An ecofriendly bleaching method for cashmere fiber with hydrogen peroxide and laccase in SCF-CO2 by avoiding heavy effluent discharge
⁎Corresponding author. longjiajie@suda.edu.cn (Jia-Jie Long)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
-
A novel and eco-friendly bleaching approach was developed for cashmere fiber production in SCF-CO2.
-
H2O2 combing with laccase was first time utilized for cashmere fiber bleaching in SCF-CO2.
-
PEG1000/SCF-CO2 system was used to transfer bleaching ingredients onto substrate fiber.
-
High efficiency in removal of various natural colorants on cashmere fiber was achieved.
-
Less integral damages to cashmere fiber were obtained by the developed SCF bleaching method.
Abstract
For the first time, we used laccase (LA) and hydrogen peroxide (H2O2) in supercritical fluid of carbon dioxide (SCF-CO2) to develop a method of bleaching cashmere fiber production in an ecofriendly way. The obtained results found that the whiteness of the cashmere was improved significantly involving a high bleaching efficient with this method, as well as influenced notably by the dosages of H2O2 and LA, bleaching temperature, pressure and duration. Various natural colorants on the cashmere sample were effectively decomposed and removed by the bleaching ingredients from the H2O2 and biologic catalyst of LA via an efficient transfer of those species onto cashmere surfaces from (H2O2 + LA+PEG1000 + H2O)/SCF-CO2 system. Particularly, this supercritical bleaching method with LA and H2O2 in SCF-CO2 reduced the number of damages to the cashmere fibers. Furthermore, we also successfully validated the supercritical removal of colorants by using X-ray energy spectroscopy (EDS) and scanning electron microscopy (SEM) analysis. Strength measurements and thermogravimetric (TG) and differential thermogravimetric (DTG) analysis demonstrate that supercritical bleaching had a reduced impact on the thermal and mechanical properties of the cashmere fiber. An optimized enzymatic-oxygen bleaching process in SCF-CO2 was further recommended by accompanying with a high whiteness value at 83.36 and an acceptable alkali solubility at 15.55 %. This developed supercritical bleaching method is prospective to be utilized for clear production of cashmere fibers by avoiding an extensive use of chemicals and the generation of heavy wastewater, as well as with less or non-effluent discharge.
Keywords
Bleaching
Cashmere fiber
Hydrogen peroxide
Laccase
Oxidative degradation
Supercritical carbon dioxide
1 Introduction
Cashmere fiber is a kind of special rare animal fibers. It has great commercial value in textile manufacturing industry due to its scarce yield and excellent characteristics such as softness and mild color (Tang, 2015; Ma et al., 2017; Li, 2005). In comparison with wool, cashmere fiber has a better strong elongation and superior moisture absorption, followed by perfect lightness, toughness, heat preservation, and elastic, etc (Yang, 2011). But as a natural material, cashmere fibers usually exhibit yellowish and other dark colors mainly due to their growth environment, and are inevitably affected by many factors to be stained and carry various impurities including different colorants. In order to ensure and meet the further process production needs, the cashmere fibers are necessary to remove those colorants, sediments and other impurities since they directly impact the physical and service properties, such as hygroscopicity, luster and drapability, etc, particularly for their whiteness (Zhu et al., 2020; Wei et al., 2013). Currently, the common methods to remove those colorants for improving the whiteness of cashmere or wool fibers are oxidative (Zhang and Zhang, 2016; Liu et al., 2011; Erdogan and Karaboyaci, 2021), reductive bleaching (He et al., 2005; Yilmazer and Kanik, 2009), or a combination of oxidative and reductive bleaching by employing various chemical oxidants and reductants (Tang et al., 2004; Cai et al., 2008). In addition, biological methods have also been successfully introduced to decompose those colorants for enhancing the substrate whiteness, which not only achieves low-temperature bleaching efficiency, but also receives good dyeing properties of the treated protein fibers (Sheng et al., 2000; Tan et al., 2008; Cao et al., 2019). Hydrogen peroxide (H2O2), as a common bleaching agent, is usually applied in various bleaching treatments for different kinds of textiles. However, most of the bleaching methods with H2O2 usually need to be carried out in a high temperature and high alkali reaction system, which easily lead to some serious problems, such as large energy consumption, heavy pollutions, undesirable fiber damages, and high costs (Xu, 2014).
Supercritical fluid of carbon dioxide (SCF-CO2) has both gas and liquid two-phase characteristics, can effectively diffuse into solid substrates for separation, and has various significant advantages such as no water consumption, zero emission, pollution-free (Bach et al., 2010; Yan et al., 2020; Zhao et al., 2023), etc., which has been widely used in textile industries (Seghini et al., 2020; Salem et al., 2021), as well as in polymers processing (Sobornova et al., 2022; Golubeva et al., 2019; Zhang et al., 2020), etc. Because reaction rates can be improved and pressure can be adjusted to control selectivity, SCF-CO2 is considered to be a suitable medium for the enzymatic reaction (Chrisochoou et al., 1995; Matsuda, 2013; Gao and Yang, 2015). According to published studies, amylase, laccase (LA), lipase, and pectinase can all maintain activity and stability under the correct pressure and appropriate temperature in an SCF-CO2 environment. These enzymatic materials are able to suitably perform numerous catalytic reactions with less water in a cleaner manner than those in a traditional aqueous bath (Shi et al., 2018; Zeljko, 2018; Maja et al., 2013). Researches to date have focused on analyzing the stability and activity of enzymes in an SCF-CO2 environment (Maja et al., 2013; Monhemi and Housaindokht, 2012; Peng et al., 2016) and in biocatalytic applications. Recently, for example, some enzymatic reactions have been applied during textile pretreatments to remove impurities in SCF-CO2 and have achieved beneficial results (Liu et al., 2016). However, very few work about the one-step bleaching of cashmere fibers with enzymes and H2O2 simultaneously presented in SCF-CO2 has been reported in literature, although it is a highly promising sustainable and green bleaching method.
In this study, we developed an enzymatic-oxygen process to bleach cashmere fibers using LA, H2O2, and polyethylene glycol 1000 (PEG1000) in SCF-CO2 in order to reduce or eliminate those heavy effluents discharged from conventional processes. We examined the impact of bleaching ingredients, including the dosage of LA, H2O2, PEG1000 and buffer solution pH, as well as system parameters such as bleaching temperature, duration and pressure, on the whiteness and the alkali solubility of cashmere fibers. We also used scanning electron microscopy (SEM), X-ray energy spectroscopy (EDS), thermogravimetric-differential thermogravimetric (TG-DTG) analysis, and fiber strength measurement to validate the supercritical enzymatic-oxygen bleaching method.
2 Experimental section
2.1 Materials
A semi-product of cashmere fiber washed by enzymatic process in SCF-CO2 in our previous work by involving a whiteness value at 72.28 and alkali solubility at 7.35 % was used for further bleaching in this work (Wang et al., 2024). We purchased 120 U g−1 of LA from Shan Ye Biology Co. Ltd (Sichuan, China). We purchased 30 % H2O2 from Yong Hua Chemical Technology Co. Ltd (Shanghai, China). Wujiang Guorong Gas Co. Ltd. (Wujiang, China) supplied industrial gas of CO2 (99.8 vol%). Other chemicals used in experiments were commercially available in an analytically pure grade, including citric acid, disodium hydrogen phosphate, PEG1000, and sodium hydroxide, etc.
2.2 Experimental equipment and instruments
The Waterless Coloration Team from Soochow University developed a supercritical bleaching system with a bleaching unit, as shown in Fig. 1. To complete this study, we also used the following instruments: (1) constant temperature oscillating water bath machine (YG871, Nantong Hong Da Experimental Instrument Co., Ltd., Nantong, China), (2) a numerical control ultrasonic instrument (KQ-50DB, Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China), (3) Ultrascan-PRO type spectrophotometer (Hunter Lab, Reston, VA, USA), (4) pH meters (C132-11, Sartorius Scientific Instruments Co., Göttingen, Germany), (5) cold field emission scanning electron microscope (S-4800, Hitachi, Tokyo, Japan), (6) universal material testing machine (Instron5967, American Instron Co., Ltd., Norwood, MA, USA), and (7) X-ray energy dispersive spectroscopy (TM3030, Hitachi), etc.
