Translate this page into:
Anti-inflammatory, analgesic and molecular docking studies of Lanostanoic acid 3-O-α-D-glycopyranoside isolated from Helichrysum stoechas
⁎Corresponding author at: Department of Pharmacognosy, RV Northland Institute, Dadri, Greater Noida, U.P., India. sarfarajpharma@gmail.com (Md. Sarfaraj Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Helichrysum stoechas has been conventionally used as herbal tea due to its anti-inflammatory, antioxidant, antimicrobial and diuretic activities. Ethanolic extract of the aerial parts of the plant (HSE) afforded a lanostane triterpenoid glycoside. The isolated compound was characterized as Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopyranoside (HS-01) with the help of UV, IR, 1H, 13C NMR and MS spectroscopic techniques. HSE (at 100 and 200 mg/kg doses) and the isolated compound, HS-01 (at 10 mg/kg dose) has been investigated for anti-inflammatory and analgesic activities against chemically challenged experimental animal. Both the HSE as well as HS-01 showed a substantial decline in paw volume when compared with the relevant control groups (p < 0.01 & p < 0.001). The HSE and HS-01 also confirms a significant prolongation of the paw licking or jumping towards the Eddy’s hot plate and reduction in quantity of writhes after the introduction of acetic acid in mice (p < 0.01 & p < 0.001). In order to have a better understanding of the binding interactions of HS-01 at molecular level, docking studies were performed with various macromolecular drug targets using AutoDock 4.2 and AutoDock Vina 1.1. Both programs predicted Galectin-3 as most favorable target for HS-01 followed by iNOS, whereas TNFα and COX-2 were among less favorable. Therefore, HS-01 could be developed as suitable therapy against inflammation and associated disorders.
Keywords
Helichrysum stoechas
Phytochemical
Lanostane triterpenoids glycosides
Molecular docking
Anti-inflammatory
1 Introduction
Every disease on earth has a cure from the grace of Almighty and mankind has been blessed by numerous medicinal plants. Natural medicinal plants have been utilized for medicinal purposes over centuries, and persist to be a medicament for various diseases even with the dramatic rise in antibiotics and other synthetic medicine in the current scientific world (Hussain et al., 2010, 2011, 2012). Helichrysum stoechas L. Moench (Asteraceae); a shrub with yellow flowers that grows widely in the Mediterranean area and also found in southern Europe, south-west Asia, various parts of southern India, Sri Lanka and Australia with majority species occurring in Africa, including Madagascar (Giuliani et al., 2016). The Helichrysum species shows incredible morphological multifariousness and hence subdivided into 30 morphological clusters (Onaran et al., 2016). The aerial parts of the plants are covered with hairs and flowers are aromatic, solitary or found in dense or dispersed inflorescences (Lourens et al., 2008). Some members of this genus have been commonly used in traditional medicine as a herbal tea comprising of ethnopharmacological uses such as has been used to treat various ailments/diseases including pain, inflammation, antioxidant, cholagogue, wounds, infections, and respiratory conditions that have been treated using this plant (Haddouchi et al., 2014). In Northern African countries usually seeds, roots and other aerial parts of the plants were traditionally used in healing of various diseases like jaundice, gall bladder stone disorders, malaria, oedema, rheumatism, gout, impotency, and in the elimination of kidney stones (Lourens et al., 2008). An immense amount of pharmacological activities are generally ascribed to this plant like anti-allergic, anti-inflammatory in cough relief, antioxidant, (Bektas et al., 2005; Sala et al., 2002) antimicrobial and in the treatment of rhinitis and wounds (Maggio et al., 2016). The chemistry of the H. stoechas has wide variety of chemical classes, among which the important ones are Flavonoids (apigenin 7-O- glucuronide, apigenin 7-O- glucoside), chalcones, alkaloids, phloroglucinol derivatives, essential oils, α-pyrones, diterpenes, sterols (stimagsterol), monoterpenes (neryl acetate, neryl propanoate, α-pinene, geraniol and geranyl acetate) triterpenes (lupeol, hentricotane, betulin, luteolin, luteolin -7-O-rutinosides) and minerals Fe, Cu, Co (Bohlmann et al., 1979; Jakupovic et al., 1989; Francisco et al., 1990). Molecular docking has been consistently exploited to underscore the molecular interactions of ligand molecules and hence, represents a valuable tool in drug discovery and development trajectory (Azam et al., 2012). It is one of the several computational methodologies intended to assist researchers to identify and investigate novel drugs candidates (Shushni et al., 2013). Hence, to elucidate the structural requirements for interaction of HS-01 with numerous anti-inflammatory drug targets, molecular docking study was employed.
In the current research work we have described the isolation and characterization of lanostane triterpenoid glycoside from the ethanolic extract of H. stoechas and successfully conducted its anti-inflammatory and analgesic potency.
2 Materials and methods
2.1 Collection and authentication of plant material
We collected fresh plant sample, weighing (3.5 kg) from the basin of the Mediterranean sea, Misurata, Libya during March 2015. It was authenticated by Dr. Huda Shaban Elgubbi, Department of Botany, College of Science, Misurata University, Misurata, Libya. A voucher specimen no. HC 55/01 has been submitted in the herbarium, Department of Botany, College of Science, Misurata University, Misurata, Libya for the proceedings.
2.2 Extraction and isolation
The aerial parts of plant H. stoechas (3.5 kg) was shade dried, coarsely powdered and further 500 g of the powder was extracted thoroughly with 95% ethanol in a Soxhlet apparatus. The ethanolic extract was then concentrated on water bath and dried under reduced pressure to get 87.9 g of dark brown mass. The extract was chemically screened out to detect the presence of different chemical constituents. About 80 g extract was placed to glass column on silica gel (60–120) using different polarities of solvents starting from petroleum ether, chloroform and methanol in different ratio. Elution of the column with chloroform: methanol (19:01) mixture produced colorless crystalline compound HS‑01 (0.89 g).
