Translate this page into:
Antitubercular and antioxidant activities of hydroxy and chloro substituted chalcone analogues: Synthesis, biological and computational studies
⁎Corresponding authors at: University College of Pharmaceutical Sciences, Acharya Nagarjuna University, Guntur, Andhra Pradesh, India (S. Ammaji). Department of Pharmaceutical Sciences, College of Pharmacy & Health Sciences, Ajman University, Ajman, PO Box 346, United Arab Emirates (R.R. Bhandare). shaik.ammaji8@gmail.com (Shaik Ammaji), r.bhandareh@ajman.ac.ae (Richie R. Bhandare), bashafoye@gmail.com (Afzal Basha Shaik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of chalcone analogues (1–15) were synthesized by Claisen-Schmidt condensation in good yields (70–95%) and characterized by FT-IR, 1H NMR and mass spectral methods. Additionally, compounds 3 and 7 were characterized by 13C NMR. Antitubercular and antioxidant activities of the chalcones were evaluated by MABA and DPPH free radical assays. In MABA assay analogues 3 (MIC = 14 ± 0.11 µM) and 11 (MIC = 14 ± 0.17 µM) bearing fluorine and methoxy groups at para and meta positions were 1.8-times more active than the standard pyrazinamide (MIC = 25.34 ± 0.22 µM). The chalcone analogues such as compound 7 (IC50 = 4 ± 1 µg/mL) containing electron releasing groups such as —OH at ortho position had slightly more antioxidant activity than Gallic acid (IC50 = 5 ± 1 µg/mL). The potential compounds 3, 7, 9 and 11 were less selective and toxic against human live cell lines-LO2. Further, molecular docking results of chalcones against anti-tubercular drug target isocitrate lyase (PDB ID: 1F8M) revealed that compound 3 and 11 shown least binding energies as −7.6, and −7.5 kcal/mol are in line with in vitro MABA assay, suggesting that these compounds 3 and 11 are strong inhibitor of isocitrate lyase. SwissADME programme estimated the drug likeliness properties of compounds 3, 7, 9 and 11. The lead molecules arisen through this study helps to develop new antitubercular and antioxidant agents.
Keywords
Hydroxy chalcones
Chlorinated chalcones
Antitubercular activity
Antioxidant activity
Autodock
SwissADME
- TB
-
Tuberculosis
- 1H NMR
-
1H Nuclear Magnetic Resonance
- LC-MS
-
Liquid Chromatography- Mass Spectrometry
- 13C NMR
-
13C Nuclear Magnetic Resonance
- TLC
-
Thin Layer Chromatography
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
Abbreviation
1 Introduction
Tuberculosis (TB) is a highly infectious airborne illness caused by the bacteria Mycobacterium tuberculosis (M.tb) which consistently affects the lungs. TB continues to pose a major health concern impacting the public even though research on the bacteria began more than a century ago (Singh et al., 2020). According to the World Health Organization (WHO) 2019 statistics, TB kills 1.4 million people each year, with 208,000 of them having HIV. In recent years, however, TB mortality has decreased as a result of better diagnosis and treatment. For example, it is estimated that between 2000 and 2019, 60 million persons were saved from death (Global Tuberculosis Report, 2020). Treatment of TB is usually initiated with a four-drug regimen of Isoniazid, Rifampin, Pyrazinamide plus Ethambutol or Streptomycin (Nolan and Goldberg, 2002). However there exists serious challenges to current treatment regimens in the form of Multidrug-resistant tuberculosis (MDR-TB), extensive drug-resistant tuberculosis (XDR-TB), and, in rare instances, fully drug-resistant tuberculosis (TDR-TB). Furthermore, the protracted and multi-drug therapy regimen has a significant disadvantage in TB patients owing to high toxicity and susceptibility. This required for more research into new molecular scaffolds that may tackle these difficulties with minimal negative effects (Chauhan et al., 2021). Researchers have evaluated several bioactive compounds in this endeavor, with chalcones emerging as one of the viable options for the discovery and development of drugs against TB.
Chalcones are a class of open-chain flavonoids containing 15 carbon framework (C6-C3-C6) with two six membered aromatic rings connected by an three carbon α,-unsaturated carbonyl skeleton (Fig. 1).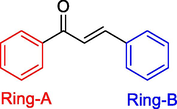
General structure of chalcone with 15 carbon (C6-C3-C6) arrangement.
Naturally available chalcones are biosynthesized from malonyl-CoA (three molecules) and p-coumaroyl-CoA (one molecule) and this reaction is catalyzed by an enzyme chalcone synthase (Ferrer et al., 1999). It is a privileged structure which can be conveniently synthesized in the laboratory by the classical Claisen-Schmidt condensation reaction (Claisen and Claparède, 1881). This class of compounds have been demonstrated to have antibacterial (Nowakowska 2007, Sashidhara et al., 2015, Zhang et al., 2018, Mahapatra et al., 2015a,b, Dan and Dai 2020), antifungal (Shaik et al., 2020, Srivastava and Pandey 2019, Lagu et al., 2020, Lokesh et al., 2017, Konidala et al., 2021, Ashburn, 2019, Sissouma et al., 2015), anti-tubercular (Kumar et al., 2020, Kishor et al., 2017, Anandam et al., 2018, Mujahid et al., 2015, Ticha et al., 2015, Shaik et al., 2019, Ramesh et al., 2020, Lagu et al., 2019, Shukla et al., 2017, Kasetti et al., 2021), cytotoxic and anti-proliferative or anticancer (Mahapatra et al., 2015a,b, Shaik et al., 2015, Karthikeyan et al., 2015, Pande et al., 2017, Li et al., 2019, Ng et al., 2017, Custodio et al., 2020, Yamali et al., 2016), and antioxidant (Niu et al., 2017, Díaz-Rubio et al., 2019, Kostopoulou et al., 2021, Sökmen and Khan, 2016, Wang et al., 2015, Xue et al., 2018, Vásquez-Martínez et al., 2019, Bhale et al., 2017) properties. The α, β-unsaturated carbonyl moiety of chalcones is the most significant structural component responsible for their qualitative activity, whilst the substituents on the two aryl rings are responsible for the intensity and range of pharmacological activities (Yazdan et al., 2015; Fig. 2)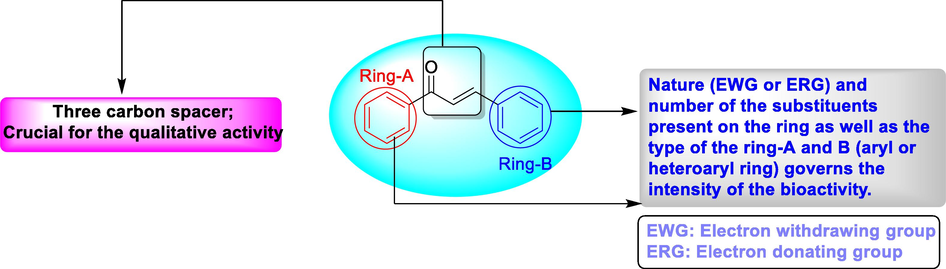
Structure of chalcone showing the importance of different structural fragments.
