Translate this page into:
A review on non-noble metal based electrocatalysis for the oxygen evolution reaction
⁎Corresponding authors at: No. 307, Ningxia Road, Qingdao, China. kaiqian2008@163.com (Qianqian Jiang), jtang951@163.com (Jianguo Tang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In order to find a clean, efficient and sustainable new energy source that can replace fossil fuels, hydrogen energy is considered to be the most ideal choice. Electrocatalytic oxygen evolution plays a vital role in the development of hydrogen energy, promotes the research of new electrocatalysts, and is dedicated to find materials with high electrocatalytic efficiency. This article discusses in detail the major developments in OER electrocatalysts, including recently reported metal and non-metal based materials. Metal-based catalysts, although having the advantages of high catalytic activity, have disadvantages such as poor stability and low selectivity, which hinder the further application of such materials. Non-metallic based materials avoid such disadvantages and exhibit very substantial performance in overall water decomposition. This review provides useful knowledge of a well-designed OER electrocatalyst and a possible strategy for OER/HER dual-function catalytic performance for future development.
Keywords
Oxygen evolution reaction (OER)
Electrocatalyst
Metal-based electrocatalysts
Carbon-based electrocatalysts
Black phosphorous (BP)
- TD
-
thermal decomposition
- EPD
-
electrophoretic deposition
- AO
-
alloy oxidation
- GCE
-
glassy carbon electrode
- SLS
-
stainless steel
- CNT
-
carbon nanotube
- FTO
-
fluorine doped tin oxide
- CFP
-
carbon fiber paper
- NP
-
nanoparticle
- NS
-
nanosheet
- NR
-
nanoribbon
- BP
-
black phosphorous
- HER
-
hydrogen evolution reaction
- OER
-
oxygen evolution reaction
- MWCNT
-
multi-walled carbon nanotube
- SWCNT
-
single-walled carbon nanotube
Abbreviations
1 Introduction
In the era of increasing energy consumption and the depletion of fossil fuels, carbon-based coal, gas fuels, and petroleum commonly used in various industrial technologies can cause serious air pollution. So, to find the efficient, clean and environmentally friendly energy has become an urgent problem. Due to the process of energy conversion releasing no carbon dioxide, hydrogen (H2) is a green energy with high energy density, which has attracted much attention. In order to achieve more efficient energy conversion or storage systems such as alkaline water electrolysis, fuel cells (FC), and metal-air batteries, numerous innovative ideas have been proposed (Suen et al., 2017; Jamesh, 2016; Li et al., 2016a; Li et al., 2019). Hydrolysis technology, as a typical hydrogen evolution technology, has been widely studied. In the past few years, noble-metal-free HER electrocatalysts have rapidly increased. Among them, transition metal carbides (TMCs) are attracted much attention due to their high conductivity, metallic band states, tunable surface/bulk architectures (Gao et al., 2019). Carbon materials are as conductive substrates and highly-dispersed support materials ; which can improve their catalytic activity by heteroatom doping (You and Sun, 2018). Carbon-based catalysts in some report have excellent HER performance even close to that of Pt. In addition, fuel cells as one of the most promising technologies gain great expectations for the commercial application in sustainable energy conversion systems. Cathode oxygen reduction reaction (ORR) kinetics of fuel cells is very slow, which lead to require a large overpotential reaction to occur, greatly limiting the energy output of fuel cell. Currently, Pt-based catalysts are the widely used cathode electrode materials to catalyze the sluggish ORR due to the best catalytic activity of the metal and oxygen binding energy (Nie et al., 2015). Carbon-based materials obtained active sites by doping are considered to be the most promising alternatives of the high-cost and scarce Pt-based catalysts for oxygen reduction reactions (ORR) (Wang et al., 2018a). Oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) are two half-reactions of the water electrolysis. Oxygen evolution reaction (OER) involves the transfer of four electrons, and the mechanism is relatively complex, which owns a slow kinetic reaction and a high overpotential. Therefore, it is the main reason to limit the water electrolysis efficiency, which has also become the focus of research.
Water electrolysis has been studied in depth since it was first proposed in 1789 (Trasatti, 1999). In fact, water electrolysis still needs to be improved in terms of efficiency and durability to become economically attractive, even though it has the advantages of flexibility, virtually zero-emissions, and production of high purity gas (Ibrahim et al., 2008). The electrodes include cathodes for the hydrogen evolution reaction (HER) and anodes for the oxygen evolution reaction (OER). According to the operating temperature of water electrolysers, low temperature electrolysers can be divided into (acidic) polymer electrolyte water electrolysers (PEWEs) and alkaline water electrolysers (AWEs). PEWE water electrolysis is one of the water electrolysis technologies available on the market. During PEWE water electrolysis, the PEWEs use a solid polymer electrolyte which selectively conducts positive ions such as protons and creates a local acidic environment. The main advantage of PEWEs is can produce pure hydrogen at high current densities (capable of achieving values above 2 A cm−2) and high pressures (over 150 bar) (Carmo et al., 2013; Wang et al., 2018b), which reduces the operational cost. However, under the typical operation conditions of a PEWE only a few electrode materials can exhibit adequate stability (Fabbri et al., 2014; Zhao et al., 2018). Therefore; the anodic and cathodic reaction is generally catalysed by noble metal based catalysts such as Pt, Ru, Ir and their oxides. On the other hand, the main advantage of AWEs is that it can show sufficient stability when the noble metals are replaced by the alternative catalysts. In addition, in the alkaline medium, it is more favorable oxygen electrocatalysis at the anode side than the acidic medium (Ramaswamy and Mukerjee, 2012; Jiang et al., 2015). Recently, Various non-noble metal catalysts have been found to have similar properties to noble metals, and some of them even show higher catalytic activity than commercial noble metal electrodes (Wang et al., 2016; Liu et al., 2017b; Tian et al., 2014a).
Cathodic HER and anodic OER are two crucial half-cell reactions of electrochemical water splitting. In general, the cathodic and anodic reactions occurring at the PEWE and AWE electrodes can be described by the following equations:
PEWEs and AWEs share a significant overpotential on the anode side of the oxygen evolution reaction (OER), as described by the following equations:
The standard oxidization potential of the OER is defined as 1.23 V related to a relative hydrogen electrode (RHE) and the standard reduction potential of HER is 0 V (vs. RHE) at standard temperature and pressure (25 °C, 1 atm). However, in the actual water electrolysis process, a larger applied potential is always required due to the involvement of complex electron and ion transfer processes, which result in sluggish kinetics and low energy efficiency (Bajdich, 2013; Zhu et al., 2018). For this reason, the OER has been studied intensively for decades to elucidate the reaction mechanism and minimize energy loss during water electrolysis. Therefore, the assistance of a suitable electrocatalyst can be sought which can greatly reduce the overpotential and increase the reaction rate. Initially, the catalyst IrO2 exhibited excellent catalytic activity and stability in both acidic and alkaline environments. However, due to the high price and limited resources of Ir, many research efforts have been directed towards the development of OER catalysts based on transition metal oxides and non-metal materials.
Currently, numerous alternative OER electrocatalysts have been exploited to improve electrode kinetics and stability under different electrolyte media. Many researchers show great interest in metal-based materials for OER since these metal-based electrocatalysts generally exhibit excellent catalytic abilities for oxygen evolution. Ru-, Ir-, and Rh-based nanomaterials catalysts (Zhao et al., 2019a, 2019b; Bai et al., 2017) exhibit most outstanding OER catalytic activity, which includes high conductivity, chemical and thermodynamic stability (Lee et al., 2015), but the disadvantages of resource shortages, high prices and poor stability limit the application of these catalysts (Fabbri et al., 2014; Li et al., 2016b; Hu et al., 2012; Xiang et al., 2016). Transition metal based OER catalysts have become the promising alternatives because of the structural and compositional active sites during the oxygen evolving complex. Such as transition-metal oxides (Lu et al., 2014; Sun et al., 2014; Kleiman-Shwarsctein et al., 2009; Han et al., 2015), hydro(oxy)oxides (Gao et al., 2014b; Zhao et al., 2014; Wu et al., 2016; Ping et al., 2016; Wang et al., 2017b), perovskites (Jung et al., 2014; Risch et al., 2014; Guo et al., 2016; Kim et al., 2015; Zhu et al., 2015b), spinels (Ponce et al., 2002; Godinho et al., 2003; Chi et al., 2005; Jin et al., 2013; Pendashteh et al., 2017). However, the main drawback of metal oxide electrocatalysts is their poor electroconductivity, which hinders the further application of materials. Metal nonoxide systems with superior conductivity such as transition-metal dichalcogenides (TMDs) (Xia et al., 2016; Ganesan et al., 2015; Tang et al., 2016; Cao et al., 2016; Liu et al., 2015b), transition-metal nitrides (TMNs) and phosphides (TMPs) (Xu et al., 2015; Chen et al., 2016b; Zhang et al., 2016; Liu and Li, 2016; Chang et al., 2015; Yu et al., 2016), have been employed and showed excellent activities for both HER and OER, which exhibits the promising potentials for bifunctional electrocatalysts toward the overall water-splitting reaction. Currently, the development bottlenecks of fuel cell and water splitting depend on the slow kinetics of oxygen electrode reactions, the high over potential and the large use of metal electrode catalysts. So, it is of great significance to develop mental-free electrocatalysts for OER with low cost, high electrocatalytic activity and outstanding durability. In particular, various mental-free electrocatalysts, such as carbon-based materials and black phosphorus, have been extensively studied because of their unique catalytic advantages (Wang et al., 2014; Lu et al., 2015b; Cai et al., 2017; Chen et al., 2014; Jiang et al., 2016; Ren et al., 2017; Zhang et al., 2015b). Naturally, non-metallic compounds have attracted the attention of researchers. Early before, Mirzakulova and co-works reported a molecule catalyst of N(5)-ethylflavinium ion (Et-Fl+) for OER and it may be the first proofed that non-metallic compounds have the ability to perform oxygen evolution reactions (Mirzakulova et al., 2012), which provides a non-metallic compounds replacement for traditional transition metal-based water oxidation catalysts. In addition, non-metallic materials show great potential in electrocatalysis for the oxygen evolution reaction in practice since no metal is needed in this kind of materials.