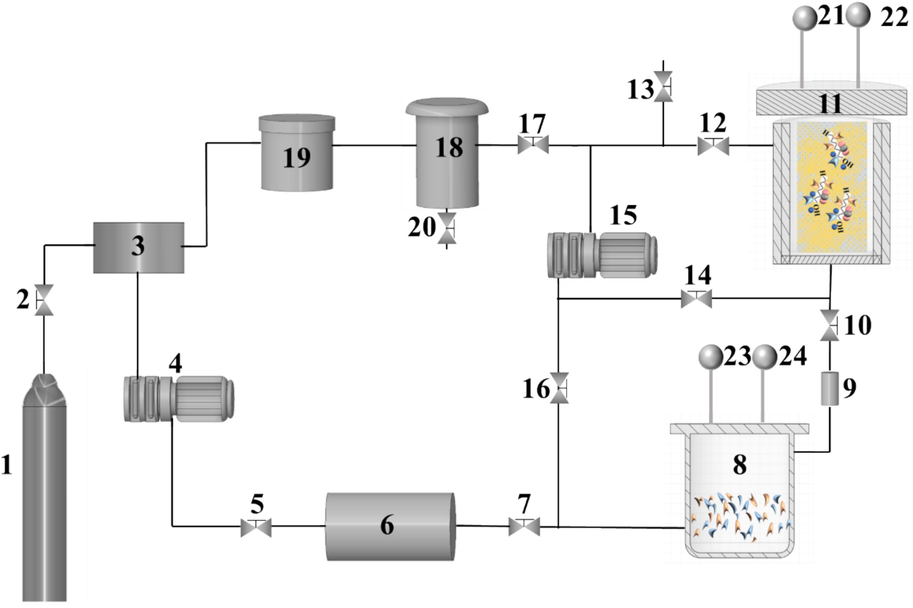
- Cashmere fiber bleaching system in supercritical CO2 equipped with the following subunits: (1) CO2 storage vessel; (2, 5, 7, 10, 12, 13, 14, 16, 17, 20) valves; (3) condenser; (4) pressurization pump; (6) heat exchanger; (8) auxiliary unit; (9) filter; (11) bleaching unit; (15) circulating pump; (18) separator; (19) purifier; (22, 24) pressure gage and (21,23) temperature gauge.
2.3 Bleaching procedure of cashmere fiber with H2O2 and LA in SCF-CO2
We used H2O2 and LA in a bleaching reactor that was internally installed in the bleaching unit (11), as shown in Fig. 1, to bleach the cashmere fiber samples in SCF-CO2. We used a buffered solution with specific dosages of citric acid and disodium hydrogen phosphate to prepare a bleaching working solution with a request volume of 30 % H2O2 and a quantitative dosage of LA and PEG1000. Then, we charged the working solution into the auxiliary unit (8) after all the additives were completely dissolved by ultrasonic treatment in the KQ-50DB ultrasonic instrument. Thereafter, we placed 3.0 g of fluffy cashmere in a sarong, which we then set it in the bleaching unit (11). The total system was sealed as the preparation work was completed. Then, we conducted the following bleaching procedure step by step. First, we slowly opened valves (2, 5, 7, 14, 16) in sequence. Second, we employed the pressurization pump (4) to charge liquid CO2 into the auxiliary unit (8) and the bleaching unit (11). Third, we used the heat exchanger (6) to increase the temperature of the cold media and used an electric heating jacket to heat the bleaching unit (11). Fourth, when the temperature and pressure of the individual experiment reached its predetermined values, the pressurization pump (4) and heat exchanger (6) stopped working and the valves (2, 5, 7, 14) were closed. Fifth, we slowly opened valves (10, 12) and let the circulating pump (15) begin working by maintaining a 1:5 min ratio of static and dynamic fluid circulation of the fluid to bleach the cashmere sample for a specific amount of duration.
Thereafter, the circulating pump (15) stopped working after the preset bleaching procedure was finished. We adjusted the valve (17) to slowly depressurize the supercritical bleaching system and, we separated the remaining working solution and CO2 gas in the separator (18). Moreover, we further purified the separated CO2 gas in the purifier (19), which was continuously recovered by the condenser (3) and stored in the CO2 vessel (1) for reuse. We ended the separation and recovery procedure when the system pressure reached an equilibrium pressure, then we closed the valve (17) and slowly opened valve (13) to continuously depressurize the remained pressure in the bleaching system to an atmospheric one. Finally, we removed the bleached cashmere sample from the bleaching unit (11) and balanced it in a desiccator for 24 h until the next test.
2.4 Evaluation of the whiteness of bleached cashmere fiber
We evaluated the efficiency of the developed method to bleach the cashmere fiber by using the sample whiteness before and after the one-step enzymatic-oxygen bleaching method. We determined chromaticity coordinate values (L*, a*, b*; CIELAB uniform chromaticity space) of each cashmere sample using the UltraScan PRO colorimeter with a D65 light source and a 10° view angle. We used the arithmetic mean value of 10 determinations at several positions on the cashmere samples for the chromaticity coordinate values (such as L*, or a* or b*). Then, we followed the criterion of “Test method for whiteness and chromaticity of textile fibers” (GB/T 17644–2008) to calculate the cashmere sample whiteness (W, %) according to Eq. (1) from the criterion:
2.5 Evaluation of the integrated damages of bleached cashmere fiber
We followed the “Determination of the solubility of wool in alkali” (GB/T 7571–2008) to conduct an evaluation of the damage caused to the cashmere fiber during the supercritical bleaching. According to the alkali solubilities of the samples before and after bleaching, we sealed a quantitative mass of cashmere fiber recorded as M0 in a conical flask with 100 mL sodium hydroxide solution (0.1 mol L−1), and reacted for 60 min at 65 °C in the YG871 type constant temperature oscillating water bath machine. When the reaction duration was over, the residual fiber sample was washed 3 times with the same concentration of sodium hydroxide solution at the same temperature, followed by a full rinsing with distilled water for 6 times. We set an oven temperature to (105 ± 2) °C and dried the sample to obtain a constant mass (recorded as M1). Thus, we used Eq. (2) to evaluate the alkali solubility (S, %) of each sample after a supercritical bleaching treatment. In general, lower value of the alkali solubility (S,%) is, less integrated damages for the cashmere fiber during the supercritical bleaching could be achieved.
2.6 Detection of fiber surface morphology and surface elemental components
We used SEM (S-4800, Hitachi) to detect the surface morphologies of the samples of cashmere fibers before and after bleaching with the one-step enzymatic-oxygen method. We set the acceleration voltage to 20.0 kV with magnifications of 3000 × and 5000×. We used an ion sputter (E-1045, Hitachi) to sputter the samples of cashmere fibers with gold for 60 s at 10.0 mA. Moreover, we used EDS, which was part of the TM3030 desktop electron microscope, to analyze the surface elements and the compositions of the cashmere fiber before and after a supercritical bleaching.
2.7 Tensile strength testing for cashmere fiber
We used a universal material testing machine (Instron5967, American Instron Co., Ltd.) to test the tensile strength of the samples of cashmere fibers before and after bleaching with the one-step enzymatic-oxygen bleaching method. We recorded the tensile load and the displacement curves by employing the balanced samples for 24 h under constant temperature (20 ± 2)°C and humidity (65 ± 3)% conditions. We repeatedly measured 20 cashmere fibers to exclude the influence of different fiber sizes on the strength test results. We took the average value as the final strength property for each individual sample of cashmere fibers.
2.8 TG-DTG analysis of cashmere fiber
We conducted a comprehensive TG-DTG analysis (SDT2960, Nippon Seiko Co., Ltd., Tokyo, Japan) to investigate the thermal properties of cashmere fibers before and after bleaching with the one-step enzymatic-oxygen method. For the TG-DTG analysis, we used 2.0 mg powder of each sample under a nitrogen flow rate of 100 mL min−1 with a heating rate of 10 °C min−1 between 25 °C and 600 °C. Then we recorded the TG-DTG curves and compared the sample decomposition temperature with its differential mass loss (the percentage mass loss per minute) and the percentage of mass loss. As we performed a thermal decomposition analysis, a replicative twice-test was utilized to control the experimental error within ± 2.0 %.
