Translate this page into:
Acid condensation products of indole-3-carbinol and their in-vitro (anti)estrogenic, (anti)androgenic and aryl hydrocarbon receptor activities
⁎Corresponding author at: Wageningen Food Safety Research, Wageningen University & Research, Akkermaalsbos 2, 6708 WB Wageningen, the Netherlands. dagnachew.eyachew@gmail.com (Dagnachew Eyachew Amare)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The objective of the study was to investigate the (anti)estrogenic, (anti)androgenic and aryl hydrocarbon receptor (AhR) agonistic activities of a mixture of acid condensation products of indole-3-carbinol, termed RXM, and to identify the compounds most responsible for the observed effects, using in vitro receptor-reporter gene transcriptional activation bioassays. For this, HPLC-fractions of RXM were prepared and tested. LC-MS/MS analysis was carried out for the identification of some of the acid condensation products. The RXM displayed weak estrogenic and anti-androgenic, and strong AhR agonistic properties. The fraction containing 3,3-diindolylmethane (DIM) displayed a weak estrogenic and relatively strong anti-androgenic activity. DIM was confirmed to be an androgen receptor (hAR) antagonist and a partial estrogen receptor (hERα) agonist. Also the fraction containing the trimer [2-(indol-3-ylmethyl)indol-3-yl]indol-3-ylmethane (LTr1) showed anti-androgenic activities. It was shown for the first time that DIM is not only estrogenic and anti-androgenic, but also possesses anti-estrogenic properties. Though indolo[3,2-b]carbazole (ICZ) is a potent AhR activator and was detected in the RXM, it did not contribute to AhR-agonist activity. Instead, fractions containing the trimers LTr1 and 5,6,11,12,17,18-hexahydrocyclonona[1,2-b:4,5-b′:7,8-b″]tri-indole (CTr), as well as some unidentified compounds showed the highest AhR activation. The fraction, containing the linear trimer LTr1, showed a weak anti-androgenic activity which has not been reported before. The study demonstrates the importance of a bioassay directed approach for identifying compounds that contribute most to the effects of mixtures.
Keywords
Estrogen receptor
Androgen receptor
Aryl hydrocarbon receptor
In vitro bioassay
Acid condensation products of I3C
1 Introduction
Epidemiological studies show that dietary intake of cruciferous vegetables is associated with reduced risks of developing cancers, e.g. breast, cervical, prostate, lung, and colorectal cancer (Aggarwal and Ichikawa, 2005; Cohen et al., 2000; Higdon et al., 2007; Kim and Milner, 2005; Minich and Bland, 2007; Mori et al., 2017; Morrison et al., 2020; Safe et al., 2008; Verhoeven et al., 1996; Weng et al., 2008; Wu et al., 2013; Yu et al., 2006; Zhang et al., 2018). It is suggested that the assumed chemo-preventive properties of cruciferous vegetables are related to the presence of indole-3-carbinol (I3C). I3C is a breakdown product of the naturally occurring dietary sulfur-containing compound glucobrassicin (3-indolylmethyl glucosinolate), which is found in cruciferous vegetables such as Brussels sprouts, broccoli, cabbage and cauliflower. When these cruciferous vegetables are chopped or chewed, the plant enzyme myrosinase, which is normally separated from glucobrassicin in intact plant cells, is liberated and quickly converts glucobrassicin into I3C (Johnson, 2002).
I3C is unstable in the acidic environment of the stomach and is rapidly converted into a number of condensation products, the best characterized being 3,3′-diindolylmethane (DIM) and indolo[3,2-b]carbazole (ICZ). In addition several trimers, i.e. a cyclic trimer 5,6,11,12,17,18-hexahydrocyclonona[1,2-b:4,5-b′:7,8-b″]tri-indole (CTr), a cyclic tetramer CTet, a symmetric linear trimer [2-(indol-3-ylmethyl)indol-3-yl]indol-3-ylmethane (LTr1) and an asymmetric linear trimer [3,3-bis(indol-3-ylmethyl)]indolenine (LTr2) have been identified (Anderton et al., 2004; Bjeldanes et al., 1991; Chang et al., 1999; Chen et al., 1998). Fig. 1 shows a putative reaction scheme for the oligomerization of I3C under acidic conditions. Upon water loss, I3C is converted to a reactive intermediate, 3-methylideneindole (3MI). This intermediate can react with another molecule of I3C at positions 2 or 3, leading to the formation of a 2,3′-adduct or a 3,3′-adduct (Grose and Bjeldanes, 1992). The latter results in the formation of DIM, after the loss of formaldehyde. The 2,3′-adduct can lose another molecule of water, resulting in a reactive dimer intermediate similar to 3MI, that can react with either I3C or DIM, leading to a wide variety of trimeric, tetrameric and higher oligomers. ICZ and the cyclic trimer CTr are stable end-products because they don’t have positions left in the indole rings that can react with 3MI and homologues.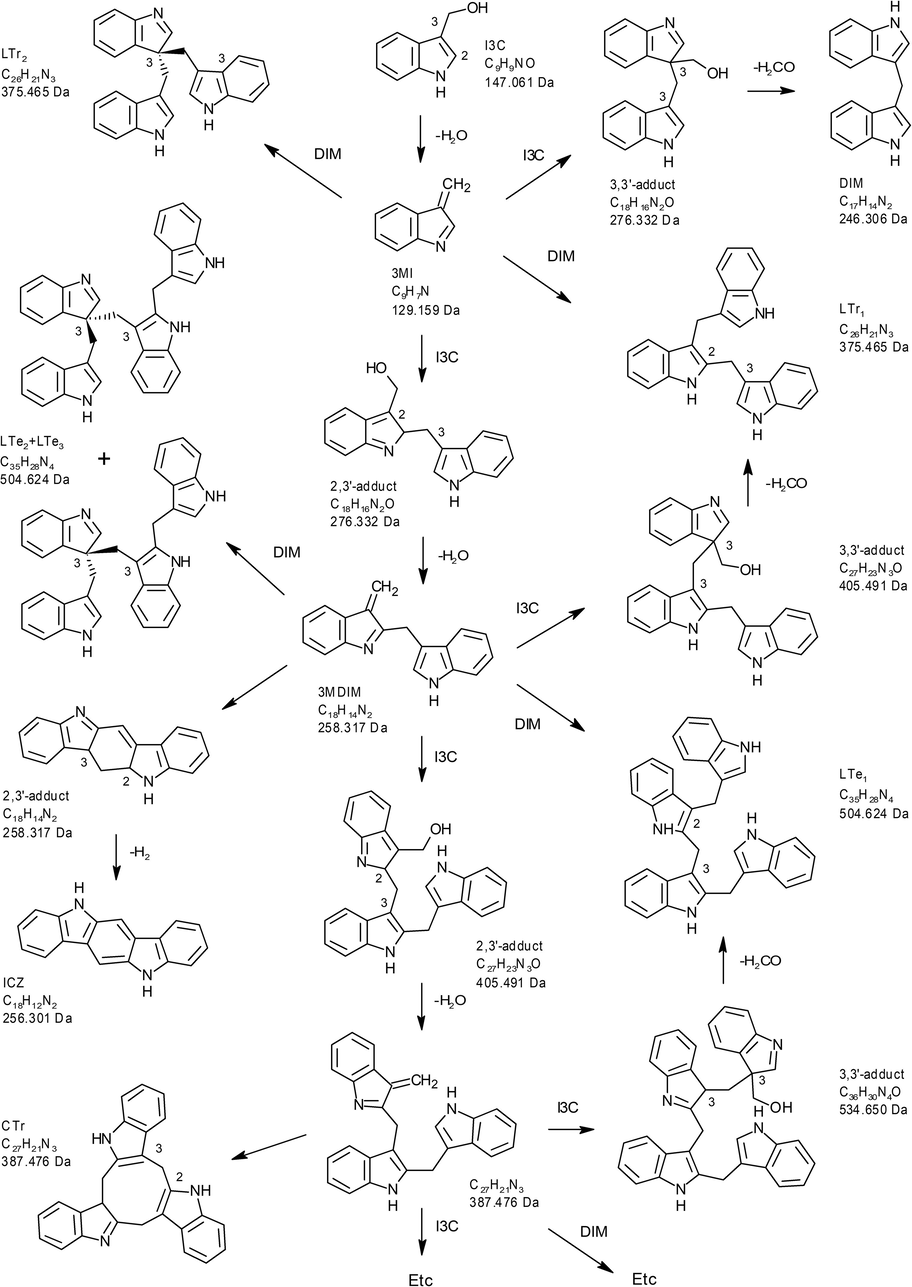
Putative scheme for the oligomerization of I3C, leading to the formation of multiple products including DIM, ICZ, CTr, LTr1, and LTr2.