Here, we demonstrate the development and applications of lower overpotential and longterm stability OER electrocatalysts in recent years and list them. The corresponding electrocatalytic parameters of these selected electrocatalysts mentioned in this article are listed in Table 1. Firstly, the reaction mechanism of OER are introduced simply, then, various metal and non-metal based OER electrocatalysts are reviewed, mainly transition metal oxides, chalcogenides, pnictides and non-metal compounds such as carbon-based materials, black phosphorus, and the like. Most of the OER electrocatalysts shows the remarkable OER catalytic performance with lower overpotential and longtime stability, also exhibits better or well comparable with commercial IrO2/RuO2 electrocatalysts. For these electrocatalysts, non-metallic catalyst shows the higher efficiency and stable performance towards both the HER and OER under alkaline conditions, which can used as a highly active electrocatalyst towards both the OER and HER. With the development study of researchers from various country and field, a new type bifunctional OER/HER electrocatalysts with novel material showing high performance will be realized to meet our requirements of practical applications in the near future.
Catalysts
Electrolytes
Substrate
Overpotential (mV) at specific current density
Current density (mA cm−2) at specific overpotential
Tafel slope (mV dec−1)
Ref.
Metal-based electrocatalysts
RuO2
0.1 M HClO4
GCE
—
∼0.01/∼0.003@ 250 mV
—
25
IrO 2
0.1 M HClO4
GCE
—
∼0.004/∼0.002@ 250 mV
—
25
PNC/Co
1.0 M KOH
MOF
410@10 mA cm−2
—
131
26
bulk Ru
0.1 M HClO4
GCE
219@0.5 mAmol−1109
—
44
74
Bulk Ir
0.05 M H2SO4
GCE
321@0.5 mAmol−1109
—
63
74
Bulk Pt
0.1 M HClO4
GCE
536@0.5 mAmol−1109
—
145
74
Ru NPs
0.1 M HClO4
GCE
274@0.5 mAmol−1109
—
—
74
Ir NPs
0.05 M H2SO4
GCE
333@0.5 mAmol−1109
—
64
74
Pt NPs
0.1 M HClO4
GCE
240@0.5 mAmol−1109
—
210
74
Co/N-CNTs
0.1 M KOH
GCE
390@10 mA cm−2
—
67
75
Fe/N-CNTs
0.1 M KOH
GCE
520@10 mA cm−2
—
76
75
Ni/N-CNTs
0.1 M KOH
GCE
590@10 mA cm−2
—
138
75
CoOx NPs/BNG
0.1 M KOH
GCE
295@10 mA cm−2
—
57
77
Co-Bi/G
0.1 M KOH
GCE
320@10 mA cm−2
—
70
77
CoOx@CN
1 M KOH
Ni foam
260@10 mA cm−2
—
—
81
Co-Fe-O/rGO
1 M KOH
GCE
340@10 mA cm−2
—
31
84
CoFe2O4/rGO
0.1 M KOH
GCE
540@29.5 mA cm−2
—
—
85
Ni0.4Co2.6O4
0.1 M KOH
Ni foil
520@10 mA cm−2
—
—
86
Ni0.6Co2.4O4
0.1 M KOH
Ni foil
530@10 mA cm−2
—
—
86
Ni0.9Co2.6O4
0.1 M KOH
Ni foil
530@10 mA cm−2
—
—
86
NiCo2O4
0.1 M KOH
GCE
490@10 mA cm−2
—
—
86
Ni-Fe oxide/Ni:Fe = 3:7
0.1 M KOH
FTO
329@2 mA cm−2
—
120
87
Ti/Co3O4
2 M KOH
Ti
—
∼0.018/∼0.02@660 mV
50
89
Ti/NiCo2O4
2 M KOH
Ti
—
∼0.44/∼0.008@660 mV
64
89
NiCo2O4
2 M NaOH
GCE
—
—
51
90
NiCo2O4(TD)
1 M KOH
Ni
453@100 mA cm−2
—
91
NiCo2O4(EPD)
1 M KOH
Ni
471@100 mA cm−2
—
91
NiCo2O4(AO)
1 M KOH
Ni
567@100 mA cm−2
—
91
NiCo2O4-G
0.1 M KOH
GCE
950@35.4 mA cm−2
—
164
92
CuCo2O4
1 M NaOH
Ti
—
—
—
94
CuCo2O4/NrGO
1 M NaOH
GCE
360@10 mA cm−2
—
64
96
CuCo2O4/NrGO
0.1 M KOH
GCE
410@10 mA cm−2
—
—
96
CuCo2O4/NrGO
0.1 M PBS
GCE
1150@10 mA cm−2
—
—
96
MnFe2O4
0.1 M KOH
GCE
470@10 mA cm−2
—
114
99
CoFe2O4
0.1 M KOH
GCE
370@10 mA cm−2
—
82
99
NiFe2O4
0.1 M KOH
GCE
440@10 mA cm−2
—
98
99
CuFe2O4
0.1 M KOH
GCE
410@10 mA cm−2
—
94
99
NiS
0.1 M KOH
SLS
297@11 mA cm−2
—
47
101
CoSe
1 M KOH
GCE
295@10 mA cm−2
—
40
102
Ni3S2
1 M NaOH
Ni foam
260@10 mA cm−2
—
—
104
Fe3O4@Co9S8/rGO
1 M KOH
GCE
340@10 mA cm−2
—
54.5
105
Co2N
1 M KOH
GCE
430@10 mA cm−2
—
80
54
Co3N
1 M KOH
GCE
410@10 mA cm−2
—
72
54
Co4N
1 M KOH
GCE
330@10 mA cm−2
—
58
54
Ni2P
1 M KOH
GCE
339@10 mA cm−2
—
—
107
Co2P
1 M KOH
GCE
367@10 mA cm−2
—
—
107
Fe2P
1 M KOH
GCE
390@10 mA cm−2
—
—
107
NiFeP
1 M KOH
GCE
277@10 mA cm−2
—
—
107
Ni2P
1 M KOH
Ni foam
200@10 mA cm−2
—
72
109
CoNiP
1 M KOH
GCE
280@10 mA cm−2
—
66.5
110
Ni3N bulk
1 M KOH
GCE
490@10 mA cm−2
—
45
53
Ni3N NSs
1 M KOH
GCE
350@10 mA cm−2
—
85
53
Ni3N NSs
1 M KOH
GC
350@52 mA cm−2
—
41
53
NiSe2-DO
1 M KOH
Ni foam
241@10 mA cm−2
—
32
28
NixFe1-xSe2-DO
1 M KOH
Ni foam
195@10 mA cm−2
—
28
28
Carbon-based electrocatalysts
NG
0.1 M KOH
GC
700@10 mA cm−2
—
—
59
N-CNT/GNR
0.1 M KOH
GC
360@10 mA cm−2
—
—
60
G-CNT
0.1 M KOH
GCE
498@5 mA cm−2
—
231
62
NG-CNT
0.1 M KOH
GCE
368@5 mA cm−2
—
141
62
NPMCa
0.1 M KOH
GCE
395@10 mA cm−2
—
—
65
N/C
0.1 M KOH
GCE
380@10 mA cm−2
—
—
115
o-MWCNTsb
0.1 M KOH
GCE
300@10 mA cm−2
—
—
118
CNT/BNc
0.1 M KOH
GCE
580@10 mA cm−2
—
122
120
N-CNTs
0.1 M KOH
GCE
390@10 mA cm−2
—
—
121
N-MWNT
1.0 M NaOH
GC
320@10 mA cm−2
—
68
122
N-G
0.1 M KOH
GC
434@10 mA cm−2
—
—
123
N-G/CNT
0.1 M KOH
GCE
400@10 mA cm−2
—
83
124
S,S-CNT
1 M KOH
GCE
350@10 mA cm−2
—
95
126
NS-GR/CNT
0.1 M KOH
GCE
560@10 mA cm−2
—
103
127
g-C3N4d
0.1 M KOH
GCE
734@10 mA cm−2
—
120.9
128
g-C3N4/G
0.1 M KOH
GCE
539@10 mA cm−2
—
68.5
128
Black phosphorous materials
BP
0.1 M KOH
Ti
370@10 mA cm−2
—
72.9
63
Few-layer BP
1 M KOH
GCE
220@10 mA cm−2
—
88
64
2 Fundamentals of the OER
Water molecules are decomposed into hydrogen and oxygen at external potential. To accelerate water splitting reaction, hydrogen and oxygen evolving catalysts are applied to the surface of cathode and anode, respectively. The reaction pathway of OER depends on the pH of the solution. In acidic medium, OER takes place by converting two water molecules into protons and oxygen molecule. However, in alkaline and neutral solutions, OER involves oxidation of four hydroxide ions to water and dioxygen.
Oxygen evolution reaction is an anodic reaction of the water splitting reaction and it is the most energy-intensive step in the electrolysis reaction because the anode reaction involve four-electron transfer. Many research groups have proposed possible mechanisms for oxygen release reactions in acidic or alkaline conditions at anode electrodes. A brief overview of OER process on the surface of transition metal oxide can help to understand the activity trends observed (Bandal et al., 2018). Oxygen evolution reactions involve the adsorption and desorption of metal active sites on a variety of oxygen containing intermediate species (Kleiman-Shwarsctein et al., 2009; Strasser et al., 2010). As can be seen in Fig. 1a, there are two different ways of producing oxygen from the MO intermediate product. One is the green route, in which oxygen is produced by the direct combination of two M-Os; the other is the generation of oxygen by the formation of MOOH intermediates followed by decomposition, including acidic and basic mechanisms (Suen et al., 2017). Despite these differences, OER's general mechanism for oxides in acidic and alkaline solutions can be summarized as follows (Fig. 1b). Generally, the OER intermediates are stabilized by the catalyst such as surface-bound peroxo- or oxo-species. The relative stabilities of these intermediates and the activation barriers between them dictate the determined step to the rate and further to the overall rate of water oxidation. The properties of the surface intermediates depend on the reaction conditions (e.g. pH) and the catalyst materials (Trotochaud and Boettcher, 2014).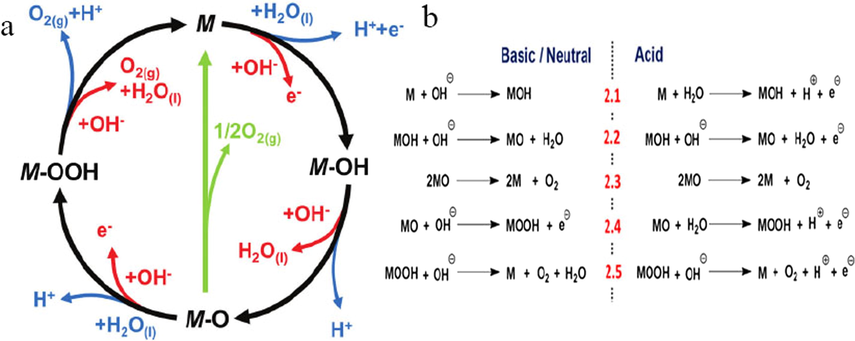
(a) The OER mechanism for acid (blue line) and alkaline (red line) conditions. The black line indicates that the oxygen evolution involves the formation of a peroxide (M-OOH) intermediate (black line). Reprinted with permission from Suen et al. (2017) copyright 2017, The Royal Society of Chemistry. (b) The scheme of proposed mechanism for OER. Reprinted with permission from Bandal et al. (2018) copyright 2018, Elsevier.