3 Results and discussion
3.1 Working solution compositions and supercritical bleaching efficiency
3.1.1 pH effect
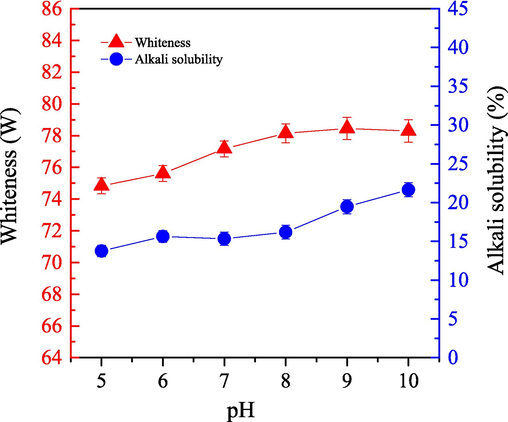
We investigated the impact of the working solution pH on the bleaching efficiency for cashmere sample by using the one-step enzymatic-oxygen bleaching method in the SCF-CO2 system. We varied the pH in the working solution from 5 to 10, which contained 0.3 g LA, 0.6 g PEG1000, 20 mL H2O2, and 20 mL buffered solution, under a pressure at 21.0 MPa and a bleaching temperature at 55 °C for a duration of 60.0 min. Fig. 2 shows the obtained whiteness and alkali solubility of the bleached samples.
- Impact of the working solution pH value on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, using a solution with 0.6 g PEG1000, 20.0 mL buffer solution, 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, using a solution with 0.6 g PEG1000, 20.0 mL buffer solution, 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.As the pH value increased from 5 to 10, Fig. 2 shows that the pH of the working solution notably affected the bleaching efficiency of the cashmere sample in the system. Particularly, Fig. 2 shows that when the working solution pH value increased from 5 to 8, the sample whiteness gradually improved; however, as the pH value increased over 8 to 10, no further improvement was observed. Therefore, we determined that by appropriately increasing the working solution pH value in the supercritical bleaching system, the removal and decomposition of natural colorants and/or other impurities had a positive effect on the cashmere fiber.
In aqueous solution, H2O2 is a weak acid to generate different active spices by changing the pH value of the bleaching system, which effectively alters the decomposition rate and stability of H2O2, and then varies its ability to bleach the cashmere fiber efficiently. Whereas, H2O2 is usually relative stable in acidic and room temperature conditions. Therefore, its removal efficiency in the supercritical bleaching treatment was poor, since its decomposition was slow to generate spices in an acidic environment to oxidize natural colorants. Consequently, the whiteness of the cashmere sample was poor when the working solution with a pH value at 5–6. After the solution pH increased to a neutral environment at about 7, in an aqueous environment, the H2O2 could easily be ionized to H+, HO2–, HO• and
As shown in Fig. 2, when the pH value increased from 5 to 8, alkali solubility slowly increased. Then as the pH value increased even more, we observed an additional enhancement, which indicated that less damage was caused to the integrated fiber under relatively mild conditions with a bleaching pH value higher than 5 to 8. Otherwise, the fibers would seriously deteriorate. In addition, these results also indicate that with the bleaching oxidation of colorants on the cashmere fiber surfaces, some components, macro-chains and/or bonds on the cashmere fiber itself could also be affected, which increase its alkali solubility; particularly, an excessive pH value in the bleaching system caused a significant damage to cashmere fibers. As s result, a pH value at about 8 was suitable to achieve relatively high whiteness while limiting deterioration in the fiber in the one-step supercritical bleaching system.
3.1.2 Effect of PEG1000
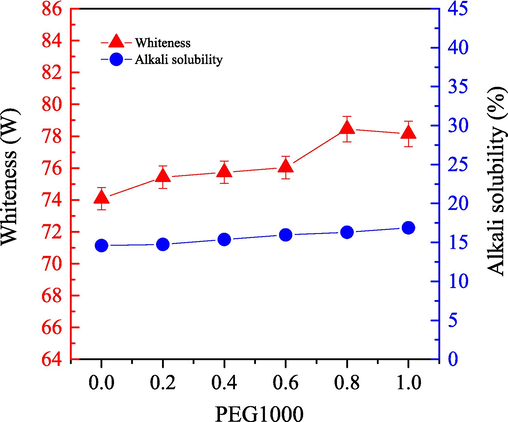
We investigated the effect of PEG1000 on the bleaching efficiency of the one-step enzymatic-oxygen system for cashmere fiber in SCF-CO2 system. We set the bleaching temperature at 55 °C for 60.0 min under a pressure at 21.0 MPa. Different PEG1000 dosages were utilized for investigation in the working solution which contained 0.3 g LA, 20 mL H2O2, and 20 mL buffered solution along with a pH value at 8. Fig. 3 shows the whiteness and the alkali solubility of the bleached samples.
- Impact of the PEG1000 dosage on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method at a temperature for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 20.0 mL buffer solution (pH=8), 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.
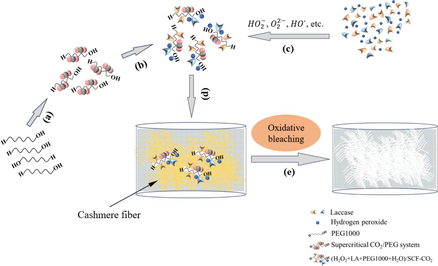
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method at a temperature for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 20.0 mL buffer solution (pH=8), 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.When we used PEG1000 in the one-step enzymatic-oxygen bleaching system in SCF-CO2 system, we observed the improved whiteness of the bleached sample by increasing the dosage from 0.0 to 0.8 g. As we increased the PEG1000 dosage from 0.8 g to 1.0 g, however, whiteness remained constant. In general, PEG1000 is not only a good solvent as well as a surfactant for extensive utilizations in industries, but also it is a nonionic phase transfer reagent possessing a certain solubility in SCF-CO2, especially with the presence of some cosolvents (Heldebrant and Jessop, 2003; Weidner et al., 1997; Mishima et al., 1999). Therefore, PEG1000 was instrumental to the formation of (H2O2 + LA+PEG1000 + H2O)/SCF-CO2 system in the supercritical fluid step by step, as the supposed supercritical bleaching mechanism in Scheme 1 (a → c). This system can be mutually soluble with a variety of organic or inorganic substances with evenly and stably distributed in the whole fluid system (Heldebrant and Jessop, 2003; Weidner et al., 1997; Mishima et al., 1999). Particularly, some interactions such as weak Coulomb forces, hydrogen bonds and van de Waals forces, etc., between the PEG1000 chains and the active species (such as the HO2–,
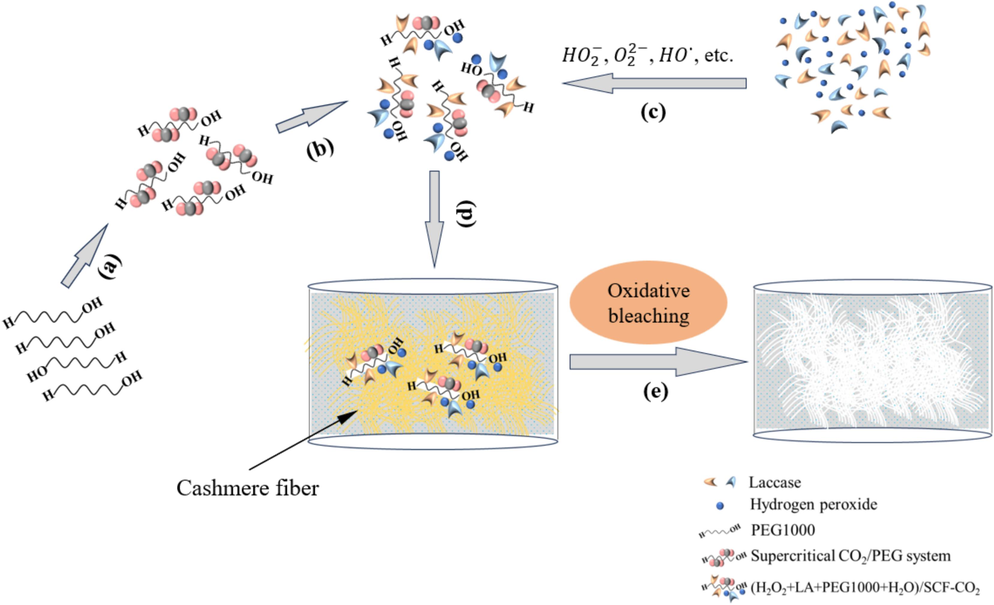
- Supercritical bleaching mechanism for cashmere according to the procedures of the proposed method: (a) PEG1000 with CO2 fluid to form the PEG1000/SCF-CO2 system; (b) the PEG1000/SCF-CO2 system with the bleaching ingredients; (c) LA, H2O2, and active spices (e.g. HO2–,
As shown in Fig. 3, when the PEG1000 dosage increased from 0.0 g to 1.0 g, the effect on the sample alkali solubility was reduced. These phenomena indicate that the PEG1000 application in the one-step enzymatic-oxygen bleaching system exhibited a protection effect on the substrate, accompanying with a mild and slight damaging on cashmere fiber. Therefore, to improve whiteness and protect the fiber from damage, a PEG1000 dosage of 0.8 is recommended in the one-step supercritical enzymatic-oxygen system.