Most of the biological activities attributed to I3C are believed to result from its acid condensation products, as it is expected that after ingestion of cruciferous vegetables, I3C is completely converted in the stomach before it reaches the intestine (Anderton et al., 2004; Bjeldanes et al., 1991; Weng et al., 2008). Bjeldanes et al. (1991) showed that ICZ, and to a lesser extent also CTr, LTR1, DIM can bind to the AhR (competitive assay), with some effect also of I3C. ICZ also induced EROD activity in mouse hepatoma cells (Nault et al., 2013), and AhR in the human colon epithelial cell line and in the mouse hepatoma cells (Faust et al., 2017). DIM induced CYP1A1 gene expression in mice (Hammerschmidt-Kamper et al., 2017), mouse hippocampal cultured cells (Rzemieniec et al., 2016) and in human MCF7 cells (Chen et al., 1998). As a result, the oral intake of the dietary compound I3C is associated with an increased activity of xenobiotic-metabolizing enzymes, which are involved in elimination of potential carcinogens and toxins (Bonnesen et al., 2001; Dietrich, 2016; Nho and Jeffery, 2001; Ociepa-Zawal et al., 2007; Wang et al., 2016). In addition, DIM has been reported for having anti-androgenic properties (Aksu et al., 2016; Bovee et al., 2008; Bjeldanes et al., 2005; Hwang et al., 2016; Le et al., 2003). It was also reported that I3C acid condensation products, DIM (Bak et al., 2016; Bovee et al., 2008; Grose and Bjeldanes, 1992; Kim et al., 2018; Thomson et al., 2016), LTr1 (Chang et al., 1999) and CTr (Xue et al., 2005), can activate the estrogen receptor.
In the case of mixtures, it is important to identify the compounds that contribute most to the biological effects. To understand the intrinsic activities of I3C and its oligomers on estrogen, androgen and aryl hydrocarbon receptors, we employed in vitro receptor-reporter gene transcriptional activation bioassays, i.e. the RIKILT yeast estrogen and androgen bioassays (Bovee et al., 2004, 2007) and the DR CALUX® bioassay, which is based on rat H4IIE hepatoma cells (Aarts et al., 1995). These bioassays were used to determine the activities of the full reaction mixture of I3C formed under acid conditions (RXM), containing many different acid condensation products, and to identify which compounds are most responsible for the observed mixture effects, by using HPLC fractionation and comparison with the effects of available standards of I3C, ICZ and DIM. Certain degradation products were not available as standards, but were tentatively identified by LC-MS/MS analysis.
2 Materials and methods
2.1 Chemicals and reagents
Indole-3-carbinol (CAS no: 700-06-01), 17ß-testosterone (CAS no: 58-22-0), 17ß-estradiol (CAS no: 50-28-2) and L-leucine (CAS no: 61-90-5) were obtained from Sigma-Aldrich (St. Louis, MO, USA), dimethyl sulfoxide (DMSO) from Merck (Germany), 3,3′-diindolylmethane (DIM) from LKT Lab. Inc. (Saint Paul, MN, USA), hydrochloric acid (HCl) from Merck (Darmstadt, Germany), 2,3,7,8-TCDD (CAS no: 1746-01-6) from Schmidt BV (Amsterdam, The Netherlands), acetonitrile (ACN, supra-HPLC-grade) and dichloromethane from Biosolve B.V. (The Netherlands), Minimal medium (MM) and alpha minimum essential medium (AMEM) were purchased from BioWhittaker (Verviers, Belgium) and fetal calf serum (FCS) and phosphate buffered saline (PBS) from Thermo Fisher Scientific (Waltham, MA, USA). Indolo[3,2-b]carbazole (ICZ) was prepared by De Waard et al. (2008) and its identity confirmed by using a reference standard provided by Prof. Bergman (Department of Chemistry, Royal Institute of Technology, and Department of Biosciences at Novum, Huddinge, Sweden).
2.2 Preparation of an I3C acidic reaction mixture
The reaction mixture (RXM) was prepared from I3C under acidic conditions according to the methods of Bjeldanes et al. (1991) and De Kruif et al. (1991). Briefly, 5 mL solution of I3C in DMSO (20 mg/mL) was added to 100 mL of 0.05 M HCl (model of stomach fluid). The mixture was swirled for 80 min at 25 °C and 140 rpm. The resulting green mixture was extracted twice with 100 mL dichloromethane and the combined organic phase was evaporated using a Buchi-Rotavapor at 40 °C, until approximately 3 mL remained. To further concentrate the RXM, 450 µL of DMSO was added and the dichloromethane was completely evaporated by nitrogen gas using a Turbovap LV at 5 mbar and 40 °C until about 450 µL DMSO remained. The volume was adjusted to 800 µL DMSO and this reddish mixture was defined as the undiluted RXM (1×) stock solution.
2.3 HPLC analysis and fractionation of I3C acid reaction products
To determine whether the expected acid condensation products were produced, a 10 times diluted RXM in ACN was analyzed with HPLC, and compared with standards of I3C, ICZ and DIM. Analysis, and also fractionation, of the RXM was performed on an HPLC system (Merck Hitachi, Germany) that consisted of two L-6200A/600B pumps, a 234 autosampler, a 900 series interface, a 783 spectroflow UV detector, and a FP-1520 fluorescence detector in combination with a SupelcosilTM LC-18-DB HPLC column (250 × 4.6 mm, 5 µm), running at room temperature (rT). The mobile phase consisted of water/ACN (v/v 95/5) (A) and ACN (B). A gradient was used that started at 50% A/50% B for 60 min, followed by a linear increase to 100% B in 5 min, and after keeping 100% B for 15 min, it was changed back to the starting conditions in 5 min. The column was equilibrated between two injections at this composition for 10 min (total run time 95 min). The flow rate was set at 1 mL/min and the injection volume was 25 µL. The peaks of standard compounds and the RXM were monitored by UV absorption (detector set at 280 nm) and fluorescence (excitation 336 nm and emission at 416 nm).
