Translate this page into:
Adsorption of chromium (VI) and iron (III) ions onto acid-modified kaolinite: Isotherm, kinetics and thermodynamics studies
⁎Corresponding author. pevdim@yahoo.com (P.E. Dim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The pollution of aquatic bodies by heavy metals effluent from many industrial activities is a significant environmental challenge affecting the ecosystem. Batch process was conducted to remove Cr (VI), and Fe (III) with hydrochloric acid modified clay (HMC) and acetic acid modified clay (AMC). The adsorbents morphology, chemical properties were measured by scanning electron microscope (SEM), X-ray fluorescence spectrometry (XRF), cation exchange capacity (CEC), X-ray diffraction (XRD) and Brunauer-Emmett-Teller (BET). The effects of time, adsorbent dose, temperature and pH of effluent on adsorption were studied. The acid activation increased the BET surface area from 84.223 m2/g raw clay (RC) to 389.37 m2/g (HMC) and 319.955 m2/g (AMC), total pore volume increased to 0.2168 and 0.2285 cm3/g, respectively. The CEC increased from 8.4 cmol/g to 22.30 cmol/g (HMC) and 20.73 cmol/g (AMC). The results of XRF, SEM and XRD studies show disintegration and a porous structure of the treated clay, and also changes in the intensity of the bands. The HMC had a maximum removal of Cr (VI), and Fe (III) of 79% and 90% at pH 7.0 and AMC recorded 60% and 57% at pH 6.0, respectively. The adsorption process equilibrium was attained at 50 and 90 min for HMC and AMC, respectively. Langmuir isotherms had the best fit. HMC and AMC adsorption capacity are, Cr (VI):18.15 mg/g and Fe (III):39.80 mg/g; Cr (VI): 10.42 mg/g and Fe (III):19.34 mg/g. The interaction of Cr (VI) and Fe (III) ions onto HMC/AMC was spontaneously endothermic. It agreed with the second-order equation. The maximum desorption efficiency recorded on HMC is 92.45 and 85.67% for Cr (VI) and Fe (III) and for AMC is 76.58 and 65.32% for Cr (VI) and Fe (III). The process is economically important because it provides an avenue for safe disposal or reuse of adsorbent. This attempt has shown the potential of modified clay as a suitable eco-friendly sorbent for removing heavy metals.
Keywords
Chromium (VI)
Iron (III)
Kaolinite
Isotherm
Adsorption
Thermodynamics
1 Introduction
The pollution of aquatic bodies by heavy metal discharged from various industrial activities is a global environmental challenge. Most industries in third world nations make little or no provision to treat their effluent before discharging it. Their presence in the water bodies in above acceptable limit is a serious hazard on human and ecosystem since they are non-biodegradable and can persist in different environmental conditions. Therefore, it is essential to protect the environment and the public from these pollutants by removing them from the aquatic ecosystem. The heavy metals in focus are chromium (VI) and Fe (III) ions. Chromium finds application in chrome plating, pigment, textile, wood preservation, and as antifouling agent in cooling towers (Li et al., 2016; Shen et al., 2012). The versatility of chromium has increased its concentration, especially in water bodies. Chromium (VI) ion presence in water above permissible level is poisonous and can cause diseases like cancer, kidney and liver failure (Rai et al., 2016, Ciopec et al., 2012). Therefore, it necessary to treat any wastewater containing chromium (VI) to the permissible level. According to the world health organization (WHO), the limit for drinkable water is 0.05 mg/L, and wastewater from industries is 0.5 mg/L (WHO, 2017). The primary source of ferric metallic ions is the ore mining activities, which comprises various minerals and heavy metals. The mining waste at oxidized state is corrosive and leads to acid mine drainage. It contains arsenic, copper, lead, cadmium, iron, sulfates. Ferric ions are highly present in acid mine drainage waters in a concentration of several 100 mg/L (Zheng et al., 2011). Therefore, it is essential to treat any ferric ion contaminated water to a level below the permissible limit of 0.3 mg/L (WHO, 2017). The overdose of iron in human body may cause anorexia, oliguria and diphasic shock, haemochromatosis, diabetes mellitus, liver cancer and cirrhosis (Li et al., 2016, Bhattacharyya and Gupta, 2006a). The adsorption of Fe (III) and Cr (VI) in wastewaters is a significant challenge. The adsorption technique is the easiest method used in the decontamination of heavy metals in discharged effluents. Some of its economic and technological advantages are, it is cheap and easy to operate, accessible and available, profitable and efficient, and effective than other techniques (Rao et al., 2014, Gupta et al., 2013).
According to Uddin (2017), different traditional and non-traditional adsorbents have been employed to remove heavy metals. They include adsorption of copper, lead, chromium and nickel (Dim et al., 2020, Ridha et al., 2017, Rai et al., 2016), and iron (Khalil et al., 2013, Zheng et al., 2011, Yeddou and Bensmaili, 2007, Bhattacharyya and Gupta, 2006b, Wan et al., 2005). Several studies have shown that natural and modified adsorbent has been used to remove heavy metal pollutants. Their use for adsorption is due to their large pore volume and surface-area (Rabie et al., 2019). Agricultural wastes and clay minerals are used to remove heavy metals and inorganic pollutants (Bakalár et al., 2020). Recently some researchers like, Bazrafshan et al. (2017) investigated the use of Cydonia oblonga seed to remove Cr (VI) ions from aqueous solution. Balarak et al. (2016b), reported the treatment of Azolla filiculoides with tetraoxosuphate (VI) acid (H2SO4) for the removal of cadmium from aqueous solution. The result showed that Azolla Filiculoides could be an efficient adsorbent to remove Cd (II) from contaminated water. Additionally, Balarak et al. (2016a) also showed that barley husk a low-cost agricultural waste could be used to remove Cd (II) from aqueous solutions. In a similar study, Azarpira and Mahdavi (2016), investigated the use of Canola biomass for the adsorption of Cd (II) from aqueous solution in a batch system. Also, removing noxious nickel (II) was reported using a novel γ-alumina nanoparticle and multiwalled carbon nanotubes (Agarwal et al., 2016).
Clay minerals are natural and effective adsorbents, and acid treatment will enhance their adsorption capacity (Dim et al., 2020, Sejie et al., 2016). As a useful adsorbent material, clay has played a crucial role in decontamination of heavy metals in aqueous media. Clay a natural resource, has a great tendency to serve as an alternative adsorbent for decontamination of aquatic bodies. Its advantages include high cation exchange capacity, renewability, low-cost, excellent affinity, non-toxic, and it can be regenerated (Sarma et al., 2016). This material is available in large quantity and also has a high surface area. Previous studies reported that acid modification of local clay samples enhanced its adsorption for heavy metals (Dim et al., 2020, Sarma et al., 2016). Therefore, it becomes necessary to improve the adsorption capacity and expand local clay application beyond fine art and local pottery work. Thus, this study will characterize clay treated with hydrochloric and acetic acid to improve its utilization and commercial value of the local clay. The sourcing of natural material from local sources is advantageous because it will support the local economy and help lower environmental impact.
The different types of acids used for clay treatment include inorganic and organic acids such as hydrochloric, sulphuric, nitric, acetic, citric, oxalic and lactic acid. The treatment of clay with hydrochloric or sulphuric generates new surface acid sites quickly. However, hydrochloric acid is more effective in activating the local clay than sulphuric acid (Mokaya and Jones, 1995, Mahmoud and Saleh, 1999) and acetic acid preserve clay structure. It is effective in the generation of surface acid sites (Mahmoud and Saleh, 1999). Their choice depends on the type and nature of clay, acid concentration and activation time (Valenzuela-Diaz and Souza-Santos, 2001). They have advantages, including lower acid costs/unit mass of clay treated, lower production costs and environmentally friendly. In this study, the clay was treated with hydrochloric and acetic acid, to date no report on the use of the local clay for the adsorption of chromium and iron in real practical industrial textile wastewater.
