Translate this page into:
An asymmetric N-rim partially substituted calix[4]pyrrole: Its affinity for Ag(I) and its destruction by Hg(II)
⁎Corresponding author at: Thermochemistry Laboratory, Chemistry Department, University of Surrey, Guildford, Surrey GU2 7XH, UK. a.danil-de-namor@surrey.ac.uk (Angela F. Danil de Namor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new partially substituted calix[4] pyrrole derivative obtained by the introduction of three thioamide functionalities in the N-rim has been synthesised and fully characterised by 1H, 13C, HSQC, ROESY NMR and mass spectroscopy. Computer modelling suggested an alternate conformation which was confirmed through ROESY 1H NMR. The receptor interacts only with the silver cation as shown by 1H NMR. The strength of interaction is quantitatively assessed by titration calorimetry. N-rim modification eliminates the possibility of interaction with anions. Unlike calix[4] pyrrole derivatives obtained by the introduction of functionalities through the meso-position, addition of Hg(NO3)2 leads to the degeneration of the receptor as demonstrated by 1H NMR, FTIR and XPS analyses. This is for the first time reported. Molecular simulation studies show significant strain in the mercury bound ligand in bonds, angles, torsions leading to the destruction of the receptor. Given the negative environmental impact produced by the availability of silver ions in aquatic organisms, the fundamental studies indicate that this receptor offers potential applications for monitoring silver (ion selective electrode) or indeed as a decontaminating material for removing silver ions from water.
Keywords
Supramolecular chemistry
Asymmetric calix[4] pyrrole
Selectivity
Silver complexation
Thermodynamics
1 Introduction
It is well established that bioavailability of ionic silver leads to greater toxicity to aquatic organisms than any other metal ion with the exception of mercury (Luoma, 2008; Howe and Dobson, 2002). As far as the negative impact of silver compounds on health is concerned, this topic was addressed in a review article (Drake and Hazelwood, 2005) which also includes effects related to the type of silver salt. Research on nano-silver compounds has led to a great degree of innovation given the number of these products available in the market. However the environmental effect of these materials has been the subject of a number of papers and review articles (Panyala and Peña-Mendez, 2008; Marambio-Jones and Hoek, 2010; Levard et al., 2012; Ylioopisto, 2014). Particularly interesting is a critical review article (Levard et al., 2012) related to the transformations that silver nanoparticles undergo in the environment and their impact on stability and toxicity. Questions which need answers and suggestions for further research in this area were carefully addressed. Extensive attention was given to the removal and recovery of silver from industrial effluents (Bachiri et al., 1996; Aamrani et al., 1999; Gherrou and Kerdjoudj, 2001, 2002; Barakat 2011).
Being a soft acid, Ag(I) exhibits a high affinity towards soft bases such as sulfur (Lai et al., 1986; Casabo et al., 1991; Chung et al., 1997; Malinowska et al., 1994; Oue et al., 1989). Therefore, many efforts have been paid toward the extraction of silver using sulfur-containing materials such as thiacrown ethers (Dietze et al., 1989), thiourea based reagents (Zuo and Muhammed, 1990), triisobutyl phosphine sulfide (Baba et al., 1986), Cyanex 272, 303,301 (Sole et al., 1994), o-dibutyl phenylamino-phenylmethanethio phosphonate (Sevdic et al., 1990) and 5,11,17,23-tetra-tert-butyl[25,27 bis(diethylthiocarbamoyl)oxy]calix[4]arene (Danil de Namor and Pawlowski, 2011).
There are two papers (Kretz et al., 1994; Jacoby et al., 1991) published in the early nineties which investigated the complexation of deprotonated meso octyl methyl calix[4]pyrrole with the iron cation. However most of the reported calix[4]pyrrole derivatives are anion receptors (Danil de Namor and Shehab, 2003, 2006) except those with pendent arms containing sulphur donor atoms (Danil de Namor, 2007a) or those in which one or two pyrrole rings were replaced by thiophene units (Danil de Namor, 2007b). Calix[4]pyrrole derivatives designed to interact with metal cations particularly silver are few in the literature.
This paper reports fundamental studies (synthesis, structural and thermodynamic characterisation) of 21,22,23-tri(N,N-diethyl-thioamide)octamethylcalix[4]pyrrole, CPII (Fig. 1) and its interaction with silver (I) and mercury (II) cations.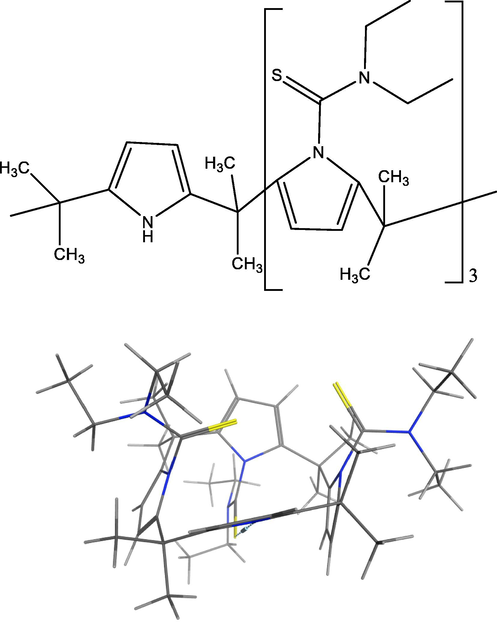
2D and 3D Structure of CPII (for 3D, (C = black, H = white, S = yellow and N = blue) showing an alternate conformation.)
2 Materials and methods
2.1 Chemicals
Pyrrole (C4H4NH, 99%), diethylthiocarbamoyl chloride ((C2H5)2NCSCl), 95%), 18-crown-6 (C12H24O6, 99%) [mercury (II) nitrate monohydrate (Hg(NO3)2·H2O, ≥99.99%), silver nitrate (AgNO3), ≥99%) and acetone (C3H6O), 99%) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl, 35–38%), acetonitrile (CH3CN, HPLC grade, 99.99%), ethanol (C2H6O, reagent grade, 99.98% v/v) and potassium carbonate (K2CO3, 99+ %) were obtained from Fisher Scientific. Salts used throughout the study were placed in a vacuum oven and then stored in vacuum desiccators over phosphorus pentoxide, P4O10 for several days to remove water, before being used for experimental purposes. Deuterated solvents used in NMR experiments, acetonitrile‑d3 (CD3CN, 99.8%) and chloroform-d (CDCl3, 99.8%) + 0.05% v/v TMS) were purchased from Cambridge Isotope Laboratories. For acetonitrile purification, the solvent was collected from PureSolv Micro solvent purification system (Inert Technology Inc. MA, USA) in a single-necked round-bottom flask containing activated 4 Å molecular sieves.
