 28.40
234.59 ±29.07
0 %
0%
Diazepam
650.00± 132.28****
1800.00 ± 0.00****
1800.00 ± 0.00****
1800.00 ± 0.00****
100 %
100%
7a
103.50 ± 15.67
173.33 ± 31.75
462.00 ± 33.17
534.25 ± 32.40
0 %
0 %
7b
311.25 ± 42.67**
518.00 ± 117.37**
1526.83 ± 273.16****
1662.50 ± 137.50****
75 %
75 %
7c
100.50 ± 8.05
127.25 ± 7.33
1044.50± 437.76**
1095.00 ± 411.61**
50 %
50 %
7d
100.25 ± 8.39
334.50 ± 170.11
644.50 ± 105.27
746.50 ± 58.02
0 %
0 %
7e
109.00 ± 3.08
104.75 ± 5.07
118.00 ± 1.22
143.75 ± 13.13
0 %
0 %
7f
110.00 ± 13.08
228.25 ± 27.32
300.00 ± 25.87
392.50 ± 46.96
0 %
0 %
The PTZ provokes convulsions by antagonizing the GABAA receptors non-competitively (Rehman et al., 2022). Gamma-aminobutyric acid type A (GABAA) receptors are responsible for most of the fast inhibitory neuronal transmission. The balance between excitatory neurotransmission via glutamate and inhibitory neurotransmission via GABA is vital for proper neurologic function and cell membrane stability. Seizure generation and epilepsy are strongly linked with decreased levels of GABA, alterations of GABAA receptor plasticity, trafficking, and expression (Fritschy, 2008). PTZ suppresses the transmission of inhibitory synapses, resulting in enhanced excitation of neuronal circuits. At a high dose, a single intraperitoneal injection of PTZ into an animal reliably evokes acute, severe generalized seizures (Shimada and Yamagata, 2018). Numerous studies have reported that compounds which enhance GABAergic neurotransmission and synaptosomal GABA concentration are effective in attenuating seizure severity in the PTZ seizure model (Rogawski et al., 2016).
Though the progression of epilepsy is complicated, the neuroinflammatory processes usually play a crucial role in the precipitation and exacerbation of seizures (Marchi et al., 2014). Besides its role as a GABAA antagonist, PTZ administration is further linked with the up-regulation of various pro-inflammatory cytokines in the hippocampus and cerebral cortex resulting in disrupted BBB and seizure induction (Faheem et al., 2021). PTZ administration causes elevated oxidative stress and increased expression of lipid peroxidase, nitric oxide and TNF-α (Alvi et al., 2021). Normally, the COX-2 expression is significantly upregulated in hippocampal neurons within 1 h after seizures showing that neuroinflammation might be the potential therapeutic target during the treatment of epilepsy (Zhang et al., 2008).
