Translate this page into:
Antiproliferative, genotoxic activities and quantification of extracts and cucurbitacin B obtained from Luffa operculata (L.) Cogn
⁎Corresponding author. natashagalucio@gmail.com (Natasha Costa da Rocha Galucio)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The medicinal plant Luffa operculata (L.) Cogn. has therapeutic properties in the treatment of sinusitis, rhinitis and abortifacient conditions. Ethnopharmacological studies report that the antitumor potential can be attributed to the presence of cucurbitacin-like compounds in the plant. This study consisted of measuring cucurbitacin in different L. operculata extracts, evaluating the antiproliferative and genotoxic activity of the extracts and the isolated substance in gastric cancer cells line, and evaluating the possible mechanism of action. The extracts were obtained by maceration, and both the acquisition of the chemical profile of the extracts and the determination of cucurbitacin were performed by high-performance liquid chromatography (HPLC). For the isolation of cucurbitacin B, column chromatography was used, and molecular identification was carried out by Nuclear Magnetic Resonance (NMR). The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay evaluated the antiproliferative activity, and the genotoxic activity was determined by the micronucleus method with cytokinesis blocking. The investigation of the possible mechanism of action was carried out by molecular docking. All tested samples caused cell death in a dose-dependent manner, but the fruit extracts were more selective for the ACP02 gastric cancer cells line than the isolated substance. The micronucleus results did not show that genomic instability reflects the greater cytotoxicity of the fruit ethanoic extract (EEF). In addition, the EEF proved to be the most selective for ACP02. The docking results showed that the isolated substance favorably inhibited the Janus kinase family proteins JAK1 and JAK2. The present work demonstrated that the use of ethanol extract can be a good alternative to fight gastric cancer.
Keywords
Cucurbitacin B
MTT
Micronucleus
Anticancer
1 Introduction
In Brazil, there are botanical records in the flora of Pará, Amazonas, Maranhão, Rio de Janeiro and Minas Gerais of Luffa operculata (L.) Cogn., which is an angiosperm and dicotyledonous plant of the Cucurbitaceae family. It is distributed throughout the Americas, and there are distribution records in Mexico, Venezuela, Costa Rica, El Salvador, Guatemala, Panama, Nicaragua, Peru, Ecuador, Colombia, Guyana, Suriname and Brazil (Tropicos.org, 2020). It is popularly known as buchinha, cabacinha, and buchinha-do-norte and is used in folk medicine to treat sinusitis and rhinitis as well as an abortifacient (Alves et al., 2018). Fruit extracts generally indicate the presence of flavonoids, tannins, saponins, steroids and/or triterpenoids (Brock et al., 2003).
The Cucurbitaceae family is rich in cucurbitacins, which belong to the class of triterpenes and comprise a wide class of natural products from the secondary metabolism of several organisms, among which plants stand out as their major producers. Due to the wide variety of triterpenes, they are grouped into subclasses according to similar structural characteristics, which leads to a better systematic structural chemical characterization (Silva et al., 2020). Cucurbitacins are characterized as triterpenoids with a tetracyclic nucleus of highly oxidized cucurbitan. They are well known for their bitterness and toxicity, in addition to having pharmacological activities such as antitumor, anti-inflammatory and hepatoprotective effects (Chen et al., 2005; Chen et al., 2012).
Curcubitacin B, a triterpene isolated from the plant, has shown good results of antitumor activity as it acts on proteins of the Janus kinase family, which are mainly expressed in tumor cells and has a direct relationship with the apoptosis of these cells when inhibited (Yar-Saglam et al., 2016).
Promising results for cancer treatment have been observed in research with medicinal plants. There is evidence of the antitumor activity of cucurbitacins in lung, liver, breast, and prostate cancer (Garg et al., 2018), and this anticancer activity occurs from several antiproliferative mechanisms, such as the inhibition of cell migration and invasion capacity, stimulation of death by apoptosis, promotion of cell cycle arrest and production of signaling pathway inhibitors (Cai et al., 2015).
In silico approaches have been used to investigate the interaction between compounds of natural origin and molecular targets of pharmacological interest (Santos et al., 2021; Oliveira et al., 2020; Araújo et al., 2020; Costa et al., 2020). Therefore, molecular docking was used to assess the interaction of cucurbitacin B with JAK1 and JAK2 proteins. These proteins were selected because they are related to the JAK/STAT pathway, which is associated with the promotion of cell life (Yar-Saglam et al., 2016; Garg et al., 2018). Studies reporting the anticancer activity of cucurbitacins in gastric cancer cells are considered insufficient (Zhang et al., 2018). Therefore, the aim of this study was to measure the major metabolites of L. operculata extracts, evaluate the antiproliferative activity of the extracts and the isolated substance in gastric, normal and cancer cells, and evaluate the genotoxic activity of the most promising extract and the isolated substance as well as its possible mechanism of action.
2 Materials and methods
2.1 Experimental procedures
2.1.1 Plant material and extraction procedure
The fruits of Luffa operculata (Fig. 1) were collected in Soure-PA, Brazil, Lat. 0°72′68″S, Log. 48°50′92″W, in the month of august 2016, and confirmation of botanical identification was performed by depositing a witness sample in the herbarium of the Brazilian Agricultural Research Corporation (EMBRAPA) under registration IAN194413. The fruits were washed with water, sanitized, and separated from the seeds. The materials were dried to a constant weight and then crushed.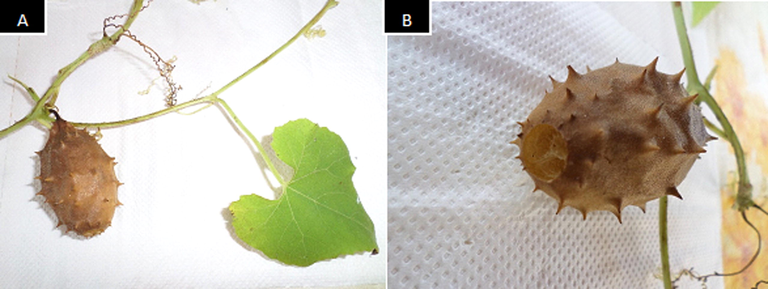
A: Fruit, leaf and stem with tendril of Luffa operculata; B: More detailed image of the fruit of L. operculata (fruit size: 3.0 × 5.5 cm).