To collect HPLC fractions of the RXM, ten injections were performed and nine fractions were collected, distributed over the total run time of 85 min. In order to investigate the in vitro estrogenic, androgenic and AhR activities of the fractions, the water and ACN solvent (volume of the fractions about 100 mL) had to be replaced by DMSO. The same procedure was followed as for the preparation of the RXM (see Section 2.2), except that 60 µL of DMSO was added to each fraction before using the Turbovap LV. This was also the final DMSO volume. All fractions were analyzed by UV and fluorescence in order to check the successful recovery of the compounds present in each time window.
2.4 LC-MS/MS analysis of the RXM
The RXM and the isolated fractions were also investigated with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). A Waters Acquity chromatographic system coupled to a Waters Xevo TQ-XS tandem mass spectrometer (Waters, Milford, MA, USA) was run in multiple reaction monitoring (MRM) mode with combined positive and negative electrospray ionisation. Cone voltage was set at 20 V, desolvation gas temperature at 600 °C, source block temperature at 150 °C, and the argon collision gas pressure at 4.3 10−3 mbar. Separation of compounds was accomplished on a Waters UPLC BEH C18 (1.7 µm, 150 × 2.1 mm) analytical column (Waters, Milford, MA, USA), kept at 50 °C and run at 0.4 mL/min. The mobile phase consisted of 10 mM ammonium carbonate in water (A) and ACN (B). A gradient was used that started at 50% A/50% B and that was changed linearly to 25% A/75% B in 10 min. The gradient was changed to 5% A/95% B in 1.5 min and after 1 min it was changed back to the starting conditions. Total run time between injections was 15 min. Mass spectrometric data were processed using Masslynx 4.1 software (Waters, USA). In Table 1 the fragmentation conditions for the most relevant products are shown. Some compounds are more sensitive in positive than in negative mode and vice versa. Therefore, both ionisation modes were used, as they provided complementary results, which assisted in the identification of the oligomeric products. Before analysis, the crude RXM was stepwise diluted in methanol/water (1:1, v:v) to obtain a 10,000-fold dilution. The fractions were diluted 100-fold in the same solvent. CE: collision energy; RT: retention time.
Compound
Precursor ion [M + H]+ (m/z)
Product ion 1 (m/z)
CE (eV)
Product ion 2 (m/z)
CE (eV)
Product ion 3 (m/z)
CE (eV)
Precursor ion [M−H]− (m/z)
Product ion 1 (m/z)
CE (eV)
Product ion 2 (m/z)
CE (eV)
Product ion 3 (m/z)
CE (eV)
RT (min)
DIM
247.2
130.0
10
–
–
–
–
245.2
116.0
20
–
–
–
–
3.15
ICZ
256.2 (M+)
128.0
50
227.2
40
255.2
40
–
–
–
–
–
–
–
3.25
LTr1, 2
376.2
130.0
10
247.0
15
259.0
15
374.2
116.0
25
257.0
20
–
–
5.26; 6.00
CTr
388.2
130.0
20
257.0
20
271.0
15
386.2
130.0
25
255.0
20
–
–
4.23
LTe1,2,3
505.3
130.0
20
259.0
15
376.0
10
503.3
116.0
30
257.0
25
374.0
20
6.80; 7.44; 7.70
2.5 Yeast estrogen and androgen bioassays
Two bioassays were employed to investigate the in vitro (anti)estrogenic and (anti)androgenic properties of the standards, the RXM and the HPLC fractions, i.e. the RIKILT yeast estrogen (REA) and androgen bioassays (RAA) (Bovee et al., 2004, 2007). The REA is based on a recombinant Saccharomyces cerevisiae cell that stably expresses the human estrogen receptor α (hERα). It also contains a stably integrated reporter construct, containing estrogen responsive elements (EREs) in the promoter region of the gene coding for a yeast-enhanced green fluorescent protein (yEGFP). This yEGFP is thus a measurable reporter protein in response to estrogens. The RAA is based on the same host yeast cell, but stably expressing the human androgen receptor (hAR) and containing a reporter construct with androgen responsive elements (AREs) that drive the expression of yEGFP, as a measurable reporter protein in response to androgens. In both REA and RAA, the fluorescence can be measured in situ without cell lysis and reagent additions. The REA and RAA were previously validated and ISO 17025 accredited for detecting estrogens and androgens in calf urine and feed (DECISION, 2002). Both bioassays have also been employed to characterize the estrogenic and androgenic potentials of several natural and synthetic steroids and plant-derived compounds (Bovee et al., 2008), showing that these bioassays are very specific and useful to investigate the (anti)estrogenic and (anti)androgenic properties of compounds.
In short, a single yeast colony from minimal medium with L-leucine and L-histidine (MM/L) agar plate was transferred into a 50 mL tube already containing 10 mL selective MM/L medium and grown overnight at 30 °C with orbital shaking at 100 rpm. The cell optical density (OD) was determined at 630 nm and the cell suspension was diluted into the range of OD 0.04–0.06. For exposure, aliquots of 200 µL of the diluted yeast culture were transferred into each well of a 96-wells plate. To examine for agonistic properties, 1 µL of a stock solution or sample extract in DMSO was added to each well. To examine the antagonistic properties, 1 µL of each compound or sample extract in DMSO was co-administered with 1 µL of estradiol (E2) or 17ß-testosterone (T) solutions that resulted in about a half maximal response in the REA and RAA, respectively. The final concentration ranges for the bioassays were: 0.003–30 nM for E2, 0.001–3000 nM for T, 0.003–100 µM for I3C, 0.003–100 µM for DIM, and 0.0015–50 µM for ICZ, 10×, 100× and 1000× dilution of the RXM, and 1×, 2×, 4× and 8× dilutions for the RXM HPLC fractions. DMSO was used as a blank control. Each compound and sample concentration was tested in triplicate. Exposure was performed for 24 h at 30 °C and orbital shaking at 100 rpm. Fluorescence (excitation at 485 nm and measuring emission at 530 nm) and OD at 630 nm were measured at 0 h and at 24 h after exposure using a SynergyTM HT Multi-Detection Microplate reader (BioTek Instruments Inc., USA). The fluorescence signal was corrected with signals obtained with MM/L medium containing DMSO solvent only. Measuring OD at 630 nm was performed in order to check for cytotoxicity (Bovee et al., 2004, 2007).
