Translate this page into:
Assessing the skin-whitening property of plant extracts from taiwanese species using zebrafish as a rapid screening platform
⁎Corresponding authors at: Ph.D. Program in Drug Discovery and Development Industry, College of Pharmacy, Taipei Medical University, Taipei 11031, Taiwan (C.-K. Lee). chcheng@tmu.edu.tw (Chia-Hsiung Cheng), hcmei@tea.ntue.edu.tw (Hui-Ching Mei), cklee@tmu.edu.tw (Ching-Kuo Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Zebrafish can be used as a phenotype-based fast-screening platform for the discovery of skin-whitening products by observing the formation of melanin in vivo through a dissecting microscope. In this study, we first evaluated commercial whitening ingredients to confirm the potency of the zebrafish model. The zebrafish screening system verified that verbascoside and 5 other commonly used commercial skin-whitening compounds– including sodium ascorbate, azelaic acid, transamin, kojic acid, and PTU- significantly reduce melanin content in a dose-dependent manner. We subsequently conducted preliminary screening of 123 natural sources to identify those with whitening property. Through a two-step screening process, anti-melanogenesis effects of 4 and 9 candidates at low and high concentration were verified respectively. The toxicity, mortality, and malformation of zebrafish embryo were also evaluated. We further investigated the components of C. manghas L. and found 6 major compounds, one of which was manghaslin, a compound known for its melanin-suppressing effect on zebrafish without cytotoxicity. With these properties, manghaslin is a promising candidate as a skin lightening agent or a treatment for melanoma.
Keywords
Zebrafish (Danio rerio)
Whitening
Phenotype-based screening
Natural extracts
Cerbera manghas
Manghaslin
1 Introduction
The darkening of the skin can be attributed to melanin secreted by melanocytes which are situated on the basal layer of skin (Hurbain et al., 2018). The pigment is initially synthesized from the amino acid L-tyrosine, which converts to dopaquinone when catalyzed by tyrosinase – a rate-limiting step of the melanogenic pathway (Ryu et al., 2015). One melanocyte provides epidermal melanin for about 36 surrounding keratinocytes (Nordlund, 2007). This phenomenon protects our skin against the harmful free radicals generated from UV radiation and thus prevents DNA damage and reduces the risks of skin cancer (Choi et al., 2007). However, dark spots, melasma, and freckles caused by excessive accumulation of melanin are considered aesthetic skin problems. Undesirable skin darkening problems make skin-whitening products with safety and effectiveness a continuous challenge and interest in the cosmetic industry.
In spite of the skin-whitening agents currently available in the cosmetic market, the industry is continuously searching for innovative skin-whitening ingredients with fewer side effects and improved outcomes (Wen et al., 2013). It is of profound importance to use effective and appropriate methods to evaluate the whitening capacity of natural products before availing to consumers. The two most common screening methods involve evaluating the inhibition of melanin production in B16 melanoma cells (Gruber and Holtz, 2013) and the enzymatic activity of tyrosinase (Uchida et al., 2014). These methods do not provide insight into the in vivo effectiveness and safety of these molecules as skin-whitening agents. The zebrafish screening platform, on the other hand, overcomes the limitation of the former methods to provide physiologically valid findings and allows for phenotypical assessment of melanin pigmentation. As a result, more researchers in the field are using zebrafish as a phenotype-based model to screen for potential molecules with skin-whitening function (Chen et al., 2015, Kim et al., 2015, Lee, S.-H. et al., 2015, Hsu et al., 2016, Lajis 2018).
Danio rerio, commonly known as zebrafish, is a well-studied model organism characterized by its small size, rapid maturation, short life span, large number of off-spring, and easy manipulation (Chen et al., 2015). From the genetic perspective, the vertebrate zebrafish has high resemblance with mammals in their genetic sequence and organ systems. For instance, the percentage similarity between zebrafish genes and human genes is approximately 70% (Li et al., 2014). Due to this, zebrafish has been widely studied and valued in molecular genetics, developmental biology, toxicology, and pharmaceutical research (Hsu et al., 2016). Both zebrafish and humans rely on gene expression and regulation of WNT, SOX10, MITF, KIT and SLF for the differentiation and proliferation of pigmented cells, leading to only minor differences in melanocytes development (Rawls et al., 2001, Hou et al., 2006, Rakers et al., 2010). Similar to mammalian skin, fish skin plays important roles in providing chemical and physical protection, sensing, as well as reacting to environmental stimuli (Rakers et al., 2010).Unlike adult zebrafish and later-stage larva (7 days post-fertilization) that rely on their mouths for ingestion and absorption, the early-stage zebrafish embryo and larva are capable of percutaneous absorption (the uptake of small molecules from their surroundings through their gills and skin) (Langheinrich, 2003). The early-stage embryo is therefore unique and advantageous for high-throughput pharmaceutical and cosmetic testing. The transparent skin of the early-stage embryos also facilitates the visualization of the whitening effect through non-invasive imaging techniques (Lee, H. J. et al., 2015).
Plant extracts with skin-whitening effects are gaining interest among consumers and researchers because they are considered milder, safer, and healthier than synthetic alternatives (Kanlayavattanakul and Lourith, 2018). For instance, pachymic acid extracted from Wolfiporia cocos, a kind of Chinese herbal medicine, has been patented as a cosmetic compound for its skin whitening function (Lee and Kim, 2014). Similarly, verbascoside, a compound derived from Plantago asiatica L. is a competitive inhibitor for mushroom tyrosinase, and was patented as a whitening hand cream (Wang, 2014). Another compound, plantamajoside was also patented for its anti-wrinkle, anti-inflammatory, anti-oxidant and skin whitening effects (Yoon et al., 2015).