2.2 Synthesis and characterization of calix[4]pyrrole, (meso octamethylcalix[4]pyrrole), CP
The synthesis of meso-octamethylcalix[4]pyrrole was originally reported by (Baeyer, 1886). The procedure used in this work was later modified by (Danil de Namor and Shehab, 2003) and is described in Scheme 1.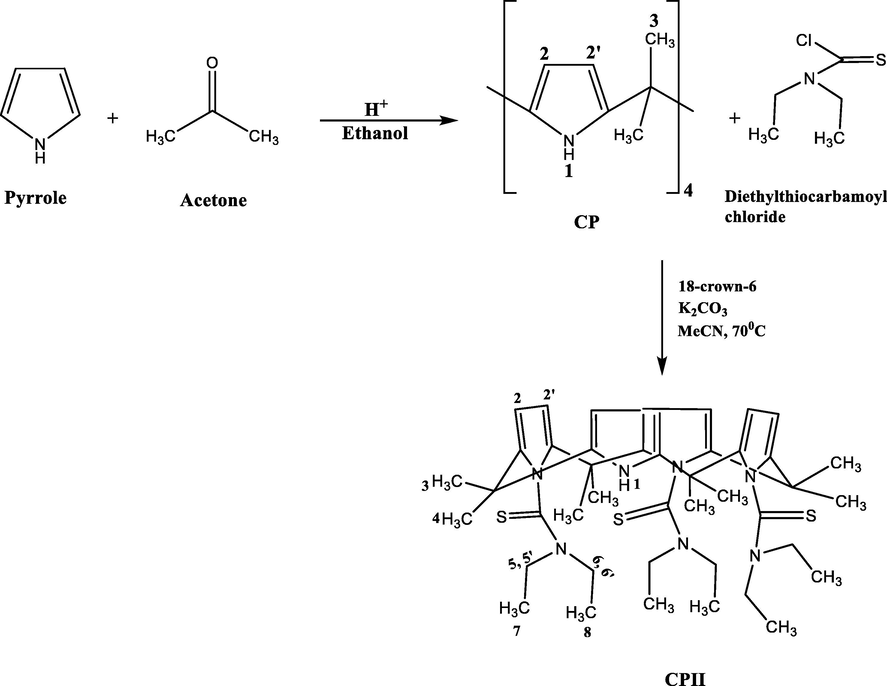
Synthetic procedure used in the preparation of CPII.
In a 250 cm3 round-bottomed flask, pyrrole (11 g, 0.149 mol) was suspended in absolute ethanol (50 cm3) for few minutes. The flask was then placed in an ice bath. An aqueous solution of hydrochloric acid (3 cm3, 37%) was added drop wise to the stirred content followed by acetone (8.7 g, 0.149 mol). Acetone addition was made over a period of 20 min. The formation of the solid was observed after complete addition of acetone where stirring was stopped. After standing at room temperature for 1 h, the product was washed with cold water (50 cm3). The solid was filtered, washed with ethanol and recrystallized from acetone. The white powder was dried under vacuum at 90 0C.
1HMR (500 MHz, CDCl3, 298 K, TMS): δ (ppm) = 7.13 (s broad, 4H; NH), 5.88 (s, 4H; ArH), 5.89 (s, 4H; ArH),1.5 (s, 24H; CH3). Microanalysis of meso-octamethylcalix[4]pyrrole was carried out in duplicate at the University of Surrey and the observed result was in good agreement with the calculated value: (C28H36N4) Mw. (428.612). Calculated %: C = 78.50, H = 8.41, N = 13.08, Found %: C = 78.90, H = 8.63, N = 12.96.
2.3 Synthesis of 21,22,23–tri(N,N-diethyl thioamide)octamethylcalix[4]pyrrole calix[4]pyrrole, CPII
The procedure used in the synthesis of CPII is described below (Scheme 1).
In a three necked round-bottomed flask (500 cm3), meso-octamethylcalix[4]pyrrole (CP) (0.98 g, 2.3 mmol) 18-crown-6 (0.5 g, 1.9 mmol) and potassium carbonate (7.5 g, 54.3 mmol) were mixed in dry acetonitrile (150 cm3) under a nitrogen atmosphere. This was followed by dropwise addition of diethylthiocarbamoyl chloride (7.58 g, 50 mmol) previously dissolved in dry acetonitrile. Then the reaction was refluxed at 70 0C for 17 h. In the meantime, the progress of the reaction was monitored by TLC using a dichloromethane/methanol/ethyl ethanoate mixture (9:0.5:0.5) as the developing solvent. After that period, the reaction was stopped and left to cool down at room temperature. Acetonitrile was removed under reduced pressure. The solid was dissolved in dichloromethane, and extracted several times with water saturated with sodium bicarbonate. The separated organic phase was dried with magnesium sulphate, then filtered. The solvent was then evaporated in vacuo to give an oily product. The oil was dissolved in cold methanol. The obtained solid was filtered and washed with cold methanol. The precipitate was collected and dried in a vacuum oven at 60 °C. (70% yield). The product was characterised by 1H NMR and 13C NMR (500 MHz) at 298 K.
1H NMR (500 MHz, CDCl3, 298 K, TMS): δ (ppm) = 9.27 (s, 1H; NH), 5.49 (s, 4H; pyrrole-H), 5.48 (s, 4H; pyrrole-H), 4.47 (q, 3H; CSNCH’2CH’3), 3.68 (q, 3H; CSNCH2CH3), 3.37 (q, 3H; CSNCH2CH3), 3.83 (q, 3H; CSNCH’2CH’3), 1.31 (t, 9H; N-CH2-CH3), 1.16 (t, 9H; N-CH2′-CH3′), 1.55 (s, 12H; CH3); 1.68 (s, 12H; CH3).
