Translate this page into:
Antimicrobial, anti-inflammatory and antioxidant activities of natural organic matter extracted from cretaceous shales in district Nowshera-Pakistan
⁎Corresponding author. fazlikhuda@uop.edu.pk (Fazli Khuda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Traditionally, Natural Organic Matter (NOM) derived from cretaceous rocks has been used for treatment of various ailments such as diabetes, inflammation and skin infections. This study evaluated the antimicrobial, antioxidant and anti-inflammatory activities of natural organic matter obtained from cretaceous shales. The shales were collected from Lumshiwal formation; located north of the main Kala Chitta range in district Nowshera-Pakistan. Isolation was done by sonicating crushed rock sample with chloroform, methanol and acetone (70: 15: 15 v/v, respectively). Antibacterial and antifungal activity of sample was determined by agar well diffusion and Agar slanting methods, respectively. In vitro anti-inflammatory activity was performed using cyclooxygenase-2 and 5-lipoxygenase enzymes. Antioxidant activity was assessed for scavenging of DPPH, superoxide anions, hydroxyl radicals, and hydrogen peroxide. In vivo anti-inflammatory activity was performed using “Carrageenan-induced paw edema model”. The sample showed significant antibacterial activity against Salmonella typhi, Pseudomonas aeruginosa and Escherichia coli with MIC values 0.82, 0.87 and 0.79 mg/ml, respectively. Considerable inhibition was observed against Bacillus subtilis (MIC; 0.93 mg/ml) and Staphylococcus aureus (MIC; 1.12 mg/ml) when compared with Imipenem as a standard. Moreover, the sample displayed significant antifungal activity against Alternaria alternata and Fusarium solani with MIC values of 0.60 and 0.68 mg/ml, respectively. Both COX-2 (IC50 31.34 µg/ml) and 5-LOX (IC50 38.45 µg/ml) enzymes were inhibited by NOM in a concentration-dependent manner. In addition, the NOM exhibited significant free radical scavenging, especially against DPPH and superoxide anions; and a moderate effect on hydroxyl and hydrogen peroxide scavenging. In vivo anti-inflammatory activity revealed that the edema volume was significantly (P < 0.001) decreased at all doses when compared with control and maximum activity (33, 47 and 54% at 50, 100 and 200 mg/kg dose, respectively) was observed at fifth hr of treatment. Likewise, the inhibition capacity was increased with dose. The present findings showed that cretaceous shales may contain a variety of medicinal agents that are traditionally believed to possess properties useful in the treatment of various ailments particularly skin and inflammatory disorders. Therefore, these shales could be a new source for activity-guided isolation of antimicrobial, antioxidant, and anti-inflammatory agents.
Keywords
Cretaceous shales
Natural organic matter
Antimicrobial
Anti-inflammatory
Antioxidant
1 Introduction
Globally, many researchers are interested in the discovery of more effective drugs from various sources. Natural sources including plants, colonizing bacteria, fungi and microalgae have been exploited for the discovery of new compounds (Ahmed et al., 2020). It has been documented that most of the marine organisms produce antimicrobial compounds as natural defence against the invading pathogens (Maria et al., 2021). Compounds isolated from different marine organisms have shown promising activity against human pathogens (Weiwei et al., 2019). However, irrational use of antibiotics has resulted in a significant increase in the number of drug-resistant microbes (Thangaraju and Venkatesan, 2019). Similarly, nonsteroidal anti-inflammatory drugs (NSAIDs) are effective in treating inflammation and pyrexia; however, they are associated with a number of side effects, particularly peptic ulcers and other GIT issues (Lawrence and Julio, 2021). Contrary to that, the NOM was extracted and subsequently dissolved for various activities using solvent systems having no associated health hazards. The complete process can be referred to the methodology section. To address these challenges, there is an urgent need to investigate new natural sources for the discovery of lead compounds with improved efficacy and minimum side effects.
Black shales are organic rich sedimentary rocks formed as a result of ocean anoxia or higher biological productivity within ocean (Fig. 1). The depositions of such shales are closely associated with periods of global warming in geological record (Naohiko et al., 2015). The enhanced hydrological cycles are closely associated with such global warming periods that may have resulted in flushing of different continental pathogens/microbes into ocean by streams and rivers. The activation of indigenous oceanic pathogens, as well as the influx of continental pathogens, could have increased the immunity of marine zooplankton and phytoplankton via production of different chemicals. These plankton are the sources of organic matter which has been preserved within the black shale (Chaoyong et al., 2021). The composition of NOM depends upon the type of precursor materials deposited during cretaceous period.
Lumshiwal rock formation located north of the main Kala Chitta range in district Nowshera-Pakistan.
Humic substances or NOM are biologically active organic macromolecules of vegetative, animal or microbiological origin that are widely spread in nature. They are predominantly found in soil, water, peat, humus, and various shales. Based on their solubility, humic substances are divided into three components: humic and fulvic acids (alkali soluble), and humin (insoluble residue). Overall, these substances contain major functional groups including carboxylic, carbonyl, phenolic, amides, amines and aliphatic moieties (Table 1) (Laurynas et al., 2021; Mao et al., 2017). Humic acid (Fig. 2A) is a major component of humic substances, dark brown in color, soluble at alkaline pH and have moderate molecular weight carboxylic and phenol groups (Maguey-Gonzalez et al., 2018). Fulvic acid (Fig. 2B) is yellow in color, soluble in a number of solvents and have low molecular weight carboxylic groups. On the other hand, the insoluble NOM is thought to be a biomacromolecules composed of variety of organic functional groups including aldehyde, ketones, carboxyl, amide, aromatic, alkyl, methoxy and amines (Agrawal and Sharma, 2018). The studied components responsible for medicinal activities of soluble NOM are humic acid and fulvic acid (Winkler and Ghosh, 2018; Robert et al., 2021; Ravin et al., 2017). NOM Natural organic matter; ArOH Phenol; –COOH Carboxylic acid; -=Ar = Conjugated aryls; -ArCOOH Aromatic carboxylic acid; –CHO Aldehydes; -C = O Carbonyls; –NH2 Amines; -RH Phenyl; –CH3O Methoxy.