3.4 Effects of 7b and 7c on PTZ-induced changes in EEG
The outcomes remained consistent with latency to myoclonic twitch induced by PTZ, the first epileptic spike which was twofold of baseline appeared in PTZ control mice within 60 s of PTZ injection. These EEG changes were followed by the increased frequency of spikes in the duration of 90 sec after the first spike. The animal group pre-treated with 7b showed a marked difference (P < 0.05) of duration from PTZ injection to the first epileptic spike appearance on the electroencephalogram. Evaluation of animals in terms of the number of epileptic spikes in post-first spike 90 sec found that administration of 100 mg/kg of compound 7b significantly reduced the seizure activity as measured by the number of spikes compared to the PTZ control group (Fig. 2A and 2B). The animals treated with 7cslightly increased the latency of the first epileptic spike from 43.75 ± 3.63 sec (PTZ control) to 52.10 ± 2.35 (7c group), the difference was not statistically significant. Similarly, the 7c could not reduce the number of spikes in the post-first spike 90 sec (Fig. 3). It is worth mentioning that though the EEG changes were noted in all four 7c animals after PTZ injection, PTZ failed to induce hind limb extension and death in two of the four animals.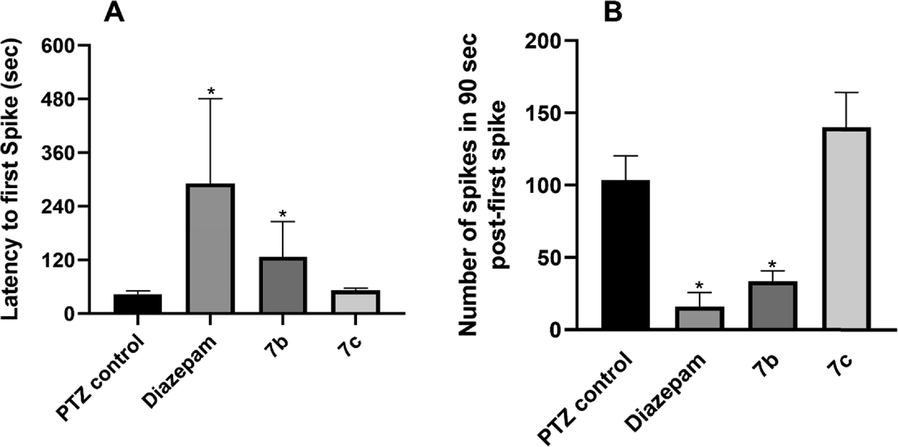
After 45–60 min of pretreatment with Diazepam, 7b and 7c to the respective groups (n = 4), the animals were injected with PTZ (80 mg/kg) and (A) latency to the first epileptic spike and (B) the number of spikes in post-first spike 90sec were monitored with EEG. The PTZ control animals exhibited showed reduced latency and increased number of epileptic spikes in comparison to Diazepam and 7b treated animals and outcomes were statistically significant when compared with the non-parametric Mann-Whitney test. All data are represented as Mean ± SEM and * indicates significance at p < 0.05.
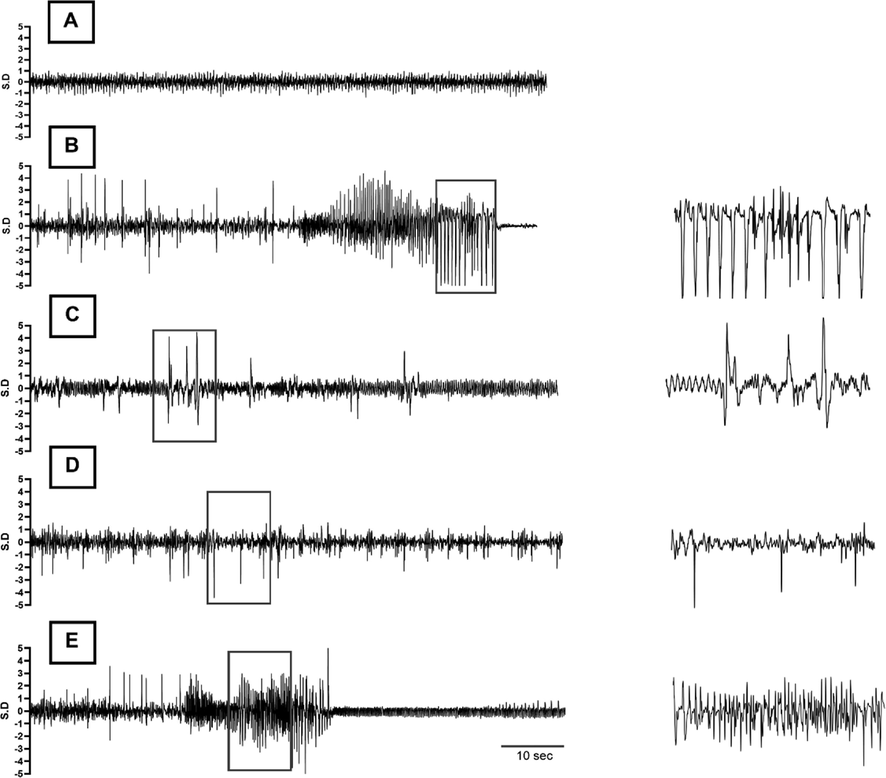
Typical EEG traces recorded during the experiment. (A) Representative portion of baseline EEG before administration of the chemo-convulsant. (B-E) 90 s of electrical activity recorded after appearance of first epileptic spike post-PTZ injection, showing EEG of (B) PTZ control (PTZ only), (C) Standard Control (Diazepam + PTZ), (D) Treatment group 1 (Compound 7b + PTZ) and (E) Treatment group 2 (Compound 7c + PTZ), respectively. A high timed resolution of spike cluster(s) (square boxes) is shown in parallel to the corresponding EEG tracings as well.
