Translate this page into:
An integrated system combining electrochemical oxidation and filtration processes to remove chlorine from pharmaceutical industry wastewater
⁎Corresponding author. hjleu@fcu.edu.tw (Hoang-Jyh Leu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This research devised an efficient method to treat wastewater generated from heparin production at a local Taiwanese company. The approach combined electrochemical techniques with filtration processes to manage the wastewater, which exhibited high levels of total dissolved solids (TDS), chemical oxygen demand (COD), organic matter, and chlorine concentration, requiring treatment before disposal or potential reuse. A specialized electrochemical oxidation (EO) system was customized for processing 500 mL of solution at a 2A current, working in tandem with an activated carbon filter chamber. Various experiments were conducted, altering potential and adsorption times, to understand their correlation in eliminating the identified pollutants in pharmaceutical wastewater. Notably, sample 4 (S4), following an electrolysis process at 2A-12 V in 5 h and subsequent adsorption by 10 g of activated carbon for 60 min, exhibited remarkable efficacy Specifically, this treatment regime facilitated the removal of over 90 % (from 14,750 to 1422 mg/L) of chlorine concentration, 91 % (from 1.12 to 0.09 a.u.) of turbidity, and 88 % (from 22,676 to 2786 mg/L) of COD from the effluent stream. These favorable outcomes were attributed to the conversion of chlorine ions into hypochlorous acid, renowned for its potent antibacterial properties in eliminating organic compounds, as well as the robust adsorption capabilities of activated carbon. This study underscores the viability of employing surface adsorption and electrochemical processes in tandem, utilizing low input currents and brief treatment durations, to treat pharmaceutical wastewater effectively.
Keywords
Heparin wastewater
Electrochemical
High chlorine ion content
1 Introduction
The issue of environmental pollution has always been a global challenge since the industrialization and modernization phase began, particularly concerning water pollution. Numerous findings have identified wastewater sources from various industries and high-salinity agricultural practices (Grattieri and Minteer, 2018; Zhou et al., 2020). Discharging large volumes of wastewater as a byproduct of industrial activities has become a significant concern, not only for the environment but also for human health (Hülsen et al., 2019; Lin et al., 2020; Shi et al., 2015; Ahmed et al., 2021). These wastewaters are generated in the production processes of different chemicals, including various pesticides, herbicides, organic peroxides, pharmaceuticals, and dyes (Dhiman and Mukherjee, 2021; Pounsamy et al., 2019; Naje et al., 2015). Reports suggest that saline-contaminated wastewater constitutes about 5 % of the total discharged wastewater and is likely to expand further (Zhang et al., 2020). Consequently, treating saline-contaminated wastewater has become one of the top-priority tasks in conserving the demand for clean water.
In fact, treating saline-contaminated wastewater has never been straightforward. Various methods and processes have been utilized to manage such wastewater stemming from multiple industries (Cui et al., 2021; Srivastava et al., 2021). Among these primary approaches, they are classified into physicochemical treatment methods and biological treatment methods. Up to now, biological treatment methods have continued to be implemented due to their cost-effectiveness, minimal chemical requirements, limited generation of by-products, and so on (Chen et al., 2021; Saidulu et al., 2021). The Spiral Symmetry Stream Anaerobic Bioreactor (SSSAB) system has found widespread use in treating different wastewater types, including saline organic wastewater (SOW). Within the SSSAB system, parameters like hydraulic retention time (HRT) and organic loading rate (OLR) play crucial roles in assessing degradation performance, especially concerning Chemical Oxygen Demand (COD) removal. As indicated by Song et al. (Song et al., 2023), the treatment of saline heparin pharmaceutical wastewater through digestion resulted in an 82 % COD removal efficiency (from 8731 mg/L to 1211 mg/L) with an initial salinity of 3.57 wt% and an OLR of 6.98 kg COD/(m3•d). Song et al. (2021) further affirmed that the pilot-scale SSSAB system exhibited excellent performance, achieving COD and NH4+-N removal rates of up to 95.2 % and 96.6 %, respectively, with an HRT of 41.7 h. However, the high osmotic pressure caused by salt ions could pose significant threats to the microorganisms utilized in conventional biological treatment technologies, limiting their application in saline organic wastewater treatment. Consequently, physicochemical treatment methods have been researched and applied to replace traditional biological treatment methods for SOW processes, emphasizing the electrolysis method.
Electrochemical Oxidation (EO) has emerged as a promising technology for efficiently treating organic pollutants and contaminants in wastewater through direct or indirect oxidation processes (Ma et al., 2021). The treatment efficiency of the EO system can be adjusted and expanded based on the number of electrodes and the active area of the reaction chamber. EO technology demonstrates remarkable efficacy in treating and breaking down non-biodegradable compounds (such as Triclosan, CBZ, pesticides, etc.) (Magro et al., 2020; Cai et al., 2022; Trellu et al., 2021). This has been validated by its ability to achieve optimal COD removal within a 360 min electrolysis period in the direct oxidation treatment of washing machine wastewater by Durán et al. (Duran et al., 2018). However, researchers have discovered that in indirect oxidation methods with the presence of chloride ions, higher electro-efficiency is achieved. Chloride, under the influence of electric current, transforms into Hypochlorite, a commonly used indirect oxidation agent in wastewater treatment (Moreira et al., 2017). Klidi et al. (Klidi et al., 2018) confirmed the existence of indirect EO with chloride, exhibiting higher electro-efficiency, reducing COD by 60 %, and chloride concentration by 87 %. Another study demonstrated that the introduction of NaCl and NaOCl into textile printing wastewater, using pairs of graphite electrodes, eliminated 86 % of COD within 150 min of EO treatment. Despite EO's effectiveness in rapidly reducing COD and organic compounds in wastewater, concerns persist regarding cost-effectiveness and chemical usage.
In this study, a Hybrid system was employed for treating saline wastewater from a pharmaceutical company based in Taichung, Taiwan, combining an EO module and an activated carbon filtration system. The EO reaction chamber (the core of the system) featured a simple design, requiring only a direct current power source, a pair of titanium electrodes coated with platinum (Pt/Ti) installed in parallel, directly connected to peripheral devices via solution pumps and conduits. Leveraging the high chlorine concentration in the solution, it was directly converted into Hypochlorous acid, serving as a potent oxidizing agent for breaking down organic compounds without the need for additional chemicals. Following the treatment cycle, the effluent showcased processing efficiencies of over 87 % for COD, 90 % for chlorine concentration, 87 % for turbidity, and a 48 % reduction in salinity compared to the input stream.
2 Material and method
2.1 Chemicals
Potassium dichromate (K2Cr2O7), Silver nitrate (AgNO3), Ammonium ferrous sulfate (FeH8N2O8S2), Ferroin indicator (C36H24FeN6O4S) used in the COD test and Sodium chloride (NaCl) for the standard potential curve in ion chlorine concentration were recorded from Union Chemical Work, Taiwan. These chemicals were obtained to ensure the utmost purity and necessitate no additional purification. The Pt/Ti electrodes used for electrolysis and activated carbon for adsorption process were procured from a local market.
2.2 The characteristics of pharmaceutical wastewater
The wastewater used in this study originates from a pharmaceutical factory located in Taichung, Taiwan. It is obtained directly from the processing of animal organs and heparin production (antibiotics from the glycosaminoglycan family of carbohydrates). The influent contains various organic and inorganic ingredients, such as intermediates, spent solvents, reactants, and catalysts. Generally, the company's wastewater output is strictly controlled, and the wastewater collected in this study is from the intermediate process, not the company's final wastewater. However, enterprises want to move towards a new treatment method that takes less time to recreate wastewater and reuse it for other uses. Large amounts of wastewater are transported directly from the factory to the Laboratory of Green Energy Science and Technology (GEST) at Feng Chia University for treatment. The basic parameters and characteristics of the input wastewater are preliminarily reported in Table 1.