Organic matter
Fractions
Identified Compounds
Chemistry
NOM
Soluble NOM
Humic acid
-ArOH and –COOH.
Fulvic acid
-ArOH, =Ar = and -ArCOOH.
Insoluble NOM
Humin
Biomacromolecules with variety of functional groups like –CHO, -C = O,–COOH, –NH2, -RH, –CH3O etc.
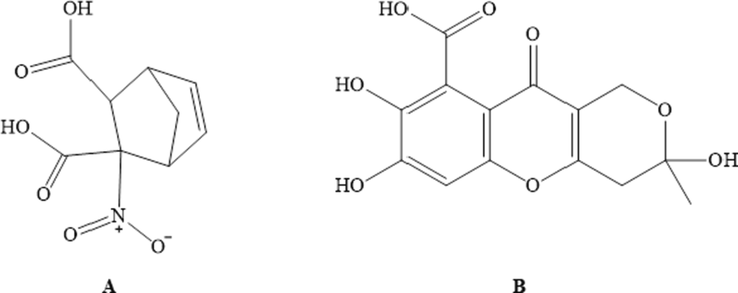
Chemical structure of (A) Humic acid and (B) Fulvic acid.
Humic acid have been used since ancient times in Indian Ayurvedic medicines for the treatment of diabetes, inflammation and cognitive disorders (Igor et al., 2009; Goel et al., 1990). Humic substances in aquatic systems and water sediments have been linked with the efficacy of balneotherapy and hydrotherapy (Senesi et al., 1991; Gadzhieva et al., 1991). In addition, these substances have been investigated for anticancer, antimicrobial, antiviral, antimutagenic, and wound healing properties (Metin et al., 2018; Maguey-Gonzalez et al., 2018; Winkler and Ghosh, 2018). Some studies have shown that humic acid can inhibit virus replication (Neyts et al., 1992). Their phenolic groups act as electron donating agents, scavenge free radicals, and inhibit chain initiation reaction in autoxidation. They can form micelles and could act as solubilizing agents for a variety of cosmetic and pharmaceutical agents (De-Melo et al., 2016). These properties are useful in formulating cosmetic preparations.
Humic substances from different sources have been investigated for a number of biological activities however; there is still a dearth of scientific validation of the traditional claims of preserved NOM, particularly of cretaceous origin. Thus, the present study was designed to assess the antimicrobial, anti-inflammatory and antioxidant effects of NOM with a view of finding alternate sources of antimicrobial, anti-inflammatory, and antioxidant agents.
2 Material and methods
2.1 Chemicals
Miconazole, Imipenem and indomethacin were donated by Ferozsons Laboratories Limited, Nowshera, KPK, Pakistan. 1,1-diphenyl 2-picryl hydrazyl, nitro blue tetrazolium, phenazine methosulfate-nicotinamide adenine dinucleotide, 1,10-phenanthroline, cyclooxygenase and 5-lipoxygenase enzymes were purchased from Sigma-Aldrich, Germany. Merck (Darmstadt, Germany) provided hydrochloric acid, hydrofluoric acid, phosphate buffer, methanol and other solvents.
2.2 Collection of cretaceous shale
The cretaceous shales were collected from Kala Chitta Range along Tora Stana in District Nowshera, Khyber Pakhtunkhwa, Pakistan (Lat. 33˚ 72′ 30″N: Long. 71˚ 96′ 65″). The sample was identified by Dr Suleiman Khan, Department of Geology, University of Peshawar, Pakistan. The samples were first washed with methanol, followed by crushing and subsequently stored in polythene bags for further processing. The sample was crushed in “Manual Processing Lab” at National Centre of Excellence (NCE), Geology, University of Peshawar.
2.3 Extraction of soluble organic matter
The powdered sample (25 g) was treated with hydrochloric acid, to remove carbonaceous matters; followed by repeated rinsing with distilled water. For removal of silicates, the residue was stirred with a mixture of hydrofluoric and hydrochloric acids (1:1 v/v) and again washed with distilled water until the rinsing become neutral. To remove heavy minerals, the soft residue was stirred with zinc bromide and distilled water. Finally, flakes of organic matter were sonicated in a mixture of chloroform, methanol and acetone (70: 15:15 v/v) and kept for 3 days for maximum extraction. It was then evaporated under vacuum using rotary evaporator. The process was done at Applied Pharmaceutical Lab, Department of Pharmacy, University of Peshawar (Agrawal and Sharma, 2018).
2.4 Isolation of humic substances
Humic substances were isolated using the following methods.
2.4.1 Humic acid
50 g of powdered sediment was treated with sodium hydroxide solution (0.4 L; 0.1 M) in distilled water. The extraction was carried out for 24 h with occasional stirring. After 24 h, the supernatant was collected and centrifuged at 10,000 × g for 15 min. The supernatant was then acidified up to pH 1 with hydrochloric acid solution (4.0 M) and left overnight to precipitate the humic acid. The precipitate was then obtained by centrifugation at 10,000 × g for 15 min (Laurynas et al., 2021).
2.4.2 Fulvic acid
The supernatant (Crude fulvic acid) was filtered through 0.2 µm cellulose nitrate membrane filter and neutralized with sodium hydroxide solution (0.1 M; pH 4 to 7). Solutions were left overnight to precipitate fulvic acid. Precipitates of fulvic acid was then obtained by centrifugation at 6000 × g for 15 min (Laurynas et al., 2021).
2.4.3 Characterization of humic and fulvic acid
2.4.3.1 Physical characterization
Both the acids were checked for physical properties including colour, odour, and solubility.