Electrocatalysis is a catalytic action accelerating the charge transfer at the interface between the electrode and the electrolyte, and also can be used as a catalyst to promote the electrochemical reaction. Electrocatalytic kinetics is a measure of electrocatalytic activity. Based on some key parameters such as overpotential, Tafel slope, stability, Faraday efficiency and Turnover frequency (TOF), it is often used to measure the catalytic activity of OER electrocatalysts. Tafel equation: η = a + b log |j|, where η is the overpotential, b is the Tafel slope, and j is the current density, which are effectively evaluate the performance of electrocatalysts. Overpotential(η) is one of the most important indicators to measure the performance of electrocatalysts. In electrochemistry, overpotential is the potential difference (voltage) between the reduction potential of the semi-reaction thermodynamic and the potential of the redox reaction observed by the experiment. Generally speaking, it is necessary to achieve the value of the specified density (10 mA cm−2), and the lower the overpotential of the electrocatalyst the better the electrocatalytic power. Exchange current density (j) is another important parameter for electrocatalytic kinetics. The exchange current density can be used to describe the ability of an electrode to react to lose electrons and to reflect the ease with which an electrode reaction can proceed. Higher exchange current density indicates better electrocatalytic capability. Tafel slope (b) is also an important indicator of the electrocatalytic kinetics. Usually smaller Tafel slope (b) indicates that the current density can increase more rapidly with a smaller overpotential (η), which illustrates good electrocatalytic kinetics. Furthermore, catalyst stability refers to the ability of a catalyst to maintain its activity, thermal stability and structure unchanged during the catalytic reaction. Faraday efficiency refers to the percentage of actual and theoretical products, which is affected by temperature, electrolyte concentration, applied voltage, solution acidity and even the purity of electrode material. Turnover frequency (TOF), is the number of transformations at a single active site per unit time, measuring the rate of a catalyst's catalytic reaction and represents the intrinsic activity of the catalyst (Liang et al., 2014). Besides, the electrocatalytic properties of the tests are mainly measured for its elemental composition, surface area, faradaic efficiency, catalytic activity, stability and so on. McCrory et al. (2013) proposes a general test method for electrocatalysts for oxygen evolution reaction including morphology test, composition test, specific surface area test, and electrochemical test in Fig. 2, which provides convenience for the evaluation of electrocatalytic performance and the assistance for the development of electrocatalysts. In recent years, relevant researchers have suggested that the phase change of the electrocatalyst during the oxygen evolution reaction will result in a different catalytic active site from the original sample preparation (Liu et al., 2015a; Seitz et al., 2016). Therefore, we should pay close attention to the structural evolution of the electrocatalyst during the oxygen evolution reaction, and develop an electrocatalyst with outstanding performance.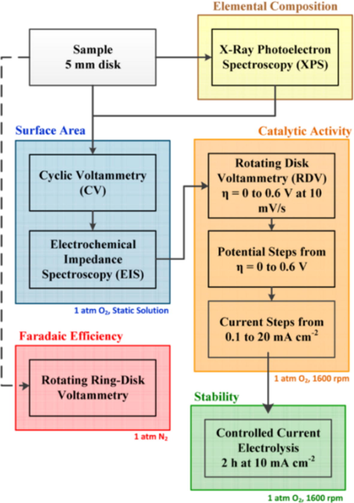
General test method for OER or ORR. Reprinted with permission from Mccrory et al. (2013) copyright © 2013, American Chemical Society.
3 Noble-metal-free based electrocatalysts for OER
Among the available metal based catalysts, Ru-, Ir-, and Rh-based catalysts exhibit most outstanding OER catalytic activity, and the OER activity catalysts decreases as Ru > Ir > Rh (Li et al., 2016a; Fabbri et al., 2014; Reier et al., 2012). Resource shortages, high prices and poor stability limit the use of these catalysts despite noble-metal materials being the best catalysts for OER. So, it is a challenge that to reduce the use of a large number of noble metal catalysts and to find alternative non-noble material with excellent performance. Transition metal based OER catalysts have become promising alternatives because of the structural and compositional active sites of the oxygen evolving complex (Liu et al., 2016), such as spinel, transition-metal oxides, sulfides, nitrides and phosphides and so on. Meanwhile, transition-metal elements have low cost, high activity and long-term stability under low oxidation conditions, which makes the whole water splitting more feasible. Among the excellent transition-metal based catalysts, cobalt oxides is one of the earlier used oxides as an oxygen evolution catalyst in an alkaline medium due to its high stability and special 3d electronic configurations (Krishtalik, 1981; Tong et al., 2017; Li et al., 2018b). With the deepening of research, it has been found that it is still inferior to noble metal oxides, although cobalt oxides has good stability and good catalytic activity. Then, it is found that doped or mixed oxides can produce synergistic effects through intra-component electronic interactions, which can effectively regulate the physicochemical properties of oxides and further improve the catalytic performance of oxides (Li et al., 2018a; Huang et al., 2017). Jin et al. (2015) have synthesized cobalt/cobalt oxide/N-doped carbon hybrids (CoOx@CN) by simple one-pot thermal treatment method, and it exhibited the better catalytic activity than cobalt oxides for both oxygen evolution and oxygen reduction reactions, reaching a current density of 20 mA cm−2 at 1.55 V.
The transition metal oxides owns the surface active sites, which can catalyze the oxygen evolution reaction (Burke et al., 2016). The process of the oxygen evolution mechanism is the continuous oxidation of the active site of the metal. However, metal cation forms the hydroxyl peroxide, leading to limit the rate of oxygen evolution (Chi et al., 2005; Strasser et al., 2010), which reflects the prosperity of transition metal oxides. Numerous kinds of transition metal oxides catalysts can be used as superior OER electrocatalysts, such as TMOs/N-doped carbon hybrids (Tong et al., 2017; Rossmeisl et al., 2007), Co-Fe-O/rGO (Geng et al., 2015; Bian et al., 2014), NixCo3-xO4 (Lambert et al., 2015); Ni-Fe oxide (Zhang et al., 2015a), MxMn3-xO4 (M = divalent metals) (Chinnusamy et al., 2011) and so on. At present, spinel-type oxides include MCo2O4, MFe2O4, MMn2O4, etc., among them, cobalt-based spinel oxides (MCo2O4, M = Ni, Zn, Cu, Mn, etc.) have high stability and activity in alkaline solution, and low price of transition metals, which makes this type of oxide electrode become one of the most potential OER the electrocatalysts. Singh et al. (1990) and Chialvo and Chialvo (2010) early studies found that the spinel NiCo2O4 with cobalt-based nickel exhibited the best catalytic activity for both oxygen evolution and oxygen reduction reactions. The effects of three preparation methods including pyrolysis, electrophoretic deposition and Ni-Co alloy oxidation on the catalytic performance of NiCo2O4 electrode were compared, which shows that the oxide catalyst prepared by pyrolysis has the best apparent catalytic activity, the catalyst prepared by electrophoretic deposition has the best stability, and the alloy oxidation method has no obvious effect on improving the performance of the catalyst. Chi et al. (2006) Besides, Dong et al. (2013) reported that a novel hybrid material composed of mesoporous NiCo2O4 nanoplatelets and graphene (NiCo2O4-G) as an active bi-functional electrocatalyst for both ORR and OER synthesized by a one-pot method. They investigated the hybrid effect of combining NiCo2O4 nanoplatelets with graphene sheets by comparing its performance to that of NiCo2O4 itself without graphene sheets, which shows that graphene sheets as a support material can facilitate charge transfer much fast. Lastly, compared with Co2O4-G, the electrocatalytic activities of NiCo2O4-G shows that Ni incorporation into the octahedral sites of the spinel crystal structure can improve electrical conductivity and generate new active sites. Meanwhile, a facile template-free co-precipitation route for the design and fabrication of well-ordered NiCo2O4 (NCO) spinel nanowire owns high specific surface area (124 m2 g−1) and good catalytic activity for the OER (Jin et al., 2013), which is higher than that of the previously reported NiCo2O4 nanoplatelet/graphene hybrid catalyst (77 m2 g−1) (in Fig. 3a, b) (Dong et al., 2013). Taking into consideration of the price and performance advantages with copper ion doping, spinel structure CuCo2O4 has become a hot spot for doped oxide catalysts. CuxCo3-xO4 prepared by pyrolysis and sol-gel method revealed that the copper ions not only selectively enter the octahedral active sites during the doping process, but also facilitate the refinement of the crystalline grains. It is also pointed out that the sol-gel method is easier to prepare pure spinel structure CuCo2O4, which has the best catalytic activity and does not contain the CuO phase (Koninck et al., 2006). It can be seen from the preparation of spinel CuxCo3-xO4 film (Berenguer et al., 2008; Rosatoro et al., 2006) that, when the stability content of Cu reaches a certain amount, the electrode will corrode, which is caused by the damage degree of the spinel surface on the degree of sharpening of Cu grain, and it is also the main causes of poor stability potential Cu in binary oxide dissolution of electrode under high anode. In order to improve the OER catalytic performance of CuCo2O4, a composite material (CuCo2O4/NrGO) consisting of CuCo2O4 nanoparticles anchored on nitrogenated reduced graphene oxide had been reported (Bikkarolla and Papakonstantinou, 2015), which greatly improves its catalytic activity. The study of MnxCo3-xO4 binary oxides shows that doping of Mn ions is not conducive to the improvement of oxygen evolution activity of oxides, but it is beneficial to the improvement of catalytic oxygen reduction activity (Restovic, 2002; Hirai et al., 2016). In addition, Li et al. (2015) have designed a series of porous M-substituted magnetite MFe2O4 nanofibers (MFe2O4NFs) with M = Co, Ni, Cu, and Mn, and also reported a trend of the OER electrocatalytic activities with CoFe2O4 > CuFe2O4 > NiFe2O4 > MnFe2O4 for MFe2O4 samples (as Fig. 3c).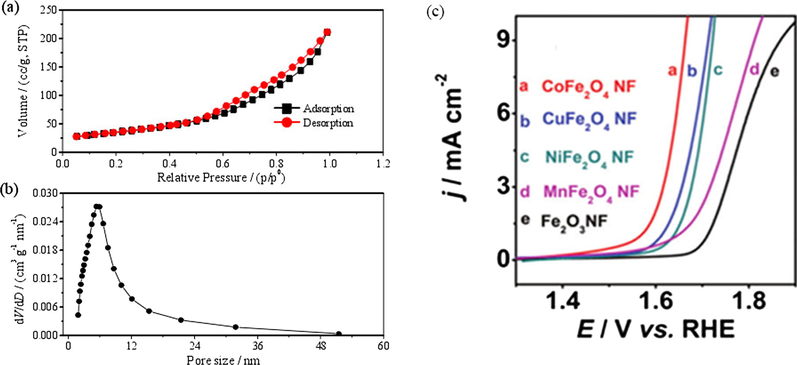
N2 adsorption-desorption isotherm (a) and pore distribution plot (b) of NCO spinel nanowire arrays. Reprinted with permission from Dong et al. (2013) copyright © 2013, The Royal Society of Chemistry. (c) Polarization curves for OER on Fe2O3, CoFe2O4, NiFe2O4, CuFe2O4, and MnFe2O4 NFs. Reprinted with permission from Li et al. (2015) copyright © 2015, The Royal Society of Chemistry.
