Translate this page into:
An overview on novel synthetic approaches and medicinal applications of benzimidazole compounds
⁎Corresponding author. heba.hashem@women.asu.edu.eg (Heba E. Hashem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Benzimidazole, a benzene-fused heterocyclic compound, has acquired significant attention in the field of contemporary medicinal chemistry because of its wide array of pharmacological activities. Nitrogen containing heterocyclic compounds are part of several therapeutically important agents with several recent patents shedding light on their worth. For these reasons, this review is concerned with different methods for synthesis of benzimidazole derivatives and their biological importance.
Keywords
O-phenylenediamines
Benzimidazoles
Synthesis
Biological activities
1 Introduction:
The benzimidazole nucleus consists of a benzene ring fused to an imidazole ring. The chemistry of benzimidazole has been an interesting field of study for a considerable time. It is one of the most promising moieties that is present in many clinically useful drugs and possesses various biological activities (Chen et al., 2015; Kamal et al., 2016).
The benzimidazole fragment fulfills the minimum structural requirements that are common for anti-inflammatory compounds (El-feky et al., 2014; Gaba et al., 2015). The natural and synthetic source of different benzimidazole derivatives play a vital role in medicinal chemistry. The structure similarity of 2-aminobenzimidazole with purine shed the light on the development of such nucleus for different biological activities (Alpan et al., 2009; Sweeney et al., 2021). It has been reported that several drugs containing the benzimidazole nucleus possess powerful analgesic properties as anti-inflammatory agents (Abdel-Alim, 2013). Moreover, there are many reports of the activity of benzimidazole-substituted molecules as anti-fungal (Küçükbay et al., 2003; KÜÇÜKBAY et al., 2011), antibacterial(Kankate et al., 2015), anti-hypertensive(Sharma, 2013), antiproliferative, anthelmintic(Karminski-zamola, 2012; Ts et al., 2013), antiviral(Evans et al., 2015), anti-infective(Zhang et al., 2012), male contraceptive(Chen et al., 2013), human glucagon receptor antagonistic(Guigón-lópez et al., 2015) and H3 antagonistic(Rizzati et al., 2016) agents. These molecules are also good inhibitors for lymphocyte tyrosine kinase (Lck)(Ella, 2016), and chemokine receptor (CXCR3)(Murayama et al., 2012) and 1H-benzimidazole derivatives are effective at blocking stomach damage caused by inflammation inhibitors(Arora et al., 2014) (Fig. 1). This review gives a recent overview of the synthesis of different benzimidazole derivatives and covers the most recent medicinal application of benzimidazole compounds.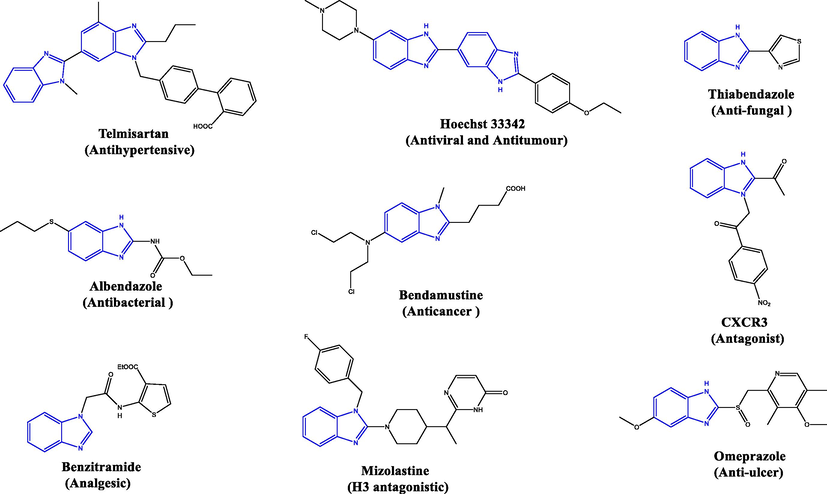
Marketed drugs having benzimidazole moiety.
2 General synthesis of benzimidazoles
Generally, benzimidazole compounds can be synthesized from the reaction of ortho-diaminobenzene derivatives with various reagents Fig. 2.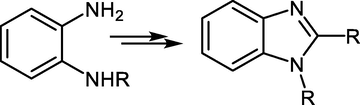
General synthetic route for benzimidazole.
Benzimidazole chemistry is of great interest since it was discovered that the 5,6-dimethylbenzimidazole moiety is a portion of the chemical structure of vitamin B12 (Brink and Folkers, 1949).
Historically the first benzimidazole was synthesized by Hoebrecker (Wright, 1951), through the reduction and dehydration of 2-nitro-4-methylacetanilide (Scheme 1).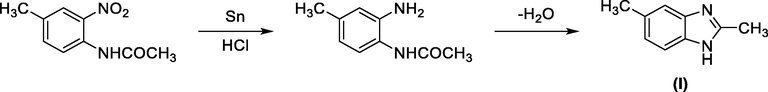
First preparation of a benzimidazole derivative.
Concurrently, Ladenburg (A. Ladenburg, 1875) obtained the same compound I, 2,5-dimethylbenzimidazole, by heating 3,4-diaminotoluene under reflux with acetic acid (Scheme 2).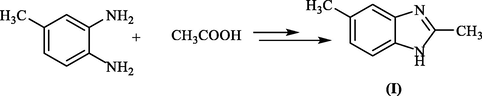
General synthesis of benzimidazole from o-phenylenediamine.
Today, benzimidazoles play a considerable role in the pharmaceutical field and there are several reports of their synthesis through different reaction media and from different active starting compounds. The presented review summarizes different methods for the preparation of benzimidazole derivatives under different conditions.
2.1 From o-phenylenediamines:
2.1.1 Through condensation with aldehydes:
Under several conditions, 2-substituted benzimidazoles can be synthesized from the reaction of substituted aldehydes and o-phenylenediamines (Scheme 3).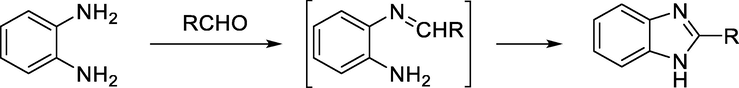
General synthesis of benzimidazole from o-phenylenediamine and aldehyde.
Since the reaction undergoes through an oxidation process this can be caused by the air or more smoothly by using other oxidizing agents.
It was reported that, palladium (Saha et al., 2009; Shashi and Krishna, 2016) and copper-based catalysts can be used for preparation of benzimidazole derivatives through cross-coupling reactions of o-phenylenediamine with alkyl, aryl, and heterocyclic aldehydes in good yields Scheme 4.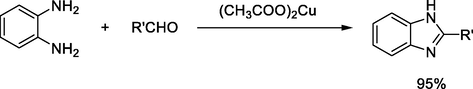
Copper acetate catalyzed synthesis of benzimidazole derivatives.
Benzimidazole derivative II is prepared via the reaction of the corresponding o-phenylenediamine derivative with 2,4-dichlorobenzaldehyde in the presence of sodium metabisulphite (Suheyla Ozbey, F. Betul Kaynak, Canan Kus, 2002) Scheme 5.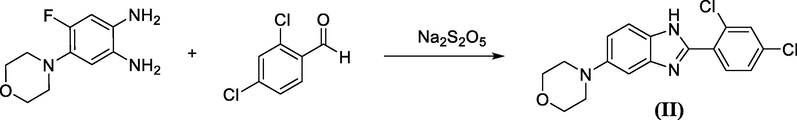
Sodium metabisulphite catalyzed synthesis of a benzimidazole.
Venkateswarlu et al. described the preparation of benzimidazole derivatives by lanthanum chloride catalysis in a one-pot synthesis from different aldehydes and o-phenylenediamine (Venkateswarlu et al., 2013) (Scheme 6).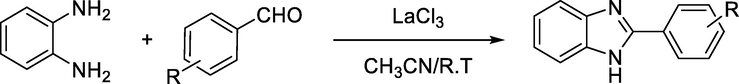
One-pot synthesis of benzimidazole derivatives by lanthanum chloride catalysis.
K.S. Jithendra Kumara et al. achieved the synthesis of benzimidazole derivatives III and IV in high yield using cobalt ferrite nanoparticles (Co@Fe2O4) and silica-coated cobalt ferrite nanoparticles (SiO2/Co@Fe2O4) as efficient catalysts for the cross-coupling reaction of o-phenylenediamine and different aldehydes (Kumara et al., 2017) Scheme 7.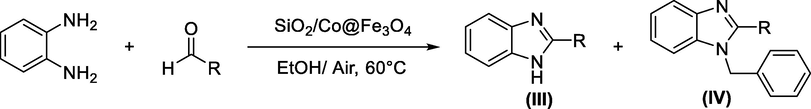
Using cobalt ferrite nanoparticles for the synthesis of benzimidazole compounds.
Rushi et al. have reported that 2-substituted benzimidazoles have been prepared in a good yields in a single pot reaction under solvent-free conditions through condensation of aldehydes and o-phenylenediamine in the presence of a catalytic amount of [In(OTf)3] at room temperature (Trivedi et al., 2006) (Scheme 8).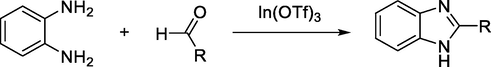
Synthesis of benzimidazole under solvent-free conditions.
Deep eutectic solvents (DES) are green solvents used in several areas of industrial chemistry. A recent study investigated the selective synthesis of benzimidazole derivatives with the DES chlorine chloride/urea as an eco-friendly solvent.
The reaction proceeded by dissolving o-phenylenediamine in ChCl:urea DES followed by addition of benzaldehyde in different ratios producing different benzimidazole derivatives (Scheme 9) (Gioia et al., 2019).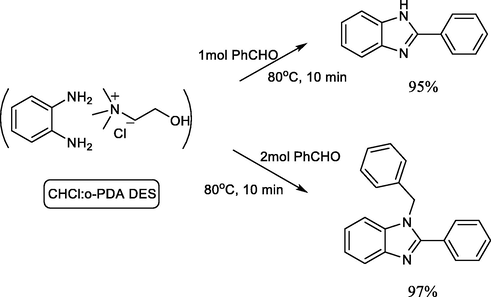
Synthesis of different benzimidazoles in DES green solvent.
