Translate this page into:
Anti-TMV activity of flavonol derivatives containing piperidine sulfonamide: Design, synthesis, mechanism study
⁎Corresponding author. wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this paper, 23 flavonols derivatives containing piperidine sulfonamide were designed and synthesized. The results of anti-TMV activity assay showed that Z6 possessed excellent curative and protective activities, EC50 values of 108.2 and 100.9 μg/mL, respectively, which were superior to those of NNM (253.8 and 203.7 μg/mL). The experimental findings indicated that Z6 was effectively combined with TMV-CP under the biosafety condition, and could to enhance the chlorophyll content and SOD activity of tobacco, alleviate the cell membrane damage, Strengthening the resistance of plants to diseases.
Abstract
A series of flavonol derivatives containing piperidine sulfonamide were designed and synthesized. The EC50 values for the curative of tobacco mosaic virus (TMV) by Z6 and Z22 were 108.2 and 102.7 μg/mL, respectively, outperforming the control agent ningenmycin (NNM) with an EC50 value of 253.8 μg/mL. Additionally, the EC50 values for the protective activity of Z6 and Z21 were 100.9 and 123.2 μg/mL, respectively, also surpassing the control agent NNM with an EC50 value of 203.7 μg/mL. Microscale thermophoresis (MST) and molecular docking test showed that Z6 bound well with tobacco mosaic virus coat protein (TMV-CP). The biological safety test showed that Z6 had almost no effect on seed germination and seedling growth. After treatment with Z6, the chlorophyll content and superoxide dismutase (SOD) activity in tobacco leaves increased, while the malondialdehyde (MDA) content decreased. Therefore, Z6 has a high probability as a potential antiviral agent.
Keywords
Flavonol
Piperidine sulfonamide
Antiviral activity
Molecular docking
Biosecurity
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR 19F
-
nuclear magnetic resonance
- HRMS
-
High-resolution mass spectrometry
- TMV
-
Tobacco mosaic virus
- EC50
-
Median effective concentration
- DMSO
-
Dimethylsulfoxide
- NNM
-
Ningnanmycin
- MST
-
Microscale thermphoresis
- TMV-CP
-
Tobacco mosaic virus coat protein
- MDA
-
Malondialdehyde
- SOD
-
Superoxide dismutase
Abbreviations
1 Introduction
Plants contribute to over 80 % of the Earth's biomass, thus playing a crucial role in the functioning and stability of ecosystems. However, plant viruses significantly diminish the yield and quality of important agricultural crops, leading to substantial damage to key cash crops (Keim and Alavanja, 2001; Zhang et al., 2024). As the earliest discovered non-cellular infectious agent, TMV has paved the way for human to study the field of plant virology (Jones, 2021; Kosamu et al., 2020; Li et al., 2022). But TMV is a single-stranded RNA virus with a unique one-dimensional structure and nanoscale monodispersity, which can infect 9 plant families and hundreds of plant species, including tobacco, orchids and tomatoes. Especially for important sources of cash crops and the provinces of tobacco production, TMV has a huge impact (Ilyas et al., 2022; Liu et al., 2015; Pan et al., 2023), Upon infection, a plant must not rely solely on its endogenous immune system for defense. At present, chemical pesticide preparations are used as the main means to control plant viruses. The common anti-plant virus control agents mainly include NNM and ribavirin, but their long-term excessive use will also cause resistance to plant viruses (Zhou et al., 2024; Cao et al., 2023a; Wang et al., 2024). Therefore, it is very important to develop a green and efficient compound for the prevention and cure of TMV.
Flavonols, widely distributed in nature, they are a class of plant secondary metabolites, that exist in red wine, coffee, tea, raspberry, ginkgo biloba and different medicinal materials (Aherne and O'Brien, 2002; Nimptsch et al., 2016; Kakigi et al., 2012), and play an important ecological role. Existing studies have shown that flavonols have a wide range of biological activities such as antibacterial (Meng et al., 2023; Gong et al., 2024a), insecticidal (Borsari et al., 2016; Shamshad et al., 2020; Landi et al., 2020), antiviral (Chen et al., 2021) and anti-tumor (Shi et al., 2014; Li et al., 2016; Du et al., 2016) effects, which have paved a new bridge for the development of green pesticides and the combination of natural products. In addition, piperidine ring fragments also have good physiological and pharmacological activities (Ding et al., 2020; Wang et al., 2023a; Zhang et al., 2019), so they play an important role in the process of creating medicine and pesticides. Sulfonamide compounds, when used as intermediates, have antibacterial (Chen et al., 2023; Meseli et al., 2021; Wu et al., 2022; Mao et al., 2024), antiviral (Wei et al., 2023; Zhou et al., 2018) and insecticidal (Jing et al., 2024) biological activities.
A series of flavonol derivatives containing piperidine sulfonamide were synthesized by using flavonol as the lead compound and structurally modified by active splicing method (Fig. 1).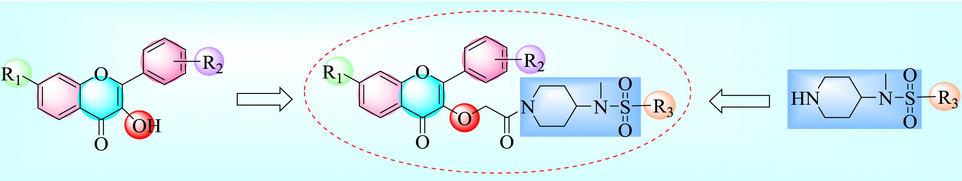
Design of target compounds.
