Translate this page into:
An integrated strategy to discover the quality control markers of herbal formulae of Danning tablet with anti-cholestasis applications
⁎Corresponding authors at: Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. nail8219@126.com (Li-Li Ding), aizhenxiong@shutcm.edu.cn (Ai-Zhen Xiong), yl7@shutcm.edu.cn (Li Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Quality makers (Q-markers) are pivotal for the standardization of quality control for traditional Chinese medicine (TCM) drugs. However, it’s a great challenge to identify the Q-markers of TCM drugs especially for TCM formulas. This study was designed to provide a method to discover Q-markers of TCM formulas and applied to identify the Q-markers responsible for the anti-cholestatic effect of Danning tablet (DNT), a TCM formulae consisting with seven herbal ingredients. In this study, the anti-cholestatic effect of DNT was evaluated in mice with cholestasis induced by α-naphthyl isothiocyanate (ANIT). An untargeted metabolomic profiling were performed using serum and liver samples of mice. Xenobiotics derived from DNT and endogenous compounds responsible for the anti-cholestatic effect of DNT were screened by statistical analysis and the Q-markers of DNT were illustrated, followed by predicting the potential targets and mechanisms via network pharmacology study. DNT exhibited a protective effect against liver injury in mice with ANIT-induced cholestasis. Three endogenous compounds, namely taurochenodeoxycholic acid (TCDCA), taurocholic acid (TCA), and glycocholic acid (GCA), were identified as the endogenous markers responsible for the anti-cholestatic effect of DNT. By setting a cutoff value of the correlation co-efficient at 0.8, 6 promising Q-markers, including luteolin, kaempferol, apigenin, emodin, luteolin-7-glucoside, and 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone, were screened out. These Q-markers were stably existed in DNT and predicted to be highly active in treating cholestasis. This study established a pipeline to screen out the potential Q-markers of TCM formulas and 6 key Q-markers responsible for the anti-cholestatic effect of DNT were identified.
Keywords
Danning Tablet
Cholestasis
Metabolomics
Quality Markers
Network Pharmacology
- ALP
-
alkaline phosphatase
- ALT
-
aminotransferase
- ANIT
-
α-Naphthylisothiocyanate
- ANOVA
-
one-way analysis of variance
- AST
-
aspartate aminotransferase
- BP
-
biological process
- CC
-
cellular component
- CF
-
Crataegi Fructus
- CMC-Na
-
Sodium carboxymethylcellulose
- CR
-
Curcumae Radix
- CRP
-
Citri Reticulatae Pericarpium
- CRPV
-
Citri Reticulatae Pericarpium Viride
- DBIL
-
direct bilirubin
- DECs
-
differential expressed components
- DNT
-
Danning Tablet
- ESI
-
electrospray ionization
- GCA
-
glycocholic acid
- GO
-
Gene Ontology
- HE
-
hematoxylin & Eosin
- HPLC
-
high performance liquid chromatography
- IDA
-
information dependent acquisition
- IR
-
Imperatae Rhizoma
- KEGG
-
Kyoto Encyclopedia of Genes and Genomes
- LOQ
-
limit of quantitation
- LC-MS
-
liquid chromatography mass spectrometry
- MF
-
molecular function
- MS
-
mass spectrometry
- OPLS-DA
-
orthogonal projections to latent structures-discriminant analysis
- PCA
-
principal component analysis
- PCRR
-
Polygoni Cuspidati Rhizoma et Radix
- PPI
-
protein–protein interaction
- QC
-
quality control
- Q-marker
-
quality marker
- RRR
-
Rhei Radix et Rhizoma
- SD
-
standard deviation
- S/N
-
signal-to-noise ratio
- SSDMC
-
single reference standard to determine multi-compounds
- SWATH
-
sequential window acquisition of all theoretical mass spectra
- TBA
-
total bile acid
- TBIL
-
total bilirubin
- TCA
-
taurocholic acid
- TCDCA
-
taurochenodeoxycholic acid
- TCM
-
Traditional Chinese Medicine
- UDCA
-
ursodeoxycholic acid
- VIP
-
variable importance for the projection
Abbreviations
1 Introduction
Traditional Chinese medicine (TCM) drugs and formulas have been used globally for preventing and treating a wide variety of human diseases, avoiding the deleterious side effects of synthetic drugs. The efficacy of TCM formulas is remarkable and is often compatible with composing principle to improve the curative effect further. TCM formulas contain many natural chemical components, and the qualitative and quantitative characterizations of these chemicals are the primary ways to control the quality and effectiveness of most TCM medicines (Liang et al., 2020). However, many of the identified components lack specificity and biological activity, which inevitably compromises the practical value of some TCM substances. In some instances, chosen indicators for assessing the efficacy and clinical potential of TCM components could be technically inappropriate for reflecting their actual therapeutic utilizations. TCM preparation is a commonly used form of decoction pieces, which is full of complex chemical components, targeting a range of molecular pathways (Gupta et al., 2022, Li et al., 2008). Hence, standardization of the quality control method for such complex biochemical systems could often be challenging. Quality markers (Q-markers) are chemical components that associated with drug effects (such as effectiveness and safety), and are transferable and traceable components in the process of production and preparation ( Liu et al., 2016; Liu et al., 2017). In recent years, Q-markers have been proposed to improve the quality and quality control standard of TCM and further promote the relevance of TCM-material basis-quality control effectiveness (Jiang et al., 2023, Yan et al., 2023, Chen et al., 2022).
Danning tablet (DNT) consists with seven herbal ingredients, namely Rhei Radix et Rhizoma (RRR), Polygoni Cuspidati Rhizoma et Radix (PCRR), Citri Reticu-latae Pericarpium (CRP), Citri Reticulatae Pericarpium Viride (CRPV), Imperatae Rhizoma (IR), Crataegi Fructus (CF), and Curcumae Radix (CR). DNT can disperse the liver qi to promote bile flow, protect the liver from inflammation, and clear heat. DNT has been clinically used to treat human liver and gallbladder disease for more than 30 years (Zhu et al., 1990, Ji et al., 2004). We previously showed that DNT could dose-dependently protect rats from α-naphthylisothiocyanate (ANIT)-induced liver injury by inhibiting oxidative stress in the liver cells and preventing neutrophil infiltration into the liver injury sites (Ding et al., 2012). DNT is also found to promote bile acid and bilirubin elimination by regulating the expressions of hepatic and renal transporters as well as hepatic metabolic enzymes (Ding et al., 2012). However, the Q-markers responsible for the anti-cholestatic effect of DNT are largely unknown, which greatly hinders the development of a reasonable and scientific quality evaluation system of DNT.
In this study, we proposed a strategy to uncover the Q-markers of TCM formulae by integrating the analysis of xenobiotics and endogenous components responsible for their pharmacodynamics. Then, we applied an established pipeline to screen out potential Q-markers of DNT based on their anti-cholestatic effects (Fig. 1). First, a well-recognized ANIT-induced cholestasis mouse model (Sundaram et al., 2017) was employed to test the anti-cholestatic effect of DNT. Second, xenobiotic compounds derived from DNT were fast identified in this mouse model. Parallelly, metabolomic studies were performed to highlight the potential endogenous markers responsible for the anti-cholestatic effect of DNT. Eventually, Pearson correlation analysis of xenobiotics and endogenous markers was utilized to explore the Q-markers, whose contributions were then validated by the network pharmacology. In conclusion, we established a method of discovering the Q-markers of DNT with anti-cholestasis applications. And the metabolomic profiling of DNT was performed to reveal exogenous chemicals. We used correlation analysis to screen the Q-markers of DNT and the contents and network pharmacology were used to measure the stability of Q-markers. In this study, the in vivo process and the pharmacodynamics were integrated to screen out the Q-markers of DNT against cholestasis. This study will beneficial the quality control of DNT in clinical application.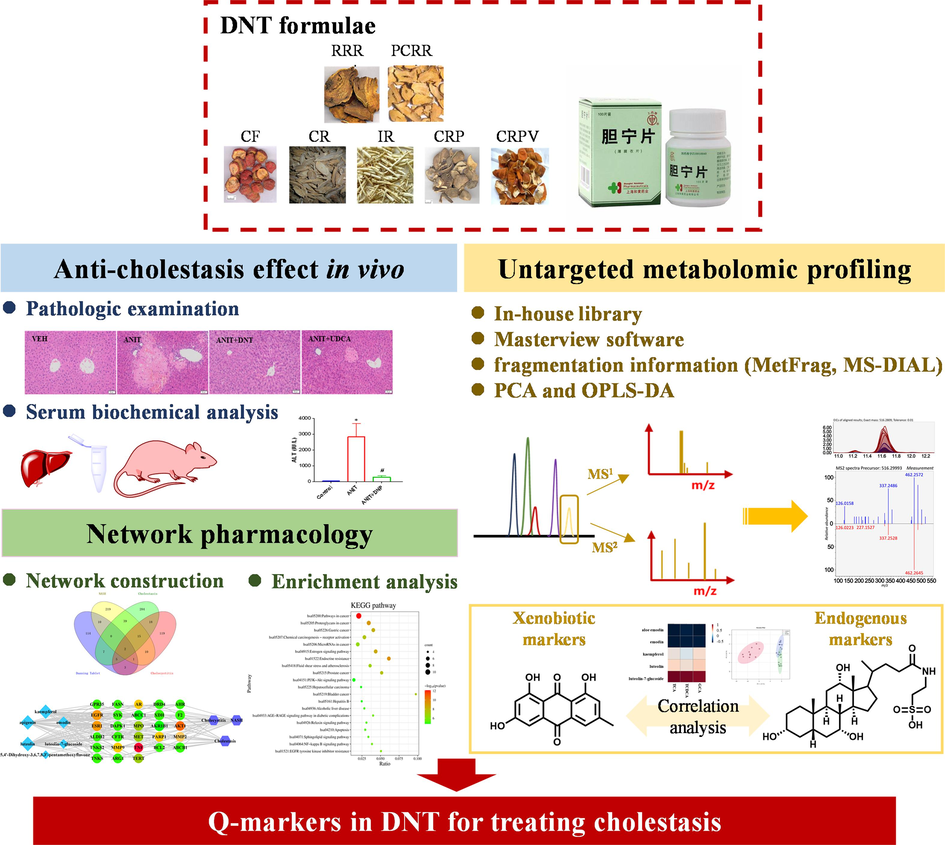
The workflow of the integrated strategy to discover Q-markers of DNT formulae in treating cholestasis.