With the quick formation and easy observation of melanin, as well as the rapid percutaneous absorption in the early-stage embryos, zebrafish model is an ideal system for screening potential skin-whitening compounds (Choi et al., 2007). In this study, we verified its validity with 8 commercial whitening compounds before establishing zebrafish as a functioning rapid screening platform. A total of 123 samples of natural ethanol extracts from 68 families and 113 genera were assessed on their skin-whitening property. Through evaluating melanin reduction activity and toxicity of extracts on zebrafishes at a high and low dose in addition to tyrosinase inhibition assay, we expect to find potential natural whitening materials that are both safe and effective for commercial and/or pharmaceutical application.
2 Materials and methods
2.1 Natural materials and chemicals
A total of 123 plants from aerial parts (Table S1) were freshly collected from Taiwan Endemic Species Research Institute from 2012 to 2019, and identified by Ms. Hsiu-Wen Huang of Taiwan Endemic Species Research Institute, Chi-Chi township, Taiwan. After 500 g of the dried material was powdered with a laboratory mill, 50 g of the powder was mixed with 500 mL of 95% EtOH and incubated at 32 °C for one day. The extraction process was three times repeated. Subsequently, the ethanolic extract was concentrated in vacuo and freeze-dried. All voucher specimens (TMU-LCK-NAT.-1–123) were deposited at the School of Pharmacy, Taipei Medical University, Taipei, Taiwan, and stored at 4 °C until use. Eight skin-whitening compounds including mangiferin, salicylic acid, verbascoside, sodium ascorbate, azelaic acid, transamin, kojic acid, and 1-phenyl-2-thiourea (PTU) were obtained from Sigma (ST. Louis, MO, USA) or AK Scientific (Ahern Avenue Union City, USA).
2.2 Screening procedures of the zebrafish model
2.2.1 Zebrafish Husbandry, Grouping, and treatment
The wild-type zebrafish (AB line) eggs were provided by Taiwan Zebrafish Core Facility at Taipei Medical University. All animal procedures were approved by Institutional Animal Care and Use Committee or Panel (IACUC/IACUP) (protocol no: LAC-2017–0311). 9 h post fertilization (hpf) eggs were used for the screening experiments. The pure compounds were first dissolved in ethanol or dimethyl sulfoxide (DMSO), and added to the E3 embryo medium (5.03 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) until the final concentration reaches 1% (v/v). The E3 embryo medium with 1% ethanol or 1% DMSO was used as control group. Twenty fertilized eggs were then cultured in each well of the 12-well plates (N = 20) with 1.5 mL of the conditioned medium to evaluate the whitening effect of the compounds. Double distilled water was also added to four empty wells to retain humidity and moisture. The plates were subsequently incubated at 28 °C for 63 h.
The screening of natural extracts was performed with the same procedure. After cross-referencing with the literature, we decided to test 100 μg/mL as the lower concentration (Cha et al., 2011), and 8.5–20.5 mg/mL as the higher concentration for evaluating the whitening effect and confirming the safety of the natural extracts. The natural extracts were first dissolved in ethanol and diluted with E3 embryo medium to obtain the final concentration of 1% ethanol. The control group consisted of E3 embryo medium with 1% ethanol, whereas 20 mM arbutin was used as the positive control. Three healthy fertilized eggs were placed in each well of the 96-well plates (N = 3), and 200 μL of the sample-containing medium was subsequently dispensed into each well. A secondary round of screening was performed with 20 eggs placed in each well of the 12-well plates (N = 20), and 1.5 mL of the effective natural extracts dispensed into each well. Once again, 1% of ethanol in E3 embryo medium was used as the control group, and 20 mM kojic acid -a melanin inhibitor- was used as the positive control group. The experimental flow diagram is shown in Fig. 1. The ingredients of medium and reagents for positive control were purchased from Sigma (ST. Louis, MO, USA).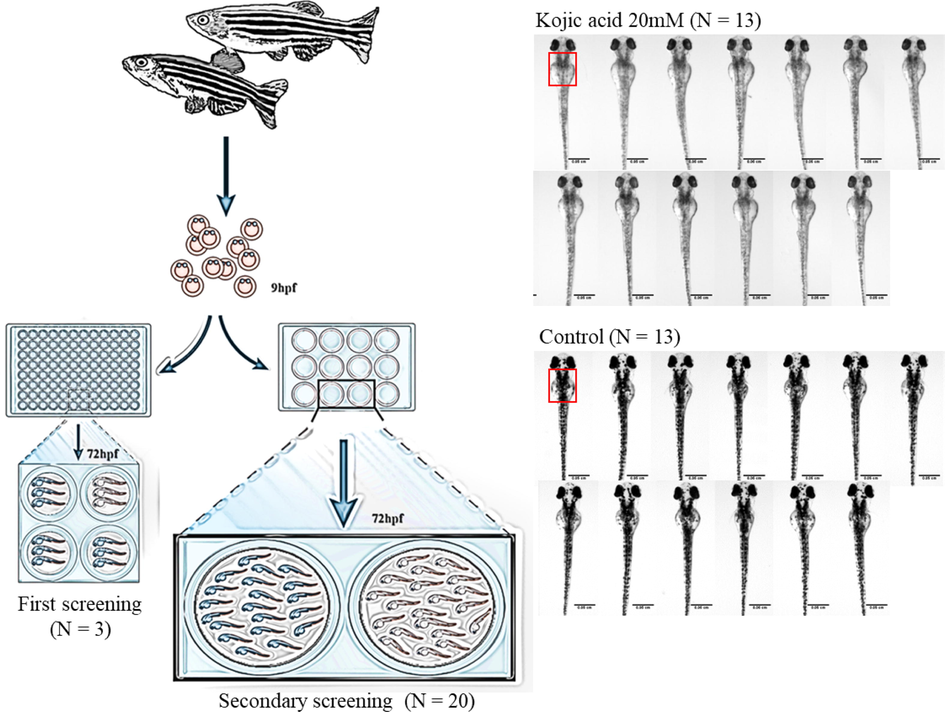
The flow diagram of zebrafish model. Three and twenty eggs were used in the first and secondary screening, respectively. 20 mM kojic acid was used as positive control. Melanin content within a fixed area (red square) was determined, and data of 13 zebrafish (N = 13) were used for statistical analysis.