The crushed fruits and seeds of L. operculata were macerated with 70% H2O:EtOH (3:7, water:ethanol, v/v) (Dynamics, BRA) and MeOH (methanol 100%) (Merck S/A, DE) for 144 h to obtain the extracts, which was performed three times. After the maceration period, simple filtration was performed, and ethanol and methanol solutions were obtained. The solutions were evaporated under reduced pressure in rotaevaporador (40 °C), from which methanol fruit extract (EMF), ethanol fruit extract (EEF), methanol seed extract (EMS) and ethanol seed extract (EES) were obtained.
2.1.2 Chromatographic profile of extracts
The extracts of L. operculata were analyzed by quaternary pump high-performance liquid chromatography (HPLC - Alliance e2695, Waters) using a C18 Sunfire™ chromatographic column (150 × 4.6 mm, 5 µm), injected with a 20 μL sample volume in a linear gradient elution of H2O:ACN (water Milli-Q® IQ 7003: acetonitrile Merck S/A, DE; v/v, 90:10 to 0:100) in 60 min a flow rate of 1.0 mL/min, and a photodiode array detector (PAD) length of 210–600 nm, and temperature of 40 °C.
2.1.3 Isolation of cucurbitacin B of the EEF extract
The EEF (500 mg) was subjected to exclusion chromatography on Sephadex LH-20 (Merck S/A, DE) using methanol (Merck S/A, DE) as the eluent in an isocratic system, resulting in fourteen fractions (1 to 14), which were analyzed by thin-layer chromatography (TLC) (Merck S/A, DE). Fraction 4 (0.150 g), which had the highest purity, was submitted to chromatographic separation by preparative HPLC-PAD Binary Pump 1525 (Waters) for isolation using a Sunfire octadecyl silane (C18) reversed-phase column (19 mm × 150 mm, 5 μm) in the isocratic mode of H2O:ACN (water: acetonitrile, v/v, 70:30) with a flow rate of 8 mL/min, and it was monitored at 230 nm at room temperature.
2.1.4 Identification of cucurbitacin B
The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Ascend 400 (400 MHz) spectrometer (Karlsruhe, DE) using the solvent signal (chlorophorme-d3) as a reference. The chemical shifts are given in delta (δ) values, and the coupling constants (J) are given in Hertz (Hz).
2.2 Quantification of cucurbitacin B
2.2.1 Standard solutions
Cucurbitacin B (CB) used in the preparation of the standard solutions was obtained from the EEF of Luffa operculata by HPLC isolation (item 2.1.3). The CB stock solution was diluted in acetonitrile in the linear range of 1 μg/mL to 100 μg/mL for the construction of the calibration curve.
2.2.2 Instrumentation and analytical conditions
The quantification analysis were carried out using a Alliance e2695 HPLC (Waters) equipped with a photodiode array detector (PAD) and a SunFire® C18 column (150 × 4.6 mm, 5 μm) as the stationary phase, a mobile phase of H2O:ACN (water: acetonitrile, v/v, 70:30) eluted isocratically for 15 min and detection at 230 nm, column heater at 40 °C.
2.2.3 Quantification of cucurbitacin B by HPLC
Quantifications of the CB in EMF and EEF extracts were performed by the standard curve method using the following equation: [CB, µg/mL]SAMPLE = PA(CB, SAMPLE) – b/δ, where PA(CB, SAMPLE) is area of the CB peak found in the extracts, b the linear coefficient and δ the angular coefficient of the standard analytical curve. In addition, the limits of detection (LD) and quantification (LQ) were calculated as follows: LD = 3 × SD/δ and LQ = 10 × SD/δ, where SD is the white standard deviation and δ the slope of the analytical curve. All analysis was performed in triplicate.
2.3 In vitro experiments
2.3.1 Cell culture
The gastric adenocarcinoma cell line ACP02 was established from a sample of a tumor removed from the cardiac region of the stomach from a cancer patient in northern Brazil (Leal et al., 2009), whereas MNP01 is a non gastric mucosa cell culture established from a sample of ten patients without gastric cancer or any other gastric disease (Maués et al., 2018). These cells were cultivated in DMEM/F12 medium (Dulbecco/F12 Modified Eagle Medium/Nutrient Mixture F-12, Gibco, Thermo Fisher Scientific, USA) supplemented with 10% FBS (Gibco, Thermo Fisher Scientific, USA) and penicillin (60 μg/mL) - streptomycin (100 μg/mL) (Gibco, Thermo Fisher Scientific, USA) in a humidified atmosphere at 37 °C under 5% CO2 (CO2 incubator Laboven, BRA) The experiments were carried out between the fourth and the tenth passage.
2.3.2 Cytotoxicity assay
Cytotoxicity was evaluated in cells lines ACP02 and MNP01 in the concentration range of 0.0019, 0.0039, 0.0078, 0.0156, 0.0312, 0.0625, 0.125 μg/mL for ethanol and methanol fruit extracts (EMF and EEF) and 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0 μg/mL for ethanol and metabolic seed extracts (EES and EMS); on the other hand, for the isolated substance, the range was from 0.0008, 0.0016, 0.0031, 0.0062 0.0125, 0.025, 0.050 μg/mL, as determined by the MTT colorimetric assay (Mosmann, 1983). Inhibitory concentrations that decreased cell viability by 50% (IC50%) at 24, 48 and 72 h (1x104, 0.6x104, 0.3x104 cells per well, respectively) were evaluated. The plates were incubated at 37 °C in a humid atmosphere with 5% CO2. Sample absorbances were measured in a SpectraMax i3 multi well scanning spectrophotometer (Molecular Devices, USA) using a reference wavelength of 570 nm. IC50% values were calculated using dose–response curves from three independent experiments.
2.3.3 Selectivity index (SI)
To calculate the selectivity index (SI), the IC50% of MNP01 was divided by the IC50% of ACP02 (Bézivin et al., 2003), according to the equation:
SI = IC50% MNP01 / IC50% ACP02
2.3.4 Cytokinesis-block micronucleus - CBMN
The MNP01 cell line was incubated with two concentrations of EEF (0.0022 μg/mL and 0.0011 μg/mL) and CB (0.0021 μg/mL and 0.0011 μg/mL). The ACP02 cell line was also incubated with two concentrations of EEF (0.0010 μg/mL and 0.0005 μg/mL) and CB (0.0027 μg/mL and 0.0014 μg/mL) that is, was used ½ IC50% and ¼ IC50% of 72 h. For the positive control (CP), 0.2 μg/mL doxorubicin (Sigma, USA) was used. After 24 h, 3 μg/mL cytochalasin-B (CitB; Sigma, USA) was added. The samples were treated with a hypotonic solution of trisodium citrate dihydrate 1% (Merck S/A, DE) and fixed with methanol (Merck S/A, DE) and acetic acid (Merck S/A, DE) at a proportion of 5:1 v/v with the addition of 37% formaldehyde (Merck S/A, DE). Later, the samples were fixed at a ratio of 3:1 v/v. Fixed samples were stained with 1% Giemsa (Merck S/A, DE) for 4 min. Finally, the analysis of the conventional micronucleus was performed using a Leica DME optical light microscope (Leica Microsystems Inc., USA) at 1000X magnification. The analysis was performed in a double-blind test with three independent experiments. (Fenech, 2000; Salvadori et al., 2003; Castro et al., 2021).