Hydroxy substituted chalcones including Isobavachalcone, Xanthohumol, butein and naringenin chalcone possess antioxidant activity (Stepanić et al., 2019). The chalcones like licochalcone A, (E)-3″,2′,4′-Trihydroxy-3′-methoxychalcone and (E)-2′,4′-Dihydroxychalcone isolated from Glycyrrhiza inflata, Galenia africana and Empetrum nigrum showed potential antitubercular activity with MIC values 7.1 µg/mL, 174.80 µM and 23.8 µM respectively (Friis-Møller et al., 2002, Mativandlela et al., 2009, Li et al., 2015). Similarly, chlorine containing chalcones were also reported with excellent anti-tubercular and antioxidant activities (Shaik et al., 2020, Kishor et al., 2017, Shaik et al., 2019, Kasetti et al., 2021, Biradar et al., 2010) (Fig. 3). Antioxidants have long been known to help reduce cancer, cardiovascular and neurological diseases that arise due to oxidative stress. However, recent studies have shown their significance in infectious disease development and control. The virulence and pathogenicity of pathogens and their metabolites is established by producing free radicals. Antioxidants either obtained through diet or supplemented externally help to alter the resistance of host to infections in many ways. Antioxidants can benefit in fighting infectious diseases not only by counteracting the deleterious effects of the reactive chemical species but also help in sustaining healthy immunological cells. The phenolic compounds and flavonoids present in the diet possess antimicrobial activity due to their capability to inhibit different kinds of enzymes and physiological processes in the microorganisms (Knight 2000, Kaur et al., 2018, Aibana et al., 2017). Taking the above facts into consideration as well as the presence of phenolic —OH and open-chain flavonoid (Chalcone) structure in our target molecules we predetermined to study the antioxidant activity of the target compounds. The biological properties of chalcones are also attributed due to their antioxidant nature (Machala et al., 2001). Chalcones exert antioxidant activity by scavenging reactive oxygen species (ROS) or free radicals directly or by inhibiting the enzyme aldose reductase (ALR2), resulting in anti-oxidative and anti-inflammatory effects (Kucerova-Chlupacova et al., 2018). On the other hand, inhibition of Mycobacterium tuberculosis protein tyrosine phosphatases (PtpA and PtpB) (Mascarello et al., 2010), 2-trans-enoyl-acyl carrier protein reductase (InhA) (Anagani et al., 2020), Fatty acid synthase type-II (FAS-II) (Shaik et al., 2019) or Decaprenyl-phosphoribose 2′-epimerase (DprE1) enzymes (Yalcin et al., 2018) may be responsible for their antitubercular effect. The presence of —OH group on the aryl rings of chalcones is essential for their antioxidant and anti-tubercular activities. In silico and in vitro studies of chalcones suggests that lipophilicity plays an imperative role in augmenting the anti-tubercular activity of chalcones (Dobchev et al., 2014, Caldwell 2015). Hence, halogenated chalcones are frequently synthesized to evaluate the anti-tubercular activity as they improve the penetrability of the compounds through the waxy cell wall barrier of the Mycobacterium tuberculosis bacteria and disrupts the cellular functions of the organism by interacting with interacting with crucial enzymes and proteins useful for the survival of the organism. In view of the above facts, we hereby report the synthesis of chalcones containing both —OH and —Cl substituents in ring-A and ring-B portion bearing different electron withdrawing and electron releasing —F, —Cl, —OH, —OCH3 groups as well as the isosteres of the phenyl ring like pyridine, thiophene and furan in order to generate novel chalcones with potential anti-tubercular and antioxidant activities.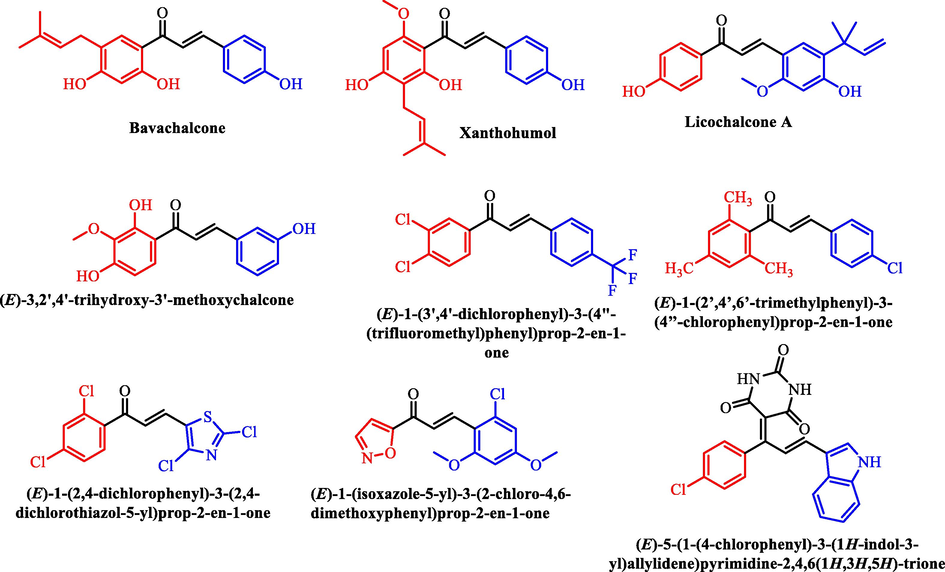
Structure of chalcone showing the biological importance of different structural fragments.
2 Materials and methods
2.1 General
The melting points of all 15 target compounds were determined using a Boetius melting point instrument (Rapido, Dresden, Germany) and are uncorrected. FT-IR spectra were recorded on Bruker alpha-T (BRUKER biospin International AG., Zug, Switzerland) and the values were represented as wavenumber in cm−1 whilst the 1H NMR and 13C NMR spectra were recorded using Bruker 400 Avance NMR spectrophotometer at 400.14 and 100.62 MHz for 1H and 13C nucleus respectively. For NMR investigations, tetramethyl silane was employed as an internal standard, and the spectral values were recorded as chemical shift (δ) values in ppm. In general, the deutered chloroform solvent is used in NMR. Mass spectra were scanned on an Agilent LC-MS spectrometer (Agilent technologies, USA). A precoated silica gel-G TLC (Merck) using 20–30% ethyl acetate-hexane as a mobile phase was utilized for monitoring the progress of the chemical reactions and assessing the purity of the compounds. A UV lamp was used to observe the spots on the TLC plate.