V. Kadu, et al., presented an ecofriendly and low-cost protocol for the synthesis of 1,2-disubstituded benzimidazoles using an aqueous extract of pods of Acacia Concinna, which reduce the hazardous effect of halogenated organic solvents (Scheme 10) (Kadu et al., 2019).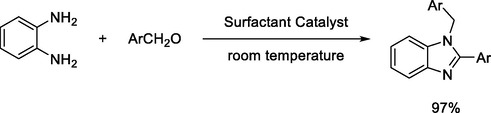
Synthesis of benzimidazole derivatives with a surfactant catalyst.
L. Hao et al., reported an effective procedure for synthesis of benzimidazoles in high yield using gold nanoparticle catalysts such as Au/TiO2, Au/Al2O3, Au/ZnO (Scheme 11) (Leiduan Hao, Yanfei Zhao, Bo Yu, Hongye Zhang, 2014).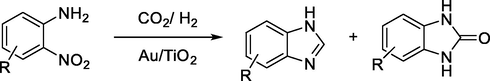
Synthesis of benzimidazole derivatives using gold nano particle catalysts.
On the other hand, recent research is focused on developing these conditions with green conditions through transition metal catalysts. A different research achieved synthesis of benzimidazole derivatives from condensation of o-phenylenediamine with different aldehydes in the presence of different transition metal nanoparticle catalysts. Inamdar et al., synthesized new benzimidazole derivatives in good yield (76–93%) in the presence of Si-CuO nanoparticle catalyst (CuOnp-SiO2 10%) (Scheme 12). While Esfahani et al., achieved the synthesis of benzimidazole derivatives in 88–97% yield using the nano catalyst CuII 0.34 mol % (Cu (II)-TD@nSiO2, nanosilica triazine dendrimers) (Mahboobeh Nasr-Esfahani, Iraj Mohammadpoor-Baltork, Ahmad Reza Khosropour, Majid Moghadam, Valliolah Mirkhani, 2013; Singhal et al., 2019)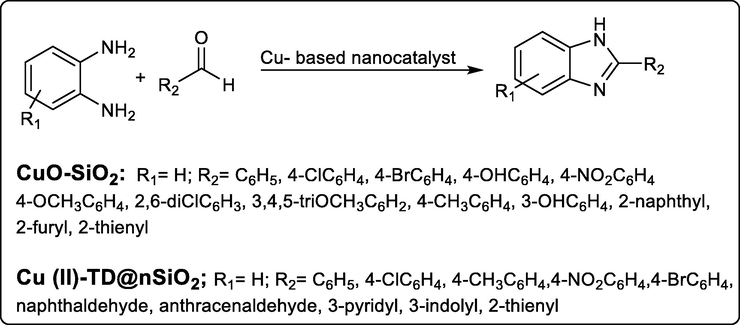
Synthesis of different benzimidazole derivatives using Cu-based nanocatalyst.
Similarly, Kommula et al. achieved synthesis of 2 arylbenzimidazoles using nano-Fe2O3 catalyzed with 70–85% yield, and Bardajee et al. performed the same reaction by using Fe (III)-Schiff base/SBA-15 as an effective catalyst in water in excellent yield (79–92%) (Ghasem Rezanejade Bardajee , Marzieh Mohammadi, Hasan Yari, 2015; Kommula et al., 2017).
2.1.2 Through condensation with Ketones:
A large number of benzimidazole derivatives have been synthesized through the condensation reaction of o-phenylenediamine with different ketones under the general proposed pathway (Scheme 13) (Alaqeel, 2017).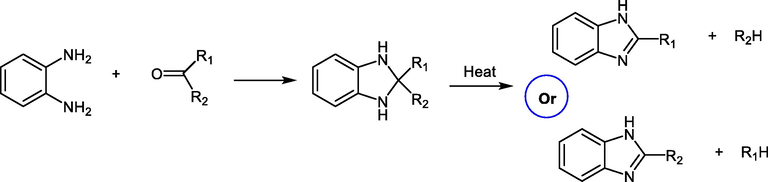
General procedure for synthesis of benzimidazole from ketones.
The reaction of o-phenylenediamine with a ketone afforded the unstable 2-disubstituted benzimidazolines, which undergo decomposition to a mixture of benzimidazole derivatives depending on which of alkyl group, is eliminated (Scheme 13).
Similarly, Ladenbrug and Rugheimer reported the synthesis of 2-phenyl-5(or 6)-methylbenzimidazole by heating 3,4-diaminotoluene with acetophenone at 180 °C. In this case, the methyl group is the one, which is eliminated affording benzimidazole V (Scheme 14).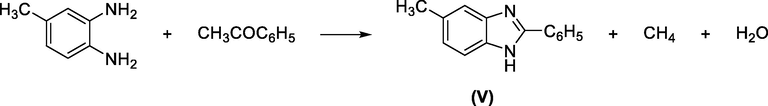
Synthesis of benzimidazole using acetophenone.
Because of several limitations of this type of reaction, such as long reaction time, difficult work up procedures, and formation of by products, better reaction conditions, such as solvent free, ionic liquid, and different catalysts have been studied.
Unfortunately, these reactions did not proceed as predicted and afforded other heterocyclic compounds rather than the desired benzimidazoles.
For instant, Catia S.Radatz et al., reported that the reaction of o-phenylenediamine and acetophenone or other ketones using glycerol as an eco-friendly and recyclable solvent at low temperature afforded benzodiazepine VI in low yield with the same product formed in high yield at higher temperatures, but no reaction occurred in absence of glycerol (Scheme 15) (Radatz et al., 2011).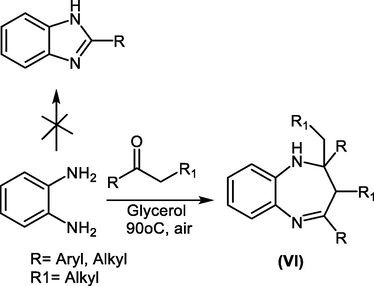
Reaction of o-phenylenediamine with ketones in glycerol as a solvent.
C. S. Digwal et al., found that the reaction of o-phenylenediamine derivatives with benzil in the presence of VOSO4 as a catalyst produced the 2,3-diphenyl quinoxaline derivatives VII instead of benzimidazole derivatives (Scheme 16) (Digwal et al., 2016).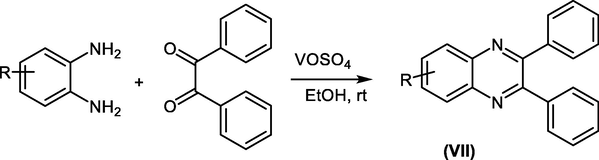
Reaction of o-phenylenediamine with ketone using VOSO4 as a catalyst.
However, P. Dhanalakshmi et al., achieved the preparation of benzimidazole derivatives from α,β-unsaturated ketones with o-phenylenediamine both thermally or with microwave irradiation in good yields (Scheme 17) [28].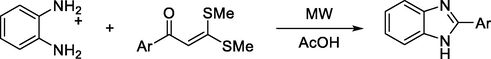
Synthesis of benzimidazoles from α,β-unsaturated ketones.
On the other hand, o-phenylenediamine undergoes cyclization when reacted with 1,3-dicarbonyl compounds under moderate heating in alcohol afforded the corresponding benzimidazole derivatives (Scheme 18) (Abdallah et al., 2015; Rafat M. Mohareb, 2020)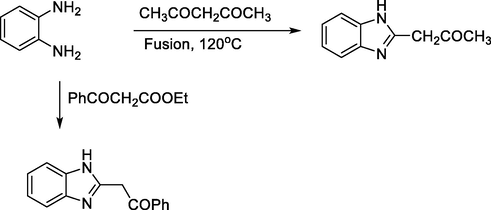
Synthesis of benzimidazole from reaction of 1,3-dicarbonyl compounds.
2.1.3 Through condensation with carboxylic acids
Several reports show that numerous benzimidazole derivatives have been synthesized from the reaction of o-phenylenediamine with carboxylic acids. The most common procedure involves condensation of these two reagents in the presence of concentrated hydrochloric acid (Scheme 19) (Alaqeel, 2017).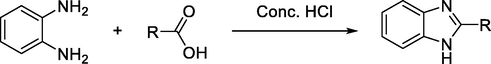
General synthesis of benzimidazoles from carboxylic acids.
T. Ahmed et al., reported the synthesis of new 2-substituted benzimidazole derivatives in good yield by refluxing an equimolar ratio of o-phenylenediamine and p-aminobenzoic acid in xylene and polyphosphoric acid for only 6 h (Ahmad et al., 2017) (Scheme 20).
Synthesis of benzimidazole from aromatic carboxylic acid.
T. Nguyen et al., reported an efficient Fe/S catalytic redox condensation reaction for the synthesis of benzimidazole derivatives from phenylacetic acid and o-nitroaniline in high yields and with no organic by-product (Scheme 21) (Nguyen et al., 2014).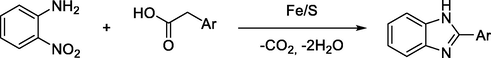
Fe/S catalytic redox condensation reaction for synthesis of benzimidazoles.
The synthesis of benzimidazole-2-thiol derivatives was achieved by the condensation of o-phenylenediamine with mercaptoacetic acid under reflux. This can be followed by cyclization with chloroacetic acid or a different aromatic aldehyde to afford biologically active polynuclear fused benzimidazole derivatives (Ranza A. Elrayess, Nagat Ghareb, Marwa M. Azab, 2013) (Scheme 22).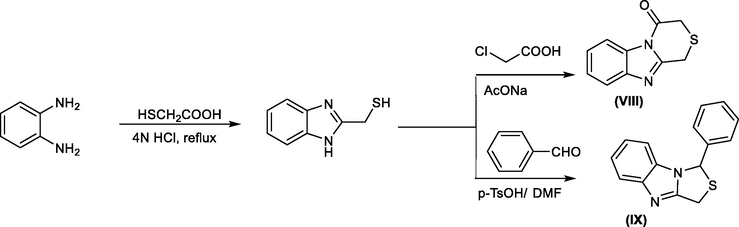
Synthesis of polynuclear fused benzimidazole derivatives.
Recently, T. Huynh et al., reported an efficient green synthesis of benzimidazole derivatives through condensation of o-phenylenediamine and mono-carboxylic acids under catalyst-free microwave irradiation (Huynh et al., 2020) (Scheme 23).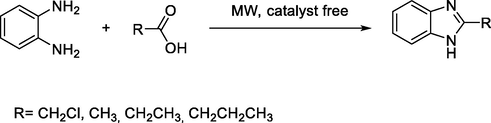
Synthesis of benzimidazole derivative under microwave irradiation.
