Translate this page into:
Antioxidant, α-glucosidase inhibitory, and cytotoxic activities of Mangifera rufocostata extract and identification of its compounds by LC-MS/MS analysis
⁎Corresponding author at: Department of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember, Sukolilo, Surabaya 60111, Indonesia. fatma@chem.its.ac.id (Sri Fatmawati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mangifera rufocostata, a member of the Mangifera genus, is a source of secondary metabolites and has been used traditionally as antidiabetic agent. Therefore, this study aims to determine the total flavonoid contens (TFC), total phenolic contents (TPC), antioxidant, α-glucosidase inhibitory, and cytotoxic activities of M. rufocostata stem bark extract. Antioxidant activity were evaluated by 2-2″-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and ferric reducing-antioxidant power (FRAP) tests. α-glucosidase inhibitory to prediction antidiabetic activity, and cytotoxic activity were studied against HT-29, HeLa, and MCF-7 cancer cell lines. The results showed that the methanol extract have the highest TFC, TPC, antioxidant, and α-glucosidase inhibitory activity. Furthermore, the methanol extract has strong cytotoxicity againts HT-29 cancer cells. Based on LC-MS/MS, the methanol extract contain several major compounds such as mangiferin, quinic acid, gallic acid, and 3-methoxy-4-hydroxy-phenyl glycol.
Keywords
M. rufocostata
Antioxidant
α-glucosidase inhibitory
Cytotoxic
LC-MS/MS
1 Introduction
The Mangifera genus consist of 69 species spread across Sumatra, Borneo, and the Malay Peninsula (Kostermans & Bomphard, 1993). They are rich in various secondary metabolites and display various bioactivities. About 23 species are found in Kalimantan (Uji, 2004), with some being active as antioxidants, antidiabetics, and anticancer.
One species that is active as an antidiabetic is M. indica as indicated by the leaf (Gazwi & Mahmoud, 2019; Mohammed & Rizvi, 2016; Ngo et al., 2019), fruit peel (Chowdhury et al., 2017), and stem bark extract (Bhowmik et al., 2009), which demonstrated significant hypoglycemic activity in diabetic-induced mice (Ojewole, 2005). Methanol extract of fruit peel with concentrations of 200 and 400 mg/kg can reduce plasma glucose by 13.95 to 26.18 % after 90–150 min, compared to standard glibenclamide (14.90––20.67 %) (Chowdhury et al., 2017). The leaves extract of M. indica is active as antioxidant with IC50 values of 3.18 to 13.37 μg/mL (Fitria et al., 2016; Itoh et al., 2020; Mohan et al., 2013; Park et al., 2015; Prommajak et al., 2014), while the peel has an antioxidant capacity of 23 ± 2.85 to 53.9 ± 4.2 (mM Trolox/100 g DW) (Marcillo-Parra et al., 2021), and 2986 ± 380 μmol QE/100 g FW (Abbasi et al., 2017). M. indica fruit extract can inhibit free radicals up to 45 % (Septembre-Malaterre et al., 2016), while the bark is active as an antioxidant with an IC50 12 μg/mL (Itoh et al., 2020). The kernel, peel, pulp, and seeds of the fruits are cytotoxic by inhibiting the growth of breast cancer cells MCF-7 and MDAMB-231 (Abdullah et al., 2014, 2015; Arbizu-Berrocal et al., 2019; Banerjee et al., 2015; Wilkinson et al., 2011), prostate LNCaP (S. Prasad et al., 2007), leukemia HL-60 (Percival et al., 2006), HepG2 (Abbasi et al., 2017), cervix HeLa (Timsina & Nadumane, 2015), lung A-549, leukemia Molt-4, and colon SW480 (Noratto et al., 2010) with IC50 15 to 30 µg/mL. M. indica pulp at a dose of 0.3 % can inhibit the growth of colon cancer in mice by up to 60 % (p = 0.05), after 10 weeks of treatment (Corrales-Bernal et al., 2014). The bark also showed toxic effects on pancreatic carcinogen PANC-1 (Nguyen, Do, et al., 2016), ovarian cell line SKOV-3, as well as breast MCF-7, and MDAMB-231 (Ediriweera et al., 2016). In contrast, M. indica leaf was less active in inhibiting gastric carcinoma Kato-III, Hepatoblastoma HepG2, ductal carcinoma BT474, colon adenocarcinoma SW 620, and bronchogenic carcinoma Chago K1 (Ganogpichayagrai et al., 2017). Other mangifera species also has been proven to be active as antioxidants, including M. casturi (Lestari et al., 2021; Pardede & Koketsu, 2017), and M. longipes (Guha et al., 2021). α-Glucosidase inhibitory from M. foetida, and M. mekongensis, with IC50 13.49 mg/mL and 1.71 µg/mL, respectively (Nguyen et al., 2021; Nguyen, Le, et al., 2016; Yusro et al., 2016).
The mangifera species usually contain similar compounds, such as mangiferin, gallic acid, and quercetin. Mangiferin is an active compound that functions as an anticancer agent (Núñez Selles et al., 2016). It actively inhibited the growth of breast cell cancer MCF-7 and MDAMB-231(Fernández-Ponce et al., 2017), lung cancer in white mice in vivo (Rajendran et al., 2014; Singh et al., 2018), leukemia carcinogenic cells (Zhang et al., 2014), and neuroblastoma IMR-32 (Das et al., 2011). The compound has been shown to strongly inhibits the activity of α-amylase and α-glucosidase (Kulkarni & Rathod, 2018; Sekar et al., 2019). α-Glucosidase is an enzyme that catalyzes the cleavage of polysaccharides into glucose. Therefore, its inhibitors have been used to treat type 2 DM (diabetes mellitus).
M. rufocostata is a Mangifera species found in Kalimantan and is also known as Asem Tanduy in South Kalimantan (Kostermans & Bomphard, 1993). Traditionally, the community use the boiled water of the bark as a medicine for diabetes and mild stroke. Although M. rufocostata shows potential benefits in traditional medicine, research on the biological activity of M. rufocostata stem bark is limited to antioxidant activity. The ethanol extract of M. rufocostata stem bark was reported to be active as an antioxidant with an IC50 of 8.254 ppm (Sutomo et al., 2023), with a total phenolic content (TPC) of 471.3126 mg GAE/g, and total flavonoid content (TFC) of 872.075 mg QE/g (Susiani et al., 2023). Phytochemical screening studies show that the extract contains tannins, phenols, flavonoids and saponins (Sutomo et al., 2023). Therefore, this study aims to evaluate TFC, TPC, the activity of antioxidants ((2-2″-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 1.1-diphenyl-2-picrylhydrazyl (DPPH), and ferric reducing-antioxidant power (FRAP)), α-glucosidase inhibitory, and cytotoxic activity against cancer cells HT-29, HeLa, MCF-7 of M. rufocostata stem bark extract comprehensively. LC-MS/MS was also used to identify the compounds present in the active extract.