Parameter
Value
Reference Value
pH
8.2
4 – 9 (Zhou et al., 2006; Shi et al., 2017; El-Gohary et al., 1995)
Concentration of chlorine (mg/L)
14,750
10,000 – 30,000 (Zhou et al., 2006; Shi et al., 2017; Li and Li, 2015; Shi et al., 2014)
Conductivity (mS/cm)
41.2
–
TS (mg/L)
19,600
–
TDS (mg/L)
14,000
20,000 – 35,000 (Zhou et al., 2006; Shi et al., 2017; El-Gohary et al., 1995; Li and Li, 2015)
TSS (mg/L)
5600
–
COD (mg/L)
22,676
5000 – 60,000 (Zhou et al., 2006; Shi et al., 2017; El-Gohary et al., 1995; Li and Li, 2015)
Salt (mg/L)
29,500
10,000 – 32,000 (Zhou et al., 2006; Shi et al., 2017; El-Gohary et al., 1995; Li and Li, 2015)
Color (a.u.)
1.12
–
Following conveyance, the wastewater was stored in a fume hood to prevent bacterial environmental influence. This approach, supported by empirical observations and parameters cited earlier, revealed distinct features of the wastewater stream: a robust odor and a green-yellow hue (Hao et al., 2000; Türker et al., 2022), indicative of elevated organic matter content. The electrochemical device operated under four distinct voltage settings, denoted by varying colors. After treatment, samples were preserved to ensure measurement precision, as depicted in Fig. 1 below.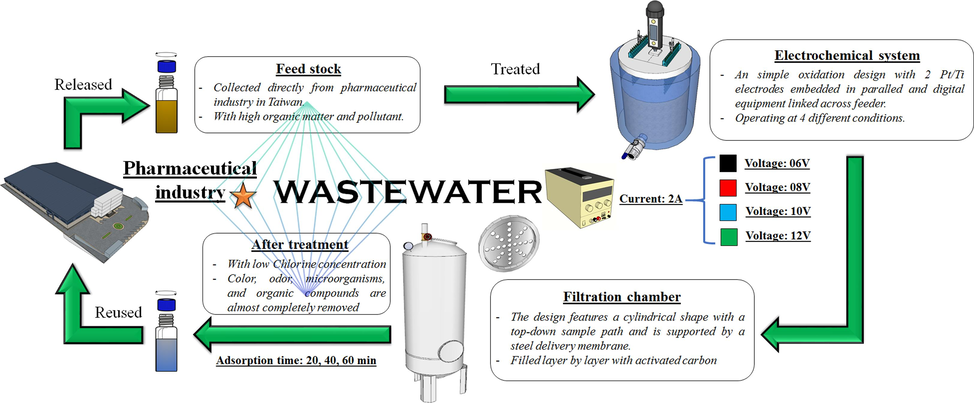
The complete model illustrates the mechanism of pharmaceutical wastewater treatment, from input to reuse, using a dual system that combines the EO system and filtration chamber.
2.3 Electrochemical reactor: Setup and mechanism
The study's design is based on fundamental theories of the laboratory-scale EO system, including a reactor housing two inert electrodes submerged in the solution and powered by a DC supply (TES electrical, Taiwan), as depicted in Fig. 2. The organic matter was loaded in by a peristaltic pump (Longer Pump, Hebei, China). Digital multi-meter measuring tool (Jenco-6173, Taipei, Taiwan) was set up at the top of the reactor via the feeder to calculate the response values. Two Pt/Ti electrodes (5.9 cm x 12 cm) with a thickness of 0.5 mm were placed in parallel, maintaining a 5.7 cm gap to meet standard operating conditions at 2A − 8 V. The electrode angle and gap were adjustable, allowing modification of the potential difference within desired values ranging from 6 V to 12 V (the maximum voltage adjustable at 2A). Each run involved applying 300 mL of influent to the reactor for a solution behavior assessment. At 30 min intervals, 5 mL of solution was extracted and stored at room temperature in a cabinet.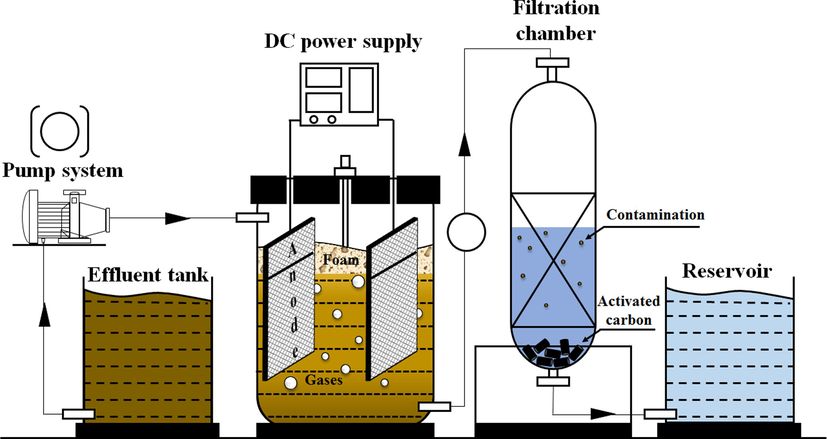
The schematic of dual system for pharmaceutical wastewater.
The airflow generated by the pump (SW-1504, Taichung, Taiwan), operating at a discharge flow rate of approximately 3500 cc/min, facilitates an even distribution of pollutants within the solution, promoting their decomposition. It simultaneously creates a lift force so that the treated organics can be removed more efficiently, thereby increasing the performance. In EO system, the organic pollutants are eliminated by chlorine and hypochlorite. During the oxidation process, the reaction of sodium chloride produces chlorine gas (Szpyrkowicz et al., 2005). The following equations presented the reactions of the anode and cathode:
-
Anode reaction
-
Cathode reaction
Because of the weak acid, hypochlorite is easy to dissociate partially.
In indirect EO, pollutants are oxidized in the bulk of the solution by means of oxidants electrochemically generated on the anode. The compounds of hypochlorite and active chlorine in bulk reactions (Eqs. (4) and (5)) were chemically reactive which decomposes organic pollutants into carbon dioxide and water (Bonfatti et al., 2000; Martínez-Huitle et al., 2008). Consequently, this oxidizing agent played a pivotal role in the pollutant removal process investigated in this study. Additionally, electroflotation, a well-known method supporting wastewater treatment through electrochemical means using inert electrodes, was employed. The production of pure hydrogen and oxygen gas bubbles, as outlined in Eqs. (2) and (3) during water electrolysis, facilitated the transportation of suspended particles, allowing them to rise to the surface within the electrolysis system (Mohtashami and Shang, 2019). Equation (6) below expresses the total reaction in EO system:
2.4 Calculation
The system's operating efficiency is measured by the combined removal efficiency of undesired pollutants and the recovery efficiency of reclaimed water. Equation (7) determine the removal efficiency for all of the investigated pollutant parameters after the treatment process:
R (%) = x 100 (7)
Here, Co represents the concentration or value of original pollutants in the input effluent (measured in mg/L), while Cp represents the concentration or value of pollutants in the treated effluent (also measured in mg/L). The concentration of chlorine, TDS, TSS, and COD was determined through APHA’s Standard Methods and specific relevant measurements.
Rv (%) = x 100 (8)
Where V0 is the intensity of input effluent (L) and VP is the bulk of the remaining solution after the treatment (L).
The DC source supply energy can be estimated by utilizing the following Equation (Elert, 2023):
Where Ee is the energy that supplies system (J), C is the supply voltage (V), I is the working current (A) and t is the working time (min).
2.5 Analytical methods
2.5.1 Concentration analysis
The APHA’s Standard Methods (Federation and Association, 2005) were used to measure the chemical oxygen demand (COD). The sample was diluted 10 to 100 times to reduce the chloride concentration and avoid the precipitation reaction with silver ions forming silver chloride, which affects the measurement result. Potassium dichromate reactant and Silver nitrate catalysts were added to the diluted samples, heated at 150 °C for 2 h, and then cooled naturally. Ferroin indicator was added before using the standard solution of Ammonium ferrous sulfate to determine the value using the calculation formula:
COD (mg/L) = (10)
Where, a represents the titrant of the blank (mL), b represents the titrant of sample (mL), M is Ferrous ammonium sulfate concentration (M), V is sample volume (mL), and X is dilution factor.