2.6 The DR CALUX® bioassay
The Dioxin Responsive Chemical Activated LUciferase gene eXpression (DR CALUX®) bioassay is based on rat hepatoma cells (H4IIE) stably transfected with a DRE driven luciferase reporter construct (H4IIE.Luc) (Murk et al., 1996; Aarts et al., 1995). The principle of the assay is based on the binding of a receptor ligand and subsequent activation of the AhR, ultimately resulting in an increased transcription of the luciferase gene, which is proportional to the amount and potency of the AhR agonists present in a sample and is measured in the form of light production. Therefore, the cells must be lysed after the exposure and luciferin substrate and ATP must be added. Briefly, H4IIE.Luc cells were grown at 37 °C, 5% CO2 in AMEM supplemented with 10% FCS, 0.5% antibiotics (penicillin (5000 U/L, Sigma) and streptomycin (95 mg/L, Sigma). Cells were seeded into 96-well plates and cultured till 90% confluence was reached for exposure. Stock solutions of test compounds and sample extracts in DMSO were added to the culture medium and the final DMSO concentration was 0.2%. The final concentration for the compounds was in the range of 0.5–500 pM for TCDD, 0.003–100 µM for I3C, 0.003–100 µM for DIM, 0.0015–50 µM for ICZ, 64×, 192× and 576× dilution of the RXM and 3×, 10× and 15× dilutions for the RXM HPLC fractions. TCDD was used as positive control and DMSO was included as a blank. After 24 h exposure of the cells, the medium was removed and the cells were washed with 200 µL PBS buffer. Then cells were lysed by adding 20 µL of lysis reagent (Promega, Leiden, The Netherlands) to each well and the plates were left for 15 min at rT. Finally, luminescence was measured with a Luminoskan Ascent (Thermo Labsystems, Finland) following addition of 100 µL assay mixture containing luciferin and ATP.
2.7 Data analysis
All the experiments were performed in triplicate and all results are presented as mean ± SD. Excel was used to process data. Statistical significance was determined by performing one sided t-test using Statistical Package for the Social Sciences version 20 software.
3 Results
3.1 Analytical characteristics of the RXM
Fig. 2 shows the HPLC elution profile of the I3C RXM for both fluorescence and UV detection. At least eight peaks were observed and in addition a bulk of peaks at the end of the chromatogram. The focus was on the smaller reaction products (di-, tri- and tetramers) and no attempt was made to separate the larger products eluting at the end of the chromatogram. Based on the various peaks, 9 fractions were collected and which were tested in the bioassays (time windows are indicated in Fig. 2A).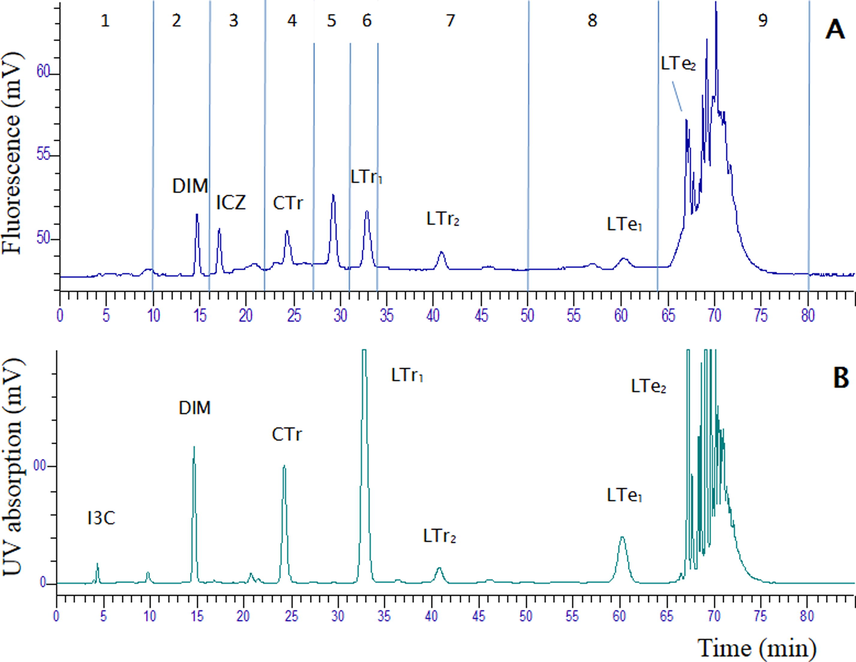
The HPLC elution profile of the I3C reaction mixture (RXM): (A) represents the profile using fluorescence detection (excitation 336 nm; emission 416 nm), and (B) represents the profile using UV detection at 280 nm. The time windows of the fractions collected by HPLC are indicated in (A).
Pure standards of I3C, ICZ and DIM were also analysed on the HPLC system and the retention times (RT) of the pure compounds were initially used to allocate the peaks in the RXM. These analyses showed that the parent compound I3C (4.4 min), is almost completely converted and that both DIM and ICZ were present in the RXM, at RT 14.5 and 16.0 min, respectively. ICZ was only detectable with fluorescence detection. The presence of DIM in fraction 2 was confirmed by LC-MS/MS analysis, but ICZ could not be detected in fraction 3 (data not shown). Further, analysis of the RXM indicated that the peaks with RTs 24.5 and 33.0 min, i.e. those in fractions 4 and 6, are the cyclic and linear CTr and LTr1 trimers, respectively. The LC-MS/MS chromatogram of the RXM is shown in Fig. 3. The presence of DIM in the RXM could be confirmed in neg ESI mode, but ICZ was not detected in the RXM, nor in fraction 3. This is an indication that the concentration of ICZ in the mixture and in fraction 3 is very low (i.e. too low to be detected by LC-MS/MS). The identity of CTr and LTr1 in the RXM was confirmed by LC-MS/MS analysis. These compounds eluted in the LC-MS/MS chromatogram at 4.25 and 5.30 min, respectively. They showed the expected signals and fragmentation behaviour in both pos and neg ESI mode (Table 1). CTr produced a protonated molecular ion with mass 388 in pos ESI and a deprotonated molecular ion with mass 386 in neg ESI. Similarly, LTr1 produced the expected protonated ion with mass 376 and deprotonated ion with mass 374 in pos and neg ESI mode, respectively. In the LC-MS/MS chromatogram at least three different linear tetramers, coded LTe1, LTe2, LTe3, eluting at 6.85, 7.45 and 7.65 min, could be detected (Fig. 3, Table 1). These compounds eluted together with higher oligomers in fractions 8 and 9 using the HPLC gradient (Fig. 2). With the LC-MS/MS gradient a much better separation was obtained for these isomers. The three linear tetramers showed the expected (de)protonated molecular ions, as well as typical fragments. In analogy with the two linear LTr trimers, the first eluting LTe1 is probably the symmetric oligomer and the other two isomers are likely the asymmetric condensation products (Fig. 1). A cyclic tetramer could not be detected.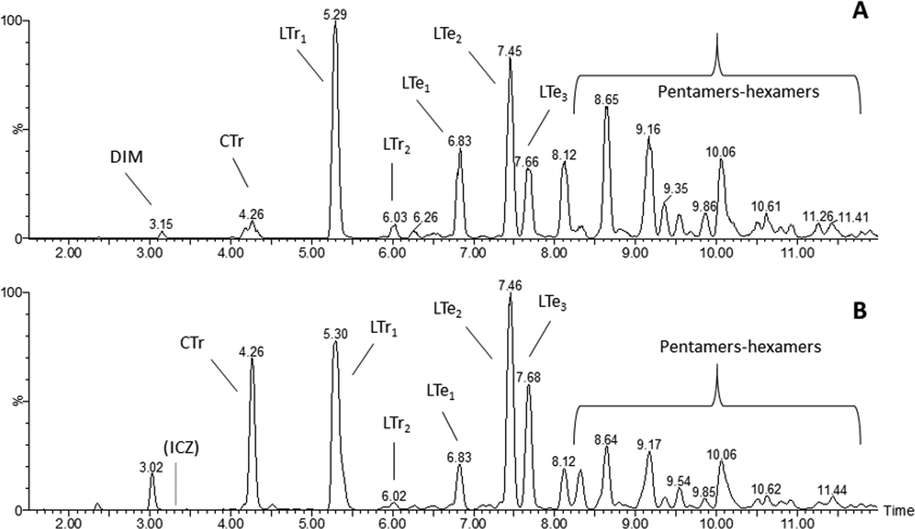
The LC-MS/MS elution profile (TIC) of the I3C reaction mixture (RXM). TIC of the MRM transitions in (A) negative or (B) positive electrospray mode. Note that the gradient used for LC-MS/MS analysis is different from the HPLC gradient used for isolation of fractions.