2.3.5 Statistical analysis
Analyzes were performed using the GraphPad Prism 5.0® software, the data were evaluated by the Shapiro-Wilk test and, in view of the result of this analysis, they were transformed to fit the normal curve. P values less than or equal to 0.05 were considered statistically significant. All tests were performed in triplicate. For cell viability assays (MTT), data analysis was performed using the percentage of inhibition × log of the concentration; thus, it was possible to determine the IC50% from nonlinear regression, followed by Tukey's test, with a significance level of 95% (p < 0.05). To compare the IC50% between the samples, the t test was used, and for comparisons of the selectivity index, two-way ANOVA followed by Bonferroni test for multiple comparisons was used. For the micronucleus assay, statistical analysis was performed using one-way ANOVA followed by Tukey's test for multiple comparisons.
2.4 In silico methodology
2.4.1 Molecular docking
The molecular structure of cucurbitacin B was designed using GaussiView 5 software (Dennington et al., 2009). Then, the molecule was optimized with B3LYP/6-31G* (Becke, 1993) using Gaussian 09 software (Frisch et al., 2009). To assess the interaction mode of cucurbitacin B with JAK1 and JAK2 proteins, the Molegro Virtual Docker (MVD) software was used (Thomsen and Christensen, 2006; Mascarenhas et al., 2020; Leão et al., 2020). For the construction of the complexes, the crystal structures of the proteins were collected in the Protein Data Bank using the PDB IDs 3EYH and 6VNK for JAK1 and JAK2 (Williams et al, 2009), respectively.
3 Results
3.1 Obtaining Luffa operculata
Maceration with organic solvents resulted in the methanol fruit extract (EMF; 1.167 g), ethanol fruit extract (EEF; 1.199 g), methanol seed extract (EMS; 2.771 g) and ethanol seed extract (EES;5.974 g). The four extracts presented the following yields: 11.67%, 11.99%, 2.77% and 5.97%.
3.2 HPLC chromatographic profile of extracts of Luffa operculata and isolation of cucurbitacin B
The chromatographic profiles of the analyzed EMF and EEF showed similar pattern. The band at a tR of 29.3 min (λmax: 230.1 nm) was observed for the extracts with high intensity, showing a predominant major compound in the EMF and EEF, which was identified as the triterpene cucurbitacin B (Fig. 2). For EMS and EES, no characteristic bands were detected for cucurbitacin B. Subsequently, the EEF was fractionated, and the compound cucurbitacin B was isolated by semi-preparative HPLC-PAD mode, giving 38.5 mg of a white crystalline solid (cucurbitacin B, λmax: 230.1 nm).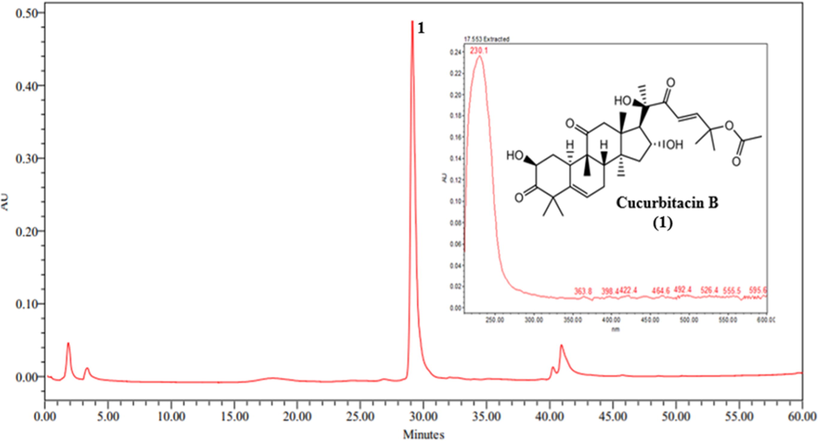
Chromatogram obtained by HPLC-PAD of the ethanol extract of the fruit of Luffa operculata. (1) cucurbitacin B.
3.3 Identification of cucurbitacin B
The compound cucurbitacin B (1) was isolated (38.5 mg) in the form of a white crystal soluble in chloroform and showed maximum absorption in UV–Vis at λmax 230.1 nm. The 1H NMR spectrum showed hydrogen signals typical of the triterpenic skeleton at δ H 2.39 (ddd, 2H, H-1), δ H 1.28 (s, 3H, H-28), δ H 1.37 (s, 3H, H- 29), δ H 2.38 (ddd, 1H, H-7a), δ HH 1.96 (ddd, 1H, H-7b) and δ H 2.00 (dd, 1H, H-8), referring to methyl hydrogens. Signals referring to oxymethine hydrogens were found at δ H 4.41 (dd, 1H, H-2); signals in the region of olefinic hydrogens were found at δ H 5.78 (d, 1H, H-5/H-6). The spectral pattern presented in the 1H NMR spectrum is typical of a substance from the class of cucurbitane triterpenes (Valente, 2004).
Through 13C NMR analysis, 32 signals referring to carbons attributed to the structure of (1) were observed, with three signals referring to C-3, C-11 and C-22 carbonyl carbons with respective chemical shifts of δ C 213.3, 212.1 and 202.3, with C-3 being adjacent to the OH group. Characteristic carbon signals of sp2-type C-5/C-6 with δ C 140.1 and 120.3 were also observed. There were also oxymethine carbon signals at δ C 71.6 (C-2), δ C 79.1 (C-20), δ C 71.0 (C-16) and δ C 78.1 (C-25). For the methyls at positions C-18, C-19, C-21, C-26, C-27, C-28, C-29, and C-30, the signals were found at δ C 19.7, 19.9, 23.5, 25.6 26.6, 29.1, 21.1 and 18.7, respectively. In addition, there were signals referring to methylene carbons at δ C 35.8 (C-1), δ C 50.2 (C-4), δ C 23.7 (C-7), δ C 48.3 (C-12), δ C 45.1 (C-15), δ C 120.1 (C-23) and δ C 151.8 (C-24). The 1H and 13C S1 NMR experimental data (Supplementary material Figure S1, Table S1) were compared with the literature and showed complete similarity for cucurbitacin B (Ayyad et al., 2011).