2 Materials and methods
2.1 Chemicals and instruments
Thin layer chromatography was performed with WFH-203B UV analyzer (Shanghai Jingke, China). The reagents and solvents used are analytical grade and are used directly without the need for further purification and drying. The nuclear magnetic resonance (NMR) spectrum was acquired by a Bruker 400 NMR instrument (Bruker Co., Germany). High resolution mass spectrometry (HRMS) was obtained using the Thermo Scientific Q Exactive instrument (Thermo Scientific, USA). The solvent is mass spectrum methanol. The melting point was determined by XT-4 binocular microscope without correction (Beijing Science and Technology Instrument Co, LTD, China). Malondialdehyde (MDA) test kit and Active oxygen species (SOD) test kit was purchased (Beijing Solarbio Techniligy Co, LTD, China).
2.2 Synthesis of compounds
2.2.1 Synthesis of intermediate 1–2
The reaction of p-hydroxyacetophenone (7.34 mmol) and substituted benzaldehyde (7.34 mmol) in the presence of NaOH (22.12 mmol) was carried out at room temperature in a 500 mL round-bottom flask with anhydrous ethanol as the solvent. After the reaction, the product was poured into ice water, the pH was adjusted to neutral by adding dilute hydrochloric acid, and the solid was precipitated, filtrate and dry to obtain intermediate 1. Intermediate 1 (4.20 mmol) was added with NaOH (16.79 mmol) and H2O2 (25.18 mmol), anhydrous methanol as solvent, the reaction at room temperature in 250 mL round bottom flask, after the reaction, the product into ice-cold water, also add HCl adjust pH to neutral, solid precipitation, filtration, drying, get intermediate 2.
2.2.2 Synthesis of intermediate 3–5
Triethylamine (5.77 mmol) was added to substituted sulfonyl chloride (5.25 mmol) and 1-tert-butyroxycarbonyl 4-methylaminopiperidine (5.77 mmol), and the reaction was performed in a 100 mL round-bottom flask containing methylene chloride solvent for 3 h in an ice bath. After the reaction, the reaction was extracted in methylene chloride solvent, get intermediate 3. Intermediate 3 (2.71 mmol) was added with HCl (32.57 mmol) in methanol solution at 80℃ for reflux for 8 h, Boc was removed, and after the reaction, it was directly steamed under pressure, get intermediate 4. Intermediate 4 (5.22 mmol) reacted with chloroacetyl chloride (10.43 mmol) in an ice bath of dichloromethane solvent. After the reaction, intermediate 5 was extracted by dichloromethane solvent.
2.2.3 Synthesis of Z1-Z23
Intermediates 2 (2.42 mmol) and K2CO3 (4.85 mmol) were first stirred in DMF solvent at room temperature for 0.5 h, and then intermediates 5 (2.42 mmol) were added, and then reflow for 2–3 h at 100℃, after the reaction, the solid was cooled to indoor temperature, and the solid was separated and purified by column chromatography (petroleum ether: ethyl acetate = 3:1, v/v) to obtain Z1-Z23.
3 Antiviral bioassays
3.1 Anti-TMV activity test of Z1-Z23
The activity of Z1-Z23 against TMV was determined by the half-leaf blip method at a concentration of 500 μg/mL. The commercial drug NNM was used as a positive control to test the activity of anti-TMV in vivo, including curative activity, protective activity and inactivation activity, The tobacco seedlings with a growth cycle of 4 weeks were used in the experiment, with left and right symmetry and uniform size of Nicotiana glutinosa (N. glutinosa). The preliminary screening results are shown in Table 1. On this basis, the EC50 values of the compounds against TMV are further tested, as shown in Table 2. The specific experimental methods have been provided in the auxiliary materials.
Compounds
R1
R2
R3
Curative (%) a
Protection (%) a
Inactivation (%) a
Z1
–OCH3
2-OCH3
Ph
61.5 ± 3.8
66.3 ± 1.7
64.5 ± 4.8
Z2
–OCH3
2-OCH3
4-F-Ph
63.2 ± 1.5
62.5 ± 2.9
58.7 ± 3.7
Z3
–OCH3
4-Br
Ph
55.7 ± 5.4
67.4 ± 5.4
56.0 ± 3.5
Z4
–OCH3
4-Cl
Ph
62.1 ± 3.8
80.2 ± 0.7
55.5 ± 4.0
Z5
–OCH3
4-OCH3
4-Cl-Ph
68.1 ± 3.0
78.5 ± 1.7
63.7 ± 0.8
Z6
–OCH3
4-OCH3
4-NO2-Ph
77.0 ± 2.9
79.4 ± 3.5
82.3 ± 2.2
Z7
–OCH3
4-Br