2.4.3.2 pH determination
The pH of the isolated acids were determined for 2% solution with the help of Mi 150 pH meter at room temperature.
2.4.3.3 UV–Vis spectral analysis
UV-visible spectroscopy was carried out for both the acids and individual spectrums were recorded from 200 to 800 nm on Perkin Elmer Lambda 35 spectrophotometer.
2.5 In vitro studies
2.5.1 Bacterial strains
The following strains of bacteria were used; Bacillus subtilisin (ATCC 6633), Staphylococcus aureus (ATCC 25924), Salmonella typhi (19430), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922). The bacterial suspension was standardized to a turbidity equivalent to 0.5 on the McFarland scale (CLSI, 2012).
2.5.2 Agar-well diffusion method
Microbial cultures were prepared using a validated method (Fazli et al., 2021). Different strains were added to Muller Hinton Agar (MHA) and poured into sterile petri dishes. Well (6 mm) were made using a sterile borer into the agar plate for both control and test samples. 3 mg of NOM was dissolved in DMSO (1 ml; 3%) and about 100 µl of it was transferred into each well. The petri dishes were then incubated for 48 h (37℃) and finally, zone of inhibition was measured (n = 3). Imipenem and DMSO were used as positive and negative control, respectively.
2.5.3 Fungal strains
Fungal strains include Aspergillus niger (ATCC 10568), Alternaria alternata (ATCC 96255), Fusarium solani (ATCC 11213) and Aspergillus terreus (ATCC 20445).
2.5.4 Agar slanting method
The selected fungal strains were grown on Sabouraud dextrose agar (SDA) and subsequently incubated for 24 h at 37℃ 4 ml of SDA along with fungal inoculum was poured into sterilized test tubes. Test sample was prepared by dissolving 23 mg in 1 ml of DMSO (3%) and 67.5 µl of it was added into each test tube. The cultured media was solidified in tilting position and then incubated for seven days at 37℃. Miconazole was used as positive control. Finally, the linear growth inhibition was measured and percent inhibition was calculated using Eq. (1). (Fazli et al., 2021)
2.5.5 Minimum inhibitory concentration (MIC)
The MIC was determined by the broth microdilution method, using 96 well microtiter plates (CLSI, 2012). Initially, NOM was dissolved in DMO and serially diluted with water (sterile) to get various dilutions (i.e. 0.1–30 mg/ml), in microtiter plates. To each well, susceptible strains of growing cultures (1.5108 cfu/ml) were added and subsequently incubated at 37℃ for 72 and 24 hr for fungi and bacteria, respectively. Tetrazolium salt was used as an indicator. It was added to each well and the appearance of violet color in culture media indicated the growth of microorganisms. Imipenem and miconazole were used as positive, while DMSO was used as a negative control.
2.5.6 Cyclooxygenase (COX-2) assay
The method of Olomola et al. was used for the determination of in vitro enzyme inhibition activity (Olomola et al., 2020). For 10 min, the enzyme (300 U/ml) and co-factor (50 µl) were preincubated on ice. In addition 50 µl of co-factor solution (1 mM Hematin, 0.9 mM glutathione and 0.24 mM N,N,N,N-tetramethyl-pphenylenediamine dihydrochloride in 0.1 M Tris HCl buffer, pH 8.0) was mixed with the enzyme solution. Subsequently, the enzyme solution and NOM (25–1000 µg/ml) were mixed and allowed to stand for 5 min at 25℃. In order to initiate the reaction, arachidonic acid (20 ml, 30 mM) was added to this mixture and incubated for 4 to 5 min. Finally, maximum absorbance was determined at 570 nm.
2.5.7 5-lipoxygenase (5-LOX) assay
Initially, solutions of montelukast, NOM (25–1000 µg/ml), phosphate buffer (50 mM, pH 6.3) and 5-LOX enzyme (10,000 U/ml) were prepared. Test samples were dissolved in buffer solution (250 µl) and the enzyme solution (250 µl) was added to it before incubation at 25℃ for 5 min. In addition, the enzyme solutions and linoleic acid (0.6 mM, 1 ml) were mixed and maximum absorbance (234 nm) was determined with a UV–vis spectrophotometer (Okur et al., 2021). Percent inhibition values were determined using Eq. (2).
2.5.8 Antioxidant activity
2.5.8.1 DPPH assay
The antioxidant activity of NOM was determined with 1,1-diphenyl 2-picryl hydrazyl radical (DPPH) spectrophotometrically at 517 nm. The DPPH was dissolved in methanol (100 ml) to get 0.01 mM solution. Stock solution of NOM (1 mg/ml) was prepared in methanol. Using suitable dilution, working solutions of the sample (25, 100, 200 and 500 µg/mL) were prepared from it. From each concentration, 100 µl was added to DPPH solution (3 ml). The absorbance was measured after 15 min of incubation at 23℃. In this assay, ascorbic acid was used as a standard drug (Ahmad et al., 2020). The percentage DPPH radical scavenging potential was measured using Eq. (3).
Where CAbs and SAbs are the absorbance of the control and test samples/standard, respectively.
2.5.8.2 Superoxide anion radical scavenging activity
This activity was measured by the reduction of nitro blue tetrazolium (NBT) based on a previous method. Initially, the superoxide radicals were generated in a phenazine methosulfate-nicotinamide adenine dinucleotide (PMS/NADH) system. In this assay the radicals were generated in sodium phosphate buffer (3 ml, 100 mM, pH 7.4) containing NBT (1 ml, 150 μM), NADH (1 ml, 468 μM) solutions, along with various concentrations of NOM (25–1000 µg/ml) in water. The reaction was initiated by the addition of PMS (1 ml, 60 μM) to the mixture. After incubation (5 min, 25℃) the maximum absorbance was measured at 562 nm, against the corresponding blank solution. Ascorbic acid was used as standard (Sourav et al., 2011).