The above-mentioned metal oxides show excellent electrocatalytic abilities for OER, but there is a problem of phase transformation of spinel during oxygen evolution. The replacement of oxides with chalcogenides (sulfides, selenides or tellurides) will increase the intrinsic conductivity of the material. It is well known that transition-metal chalcogenides have prominent electronic structures and physical appearance, for instance, excellent conductivity, magnetic property and halfmetallicity (Xiang et al., 2016; Xia et al., 2016; Ganesan et al., 2015; Tang et al., 2016; Cao et al., 2016; Liu et al., 2015b; Kakade et al., 2016; Chen et al., 2016a; Liao et al., 2016; Gao et al., 2014a; Feng et al., 2015; Yang et al., 2016). Fe doped NiSe2 derived oxide catalysts (NixFe1-xSe2-DO) with an overpotential of ∼195 mV, a Tafel slope of 28 mV dec−1 (as Fig. 4), and the derived oxide (NixFe1-xSe2-DO) exhibits much better OER activity than that of NiFe-LDH (Xiang et al., 2016). Due to their distinct electronic structure especially in as electrocatalysts, similar to metal chalcogenides, transition metal nitrides (TMNs) also show excellent catalytic activities in many fields (Xu et al., 2015; Chen et al., 2016b; Zhang et al., 2016; Liu and Li, 2016). A series of metallic cobalt nitrides (Co2N, Co3N and Co4N) arising from electron delocalization modulation indicated that the materials with higher amounts of cobalt are more conductive and more active for OER (Chen et al., 2016b). Likewise, Xu et al. (2015) pointed that the electroconductivity of Ni3N sheet is higher than that of bulk Ni3N. The calculated density of states (DOS) of Ni3N sheet (Fig. 5a) nearing the Fermi level is more intense than bulk Ni3N, which improves the OER activity greatly. Recently, transition metal phosphides (TMPs) have been extensively identified as new promising earth-abundant and highly active catalysts for hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) (Liu and Li, 2016; Chang et al., 2015; Yu et al., 2016; Read et al., 2016; Ryu et al., 2015; You et al., 2016; Fu et al., 2016). A series of transition metal phosphide electrodes as electrocatalysts for the HER and OER are fabricated by thin film phosphidation strategy, which have highly actives and excellent electrocatalytic performance (as Fig. 5b) (Read et al., 2016). In a word, metal phosphides have shown great potential in both HER and OER (Liu et al., 2017a). The combination of transition metals and metal phosphides catalysts can improve their OER activity, but their performance will deteriorate rapidly after long-term use, which is worthy of our consideration.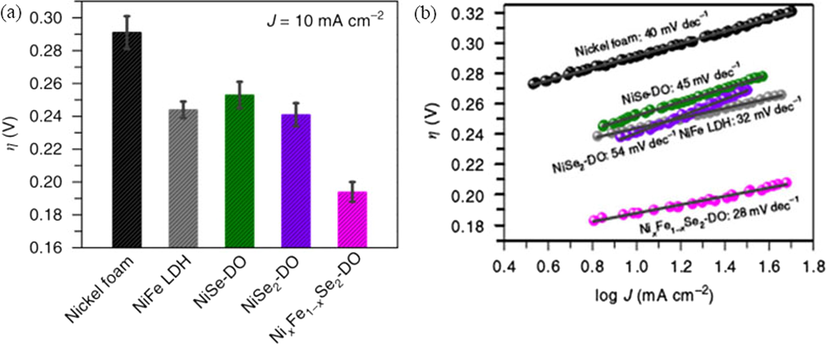
(a) Overpotential (η) at 10 mA cm−2 and (b) Tafel plots for Ni foam, NiFe LHD, NiSe-DO, NiSe2-DO and NixFe1-xSe2-DO. Reprinted with permission from Xiang et al. (2016) copyright © 2016, Nature Communications.
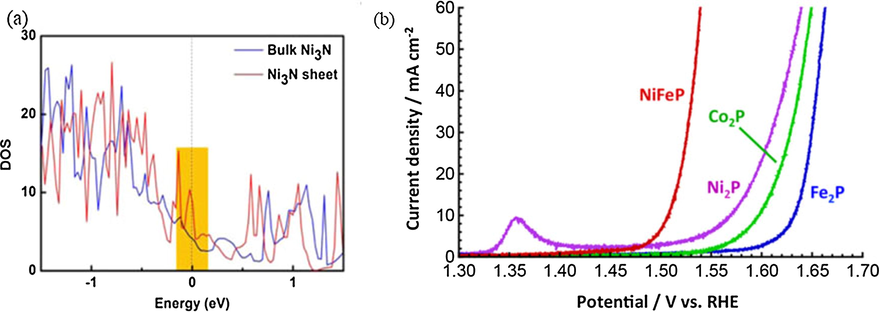
(a) Calculated density of states for bulk Ni3N and Ni3N sheet. The Fermi level is set at 0 eV. Reprinted with permission from Xu et al. (2015) copyright © 2015, American Chemical Society. (b) Polarization data of NiFeP, Co2P, Ni2P, Fe2P films for the OER in 1 M KOH. Reprinted with permission from Read et al. (2016) copyright © 2016, American Chemical Society.
4 Carbon-based electrocatalysts for OER
Metal-based catalysts suffer from many drawbacks, such as impurity interference, low selectivity, poor stability, unclear active sites and so on (Frydendal et al., 2015. Therefore, it is will be a particularly challenge to find a category of OER catalyst, which has a sufficiently high activity, with more electrocatalytic active sites, larger specific surface area and stable lifetime. Since Mirzakulova et al. (2012) proved that the non-metallic compounds has the ability to perform oxygen evolution reactions, carbon-based materials such as carbon black, carbon nanotubes and graphene with N, P, B and S doped as good conductive material and in acidic or alkaline electrolyte showing chemical inertness, have been studied and widely used in electric catalytic. Carbon-based materials can be used as promising electrocatalysts, and doping mixed atoms such as nitrogen or boron can effectively improve their catalytic activity. Moreover, the presence of N in N-doped carbon results in more chemically active sites, high density defects and high electrochemical activity (Chi et al., 2018). Due to these enhanced electronic properties, N-doped catalysts in the Carbon network are attractive for a wide range of applications, as non-metallic catalysts for OER/ORR in fuel cell systems (Yang et al., 2017) For example, Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for OER with a current density reached to 10 mA cm−2 at 0.38 V in alkaline media (Fig. 6a), this value is equivalent to iridium and cobalt oxide catalysts and is the best among non-metal oxygen evolution catalysts. The evolved O2 gas was detected at an onset potential (Fig. 6b), clearly showing that the N/C catalyst has OER activity (Zhao et al., 2013).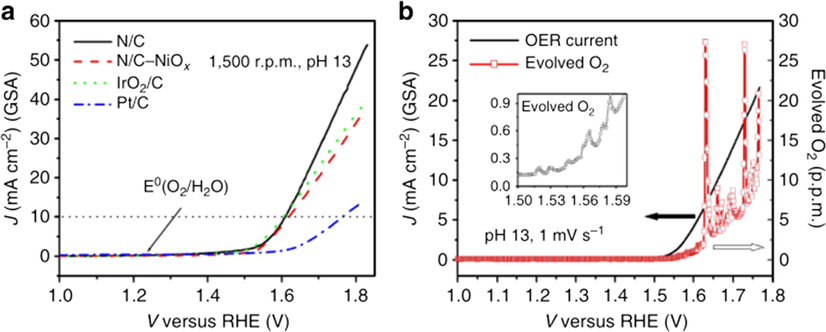
(a) Oxygen evolution activities of the N/C electrodes with KOH electrolyte (pH 13) analyzed from RRDE system (b) The amount of evolved O2 (curve 1) and current density on the N/C electrode (curve 2) during the positive potential sweep with the electrochemical cell (inset: the evolved O2 in the potential range from 1.5 to 1.6 V). Reprinted with permission from Zhao et al. (2013) copyright © 2013, 2013 Macmillan Publishers Limited.