2.2 Via rearrangement
In addition to the condensation reactions described in the preceding section, some more efficient and convenient routes to benzimidazoles and benzimidazole-2-ones have been achieved through the rearrangement of different heterocyclic compounds as described below.
2.2.1 Rearrangement of Benzodiazepinones
Refluxing a mixture of ortho-diaminobenzene with ethyl acetoacetate in boiling xylene afforded 4,7-dihydro-5-methyl-1H- 2,3-benzo-1,4-diazepin-7-one X but when the same reaction was carried out in the presence of potassium hydroxide the product was 1-isopropenylbenzimidazol-2-one (XI). It was proposed that the reaction takes place through elimination of molecule of ethanol followed by C-2-C-3 bond breakage with a subsequent intramolecular nucleophilic reaction (cf. Scheme 24) (Mamedov and Zhukova, 2017).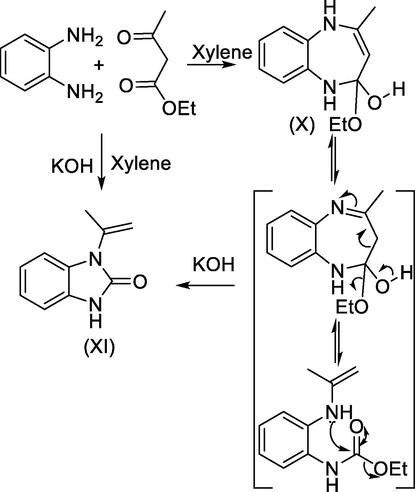
General procedure for synthesis of benzimidazolone via rearrangement of benzodiazepinones.
It was mentioned that this type of reaction could also occur with other dicarbonyl compounds.
El eftheriadis et.al., reported that benzimidazolone derivative XIV was prepared from the reaction of o-phenylenediamine with dimethylacetone dicarboxylate (XII) through the formation of diazepine derivative XIII, which undergoes rearrangement via action of mercaptoacetic acid to afford the benzimidazolone XIV (cf. Scheme 25) (Eleftheriadis et al., 2013).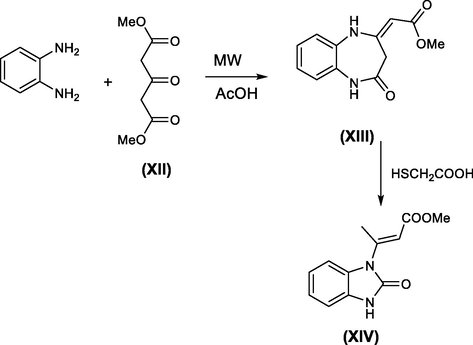
Synthesis of benzimidazolone via rearrangement of benzodiazepinones.
2.2.2 Rearrangement of quinoxaline derivatives
It was reported some time ago that different benzimidazole derivatives can be synthesized through rearrangement of quinoxaline-1,4-dioxides. Recent studies revealed that the rearrangement process occurs through the formation of quinoxaline-2-one as an intermediate (Scheme 26). This study prompted a search for more effective methods for the preparation of benzimidazole derivatives from quinoxalinone compounds in good yield with high efficiency and broad functional group tolerance (Mamedov and Zhukova, 2017).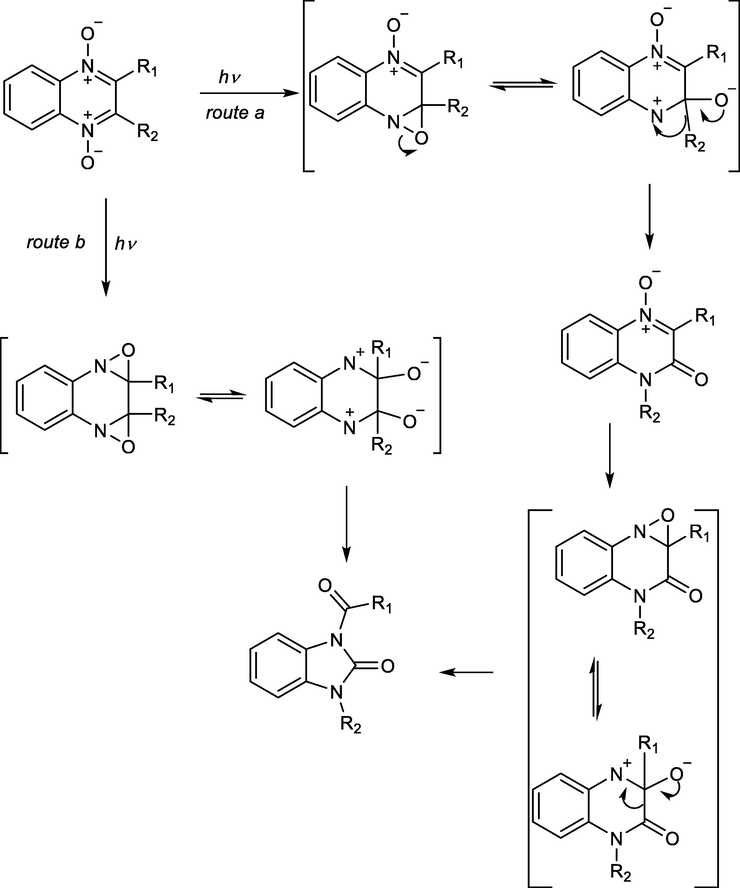
Mechanistic pathway of imidazolone preparation from quinoxaline-1,4-dioxides.
On the basis of the previous studies, recent work uses quinoxalin-2(1H)-ones for synthesis of different benzimidazole derivatives through an acid-catalyzed rearrangement pathway as shown in Scheme 27. (Mamedov and Zhukova, 2017; Zhukova and Mamedov, 2017) It was shown that benzimidazole derivative A was formed only in the presence of at least one hydrogen atom in the spiro-forming intermediate. Without presence of a hydrogen atom, benzimidazolone B will form with migration of the spiro-forming moiety to the 1-position.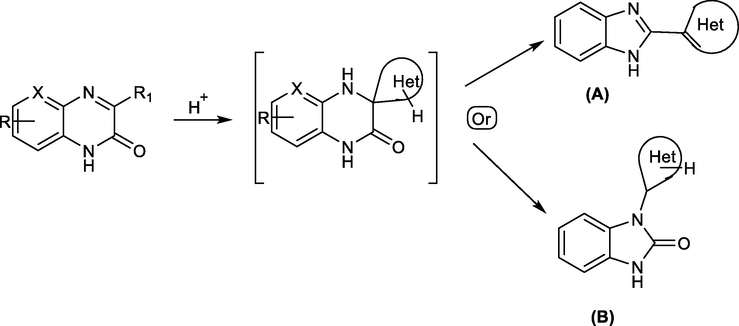
Rearrangement of quinoxalin-2(1H)-ones to the related benzimidazole and benzimidazolone derivatives.
This method possesses great advantages; for instance, it proceeds at a high rate and in high yield even under mild conditions. Moreover, it can be used with a broad range of substrates.
V. A. Mamedov et al., developed an efficient procedure for synthesis 4-(benzimidazol-2-yl)quinolines (XV) from the reaction of 3-(2-aminophenyl)-quinoxalin-2(1H)-one with acetophenone (cf. Scheme 28) (Mamedov et al., 2014).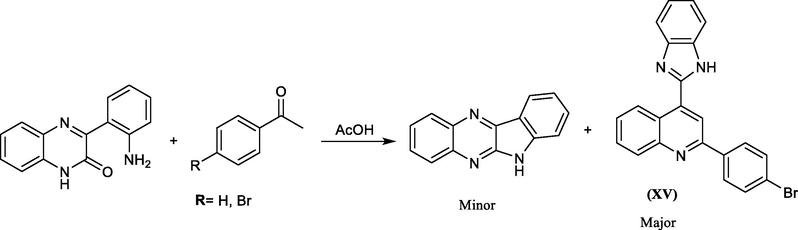
Synthesis of benzimidazole derivative from quinoxalinone and acetophenone.
It was suggested that compound XV formed through intramolecular nucleophilic addition of the imine formed as shown in Scheme 29.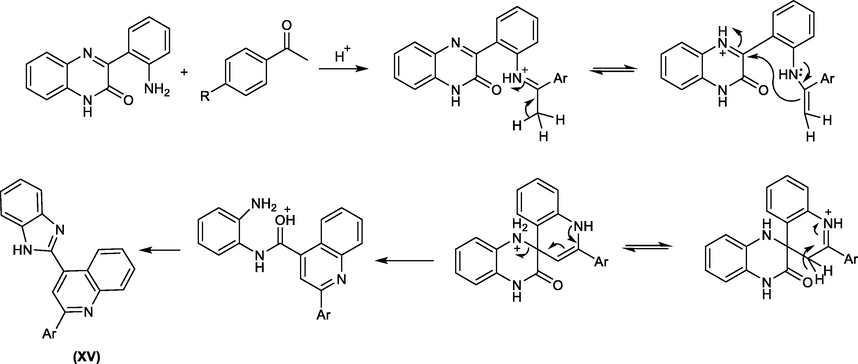
Suggested mechanism for the synthesis of a benzimidazole derivative from quinoxalinone and acetophenone.
The structure of imidazolone XV has been determined by X-ray crystallography (Fig. 3).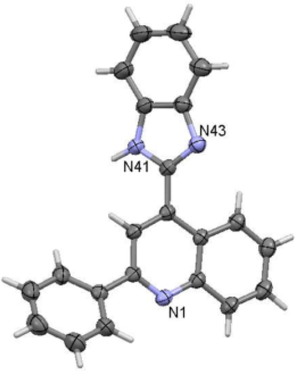
Crystal structure of imidazole XV.
This approach was later extended to the reaction of 3-(2-aminophenyl)-quinoxalin-2(1H)-one with ethyl acetoacetate in the presence of acetic acid to construct the polynuclear fused benzimidazol derivative XVI, which is difficult to prepare by any other process(Mamedov et al., 2014) Scheme 30.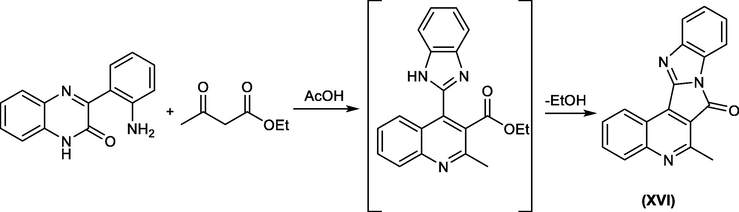
Synthesis of polynuclear fused imidazole derivatives via rearrangement of quinoxalinone compounds.