2.5.8.3 Hydroxyl scavenging activity
This activity was determined with Fenton reaction. Reaction mixture contained FeCl2 (60 μl, 1.0 mM), H2O2 (150 μl, 0.17 M), 1,10-phenanthroline (90 μl, 1 mM), phosphate buffer (2.4 ml, 0.2 M) and 1.5 ml of NOM at various concentrations (25–1000 µg/ml). The reaction was started by the addition of H2O2. After incubation (25℃, 5 min), the maximum absorbance was measured at 560 nm (Yupiao et al., 2018).
2.5.8.4 Hydrogen peroxide scavenging
An aliquot of H2O2 (50 mM) and various concentration of NOM (25–1000 µg/ml) were mixed (1:1 v/v) and subsequently incubated for 25 min at 25℃. Following incubation, 80 µl of H2O2 and NOM solution was mixed with methanol (10 μl) and FOX reagent (0.9 ml) was added to it (FOX reagent was prepared by mixing BHT (9 volumes, 4.4 mM) in methanol with xylenol orange (1 vol, 1 mM) and ammonium ferrous sulfate (2.56 mM) in 0.25 M H2SO4). The reaction mixture was vortexed (25 min) before incubation at 25℃. The absorbance of the complex (ferric-xylenol orange) was measured at 560 nm. Sodium pyruvate was used as standard (Shumaila et al., 2013).
2.6 In vivo studies: Anti-inflammatory activity
2.6.1 Animals
Healthy BALB/c mice (25–30 g) of either sex were used for the in vivo anti-inflammatory activity. Standard protocol was adopted for conducting the experiments. The experiment was approved by the “Ethical Committee of the Department of Pharmacy, University of Peshawar” (Application No. 03/EC-18/Phar).
2.6.2 Carrageenan-induced paw edema
The in vivo anti-inflammatory activity was determined using carrageenan-induced paw edema model (Lingadurai et al., 2007). The animals were divided into five groups (n = 5). They were treated with NOM (50, 100 and 200 mg/kg, i.p), indomethacin (10 mg/kg, i.p), and normal saline. Paw edema was induced by injecting carrageenan solution (0.05 ml; 1%), 1 hr after the administration of NOM, into the left hind paw’s subplantar region. The standard and control groups received indomethacin and normal saline, respectively. The linear paw circumference was measured before and 1, 2, 3, 4 and 5 h after the injection of carrageenan, using a digital plethysmometer (model 7150, Ugo Basile, Italy). Percent inhibition was measured using Eq. (4).
2.7 Statistical analysis
Statistical analysis was conducted using One-way ANOVA along with Dunnet post hoc test (GraphPad Software Inc., San Diego, California, USA); data was presented as mean ± SEM for each group of five animals.
3 Results and discussion
3.1 Isolation of humic substances
Two fractions of NOM were isolated from organic rich cretaceous shale employing repeated sonication with polar solvents followed by subsequent treatment with acids (HCL/HF). The yield of isolated soluble NOM was<0.1 %. Furthermore, individual compounds (humic and fulvic acids) thought to be responsible for medicinal activities, were isolated using pH derived precipitation methods and identification was carried out by physical characterization.
3.2 Physical characterization
In present study, soluble NOM appears as yellowish brown, while insoluble NOM as black powder. Fulvic and humic acid was golden-yellow and brown in color, respectively (Fig. S1). Fulvic acid exhibited muddy or candy like odour while humic acid possess no odour (Table S1).
3.2.1 pH determination
The pH of fulvic and humic acid solution (2%) was 3.2 and 3.8, respectively.
3.2.2 UV–Vis spectral analysis
UV–Vis spectrums of fulvic and humic acid was in the range of 200 to 600 nm and 200 to 550 nm, respectively (Fig. S2). Both the acids exhibit wide uncharacteristic spectra consistent with earlier reported studies conducted on structural elucidation of natural organic matter (Kyoichi, 1987).
3.3 In vitro studies
3.3.1 Agar-well diffusion method
The cretaceous period was the warmest period in earth history. During that period, the temperature raised three times than the current global temperature (Ohkouchi et al., 2015). As a result, the polar glaciers melted and all water was added to oceans. The high global temperature and water on continents and ocean might have activated different strains of pathogens because they are sensitive to high temperature and water. The diverse pathogens from continents were shredded into oceans which might have further added to the diversity of oceanic pathogens. In response, the immunity of phytoplankton and zooplankton might have enhanced by producing different chemicals. The same algae and cyanobacteria are now preserved in rock record. Therefore, this study was designed to preliminary investigate the antimicrobial potential of NOM which may lead to the discovery of new lead compounds.
In present study, the NOM was tested for antimicrobial activity against some well-known pathogens, including P. aeruginosa, S. typhi, S. aureus, B. subtilis and E. coli. Literature review showed that typhoid fever, skin and other frequent infections are caused by these bacteria (Eng et al., 2015). E. coli is the most common facultative anaerobe in humans, causing illnesses such as gastroenteritis, urinary tract infection, meningitis and peritonitis (Arwyn-Jones and Surjo, 2019).
NOM exhibited significant activity against S. typhi, P. aeruginosa, E. coli and B. subtilis with MIC values 0.82, 0.87, 0.79 and 0.93 mg/ml, respectively. In addition, it showed considerable activity against S. aureus with MIC values 1.12 mg/ml (Table 2). MIC: Minimum inhibitory concentration (mg/ml) Standard: Imipenem
Test microorganisms
NOM
Standard
Inhibition zone (mm)
MIC
% Inhibition
Inhibition zone
MIC
Pseudomonas aeruginosa
16
0.87
48
33
0.0023
Salmonella typhi
17
0.82
49
35
0.0012
Staphylococcus aureus
9
1.12
32
28
0.0011
Bacillus subtilis
11
0.93
42
26
0.0014
Escherichia coli
15
0.79
49
32
0.0022
The antibacterial activity shown by NOM may involve several mechanisms including, but not limited to, pore formation, nucleic acid synthesis, DNA gyrase inhibition, and change in physiochemical properties of the plasma membrane (JJose and Cesar, 2016). It has been previously reported that humic and fulvic acids possess significant antibacterial activity particularly against methicillin-sensitive S. aureus, S. thyphi, MRSA, E. coli, and P. aeruginosa (Maguey-Gonzalez et al., 2018; Winkler and Ghosh, 2018). According to literature review, both fulvic and humic acids are the prominent constituents of NOM. Therefore, the antibacterial activity of NOM in this study against the mentioned pathogens may be partially due to these compounds.