2.2.2 Image capture and quantification of melanin distribution on zebrafish
After the incubation period, the 72-hour post fertilization zebrafish larvae were transferred to a plastic plate, anesthetized using tricaine methanesulfonate (Sigma, ST. Louis, MO, USA), and suspended in 1% low melting point agarose (BioShop, Canada) containing tricaine methanesulfonate solution. The larvae were placed under the stereo/dissecting microscope (Stemi 508, SEISS, Germany) and visually examined using a dynamic image capture lens (Forever Plus Corp. FPC-U130037300, Taipei, Taiwan). The melanin distribution along the dorsal side of the larvae was analyzed and quantified using Fiji ImageJ by converting the image into 8-bit format, setting the threshold to 30, and sampling a fixed area under the eyes of the larvae to determine the black color ratios. The outcomes were recorded for statistical analysis.
2.2.3 Statistics
To statistically evaluate the dataset, the statistical standard was set at the conditions with the lowest number of surviving zebrafishes, they are: N = 3 for the first screening and N = 13 for secondary screening. When more than 13 zebrafishes were analyzed, the medium values of melanin content were prioritized selected. The control group was used as the standard for 100% melanin content, to which the treatment groups were compared. Since the sample size is small, we performed independent sample t-test. F-test was used to first assess the homogeneity of the population variance (p-value < 0.05) between the control and treatment groups. A two-tailed t-test was performed subsequently to determine if there was a significant difference between the means of two groups (p-value < 0.05).
2.3 Evaluation of melanin content in B16-F10 cells
The B16-F10 melanoma cells (CRL6475) were purchased from the Food Industry Research and Development Institute (FIRDI, Hsinchu, Taiwan). The cells were cultured in 90% Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA) and 1% penicillin–streptomycin. Cells were incubated with humidified air containing 5% CO2 at 37 °C. Melanin content was measured as described previously with slight modifications (Cheng et al., 2018). The B16-F10 cells were seeded with 1 × 104 cells/well in 1 mL of medium in 12-well culture plates, and incubated for 24 h for cell adhesion. The crude extract of C. manghas was dissolved in DMSO and diluted with PBS to the final concentration of 1% DMSO (v/v). 1% DMSO was used as blank, and 50 μM of arbutin was used as positive control. The cells were co-cultured with various concentrations of the extracts (25, 50 and 100 μg/mL) for 72 h in the presence of 100 nM α-melanocyte-stimulating hormone (α-MSH). The melanin content was measured by detecting the absorbance at 405 nm. The cell viability was evaluated by MTT assay.
2.4 Two-dimensional preparative HPLC analysis of C. manghas extract
Sepbox 2D-2000 is a combination of high-performance liquid chromatography (HPLC) and solid-phase extraction (SPE), which is suitable for the separation of polar and non-polar components, and is equipped with ultraviolet–visible light detector (UV detector) and evaporative light Scattering detector (ELSD). Compared with traditional separation methods, Sepbox 2D-2000 has higher separation efficiency and shorter separation time, so it can make sample purification more convenient. Therefore, 2 g of C. manghas extract was coated with 10 g of C4 reversed-phase silicone gel, then eluted with H2O/MeOH mixture (H2O/MeOH = 95/5–0/100, 70 min, flow rate 40 mL/min) through the stepwise gradient method. The eluent with different ratios of methanol and water was flowed into the main column of Sepiatec® Sepbox 2D-2000 (Seipatec, Germany). Based on different elution time, the eluents were collected into 15 fractions through solid phase extraction column (Trap column). Subsequently, the eluent was flushed into the C18-reversed phase column with different ratios of methanol and acetonitrile as mobile phases for the second separation. A dual detector approach with DAD and ELSD was utilized to record the signal. Eventually, a total of 352 fractions were collected and trace merged by TLC method. The above procedures were repeated three times to combine the three batches of C. manghas extract. The collected solution was then vacuum-concentrated and freeze-dried. After weighing, NMR was used for structural identification of components in fractions 193 to 195 containing major peaks with large areas.
3 Results
3.1 Evaluation of the melanin inhibitory effect of skin-whitening compounds using the zebrafish model
In order to assess the feasibility of using zebrafish as a screening platform for whitening compounds, six commercially available whitening compounds and additives (salicylic acid, sodium ascorbate, azelaic acid, transamin, kojic acid, and phenylthiourea (PTU)), as well as two ingredients with anti-oxidative property for preventing skin aging (mangiferin [25] and verbascoside [26]) were subjected to the zebrafish screening system. Various concentrations of different compounds were applied to explore the safe dosage for embryos. The embryo and larva stages of zebrafish are the most vulnerable and thus susceptible to physical and chemical distress. When exposed to toxins, they often experience developmental abnormalities, delays, and death. Cytotoxicity can therefore be evaluated through malformation, non-hatching, and mortality rate. Based on the results, four compounds (azelaic acid, transamin, kojic acid and PTU) showed significant reduction in melanin contents, which demonstrated their skin whitening effect in relation to their dose–response relationship (Table 1). The safe and effective dose range for each of these four compounds were indicated in the table as well. The other two compounds (sodium ascorbate and verbascoside) also had skin-whitening effects on zebrafish, while mangiferin showed little effect. On the other hand, 250 μM of salicylic acid significantly increased the melanin content in contrast. Based on the experimental results, zebrafish model corroborated the effects of known skin-whitening compounds currently used in the cosmetic industry, therefore proving its suitability for screening whitening compounds.