2 Materials and methods
2.1 Materials and reagents
DNT formulae samples (batch number: 170105, 231225, 231224) were purchased from Shanghai Hutchison Pharmaceuticals (Shanghai, China). Kaempferol (purity ≥ 98 %, batch number: 01–2007) and emodin (purity ≥ 98 %, batch number: 05–2004) were purchased from Shanghai Standard Technical Service Co., Ltd. (Shanghai, China). Luteolin (purity ≥ 98 %, batch number: C29N10Q104574) and apigenin (purity ≥ 98 %, batch number: M29GB150104) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Ursodeoxycholic acid (UDCA) was purchased from Sigma-Aldrich (Merck, Darmstadt, Germany). ANIT was purchased from Sigma-Aldrich (Merck, Darmstadt, Germany).
High-performance liquid chromatography (HPLC) grade methanol, acetonitrile, and formic acid were purchased from Fisher Scientific Co. (Santa Clara, CA, USA). Deionized water was purified using a Milli-Q Academic System made by Millipore (Billerica, MA, USA). Sodium carboxymethylcellulose (CMC-Na) was obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
2.2 Animal experiment
All animal studies were conducted by regulations of experimental animal administration issued by the State Committee of Science and Technology of the People's Re-public of China as well as the Ethics Committee of Shanghai University of Traditional Chinese Medicine (PZSHUTCM201023007). Male C57BL/6 J mice (20 ± 2 g) were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China). The mice were maintained on a standard chow diet and water with ad libitum access and housed in a 12-h light/12-h dark cycle environment. All animals were allowed for a 1-week quarantine and acclimation period before the experiment.
The mice were randomly divided into 4 groups, namely the vehicle control group (VEH), ANIT-induced cholestasis group (ANIT), DNT (3 g/kg) treatment group (ANIT + DNT), and UDCA (90 mg/kg) treatment group (ANIT + UDCA), with 10 mice per group. DNT formulae sample was ground into powder (100 mesh, 165 mm) and suspended in 0.5 % CMC-Na aqueous solution to obtain a final solution of 0.3 g/mL of DNT. Similarly, UDCA was dissolved in 0.5 % CMC-Na aqueous solution to obtain a final solution of 9 mg/mL. ANIT was dissolved in olive oil to obtain a final solution of 10 mg/mL. Mice in ANIT + DNT and ANIT + UDCA groups were respectively treated with DNT (3 g/kg) and UDCA (90 mg/kg) by intragastric administration for seven consecutive days, while mice in the VEH and ANIT groups received an equal volume of 0.5 % CMC-Na aqueous solution each day. On the fifth day (12 h after DNT or UDCA treatment), mice in the ANIT, ANIT + DNT, and ANIT + UDCA groups received a single oral administration of ANIT (100 mg/kg), while mice in the VEH group received an equal volume of olive oil.
At 48 h after the ANIT treatment, mice were euthanized under anesthesia for the collection of blood and liver samples. Blood samples were kept undisturbed for 1 h at room temperature, then the sera were collected by centrifugation at 4,000 g for 15 min at 4°C. An aliquot of the left lobe of the liver was immersed in 10 % PBS-buffered formalin at room temperature, and all other samples were stored at −80°C for future use.
2.3 Serum biochemical analysis and the liver histological examination
Serum liver function indexes, including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL), and total bile acid (TBA), were determined with an automatic biochemical analyzer (Hitachi 7080, Japan).
After fixing in 10 % PBS-buffered formalin for 24 h, the liver tissue was embedded in paraffin. Samples were subsequently sectioned (3 μm) and applied for hematoxylin & eosin (H&E) staining. Images were captured from six randomly selected microscopic fields on each slide using a Leica microsystem (Wetzlar, Germany).
2.4 LC-MS analysis
2.4.1 Sample preparation
A 50 μL aliquot of serum sample was extracted with 500 μL of a pre-cooled mixture of acetonitrile-methanol–water (2:2:1, v/v). After vortex for 1 min and ultrasonication extraction for 5 min, the mixture was centrifuged at 20,000 g for 10 min at 4°C. The supernatant was collected and evaporated to dryness with a vacuum dryer. The residue was reconstituted in 50 μL of acetonitrile–water (1:1, v/v) mixture and then applied to the liquid chromatography-mass spectrometry (LC-MS) analysis.
About 50 mg aliquot of liver tissue sample was weighed and mixed with 100 μL of pre-cooled normal saline by swirling for 1 min, then the mixture was extracted with 400 μL of pre-cooled acetonitrile-methanol solution (2:2, v/v). The homogenate was further vortexed for 1 min, followed by ultrasonic extraction for 5 min in an ice bath, and centrifugation at 20,000 g for 10 min at 4°C. The supernatant was collected and evaporated to dryness, and reconstituted with acetonitrile–water (1:1, v/v) solution. After filtering through a 0.22 μm nylon filter, the supernatant was applied for the LC-MS analysis following the LC-MS method described above.
DNT formulae samples (0.5 g) were powdered and extracted with 50 mL of methanol by ultrasonicating for 1 h and centrifuged at 20,000 g for 5 min.
2.4.2 LC-MS parameters
The LC-MS based metabolic profiling analysis was performed using a Shimadzu LC-30A HPLC system (Japan) and an Applied Biosystems Sciex Triple TOF 5600+ (USA) with an electrospray ionization (ESI) source. Data acquisition and processing were performed using Analyst TF (version 1.7.1), and PeakView (version 2.0) with the MasterView plugin (Applied Biosystems, Foster City, CA, USA).
Chromatographic separations were carried out on a Waters Acquity UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) with the column temperature at 35°C. The mobile phase was composed of 0.1 % aqueous formic acid (phase A), and methanol (phase B) and gradient elution were performed as follows: 0–3 min, 5–16 % B; 3–10 min, 16–70 % B; 10–15 min, 70–95 % B; 15–16 min, 95–5 % B; 16–20 min, 5 % B. The flow rate was set at 0.3 mL/min. The auto-sampler was set at 10°C, and the injection volume was 5 μL.
The MS data acquisition was performed in the information-dependent acquisition (IDA) mode, including positive and negative ion modes, via a DuoSpray ESI system. The optimized MS parameters were as follows: Gas 1, Gas 2, curtain gas, and collision gas (nitrogen) were set at 50, 50, 45, and 12 psi, respectively; source temperature, 350°C; ion spray voltage, 5500 V in the positive ion mode and −4500 V in the negative ion mode. To determine the mass of fragments of target compounds, product ion mode was further performed with the collision energy at 35 V and collision energy at 15 V.
2.5 Identification of DNT-derived xenobiotic chemicals in mice
The samples, including DNT formulae samples, serum and liver samples from VEH, ANIT, and ANIT + DNT groups, were applied to the LC-MS analysis as described previously. High-resolution LC-MS data, including retention time, accurate mass, peak intensity, and MS/MS spectra, were obtained from PeakView with the MasterView plugin. The DNT-derived xenobiotic chemicals in mice were fast identified by comparing these data with available chemical reference standards and those reported in public references.