Oxadiazoles are compounds of synthetic origin that have been studied broadly in the last few decades (Wang et al., 2020). Their anti-inflammatory (Akhter et al., 2009), analgesic (Kaur et al., 2019) and anti-oxidant (Mihailović et al., 2017) potential have been widely reported. In preclinical studies, the administration of oxadiazole-derived compound resulted in the correction of the post-PTZ behavioural deficit by reducing the seizure's duration and delaying the seizure onset.
Ibuprofen is a widely used anti-inflammatory drug that mediates its major pharmacological effects through the inhibition of COX-2. A previous study by Liu et al reported that expression of COX-2 was reduced in rats pretreated with ibuprofen as compared to PTZ control animals (Liu et al., 2020). Our current results suggested that 7b and 7c worked fairly well in PTZ model. The EEG epileptiform activity, the number of epileptic spikes and overall mortality in PTZ-treated mice were reduced in 7b treated animals. These findings are in-line with the previously reported literature where seizure latency was increased and duration as well as frequency were decreased by the anti-inflammatory drug, ibuprofen (Liu et al., 2020).
3.5 Docking studies
Epilepsy is a neurological disease that characterized by recurrent seizures. This disease originates due to abnormal electrical discharge from the brain neurons and it affects almost 1 % of the world’s population. The invention of new targeted anticonvulsant drugs provides a very effective approach in epilepsy treatment. The γ-Aminobutyric acid type A (GABAA) receptors are very crucial targets of the central nervous system drugs and benzodiazepine sites on the GABAA receptor are important active sites for the development of anticonvulsant drugs (Rudolph and Knoflach, 2011). The synaptic vesicle protein 2A is an essential membrane protein that present on all synaptic vesicles and responsible for many important functions in the central nervous system. Moreover, scientist also found out that the reduction of SV2A protein contributes in progression of epilepsy. Synaptic vesical protein 2A is the molecular target of the second-generation antiepileptic drug such as levetiracetam and its structural analogs brivaracetam and seletracetam. In recent years, molecular docking has become an important technology in the field of computer aided drug study. Therefore, an in-silico study was performed to identify the binding modes of the synthesized compounds with receptor proteins.