3.1.3 Effect of buffer solution
We examined the impact of buffer solution dosage on the bleaching efficiency for cashmere fiber by using the one-step supercritical enzymatic-oxygen bleaching method. We set the bleaching temperature at 55 °C for 60.0 min under a system pressure at 21.0 MPa. We changed the dosage of the buffer solution (adjusted pH value to 8), which included 0.3 g LA, 0.8 g PEG1000, and 20 mL H2O2. The results are shown in Fig. 4.
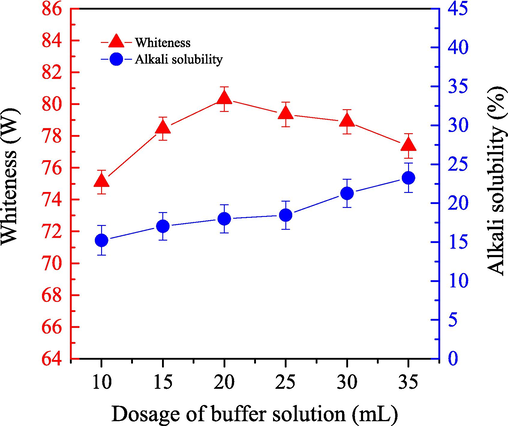
- Impact of the buffer solution dosage on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 0.3 g LA, and 20.0 mL H2O2 in SCF-CO2.Fig. 4 shows that the whiteness of the cashmere samples was enhanced when the buffer solution dosage increased to 20 mL in the supercritical bleaching system. We then observed a tendence to decrease as the buffer solution dosage continued to 35 mL. Theoretically, buffer solution can stabilize the pH of the bleaching working solution and supply enough concentrations of bleaching substances and/or active species in the system. In the one-step supercritical enzyme-oxygen bleaching system, the buffer solution should be able to stabilize the pH of the working solution to stabilize the bleaching substances. This is particularly true for generated reaction species in the working solution, as well as in the supercritical fluid media. Additionally, LA expression requires a small amount of water to trigger biological oxidation. Therefore, by increasing the buffer solution dosage from 10 mL to 20 mL, the bleaching working solution not only efficiently generated stable active bleaching spices by promoting the decomposition of H2O2 but also improved the activity of LA. As a result, the whiteness was significantly increased and improved the bleaching efficiency, as shown in Fig. 4. Sample whiteness did gradually reduce, however, as the buffer solution dosage increased to more than 20 mL. Excess water likely caused the concentrations of the bleaching ingredients to decrease gradually in the working solution and in the inner cores of the supercritical microemulsion. Therefore, their bleaching activities, as well as LA activity, were hindered on the sample surfaces.
Fig. 4 also shows a slow uptick in alkali solubility when the buffer solution dosage increased to 25 mL. We observed an even more significant enhancement when the buffer solution was greater than 25 mL. When the application of the buffer solution was appropriate, it not only removed various colorants on the fibers but also reduced the damages to the cashmere fibers. It is likely that the LA activity and the rate of H2O2 decomposition remained relatively stable, which enabled the bleaching reactions to remove colorants and other impurities from the cashmere fiber. However, the obvious enhancement in the sample alkali solubility with the buffer solution dosage further increased was probably due to the more excess alkali buffer solution, particularly some excess active species, transferred on the fiber sample which might cause some more decompositions on the macrochains of the fiber. To sum up, an application and 20 mL dosage of buffer solution could be implemented according to the sample whiteness and the alkali solubility during the supercritical bleaching for cashmere fibers with the one-step enzymatic-oxygen method.
3.1.4 H2O2 effect
We investigated the impact of H2O2 on the bleaching efficiency for cashmere sample in the one-step enzymatic-oxygen bleaching system. We set the bleaching temperature at 55 °C for 60.0 min under a bleaching pressure of 21.0 MPa. We used different H2O2 (30 %) dosages in the working solution (adjusted pH value to 8), which included 0.3 g LA, 0.8 g PEG1000, and 20 mL buffered solution. The achieved results are as shown in Fig. 5.
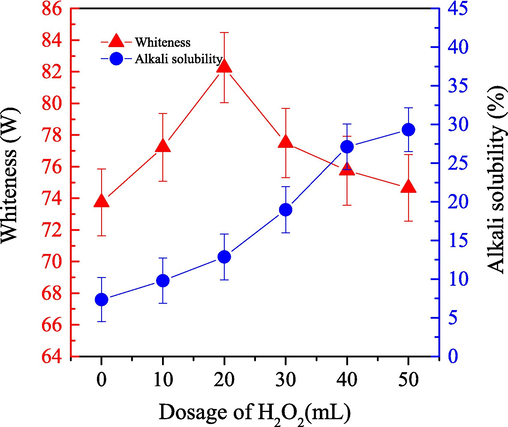
- Impact of the H2O2 dosage on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, and 0.3 g LA in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, and 0.3 g LA in SCF-CO2.Fig. 5 illustrates the remarkable improvement in whiteness when the H2O2 dosage increased to 20.0 mL. When the H2O2 dosage increased from 20.0 mL to 50.0 mL, however, whiteness decreased. H2O2 was present in the aqueous solution primarily in an alkaline environment as HO2– through the ionization shown in Eq. (3), which effectively oxidized natural colorants thanks to its strong oxidizing power. The formation of more HO2–, HO• and
Fig. 5 also demonstrates the sample alkali solubility notably improved when the H2O2 dosage increased to 50.0 mL, in particular, when the H2O2 dosage exceeded 20.0 mL. The H2O2 dosage likely increased the alkali solubility because of an adverse oxidation effect on the fiber, which might have been caused by an excessive amount of H2O2, thus damaging the cashmere fiber. Therefore, an appropriate dosage of H2O2 is preferred to protect the cashmere in the supercritical enzymatic-oxygen bleaching process. To achieve whiteness and limit fiber damage, as an oxidizing agent, an H2O2 dosage of 20.0 mL is recommended to achieve high bleaching efficiency for cashmere fiber by using the one-step supercritical enzymatic-oxygen bleaching method.
3.1.5 LA effect
We examined the impact of LA application on the bleaching efficiency for cashmere sample by using the one-step enzymatic-oxygen bleaching method in the system. We set the bleaching temperature to 55 °C for 60.0 min under a pressure of 21.0 MPa. We changed the LA dosage in the working solution (adjusted pH value to 8) which included 0.8 g PEG1000, 20 mL H2O2, and 20 mL buffered solution. The results are as shown in Fig. 6.