4-NO2-Ph
64.0 ± 2.6
65.0 ± 2.6
66.4 ± 3.4
Z8
–OCH3
4-F
4-Cl-Ph
52.2 ± 1.4
72.5 ± 4.4
65.2 ± 5.7
Z9
–OCH3
4-OCH3
ethyl
59.6 ± 5.5
57.0 ± 3.2
51.9 ± 2.5
Z10
–OCH3
4-Cl
ethyl
66.4 ± 4.1
63.0 ± 3.4
54.9 ± 4.7
Z11
H
2-OCH3
4-Cl-Ph
66.6 ± 1.8
52.3 ± 4.6
67.8 ± 2.0
Z12
H
3-CH3
4-Cl-Ph
71.7 ± 4.0
57.1 ± 2.0
58.2 ± 4.7
Z13
H
3-CH3
ethyl
64.5 ± 3.9
71.9 ± 2.1
45.5 ± 2.1
Z14
H
2-OCH3
4-F-Ph
70.2 ± 3.1
55.7 ± 5.1
51.0 ± 3.5
Z15
H
4-CH3
4-F-Ph
68.9 ± 2.3
65.0 ± 5.2
21.9 ± 2.4
Z16
H
2-Br
4-CH3-Ph
54.3 ± 3.4
50.9 ± 4.9
53.9 ± 2.3
Z17
H
2-Br
ethyl
65.8 ± 2.0
55.7 ± 4.2
55.8 ± 0.3
Z18
H
4-OCH3
4-CH3-Ph
57.3 ± 4.7
72.0 ± 4.1
66.8 ± 5.2
Z19
H
4-CH3
4-CF3-Ph
68.0 ± 1.7
35.2 ± 1.5
67.7 ± 0.9
Z20
H
4-Cl
4-CF3-Ph
56.7 ± 3.2
63.6 ± 5.7
77.3 ± 4.9
Z21
H
4-CH3
Ph
78.5 ± 3.3
73.1 ± 1.5
55.9 ± 1.2
Z22
H
4-OCH3
Ph
75.0 ± 2.8
51.7 ± 0.8
80.0 ± 2.2
Z23
H
3-F
4-NO2-Ph
62.1 ± 1.6
56.5 ± 3.9
52.6 ± 1.9
Flavonol
–OCH3
4-OCH3
−
25.3 ± 4.0
38.4 ± 5.2
−
NNMb
−
−
−
62.5 ± 2.4
67.1 ± 2.8
91.8 ± 2.6
Activity
Compounds
Regression equation
r
EC50 (μg/mL)
Curative activity
Z6
y = 0.9990x + 2.9680
0.9846
108.2
Z21
y = 0.9614x + 3.0335
0.9816
111.0
Z22
y = 1.0372x + 2.9137
0.9846
102.7
NNMb
y = 1.0685x + 2.4308
0.9937
253.8
Protective activity
Z4
y = 1.0471x + 2.8146
0.9846
122.2
Z5
y = 1.0103x + 2.8414
0.9880
136.9
Z6
y = 1.1013x + 2.7932
0.9885
100.9
Z21
y = 0.9842x + 2.9425
0.9871
123.2
NNMb
y = 0.9920x + 2.7545
0.9862
203.7
3.2 Microscale thermophoresis (MST) test
According to the MST test method (Wang et al., 2023b; Cao et al., 2023b) in the literature, the correlation between Z6, Z15 and Z21 with TMV-CP was tested respectively. The specific test method has been given in the supporting material.
3.3 Molecular docking experiment
In order to further study the binding mode of the target compound with TMV-CP (PDB code:1EI7) at the molecular level, Z6, Z15 and Z21 were selected to perform molecular docking with TMV-CP in Discovery Studio software according to the method reported in literature (Xing et al., 2023). In supporting material describes the specific experimental methods.
3.4 Biological safety of Z6
In order to study the effect of Z6 on tobacco seeds and seedlings, with the same growth cycle, different concentrations of Z6 were selected for spraying (Lu et al., 2024).
3.5 Chlorophyll content test
The presence of chlorophyll lays the foundation for plant photosynthesis (Wei et al., 2019). Literature reports have indicated that the TMV infection can impact changes in plant chlorophyll content (Liu et al., 2022; Luo et al., 2023). Therefore, the variations in chlorophyll a, chlorophyll b, and total chlorophyll content of tobacco leaves after treatment with Z6 were further investigated to elucidate its mechanism of action. Specific experimental methods are detailed in the supporting materials.
3.6 Malondialdehyde (MDA) content test
MDA is a biomarker produced by lipid peroxidation and intracellular oxidative stress. The content in MDA can indicate the degree of lipid peroxidation in cell membranes and the extent of membrane damage (Heath and Packer, 2022; Gong et al., 2024b). Therefore, the alteration in MDA content in tobacco leaves treated with Z6 was chosen for further investigation into its mechanism of action. Specific experimental methods are detailed in the supporting materials.
3.7 Defense enzyme activity assay
SOD is an antioxidant enzyme that converts superoxide radicals in plant cells into hydrogen peroxide (H2O2) and oxygen molecules (O2), thus protecting cells from damage (Hunter et al., 1997). Accordingly, the change in SOD activity in tobacco leaves treated with Z6 was recorded to further study its mechanism of action. Specific experimental methods are described in the supporting materials.
4 Results and discussion
4.1 Chemistry
The synthesis pathway of the target compound has been given in Scheme 1, and the specific spectral characterization data of Z1-Z23 are given in the supporting information.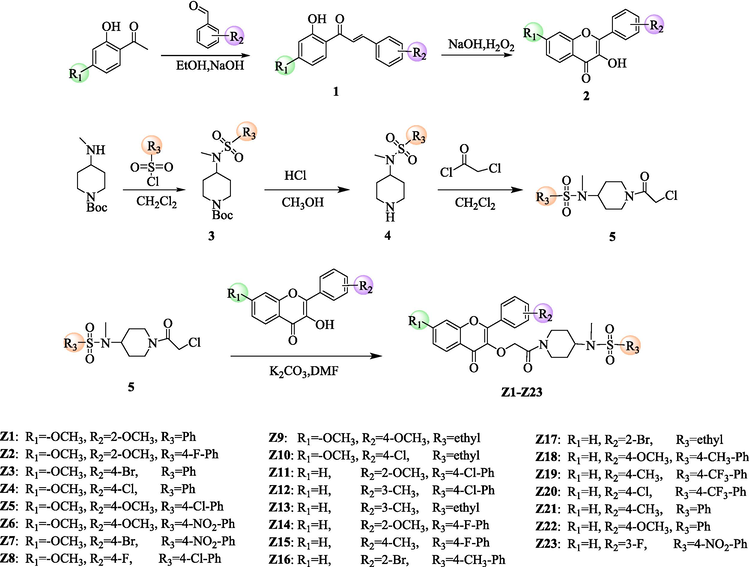
Synthetic route of the target compounds Z1-Z23.