Compounds
Concentration
Mortality rate (%)
Non-hatching rate (%)
Malformation rate (%)
Area of pigmentation (%)
Mean ± SEM1
N = 20
N = 13
Azelaic acid
750 μM
100
0
0
NA
500 μM
0
0
0
84.24 ± 1.82***
250 μM
0
0
0
89.65 ± 2.65*
50 μM
0
0
0
90.68 ± 3.15
Transamin
20 mM
0
0
0
64.32 ± 1.59***
10 mM
0
0
0
86.64 ± 3.05***
2 mM
0
0
0
91.05 ± 3.28*
Kojic acid
20 mM
0
0
0
0.00 ± 0.00***
10 mM
0
0
0
0.37 ± 0.18***
2 mM
0
0
0
58.70 ± 1.75***
PTU
200 μM
0
0
0
0.00 ± 0.00***
100 μM
0
0
0
6.02 ± 0.80***
20 μM
0
0
0
62.25 ± 4.80***
Sodium ascorbate
20 mM
0
0
0
88.24 ± 2.84**
Salicylic acid
500 μM
100
0
0
NA
250 μM
0
0
0
157.45 ± 5.15***
Mangiferin
500 μM
100
0
0
NA
250 μM
0
0
0
105.59 ± 5.37
Verbascoside
750 μM
0
100
0
NA
500 μM
0
0
0
93.49 ± 1.51*
3.2 Rapid screening of natural extracts on three zebrafish embryos
As supported by the previous experiment, we established zebrafish as a viable rapid screening platform that evaluates the potential whitening property of 123 natural extracts. Low (100 μg/mL) and high (8.5–20.5 mg/mL) concentrations of natural extracts (Table S1) were used to analyze the embryogenesis and melanin distribution of zebrafish larvae (N = 3).
Based on our preliminary data (refer to Table S1), 26 samples (# 26, 31, 32, 35, 37, 40, 41, 43, 48, 51, 56, 60, 63, 65, 69, 82, 96, 101, 102, 113, 114, 115, 116, 118, 119, and 121) showed slight whitening effect without toxicity at the lower concentration (refer to Table S1 for scientific names). Meanwhile, 9 extracts (# 11, 12, 13, 21, 25, 93, 94, 99, and 108) increased melanin production significantly with no toxic effect. Results also showed that extracts from 5 samples significantly increased the mortality rates from 33 to 100% (# 34, 36, 44, 78, and 123). Moreover, 9 extracts led to developmental abnormalities of the zebrafish embryos (#1, 4, 27, 28, 30, 38, 43, 57, and 79). The rest of the extracts neither showed toxicity nor demonstrated whitening effect.
At higher concentrations (refer to Table S1), extracts from 11 natural sources significantly reduced melanin production with no toxic effect on the zebrafish embryos (#7, 10, 29, 42, 47, 53, 77, 81, 82, 103, 119). Extracts from 2 samples showed significant inhibitory effect on melanogenesis, but had toxicity and impeded embryo development (#32 and 76). There were 3 extracts with non-toxic and non-significant melanin reduction effects (#79, 92, and 94). In total, we found 91 natural extracts lethal, 13 natural extracts mutagenic that caused developmental delays, and 7 led to developmental abnormalities at a high concentration.
3.3 Rapid screening of natural extracts on twenty zebrafish embryos
According to the initial results, we observed that none of the extracts had a significant skin-whitening effect at a low concentration. Despite some extracts showing a significant reduction in melanin production at higher concentrations, most extracts possessed toxicity. These preliminary results indicated the need for further investigation on the effectiveness and safety with a larger sample size.
Based on the results of preliminary screening at a lower concentration, the top 16 candidates that showed melanin inhibitory effect and no detectable lethal toxicity were chosen to repeat the experiments not only to increase the sample size, but also to reevaluate their respective toxicity and whitening property (Table 2 and Fig. 2). Among the 16 natural extracts, 4 (#31, 40, 79, and 96) were non-toxic and significantly reduced the melanogenesis at a concentration of 100 μg/mL. 4 extracts proved to be toxic to zebrafish embryos with 5–40% mortality or abnormality rate (#26, 35, 102, and 118), and 2 extracts conversely increased the production of melanin significantly (#60 and 69). The other 6 extracts showed no effects. Even though samples number 31, 40, and 96 presented whitening effects at low concentration with no harmful effects on zebrafish embryogenesis, they were cytotoxic at a higher concentration in the preliminary results, which engendered the need to determine the optimal concentration for usage.
Compound Number
Scientific Name of the Compounds
Mortality rate (%)
Non-hatching rate (%)
Malformation rate (%)
Area of pigmentation (%) Mean ± SEM1
N = 20
N = 13
26
Ludwigia octovalvis (Jacq.) P. H. Raven
0
0
5
98.55 ± 3.93
31
Ixeris chinensis (Thunb.) Nakai
0
0
0
74.86 ± 9.88*
35
Peperomia dindigulensis Miq.
5
0
0
108.41 ± 4.58
40
Lindera communis Hemsl.
0
0
0
88.06 ± 4.22*
41
Glossogyne tenuifolia (Labill.) Cass.
0
0
0
94.52 ± 2.79
60
Celtis sinensis Pers
0
0
0
123.82 ± 4.90***
69
Duchesnea indica (Andr.) Focke
0
0
0
150.73 ± 2.57***
79
Kalanchoe garambiensis Kudo
0
0
0
80.64 ± 4.39***
82
Hexagonia apiaria Pers.