2 Material and methods
2.1 Plant material
The stem bark of M. rufocostata was obtained in September 2021 at Barabai, Hulu Sungai Tengah, South Kalimantan. The samples were identified by Dr. Gunawan, M.Si the Plant botanist of the Faculty of Mathematics and Science, Lambung Mangkurat University, Banjarbaru, Indonesia.
2.2 Extraction
M. rufocostata bark is dried at 40 °C, then ground into powder with a size of 60–80 mesh. The dry powder 25.0 g each was extracted with 300 mL n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and methanol (MeOH) (Merck) for 24 h at room temperature. Each extract was concentrated using a rotary evaporator to produce dry extracts.
2.3 Total flavonoid contents (TFC)
The TFC value of M. rufocostata stem bark extract was measured using the method of Idris et al. (2022). Extracts including n-hexane, CH2Cl2, EtOAc, and MeOH with a certain concentration were taken up to 1 mL and mixed with 1 mL of AlCl3 solution (2 %) in methanol (Merck), then the mixture was incubated for 1 h. The absorbance of the mixture was measured using a UV–vis spectrophotometer (415 nm). The standard curve of quercetin (Sigma-Aldrich) ranged from a concentration of 0–60 mg/L and the TFC value is equivalent to quercetin (mg QE)/g dry extract.
2.4 Total phenolic content (TPC)
The total phenolic content of M. rufocostata stem bark extract was measured using the Follin-Ciocalteu method (Idris et al., 2022). Each extract including n-hexane, CH2Cl2, EtOAc, and MeOH with a certain concentration was taken up to 0.5 mL each, then added with sodium carbonate solution (7.5 %; 2.0 mL), and 2.5 mL of Follin–Ciocalteu solution (10 %) (Sigma-Aldrich). After the mixture was incubated (1 h), it was measured using a UV–vis spectrophotometer (765 nm) to determine the absorbance. The standard curve of gallic acid (Sigma-Aldrich) has a 0–130 mg/L concentration. The calculated TPC value was equivalent to gallic acid (mg GAE)/g dry extract.
2.5 ABTS radical scavenging assay
Free radical scavenging activity of M. rufocostata stem extract was performed using the ABTS method, as reported by Idris et al. (2022) with slight modifications. About 5 mL of 7 mM ABTS (Sigma-Aldrich) was mixed with 8.8 mL of 1.40 mM potassium persulfate (Merck) and stored in a dark place at room temperature for 12 to 16 h. The mixture was diluted with ethanol (Merck) to obtain an absorbance of 0.700 ± 0.015 at a wavelength of 734 nm and was referred to as the ABTS working solution. Furthermore, 3 mL of ABTS working solution and 30 µL of the extract with various concentrations were vortexed for 10 s and incubated at 30 °C for 4 min. The absorbance of each solution was measured with a UV–vis spectrophotometer at a wavelength of 734 nm. Gallic acid (Sigma-Aldrich) was used as a positive control.
2.6 DPPH radical scavenging assay
The antioxidant activity of M. rufocostata stem bark extract was analyzed using the modified DPPH method (Kuntorini et al., 2022). The n-hexane, CH2Cl2, EtOAc, and MeOH extracts were diluted to a certain concentration in methanol. Afterward, 2 mL of DPPH (0.15 mM) (Sigma-Aldrich) was mixed with 2 mL of sample, then the mixture was incubated for 30 min in the dark at room temperature. The absorbance of the mixture was measured using a UV/Vis spectrophotometer (517 nm). The antioxidant activity was measured in moles of Trolox equivalent (Sigma-Aldrich)/g dry extract (μg TE/g). Gallic acid (Sigma-Aldrich) was used as a positive control.
2.7 Ferric-reducing antioxidant power (FRAP) assay
The antioxidant activity of M. rufocostata stem bark was also measured using the FRAP method modified by Kuntorini et al. (2022). The TPTZ in HCl (2.5 mL; 10 mM) (Sigma Aldrich), 25 mL of acetate buffer (300 mM) with a pH of 3.6, and 2.5 mL of FeCl3·6H2O (20 mM) solution (Merck) is referred to as FRAP solution. Extracts including n-hexane, CH2Cl2, EtOAc, and MeOH with a certain concentration of 1 mL were reacted with the FRAP solution of 3 mL. The mixture was incubated for 15 min at room temperature in a dark place and the absorbance of the mixture was measured using a UV/Vis spectrophotometer at a wavelength of 597 nm. FeSO4·7H2O (Merck) and Trolox (Sigma-Aldrich) were used as standard curves, while the antioxidant capacity was expressed as the mole equivalent of Trolox (TE)/g dry extract (μg TE/g). Gallic acid (Sigma-Aldrich) was used as a positive control.
2.8 a-glucosidase inhibitory activity
Stem bark of M. rufocostata was carried out using in vitro based assay againts the inhibition of α-glucosidase enzymes (rat intestinal acetone powder, Sigma) (Fatmawati et al., 2011; Idris et al., 2022). About 10 µL of M. rufocostata stem bark extract (10 mg/mL in DMSO) was reacted with phosphate buffer (50 µL; 0.1 M) at pH 6.9 and maltose substrate (20 µL; 10 mM) in 0.1 M phosphate buffer (Merck). Then 80 µL of glucose kit (Human) and α-glukosidase enzyme solution (20 µL) were added. Then the mixture was incubated (37 °C; 10 min) and the absorbance was measured using a microplate reader at 520 nm (Spectrostar nano, BMG Labtech).