3.4 Quantification of cucurbitacin B by HPLC
HPLC analysis showed CB were present in the EMF and EEF extracts as compared their chromatograms (Supplementary material Figure S2). Thus, quantification of the CB in these extracts was performed in order to determine CB amount in µg/mL present in each extract studied. The data (Table 1) showed that CB are present in large amounts in the EMF and EEF of Luffa operculata, 44.2 µg/mL and 79.1 µg/mL, respectively. Furthermore, the adjustments obtained in the calibration curve (Fig. 3) showed satisfactory linearity in the working range with good limits of detection and quantification.
Curve equation
R2
LD
LQ
Range (µg/mL)
[CB, µg/mL]Extracts
(µg/mL)
(PA(CB), AU1) = 29216.4 [CB, µg/mL] + 22136.5
0.999
0.03
0.10
1.0 – 100
EMF
44.2 (±0.16)
EEF
79.1 (±0.57)
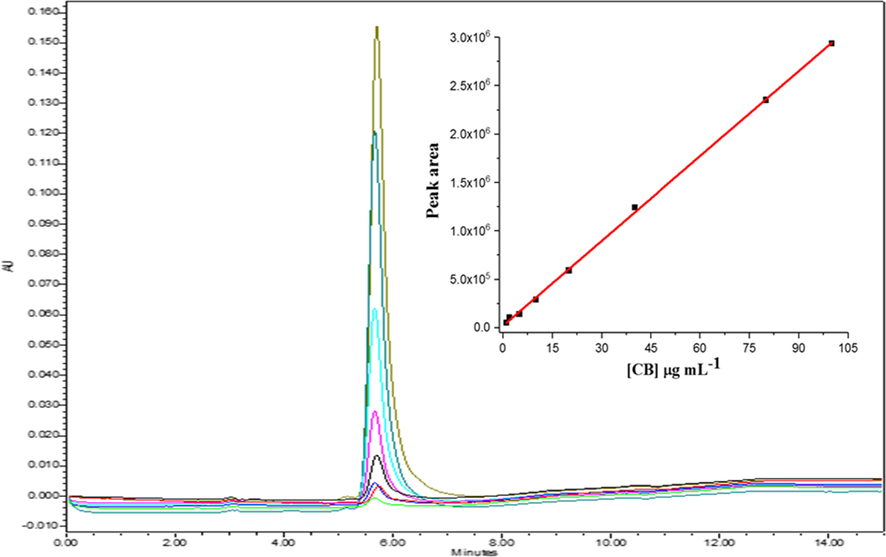
Analytical curve obtained for CB in the linear range from 1 to 100 µg/mL.
3.5 Cytotoxicity assay and selectivity index (SI)
The extracts of the fruits of Luffa operculata caused significant cell death in the two cell lines tested (Table 2) in a dose- and time-dependent manner. The EMF and EEF were more selective for the ACP02 cell line, demonstrating greater cytotoxicity at all times of treatment than the other extracts. The EEF (Table 2) showed a 50% lower IC in the 48 h exposure for both the MNP01 (0.00905 µg/mL) and ACP02 (0.00288 µg/mL), and in the 72 h period for the MNP01 (0.00444 µg/mL) and ACP02 (0.00210 µg/mL), compared to EMF in the same treatment period, thus demonstrating a more cytotoxic activity. Legend: EMF- methanol fruit extract; EEF- ethanol fruit extract; EMS- methanol seed extract; EES- ethanol seed extract; CB- Cucurbitacin B; IC50- inhibitory concentration 50%; SI- selective index. *p < 0.05 e R2 > 0.96.
Time
IC50% (µg/mL)
SI
MNP01
ACP02
EMF
24H
0.02229
0.00766
2.91
48H
0.01737
0.00498
3.49
72H
0.00930
0.00328
2.83
EEF
24H
0.01471
0.00802
1.83
48H
0.00905
0.00288
3.14
72H
0.00444
0.00210
2.11
EMS
24H
30.66
61.98
0.49
48H
15.71
22.49
0.70
72H
14.49
15.60
0.93
EES
24H
6.41
11.18
0.57
48H
5.06
8.69
0.58
72H
2.26
5.56
0.41
CB
24H
0.00978
0.00724
1.35
48H
0.00659
0.00618
1.07
72H
0.00427
0.00548
0.78
Cucurbitacin B (CB) isolated in this study caused cell death in a dose-dependent manner, as observed in L. operculata extracts, but no selectivity was observed for ACP02 (Table 2).
Statistically comparing the EEF IC50% values between the two cell lines, EEF showed significantly lower cytotoxicity for MNP01 and higher cytotoxicity for ACP02 (p < 0.05), but when comparing the IC50% CB values between the different cell types, it did not there was a statistically significant difference (p < 0.05), highlighting the biological activity of the extract, as the cytotoxic concentration was three times lower for the cancer cells than for the nonneoplastic cells. The selectivity index (SI) corroborates this observation (Supplementary material Figure S3; Figure S4), as the EEF presented a selectivity>3 for the 48 h treatment (SI = 3.14) (Bézivin et al., 2003).
3.6 Cytokinesis-block micronucleus
The micronucleus tests (MNs) showed a statistically significant difference for the two cell lines (MNP01 and ACP02) for all concentrations (of the extract and isolated substance) when compared to the positive control, and all induced a lower formation of MNs, as shown in Fig. 4. The results for EEF and CB showed that as the concentration increased, it induced a small increase in the number of MNs, but there was no statistically significant difference between different concentrations of EEF and CB.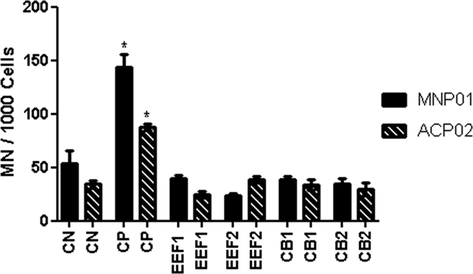
Frequency of micronuclei observed in cell lines MNP01 and ACP02. * Statistically significant compared to the positive control in both cell lines p < 0.05;
When comparing the results of MNs from the same samples (EEF and CB) in proportional concentrations, no statistically significant difference was observed in the different cells lines (MNP01 and ACP02) (p < 0.05).