1H NMR (500 MHz, CD3CN, 298 K, TMS): δ (ppm) = 9.18 (s, 1H; NH), 5.48 (s, 4H; pyrrole-H), 5.47 (s, 4H; pyrrole-H), 4.39 (q, 3H; CSNCH’2CH’3), 3.48 (q, 3H; CSNCH2CH3), 3.30 (q, 3H; CSNCH2CH3), 3.78 (q, 3H; CSNCH’2CH’3), 1.29 (t, 9H; N-CH2-CH3), 1.18 (t, 9H; N-CH2′-CH3′), 1.57 (s, 12H; CH3), 1.67 (s, 12H; CH3).
13C NMR (100 MHz, CDCl3, 298 K, TMS): δ (ppm) = 200 (C10), 140 (C1), 130 (C1′), 120.5 (C1′’), 100 (C2), 47.7 (C6), 45.3 (C5), 36.5 (C9), 29 (C4), 27.6 (C3), 13.5 (C7), 10.4 (C8). (See SI for C labelling).
FAB MS calculated for C43H63N7S3: 774.21; Found 775.2 (M+H+).
2.4 1H NMR complexation studies on the interaction of CP (II) with metal ions at 298 K
1H NMR complexation experiments were conducted at 298 K on a Bruker DRX-500 pulse Fourier Transform NMR Spectrometer by dissolving the ligand (CPII) (1-5x10−4 mol·dm−3) in deuterated CD3CN, then adding a known amount of metal cation salt (1–2 × 10−3 mol·dm−3) into an NMR tube using TMS as an internal standard. Chemical shift changes (Δδ, ppm) were calculated by subtracting the chemical shift of the free ligand (reference spectrum) from the chemical shift of the complex.
2.5 Calorimetric titration experiments
Calorimetric titration experiments were carried out using Nano ITC2G calorimeter instrument, model 5300, TA Co. The ligand (CPII) and the salt were prepared in an anhydrous acetonitrile at 298.15 K. The measurement cell and the syringe were rinsed with freshly distilled solvent and CPII solution three times prior to ligand loading. The reference cell was filled with the solvent (acetonitrile) and the measurement cell was loaded with the ligand solution (950 µl, 1 mmol dm−3). The injection syringe was filled with the silver nitrate solution (250 µl, 20 mmol dm−3) and the equipment was operated to make twenty five to thirty five incremental additions (5 µl each) for each run with an injection interval of 300 s. During the guest addition to the ligand, the heat of dilution was recorded. Heat was either released (exothermic) or absorbed (endothermic) to the system. This process continued until all the binding sites of the ligand were saturated by the guest solution. Consequently, the heat signal was diminished until the heat of dilution background was noticed. Afterward, data were analysed using the appropriate model system (independent or multiple sites model) provided by the NanoAnalyseTM software.
2.6 Scanning electron microscopy and energy dispersive X-ray analysis (SEM-EDX)
A scanning electron microscope (SEM) JEOL JSM-7100F, equipped with secondary and backscattered imaging coupled with Ultradry energy dispersive X-ray (EDX) was used for morphological investigations and elemental analysis. Samples of CP II and CPII treated with the investigated metal ions were mounted on aluminum stub. For electron microscope imaging, samples were coated with a thin film of gold. Working conditions were 15 KeV for accelerating voltage and 10 mm for the detector working distance.
2.7 Analyses of CP II loaded with Hg (II) precipitate
The obtained precipitate (CP II with Hg (II)) was analysed using Fourier transform infrared spectroscopy, Agilent Cary 600 Series FT-IR spectrometer with MKII Golden Gate Single Reflection ATR System. The infrared spectra were recorded by averaging 32 scans at a resolution of 4 cm−1 in the region of 500–4000 cm−1 and X-ray photoelectron spectroscopy (XPS) Thermoscientific Theta probe with a monochromatic (Al Kα X-ray source). The X-ray spot size was 400 µm, the pass energy (PE) used was 50 eV and a 0.1 eV step size. Data was processed with Thermo Avantage 5934 software. The characterisation of the precipitate is detailed under Results and Discussion Section.
2.8 Molecular simulation and modelling
The structure of CP II was modulated using MOE 2014.09 software (CCGI, 2014). Steric energies of the ligand and the complexes were evaluated by Merck Molecular Force Field parameters (MMFF94x).
The strain energy of the complex was calculated by using a potential energy function, which contains terms for the strain in the bonds, angles, torsion angles, non-bonded interactions and charge interactions. e.g. where: Etc.
Hence any deviation from the expected bond lengths, angles, etc. leads to strain. Complexes tend to be stable if they do not have any strain energy, those that do are unstable. The molecular mechanics modelling shows in this case that the complex has strained angles and torsions and hence will prefer to relieve this strain by reacting.
3 Results and discussion
Substitution of meso-octylmethyl calix[4]pyrrole has taken place at the N-rim position. However, a partial substitution has been attained due to the applied rate of the reaction (17 h) which led to the introduction of thioamide pendant arm into three pyrrolic NH. This partially substituted ligand was structurally characterised by 1H NMR, 13C NMR, HSQC, HMBC and ROESY NMR (Supplementary Material) this was followed by complexation studies with various metal cations and anions which are described in the following section.
3.1 1D and 2D NMR characterisation in deuterated chloroform at 298 K
1H NMR findings in deuterated chloroform (Supplementary Material, S1) show that the receptor has eight different sets of protons resonating in different environments. The signal displayed at 9.27 ppm (singlet) is assigned to H-1 which represents the NH pyrrole. The two singlets at 5.48 and 5.49 ppm correspond to the 1H NMR chemical shifts of the β-carbon of the pyrrole ring. The two singlets observed at 1.68 and 1.55 ppm are assigned to H-3 and H-4 corresponding to the methyl group located at the meso position between the pyrrole rings. However, the singlet at 1.61 ppm peak represents trace water in CDCl3 where it did not appear in the 1H NMR spectrum of the ligand in CD3CN. The four signals between 3.25 and 5.25 ppm correspond to H-5,H-5′ (3.68 & 4.47 ppm) and H-6,H-6′ (3.37 & 3.83 ppm). This indicates that the arms are not symmetrical as these protons resonate in different environments due to diastereotopic effect. The signals recorded at 1.16 and 1.31 ppm are assigned to H-7 and H-8 respectively.