3.2 In vitro (anti)estrogenic, (anti) androgenic and AhR activities of RXM, I3C, ICZ and DIM
The estrogenic and anti-estrogenic activities of the RXM, as well as I3C and its digestive products DIM and ICZ were examined in the yeast estrogen bioassay (REA). Fig. 4A (white bars) shows that the RXM displayed a weak estrogenic potency at 10× dilution (undiluted RXM interfered with the fluorescent measurement of both the REA and RAA (data not shown)). When tested in co-exposures with concentrations of E2 at effective concentration (EC50) (faint green bars) and EC100 (green bars), the RXM did not result in a response, indicating no anti-estrogenic effects. As shown in Fig. S1 (Supplement), DIM also displayed clear estrogenic activity, showing an additive effect when co-exposed with low concentrations of E2 (below the EC50). However, an anti-estrogenic activity was observed when DIM was co-exposed with higher concentrations of E2, i.e. above the EC50. DIM is thus classified by this in vitro yeast estrogen bioassay as a partial ERα-agonist. I3C did not show an estrogenic activity, but a very weak anti-estrogenic activity. ICZ was neither estrogenic nor anti-estrogenic.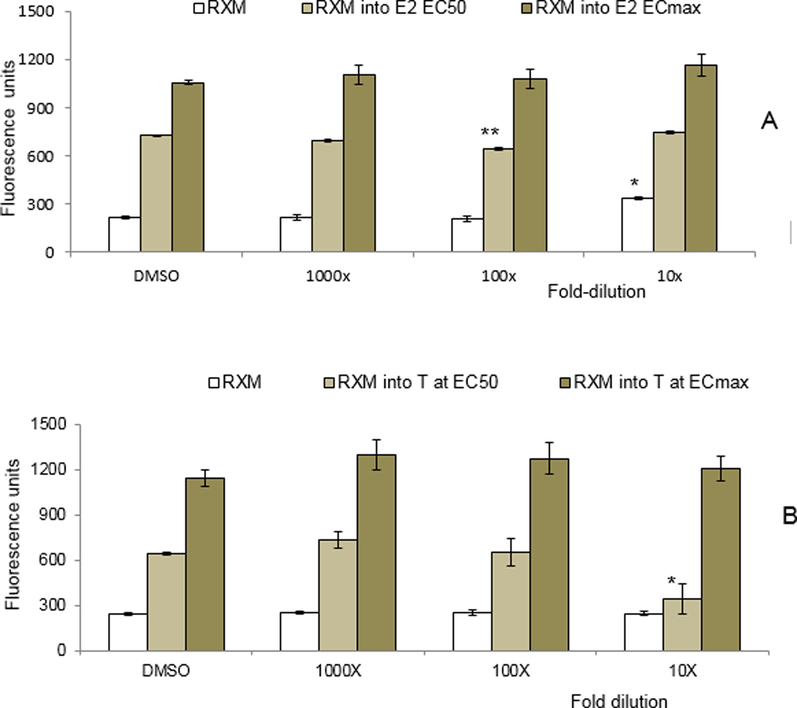
Response of the RXM in (A) the yeast estrogen bioassay (REA), and (B) the yeast androgen bioassay (RAA). RXM dilutions of 10x, 100x and 1000x were tested without and in combination with A) estradiol or B) 17β-testosterone at the EC50 or ECmax. Each data point represents the mean ± SD for triplicate measurements. * (at P ≤ 0.05), ** (at P ≤ 0.001) significant difference between treatment and control (DMSO).
The androgenic and anti-androgenic activities of the RXM, as well as I3C, ICZ and DIM were examined in the RAA. Neither the RXM (Fig. 4B, white bars), nor the pure compounds (Fig. S2; data not shown for ICZ) showed androgenic activity. However, at the highest concentration, a strong anti-androgenic effect of the RXM was observed in combination with a concentration of 17β-testosterone (T) at the EC50 level (faint green bars) at the level of P ≤ 0.001, but not when co-exposed with a concentration of T giving a maximal response (green bars). I3C and DIM showed clear anti-androgenic activities when co-exposed with concentrations of T at the EC50 or ECmax (Fig. S2). DIM showed a relatively strong antagonistic activity and was able to completely inhibit the response of T. DIM is thus classified by this in vitro yeast androgen bioassay as a strong AR-antagonist.
When tested in the DR CALUX assay, the results show that both the RXM and ICZ (Fig. S3) display AhR agonist activities. RXM-dilutions of 576-, 192- and 64-fold showed responses in the DR CALUX assay with means ± SD of 95 ± 2, 265 ± 8 and 830 ± 33, respectively, as compared to 23 ± 1 RLUs for the controls. The 64 times diluted RXM extract still resulted in a maximal response, i.e. the same maximum as obtained with a high concentration (50 pM) of TCDD. More concentrated RXM could not be tested in the DR CALUX, as these were toxic for the cells. ICZ is a rather potent AhR agonist, having a relative potency of 0.003 when compared to TCDD, one of the most potent AhR agonists. Neither DIM nor I3C showed an activity in the DR CALUX bioassay (Fig. S3).
3.3 In vitro activities of the HPLC fractions from the RXM
To further investigate to what extent the observed activities of the RXM can be explained by the presence of DIM and ICZ, or to what extent other compounds contribute to the observed effects, HPLC fractionation was performed on the basis of the peak profiles shown in Fig. 2A. Nine fractions were collected and used to expose the cells in the above mentioned bioassays. Their characteristics are summarized in Table 2. NA not applicable, NT not tested, + positive response as agonist or antagonist, - negative response as an agonist or antagonist; Antagonism tested in REA by co-exposure with 17ß-estradiol at 0.7 nM (EC50) and 1.5 nM (ECmax); Antagonism tested in RAA by co-exposure with testosterone at 70 nM (EC50) and 1000 nM (ECmax).