In the crystal structure of SV2A, the co-crystallized ligand is not present, therefore, the antiepileptic drug such as levetiracetam (LEV) was docked inside the SV2A protein. The docking score of the reference drug was −4.34 Kcal/mol and showed hydrogen bonding interactions with important residues Asn579, Gly687, Asn690, Ala691 and Lys694 in green color. LEV exhibited alkyl interactions with Lys580, Cys583 and Leu585 (Fig. 4A). All compounds were docked inside SV2A protein and only compounds 7b and 7c showed good results their binding energies are presented in Table 3. These compounds showed numerous interactions with residues such as hydrogen bonding, π-alkyl, π-stacking, and π-sulfur interactions and their bonding distance also presented in corresponding docking diagrams. Compound IOZ 3 showed better activity with its comparatively lower binding energy of −3.68 Kcal/mol. In SV2A compound 7b forms hydrogen bonding interaction with amino acid residues Asn690 and Lys694. The π-alkyl interactions with amino acids Leu657, Ala664, and Ala691. The π-sulfur interactions are also found with amino acid residues Cys583, Cys656, and Phe686 (Fig. 4B). The 7c also interacted with amino acid residues located at active sites of the SV2A and all possible interactions are presented (Fig. 4C). The docking study of remaining compounds is also added in supplementary data (supplementary data, S29-S30).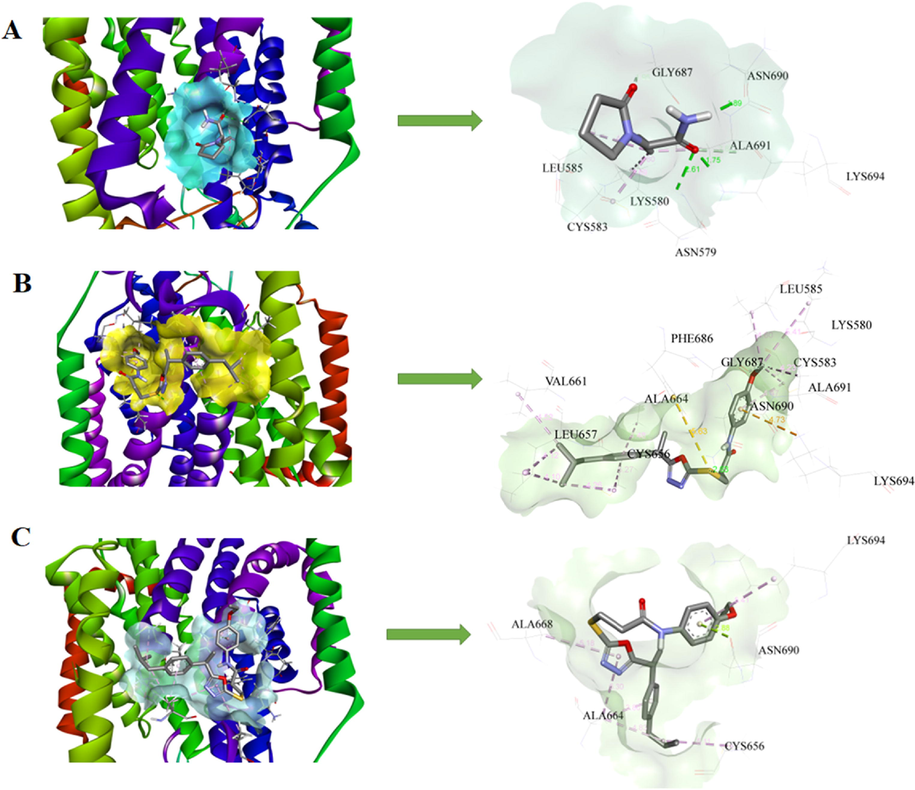
3D models and schematic representations of the interactions between SV2A and (A) LEV, (B) 7b and (C) 7c with their bond distances. Green dashed lines represent hydrogen bonding interactions. The π-sulfur interactions are represented by yellow dashed lines. Light pink and purple lines represent π-alkyl and π-π stacked interactions respectively.
Compounds
Binding Energies against SV2A Protein
(Kcal/mol)Binding Energies against GABAA Receptors (6HUP)
(Kcal/mol)
7a
−3.55
−6.39
7b
−3.58
−6.57
7c
−3.68
−6.64
7d
−3.49
−6.29
7e
−3.38
−5.99
7f
−3.53
−6.04
Levetiracetam
−4.34
–
Diazepam
–
−6.44
The SV2A is an essential membrane protein located on all synaptic vesicles and responsible for many important functions in the central nervous system. Moreover, the scientist also found out that the reduction of SV2A protein results into the progression of epilepsy. The second-generation antiepileptic drug i.e. levetiracetam and its structural analog brivaracetam interact with this synaptic vesical protein to produce their antiseizure effects (Lynch et al., 2008). Thus, beneficial outcomes of 7b and 7c compounds in 6 Hz test might be attributed to the lower binding energies and modulating the SV2A protein levels in brain.