As far as the 13C NMR is concerned, six quaternary carbons with two sp3 and four sp2 including a thioamide functionality at 200 ppm and another ones C1, C1′ and C1′’ at 140, 130 and 120 ppm respectively have been identified. In addition, signals attributed to four methyl [(CH3)] (29, 27, 13 and 10 ppm), two methylene (48 and 45 ppm) and one methine (100 ppm) have been also identified (Supplementary Material, S2).
The 1H–13C correlation (Supplementary Material, S3 & S4), was conducted to assign the protons to carbons in a straight forward manner.
The conformation of the ligand was investigated through ROESY 1H NMR (Supplementary Material, S5) where the spectrum revealed a correlation between the signals assigned to H-5, H-5′, H-6, H-6′, H-7 and H-8 whereas no correlation was found between these protons and H-1 indicating that the receptor adopts an alternate conformation as suggested from Molecular Simulation calculations (Fig. 1).
3.2 1H NMR complexation of CPII with Ag(I) and Hg(II) as nitrate salts in deuterated acetonitrile at 298 K
1H NMR measurements of CPII with alkali, alkaline-earth, transition and halide anions in CD3CN at 298 K were conducted to investigate the interactions of the ligand with these ion salts. Results revealed no interaction of the ligand with alkali, alkaline-earth, some transition metal cations and halide anions. The latter is attributed to the replacement of three hydrogen atoms of the N-rim by thioamide functionalities which eliminate the possibility of anion-receptor interactions. On the other hand, alkali and alkaline earth metal cations are hard cations which have a tendency to interact with receptors containing hard donor atoms.
The ligand showed strong interaction with the Ag(I) cation in CD3CN. The chemical shift changes of the ligand protons induced by the Ag(I) cation relative to corresponding values of CPII (free ligand) are listed in Table 1, 1H NMR spectra are shown in Supplementary Material (S6). Inspection of this Table shows that most of the ligand protons were remarkably deshielded (H-2, H-2′, H-5, H-5′, H-6, H-6′ & H-8) except for H-1 as it was shielded upon the addition of the Ag(I) cation. The deshielding in H-2 and H-2′ protons are attributed to the change in the ligand conformation. Moreover, the downfield shifts in H-5, H-5′, H-6 and H-6′ protons are attributed to the interaction of the Ag(I) cation with the thioamide moiety which suggests that sulphur donor atoms are the sites of cation-receptor interaction as suggested by Molecular Simulation studies (Fig. 2). [Mn+] = 1.10 × 10−3 mol·dm−3; V = 0.5 cm3. [CPII] = 2.17 × 10−4 mol·dm−3; V = 0.5 cm3.
δ (ppm)
H-1
H-2
H-2′
H-3
H-4
H-5
H-5′
H-6
H-6′
H-7
H-8
δ Ref
9.18
5.48
5.47
1.67
1.57
3.48
4.39
3.30
3.78
1.18
1.29
Ag(I)
−0.39
0.71
0.71
−0.05
−0.08
0.38
0.21
0.34
0.23
0.09
0.11
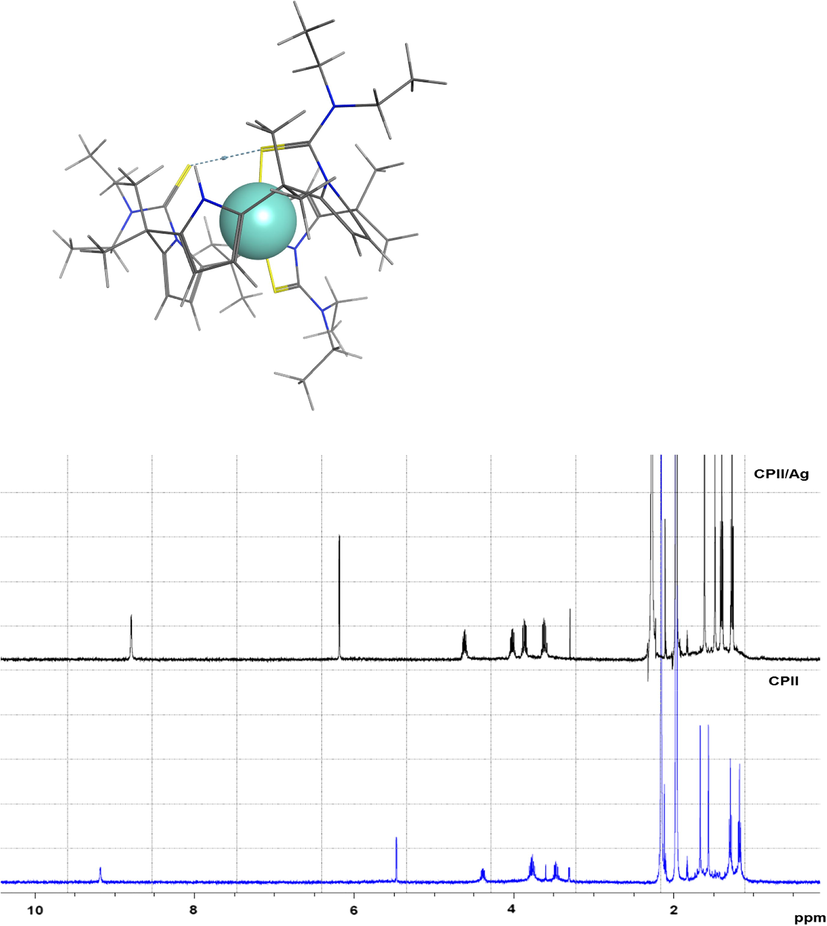
Stacked 1H NMR spectra of CPII and CPII/Ag(I) (as nitrate) in CD3CN at 298 K and molecular modeling of CPII with Ag(I) bound, in and stick rendering with Ag in Space filled; Representation (C = black, H = white, S = yellow and N = blue, Ag = light blue).
An unexpected result was observed upon addition of Hg(NO3)2 to the receptor where all aromatics along with most of ligand protons disappeared from the 1H NMR spectrum. Moreover, a dark brown precipitate was observed in the NMR tube. This was further investigated as discussed in the following sections.
Therefore, the findings reveal that CPII displays a high selectivity for the Ag(I) cation and these findings opens the need for future studies on its applications for the development of ion selective electrodes or indeed for its use as decontaminating material for the removal of silver (I) ion from water. Accordingly thermodynamic studies were carried out. The results are presented in the next section.