2.2 Experimental
2.2.1 General procedure for the synthesis of target chalcones (1–15)
These chalcones were synthesized by Claisen-Schmidt condensation. In 7.5 mL of ethanol, equal quantities of 5′-chloro-2′-hydroxy acetophenone (1 mmol) and substituted aldehydes (1 mmol) were dissolved. To the above mixture, 7.5 mL of 50% alcoholic KOH was added dropwise, and the reaction mixture was allowed to stay at room temperature for 24 h. It was then acidified using a 1:1 solution of conc. hydrochloric acid and water, resulting in the precipitation of the desired compounds (1–15). After that, the chalcones were vacuum filtered, washed in cold water, dried, and recrystallized from either ethanol or chloroform. The physicochemical and spectral properties of the compounds can be found in the supplementary material.
2.2.2 In vitro Anti-tubercular activity:
The target compounds (1–15) were investigated for anti-tubercular activity against the Mycobacterium tuberculosis H37Rv strain using pyrazinamide as a reference standard. The current investigation followed the protocol provided in the literature by MABA assay (Lokesh et al., 2019). The frosty culture of Mycobacterium tuberculosis H37Rv strain in Middlebrook 7H9 broth was defrosted by the addition of 0.2 percent glycerol and 10% albumin-dextrose-catalase and then diluted in broth to 105 CFU mL−1 (colony forming unit/mL) dilutions. Individually the test compounds were dissolved in Dimethyl sulfoxide (DMSO) and then diluted with broth to obtain a two-fold concentration. Throughout the experiment, the final concentration of DMSO in the test was 1.3%. The test tubes were then inoculated with 0.05 mL of standardized culture and cultured for 21 days at 37 °C. The growth of the bacteria in the test tubes was compared to the positive control, pyrazinamide, and the negative control, i.e., without inoculum and the standard drug. The minimal inhibitory concentration (MIC) of each target compound was determined using a broth dilution experiment. The MIC values obtained in µg/mL were converted to micromoles (µM) by considering the structural variation of the pyrazinamide and target molecules to arrive at a more acceptable conclusion.
2.2.3 In vitro antioxidant activity
The DPPH assay is a quick and easy way to assess antioxidants using spectrophotometry, and it may be used to analyse a large number of samples at once. The purpose of this study was to evaluate the antioxidant activity (AA%) of the target chalcones (1–15) using the DPPH free radical test. The activity of DPPH radical scavenging was determined using the protocol designated by Brand-Williams et al. The samples were treated with the stable DPPH radical in an ethanol. By dissolving DPPH in methanol, a 0.1 mM solution of DPPH was obtained. In an ethanol solution, the samples were treated with the stable DPPH radical. Gallic acid was used as a reference standard, and methanol was used to generate various concentration of test samples (5–100 g/mL) and standard (1.0, 2.5, and 5.0 g/mL). In 3 mL of each concentration of test samples and standard, one millilitre of 0.1 mM DPPH solution was added separately. After being maintained in the dark for about 30 min, the absorbance of these combinations was measured at 517 nm (Garcia et al., 2012). The ability to scavenge the DPPH radical was calculated using the formula below:
The reduction of DPPH with antioxidant molecules resulted in a color change from deep violet to light yellow, which was quantified using a UV–VIS spectrophotometer at 517 nm after 100 min of DPPH reaction.
2.2.4 Cytotoxicity assay
Cytotoxicity is usually screened for most active compounds in a series of synthetic analogues as this is critical for evaluating whether or not additional studies need to be done. Hence, the potent compounds identified out of the anti-tubercular and antioxidant studies i.e., 3, 7, 9 and 11 were screened for cytotoxic assay with the aid of MTT assay prescribed in the literature against LO2 (Human liver normal) cell lines (obtained from National Centre for Cell Science (NCCS), Pune, India (Palleapati et al., 2019).
2.2.5 Molecular docking studies
Isocitrate Lyase (PDB ID: 1F8M) X-ray crystal structure was obtained from the protein data bank (rcsb.com/pdb database). Water molecules were removed, hydrogens were added, and co-crystal ligands were extracted and saved in mol2 format using PyMOL 2.3.4. Using the Autodock module Macromolecule tool in PyRx Virtual screening program 0.8, a mol2 format file of protein was loaded and subsequently converted to pdbqt format. In ChemDraw ultra 12.0, the 2D structures of the target compounds (1–15) and the reference drug pyrazinamide were drawn and saved as sdf files. The ligand files were subjected to energy minimization (force field-uff) through Open babel tool and then conformers for the selected ligands were generated through AutoDock pdbqt files in PyRx Virtual screening software 0.8. The docking was then performed through PyRx Virtual screening software 0.8 combined with AutoDock Vina, Open babel, Python shell tools. The prepared protein file and ligand files were selected through Vina module and the grid box was selected according to the previously reported amino acid residues by adjusting the x, y, z coordinates of grid box, then run the Vina. The results were analyzed by using DS visualizer software to visualize the interactions between ligands and amino acid residues of active site of protein (Kwofie et al., 2018).
2.2.6 In silico drug likeliness studies
To meet the requirements of the drug-likeliness, the properties of the most potent compounds 7, 14 and 20 were evaluated for their in silico parameters including GI absorption, Lipinski rule of five as well as CYP2C19 CYP2D6 inhibition using SwissADME web [http://www.swissadme.ch/ (accessed on 15th April 2021)] (SwissADME, 2021).
3 Results and discussion
3.1 Chemistry
The target chalcones (1–15) were obtained by the Claisen-Schmidt condensation of 5-chloro-2-hydroxy acetophenone with substituted aryl aldehydes and unsubstituted heteroaryl aldehydes (Scheme 1) (Claisen and Claparède, 1881). Recrystallization was used to purify all of the compounds, with either ethanol or chloroform as the recrystallizing solvents. Except compound 13 (cream color) all the compounds were yellow in color. Chalcones get their color from the considerable conjugation of the ketovinyl scaffold with the A and B rings, as well as the additional electronic effects of the substituents on the two rings. All the synthesized compounds were characterized by FT-IR, 1H NMR and mass spectroscopy. Additionally, the structures of compounds 3 and 7 were also deduced by 13C NMR technique.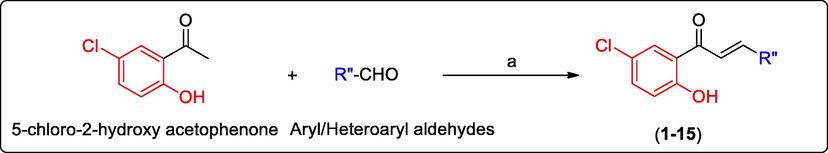
Synthesis of chalcones (1–15). Reagents and conditions: (a) ethanol, KOH, and room temperature; (1) 5-chloro-2-hydroxy acetophenone; R”—CHO aryl or heteroaryl aldehydes. R” = ring B; 1: 2″-fluorophenyl (Yield: 74%); 2: 3″-fluorophenyl (Yield: 84%); 3: 4″-fluorophenyl (Yield: 85%); 4: 2″-chlorophenyl (Yield: 75%); 5: 3″-chlorophenyl (Yield: 80%); 6: 4″-chlorophenyl (Yield: 82%); 7: 2″-methoxyphenyl (Yield: 80%); 8: 3″-methoxyphenyl (Yield: 75%); 9: 4″-methoxyphenyl (Yield: 70%); 10: 2″-hydroxyphenyl (Yield: 85%); 11: 3″-hydroxyphenyl (Yield: 80%); 12: 4″-hydroxyphenyl (Yield: 85%); 13: 4″-pyridinyl (Yield 95%); 14: 2″-thienyl (Yield: 95%); 15: 2″-furfuryl (Yield: 95%).