2.9 Cytotoxic activity
The in vitro cytotoxicity assay was carried using MTT assay againts HT29 (human colon cancer from ATCC, HTB-38), HeLa (human cervical adenocarcinoma from ATCC, CCL-2), and MCF-7 (human breast adenocarcinoma from ATCC, HTB-22) cells (Ali et al., 2021). HT29 was grown in McCoy's 5A Medium (Gibco), while HeLa and MCF-7 were grown in DMEM (Gibco). They were supplemented with 10 % fetal bovine serum (FBS, Gibco), sodium pyruvate 1 % (Gibco), and penicillin/streptomycin 1 % (Gibco). They were preserved in complete medium containing 10 % (v/v) PBS in 96 well plate cultures at a density of 185,000 cells per mL of medium, then incubated for 24 h at 37 °C (5 % CO2). Furthermore, the MeOH extract with a concentration of 7.8 to 1000 µg mL−1 was added to the media and incubated for 48 h. At 42 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) solution (20 µL, 5 mg mL−1) was added to each well and incubation continued for six hours at 37 °C. The absorbance of the mixture was measured at 570 nm using an ELISA reader (Zenix). The negative control was in the form of media, while cisplatin (Sigma-Aldrich) was used as the positive control. Cell morphology was observed using an inverted microscope.
2.10 LC-MS/MS analysis
About 10 mg/mL of the MeOH extract was dissolved in methanol and filtered through a 0.2 μm PTFE membrane, then measured with UHPLC Vanquish Tandem Q Exactive Plus Orbitrap HRMS ThermoScientific at column temperature of 30 °C; flow rate 0.2 mL/min, mobile phase H2O + 0.1 % formic acid (A) and acetonitrile + 0.1 % formic acid (B), and elution gradient, 0–1 min (5 % B), 1–25 min (5–95 % B), 25–28 min (95 % B), and 28–30 min (5 % B). The mass spectrometry conditions electrospray ionization (ESI) source, scanning range of 100–1500 m/z with the ChemSpider and Mzcloud database (Resida et al., 2021).
2.11 Statistical analysis
The data obtained were summarized using the standard deviation formula including antioxidant activity, TFC and TPC values, α-glucosidase inhibitory which were calculated with the linear regression equation. Meanwhile, cytotoxic activity against several cancer cells was calculated using Probit analysis with p < 0.05. Differences in the antioxidant, TPC, TFC, and α-glucosidase inhibitory of each extract were analyzed by Student's t-test with p < 0.01. The relationship between antioxidant, TPC, TFC, and, α-glucosidase inhibitory was analyzed using the Pearson Correlation test with p < 0.01.
3 Results and discussion
3.1 Extraction yield
The solvents chosen for extraction of M. rufocostata stem bark were n-hexane, dichloromethane, ethyl acetate and methanol. The solvents were chosen based on differences in polarity, because the polarity of the solvent greatly influences the yield of the extract. The order of polarity of the solvent used is n-hexane < CH2Cl2 < EtOAc < MeOH. The extraction results of the four solvents are presented in Table 1. It can be seen that the greatest extract yield was obtained from extraction with methanol in the order MeOH > EtOAc > CH2Cl2 > n-hexane. This is caused by the polar protic nature and dielectric constant of methanol which is greater than other solvents. This shows that the secondary metabolite compounds in the stem bark of M.rufocostata are dominated by polar compounds. This trend is in line with the results of extraction of N. leucophylla plants (Sharma & Cannoo, 2017).
Extract
Yield
weight (g)
w/w (%)
n-Hexane
0.50
2.00
CH2Cl2
0.75
3.00
EtOAc
1.50
6.00
MeOH
2.47
9.88
However, the opposite results were found in the extraction results of the D. pentandra (L.) plant, where the extract from the non-polar solvent (n-hexane) was greater than the polar solvent (ethanol) (Kristiningrum et al., 2018). Therefore, the choice of solvent for extraction depends on the type and polarity of the secondary metabolite compounds of a plant material (El Mannoubi, 2023).
3.2 Total flavonoids and phenolics contents
The total flavonoids and phenolic content of M. rufocostata stem bark extract is shown in Table 2.
Extract
TFC (mg QE g−1 dry extract)
TPC (mg GAE g−1 dry extract)
n-Hexane
1.74 ± 0.05b
19.29 ± 0.08d
CH2Cl2
1.93 ± 0.03c
22.72 ± 0.26d
EtOAc
9.18 ± 0.07b,c
27.05 ± 0.14d
MeOH
195.44 ± 0.71b,c
446.07 ± 3.01d
The TFC value of the M. rufocostata extract is equivalent to that of quercetin, hence, it was determined by the linear standard curve of quercetin. The results of the TFC showed significant differences among all extracts, except between n-hexane and CH2Cl2 extracts (t-Test; p < 0.01). Moreover, the MeOH extract showed the highest flavonoid content compared to other extract, in the order MeOH > EtOAc > CH2Cl2 > n-hexane. These results indicate that M. rufocostata flavonoids accumulated more in polar extracts and less in the semipolar and non-polar. This trend is in line with several other research results, including on the flavonoid content in plants Gracilaria changii (Chan et al., 2015) and N. leucophylla (Sharma & Cannoo, 2016). The results of research by Susiani et al. (2023) regarding the TFC test on M. rufocostata stem bark using EtOH solvent produced TFC levels of 872.075 mg QE/g. The results of this study were greater when compared with the MeOH extract in this study. This condition is possible if the flavonoid compounds in the bark of M. rufocostata are more easily extracted into EtOH. However, the flavonoid content in the stem bark of M. rufocostata MeOH extract in this study was greater when compared to other mangifera species (M. foetida and M. kemanga) even though EtOH solvent was used. The TFC values of M. foetida and M. kemanga EtOH extracts were 94.34 ± 0.24 and 87.46 ± 0.35 mg QE/g dry extract, respectively (Fitmawati et al., 2020).
The TPC was determined from a linear gallic acid standard curve and the results of each extract showed a significant difference (t-test; p < 0.01). TPC value trends are in line with TFC. The trend in TPC values is in line with TFC, where the phenolic compounds also accumulates more in MeOH as a polar solvent, and becomes smaller with decreasing polarity (MeOH > EtOAc > CH2Cl2 > n-hexane). This result is also in line with the results of TPC analysis on plants G. changii (Chan et al., 2015) and N. leucophylla (Sharma & Cannoo, 2016). The phenolic content in the MeOH extract of M. rufocostata stem bark in this study was also lower when compared to the EtOH extract (471.3126 mg GAE/g) reported by Susiani et al. (2023). This result is in line with the TPC value of the EtOH extract of M. caesia fruit which is greater than the MeOH extract (Yunus et al., 2021). The TPC value of the MeOH extract of M. rufocostata stem bark in this study was also greater than the EtOH extract of stem bark M. caesia (48.54 ± 1.75 mg GAE/crude extract) (Yunus et al., 2021), M. foetida (100.65 ± 1.94 mg GAE/g) and M. kemanga (90.65 ± 0.59 mg GAE/g) (Fitmawati et al., 2020).