4.2 Virus activity analysis
The resistance test of Z1-Z23 to TMV at 500 μg/mL concentration was determined, and the commercial drug NNM was used as a positive control. The preliminary screening data were shown in Table 1. The curative activities of Z5, Z6, Z12, Z14, Z15, Z19, Z21 and Z22 on TMV were 68.1, 77.0, 71.7, 70.2, 68.9, 68.0, 78.5 and 75.0 %, respectively, which were superior to the control drug NNM (62.5 %). The protective activities of Z4, Z5, Z6, Z8, Z13, Z18 and Z21 against TMV were 80.2, 78.5, 79.4, 72.5, 71.9, 72.0 and 73.1 %, which were superior to the control drug NNM (67.1 %).
Based on the analysis of the activity data in Table 1, it was found that the different structure of substituents structures also had a certain influence on the anti-TMV activity of the compound. Specifically, when substituents R1 = –OCH3, R2 = 4-OCH3, the curative and protective activity of the target compound is higher than that of the electron-donating group when R3 is a strong electron-withdrawing group. For example, Curative and protective activity of Z6 (R3 = 4-NO2-Ph) > Z5 (R3 = 4-Cl-Ph) > Z9 (R3 = ethyl). In summary, Z6 (R1 = –OCH3, R2 = 4-OCH3, R3 = 4-NO2-Ph) exhibits good inhibitory activity in both curative and protection. The leaf characterization of Z6 and NNM against TMV is shown in Fig. 2.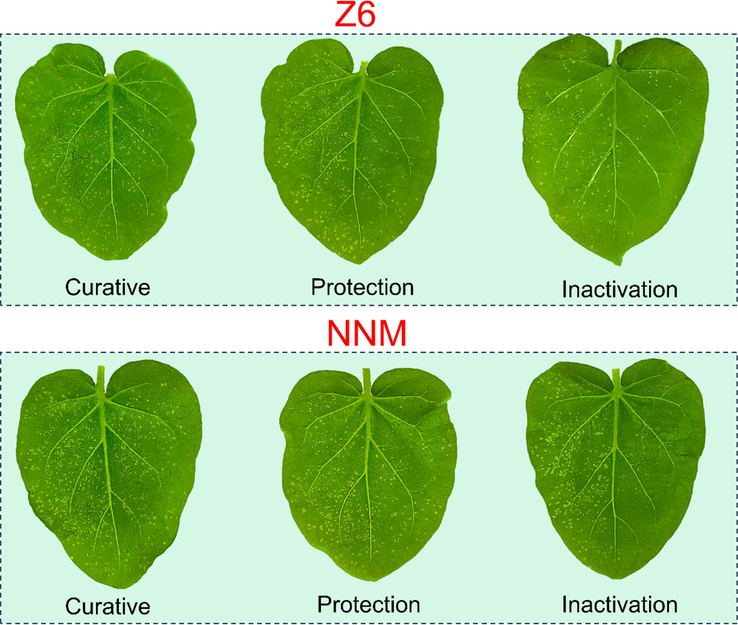
Phenotypic study of TMV infection in tobacco treated with Z6 and NNM (left leaf: untreated with compound; right leaf: treated with compound).
4.3 Binding ability of Z6 and NNM to TMV‑CP
The Fig. 3 shows the MST results of Z6, Z15, Z21 and tobacco Mosaic virus coat protein (TMV-CP). The Kd value can be used to evaluate the binding affinity with TMV-CP. The Kd values of the three compounds binding to TMV-CP were 0.4651 ± 0.1227, 27.3606 ± 7.4426 and 0.9720 ± 0.4015 μmol/L, respectively. The results showed that the binding ability of Z6 to TMV-CP was better than that of Z15 and Z21, further suggesting its good antiviral activity.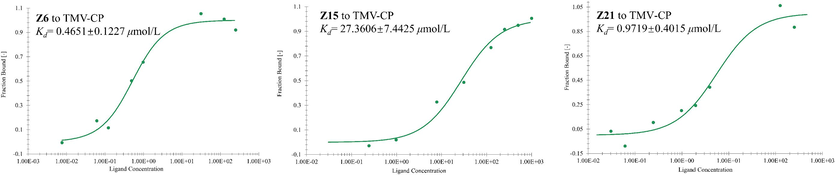
Microscale thermophoresis (MST) of Z6, Z15 and Z21.