Legend: EEF- ethanol fruit extract; CB- cucurbitacin B. The MNP01cell line was incubated with two concentrations of EEF1 = 0.0022 μg/mL, EE2 = 0.0011 μg/mL, CB1 = 0.0021 μg/mL and CB2 = 0.0011 μg/mL. In the ACP02 cell line, EEF1 = 0.0010 μg/mL, EEF2 = 0.0005 μg/mL, CB1 = 0.0027 μg/mL and CB2 = 0.0014 μg/mL.
Along with the frequency of micronuclei, the nuclear division index (NDI) was evaluated, but the results of EEF e CB were not statistically significant (p > 0.05; supplementary material Figure S5).
3.7 Molecular docking
The docking results showed that CB binds favorably, forming a stable complex with an affinity energy (Moldock Score) of −123.75 kJ/mol for JAK1, while JAK2 presented an affinity energy of −148.19 kJ/mol.
Fig. 5 shows the interactions between CB and JAK1, showing that the main bonds were hydrogen bonds with the amino acid residues Leu-959, Asp-1021, Glu-883, Gly-962, Ser-963 and Gly-884. Alkyl-type bonds were further visualized with Leu-1010, Leu-881, Ala-906, Val-938, Val-889 and Arg-1007. Fig. 6 shows the molecular interactions with JAK2, revealing hydrogen bonds with Lys-857, Ser-936, and Leu-932 and alky-like interactions with Leu-983, Val-911, Ala-880, Met-929, and Val-863. These results confirm that the substance binds to amino acids of interest for protein inhibition (Williams et al., 2009; Davis et al., 2021).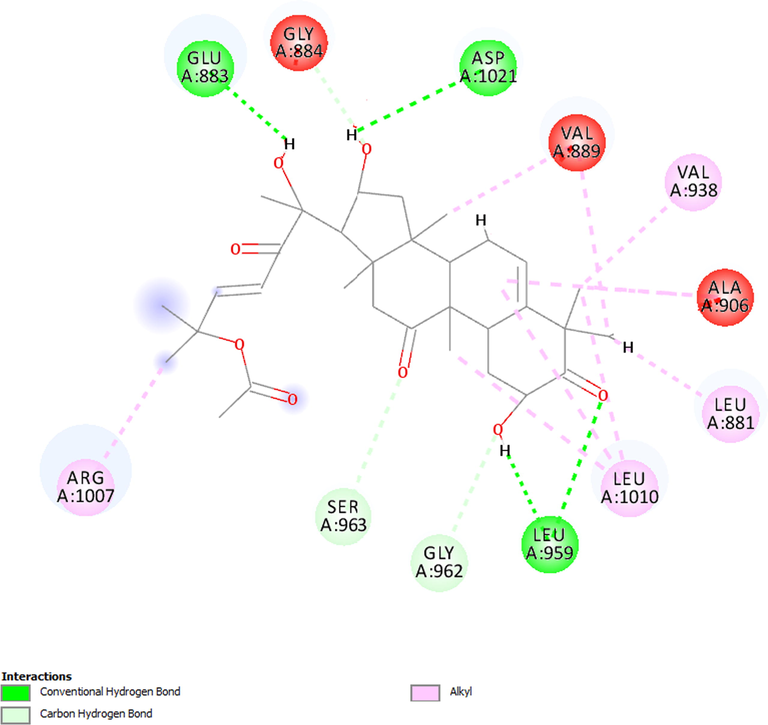
Molecular interactions established between cucurbitacin B and JAK1.
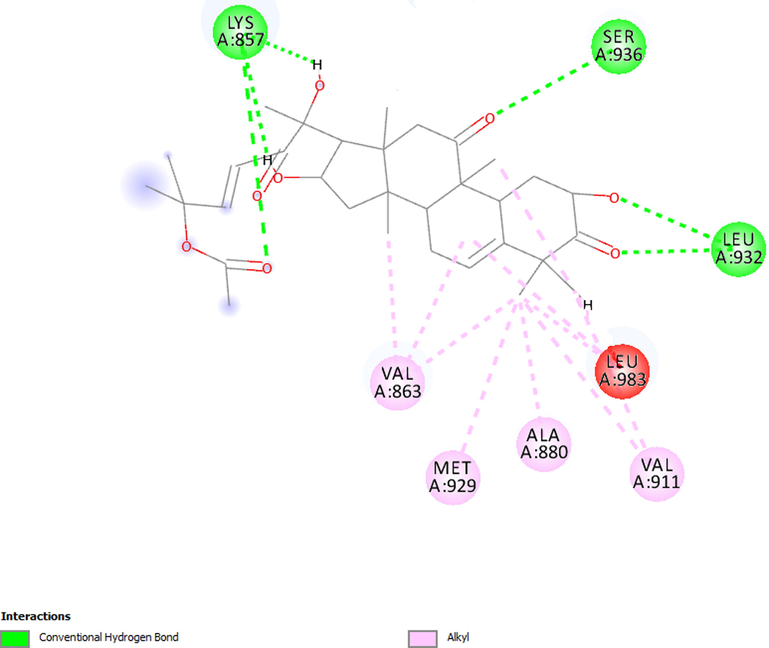
Molecular interactions established between cucurbitacin B and JAK2.
4 Discussion
The chemical compositions of the EMF and EEF analyzed by HPLC-PAD exhibited similarities, with both presenting chromatographic bands suggestive of compounds of the triterpene class, after isolation the majority compound was identified as CB by NMR.
In the EMS and EES extracts, no characteristic cucurbitacin B band was detected in the HPLC-PAD analyses; therefore, the Luffa operculata seed from the collected sample did not present in its chemical constitute, the cucurbitacin B triterpene, corroborating the findings of Lang et al. (2012). The choice of fruit extracts over seed extracts was due to the fact that the former presented peaks suggestive of cucurbitacin B, a substance of interest in this study.
A major band at a tR of 29.3 min, which in the UV spectrum (Fig. 2) has a λmax of 230.1 nm, was compared with literature data, and it is suggested that band 1 (tR: 29.3 min and λmax: 230.1 nm) contains cucurbitacin B (CB) this information was confirmed by NMR analysis (Krepsky et al., 2009; Ayyad et al., 2011; Kaushik et al., 2015). Furthermore, EMF and EEF presented band 1 as the major constituent.