The total solid (TS), total suspended solids (TSS), and total dissolved solids (TDS) values of the effluent in this work were measured by evaporated method. The TS values were determined by weighing the material left after evaporation and drying of the sample in the oven at 180 °C for 2 h. The values of TDS were determined in a similar manner as TS; sample filtrate passing through filter paper (90 mm) was evaporated in a weighed dish and dried in the oven at 180 °C for 2 h. The values of TSS were obtained from the difference between TS and TDS (Adjovu et al., 2023; Taylor et al., 2018). Furthermore, the values of TDS and TSS were determined using an intermediary method by establishing the relationship between electrical conductivity-total dissolved solids (EC-TDS) through the following equations (Walton, 1989):
Where, TDS* is in mg/L, TSS* is in mg/L, EC is in μS/cm at 25 °C, and ke is a constant of proportionality = 0.55 ∼ 0.85 (0.7 is chosen in this study for the recommended appropriate factor) (Walton, 1989). The application of intermediate measurement methods using EC values helps reduce the waiting time for sample processing and provides real-time quantification of liquid intensity (Fallatah and Khattab, 2023). Therefore, these techniques are commonly used in educational settings for scientific analysis and research.
The chloride ion concentration was calculated indirectly through a standard solution prepared by diluting sodium chlorine with deionized water to specific concentrations to build a standard electrode potential curve. The chloride ion molarity can be determined from the curve with practical values. Salinity was measured using the conductivity method using refractometer equipment (TR-055, Taichung, Taiwan).
The UV/vis spectrophotometer with a model SPECTRONIC GENESYSTM 2PC, and slit width 2 nm with quartz cuvette 1 cm used to determine the color change of solution after treatment.
2.5.2 Material characterization
The activated carbon's morphology was analyzed using scanning electron microscopy (SEM, Hitachi S4800 type 2) equipped with Thermo NORAN NSS EDS. The cold field electron gun allowed for magnification ranging from 20 to 800,000 times, achieving a resolution of 1 nm at 1 kV and 1.4 nm at 15 kV. The SEM analysis of the activated carbon involved a sample preparation process: drying the sample in an oven at 80 °C for 2 h followed by coating with a platinum layer for 30 s. This SEM method facilitated the examination of the activated carbon's microscopic structure and surface morphology.
3 Results and discussion
3.1 Effects of voltage on chloride removal and operating temperature
Such as the main goal of the study, 300 mL of saline wastewater was directly treated in the EO reaction chamber at four different voltage values under the same current intensity of 2 Amperes, labeled as S1 to S4. Fig. 3A distinctly illustrates the variation in chlorine ion concentration in the solution before and after 5 h treatment, with the highest removal efficiency reaching 76.4 % (from 14,750 to 3434 mg/L) observed in S4, operating at 2 Amperes and maintaining a consistent voltage of 12 Volts in the EO reactor. The variation in voltage directly impacted the treatment efficiency of the entire oxidative reduction process, gradually increasing from 33.4 % to 76.4 % after the completion of the reaction. The results indicate that the EO method is suitable for removing chlorine ions from saline solutions by transforming them into potent oxidizing compounds for decomposing organic substances or converting them into gaseous forms. This aligns well with the previously discussed reaction mechanisms. In comparison to reports by Darvishmotevalli et al. (2019) and Ye et al. (2020), the chlorine ion removal efficiency in this study (76.4 %) exceeds the figures mentioned in those reports (73 % and 50 %, respectively). However, observers noted a nearly 40 % reduction in the recovered water volume in S4 after the electrolysis reaction, which prompted the application of activated carbon adsorption to prevent wastage.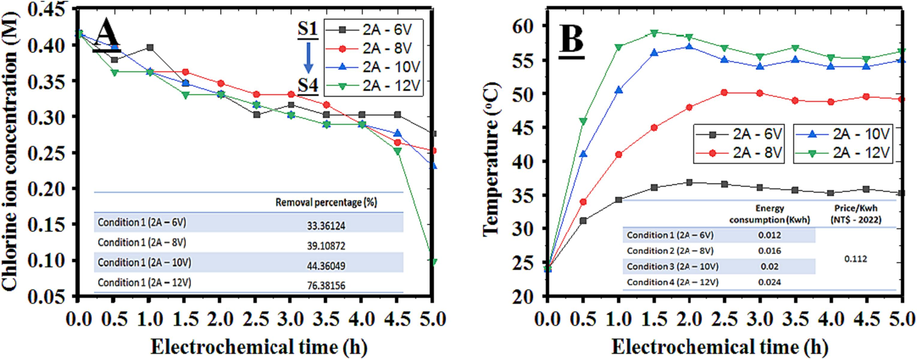
The ability to remove chlorine (A) and the increase in the heat index (B) under the effect of electric potential.
In addition to the variation in ion concentration, temperature was also among the parameters examined in this investigation. The temperature rises for a conventional EO system as more energy accumulates within the same survey time frame (Phan et al., 2023). Additionally, heat released from the decomposition of organic compounds also contributes to temperature variations. Under current operating conditions, a noticeable temperature difference has emerged after 30 min of operation, peaking after a specific duration and stabilizing after that. This temperature variance correlates with ongoing metabolic reactions. Consequently, the pH values in Fig. 4 also exhibit pronounced fluctuations. Unlike the observations made by Sun et al. (Sun et al., 2020), the temperature does not demonstrate a tendency to rise continuously during prolonged processing; instead, it stabilizes after a certain period and remains constant throughout the oxidation–reduction reactions, provided that the current intensity is controlled. The peak temperatures observed were approximately 35 °C, 47 °C, 55 °C, and 58 °C at respective voltage values of 6 V, 8 V, 10 V, and 12 V, as depicted in Fig. 3B. Compared to Nguyen et al. study (Phan et al., 2023), the temperatures during the stable phase in this research are significantly lower than 84 °C after 3 h of reaction under 3.05 A and 30 V conditions. This divergence results from employing higher voltage to reduce processing time. Consequently, supplying additional energy to the electrolytic cell has led to increased current and temperature. These factors could potentially impact the durability and safety of the system.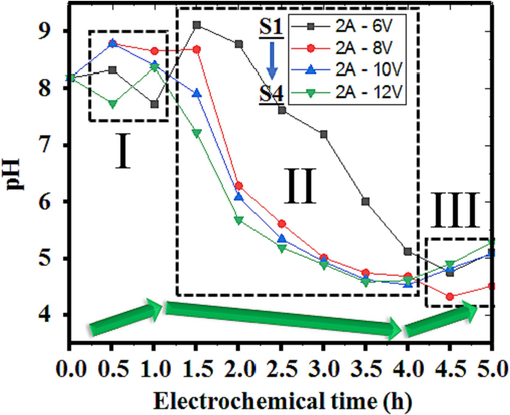
Change in pH of the solution under voltage variation in the EO system.
3.2 Effects of voltage on pH
The pH level of a solution stands as a crucial parameter influencing the generation of hypochlorous acid (HClO) and hypochlorite ions (ClO-) pivotal for pollutant removal efficiency (Cheng et al., 2022; Belghit et al., 2020). This value is contingent upon reaction mechanisms, electrode utilization, and specific pollutants (Prazeres et al., 2019). George Bowman's previous studies at The Wisconsin State Lab of Hygiene revealed a gradual decline in hypochlorous acid concentration from nearly 100 % to almost 0 % when the pH reached 9.0 (Bowman and Mealy, 2007). Optimal pollutant elimination occurred within a pH range of 5 to 8 due to a significantly heightened conversion efficiency into HClO/ClO- compounds (Jiang et al., 2020). Typically, pH levels tend to increase over the reaction period, making pH control imperative to sustain an optimal pH for effective pollutant eradication (Takabe et al., 2022). One can adjust pH by introducing solid acids like sulfuric, hydrochloric, or sodium hydroxide bases (Nidheesh et al., 2020). Fortunately, the wastewater in this study demonstrates a high chlorine ion concentration, allowing for direct conversion into potent oxidative compounds without the need for extra chemicals to sustain the optimal pH level. At the anode and under the influence of direct current, free chlorine ion molecules transform into chlorine in the first stage. Subsequently, upon combination with water molecules, this chlorine forms hypochlorous acid, a highly bactericidal compound surpassing hypochlorite by nearly 80 times in potency (Block and Rowan, 2020). Due to its nature as a weak acid, it partially dissociates, forming hypochlorite ions. Additionally, the reaction between chlorine and water, along with the metabolism of hypochlorous acid, generates free H+ ions, thereby reducing the pH of the solution post-treatment. After a 5 h treatment, there is a slight tendency for the pH to increase, typically within the range of 4.5 to 5.5. This increase occurs because the remaining Na+ ions in the solution act as a charge balance alongside ClO- ions, stabilizing the overall reaction.