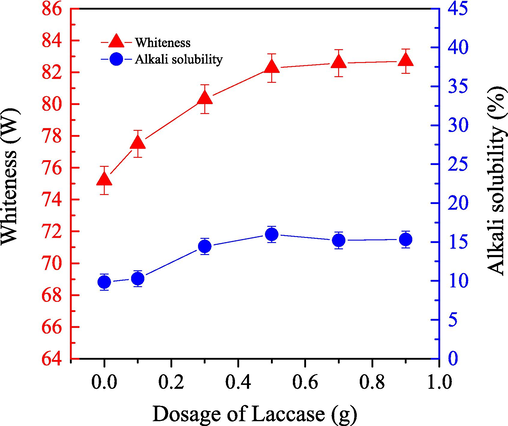
- Impact of the LA dosage on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 21.0 MPa and a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, and 20.0 mL H2O2 in SCF-CO2.Fig. 6 shows that sample whiteness was enhanced significantly with the application of LA. We increased its dosage to 0.5 g by achieving a constant value until we utilized a dosage of 0.9 g. In general, some of the colorants on the cashmere fibers were covered by the scale layers, which can’t be oxidized and destroyed efficiently and completely by those transferred active species. Additionally, the chemical compositions and structures for most of the colorants are relative to phospholipid-sterol bilayer envelopes and carrier membranes. Thus, as a redox enzyme, LA can readily change the structures of the colorants as well as those envelopes or membranes to a certain degree at an appropriate condition (Wang et al., 2018; Lan et al., 2010), which help those colorants can be easily oxidized and removed by the various bleaching ingredients in the system. When the LA dosage was increased to 0.5 g, efficient removal of those colorants was achieved through oxidative degradation, by reaching a maximum whiteness value at 82.26 for the cashmere sample in the one-step supercritical enzymatic-oxygen bleaching system.
As shown in Fig. 6, alkali solubility of the sample followed an upward trend as the LA dosage increased to 0.5 g. With the application of LA in the supercritical enzymatic-oxygen bleaching system, natural colorants were efficiently oxidized and removed from the surfaces of the cashmere fiber sample and the cashmere fibers also had less damage. Obviously, an appropriate application of LA in this developed supercritical bleaching system is also necessary and important. Thus, to achieve satisfactory whiteness and limit damage to the cashmere fiber, an LA dosage of 0.5 g is recommended.
3.2 Influence of system parameters on bleaching efficiency for cashmere
3.2.1 System pressure
We explored the effect of system pressure on the bleaching efficiency of cashmere sample by employing the one-step enzymatic-oxygen bleaching method. We set a system temperature of 55 °C for 60.0 min and applied different pressures with the working solution (adjusted pH value to 8) which included 0.5 g LA, 0.8 g PEG1000, 20 mL H2O2 (30 %), and 20 mL buffer solution. The results are shown as in Fig. 7.
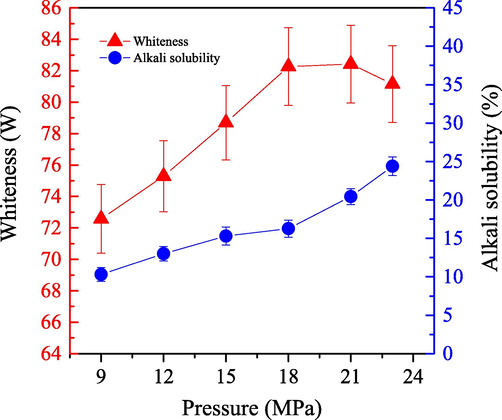
- Impact of the bleached pressure on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution (pH=8), 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution (pH=8), 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.Fig. 7 illustrates the significant improvement in bleaching whiteness of the cashmere sample when the system pressure increased from 9.0 MPa to 18.0 MPa. We observed a constant value and then a small decrease in whiteness when the system pressure increased to 23.0 MPa. System pressure has a significant effect on solute solubility, which forms the transfer system easily because of enhanced density (Heldebrant and Jessop, 2003; Weidner et al., 1997; Mishima et al., 1999). Therefore; a higher system pressure increased the solubility of PEG1000 in SCF-CO2, and resulted in the improving transfer efficiency of the active bleaching ingredients onto the fiber substrate. Moreover, the PEG1000 could be distributed more evenly in the treatment unit under higher system pressure, which resulted in more active spices accessed uniformly onto the surfaces of cashmere fibers for the oxidation of colorants. However, no continuous enhancement or even a slight decrease in the whiteness as a further increase in the system pressure over 18 MPa, was probably due to a fact that the excessive pressure was not contributed to further improve the solubility of the PEG1000, and/or showed some affects on the generation of the bleaching ingredients as well as on the LA activity in some degree.
Fig. 7 also shows that the alkali solubility increased by indicating some more damages to the cashmere fiber as pressure increased to 23.0 MPa. Particularly, if system pressure was higher than 18 MPa, which notably increased the sample alkali solubility. At an excessive system pressure, it is likely that undesirable interactions occurred between the fiber macrochains and the active oxidative spices as well as the system media. In brief, the system pressure had a remarkable influence on bleaching efficiency of the cashmere fiber. If the bleaching pressure was appropriate, it was able to remove the colorants and protect the substrate fiber simultaneously by employing the one-step enzymatic-oxygen bleaching method. Therefore, a bleaching pressure at 18.0 MPa is recommended according to sample whiteness and alkali solubility.
3.2.2 System temperature
We examined the impact of system temperature on the bleaching efficiency for cashmere fiber by using the one-step enzymatic-oxygen bleaching method in the SCF-CO2 system. We applied different system temperatures under a pressure of 18.0 MPa for 60.0 min, by using a working solution (adjusted pH value to 8) that included 0.5 g LA, 0.8 g PEG1000, 20 mL H2O2 (30 %), and 20 mL buffered solution. The results are depicted in Fig. 8.
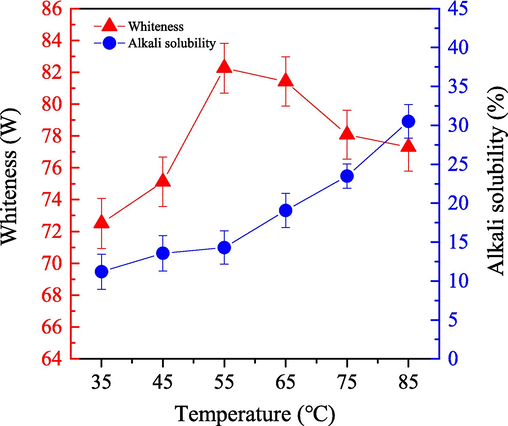
- Impact of the bleached temperature on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 18.0 MPa, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method for 60 min at a pressure of 18.0 MPa, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.Fig. 8 illustrates that the whiteness of the bleached cashmere sample increased from 72.51 to 82.26 as system temperature increased from 35 °C to 55 °C. When the temperature exceeded 55 °C, however, whiteness decreased. When the bleaching temperature was appropriate, it had a positive effect on the decomposition and removal of colorants on the cashmere sample. The generation rate of active spices from LA and H2O2 in the supercritical system was improved by increasing the temperature appropriately. Then it was possible to transfer powerful oxidative spices onto the cashmere fiber surfaces to oxidize and remove colorants and other impurities, which enhanced the supercritical bleaching efficiency, as shown in Fig. 8. Whereas, the sample whiteness likely decreased when the system temperature was higher than 55 °C because the excess temperature inhibited LA activities and enhanced fiber damage as well as made it yellow via some serious oxidative attacks from the active spices. Obviously, applying an appropriate temperature plays a crucial role in the cashmere bleaching by employing the one-step enzymatic-oxygen bleaching method in SCF-CO2.
Fig. 8 also illustrates the increase in alkali solubility of the bleached cashmere samples when the system temperature was between 35 °C and 55 °C. Especially, when the system temperature rose from 55 °C to 85 °C, alkali solubility increased rapidly. The rate of H2O2 decomposition and its active spices generation increased with system temperature. Then various oxidants were produced in the bleaching system quite quickly. Therefore, increasingly intense oxidation reactions and attacks damaged the fiber substrate other than the colorants by the transferred active spices at the overhigh system temperatures. As a result, alkali solubility tended to increase as bleaching temperature increased, which was particularly notable when the bleaching temperature exceeded 55 °C. Additionally, system temperature could show remarkable influences not only on the removing various colorants of the cashmere sample but also on the fiber itself damages simultaneously. Thus, an appropriate temperature at 55 °C is recommended for the cashmere by utilizing the one-step enzymatic-oxygen bleaching method in SCF-CO2 system.