Compound
Yeast estrogen bioassay (REA)
Yeast androgen bioassay (RAA)
DR CALUX bioassay
Agonist
EC50 E2 antagonist
ECmax E2 antagonist
Comment
Agonist
EC50 T antagonist
ECmax T antagonist
Comment
Agonist
Comment
17β-Estradiol
+
NA
NA
Strong agonist
NA
NA
NA
NA
17β-Testosterone
NA
NA
NA
+
NA
NA
Strong agonist
NA
2,3,7,8-TCDD
NA
NA
NA
NA
NA
NA
+
Strong agonist
Indole-3-carbinol
–
+
+
Weak antagonist
–
+
+
Weak antagonist
–
Diindolylmethane (DIM)
+
+
+
Agonist < EC50;
Antagonist ≥ EC50
–
+
+
Complete antagonist at both EC50 & max
–
Indolo[3,2-b] carbazole (ICZ)
–
–
–
–
–
–
+
Strong agonist
RXM
+
–
–
Weak agonistic
–
+
–
Strong antagonistic
+
Strong agonistic
Fractions (1–9)
+ (2, 6)
NT
–
Fractions 2 & 6 weak agonistic, other fractions inactive
–
NT
+ (2, 6)
Fraction 2 (strong) & 6 (weak) antagonistic at T ECmax
+ (4, 6, 8, 9)
Fractions 4, 6, 8 & 9 strong agonistic activity, other fractions weak
Only fractions 2 and 6 showed an estrogenic activity in the REA, although with fraction 6 no clear dose–response was observed (Fig. 5A). None of the nine fractions was able to inhibit the response of E2 in the REA (results not shown). The agonistic activity of fraction 2 was expected, as this fraction contains DIM, shown to display estrogenic activity in the REA. The observed ER-agonist effect of fraction 6 is probably caused by LTr1, as this compound was found to be a weak ligand for the estrogen receptor (Chang et al., 1999).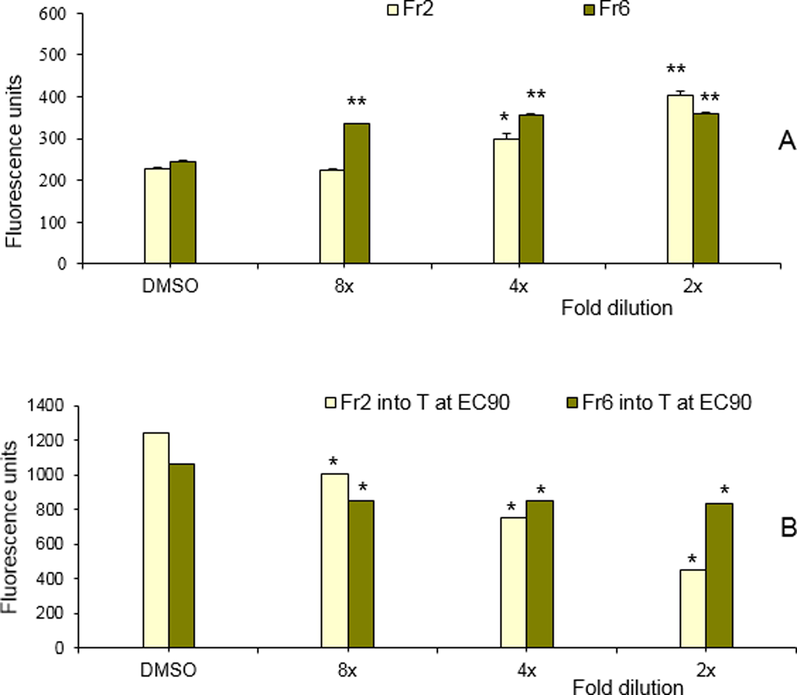
Estrogenic and anti-androgenic response of the HPLC fractions 2 and 6 in (A) the REA and (B) the RAA, respectively. For the RAA, both fractions were tested in combination with 17β-testosterone at the ECmax. Each data point represents the mean ± SD for n = 3. * (at P ≤ 0.05), ** (at P ≤ 0.001) significant difference between treatment and control (DMSO).
When tested for androgenic and anti-androgenic activity in the RAA, none of the fractions displayed an agonistic activity (results not shown), but when these fractions were tested in co-exposure with testosterone, fractions 2 and, to a lesser extent, 6 displayed anti-androgenic activities (Fig. 5B). The AR-antagonistic effect of fraction 2 was expected, as this fraction contains DIM, shown to be a strong AR-antagonist (Fig. S2). The observed AR-antagonistic effect of fraction 6, also showing a weak estrogenic activity, is probably caused by LTr1.
When tested in the DR CALUX bioassay, all fractions displayed an AhR agonist activity (Fig. 6). However, fractions 6 (LTr1) and 8 (LTe1) turned out to be the most active fractions, followed by fractions 9, 4 (CTr) and 1. At lower dilution, also fraction 7 showed some activity. Fractions 2, 3 and 5 were only slightly active. The poor activity of fraction 3 seems surprising, as it contains ICZ, which was shown to be a strong AhR agonist (Fig. S3). This is a strong indication that ICZ is only present at a very low concentration, too low to be detected in either fraction 3 or in the RXM by LC-MS/MS. When fraction 3 was spiked with an amount of ICZ that increased the HPLC-fluorescence peak by a factor 2, this still did not result in an increased DR CALUX response. Compared to fraction 3, other fractions containing unknowns showed higher AhR agonist activity. Fraction 1 also showed a response and contains I3C, but this compound did not show any activity by itself.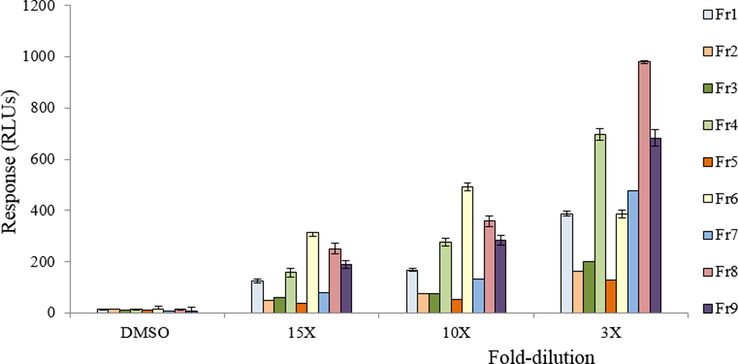
Response of the nine RXM HPLC fractions in the DR CALUX bioassay. Each data point represents the mean ± SD for triplicate measurements. Significant difference was observed between treatment (for all concentrations of the RXM) and control (DMSO) at a level of P ≤ 0.05.
4 Discussion
It has been reported that in cultured rat hepatoma cells (Bjeldanes et al., 1991, Bradfield and Bjeldanes, 1987), the crude I3C RXM weakly induces the CYP1A1 enzyme, suggesting activation of the Ah receptor. In studies in humans, oral administration of 500 mg I3C daily for 1 week (Michnovicz and Bradlow, 1990) or 400 mg I3C daily for 4 weeks (Reed et al., 2005) revealed a significant increase in estradiol metabolism, proposed as a new chemopreventive strategy to estrogen dependent diseases. Further, human clinical trial studies showed that the I3C oligomer DIM has increased estradiol metabolism (Amare, 2020; Gee et al., 2016; Rajoria et al., 2011; Thomson et al., 2017).
