Translate this page into:
A molecular docking study: Cepharanthine protects articular cartilage against arthritis by Wnt/PI3K/TLR-3 signaling
⁎Corresponding author at: Department of Rheumatology, Jiaozuo people's hospital, No. 267 Jiefang Middle Road, Jiaozuo, Henan Province 454002, China. ErinNoahwPqZhB@hotmail.com (Wenmin Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Rheumatoid arthritis (RA) is an autoimmune disorder that causes joint inflammation and permanent disability in affected patients. This study investigated the protective effect of cepharanthine treatment against Freund's adjuvant-induced RA in a rat model. RA was induced in rats by injection of incomplete Freund’s adjuvant (IFA) into the plantar surface on days 0 and 9. Cepharanthine at a dose of 10 or 20 mg/kg was administered intraperitoneally to separate groups of rats 12 days after the administration of IFA. The effects of cepharanthine on RA were determined by measuring serum biochemical parameters, blood cell counts and serum inflammatory and oxidative stress mediators. The expression levels of several proteins that regulate the Wnt/PI3K/Toll-like receptor (TLR)-3 pathway were measured via immunohistochemistry, western blotting and quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) assays. Molecular docking was performed to assess the interactions of cepharanthine with Wnt, PI3K and TLR-3 proteins. Our data revealed that cepharanthine treatment attenuated serum biochemical parameters, blood cell counts and serum inflammatory and oxidative stress mediators in RA rats. Analyses with qRT-PCR and western blotting revealed that the mRNA expression levels of Wnt/PI3K/TLR-3 pathway components were attenuated in cepharanthine-treated RA rats. Cepharanthine also reduced chondrocyte apoptosis and autophagy. Molecular docking analysis revealed significant interactions of cepharanthine with Wnt, PI3K and TLR-3 proteins. In conclusion, the findings implied that cepharanthine treatment attenuates chondrocyte autophagy, apoptosis and proliferation by regulating the Wnt/PI3K/TLR-3 pathway in RA rats.
Keywords
Cepharanthine
Rheumatoid arthritis
Chondrocytes
Autophagy
Inflammation
1 Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder that results in progressive joint degeneration (Guo et al., 2018). RA is characterised by the formation of osteophytes, loss of articular cartilage and degradation of extracellular matrix (Maldonado and Nam, 2013). RA is an important cause of disability worldwide (Krause et al., 2015). The etiopathogenesis of RA is not fully elucidated, but the following factors contribute to its development: increased growth factor, chemokine and cytokine levels, as well as altered synovial factor expression (Yap et al., 2018). Enhanced secretion of matrix metalloproteinases occurs due to the increased presence of inflammatory mediators, following synovial factor upregulation (Sokolove and Lepus, 2013). Moreover, bone degeneration occurs in RA due to the altered differentiation of osteoclasts and osteoblasts (Corrado et al., 2017). Conventional therapies for RA treatment have various limitations, and further options are needed.
Cell adhesion, limb development and embryogenesis are controlled by the Wnt/β-catenin signalling pathway (Guo et al., 2004). Moreover, the production and proliferation of chemokines and cytokines are regulated by the Wnt/β-catenin pathway (Du et al., 2019). Wnt protein expression is enhanced in the synovial tissues of patients with RA (Tanaka et al., 2005). Inflammatory cytokine levels (e.g., interleukin [IL]-6, IL-8, IL-15 and tumour necrosis factor [TNF]-α) are enhanced due to elevated levels of Wnt3a, Wnt7a and Wnt10b proteins in patients with RA (Hwang et al., 2004). Thus, regulation of the Wnt/β-catenin signalling pathway could aid in the management of RA.
Cepharanthine is an alkaloid isolated from the stem of Stephania cepharantha (Huang et al., 2014). This chemical reportedly can be used to treat thrombocytopenic purpura, alopecia areata and leukopenia (Huang et al., 2014; Rogosnitzky and Danks, 2011; Zhou et al., 2018). Notably, cepharanthine exhibits anti-allergic, anti-viral and anti-inflammatory activities (Kohno et al., 1987; Uto et al., 2011; Okamoto et al., 2001). It inhibits bone resorption, thereby protecting against bone loss and osteoporosis (Zhou et al., 2018). Cepharanthine also has been reported to reduce the levels of inflammatory cytokines (e.g., IL-1β, IL-6 and TNF-α) and inhibit the activity of nuclear factor (NF)-κB (Kudo et al., 2011). This study was performed to investigate the protective effects of cepharanthine against RA.
2 Material and methods
2.1 Animals
Sprague–Dawley rats (body weight 180–225 g) were used in this study. All rats were housed under a 12-h light/12-h dark cycle, with a humidity of 60 ± 5% and temperature of 24 ± 3 °C. All animal experiments in this study were approved by the Institutional Animal Ethical Committee of Jiaozuo People's Hospital, China (IAEC/JPH/2018-19/09).
2.2 Chemicals
Cepharanthine and LY294002, a PI3K inhibitor, were procured from Sigma-Aldrich (St. Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kits for the assessment of inflammatory and oxidative stress mediators were procured from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies for western blotting were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.3 Experimental design and treatment protocol
Forty rats were divided into the following four groups: control, RA, cepharanthine 10 mg/kg, and cepharanthine 20 mg/kg. RA was induced as previously described (Zhang et al., 1999). Isometric incomplete Freund’s adjuvant (IFA) was prepared by emulsification with 2 mL of bovine type II collagen and acetic acid. IFA (0.1 mL of emulsion) was administered on days 0 and 9 by injection into the plantar surface. Establishment of RA was assessed on the basis of hind-paw swelling. Cepharanthine at a dose of 10 or 20 mg/kg was administered intraperitoneally to the respective groups of rats over 12 days after the administration of IFA.
2.3.1 Assessment of serum biochemical parameters
Blood samples were isolated from each rat and used for the assessment of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, as well as platelet and blood cell counts. Levels of AST and ALT were measured using appropriate commercial kits, in accordance with the manufacturer’s instructions. Quantitative turbidimetry was performed to measure levels of C-reactive protein. Inflammatory mediators (e.g., IL-1β, IL-6 and TNF-α) were measured in serum samples of IFA induced RA rats using ELISA kits, in accordance with the manufacturer’s instructions.
2.3.2 Determination of oxidative stress
Levels of malondialdehyde (MDA), reduced glutathione (GSH) and superoxide dismutase (SOD) were measured in serum samples using ELISA, in accordance with the manufacturer’s instructions.