Analyzed by the chromatographic profile, EMF and EEF showed CB contents of 79.1 and 44.5 µg/ml in the EEF and EMF, respectively. Therefore, ethanol is the solvent with the highest yield in the extraction of CB from Luffa operculata using the maceration technique, corroborating the findings by Kaushik et al. (2015) on the efficiency of ethanol as a CB extraction solvent, therefore, the EEF was used in the fractionation to obtain CB. Additionally, it is known that ethanol has greater safety for use in humans (Do et al., 2014; USP, 2007).
Given the good cytotoxicity results and the higher yield of CB in this extract, the EEF was selected to perform the cytokinesis-block micronucleus assay due to the good IS presented and taking into account that ethanol was used as an extraction solvent in the EEF. Therefore, from the fractionation of EEF and isolation of the CB substance, the cytotoxicity assay was continued, in addition to a later genotoxicity study.
Cytotoxicity assays are used to screen molecules (Adan et al., 2016). These assays are related to cell proliferative inhibition, and generally the most common type of cell death that occurs is the induction of apoptosis. Apoptosis, consisting of extrinsic and intrinsic pathways, is the main cell death response to chemotherapy (Chan et al., 2010). Other studies usually evaluate isolated substances, but this study chose to carry out a comparison between the precursor extract and the CB; with this, it was possible to discover greater selectivity in relation to the extract.
One of the characteristics of cancer cells is their great proliferative potential and several signaling pathways that act to promote the cell cycle; that is, these cells can adapt to high levels of oncogenic signaling, deactivating their senescence or apoptosis-inducing pathways (Hanahan and Weinberg, 2011). Therefore, substances that induce cell death by apoptosis and cell cycle arrest can be research targets for the treatment of cancer.
The cytotoxic potential induced by extracts containing cucurbitacins and isolated cucurbitacins has been widely researched in several cancer cell models, such as lung (H1975, H820, A549 and H1299) (Feng et al., 2014; Liu et al., 2019), cervical (CaSki and SiHa) (Sikander et al., 2016), breast (Dittharot et al., 2019), colon (SW480) (Feng et al., 2014), hepatocellular (HepG-2) (Ge et al., 2018) (Huh7) (Liu et al., 2020), prostate (He et al., 2017), ovarian (SKOV3) (Li et al., 2018), pancreatic (Sikander et al., 2019), murine intracranial glioma (GL261) (Fujita et al., 2008), mesenchymal glioblastoma multiforme (GBM) (Park et al., 2014) and leukemia (K562) (Chan et al., 2010). A study with CB and its synthesized derivatives evaluated their cytotoxicity in HepG-2 cells with IC50% values ranging from 0.033522 to 5.743436 µg/ml (Ge et al., 2018). In another study, the effect of CB on cell proliferation was evaluated in H1975, H820, A549 and H1299 cells, observing a cytotoxic effect for all four cell lines, with an IC50% value between 0.106153 and 2.363301 µg/ml (Liu et al., 2019). The IC50% results for cell types in these studies demonstrated much higher values than the results obtained in ACP02 and MNP01 (Table 2). This means that both the extract and CB induced greater cytotoxicity in the gastric cells studied, generating research potential promise for a possible chemotherapy treatment for gastric cancer.
A study (Liu et al., 2017) with gastric cancer cells (SGC7901 and SGC7901/DDP) resulted in high cytotoxicity with IC50% values of 0.095 and 0.121 µg/mL; however, the IC50% of the fruit extracts and cucurbitacins in the present study demonstrates a ten times greater cytotoxicity (Table 2). In another study, the antiproliferative effect of CB in the nonneoplastic gastric lineage (GES-1) and neoplastic gastric lines (MKN-74, BGC823, SGC7901 and MGC803) was compared, and the induction of cytotoxicity in neoplastic cells was observed with an IC50% of 0.0626 at 0.205 µg/mL, exhibiting a dose-dependent inhibitory effect and being more selective in cancer cells (Xu et al., 2020). Therefore, this study corroborates the findings of the current research, as greater selectivity was also observed in the gastric cancer cell line, but only in the EEF and EMF results. Another pathway associated with the cytotoxic activity of cucurbitacins is the inhibition of STAT3 phosphorylation, which impacts the low expression of genes such as c-Myc and Bcl-xL, resulting in the growth inhibition effect attributed to cell cycle arrest in the G2/M phase and induction of apoptosis (Chan et al., 2010; Xu et al., 2020).
The micronucleus results from unrepaired genomic damage, and it is known that damage such as this can cause cell cycle arrest as well as apoptosis and accumulate mutations in the genome (Tian et al., 2015; Sylvia et al., 2018). In the present study, the highest concentrations of EEF (MNP01: 0.0022 μg/mL and ACP02 0.0010 μg/mL) and CB (MNP01: 0.0021 μg/mL and ACP02 0.0027 μg/mL) induced a small increase in the formation of MNs in the two cell lines evaluated but without statistical significance. The results of MNs do not show that genomic instability reflects the greater cytotoxicity of EEF, it is believed that the concentrations tested were not sufficient to induce the formation of micronuclei, or the second hypothesis is that if a genomic instability was really induced, the Cell repair mechanisms were efficient before the new cell cycle was concluded, it is noteworthy that the micronucleus only forms if an irreparable DNA break occurs (Fenech, 2020). Also in this assay, the result of the nuclear division index did not show that EEF or CB had cytostatic activity at the concentrations tested.
For the docking study, the choice of target was based on the fact that previous studies stated that CB has the ability to inhibit proteins from the Janus kinase family (Yar-Saglam et al., 2016). Alghasham (2013) reported that cucurbitacins can induce apoptosis of a series of human cancer cells, such as leukemia, lymphomas, liver, breast, lung, pancreas and colon, and this effect is possibly linked to the fact that this molecule inhibits the phosphorylation of JAK2 and consequently activation of the apoptosis cascade and cell cycle arrest.
It is known that proteins from the Janus kinase family are responsible for activating STAT3 before this protein successfully binds to the receptor, and phosphorylated STAT3, when migrating to the cell nucleus, has the ability to activate the transcription of target genes. There is at least ten times more activated STAT3 in gastric cancer cells than in normal stomach epithelial cells (Deng et al, 2010), which supports the claim that the phosphorylation of STAT3 is associated with promoting the survival of cancer cells and the inhibition of apoptosis (Liu et al., 2015).