3.3 Antioxidant activity
The antioxidant activity of the extract was analyzed by ABTS, DPPH, and FRAP methods. The test results are shown in Table 3.
Extracts
ABTS
DPPH
FRAP
IC50 (µg mL−1)
Antioxidant Capacity (µg TE/g dry extract)
Antioxidant Capacity (µg TE/g dry extract)
n-Hexane
10,780 ± 255.85b
2,789.17 ± 57.50c
7,356.95 ± 34.55d
CH2Cl2
2,035.00 ± 25.47b
3,317.73 ± 99.59c
7,680.18 ± 22.62d
EtOAc
1,137.44 ± 4.07b
6,407.78 ± 152.13c
19,805.85 ± 56.93d
MeOH
30.84 ± 0.05b
377,961.70 ± 18,426.9c
173,031.40 ± 345.55d
Gallic Acid
2.02 ± 0.01b
560,925.10 ± 6,003.2c
203,998.10 ± 513.32d
As shown in Table 3, the test results between extracts indicated significant differences in antioxidant activity (t-test; p < 0.01).
The ABTS method is used to evaluate the antioxidant activity through free radical scavenging with a proton donor (Aguirre-Becerra et al., 2020; Chohra et al., 2020). The test results showed that the MeOH extract have the highest antioxidant activity, with IC50 value of 30.84 ± 0.05 µg mL−1. This value indicates that the antioxidant activity of the MeOH extract is very strong, although it is still weaker compared to the positive control of gallic acid. Gallic acid is a polyphenolic compound that is very active as an antioxidant which can act as a neuroprotective agent in oxidative stress, neurodegeneration and neurotoxicity (Daglia et al., 2014). Antioxidant activity decreased in descending order MeOH > EtOAc > CH2Cl2 > n-hexane.
The DPPH method was used to test the antioxidant activity using free radicals derived from DPPH compounds. The radical derived electrons or hydroxyl radicals from the extract's antioxidant compounds and became stable diamagnetic compounds. The presence of antioxidant activity was indicated by the change in color of the solution, from purple to yellow. Based on the results, the MeOH extract had the greatest antioxidant activity as shown in Table 3. The antioxidant capacity of MeOH extract is almost 63 times the antioxidant capacity of EtOAc, although it is smaller compared to the antioxidant capacity of gallic acid (1.5 x).
The FRAP method was used to evaluate the antioxidant activity based on the capacity of the sample to participate in a redox reaction with the FRAP reagent. The MeOH extract also showed the highest activity as shown in Table 3. In contrast, the smallest antioxidant activity was obtained in the other extract (EtOAc > CH2Cl2 > n-hexane), although it was smaller than the antioxidant capacity of gallic acid (1.2 x).
The results of antioxidant tests using three different methods (ABTS, DPPH, FRAP) in this study showed the same trend. These results are in line with the results of the antioxidant test of the Garciinia forbesi King plant (Wairata et al., 2022). MeOH extract has the highest inhibitory activity against free radicals, because MeOH extract contains the most flavonoid and phenolic compounds compared to other extracts, as evidenced by the high TFC and TPC (Table 2).
3.4 α-glucosidase inhibitory
The results of the α-glucosidase inhibitory activity assay of stem bark M. rufocostata extract are shown in Table 4.
Extracts
α-glukosidase
% inhibition ± SD (10 mg mL−1)
IC50 (µg/mL)
n-Hexane
16.64 ± 3.09b,c
nt
CH2Cl2
40.28 ± 6.04b,c
nt
EtOAc
67.24 ± 6.45b,c
nt
MeOH
99.07 ± 0.50b
49.57 ± 15.56
Acarbose
99.47 ± 0.19c
10.42 ± 2.39
Table 4 shows the α-glucosidase inhibitory activity screening results of n-hexane, CH2Cl2, EtOAc, MeOH extracts, and acarbose as positive controls. The MeOH extract showed the highest activity with inhibition of 99.07 ± 0.50 % in concentration 10 mg mL−1 but not significantly different compared to acarbose as a positive control (t-test, p < 0.01). The EtOAc, CH2Cl2 and n-hexane extracts showed less inhibitory activity compared to acarbose in the same concentration but have a significant difference (t-test; p < 0.01). Several concentrations series of MeOH extract in the α-glucosidase enzyme inhibition produced an IC50 value of 49.57 ± 15.56 µg mL−1, although the value is weaker compared to acarbose with IC50 = 10.42 ± 2.39 µg mL−1. The results of α-Glucosidase inhibition from M. rufocostata stem bark extract are smaller when compared to other mangifera species, such as M. mekongensis and M. foetida. The n-hexane extract of M. mekongensis stem bark was able to inhibit the α-glucosidase enzyme with an IC50 of 1.71 µg/mL, while the MeOH extract of M. foetida had an IC50 of 13.49 mg/mL. Several compounds that were isolated from M. reba, and M. gedebe were also active in inhibiting α-glucosidase with IC50 28.5 to 162.8 mM, and 45.3 to 142.6 µM, respectively (Duong et al., 2017; Nguyen et al., 2021; Nguyen, Le, et al., 2016; Yusro et al., 2016). This shows that plants from the genus Mangifera have great potential as sources of phytopharmaceuticals.
This study is the first to report on the inhibitory activity of α-glucosidase in vitro by the stem bark extract of M. rufocostata. The results provide support for further investigations on the utilization of M. rufocostata stem bark extract as an antidiabetic therapy, through in vivo study and isolation of active compounds. Based on the screening results, MeOH extract showed the most potential with the highest inhibition. One therapy for treating type 2 DM is through the inhibitory activity of the α-glucosidase enzyme. The enzymes located on the border of the small intestine function to break down complex carbohydrates into glucose. When the α-glucosidase enzyme is inhibited, then the metabolism of complex carbohydrates will be delayed, thereby reducing glucose levels in the blood.