3.3 Thermodynamic parameters of complexation of CPII with Ag(I) as nitrate salts in acetonitrile at 298.15 K
CPII-Ag(I) ion complexation in acetonitrile at 298.15 K was further investigated calometrically. Thus stability constant (log Ks), derived standard Gibbs energy of complexation (ΔcG0), enthalpy (ΔcH0) and entropy (ΔcS0) values are listed in Table 2. These data are referred to the standard state for the reactants and the product of 1 mol dm−3. The thermodynamic data for CPII with Ag(I) cation fit into a 1:1 (ligand: metal cation) model.
log Ks
ΔcGo (kJ mol−1)
ΔcHo (kJ mol−1)
ΔcSo (J mol−1 K−1)
4.67 ± 0.01
−26.65 ± 0.06
−42.1 ± 0.1
−51
Inspection of Table 2 reveals that the complexation process of the ligand with Ag(I) cation in acetonitrile is enthalpically controlled ( as the ΔcH0 value indicates an exothermic complexation process. Moreover, unfavourable contribution of the entropy to the stability of the complex in acetonitrile was obtained. It is well established that a specific interaction takes place between the silver cation and acetonitrile due to the back bonding of the d electrons of the cation to the solvent (Popovych and Tomkins, 1981). This is clearly reflected in the Gibbs energy of transfer of the silver cation from water to acetonitrile ( tG˚ = −21.75 kJ·mol−1, data based on the Ph4As Ph4B convention) (Cox et al., 1974). However the receptor is able to compete successfully with the solvent for the cation and complexation takes place (Danil de Namor et al., 1998).
The unusual NMR results obtained by the addition of Hg(II) (nitrate as counter-ion) to the receptor in acetonitrile led to further investigations to get an insight into the nature of the problem given that this was not observed with other calix[4]pyrrole derivatives (Danil de Namor et al., 2015). This is detailed below.
3.4 SEM-EDX analysis of CPII with Ag (I) and Hg (II)
The scanning electron micrographs of CPII and complexes (CPII-Ag(I) and CPII-Hg (II)) showed a clear change in their morphological appearances (Fig. 3) which indicates a different interaction behavior of the receptor with the two investigated metal ions. This finding was supported with EDX analysis of the receptor and the complexes. The EDX spectrum (Fig. 3b) confirms the complexation of Ag (I) to CPII whereas the one for Hg (II) complex shows a significant reduction in the carbon peak and a disappearance of the sulfur peak compared to the control spectrum (Fig. 3a). The strange variation in the EDX spectrum of Hg (II) complex was further explored and is detailed in the following section.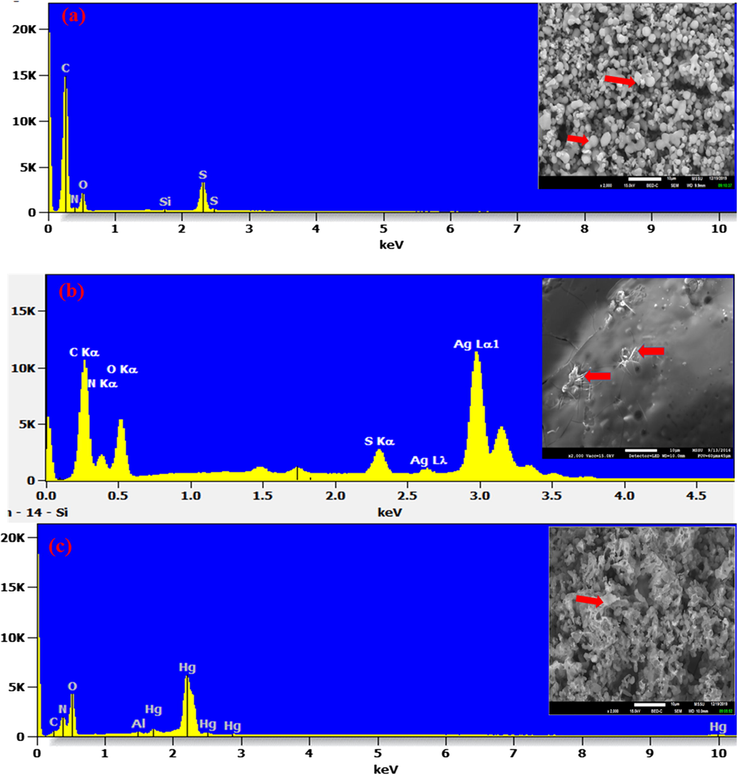
SEM images and EDX spectra showing the microstructure and the elemental composition of CPII (a); CPII treated with Ag (I) (b) and CPII treated with Hg (II) (c). Al peak referred to aluminum stub used for samples’ mounting.
3.5 Degeneration of CPII by Hg(NO3)2 in non-aqueous solvents
The precipitate obtained by the addition of Hg(II) nitrate to the receptor was investigated by 1H NMR, FTIR and XPS analyses, in a trial to gain more insights on the nature of interaction that led to the degeneration of the calix[4] pyrrole derivative.