2.3 Instrumentation, chemicals and drugs
Melting point apparatus (Perfit), ultraviolet (UV) spectra (Lambda Bio 20 Spectrophotometer Shimadzu-U Singapore) scanned in chloroform, infrared (IR) spectra (Win IR FTS 135 instrument, Biorad, USA), 1H nuclear magnetic resonance (NMR) 300 MHz and 13C NMR 75 MHz spectra in CD3OD solvent (Brucker Spectrometer, Brucker, USA), MS (DART dry Helium, JEOL-Accu TOF JMS-T100LC), plethysmometer (UGO Basil, Italy). Carrageenan, were procured from Sigma Chemical Co., St. Louis, MO. Standard drugs Indomethacin, Diclofenac and Tramadol was received as a gift sample from Ranbaxy labs, New Delhi. The solvents for isolation were acquired from Merck Germany. Silica gel (60–120) mesh, Merck, Germany) for column chromatography and for thin-layer chromatography, silica gel G coated TLC plates (Merck, Germany) was utilized. Spots were visualized by exposure to iodine vapors, UV Lamp 254 nm and by spraying with anisaldehyde sulphuric acid reagents or with iodine vapors. The percentage yield of the isolated compound was calculated on the basis of dried plant material (500 g) used for extraction (Hussain et al., 2019).
2.4 Animals
Healthy Wistar albino-rats of both sexes weighing between 140 and 160 g were preferred for anti-inflammatory activity and adult Swiss albino mice of each sex (25–30 g) for analgesic activity. They were maintained in clean, sterile, polypropylene cages maintained at room temperature (21 ± 2 °C) in 12 h dark/light control and fed with commercial pellet and water ad libitum. After randomization into various groups, the mice were quarantined for a period of a week for environmental and handling acclimatization before initiation of the experiments. The experimental procedures were in agreement by the Institutional Ethical Committee, Faculty of Pharmacy, Misurata University, Misurata, Libya and their guidelines were followed for the investigations (MU/COP/PHAR-03/2015).
2.4.1 Administration of drugs
The ethanolic extract of H. stoechas (HSE) at doses 100 and 200 mg/kg and its isolated constituent HS-01 (10 mg/kg) was used in all the experimental models. Indomethacin 10 mg/kg was used as a standard anti-inflammatory drug, Tramadol 0.1 ml (40 mg/kg. s.c) was used as pain inhibiting drug in hot plate method and Diclofenac 5 mg/kg was used as pain inhibiting drug in acetic acid influenced writhing in mice. All the test and standard drugs were formulated into an emulsion using 0.3% Carboxy Methyl Cellulose (CMC) to obtain the desired dose on body weight basis (mg/kg) of the animal and administered orally using a ball ended feeding needle. The animals were allowed for free access to water and food after dosing (Iqbal et al., 2016).
2.4.2 Safety profile study
An acute toxicity study was conducted for the determination of LD50 by implementing the scheme (Annexure 2d) of CPCSEA, OECD guideline No. 423. Swiss albino mice of either of the each sex were alienated into six groups with six animals in each group. Ethanolic extract of H. stoechas at different dose levels of 500, 1000, 1500, 2000 and 2500 mg/kg were administered orally as a single dose to mice. The animals were watched periodically for the indication of toxicity and death for 24 h and then on a daily basis for 14 days (Hussain et al., 2016).
2.4.3 Carrageenan-induced rat hind paw edema
Acute inflammation was provoked by injection of 0.1 ml of 1% freshly prepared suspension of carrageenan in normal saline at the sub-plantar region of the right hind paw of all groups of animals (Mukherjee et al., 1997). At 1, 2, 3, 4 and 5 hr intervals the volume of the injected paws was measured using a plethysmometer. The animals were premedicated with vehicle (0.3% CMC p.o.), ethanolic extract of H. stoechas (HSE) at doses 100 and 200 mg/kg along with the isolated compound HS-01 (10 mg/kg) and standard drug indomethacin (10 mg/kg) 1hr before carrageenan challenge (Sachan et al., 2011). The percentage inhibition of inflammation was intended according to the following formula: % inhibition = 100 (1-Vt/Vc), where ‘Vc’ stand for inflammation volume in control and ‘Vt’ inflammation volume in group treated with tested drugs.
2.4.4 Analgesic activity
Analgesic activity of the ethanolic extracts of H. stoechas and isolated compound was determined by both chemical (acetic acid induced writhing response) and thermal method (hot plate reaction time).
2.4.4.1 Hot plate test
Eddy’s hot-plate method was used for determining the analgesic activity. The mice were divided into five groups (n = 6 per group): vehicle (0.3% CMC p.o.), HSE (100 and 200 mg/kg), HS-01 (10 mg/kg) and standard drug Tramadol 0.1 ml (40 mg/kg. s.c). After 60 min of the test and standard drug administration, the mice in all groups were gently handled and individually placed on the hot plate surface, maintained at 55 ± 0.5 °C. The duration of time taken in seconds for paw licking or jumping was recorded as response time at 0, 30, 60 and 120 min. A cut off stage of 30 s is maintained to circumvent the damage in the paws. The pain inhibition percentage (PIP) was calculated according to the following formula (Ahmed et al., 2004; Delporte et al., 2005). where, T1 is post-drug latency and T0 is pre-drug latency time.
2.4.4.2 Acetic acid induced writhing test
The animals were pre-medicated with vehicle (0.3% CMC p.o.), HSE (100 and 200 mg/kg), HS-01 (10 mg/kg) and standard drug Diclofenac (5 mg/kg). Acetic acid (1% v/v) at the dose of 1 ml/kg body weight was injected intra-peritoneally to all the groups of animals 1hr after the dosing of test and standard drugs. Writhing was recorded by counting the quantity of writhes followed by the injection of acetic acid for a period of 30 min. The writhe is signaled by abdominal constriction and full extension of hind limb (Sajad et al., 2009).