The objectives of study are to examine the adsorption characteristic of clay treated with acids for Fe (III) and Cr (VI) removal in batch system. To explore the effect of treatment on the structure of clay; and investigate the effect of contact time, dosage, pH and temperature, and the isotherms, kinetics, and thermodynamics on the practical application of HMC and AMC in the removal of heavy metals from real industrial wastewater.
2 Materials and methods
2.1 Materials
The clay was obtained from Umunze, Anambra State, Nigeria, is mainly used by the locals for pottery and artwork. Analytical grade reagents include phosphoric acid, sodium hydroxide, hydrochloric acid, acetic acid and potassium iodide were purchased from merck chemical company. Textile industry in the community supplied the effluent used. The standard solutions of Cr and Fe were used as purchased from Sigma-aldrich Germany (99.99%) (1000 mg/L Cr in 2% nitric acid, prepared with high purity (NH4)2Cr2O7, HNO3 and water and, 1000 mg/L Fe in 2% nitric acid, prepared with high purity Fe metal, HNO3 and water).
2.2 Preparation of clay adsorbent
The raw clay (RC) treated with hydrochloric acid is (HMC), and acetic acid is (AMC). The clay minerals after crushing with a wooden mortar and a pestle. It was washed thoroughly with distilled water to remove any particles attached to its surface. The ground clay minerals were dried in an oven at 110 °C for 4 h. After pretreatment, 50 g of dried clay with 100 ml of 4 M hydrochloric and 2.5 M acetic acid solution, were stirred continuously for 30 min and left for 24 h. The suspension of clay was filtered, washed thoroughly and then dried at 85 °C overnight. After that, it was sieved to particle sizes of approximately 125 um and kept safe in a desiccator.
2.3 Characterization of modified clay
The Micromeritics ASAP 2020 measured the surface area and pore volume of the adsorbents. SEM-EDX (JEOLJSM 7600F) determined the morphology and elemental composition. X-Ray Fluorescence (XRF) and X-ray diffraction (XRD) analysis was done with a Model-PW2400 and MD 10 Randicon diffractometer, respectively. The cation exchange capacity (CEC) of clay was measured by mixing 5 ml of copper bisethylenediamine complex solution with 0.5 g clay in a 100 ml flask. After that diluted with distilled water to 25 ml. The mixture was agitated for 30 min in a thermostatic water bath shaker and centrifuged. The residual concentration was determined by mixing 5 ml of the complex with 5 ml of 0.1 M HCl to destroy the complex, followed by addition of 0.5 g KI per ml and then titrating it with 0.02 M Na2S2O3 in the presence of starch as indicator. The CEC was calculated from values obtained (Bergaya and Vayer, 1997).
2.4 Adsorption study
The adsorption was conducted with batch method. 0.1 g of adsorbent was mixed in 250 ml conical flasks containing 50 ml of real wastewater and adjusted with 1.0 M NaOH or 1.0 M H3PO4. The content was agitated using water-bath shaker equipped with the controller of shaking and temperature (Jisico, model J-NSIL-R) at 190 rpm at room temperature for 120 min. After which is filtered, and the total iron and chromium concentration was determined by Atomic Absorption Spectroscopy (AAS) (Buck Scientific, model 210VGP) (Dokmaji et al., 2020). It is worthy to note that this method measures total Cr or Fe in solution, regardless of the oxidation state of the metal.
The content of Cr (VI) and Fe (III) were analysed by UV–vis spectrophotometer (Model UV-1601 Shimadzu) (Ahmed and Roy, 2009, Khamis et al., 2020). These parameters, as studied at the following experimental conditions. Contact time used are 10, 20, 30, 40, 50, 60, 90, and 120 min, adsorbent dose used are 0.1, 0.2, 0.3, 0.4 and 0.5 g, pH values was tested from 2 to 12 and temperatures were 25, 30, 35, 40 and 45 °C. Eq. (1) was applied to calculate the amount of heavy metals adsorbed, qe (mg g−1):
2.5 Desorption study
The batch desorption studies tested the recoverability and reusability of HMC and AMC adsorbent. 30 ml of 0.1 N HCl solution was contacted with adsorbents saturated with metal ions after the adsorption process. The mixture was shaken at 180 rpm for 120 mins, after which each sample was analysed for desorbed metal concentration (Fernández-Pazos et al., 2013). The percentage of desorption (Ds) of metal ions was expressed as Eq. (3), M1 = amount of metal ions desorbed (mg), M2 = amount of metal ions adsorbed (mg).
3 Results and discussion
3.1 XRF results
XRF results, as presented in Table 1. The finding reveals that the major constituents in the clay are silica (SiO2), alumina (Al2O3) and iron oxide (Fe2O3) containing an approximate percentage of 49.75, 9.20, and 6.94% respectively. The composition of copper, nickel, zinc, chromium, titanium, calcium, and manganese oxides was low, which conform to clay's chemical analysis (Bakhtyar and Shareef, 2013). From Table 1, the contents of the raw clay Al2O3, Fe2O3, MgO, CaO were 12.88, 6.88, 1.50, and 0.36 wt%. As can be seen that Al2O3, Fe2O3, MgO, CaO contents decreased; on the other hand, the content of silica oxide (SiO2) content changed after treatment. The percentage of SiO2 is 67.82% for the raw clay. However, it increased to 70.27% and 68.21% with treatment by hydrochloric acid and acetic acid. The increase resulted from removing impurities, replacing the exchangeable cations (K+, Na+ and Ca2+) with hydrogen ions and leaching of Al3+, Fe3+ and Mg2+ octahedral tetrahedral sites which exposes the edges of the clay particles (Tsai et al., 2007). It can also suggest that acid modification must have depleted the octahedral and interlayer and cations, affecting adsorbent efficiency (Nweke et al., 2015). The increase is mainly due to the elimination of exchangeable cations and generation of silica (Barrios et al., 1995). The significant increase in the surface area resulted from the treatment with mineral acids (Komadel and Madejova, 2006). The increase also caused the dissolution of the octahedral cations. The Fe3+ and Mg2+ were easy to dissolve by the acids, while Al3+ was difficult to dissolve. This behaviour is as a result of their positions in the structure of the clay. Mg2+ is found on the ribbons' edges, while Al3+ is at the octahedral ribbon centre (Güven, 1992). Besides, solubility plays a significant role in the changes; Al2O3 is less soluble in the acid medium than the MgO. The adsorbents' high mineral content and low carbonaceous matter confirmed the value of a loss on ignition obtained, 7.92 for RC, 7.79 for HMC and 8.31% for AMC (Yusuff et al., 2015).
Composition
Weight (%) RC
Weight (%) HMC
Weight (%) AMC
SiO2
67.82
70.27
68.21
Al2O3
12.88
12.32
12.55
Fe2O3
6.68
5.17
6.42
Cr2O3
0.02
0.01
0.02
TiO2
1.48
1.42
1.42
CaO
0.36
0.24
0.26
MgO
1.50
1.17
1.32
Na2O
0.15
0.16
0.00
MnO
0.14
0.02
0.10
V2O5
0.03
0.01
0.04
K2O
0.51
0.49
0.51
P2O5
0.12
0.12
0.13
LOI
7.92
7.79
8.31
3.2 BET analysis
The primary aim of acid activation of clay is to increase the adsorption area and void capacity because they affect adsorbent behaviour significantly. As exhibited by clay materials, the adsorption tendency corresponds to the available surface area (Elmoubarki et al., 2015). Furthermore, studies have shown that the relationship existing between a clay available area and its adsorption ability is directly proportional (Sarma et al., 2016, Elmoubarki et al., 2015).