1H NMR spectra of CPII upon the addition of Hg(NO3)2 relative to the free receptor in CD3CN (protophobic dipolar aprotic) and in CDCl3 (inert solvent) at 298 K are shown in Fig. 4. The effect of Hg(NO3)2 is clearly seen in the spectra of the ligand in both deuterated solvents where the signals corresponding to the NH pyrrole, the β-carbon of the pyrrole ring and the methyl group located at the meso position between the pyrrole rings disappeared from the spectra in addition to the characteristic signals of the thioamide moiety.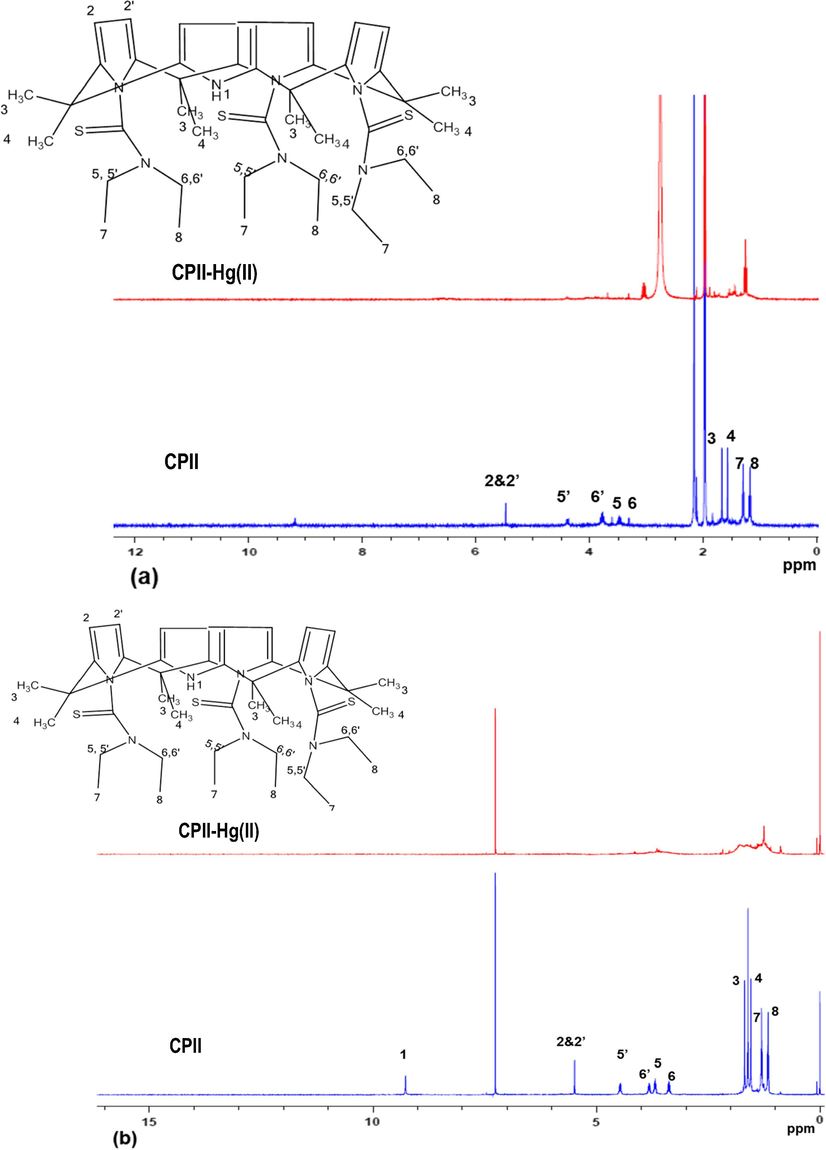
Stacked 1H NMR spectra of CPII and CPII treated with Hg(NO3)2 in CD3CN (a) and CDCl3 (b) at 298 K.
This finding was further investigated using ATR-FTIR analysis. Insets of CPII and CPII treated with Hg(NO3)2 are shown in Fig. 5. Inspection of both spectra reveals the disappearance of N-H stretching vibration at 3235 cm−1 and the C⚌C stretching mode of the pyrrole ring at 1584 cm−1 from the CPII treated with Hg(NO3)2 (Fig. 5b). Moreover, C—O stretching vibration at 1072 cm−1 is shown in Fig. 5b. However, this finding was further investigated by X-ray photoelectron spectroscopy (XPS). Although the technique deals with surface analysis, but its lateral resolution as well as real time imaging capability make it comparable to the FTIR technique.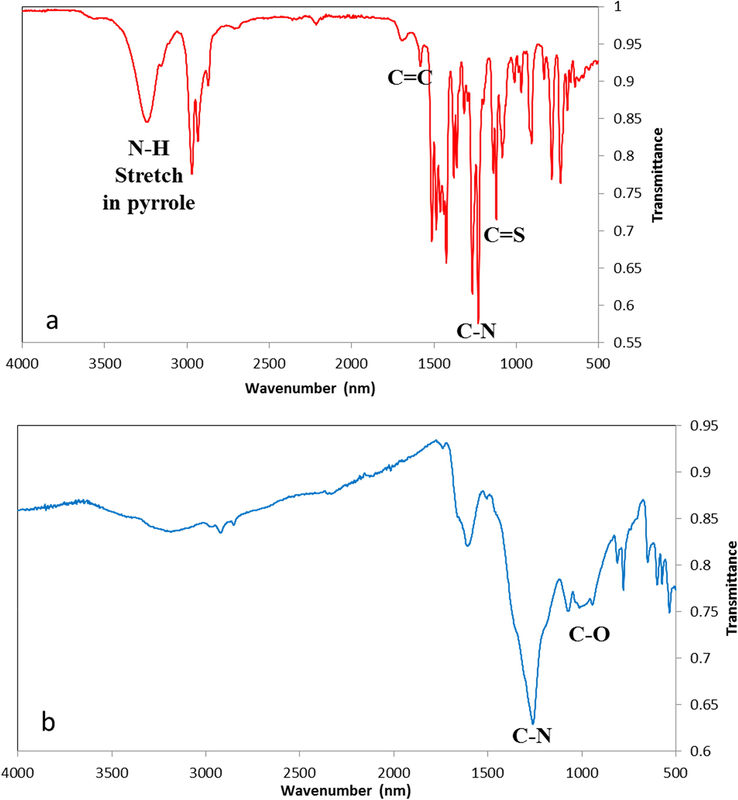
IR spectra of (a) CPII and (b) CPII treated with Hg(NO3)2.