The crystal structure of GABAA receptors (PDB ID 6HUP, co-crystallized with Diazepam (DZP502)) was downloaded from the protein data bank. The co-crystallized ligand was re-docked in the active sites of the GABAA receptor and Autodock4.2 was capable to reproduce the same binding modes with < 1.5 Å RMSD. The docking score of the reference ligand was −6.44 Kcal/mol and showed hydrogen bonding interactions with important residues Thr262 and Asn265 represented in green color. A π-sulfur interaction was found Met261 represented in yellow color. The chlorine atom of the reference ligand exhibited alkyl interactions with Leu285 and Met286. The π-stacked interactions were also found with Phe289 (Fig. 5A). All compounds were docked in the active site of GABAA receptor sire and only 7b and 7c showed good results and their lowest binding energies are presented in Table 3. These compounds showed numerous interactions with residues such as hydrogen bonding, π-alkyl, π-stacking, and π-sulfur interactions and their bonding distance also presented in corresponding docking diagrams. Compound 7b showed the best protection in the PTZ test which might be due to its lower binding energy of −6.57 Kcal/mol. In 6HUP compound 7b forms hydrogen bonding interaction with amino acid residues Thr262 and Asn265. The π-alkyl interactions with amino acid Met261, Leu285 and Met286, π-π staking interaction with Phe289 were also observed (Fig. 5B). The 7c also interacted with amino acid residues located at active sites of the GABAA receptor and all-possible interactions are presented in Fig. 5C. The study revealed the brain permeability and lower binding energy of these compounds showing the increased interaction with the receptor. These observed outcomes might be attributed to the GABAA agonistic effects of these compounds. The docking study of remaining compounds is also added in supplementary data.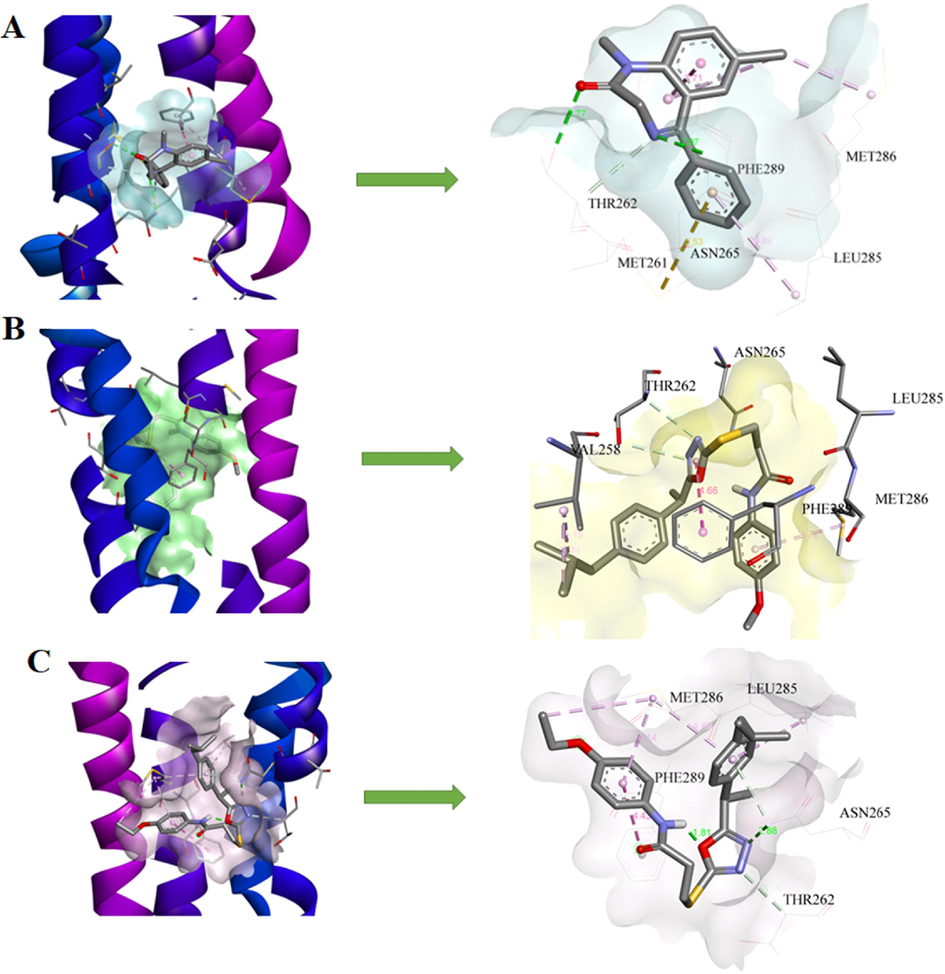
3D models and schematic representations of the interactions between 6HUP and (A) Diazepam (B) 7b and (C) 7c with their bond distances. Green dashed lines represent hydrogen bonding interactions. The π-sulfur interactions are represented by yellow dashed lines. Light pink and purple lines represent π-alkyl and π-π stacked interactions respectively.