4.4 Molecular docking of Z6 with TMV‑CP
As shown in the Fig. 4, The results of molecular docking demonstrated the ability of Z6, Z15, and Z21 to establish hydrogen bonds with TMV-CP. However, there is variation in the amino acid residues involved in these interactions, with 6, 2, and 3 amino acids respectively. The hydrogen bond lengths for the conserved amino acid residue ARG-134 are measured at 1.85 Å, 2.07 Å, and 3.79 Å for Z6, Z15, and Z21 respectively. Henceforth, the differences in anti-TMV activity among Z6, Z15 and Z21 may be attributed to their distinct binding affinities with TMV-CP. Z6 binds to TMV-CP amino acid residues through six conventional hydrogen bonds, The oxygen atoms on the phenyl nitro group of substituent R3 form hydrogen bonds with the TMV-CP amino acid residues ARG-261 (2.53 Å) and SER-146 (2.91 Å). The oxygen atom on the sulfonyl group forms hydrogen bonds with the TMV-CP amino acid residues ARG-341 (1.84 Å) and ASP-146 (2.32 Å). The oxygen atom on the methoxy group of the substituent R2 forms a hydrogen bond with the TMV-CP amino acid residue GLN-257 (2.79 Å). The oxygen atom on the flavonol carbon–oxygen double bond forms a hydrogen bond with the TMV-CP amino acid residue ARG-134(1.86 Å). In addition, Z6 interacts with nine other TMV-CP amino acid residues: (VAL-260, PRO-263, THR-136, LYS-253, VAL-75, LYS-26, GLU-131, GLU-222, and VAL-251). The experimental results further prove that Z6 has a good binding affinity with TMV-CP.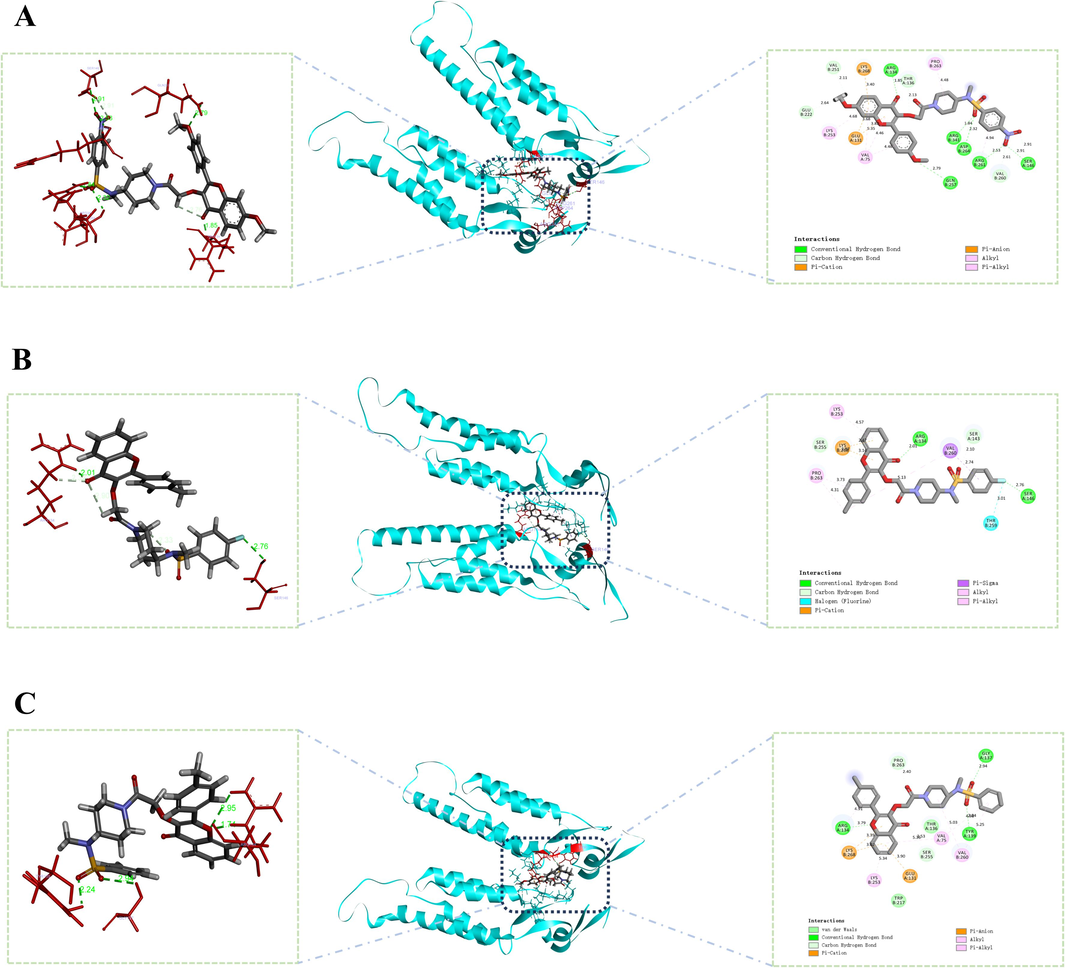
The binding mode of Z6(A), Z15(B) and Z21(C) docked with TMV-CP.
4.5 Biological safety of Z6
As shown in the Fig. 5, Z6 and DMSO at concentrations of 250, 500 and 1000 μg/mL were sprayed on the seeds of N. glutinosa. It was found that the seed germination was almost unaffected. Similarly, sprays of Z6 and DMSO at concentrations of 250, 500, and 1000 μg/mL had almost no effect on the growth of N. glutinosa with the same growth cycle. The results indicated that Z6 had no significant effect on the germination or growth of the seeds, which proved that Z6 had a non-significant effect on plant growth and good biosafety.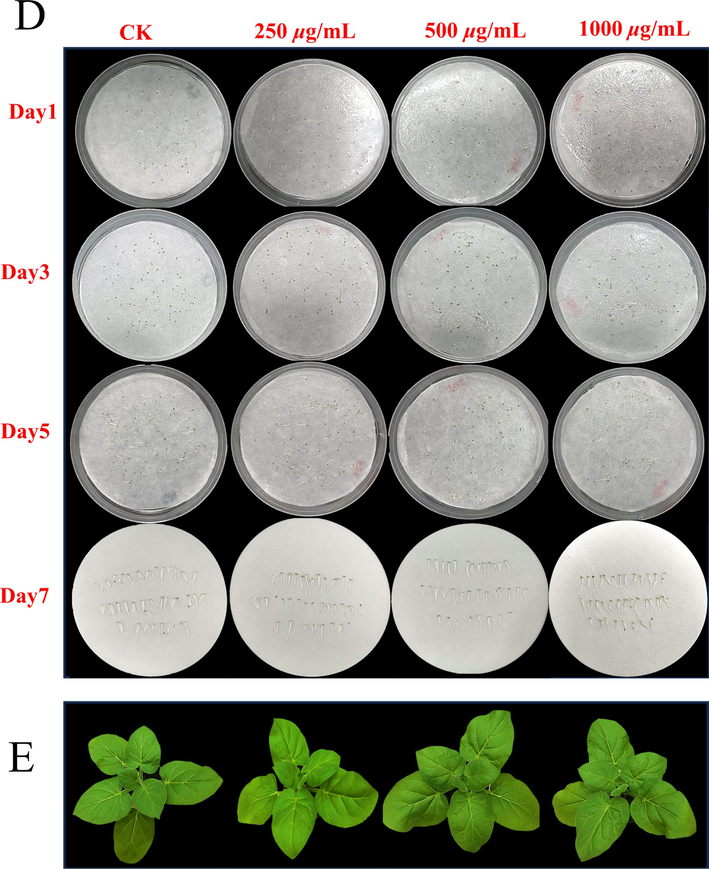
Biological safety of Z6 (D) Photos of tobacco seeds soaked in Z6 solution of different concentrations for 1, 3, 5, and 7 d. (E) Photos taken after 7 d of growth of seedlings sprayed with different concentrations of Z6 solution.