The original features of carbon nanotubes (single wall and multiple walls, SWNTs and MWNTs) and the correlation between the numbers of the walls and their corresponding OER activities, in which the outer wall was responsible for OER and the function of the inner wall is to increase the electron transfer rate, thus the OER activity will be enhanced with the numbers of walls increased. Recently, the increased number of walls has a lower OER activity due to the diminishment of dc bias influence (Cheng et al., 2015). Later, some people successfully used MWCNT without any treatment as a high-efficiency electrocatalyst in OER, and proposed the dual function of MWCNT with outer wall and inner wall (Ali et al., 2018). At the same time, the surface oxidation of MWCNTs to generate oxygen-containing groups and defect sites to dope the heteroatoms to achieve unexpectedly high OER catalytic activity was also firstly proposed (Lu et al., 2015a). In addition, the composite of carbon nanotubes with other nanomaterials has also proven to be an important catalyst (Patil et al., 2016; Zhu et al., 2015a). Particularly, due to the unique graphene-like structure of hexagonal boron nitride (h-BN) and its synergy with carbon nanotubes, the composites exhibits an excellent OER electrocatalytic activity towards, which owns better stability than that of the state-of-the-art Pt/C catalyst (as Fig. 7) (Patil et al., 2016). In the research field of carbon nanotubes, large surface area N-doped porous carbon nanotubes (NCNT) obtained by nitrogen doping have in situ N-doped graded porous structures, which can promote the transport of gas molecules, and N-doping can provide abundant active sites to enhance catalytic activity, which was proved by Pan et al. (2016). Nitrogen-doped multi-walled carbon nanotubes (N-MWNTs) are controlling the nitrogen active sites, in which shows remarkable OER activity, owing the most superior active non-metallic OER electrocatalyst (Davodi et al., 2017).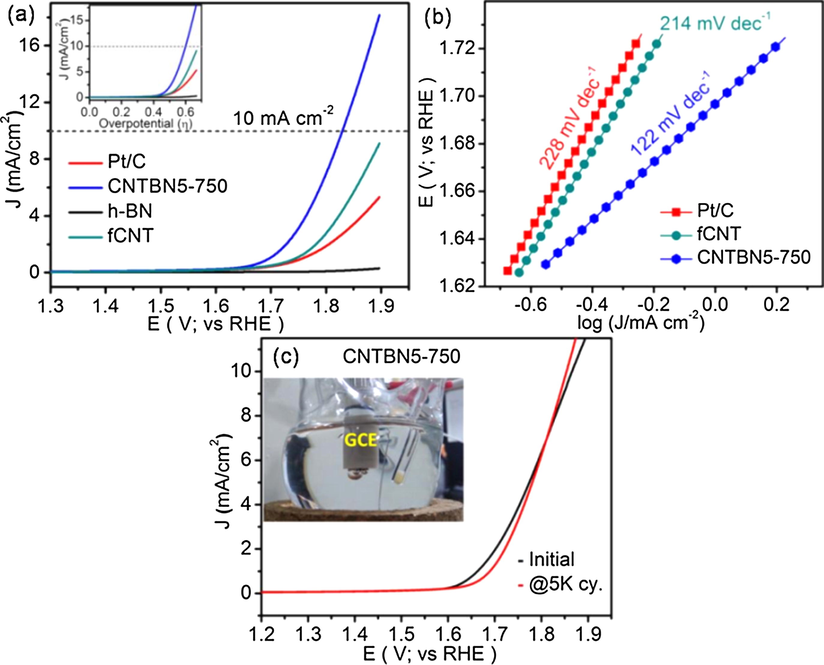
(a) OER voltammograms of CNTBN5-750, bare h-BN, fCNT and Pt/C catalyst; inset shows plot of standard thermodynamic potential for OER; (b) Tafel plots of CNTBN5-750, fCNT and Pt/C. (c) Comparison of durability of CNTBN5-750; inset shows a picture of the oxygen bubbles generated on the CNTBN5-750 modified electrode during OER. Reprinted with permission from Patil et al. (2016) copyright © 2016, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Except that, graphene-based materials such as graphene, graphene-doped or graphene-like materials also have the excellent OER activity (Chen et al., 2014; Chi et al., 2018; Wang et al., 2017a; Tian et al., 2014b, 2015a, 2015b; El-Sawy et al., 2016; Zhao et al., 2016). Chen et al. (2014) reported a N,O-dual doped graphene-CNT hydrogel film with excellent electrochemical durability because of the higher surface area and more active sites. While the Tafel slope was around 141 mV dec−1, the slightly higher Tafel slope indicates that OER dynamics need to be improved, and a more active site may improve OER dynamics. In order to develop an effective catalyst with abundant highly active sites and full exposure to reactants, a nitrogen-doped mesoporous graphene framework (NMGF) was synthesized by a CVD method on 3D MgO templates (in Fig. 8a). The as-obtained NMGF has unique structural features with plentiful active centers due to defects and heteroatoms, a large specific surface area and a high electrical conductivity, which improves utilization efficiency due to the high electrochemically active surface area and hydrophilic surface (as Fig. 8c), thereby leading to superior ORR and OER bifunctional activities (as Fig. 8b) (Wang et al., 2017a). Meanwhile, a new N-doped graphene/single-walled carbon nanotube (SWCNT) hybrids is synthesized by one-step CVD method as a bifunctional electrocatalyst, which forms a three-dimensional interconnect network of graphene and SWCNT. Meanwhile, the NGSHs have a large specific surface area of 812.9 m2 g−1 and a high conductivity of 53.8 S cm−1 (Tian et al., 2014b). Controlling active sites of metal-free catalysts is an important method to improve activity of the oxygen evolution reaction (OER) (Tian et al., 2015b). El-Sawy et al. (2016) developed a sequential two-step strategy to dope sulfur into carbon nanotube-graphene nanolobes and the strategy introduces stable sulfur-carbon active-sites and enhances catalytic activity of bidoped carbon nanotubes. Moreover, compared the 3D structure of nitrogen and sulfur co-doped graphene/carbon nanotube (NS-GR/CNT) with single N-doped graphene/carbon nanotubes (N-GR/CNT), NS-GR/CNT has more negative onset potential and lower Tafel slope (in Fig. 9), which indicates S doping provides more active site and further significantly improves OER performance (Zhao et al., 2016).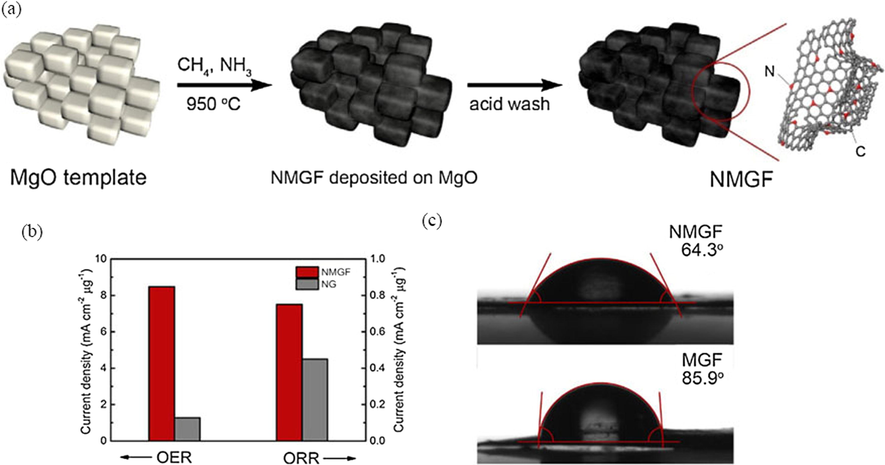
(a) Schematic illustration of NMGF formation on porous MgO template. (b) The specific current density of NMGF and NG. (c) The contact angles of NMGF and MGF, With the introduction of N heteroatoms, contact Angle from 85.9° greatly reduced to 64.3°, show that the surface wettability significantly increased, thus improve the permeability of the electrolyte and affinity. Reprinted with permission from Wang et al. (2017a) copyright © 2017, Elsevier.
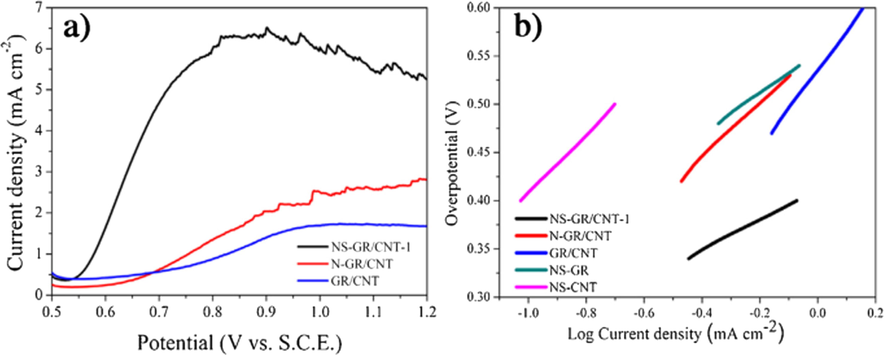
(a) Linear sweep voltammograms of NS-GR/CNT-1, N-GR/CNT and GR/CNT. (b) The Tafel plots of NS-GR/CNT-1, N-GR/CNT, GR/CNT-NS-GR and NS-CNT. Reprinted with permission from Zhao et al. (2016) copyright © 2016, Elsevier.