The FT-IR spectrum of compounds shows two diagnostic absorption bands corresponding to —C⚌C— and —C⚌O at wavenumber 1610–1685 cm−1 and 1704–1787 cm−1 respectively. Similarly, the vinylic protons (Hα and Hβ) of chalcones in their 1H NMR spectrum displayed two distinctive doublet peaks with chemical shift values ranging between 6.96 and 7.95 and 7.09–8.06 ppm respectively. The polarization of —C⚌C— by the carbonyl group of chalcones that may result in increase in the electron density at Hα might be responsible for the high chemical shift values of Hβ over Hα (Thirunarayanan et al., 2007). The other aromatic protons displayed multiplet peaks around the chemical shift values ranging between 6.72 and 8.06 ppm and the —OH proton showed a singlet peak at a chemical shift value above 12 ppm. All the compounds exhibited an M+ peak corresponding to their molecular weight as well as an isotopic M + 2 peak related to the isotope of chlorine (37Cl) atom present in these molecules. Table 1 shows the spectral characterization (1H and 13c NMR) for compounds 3 and 7.
Compound #
Compound structure
1H NMR δ (ppm)
13C NMR δ (ppm)
3
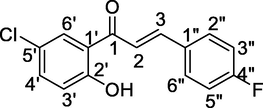
7.26 (d, 1H, Hα, J = 16 Hz), 7.52 (d, 1H, Hβ, J = 16 Hz), 7.24–7.69 (m, 7H, Ar-H), 12.11 (s, Ar-OH)
116.2 (C-3‘’ and C-5‘’), 119.6 (C-2), 121.8 (C-3′), 125.4 (C-1′), 131.6 (C-2″ and C-6″), 132.4 (C-6′), 134.8 (C-5′), 138.7 (C-4′), 147.4 (C-3), 162.6 (C-2′), 164.5 (C-4″), 192.8 (C-1)
7
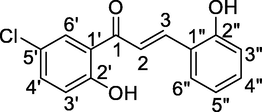
5.45 (s, Ar-OH), 7.57 (d, 1H, Hα, J = 16.5 Hz), 7.84 (d, 1H, Hβ, J = 16 Hz), 6.72–8.08 (m, 7H, Ar-H), 12.35 (s, Ar-OH)
117.3 (C-3″), 119.8 (C-3′), 122.4 (C-5″), 123.3 (C-2), 125.8 (C-1″), 127.5 (C-1′), 133.6 (C-5′), 135.5 (C-6″), 136.6 (C-4″), 137.8 (C-6′), 138.6 (C-4′), 143.2 (C-3), 154.3 (C-2″), 162.4 (C-2′), 191.5 (C-1)
The compound 3 analyzed for molecular formula C15H10ClFO2, m.p: 164–166 °C was well supported by a M+ peak (m/z 276.69, 99.08%) and an additional satellite M + 2 peak of intensity 33.03% at m/z value 278.69 due to chlorine atom in its mass spectrum. The FT-IR spectrum of compound 3 revealed two intense, characteristic stretching absorption bands pertaining to —C⚌C— and —C⚌O of chalcones at 1623 and 1720 cm−1 respectively. The two doublet peaks at 7.26 and 7.52 ppm corresponding to Hα and Hβ respectively with coupling constant (J) value 16 Hz confirmed the construction of chalcone bridge with trans or E configuration. In addition, the 1H NMR also showed multiplet peaks corresponding to the seven aromatic protons around 7.24–7.69 ppm and at 11.89 ppm accounting for the aromatic —OH proton. The 13C NMR of compound 3 displayed diagnostic peaks of the three-carbon spacer of chalcones at chemical shifts 192.8 (C-1), 119.6 (C-2) and 147.4 (C-3) respectively. The other 13C NMR peaks seen were for the other 12 carbons at chemical shifts as represented in Table 1.
The compound 7 analyzed for molecular formula C15H11ClO2, m.p: 198–200 °C was well supported by a M+ peak (m/z 274.70, 99.09%) and a supplementary M + 2 satellite peak of intensity 33.03% at m/z value 276.70 due to chlorine atom in its mass spectrum. Compound 7 showed two characteristic stretching absorption bands in FT-IR spectrum corresponding to —C⚌C— and —C⚌O of chalcones at 1665 and 1756 cm−1 respectively. The two doublet peaks at 7.57 and 7.84 ppm corresponding to Hα and Hβ respectively with coupling constant (J) value 16.5 and 16 Hz confirmed the formation of chalcone bridge with trans or E configuration. Additionally, the 1H NMR also disclosed multiplet peaks corresponding to the seven aromatic protons around 6.72–8.08 ppm and at 5.45 and 11.68 ppm pertaining to the two aromatic —OH groups. The 13C NMR of compound 7 displayed diagnostic peaks of the three-carbon spacer of chalcones at chemical shifts 191.5 (C-1), 123.3 (C-2) and 143.2 (C-3) respectively. The other 13C NMR peaks seen were for the other 12 carbons at chemical shifts as displayed in Table 1.
Based on the above spectral data compounds 3 and 7 were confirmed as (E)-1-(5′-Chloro-2′-hydroxyphenyl)-3-(4″-fluorophenyl)prop-2-ene-1-one and (E)-1-(5′-Chloro-2′-hydroxyphenyl)-3-(2″-hydroxyphenyl)prop-2-ene-1-one respectively (Fig. 4).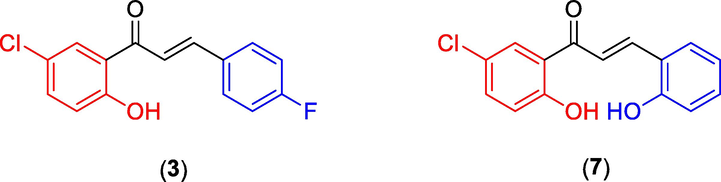
Structures of compounds (3) and (7).