4.6 Chlorophyll content of Z6
The change of chlorophyll content directly affects the progress of plant photosynthesis and is closely related to plant growth. It can be seen from the Fig. 6 that Z6 can change the content of chlorophyll in tobacco leaves to a certain extent. The chlorophyll content of tobacco leaves treated with Z6 was significantly higher than that of the blank control group, which proved that Z6 could promote the photosynthesis of plants to a certain extent and improve the ability of plants to anti-TMV.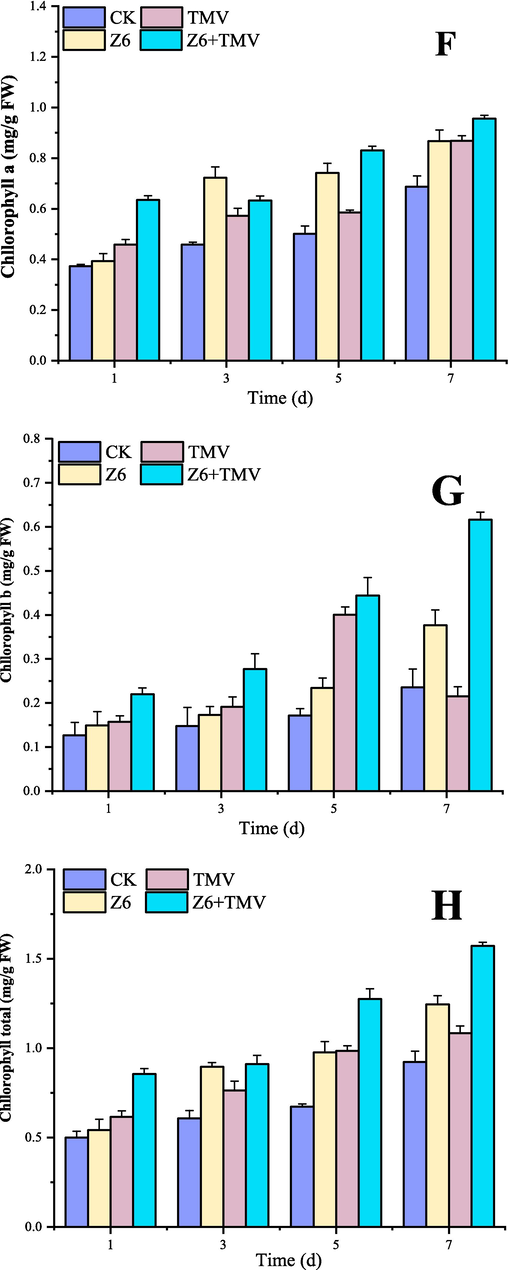
Changes of chlorophyll content in tobacco after Z6 treatment.
4.7 MDA content analysis of Z6
As shown in the Fig. 7, MDA content in treated tobacco leaves was measured after 1, 3, 5, and 7 d. MDA content was higher than that in healthy tobacco leaves after 3–7 d of TMV infection, indicating that TMV seriously damaged the integrity of cell membrane. However, the MDA content of tobacco leaves treated with Z6 was significantly lower than that of CK group, especially on the 7th day. With the aging of leaves, the phenomenon was more obvious, indicating that Z6 could inhibit membrane lipid peroxidation and membrane damage to a certain extent, thus resisting the infection of TMV.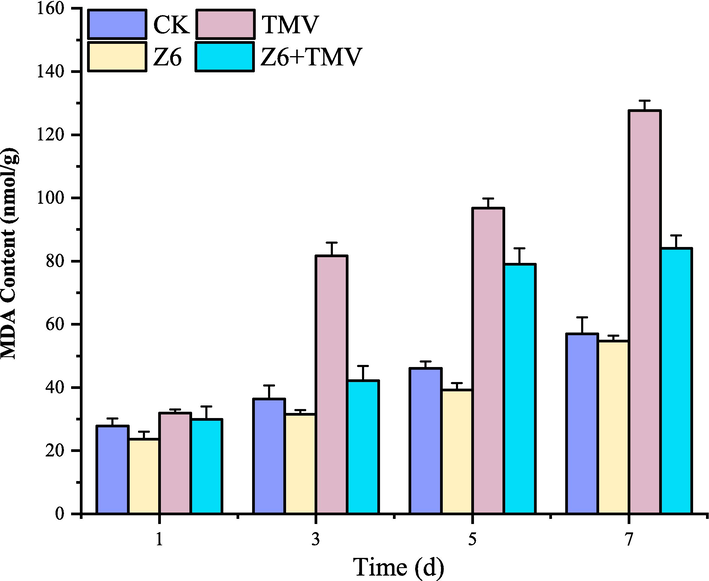
Changes of MDA content in tobacco after Z6 treatment.