0
0
0
107.04 ± 3.20
96
Pinus morrisonicola Hayata
0
0
0
85.00 ± 1.88*
101
Arenga tremula (Blanco) Becc.
0
0
0
94.64 ± 5.91
102
Siegesbeckia orientalis L.
0
10
0
95.67 ± 5.02
114
Tinospora dentata Diels
0
0
0
103.50 ± 4.68
115
Actinostemma tenerum Griff.
0
0
0
105.97 ± 6.05
116
Liquidambar formosana Hance
0
0
0
93.76 ± 2.47
118
Myristica ceylanica A. DC. var. cagayanensis (Merr.) J. Sinclair
0
35
5
80.61 ± 11.46
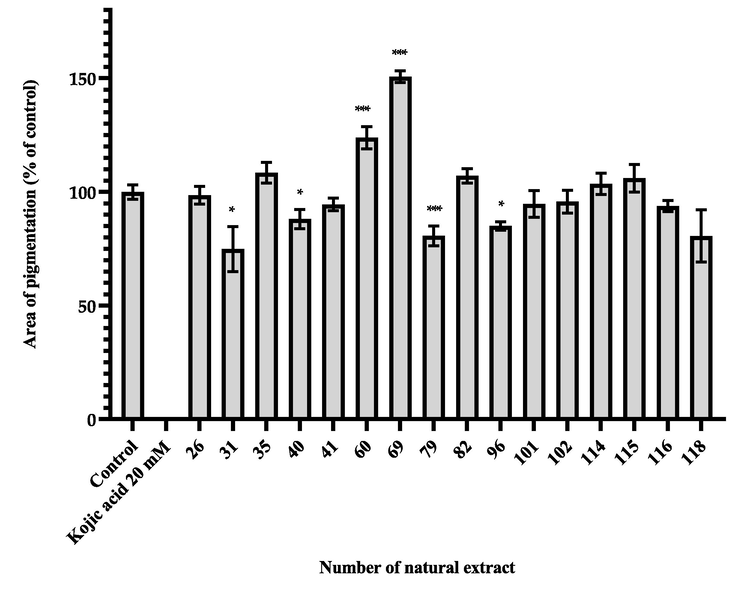
The area of pigmentation of zebrafish treated with natural extracts at low concentration (100 μg/mL). Results were expressed as % of control (100%) and mean ± SEM, N = 13, * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
At the high concentration, we selected 13 natural extracts which exhibited significant whitening effects with no lethal toxicity in the preliminary screening to subject to similar procedures with larger sample sizes as well. 20 fertilized eggs were incubated with extracts at a concentration of 10 mg/mL. As shown in Table 3 and Fig. 3, 9 extracts (#7, 10, 29, 42, 47, 53, 76, 77, and 81) significantly reduced the melanin content on the larvae with no signs of toxicity. The other 4 extracts (#32, 82, 103 and 119), however, significantly reduced the melanin content while suggesting toxicity (see Table 3).
CompoundNumber
Scientific Name of the Compounds
Mortality rate (%)
Non-hatching rate (%)
Malformation rate (%)
Area of pigmentation (%) Mean ± SEM1
N = 20
N = 13
7
Wolfiporia cocos (Schw.) Ryv. & Gilbn.
0
0
0
39.06 ± 3.98***
10
Pinellia ternata (Thunb.) Breit.
0
0
0
70.19 ± 1.47***
29
Anoectochilus formosanus Hayata
0
0
0
73.53 ± 3.72***
32
Vernonia patula (Dryand.) Merr.
0
25
55
23.63 ± 2.67***
42
Urena lobata L.
0
0
0
71.75 ± 6.83**
47
Ageratum houstonianum Mill.
0
0
0
53.6 ± 4.46***
53
Pennisetum purpureum Schumach.
0
0
0
46.83 ± 3.33***
76
Plantago asiatica L.
0
0
0
61.99 ± 2.42***
77
Paederia foetida L.
0
0
0
51.89 ± 1.84***
81
Cerbera manghas L.
0
0
0
58.27 ± 3.65***
82
Hexagonia apiaria Pers.
0
5
0
45.91 ± 4.38***
103
Sedum formosanum N.E. Br.