3.2.3 Bleaching duration
We examined the impact of bleaching duration on the bleaching efficiency for cashmere fiber by using the one-step enzymatic-oxygen bleaching method. We set the temperature to 55 °C under a pressure of 18.0 MPa with different bleaching durations in a working solution (adjusted pH value to 8) including 0.5 g LA, 0.8 g PEG1000, 20 mL H2O2 (30 %), and 20 mL buffered solution. All results are shown in Fig. 9.
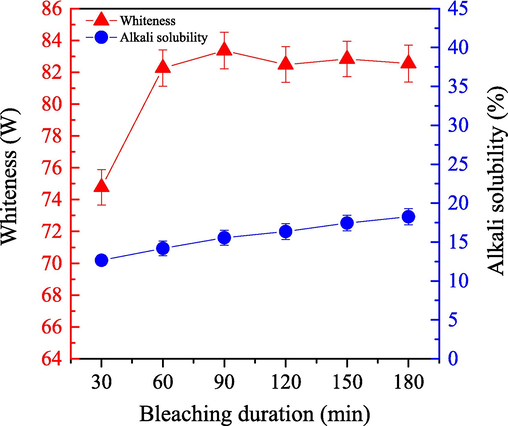
- Impact of the bleached duration on the alkali solubility (
 ) and whiteness (
) and whiteness (
 ) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method at a pressure of 18.0 MPa and at a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.
) of cashmere fiber bleaching according to the one-step supercritical enzymatic-oxygen method at a pressure of 18.0 MPa and at a temperature of 55 °C, by using a solution with an adjusted pH value to 8 with 0.8 g PEG1000, 20.0 mL buffer solution, 0.5 g LA, and 20.0 mL H2O2 in SCF-CO2.Fig. 9 illustrates that a prolonged bleaching duration from 30.0 min to 180.0 min caused a significant influence on bleaching whiteness. When the bleaching duration increased to 60.0 min, we achieved a remarkable enhancement in the whiteness. Further improvement was also observed and then a constant value was maintained as the bleaching duration reached and then exceeded 90.0 min. Generally, with a longer bleaching duration, it was possible to generate more active spices and additional cycles of supercritical fluid circulation. Thus, it was also possible to transfer more LA and H2O2 active spices for bleaching to the supercritical system and then onto the surfaces of the samples. As a result, additional oxidative reactions occurred between the colorants (as well as other impurities) and the active spices for degradation and removal, which enhanced bleaching whiteness significantly when duration increased from 30.0 min to 60.0 min and then from 60.0 min to 90.0 min. After 90.0 min, however, the bleaching efficiency did not improve, probably because the bleaching reaction reached equilibrium between the colorants (as well as other impurities) and the active ingredients. Fig. 9 also shows that alkali solubility of the sample was gradually enhanced as the bleaching extended from 30.0 min to 180.0 min. By ensuring that the bleaching duration was suitable, a relatively low degree of fiber damage could be considered. In conclusion, bleaching duration had a significant effect on the whiteness of the cashmere fiber in the supercritical enzymatic-oxygen bleaching system, and then caused a mild degree of fiber deterioration. A bleaching duration of 60.0 min or 90.0 min not only improved the sample whiteness but also reduced fiber damage for the cashmere substrate by employing the one-step supercritical enzymatic-oxygen method.
3.3 Surface morphology, composition, and fiber property by following supercritical bleaching
3.3.1 Surface morphology of the bleached cashmere fiber
We used SEM (S-4800, Hitachi) to further validate the efficiency of the one-step supercritical enzymatic-oxygen bleaching method. We examined the morphologies of the cashmere fibers before and after bleaching, as shown in Fig. 10(a1–a3) and Fig. 10(b1–b3).
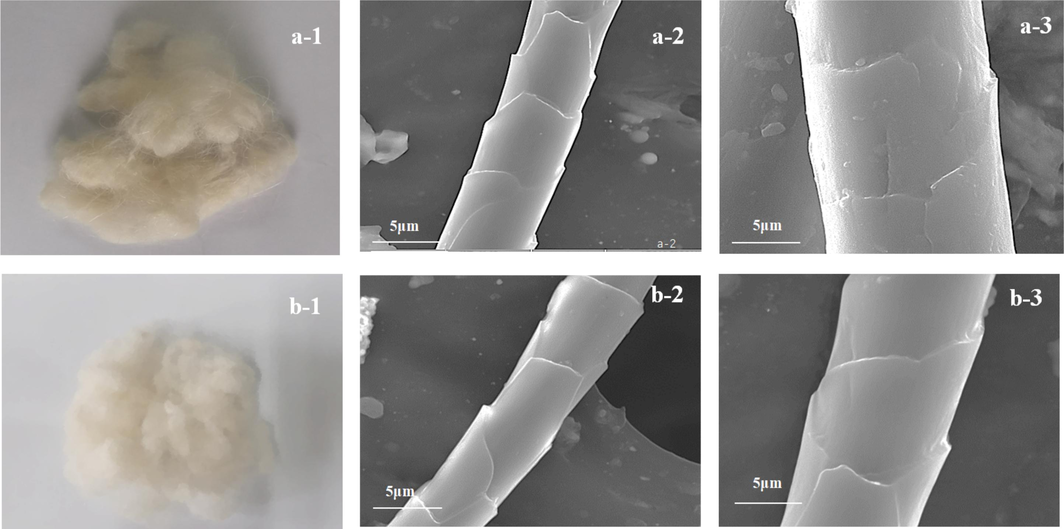
- Control cashmere fiber samples (a-1) before bleaching and SEM images at a magnification of (a-2) × 3000 and (a-3) × 5000; (b) Supercritically bleached fibers at a temperature of 55 °C and a pressure at 18.0 MPa for 90 min with the optimized working solution in the (b-1) SCF-CO2 system and the SEM images at a magnification of (b-2) × 3000 and (b-3) × 5000.
Fig. 10(a-1), (a-2), and (a-3) show that the control cashmere fibers before bleaching exhibited a yellowish color and their surfaces were relative clean by involving less other impurities such as some sediments, natural oils and animal dander, after a previous supercritical pretreatment in our previous work (Wang et al., 2024). Natural surface stripes and special scale layers of the control cashmere fiber were evident. Fig. 10(b-1), (b-2), and (b-3) show that the cashmere fiber surfaces featured thin circular scales and neat, clean scale surfaces along with natural stripes after being bleached in SCF-CO2. Particularly, the heavy yellowish colors on the control fiber sample were disappeared, and the cashmere sample became pure white, which indicate that this developed supercritical one-step enzymatic-oxygen bleaching method played a fantastic role in the degradation and removal of those colorants and/or other impurities as well as imparted less damages on the fiber surfaces.
Because PEG1000 is an amphiphilic phase transfer reagent, it can form a special type of PEG1000/SCF-CO2 dispersion system in SCF-CO2. Accordingly, this system can dissolve and disperse organic and inorganic substances with a stable distribution across the supercritical system to achieve the efficient transfer of active spices from the working solution into the supercritical hydrophobic media. Along with PEG1000 and under appropriate conditions, LA and H2O2 as well as their generated active spices transferred onto the cashmere fiber surfaces and triggered oxidative bleaching reactions to degrade and remove colorants and/or other impurities during supercritical fluid circulation, as shown in Scheme 1. Briefly, we further validate that pure whiteness of bleached cashmere and intact fiber surface morphologies are possible as cashmere fibers are bleached by following the one-step enzymatic-oxygen bleaching method in SCF-CO2.