3.5 Cytotoxic activity
Cytotoxic activity of M. rufocostata stem bark was evaluated using the cell viability of the MTT method against three cancer cells, namely HT-29 (colon), HeLa (cervix), and MCF-7 (breast). The reason for selecting these three cancer cells in this research is based on literature studies which state that extracts from the other plants from genus mangifera, consist of M. indica and M. Pajang are toxic to colon, breast and cervical cancer cells line (Ahmad et al., 2015; Navarro et al., 2019). The MeOH extract of stem bark M. rufocostata was evaluated for its toxicity effect on these three cells and the test results are shown in Table 5.
Table 5 shows that the MeOH extract has less citotoxic against HeLa cancer cells with an IC50 value of 801.93 ± 0.02 µg/mL, less potential when compared to cisplatin as a positive control. This result is also less potent when compared with the cytotoxicity of MeOH extract of M. pajang stem bark against HeLa cells (>30 μg/mL) (Ahmad et al., 2015). However, it was more potent against HT-29 with an IC50 value of 0.25 ± 2.74 µg/mL. It was also more active than cisplatin as a positive control. This result is also more toxic when compared with the toxicity of MeOH extract of M.pajang stem bark against HT-29 (>30 μg/mL). These results are also very potential when compared with Cetuximab, the drug for colorectal cancer accepted by the FDA (Wu et al., 2022). Cetuximab toxicity test results against colon cancer cells (E705) with IC50 0.165 ± 0.047 µg/mL (Bovio et al., 2020). But, the MeOH extract did not potentially inhibit MCF-7 cells, as indicated by the high IC50 value, while the MeOH extracts of M. pajang and M. indica are toxic with IC50 > 30 and 15 µg/mL, respectively (Ahmad et al., 2015; Navarro et al., 2019).
Several investigations have been carried out on the development of effective source of anticancer drugs using plants with diverse chemical structures. This study shows that M. rufocostata has potential as a source of new phytopharmaceuticals. The toxycity of M. rufocostata methanol extract originates from the combination of the phenolic and flavonoid content. These compounds including mangiferin, gallic acid, and quercetin are commonly found in the Mangifera genus.
3.6 LC-MS/MS analysis
The compounds in the methanol extract of M. rufocostata stem bark were analyzed using LC-MS/MS. The total ion current chromatogram in negative ESI mode is displayed in Fig. 1, and the compounds tentatively detected are summarized in Table 6.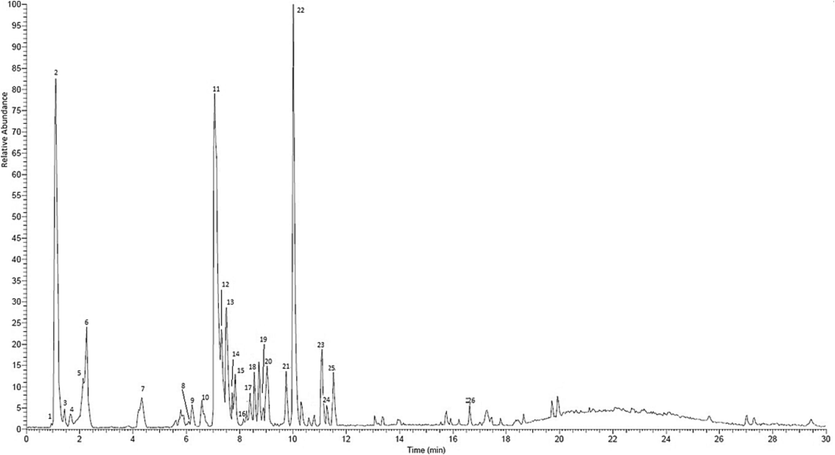
LC-MS/MS Chromatogram of methanol extract stem bark M. rufocostata in a negative ion mode.
No
TR (min)
Molecular formula
Major ion [M−H]- (m/z)
Calculate Molecular Weight
Tentative Assignment
1
1.513
C14H24O12
383.1268
384.12678
1-O-acetyl-alpha-maltose
2
1.522
C7H12O6
191.0626
192.06264
Quinic acid
3
1.529
C6H8O7
191.0267
192.02669
Citric acid
4
1.543
C4H6O5
133.0206
134.02057
Malic acid
5
2.088
C4H6O4
117.0257
118.02571
Methylmalonic acid
6
2.670
C7H6O5
169.0208
170.02075
Gallic acid
7
4.750
C7H6O4
153.0259
154.02589
Gentisic acid
9
6.305
C22H28N4O6
443.2000
444.19996
Mitoxantrone
10
6.650
C8H8O5
183.0367
184.03667
3.4-Dihydroxyphenyl glycolic acid
11
7.507
C19H18O11
421.0849
422.08494
Mangiferin
13
7.658
C20H22O10
421.1193
422.11925
Catechin 7-O-ß-D-xyloside
14
7.730
C11H14O8S
305.0411
306.04109
4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-sulphate
15
7.929
C20H20O11
435.1006
436.1006
Irisxanthone
16
8.150
C13H16O7
283.0898
284.0898
4-Methylphenyl-ß-D-glucopyranosiduronic acid
17
8.679
C8H8O4
167.0416
168.04162
5-Methoxysalicylic acid
18
8.970
C21H20O10
431.1061
432.10609
Apigetrin
19
9.149
C22H22O10
445.1215
446.12153
NP-018731
20
9.413
C19H16O10
403.0745
404.07451
Urolithin A-8-O-glucuronide
21
9.466
C22H22O10
445.1213
446.12153
Glycitin
22
10.450
C9H12O4
183.0728
184.07281
3-Methoxy-4-hydroxy-phenylglycol
23
11.495
C9H14O4
185.0886
186.08859
1-(Carboxymethyl) cyclohexane carboxylic acid
24
11.557
C15H10O8S
349.0098
350.00981
Apigenin 7-sulfate
25
11.940
C15H10O7
301.0428
302.0428
Quercetin
26
17.047
C18H2206
333.1418
334.14183
Adlerol
Based on the LC-MS/MS results, the dominant compounds present in the MeOH extract are mangiferin, quinic acid, gallic acid, 3-methoxy-4-hydroxy-phenyl glycol, as well as a quercetin compound. These compounds play a role in antioxidant, antidiabetic, and anticancer activities. According to previous studies, mangiferin is active as an anticancer (du Plessis-Stoman et al., 2011; Jung et al., 2012; Núñez Selles et al., 2016; Peng et al., 2004; Shoji et al., 2011), antidiabetic (Ganogpichayagrai et al., 2017; Kulkarni & Rathod, 2018; Muruganandan et al., 2005), while quinic acid also has bioactivity as an antioxidant, antidiabetic, and anticancer (Benali et al., 2022). Moreover, gallic acid is active as an antioxidant (Badhani et al., 2015), anticancer (Subramanian et al., 2015), and antidiabetic (Variya et al., 2020). Another polyphenol compound, quercetin, is also an antioxidant and anticancer agent (Baghel et al., 2012). Mangiferin, gallic acid, and quercetin compounds were also identified in other Mangifera species, such as M. indica (Gu et al., 2019; Ronchi et al., 2015) and M. pajang (Bakar et al., 2010 (a); 2010 (b); Prasad et al., 2011). Based on taxonomy, some species have close kinship relationships because they belong to the same genus.