0
0
15
45.71 ± 3.59***
119
Alpinia shimadae Hayata
0
20
5
31.28 ± 4.22***
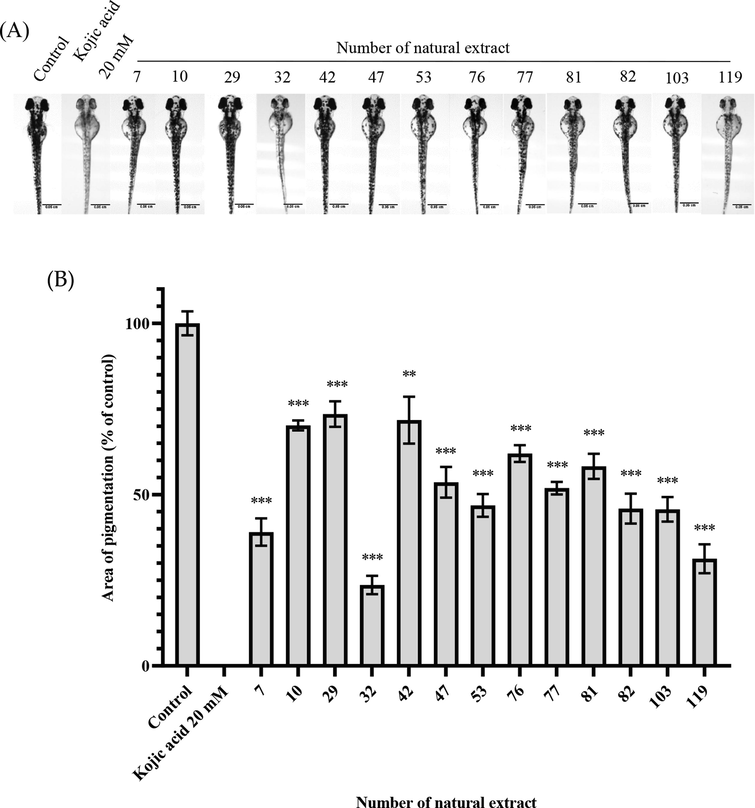
The whitening effect of natural extracts at high concentration (10 mg/mL) (A) Scale of zebrafish photo = 0.05 cm. (B)The area of pigmentation of zebrafish treated with the extracts. Results were expressed as % of control (100%) and mean ± SEM, N = 13, * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
3.4 Effect of C. manghas extract on melanogenesis in B16-F10 cells
Through the zebrafish fast screening platform, 4 natural extracts selected from low concentrations and 9 from high concentrations indicated non-toxic whitening effects. Considering that the 9 highly concentrated, non-toxic extracts are safer to use on the skin and that there have only been a few studies published about C. manghas, we decided to focus on it and used a well-established α-MSH-stimulated B16-F10 melanoma cell model for evaluating its anti-melanogenic property. The melanin content and cell viability of B16-F10 cells treated with different concentrations of C. manghas extracts were compared with the control group, which was assigned at 100%. Arbutin was used as positive control at the concentration of 50 μM. As a result, the extracts at all of the three concentration significantly reduced the melanin content in B16-F10 cells (p-value < 0.05), and the melanogenesis inhibitory rate of 25, 50, and 100 μg/mL of extract were 13.20 ± 4.55%, 20.70 ± 10.11%, and 46.87 ± 5.55% (Mean ± SEM), respectively (Fig. 4). The cell viabilities were all higher than 80%, indicating a moderate level of cytotoxicity for C. manghas at the concentrations tested.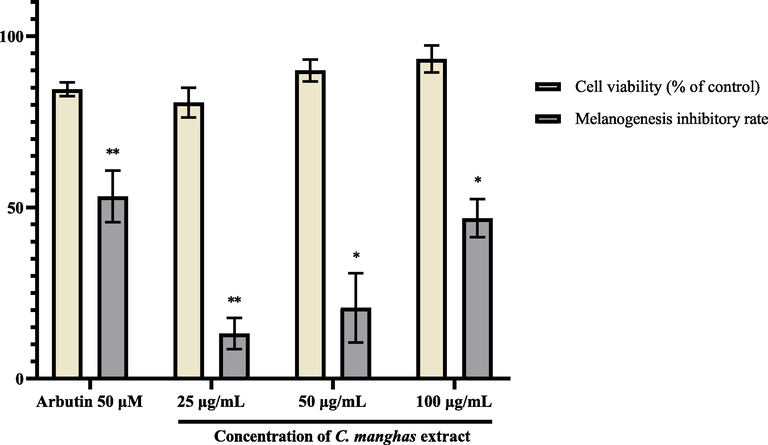
Cell viability and melanogenesis inhibitory rate of B16-F10 cells treated with different concentrations of C. manghas extract (25–100 μg/mL). Results were expressed as % of control and mean ± SEM, N = 3, * p-value < 0.05, ** p-value < 0.01.
3.5 Plant material
The leaves of Cerbera manghas were collected from Yilan Sports Park in Yilan City in July 2018 and were identified by Dr. S. Y. Chen at the Council of Agriculture, Executive Yuan. Voucher Specimens (No. CKL07012018) were deposited at the School of Pharmacy, Taipei Medical University, Taipei, Taiwan.
3.5.1 Extraction and isolation of C. manghas leaf
The fresh leaves (600 g) of C. manghas (mango) were extracted three times with 6 L of EtOH. Subsequently, 2 g of the mango extract (out of 42 g) was taken out for separation and purification experiments using the two-dimensional chromatography Sepiatec Sepbox stepwise gradient mode.
3.5.2 Two-dimensional chromatography Sepiatec Sepbox 2D-2000 separation of C. manghas leaf crude extract
2 g of C. manghas extract was coated with 10 g of C4 reversed-phase silicone gel, then eluted with H2O/MeOH mixture (H2O/MeOH = 95/5–0/100, 70 min, flow rate 40 mL/min) through the stepwise gradient method. The eluent with different ratios of methanol and water was flowed into the main column of Sepiatec®Sepbox 2D-2000 (Seipatec, Germany). Based on different elution time, the eluents were collected into 15 fractions through solid phase extraction column (Trap column). Afterwards, the eluent was flushed into the C18-reversed phase column with different ratios of methanol and acetonitrile as mobile phases for the second separation. Finally, a total of 352 fractions were collected and analyzed by TLC method. The above procedures were repeated three times to combine the three batches of C. manghas extract. The collected solution was then vacuum-concentrated and freeze-dried.
3.5.3 Separation and identification of the compounds extracted from C. manghas
In order to find the compounds with potential bioactivity in a complex mixture, we used Sepbox® to efficiently separate 6 g of the C. manghas methanol extract into 352 fractions, fractions with similar TLC results were combined and NMR was used to confirm the purity and structure. Six major compounds (Fig. 5) were identified in fractions 191–193, 195–196, 247–250, and 342–343.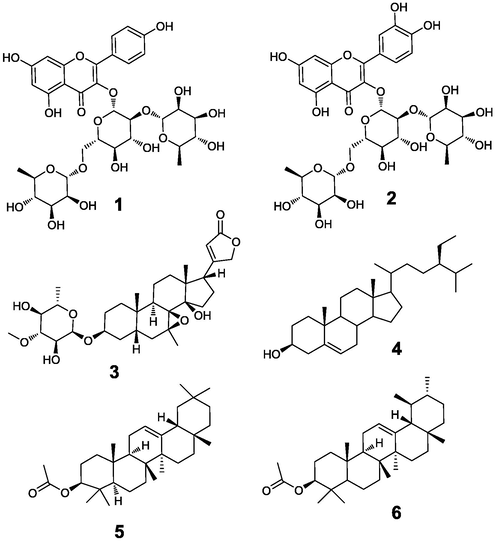
Structure of 1–6.