4 Conclusion
Target compounds (7a-f) were synthesized by coupling compound (4) with different electrophiles under different reaction conditions, that eventually led to the synthesis of various carboxamide based oxadiazole derivatives (7a-f) in moderate to good yields (77–88 %). The series of compounds were tested for anticonvulsant potential in acute 6 Hz and PTZ seizures models in mice. The significantly noticeable results were observed in animals treated with 7b and 7c. The EEGs findings showed the reduced epileptic spiking activity in brains of 7b-treated mice. The molecular docking studies showed that these pharmacological outcomes might be attributed to the SV2A and GABAA modulating potentials of 7b and 7c, both contained electron donation groups at position 4.
Acknowledgements
We are thankful to Mr. Muhammad Imran, an animal house attendant who is responsible for the care of animals in the animal house facility of Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan.
Funding
This work was funded by Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia through research supporting project Number (RSP2023R131).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. Beni-Suef Univ. J. Basic Appl. Sci.. 2016;5:85-96.
- [Google Scholar]
- Synthesis, in-vitro cholinesterase inhibition, in-vivo anticonvulsant activity and in-silico exploration of N-(4-methylpyridin-2-yl)thiophene-2-carboxamide analogs. Bioorg. Chem.. 2019;92:103216
- [Google Scholar]
- Synthesis and anti-HIV activity of new chiral 1, 2, 4-triazoles and 1, 3, 4-thiadiazoles. Heteroat. Chem.. 2007;18:316-322.
- [Google Scholar]
- Aroylpropionic acid based 2,5-disubstituted-1,3,4-oxadiazoles: synthesis and their anti-inflammatory and analgesic activities. Eur. J. Med. Chem.. 2009;44:2372-2378.
- [Google Scholar]
- Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. Daru. 2014;22:22.
- [Google Scholar]
- 1,3,4, Oxadiazole compound A3 provides robust protection against PTZ-induced neuroinflammation and oxidative stress by regulating Nrf2-pathway. J. Inflamm. Res.. 2021;14:7393-7409.
- [Google Scholar]
- Automated quantification of EEG spikes and spike clusters as a new read out in Theiler’s virus mouse model of encephalitis-induced epilepsy. Epilepsy Behav.. 2018;88:189-204.
- [Google Scholar]
- Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res.. 2001;47:217-227.
- [Google Scholar]
- The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res.. 2007;35:D301-D303.
- [Google Scholar]
- A standardized protocol for stereotaxic intrahippocampal administration of kainic acid combined with electroencephalographic seizure monitoring in mice. Front. Neurosci.. 2017;11:160.
- [Google Scholar]
- Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp. Neurol.. 2016;279:57-74.
- [Google Scholar]
- Exploring 1, 3, 4-oxadiazole scaffold for anti-inflammatory and analgesic activities: a review of literature from 2005–2016. Mini rev. Med. Chem.. 2018;18:216-233.