2.3.3 Immunohistochemistry
Immunohistochemical analysis was performed to measure the protein expression patterns of PI3K, mTOR and AKT in cartilage tissue. Cartilage tissue was fixed in 10% formalin and embedded in paraffin. The tissue was then sectioned at a thickness of 5 μm and incubated for 60 min at 60 °C. Hydrogen peroxide solution was used to block endogenous peroxidase activity. Primary antibodies (anti-Wnt, anti-β-catenin and anti-AKT) were diluted in phosphate-buffered saline and incubated overnight at 4 °C with the tissue sections. Subsequently, secondary antibody (horseradish peroxidase-labelled goat anti-rabbit IgG) was incubated with tissue sections for 60 min at room temperature. Diaminobenzidine stain was applied, and slices were counterstained with haematoxylin. Slices were hydrated, sealed and observed using an inverted microscope.
2.3.4 Assessment of apoptosis
TUNEL assays were performed to measure chondrocyte apoptosis as in a previous study. The ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Sigma-Aldrich) was used, following the manufacturer’s instructions. TUNEL-positive cells were identified using a trinocular microscope.
2.3.5 Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis
RNA was isolated from cartilage tissue using TRIzol reagent (ThermoFisher Ltd., USA). The RevertAid First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada) was used to reverse-transcribe RNA. The primers listed below were mixed with RT2 SYBR Green Master Mix (SuperArray, Frederick, MD, USA) to determine gene expression by means of Quantitative SYBR Green PCR assays.
Primer
Forward
Reverse
Wnt3a
5′-TCAGCTGCCAGGAGTGCACG-3′
5′-CGCCCTCAGGGAGCAGCCTAC-3′
GRP78
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
CHOP
5′-AATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
TLR-3
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
NF-κB
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
PI3K
5′-GGTTGTCTGTCAATCGGTGACTGT-3′
5′-GAACTGCAGTGCACCTTTCAAGC-3′
AKT
5′-TTCTGCAGCTATGCGCAATGTG-3′
5′-TGGCCAGCATACCATAGTGAGGTT-3′
mTOR
5′-GCTTGATTTGGTTCCCAGGACAGT-3′
5′-GTGCTGAGTTTGCTGTACCCATGT-3′
β-catenin
5′-CACAAGCAGAGTGCTGAAGGTG-3′
5′-GATTCCTGAGAGTCCAAAGACAG-3′
Caspase-3
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
Bax
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
Bcl-2
5′-TAATACGACTCACTATAGGG-3′
5′-TAGAAGGCACAGTCGAGG-3′
2.3.6 Western blotting assay
Extraction of total protein from cartilage tissue was performed by treatment with ice-cold radioimmunoprecipitation lysis buffer. Total protein concentration was measured using bicinchoninic acid. Proteins were separated via sodium dodecyl sulphate polyacrylamide gel electrophoresis (12.5%) and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% milk at 30 °C for 30 min, then incubated overnight at 4 °C with the following primary antibodies: anti-Wnt3a (1:200), anti-PI3K (1:500), anti-AKT (1:500), anti-Toll-like receptor (TLR)-3 (1:200) and anti-β-actin (1:1000). Membranes were subsequently incubated with secondary antibodies for 60 min at room temperature. After the final wash, signals were developed using a Western Lightning ECL kit (PerkinElmer, Waltham, MA, USA). The results were analysed using Gel-Pro Analyzer 4.0 software. Densitometric levels of target proteins were quantified and normalised to that of β-actin.
2.3.7 Histopathological analysis
Cartilage tissue was fixed in 10% formalin and embedded in paraffin. The tissue was then sectioned at a thickness of 5 μm and stained with haematoxylin for 5 min at 37 °C. Thereafter, the tissue was washed for 10 min with water, then counterstained with 5% eosin for 3 min and dehydrated with alcohol. Histopathological changes in cartilage tissue were measured under a light microscope.
2.3.8 Chondrocyte culture and identification
Cartilage tissue was isolated from the ankle bone and immersed in phosphate-buffered saline with 10% penicillin and streptomycin. Tissue sections were cut into 1-mm3 pieces and placed into microcentrifuge tubes. Sections were then digested by overnight incubation in 0.2% type II collagenase at 37 °C. Cells were pelleted by centrifugation and resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/F-12. Cells were incubated in a CO2 incubator at 37 °C and provided fresh medium at 3-day intervals. Cell growth measurements were also performed. Cell protein digestion was performed by treating the cells for 5 min with 0.25% trypsin. Cells were observed by microscopy for changes in shape during incubation.
2.3.9 CCK-8 assay
Cells (8 × 103 cells/100 μL) were seeded in 96-well plates and divided into five groups: control, RA, cepharanthine (10 μmol/L cepharanthine), LY294002 (10 μmol/L LY294002) and cepharanthine + LY294002 (10 μmol/L cepharanthine and 10 μmol/L LY294002). After 0, 24, 48 or 72 h of growth, cells were treated with CCK-8 reagent and incubated at 37 °C for an additional 2 h. ELISA was used to observe optical density at 450 nm.
2.3.10 Transmission electron microscopy
Cell morphology was observed after centrifugation of trypsinised cells at 1500 RPM for 5 min at 4 °C. Osmic acid (1%) was used to fix the cells, and the cells were then polymerised in 60% epoxy resin at 37 °C for 16 h, 24 °C for 12 h and 60 °C for 14 h. Embedded cells were sliced and observed using transmission electron microscopy.
2.3.11 Homology modelling of Wnt, PI3K and TLR-3
The Wnt protein sequence was obtained from the NCBI database for use in homology modelling. The SWISS-Model server was used to develop the Wnt homology model. Software used for this investigation included ACD, ChemSketch (acdlabs), PyMol molecular graphics system version 2.4.0 (Schrodinger LLC) and AutoDock Vina tools 1.5.6 (Scripps Research Institute). Target sequences were compared by performing BLAST searches using the primary amino-acid sequence. The best quality templates were selected for building the homology model. AutoDock Vina MGL tools was used to prepare the ligand for molecular docking analysis.
2.3.12 Preparation of proteins and ligand and molecular docking
The two-dimensional structure of cepharanthine was obtained from the PubChem database and converted into a pdb file using openBabel software. AutoDock Vina MGL tools was used to prepare the ligand for docking analysis by removing water molecules and adding hydrogen molecules, and by modulating the charges through the Kollman approach. A pdbqt file was then obtained. Moreover, molecular docking simulation of the cepharanthine ligand was performed using AutoDock Kollman and Gasteiger charges for both the ligand and protein. The grid map was created using Autogrid 4. The grid box was prepared and the area of protein structure to be mapped was defined by providing appropriate coordinates. The grid box dimensions (x, y and z coordinates) for Wnt were 31.075552, 8.515565 and −18.953583; those for TLR-3 were −36.929849, −18.411961 and 25.138303; and those for PI3K were 20.951652, 62.328609 and 21.408522. The Lamarckian genetic algorithm was used for the minimisation and optimisation of energy during the docking simulation process.