In this study, the input solution with a pH value of 8.2 was used to evaluate the effect of voltage on the conversion of agents in the solution. In Fig. 4, the results are divided into different data regions showing two main mechanisms throughout the reaction: conversation and decomposition of organic matter. With the voltage change, the data has an apparent variation in the electrochemical treatment at the same reaction time. In general, the pH decreases sharply and is regular when the voltage changes from 6 V to 12 V. This phenomenon explains the effect of voltage on the production rate of hypochlorous acid in the reaction solution, thereby affecting the decomposition efficiency of organic substances. In addition, the amount of syngas at both electrodes will also be subject to variation under the change of the applied voltage. Several prior studies align with similar findings, demonstrating enhanced pollutant removal efficiencies within the pH range of 5 to 8. Al-Raad et al. (Al-Raad et al., 2019) observed an increase in pollutant removal efficiencies, rising from 86 % at pH 6.5 to 92 % at pH 8 when employing aluminum-aluminum (Al-Al) electrode pairs in Lake water-electrolyte with a conductivity of approximately 38.6 mS/cm. Similarly, Kermet-Said et al. (Kermet-Said and Moulai-Mostefa, 2015) noted the highest removal efficiencies (70.8 % for COD and 96.7 % for turbidity) at a pH value of 5.31 for pharmaceutical effluents, with a subsequent decrease in efficiency observed at pH values exceeding 10.
3.3 Effects of voltage on color removal
Fig. 5A displays a representative UV–VIS spectrum of heparin saline wastewater before and after electrolysis at different voltage levels. The prominent absorption peaks detected at 422 nm, indicative of specific pollutants, and a UV absorption peak centered around 302 nm were particularly noteworthy. Analysis of UV–VIS spectra demonstrated a substantial reduction in the absorbance characteristics of wastewater pollutants upon employing Pt/Ti electrode pairs for SOW treatment through EO methods. This reduction signifies the successful removal of pollutants, including the previously mentioned peaks. After 5 h treatment, removal efficiency progressively increased from 40 % to 72 %, 78 %, and reached up to 87 % at 6 V, 8 V, 10 V, and 12 V, respectively. These outcomes indicate that the increment in voltage directly impacts the degradation of organic compounds present in the solution, a finding consistent with Wang et al. confirmation (Wang et al., 2023). Concurrently, as Xie et al. noted (Xie et al., 2012), heparin wastewater can also be treated using a surface adsorption method employing nickel zinc ferrite nanoparticles, achieving an approximate 90 % treatment efficiency when combined with hydrogen peroxide oxidation and coagulation filtration. The negligible difference in peak absorption wavelengths (approximately 4–5 nm) is attributed to variations in pollutant concentration levels.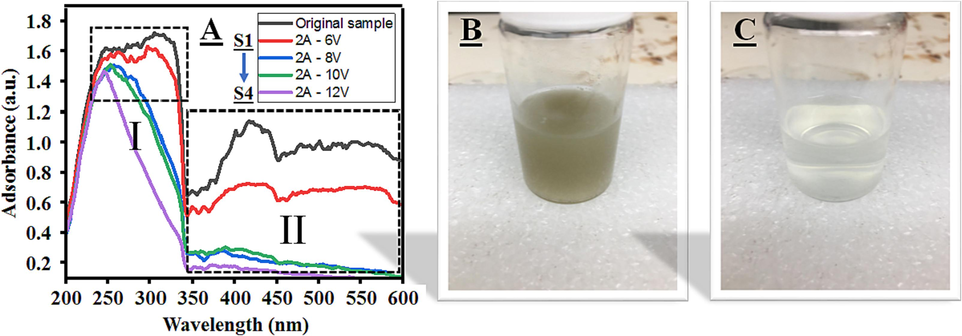
(A) UV–VIS spectra of four difference samples, (B) influents flow coming with yellow-green color, (C) effluents flow out without color at 2A-12 V.
Unlike heavy industrial wastewater, pharmaceutical wastewater's color and odor indicate the presence of organic compounds and bacteria in the solution (Chakrabortty et al., 2020; Moideen et al., 2023; Gupta et al., 2019). Following the reaction, the wastewater undergoes nearly complete removal of organic compounds and bacteria, producing a clear solution, as depicted in Fig. 5C. As a result, there are no significant changes in the surface material of the electrodes after the EO treatment. The changes referred to here are the corrosion from the clip and ions adhering to the surface, which alters the shape and color of the original material. As inert electrodes, the primary function of the cathode and anode in the entire reaction is to allow the movement of electrons to transfer without directly participating in the chemical reactions throughout the reaction process (Isik et al., 2021; Pourbaix et al., 1959).
3.4 Effects of voltage on electrical conductivity
Current flow density directly correlates with the electrolyte solution's electrical conductivity and ion concentration, influencing the pollutants' degradation time. Higher electrical conductivity leads to shorter treatment durations for specific pollutants, reducing energy consumption (Chou, 2010). In typical wastewater treatment practices, augmenting the electrolytic solution's conductivity involves using NaCl, as indicated in previous studies (Alam et al., 2022). However, the solution's salt concentration was already sufficient for conductivity enhancement in this investigation. Fig. 6A demonstrates the direct impact of voltage fluctuations on the variation in current density during electrolysis. Elevated voltage levels prompt the oxidation of chloride ions in the saline solution, transforming them into more active chlorine forms, potent oxidizers that significantly aid in disinfection. The initial wastewater, with a pH of 8.2 and a conductivity of 41.2 mS/cm, served to assess the impact of voltage on treatment effectiveness and conductivity metrics. Throughout the treatment process, shifts in the wastewater's electrical conductivity were observed, ranging between 35.4 and 42.6 mS/cm under electrochemical treatment. Specifically, there was a noticeable change in electrical conductivity values after 1.5 to 2 h of treatment. During this period, the reaction process significantly altered parameters such as pH, temperature, and chlorine ion concentration. Notably, under conditions of 2A-12 V, the maximum removal efficiency of chlorine ions (76.4 %) was achieved after 5 h treatment period, getting current density higher than the initial value (approximately 3.3 %). This phenomenon is attributed to the corrosion of the iron alligator clip upon direct contact with the foam layer formed from the breakdown of organic impurities. Under the high impact of voltage and the thermal energy generated by the reaction, metal ions from the clip corrode, reintroducing themselves into the solution and thus increasing its conductivity. Sazou and Pagitsas (2006) documented that the iron electrode surface exhibited signs of corrosion across various dissolved states under the influence of chlorine ions, resulting in electrochemical instability and impacting treatment efficiency. Therefore, careful consideration in selecting electrode materials and designing EO systems is crucial to mitigate the undesirable influence of ions on treatment effectiveness.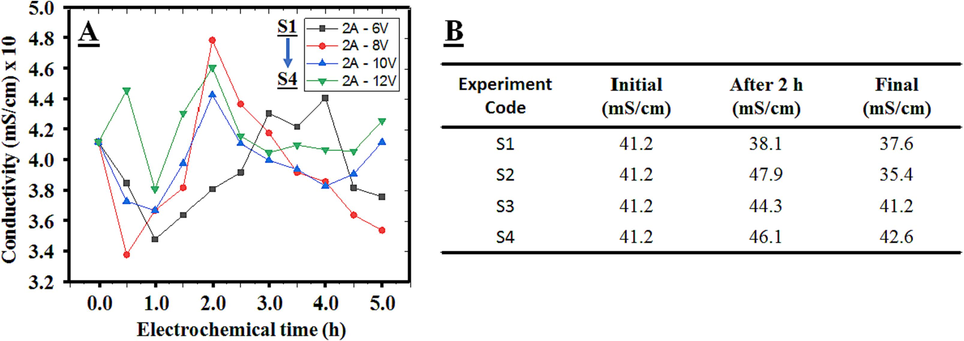
Experimental Conductivity Data (A) for fixed time intervals, (B) for final section.