3.2 Biological studies
3.2.1 Antitubercular and antioxidant studies
The anti-tubercular and antioxidant activities of all the synthesized compounds (1–15) are shown in Tables 2 and 3 (see supporting information), respectively. The target compounds (1–15) had electron withdrawing (Cl and F) and donating (OH and OCH3) groups substituted at ortho (1, 4, 7 and 10), meta (2, 5, 8 and 11) and para (3, 6, 9 and 12) position of the phenyl ring. Unsubstituted heteroaryl derivatives [3′-pyridyl (13), 2′-thienyl (14) and 2′-furfuryl (15)] were utilized in compounds 13–15 to evaluate the changes in physicochemical properties with bioisosteric replacement of phenyl ring. aIC50 are the mean values of three independent experiments.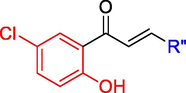
Compound #
R
Mtb (H37Rv Strain) (MIC in µM)
1
2″-fluorophenyl
116 ± 0.53
2
3″-fluorophenyl
116 ± 0.19
3
4″-fluorophenyl
14 ± 0.11
4
2″-chlorophenyl
218 ± 0.68
5
3″-chlorophenyl
109 ± 0.38
6
4″-chlorophenyl
55 ± 0.55
7
2″-hydroxyphenyl
466 ± 0.42
8
3″-hydroxyphenyl
29 ± 0.21
9
4″-hydroxyphenyl
233 ± 0.58
10
2″-methoxyphenyl
55 ± 0.33
11
3″-methoxyphenyl
14 ± 0.17
12
4″-methoxyphenyl
28 ± 0.16
13
3″-pyridinyl
985 ± 24
14
2″-thienyl
967 ± 0.66
15
2″-furfuryl
257 ± 0.51
Pyrazinamide
–
25.34 ± 0.22
Compound #
R
Antioxidant activity IC50 (µg/mL)
1
2′-fluorophenyl
22 ± 1
2
3′-fluorophenyl
32 ± 1
3
4′-fluorophenyl
46 ± 2
4
2′-chlorophenyl
18 ± 1
5
3′-chlorophenyl
26 ± 2
6
4′-chlorophenyl
38 ± 2
7
2′-hydroxyphenyl
4 ± 1
8
3′-hydroxyphenyl
10 ± 2
9
4′-hydroxyphenyl
5 ± 1
10
2′-methoxyphenyl
12 ± 2
11
3′-methoxyphenyl
17 ± 1
12
4′-methoxyphenyl
14 ± 1
13
3′-pyridinyl
25 ± 2
14
2′-thienyl
28 ± 1
15
2″-furfuryl
32 ± 1
Gallic acid
–
5 ± 1
The Minimum inhibitory concentration (MIC) values for anti-tubercular activity ranged from 14 to 985 µM (Table 2). Among the fifteen compounds, compounds 3 and 11 displayed 1.8-fold better activity than the standard, pyrazinamide (MIC = 25.34 ± 0.22 µM). This clearly reveals the importance of incorporating lipophilic groups like halogens and alkoxy groups (in this case —F and —OCH3) will enhance the penetrability of the compounds and support in the antitubercular activity of chalcones. On the other hand, compounds 8 and 12 had equivalent activity with pyrazinamide. In compounds (1–6) having electron withdrawing groups (F and Cl), the activity ranged from 14 to 218 µM. Substitution at the para position was found to be better over ortho and meta [3 (14 ± 0.11 µM); 6 (55 ± 0.55 µM) Vs 1 (116 ± 0.53 µM), 2 (116 ± 0.19 µM); 4 (218 ± 0.68 µM), 5 (109 ± 0.38 µM)]. The “F” substituent at para (3) position was found to exhibit 4-fold better activity over “Cl” substituent (6) where as “OCH3” substituent at meta position 11 displayed 2 fold better activity over compounds 8 and 12. In case of compounds having electron donating groups (7–12), the MIC ranged from 14 to 466 µM. Substitution with methoxy over hydroxy at the meta position was found to be better than ortho and para [11 (14 ± 0.17 µM) Vs 8 (29 ± 0.21 µM); 7 (466 ± 0.42 µM); 9 (233 ± 0.58 µM); 10 (55 ± 0.33 µM); 12 (28 ± 0.16 µM)]. Bioisosteric substitution did not improve the anti-tubercular activity with compounds 13–15 having low or loss of activity (MICs 257–985 µM).
The antioxidant activity of all fifteen compounds was determined using the DPPH free radical assay, with the results based on three independent experiments (Table 3). Gallic acid was employed as the positive control (IC50 5 ± 1 µg/mL). The target chalcones exhibited significant antioxidant activity ranging from 4 ± 1 to 46 ± 1 µg/mL. Electron withdrawing substituents [F (1–3) and Cl (4–6)) displayed IC50 between 18 ± 1 to 46 ± 2 µg/mL but was found to be lower than Gallic acid. Electron donating substituents [OH (7–9) and Cl (10–12)] displayed IC50 between 4 ± 1 to 17 ± 2 µg/mL and fared better than compounds 1–6. Bioisosteres 13–15 showed antioxidant activity (IC50 25 ± 2 to 32 ± 2 µg/mL) similar to compounds 1–6 and had not shown any improvement in activity over Gallic acid. It can be inferred from the data that the antioxidant activity was found to be improved on substitution of electron donating group (—OH) on the phenyl ring at positions ortho and para.
Summary of the anti-tubercular and antioxidant activities and the structure activity relationships of these of chalcones (1–15) is depicted in Fig. 5.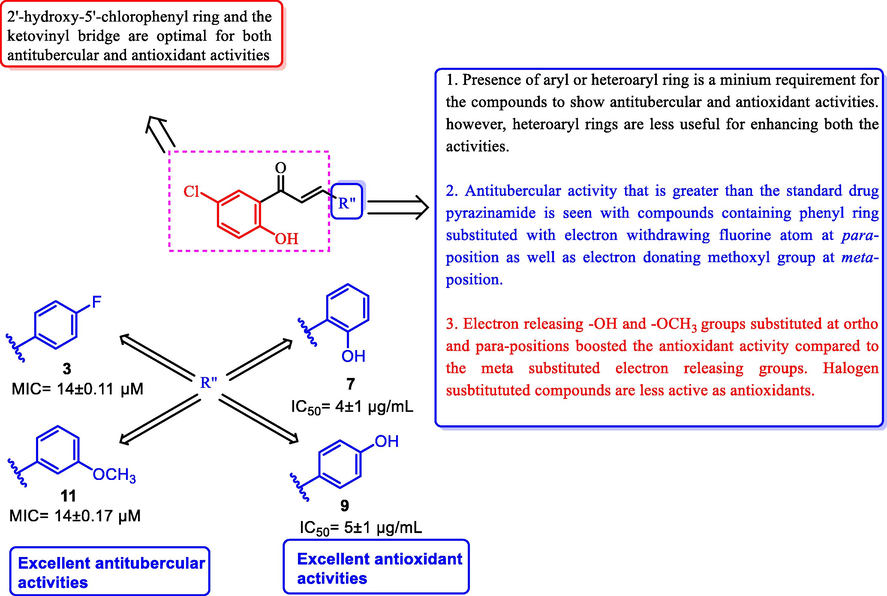
Summary of the anti-tubercular and antioxidant activities and structure activity relationships of chalcones (1–15).