The present study revealed that acid condensation products of I3C (RXM) displayed strong AhR agonist activity, as well as estrogenic (weak), and anti-androgenic activity (Table 2). The question is which condensation products are responsible for these observed effects, as I3C itself cannot account for the observed RXM effects. Therefore, nine HPLC fractions prepared from the RXM were tested. Using available standards of I3C, ICZ and DIM, and applying HPLC UV, fluorescence and LC-MS/MS analysis, several of the acid condensation products were identified. The observed estrogenic and anti-androgenic effects of the RXM are most likely due to DIM, as both fraction 2, containing DIM, and the pure standard of DIM displayed agonistic effects in the REA and antagonistic effects in the RAA. The ER agonist activity of DIM is in agreement with our previous finding (Bovee et al., 2008), and other previous in vitro studies (Li, 2018; Yoo and Allred, 2016). It is also in line with in vivo observations, as DIM has been reported to be a potent estrogen in the rainbow trout (Shilling et al., 2001) and rats (Aksu et al., 2016). However, the present study is the first that demonstrates that DIM also possesses anti-estrogenic properties, i.e. when co-administered with E2 concentrations above the EC50 (Fig. S1). The results showed that DIM inhibits the maximal response of E2 to a level that equals their own maximal response (about 50% of the maximal response of E2). DIM could thus be classified as a partial ER-agonist. ICZ, previously shown to bind to the estrogen receptor in MCF-7 cells (Liu et al., 1994), was unable to activate or inhibit the estrogen receptor in the yeast estrogen bioassay. This may be due to the fact that ICZ is only weakly estrogenic and that the MCF-7 assay is more sensitive. Chang et al. (1999) showed that LTr1 has anti-estrogenic activity. In the present study RXM did not show anti-estrogenic effects, nor did fraction 6 containing LTr1. However, fraction 6 was able to exert a slight agonistic effect on the estrogen receptor.
The present study also demonstrates that DIM exhibits a rather strong anti-androgenic property, while I3C was a very weak androgen receptor antagonist. This finding is in agreement with our previous findings (Bovee et al., 2008) and correlates well with other previous findings that reported DIM inhibits the growth of androgen dependent prostate cancer cells in vitro (Le et al., 2003) and in human clinical trial studies (Hwang et al., 2016; Kallifatidis et al., 2016). Interestingly, fraction 6, containing the linear trimer LTr1, showed a weak anti-androgenic activity which has not been reported before.
It is widely reported that AhR ligands mediate the expression of different phase I and II metabolizing enzymes. In the present study, ICZ was confirmed to be a very potent AhR agonist with a potency that was only 300-fold lower than that of TCDD. This result correlates well with the findings of Bjeldanes et al., (1991). However, the data of the RXM fractions show that the fraction containing ICZ (fraction 3) showed a rather poor effect and that almost all fractions (1, 4, 6, 7, 8 and 9) are relatively more active than this ICZ fraction. This is in line with other studies that reported only low formation of ICZ in the I3C acid condensation reaction process (Bjeldanes et al., 1991; Chang et al., 1999; De Waard et al., 2008). Several previous in vitro and in vivo studies have shown that DIM and I3C can activate the AhR, thereby inducing CYP1A1 and other enzymes (Bonnesen et al., 2001; Ociepa-Zawal et al., 2007; Hestermann and Brown, 2003; Jellinck et al., 1993; Manson et al., 1998). However, these results contradict with our findings using the DR CALUX bioassay in which pure DIM and I3C failed to activate the AhR. On the other hand, the activity of LTr1 (fraction 6) in the DR CALUX bioassay is in agreement with a previous study by Chang et al., (1999) that reported AhR agonist activity for this compound. In addition, also fraction 4 containing CTr and fraction 8 containing LTe1 contributed to the overall response.
Considering the relevance for in vivo, Anderton et al. (2004) showed that I3C itself is rapidly absorbed and present at sufficient levels in rat liver, which suggested that I3C was possibly responsible for some of the biological effects (Anderton et al., 2004). Their study contradicts Stresser et al. (1995) and Reed et al. (2006) who reported that I3C could not be detected in liver and human plasma, respectively. It has also been reported that LTr1 and DIM are the major products detected in blood samples after I3C oral administration to rats (Chang et al., 1999; Stresser et al., 1995).
Conclusion
Mainly DIM seems responsible for the observed in vitro estrogenic and anti-androgenic effects of the RXM, and LTr1 most likely contributes to this profile. Rather than ICZ, the LTr1 and several other compounds present in fractions 1 and 4 (CTr), and larger molecules present in fractions 7, 8 (LTe1) and 9 seem responsible for the observed AhR activity of the RXM. Especially a structural elucidation of unknowns in fractions 8 and 9 may be important to conduct in the future. Overall, this work shows the importance of evaluating the relative contribution of individual compounds to the effect of the mixture.
CRediT authorship contribution statement
Dagnachew Eyachew Amare: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Toine F.H. Bovee: Conceptualization, Data curation, Methodology, Resources, Supervision, Writing - review & editing. Patrick P.J. Mulder: Formal analysis, Software, Writing - original draft, Writing - review & editing. Astrid Hamers: Methodology, Resources, Supervision, Writing - review & editing. Ron L.A.P. Hoogenboom: Methodology, Supervision, Visualization, Writing - review & editing.
Acknowledgments
The authors would also like to thank Elsa Antunes-Fernandes and Richard Helsdingen for their work on this research topic.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Species-specific antagonism of Ah receptor action by 2,2′,5,5′-tetrachloro-and 2,2′,3,3′,4,4′-hexachlorobiphenyl. Eur. J. Pharmacol.: Environ Toxicol. Pharmacol.. 1995;293:463-474.
- [CrossRef] [Google Scholar]
- Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201-1215.
- [CrossRef] [Google Scholar]
- 3,3 diindolylmethane leads to apoptosis, decreases sperm quality, affects blood estradiol 17 β and testosterone, oestrogen (α and β) and androgen receptor levels in the reproductive system in male rats. Andrologia. 2016;48(10):1155-1165. 10.1111/and.2016.48.issue-1010.1111/and.12554
- [Google Scholar]
- Anti-cancer and other biological effects of a dietary compound 3,3’-Diindolylmethane supplementation: A systematic review of human clinical trials. Nutrit. Dietary Suppl.. 2020;12:1-15.
- [Google Scholar]
- Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res.. 2004;10:5233-5241.
- [CrossRef] [Google Scholar]
- Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Biol.. 2016;40-41:170-191.
- [CrossRef] [Google Scholar]
- Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci.. 1991;88:9543-9547.
- [CrossRef] [Google Scholar]
- Bjeldanes, L.F., Le, H.T., Gary, L., 2005. 3,3'-Diindolylmethane antiandrogenic compositions: Google patents, 2005.
- Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res.. 2001;61:6120-6130.
- [Google Scholar]
- A new highly specific and robust yeast androgen bioassay for the detection of agonists and antagonists. Anal. Bioanal. Chem.. 2007;389:1549-1558.
- [CrossRef] [Google Scholar]
- Rapid yeast estrogen bioassays stably expressing human estrogen receptors α and β, and green fluorescent protein: a comparison of different compounds with both receptor types. The Journal of steroid biochemistry and molecular biology. 2004;91:99-109.
- [CrossRef] [Google Scholar]
- Screening of synthetic and plant-derived compounds for (anti) estrogenic and (anti) androgenic activities. Anal. Bioanal. Chem.. 2008;390:1111-1119.
- [CrossRef] [Google Scholar]
- Structure—activity relationships of dietary indoles: A proposed mechanism of action as modifiers of xenobiotic metabolism. Journal of Toxicology and Environmental Health, Part A Current Issues. 1987;21:311-323.
- [Google Scholar]
- Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3, 3′-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochemical pharmacology. 1999;58:825-834.
- [Google Scholar]
- Chen, I., MCDougal, A., Wang, F., Safe, S., 1998. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis, 19, 1631-1639. 10.1093/carcin/19.9.1631
- Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Instit.. 2000;92:61-68.
- [CrossRef] [Google Scholar]
- Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem.-Biol. Interact.. 1991;80:303-315.
- [CrossRef] [Google Scholar]
- Ah receptor agonist activity in frequently consumed food items. Food Additiv. Contamin.. 2008;25:779-787.