3.5 Effects of voltage on TDS and TSS
Determining TDS values before and after electrochemical treatment aids in assessing the treatment efficiency of an EO system. The abundant presence of certain salt species in SOW plays a crucial role in selecting the treatment method. Besides non-ionized solutes, TDS measurement encompasses major cations like sodium, potassium, calcium, and magnesium, along with significant anions such as bicarbonate, sulfate, chloride, and nitrate (Das and Nandi, 2019). In this scenario, the primary salt ions present in the wastewater are sodium and chlorine. Therefore, employing the EO method with inert electrode pairs is suitable to rapidly enhance treatment capabilities while averting electrode corrosion After 5 h of electrolysis treatment, the TDS values in the initial solution ranged from 14 g/L and gradually escalated to 23.9 g/L, 26 g/L, 29.1 g/L, and 31.9 g/L for voltage values of 6 V, 8 V, 10 V, and 12 V, respectively, as depicted in Fig. 7A. This phenomenon once again indicates that Fe ions are the primary cause of TDS value alterations post-electrochemical treatment. As Ghosh et al. (2008), reported that eliminating iron ions from the wastewater during electrochemical treatment poses challenges, as Fe2+ ions convert to Fe3+ and form stable compounds when the pH is below 6.0. Additionally, Streche et al. (2018) mentioned an increase in TDS values when intensifying the current density at positive polar regions and subsequently across the entire solution while remediating oil-contaminated soil at a pH of 6.97. However, when comparing the TDS*/TDS ratio among samples, it becomes evident that the increase in TDS values post-treatment does not necessarily correlate with the effective treatment of affected organic pollutants. This can be explained by the conversion mechanism from EC to TDS*, which directly hinges on the quantity, type of ions, and the sample's vaporization process (Rusydi, 2018). For instance, in original sample (ORL), the TDS value obtained from water evaporation at a constant mass by 180 °C (Red line) will be significantly lower than the EC-to-TDS* conversion (Black line) due to the evaporation of chlorine ions and organic matter. Consequently, for samples post-eletrolysis treatment (from S1 to S4), the discrepancy between the TDS*/TDS values notably narrows due to the breakdown of organic compounds during electrolysis, demonstrating an R2 value of 0.994.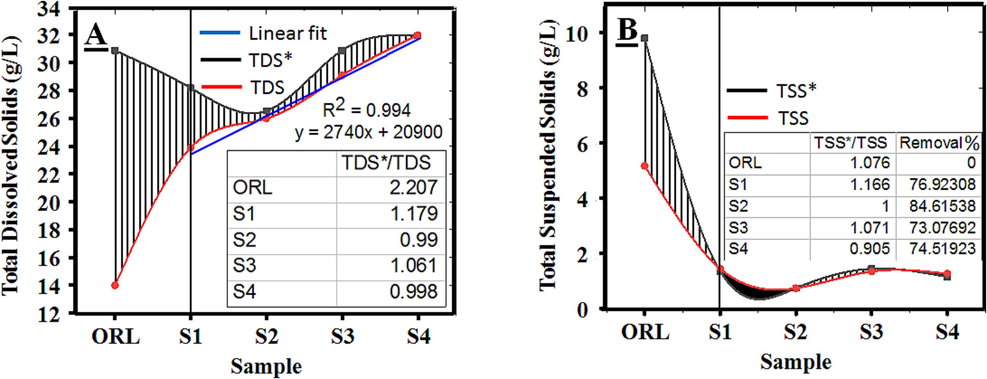
Relationship between (A) TDS*/TDS, and (B) TSS*/TSS.
Similar to TDS, TSS is also computed through separate processes to evaluate the percentage error of the index, thereby determining the accuracy of the measured values. As indicated by Ritchie et al. (2003), TSS stands as the most characteristic and prevalent indicator for pollutant content in both weight and volume. The impact of the oxidation process on the degradation of organic substances within the SOW is undeniable, evidenced by the TSS value fluctuations. After EO treatment, the initial TSS value fluctuated from 5.2 g/L, gradually decreasing to 1.2, 0.8, 1.4, and 1.3 g/L with variations in voltage to 6 V, 8 V, 10 V, and 12 V, respectively. These outcomes align with findings from previous studies. Ozturk et al. (Ozturk and Yilmaz, 2019) reported an efficient removal of TSS in real slaughterhouse wastewater, achieving over 99.5 % efficiency after 4 h of EO treatment using 3 pairs of Pt/Ti electrodes at a pH of 7.03. Additionally, AlJaberi et al. (AlJaberi et al., 2020) indicated successful removal of TSS from real oily saline wastewater, reaching up to 83 % removal efficiency. Moreover, the correlation ratio between TSS*/TSS once again affirms the EO system's efficacy in eliminating pollutant impurities through the electrolysis process. The discrepancy in the TSS*/TSS ratio is negligible across all samples post-treatment, affirming the compatibility of the obtained practical values with the calculated ones.
Table 2 provides a summary of the data obtained from Heparin pharmaceutical wastewater after the electrolysis process. Overall, as the primary purpose of this study, chlorine in the waste solution was removed clearly and efficiently with an increasing efficiency from 33 % to 78 % after 5 h of treatment. Additionally, the percentage of turbidity removal and the concentration of organic matter are also very promising. However, some parameters have not been effectively treated, such as total solids, which not only did not decrease but increased after the aforementioned electrochemical process. Therefore, the filtration process is a necessary stepping stone to increase the treatment efficiency of the input wastewater, as summarized in Table 3.
Parameter
Bulk manufacturing
After treatment
S1
S2
S3
S4
pH
8.19
5.1
4.51
5.08
5.28
Concentation of chlorine (mg/L)
14,750
9690
8854
8090
3434
Conductivity (mS/cm)
41.2
37.6
35.4
41.2
42.6
TS (mg/L)
19,600
25,300
26,800
30,600
33,200
TDS (mg/L)
14,000
23,900
26,000
29,100
32,000
TDS* (mg/L)
30,900
28,200
26,550
30,900
31,950
TSS (mg/L)
5200
1200
800
1400
1325
TSS* (mg/L)
5600
1400
800
1500
1200
TDS*/TDS ratio
2.207
1.179
0.99
1.061
0.998
TSS*/TSS ratio
1.076
1.166
1
1.071
0.905
COD (mg/L)
22,676
11,024
6425
6250
4889
Salinity (mg/L)
29,500
21,000
22,500
26,000
27,000
Recovery efficiency (mL)
300
210
185
160
155
Color (a.u.) (at 422 nm)
1.121
0.731
0.297
0.292
0.162
Parameter
S4
S420
S440
S460
pH
5.28
5.38
5.56
5.88
Concentation of chlorine (mg/L)
3434
2300
1800
1422
Conductivity (mS/cm)
42.6
35.9
36
29.3
TS (mg/L)
33,200
21,867
15,622
13,269
TDS (mg/L)
32,000
20,947
14,963
12,716
TSS (mg/L)
1325
920
659
553
COD (mg/L)
4889
3764
3177
2786
Salinity (mg/L)
27,000
21,633
17,894
15,266
Color (a.u.) (at 422 nm)
0.162
0.086
0.114
0.099
3.6 Effects of activated carbon on parameter after adsorption treatment
Previous studies have demonstrated successful use of activated carbon, prepared from various agricultural wastes, for preliminary adsorption processes in reducing wastewater salinity (Abdullah et al., 2022; Mita et al., 2021; Jorfi et al., 2019). In addition to significantly impacting the salinity of wastewater during removal, activated carbon has been shown to also affect remaining pollutants in the solution following electrochemical process (Budhiary and Sumantri, 2021; Sia et al., 2017; Sher et al., 2021). Based on data from Table 2, S4 exhibited the most effective removal of chlorine components from pharmaceutical wastewater, and was therefore selected for further testing with activated carbon under varying adsoprtion time conditions of 20, 40, and 60 min, denoted as S420, S440, and S460 respectively. In this study, the use of 10 g of activated carbon has been found to increase the removal efficiency of solid waste from 34 % to 60 %, while the COD ranges from 23 % to 43 %, and increases correspondingly with adsorption time.