3.2.2 Cytotoxicity studies
We performed the cytotoxicity assay of compounds 3, 7, 9 and 11 using MTT assay against LO2 (Human liver normal) cell lines. Table 4 (see supporting information) summarized the results of the experiment. All the four compounds shown had an IC50 value more than 75 μg/mL, indicating that these compounds are less selective towards the typical human cell lines and can be considered as safe compounds to move into the next phase of the drug discovery cycle.
Compound #
R
Cytotoxic activity IC50 (µg/mL)
3
4′-fluorophenyl
76 ± 2
7
2′-hydroxyphenyl
82 ± 2
9
4′-hydroxyphenyl
77 ± 2
11
3′-methoxyphenyl
79 ± 1
Docetaxel
–
5 ± 1
3.2.3 Molecular docking studies
The in silico antitubercular activity results of selected ligands against Isocitrate Lyase were reported in terms of binding energy and ligand interactions (Tables 5 and 6; Fig. 6 (see supporting information) and with amino acid residues at active pocket of proteins. In comparison to the standard drugs Pyrazinamide and Isoniazid, the in silico anti-tubercular results showed that all compounds (1–15) had strong binding affinity (ranges from −6.2 to −7.6 given in Tables 4 and 5) towards the amino acid residues in active pocket of Isocitrate Lyase protein through H-bond and hydrophobic interactions. Specifically compounds 3, 11, 1, 2, 5, and 4 having 4-fluoro, 4-methoxy, 2-fluoro, 3-fluoro, 2-methyl, and 1-methyl substitutions respectively on phenyl ring of compounds in place of amine group that may be the reason for the high affinity of these compounds compared to the standard drug pyrazinamide and Isoniazid. Compound 3 had the highest binding affinity of all the compounds, with a value of −7.6. This could be because it interacts with Trp320 amino acid residue by H-bonding and with Leu69, Cys314, Pro316, Ile346, Ala349 amino acid residue via hydrophobic interaction. Similarly compounds 11, 1, 2, 5, and 4 also showed H-bonding with Trp320 and hydrophobic interaction with amino acid residues given in Table 5 and depicted in Figs. 6 (see supporting information) and 7, but the standard drug pyrazinamide has H-bond interaction with Asp153, Arg228, Glu285, Asp108, Ser91, Leu348 amino acid residues and has hydrophobic interaction with Cys191, Thr347, His180 amino acid residues. Similarly, isoniazid has only H bond interactions with Trp17, Asp18, Trp23, Val26, Arg28. Furthermore, hydrophobic interactions between functional groups of substances and amino acid residues are higher than hydrophobic interactions between functional groups of conventional drugs pyrazinamide and isoniazid and amino acid residues of target proteins. Compounds with an electron withdrawing group substituted on the aromatic ring in place of an amine group, compounds with H-bond interactions with Trp320 amino acid residues, and a greater number of hydrophobic interaction amino acids present between compounds and amino acid residues in the active site of the target protein could all be contributing to the compounds' high affinity when compared to standard drug pyrazinamide and Isoniazid. The in silico docking results supporting in vivo MABA assay that the compounds 3, and 11 with 4-fluoro and 3-methoxy substituents respectively on phenyl ring shown maximum binding affinity with least binding energies which can greatly inhibits the target protein. The electron withdrawing and electron donating substituents on phenyl ring at different positions will greatly influence the in silico and in vitro antitubercular activity of chalcone analogues.
Compound #
Binding Affinity against 1F8M
1
−7.3
2
−7.3
3
−7.6
4
−7.2
5
−7.3
6
−6.8
7
−6.4
8
−7.2
9
−7.2
10
−7
11
−7.5
12
−7
13
−6.6
14
−6.2
15
−7.2
Isoniazid
−4.8
Pyrazinamide
−5.4
Compound #
Docking Score
Amino Acids Interacted Through
H-Bond
Hydrophobic
3
−7.6
Trp320
Leu69, Cys314, Pro316, Ile346, Ala349
11
−7.5
Pro316, Trp320
Cys314, Phe332, Ile346, Ala349, Ala353
1
−7.3
Trp320
Leu69, Cys314, Pro316, Ile346, Ala349
2
−7.3
Trp320
Cys314, Trp320, Phe332, Ala349
5
−7.3
Trp320
Leu69, Cys314, Trp320, Phe332, Ala349, Ala353
4
−7.2
Trp320
Leu69, Cys314, Ile329, Pro316, Ile346, Ala349
Pyrazinamide
−5.4
Asp153, Arg228, Glu285, Asp108, Ser91, Leu348
Cys191, Thr347, His180
Isoniazid
−4.8
Trp17, Asp18, Trp23, Val26, Arg28
–
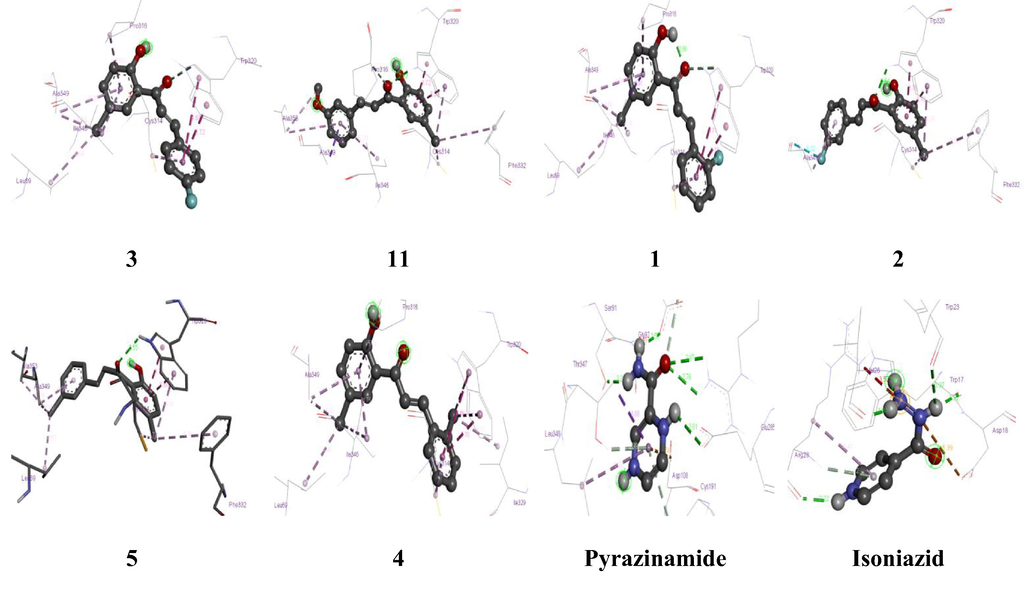
3D Interactions of selected compounds with Isocitrate Lyase protein amino acid residues.