2.3.13 Statistical analysis
All data are expressed as the mean ± standard error of the mean (n = 10). Statistical analysis was performed using one-way analysis of variance. Post hoc comparisons were performed by means of Dunnett’s post hoc test using GraphPad Prism software (ver. 6.1; San Diego, CA, USA). P-values < 0.05 were considered to indicate statistical significance.
3 Results
3.1 Effects of cepharanthine on hind paw volume
Oedema in the joint was confirmed by determining the hind paw volume in IFA induced RA rats as shown in Fig. 1. There was increase in the hind paw volume of all the IFA group than Control group of rats. Moreover RA group shows further significant increase hind paw volume than Control group. Treatment with cepharanthine significantly (p < 0.01) reduces the hind paw volume than RA group of rats.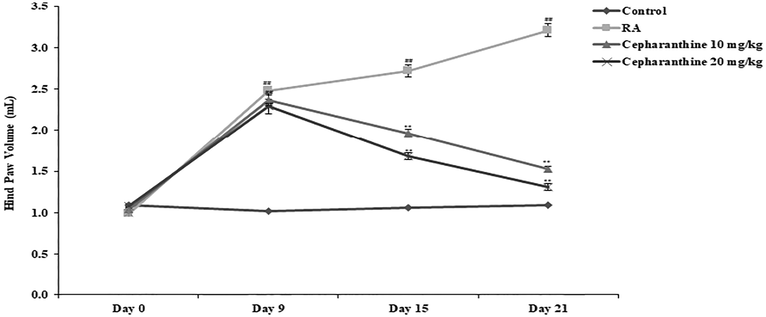
Cepharanthine reduces the hind paw volume in IFA induced RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.2 Effects of cepharanthine on serum biochemical parameters
Biochemical parameters and cell counts were measured in the serum and blood, respectively. Activities of the AST and ALT enzymes and levels of C-reactive protein were enhanced in the serum of RA rats, compared with control rats. Moreover, white blood cell and platelet counts were enhanced in RA rats, compared with control rats. Notably, cepharanthine treatment attenuated the altered serum levels of C-reactive protein and AST and ALT activities in RA rats. It also reduced the white blood cell and platelet counts in RA rats (Fig. 2).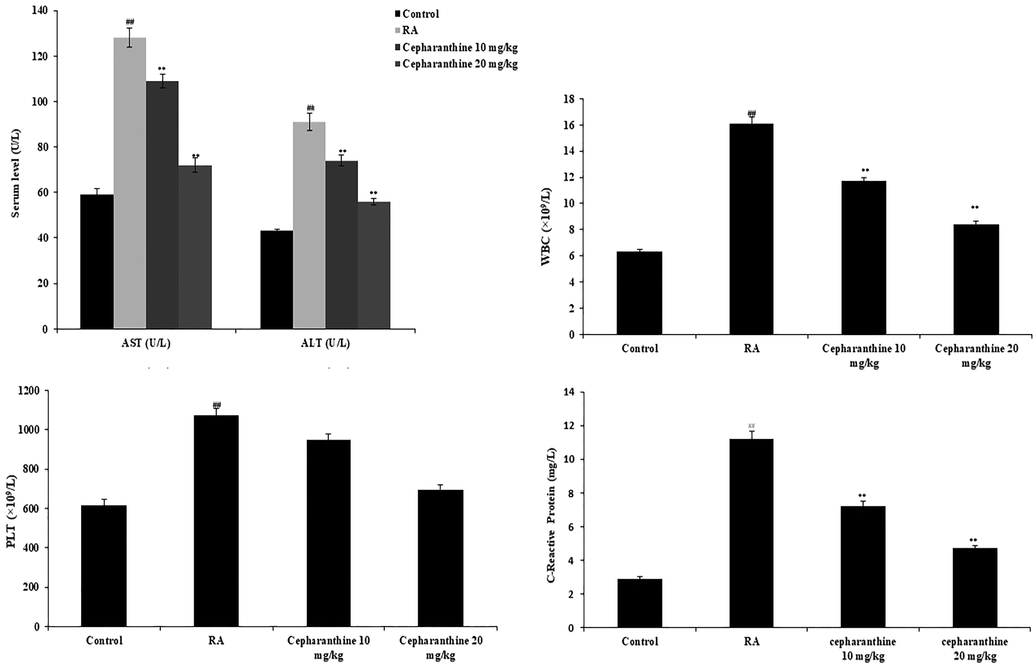
Cepharanthine ameliorated changes in serum biochemical parameters in IFA induced RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.3 Effects of cepharanthine on inflammatory mediators
ELISA was used to measure the levels of inflammatory mediators in the serum. Notably, serum levels of IL-1β, IL-6 and TNF-α were higher in RA rats than in control rats. Serum levels of inflammatory mediators were significantly lower in cepharanthine-treated rats than in RA rats (p < 0.01) (Fig. 3).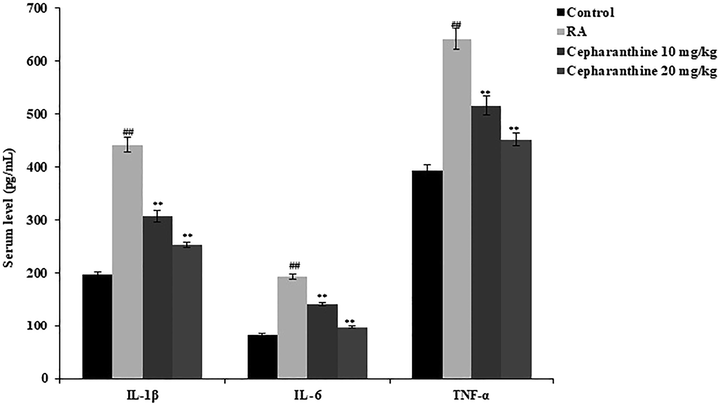