In the context of work toward the quinoxaline–benzimidazole rearrangement, Vakhid A. Mamedov et al., developed a new method based on the acid-catalyzed rearrangement of 3′-aryl-1,2,3,4,4′,5′-hexahydrospiro[quinoxalin-2,50- pyrazol]-3-ones XVIIIa-f, which are obtained from the reaction of 3-arylacylidene-3,4-dihydroquinoxalin-2(1H)-ones with hydrazine hydrate (Scheme 31) (Mamedov et al., 2009).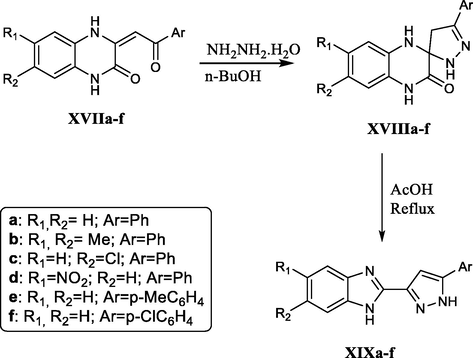
Acid catalyzed quinoxaline–benzimidazole rearrangements.
The molecular structures of the spiro-compound XVIIIe and imidazole XIXa were determined by x-ray crystallography (Fig. 4).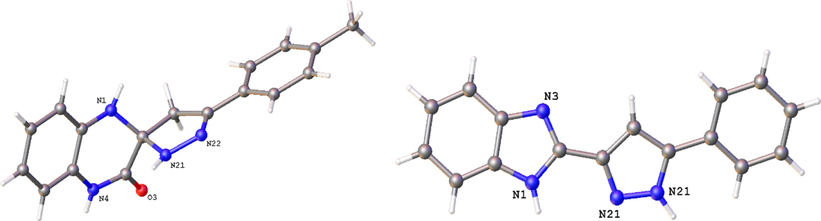
ORTEP drawing of XVIIIe and XIXa. Displacement ellipsoids are drawn at the 30% probability level. H atoms are represented by circles of arbitrary size.
In a related transformation, the 3-aroylquinoxalin-2(1H)-ones XXa,b behave in a similar way when condensed with o-phenylenediamine under refluxing acetic acid (Mamedov et al., 2010). Scheme 32.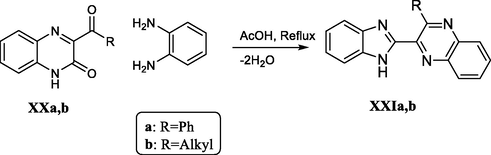
Synthesis of benzimidazole derivatives through acid-catalyzed rearrangement in the quinoxalinone-o-phenylenediamine system.
2.2.3 Rearrangement of 1,2,3-triazole
A further approach for an efficient synthesis of benzimidazolone derivatives via a rearrangement process was demonstrated for active 1,2,3-triazole derivatives.
It was reported that benzimidazolone derivative XXIII was formed upon refluxing a solution of 5-amino-4-carbamoyl-1-(2-nitrophenyl)-1H-1,2,3-triazol (XXII) for 4 h (Scheme 33 )(Mamedov and Zhukova, 2017).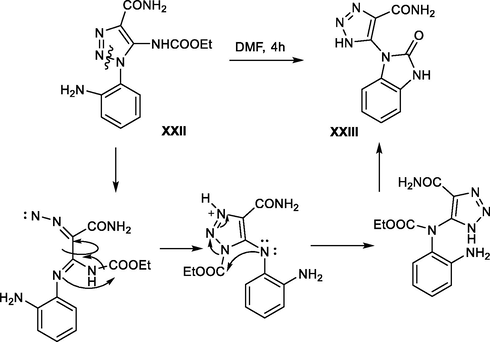
Synthesis of benzimidazole XXIII from the triazole derivative XXII.
3 Benzimidazole based bioactive compounds
The biological importance of benzimidazole derivatives began in earnest in 1994 when it was noted that the benzimidazole moiety resembled the purine structure. Then it was discovered by Brink that 5,6-dimethylbenzimidazole is produced from Vitamin B12 degradation. According to these initial studies, it was reported that compounds containing the benzimidazole moiety possess important pharmacological applications including as anticancer, antimicrobial, analgesic, antiviral, and antifungal agents(Bansal and Silakari, 2012; Durmaz et al., 1997; Küçükbay and Durmaz, 1997; Narasimhan et al., 2012; Yadav and Ganguly, 2015). On the other hand, there are recent studies which revealed that different benzimidazole carbamates (BZC) such as Albendazole, Mebendazole, Flubendazole and Fenbendazole have an inhibition effect on in vitro growth of Trichomonas vaginalis and G. lamblia. Furthermore, the clinical reports have shown their treatment of giardiasis (Valdez et al., 2002). Literature indicates that the benzimidazole derivatives are active agents against many micro-organisms and useful for the development of new drugs in the pharmaceutical field (Fig. 5) (Hiroyuki Nakano, Tsutomu Inoue, Nobuhide Kawasaki, Hideki Miyataka, Hitoshi Matsumoto, Takeo Taguchi, Naoki Inagaki, Hiroichi Nagai, 1999; Valdez et al., 2002).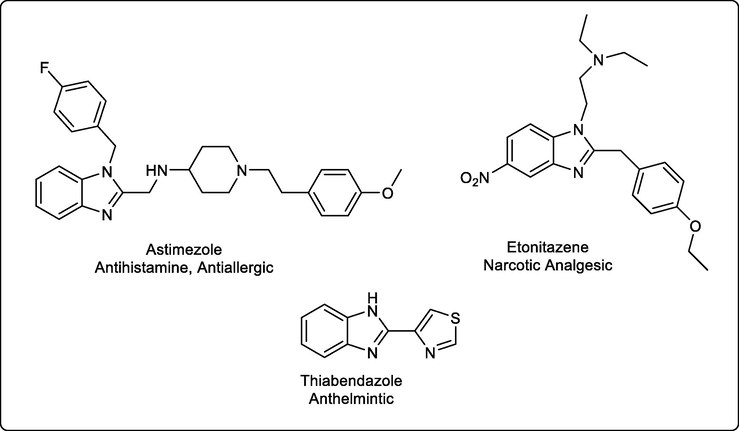
Different benzimidazole derivatives as drugs for different therapeutic field.
3.1 Antimicrobial activities of benzimidazole derivatives
A large series of benzimidazole derivatives have shown broad effects against different types of microbes including bacteria and fungi. Several studies of the effective benzimidazole derivatives revealed that the presence of bulky groups at position 1 and 2 and small alkyl groups at other positions give the optimum antimicrobial effect (Bansal and Silakari, 2012; Güngördü et al., 2013) (Fig. 6).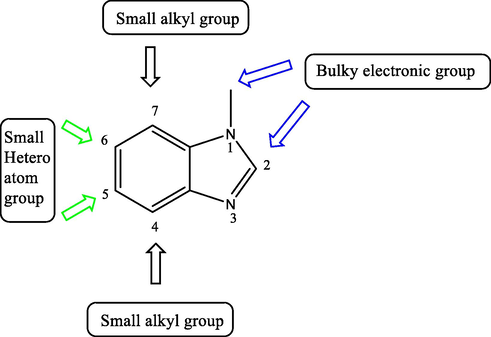
Benzimidazole structure for optimum antimicrobial activity.
Gowda et al., developed a new series of 2-substituted-1-[(5-substituted-phenyl-1,3,4-oxadiazole-2-yl)methyl-1H-benzimidazoles (XXIV) with high antibacterial activity with 50- and 250 μg/mL MBC (minimum bactericidal concentration) against Gram negative bacteria E. coli and Gram positive bacteria S. aureus and Pseudomonas aeruginosa compared with the standard Ampicillin which showed 50- and 100 μg/mL MBC (Gowda et al., 2010). Scheme 34.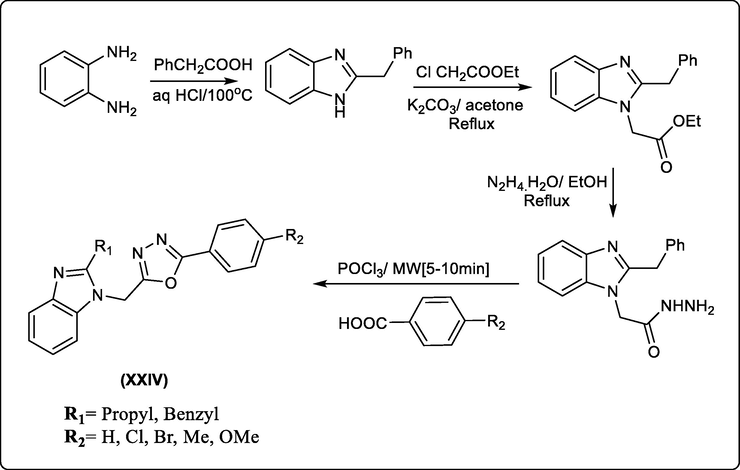
Benzimidazoles bearing oxazole moieties as antibacterial compounds.
Mehlika Dilek et al., reported a new class of benzimidazole compounds and screened them against the bacterial species Pseudomonas Aeruginosa, Staphylococcus aureus and Escherichia coli, and for antifungal activity against Aspergillus flavus, Aspergillus Niger and Fusarium Solani (Altintop et al., 2015) (Fig. 7). These showed good activity as compared with the standard ketoconazole as an antifungal and streptomycin as an antibacterial.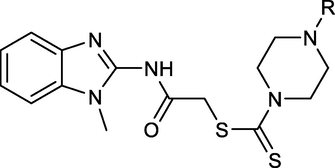
1,2-disubstituted benzimidazole derivatives as antimicrobial compounds.