4.8 Defense enzyme activity of Z6
As shown in the Fig. 8, SOD enzyme activity in the blank group and drug group infected by TMV was higher than that in the blank group and drug group not infected by TMV, because the cell defense mechanism would be activated after TMV infection, thus increasing SOD activity. In general, SOD activity in drug group was higher than that in blank control group. It can be concluded that Z6 can improve SOD activity, promote SOD generation and eliminate O2– in cells to a certain extent. Thereby improving the disease resistance of the plant.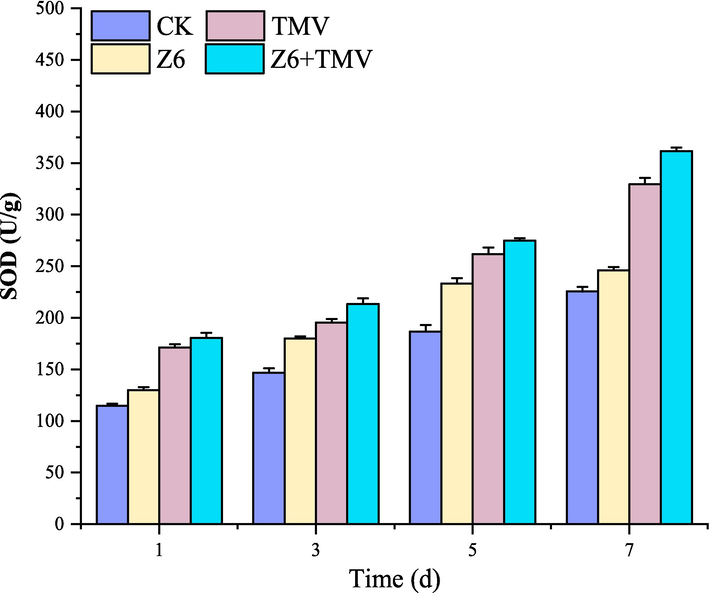
Changes of SOD activity in tobacco after Z6 treatment.
5 Conclusions
In summary, 23 flavonol derivatives containing piperidine sulfonamide were designed and synthesized in this paper, and their antiviral activities were evaluated, and Z6 was found to have good therapeutic and protective activities. EC50 values of 102.7 and 100.9 μg/mL were better than those of commercial agents NNM (253.8 and 203.7 μg/mL). The results of MST and molecular docking showed that Z6 had a good binding force with TMV-CP, with a Kd value of 0.4651 μmol/L, and it was bound to TMV-CP amino acid residues by six conventional hydrogen bonds. At the same time, Z6 has little effect on the germination and growth of tobacco seeds, and has good biosafety. To a certain extent, it can also increase chlorophyll content and promote plant photosynthesis. Leaves treated with Z6 can slow the increase of MDA content in plants, improve SOD enzyme activity to a certain extent, inhibit the damage to tobacco leaves, promote the generation of SOD and the clearance of O2– in cells. This improves the disease resistance of the plant. Therefore, Z6 is expected to be a potential antiviral agent in the future.
CRediT authorship contribution statement
Zhiling Sun: Writing – original draft, Visualization, Data curation, Conceptualization. Wei Zeng: Visualization, Formal analysis, Conceptualization. Qing Zhou: Software, Formal analysis, Conceptualization. Yujiao Qiu: Formal analysis, Conceptualization. Yuzhi Hu: Investigation, Formal analysis. Jieyu Li: Methodology, Formal analysis. Hong Fu: Investigation, Formal analysis. Hongqian Zou: Methodology, Formal analysis. Wei Xue: Conceptualization, Formal analysis, Visualization.
Acknowledgements
Authors gratefully acknowledge the Science Foundation of Guizhou Province (No. ZK2024008), The Key Research and Development Program of Hainan Province (No. ZDYF2024XDNY202).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18(1):75-81.
- [CrossRef] [Google Scholar]
- Profiling of flavonol derivatives for the development of antitrypanosomatidic drugs. J. Med. Chem.. 2016;59(16):7598-7616.
- [CrossRef] [Google Scholar]
- Design, synthesis and bioactivity of myricetin derivatives for control of fungal disease and tobacco mosaic virus disease. RSC Adv.. 2023;13(10):6459-6465.
- [CrossRef] [Google Scholar]
- Design, synthesis, bioactivity and mechanism of action of novel myricetin derivatives containing amide and hydrazide. Arab. J. Chem.. 2023;16(4):104588
- [CrossRef] [Google Scholar]
- Design, synthesis and antibacterial activity of 1,3,4-Oxadiazole sufones containing sulfonamide structure. Chin. J. Org. Chem.. 2023;43(1):274-284.
- [CrossRef] [Google Scholar]
- Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety. J. Saudi Chem. Soc.. 2021;25(2):101194-101208.
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of aryl thiazole piperidine amide compounds. Chin. J. Org. Chem.. 2020;40(2):528-535.
- [CrossRef] [Google Scholar]
- Antitumor activity of total flavonoids from daphne genkwa in colorectal cancer. Phytother. Res.. 2016;30(2):323-330.
- [CrossRef] [Google Scholar]
- Flavonol derivatives containing a quinazolinone moiety: design, synthesis, and antiviral activity. Chem. Biodiversity.. 2024;21(2)
- [CrossRef] [Google Scholar]
- Novel flavonoid derivatives containing 1,2,4-triazolo [4,3-a] pyridine as potential antifungal agents: design, synthesis, and biological evaluation. J. Saudi Chem. Soc.. 2024;28(2):101797-101812.
- [CrossRef] [Google Scholar]
- Photoperoxidation in isolated chloroplasts i. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 2022;726(125):109248-109255.
- [CrossRef] [Google Scholar]
- Cloning, expression, and characterization of two manganese superoxide dismutases from caenorhabditis elegans. J. Biol. Chem.. 1997;272(2):28652-28659.