The significant alterations on the activated carbon surface post-adsorption were identified through SEM results at a magnification of 100 μm. Fig. 8 illustrates the surface structure of activated carbon before and after adsorption across four different stages. Fig. 8A depicts the pre-adsorption surface, rugged and uneven with numerous small pores enhancing the adsorption surface area. Fig. 8B, C, and D exhibit signs of pollutant impurities increasingly evident over the course of adsorption. Most notably, after 60 min of adsorption, scattered white streaks became prominently visible across the entire material surface. Several similar studies have highlighted the excellent surface area and adsorption capabilities of activated carbon, making it highly suitable for wastewater treatment applications (Kosheleva et al., 2019; Yousefi et al., 2018; Morin-Crini et al., 2019). As per Wang et al. (Wang et al., 2022), the specific surface area and average pore size of Coal-based activated carbon were 4.0697 m2/g and 13.388 nm respectively, achieving up to 90 % Methylene Blue adsorption efficiency in High-Salt Wastewater. In another study, Wang emphasized that the rugged and uneven surface morphology of the adsorption material is considered favorable and ideal for adsorption processes in wastewater treatment (Wang et al., 2022). In addition to the SEM analysis, EDS results were provided to detect the adsorbed ions on the activated carbon surface. Following the adsorption process, the concentrations of Na+ and Cl- ions gradually increased with adsorption time.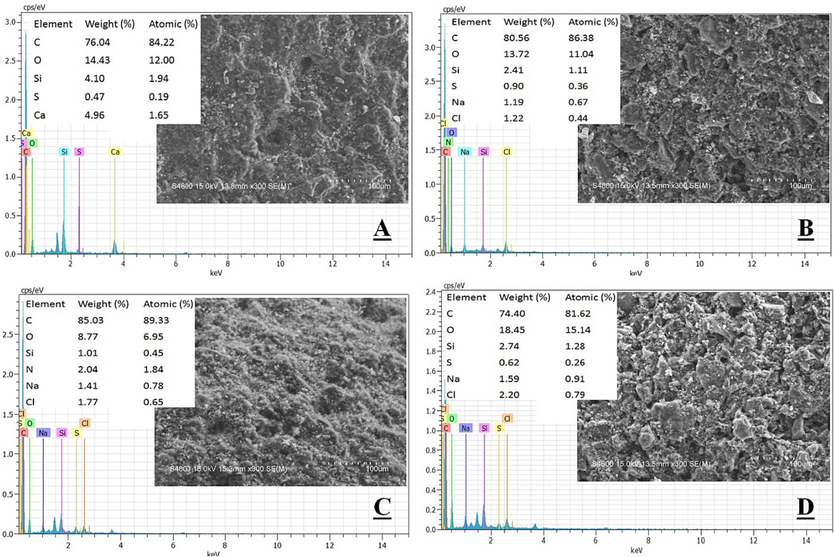
SEM/EDS result of the activated carbon for (A) original stage, (B) after adsorption 20 min, (C) after adsorption 40 min, and (D) after adsorption 60 min.
Although the adsorption efficiency is high, a comparison of the collected data in Table 1 shows that the effluent salinity is still very high (>15,000 mg/L). This means that the amount of activated carbon used in this study is still not suitable for the input stream, which reduces the adsorption efficiency and increases the waiting time. Additionally, the dropwise method may be more suitable for the type of solution used in this study than the immersion method. Therefore, besides studying the electrochemical treatment mechanism, the adsorption mechanism is also an important factor throughout the treatment process.
In addition to surface SEM analysis, the UV–VIS results depicted in Fig. 9A demonstrate substantial changes in the analyzed spectra compared to S4 following the adsorption time intervals. The reduced spectra suggest that a significant proportion of impurities and suspended particles were trapped after the activated carbon filtration process. Nonetheless, the results also indicate that the differences in spectra after adsorption intervals are insignificant, suggesting that the adsorption capacity has reached its limit for 10 g of activated carbon in this study. Despite the relatively high salinity levels, other parameters have exhibited considerable improvement. In particular, chlorine concentration decreased by 58 %, 61 % for TS, 43 % for COD, 45 % for salinity, and over 30 % for conductivity after filtration. These findings demonstrate the excellent adsorption capacity of activated carbon in this study when comparing the ratio between the amount of carbon and input solution.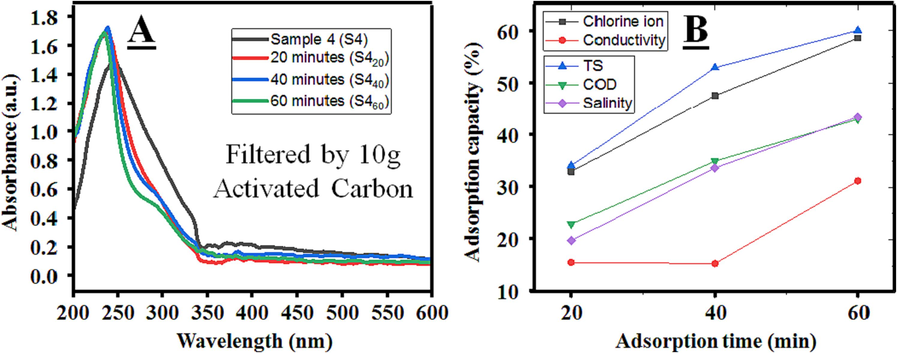
Working efficiency of activated carbon after adsorption on (A) UV–VIS spectrum, and (B) other parameters.
Therefore, overall, at the end of the dual treatment system, the pharmaceutical company's input solution achieved substantial results. The summarized results in Table 4 suggest that Cl-, COD, and color parameters have significantly improved (>87 %). However, conductivity and salinity remain a concern, as salinity directly affects the conductivity of the solution. Thus, in this case, both parameters are correlated with each other for changes, whether increasing or decreasing. The final ion chlorine removal efficiency obtained in this study was compared with that reported in recent studies at different initial conditions, as summarized in Table 5.
No.
Removal efficiency (%)
Cl-
Conductivity
COD
Salinity
Color
S420
84.40
12.86
83.40
26.66
90.60
S440
87.79
12.62
85.98
39.34
87.55
S460
90.35
28.88
87.71
48.25
91.23
Methods
Types of wastewater
Initial Cl- concentration
Experimental conditions
Removal Efficiency
Reference
Electrochemical oxidation (EO)
and activated carbon filtrationHeparin pharmaceutical wastewater
14750 mg/L
Temperature: 24 ◦C – 58 °C; pH: 4.5 – 8.3; Initial COD: 22676 mg/L.
90.3 %
This work
Reverse osmosis
Synthetic wastewater
2000 mg/L
Operating pressure: 1.6 MPa; Filtration area: 12.57 cm2; Water flux: 18.42 L/m2·h
89.3 %
(Pei et al., 2018)
Chemical precipitation
Strongly acidic wastewater
428.8 mg/L
Precipitant dose: 1.2 g/L; Temperature: 25 °C
87.9 %
(Peng et al., 2018)
Membrane separation
Aqueous solution
2000 mg/L
Membrane flux: 5.0 L/m2·h
98 %
(Welch et al., 2021)
Ozone oxidation
Simulated acidic wastewater
10000 mg/L
Temperature: 80 °C; Oxygen gas velocity: 0.5 L·min−1
80 %
(Liu et al., 2019)
Adsorption
Alkaline process mining water
20 mg/L
Temperature: 20 °C; pH: 12
45 %
(Iakovleva et al., 2015)
4 Conclusion
The combination of a simple EO system with an activated carbon filtration chamber is an effective solution for directly treating pharmaceutical wastewater containing high-concentration organic and salinity. The experimentation primarily focuses on evaluating the feasibility of the EO system and the impact of voltage on experiment performance. It is important to note that the wastewater samples are directly collected from pharmaceutical factories and treated within 1 h to avoid any impact on the final results. Throughout the experiment, the voltage was adjusted to different levels: 6 V, 8 V, 10 V, 12 V, and producing satisfactory results. Based on collected data, It was found that the removal efficiency of chloride ions is significantly improved (more than 90 %) when the system operates at 2A − 12 V in EO and with the use of 10 g of activated carbon in filtration process. However, the heat generated from the reaction and energy required to maintain the system is also an issue that needs to be considered. Additionally, under the influence of the electrical current, the potent oxidizing agent (HClO) generated from the transformation of chlorine ions aids in removing most color and odor-causing pollutants. However, despite the current material and design configurations, corrosion persists on the clip surface, directly impacting the TS or TDS parameters and consequently affecting treatment efficiency. Hence, altering the material or adjusting the design better to suit the connection between the alligator clip and electrode is crucial to avoid direct contact with the foam layer formed during organic matter decomposition.