2.6 Identification of potential endogenous markers in mice responsible for the anti-cholestatic effect of DNT
Raw LC-MS data in the “(dot) wiff” format was preprocessed using the PeakView with MasterView plugin. The program XCMS was used for peak alignment, peak picking, and data normalization. The missing values of metabolic data were removed according to the modified 80% rule. Principal component analysis (PCA), an unsupervised pattern recognition approach, was applied to obtain trends in the overall metabolic profile across the groups. Metabolic data matrices between any two groups were analyzed by orthogonal projections to latent structures-discriminant analysis (OPLS-DA), a supervised pattern recognition approach. A preliminary variable of importance in the projection (VIP) value was selected for primary screening. Peak intensities with a significant fold change of > 1.5 and a P-value (by Student’s t-test) of < 0.05 indicated potential endogenous markers. An online biochemical database service METLIN and Chemspider were applied to identify and verify those potential endogenous markers. The MS2 fragmentation information was matched by MetFrag and MS-DIAL, combining the network MS/MS information databases, including MassBank.
2.7 Pearson’s correlation analysis
Pearson’s correlation analysis was performed to explore the Q-markers, i.e., the xenobiotics derived from DNT responsible for the anti-cholestatic effect of DNT in ANIT-induced cholestasis mice. Pearson’s correlation analysis was performed using the DNT-derived xenobiotics and potential endogenous markers in rodent samples by MetaboAnalyst. The correlation coefficient (r), reflecting the intrinsic relations between different variable groups, was obtained and used as the indicator to establish the correlation between the endogenous biomarkers and the chemical components in DNT from serum and liver samples. In this study, a value of |r| > 0.8, which suggests a high correlation, was set as the criteria.
2.8 Network pharmacology study
The target prediction of the Q-markers was performed by the Swiss Target Pre-diction database (https://www.swisstargetprediction.ch/). The GeneCards (https://www.genecards.org/) and DisGeNET database (https://www.disgenet.org/) with “NASH”, “Cholestasis”, and “Cholecystitis” as keywords were used to search for disease targets. The intersection of data revealed the targets related to the efficacy of DNT. Then, a compound-target-disease network was constructed using the Cytoscape software to suggest the linkage of Q-markers to pharmacodynamic-related targets. Through the protein–protein interaction (PPI) analysis of the String database (https://string-db.org/), the PPI network diagram of the therapeutic effect of DNT was obtained to explore any therapy-associated protein factors. Metascape (https://metascape.org/), a platform for genetic function annotation, was applied for Gene Ontology (GO) as well as the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The top 10 levels of biological process (BP), cellular component (CC), and molecular function (MF) were described for GO enrichment analysis. KEGG is a database that integrates genome, chemical, and system function information, which can associate gene catalogs obtained from fully sequenced genomes with higher-level system functions at the cellular, species, and ecosystem levels. A target-pathway network was constructed using the top 20 enriched KEGG pathway.
2.9 Quantitative real-time PCR
Total RNA was extracted from liver tissue by using an RNA Faster200 reagent kit (Fastagen, Shanghai, China), and cDNA was synthesized by applying the PrimeScript™ RT Master Mix Kit (Takara, Shiga, Japan) in accordance with the manufacturer’s instructions. The Real-time PCR analysis was performed on a 6000 Real-Time PCR System (Thermo Fisher Scientific) using the SYBR Premix Ex Taq™ Kit (Takara, Shiga, Japan). And the relative expression of target genes was quantified using the 2−ΔΔCt method, normalized to that of Gapdh. The primer sequences are shown in Supplementary Table S1.
2.10 Statistical analysis
Experimental data were expressed as mean ± standard deviation (SD). Statistical analysis was performed with the two-tailed Student’s t-test or one-way analysis of variance (ANOVA) using Prism 7.0 (GraphPad Software, Inc., USA). The normality test was performed first in data analysis, and when the normality test failed, the nonparametric test was selected. The F-test was used to judge the homogeneity of variance, and different test methods were selected. A p-value < 0.05 was considered statistically significant.
3 Results
3.1 DNT attenuates ANIT-induced liver injury in mice
ANIT is a well-recognized hepatotoxin that can efficiently induce cholestasis in rodents (Sundaram et al., 2017). According to our previous study (Ding et al., 2012), DNT showed a dose-dependent protective effect on liver injury in rats. Therefore, DNT (3 g/kg) was chosen to test the anti-cholestatic effect of DNT in present study. The two central veins were located in the center of the liver slices of the VEH group animals, and the structure of the portal area was typical, without any pathological changes in morphology (Fig. 2A). In our present study, mice treated with ANIT (100 mg/kg) presented focal hepatic necrosis and acute infiltration of polymorphonuclear neutrophils in the portal area, as well as enlarged hepatocytes and loose cytoplasm with balloon-like or feather-like changes, which were consistent with previous reports. No apparent necrosis of hepatocytes and infiltration of immune-active cells were observed in the ANIT + DNT (3 g/kg) treated group compared with the VEH group, suggesting that DNT can ameliorate the ANIT-induced damage to liver tissues. Moreover, UDCA (90 mg/kg), an effective clinical drug for treating cholestasis, also decreased the extent of damage to hepatocytes as well as neutrophil infiltration induced by ANIT but with occasional hepatic necrosis.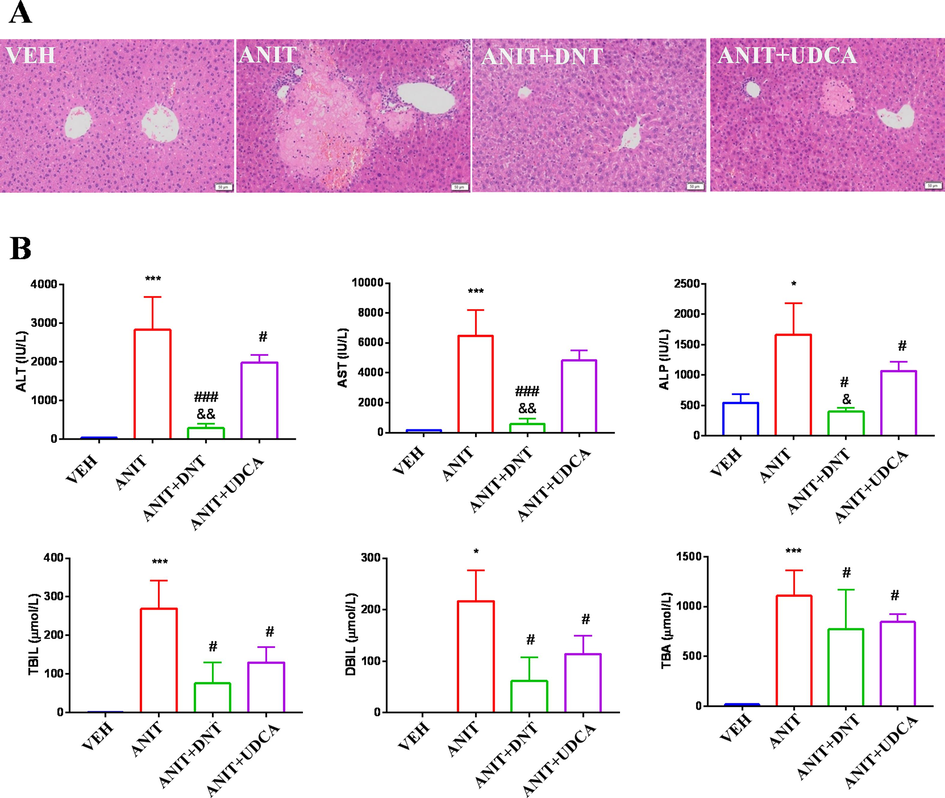
DNT attenuates ANIT-induced liver injury in mice. (A) Representative H&E staining (magnification × 400) images of liver sections of mice. (B) Serum levels of ALT, AST, ALP, TBIL, DBIL, and TBA. Compared with the VEH (control) group, *P < 0.05, **P < 0.01, ***P < 0.001; Compared with the ANIT (model) group, #P < 0.05, ##P < 0.01, ###P < 0.001; Compared with the ANIT + UDCA (positive) group, &P < 0.05, &&P < 0.01.
In addition, a dose of 100 mg/kg of ANIT drastically elevated the serum levels of ALT, AST, ALP, TBIL, DBIL, and TBA in mice (Fig. 2B; P < 0.05). These results suggested that ANIT might cause hepatotoxicity both by direct damage to hepatocytes and by inducing cholestasis, which was consistent with the histological changes observed in our present study. Conversely, the serum levels of these indicators were significantly reduced (P < 0.05) in the DNT (3 g/kg) treated group. UDCA (90 mg/kg) treatment also reduced the levels of ALT, ALP, TBIL, DBIL, and TBA (P < 0.05) in vivo. Notably, ALT, AST, and ALP levels were significantly lower in the DNT group compared to that in the UDCA group, implying that 3 g/kg of DNT could exert a better effect than 90 mg/kg of UDCA in recovering hepatocytes injuries caused by ANIT. Taken together, these results indicate that DNT can protect mice from ANIT-induced liver injury.