- [CrossRef] [Google Scholar]
- To be seen or not to be seen: latent infection by tobamoviruses. Plants.. 2022;11(16):2166-2179.
- [CrossRef] [Google Scholar]
- UGT89AC1-mediated quercetin glucosylation is induced upon herbivore damage and enhances Camellia sinensis resistance to insect feeding. Plant Cell Environ.. 2024;47(2):682-697.
- [CrossRef] [Google Scholar]
- Global plant virus disease pandemics and epidemics. Plants.. 2021;10(2):233-275.
- [CrossRef] [Google Scholar]
- Comprehensive analysis of flavonols in ginkgo biloba products by Ultra-High-Performance liquid chromatography coupled with ultra-violet detection and time-of-flight mass spectrometry. Biosci. Biotechnol. Biochem.. 2012;76(5):1003-1007.
- [CrossRef] [Google Scholar]
- Pesticide use by persons who reported a high pesticide exposure event in the agricultural health study. Environ. Res.. 2001;85(3):256-259.
- [CrossRef] [Google Scholar]
- A critical review of the status of pesticide exposure management in malawi. Int. J. Environ. Res.. 2020;17(18):6727-6740.
- [CrossRef] [Google Scholar]
- High-resolution crystal structure of trypanosoma brucei pteridine reductase 1 in complex with an innovative tricyclic-based inhibitor. Acta Cryst. 2020;76(6):558-564.
- [CrossRef] [Google Scholar]
- A new class of flavonol-based anti-prostate cancer agents: design, synthesis, and evaluation in cell models. Bioorg. Med. Chem. Lett.. 2016;26(17):4241-4245.
- [CrossRef] [Google Scholar]
- Governmental regulation induced pesticide retailers to provide more accurate advice on pesticide use to farmers in China. Pest Manage. Sci.. 2022;78(1):184-192.
- [CrossRef] [Google Scholar]
- One-dimensional rod-like tobacco mosaic virus self-assembly and applications. Prog. Chem.. 2015;27(10):1425-1434.
- [CrossRef] [Google Scholar]
- Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone. Arabian J. Chem.. 2022;15(8):104019-104030.
- [CrossRef] [Google Scholar]
- Multidimensional response of dopamine nano-system for on-demand fungicides delivery: reduced toxicity and synergistic antibacterial effects. Chem. Eng. J.. 2024;482:148990-149007.
- [CrossRef] [Google Scholar]
- Limonene anti-TMV activity and its mode of action. Pestic. Biochem. Physiol.. 2023;194:105512-105523.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antifungal activities of chalcone derivatives containing piperidine and sulfonamide moiety. J. Saudi Chem. Soc.. 2024;28(1):101791-101801.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antifungal activity of flavonoid derivatives containing thiazole moiety. Chem. Pap.. 2023;77(2):877-885.
- [CrossRef] [Google Scholar]
- Design, synthesis, antibacterial activity evaluation and molecular modeling studies of new sulfonamides containing a sulfathiazole moiety. New J. Chem.. 2021;45(18):8166-8177.
- [CrossRef] [Google Scholar]
- Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am. J. Clin. Nutr.. 2016;103(1):184-191.
- [CrossRef] [Google Scholar]
- Tobacco disease detection model based on band selection. Spectrosc. Spectral Anal.. 2023;43(4):1023-1029.
- [CrossRef] [Google Scholar]
- Characterization of the trypanosonta brucei pteridine reductase active-site using computational docking and virtual screening techniques. Curr. Comput. Aided Drug Des.. 2020;16(5):583-598.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and SAR analysis of O-alkylated analogs of quercetin for anticancer. Bioorg. Med. Chem. Lett.. 2014;24(18):4424-4427.
- [CrossRef] [Google Scholar]
- Design, synthesis and antifungal activities of novel 1,2,4-oxadiazole derivatives containing piperidine. Chin. J. Org. Chem.. 2023;43(8):2826-2836.
- [CrossRef] [Google Scholar]
- Antiviral activity evaluation and action mechanism of myricetin derivatives containing thioether quinoline moiety. Mol Divers 2023
- [CrossRef] [Google Scholar]
- Plant antiviral compounds containing pyrazolo [3,4-d] pyrimidine based on the systemin receptor model. Arab. J. Chem.. 2024;17(8):105849
- [CrossRef] [Google Scholar]
- Synthesis, antiviral activity, and induction of plant resistance of indole analogues bearing dithioacetal moiety. J. Agric. Food Chem.. 2019;67(50):13882-13891.
- [CrossRef] [Google Scholar]
- 3-hydroxy-2-oxindole derivatives containing sulfonamide motif: synthesis, antiviral activity, and modes of action. J. Agric. Food Chem.. 2023;71(1):267-275.
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial activity, and action mechanism of novel sulfonamides containing oxyacetal and pyrimidine. J. Agric. Food Chem.. 2022;70(30):9305-9318.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antiviral activities of myricetin derivatives containing phenoxypyridine. J. Saudi Chem. Soc.. 2023;27(6):101751-101763.
- [CrossRef] [Google Scholar]
- Design, synthesis and fungicidal activity of novel piperidine containing cinnamaldehyde thiosemicarbazide derivatives. Chin. J. Org. Chem.. 2019;39(10):2965-2972.
- [CrossRef] [Google Scholar]
- Design, synthesis, antibacterial and antiviral evaluation of chalcone derivatives containing benzoxazole. Arab. J. Chem.. 2024;17(1):105368-105381.
- [CrossRef] [Google Scholar]
- Novel flavonol derivatives containing benzoxazole as potential antiviral agents: design, synthesis, and biological evaluation. Mol. Diversity. 2024
- [CrossRef] [Google Scholar]
- Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg. Med. Chem. Lett.. 2018;28(11):2091-2097.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105944.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