3.3.2 Agar slanting method
Despite the fact that there are few studies in the literature regarding the antifungal activity of NOM. Here, it proved to be active against all the tested fungi especially against A. alternata and F. solani with MIC values 0.60 and 0.68 mg/ml, respectively, whereas it showed considerable activity against A. niger and A. terreus with MIC values 0.82 and 1.10 mg/ml, respectively (Table 3). Fusarium and Alternaria are well-known plant pathogens responsible for significant productivity losses in agricultural crops globally. These fungi causes early blight and wilt of tomato (Haytham et al., 2021). It has been documented that synthetic fungicides are extensively used to control plant pathogens; however, in addition to its toxicity to consumers they are considered the most lethal pollutants for the environment. Contrary to that, natural pesticides are environment friendly with minimal or no health hazards or pollution (Asmaa et al., 2020). Based on our results NOM may be a potential source for developing an eco-friendly phytofungicides. Antifungal activity of NOM against selected fungal strains MIC: Minimum inhibitory concentration (mg/ml) Standard: Miconazole
Test microorganisms
NOM
Standard
% Inhibition
MIC
% Inhibition
MIC
Fusarium solani
53
0.68
68
0.0017
Alternaria alternata
62
0.60
76
0.0001
Aspergillus niger
38
0.82
69
0.0004
Aspergillus terreus
25
1.10
55
0.0019
3.3.3 Cyclooxygenase and lipoxygenase assays
Both COX-2 and 5-LOX are considered as pro-inflammatory enzymes, involved in arachidonic acid metabolism (Violette and Ebtisam, 2018). They are also involved in the production of eicosanoids, including thromboxane, leukotrienes and prostaglandins that primarily cause inflammation. Therefore, these enzymes are the rate-limiting factors in the production of mediators of inflammation and commonly used for screening anti-inflammatory drugs. It is pertinent to mention that co-inhibition of COX/5-LOX potentially reduce side effects preferably on the GIT and cardiovascular system, while retaining the activity of COX-1/2 inhibitors (Martel-Pelletier et al., 2003).
Based on its traditional uses, we investigated the anti-inflammatory effect of NOM using in vitro models. In this study, COX-2 and 5-LOX enzymes were inhibited by NOM in a dose-dependent manner. The COX-2 inhibition results showed that NOM had significant activity with IC50 value of 31.34 µg/ml, compared to reference drugs Celecoxib (18.23 µg/ml). Concerning 5-LOX inhibition capacity, the sample showed considerable activity with IC50 value of 38.45 µg/ml, compared to Montelukast (25.34 µg/ml) (Fig. 3).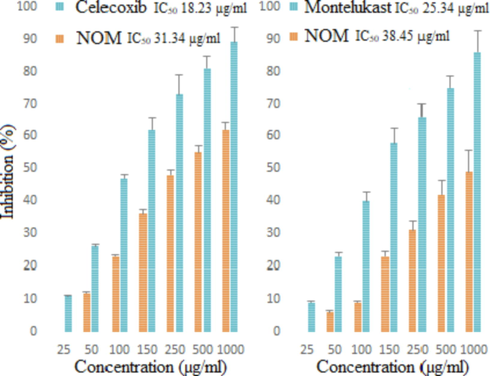
The relative inhibition of COX-2 and 5-LOX enzymes by NOM and standard.
Inflammatory reactions are commonly triggered by higher level of prostaglandins and leukotrienes, which are produced by 5-LOX and COX-1/2 enzymes, respectively. Prostaglandins sensitize nociceptive fibres and facilitates the passage of pain stimuli, while leukotrienes stimulates cell adhesion to vascular endothelium (Felipe et al., 2017). Therefore, inhibition of COX-1/2, reduce prostaglandin production, which subsequently reduce pain and inflammation. However, inhibition of prostaglandins may lead to activation of 5-LOX pathway, which in turn raise gastrotoxic leukotrienes. The use of dual inhibitors of COX-2/5-LOX, help to reduce these problems (Afshin and Sara, 2011). The results from the current study clearly exhibited that NOM had a high potency against COX and LOX and thus may be considered in anti-inflammatory drug discovery and development.
3.3.4 Antioxidant activity
According to WHO, nearly 80% of the world population rely on herbal traditional medicines for their primary health care needs and most of these therapies involves the use of herbal extracts (Nisa et al., 2013). It has been documented that our body produces more reactive oxygen species (ROS) than enzymatic antioxidants under stress condition. These includes superoxide anion radical (O2·–), perhydroxyl radical (HO2·) and hydroxyl radical (·OH) as well as some non-free radicals i.e·H2O2 (Duduku et al., 2011). In the absence of antioxidants, these free radicals facilitates the development of several diseases which includes but not limited to cancer, liver cirrhosis, diabetes, atherosclerosis, cardiovascular diseases, inflammation, and a variety of neurodegenerative disorders (Nisa et al., 2013; Sajjad et al., 2015). Synthetic antioxidants such as butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) have been widely used in food industry but these agents are reported to be associated with carcinogenesis and liver cirrhosis (Duduku et al., 2011). Therefore, in last few decades the use of natural antioxidants have got much attention.