3.5.3.1 Characterization of clitorin (1)
The compound was obtained as a yellowish solid from the195-196th fraction separated by the Sepbox®. The results of 1H- and 13C NMR of 1 were as follows: 1H NMR (600 MHz, methanol‑d4): δ 8.03 (2H, d, J = 7.9 Hz, H-2′and H-6′), 6.89 (2H, d, J = 7.9 Hz, H-3′ and H-5′), 6.37 (1H, d, J = 2.0 Hz, H-8), 6.18 (1H, d, J = 2.0 Hz, H-6), 5.60 (1H, d, J = 7.7 Hz, H-1 glc), 5.22 (1H, s, H-1 rha), 4.50 (1H, s, H-1 rha'), 4.10 to 3.20 (14H, sugar protons), 1.05 (3H, d, J = 6.0 Hz, Me-rha'), 0.98 (3H, d, J = 6.4 Hz, Me-rha). 13C NMR (150 MHz, methanol‑d4): δC 179.3, 166.3, 163.3, 161.4, 159.1, 158.7, 134.4, 132.3, 123.3, 116.3, 105.9, 102.7, 102.4, 100.6, 100.1, 95.0, 80.0, 79.1, 77.2, 74.2, 74.0, 72.5, 72.4, 72.2, 72.1, 70.0, 69.9, 68.4, 18.0, 17.7. The compound was identified as kaempferol 3-O-(2′’,6′’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (Clitorin) (1) and confirmed by literature (Yoshitama et al., 1997).
3.5.3.2 Characterization of manghaslin (2)
The compound was obtained as a yellowish solid from the 191st-193rd fraction separated by the Sepbox®. The results of 1H - and 13C -NMR of 2 were as follows: 1H NMR (600 MHz, Methanol‑d4): δ 7.59 (1H, dd, J = 8.2, 2.3 Hz, H-6′), 7.58 (1H, d, J = 2.3 Hz, H-2′), 6.87 (1H, d, J = 8.2 Hz, H-5′), 6.36 (1H, d, J = 1.8 Hz, H-8), 6.18 (1H, d, J = 1.8 Hz, H-6), 5.58 (1H, d, J = 7.6 Hz, H-1′’), 5.22 (1H, br s, 2′’-Rha-1), 4.29 (1H, br s, 1H, 6′’-Rha-1), 4.220–3.20 (m, 14H, remaining sugar signals)*, 1.07 (3H, d, J = 6.5 Hz, 2′’-Rha-6), 1.00 (3H, d, J = 6.5 Hz, 6′’-Rha-6). 13C NMR (150 MHz, Methanol‑d4): δC 179.0 (C-4), 164.2 (C-7), 163.0 (C-5), 158.6 (C-2), 158.3 (C-9), 149.5 (C-4′), 145.7 (C-3′), 132.3 (C-3), 123.3 (C-1′), 123.1 (C-6′), 117.1 (C-2,), 116.0 (C-5′), 105.3 (C-10), 102.4 (C-2′’-Rha-1), 102.0 (C-6′’-Rha-1), 100.3 (C-1′’), 99.7 (C-6), 94.3 (C-8), 80.0 (C-2′’), 78.7 (C-3′’), 76.7 (C- 5′’), 73.8 (C-6′’-Rha-4), 73.8 (C-2′’-Rha-4), 72.3 (C-2′’-Rha-2), 72. 1 (C- 2′’-Rha-3), 72.0 (C-6′’-Rha-3), 71.9 (C-6′’-Rha-2), 71.5 (C-4′’), 69.8 (C- 2′’-Rha-5), 69.6 (C-6′’-Rha-5), 68.0 (C-6′’), 17.7 (C-6′’-Rha-6), 17.3 (C- 2′’-Rha-6). The compound was identified as Quercetin 3-O-(2′’,6′’-di-O-α-L-rhamnopyranosyl)- β-D-glucopyr-anoside (manghaslin) (2) and confirmed by literature (Gürbüz et al., 2021).
3.5.3.3 Characterization of deacetyltanghinin (3)
The compound was obtained as a white solid from the 247-250th fraction separated by the Sepbox®. The 1H - and 13C -NMR spectra of compound 3 were as follows:
1H NMR (600 MHz, CDCl3): δH 5.86 (1H, br t, H-6′), 4.93 (1H, dd, J = 2.3 Hz, H-21a), 4.82 (1H, d, J = 4.2 Hz, H-1′), 4.77 (1H, dd, J = 18.0, 1.8 Hz, H-21b), 3.91 (1H, brs, H-3), 3.66 (3H, s, H-3′ OMe), 3.22 (1H, d, J = 9.6 Hz, 3′), 3.20 (1H, d, J = 5.2 Hz, H-7), 3.13 (1H, t, J = 9.6 Hz), 2.80 (1H, dd, J = 8.0,6.0 Hz, H-17), 1.23 (3H, d, J = 6.0 Hz, H-6′), 0.97 (3H, s, H-19), 0.90 (3H, s, H-18).