As shown in Table 2, HMC and AMC have a surface area of 389.37 and 319.955 m2/g, respectively. The effect of acid activation caused more surface creation, which resulted in about four-fold increment compared to RC (84.223 m2/g). A similar effect in total pore volume (0.2168 and 0.2285 ml/g) for the modified clay in the ratio of 4:1 with raw clay. The surface area increased resulted from the removal of impurities, generation of silica, replacement of the exchangeable cations (K+, Na+ and Ca2+) with hydrogen ions. The leaching of Al3+, Fe3+ and Mg2+ from the octahedral and tetrahedral sites exposes the edges of clay particles (Edama et al., 2014, Tsai et al., 2007).
Samples
Surface area (m2/g)
Pore volume (cc/g)
Pore size (nm)
CEC
RC
84.223
0.0527
2.115
8.41
HMC
389.370
0.2168
2.116
22.29
AMC
319.955
0.2285
2.126
20.73
The CEC of the raw clay (RC) is 8.41 cmol/g, and acid treatment increased it to 22.29 cmol/g for HMC and 20.73 cmol/g for AMC, which is almost thrice the value of RC. The treatment of the clay resulted in the replacement of different cations with H+ ions. The ion exchange capacity of clay minerals resulted in structural defects, broken bonds and structural hydroxyl transfers. Acid treatment increased the total number of exchange sites marginally (Bhattacharyya and Gupta, 2006a, Rodrigues, 2003).
3.3 SEM analysis
The SEM morphology of RC, HMC and AMC are shown in Fig. 1. The micrograph of RC (Fig. 1a) shows the presence of a mixture of large plates that appeared to have been formed by several flaky particles stacked together in the form of agglomerates. The HMC (Fig. 1b) and AMC (Fig. 1c) clay show a clear difference in structure with RC (Fig. 1a). Fig. 1b and c showed the effect of acid treatment on the clay samples. This acid opened the platelets and produced a more porous structure which caused an increase in surface area (Kumar et al. 2013). Their surfaces are irregular, rough, porous, and heterogeneous, a suitable property of an adsorbent (Mudzielwana et al., 2016). The significant changes in HMC and AMC after activation compared to RC, can be observed from their respective images. This observation is similar to reports in the literature on the treatment of clay (Sarma et al., 2016, Edama et al., 2014).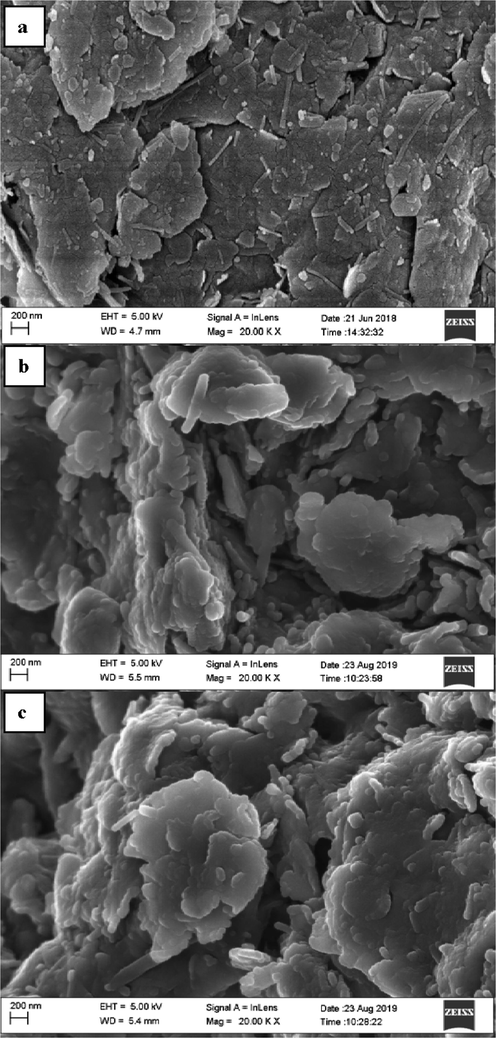
SEM micrographs (a) RC (b) HMC and (c) AMC adsorbent.
3.4 XRD analysis
Fig. 2 show the diffractograms of the adsorbents. It displays spectra of the clay, (A) is montmorillonite, (B) is kaolinite and (C) is quartz, these species are related to clay fractions (Ayari et al., 2019, Sejie et al., 2016). The prominent peaks at 2θ = 25.2° and 28.65°, 30.2° and 53.45° are characteristic bands for quartz SiO2 which is silica consisting of interconnected SiO2 tetrahedra that build up a rigid three-dimensional network. The peaks at 2θ = 21.41°, 36.13°, 41.45°, 45.70°, 60.21° and 68.32° are characteristic of a kaolinite clay mineral (Sarma et al., 2016). The appearance of more kaolinite indicates the increase in the pore size of the treated clay. The transformation of the crystalline structure of clay leads to a decrease in peak intensity (Eren and Afsin, 2009).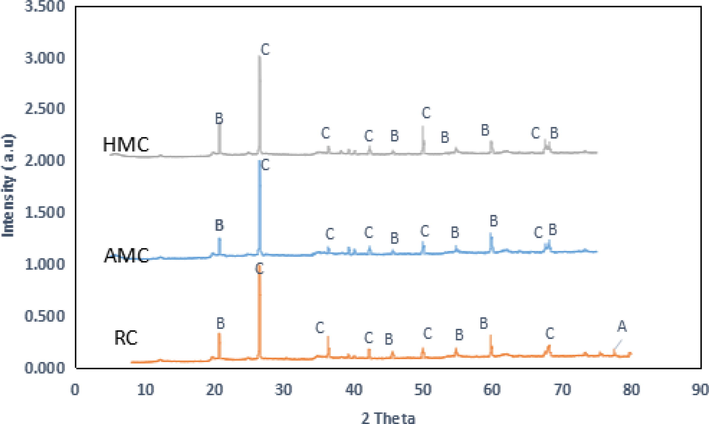
XRD pattern of RC, AMC and HMC.
3.5 Adsorption study
3.5.1 Effect of contact time
Sorption process depends on interaction time between adsorbent and adsorbates. The adsorption process, as performed in a time range of 10–120 min, and the results are shown in Fig. 3. The metal uptake increases rapidly with time up to saturation capacity at 50 min for HMC and gradually for 90 min for AMC. After that, the metal ion uptake became slow and significantly constant, suggesting the equilibrium time as 50 and 90 min for HMC and AMC. The maximum adsorption achieved at equilibrium time of 50 min for HMC is Cr (VI) (74.39%) and Fe (III) (89.93%), and 90 min for AMC are Cr (61.23%) and Fe (79.74%). In the adsorption process, equilibrium time is crucial for metal ion removal from solution. Its implementation can improve cost efficiency in adsorption technology. Rapid increase at the onset of adsorption process occurred because of the high solute concentration gradient and vacant pore voids. As the contact time increased metal uptake slows down because of the occupation of the available adsorption sites. The heavy metal movement into the pore surface will be slow due to few sites available; this will continue until equilibrium is achieved (Azouaou et al., 2010).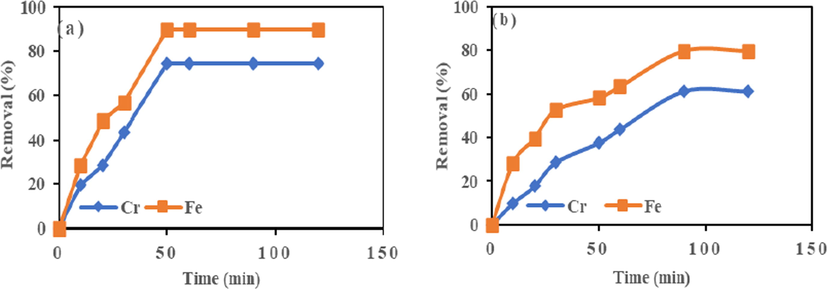
Effect of contact time on adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC at agitation speed = 190 rpm, Mass = 0.1 g, and temperature = 25 °C.