Cepharanthine treatment ameliorated changes in serum inflammatory mediators in RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.4 Effects of cepharanthine on oxidative stress mediators
Oxidative stress mediators were measured in the serum, as shown in Fig. 4. GSH and MDA levels were significantly reduced and SOD activity was significantly enhanced in the serum of RA rats, compared with control rats. However, cepharanthine treatment enhanced GSH and MDA levels, whereas it reduced SOD activity, in the serum of RA rats.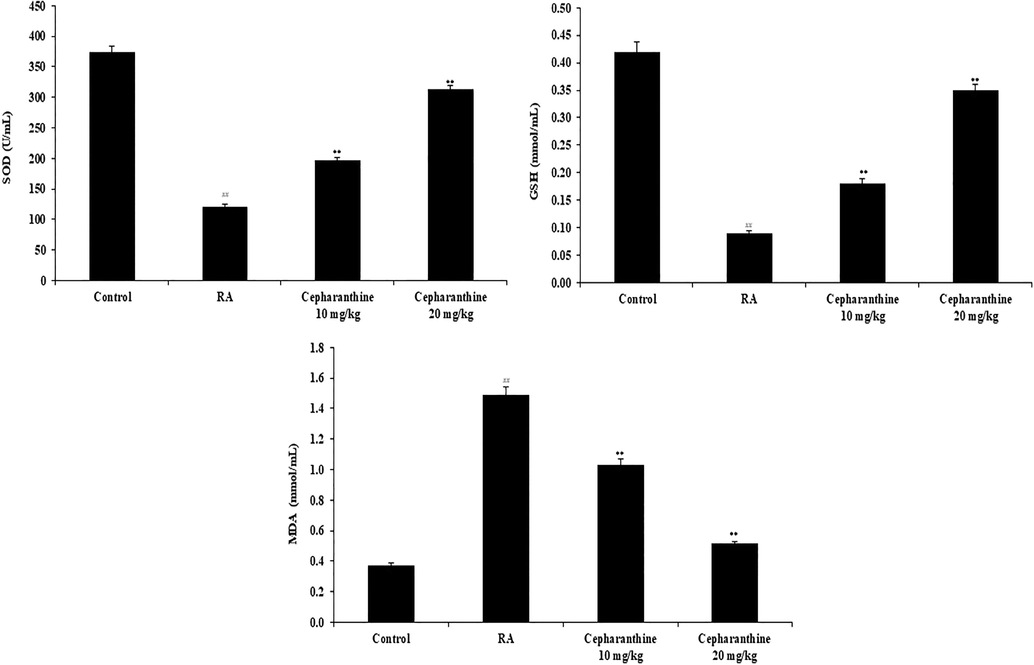
Cepharanthine treatment ameliorated serum oxidative stress mediators in RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.5 Effects of cepharanthine on protein expression levels of mTOR, AKT and PI3K
Protein expression levels of mTOR, AKT and PI3K in cartilage were determined using immunohistochemistry, as shown in Fig. 5. The protein expression levels of AKT, PI3K and Wnt were significantly enhanced in RA rats, compared with control rats (p < 0.01). The protein expression levels of AKT, PI3K and mTOR were reduced in the cartilage tissue of cepharanthine-treated rats, compared with RA rats.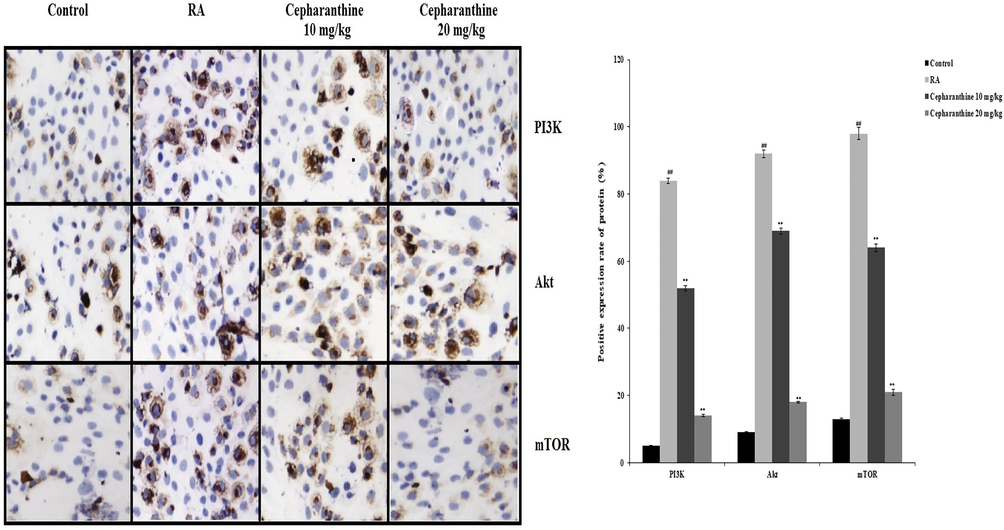
Cepharanthine treatment ameliorated alterations in protein expression levels of Wnt, AKT and PI3K in cartilage tissue of RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.6 Effects of cepharanthine on chondrocyte apoptosis
Chondrocyte apoptosis was determined by analysing TUNEL-positive cells and the mRNA expression levels of caspase-3, Bax and Bcl-2. The proportion of apoptotic chondrocytes was enhanced in RA rats, compared with control rats. However, cepharanthine treatment reduced the proportion of apoptotic chondrocytes in RA rats. Moreover, mRNA expression levels of caspase-3 and Bcl-2 were enhanced in RA rats, compared with control rats, whereas mRNA expression levels of Bax were reduced in RA rats. Cepharanthine treatment attenuated the altered mRNA expression levels of Bcl-2, Bax and caspase-3 in RA rats (Fig. 6).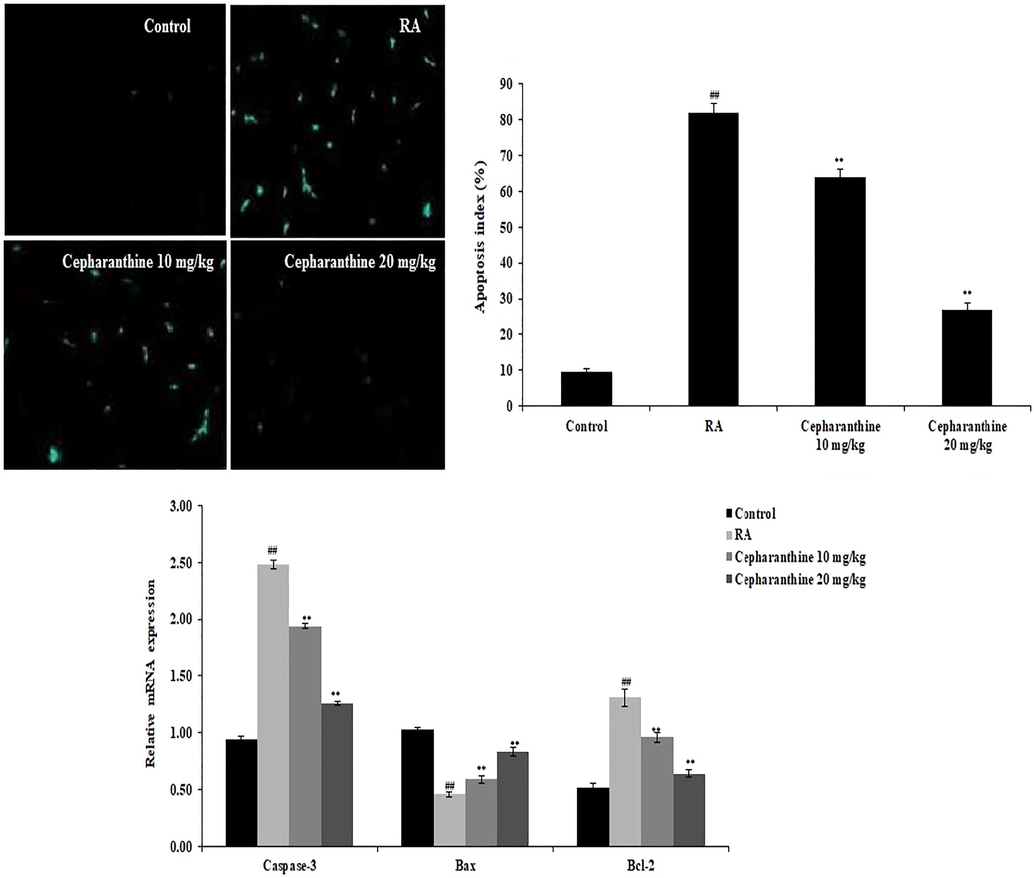
Cepharanthine treatment ameliorated chondrocyte apoptosis in RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.7 Effects of cepharanthine on mRNA expression levels of Wnt/PI3K/TLR-3 pathway components