The results show that CB was able to inhibit the growth of gastric lines with a better result in 48 h, possibly because the pharmacological effect is due to the inhibition of these proteins, since in the docking results, CB was able to interact favorably with JAK1 and JAK2, being able to form bonds with the amino acid residues of interest in the catalytic site. This type of interaction can prevent JAK from having the ability to autophosphorylate, thus causing JAK inhibition, which would prevent STAT3 activation through this pathway (JAK/STAT) (Yar-Saglam et al., 2016). It must also be taken into account that CB belongs to the class of triterpenes, and as such, it has high lipophilicity (Garg et al., 2018), which gives the molecules an ability to passively cross membrane barriers, thus acting in intracellular complexes (Lipinski, 2004).
It is interesting to note that the isolation of CB from the EEF did not generate an important selective effect on the cytotoxicity, which reinforces the idea that crude extracts can often be more effective than their chemical components isolated at an equivalent dose. A possible explanation for this more effective result would be the hypothesis of synergy, that is, other compounds in the extract, despite not being the majority, help the substance with a specific pharmacological effect to reach the target, either by improving solubility, absorption, and distribution or decreasing metabolism and excretion, thereby increasing the bioavailability and effectiveness of this extract (Rasoanaivo et al., 2011; Caesar and Cech, 2019).
5 Conclusions
The phytochemical study of Luffa operculata resulted in the isolation and identification of cucurbitacin B, whose assay showed a higher concentration in the EEF, being the major chemical compound. Regarding the cytotoxicity index in the cell lines tested, the EEF result was more promising (p < 0.05) for antiproliferative activity in the cancer cells due to a selectivity index of 3.14. CB had no significant selectivity index in the cells of this study. The trial to assess the formation of micronucleus showed that the EEF and CB results were not significant, so their cytotoxicity could not be associated with genomic instability. It is suggested that CB is the pharmacological marker of L. operculata, and its possible mechanism of action is also related to the inhibition of JAK1 and JAK2 proteins that are associated with the JAK/STAT pathway and related to apoptosis. Given these findings, the EEF, which is rich in cucurbitacin, is considered promising for further studies aimed at the development of a herbal medicine with potential clinical applicability.
Acknowledgements
This study was financed in part by the Coordination of Superior Level Staff Improvement (CAPES), National Council for Scientific and Technological Development (CNPq) and PROPESP-UFPA. To the Human Cytogenetics Laboratory of the Federal University of Pará, for the donation of cell lines ACP02 and MNP01.
References
- Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol.. 2016;17(14):1213-1221.
- [CrossRef] [Google Scholar]
- Cucurbitacins - a promising target for cancer therapy. Int. J. Health Sci. (Qassim).. 2013;7(1):77-89.
- [CrossRef] [Google Scholar]
- Luffa operculata fruit aqueous extract induces motor impairments, anxiety-like behavior, and testis damage in rats. J. Ethnopharmacol.. 2018;222:52-60.
- [CrossRef] [Google Scholar]
- Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules. 2020;25:4183.
- [CrossRef] [Google Scholar]
- Cucurbitacins-type triterpene with potent activity on mouse embryonic fibroblast from Cucumis prophetarum, Cucurbitaceae. Pharmacognosy Res.. 2011;3(3):189-193.
- [CrossRef] [Google Scholar]
- Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys.. 1993;98:5648-5652.
- [CrossRef] [Google Scholar]
- Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomed. Int. J. Phytotherapy Phytopharmacol.. 2003;10(6–7):499-503.
- [CrossRef] [Google Scholar]
- Estudo morfo-anatômico e abordagem fitoquímica de frutos e sementes de Luffa operculata (l.) Cogn., Cucurbitaceae. Visão Acadêmica.. 2003;4(1):31-37.
- [CrossRef] [Google Scholar]
- Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep.. 2019;36(6):869-888.
- [CrossRef] [Google Scholar]
- Cai, Y., Fang, X., He, C., Li, P., Xiao, F., Wang, Y., 2015. Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chinese Medicine. 43(7), 1331–1350. https://doi.org/10.1142/S0192415X15500755
- Castro, A.L.G., Cruz, J.N., Sodré, D,F., Correa-Barbosa, J., Azonsivo, R., Oliveira, M.S., Siqueira, J.E.S., Galucio, N.C.R., Bahia, M.O., Burbano, R.M.R., Marinho, A.M.R., Percario, S., Dolabela, M.F., Vale, V.V., 2021. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. using bioassays and in silico approaches. Arabian Journal of Chemistry. 14, 103084. https://doi.org/10.1016/j.arabjc.2021.103084
- Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett.. 2010;289(1):46-52.
- [CrossRef] [Google Scholar]
- Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep.. 2005;22(3):386-399.
- [CrossRef] [Google Scholar]
- Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs. 2012;23(8):777-787.
- [CrossRef] [Google Scholar]
- Chemometric methods in antimalarial drug design from 1,2,4,5-tetraoxanes analogues. SAR QSAR Environ. Res.. 2020;31(9):677-695.
- [CrossRef] [Google Scholar]
- Structural Insights into JAK2 Inhibition by Ruxolitinib, Fedratinib, and Derivatives Thereof. J. Med. Chem.. 2021;25;64(4):2228-2241.
- [CrossRef] [Google Scholar]
- STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J. Gastroenterol.. 2010;16(42):5380-5387.
- [CrossRef] [Google Scholar]
- Dennington, R., Keith, T., Millam, J., 2009. GaussView, Version 5. Semichem Inc. Shawnee Mission. KS.
- Dennington, R., Keith, T., Millam, J., 2009. GaussView, Version 5., Semichem Inc. , Shawnee Mission. KS. Semichem Inc.
- Dittharot, K., Dakeng, S., Suebsakwong, P., Suksamrarn, A., Patmasiriwat, P., Promkan, M., 2019. Cucurbitacin B Induces Hypermethylation of Oncogenes in Breast Cancer Cells. Planta medica. 85(5), 370–378. https://doi.org/10.1055/a-0791-1591
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22(3):296-302.
- [CrossRef] [Google Scholar]
- Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes. 2020;11(10):1203.
- [CrossRef] [Google Scholar]
- Cucurbitacin-E inhibits multiple cancer cells proliferation through attenuation of Wnt/β-catenin signaling. Cancer Biother. Radiopharm.. 2014;29(5):210-214.
- [CrossRef] [Google Scholar]
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K.A., Rendell, K., Burant, J.C. S, Iyengar, S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L. Morokuma, K., Zakrzewski, V.G., Voth, A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J., Gaussian, D.J., Bloino, J., Honda, Y., Kudin, K.N., Stratmann, R.E., Voth, G.A. 2009. Gaussian 09, 2–3.
- Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J. Immunol.. 2008;180(4):2089-2098.