3.3.4.1 DPPH assay
Several in vitro models have been documented for investigating the scavenging activity in various compounds. The DPPH is a stable organic radical commonly used to assess the free radical scavenging potential of phytochemicals and extracts. When the stable DPPH accepts an electron from the antioxidant specie, its violet color is reduced to yellow diphenylpicrylhydrazine radical, which is colorimetrically measured (Sagar and Sing, 2011). In present study, NOM showed significant activity 18, 31, 42, 51, 63 and 72% at concentrations of 25, 50, 100, 250, 500 and 1000 µg/ml, respectively. As shown in Table 4, the sample depicted a dose dependent activity, compared to ascorbic acid, which showed 95% DPPH scavenging at 1000 µg/ml. Traditional medicines have been used since long time for the treatment of a variety of ailments and their scientific validation have led to the development of many novel drugs. Results of the present study suggests that NOM may be considered as a potential source for the discovery of natural antioxidants.
Concentrations (µg/ ml)
DPPH Scavenging activity (%)
Superoxide anion radical scavenging (%)
NOM
Ascorbic acid
NOM
Ascorbic acid
25
18.12 ± 0.32
28.22 ± 0.33
11.20 ± 0.20
19.05 ± 0.18
50
31.22 ± 0.55
43.14 ± 0.56
28.10 ± 0.25
39.18 ± 0.45
100
42.12 ± 1.22
55.10 ± 1.21
35.12 ± 0.90
47.16 ± 1.20
250
51.45 ± 1.50
71.25 ± 1.32
44.12 ± 0.78
62.15 ± 1.66
500
63.40 ± 1.65
82.12 ± 1.50
53.23 ± 1.13
74.21 ± 1.34
1000
72.15 ± 1.88
95.18 ± 1.65
68.10 ± 1.0
88.12 ± 1.34
3.3.4.2 Superoxide anion radical scavenging activity
It has been reported that superoxide anions directly or indirectly damage biomolecules during pathological events such as inflammation by forming H2O2, singlet oxygen or –OH. They can also directly initiate lipid peroxidation (Małgorzata and Andrzej, 2016). The superoxide radicals (produced from dissolved oxygen by PMS-NADH coupling) can be quantified by their ability to reduce NBT. The reduction in absorbance at 560 nm caused by NOM shows its potential to quench superoxide radicals in the reaction. As shown in Table 4, the radical scavenging activity of NOM markedly increased with increase in concentration. In this assay, the sample and standard showed 68 and 88% superoxide anion scavenging at 1000 µg/ml, respectively. The results suggest that NOM can be further investigated for the isolation of specific compounds responsible for superoxide radical scavenging effects.
3.3.4.3 Hydroxyl scavenging activity
Hydroxyl radicals are the primary active oxygen species responsible for lipid peroxidation and massive biological injuries. These radicals are generated from the reaction of transition metals with various hydroperoxides. It damages unsaturated fatty acids in membrane, proteins, DNA and other biological molecules (Anju et al., 2019). In this study, NOM depicted concentration dependent activity against hydroxyl radicals produced in the Fenton reaction. As shown in Fig. 4, the sample and standard exhibited 73 and 92% hydroxyl scavenging activity at 1000 µg/ml, respectively.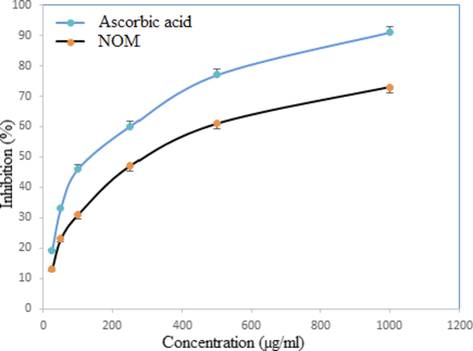
Hydroxyl scavenging activity of NOM and standard at different concentrations. Each value represents mean ± SD (n = 3).
3.3.4.4 Hydrogen peroxide scavenging
This is a weak oxidizing agent that directly inactivates few enzymes, typically by oxidation of essential thiol (-SH) groups. It has the ability to rapidly cross cell membranes; once inside the cell, it is likely to react with Fe2+ and possibly Cu2+ ions to form –OH radicals, which may be the source of many of its toxic effects (Ergul, 2016). NOM showed weak hydrogen peroxide scavenging activity i.e. 40 % at the highest tested dose, compared to standard, which depicted 73% activity at the same dose (Fig. 5).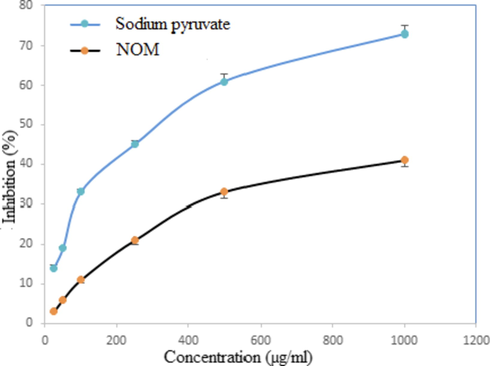
Hydrogen peroxide scavenging activity of NOM and standard at different concentrations. Each value represents mean ± SD (n = 3).
These in vitro tests show that NOM contains a significant amount of natural antioxidants, which may aid in the prevention of a variety of oxidative stresses. However, the compounds responsible for the antioxidant activity are unknown at this time. As a result, additional research is required to isolate and identify the antioxidant compounds present in NOM. Furthermore, the antioxidant activity of NOM in vivo must be determined before it can be used in clinical trials.