The combination of several secondary metabolite compounds in the flavonoid and polyphenol groups such as mangiferin, gallic acid quinic acid and quercetin in the MeOH extract of M. rufocostata allows these compounds to jointly inhibit cancer cells. The ability of flavonoids and polyphenols to capture free radicals can help regulate cell metabolism and prevent diseases caused by oxidative stress. There is a lot of evidence showing that these two groups of compounds have anticancer activity, but the molecular mechanism is not completely clear. Cancer is a disease caused by uncontrolled cell proliferation processes, resulting in abnormal cell growth. Oxidative stress, and reduced apoptotic function are the main causes of internal cancer. Therefore, the ability of flavonoid and phenolic compounds to capture free radicals such as ROS can prevent cell damage (Kopustinskiene et al., 2020).
3.7 Correlation of TFC, TPC, antioxidant and antidiabetic activity
Pearson's test was used to determine the correlation between TFC, TPC, as well as the antioxidant and antidiabetic activities of M. rufocostata stem bark extract. As shown in Table 7, the total flavonoids positively correlated with total phenolics (r = 1.000, p < 0.01), meaning that the higher the TFC, the higher the TPC content. Both TFC and TPC play an important role in antioxidant activity as indicated by a strong positive correlation between TFC vs DPPH and TPC vs DPPH r = 0.998 (p < 0.01). There was also a strong positive correlation between TFC vs FRAP (r = 0.999; p < 0.01) as well as TPC vs FRAP with r = 0.998 (p < 0.01). Meanwhile, the correlation between TFC and TPC with ABTS was negative. The greater the flavonoid and phenolic content, the smaller the IC50 (ABTS) value and the more active the extract is as an antioxidant. The level of correlation obtained was moderate in TFC vs ABTS (r = -0.489; p < 0.01) and TPC vs ABTS with r = -0.482 (p < 0.01). Furthermore, DPPH, FRAP, and ABTS were strongly correlated with the inhibitory activity of α-glucosidase enzymes, with Pearson's coefficient (r) of 0.807, 0.838, and −0.830 (p < 0.01), respectively. This data shows that there is a correlation between antioxidant and antidiabetic activity as also reported by Wairata et al.,(2022). *Correlation is moderate at p < 0.01, ** correlation is strongly at p < 0.01.
TFC
TPC
DPPH
FRAP
ABTS
α-Glukosidase
TFC
1
TPC
1.000**
1
DPPH
0.998**
0.998**
1
FRAP
0.999**
0.998**
0.997**
1
ABTS
−0.489*
−0.482*
−0.475*
−0.507*
1
α-Glucosidase
0.822**
0.812**
0.807**
0.838**
−0.830**
1
A correlation between antioxidants and antidiabetes is possible, because as an antioxidant a plant can neutralize cell damage caused by reactive nitrogen and reactive oxygen species (ROS) such as singlet oxygen, free radicals and hydroperoxides. Cells damaged by free radicals appear to be the main cause of diabetes mellitus. Apart from that, it also has an impact on degenerative diseases such as cancer. Secondary metabolite compounds may help enzymes in the body to reduce free radicals.
4 Conclusions
This study showed that the methanol extract of M. rufocostata stems bark had the highest TFC and TPC content compared to other extracts. These results affected the highest antioxidant activity. The methanol extract inhibited the α-glucosidase enzyme, and strong cytotoxicity against HT-29 cells. Based on the LC/MS-MS results, the extract contains main compounds such as mangiferin, quinic acid, gallic acid, and 3-methoxy-4-hydroxy-phenyl glycol. This suggests that the methanol extract of M. rufocostata stem bark can be utilized as a source of biopharmaceutical in the future.
Acknowledgments
The authors would like to acknowledge the Directorate of Research and Community Service and Directorate General of Strengthening Research and Development, Ministry of Research, Technology and Higher Education of Republic Indonesia for the financial support for Doctoral Dissertation Research with contract number 1914/PKS/ITS/2023.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical composition, cellular antioxidant capacity and antiproliferative activity in mango (Mangifera indica L.) pulp and peel. Int. J. Food Sci. Technol.. 2017;52(3):817-826.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement. Altern. Med.. 2014;14(1):1-10.
- [CrossRef] [Google Scholar]
- Oxidative stress-mediated apoptosis induced by ethanolic mango seed extract in cultured estrogen receptor positive breast cancer MCF-7 cells. Int. J. Mol. Sci.. 2015;16(2):3528-3536.
- [CrossRef] [Google Scholar]
- Jacaranda flower (Jacaranda mimosifolia) as an alternative for antioxidant and antimicrobial use. Heliyon. 2020;6(12):e05802.
- [Google Scholar]
- Phytochemicals from Mangifera pajang Kosterm and their biological activities. BMC Complement. Altern. Med.. 2015;15(1):1-8.
- [Google Scholar]
- Ligand substituent effect on the cytotoxicity activity of two new copper (ii) complexes bearing 8-hydroxyquinoline derivatives: Validated by MTT assay and apoptosis in MCF-7 cancer cell line (human breast cancer) RSC Adv.. 2021;11(24):14362-14373.
- [CrossRef] [Google Scholar]
- Polyphenols from mango (Mangifera indica L.) modulate PI3K/AKT/mTOR-associated micro-RNAs and reduce inflammation in non-cancer and induce cell death in breast cancer cells. J. Funct. Foods. 2019;55:9-16.
- [CrossRef] [Google Scholar]
- Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv.. 2015;5(35):27540-27557.
- [CrossRef] [Google Scholar]
- A review of quercetin: antioxidant and anticancer properties. World J. Pharm. Pharma. Sci.. 2012;1(1):146-160.