Andrea Bistrović et al., designed new biologically active benzimidazoles linked with a triazole ring and evaluated the for their in vitro anti-bacterial activity against Gram-positive bacteria: S. aureus (ATCC 25923), MRSA, methicillin-sensitive S. aureus (MSSA), E. faecalis, vanco- mycin-resistant E. faecium (VREF), and Gram-negative bacteria: E. coli (ATCC 25925), P. aeruginosa (ATCC 27853), A. baumannii (ATCC 19606) and ESBL-producing K. pneumoniae. Structure-activity relationship studies showed that the non-substituted amidino benzimidazoles have the highest overall activities (Bistrović et al., 2018) (Fig. 8).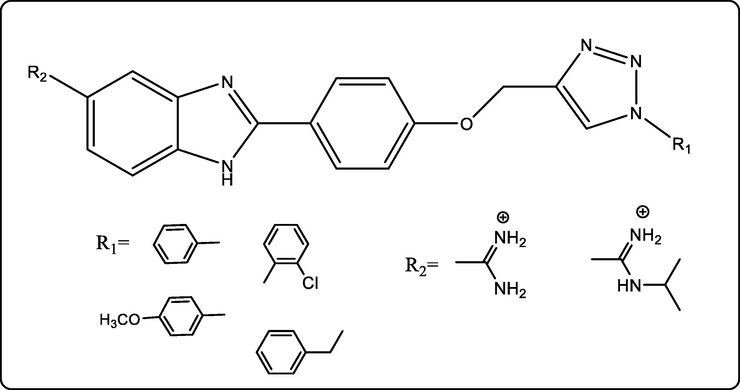
Biologically active benzimidazoles linked to a triazole ring.
Sravanthi A. et al., synthesized new benzimidazole clubbed chalcone derivatives and evaluated them against Bacillus subtilis, Staphylococcus aureus, Escherichia Coli, Streptococcus pyogenes and Pseudomonas aeruginosa organisms for possible antibacterial effects. The SAR studies revealed that compounds possessing electron withdrawing groups exhibited significantly higher activity than the rest (AVUNOORI, 2020) (Fig. 9).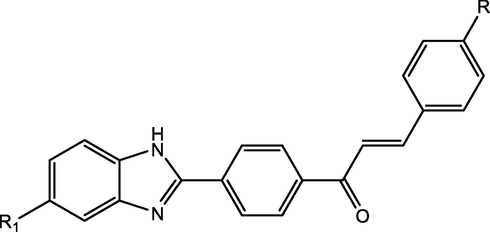
Benzimidazole clubbed chalcone derivatives as antibacterial compounds.
Eman M. E. Dokla et al., developed new 1,2-disubstituted benzimidazole derivatives as antimicrobial agents and studied their synergistic effect with colistin against Gram-negative bacteria. Out of thirty-three derivatives studied, seventeen exhibited moderate to potent activity against E. coli strain by varying the substituents at ring A and B or replacing ring B with a naphthyl or cyclohexyl moiety. The structure–activity relationship for these derivatives is shown in Fig. 10 (Dokla et al., 2020)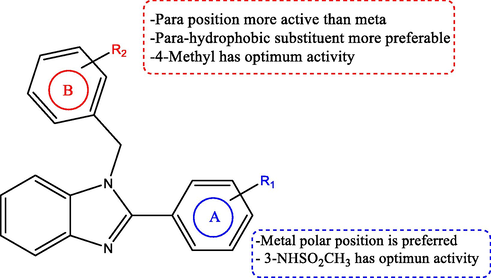
The structure–activity relationship of antibacterial 1,2-disubstituted benzimidazole derivatives.
On the other hand, Nesrin Buğday et al., synthesized new benzimidazole incorporating amino acid and dipeptide moieties with anticipated antimicrobial activities. The resulted study revealed that the new amino acid/ dipeptide conjugates possess from moderate to good effect as antifungal against C. albicans and C. tropicalis with a range of MICs between
100 and 400 μg/mL compared to the standard Fluconazole, while the antibacterial activities of these compounds on bacteria were low (Fig. 11) (Bugday et al., 2017)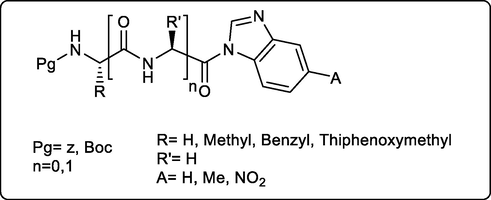
Amino acid/peptide-benzimidazole conjugates as potent antifungal compounds.
Recently, Hasan Küçükbay et al., achieved new 1,3-benzimidazole salts and 1,3- benzimidazole metal complexes as potent antibacterial and antifungal compounds, and some of them exhibit great cytotoxicity against the lung cancer cell line (A549) and healthy lung epithelial cell line (BEAS-2B). The structure activity relationship study of the tested compounds revealed that compounds with 5-position electron withdrawing or electron releasing groups possess a higher antimicrobial effect compared with those without any substituent. While the benzimidazole derivative with metal and without any substituent enhance the antimicrobial and cytotoxic properties (scheme 35) (Küçükbay et al., 2021).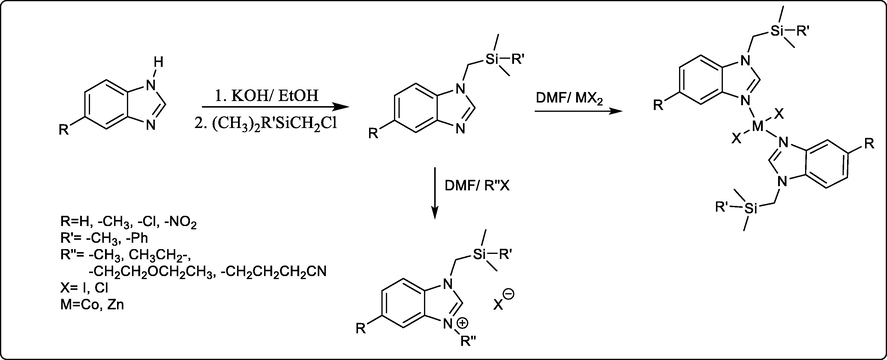
Synthesis of 1,3-benzimidazole salts and 1,3-benzimidazole metal complexes.
3.2 Antiviral activity of benzimidazole derivatives
Several studies have shown that some benzimidazole derivatives possess important antiviral activity.
Martin Tremblay et al., synthesized numerous 5-(5-furan-2-yl-pyrazol-1-yl)-1H-benzimidazole derivatives as HIV capsid assembly inhibitors. They showed that introduction of a pyridine side chain at N1 in XXV, a 2-hydroxyphenyl appendage at C2 and a C16 trifluoromethyl group (XXVI) were crucial for improving the antiviral potency (Fig. 12) (Tremblay et al., 2012)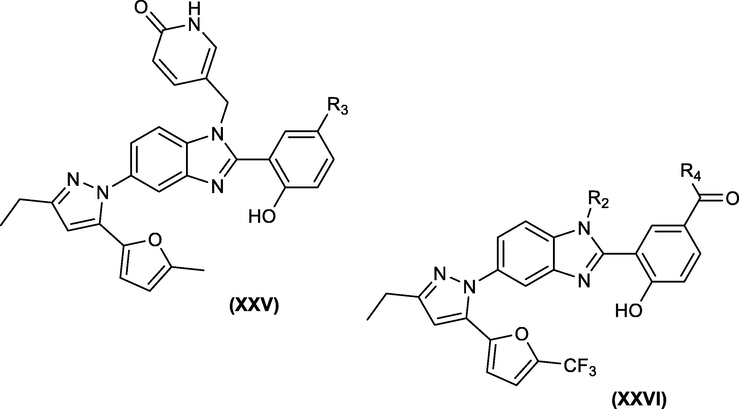
Modified 5-(5-furan-2-yl-pyrazol-1-yl)-1H-benzimidazole at N1, C2 and C16 for HIV-1 inhibition.
Michele Tonelli et al., developed novel benzimidazole derivatives as anti-coxsackie virus B5, anti-respiratory syncytial viru, and anti-staphylococcal bacteriophage-1 agents. The study showed that 2-[(benzotriazol-1/2-yl) methyl] benzimidazoles, bearing at position 1 a di-alkylaminoalkyl or quinolizidin-1-ylalkyl moiety exhibited potent activity against respiratory syncytial virus (Fig. 13).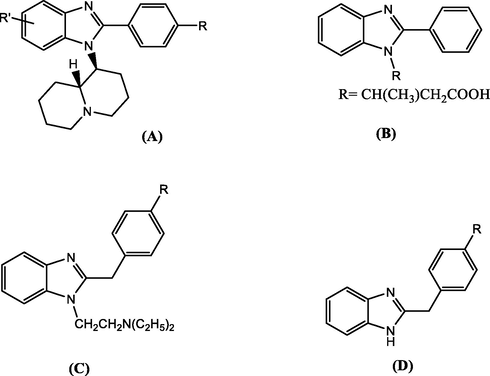
Benzimidazole derivatives investigated as antiviral compounds; (A) 2-phenylbenzimidazole with basic sidechain; (B) 2-phenylbenzimidazole without basic sidechain; (C) 2-benzylbenzimidazole with basic sidechain; (D) 2-benzylbenzimidazole without basic sidechain.
It was observed that compounds bearing a basic sidechain were more frequently active than those devoid of it, and 1/2-phenyl and 2-benzylbenzimidazole derivatives (with and without the sidechain) were more active than benzimidazole derivatives devoid of any aromatic moiety in position 1 or 2 (Tonelli et al., 2014).
Lei Liu et al., designed a new 7-(4-benzimidazole-butoxy)-coumarin (BBC) having an antiviral effect against SVCV infection in zebrafish.
The study found that BBC has an intense response on SVCV replication through the activation of PKCα/β-Nrf2 signaling which enhances HO-1 expression to suppress viral infection (Fig. 14) (Tonelli et al., 2014).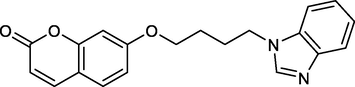
Antiviral benzimidazole bearing a coumarin moiety.
Recently, Mei Chen et al., constructed new benzimidazole-containing flavonoids having remarkable antiviral effects against tobacco mosaic virus (TMV).