2.5 Molecular docking
Three-dimensional coordinates of numerous drug targets associated with inflammation were obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB, http://www.rcsb.org/pdb/home/home.do). All the input files for docking were prepared using graphical user interface of Biovia Discovery Studio Visualizer and MGLTools packages. Native co-crystalized ligands, water molecules and cofactors were removed from each PDB file and Gasteiger method was used for assigning partial atomic charges. Each rotatable bond of ligand was assigned after merging non-polar hydrogens. The 2D-structure of the HS-01 was converted to its 3D-coordinate and energy minimized by MM2 method implemented in ChemBio3D Ultra 12.0. AutoDock 4.2 (Morris et al., 1998) and AutoDock Vina 1.1 (Trott and Olson, 2010) was used for molecular docking simulation employing Lamarckian genetic algorithm methodology. Default docking protocol was adopted for a rigid protein and a flexible ligand with 100 independent runs. A grid box having dimension of 60, 60, and 60 points in x, y, and z directions was built with grid spacing of 0.375 Å. Distance-dependent function of the dielectric constant was used for the calculation of the energetic map. The default settings were used for all other parameters. Upon completion of docking, the best poses were screened by examination of binding energy (ΔGbinding, kcal/mol) and number in cluster. Molecular interactions of both hydrophobic and hydrophilic types and root mean square deviation (RMSD) were studied using Biovia Discovery Studio Visualizer, LigPlot+ and PyMol programs.
2.6 Evaluation of in-silico toxicity
The assessment of toxicity of the HS-01 was performed by using the T.E.S.T. tool, Version 4.2.1, a program from the US Environmental Protection Agency (Martin 2016). This tool is based on advanced quantitative structure–activity relationship models to predict the toxicity of natural or synthetic compounds based on molecular structures, and their applicability has been well documented (Claeys et al., 2013; Li et al., 2020; Sripriya et al., 2019).
2.7 Statistical analysis
A one-way analysis of variance followed by Dunnett’s post-hoc test was performed by using Graph Pad Prism V2.01 (GraphPad Software, Inc., San Diego, California, USA). The data were expressed as the mean ± standard error of the mean and P < 0.05 and < 0.01 was considered as statistically substantial.
3 Results and discussion
3.1 Structural interpretation of isolated compound (HS-01)
Colorless crystalline compound; yield 0.24% w/w; RF 0.43 (MeOH: CHCl3, 1:2); mp 92–93 °C; UV–visible λmax (MeOH): 233 nm; IR νmax (KBr): 3375, 3260, 2923, 2856, 1690, 1639, 1461, 1383, 1277, 1215, 1171, 1031, 774/cm; 1H NMR (300 MHz, MeOD) and 13C NMR (75 MHz, MeOD) [data: Table 1]; MS DART m/z (rel. int) : 622 [M]+ (C36H62O8), 479, 459, 443, 316. Compound HS-01 is a Lanosten triterpenoid glucoside, has been found as a monotonous crystalline mass from chloroform: methanol (19:1) eluants, which produced fizziness with sodium bicarbonate solution and retaliated positive Fehling’s test for glycoside. It has distinct IR absorption bands for OH (3375, 3260 cm−1), COOH (1690 cm−1) and C = C (1639 cm−1). The 1HNMR spectrum of HS-01 displayed a single proton double doublet at δH 3.89 (J = 5.5, 8.9 Hz) which is was attributed to oxygenated methine H-3α proton. A one-proton doublet at δH 5.24 (J = 3.9 Hz) is due to anomeric H-1ꞌ protons and another two one proton doublet’s proton appears at 3.16 (J = 5.1 Hz) and 3.11 (J = 4.2 Hz) has been ascribed to H2- 6′a and H2- 6′b position respectively. Four broad singlets at δH 1.11, 0.97, 0.90, and 0.77 and two doublets at δH 1.29 (J = 6.3 Hz), 1.05 (J = 6.3 Hz) are ascribed to tertiary C-19, C-27, C-30 and C-18 and secondary C-28 and C-21 methyl protons, all including three-protons each respectively. The additional sugar protons materialize as four single proton mulitiplets found respectively at 3.99, 3.76, 3.58 and 3.54. The 13C NMR signals of HS-01 exhibited by the COOH group at δ 181.26 (C-26), oxygenated CH at δ 80.19 (C-3) and 119.71(C-6) anomeric carbons at δ 104.86 (C-1ꞌ), and additional sugar carbons involving 72.84–64.74 has been observed. These 1H and 13C NMR data of the lanostene unit has been correlated with triterpenoids (Ansari et al., 2016). Acidic hydrolysis of isolated compound HS-01 produced D-glucose and capric acid in proportional quantity (Co- TLC). The mass and 13C NMR spectra of the HS-01 molecular peak establishes m/z 622.39 [M]+ in agreement to the molecular formula C36H62O8. It may be due to the breaking of glycoside bond during the ionization of the compound at position C3 that the molecular ions found in the mass spectra were corresponding to the mass peak at m/z 459, corresponding to the molecular formula C30H49O3 (Arif et al., 2013). On the basis of mass fragmentation and spectral analysis the structure of HS-01 has been elucidated as Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopyranoside (Figs. 1 and 2). This is found to be a new lanostane triterpenic glycoside phytoconstituents in this plant species. Coupling constants in Hz are provided in parentheses.