- [CrossRef] [Google Scholar]
- Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review) Int. J. Oncol.. 2018;52(1):19-37.
- [CrossRef] [Google Scholar]
- Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents. Molecules (Basel, Switzerland).. 2018;23(12):3345.
- [CrossRef] [Google Scholar]
- Cucurbitacin E induces apoptosis of human prostate cancer cells via cofilin-1 and mTORC1. Oncology letters.. 2017;13(6):4905-4910.
- [CrossRef] [Google Scholar]
- Cucurbitacins - An insight into medicinal leads from nature. Pharmacogn. Rev.. 2015;9(17):12-18.
- [CrossRef] [Google Scholar]
- High performance liquid chromatography determination of cucurbitacins in the roots of Wilbrandia ebracteata Cogn. Revista Brasileira de Farmacognosia. 2009;19(3):715-719.
- [CrossRef] [Google Scholar]
- Synthesis and cytotoxic activity evaluation of dihydrocucurbitacin B and cucurbitacin B derivatives. Química Bioorg. Med.. 2012;20(9):3016-3030.
- [CrossRef] [Google Scholar]
- Establishment and conventional cytogenetic characterization of three gastric cancer cell lines. Cancer Genet. Cytogenet.. 2009;195(1):85-91.
- [CrossRef] [Google Scholar]
- Identification of New Rofecoxib-Based Cyclooxygenase-2 Inhibitors: A Bioinformatics Approach. Pharmaceuticals (Basel). 2020;26:209.
- [CrossRef] [Google Scholar]
- Cucurbitacin I induces cancer cell death through the endoplasmic reticulum stress pathway. J. Cell. Biochem.. 2018;1–13:27570.
- [CrossRef] [Google Scholar]
- Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol.. 2004;1(4):337-341.
- [CrossRef] [Google Scholar]
- Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules (Basel, Switzerland).. 2019;24(3):647.
- [CrossRef] [Google Scholar]
- Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol. Rep.. 2017;38(1):271-278.
- [CrossRef] [Google Scholar]
- Cucurbitacin E Inhibits Huh7 Hepatoma Carcinoma Cell Proliferation and Metastasis via Suppressing MAPKs and JAK/STAT3 Pathways. Molecules (Basel, Switzerland).. 2020;25(3):560.
- [CrossRef] [Google Scholar]
- Ponicidin induces apoptosis via JAK2 and STAT3 signaling pathways in gastric carcinoma. Int. J. Mol. Sci.. 2015;16(1):1576-1589.
- [CrossRef] [Google Scholar]
- Pharmacophore-based virtual screening and molecular docking to identify promising dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. J. Biomol. Struct. Dyn.. 2020;1–10
- [CrossRef] [Google Scholar]
- Gastric Cancer Cell Lines Have Different MYC-Regulated Expression Patterns but Share a Common Core of Altered Genes. Can J Gastroenterol Hepatol.. 2018;2018:5804376.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [CrossRef] [Google Scholar]
- Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules. 2020;25(17):3852.
- [CrossRef] [Google Scholar]
- Park, J.B., Agnihotri, S., Golbourn, B., Bertrand, K.C., Luck, A., Sabha, N., Smith, C.A., Byron, S., Zadeh, G., Croul, S., Berens, M., Rutka, J. T., 2014. Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget, 2014, 5(19), 9382–9395. https://doi.org/10.18632/oncotarget.2412.
- Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar. J.. 2011;10(1):S4.
- [CrossRef] [Google Scholar]
- Salvadori, D.M.F., Ribeiro, L.R., Fenech, M. 2003. Teste do Micronúcleo em células humanas in vitro, in: Ribeiro, L.R., Salvadori, D.M.F., Marques, E.K., Mutagênese Ambiental. Ed. ULBRA, Canoas, RS, Brazil, 201-210.
- Molecular modeling approaches of selective adenosine receptor type 2A agonists as potential anti-inflammatory drugs. J. Biomol. Struct. Dyn.. 2021;39(9):3115-3127.
- [CrossRef] [Google Scholar]
- Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci. Rep.. 2016;6:36594.
- [CrossRef] [Google Scholar]
- Novel Mechanistic Insight into the Anticancer Activity of Cucurbitacin D against Pancreatic Cancer (Cuc D Attenuates Pancreatic Cancer) Cells.. 2019;9(1):103.
- [CrossRef] [Google Scholar]
- Bioativities of plant-isolated Triterpenes: A brief review. Revista Virtual de Química.. 2020;12:234-247.
- [CrossRef] [Google Scholar]
- Micronucleus Study on Breast Cytology Aspirate Smears and its Diagnostic Utility. J Cytol.. 2018;35(1):22-26.
- [CrossRef] [Google Scholar]
- MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem.. 2006;49:3315-3321.
- [CrossRef] [Google Scholar]
- DNA damage response–a double-edged sword in cancer prevention and cancer therapy. Cancer Lett.. 2015;358(1):8-16.
- [CrossRef] [Google Scholar]
- Tropicos.org., 2020. Missouri Botanical Gardem. http://www.tropicos.org/Name/9200612 (accessed 11 june 2020)
- USP 30-NF 25, 2007. General Chapter USP <467> Residual Solvents / Organic volatile impurities, United States Pharmacopeia. Pharmacopoeia Convention Inc., Rockville, MD, USA, 1-10. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/generalChapter467Current.pdf (accessed 03 October 2021).
- Cucurbitacinas e suas principais características estruturais. Quím. Nova.. 2004;27(6):944-948.
- [CrossRef] [Google Scholar]
- Dissecting specificity in the Janus kinases: the structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J. Mol. Biol.. 2009;20;387(1):219-232.
- [CrossRef] [Google Scholar]
- Cucurbitacin B inhibits gastric cancer progression by suppressing STAT3 activity. Arch. Biochem. Biophys.. 2020;684:108314.
- [CrossRef] [Google Scholar]
- Treatment with cucurbitacin B alone and in combination with gefitinib induces cell cycle inhibition and apoptosis via EGFR and JAK/STAT pathway in human colorectal cancer cell lines. Hum. Exp. Toxicol.. 2016;35(5):526-543.
- [CrossRef] [Google Scholar]
- Zhang, Y. Z., Wang, C. F., Zhang, L. F., 2018. Cucurbitacin D impedes gastric cancer cell survival via activation of the iNOS/NO and inhibition of the Akt signalling pathway. Oncology reports. 39(6), 2595–2603. https://doi.org/10.3892/or.2018.6361
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103589.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