3.2 Identification of DNT-derived xenobiotics in mice
DNT consists of 7 herb ingredients according to TCM formulation theory, among which RRR and PCRR are regarded as the Emperor (Jun) drugs. An in-house database of DNT containing 732 chemical compounds was established by a wide-scale literature mining to help identify compounds. Our previous study has reported various components in the formula (Zhan et al., 2016), including terpenoids, flavonoids, aliphatic compounds, anthraquinone derivatives, and stilbenes, etc. In present study, serum and liver samples of mice from both VEH, ANIT and ANIT + DNT groups were analyzed both in positive and negative ion modes (Supplementary Fig. S1), respectively, to screen the DNT-derived xenobiotic chemicals.
The candidates should meet the following criteria: 1) be present in the samples from ANIT + DNT mice; 2) be present in the DNT formulae sample; and 3) be absent in the samples from VEH and ANIT mice. The total ion chromatography (TIC) was obtained using the PeakView with MasterView plugin. And the DNT-derived xenobiotic chemicals were identified by comparing the reference information (including accurate molecular weight, retention time, and MS2 fragmentation, etc.) with the chemical references available and those reported in references. As result, a total of 45 xenobiotic chemicals derived from DNT formulae were identified in mice. A summary of these compounds and the source herbal ingredient were shown in Supplementary Table S2. Here, isomer constituents with m/z 271.0599 Da in ESI+ mode were taken as an example to illustrate the identification process (Fig. 3). Three peaks with m/z 271.0599 Da were eluted at 11.3 min, 12.4 min, and 14.5 min, respectively. First, the exact mass of the components, i.e., m/z 271.0599, was automatic searched in the compound database of DNT. As results, a list of possible candidates was generated, including apigenin, aloe emodin, and emodin. Second, the MS2 fragments of these components were extracted and compared with reported data and with those generated by their corresponding standard chemical references. Third, tR (retention time) values of candidates and standard chemical references were matched. Finally, the three compounds of m/z 271.0599 were identified as apigenin (tR 11.3 min), aloe emodin (tR 12.4 min), and emodin (tR 14.5 min), respectively.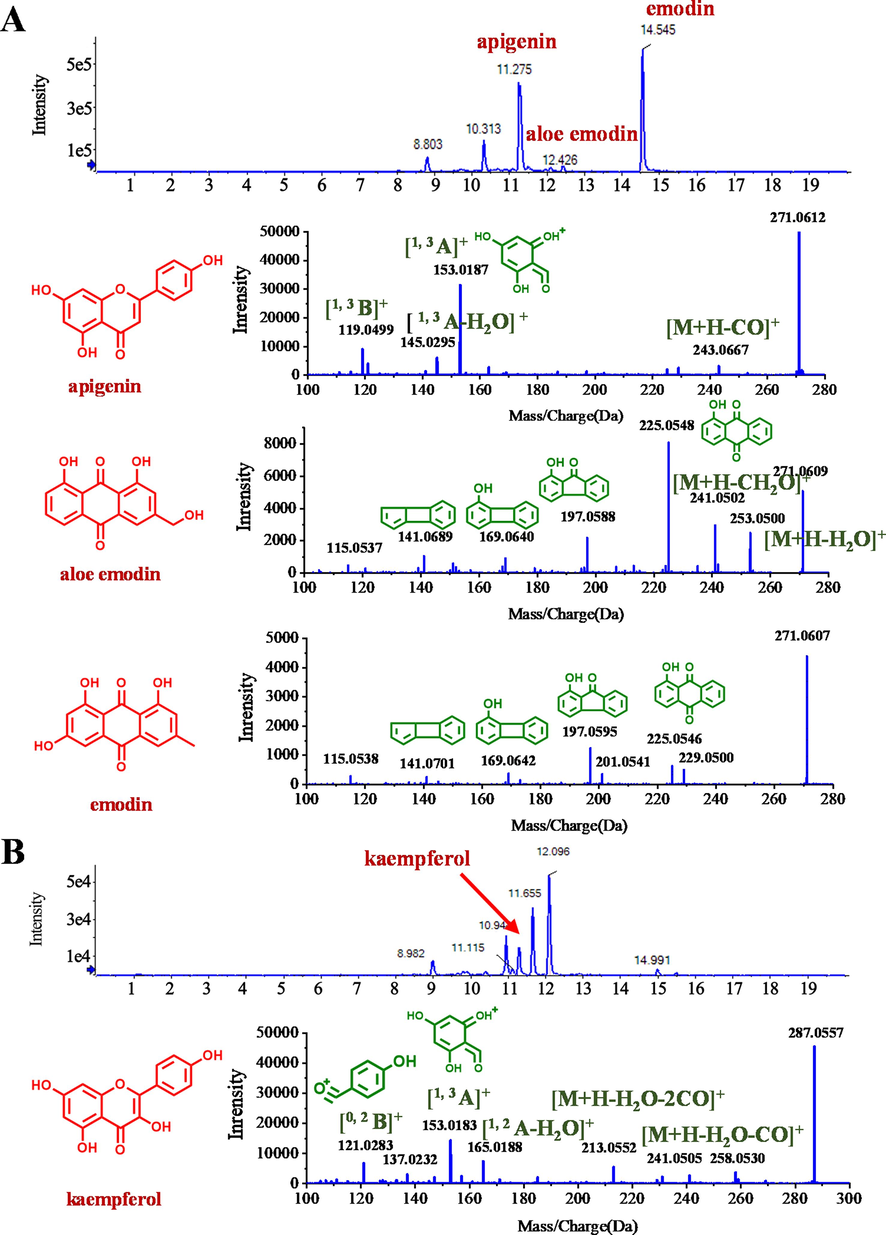
Identification of DNT-derived xenobiotic compounds in mice. (A) Total ion chromatogram (upper panel) and MS2 fragmentaion (lower panel) of apigenin, aloe emodin, and emodin in ESI+ mode. (B) Total ion chromatogram (upper panel) and MS2 fragmentation (lower panel) of kaempferol in ESI+ mode.
3.3 DNT recovers alteration in the metabolic profiling in ANIT-treated mice
Untargeted metabolomic study was performed by using serum and liver samples from mice in the VEH group, ANIT group, and ANIT + DNT groups. The LC-MS spectrum was processed using XCMS and pre-treated before performing the multivariate data analysis, including the unsupervised principal components analysis (PCA) and the supervised orthogonal partial least squares discrimination analysis (OPLS-DA). The results for serum and liver metabolic profiles are shown in Fig. 4.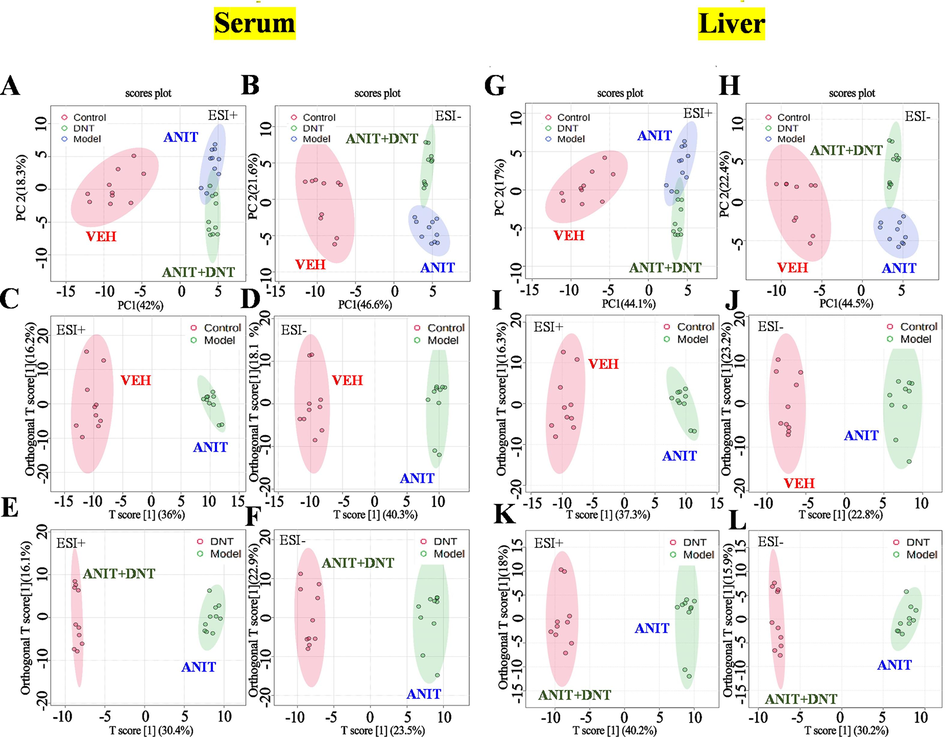
DNT recovers alteration in the metabolic profiling in ANIT-treated mice. The PCA score plots of serum samples (A, B) and liver samples (G, H) both in the positive and negative modes. The OPLS-DA score plots of serum samples (C-F) and liver samples (I-L) both in the positive and negative modes.