Considering the excellent chemical and thermal stability, graphite C3N4 (g-C3N4) owing high nitrogen content has attracted much attention. Tian et al. (2015a further demonstrated that g-C3N4 nanosheets/graphene composites are synthesized by the integration of ultrathin g-C3N4 nanosheets and graphene, which can be used as an efficient OER catalyst with good durability. It is the first demonstration of the use of g-C3N4 as a metal-free OER electrocatalyst. In addition, g-C3N4 as a good composite material can be doped with P or S, which has shown promising catalytic activities in OER. Recently, P and S co-doped C3N4 (PCSC-C3N4, C3N4 with P and S co-doped at the carbon site) shows superior OER/ORR activity due to the synergistic effects between electronic and geometric factors, which improves electrical conductance by modulating the electronic structure with extra electrons from dopants (in Fig. 10) (Chi et al., 2018).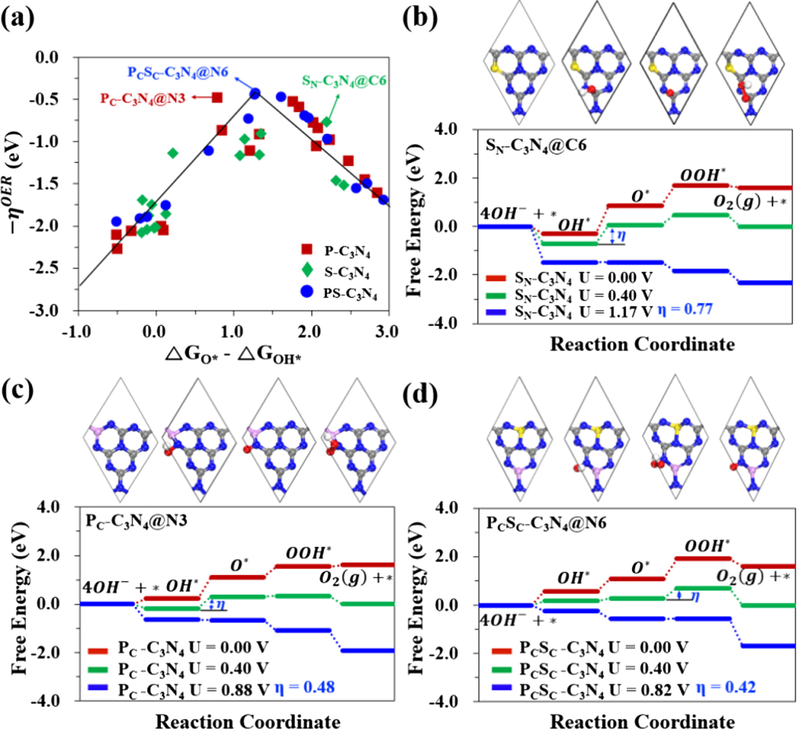
(a) The volcano plots of OER at all possible active sites on XY- C3N4. (b)–(d) The free energy diagrams (FEDs) of SN-C3N4, PC-C3N4 and PCSC-C3N4 structures having the best catalytic activity. (The order of OER activity is PS-C3N4 > P-C3N4 > S-C3N4.) Reprinted with permission from Chi et al. (2018) copyright © 2018, American Chemical Society.
As mentioned above, carbon-based materials not only provide higher electron conductivity and improve the electrochemical performance of the catalysts compared with metal oxide-based OER catalysts, but also can be mass produced due to lower manufacturing costs. Moreover, such metal-free catalysts can avoid the release of metal ions, thereby reducing the environmental impact.
5 Black phosphorus materials
Recently, black phosphorus as a new 2D material with 2D puckered layer structure, which can be regulated its electroconductivity by adjusting the film thickness. The black phosphorus layers have an anisotropic structure, which is unique among layered materials (Churchill and Jarilloherrero, 2014; Li et al., 2014). In the recent work of Jiang et al. (2016), black phosphorus (BP) prepared by the thermal-vaporization transformation (TVT) method exhibits a highly efficient OER activity with an onset potential of about 1.48 V (vs. RHE) and a current density of 10 mA cm−2 at an applied voltage of around 1.6 V. The stability performance of BP grown on carbon nanotube network (BP-CNT) is better than that of BP supported on Ti foil (BP-Ti) (as Fig. 11b, c), which is the first time the application of BP in electrocatalysis has been proposed. However, the crystal structure of bulk BP owns little active sites, which leads to the inferior OER catalytic performances, thus few-layer BP nanosheets prepared by facile liquid exfoliation (Fig. 11a) with excellent OER catalytic performance compared with that of bulk BP (Ren et al., 2017). The synergy of few-layered exfoliated black phosphorus (EBP) and N-doped graphene (NG) is fully exploited by interface engineering, which not only improves the stability of EBP, but also effectively boosts their intrinsic activities. EBP@NG as an efficient bifunctional catalysts towards hydrogen evolution and oxygen evolution reactions (HER/OER) achieves significant catalytic performance and an excellent durability (Yuan et al., 2019). At present, there are few studies on BP, it is both an opportunity and a challenge for us to continue to the study based on black phosphorus.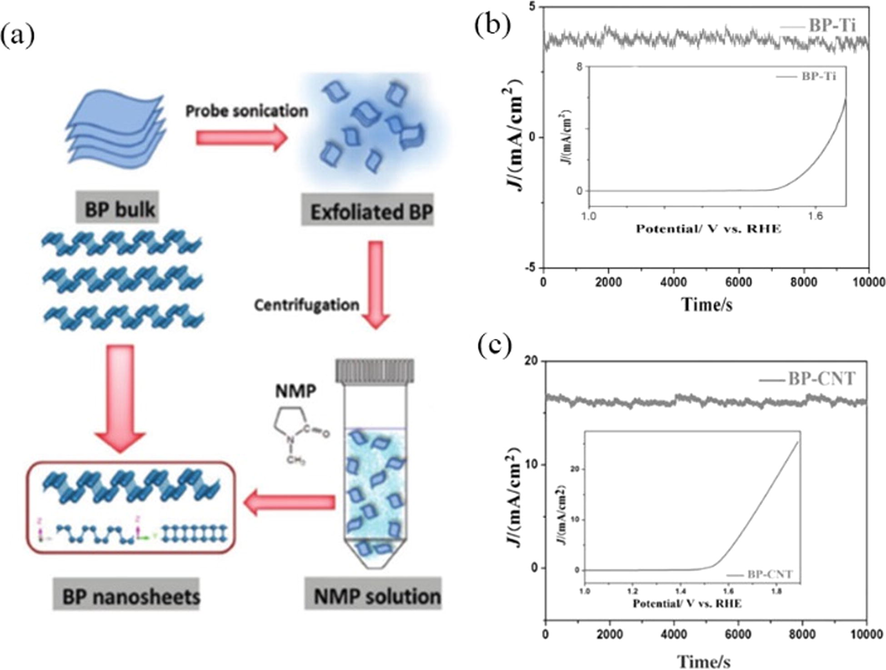
(a) Schematic illustration of solution-phase exfoliation route to prepare few-layer BP nanosheets. Reprinted with permission from Ren et al. (2017) copyright © 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b and c) Chronoamperometric response at 1.65 V and 1.77 V (E j = 16.5), insert shows polarization curves of BP-Ti and BP-CNT before chronoamperometric response. Reprinted with permission from Jiang et al. (2016) copyright © 2016, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
6 Conclusion and outlook
Water electrolysis to hydrogen and fuel cells are two key technologies in the field of hydrogen energy, the development of highly efficient materials to electrocatalyze water splitting plays a crucial role in realizing high energy conversion efficiency. However, the use of rare precious metal catalysts has hindered the smooth development with these two technologies because of their high price and scarce resources. Therefore, the development of efficient and low-cost non-noble metal catalysts and non-metallic catalysts are become the key problem to solve. For many catalyst materials proposed in the present review have their advantages and disadvantages. For example, the drawback of metal oxide electrocatalysts is the poor electroconductivity. Non-metallic oxide systems with excellent conductivity such as metal chalcogenides and pnictides have been employed and have exhibited excellent activities for both HER and OER, which makes it promise potentials for bifunctional electrocatalysts toward the overall water-splitting reaction. In addition, non-metal compounds and black phosphorus electrocatalysts have demonstrated excellent oxygen evolution catalytic capabilities.
Many researchers initially were very interested in metal-based electrocatalysts, because these metal-based electrocatalysts exhibit excellent electrocatalytic effects and the catalyst is of great practical value based on inexpensive transition metals such as nickel, iron, and cobalt. For such transition metal oxide catalysts, including spinel structures, perovskite structures, and pyrochlore structures, the most widely studied are spinel electrocatalysts. But some literature indicates that there are some problems with such catalysts. For example, the effects of the preparation methods, the doping of excess elements in the spinel material results in structural instability and the poor conductivity of ubiquitous metal oxides. Therefore, it is especially important to find new types of doped oxide electrocatalysts to break the limitations. While, for non-metal oxide systems, such as metal chalcogenides and pnictides, have excellent electrical conductivity and exhibit excellent activity for both HER and OER. This bifunctional electrocatalyst becomes a potential share of the overall hydrolysis reaction. The catalytic mechanism of this catalyst is that the thin oxide layer formed in situ on the surface is responsible for performing the OER, while the material itself acts as a conductive support. Similarly, designing a non-metallic compounds catalyst requires only a large number of active sites to increase its catalytic activity, which can avoid impurities introduced by metal doping.
In summary, future efforts to design high-activity OER catalysts should pay much attention on the material composition, dynamic structures of the catalysts and purity materials during the synthesis process. Therefore, designing an efficient, stable, economic and environmental electrocatalyst is a urgent challenge to be faced. In this case, the electrocatalyst can be designed with high efficiency by introducing foreign elements, doping conductive materials, and changing the morphology, which can improve its electrocatalytic ability. Most importantly, most works in the design of nonprecious electrocatalysts have focused on developing HER catalysts for acidic conditions. But for OER catalysts, due to their thermodynamic convenience are used for alkaline conditions, which may lead to incompatible integration of the two types of catalysts and resulting in poor overall performance. Thus, in order to develop more mature cathode and anode catalysts with excellent electrocatalytic performance, more and more research on combination of HER and OER catalyst should be done, which will satisfy the need of practical application requirements in the future.
Acknowledgements
The work is supported by the National Natural Science Foundation of China (Grant No.: 51473082, 51273096 and 51603109), the programme of Introducing Talents to the Universities (111 plan) and the National One-Thousand Foreign Expert Program (No. WQ20123700111), the Natural Science Foundation of Shandong province (No. ZR2017BEM047) and the postdoctoral science foundation of China (No. 2017M610408).