Position of proton
Nature of proton
δH (ppm)
δC (ppm)
α
β
C1
CH2
1.25 (brs)
1.29
37.79
C2
CH2
1.43 (brs)
1.50
30.84
C3
CH
3.89 (dd, J = 5.5, 8.9)
–
80.19
C4
C
–
–
42.36
C5
CH
4.28 (d, J = 7.99)
–
139.24
C6
CH2
1.28 (brs)
1.25
119.71
C7
CH2
2.27
2.31(m)
30.55
C8
CH
2.34 (d, J = 6.0)
–
40.71
C9
CH
2.13
–
48.28
C10
C
–
–
36.68
C11
CH2
2.17
2.20
22.95
C12
CH2
1.98
2.02
28.91
C13
C
–
–
44.52
C14
C
–
–
52.25
C15
CH2
1.87 (brs)
1.90
34.99
C16
CH2
1.62 (brs)
1.68
42.83
C17
CH
1.81(m)
–
51.18
C18
CH3
0.77 (brs)
–
18.49
C19
CH3
1.11(brs)
–
20.46
C20
CH
2.49 (d, J = 5.7)
–
35.13
C21
CH3
1.05 (d, J = 6.5)
–
19.17
C22
CH2
1.17
–
33.15
C23
CH2
1.13
1.08
25.74
C24
CH2
1.03
–
45.16
C25
CH
1.54 (d)
–
30.23
C26
C
–
–
181.26
C27
CH3
0.97 (brs)
–
24.87
C28
CH3
1.29 (d, J = 6.3)
–
26.14
C29
CH3
0.90 (brs)
–
26.87
C30
CH3
0.88 (brs)
–
19.11
C1ꞌ
CH
5.24 (d, J = 3.9)
–
104.86
C2ꞌ
CH
3.76 (m)
–
74.84
C3ꞌ
CH
3.58 (m)
–
72.41
C4ꞌ
CH
3.54 (m)
–
71.65
C5ꞌ
CH
3.99 (m)
–
79.70
C6ꞌ
CH2
3.16 (d, J = 5.1)
3.11 (d, J = 4.2)
64.74
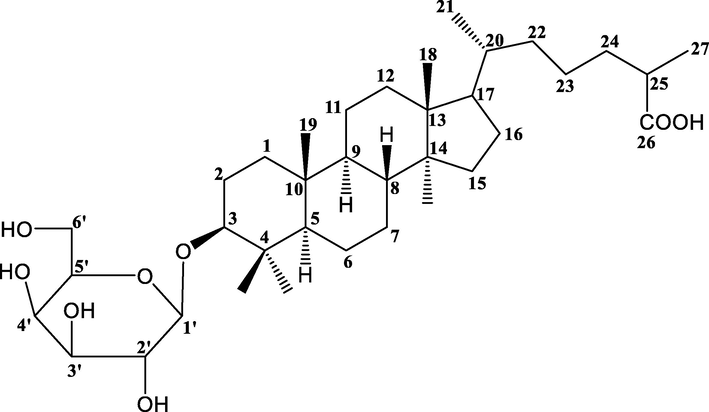
Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopyranoside (HS-01).
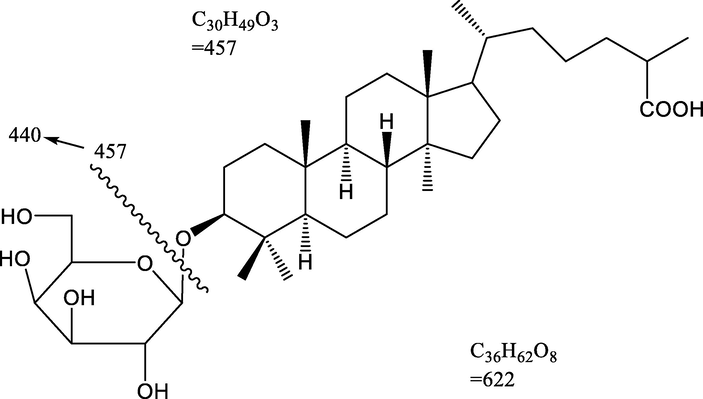
Mass fragmentation pattern of Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopyranoside (HS-01).
3.2 Safety profile study
It has been observed that the dosing of HSE up to 2.5 gm/kg orally to the mice did not induce drug related toxicity and mortality. The mice tolerated the drug well and exhibited normal behavior up to 2.5 gm/kg orally. All the animals were observed to be vigilant with normal spruce, touch and pain response, and there was no signal of meekness and vocalization.
3.2.1 Carrageenan induced rat paw edema
Anti-inflammatory effect of Indomethacin at dose (10 mg/kg), HSE at two different dose levels (100 and 200 mg/kg) and the isolated compound HS-01 at (10 mg/kg) on carrageenan aggravated rat paw is shown in Table 2. The anti-inflammatory effects of all tested drugs were observed from 60 min. after carrageenan challenge. Paw edema in rats was attained at its peak at 4 hrs following carrageenan challenge and the animal which was treated with Indomethacin showed significant (P < 0.05) reduction in paw volume of rats from 2 hr. Similarly treatment with HS-01 (10 mg/kg) and HSE (200 mg/kg) showed a significant reduction (P < 0.05) of paw volume in rats provoked by carrageenan. The effect of HS-01 at dose 10 mg/kg was found advantageous in reducing the swollen paw volume than higher dose of HSE (200 mg/kg). Inflammation is coupled with a lot of patho-physiologies of different clinical conditions like arthritis, cancer, gout and vascular diseases. A lot of medicinal plants are used in a range of traditional medical systems for relief from the warning sign of pain and inflammation. In this study the Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopryranoside (HS-01) isolated from H. stoechas extract demonstrated anti-inflammatory-analgesic activity at dose 10 mg/kg. Carrageenan, which is known to be a Phlogestic agent and augmented the prostaglandins and bradykinins synthesis at various time intervals (Arif et al., 2016). HS-01 at 10 mg/kg dose exhibited a decline in paw edema volume from 1hr to 5hr and prolonged its anti-inflammatory effect after a duration of 3 hr. This study clearly illustrates that the effect of lanostane-triterpenoid glycoside (HS-01) may interact with the prostaglandins spurt. Interestingly both dose levels of HSE extract exhibited similar pattern in reducing carrageenan induced paw edema from 1hr to 5hr. Carrageenan-induced inflammation is caused by the commencement of prostaglandins, platelet activating factors (PAF) and other inflammatory mediators. The first phase (0-1hr) is endorsed to the release of histamine, 5-HT and kinin, whereas the second phase (3–5 hr) is attributed to the discharge of prostaglandin and bradykinin. Nevertheless the anti-inflammatory reaction found in 200 mg/kg was greater than in the 100 mg/kg. This result directly correlated with the results observed in carrageenan induced paw edema where single dose of HS-01 (10 mg/kg) and both doses of HSE (100 and 200 mg/kg) extracts significantly alter the carrageenan induced prostaglandins mediated inflammatory response (Mukherjee et al., 1997). Each values are expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: *p < 0.05 & **p < 0.01compare to respective control group.