As for serum, the three groups formed a well-clustered inner group and were significantly separated in the PCA score plot from each other as indicated by LC-MS data acquired in the positive (Fig. 4A) and negative (Fig. 4B) modes. An OPLS-DA was further performed to mine out the markers that might contribute to the between-group differences. The values for R2Y and Q2 were all above 0.8 (Supplementary Table S3), suggesting satisfactory modeling and predictive abilities of the model. The score plots of OPLS-DA exhibited a distinguishable separation between the VEH and ANIT groups (Fig. 4C and 4D), like between the ANIT and ANIT + DNT groups (Fig. 4E and 4F). Additionally, a volcano plot of endogenous compounds also high-lighted alterations in the metabolic profiling in serum samples of mice from the VEH versus ANIT, and ANIT versus ANIT + DNT (Supplementary Fig. S2) pairs of groups. Volcano plots of differential compounds in the liver of cholestatic mice were presented in Supplementary Fig. S2E-H. While the three groups of liver samples were separated from each other based on their PCA score, acquired in both positive (Fig. 4G) and negative (Fig. 4H) modes. Besides, the score plots of OPLS-DA suggested distinguishable separation between the VEH and ANIT groups (Fig. 4I and 4 J), as well as the ANIT and ANIT + DNT groups (Fig. 4K and 4L). Following were the criteria of fold change > 1.5, P < 0.05 (by Student’s t-test), and a VIP value (in OPLS-DA) of > 1, differentially annotated peaks were screened and identified. Fragments matching of three bile acids compounds, namely taurocholic acid (TCA), glycocholic acid (GCA), and taurochenodeoxycholic acid (TCDCA), with the METLIN databases are shown in Fig. 5. The detailed endogenous metabolite identification information is supplied in Supplementary Table S4.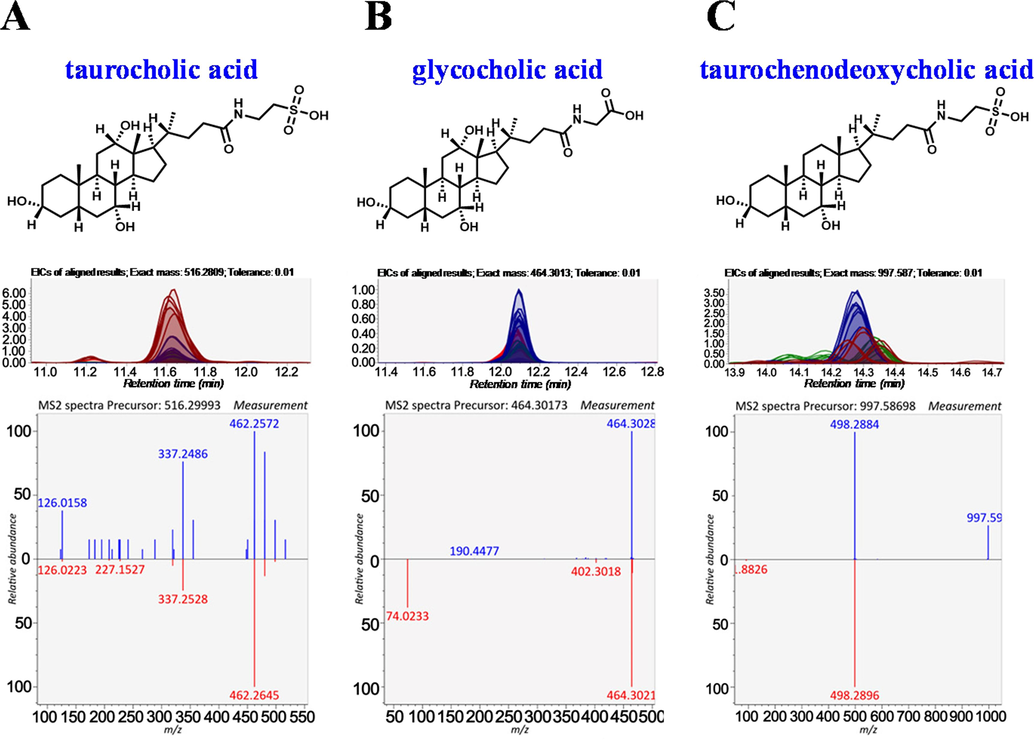
Identification of three potential endogenous biomarkers associated with the anti-cholestatic effect of DNT in mice. (A) Taurocholic acid, (B) Glycocholic acid, and (C) Taurochenodeoxycholic acid.
3.4 Correlations between the DNT-derived xenobiotics and endogenous biomarkers in mice
Correlation analysis is a statistical method to study the correlation between two or more random variables. The correlation coefficient obtained from Pearson’s correlation analysis is the covariance ratio of the standard deviations. It can reflect the intrinsic relationships between the variable groups. Thus, to further discover the pharmacodynamic chemicals responsible for the anti-cholestatic effect of DNT against ANIT-induced liver injuries, Pearson’s correlation analysis was performed between the DNT-derived xenobiotics and potential endogenous markers in rodent samples by using their intensities.
A cutoff value of the correlation coefficient at 0.8 was set to screen the DNT-derived xenobiotics and potential endogenous markers those were highly correlated. As results, TCA, GCA, and TCDCA were found to be highly correlated with the xenobiotics from DNT and identified as potential biomarkers associated with the anticholestatic effect of DNT in serum samples (Supplementary Table S5) as well as in liver samples (Supplementary Table S6). Briefly, in serum samples, TCA was positively correlated with luteolin (r = 0.85) (Fig. 6A) and luteolin-7-glucoside (r = 0.97) (Fig. 6B). While GCA and TCDCA were positively correlated with luteolin-7-glucoside (r = 0.92 and 0.95, respectively) (Fig. 6B). We also identified that in the liver, TCA was positively correlated with kaempferol (r = 0.81), luteolin (r = 0.83), and luteolin-7-glucoside (r = 0.95) but negatively correlated with apigenin (r = -0.81) and emodin (r = -0.81) (Fig. 6C and 6D). The TCDCA was positively correlated with 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone (DHPF) (r = 0.84) (Fig. 6D). In addition, hepatic mRNA expression of genes involved in bile acid synthesis and bile acid transport were further determined by q-PCR (Supplementary Figure S3). ANIT exposure decreased the levels of Farnesoid X receptor (Fxr), a key nuclear receptor modulating bile acid homeostasis via a feedback mechanism (Song et al., 2022; Liu et al., 2022). As those have been reported (Shi et al., 2022), ANIT exposure also decreased several genes involved in bile acid biosynthesis (including Fxr, Shp and Cyp8b1) and increased several genes involved in bile acid transport (including Ntcp and Bsep). DNT treatment reversed the changes of these genes, supporting that DNT modulates ANIT-induced bile acids impairment and bile acids are important endogenous biomarkers responsible for the anti-cholestasis effect of DNT.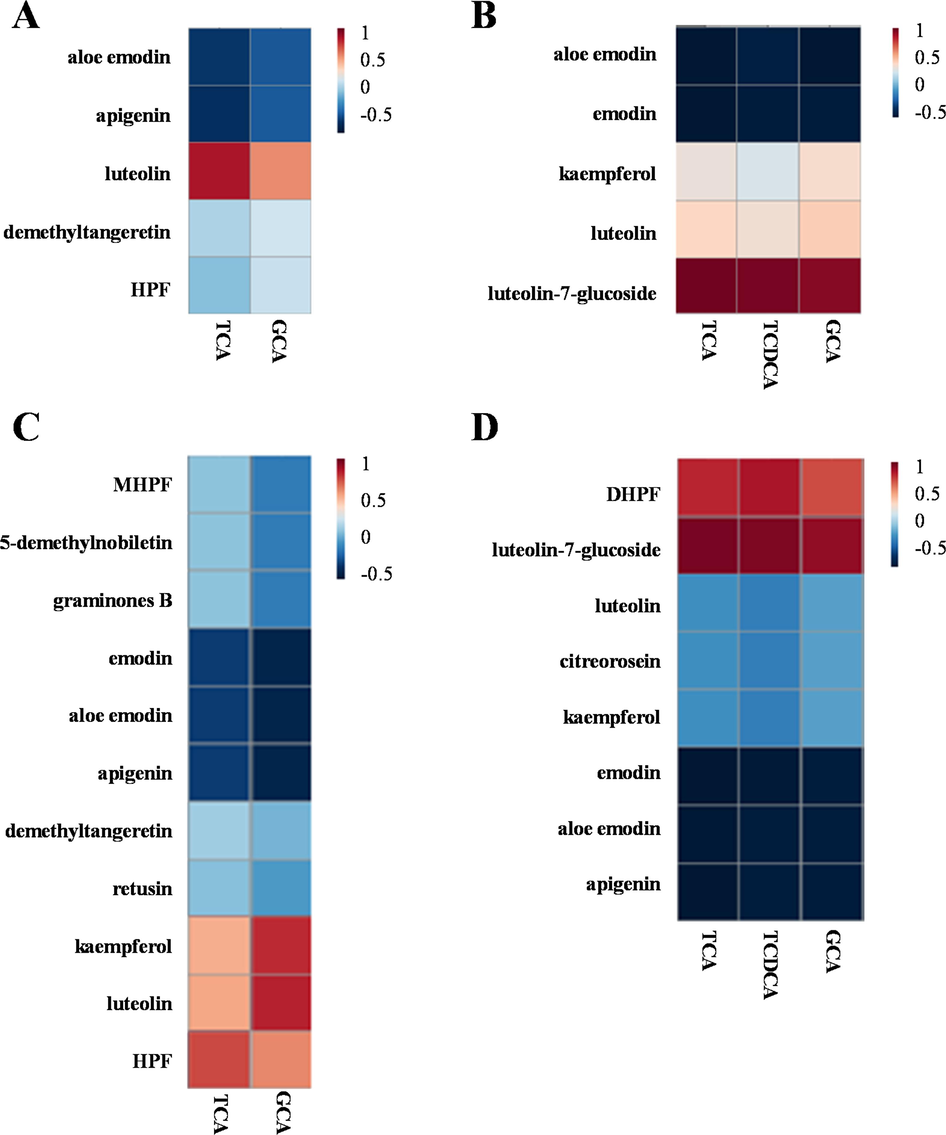
Correlation analyses of xenobiotic compounds and biomarkers associated with the anti-cholestatic effect of DNT in mice with ANIT-induced cholestasis. Correlation coefficients of them in serum samples in positive (A) and negative (B) ion modes. Correlation coefficients of them in liver samples in positive (C) and negative (D) ion modes.