Overall, the wastewater treatment process involving high salt concentrations, as explored in this study, achieved commendable efficiencies. Unlike conventional biological treatment methods, the high osmotic pressure caused by salt ions isn't a significant concern or limitation for EO systems equipped with inert electrodes; instead, it becomes an asset in removing pollutants. Apart from leveraging the high concentration of chlorine ions in the solution, the addition of auxiliary agents to maintain pH isn't necessary to sustain EO system operations. Due to its high flexibility, the EO system can be adjusted and expanded based on electrode quantity and the active area of the reaction chamber. In order to achieve industrial-scale efficiency and sustainability, future research should prioritize scaling up parallel system designs while incorporating appropriate measures to prevent potential corrosion risks that might occur during electrode connections.
Acknowledgment
The primary author is grateful to the local company and Feng Chia University for their assistance with the e.p.r. studies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdullah, N.O., Zubair, A., Sari, K. and Nursyawal, M.A.R., 2022, November. Effectiveness of coconut shell activated carbon for decreasing water salinity. In: AIP Conference Proceedings (Vol. 2543, No. 1, p. 030010). AIP Publishing LLC.
- Measurement of total dissolved solids and total suspended solids in water systems: a review of the issues, conventional, and remote sensing techniques. Remote Sens. (Basel). 2023;15(14):3534.
- [Google Scholar]
- Ahmed, J., Thakur, A. and Goyal, A., 2021. Industrial wastewater and its toxic effects.
- A critical review on treatment of saline wastewater with emphasis on electrochemical based approaches. Process Saf. Environ. Prot.. 2022;158:625-643.
- [Google Scholar]
- Electrocoagulation treatment of high saline oily wastewater: evaluation and optimization. Heliyon. 2020;6(6)
- [Google Scholar]
- A. Al-Raad, A., Hanafiah, M.M., Naje, A.S., Ajeel, M.A., O. Basheer, A., Ali Aljayashi, T., Ekhwan Toriman, M., 2019. Treatment of saline water using electrocoagulation with combined electrical connection of electrodes. Processes, 7(5), p.242.
- Influence of processing conditions on the synergism between UV irradiation and chlorine toward the degradation of refractory organic pollutants in UV/chlorine advanced oxidation system. Sci. Total Environ.. 2020;736:139623
- [Google Scholar]
- Electrochemical incineration of glucose as a model organic substrate. II. Role of active chlorine mediation. J. Electrochem. Soc.. 2000;147(2):592.
- [Google Scholar]
- Bowman, G. and Mealy, R., 2007. The Fundamentals of Chlorine Chemistry and Disinfection. The Wisconsin State Lab of Hygiene and The Wisconsin Dept. of Natural Resources: Madison, WI, USA.
- Budhiary, K.N.S. and Sumantri, I., 2021, February. Langmuir and Freundlich isotherm adsorption using activated charcoal from banana peel to reduce total suspended solid (TSS) levels in tofu industry liquid waste. In IOP Conference Series: Materials Science and Engineering (Vol. 1053, No. 1, p. 012113). IOP Publishing.
- Enhanced mechanism of carbamazepine degradation by electrochemical activation of persulfate in flow-through system. Sep. Purif. Technol.. 2022;301:122021
- [Google Scholar]
- Separation of COD, sulphate and chloride from pharmaceutical wastewater using membrane integrated system: Transport modeling towards scale-up. J. Environ. Chem. Eng.. 2020;8(5):104275
- [Google Scholar]
- Anaerobic biodegradation of soybean-process wastewater: Operation strategy and sludge bed characteristics of a high-performance Spiral Symmetric Stream Anaerobic Bioreactor. Water Res.. 2021;197:117095
- [Google Scholar]
- Current status of hypochlorite technology on the wastewater treatment and sludge disposal: Performance, principals and prospects. Sci. Total Environ.. 2022;803:150085
- [Google Scholar]
- Removal and adsorption characteristics of polyvinyl alcohol from aqueous solutions using electrocoagulation. J. Hazard. Mater.. 2010;177(1–3):842-850.
- [Google Scholar]
- Fast granulation of halophilic activated sludge treating low-strength organic saline wastewater via addition of divalent cations. Chemosphere. 2021;264:128396
- [Google Scholar]
- Optimization of saline wastewater treatment using electrochemical oxidation process: prediction by RSM method. MethodsX. 2019;6:1101-1113.
- [Google Scholar]
- Removal of Fe (II) ions from drinking water using Electrocoagulation (EC) process: Parametric optimization and kinetic study. J. Environ. Chem. Eng.. 2019;7(3):103116
- [Google Scholar]
- Biotechnological approaches towards treatment and recycling of wastewater from tanneries and leather industry. In: Microbial Ecology of Wastewater Treatment Plants. Elsevier; 2021. p. :249-268.
- [Google Scholar]
- Duran, F.E., de Araujo, D.M., do Nascimento Brito, C., Santos, E.V., Ganiyu, S.O., Martinez-Huitle, C.A., 2018. Electrochemical technology for the treatment of real washing machine effluent at pre-pilot plant scale by using active and non-active anodes. J. Electroanal. Chem., 818, pp.216-222.
- Evaluation of biological technologies for wastewater treatment in the pharmaceutical industry. Water Sci. Technol.. 1995;32(11):13-20.
- [Google Scholar]
- Evaluation of Groundwater Quality and Suitability for Irrigation Purposes and Human Consumption in Saudi Arabia. Water. 2023;15(13):2352.
- [Google Scholar]
- Removal of Fe (II) from tap water by electrocoagulation technique. J. Hazard. Mater.. 2008;155(1–2):135-143.
- [Google Scholar]
- Microbial fuel cells in saline and hypersaline environments: Advancements, challenges and future perspectives. Bioelectrochemistry. 2018;120:127-137.
- [Google Scholar]
- Treatment and recycling of wastewater from pharmaceutical industry. Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future 2019:267-302.
- [Google Scholar]
- Decolorization of wastewater. Crit. Rev. Environ. Sci. Technol.. 2000;30(4):449-505.
- [Google Scholar]
- Saline wastewater treatment with purple phototrophic bacteria. Water Res.. 2019;160:259-267.
- [Google Scholar]
- Industrial products and wastes as adsorbents for sulphate and chloride removal from synthetic alkaline solution and mine process water. Chem. Eng. J.. 2015;259:364-371.
- [Google Scholar]
- Investigation of sesame processing wastewater treatment with combined electrochemical and membrane processes. Water Sci. Technol.. 2021;84(10–11):2652-2660.
- [Google Scholar]
- Radical attack and mineralization mechanisms on electrochemical oxidation of p-substituted phenols at boron-doped diamond anodes. Chemosphere. 2020;248:126033
- [Google Scholar]
- Biodegradation of high saline petrochemical wastewater by novel isolated halotolerant bacterial strains using integrated powder activated carbon/activated sludge bioreactor. Environ. Prog. Sustain. Energy. 2019;38(4):13088.
- [Google Scholar]
- Optimization of Turbidity and COD Removal from Pharmaceutical Wastewater by Electrocoagulation. Isotherm Modeling and Cost Analysis. Pol. J. Environ. Stud.. 2015;24(3)
- [Google Scholar]
- Applicability of electrochemical methods to paper mill wastewater for reuse. Anodic oxidation with BDD and TiRuSnO2 anodes. J. Electroanal. Chem.. 2018;815:16-23.
- [Google Scholar]
- Synthesis of activated carbon from food waste. Environ. Chem. Lett.. 2019;17:429-438.
- [Google Scholar]
- June. A review: pharmaceutical wastewater treatment technology and research in China. In 2015 Asia-Pacific Energy Equipment Engineering Research Conference. Atlantis Press; 2015. p. :345-348.
- Individual and combined effect of salinity and nitrite on freshwater Anammox bacteria (FAB) Water Res.. 2020;169:114931
- [Google Scholar]
- Removal of chloride from simulated acidic wastewater in the zinc production. Chin. J. Chem. Eng.. 2019;27(5):1037-1043.