Group (n = 6)
Increase in paw volume (ml) (mean ± SEM)
Dose (mg/kg p.o)
0 h
1 h
2 h
3 h
4 h
5 h
Control 0.3% CMC
1.28 ± 0.03
1.51 ± 0.02
1.73 ± 0.01
1.85 ± 0.019
1.93 ± 0.025
1.86 ± 0.03
IDM (10 mg)
1.26 ± 0.034
1.46 ± 0.025
1.51 ± 0.029*
1.56 ± 0.015*
1.49 ± 0.018*
1.34 ± 0.02**
HSE (100 mg)
1.27 ± 0.02
1.52 ± 0.01
1.66 ± 0.02
1.72 ± 0.027
1.79 ± 0.033
1.62 ± 0.034
HSE (200 mg)
1.28 ± 0.01
1.48 ± 0.03
1.55 ± 0.025
1.64 ± 0.024*
1.60 ± 0.012*
1.53 ± 0.01*
HS-01 (10 mg)
1.26 ± 0.02
1.44 ± 0.029
1.56 ± 0.03*
1.48 ± 0.036*
1.43 ± 0.027**
1.36 ± 0.01**
3.2.2 Hot plate test
HSE 100 and 200 mg/kg and isolated compound HS-01 (10 mg) considerably (P < 0.001) amplified the response time of animals towards the thermal source with different time periods (Fig. 3). In hot plate test HSE at 100 mg exhibited a pain inhibition percentage (PIP) of 57.26% and HSE at 200 mg showed 59.76%, whereas HS-01 at 10 mg showed a PIP of 63.94% respectively.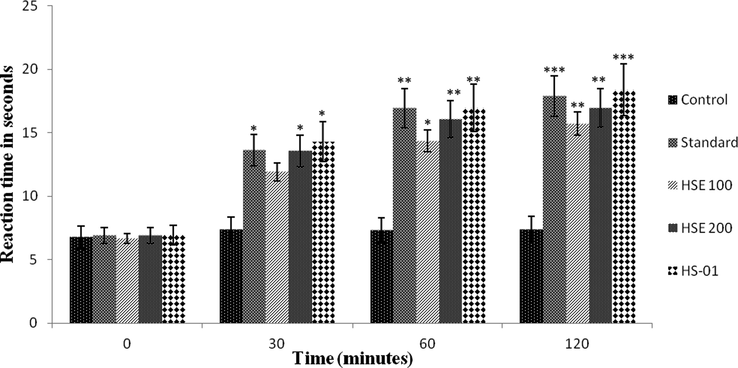
Effect of ethanolic extract of H. stoechas (HSE 100 mg and HSE 200 mg), isolated compound (HS-01) and Tramadol 0.1 ml (Standard) on reaction time of mice exposed to hot plate. Each value is expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: *p < 0.05, **p < 0.01 and ***p < 0.001compare to respective control group.
3.2.3 Acetic acid induced writhing methods
Acetic acid-induced writhing test in mice was used to evaluate the analgesic activity of HSE (at doses of 100 and 200 mg/kg) and HS-01 (at 10 mg/kg dose). Both HSE 200 mg/kg and HS-01, 10 mg/kg significantly (P < 0.001) lowered the quantity of abdominal constriction and draw out of hind limbs provoked by the injection of acetic acid (Fig. 4). HSE 100 mg/kg exhibited a significant (P < 0.01) writhing inhibition. The percentage of inhibition by HSE at 100 mg/kg and 200 mg/kg were 58.46% and 65.69%, respectively, whereas for the isolated compound HS-01 it was 73.36% and for the standard drug Diclofenac it showed 66.15%. The abdominal constrictions observed after challenge of acetic acid is associated to sensitization of nociceptive receptors to prostaglandins. Hence the possibilities of the extracts exert their analgesic effect perhaps by slowing down the synthesis of prostaglandins.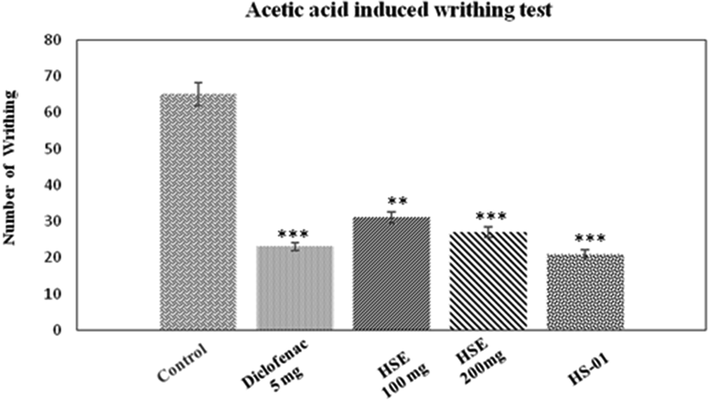
Effect of ethanolic extract of H. stoechas (HSE 100 mg and HSE 200 mg), isolated compound HS-01 (10 mg) and diclofenac 5 mg (Standard) on acetic acid induced writhing in mice. Each value is expressed in Mean ± S.E.M. one way ANOVA followed by Dunnett’s test. P: **p < 0.01 and ***p < 0.001compare to respective control group.