3.3.2 Surface elemental compositions of the bleached cashmere fiber
To explore the efficiency of the one-step supercritical enzyme-oxygen bleaching method further, we also used EDS to investigate the surface chemical components of cashmere fiber before and after bleaching, as shown in Fig. S1(A) and Fig. S1(B) and Table 1.
| Control cashmere fiber | Bleached by the supercritical method | |||||
|---|---|---|---|---|---|---|
| Elements | wt% | δ, wt% | Percentage of atoms, % | wt% | δ, wt% | Percentage of atoms, % |
| C | 72.96 | 0.34 | 77.71 | 82.23 | 0.28 | 85.82 |
| O | 11.04 | 0.17 | 8.82 | 11.59 | 0.15 | 9.08 |
| N | 13.61 | 0.35 | 12.43 | 5.33 | 0.27 | 4.77 |
| S | 1.35 | 0.07 | 0.55 | 0.85 | 0.07 | 0.33 |
| Al | 1.04 | 0.05 | 0.49 | / | / | / |
| Total | 100.00 | / | 100.00 | 100.00 | / | 100.00 |
Fig. S1(A) and Fig. S1(B) show the different element distributions on the control and on the bleached cashmere fiber surfaces, respectively. According to Fig. S1(A, a → g) the elements C, N, O, Si, S, and Al were distributed on the cashmere fiber surface, which indicated that these relative clean and neat fiber surfaces had very fewer impurities still. According to Fig. S1 (B, a → f), the cashmere surface was increasingly pure after bleaching with the one-step supercritical enzymatic-oxygen method. Then we observed a significant reduction in the remaining elemental species distributions and only detected C, N, O, and S on the bleached fiber surface. Table 1 shows that other than the basic fiber elements C, H, O, N, and S, only Al, the extraneous element, was identified on the control cashmere fiber surface, which indicating that some inorganics, etc., still remained on the control fiber. However, compared with the control sample in Table 1, we did not detect the extraneous element or its relative compounds on the cashmere sample after supercritical bleaching. In addition, we also found that N, and S were reduced significantly, which likely was due to the removal and decomposition of natural colorants and/or other impurities from the cashmere fibers. Therefore, the developed bleaching method provided excellent efficiency in the degradation and removal of colorants and other impurities from the cashmere sample.
3.3.3 Tensile strength property of the bleached cashmere fiber
We used an Instron5967 universal material testing machine, to evaluate tensile strength of the cashmere fiber before and after bleaching with the developed method in SCF-CO2. Then we further validated the methodology according to properties of the bleached cashmere fiber, as shown in Fig. S2 and Table 2.
| Samples | Breaking strength, cN | Percentage of breaking elongation, % |
|---|---|---|
| Control Cashmere fiber | 6.82 | 46.76 |
| Bleached in supercritical enzymatic-oxygen system | 6.01 | 47.39 |
Fig. S2 shows the tensile fracture of cashmere fibers before and after bleaching in the SCF-CO2 system, which satisfied Hooke's law. Tensile fracture of cashmere fibers occurred in three stages. First, the large molecular chain of cashmere fiber was deformed in a relatively low stress range, which produced a small strain and caused the tensile curve of the cashmere fiber to from a straight line, as the A district depicted in Fig. S2. Second, when stress increased, a continuous and prolonged strain occurred, which broke the secondary valence bonds of the cashmere fiber in the amorphous regions and caused evident macrochain slips, as the B district described in Fig. S2. Third, the cashmere fiber underwent a tensile breaking as stress increased because the macromolecular backbone and secondary bonds completely broke, as the C district shown in Fig. S2. The stress–strain curve of the bleached fiber moved to a lower position along the strain axes than the control cashmere fiber. Except for a slight lower breaking stress recorded in Fig. S2, this shift was accompanied by nearly closed stress–strain tendency with the control fiber. These phenomena were probably due to some slightly integral damages occurred to the cashmere fiber, particularly for its internal macrochain compactness, which led to the slightly reduced strength and the shift of the fracture curve.
According to the results in Table 2, the tensile breaking strength of the bleached fiber decreased from 6.82 N to 6.01 N compared with the control. This decreased in breaking strength was accompanied by an improvement in elongation from 46.76 % to 47.39 %. Accordingly, we verify that the one-step supercritical enzymatic-oxygen method for cashmere bleaching only slightly affected the mechanic property of the fiber. As a result, under suitable conditions, this developed supercritical bleaching method could achieve efficient bleaching efficiency and satisfactory mechanic property for cashmere fibers in SCF-CO2.
3.3.4 Thermal property of the bleached cashmere fiber
We also used TG-DTG (SDT2960) to investigate the thermal properties of cashmere samples before and after bleaching according to the developed method, in order to further validate the applicability of this methodology in practice, as shown in Fig. S3 and Table 3.
| Samples | Fastest decomposition temperature, Tp, °C | Fastest decomposition rate, mg min−1 | Weight loss at 550 °C, % | Residual weight at 600 °C, % |
|---|---|---|---|---|
| Control Cashmere fiber | 322.6 | 0.48 | 76.06 | 23.94 |
| Bleached in supercritical enzymatic-oxygen system | 323.7 | 0.50 | 76.08 | 23.92 |
According to Fig. S3(a) and Fig. S3(b), the TG-DTG analysis shows that the thermal property of the bleached cashmere fiber was similar to the control. Additionally, the thermal decomposition curves and stages were closely aligned without any obvious changes. Additionally, Fig. S3(a) and Fig. S3(b) also show that the mass fractions of the cashmere samples slightly decreased in the temperature range of 25 °C to 100 °C, and we also observed that each sample had a weak peak in the DTG curve which was caused primarily by water evaporation. We also observed that weight loss in each sample was significantly accelerated in the temperature range of 200 °C to 400 °C. Because of the large number of internal chemical bonds that were broken, and then the fiber macrochains were seriously degraded, which caused the low molecular substances to become volatile and finally led to the significantly improved weight loss. Moreover, between 250 °C and 350 °C, we observed that each sample had a similar thermal decomposition rate and shape. Additionally, the fastest rate of weight loss was also indicated by a strong decomposition peak with the temperature over 200 °C to 400 °C in the corresponding DTG curves.
Table 3 shows the detailed results for the TG-DTG analysis. We obtained similar results for the bleached cashmere sample and the control one with a weight loss at 550 °C (%), a residual weight at 600 °C, and the fastest decomposition point temperature (Tp). These results clearly demonstrate that the generated and transferred active bleaching spices in the supercritical system showed some good selection to those natural colorants and/or other impurities on the cashmere substrate, which imparted very less effect on the fiber thermal properties on the whole. Consequently, the results of this study also verified the possible application of this developed method for cashmere bleaching in SCF-CO2.
4 Conclusions
In this work, we employed H2O2 and LA in SCF-CO2 to develop an environmentally friendly and efficient one-step enzymatic-oxygen bleaching method for the ecofriendly production of cashmere fiber as an alternative to conventional ones. We found that the LA and H2O2 dosages, as well as bleaching temperature, duration, and pressure, had a significant impact on the sample whiteness as well as to the damage caused to the cashmere fiber. We optimized and recommended a bleaching process by using a one-step supercritical enzymatic-oxygen system with 0.8 g PEG1000, 20 mL buffer solution (pH=8), 20 mL H2O2, and 0.5 g LA in a working solution, and a pressure at 18.0 MPa at a system temperature of 55 °C with a duration of 60–90 min for the supercritical bleaching, by achieving sample whiteness as high at 82.3–83.4 with limited damage to the fiber with an alkali solubility at 15.55 %. By following SEM, EDS, TG-DTG, and strength measurements, we further verified the applicability and performance of this method. Overall, we successfully validated this supercritical bleaching method, which not only demonstrated the high efficiency in the whiteness of the cashmere but also showed limited damages on the substrate fiber. We also found that this proposed method to bleach cashmere fibers in SCF-CO2 medium was more environmentally-friendly and cost effective than the traditional aqueous method, by avoiding some extensive uses of chemicals and a large generation of wastewater or heavy discharge of effluents.
CRediT authorship contribution statement
Fan Wang: Writing – original draft, Visualization, Investigation, Data curation. Yuan-Bin Zheng: Visualization, Investigation, Data curation. Xin-Xin Cao: Visualization, Data curation. Zi-Qing Du: Visualization, Data curation. Jia-Jie Long: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Acknowledgments
The authors acknowledge gratefully the financial supports from the Project of Jiangsu Engineering Research Center of Textile Dyeing and Printing for Energy Conservation, Discharge Reduction and Cleaner Production, P. R. China (ERC, PRC) (Grant No. 2024-ERC(PRC)-9011580324).