- [Google Scholar]
- Cytotoxicity, cell cycle arrest, and apoptosis in breast cancer cell lines exposed to an extract of the seed kernel of Mangifera pajang (bambangan) Food Chem. Toxicol.. 2010;48(6):1688-1697.
- [CrossRef] [Google Scholar]
- Cytotoxicity and polyphenol diversity in selected parts of Mangifera pajang and Artocarpus odoratissimus fruits. Nutr. Food Sci. 2010
- [Google Scholar]
- Mango polyphenolics suppressed tumor growth in breast cancer xenografts in mice: Role of the PI3K/AKT pathway and associated microRNAs. Nutr. Res.. 2015;35(8):744-751.
- [CrossRef] [Google Scholar]
- Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev.. 2022;1–30
- [CrossRef] [Google Scholar]
- Bhowmik, A., Khan, L. A., Akhter, M., & Rokeya, B. (2009). Studies on the antidiabetic effects of Mangifera indica stem-barks and leaves on nondiabetic, type 1 and 2 diabetic model rats. ||| Bangladesh Journal of Pharmacology|||, 4(2), 110–114. https://doi.org/10.3329/bjp.v4i2.2488.
- Role of NEU3 overexpression in the prediction of efficacy of EGFR-targeted therapies in colon cancer cell lines. Int. J. Mol. Sci.. 2020;21(22):8805.
- [Google Scholar]
- Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol.. 2015;27:2377-2386.
- [Google Scholar]
- Phenolic profiles, antioxidant activities and enzyme inhibitory effects of an Algerian medicinal plant (Clematis cirrhosa L.) S. Afr. J. Bot.. 2020;132:164-170.
- [CrossRef] [Google Scholar]
- Phytochemical screening and evaluation of cytotoxic and hypoglycemic properties of Mangifera indica peels. Asian Pac. J. Trop. Biomed.. 2017;7(1):49-52.
- [CrossRef] [Google Scholar]
- In vitro and in vivo effects of mango pulp (Mangifera indica cv. Azucar) in colon carcinogenesis. Arch. Latinoam. Nutr.. 2014;64(1):16-23.
- [Google Scholar]
- Daglia, M., Di Lorenzo, A., F Nabavi, S., S Talas, Z., & M Nabavi, S. (2014). Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Current Pharmaceutical Biotechnology, 15(4), 362–372.
- Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem. Biol. Interact.. 2011;193(2):129-140.
- [CrossRef] [Google Scholar]
- Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. Afr. J. Trad. Complement. Altern. Med.. 2011;8(2)
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitors from the stem of Mangifera reba. Tetrahedron Lett.. 2017;58(23):2280-2283.
- [Google Scholar]
- Cytotoxic and apoptotic effects of the bark of two common mango (Mangifera indica) varieties from Sri Lanka on breast and ovarian cancer cells. Br. J. Pharma. Res.. 2016;10(2):1-7.
- [CrossRef] [Google Scholar]
- Impact of different solvents on extraction yield, phenolic composition, in vitro antioxidant and antibacterial activities of deseeded Opuntia stricta fruit. J. Umm Al-Qura Univ. Appl. Sci. 2023:1-9.
- [Google Scholar]
- Ganoderol B: A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18(12):1053-1055.
- [CrossRef] [Google Scholar]
- Selective antitumoural action of pressurized mango leaf extracts against minimally and highly invasive breast cancer. Food Funct.. 2017;8(10):3610-3620.
- [CrossRef] [Google Scholar]
- Fitmawati, F., Resida, E., Kholifah, S. N., Roza, R. M., Almurdani, M., & Emrizal, E. (2020). Phytochemical screening and antioxidant profiling of Sumatran wild mangoes (Mangifera spp.): a potential source for medicine antidegenerative effects. F1000Research, 9. https://doi.org/10.12688/f1000research.22380.3.
- Isolation of antioxidant compounds from Mangiferaindica L. leaves. IPTEK J. Proc. Ser.. 2016;2(1)
- [CrossRef] [Google Scholar]
- Antidiabetic and anticancer activities of Mangifera indica cv. Okrong leaves. J. Adv. Pharm. Technol. Res.. 2017;8(1):19.
- [CrossRef] [Google Scholar]
- Restorative activity of aqueous extract Mangifera indica leaves against CCl4 induced hepatic damage in rats. J. Pharm. Biomed. Anal.. 2019;164:112-118.
- [CrossRef] [Google Scholar]
- Purification and characterization of four benzophenone derivatives from Mangifera indica L. leaves and their antioxidant, immunosuppressive and α-glucosidase inhibitory activities. J. Funct. Foods. 2019;52:709-714.
- [CrossRef] [Google Scholar]
- Antidiabetic, cytotoxic and antioxidant activities of Rhodomyrtus tomentosa leaf extracts. RSC Adv.. 2022;12(39):25697-25710.
- [CrossRef] [Google Scholar]
- In vitro antioxidant activity of Mangifera indica leaf extracts. J. Plant Stud.. 2020;9(2)
- [CrossRef] [Google Scholar]
- Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res.. 2012;66(1):95-103.
- [CrossRef] [Google Scholar]
- The mangoes: their botany, nomenclature, horticulture and utilization. Academic Press; 1993.
- Kristiningrum, N., Wulandari, L., & Zuhriyah, A. (2018). Phytochemical screening, total phenolic content, and antioxidant activity of water, ethyl acetate, and n-hexane fractions from mistletoe moringa oleifera lam.(dendrophthoe pentandra (L.) Miq.). Dendrophthoe Pentandra, 104–106.
- Exploring the potential of Mangifera indica leaves extract versus mangiferin for therapeutic application. Agric. Nat. Resour.. 2018;52(2):155-161.
- [CrossRef] [Google Scholar]
- Kuntorini, E. M., Nugroho, L. H., Maryani, & Nuringtyas, T. R. (2022). Maturity effect on the antioxidant activity of leaves and fruits of Rhodomyrtus tomentosa (Aiton.) Hassk. AIMS Agriculture and Food, 7(2), 282–296. https://doi.org/10.3934/agrfood.2022018.
- Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J.. 2021;23:1-7.
- [Google Scholar]
- Anti-diabetic efficacy of young and mature leaf extract of Mangifera indica. Asian J. Trad. Med.. 2016;12(1):1-11.