Therefore, 6 xenobiotics from DNT formulae, namely, luteolin (source herb: CR), kaempferol (source herb: RRR, PCRR, CR, and CF), apigenin (source herb: PCRR), emodin (source herb: RRR, and PCRR), luteolin-7-glucoside (source herb: PCRR) and 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone (source herb: CRP), were selected as the potential Q-markers for the anti-cholestatic effect of DNT. Q-markers of TCM herbs and formulas should be stably existed in the herbs and formulas, and have readily available references. Therefore, the contents of 6 active Q-markers (luteolin, kaempferol, apigenin, emodin, luteolin-7-glucoside, and 5, 4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone) in three different batches of DNT were further determined. As results, the contents of luteolin, kaempferol, apigenin, and emodin in different batches of DNT preparations were stable, but the relative contents of different ingredients were quite different. In three batches of DNT, the average contents of luteolin (source herb: CR), kaempferol (source herb: RRR, PCRR, CR, and CF), apigenin (source herb: PCRR), emodin (source herb: RRR, and PCRR), luteolin-7-glucoside (source herb: PCRR), and 5, 4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone (source herb: CRP) were calculated as 0.0505 ± 0.0080, 0.0896 ± 0.0209, 1.0184 ± 0.1301, 3.7336 ± 0.3608, 1.3540 ± 1.1354, and 0.0148 ± 0.0137 mg/g, respectively. These results suggested that these six components could serve as the Q-markers for establishing the content determination method of DNT.
3.5 Network pharmacology suggests the possible mechanism and targets for the anti-cholestatic effect of the potential Q-markers from DNT formulae
A total of 142 predicted drug targets of the 6 potential Q-markers (including luteolin, kaempferol, apigenin, emodin, luteolin-7-glucoside, and 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone) were obtained. On the other hand, a total of 732 predicted disease targets associated with cholestasis-related diseases (including “NASH”, “Cholestasis”, and “Cholecystitis”) were acquired. The drug targets of the 6 potential Q-markers were matched with the disease targets, and a total of 28 targets were identified (Fig. 7A) and subjected to KEGG and GO pathway enrichment analyses. As results, a total of 60 KEGG pathways were obtained (Supplementary Fig. S4A), related to various cancer pathways, including the PI3K-Akt, AGE-RAGE, and NF-κB signaling pathways. Natural chemicals from herbs have been shown to protect against liver damage by modulating these signaling pathways (Wang et al., 2021, Wu et al., 2017). Furthermore, a total of 536 GO enrichment analysis items, including 478 biological process (BP), 15 cellular component (CC), and 43 molecular function (MF), were found. The top 10 items of each enrichment process are shown in Supplementary Fig. S4B. BP included the regulation of cellular responses to chemical stress, oxidative stress, trans-membrane transport, and ion transport. CC mainly involved the chromosomal telomeric regions, nuclear envelope, and endocytic vesicle. MF was focused on the regulation of nitric-oxide synthase activity, the binding of transcriptional coactivators, the response of NAD + ADP-ribosyltransferase activity, and ABC transporter activity. In addition, protein–protein interaction (PPI) analysis was further performed to screen the essential targets of the 6 Q-markers responsible for their cholestasis-related effects. According to the connectivity scores, four core regulatory genes, including TNF, AKT1, EGFR and ESR1, were highlighted as the key targets involved in the anti-cholestatic effect of the 6 potential Q-markers in DNT (Fig. 7B). The q-PCR analysis of the liver tissue suggested that hepatic mRNA expression of these genes was upregulated in mice after ANIT exposure and their expression was attenuated after DNT treatment (Fig. 7C).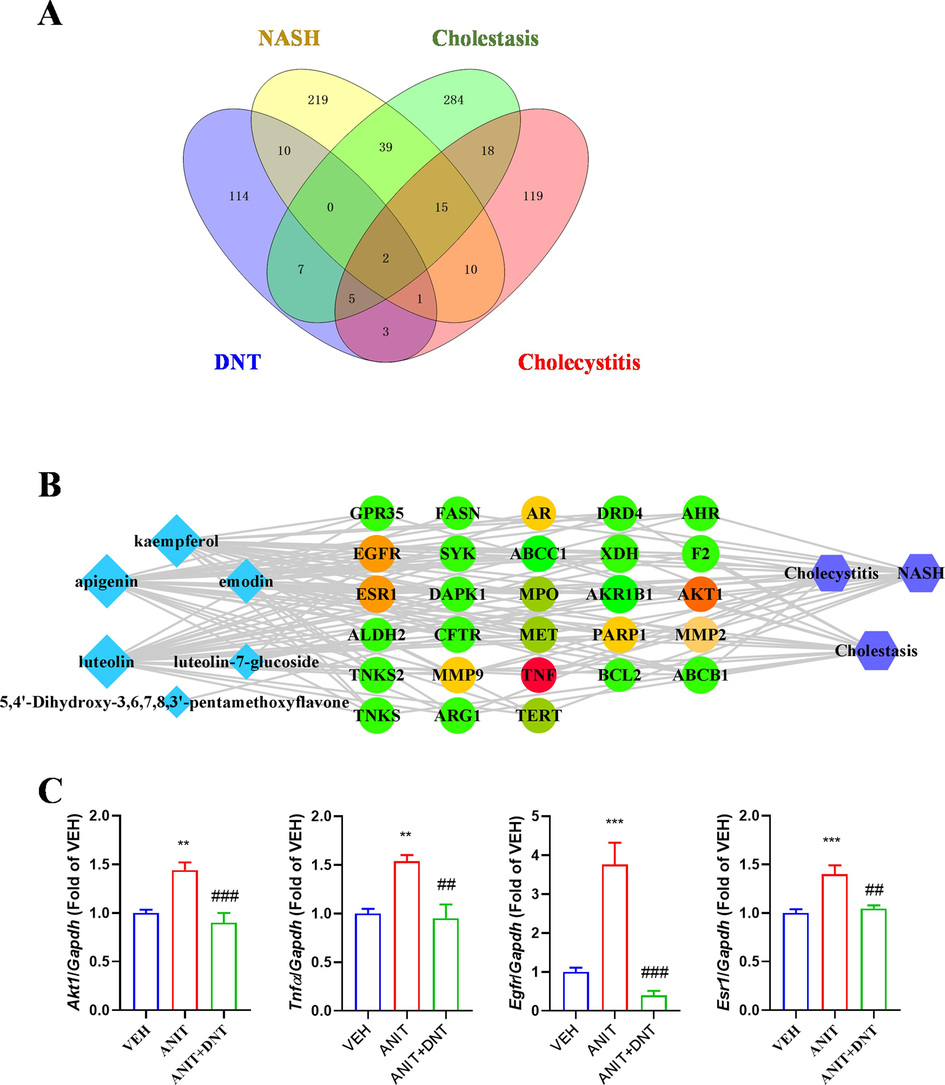
Confirmation of Q-markers of DNT by network pharmacology. (A) The number of potential targets for DNT Q-markers in the treatment of cholestasis-related disease. (B)The interaction network of the compound-target-disease. The light blue diamonds represent compounds, the green rotundities represent intersection targets, and the purple blue hexagons represent diseases. The grey edges indicate the link be-tween the compounds and the target. (C) Changes of hepatic mRNA expression of Tnfa, Akt1, Egfr and Esr1 in mice after DNT treatment. Compared with the VEH (control) group, *P < 0.05, **P < 0.01, ***P < 0.001; Compared with the ANIT (model) group, #P < 0.05, ##P < 0.01, ###P < 0.001.