Myricetin, is a polyphenolic flavonoid compound (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one) which possesses different biological activities although most of them are limited to applied research in medicine. The recent study provides an active antiviral benzimidazole-containing a 4-H–chromen-4-one derivative (XXVII) (Fig. 15) (Chen et al., 2021).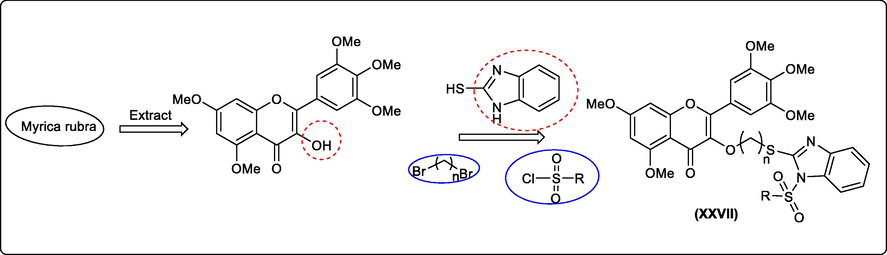
Synthesis of an antiviral benzimidazole-containing 4-H–chromen-4-one derivative.
3.3 Anticancer activities of benzimidazole derivatives
Several studies reported an effective anticancer effect of various benzimidazole derivatives with 2-substituted benzimidazoles known to act as potential anticancer agents. For instant, bis-benzimidazole derivatives showed an active effect in interfering with DNA topoisomerase I and possess cytotoxic effects against breast adenocarcinoma (MCF7) and skin epidermoid carcinoma (A431).
Based on this study, Hanan M Refaat achieved synthesis of certain novel 2-substituted benzimidazole derivatives that contain the 5-chloro or 5-underivatized carboxylic acid group in their framework which are potent antitumor compounds (Fig. 16) (Refaat, 2010).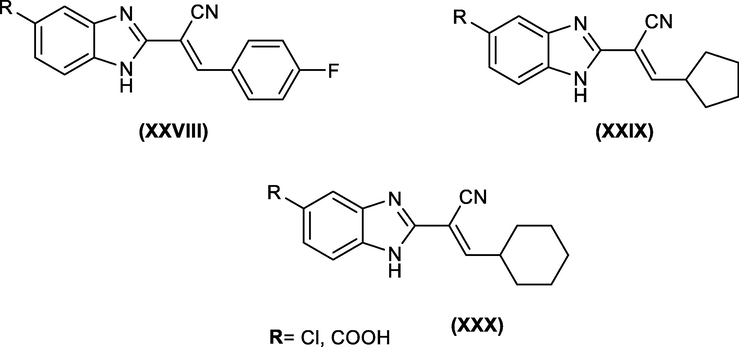
Novel 2-substituted benzimidazole derivatives as active anticancer compounds.
The anti-helmitics Benzimidazole methyl carbamate drugs have been suggested to possess anticancer activity but have poor water solubility and poor suitability for systemic delivery to disseminated cancers. Jae Eun Cheong et al., developed new benzimidzoles containing an oxetane or an amine group to enhance solubility and showed them to exhibit significant inhibition of the growth of prostate and lung tumors without noticeable toxicity (Fig. 17) (Cheong et al., 2018). The solubility of anticancer compounds containing the benzimidazole carbamate moiety was achieved by incorporation of the polar functional group amine and 4-membered cyclic oxetane.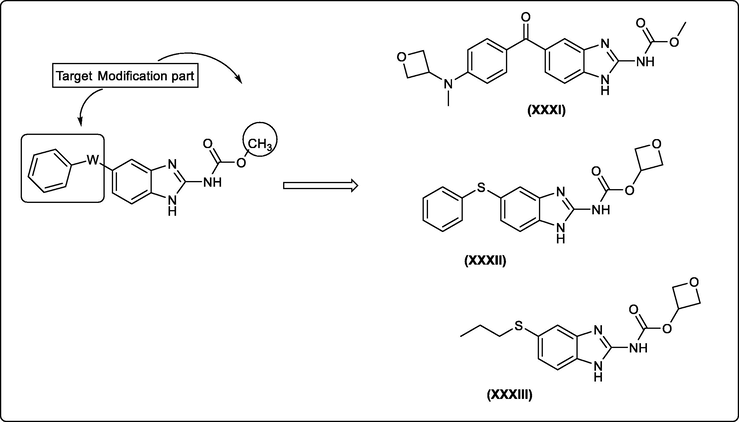
New water soluble benzimidazole carbamates as anticancer drugs.
Indole and benzimidazole rings are bio-valuable molecules found in current drugs which are effective agents against different cancer cells. Phenylindole derivatives have been shown to inhibit breast cancer and recent studies show the different 2-benzimidazole derivatives have potent anticancer effects against various cancer cell lines. Fikriye Zengin Karadayi et al., demonstrated that new indole benzimidazoles with p-fluorobenzyl or small alkyl groups at the R1-position and electron withdrawing groups at the R2-position have highly effective anticancer activities (Fig. 18) (Karadayi et al., 2020).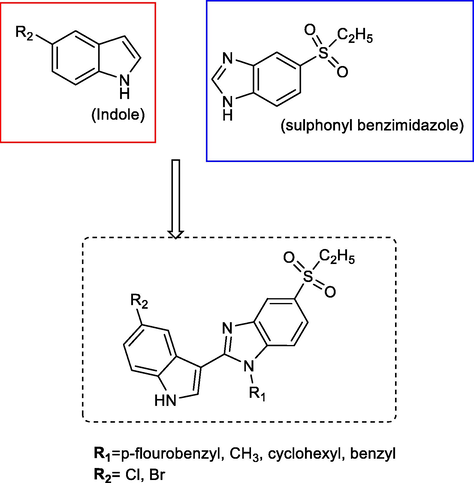
Novel anticancer indole-benzimidazole derivatives.
Modified nucleosides have valuable applications in bioorganic and medicinal chemistry. There are different nucleoside analogs that have been synthesized and evaluated for their biological activities as well as non-natural nucleosides which are of importance in medicinal chemistry and synthetic biology. Currently, efforts are underway to modify analogs of purine and pyrimidine nucleosides and to evaluate their biological activities. Benzimidazole, a structural mimic of the purine base, possesses a great spectrum of biological activities by interacting with DNA and RNA. In this direction, V. Shinde et al., synthesized new sugar-modified benzimidazole nucleosides XXXIV with different substituents at the 2- position which displayed promising anti-cancer activities (Fig. 19) (Shinde et al., 2020).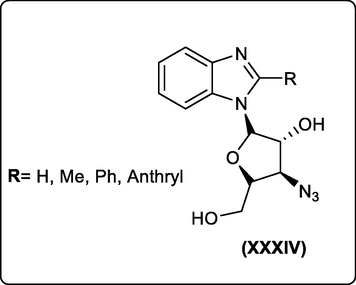
Anti-cancer benzimidazole nucleosides.
Recently, new benzimidazole metal complexes have been synthesized and achieved great antitumor activity against A-2780 cell lines compared to the standard drug docetaxel with a LogIC50 value of − 0.81 μM (p < 0.05). and it was revealed that metalation of benzimidazole derivatives enhance their anticancer activity against different cell line including A-2780 (human ovarian) and DU-145 (human prostate) (scheme 36) (Yılmaz et al., 2019)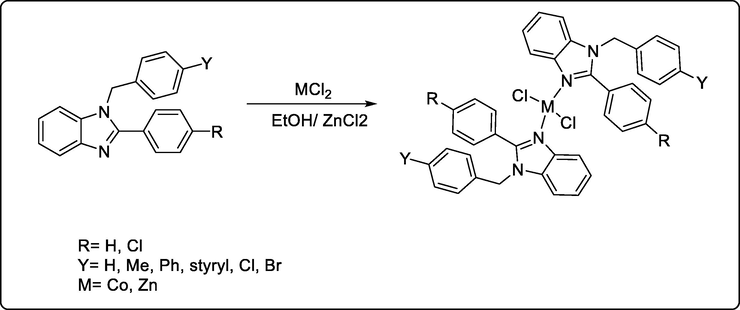
Synthesis of new benzimidazole metal complexes as anticancer compounds.
3.4 Analgesic properties of benzimidazole derivatives
There are several recent researches which revealed that different benzimidazole derivatives have remarkable pharmacological activities including analgesic and anti-inflammatory activities (Achar et al., 2010; Asma Eswayah, Souad Khaliel, Shaban Saad et al., 2017; Datar and Limaye, 2015; Kamil et al., 2016; Srivastava et al., 2013).
Asma Eswayah et al., achieved a series of N-substituted benzimidazole derivatives as analgesic active potent compounds, through the reaction of benzimidazole and benzoyl chloride followed by addition of different amines producing the desired active compounds (Scheme 37) (Asma Eswayah, Souad Khaliel, Shaban Saad et al., 2017).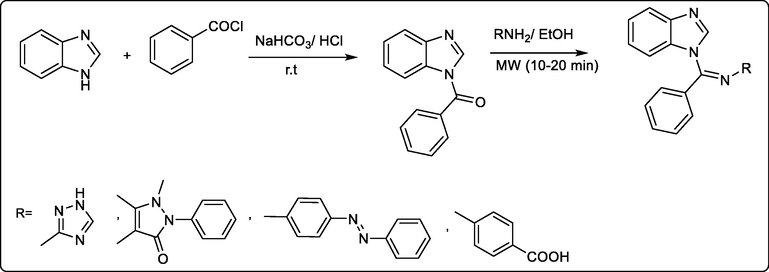
Synthetic pathway for the preparation of active analgesic N-substituted benzimidazole derivatives.
The tested compounds gave good inhibiting activity compared to the standard aspirin (non steroidal anti-inflammatory drug (NSAID)) at the same dose.
Monika Gaba et al., reported a series of new benzimidazole derivatives as GI (gastrointestinal)-friendly anti-inflammatory analgesic treatment (scheme 38). The in vitro and in vivo studies of these compounds showed encouraging anti-inflammatory activity ranging from 52.84% to 57.58% compared to the standard drug acetyl salicylic acid. It was revealed that the presence of electron donating group in the tested compounds enhanced the analgesic activity (Asma Eswayah, Souad Khaliel, Shaban Saad et al., 2017).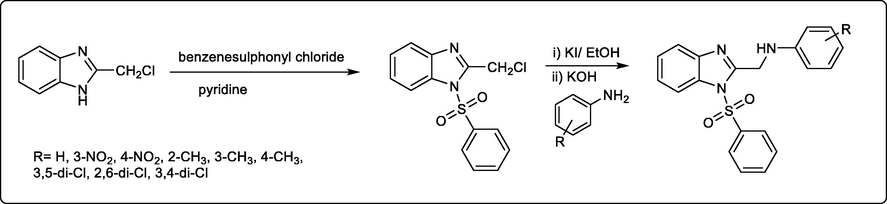
synthesis of new GI-friendly anti-inflammatory analgesic benzimidazole derivatives.