3.2.4 In silico drug likeliness studies
Some selected compounds 3, 8, 11 and 12 which showed the best activity in anti-tubercular activity were computed for certain properties using web based SwissADME software (Table 7). It can be observed that compounds 3, 8, 11 and 12 inhibited CYP2C19, but compounds 3 and 8 did not inhibit CYP2D6. However, they showed high GI absorption and passed Lipinski Rule of five. Hence, these molecules have good drug-like properties and they can be taken as leads for further in vivo investigation.
Compound #
GI absorption
CYP2C19 inhibitor
CYP2D6 inhibitor
Lipinski #violations
3
High
Yes
No
0
8
High
Yes
No
0
11
High
Yes
Yes
0
12
high
Yes
Yes
0
4 Conclusion
We synthesized 15 chalcones and evaluated all the compounds for their anti-tubercular and antioxidant activities by standard protocols. Compounds 3 and 11 containing 4″-fluorophenyl and 3″-methoxyphenyl as ring-B component elicited excellent anti-tubercular activity against Mycobacterium tuberculosis H37Rv strain than pyrazinamide whereas compound 7 containing 2″-hydroxyphenyl group had shown greater antioxidant activity than Gallic acid. The most potent compounds 3, 7, 9 and 11 screened for their cytotoxic effects were less selective towards the normal cell lines indicating the safety profile of these compounds. Molecular docking results were much in correlation with the in vitro anti-tubercular activity as well as the SwissADME results of 3, 7, 9 and 11 suggesting the usefulness of computational studies in the development of new drug like candidates. The potential lead compounds identified through this work will be useful in the design and optimization of drug candidates against tuberculosis and oxidative stress.
Acknowledgements
The authors thank the Acharya Nagarjuna University College of Pharmaceutical Sciences, Acharya Nagarjuna University, Guntur, for providing the lab facilities and chemicals for this work. The authors like to acknowledge Vignan Pharmacy College, Vadlamudi, Andhra Pradesh, India, Dean’s office of College of Pharmacy and Health Sciences, Ajman University, UAE for their support in providing partial assistance in article processing charges of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin. Infect. Dis.. 2017;65:900-909.
- [CrossRef] [Google Scholar]
- Synthesis of new C- dimethylated chalcones as potent antitubercular agents. Med. Chem. Res.. 2018;27:1690-1704.
- [CrossRef] [Google Scholar]
- Identification and validation of the mode of action of the chalcone anti- mycobacterial compounds. The. Cell. Surface.. 2020;6:100041.
- [CrossRef] [Google Scholar]
- Computational analysis of a series of chlorinated Chalcone derivatives. Comput. Chem.. 2019;7:106-120.
- [CrossRef] [Google Scholar]
- Synthesis of extended conjugated indolyl chalcones as potent anti-breast cancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett.. 2017;27:1502-1507.
- [CrossRef] [Google Scholar]
- Synthesis, antioxidant and DNA cleavage activities of novel indole derivatives. Eur. J. Med. Chem.. 2010;45:4074-4078.
- [CrossRef] [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol.. 1995;28:25-30.
- [CrossRef] [Google Scholar]
- Comprehensive review on mechanism of action, resistance and evolution of antimycobacterial drugs. Life. Sci.. 2021;119301
- [CrossRef] [Google Scholar]
- Condensationen von Ketonen mit Aldehyden reports of the German. Chem. Soc.. 1881;14:2460-2468.
- [CrossRef] [Google Scholar]
- On the in silico and in vitro anticancer activity of sulfonamide chalcones: potential JNKK3 inhibitors. New. J. Chem.. 2020;44:3294-3309.
- [CrossRef] [Google Scholar]
- In silico tools used for compound selection during target-based drug discovery and development. Expert Opin. Drug Discov.. 2015;10:901-923.
- [CrossRef] [Google Scholar]
- Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem.. 2020;187:111980.
- [CrossRef] [Google Scholar]
- In silico machine learning methods in drug development. Curr. Top. Med. Chem.. 2014;14:1913-1922.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and docking studies of chalcone and flavone analogs as antioxidants and acetylcholinesterase inhibitors. Appl. Sci.. 2019;9:410.
- [CrossRef] [Google Scholar]
- In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med.. 2002;68:416-419.
- [CrossRef] [Google Scholar]
- Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol.. 1999;6:775-784.
- [CrossRef] [Google Scholar]
- Global Tuberculosis Report, 2020. World Health Organisation. October 15. Available from https://www.who.int/publications/i/item/9789240013131.
- Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J.. 2012;23:22-27.
- [CrossRef] [Google Scholar]
- Design, multistep synthesis and in-vitro antimicrobial and antioxidant screening of coumarin clubbed chalcone hybrids through molecular hybridization approach. Arab. J. Chem.. 2021;14:103154.
- [CrossRef] [Google Scholar]
- Pyrazole–coumarin and pyrazole–quinoline chalcones as potential antitubercular agents. Archiv. Der. Pharmazie.. 2020;353:2000077.
- [CrossRef] [Google Scholar]
- Thiazole-Chalcone hybrids as prospective antitubercular and antiproliferative agents: design, synthesis, biological, molecular docking studies and In Silico ADME evaluation. Molecules. 2021;26:2847.
- [CrossRef] [Google Scholar]
- Advances in chalcones with anticancer activities. Recent. Pat. Anticancer. Drug. Discov.. 2015;10:97-115.
- [CrossRef] [Google Scholar]
- Exploring the 2′-hydroxy- chalcone framework for the development of dual antioxidant and soybean lipoxygenase inhibitory agents. Molecules. 2021;26:2777.
- [CrossRef] [Google Scholar]
- Chalcones and their pyrazine analogs: synthesis, inhibition of aldose reductase, antioxidant activity, and molecular docking study. Monatsh. Chem.. 2018;149:921-929.
- [CrossRef] [Google Scholar]
- Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci.. 2000;30:145-158. PMID: 10807157
- [Google Scholar]
- Kaur, J., Kaur, R., Kaur, A., 2018. Dietary antioxidants and infectious diseases. In: Infectious Diseases and Your Health. Singapore: Springer, pp. 307–316.
- In Silico screening of isocitrate lyase for novel anti-buruli ulcer natural products originating from Africa. Molecules. 2018;23:1550.
- [CrossRef] [Google Scholar]
- Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals. 2020;13:375.
- [CrossRef] [Google Scholar]
- Synthesis, antibacterial, antifungal, antitubercular activites and molecular docking studies of nitrophenyl derivatives. Int. J. Life. Sci. Pharma. Res.. 2019;9:54-64.
- [CrossRef] [Google Scholar]
- Discovery of novel quinoline-chalcone derivatives as potent antitumor agents with microtubule polymerization inhibitory activity. J. Med. Chem.. 2019;62:993-1013.