- [Google Scholar]
- Anti-oxidant and anti-inflammatory activity of leaf extracts and fractions of Mangifera indica. Asian Pac. J. Trop. Med.. 2013;6(4):311-314.
- [CrossRef] [Google Scholar]
- Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol.. 2005;97(3):497-501.
- [CrossRef] [Google Scholar]
- Polyphenolic characterization, antioxidant, and cytotoxic activities of Mangifera indica cultivars from Costa Rica. Foods. 2019;8(9):384.
- [Google Scholar]
- Mechanism of action of Mangifera indica leaves for anti-diabetic activity. Sci. Pharm.. 2019;87(2):13.
- [CrossRef] [Google Scholar]
- Chemical constituents of Mangifera indica and their antiausterity activity against the PANC-1 Human pancreatic cancer cell line. J. Nat. Prod.. 2016;79(8):2053-2059.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitors from the bark of Mangifera mekongensis. Chem. Cent. J.. 2016;10(1):1-6.
- [Google Scholar]
- A new phenolic acid from the wood of Mangifera gedebe. Nat. Prod. Res.. 2021;35(15):2579-2582.
- [Google Scholar]
- Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem.. 2010;58(7):4104-4112.
- [CrossRef] [Google Scholar]
- The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors. 2016;42(5):475-491.
- [CrossRef] [Google Scholar]
- Antiinflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem-bark aqueous extract. Methods Find. Exp. Clin. Pharmacol.. 2005;27(8):547-554.
- [CrossRef] [Google Scholar]
- Antioxidant properties of fermented mango leaf extracts. J. Cosmet. Sci.. 2015;66(1):1-13.
- [Google Scholar]
- Peng, Z.-G., Luo, J., Xia, L.-H., Chen, Y., & Song, S.-J. (2004). CML cell line K562 cell apoptosis induced by mangiferin. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 12(5), 590–594. https://doi.org/15498116.
- Neoplastic transformation of BALB/3T3 cells and cell cycle of HL-60 cells are inhibited by mango (Mangifera indica L.) juice and mango juice extracts. J. Nutr.. 2006;136(5):1300-1304.
- [CrossRef] [Google Scholar]
- Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chem.. 2011;128(4):1121-1127.
- [Google Scholar]
- Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr. Cancer. 2007;60(1):120-130.
- [CrossRef] [Google Scholar]
- Identification of antioxidants in young mango leaves by LC-ABTS and LC-MS. Chiang Mai Univ. J. Nat. Sci.. 2014;13(3):317-330.
- [CrossRef] [Google Scholar]
- Potent chemopreventive effect of mangiferin on lung carcinogenesis in experimental Swiss albino mice. J. Cancer Res. Ther.. 2014;10(4):1033.
- [CrossRef] [Google Scholar]
- Chemotaxonomic study of Sumatran wild mangoes (Mangifera Spp.) based on liquid chromatography mass-spectrometry (Lc-Ms) SABRAO J. Breed. Genet.. 2021;53(1)
- [Google Scholar]
- Ronchi, S. N., Brasil, G. A., do Nascimento, A. M., de Lima, E. M., Scherer, R., Costa, H. B., Romão, W., Boëchat, G. A. P., Lenz, D., & Fronza, M. (2015). Phytochemical and in vitro and in vivo biological investigation on the antihypertensive activity of mango leaves (Mangifera indica L.). Therapeutic Advances in Cardiovascular Disease, 9(5), 244–256. https://doi.org/10.1177/ 1753944715572958.
- Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot.. 2019;120:129-134.
- [Google Scholar]
- Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem.. 2016;212:225-233.
- [CrossRef] [Google Scholar]
- Effect of extraction solvents/techniques on polyphenolic contents and antioxidant potential of the aerial parts of Nepeta leucophylla and the analysis of their phytoconstituents using RP-HPLC-DAD and GC-MS. RSC Adv.. 2016;6(81):78151-78160.
- [Google Scholar]
- A comparative study of effects of extraction solvents/techniques on percentage yield, polyhenolic composition, and antioxidant potential of various extracts obtained from stems of N epeta leucophylla: RP-HPLC-DAD assessment of its polyhenolic constituents. J. Food Biochem.. 2017;41(2):e12337.
- [Google Scholar]
- Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-κB in HL-60 cells. Arch. Pharm. Res.. 2011;34(3):469-475.
- [CrossRef] [Google Scholar]
- Isolated mangiferin and naringenin exert antidiabetic effect via PPARγ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact.. 2018;280:33-44.
- [CrossRef] [Google Scholar]
- Gallic acid: prospects and molecular mechanisms of its anticancer activity. RSC Adv.. 2015;5(45):35608-35621.
- [CrossRef] [Google Scholar]
- Penetapan Kadar Total Fenolik-Flavonoid Ekstrak Etanol 70% Kulit Batang Tandui (Mangifera rufocostata Kosterm.) Jurnal Ilmiah Manuntung. 2023;9(1):102-110.
- [Google Scholar]
- Mango seeds: A potential source for the isolation of bioactive compounds with anti-cancer activity. Int. J. Pharm. Pharma. Sci.. 2015;7(3):89-95.
- [Google Scholar]
- Keanekaragaman jenis, plasma nutfah, dan potensi buah-buahan asli Kalimantan. BioSmart. 2004;6(2):117-125.
- [Google Scholar]
- Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine. 2020;73:152906
- [CrossRef] [Google Scholar]
- Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King: A correlation study. Arab. J. Chem.. 2022;15(2):103541
- [CrossRef] [Google Scholar]
- Bioactivity of mango flesh and peel extracts on peroxisome proliferator-activated receptor γ [PPARγ] activation and MCF-7 cell proliferation: fraction and fruit variability. J. Food Sci.. 2011;76(1):H11-H18.
- [CrossRef] [Google Scholar]
- Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J. Hematol. Oncol.. 2022;15(1):1-63.
- [Google Scholar]
- Antioxidant and α-glucosidase inhibitory activities of eight neglected fruit extracts and UHPLC-MS/MS profile of the active extracts. Food Sci. Biotechnol.. 2021;30:195-208.
- [Google Scholar]
- Yusro, F., Ohtani, K., & Kubota, S. (2016). Inhibition of α-glucosidase by methanol extracts from wood bark of Anacardiaceae, Fabaceae, Malvaceae and Phyllanthaceae plants family in West Kalimantan, Indonesia.
- Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacol. Sin.. 2014;35(2):257-266.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105391.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