4 Discussion
Cholestasis is caused either by an obstruction within the liver (intrahepatic) or outside the liver (extrahepatic) and is characterized by decreased bile flow and the concurrent accumulation of bile acids. Cholestasis has been involved in various hereditary and acquired liver diseases, yet the outcomes are poor and often without proper treatments. Currently, UDCA, the first-line anti-cholelithic and choleretic agent, is used to facilitate the bile flow through the liver to protect liver cells. However, diverse side effects caused by UDCA have been reported, including fever, hepatitis, cholangitis, vanishing bile duct syndrome, liver cell failure, severe watery diarrhea, pneumonia, interstitial lung disease, convulsions, and mutagenic effects. Several TCM herbs and formulas are reported to be effective in treating liver diseases (Wu et al., 2017, Ma et al., 2020). Zhuyu pills (Coptis-Evodia herb couples) can improve ANIT-induced fecal microbiota dysbiosis and effectively restore blood biochemical and metabolic profiles (Wei et al., 2022). Yinchenhao decoction has been used for treating cholestasis over 2,000 years (Shi et al., 2021; Yu et al., 2021). Huangqi decoction, which nourishes qi and strengthens the body, has broad application in the prevention and treatment of cholestatic, as well as in blocking the progression of the disease to liver cirrhosis (Ma et al., 2020). Although TCM has demonstrated great clinical achievements in treating cholestatic, it still has some underlying problems, such as ambiguous pharmacodynamic-related Q-markers, vague indications, unclear mechanisms of actions, and unknown influence on the bile components.
During cholestasis, bile acids accumulate in hepatocytes, inducing cytotoxic reactions (Woolbright et al., 2014). Several studies have also shown that increased oxidative stress plays an essential role in the pathogenesis of cholestatic injury. Bilirubin is a potent antioxidant, and TCA reduces intracellular bilirubin levels (Muchova et al., 2011). TCA significantly increases the expression of pro-inflammatory cytokines in mouse and human hepatocytes, and these pro-inflammatory cytokines, in turn, lead to further progression of neutrophil chemotaxis and liver injury (Chatterjee et al., 2018). Conjugated bile acids are generally more hydrophilic and can counteract the cytotoxicity of hydrophobic primary bile acids, e.g., pretreatment with low doses of TCA and TUDCA-protected GCDCA-induced apoptosis (Verhaag et al., 2016). In this study, the contents of TCA, TCDCA, and GCA were significantly increased in the ANIT group compared with the VEH group but were lowered in the ANIT + DNT group mice. These data suggest that DNT may alleviate ANIT-induced cholestatic liver injuries by reducing the concentration of bile acids in vivo.
Markedly elevated total bile acids levels are the predictive indicators of cholestatic jaundice. When cholestasis occurs, bile secretion decreases that rapidly changing the distribution of bile acid stores and increasing serum and urinary bile acid concentrations (Li et al., 2017). Our previous study has proved that DNT could promote the clearance of bile acids and bilirubin to prevent ANIT-induced cholestasis (Ding et al., 2014). DNT supplement following cholecystectomy operation also alleviated the cholestasis symptoms and reduced levels of several bile acids in cholecystitis patients after cholecystectomy surgery (Yang et al., 2016). Curcumin in DNT can exert better anti-cholestatic effects through the Farnesoid X receptor (FXR) signaling pathway (Yang et al., 2016). In our present study, DNT attenuated the changes of hepatic mRNA expression of Fxr and several downstream genes involved in bile acid biosynthesis and transport, which further proved that DNT can modulate ANIT-induced bile acids impairment.
A total of 6 DNT-derived xenobiotics compounds, including luteolin, kaempferol, apigenin, emodin, luteolin-7-glucoside, and 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone, were found to correlate with three specific bile acids, namely TCDCA, TCA, and GCA. Luteolin is a tetrahydroxyflavonoid, which has been reported to attenuate doxorubicin-induced derangements of the liver by reducing oxidative stress, inflammatory stress, and apoptosis (Owumi et al., 2021). Moreover, the anti-inflammatory and antioxidative activities of luteolin and luteolin-7-glucoside are also well characterized. These compounds could protect the liver from acute injuries by regulating the inflammatory mediators and antioxidative enzymes in hepatocytes (Fan et al., 2014, Park et al., 2019). Kaempferol is also a flavonoid with various biological effects, such as anti-inflammatory, antioxidant, and cardiovascular protection (Chen et al., 2019). Some studies have found that kaempferol may bind to PPARα. Another study has confirmed that kaempferol could ameliorate liver damage by regulating the LXRα-LPCAT3 axis and inhibiting ERS (Xiang et al., 2021). Apigenin is an effective flavonoid with anti-inflammatory, anti-tumor, and antioxidant activities (Berköz et al., 2021, Janda et al., 2021). In mice, pretreatment with apigenin attenuates methotrexate-induced liver injury by restoring the antioxidant defense mechanism (Sahindokuyucu-Kocasari et al., 2021). Besides, it is reported that apigenin can block gallbladder atrophy and associated liver injuries by modulating the FXR signaling pathway (Zheng et al., 2021). Emodin is a widely studied anthraquinone with an excellent preventive effect against various liver damage such as hepatitis, liver fibrosis, and hepatocarcinoma (Ding et al., 2018, Jia et al., 2014, Xie et al., 2019). It has been reported that emodin can rescue liver activity from cholestasis by stimulating the FXR/BSEP pathways and promoting the canalicular export of accumulated bile (Xiong et al., 2019). As reported, luteolin-7-glucoside, 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone, kaempferol, luteolin, and apigenin were flavonoid compounds that are reportedly biologically active in the human body with an important role in counteracting inflammatory processes by blocking the expression of pro-inflammatory effector molecules (Serafini et al., 2010, Yi et al., 2018). Luteolin-7-glucoside has demonstrated its potential in attenuating oxidative stress (Caporali et al., 2022, De Stefano et al., 2021). Wang et al. (Wang et al., 2021) have investigated the underlying mechanism of luteolin-7-glucoside in an in vitro inflammation model, which shows that it can strongly inhibit inflammatory responses and might be an effective substance against sepsis. Polymethoxylated flavones (such as 5,4′-dihydroxy-3,6,7,8,3′-pentamethoxyflavone) from citrus fruits are reported to have anti-inflammatory, antibacterial and neuroprotective activities, enabling them as active Q-markers (Peng et al., 2021, Singh et al.,2020). These ingredients were derived from different raw medicinal materials in DNT and stably existed with relatively higher contents in DNT formulae, indicating that they can be used as representative and valuable markers for the quality control of DNT formulae.
5 Conclusions
We proposed a strategy by integrating analysis of xenobiotics and endogenous components responsible for the pharmacodynamics and applied the established pipeline to uncover the Q-markers for the anti-cholestasis effect of DNT formulae. Six Q-markers were identified that were highly correlated with changes in endogenous bile acids, namely, TCA, TCDCA, and GCA levels, suggesting that they were the key components responsible for the pharmacological mechanism of DNT formulae in the treatment of cholestatic liver injury. The network pharmacology and content determination were designed to verify their participation in the pharmacodynamic activities related to cholestatic liver injury in DNT-treated mice.
This study demonstrated that DNT formulae had a potential protective effect on liver injury in mice and first systematically characterized six Q-markers responsible for the anti-cholestasis effect of DNT formulae, thus providing a valuable reference for screening the Q-markers of TCM formulas.
CRediT authorship contribution statement
Jie-Jing Yu: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft. Yu Wang: Methodology, Investigation, Data curation, Writing – original draft, Visualization. Xing Yan: Investigation. Xue Yan: Investigation. Yi Zhang: Investigation. Jiao-Jiao Wei: Investigation. Long-Chan Liu: Investigation. Li-Li Ding: Investigation, Visualization, Project administration, Funding acquisition. Ai-Zhen Xiong: Visualization, Conceptualization, Investigation, Writing – review & editing, Funding acquisition. Lin-Shan Jiang: Investigation. Zheng-Tao Wang: Conceptualization, Investigation, Supervision. Li Yang: Conceptualization, Investigation, Data curation, Writing – review & editing, Supervision, Funding acquisition.