3.5.2 Solution pH
The pH was tested at 190 rpm, adsorbent dosage of 0.1 g and 25 °C., as shown in Fig. 4. The pH varied from 2 to 12. The percentage removal of the species increased as the pH increased for both adsorbents. The removal by adsorbent HMC and AMC shows a steady increase in both metal ions' pH value. The changing of the pH from 2 to 12 caused an increase in the removal of metals. HMC achieved the maximum removal of 79% Cr (VI) and 90% Fe (III) at pH 7.0 and AMC is 60% and 57% at pH 6.0, respectively. Maximum metal removal peaked at pH of 7.0 and 6.0 for HMC and AMC, suggesting the optimum pH values. At any pH, the HMC has a higher removal percent compared to the AMC.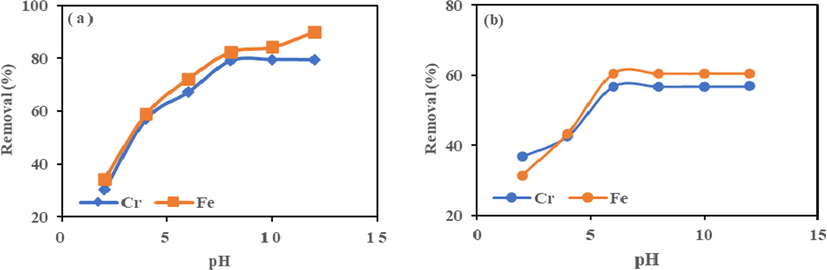
Effect of pH on adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC at agitation speed = 190 rpm, Mass = 0.1 g, and temperature = 25 °C.
However, beyond the maximum removal, the AMC equilibrium behaviour in removing metal species was constant. However, for HMC, Cr (VI) ion removal was constant, and Fe (III) showed a moment of stability, and a sudden rise in removal was observed (Boonamnuayvitaya et al., 2004). This behaviour could be the effect of competition from other contaminants, precipitation of complexes of chromium, Cr (OH)3 and iron, Fe (OH)3 at pH beyond 6 and 7. The insoluble complexes of precipitated metal hydroxides may appear as apparently higher metal ion removal (Srivastava et al., 2008, Hizal and Apak, 2006). According to Azouaou et al. (2010), the binding of metal ions is based on the theory of acid-base equilibria which is the dissociation of weak acid bonds within a pH range of 2–7. The formation of complexes of chromium and iron beyond pH 6.0 and 7.0 must have hindered the diffusion of Cr (VI) and Fe (III) ions to the clay surface resulting to concurrent adsorption and co-deposition (Bhattacharyya and Gupta, 2008). The increase in species adsorption in acidic medium was by electrostatic attraction between positively charged groups on the clay surface and oxyanions of Cr (VI), i.e., HCrO4− anion and Fe (OH)3+ (Padmavathy et al., 2016). At low pH, Fe (III) and Cr (VI) ions face stiff competition from H3O+ ions for the adsorption sites and consequently lead to low adsorption. The active sites on clay surface have weakly acidic properties (Boonamnuayvitaya et al., 2004; Padmavathy et al., 2016). These sites will gradually deprotonate at comparatively higher pH resulting in high metal ions uptake (Rai et al., 2016, Rangabhashiyam and Selvaraju, 2015, Taffarel and Rubio, 2009). The adsorption behaviour, as displayed by HMC and AMC, resulted from mechanisms such as ion exchange, chemical complexation, and electrostatic forces (Bhattacharyya and Gupta, 2008).
3.5.3 Effect of adsorbent dose
The effect of adsorbent dose test was chosen within 0.1–0.5 g, as seen in Fig. 5. The removal of metal ions increased with dosage for HMC and AMC adsorbent. The metal ion adsorbed by HMC increased with the amount of adsorbent until at 0.3 g, the maximum value was attained. However, the removal became steady beyond this point, suggesting an optimum dosage of 0.3 g for HMC, with maximum adsorption of 45.69% for Cr (VI) and 42.86% for Fe (III). On the other hand, for AMC, the removal percent also increased with the amount of adsorbent. The maximum removal of 55.69% for Cr (VI) and 52.91% for Fe (III) at 0.3 g is the optimum dosage after which removal percent decreased. From Fig. 5, percentage adsorption increased between 0.1 and 0.3 g; the dose increased the availability of adsorption sites. However, beyond 0.3 g, Cr (VI) and Fe (III) were few in the reaction medium to interact with the available surface area. Hence the adsorption gradually becomes constant or decreased, and additional adsorbent made no contribution (Simha et al., 2016).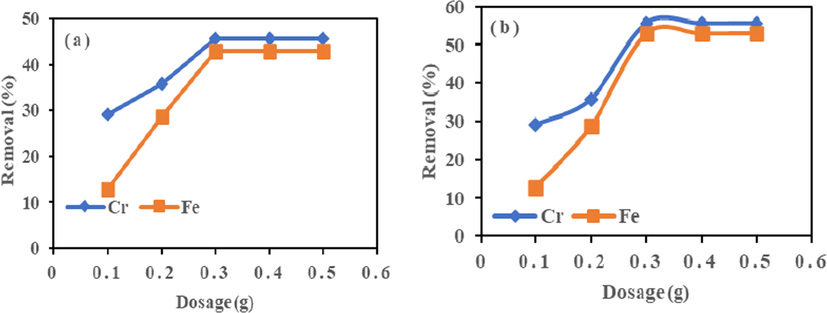
Effect of adsorbent dosage on adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC at agitation speed = 190 rpm, Mass = 0.1 g, and temperature = 25 °C.
3.5.4 Effect of temperature
As seen from Fig. 6, temperatures chosen for the test are 25, 30, 35, 40 and 45 °C. The sorbent HMC showed an increase in metal ions adsorption from 25 to 35 °C, (87.49–97.56%) for Fe (III) and (82.37–92.35%) for Cr (VI). The increased kinetic effect led to increased mobility of the adsorbates molecule as temperature increased. The high adsorption recorded indicates the process is endothermic. A further increase in temperature beyond 35 °C had no or insignificant effect, suggesting the optimum temperature of HMC as 35 °C.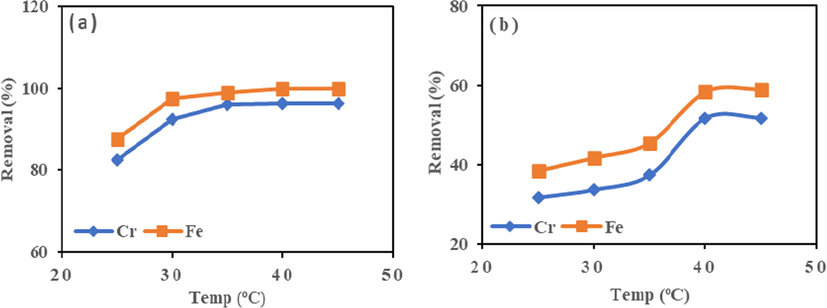
Effect of temperature on adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC at agitation speed = 190 rpm, Mass = 0.1 g.