The previous literature SAR studies revealed that the presence of different heterocycle substituents at N1 position enhance the anti-inflammatory analgesic activity, as well as that the presence of different substituents at C2, C5, and C6 of benzimidazole influence the anti-inflammatory activity. Structure activity relationship study of various literature synthesized benzimidazole as analgesic drug as illustrated in Fig. 20 (Gaba et al., 2015; Gijsen et al., 2012; Paramashivappa et al., 2003; Veerasamy et al., 2021).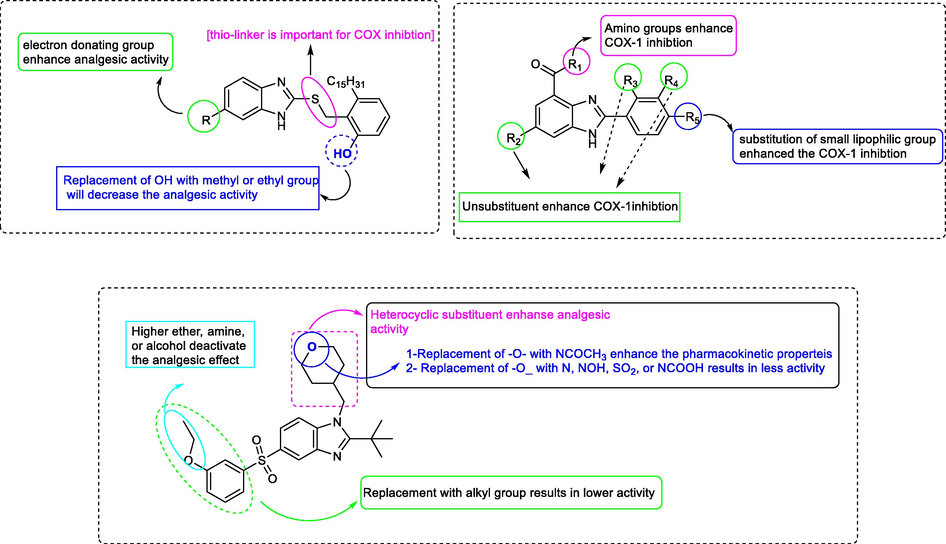
Structure activity relationship studies of benzimidazole as an active analgesic compound.
4 Conclusion
Benzimidazole is the most important heterocycle made up imidazole and phenyl ring, which is widely used by the drug discovery and pharmaceutical industry. Based on the literature survey done, a lot of evidence has been provided that benzimidazole have a vast range of applications in the field of medicine and chemistry. They can be prepared via various routes of synthesis by using different starting materials. They can be synthesized by using a variety of different catalysts in solvent free conditions and by employing numerous solvents. All the synthesized benzimidazoles derivatives display a wide range of biological activities. The aim of this review was to demonstrate the wide synthetic strategies to benzimidazoles derivatives and their biological activities.
References
- Heterocyclization, dyeing applications and anticancer evaluations of benzimidazole derivatives: Novel synthesis of thiophene, triazole and pyrimidine derivatives. Egypt J. Chem.. 2015;58:699-719.
- [CrossRef] [Google Scholar]
- Anber F. Mohammed, Samia G. Abdel-Moty, Mostafa A. Hussein & Abdel-Alim. Arch. Pharm. Res.. 2013;36:1465-1479.
- [Google Scholar]
- European Journal of Medicinal Chemistry In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem.. 2010;45(5):2048-2054.
- [CrossRef] [Google Scholar]
- Synthesis of Benzimidazole Derivatives Containing Schiff Base Exhibiting Antimicrobial Activities. Int. J. Res. Stud. Biosci.. 2017;5
- [CrossRef] [Google Scholar]
- Synthetic approaches to benzimidazoles from o-phenylenediamine: A literature review. J. Saudi Chem. Soc.. 2017;21(2):229-237.
- [CrossRef] [Google Scholar]
- Alpan, A.S., Zencir, S., Zupkó, I., Coban, G., Réthy, B., Gunes, S., Topcu, Z., 2009. Biological activity of bis-benzimidazole derivatives on DNA topoisomerase I and HeLa , MCF7 and A431 cells 6366. https://doi.org/10.1080/14756360802420831.
- Synthesis and antimicrobial activity of benzimidazole-based acetamide derivatives. Turkish J. Pharm. Sci.. 2015;12:29-38.
- [Google Scholar]
- Novel coumarin – benzimidazole derivatives as antioxidants and safer anti-in fl ammatory agents. Acta Pharm. Sin. B. 2014;4(5):368-375.
- [CrossRef] [Google Scholar]
- Asma Eswayah, Souad Khaliel, Shaban Saad, N.S., Omran Fhid, Amal Belaid, Tawssul Alsharif, H.E., Yousra Saadalla, E.B., 2017. Synthesis and Analgesic Activity Evaluation of Some New Benzimidazole Derivatives. Am J Chem Appl 4, 30–35.
- Synthesis, Structural Characterisation and Biological Evaluation of Novel Series of Benzimidazole Clubbed Chalcone Derivatives. J. Bio. Innov.. 2020;09:457-465.
- [CrossRef] [Google Scholar]
- The therapeutic journey of benzimidazoles: A review. Bioorganic Med. Chem.. 2012;20(21):6208-6236.
- [CrossRef] [Google Scholar]
- Synthesis, anti-bacterial and anti-protozoal activities of amidinobenzimidazole derivatives and their interactions with DNA and RNA. J. Enzyme Inhib. Med. Chem.. 2018;33(1):1323-1334.
- [CrossRef] [Google Scholar]
- Brink, N.G., Folkers, K., 1949. Vitamin B12. VI. 5, 6-Dimethylbenzimidazole, a degradation product of vitamin B12. J. Am. Chem. Soc. 71, 2951.
- Synthesis and Evaluation of Novel Benzimidazole Conjugates Incorporating Amino Acids and Dipeptide Moieties. Lett. Org. Chem.. 2017;14(3):198-206.
- [CrossRef] [Google Scholar]
- Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety. J. Saudi Chem. Soc.. 2021;25(2):101194.
- [CrossRef] [Google Scholar]
- European Journal of Medicinal Chemistry Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. Eur. J. Med. Chem.. 2013;59:176-182.
- [CrossRef] [Google Scholar]
- Ef fi cient method for the synthesis of fused benzimidazole e imidazoles via deprotection and cyclization reactions. Tetrahedron. 2015;45–48
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of novel water soluble benzimidazole carbamates. Eur. J. Med. Chem.. 2018;144:372-385.
- [CrossRef] [Google Scholar]
- Design and Synthesis of Mannich bases as Benzimidazole Deriva- tives as Analgesic Agents. Antiinflamm Antiallergy Agents Med. Chem.. 2015;14:35-46.
- [Google Scholar]
- VOSO4catalyzed highly efficient synthesis of benzimidazoles, benzothiazoles, and quinoxalines. Tetrahedron Lett.. 2016;57(36):4012-4016.
- [CrossRef] [Google Scholar]
- Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem.. 2020;186:111850.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of rhodium (I) and ruthenium (II) carbene complexes derived from benzimidazole against Staphylococcus aureus isolates. Turkish J. Med. Sci.. 1997;27:59-61.
- [Google Scholar]
- Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg. Chem.. 2014;58:104-116.
- [CrossRef] [Google Scholar]
- One-pot microwave assisted synthesis of new 2-alkoxycarbonylmethylene-4- oxo-1,5-benzo-, naphtho-, and pyridodiazepines and assessment of their cytogenetic activity. Eur. J. Med. Chem.. 2013;67:302-309.
- [CrossRef] [Google Scholar]
- Benzimidazole analogs inhibit respiratory syncytial virus G protein function. Antiviral Res. 2015;121:31-38.
- [CrossRef] [Google Scholar]
- Gaba, M., Gaba, P., Uppal, D., Dhingra, N., Singh, M., Silakari, O., Mohan, C., 2015. Benzimidazole derivatives : search for GI-friendly anti-in fl ammatory analgesic agents 5, 337–342. https://doi.org/10.1016/j.apsb.2015.05.003.
- Simple and efficient protocol for the synthesis of benzoxazole, benzoimidazole and benzothiazole heterocycles using Fe (III)– Schiff base / SBA-15 as a nanocatalyst. Chinese Chem. Lett.. 2016;27(2):265-270.
- [CrossRef] [Google Scholar]
- Bioorganic & Medicinal Chemistry Letters 5-Sulfonyl-benzimidazoles as selective CB2 agonists-Part 2. Bioorg. Med. Chem. Lett.. 2012;22(1):547-552.
- [CrossRef] [Google Scholar]
- Green synthesis of privileged benzimidazole scaffolds using active deep eutectic solvent. Molecules. 2019;24(16):2885.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis of 1,3,4-oxadiazoles carrying benzimidazole moiety and their antimicrobial properties. Indian J. Chem. Sect. B Org. Med. Chem.. 2010;49:1130-1134.
- [CrossRef] [Google Scholar]
- Changes in Trichoderma asperellum enzyme expression during parasitism of the cotton root rot pathogen Phymatotrichopsis omnivora. Fungal Biol.. 2015;119(4):264-273.
- [CrossRef] [Google Scholar]
- Evaluation of in vitro and in vivo toxic effects of newly synthesized benzimidazole-based organophosphorus compounds. Ecotoxicol. Environ. Saf.. 2013;87:23-32.
- [CrossRef] [Google Scholar]
- Synthesis of Benzimidazole Derivatives as Antiallergic Agents with 5-Lipoxygenase Inhibiting Action. Chem. Pharm. Bull (Tokyo). 1999;47(11):1573-1578.
- [Google Scholar]
- A facile and efficient synthesis of benzimidazole as potential anticancer agents. J. Chem. Sci.. 2020;132(1)
- [CrossRef] [Google Scholar]
- Additive Free Greener Synthesis of 1,2-Disubstituted Benzimidazoles Using Aqueous Extract of Acacia concinna Pods as an Efficient Surfactant Type Catalyst. Polycycl. Aromat. Compd. 2019;41(6):1263-1273.