- [CrossRef] [Google Scholar]
- Dibenz[b, f]oxepin and antimycobacterial chalcone constituents of empetrum nigrum. J. Nat. Prod.. 2015;78:2837-2840.
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of novel 2,5-dichloro-3-acetylthiophene chalcone derivatives, Indian. J. Pharm. Educ. Res.. 2017;51:s679-s690.
- [Google Scholar]
- Synthesis, Biological evaluation and molecular docking studies of new pyrazolines as an antitubercular and cytotoxic agents. IDDT (Formerly Curr. Drug Targets-Infect. Dis.). 2019;19:310-321.
- [Google Scholar]
- Chalcone scaffolds as anti-infective agents: structural and molecular target perspectives. Eur. J. Med. Chem.. 2015;101:496-524.
- [CrossRef] [Google Scholar]
- Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: modulations of drug- metabolizing enzymes and antioxidant activity. Phytother. Res.. 2001;15:114-118.
- [CrossRef] [Google Scholar]
- Anti-cancer chalcones: structural and molecular target perspectives. Eur. J. Med. Chem.. 2015;98:69-114.
- [CrossRef] [Google Scholar]
- Inhibition of Mycobacterium tuberculosis tyrosine phosphatase PtpA by synthetic chalcones: kinetics, molecular modeling, toxicity and effect on growth. Bioorg. Med. Chem.. 2010;18:3783-3789.
- [CrossRef] [Google Scholar]
- Antimycobacterial flavonoids from the leaf extract of Galenia Africana. J Nat Prod.. 2009;72:2169-2171.
- [CrossRef] [Google Scholar]
- Spirochromone-chalcone conjugates as antitubercular agents: synthesis, bio evaluation and molecular modeling studies. RSC Adv.. 2015;5:106448-106460.
- [CrossRef] [Google Scholar]
- Treatment of isoniazid-resistant tuberculosis with isoniazid, rifampin, ethambutol, and pyrazinamide for 6 months. Int. J. Tuberc. Lung. Dis.. 2002;6(11):952-958.
- [Google Scholar]
- A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem.. 2007;42:125-137.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of coupled bioactive scaffolds as potential anticancer agents for dual targeting of dihydrofolate reductase and thioredoxin reductase. J. Med. Chem.. 2017;60:1734-1745.
- [CrossRef] [Google Scholar]
- A novel structural class of coumarin-chalcone fibrates as PPARα/γ agonists with potent antioxidant activities: design, synthesis, biological evaluation and molecular docking studies. Eur. J. Med. Chem.. 2017;138:212-220.
- [CrossRef] [Google Scholar]
- In vitro and in vivo anticancer studies of 2'-hydroxy chalcone derivatives exhibit apoptosis in colon cancer cells by HDAC inhibition and cell cycle arrest. Excli. J.. 2017;16:448.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antitubercular evaluation of some new isoxazole appended 1-carboxamido-4,5-dihydro- 1H-pyrazoles. J. Res. Pharm.. 2019;23:156-163.
- [CrossRef] [Google Scholar]
- Indole chalcones: design, synthesis, in vitro and in silico evaluation against Mycobacterium tuberculosis. Eur. J. Med. Chem.. 2020;198:112358.
- [CrossRef] [Google Scholar]
- Novel Chalcone-Thiazole hybrids as potent inhibitors of drug resistant staphylococcus aureus. ACS Med. Chem. Lett.. 2015;6:809-813.
- [CrossRef] [Google Scholar]
- Synthesis and screening of novel lipophilic diarylpropeones as prospective antitubercular, antibacterial and antifungal agents. Biointerface Res. Appl. Chem.. 2019;9:3912-3918.
- [CrossRef] [Google Scholar]
- Design, facile synthesis and characterization of dichloro substituted chalcones and dihydropyrazole derivatives for their antifungal, antitubercular and antiproliferative activities. Molecules. 2020;25:3188.
- [CrossRef] [Google Scholar]
- Design, facile synthesis, characterization and computational evaluation of novel isobutylchalcones as cytotoxic agents: part-a. Fabad. J. Pharm. Sci.. 2015;40:1-6.
- [Google Scholar]
- Exploring coumarin and chalcone analogues as potential antimycobacterial agents. Antiinfect. Agents. 2017;15:69-86.
- [CrossRef] [Google Scholar]
- Synthesis of chalcones and nucleosides incorporating [1, 3, 4] oxadiazolenone core and evaluation of their antifungal and antibacterial activities. Curr. Bioact. Compd.. 2019;15:665-679.
- [CrossRef] [Google Scholar]
- Singh, R., Kumar, P., Tahlan, K., Drugs against Mycobacterium tuberculosis, Drug Discovery Targeting Drug-Resistant Bacteria, Academic Press, 2020, pp. 139–170. ISBN 9780128184806.
- Synthesis and antifungal activities of some benzimidazolyl-chalcones, analogues of chlormidazole. Afr. J. Pharm. Pharmacol.. 2015;9:418-423.
- [CrossRef] [Google Scholar]
- The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology. 2016;24:81-86.
- [CrossRef] [Google Scholar]
- Antioxidant activities of alkyl substituted pyrazine derivatives of chalcones—in vitro and in silico study. Antioxidants. 2019;8:90.
- [CrossRef] [Google Scholar]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 15th April 2021).
- IR and NMR spectral studies of 4-bromo-1-naphthyl chalcones-assessment of substituent effects. Spectrochim Acta A.. 2007;67:1106-1112.
- [CrossRef] [Google Scholar]
- Phytochemical and antimicrobial screening of flavanones and chalcones from galenia africana and dicerothamnus rhinocerotis. Nat. Prod. Commun.. 2015;10:1185-1190. PMID: 26411007
- [Google Scholar]
- Antimicrobial, anti-inflammatory and antioxidant activities of polyoxygenated chalcones. J. Braz.. 2019;30:286-304.
- [CrossRef] [Google Scholar]
- Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5- trimethoxy chalcones. Food. Chem.. 2015;171:89-97.
- [CrossRef] [Google Scholar]
- Antiradical activity and mechanism of Coumarin-Chalcone hybrids: theoretical insights. J. Phys. Chem. A.. 2018;122:8520-8529.
- [CrossRef] [Google Scholar]
- Molecular docking studies on fluoro- substituted chalcones as potential DprE1 enzyme inhibitors. J. Mol. Struct.. 2018;1164:50-56.
- [CrossRef] [Google Scholar]
- Synthesis and bioactivities of halogen bearing phenolic chalcones and their corresponding bis Mannich bases. J. Enzyme. Inhib. Med. Chem.. 2016;31:125-131.
- [CrossRef] [Google Scholar]
- Biological and synthetic potentiality of chalcones. J. Chem. Pharm. Res.. 2015;7(11):829-842.
- [Google Scholar]
- Pharmacophore modeling, synthesis, and antibacterial evaluation of Chalcones and derivatives. ACS Omega. 2018;3:18343-18360.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103581.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