Conversely, for AMC the removal of Fe (III) and Cr (VI) increased gradually. They reached percentage removal of (38.34–45.65%) for Fe (III) and (31.67–37.64%) for Cr (VI) from 25 to 35 °C, and sudden increase in percentage adsorption was recorded of (45.43–58.37%) for Fe (III) and (37.64–51.68%) for Cr (VI), from 35 to 40 °C. The penetration of vacant active sites caused an increase in temperature; this suggests an endothermic process (Yusuff et al., 2017). The maximum percent removed for Fe (III) and Cr (VI) are 58.97% and 51.72% with AMC at the optimum temperature of 40 °C. Fig. 6a and 6b show 35 °C and 40 °C as the optimum value for metal ion removal by HMC and AMC, respectively.
3.6 Kinetic study
The pseudo-first-order Eq. (4) and pseudo-second-order Eq. (5) was used to understand the adsorption dynamics with time for the ions system. As presented in Fig. 7 and Table 3, the R2 value of 0.9481 and 0.9568 (Cr (VI) and Fe (III)), and 0.9328 and 0.9363 (Cr (VI) and Fe (III)) as obtained for the pseudo-first-order model. The correlation coefficients (R2) values for the pseudo-first-order kinetic model were lower than second order. The value indicates that the first-order model did not accurately describe Cr (VI) and Fe (III) adsorption onto HMC and AMC. While R2 values of pseudo-second-order for HMC and AMC adsorbents were 0.999 and 0.998 (Cr (VI) and Fe (III)), and 0.999 and 0.999 (Cr (VI) and Fe (III)) respectively (Fig. 8 and Table 3). The calculated correlation coefficient (R2) was high and very close to one for the pseudo-second-order kinetic model. The value illustrated good linearity with R2 above 0.99. It demonstrated that the adsorption capacity was proportional to the number of active sites occupied on HMC and AMC. High regression coefficient (R2) value conformed with the pseudo-second-order kinetic model (Bhattacharyya and Gupta, 2006a; Bhattacharyya and Gupta, 2006b; Al-Anber, 2015). Besides the higher regression coefficient (R2) value, the lowest SSE and □2 values in the two error functions support the R2 values (Table 3). This confirmed pseudo-second-order equation had the best fit; and are consistent with other reports in the literature (Akpomie and Dawodu, 2015).
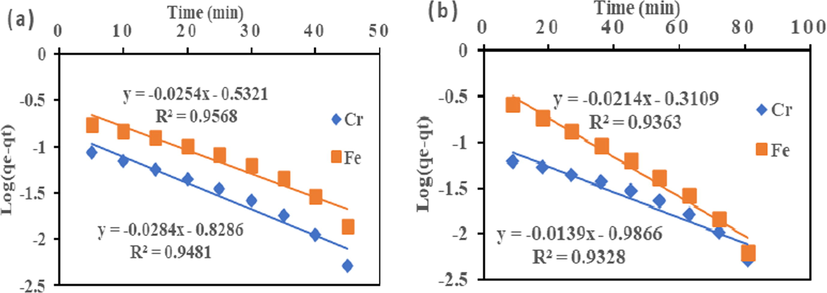
Pseudo-first order for adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC.
Pseudo-first-order kinetics
Metal ions
HMC
AMC
qeexp (mg/g)
qecal (mg/g)
k1 (min−1)
R2
SSE
χ2
qeexp (mg/g)
qecal (mg/g)
k1 (min−1)
R2
SSE
χ2
Cr (VI)
0.1034
0.4367
0.0654
0.9481
0.1111
0.254
0.0713
0.2682
0.0320
0.9328
0.03876
0.1445
Fe (III)
0.2030
0.3504
0.0707
0.9568
0.0217
0.0619
0.0356
0.2641
0.0329
0.9363
0.05221
0.1976
Pseudo-second-order kinetics
qeexp (mg/g)
qecal (mg/g)
k2 (g.mg−1mn-1)
R2
SSE
χ2
qeexp (mg/g)
qecal (mg/g)
k2 (g.mg−1mn-1)
R2
SSE
χ2
Cr (VI)
0.0713
0.0729
1.679
0.999
2.56E-6
3.51E-5
0.0322
0.0582
0.0172
0.999
0.000676
0.0116
Fe (III)
0.2030
0.1334
0.526
0.998
0.00484
0.0362
0.3577
0.1394
0.0833
0.999
0.0476
0.1333
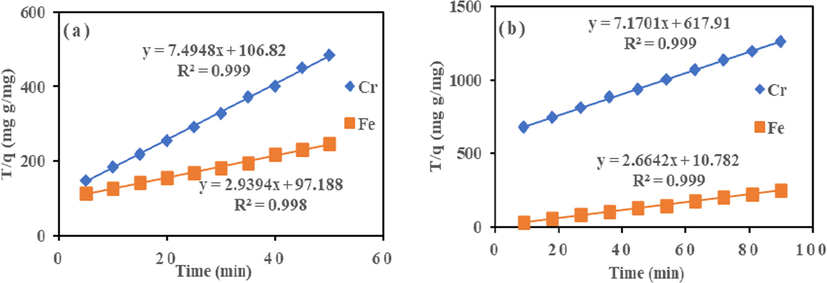
Pseudo-second order for adsorption of Cr (VI) and Fe (III) onto (a) HMC and (b) AMC.
3.7 Adsorption isotherms study
The Langmuir model Eq. (6) and Freundlich model Eq. (7) in their linear form were employed to analyze the isotherm data to remove Cr (VI) and Fe (III) by HMC and AMC. The isotherms plots shown in Figs. 9–12 and the calculated parameters are in Table 4. High regression coefficient (R2) values showed that Langmuir isotherm had a better fit than Freundlich. The adsorption occurred on a monolayer surface for all identical sorption sites (Rao and Uddin, 2014). The highest removal recorded for Cr (VI) and Fe (III) are 18.15 and 39.80 mg/g (HMC), and 10.42 and 19.34 mg/g (AMC), this suggests HMC has adsorptive advantage than AMC. The RL values show that HMC has a greater affinity for the two metals than AMC. The adsorption is favourable because all the RL obtained lies between zero and one (0 < RL < 1) (Ayari et al., 2019, Goher et al., 2015).
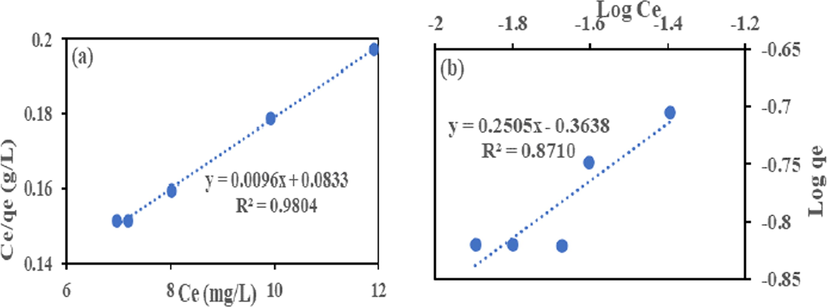
(a) Langmuir and (b) Freundlich Isotherm for Cr (VI) adsorption onto HMC.
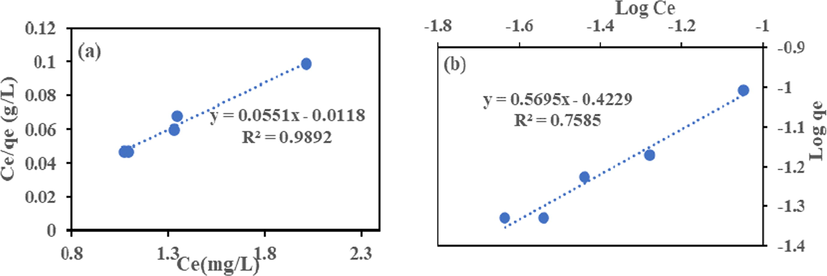
(a) Langmuir and (b) Freundlich Isotherm for Fe (III) adsorption onto HMC.