3.4 In vivo studies: Anti-inflammatory activity
3.4.1 Carrageenan-induced paw edema
The anti-inflammatory activity of NOM against carrageenan-induced paw edema is shown in Table 5. The NOM at all doses significantly (P < 0.001) decreased the edema volume against control and maximum activity (33, 47 and 54% at 50, 100 and 200 mg/kg dose, respectively) was observed at fifth hr. of treatment. The standard indomethacin showed maximum inhibition (58%) at fourth hr. of administration. Values are expressed as mean ± SEM, n = 5, ap ≤ 0.01, bp ≤ 0.001 compared with control, analyzed by one way ANOVA followed by Dunnet’s test for multiple comparison; BV: Basal volume; Std; Indomethacin; cValues in parentheses indicates the percentage inhibition rate
Groups
Mean change in the hind paw volume (ml)
Dose (mg/kg,i.p)
BV
1 h
2 h
3 h
4 h
5 h
Control
–
0.71 ± 0.02
1.19 ± 0.09
1.20 ± 0.08
1.21 ± 0.08
1.19 ± 0.08
1.18 ± 0.07
NOM
50
0.70 ± 0.02
0.97 ± 0.03 (17.31 %)c
0.98 ± 0.12 (21.1%)
1.10 ± 0.09 (13.98%)
0.93 ± 0.10b (25.99%)
0.80 ± 0.05b (33.89%)
NOM
100
0.65 ± 0.02
0.94 ± 0.04 (23.2%)
0.94 ± 0.07 (29%)
0.88 ± 0.07a (39%)
0.76 ± 0.04b (44%)
0.71 ± 0.01b (47%)
NOM
200
0.60 ± 0.04
0.80 ± 0.07 (27%)
0.77 ± 0.03a (35%)
0.75 ± 0. 03a (43%)
0.69 ± 0.05b (48%)
0.57 ± 0.2b (54%)
Std
10
0.58 ± 0.2
0.75 ± 0.03b (39%)
0.73 ± 0.04b (45%)
0.60 ± 0.02b (52%)
0.53 ± 0.01b (58%)
0.57 ± 0.04b (55%)
It is evident from the above results that NOM has significant anti-inflammatory potential by controlling biphasic inflammatory events. Carrageenan-induced inflammation in animals occur in two phases. The early phase (90–180 min) is characterized by the release of histamine, serotonin and similar substance whereas; the later phase (270–360 min) is associated with the release of prostaglandins, lysosome and proteases. In this study, NOM showed significant inhibitory effect particularly in the later phase of inflammation. This suggests that NOM possibly acts by inhibiting the release and/or actions of vasoactive substances (serotonin, histamine) and prostaglandins. The present results corroborate the importance for screening cretaceous shales as a potential source for bioactive compounds. Phytochemical composition of NOM showed the presence of humic substances including fulvic acid and some complex organic matters mainly produced by cyanobacteria (Ohkouchi et al., 2015). Literature review showed that humic substances possess significant anti-inflammatory activity (De-Melo et al,. 2016). In light of the abovementioned facts, this preliminary study could lead to the discovery of novel anti-inflammatory drugs derived from cretaceous shales that can be used for the rapid and effective management of infectious diseases.
4 Conclusion
This study for the first time confirms the antimicrobial as well as antioxidant and anti-inflammatory potential of NOM extracted from cretaceous shales. The NOM showed significant antimicrobial activity against S. typhi, P. aeruginosa, E. coli, A. alternata and F. solani. In vitro models depicted concentration dependent inhibition of COX-2 and 5-LOX enzymes. The NOM revealed good antioxidant activity against common free radicals i.e. DPPH, superoxide and hydroxyl. It also exhibited significant anti-inflammatory activity particularly at late hrs. of inflammation. These findings suggest that NOM could be a novel source for antimicrobial, anti-inflammatory and antioxidant agents. However, it is important to determine the specific compound(s) responsible for the said activities; and to establish its proper mechanism in order to come to a definite conclusion.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Group Research Project under grant number (R.G.P.2/33/42).
Declaration of Competing Interest
The authors have no conflict of interest to declare.
References
- Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res.. 2011;10:655-683.
- [Google Scholar]
- Molecular characterization of kerogen and its implications for determining hydrocarbon potential, organic matter sources and thermal maturity in marcellus shale. Fuel.. 2018;228:429-437.
- [CrossRef] [Google Scholar]
- Synthetic b-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol. Biol. Rep.. 2020;47:9553-9566.
- [CrossRef] [Google Scholar]
- Ahmed, S., Abdel-Razek, Mehrez, E.E., Ahmed A., Osama, M.M., Sarah, I.O., 2020. Microbial Natural Products in Drug Discovery. Processes. 8, 470. doi:10.3390/pr8040470.
- Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583.
- [CrossRef] [Google Scholar]
- The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy. 2020;10:1402.
- [CrossRef] [Google Scholar]
- Origin and paleoenvironment of organic matter in the Wufeng-Longmaxi shales in the northeastern Sichuan basin. Energy Explor. Exploit.. 2021;39:134-155.
- [CrossRef] [Google Scholar]
- CLSI, 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard — Ninth Edition. Clinical and Laboratory Standards Institute, CLSI Document M07A9. Vol. 32.
- Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C. 2016;62:967-974.
- [CrossRef] [Google Scholar]
- A review of the antioxidant potential of medicinal plant species. Food. Bioprod. Process.. 2011;8:217-233.
- [CrossRef] [Google Scholar]
- Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life. Sci.. 2015;8:284-293.
- [CrossRef] [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J.. 2016;15:71.
- [CrossRef] [Google Scholar]
- Synthesis of gold nanoparticles using Sambucus wightiana extract and investigation of its antimicrobial, anti-inflammatory, antioxidant and analgesic activities. Arab. J. Chem.. 2021;14:103344
- [CrossRef] [Google Scholar]
- Felipe, A., Pinho-Ribeiro, Waldiceu, A., Verri, J., Isaac, M.C., 2017. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends. Immunol. 38, 5–19.doi: 10.1016/j.it.2016.10.001.
- Gadzhieva, N.Z., Tsŏı, E.P., Turovskaia, S.I., Ammosova, I., 1991. The antibacterial activity of a humic preparation made from the therapeutic peat mud of the Dzalal Abad deposit in Kirghizia. Nauchnye Dokl. Vyss. Shkoly. Biol. Nauk. 10, 109–113.