Acknowledgments
This study is supported by the National Nature Science Foundation of China (82130115 and 82122074) and the Shanghai Nature Science Foundation (17DZ1920100 and 23ZR1463200).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee) of Shanghai University of Traditional Chinese Medicine (PZSHUTCM201023007).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Prophylactic effect of myricetin and apigenin against lipopolysaccharide-induced acute liver injury. Mol. Biol. Rep.. 2021;48:6363-6373.
- [Google Scholar]
- Caporali, S., De Stefano, A., Calabrese, et al, 2022. Anti-inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-glucoside. Nutrients. 14(6), 1155.
- Drug-induced cholestasis: Mechanisms, models, and markers. Curr. Drug Metab.. 2018;19:808-818.
- [Google Scholar]
- Screening and evaluation of quality markers from Shuangshen Pingfei formula for idiopathic pulmonary fibrosis using network pharmacology and pharmacodynamic, phytochemical, and pharmacokinetic analyses. Phytomedicine. 2022;100:154040
- [Google Scholar]
- Kaempferol alleviates acute alcoholic liver injury in mice by regulating intestinal tight junction proteins and butyrate receptors and transporters. Toxicology. 2019;429:152338
- [Google Scholar]
- Anti-inflammatory and proliferative properties of luteolin-7-O-glucoside. Int. J. Mol. Sci.. 2021;22(3):1321.
- [Google Scholar]
- Emodin attenuates lipopolysaccharide-induced acute liver injury via inhibiting the TLR4 signaling pathway in vitro and in vivo. Front. Pharmacol.. 2018;9:962.
- [Google Scholar]
- Protective effect of Danning tablet on acute livery injury with cholestasis induced by α-naphthylisothiocyanate in rats. J. Ethnopharmacol.. 2012;140:222-229.
- [Google Scholar]
- Danning tablets attenuates α-naphthylisothiocyanate-induced cholestasis by modulating the expression of transporters and metabolic enzymes. BMC Complement. Altern. Med.. 2014;14:249.
- [Google Scholar]
- Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One. 2014;9:e89961.
- [Google Scholar]
- Simultaneous spectrophotometric determination of drug components from their dosage formulations. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2022;270:120819
- [Google Scholar]
- Apigenin and luteolin regulate autophagy by targeting NRH-quinone oxidoreductase 2 in liver cells. Antioxidants. 2021;10:776.
- [Google Scholar]
- Preventive effect and mechanism of Danning Tablet on the cholesterol gallstone. J. Hepatopanereatobiliay Surg.. 2004;16:44-46.
- [Google Scholar]
- Emodin attenuates systemic and liver inflammation in hyperlipidemic mice administrated with lipopolysaccharides. Exp. Biol. Med.. 2014;239:1025-1035.
- [Google Scholar]
- A funnel-type stepwise filtering strategy for identification of potential Q-markers of traditional Chinese medicine formulas. Front. Pharmacol.. 2023;14:1143768.
- [Google Scholar]
- Mechanisms of bile acid mediated inflammation in the liver. Mol. Aspects Med.. 2017;56:45-53.
- [Google Scholar]
- Chemical markers for the quality control of herbal medicines: An overview. Chin. Med.. 2008;3:7.
- [Google Scholar]
- The potential effects and use of Chinese herbal medicine pine pollen (pinus pollen): A bibliometric analysis of pharmacological and clinical studies. World J. Tradit. Chin. Med.. 2020;6:163.
- [Google Scholar]
- Construction of traceability system of Chinese materia medica product quality based on quality marker of Chinese materia medica. Chin. Tradit. Herb. Drugs. 2017;48:3669-3676.
- [Google Scholar]
- A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs. 2016;47:1443-1457.
- [Google Scholar]
- Oleanolic acid alleviates ANIT-induced cholestatic liver injury by activating Fxr and Nrf2 pathways to ameliorate disordered bile acids homeostasis. Phytomedicine. 2022;102:154173
- [Google Scholar]
- Natural products for the prevention and treatment of cholestasis: A review. Phytother. Res.. 2020;34:1291-1309.
- [Google Scholar]
- Bile acids decrease intracellular bilirubin levels in the cholestatic liver: Implications for bile acid-mediated oxidative stress. J. Cell Mol. Med.. 2011;15:1156-1165.
- [Google Scholar]
- Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol.. 2021;40:1656-1672.
- [Google Scholar]
- Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice. Nutr. Res. Pract.. 2019;13:473-479.
- [Google Scholar]
- Comparative profiling and natural variation of polymethoxylated flavones in various citrus germplasms. Food Chem.. 2021;354:129499
- [Google Scholar]
- Apigenin alleviates methotrexate-induced liver and kidney injury in mice. Hum. Exp. Toxicol.. 2021;40:1721-1731.
- [Google Scholar]
- Shi, M.G., Tang, J., Zhang, t., et al, 2022. Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway. J Ethnopharmacol. 291, 115164.
- Preclinical evidence of Yinchenhao decoction on cholestasis: A systematic review and meta-analysis of animal studies. Phytother. Res.. 2021;35:138-154.
- [Google Scholar]
- Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int.. 2020;132:109114
- [Google Scholar]
- Potential therapeutic action of tauroursodeoxycholic acid against cholestatic liver injury via hepatic Fxr/Nrf2 and CHOP-DR5-caspase-8 pathway. Clin. Sci.. 2022;137(7):561-577.
- [Google Scholar]
- Verhaag, E.M., Buist-Homan, M., Koehorst, et al, 2016. Hormesis in cholestatic liver disease; Preconditioning with low bile acid concentrations protects against bile acid-induced toxicity. Plos One. 11, e149782.
- Schisantherin A ameliorates liver fibrosis through TGF-β1mediated activation of TAK1/MAPK and NF-κB pathways in vitro and in vivo. Phytomedicine. 2021;88:153609
- [Google Scholar]
- Reduning injection and its effective constituent luteoloside protect against sepsis partly via inhibition of HMGB1/TLR4/NF-κB/MAPKs signaling pathways. J. Ethnopharmacol.. 2021;270:113783
- [Google Scholar]
- Promising traditional Chinese medicine for the treatment of cholestatic liver disease process (cholestasis, hepatitis, liver fibrosis, liver cirrhosis) J. Ethnopharmacol.. 2022;297:115550
- [Google Scholar]
- Lithocholic acid feeding results in direct hepatotoxicity independent of neutrophil function in mice. Toxicol. Lett.. 2014;228:56-66.
- [Google Scholar]
- Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci. Rep.. 2017;7:9289.
- [Google Scholar]
- Kaempferol alleviates steatosis and inflammation during early non-alcoholic steatohepatitis associated with liver X receptor α-lysophosphatidylcholine acyltransferase 3 signaling pathway. Front. Pharmacol.. 2021;12:690736
- [Google Scholar]
- Emodin weakens liver inflammatory injury triggered by lipopolysaccharide through elevating microRNA-145 in vitro and in vivo. Artif. Cells Nanomed. Biotechnol.. 2019;47:1877-1887.
- [Google Scholar]
- Emodin rescues intrahepatic cholestasis via stimulating FXR/BSEP pathway in promoting the canalicular export of accumulated bile. Front. Pharmacol.. 2019;10:522.
- [Google Scholar]
- Screening the effective components of Suanzaoren decoction on the treatment of chronic restraint stress induced anxiety-like mice by integrated chinmedomics and network pharmacology. Phytomedicine. 2023;115:154853
- [Google Scholar]
- Curcumin protects ANIT-induced cholestasis through signaling pathway of FXR-regulated bile acid and inflammation. Sci. Rep.. 2016;6:33052.
- [Google Scholar]
- Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Mol. Nutr. Food Res.. 2018;62:e1800147.
- [Google Scholar]
- Efficacy of Zhuyu pill intervention in a cholestasis rat model: Mutual effects on fecal metabolism and microbial diversity. Front. Pharmacol.. 2021;12:695035
- [Google Scholar]
- Characterization of the principal constituents of Danning Tablets, a Chinese formula consisting of seven herbs, by an UPLC-DAD-MS/MS approach. Molecules. 2016;21(5):631.
- [Google Scholar]
- Apigenin protects mice against 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced cholestasis. Food Funct.. 2021;12:2323-2334.
- [Google Scholar]
- Clinical study on treatment of chronic cholecystitis, gallstones by Danning Tablets. Shanghai J. Tradit. Chin. Med.. 1990;24:18-20.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105640.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