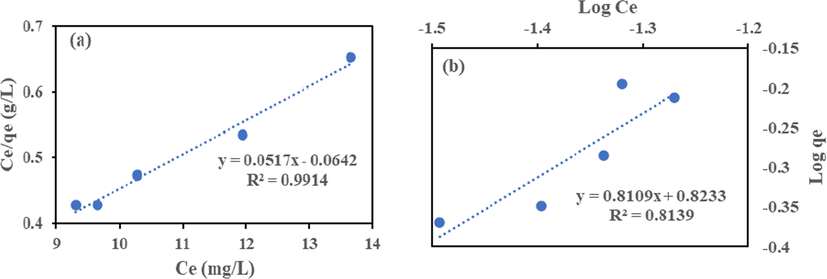
Langmuir (a) and Freundlich (b) Isotherm for Cr (VI) adsorption onto AMC.
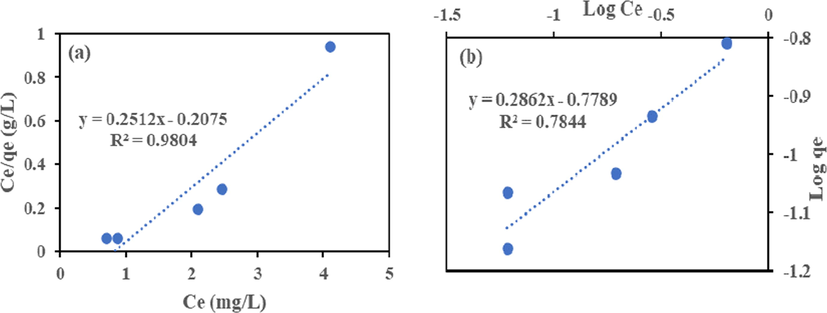
Langmuir (a) and Freundlich (b) Isotherm for Fe (III) adsorption onto AMC.
Isotherm Parameter
HMC
AMC
Cr (VI)
Fe (III)
Cr (VI)
Fe (III)
Langmuir
qm(mg/g)
18.15
39.80
10.42
19.34
KL (L/mg)
0.056
0.028
0.961
0.788
RL
0.984
0.979
0.788
0.979
R2
0.9804
0.9892
0.9914
0.9804
SSE
0.01137
0.00045
0.0179
0.0073
χ2
0.03281
0.00132
0.1296
0.0190
Freundlich
KF (mg/g)(L/g)
1.526
7.264
1.439
2.278
n
1.76
0.356
3.991
1.233
R2
0.8710
0.7585
0.8139
0.7844
SSE
0.0135
0.00345
0.0288
0.0097
χ2
0.03782
0.0141
0.9936
0.0217
The value of separation factor (RL) indicates the effectiveness of adsorbent, and this is defined as represented in Eq. (8),
3.8 Error analysis
The fitting for best isotherm and kinetic model calculated by analyzing the regression coefficient (R2). Nevertheless, the models' linearization has resulted in inherent bias to confirm the good fit (R2) of models. We employed error functions, chi-square test (χ2) and sum square error (SSE) to confirm the best-fit isotherm and kinetic models using Eqs. (9) and (10) (Batool et al., 2018). The experimental value is expressed as qe, exp, the calculated value as qe, cal, and the number of data points (n).
Beside higher R2 (Table 4 and Figs. 9 and 10), the confirmation of isotherms and kinetic models using error analysis was investigated. The lowest SSE and χ2 value supported the R2 values (Table 4). The value further suggests that the Langmuir model is very suitable (Akpomie and Dawodu, 2015).
A comparison of clay materials and their adsorption capacity for Fe (III) and Cr (VI) ions, are presented in Table 5. In Table 5, the Langmuir value for HMC and AMC compared with prior studies in the literature. It showed that both HMC and AMC displayed high adsorption capacity to signify improvement and suitability to remove heavy metals from wastewater.
Adsorbent
Metal ion
Langmuir qm (mg/g)
References
HMC
Fe (III)
39.80
This study
AMC
Fe (III)
19.34
This study
Natural clay
Fe (III)
5.7
El-Maghrabi and Mikhail, 2014.
Brown algae
Fe (III)
60.67
Benaisa et al., 2016
Metal oxide nanocomposite
Fe (III)
22.03
Langeroodi et al., 2018
Acid activated clays
Fe (III)
30
Bhattacharyya and Gupta, 2006b.
Natural feldspar
Fe (III)
25
Al-Anber, 2015.
Zeolite
Fe (III)
10
Bakalár et al., 2020
Bentonite
Fe (III)
14
Bakalár et al., 2020
HMC
Cr (VI)
18.15
This study
AMC
Cr (VI)
10.42
This study
Purified carbon naontube
Cr (VI)
35.60
Hamzat et al., 2019
Leucocephala seed pod AC
Cr (VI)
26.94
Yusuff, 2019
Date press cake
Cr (VI)
28.28
Norouzi et al., 2018
Mango kernel
Cr (VI)
7.8
Rai et al., 2016
Natural material
Cr (VI)
15.67
Ksakas et al., 2015
Kaolin
Cr (VI)
11.60
Bhattacharyya and Gupta, 2006a.
Bentonite
Cr (VI)
48.83
Wanees et al., 2012.
Montmorillonite
Cr (VI)
28.9
Gupta and Bhattacharyya, 2012.
3.9 Thermodynamic study
The thermodynamic parameters depend on the reaction process, and they can help in the detailed study of adsorption process mechanism. This experimental study considered different temperature values. The enthalpy (ΔHo) in kJ mol−1, entropy (ΔSo) in kJ mol−1 K−1 and standard free energy (ΔGo) in kJ mol−1, for sorption of metals onto adsorbents was determined with Eqs. (11)–(13). Eq. (13) was applied to analyze ln KC against 1/T plot, the slope obtained as enthalpy (ΔH°) and intercept as the entropy (ΔS°) respectively.
The reaction's thermal conditions examined at different temperatures of 25, 30, 35, 40 and 45 °C. The value of enthalpy change (ΔHo) is positive, which indicates an endothermic process. The enthalpy of HMC is 26.3 and 36.1 kJ mol−1, and AMC is 66.7 and 27.9 kJ mol−1 respectively. This suggests that the interaction of metal ions with activated clay is endothermic (Bhattacharyya and Gupta, 2006a). The entropy change (ΔSo) is positive, as measured at 298 K for Cr (VI) and Fe (III), HMC (0.058 and 0.159 kJ mol−1 K−1) and AMC (0.239 and 0.154 kJ mol−1 K−1). This signifies a strong affinity of ions for adsorbent and a high degree of randomness. The value of Gibbs free energy (ΔGo) is negative, and it measures the spontaneity of a reaction process.
Table 6 shows that as temperature increased from 298 to 318 K, the free energy increased (become more negative) for both adsorbents. The increment indicates a favourable and energetically spontaneous reaction (Rao and Uddin, 2014). The analysis shows that the process was characterized by high randomness and spontaneous endothermic (Gupta and Bhattacharyya, 2012, Bhattacharyya and Gupta, 2012).