3.3 Molecular docking
In the present study, molecular interaction of HS-01 with numerous anti-inflammatory drug targets was studied by molecular docking using two different programs namely, AutoDock 4.2 and AutoDock Vina 1.1. A number of macromolecular targets associated with inflammatory cascade such as cyclooxygenase-2, tumour necrosis factor-α (He et al., 2005), inducible nitric oxide synthase (iNOS) (Fischmann et al., 1999) and galectin-3 (Williams et al., 2003) has been exploited for in silico study. Prior to the docking of test compound, all co-crystallized ligands were redocked into their respective proteins (PDB ID: 3LN1, 2AZ5, 4NOS and 1P8D). In each protein, the observed root mean square deviation (RMSD) of the docked ligands having minimum energy of binding were ≤ 2.0 Å in comparison with the co-crystallized ones, indicating that the scoring parameters implemented in both AutoDock 4.2 and AutoDock Vina 1.1 are reliable (Ahmed et al., 2012; Azam et al., 2019, 2015).
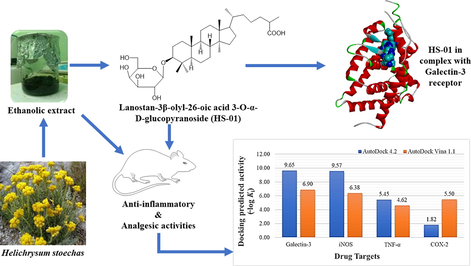
The unique chemical structure of HS-01 consists of a glycoside of lanostanol-based steroidal nucleus, making it an invaluable drug contender. Fortunately, the compound owns a large hydrophobic skeleton with appended hydrophilic groups participating in both non-polar and polar interactions. In-silico screening results furnished a large disparity of the predicted inhibition constants (Ki) and hence, all values were converted into negative logarithmic scale (−log Ki) in order to have uniformity in data, and plotted against protein targets (Fig. 5). The results portrayed in Table 3 clearly implicate that HS-01 interacts with diverse molecular targets showing variable affinities and equivalent alignment to the co-crystallized molecules of each protein. However, comparative evaluation of the docking results obtained from both AutoDock 4.2 and AutoDock Vina 1.1 (Fig. 5) showing binding energies of −13.16 and −9.4 kcal/mol, respectively, demonstrate that galectin-3 receptor is the most preferable target for HS-01 which accommodates itself most contentedly in the binding pocket (Fig. 6). Remarkable affinity was observed with iNOS while moderate affection was envisaged for both TNF-α and COX-2. Galectins are a group of β-galactoside-binding proteins which regulate multiple functions at cellular level, such as inflammation, cell proliferation, immune surveillance and apoptosis. In particular, galectin-3 is endowed with distinct affinity for glycosides-based compounds, making it a fascinating therapeutic target for treating inflammation-mediated disorders by blocking the active site by natural glycosides (Arifuzzaman et al., 2020; Murphy et al., 2020). Binding pocket of galectin-3 consists of large hydrophobic cavity encircled by residues Phe243, Phe271, Leu274, Met312, Glu315, Thr316, Phe329, His435, Leu442 and Trp457. Docked HS-01 occupies this pocket and exploits these residues for non-polar interactions while polar interactions were conferred by Ser278, Glu281 and Arg319 (Fig. 6). These results are expected to be vital for various drug design attributes of lead development based on HS-01 molecule for the cure of inflammation-mediated ailments. Values corresponding to AutoDock Vina 1.1 are given in brackets.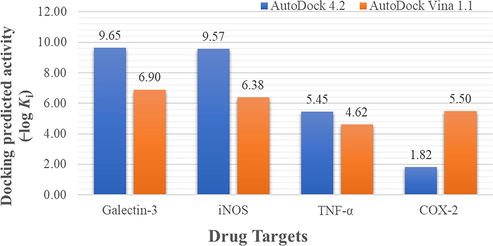
Docking predicted activity plotted against various probable drug targets.
Target; PDB code
ΔGba
Kib
RMSDc (Å)
Residues involved in interactions
H-bonds
Hydrophobic
Galectin-3; 1P8D
−13.16 (−9.4)
224.74 pM (12.6 μM)
0.45 (0.98)
Ser278, Glu281, Arg319, Leu345
Phe243, Phe268, Phe271, Thr272, Ala275, Met312, Glu315, Thr316, Phe329, Ala343, Gly344, Phe349, His435, Leu442, Leu449, Leu453, Trp457
Inducible Nitric Oxide Synthase; 4NOS
−13.05 (−8.7)
269.62 pM (41.8 μM)
4.63 (5.04)
Arg199, Trp372, Tyr489
Trp194, Ala197, Pro198, Gly202, Ser242, Val352, Met355, Phe369, Asn370, Gly371, Met374, Met434, Ala439, Trp463, Phe488, Tyr491
TNF-α; 2AZ5
−7.43 (−6.3)
3.55 µM (0.24 μM)
3.20 (5.81)
Tyr119, Gly121, Gly148
His15, Tyr59, Leu94, Leu120, Tyr151
COX-2; 3LN1
−2.48 (−7.5)
15.28 mM (3.17 μM)
1.99 (11.92)
Pro71
Val74, Leu78, Val102, Arg106, Val335, Leu338, Ser339, Tyr341, Leu345, Phe504, Val509, Glu510, Gly512, Ala513, Ser516, Leu517
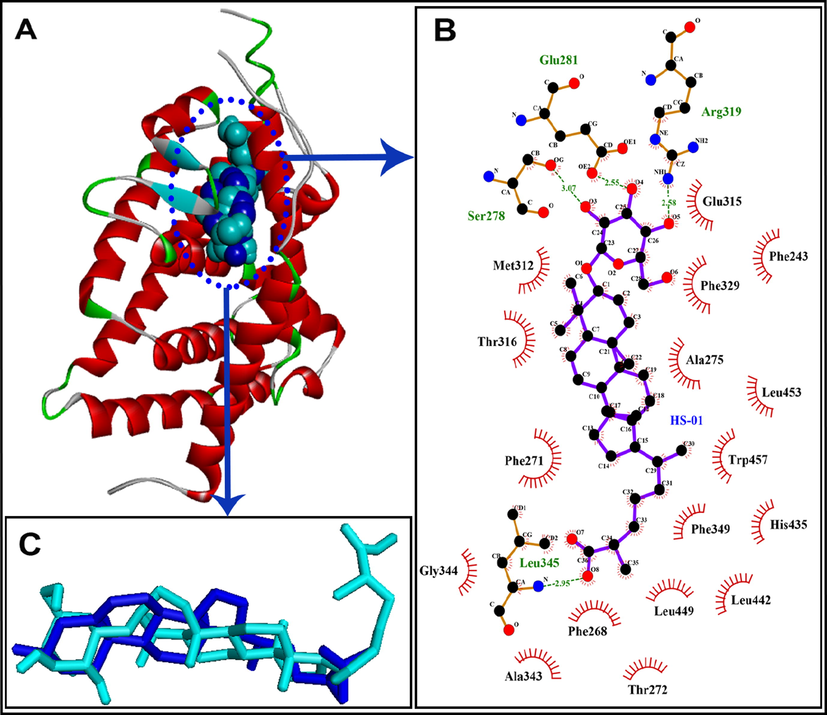
A: HS-01 (shown as CPK in cyan color) docked into the binding pocket of galectin-3 (shown as ribbon), native cocrystallized ligand is shown as CPK style in blue color; B: Ligplot diagram of docked HS-01 in the binding pocket of galectin-3 showing key interacting residues; C: Docked HS-01 (shown in cyan color) with native co-crystallized ligand (shown in blue color) exhibiting RMSD of 0.45 Å.