References
- One-step bleaching process for cotton fabrics using activated hydrogen peroxide. Carbohyd. Polym.. 2013;92:1844-1849.
- [CrossRef] [Google Scholar]
- Past, present and future of supercritical fluid dyeing technology an overview. Color. Technol.. 2010;32:88-102.
- [CrossRef] [Google Scholar]
- Amine borane: a unique reductive bleaching agent that protects cystine disulphide bonds in keratins. J. Color Technol.. 2008;124:318-323.
- [CrossRef] [Google Scholar]
- A low temperature neutral reduction bleaching method for protein fibers. P. China Patent, CN109183387A.. 2019;01(11)
- [Google Scholar]
- Phase equilibria for enzyme-catalyzed reactions in supercritical carbon dioxide. Fluid Phase Equilib.. 1995;108:1-14.
- [CrossRef] [Google Scholar]
- Investigation of alternative ecologic bleaching methods for the wool fibers. J. Nat. Fibers. 2021;18:1229-1246.
- [CrossRef] [Google Scholar]
- Influence of pre-treatment on enzymatic degumming of apocynum venetum bast fibers in supercritical carbon dioxide. J. Thermal Science.. 2015;4:19.
- [CrossRef] [Google Scholar]
- Paramagnetic bioactives encapsulated in poly (D, L-lactide) microparticules: spatial distribution and in vitro release kinetics. J. Supercrit. Fluids. 2019;158(C):104748.
- [CrossRef] [Google Scholar]
- Sonochemical and hydrodynamic cavitation reactors for laccase/hydrogen peroxide cotton bleaching. Ultrason. Sonochem.. 2014;21:774-781.
- [CrossRef] [Google Scholar]
- Liquid poly (ethylene glycol) and supercritical carbon dioxide: a benign biphasic solvent system for use and recycling of homogeneous catalysts. J. Am. Chem. Soc.. 2003;125:5600-5601.
- [CrossRef] [Google Scholar]
- Progress of applications of laccase in organic synthesis. Chem. Eng. Prog.. 2010;7:300-301.
- [CrossRef] [Google Scholar]
- The development of cashmere products dyeing and finishing process. J. China Text. Leader.. 2005;98:10-124.
- [CrossRef] [Google Scholar]
- Ecofriendly pretreatment of grey cotton fabric with enzymes in supercritical carbon dioxide fluid. J. Clean. Prod.. 2016;120:85-94.
- [CrossRef] [Google Scholar]
- Bleaching process to wool/flax blended yarn with peracetic acid. Wool Text. J.. 2011;39:13-16.
- [CrossRef] [Google Scholar]
- Mechanism of H2O2/bleach activators and related factors. Cellul.. 2019;26:2743-2757.
- [CrossRef] [Google Scholar]
- Current situation and development trend of cashmere identification technology. J. China Text. Leader.. 2017;3:45-46.
- [CrossRef] [Google Scholar]
- Activity of cellulase and a-amylase from Hortaea werneckii after cell treatment with supercritical carbon dioxide. J. Supercrit. Fluids. 2013;78:143-148.
- [CrossRef] [Google Scholar]
- Recent progress in biocatalysis using supercritical carbon dioxide. J. Biosci. Bioeng.. 2013;115:233-241.
- [CrossRef] [Google Scholar]
- Solubilities of poly (ethylene glycol) in the mixtures of supercritical carbon dioxide and cosolvent. Fluid Phase Equilibr.. 1999;161:315-324.
- [CrossRef] [Google Scholar]
- How enzymes can remain active and stable in a compressed gas? New insights into the conformational stability of Candida Antarctica lipase B in near-critical propane. J. Supercrit. Fluids. 2012;72:161-167.
- [CrossRef] [Google Scholar]
- Investigation of enzymatic activity, stability and structure changes of pectinase treated in supercritical carbon dioxide. J. Clean. Prod.. 2016;125:331-340.
- [CrossRef] [Google Scholar]
- Waterless processing of sheep wool fiber in textile industry with supercritical CO2: Potential and challenges. J. Clean. Prod.. 2021;41:124-129.
- [CrossRef] [Google Scholar]
- Environmentally friendly surface modlifcation treatment of flax fibers by supercritical carbon dioxide. J. Molecules.. 2020;25:438.
- [CrossRef] [Google Scholar]
- Research on wool bleaching with hydrogen peroxide and catalyzing with enzyme. Text. Dyeing Finish. J.. 2000;22:1-5.
- [CrossRef] [Google Scholar]
- A strategy for environmentally-friendly removal of impurities from cotton based on biocatalytic reaction in supercritical carbon dioxide. J. Cellulose.. 2018;11:6771-6792.
- [CrossRef] [Google Scholar]
- Molecular dynamics and nuclear magnetic resonance studies of supercritical CO2 sorption in poly (methyl methacrylate) Polymers. 2022;14(23):5332.
- [CrossRef] [Google Scholar]
- The influence of biological enzyme on hydrogen peroxide bleaching wastepaper pulp. China Pulp Pap. Ind.. 2008;12:26-28.
- [CrossRef] [Google Scholar]
- The development of milk protein fiber, cashmere and PTT blended fabric. J. China Fiber Inspection (CFI). 2015;3:82-85.
- [CrossRef] [Google Scholar]
- Influence of oxidation and reduction bleaching on structure and properties of soybean fibers. Dyeing Finish (Shanghai, China). 2004;13:1-6.
- [CrossRef] [Google Scholar]
- An ecofriendly and sustainable method for efficient cleaning of raw cashmere fiber with enzymes in SCF-CO2. J. CO2 Util.. 2024;80:102662
- [CrossRef] [Google Scholar]
- Application of laccase in Chinese hair bleaching. Text. Aux.. 2018;9:43-45.
- [CrossRef] [Google Scholar]
- Morphology and performance analysis of alpaca and cashmere fiber. J. Appl. Mech. Mater.. 2013;477:1336-1339.
- [CrossRef] [Google Scholar]
- Phase equilibrium (solid-liquid-gas) in polyethyleneglycol-carbon dioxide systems. J. Supercrit. Fluids. 1997;10:139-147.
- [CrossRef] [Google Scholar]
- Views and suggestions on cotton fabric pre-treatment new process of low temperature & low alkali-activated and complex enzyme scouring & bleaching. J. Prog. Text. Sci. Technol.. 2014;1:8-13.
- [CrossRef] [Google Scholar]
- Development of a special SCFX-AnB3L dye and its application in ecological dyeing of silk with supercritical carbon dioxide. J. CO2 Util.. 2020;35:67-78.
- [CrossRef] [Google Scholar]
- The new standard of the fineness and length test method for cashmere and wool and correlative equipment. J. China Fiber Inspection (CFI). 2011;3:54-56.
- [CrossRef] [Google Scholar]
- Bleaching of wool with sodium borohydride. J Een Fiber Fabr.. 2009;4:45-50.
- [CrossRef] [Google Scholar]
- Enzymatic reactions in subcritical and supercritical fluids. J. Supercrit. Fluid.. 2018;23:134-136.
- [CrossRef] [Google Scholar]
- Investigation of the influence of supercritical carbon dioxide treatment on meta-aramid fiber: thermal decomposition behavior and kinetics. J. CO2 Util.. 2020;37:85-96.
- [CrossRef] [Google Scholar]
- Study on wool nondestructive decoloring process. Wool Text. J.. 2016;44:24-27.
- [CrossRef] [Google Scholar]
- Investigation of the uptake and compatibility behaviors of special disperse dyes developed for sustainable color matching in supercritical carbon dioxide. J. CO2 Util.. 2023;72:102478
- [CrossRef] [Google Scholar]
- A sustainable one-step pretreatment of cotton grey fabric with 18-crown-6 as phase transfer in supercritical carbon dioxide. J. Supercrit. Fluid.. 2021;105269
- [CrossRef] [Google Scholar]
- Research status of cashmere fiber structure and properties. Prog. Text. Sci. Technol.. 2020;10:15-18.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105953.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