The mRNA expression levels of Wnt/PI3K/TLR-3 pathway components were measured in cartilage tissue (Fig. 7A–C). The mRNA expression levels of Wnt3a, GRP78, CHOP and β-catenin were assessed to monitor the Wnt pathway, as shown in Fig. 7A. The mRNA expression levels of Wnt3a and β-catenin in cartilage tissue were elevated in RA rats, compared with control rats, whereas mRNA expression levels of CHOP and GRP78 were reduced in cartilage tissue in RA rats. However, cepharanthine treatment ameliorated the altered mRNA expression levels of Wnt3a, GRP78, CHOP and β-catenin in RA rats. Furthermore, cepharanthine treatment attenuated inflammatory pathway activity by reducing the mRNA expression levels of TLR-3 and NF-κB in cartilage tissue in RA rats (Fig. 7B). The mRNA expression levels of PI3K, AKT and mTOR were enhanced in cartilage tissue in RA rats, compared with control rats. Finally, cepharanthine treatment attenuated the altered mRNA expression levels of PI3K, AKT and mTOR in cartilage tissue in RA rats (Fig. 7C).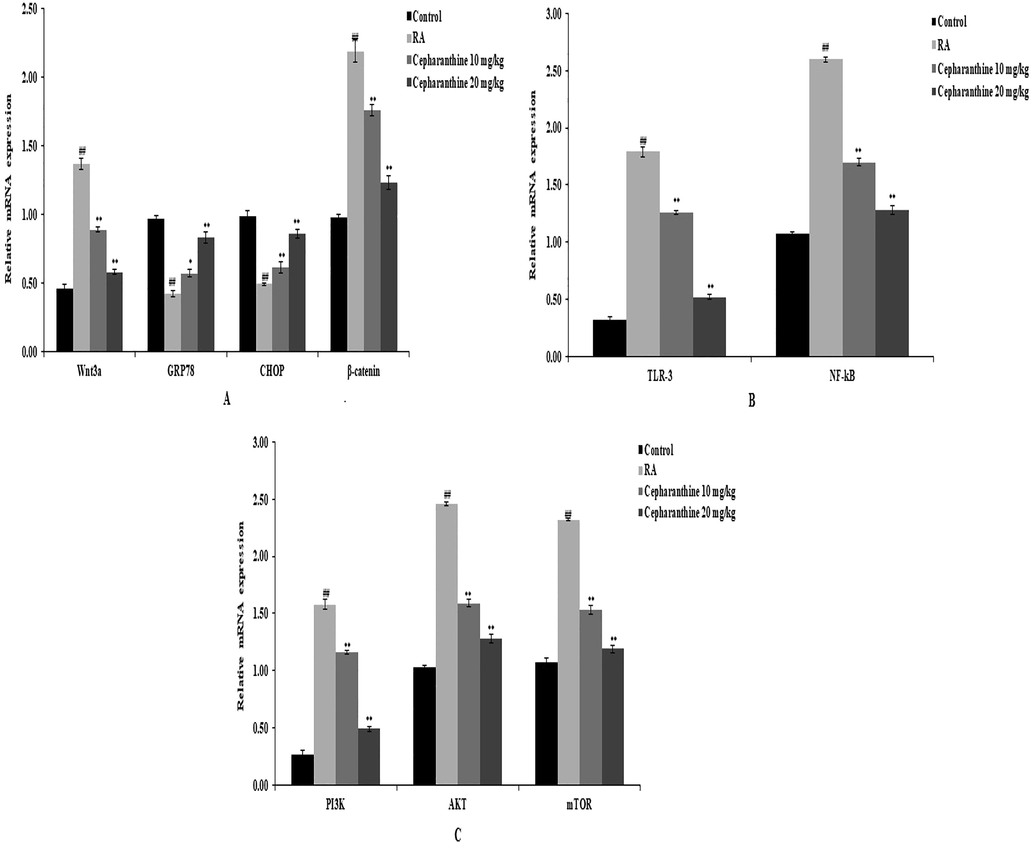
Cepharanthine treatment ameliorated alterations in the mRNA expression levels of Wnt, PI3K and TLR-3 in cartilage tissue of RA rats, as measured by qRT-PCR. A: mRNA expression levels of Wnt3a, GRP78, CHOP and β-catenin. B: mRNA expression levels of TLR-3 and NF-kB. C: mRNA expression levels of PI3K, AKT and mTOR. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.8 Effects of cepharanthine on protein expression levels of Wnt, PI3K, AKT and TLR-3
Fig. 8. shows the effects of cepharanthine treatment on the protein expression levels of Wnt3a, PI3K, AKT and TLR-3 in the cartilage tissue of RA rats, as determined by western blotting. There were significant increases in the protein expression levels of Wnt, PI3K, AKT and TLR-3 in the cartilage tissue of RA rats, compared with control rats. However, cepharanthine treatment attenuated the altered protein expression levels of Wnt, PI3K, AKT and TLR-3 in the cartilage tissue of RA rats.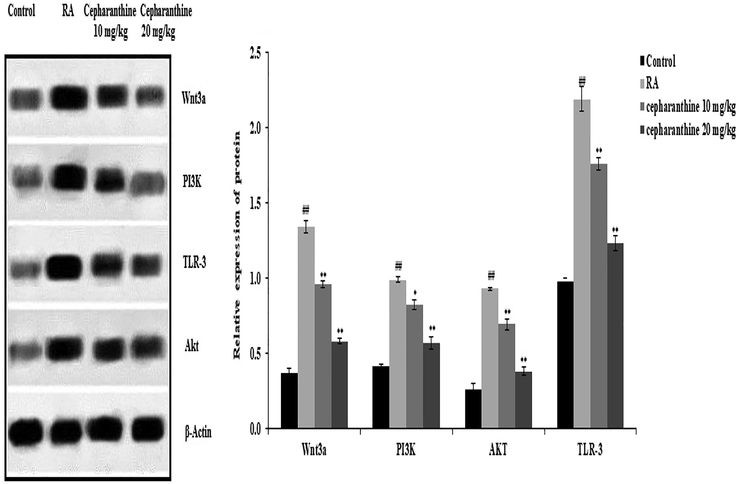
Cepharanthine treatment ameliorated alterations in protein expression levels of Wnt, PI3K, AKT and TLR-3 in cartilage tissue of RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.9 Effects of cepharanthine on histopathological changes in cartilage tissue