- [Google Scholar]
- Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere. 2021;275:130104
- [Google Scholar]
- Emerging organic contaminants in wastewater: Understanding electrochemical reactors for triclosan and its by-products degradation. Chemosphere. 2020;247:125758
- [Google Scholar]
- Electrochemical oxidation of oxalic acid in the presence of halides at boron doped diamond electrode. J. Braz. Chem. Soc.. 2008;19:150-156.
- [Google Scholar]
- February. Synthesis of Activated Carbon from Locally Available Rice Husk for Treating Saline Drinking Water. Sylhet, Bangladesh: Innovation and Education; 2021. p. :26-28.
- Electroflotation for treatment of industrial wastewaters: a focused review. Environmental Processes. 2019;6(2):325-353.
- [Google Scholar]
- Performance evaluation and energy potential analysis of anaerobic membrane bioreactor (AnMBR) in the treatment of simulated milk wastewater. Chemosphere 2023137923
- [Google Scholar]
- Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B. 2017;202:217-261.
- [Google Scholar]
- Hemp-based adsorbents for sequestration of metals: a review. Environ. Chem. Lett.. 2019;17:393-408.
- [Google Scholar]
- Treatment performance of textile wastewater using electrocoagulation (EC) process under combined electrical connection of electrodes. Int. J. Electrochem. Sci.. 2015;10(7):5924-5941.
- [Google Scholar]
- Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation. Chemosphere. 2020;251:126437
- [Google Scholar]
- Treatment of slaughterhouse wastewater with the electrochemical oxidation process: Role of operating parameters on treatment efficiency and energy consumption. J. Water Process Eng.. 2019;31:100834
- [Google Scholar]
- Hyperbranched poly (amidoamine)/TMC reverse osmosis membrane for oily saline water treatment. Environ. Technol. 2018
- [Google Scholar]
- Removal of chloride ions from strongly acidic wastewater using Cu (0)/Cu (II): Efficiency enhancement by UV irradiation and the mechanism for chloride ions removal. Environ. Sci. Tech.. 2018;53(1):383-389.
- [Google Scholar]
- The Combination of Anaerobic Digestion and Electro-Oxidation for Efficient COD Removal in Beverage Wastewater: Investigation of Electrolytic Cells. Sustainability. 2023;15(6):5551.
- [Google Scholar]
- A novel protease-immobilized carbon catalyst for the effective fragmentation of proteins in high-TDS wastewater generated in tanneries: Spectral and electrochemical studies. Environ. Res.. 2019;172:408-419.
- [Google Scholar]
- Electrochemical properties of the platinum metals. Platin. Met. Rev.. 1959;3(2):47-53.
- [Google Scholar]
- Treatment of slaughterhouse wastewater by acid precipitation (H2SO4, HCl and HNO3) and oxidation (Ca (ClO) ₂, H2O2 and CaO₂) J. Environ. Manage.. 2019;250:109558
- [Google Scholar]
- Remote sensing techniques to assess water quality. Photogramm. Eng. Remote Sens.. 2003;69(6):695-704.
- [Google Scholar]
- Rusydi, A.F., 2018, February. Correlation between conductivity and total dissolved solid in various type of water: A review. In IOP conference series: earth and environmental science (Vol. 118, p. 012019). IOP Publishing.
- A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater: special emphasis on biological treatment based hybrid systems. J. Environ. Chem. Eng.. 2021;9(4):105282
- [Google Scholar]
- Electrochemical instabilities due to pitting corrosion of iron. Russ. J. Electrochem.. 2006;42:476-490.
- [Google Scholar]
- Removal of micropollutants from municipal wastewater using different types of activated carbons. J. Environ. Manage.. 2021;278:111302
- [Google Scholar]
- Sequential anaerobic–aerobic treatment of pharmaceutical wastewater with high salinity. Bioresour. Technol.. 2014;153:79-86.
- [Google Scholar]
- Investigation of intertidal wetland sediment as a novel inoculation source for anaerobic saline wastewater treatment. Environ. Sci. Tech.. 2015;49(10):6231-6239.
- [Google Scholar]
- Anaerobic treatment of pharmaceutical wastewater: a critical review. Bioresour. Technol.. 2017;245:1238-1244.
- [Google Scholar]
- Adsorption of Colour, TSS and COD from Palm Oil Mill Effluent (POME) Using Acid-Washed Coconut Shell Activated Carbon: Kinetic and Mechanism Studies. in MATEC Web of Conferences. 2017;87:03010.
- Song, Q., Chen, X., Hua, Y., Chen, S., Ren, L. and Dai, X., 2023. Biological treatment processes for saline organic wastewater and related inhibition mechanisms and facilitation techniques: A comprehensive review. Environmental Research, p.117404.
- Efficient degradation of polyacrylate containing wastewater by combined anaerobic–aerobic fluidized bed bioreactors. Bioresour. Technol.. 2021;332:125108
- [Google Scholar]
- Treatment of saline wastewater using physicochemical, biological, and hybrid processes: Insights into inhibition mechanisms, treatment efficiencies and performance enhancement. J. Environ. Chem. Eng.. 2021;9(4):105775
- [Google Scholar]
- Decontamination of petroleum-contaminated soils using the electrochemical technique: remediation degree and energy consumption. Sci. Rep.. 2018;8(1):3272.
- [Google Scholar]
- Treatment of landfill leachate using magnetically attracted zero-valent iron powder electrode in an electric field. J. Hazard. Mater.. 2020;388:121768
- [Google Scholar]
- Influence of anode material on electrochemical oxidation for the treatment of tannery wastewater. Water Res.. 2005;39(8):1601-1613.
- [Google Scholar]
- Influences of electrode distance and electrolysis time on phosphorus precipitation and composition during electrolysis of anaerobic digestion effluent. Sci. Total Environ.. 2022;803:150114
- [Google Scholar]
- Relationship between total dissolved solids and electrical conductivity in Marcellus hydraulic fracturing fluids. Water Sci. Technol.. 2018;77(8):1998-2004.
- [Google Scholar]
- Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem.. 2021;26:100677
- [Google Scholar]
- Waste (water) to feed protein: effluent characteristics, protein recovery, and single-cell protein production from food industry waste streams. Clean Energy and Resource Recovery 2022:201-244.
- [Google Scholar]
- Electrical conductivity and total dissolved solids—what is their precise relationship? Desalination. 1989;72(3):275-292.
- [Google Scholar]
- Adsorption of methylene blue by coal-based activated carbon in high-salt wastewater. Water. 2022;14(21):3576.
- [Google Scholar]
- Study on the application of shell-activated carbon for the adsorption of dyes and antibiotics. Water. 2022;14(22):3752.
- [Google Scholar]
- High-efficiency removal of tetracycline from water by electrolysis-assisted NZVI: mechanism of electron transfer and redox of iron. RSC Adv.. 2023;13(23):15881-15891.
- [Google Scholar]
- Molecular layer deposition for the fabrication of desalination membranes with tunable metrics. Desalination. 2021;520:115334
- [Google Scholar]
- Characterization of nickel zinc ferrite and application in treating heparin wastewater. Environmental Science & Technology (china). 2012;35(11):126-130.
- [Google Scholar]
- Loose nanofiltration-based electrodialysis for highly efficient textile wastewater treatment. J. Membr. Sci.. 2020;608:118182
- [Google Scholar]
- Modification of pumice with HCl and NaOH enhancing its fluoride adsorption capacity: kinetic and isotherm studies. Human and Ecological Risk Assessment: An. Int. J. 2018
- [Google Scholar]
- Hybrid electrocatalytic ozonation treatment of high-salinity organic wastewater using Ni–Ce/OMC particle electrodes. Sci. Total Environ.. 2020;724:138170
- [Google Scholar]
- Treatment of high-strength pharmaceutical wastewater and removal of antibiotics in anaerobic and aerobic biological treatment processes. J. Environ. Eng.. 2006;132(1):129-136.
- [Google Scholar]
- Isolation of two salt-tolerant strains from activated sludge and its COD degradation characteristics from saline organic wastewater. Sci. Rep.. 2020;10(1):18421.
- [Google Scholar]