- [Google Scholar]
- Identification of the antiepileptic racetam binding site in the synaptic vesicle protein 2A by molecular dynamics and docking simulations. Front. Cell. Neurosci.. 2015;9:125.
- [Google Scholar]
- Investigation of 1, 3, 4 Oxadiazole Derivative in PTZ-Induced Neurodegeneration: A Simulation and Molecular Approach. J. Inflamm. Res.. 2021;14:5659.
- [Google Scholar]
- The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front. Neurol.. 2021;12:1030.
- [Google Scholar]
- Epilepsy, E/I balance and GABAA receptor plasticity. Front. Mol. Neurosci.. 2008;1:5.
- [Google Scholar]
- Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1, 3, 4-oxadiazol-2-yl] thio]-N-[4-(4-morpholinyl) phenyl] acetamide derivatives. J. Saudi Chem. Soc.. 2017;21:S425-S433.
- [Google Scholar]
- Design, synthesis and molecular docking of benzophenone conjugated with oxadiazole sulphur bridge pyrazole pharmacophores as anti inflammatory and analgesic agents. Bioorg. Chem.. 2019;92:103220
- [Google Scholar]
- Anticonvulsant effect of ethanolic extract of Cyperus articulatus L. leaves on pentylenetetrazol induced seizure in mice. J. Tradit. Complement. Med.. 2018;8:95-99.
- [Google Scholar]
- Synthesis of N-substituted acetamide derivatives of azinane-bearing 1, 3, 4-oxadiazole nucleus and screening for antibacterial activity. Trop. J. Pharm. Res.. 2017;16:429-437.
- [Google Scholar]
- Synthesis and antifungal potential of some novel benzimidazole-1, 3, 4-oxadiazole compounds. Molecules. 2019;24:191.
- [Google Scholar]
- Optimization of a 1,3,4-oxadiazole series for inhibition of Ca 2+/calmodulin-stimulated activity of adenylyl cyclases 1 and 8 for the treatment of chronic pain. Eur. J. Med. Chem.. 2019;162:568-585.
- [Google Scholar]
- Synthesis, characterization, antibacterial, hemolytic and thrombolytic activity evaluation of 5-(3-chlorophenyl)-2-((N-(substituted)-2-acetamoyl) sulfanyl)-1, 3, 4-oxadiazole derivatives. Pak. J. Pharm. Sci.. 2020;33:871-876.
- [Google Scholar]
- Synthesis, spectral analysis and biological evaluation of 2-{[(morpholin-4-yl) ethyl] thio}-5-phenyl/aryl-1, 3, 4-oxadiazole derivatives. Pak. J. Pharm. Sci.. 2021;34:441-446.
- [Google Scholar]
- Increased seizure latency and decreased severity of pentylenetetrazol-induced seizures in mice after essential oil administration. Epilepsy Res. Treat.. 2013;2013:1-6.
- [Google Scholar]
- Refractory epilepsy: a progressive, intractable but preventable condition? Seizure. 2002;11:77-84.
- [Google Scholar]
- N-terminal alternative splicing of GluN1 regulates the maturation of excitatory synapses and seizure susceptibility. Proc. Natl. Acad. Sci. U. S. A.. 2019;116:21207-21212.
- [Google Scholar]
- Ibuprofen exerts antiepileptic and neuroprotective effects in the rat model of pentylenetetrazol-induced epilepsy via the COX-2/NLRP3/IL-18 pathway. Neurochem. Res.. 2020;45:2516-2526.
- [Google Scholar]
- Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359-368.
- [Google Scholar]
- Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev.. 2020;72:606-638.
- [Google Scholar]
- Löscher, W., 1999. Animal Models of Epilepsy and Epileptic Seizures in Antiepileptic Drugs. 138, 19–62.
- Visualization of SV2A conformations in situ by the use of Protein Tomography. Biochem. Biophys. Res. Commun.. 2008;375:491-495.