- [CrossRef] [Google Scholar]
- Kamal, A., Digwal, C.S., Yadav, U., Sakla, A.P., Ramya, P.V.S., Aaghaz, S., Kamal, A., 2016. Tetrahedron Letters VOSO 4 catalyzed highly efficient synthesis of benzimidazoles , benzothiazoles and quinoxalines. Tetrahedron Lett. https://doi.org/10.1016/j.tetlet.2016.06.074.
- CHEMISTRY Synthesis, structure – activity relationship and antinociceptive activities of some 2- (2 0 -pyridyl) benzimidazole derivatives. Med. Chem. Res.. 2016;25:1216-1228.
- [CrossRef] [Google Scholar]
- Title : Design, Synthesis and Antifungal Evaluation of Novel Benzimidazole Tertiary Amine Department of Pharmaceutical Chemistry, Pune University, NDMVPS college of Pharmacy. Arab. J. Chem. 2015
- [CrossRef] [Google Scholar]
- Design, synthesis and anticancer/antiestrogenic activities of novel indole-benzimidazoles. Bioorg. Chem.. 2020;100:103929.
- [CrossRef] [Google Scholar]
- Karminski-zamola, G., 2012. Synthesis , crystal structure determination and antiproliferative activity of novel 1007, 242–251. https://doi.org/10.1016/j.molstruc.2011.10.054.
- Synthesis of benzimidazoles / benzothiazoles by using recyclable, magnetically separable nano - Fe 2 O 3 in aqueous medium. J. Iran Chem. Soc.. 2017;14:1665-1671.
- [CrossRef] [Google Scholar]
- Antifungal activity of organic and organometallic derivatives of benzimidazole and benzothiazole. Arzneimittel-Forschung/Drug Res. 1997;47:667-670.
- [Google Scholar]
- Antifungal activity of some bis-5-methylbenzimidazole compounds. Folia Microbiol (Praha). 2003;48(5):679-681.
- [Google Scholar]
- Cytotoxic and antimicrobial potential of benzimidazole derivatives. Arch Pharm (Weinheim). 2021;354(8):2100076.
- [CrossRef] [Google Scholar]
- KÜÇÜKBAY, H., Yilmaz, Ü., ŞİRECİ, N., GÜVENÇ, A.N., 2011. Synthesis and antimicrobial activities of some bridged bis-benzimidazole derivatives. Turkish J. Chem. 35, 561–571.
- Kumara, K.S.J., Krishnamurthy, G., Kumar, N.S., Naik, N., Praveen, T.M., 2017. Sustainable Synthesis of magnetically separable SiO 2 / Co @ Fe 2 O 4 Nanocomposite and its Catalytic applications for the Benzimidazole Synthesis. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2017.10.125
- Leiduan Hao, Yanfei Zhao, Bo Yu, Hongye Zhang, H.X. and Z.L., 2014. Au catalyzed synthesis of benzimidazoles from. Green Chem. https://doi.org/10.1039/c4gc00153b
- Synthesis and characterization of Cu(II) containing nanosilica triazine dendrimer: A recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bis-benzimidazoles and bisbenzothiazoles. J. Mol. Catal. A, Chem.. 2013;379:243-254.
- [CrossRef] [Google Scholar]
- Quinoxalinone-benzimidazole rearrangement: An efficient strategy for the synthesis of structurally diverse quinoline derivatives with benzimidazole moieties Dedicated to the 80th anniversary of Academician Oleg Nikolaevich Chupakhin. Tetrahedron Lett.. 2014;55(31):4319-4324.
- [CrossRef] [Google Scholar]
- Efficient synthesis of 2-(pyrazol-3-yl)benzimidazoles from 3-arylacylidene-3,4-dihydroquinoxalin-2(1H)-ones and hydrazine hydrate via a novel rearrangement. Tetrahedron Lett.. 2009;50(37):5186-5189.
- [CrossRef] [Google Scholar]
- Recent Advances in the Synthesis of Benzimidazol-2-ones via Rearrangements, Progress in Heterocyclic Chemistry. Elsevier. 2017
- [CrossRef] [Google Scholar]
- A reaction for the synthesis of benzimidazoles and 1H-imidazo[4,5-b] pyridines via a novel rearrangement of quinoxalinones and their aza-analogues when exposed to 1,2-arylenediamines. Tetrahedron. 2010;66(51):9745-9753.
- [CrossRef] [Google Scholar]
- Anti-cytomegalovirus effects of tricin are dependent on CXCL11. Microbes Infect.. 2012;14(12):1086-1092.
- [CrossRef] [Google Scholar]
- Benzimidazole: A medicinally important heterocyclic moiety. Med. Chem. Res.. 2012;21(3):269-283.
- [CrossRef] [Google Scholar]
- Fe/S-catalyzed decarboxylative redox condensation of arylacetic acids with nitroarenes. Org. Chem. Front.. 2014;1(10):1157-1160.
- [CrossRef] [Google Scholar]
- Design, Synthesis and Biological Evaluation of Benzimidazole / Benzothiazole and Benzoxazole Derivatives as Cyclooxygenase Inhibitors. Bioorg. Med. Chem. Lett.. 2003;13:657-660.
- [CrossRef] [Google Scholar]
- Catalyst-free synthesis of benzodiazepines and benzimidazoles using glycerol as recyclable solvent. Tetrahedron Lett.. 2011;52(32):4132-4136.
- [CrossRef] [Google Scholar]
- Rafat M. Mohareb, M.M.K. and Y.R.M., 2020. USES OF ?-DIKETONES FOR THE SYNTHESIS OF NOVEL HETEROCYCLIC COMPOUNDS AND THEIR ANTITUMOR EVALUATIONS. Bull Chem Soc Ethiop 34, 385–405.
- Ranza A. Elrayess, Nagat Ghareb, Marwa M. Azab, M.M.S., 2013. Synthesis and Antimicrobial Activities of Some Novel Benzimidazole and Benzotriazole Derivatives containing β-Lactam Moiety. Life Sci J 10.
- Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur. J. Med. Chem.. 2010;45(7):2949-2956.
- [CrossRef] [Google Scholar]
- Chemico-Biological Interactions Effects of pesticide mixtures in human and animal models : An update of the recent literature. Chem Biol Interact. 2016;254:231-246.
- [CrossRef] [Google Scholar]
- Saha, P., Ramana, T., Purkait, N., Ali, A., Paul, R., Punniyamurthy, T., 2009. Ligand-Free Copper-Catalyzed Synthesis of Substituted Benzimidazoles , 8719–8725. https://doi.org/10.1021/jo901813g.
- 3D QSAR k NN-MFA studies on 6-substituted benzimidazoles derivatives as Nonpeptide Angiotensin II Receptor Antagonists : A rational approach to antihypertensive agents. J. Saudi Chem. Soc.. 2013;17:167-176.
- [CrossRef] [Google Scholar]
- Shashi, N.D., Krishna, B.S., 2016. Terephthalic acid derived ligand-stabilized palladium nanocomposite catalyst for Heck coupling reaction : without surface-modified heterogeneous catalyst. https://doi.org/10.1002/aoc.3549.
- Synthesis of benzimidazole nucleosides and their anticancer activity. Carbohydr. Res.. 2020;498:108178.
- [CrossRef] [Google Scholar]
- Recent Trends in the Synthesis of Benzimidazoles From o -Phenylenediamine via Nanoparticles and Green Strategies Using Transition Metal Catalysts. J. Heterocycl. Chem.. 2019;56(10):2702-2729.
- [CrossRef] [Google Scholar]
- Synthesis and Analgesic Activity of Novel Derivatives of. J Chem. 2013;2013
- [CrossRef] [Google Scholar]
- Suheyla Ozbey, F. Betul Kaynak, Canan Kus, and H.G., 2002. 2-(2,4-Dichlorophenyl)-5-fluoro-6-morpholin-4-yl-1H-benzimidazole monohydrate. Org Pap 1062–1064. https://doi.org/10.1107/S1600536802015842.
- Advances in the Synthesis of Ring-Fused Benzimidazoles and Imidazobenzimidazoles. Molecules. 2021;26(9):2684.
- [CrossRef] [Google Scholar]
- Antiviral activity of benzimidazole derivatives. III. Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem.. 2014;22(17):4893-4909.
- [CrossRef] [Google Scholar]
- Inhibition of HIV-1 capsid assembly: Optimization of the antiviral potency by site selective modifications at N1, C2 and C16 of a 5-(5-furan-2-yl-pyrazol- 1-yl)-1H-benzimidazole scaffold. Bioorg. Med. Chem. Lett.. 2012;22(24):7512-7517.
- [CrossRef] [Google Scholar]
- Trivedi, R., De, S.K., Gibbs, R.A., 2006. A convenient one-pot synthesis of 2-substituted benzimidazoles 245, 8–11. https://doi.org/10.1016/j.molcata.2005.09.025.
- European Journal of Medicinal Chemistry Design, synthesis and antiproliferative properties of some new 5-substituted-2-iminobenzimidazole derivatives. Eur. J. Med. Chem.. 2013;63:696-701.
- [CrossRef] [Google Scholar]
- Synthesis and Antiparasitic Activity of 1 H -Benzimidazole Derivatives. Bioorg. Med. Chem. Lett.. 2002;12(16):2221-2224.
- [Google Scholar]
- Structure – Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents : An Overview. pharmaceuticals. 2021;14:1-31.
- [CrossRef] [Google Scholar]
- Venkateswarlu, Y., Kumar, S.R., Leelavathi, P., 2013. Facile and efficient one-pot synthesis of benzimidazoles using lanthanum chloride 1–8.
- Wright, J.B., 1951. The chemistry of the benzimidazoles, in: Rsesarch Laboratories, The Upjohn Company, Kalamazoo, Michigan.
- Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem.. 2015;97:419-443.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of anticancer properties of novel benzimidazole ligand and their cobalt(II) and zinc(II) complexes against cancer cell lines A-2780 and DU-145. Inorganica Chim. Acta. 2019;495:118977.
- [CrossRef] [Google Scholar]
- Bioorganic & Medicinal Chemistry Letters Benzoylbenzimidazole-based selective inhibitors targeting Cryptosporidium parvum and Toxoplasma gondii calcium-dependent protein kinase-1. Bioorg. Med. Chem. Lett.. 2012;22(16):5264-5267.
- [CrossRef] [Google Scholar]
- Advances in the synthesis of benzimidazol-2-ones. Russ. Chem. Rev.. 2017;86(10):968-997.
- [CrossRef] [Google Scholar]