Histopathological changes in cartilage tissue were measured in cepharanthine-treated RA rats, as shown in Fig. 9. The articular cartilage layer was thick and smooth in control rats, and the ankle was also intact in those rats. Moreover, no trabecular bone absorption was observed in the distal phalanx in control rats. RA rats exhibited cartilaginous cap disappearance and collagen fibres fracture, as well as inflammatory cell infiltration and a rough surface. Importantly, cepharanthine treatment reversed these pathological changes in RA rats.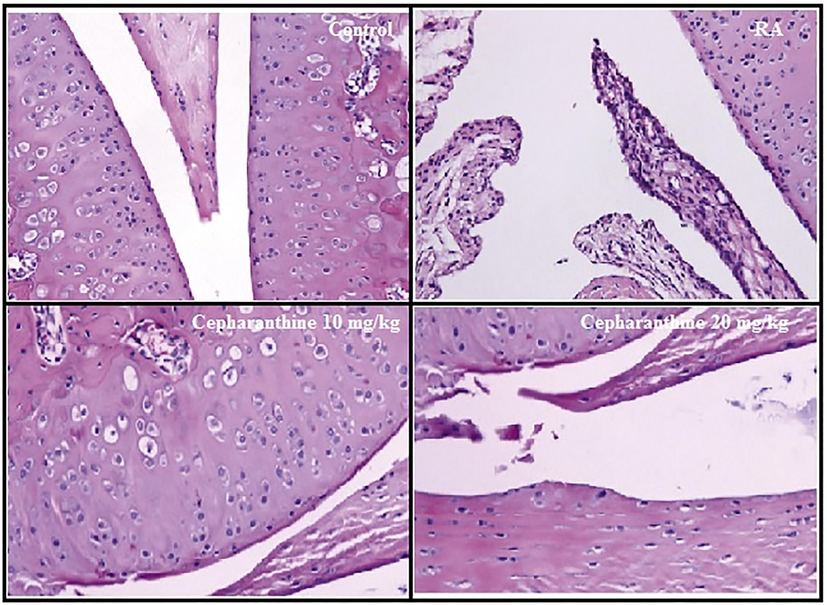
Cepharanthine treatment ameliorated histopathological changes in cartilage tissue of RA rats.
3.10 Effects of cepharanthine and LY294002 on chondrocyte proliferation
The effects of cepharanthine on chondrocyte proliferation were observed using optical density assessment, as shown in Fig. 10. Chondrocyte proliferation was enhanced after 24, 48 and 72 h of culture in the RA group, compared with the control group. Treatment with cepharanthine and LY294002, alone or in combination, negatively regulated the proliferation of chondrocytes, compared with the RA group, after 24, 48 and 72 h of culture.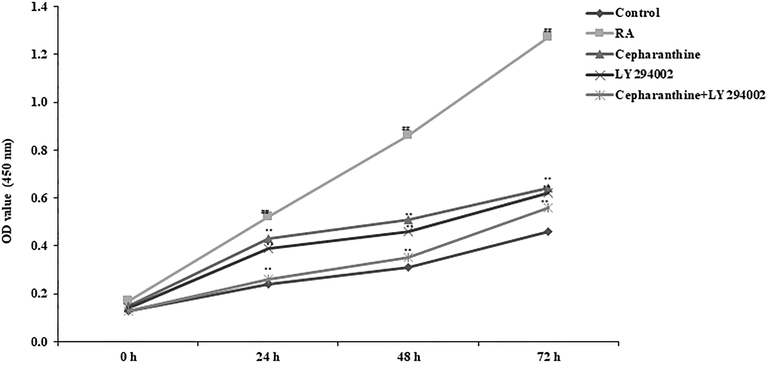
Cepharanthine and LY294002 treatment ameliorated chondrocyte proliferation in RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group.
3.11 Effects of cepharanthine and LY294002 on chondrocyte autophagy
Transmission electron microscopy was used to observe autophagic bodies in chondrocytes. Chondrocytes in the RA group exhibited autophagic bodies with a bilayer membrane structure, as well as swollen mitochondria, cytoplasmic vacuolar degeneration and fewer organelles. Treatment with cepharanthine and LY294002, alone or in combination, resulted in greater numbers of autophagic bodies in chondrocytes, compared with the RA group (Fig. 11).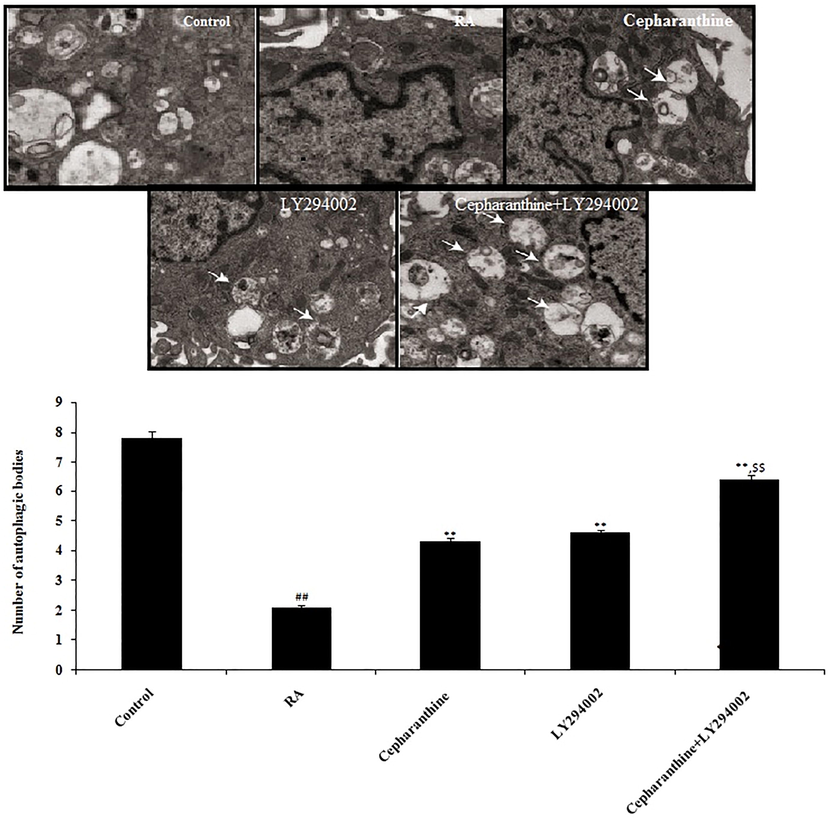
Cepharanthine and LY294002 treatment ameliorated chondrocyte autophagy in cartilage tissue of RA rats. Mean ± SEM (n = 10); ##p < 0.01 compared with control group, **p < 0.01 compared with RA group, $$p < 0.01 compared to cepharanthine-treated and LY294002-treated groups.