- [CrossRef] [Google Scholar]
- DECISION, C., 2002. 657/EC implementing Council Directive 96/23. EC concerning.
- Antioxidant functions of the aryl hydrocarbon receptor. Stem Cells Int. 2016
- [CrossRef] [Google Scholar]
- The Brassica-derived phytochemical indolo[3,2-b] carbazole protects against oxidative DNA damage by aryl hydrocarbon receptor activation. Archiv. Toxicol.. 2017;91(2):967-982.
- [CrossRef] [Google Scholar]
- Phase Ib placebo-controlled, tissue biomarker trial of diindolylmethane (BR-DIMNG) in patients with prostate cancer who are undergoing prostatectomy. Eur. J. Cancer Prev.. 2016;25(4):312-320.
- [Google Scholar]
- Oligomerization of indole-3-carbinol in aqueous acid. Chem. Res. Toxicol.. 1992;5:188-193.
- [CrossRef] [Google Scholar]
- Indole-3-carbinol, a plant nutrient and AhR-Ligand precursor, supports oral tolerance against OVA and improves peanut allergy symptoms in mice. PLoS One. 2017;12(6):e0180321.
- [CrossRef] [Google Scholar]
- Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol. Cell. Biol.. 2003;23:7920-7925.
- [CrossRef] [Google Scholar]
- Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol. Res.. 2007;55:224-236.
- [CrossRef] [Google Scholar]
- Anti-androgenic activity of absorption-enhanced 3, 3′-diindolylmethane in prostatectomy patients. Am. J. Transl. Res.. 2016;8(1):166-176.
- [Google Scholar]
- Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem. Pharmacol.y. 1993;45:1129-1136.
- [CrossRef] [Google Scholar]
- Glucosinolates: bioavailability and importance to health. Int. J. Vitamin Nutrit. Res.. 2002;72:26-31.
- [CrossRef] [Google Scholar]
- Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol.. 2016;40–41:160-169.
- [CrossRef] [Google Scholar]
- 3, 3′-Diindolylmethane suppressed cyprodinil-induced epithelial-mesenchymal transition and metastatic-related behaviors of human endometrial ishikawa cells via an estrogen receptor-dependent pathway. Int. J. Mol. Sci.. 2018;19(1):189.
- [CrossRef] [Google Scholar]
- Targets for indole-3-carbinol in cancer prevention. J. Nutrit. Biochem.. 2005;16:65-73.
- [CrossRef] [Google Scholar]
- Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem.. 2003;278(23):21136-21145.
- [CrossRef] [Google Scholar]
- Li, Y., 2018. Characterization of the effects of 3,3′-diindolylmethane (DIM) in the TRAMP mouse prostate cancer model and DIM’s interaction with estrogen receptor signalling.
- Indolo[3,2-b]carbazole: a dietary-derived factor that exhibits both antiestrogenic and estrogenic activity. JNCI: J. Natl. Cancer Instit.. 1994;86(23):1758-1765.
- [CrossRef] [Google Scholar]
- Chemoprevention of aflatoxin B1-induced carcinogenesis by indole-3-carbinol in rat liver–predicting the outcome using early biomarkers. Carcinogenesis. 1998;19:1829-1836.
- [CrossRef] [Google Scholar]
- Induction of estradiol metabolism by dietary indole-3-carbinol in humans. JNCI: J. Natl. Cancer Instit.. 1990;82:947-949.
- [CrossRef] [Google Scholar]
- A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutrit. Rev.. 2007;65:259-267.
- [CrossRef] [Google Scholar]
- Chemical-Activated Luciferase Gene Expression (CALUX): A novelin vitrobioassay for Ah receptor active compounds in sediments and pore water. Fundam. Appl. Toxicol.. 1996;33:149-160.
- [CrossRef] [Google Scholar]
- Cruciferous vegetable intake is inversely associated with lung cancer risk among current nonsmoking men in the Japan Public Health Center (JPHC) Study. J. Nutrit.. 2017;147(5):841-849.
- [CrossRef] [Google Scholar]
- Cruciferous vegetable consumption and stomach cancer: a case-control study. Nutrit. Cancer. 2020;72(1):52-61.
- [CrossRef] [Google Scholar]
- Comparisons of differential gene expression elicited by TCDD, PCB126, βNF, or ICZ in mouse hepatoma Hepa1c1c7 cells and C57BL/6 mouse liver. Toxicol. Lett.. 2013;223(1):52-59.
- [CrossRef] [Google Scholar]
- The synergistic upregulation of phase II detoxification enzymes by glucosinolate breakdown products in cruciferous vegetables. Toxicol. Appl. Pharmacol.. 2001;174:146-152.
- [CrossRef] [Google Scholar]
- The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim. Polon.-English Ed.. 2007;54:113.
- [Google Scholar]
- 3,3′-Diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21(3):299-304.
- [CrossRef] [Google Scholar]
- Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3, 3′-diindolylmethane. Cancer Epidemiol. Prevent. Biomark.. 2006;15(12):2477-2481.
- [CrossRef] [Google Scholar]
- A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol. Prevent. Biomark.. 2005;14:1953-1960.
- [CrossRef] [Google Scholar]
- Selective aryl hydrocarbon receptor modulator 3,3′-diindolylmethane impairs AhR and ARNT signaling and protects mouse neuronal cells against hypoxia. Mol. Neurobiol.. 2016;53(8):5591-5606.
- [CrossRef] [Google Scholar]
- Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett.. 2008;269(2):326-338.
- [CrossRef] [Google Scholar]
- 3, 3′-Diindolylmethane, a major condensation product of indole-3-carbinol, is a potent estrogen in the rainbow trout. Toxicol. Appl. Pharmacol.. 2001;170:191-200.
- [CrossRef] [Google Scholar]
- Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion in male Fischer 344 rats. Drug Metabol. Disposit.. 1995;23:965-975.
- [Google Scholar]
- A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat.. 2017;165(1):97-107.
- [CrossRef] [Google Scholar]
- Chemopreventive properties of 3,3'-diindolylmethane in breast cancer: evidence from experimental and human studies. Nutr. Rev.. 2016;74(7):432-443.
- [CrossRef] [Google Scholar]
- Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Prevent. Biomark.. 1996;5:733-748.
- [Google Scholar]
- Indole-3-Carbinol (I3C) and its major derivatives: their pharmacokinetics and important roles in hepatic protection. Curr. Drug Metab.. 2016;17(4):401-409.
- [CrossRef] [Google Scholar]
- Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett.. 2008;262:153-163.
- [CrossRef] [Google Scholar]
- Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann. Oncol.. 2013;24(4):1079-1087.
- [CrossRef] [Google Scholar]
- Effects of analogs of indole-3-carbinol cyclic trimerization product in human breast cancer cells. Chem.-Biol. Interact.. 2005;152:119-129.
- [Google Scholar]
- The estrogenic effect of trigonelline and 3,3-diindolymethane on cell growth in non-malignant colonocytes. Food Chem. Toxicol.. 2016;87:23-30.
- [CrossRef] [Google Scholar]
- Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo [a, l] pyrene. Carcinogenesis. 2006;27:2116-2123.
- [CrossRef] [Google Scholar]
- Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case–control study in China. Br. J. 2018
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.08.002.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