- [Google Scholar]
- Design, synthesis, biological evaluation and molecular docking of new 1, 3, 4-oxadiazole homonucleosides and their double-headed analogs as antitumor agents. Bioorg. Chem.. 2021;108:104558
- [Google Scholar]
- Development and Pharmacological Characterization of the Rat 6 Hz Model of Partial Seizures. Epilepsia. 2017;58:1073-1084.
- [Google Scholar]
- Synthesis and antioxidant activity of 1,3,4-oxadiazoles and their diacylhydrazine precursors derived from phenolic acids. RSC Adv.. 2017;7:8550-8560.
- [Google Scholar]
- Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30:2785-2791.
- [Google Scholar]
- Effect of sildenafil, a selective phosphodiesterase 5 inhibitor, on the anticonvulsant action of some antiepileptic drugs in the mouse 6-Hz psychomotor seizure model. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:104-110.
- [Google Scholar]
- Synthesis, anticonvulsant activity and 5-HT1A/5-HT7 receptors affinity of 1-[(4-arylpiperazin-1-yl)-propyl]-succinimides. Pharmacol. Rep.. 2012;64:326-335.
- [Google Scholar]
- The pharmacological treatment of epilepsy: recent advances and future perspectives. Acta Epileptol.. 2021;3:1-11.
- [Google Scholar]
- Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi Pharm. J.. 2022;30:494-507.
- [Google Scholar]
- Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med.. 2016;6:a022780
- [Google Scholar]
- I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc.. 2010;5:725-738.
- [Google Scholar]
- Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov.. 2011;10:685-697.
- [Google Scholar]
- Pharmacoresistant epilepsy: a current update on non-conventional pharmacological and non-pharmacological interventions. J. Epilepsy Res.. 2015;5:1-8.
- [Google Scholar]
- Combining modelling and mutagenesis studies of synaptic vesicle protein 2A to identify a series of residues involved in racetam binding. Biochem. Soc. Trans.. 2011;39:1341-1347.
- [Google Scholar]
- Lead finder: an approach to improve accuracy of protein-ligand docking, binding energy estimation, and virtual screening. J. Chem. Inf. Model.. 2008;48:2371-2385.
- [Google Scholar]
- Synthesis of 1,3,4-oxadiazole derivatives with anticonvulsant activity and their binding to the GABA A receptor. Eur. J. Med. Chem.. 2020;206:112672
- [Google Scholar]
- Design, synthesis, insecticidal activity and 3D-QSR study for novel trifluoromethyl pyridine derivatives containing an 1,3,4-oxadiazole moiety. RSC Adv. 2018;8:6306-6314.
- [Google Scholar]
- Development of 1,3,4-oxadiazole thione based novel anticancer agents: Design, synthesis and in-vitro studies. Biomed. Pharmacother.. 2017;95:721-730.
- [Google Scholar]
- Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48:1223-1244.
- [Google Scholar]
- Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res.. 2011;10:655.
- [Google Scholar]
- Cyclooxygenase-2 inhibitor inhibits hippocampal synaptic reorganization in pilocarpine-induced status epilepticus rats. J. Zhejiang Univ. Sci. B. 2008;9:903-915.
- [Google Scholar]
- Antimicrobial activity of 1, 3, 4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med. Chem.. 2018;10:283-296.
- [Google Scholar]
- Bala, S., Kamboj, S., Kajal, A., Saini, V., Prasad, D.N., 2014. 1,3,4-Oxadiazole Derivatives: Synthesis, Characterization, Antimicrobial Potential, and Computational Studies. Biomed Res. Int. 2014, 172791.
- Khan, M. ul H., Hameed, S., Akhtar, T., Al-Masoudi, N.A., Al-Masoudi, W.A., Jones, P.G., Pannecouque, C., 2016. Synthesis, crystal structure, anti-HIV, and antiproliferative activity of new oxadiazole and thiazole analogs. Med. Chem. Res. 25, 2399–2409.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104610.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