Adsorbent
Adsorbate
Temp (K)
ΔHo (kJ mol−1)
ΔSo (KJ mol−1 K−1)
ΔGo (kJ mol−1)
HMC
Cr (VI)
298
26.30292
0.057807
−0.9236
303
−1.21267
308
−1.5017
313
−1.7907
318
−2.0798
Fe (III)
298
36.0811
0.159155
−11.3471
303
−12.1428
308
−12.9386
313
−13.7344
318
−14.5302
AMC
Cr (VI)
298
66.664
0.239359
−4.6643
303
−5.8611
308
−7.05789
313
−8.25468
318
−9.45148
Fe (III)
298
27.8884
0.15356
−0.15356
303
−0.27436
308
−0.57408
313
−0.87379
318
−1.17351
3.10 Desorption study
The reusability and stability of HMC and AMC are essential in terms of large-scale application. Table 7 shows that the amount of Cr (VI) and Fe (III) removed after first and second cycle decreased slightly, but decreased significantly after the second cycle for the adsorbents. The reduction in efficiency is probably due to the adsorption process's repeated desorption process, which decreased its adsorption capacity (Yussuf, 2019). The maximum desorption efficiency recorded on HMC is 92.45 and 85.67% for Cr (VI) and Fe (III) and for AMC is 76.58 and 65.32% for Cr (VI) and Fe (III). The results show HMC and AMC's recyclability potential in removing Fe (III) and Cr (VI) ions from real wastewater. The process is economical because it provides an avenue for safe disposal or reuse of adsorbent (Fernández-Pazos et al., 2013).
Desorption efficiency (%)
Adsorbents
Adsorbates
Cycle 1
Cycle 2
Cycle 3
Cycle 4
HMC
Cr (VI)
92.45
85.46
64.24
48.54
Fe (III)
86.32
80.87
59.76
42.43
AMC
Cr (VI)
76.58
65.46
44.23
40.45
Fe (III)
65.32
58.67
41.97
39.89
4 Conclusions
In this study, the effect of hydrochloric acid and acetic acid on the locally sourced clay has been investigated successfully. The BET analysis indicates an increase in surface area and pore volume after treatment. The surface area increased from 84.223 m2/g for (RC) to 389.37 m2/g (HMC) and 319.955 m2/g (AMC), total pore volume increased to 0.2168 and 0.2285 cm3/g, respectively. The CEC value was 22.30 cmol/g (HMC) and 20.73 cmol/g (AMC). These results show similarity with XRF and SEM studies which shows disintegration and a porous structure of the treated clay. XRD study of the treated clay also shows changes in the intensity of the bands. Langmuir isotherm conformed best for both adsorbate on HMC and AMC adsorbent. The high regression coefficient (R2) value shows the pseudo-second-order model as the better fit. Also, the lowest value of SSE and χ2 supported the claim. The Langmuir adsorption capacity of Cr (VI) and Fe (III) for HMC was 18.15 and 39.80 mg/g, for AMC was 10.42 and 19.34 mg/g. The maximum desorption efficiency recorded on HMC is 92.45 and 85.67% for Cr (VI) and Fe (III) and for AMC is 76.58 and 65.32% for Cr (VI) and Fe (III). The process is important because it provides an avenue for safe disposal or reuse of adsorbent. Acid modified clay has the potential to treat heavy metal contaminated wastewater.
Acknowledgements
The authors will like to appreciate the following for their contribution in sample analysis: Dr François Cummings (SEM/EDS, Physics Department), University of Western Cape (UWC), South Africa, Dr Remy Bucher (XRD, ithemba Labs), and Prof. S. J. Moir (XRF, Scientific Services CC), Cape Town, South Africa.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rapid removal of noxious nickel (II) using novel γ-alumina nanoparticles and multiwalled carbon nanotubes: kinetic and isotherm studies. J. Mole. Liquids. 2016;224:618-623.
- [Google Scholar]
- A simple spectrophotometric method for the determination of iron (II) aqueous solutions. Turk. J. Chem.. 2009;33:709-726.
- [Google Scholar]
- Treatment of an automobile effluent from heavy metals contamination by an eco-friendly montmorillonite. J. Adv. Res.. 2015;6:1003-1013.
- [Google Scholar]
- Adsorption of ferric ions onto natural feldspar: kinetic modelling and adsorption isotherm. Int. J. Environ. Sci. Technol.. 2015;2:139-150.
- [Google Scholar]
- Treatment of anionic dye aqueous solution using Ti, HDTMA and Al/Fe pillard bentonite. Essay to regenerate the adsorbent. J. Saudi Chem. Soc.. 2019;23:294-306.
- [Google Scholar]
- Removal of Cd (II) by adsorption on agricultural waste biomass. Der Pharma Chemica.. 2016;8(12):61-67.
- [Google Scholar]
- Adsorption of cadmium from aqueous solution onto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J. Hazard. Mater.. 2010;184:126-134.
- [Google Scholar]
- Characterization of Fe (III) adsorption onto zeolite and bentonite. Int. J. Environ. Res. Public Health. 2020;17:5718-5727.
- [Google Scholar]
- Using natural clays and spent bleaching clay as a cheap adsorbent for the removal of phenol in aqueous media. Int. J. Basic Appl. Sci.. 2013;13:45-49.
- [Google Scholar]
- Thermodynamics of removal of cadmium by adsorption on Barley husk biomass. Der Pharma Chemica.. 2016;8(10):243-247.
- [Google Scholar]
- Isotherms and thermodynamics of cd (II) ion removal by adsorption onto azolla filiculoides. Int. J. Pharm. Technol.. 2016;8(3):15780-15788.
- [Google Scholar]
- Acid activation of a polygorskite with HCl: Development of physic-chemical textural and surface properties. Appl. Clay Sci.. 1995;10:247-258.
- [Google Scholar]
- Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: an overview of linear and nonlinear approach and error analysis. Bioinorganic Chem. Appl.. 2018;2018 Article ID 3463724, 11 pages
- [CrossRef] [Google Scholar]
- Chromium biosorption from aqueous environments by mucilaginous seeds of Cydonia oblonga: kinetic and thermodynamic studies. Global NEST J.. 2017;19(2):269-277.
- [Google Scholar]
- Biosorption of Fe (III) from aqueous solution using brown algae Sargassum Vulgare. J. Mater. Environ. Sci.. 2016;7(5):1461-1468.
- [Google Scholar]
- CEC of clays: Measurement by adsorption of a copper ethylendiamine complex. Appl. Clay Sci.. 1997;12:275.
- [Google Scholar]
- Adsorption of a few heavy metals on natural and modified kaolinite & montmorillonite. A review. Adv. Colloid Interface Sci.. 2012;140:114-131.
- [Google Scholar]
- Adsorption of Fe (III), Co (II) and Ni (II) on ZrO kaolinite and ZrO-montmorillonite surfaces in aqueous medium. Colloids Surf. A. 2008;317:71-79.
- [Google Scholar]
- Adsorption of chromium (VI) from water by clays. Ind. Eng. Chem. Res.. 2006;45:7232-7240.
- [Google Scholar]
- Adsorption of Fe (III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption. 2006;12:185-204.
- [Google Scholar]
- Removal of heavy metals by adsorbents prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol.. 2004;35:11-22.
- [Google Scholar]
- Adsorption studies of Cr (III) ions from aqueous solutions by DEHPA impregnated onto Amberlite XAD7-factorial design analysis. Chem. Eng. J.. 2012;90:1660-1670.
- [Google Scholar]
- Kinetic and thermodynamic study of Adsorption of Cu (II) and Cr (VI) ion from industrial effluent onto kaolinite clay. J. Chem. Technol. Metall.. 2020;55:1057-1067.
- [Google Scholar]
- Chemically modified nanoparticles usage for removal of chromium from sewer water. Environ. Nanotechnol. Monit. Manage.. 2020;14:103-110.
- [Google Scholar]
- The effect of hydrochloric acid on the surface area, morphology and physico-chemical properties of Sayong Kaolinite clay. Key Eng. Mater.. 2014;594:49-56.
- [Google Scholar]
- Removal of heavy metals via adsorption using natural clay material. J. Environ. Earth Sci.. 2014;4:38-46.
- [Google Scholar]
- Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour. Industry.. 2015;9:16-29.