XPS survey spectra recorded for C1s, N1s, O1s, Cl2p and Hg4f are displayed in Fig. 6a. Peak fitting of C1s revealed four signals at 285.01, 286.56, 288.06 and 289.47 eV. These four signals correspond to C—C (aliphatic carbon), C—O, C⚌O and COO− respectively. The carbon-oxygen compounds like C—OH, C⚌O, COOH in C1s are usually displayed in the XPS spectra as small signals with high binding energy (Yumitori, 2000). Aromatic carbons are absent from the C1s spectrum and that was confirmed by the 1H NMR analysis. N1s peak fitting showed six signals with centered binding energies at 398.51, 399.47, 400.84, 402.99, 404.46 and 406.64 eV which correspond to —N⚌ (N-imine), CN, bipolaron charge carrier species (⚌NH+—, NH3+) and NO3 respectively. The signal corresponding to NO3 at 406.64 eV is related to the nitrate ion that came from Hg(NO3)2 whereas the other five signals correspond to CPII treated with Hg(NO3)2. The O1s in the XPS spectrum revealed two signals with centered binding energies at 530.78 and 534.17 eV which correspond to C⚌O moiety in the structure. Regarding the Cl2p, the Cl signal at 200.77 eV is an impurity from the chloroform solvent. The Hg4f peak fitting revealed one signal at 101.61 eV that corresponds to the added Hg(NO3)2 to the receptor. A possible explanation for this outcome is that the proximity of the Hg(II) cation to the sulfur donor atoms of the receptor leads to a reduction of mercury (II) with oxidation of the C atoms of the pyrrole rings and subsequent degeneration. This statement is based on the reduction of molar conductance observed upon the addition of the receptor to the mercury (II) salt. In our knowledge this is the first example of the decomposition of calix[4]pyrrole derivative in the presence of a mercury (II) salt. This was not observed for with other calix[4] pyrrole derivative obtained by the introduction of functionalities through the meso-position (Danil de Namor et al., 2015).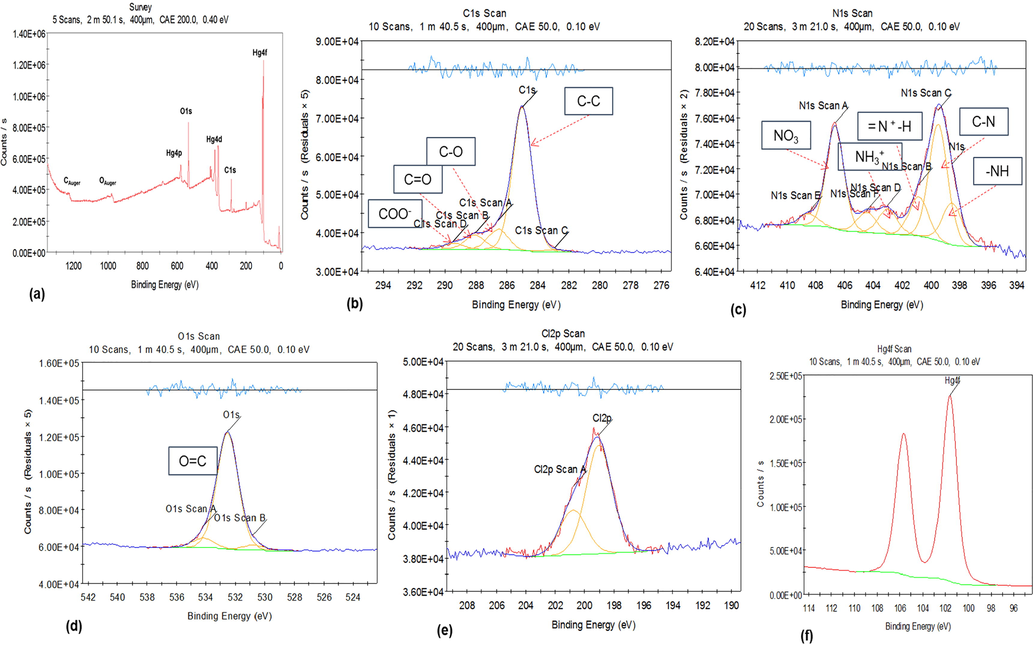
XPS spectra of (a) region spectroscopy (b) C1s, (c) N1s, (d) O1s, (e) Cl2p and (f) Hg4f of CPII treated with Hg(NO3)2. Data was processed using Thermo Avantage 5934 software.
Molecular simulation studies suggest distortions of five membered pyrrole rings ranging from C—N—C angles of 104 degrees as the pyrrole rings are not flat. Indeed the significant strain in mercury bound ligand in bonds, angles, torsions leads to the destruction of the receptor. The lowest energy conformation of the ligand was explored by low mode molecular dynamics and is shown in Fig. 7a. The lowest energy conformation has two sulfur atoms and the unsubstituted pyrrole nitrogen on one side of the calixarene and the other sulfur the ‘down’ position. Mercury was added to the two closest sulfurs and the resulting structure is shown in Fig. 7b. The angle strain is increased by 33 kJ/mol and the torsional strain is increased by 42 kJ/mol from the lowest energy conformation of the ligand on binding mercury.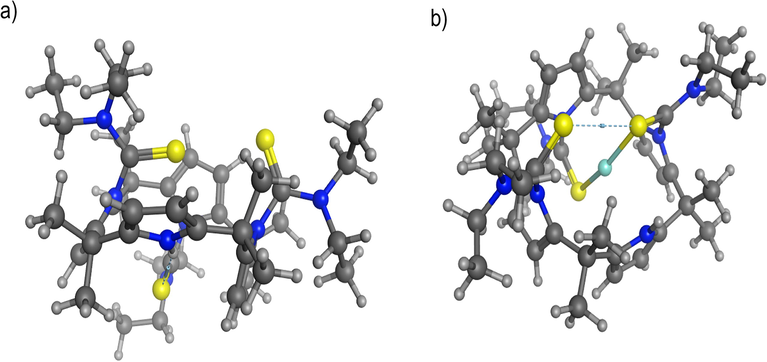
(a) Lowest energy conformation of the ligand after low mode MD search (energy = 1061 kJ/mol) in ball and stick rendering (C = black, H = white, S = yellow and N = blue). (b) CP II with mercury bound, also in ball and stick rendering (Hg = light blue) (energy = 1096 kJ/mol). Energies calculated using the MMFF94X force field.
4 Conclusions
The synthesis of new asymmetric calix[4]pyrrole derivative obtained by modification of meso-octylmethyl calix[4]pyrrole by the introduction of thioamide pendant arm functionality at the N-rim positions (CPII) have been achieved.
1H NMR measurements of this receptor show its interaction with Ag(I) cation in CD3CN at 298 K through the sulfur donor atoms of the thioamide moiety where significant downfield chemical shift changes were observed in these protons. On the other hand, the 1H NMR findings of this receptor with Hg(II) (as nitrate) cation in CD3CN and CDCl3 show a degeneration of the pyrrole rings as brownish colour precipitates were seen in the NMR tube. This case was further investigated by FTIR and XPS analyses of the precipitate; the XPS spectrum of the carbon (C1s) of the receptor suggests that the interaction with mercury (II) nitrate salt led to a redox reaction.