3.12 Effects of cepharanthine on Wnt, PI3K and TLR-3 protein interactions
Cepharanthine exhibited high binding affinities with Wnt, PI3K and TLR-3 proteins. In vivo and in vitro molecular docking analyses were performed using BLAST and homology modelling followed by ligand and protein preparation. Molecular docking simulations of cepharanthine were performed on the basis of AutoDock Kollman and Gasteiger charges. Docking analyses revealed high binding affinity of cepharanthine for Wnt, PI3K and TLR-3 proteins. Binding energy analyses revealed that cepharanthine had low binding energies with PI3K and Wnt (Table 1). Three-dimensional protein structure analyses revealed solid areas representing the ligand binding region in Wnt, PI3K and TLR-3 proteins (Fig. 12). RMSD, root mean square deviation; l.b., lower bound; u.b., upper bound.
Docking scores for Wnt with Cepharanthine
Mode of ligand
Affinity (kcal/mol)
Distance from RMSD l.b.
Best mode RMSD u.b.
1
−8.9
0.000
0.000
2
−8.0
3.069
6.957
3
−7.8
2.562
6.901
4
−7.7
2.992
6.594
5
−7.5
3.685
6.277
6
−7.4
3.666
7.609
7
−7.3
2.157
8.283
8
−7.2
4.130
7.039
9
−7.2
2.617
7.295
Docking scores for TLR-3 with cepharanthine
Mode of ligand
Affinity (kcal/mol)
Distance from RMSD l.b.
Best mode RMSD u.b.
1
−7.4
0.000
0.000
2
−7.3
3.023
7.091
3
−7.2
3.427
6.886
4
−7.1
7.408
11.274
5
−7.0
10.238
14.649
6
−6.8
8.014
11.570
7
−6.6
9.722
14.778
8
−6.6
12.007
16.503
9
−6.5
2.599
6.593
Docking scores for PI3K with cepharanthine
Mode of ligand
Affinity (kcal/mol)
Distance from RMSD l.b.
Best mode RMSD u.b.
1
−10.6
0.000
0.000
2
−8.4
2.994
6.620
3
−8.2
3.301
7.983
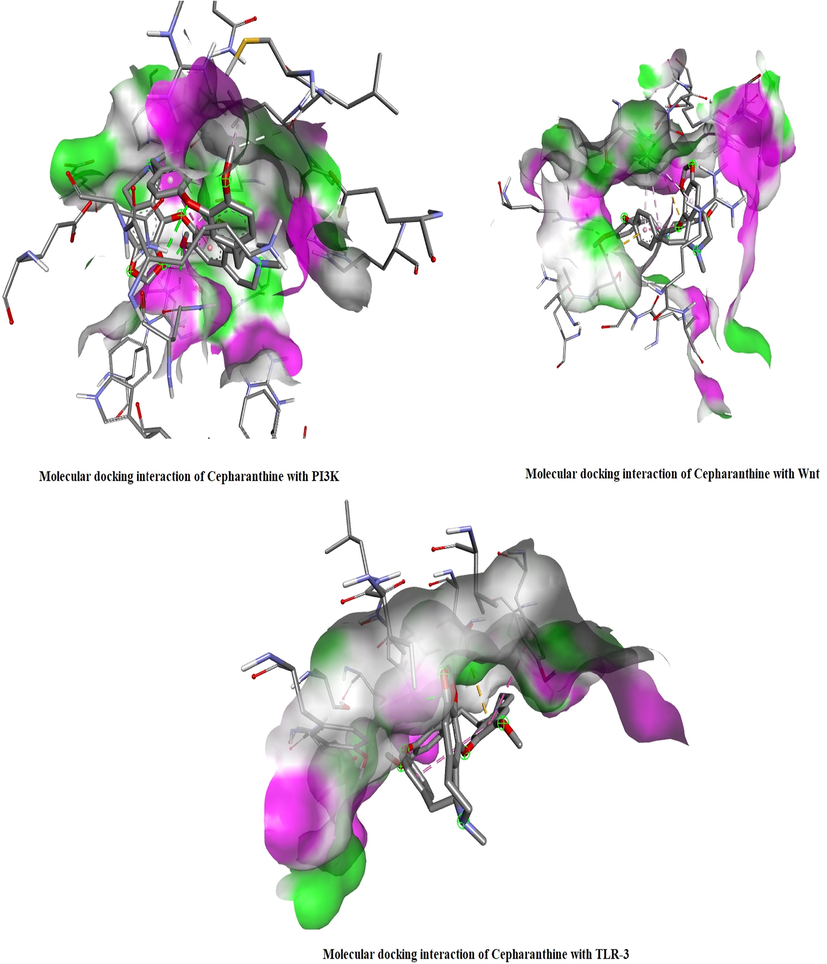
In silico molecular docking analysis revealed interactions of PI3K, Wnt and TLR-3 with Cepharanthine. The solid areas in the protein structures represent the zone of interaction with cepharanthine.
4 Discussion
RA is an autoimmune disorder that causes joint inflammation and permanent disability in affected patients (Bullock et al., 2018). Conventional therapy for the management of RA has various limitations, and it remains challenging to prevent bone degeneration in patients with RA. This study examined the protective effects of cepharanthine treatment against RA and aimed to elucidate its underlying molecular mechanism. The effects of cepharanthine on chondrocyte autophagy, apoptosis and proliferation via the regulation the Wnt/PI3K/TLR-3 pathway was examined in a rat model of RA. The effects of cepharanthine were assessed by measuring serum biochemical parameters, blood cell counts and serum inflammatory and oxidative stress mediators. Moreover, the expression levels of several proteins that regulate the Wnt/PI3K/TLR-3 pathway were measured using immunohistochemistry, western blotting and qRT-PCR.