3.4 In-silico toxicity prediction of HS-01
Molecular features of compounds are utilized for the development of models in quantitative structure–activity relationship (QSAR) studies for prediction of toxicity and biological activity as well. These models have proven very useful tools for predicting the QSAR of various harmful substances (Puzyn et al., 2011; Roy and Das, 2014). The acute toxicity of isolated compound HS-01 was evaluated by using the T.E.S.T. software which predicts LD50 (oral lethal dose for 50% of test rats) as well as mutagenicity. The results so obtained are presented in Table 4. The prediction by T.E.S.T. program is based on four different methods such as consensus, FDA, hierarchical clustering and nearest neighbor. The predicted oral rat LD50 was observed in the range of 88.32 mg/kg to570.35 mg/kg in hierarchical clustering and nearest neighbor methods, respectively. All of the employed methods predicted negative mutagenicity except nearest neighbor method, which estimated positive mutagenicity for HS-01 compound (see Table 4).
Toxicity parameters
Toxicity results
Consensus method
Oral rat LD50 mg/kg
214.38
Mutagenicity value
0.26
Mutagenicity result
Negative
FDA method
Oral rat LD50 mg/kg
195.60
Mutagenicity value
0.04
Mutagenicity result
Negative
Hierarchical clustering method
Oral rat LD50 mg/kg
88.32
Mutagenicity value
0.06
Mutagenicity result
Negative
Nearest neighbor method
Oral rat LD50 mg/kg
570.35
Mutagenicity value
0.67
Mutagenicity result
Positive
4 Conclusion
The present study suggested that HSE at doses 100 and 200 mg/kg in combination with isolated compound HS-01 (10 mg/kg) showed significant anti-inflammatory, analgesic activity. Carrageenan activated edema is the main cause for the activation of prostaglandins, platelet activating factors (PAF) and other inflammatory intermediaries. In conclusion Lanostan-3β-olyl-26-oic acid 3-O-α-D-glycopyranoside isolated from H. stoechas extract showed protection against inflammation; pain and cytokine like pro-inflammatory mediators release might be beneficial in the treatment of endotoxin associated systemic inflammation. To the best of our knowledge there is no scientific report on the presence of this lanostane triterpenoid from ethanolic extract of H. stoechas through several triterpenoids and triterpenoidal glycosides. The study also affirms that the effectiveness of ethanolic extract is due to the presence of lanostane triterpenoid (HS-01) which is useful marker for Helichrysum genus. Molecular docking results highlighted the probable mechanism by which HS-01 is expected to elicit anti-inflammatory effects.
Acknowledgments
The authors are thankful to the Honorable President, Misurata University Misurata, Libya, for providing necessary facilities in University premises for this research. Dr. Huda Elgubbi, Department of Botany, College of Science, Misurata University, Misurata, Libya is kindly acknowledged for assisting in authentication of plant material.
Compliance with ethical standards
Ethical statement
The experimental protocols were approved by Institutional Ethical Committee of Faculty of Pharmacy, Misurata University, Misurata, Libya and their guidelines were followed for the studies (Phar-03/2015)
Author contributions
Md. Sarfaraj Hussain designed and supervised the entire work and wrote the chemistry part of the manuscript, Faizul Azam performed and wrote the results of docking study, Hanan Ahmed Eldarrat isolated and identified Lanostanoic acid 3-O-α-D-glycopyranoside, Ismail Alkskas, Jamal Abdurahman Mayoof and Hend Ismail designed and supervised the chemistry work, Mohammed Ali interpreted the spectroscopic data and revised the manuscript, Jamal Mohammed Damoona provided the carrageenan and helped in the anti-inflammatory, analgesic study, Muhammad Arif & Anzarul Haque designed and revised the submitted manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Structure-based design, synthesis, molecular docking, and biological activities of 2-(3-benzoylphenyl) propanoic acid derivatives as dual mechanism drugs. J. Pharm. Bioall. Sci.. 2012;4(1):43.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-nociceptive activities of Lippianodiflora Linn. Pharmazie. 2004;59:329-330.
- [Google Scholar]
- Antiplatelet activity and isolation of triterpenoidal glycoside from ethanolic extract of Nepeta hindostana. Ori. Pharm. Exp. Med.. 2016;16(4):333-337.
- [Google Scholar]
- Adaptogenic activity of lanostane triterpenoid isolated from Carissa carandas fruit against physically and chemically challenged experimental mice. Pharmacog. J.. 2013;5(5):216-220.
- [Google Scholar]
- Stress relaxant and antioxidant activities of acid glycoside from Spondias mangifera fruit against physically and chemically challenged albino mice. J. Pharm. Bioall. Sci.. 2016;8(1):58.