Fundamental studies show that this receptor has potential for the design of a silver ion selective electrode and indeed as a decontaminating agent for the removal of this cation from water. Work is now in progress.
Acknowledgements
The authors thank the Leverhulme Trust (Emeritus Fellowship awarded to AFDdeN) and Prof. John F Watts and staff for invaluable assistance in the use of the X-ray Photoelectron Spectroscopy, XPS equipment available in the Micro Structural Unit and Surface Analysis Laboratory (University of Surrey).
References
- Mechanistic study of active transport of silver(I) using sulfur containing novel carriers across a liquid membrane. J. Membr. Sci.. 1999;152:263-275.
- [Google Scholar]
- Extraction equilibrium of silver with triisobutylphosphine sulfide from nitrate media. J. Chem. Eng. Jpn.. 1986;19:27-30.
- [Google Scholar]
- Recovery of silver nitrate by transport across a liquid membrane containing dicyclohexano 18 crown 6 as a carrier. J. Membr. Sci.. 1996;121:159-168.
- [Google Scholar]
- Ueber ein Condensationsproduct von Pyrrol mit Aceton. Ber. Dtsch. Chem. Ges.. 1886;19:2184-2185.
- [Google Scholar]
- New trends in removing heavy metals from industrial wastewater. Arab. J. Chem.. 2011;4:361-377.
- [Google Scholar]
- Silver(I) ion-selective electrodes based on polythiamacrocycles. Chem. Soc. Dalton Trans. 1991:1969-1971.
- [Google Scholar]
- CCGI, M., 2014. Molecular Operating Environment (MOE), 2013.08. Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7.
- Sensor molecules for silver(i)-selective membranes based on mono to quadri-dentate podands. J. Chem. Soc. Chem. Commun. 1997:965-966.
- [Google Scholar]
- Solvation of Ions. XIX. Thermodynamic properties for transfer of single ions between protic and dipolar aprotic solvents. Aust. J. Chem.. 1974;27:477-501.
- [Google Scholar]
- Selective recognition of halide anions by Calix[4]pyrrole: a detailed thermodynamic study. J. Phys. Chem. B. 2003;107:6462-6468.
- [Google Scholar]
- New insights on anion recognition by isomers of a calix pyrrole derivative. J. Phys. Chem. B. 2006;110:12653-12659.
- [Google Scholar]
- A New Calix[4]pyrrole derivative and its anion (fluoride)/cation (mercury and silver) recognition. J. Phys. Chem.. 2007;111:3098-3105.
- [Google Scholar]
- Sulfur-containing hetero-calix[4]pyrroles as mercury(II) cation-selective receptors: thermodynamic aspects. J. Phys. Chem. B. 2007;111:5803-5810.
- [Google Scholar]
- A new calix[4]arene derivative and its ionic recognition for silver(I) and mercury(II): the solvent effect. New J. Chem.. 2011;35:375-384.
- [Google Scholar]
- A ditopic calix[4]pyrrole amide derivative: highlighting the importance of fundamental studies and the use of NaPh4B as additive in the design and applications of mercury(II) ion selective electrodes. J. Mater. Chem.. 2015;3:13016-13578.
- [Google Scholar]
- Complex formation and liquid-liquid extraction of silver with cyclic and open-chain oxathia alkanes. Solvent Extr. Ion Exch.. 1989;7:223-247.
- [Google Scholar]
- Exposure-related health effects of silver and silver compounds: a review. Ann. Occup. Hyg.. 2005;49:575-585.
- [Google Scholar]
- Effect of thiourea on the facilitated transport of silver and copper using a crown ether as carrier. Sep. Sci. Technol.. 2001;571:22-23.
- [Google Scholar]
- Specific membrane transport of silver and copper as Ag(CN)3 2- and Cu(CN)4 3- ions through a supported liquid membrane using K+-crown ether as a carrier. Desalination. 2002;151:87-94.
- [Google Scholar]
- Howe, P.D., Dobson, S., 2002. World Health Organisation & International Programme on Chemical Safety, Geneva (Switzerland).
- meso-Octamethyl-porphyrinogen metal complexes: an entry to high valent unsaturated metal centres. J. Chem. Soc., Chem. Commun. 1991:220-222.
- [Google Scholar]
- Silver complexation by sigma CH Bonds: interaction of silver with meso-octaethyltetraoxaporphyrinogen. J. Am. Chem. Soc.. 1994;116:10775-10776.
- [Google Scholar]
- Mercury (II) and silver (I) ion-selective electrodes based on dithia crown ethers. Analyst. 1986;111:891-895.
- [Google Scholar]
- Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol.. 2012;46:6900-6914.
- [Google Scholar]
- Luoma, S.N., 2008. Silver nanotechnologies and the environment: old problems or new challenges? Woodrow Wilson International Center for Scholars, Washington, D.C. (United States).
- Silver selective electrodes based on thioether functionalized calix[4]arenes as ionophores. N. Anal. Chim. Acta.. 1994;298:245-251.
- [Google Scholar]
- A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart.. 2010;12:1531-1551.
- [Google Scholar]
- Lipophilic thiacrown ether derivatives as neutral silver-ion selective carriers. J. Chem. Soc. Perkin Trans.. 1989;1:1675-1678.
- [Google Scholar]
- Silver or silver nanoparticles: a hazardous threat to the environment and human health. J. Appl. Biomed.. 2008;6:117-129.
- [Google Scholar]
- Nonaqueous Solution Chemistry. New York: John Wiley & Sons; 1981. p. :192.
- Solvent extraction of silver and mercury by O-dibutylphenylamino-phenylmethanethiophosphonate from chloride solutions. Solvent Extr. Ion Exch.. 1990;8:643-658.
- [Google Scholar]
- Solvent extraction of silver by cyanex 272, cyanex 302 and cyanex 301. Solvent Extr. Ion Exch.. 1994;12:1033-1050.
- [Google Scholar]
- Mater. Sci.. 2014;37:797-803.
- Correlation of C1s chemical state intensities with the O1s intensity in the XPS analysis of anodically oxidized glass-like carbon samples. J. Mater. Sci.. 2000;35:139-146.
- [Google Scholar]
- Extraction of gold and silver by thiourea-based reagents. Sep. Sci. Technol.. 1990;25:1785-1802.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.12.015.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