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Free-standing carbon nanotubes as non-metal electrocatalyst for oxygen evolution reaction in water splitting. Int. J. Hydrogen Energy. 2018;43(2):1123-1128.
- [Google Scholar]
- Ultrathin rhodium oxide nanosheet nanoassemblies: synthesis, morphological stability, and electrocatalytic application. ACS Appl. Mater. Interfaces. 2017;9(20):17195-17200.
- [Google Scholar]
- Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc.. 2013;135(36):13521-13530.
- [Google Scholar]
- Iron-based heterogeneous catalysts for oxygen evolution reaction; change in perspective from activity promoter to active catalyst. J. Power Source. 2018;395:106-127.
- [Google Scholar]
- Origin of the deactivation of Spinel CuxCo3−xO4/Ti anodes prepared by thermal decomposition. J. Phys. Chem. C. 2008;112(43):16945-16952.
- [Google Scholar]
- A CoFe2O4/graphene nanohybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J. Power Source. 2014;250(3):196-203.
- [Google Scholar]
- CuCo2O4 nanoparticles on nitrogenated graphene as highly efficient oxygen evolution catalyst. J. Power Source. 2015;281:243-251.
- [Google Scholar]
- Oxygen evolution reaction electrocatalysis on transition metal oxides and (Oxy)hydroxides: activity trends and design principles. Cheminform. 2016;47(4) 151014123904001
- [Google Scholar]
- 3D Co-N-doped hollow carbon spheres as excellent bifunctional electrocatalysts for oxygen reduction reaction and oxygen evolution reaction. Appl. Catal. B: Environ.. 2017;217:477-484.
- [Google Scholar]
- Cobalt sulfide embedded in porous nitrogen-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Electrochim. Acta. 2016;191:776-783.
- [Google Scholar]
- A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy. 2013;38(12):4901-4934.
- [Google Scholar]
- Surface oxidized cobalt-phosphide nanorods As an advanced oxygen evolution catalyst in alkaline solution. ACS Catal.. 2015;5(11):6874-6878.
- [Google Scholar]
- Nitrogen and oxygen dual-doped carbon hydrogel film as a substrate-free electrode for highly efficient oxygen evolution reaction. Adv. Mater.. 2014;26(18):2925-2930.
- [Google Scholar]
- Stainless steel mesh-supported NiS nanosheet array as highly efficient catalyst for oxygen evolution reaction. ACS Appl. Mater. Interfaces. 2016;8(8):5509-5516.
- [Google Scholar]
- ChemInform abstract: cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction. Cheminform. 2016;3(2):236-242.
- [Google Scholar]
- Pristine carbon nanotubes as non-metal electrocatalysts for oxygen evolution reaction of water splitting. Appl. Catal. B Environ.. 2015;163:96-104.
- [Google Scholar]
- A metal-free OER/ORR bifunctional electrocatalyst in alkaline media: from mechanisms to structure-catalytic activity relationship. ACS Sustain. Chem. Eng.. 2018;6(4)
- [Google Scholar]
- Electrophoretic deposition of ZnCoO spinel and its electrocatalytic properties for oxygen evolution reaction. Electrochim. Acta. 2005;50(10):2059-2064.
- [Google Scholar]
- Comparison of three preparation methods of NiCo2O4 electrodes. Int. J. Hydrogen Energy. 2006;31(9):1210-1214.
- [Google Scholar]
- Oxygen evolution reaction on NixCo3-xO4 electrodes with spinel structure. Cheminform. 2010;24(50):2247-2252.
- [Google Scholar]
- Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem.. 2011;3(1):79-84.
- [Google Scholar]
- Two-dimensional crystals: phosphorus joins the family. Nat. Nanotechnol.. 2014;9(5):330-331.
- [Google Scholar]
- Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal.. 2017;353:19-27.
- [Google Scholar]
- One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A. 2013;1(15):4754-4762.
- [Google Scholar]
- Controlling the active sites of sulfur-doped carbon nanotube-graphene nanolobes for highly efficient oxygen evolution and reduction catalysis. Adv. Energy Mater.. 2016;6(5):1501966.
- [Google Scholar]
- Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol.. 2014;4(11):3800-3821.
- [Google Scholar]
- High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc.. 2015;137(44):14023-14026.
- [Google Scholar]
- Benchmarking the stability of oxygen evolution reaction catalysts: the importance of monitoring mass losses. Chemelectrochem. 2015;1(12):2075-2081.
- [Google Scholar]
- Highly ordered mesoporous bimetallic phosphides as efficient oxygen evolution electrocatalysts. ACS Energy Lett.. 2016;1:792-796.
- [Google Scholar]
- Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: an efficient electrocatalyst for oxygen reduction and evolution reactions. ACS Catal.. 2015;5(6):3625-3637.
- [Google Scholar]
- Nitrogen-doped graphene supported CoSe₂ nanobelt composite catalyst for efficient water oxidation. ACS Nano. 2014;8(4):3970-3978.
- [Google Scholar]
- Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc.. 2014;136(19):7077-7084.
- [Google Scholar]
- Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv. Mater.. 2019;31(2):e1802880.
- [Google Scholar]
- Precious-metal-free Co-Fe-O/rGO synergetic electrocatalysts for oxygen evolution reaction by a facile hydrothermal route. Chemsuschem. 2015;8(4):659-664.
- [Google Scholar]
- Effect of the partial replacement of Fe by Ni and/or Mn on the electrocatalytic activity for oxygen evolution of the CoFeO spinel oxide electrode. Electrochim. Acta. 2003;47(27):4307-4314.
- [Google Scholar]
- ChemInform abstract: engineering the electronic state of a perovskite electrocatalyst for synergistically enhanced oxygen evolution reaction. Adv. Mater.. 2016;46(50):5989-5994.
- [Google Scholar]
- Three dimensional nickel oxides/nickel structure by in situ electro-oxidation of nickel foam as robust electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci.. 2015;359:172-176.
- [Google Scholar]
- Enhancement of the oxygen evolution reaction in Mn3+-based electrocatalysts: correlation between Jahn-Teller distortion and catalytic activity. RSC Adv.. 2016;6(3):2019-2023.
- [Google Scholar]
- Three-dimensional ordered macroporous IrO2 as electrocatalyst for oxygen evolution reaction in acidic medium. J. Mater. Chem.. 2012;22(13):6010-6016.
- [Google Scholar]
- A microribbon hybrid structure of CoOx-MoC encapsulated in N-doped carbon nanowire derived from MOF as efficient oxygen evolution electrocatalysts. Small. 2017;13(48):1702753.
- [Google Scholar]
- Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev.. 2008;12(5):1221-1250.
- [Google Scholar]
- Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Source. 2016;333:213-236.
- [Google Scholar]
- A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew. Chem.. 2015;126(47):13069-13073.
- [Google Scholar]
- Facile synthesis of black phosphorus: an efficient electrocatalyst for the oxygen evolving reaction. Angew. Chem. Int. Ed.. 2016;55(44):13849-13853.
- [Google Scholar]
- Facile synthesis and excellent electrochemical properties of NiCo2O4 spinel nanowire arrays as a bifunctional catalyst for the oxygen reduction and evolution reaction. J. Mater. Chem. A. 2013;1(39):12170-12177.
- [Google Scholar]
- In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc.. 2015;137(7):2688-2694.
- [Google Scholar]
- A bifunctional perovskite catalyst for oxygen reduction and evolution. Angew. Chem.. 2014;126(18):4670-4674.
- [Google Scholar]
- Carbon Nanotube/boron nitride nanocomposite as a significant bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Chemistry (Easton). 2016;23(3):676.
- [Google Scholar]
- ChemInform abstract: Ca2Mn2O5 as oxygen-deficient perovskite electrocatalyst for oxygen evolution reaction. Cheminform. 2015;46(11):14646-14649.
- [Google Scholar]
- NiFe-oxide electrocatalysts for the oxygen evolution reaction on Ti doped hematite photoelectrodes. Electrochem. Commun.. 2009;11(6):1150-1153.
- [Google Scholar]
- CuxCo3-xO4 used as bifunctional electrocatalyst. J. Electrochem. Soc.. 2006;153(12):A381-A388.
- [Google Scholar]
- ChemInform Abstract: Kinetics and mechanism of anodic chlorine and oxygen evolution reactions on transition metal oxide electrodes. Electrochim. Acta. 1981;26(3):329-337.
- [Google Scholar]
- Electrodeposited NixCo3-xO4 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun.. 2015;51(46):9511-9514.
- [Google Scholar]
- Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett.. 2015;3(3):399-404.
- [Google Scholar]
- Nanostructured catalysts for electrochemical water splitting: current state and prospects. J. Mater. Chem. A. 2016;4(31):11973-12000.
- [Google Scholar]
- Cobalt nanoparticles embedded in porous N-rich carbon as an efficient bifunctional electrocatalyst for water splitting. J. Mater. Chem. A. 2016;4(9):3204-3209.
- [Google Scholar]
- Direct chemical synthesis of ultrathin holey iron doped cobalt oxide nanosheets on nickel foam for oxygen evolution reaction. Nano Energy 2018
- [Google Scholar]
- Ultrathin Co3O4 nanomeshes for the oxygen evolution reaction. ACS Catal.. 2018;8(3) acscatal.7b03949
- [Google Scholar]
- Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale. 2015;7(19):8920-8930.
- [Google Scholar]
- Recent advances in the development of water oxidation electrocatalysts at mild pH. Small. 2019;15(13):e1805103.
- [Google Scholar]
- Self-supported FeP nanorod arrays: a cost-effective 3D hydrogen evolution cathode with high catalytic activity. ACS Catal.. 2014;4(11):4065-4069.
- [Google Scholar]
- Three-dimensional coral-like cobalt selenide as an advanced electrocatalyst for highly efficient oxygen evolution reaction. Electrochim. Acta. 2016;194:59-66.
- [Google Scholar]
- Ultrathin Co3S4 nanosheets that synergistically engineer spin states and exposed polyhedra that promote water oxidation under neutral conditions. Angew. Chem.. 2015;127(38):11383-11387.