- [CrossRef] [Google Scholar]
- Targeting galectin-3 by natural glycosides: a computational approach. Netw Model Anal. Health Inform. Bioinforma.. 2020;9(1)
- [CrossRef] [Google Scholar]
- Rutin as promising drug for the treatment of Parkinson’s disease: an assessment of MAO-B inhibitory potential by docking, molecular dynamics and DFT studies. Mol. Simul.. 2019;45(18):1563-1571.
- [Google Scholar]
- Molecular interaction studies of green tea catechins as multitarget drug candidates for the treatment of Parkinson’s disease: computational and structural insights. Network: Comput. Neural Syst.. 2015;26(3-4):97-115.
- [Google Scholar]
- ‘Structure-based design, synthesis and molecular modeling studies of thiazolyl urea derivatives as novel anti-parkinsonian agents. Med. Chem.. 2012;8(6):1057-1068.
- [Google Scholar]
- In vitro antioxidant activities of the methanol extracts of four Helichrysum species from Turkey. Food Chem.. 2005;90(4):685-689.
- [Google Scholar]
- A New Flavone and Phloroglucinol derivative Helichrysum herbaceum and Helichrysum chrysargyrum. Phytochem.. 1979;18:1375-1378.
- [Google Scholar]
- Development and validation of a quantitative structure-activity relationship for chronic narcosis to fish: QSAR for chronic narcosis to fish. Environ. Toxicol. Chem.. 2013;32(10):2217-2225.
- [Google Scholar]
- Analgesic–antiinflammatory properties of Proustia pyrifolia. J. Ethnopharmacol.. 2005;99(1):119-124.
- [Google Scholar]
- Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Biol.. 1999;6(3):233-242.
- [Google Scholar]
- Antimicrobial phenolic compounds from three Spanish Helichrysum species. Phytochem.. 1990;29(4):1093-1095.
- [Google Scholar]
- A volatolomic approach for studying plant variability: the case of selected Helichrysum species (Asteraceae) Phytochemistry. 2016;130:128-143.
- [Google Scholar]
- Antioxidant activity profiling by spectrophotometric methods of aqueous methanolic extracts of Helichrysum stoechas subsp. rupestre and Phagnalon saxatile subsp. saxatile. Chin. J. Natl. Med.. 2014;12(6):415-422.
- [Google Scholar]
- Anti-endotoxin effects of terpenoids fraction from Hygrophila auriculata in lipopolysaccharide-induced septic shock in rats. Pharm. Biol.. 2016;54(4):628-636.
- [Google Scholar]
- Hygrophila auriculata (K. Schum) Heine: Ethnobotany, phytochemistry and pharmacology. Asian J. Tradit. Med.. 2010;5(4):122-131.
- [Google Scholar]
- Preliminary phytochemical and pharmacognostical screening of the ayurvedic drug Hygrophila auriculata (K. Schum) heine. Pharmacog. J.. 2011;3(23):28-40.
- [Google Scholar]
- Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci.. 2012;4(1):10-20.
- [Google Scholar]
- New aliphatic ester constituents from Hygrophila auriculata (K.Schum) Heine from the basin area of Koshi River. Ori. Pharm. Exp. Med.. 2019;19(3):251-258.
- [Google Scholar]
- Iqbal, S.S., Mujahid, M., Kashif, S.M., Khalid, M., Badruddeen, Arif, M., Bagga, P., Akhtar, J., Rahman M.A., 2016. Protection of hepatotoxicity using Spondias pinnata by prevention of ethanol induced oxidative stress, DNA-damage and altered biochemical markers in Wistar rat. Integ. Med. Res. 5: 267–275.
- Diterpenes and other constituents from Australian Helichrysum and related species. Phytochemistry. 1989;28(2):543-551.
- [Google Scholar]
- Degradation of fluopyram in water under ozone enhanced microbubbles: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Chemosphere. 2020;258:127216.
- [CrossRef] [Google Scholar]
- South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol.. 2008;119(3):630-652.
- [Google Scholar]
- Contribution to a taxonomic revision of the Sicilian Helichrysum taxa by PCA analysis of their essential-oil compositions. Chem. Biodivers.. 2016;13(2):151-159.
- [Google Scholar]
- Toxicity estimation software tool (TEST). Washington DC: US Environmental Protection Agency; 2016.
- Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem.. 1998;19(14):1639-1662.
- [Google Scholar]
- Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med.. 1997;63(04):367-369.
- [Google Scholar]
- Successful treatment of sodium oxalate induced urolithiasis with Helichrysum flowers. J. Ethnopharmacol.. 2016;186:322-328.
- [Google Scholar]
- Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotech.. 2011;6(3):175-178.
- [Google Scholar]
- A review on principles, theory and practices of 2D-QSAR. Curr. Drug Metabol.. 2014;15(4):346-379.
- [Google Scholar]
- Anti-inflammatory, analgesic and antioxidant potential of the stem bark of Spondias mangifera Willd. Arch. Boil. Sci. (Beogr.). 2011;63(2):413-419.
- [Google Scholar]
- Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): potential role of inflammation activated myeloperoxidase. Mol. Cell. Biochem.. 2009;328(1-2):183-188.
- [Google Scholar]
- Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. & Pharmacol.. 2002;54(3):365-371.
- [Google Scholar]
- Shushni, S.M.A., Azam F., Lindequist U., 2013. Oxasetin from Lophiostoma sp. of the Baltic Sea: identification, in silico binding mode prediction and antibacterial evaluation against fish pathogenic bacteria. Nat. Prod. Commun. 8(9), 1223–1226.
- Sripriya., N., M., R.K., N., A.K., S., B., N. K., U.P., 2019. In silico evaluation of multispecies toxicity of natural compounds. Drug Chem. Toxicol. 1–7.
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2010;31(2):455-461.
- [Google Scholar]
- X-ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine-tryptophan switch. J. Biol. Chem.. 2003;278(29):27138-27143.
- [Google Scholar]