- Antiulcerogenic and antiinflammatory studies with shilajit. J. Ethnopharmacol.. 1990;29:95-103.
- [CrossRef] [Google Scholar]
- Antifungal Effect of Different Plant Extracts against Phytopathogenic Fungi Alternaria alternata and Fusarium oxysporum Isolated from Tomato Plant. JPRI. 2021;33:188-197.
- [CrossRef] [Google Scholar]
- Complement-fixing Activity of Fulvic Acid from Shilajit and Other Natural Sources. Phytother. Res.. 2009;23:373-384.
- [CrossRef] [Google Scholar]
- Spectroscopic Characteristics of Humic Acids and Fulvic Acids. Developments Soil Sci.. 1987;17:34-56.
- [CrossRef] [Google Scholar]
- Laurynas, J., Liudas, I., Giedre, K., Juste B., Mindaugas, M., Jurga, B. 2021. Determination of Organic Compounds, Fulvic Acid, Humic Acid, and Humin in Peat and Sapropel Alkaline Extracts. Molecules. 26, 2995. https://doi.org/ 10.3390/molecules26102995.
- NSAIDs and anastomotic leak: What's the evidence? Semin. Colon Rectal. Surg.. 2021;32:100833
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti- nociceptive activities of methanolic extract of the leaves of Fraxinus floribunda Wallich. Afr. J. Tradit. Complement. Altern. Med.. 2007;4:411-416.
- [CrossRef] [Google Scholar]
- Effects of humic acids on recovery of Salmonella enterica serovar Enteritidis. Ann. Anim. Sci.. 2018;18:387-399.
- [CrossRef] [Google Scholar]
- The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev.. 2016;2016:3164734.
- [CrossRef] [Google Scholar]
- Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Nucr. Mag. Res. Spec.. 2017;100:17-51.
- [CrossRef] [Google Scholar]
- Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci.. 2021;8
- [CrossRef] [Google Scholar]
- Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis.. 2003;62:501-509.
- [CrossRef] [Google Scholar]
- Humic Acid Enhances Wound Healing in the Rat Palate. Evid. Based. Complement. Alternat. Med.. 2018;2018:1783513.
- [CrossRef] [Google Scholar]
- The origin of Cretaceous black shales: a change in the surface ocean ecosystem and its triggers. Proc. Jpn Acad. Ser. B Phys. Biol. Sci.. 2015;91:273-291.
- [CrossRef] [Google Scholar]
- Poly (hydroxy) carboxylates as selective inhibitors of cytomegalovirus and herpes simplex virus replication. Antivir. Chem. Chemother.. 1992;3:215-222.
- [Google Scholar]
- Nisa, H., Azra, N., Kamili, S.A., Shajr-ul-Amin, Bashir, A., Nisa, G., 2013. Antimicrobial and antioxidant activities of alcoholic extracts of Rumex dentatus L. Microb. Pathog. 57, 17-20. doi: 10.1016/j.micpath.2013.02.001.
- The origin of Cretaceous black shales: a change in the surface ocean ecosystem and its triggers. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci.. 2015;91:273-291.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, analgesic and in vivo-in vitro wound healing potential of the Phlomis rigida Labill. extract. J. Ethnopharmacol.. 2021;266
- [CrossRef] [Google Scholar]
- Benzofuran selenadiazole hybrids as novel a-glucosidase and cyclooxygenase-2 inhibitors with antioxidant and cytotoxic properties. Bioorg. Med. Chem.. 2020;100:103945
- [CrossRef] [Google Scholar]
- Determination of organic degradation rates in 100 My old sediments: Application to Cretaceous black shale intervals from Demerara Rise, ODP Leg 207. Org. Geochem.. 2017;113:128-140.
- [CrossRef] [Google Scholar]
- Robert, W.N., John, P.N., Jakob, R., 2021. Humate in the Upper Cretaceous Fruitland Formation in northwestern .New Mexico Geological Society Guidebook, 71st Field Conference, Geology of the Mount Taylor Area, p. 153-158. https://nmgs.nmt.edu/publications/guidebooks/72.
- Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.. 2011;48:412-422.
- [CrossRef] [Google Scholar]
- Antioxidant and anticholinesterase investigations of Rumex hastatus D. Don: potential effectiveness in oxidative stress and neurological disorders. Biol. Res.. 2015;48:20.
- [CrossRef] [Google Scholar]
- Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil. Sci.. 1991;152:259-271.
- [CrossRef] [Google Scholar]
- Assessment of Antioxidant Potential, Total Phenolics and Flavonoids of Different Solvent Fractions of Monotheca Buxifolia Fruit. Osong. Public Health Res. Perspect.. 2013;4:246-254.
- [CrossRef] [Google Scholar]
- Assessment of the Antioxidant and Reactive Oxygen Species Scavenging Activity of Methanolic Extract of Caesalpinia crista Leaf. Evid. Based Complement Alternat. Med.. 2011;2011:173768
- [CrossRef] [Google Scholar]
- Thangaraju, P., Venkatesan, S., 2019. WHO Ten threats to global health in 2019: Antimicrob. Resist. 44, 1150-1151. https://doi.org/10.17826/cumj.514157.
- Violette, S.h., Ebtisam, A.A.H., 2018. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 11, 23–32. doi: 10.1016/j.jare.2018.03.005
- Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009–2018. Mar Drugs.. 2019;17:339.
- [CrossRef] [Google Scholar]
- Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes. Res.. 2018;1–7
- [CrossRef] [Google Scholar]
- Optimisation of Ethanol-Reflux Extraction of Saponins from Steamed Panax notoginseng by Response Surface Methodology and Evaluation of Hematopoiesis Effect. Molecules.. 2018;23:1206.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103633.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