- [Google Scholar]
- Electrodeposition of cobalt-sulfide nanosheets film as an efficient electrocatalyst for oxygen evolution reaction. Electrochem. Commun.. 2015;60:92-96.
- [Google Scholar]
- Transition metals (Fe Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A. 2016;4(5):1694-1701.
- [Google Scholar]
- Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl. Mater. Interfaces. 2016;8(3):2158-2165.
- [Google Scholar]
- Self-standing CoP nanosheets array: a three-dimensional bifunctional catalyst electrode for overall water splitting in both neutral and alkaline media. ChemElectroChem. 2017;4(8):1840-1845.
- [Google Scholar]
- A Zn-doped Ni3S2 nanosheet array as a high-performance electrochemical water oxidation catalyst in alkaline solution. Chem. Commun.. 2017;53(92):12446-12449.
- [Google Scholar]
- Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J. Am. Chem. Soc.. 2015;137(8):2901-2907.
- [Google Scholar]
- Interconnected core-shell carbon nanotube/graphene nanoribbon scaffolds for anchorng cobalt oxides as bifunctional electrocatalysts for oxygen evolution and reduction. J. Mater. Chem. A. 2015;3(25):13371-13376.
- [Google Scholar]
- Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat. Commun.. 2014;5(11):4345-4352.
- [Google Scholar]
- Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc.. 2013;135(45):16977-16987.
- [Google Scholar]
- Electrode-assisted catalytic water oxidation by a flavin derivative. Nat. Chem.. 2012;4(10):794-801.
- [Google Scholar]
- Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev.. 2015;44(8):2168-2201.
- [Google Scholar]
- Metal-free porous nitrogen-doped carbon nanotubes for enhanced oxygen reduction and evolution reactions. Sci. Bull.. 2016;61(11):889-896.
- [Google Scholar]
- Carbon nanotube/boron nitride nanocomposite as a significant bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Chemistry (Easton). 2016;23(3):676.
- [Google Scholar]
- NiCoMnO4 nanoparticles on N-doped graphene: highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions. Appl. Catal. B: Environ.. 2017;201:241-252.
- [Google Scholar]
- Self-assembly of single-layer CoAl-layered double hydroxide nanosheets on 3D graphene network used as highly efficient electrocatalyst for oxygen evolution reaction. Adv. Mater.. 2016;28(35):7640-7645.
- [Google Scholar]
- Electrochemical study of nickel-aluminium-manganese spinel NixAlxMnO. Electrocatalytical properties for the oxygen evolution reaction and oxygen reduction reaction in alkaline media. Electrochim. Acta. 2002;46(22):3373-3380.
- [Google Scholar]
- Fundamental mechanistic understanding of electrocatalysis of oxygen reduction on Pt and non-Pt surfaces: acid versus alkaline media. Adv. Phys. Chem.. 2012;2012:1-17.
- [Google Scholar]
- General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces. 2016;8(20):12798-12803.
- [Google Scholar]
- Electrocatalytic Oxygen Evolution Reaction (OER) on Ru Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal.. 2012;2(8):1765-1772.
- [Google Scholar]
- Few-layer black phosphorus nanosheets as electrocatalysts for highly efficient oxygen evolution reaction. Adv. Energy Mater.. 2017;7(19):1700396-1700403.
- [Google Scholar]
- Oxygen reduction in alkaline medium at thin MnxCo3-xO4 (0≤x≤1) spinel films prepared by spray pyrolysis. Effect of oxide cation composition on the reaction kinetics. J. Electroanal. Chem.. 2002;522(2):141-151.
- [Google Scholar]
- La0.8Sr0.2MnO3−δ decorated with Ba0. 5Sr0.5Co0.8Fe0.2O3−δ: a bifunctional surface for oxygen electrocatalysis with enhanced stability and activity. J. Am. Chem. Soc.. 2014;136(14):5229-5232.
- [Google Scholar]
- Preparation and characterization of copper-doped cobalt oxide electrodes. J. Phys. Chem. B. 2006;110(47):24021-24029.
- [Google Scholar]
- In situ transformation of hydrogen-evolving CoP nanoparticles: toward efficient oxygen evolution catalysts bearing dispersed morphologies with Co-oxo/hydroxo molecular units. ACS Catal.. 2015;5(7):4066-4074.
- [Google Scholar]
- A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science. 2016;353(6303):1011-1014.
- [Google Scholar]
- Electrochemical studies on protective thin cobalt oxide (Co3O4) and nickel cobaltate (NiCo2O4) films prepared on titanium by spray pyrolysis for oxygen evolution. J. Electrochem. Soc.. 1990;137(5):1408-1413.
- [Google Scholar]
- Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem.. 2010;2(6):454-460.
- [Google Scholar]
- Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev.. 2017;46(2):337-365.
- [Google Scholar]
- Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci.. 2014;5(10):3976-3982.
- [Google Scholar]
- Highly-active oxygen evolution electrocatalyzed by a Fe-doped NiSe nanoflake array electrode. Chem. Commun.. 2016;52(24):4529-4532.
- [Google Scholar]
- Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc.. 2014;136(21):7587-7590.
- [Google Scholar]
- Nitrogen-doped graphene/carbon nanotube hybrids: in situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small. 2014;10(11):2251-2259.
- [Google Scholar]
- Ultrathin graphitic C3N4 nanosheets/graphene composites: efficient organic electrocatalyst for oxygen evolution reaction. Chemsuschem. 2015;7(8):2125-2130.
- [Google Scholar]
- Toward full exposure of “active sites”: nanocarbon electrocatalyst with surface enriched nitrogen for superior oxygen reduction and evolution reactivity. Adv. Funct. Mater.. 2015;24(38):5956-5961.
- [Google Scholar]
- A bifunctional hybrid electrocatalyst for oxygen reduction and evolution: cobalt oxide nanoparticles strongly coupled to B, N-decorated graphene. Angew. Chem. Int. Ed.. 2017;56(25):7121.
- [Google Scholar]
- Precise oxygen evolution catalysts: status and opportunities. Scripta Mater.. 2014;74(74):25-32.
- [Google Scholar]
- Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater.. 2016;28(2):215-230.
- [Google Scholar]
- Recent progress of carbon-based materials in oxygen reduction reaction catalysis. ChemElectroChem. 2018;5(14):1764-1774.
- [Google Scholar]
- A hierarchical CoTe2–MnTe2 hybrid nanowire array enables high activity for oxygen evolution reactions. Chem. Commun.. 2018;54(78)
- [Google Scholar]
- Template growth of nitrogen-doped mesoporous graphene on metal oxides and its use as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Catal. Today 2017
- [Google Scholar]
- Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem.. 2017;129(21):5867.
- [Google Scholar]
- N-doped graphene as a bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions in an alkaline electrolyte. Int. J. Hydrogen Energy. 2014;39(28):15913-15919.
- [Google Scholar]
- A highly active oxygen evolution electrocatalyst: ultrathin CoNi double hydroxide/CoO nanosheets synthesized via interface-directed assembly. Nano Res.. 2016;9(3):713-725.
- [Google Scholar]
- Selenide-based electrocatalysts and scaffolds for water oxidation applications. Adv. Mater.. 2016;28(1):77-85.
- [Google Scholar]
- A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun.. 2016;7:12324-12330.
- [Google Scholar]
- Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc.. 2015;137(12):4119-4125.
- [Google Scholar]
- Fe3O4-decorated Co9S8 nanoparticles in situ grown on reduced graphene oxide: a new and efficient electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater.. 2016;26(26):4712-4721.
- [Google Scholar]
- Decorating unoxidized-carbon nanotubes with homogeneous Ni-Co spinel nanocrystals show superior performance for oxygen evolution/reduction reactions. Sci. Rep.. 2017;7:45384.
- [Google Scholar]
- Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res.. 2018;51(7):1571-1580.
- [Google Scholar]
- Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. ACS Catal.. 2016;6(2):714-721.
- [Google Scholar]
- Carbon coated nickel phosphides porous nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci.. 2016;9(4):1246-1250.
- [Google Scholar]
- Ultrathin black phosphorus-on-nitrogen doped graphene for efficient overall water splitting: dual modulation roles of directional interfacial charge transfer. J. Am. Chem. Soc.. 2019;141(12):4972-4979.
- [Google Scholar]
- One-step preparation of optically transparent Ni-Fe oxide film electrocatalyst for oxygen evolution reaction. Electrochim. Acta. 2015;169:402-408.
- [Google Scholar]
- A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol.. 2015;10(5):444-452.
- [Google Scholar]
- Rapid synthesis of cobalt nitride nanowires: highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem.. 2016;55(30):8670-8674.
- [Google Scholar]
- Atomically ultrathin RhCo alloy nanosheet aggregates for efficient water electrolysis in broad pH range. J. Mater. Chem. A. 2019;7(27):16437-16446.
- [Google Scholar]
- Ultrathin Rh nanosheets as a highly efficient bifunctional electrocatalyst for isopropanol-assisted overall water splitting. Nanoscale 2019
- [Google Scholar]
- J. Zhao, X. Li, G. Cui, X. Sun, 2018. Highly-active oxygen evolution electrocatalyzed by an Fe-doped NiCr2O4 nanoparticle film.
- Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: the enhanced performance by sulfur doping. Electrochim. Acta. 2016;204:169-175.
- [Google Scholar]
- Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun.. 2013;4(2):2390-2396.
- [Google Scholar]
- A high-performance binary Ni-Co hydroxide-based water oxidation electrode with three-dimensional coaxial nanotube array structure. Adv. Funct. Mater.. 2014;24(29):4698-4705.
- [Google Scholar]
- Carbon nanotube/S-N-C nanohybrids as high performance bifunctional electrocatalysts for both oxygen reduction and evolution reactions. New J. Chem.. 2015;39(8):6289-6296.
- [Google Scholar]
- SrNb0.1Co0.7Fe0.2O3-δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. Int. Ed.. 2015;127(13):3969-3973.
- [Google Scholar]
- Efficient oxygen evolution electrocatalyzed by Cu nanoparticles-embedded N-doped carbon nanowire array. Inorg. Chem. Front.. 2018;5(5)
- [CrossRef] [Google Scholar]