- [Google Scholar]
- An investigation of Cu (II) adsorption by raw and acid activated bentonite. A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazardous Mater.. 2009;151:682-691.
- [Google Scholar]
- Cr (VI) adsorption and desorption on soils and biosorbents. Water Air Soil Pollut.. 2013;224:1366.
- [CrossRef] [Google Scholar]
- Removal of aluminium, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H, Egypt. J. Aquat. Res.. 2015;41:155-164.
- [Google Scholar]
- Adsorption of heavy metals on kaolinite and montmorillonite: a review. PCCP. 2012;14:6698-6723.
- [Google Scholar]
- Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci.. 2013;193:24-34.
- [Google Scholar]
- Molecular aspects of clay-water interactions, in the clay-water interface and its rheological implications. In N. Güven, Clay Mineral. Society. Workshop Lecture. 1992;4:1-79.
- [Google Scholar]
- Adsorption studies on the treatment of battery wastewater by purified carbon nanotubes (P-CNTs) and polyethylene glycol carbon nanotubes (PEG-CNTs) J. Environ. Sci. Health, Part A 2019
- [CrossRef] [Google Scholar]
- Modelling of cadmium (II) adsorption on kaolinite-based clays in the absence and presence of humic acid. Appl. Clay Sci.. 2006;32:232-244.
- [Google Scholar]
- Efficient removal of ferric ions from aqueous medium by amine modified chitosan resins. J. Environ. Chem. Eng.. 2013;15:66-573.
- [Google Scholar]
- Cyclic sequential removal of Alizarin Red S Dye and Cr (VI) Ions using wool as a low-cost adsorbent. Processes. 2020;8:556-564.
- [CrossRef] [Google Scholar]
- Komadel, P., Madejova, J., 2006. Acid activation of clay minerals. In: Bergaya, F., Theng, B.K.G., Lagalay, G. (Eds.), Developments in clay science: Handbook of clay science, vol. 1. Netherlands: Elsevier, Oxford. pp. 263–287.
- The adsorption of Cr (VI) from aqueous solution by natural materials. J. Mater. Environ. Sci.. 2015;6(7):2028-2037.
- [Google Scholar]
- Preparation and characterisation of acids and alkali treated kaolin clay. Bullet. Chem. React. Eng. Catal.. 2013;8(1):61-69.
- [Google Scholar]
- Optimisation of adsorption parameters for Fe (III) ions removal from aqueous solutions by transition metal oxide nanocomposite. Green Chem. Lett. Rev.. 2018;11(4):404-413.
- [Google Scholar]
- Removal of high-concentration Fe (III) by oxidized multiwall carbon nanotubes in a fixed bed column. Am. Chem. Sci. J.. 2016;10:1-9.
- [Google Scholar]
- Effect of acid activation on the de-tert-butylation activity of some Jordian clays. Clays Clay Miner.. 1999;47:481-486.
- [Google Scholar]
- Pillared clays and pillared acid-activated clay: a comparative study of physical, acidic and catalytic properties. J. Catal.. 1995;153:76-85.
- [Google Scholar]
- Characterisation of smectite rich clay soils: implication for groundwater defluoridation. South Afr. J. Sci.. 2016;112:1-8.
- [Google Scholar]
- Preparation, characterisation and Cr (VI) adsorption evaluation of NaOH activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol.. 2018;258:48-56.
- [Google Scholar]
- Comparative evaluation of clays from abakaliki formation with commercial bentonite clays for use as drilling mud. Afr. J. Environ. Sci. Technol.. 2015;9(6):508-518.
- [Google Scholar]
- A study on effects of ph, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. J. Environ. Technol.. 2016;24:585-594.
- [Google Scholar]
- Mercury removal from aqueous solution via functionalized mesoporous silica nanoparticles with the amine compound, Egypt. J. Pet.. 2019;28:289-296.
- [Google Scholar]
- Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. -Eff. Technol.. 2016;2:63-70.
- [Google Scholar]
- Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J. Mole. Liquid. 2015;207:39-49.
- [Google Scholar]
- Kinetics and isotherm studies of Cd (II) adsorption from aqueous solution utilising seeds of bottlebrush plant (Callistemon chisholmii) Appl. Water Sci. 2014
- [CrossRef] [Google Scholar]
- Removal of Cr (VI) from aqueous solution on seeds of Artimisia absinthium (novel plant material) Desalin. Water Treat.. 2014;54:3358-3371.
- [Google Scholar]
- Investigation of the thermodynamic, kinetic and equilibrium parameters of batch biosorption of Pb (II), Cu (II) and Ni (II) from aqueous phase using low-cost biosorbent. Al-Nahrain J. Eng. Sci.. 2017;201(1):298-310.
- [Google Scholar]
- Physical and catalytic characterisation of smectites from Boa-Vista, Paraiba, Brazil. Ceramica. 2003;49:146-150.
- [Google Scholar]
- Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag.. 2016;171:1-10.
- [Google Scholar]
- Removal of methyl Orange from water by Adsorption onto modified local clay (kaolinite) Phys. Chem.. 2016;6(2):39-48.
- [Google Scholar]
- Biosorption of Cr (VI) by coconut coir: spectroscopic investigation on the reaction mechanism of Cr (VI) with lignocellulosic material. J. Hazard. Mater.. 2012;179:160-165.
- [Google Scholar]
- Adsorptive resource recovery from human urine: system design, parametric considerations and response surface optimisation. Procedia Eng.. 2016;148:779-786.
- [Google Scholar]
- Removal of cadmium (II) and zinc (II) metal ions from binary aqueous solution by rice husk ash. Colloids Surf. A: Physicochem. Eng. Asp.. 2008;312:172-184.
- [Google Scholar]
- The removal of Mn (II) ions by Adsorption onto natural & activated Chilean zeolites. J. Miner. Eng... 2009;22:336-343.
- [Google Scholar]
- The adsorption of cationic dye from aqueous solution onto acid-activated. J. Hazard. Mater.. 2007;147:1056-1062.
- [Google Scholar]
- A review on the Adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J.. 2017;308:438-462.
- [Google Scholar]
- Studies on the acid activation of Brazilian smectite clays. Quim. Nova. 2001;24:345-353.
- [Google Scholar]
- Adsorption behaviour of Fe (II) and Fe (III) ions in aqueous solution on chitosan and crosslinked chitosan beads. Biore Technol.. 2005;96:443-450.
- [Google Scholar]
- Adsorption studies on the removal of hexavalent chromium-contaminated wastewater using activated carbon and bentonite. Chem. J.. 2012;2:95-105.
- [Google Scholar]
- WHO, 2017. Guidelines for Drinking-Water Quality, fourth ed., WHO, Press, Geneva.
- Equilibrium and kinetic of iron adsorption by eggshells in a batch system: Effect of temperature. Desalination. 2007;206:127-134.
- [Google Scholar]
- Adsorption of hexavalent chromium from aqueous solution by Leucaena leucocephala seed pod activated carbon: equilibrium, kinetic and thermodynamic studies. Arab J. Basic Appl. Sci.. 2019;26(1):89-102.
- [Google Scholar]
- Synthesis and characterisation of anthill-egg Shell-Ni-Co mixed oxides composite catalyst for biodiesel production from waste frying oil. J. Bioprod. Bio. Refin.. 2015;12:37-45.
- [Google Scholar]
- Equilibrium, kinetic and thermodynamic studies of the Adsorption of heavy metals from aqueous solution by thermally treated quail eggshell. J. Environ. Sci. Technol.. 2017;10(5):246-257.
- [Google Scholar]
- Separation and recovery of Zn, Fe and Mn in acid mine drainage. J. Central South Univ. Sci. Technol.. 2011;42:1858-1864.
- [Google Scholar]