There are notable alterations in blood cell counts and some serum biochemical parameters in RA. For instance, C-reactive protein levels are reportedly enhanced in patients with autoimmune disorders (Sproston and Ashworth, 2018). The data in this study showed that blood cell counts and C-reactive protein levels were enhanced in RA rats, but were reversed by cepharanthine treatment. In RA, cartilage degeneration occurs, leading to elevated levels of inflammatory and oxidative stress mediators, as well as enhanced apoptosis in cartilage tissue (Riegger and Brenner, 2019). TLR-3 activates immune cells and stimulates the expression of NF-κB (D'Atri et al., 2015). NF-κB plays an important role in stimulating the mediators of inflammation and in enhancing the production of reactive oxygen species (Lingappan, 2018). In this study, cepharanthine treatment attenuated the mRNA expression levels of TLR-3 and NF-kB, while reducing the levels of inflammatory and oxidative stress mediators in RA rats.
The PI3K/AKT/mTOR pathway is important in the development of several chronic disorders, including RA (Dai et al., 2018). This pathway is crucial for survival and cell growth in both pathological and normal physiological states (Elmore, 2007). Our findings suggest that the PI3K/AKT pathway inhibits chondrocyte proliferation by downregulating glycolysis. Cepharanthine treatment ameliorated alterations in the PI3K/AKT/mTOR pathway and protected against cartilage destruction in RA.
Bax, Bcl-2 and caspase-3 proteins reportedly regulate mitochondrial activity. Thus, alterations in their expression levels lead to apoptosis due to mitochondrial dysfunction (Chen et al., 2018). Changes in mitochondrial function from modifications of Bax, Bcl-2 and caspase-3 can reduce apoptosis (Park et al., 2018), consistent with our findings regarding cepharanthine treatment.
Importantly, the Wnt pathway is also involved in the development of RA, through activation of NF-κB (Du and Geller, 2010). In our study, cepharanthine attenuated the altered Wnt pathway in RA rats.
5 Conclusions
The findings in this study suggest that cepharanthine treatment attenuates chondrocyte autophagy, apoptosis and proliferation by regulating the Wnt/PI3K/TLR-3 pathway in RA rats. Therefore, cepharanthine may be useful for the clinical treatment of RA.
Author Contributions
Wenmin Zhao performed the experimental work, collected the data and writes the manuscript. Dongyun Yao, Ru Yang and Heshui Huo contribute performing western blot assay and qRT-PCR and also helps in statically analysis. Chenman Qin and Kai Sun contribute for the histopathological study.
Acknowledgement
All the author of this manuscript are thankful to Jiaozuo people's hospital, China for providing necessary facility to conduct the presented work.
Declaration of Competing Interest
No.
References
- Rheumatoid arthritis: A brief overview of the treatment. Med. Princ. Pract.. 2018;27(6):501-507.
- [Google Scholar]
- XIAP impairs mitochondrial function during apoptosis by regulating the Bcl-2 family in renal cell carcinoma. Exp Ther Med.. 2018;15(5):4587-4593.
- [Google Scholar]
- Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther.. 2018;12:4095-4105.
- [Google Scholar]
- Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J. Thromb. Haemost.. 2015;13(5):839-850.
- [Google Scholar]
- Cross-Regulation between Wnt and NF-κB signaling pathways. For Immunopathol Dis Therap.. 2010;1(3):155-181.
- [Google Scholar]
- A Novel Phytochemical, DIM, inhibits proliferation, migration, invasion and TNF-α induced inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal pathway. Front. Immunol.. 2019;10:1620.
- [Google Scholar]
- Apoptosis: a review of programmed cell death. Toxicol. Pathol.. 2007;35(4):495-516.
- [Google Scholar]
- Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev.. 2004;18(19):2404-2417.
- [Google Scholar]
- Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res.. 2018;6:15.
- [Google Scholar]
- Cepharanthine, an alkaloid from Stephania cepharantha Hayata, inhibits the inflammatory response in the RAW264.7 cell and mouse models. Inflammation.. 2014;37(1):235-246.
- [Google Scholar]
- IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res. Ther.. 2004;6(2):R120-R128.
- [Google Scholar]
- Mode of the anti-allergic action of cepharanthine on an experimental model of allergic rhinitis. Nihon Yakurigaku Zasshi.. 1987;90(4):205-211.
- [Google Scholar]
- Determinants of disability in rheumatoid arthritis: A community-based cohort study. Open Rheumatol. J.. 2015;9:88-93.
- [Google Scholar]
- Cepharanthine exerts anti-inflammatory effects via NF-κB inhibition in a LPS-induced rat model of systemic inflammation. J. Surg. Res.. 2011;171(1):199-204.
- [Google Scholar]
- The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int.. 2013;2013:284873.
- [Google Scholar]
- Suppression of cytokine production and neural cell death by the anti-inflammatory alkaloid cepharanthine: a potential agent against HIV-1 encephalopathy. Biochem. Pharmacol.. 2001;62(6):747-753.
- [Google Scholar]
- Evidence of necroptosis in osteoarthritic disease: investigation of blunt mechanical impact as possible trigger in regulated necrosis. Cell Death Dis.. 2019;10(10):683.
- [Google Scholar]
- Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions. Pharmacol. Rep.. 2011;63(2):337-347.
- [Google Scholar]
- Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskelet. Dis.. 2013;5(2):77-94.
- [Google Scholar]
- Role of C-reactive protein at sites of inflammation and infection. Front. Immunol.. 2018;9:754.
- [Google Scholar]
- Expression and regulation of WISP2 in rheumatoid arthritic synovium. Biochem. Biophys. Res. Commun.. 2005;334(4):973-978.
- [Google Scholar]
- Inhibitory effect of cepharanthine on dendritic cell activation and function. Int. Immunopharmacol.. 2011;11(11):1932-1938.
- [Google Scholar]
- Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells.. 2018;7(10):161.
- [Google Scholar]
- The preventive effects of incomplete Freund's adjuvant and other vehicles on the development of adjuvant-induced arthritis in Lewis rats. Immunology. 1999;98(2):267-272.
- [Google Scholar]
- Cepharanthine prevents estrogen deficiency-induced bone loss by inhibiting bone resorption. Front. Pharmacol.. 2018;9:210.
- [Google Scholar]