13C NMR (150 MHz, CDCl3): δC 174.2 (C-23), 173.5 (C-20), 117.9 (C-22), 97.3 (C-1′), 84.5 (C-3′), 81.0 (C-14), 74.7 (C-4′), 73.3 (C-21), 72.9 (C-3), 72.7 (C-2′), 67.6 (C-5′), 63.9 (C-8), 60.6 (C-3′ OMe), 52.2 (C-13), 51.1 (C-7), 50.6 (C-17), 41.0 (C-12), 34.4 (C-15), 34.2 (C-5), 33.6 (C-10), 31.6 (C-1), 31.5 (C-9), 28.3 (C-6), 27.8 (C-16), 27.0 (C-2), 24.3 (C-19), 20.3 (C-11), 17.5 (C-6′), 17.0 (C-18).
The compound was identified as deacetyltanghinin (3) and confirmed by Abe et al. in literature.
3.5.3.4 Characterization of β-sitosterol (4), β-amyrin acetate (5), and α-amyrin acetate (6)
The mixture compounds were obtained as a white solid from the 342nd-343rd fraction separated by the Sepbox®. The NMR spectra of compound 4–6 were as follows:
The 1H - and 13C -NMR spectra of compound 4:
1H NMR (600 MHz, CDCl3): δH 5.34 (1H, t, J = 6.4 Hz, H-5), 3.51 (1H, m, H-3), 1.01 (3H, s, H-29), 0.91 (3H, d, J = 6.2 Hz), H-19), 0.84 (3H, t, J = 7.2, H-24), 0.82 (3H, d, J = 6.4 Hz, H-26), 0.80 (3H, d, J = 6.4, H-27), 0.67 (3H, s, H-28).
13C NMR (150 MHz, CDCl3): δC 140.8 (C-5), 121.6 (C-6), 71.8 (C-3), 56.8 (C-14), 56.1 (C-17), 50.2 (C-9), 46.1 (C-22), 42.3 (C-13), 42.3 (C-4), 40.0 (C-18), 39.9 (C-12), 37.3 (C-1), 36.5 (C-10), 32.5 (C-2), 31.9 (C-7), 31.9 (C-8), 29.7 (C-25), 29.2 (C-16), 26.1 (C-15), 26.1 (C-21), 25.9 (C-15), 23.3 (C-23), 21.3 (C-11), 19.9 (C-26),19.4 (C-19), 19.8 (C-27),19.0 (C-28), 12.0 (C-29). The compound was identified as β-sitosterol (4) and we confirmed this with published literature (Chaturvedula, et al., 2012).
The 1H - and 13C -NMR spectra of compound 5:
1H NMR (600 MHz, CDCl3): δH 5.17 (1H, t, J = 3.8 Hz, H-12), 4.48 (1H, t, J = 8.2 Hz, H-3), 1.12 (3H, s, H-27), 1.00 (3H, s, H-26), 0.96 (3H, s, H-25), 0.86 (3H, s, H-23), 0.86 (3H, s, H-24), 0.86 (3H, s, H-29), 0.856 (3H, s, H-30), 0.852 (3H, s, H-28), 2.02 (3H, s, OCOCH3).
13C NMR (1 (150 MHz, CDCl3): δC 171.0(OC = O), 145.2 (C-13), 121.7 (C-12), 81.0 (C-3), 55.2 (C-5), 47.6 (C-9), 46.8 (C-19), 41.7 (C-14), 39.8 (C-4), 37.7 (C-8), 37.1 (C-22), 36.8 (C-10), 34.7 (C-21), 33.4 (C-29), 32.6 (C-7), 32.5 (C-17), 31.1 (C-20), 28.4 (C-28), 28.0 (C-23), 26.9 (C-16), 26.1 (C-15), 25.9 (C-27), 23.7 (C-30), 23.6 (C-11), 23.5 (C-2), 21.3 (OOCCH3), 18.2 (C-6), 16.8 (C-26), 15.6 (C-24), 15.5 (C-25). The compound was identified as β-amyrin acetate (5) and we confirmed this with published literature (Atiku et al., 2022).
The 1H - and 13C -NMR spectra of compound 6:
1H NMR (600 MHz, CDCl3): δH 5.11 (1H, t, J = 3.4 Hz, H-12), 4.48 (1H, t, J = 8.2 Hz, H-3), 1.00 (3H, s, H-27), 0.96 (3H, s, H-26), 0.95 (3H, s, H-25), 0.86 (3H, s, H-23), 0.86 (3H, s, H-24), 0.81 (3H, d, H-30), 0.80 (3H, s, H-28), 0.79 (3H, d, H-28), 2.02 (3H, s, OCOCH3).
13C NMR (150 MHz, CDCl3): δC 171.0 (OC = O), 139.6 (C-13), 124.3 (C-12), 81.0 (C-3), 59.1 (C-18), 55.3 (C-5), 47.7 (C-9), 42.1 (C-14), 41.5 (C-22), 40.0 (C-8), 39.65 (C-20), 39.61(C-19), 38.5 (C-1), 37.7 (C-4), 36.8 (C-10), 33.8 (C-17), 32.9 (C-7), 31.3 (C-21), 28.7 (C-28), 28.10 (C-15), 28.07 (C-23), 26.6 (C-16), 23.6 (C-2), 23.4 (C-11), 23.2 (C-27), 21.4 (C-30), 21.4 (OOCCH3), 18.2 (C-6), 17.5 (C-26),16.9 (C-29),16.7 (C-24),15.8 (C-25). The compound was identified as α-amyrin acetate (6) and confirmed by literature (Nnamonu et al., 2016).
3.6 Effect of the isolated compounds on zebrafish
We conducted tests on zebrafish in order to verify the whitening property of clitorin, manghaslin and deacetyltanghinin. The results showed inconspicuously that clitorin reduces melanin to 77.05 ± 11.37% at the concentration of 50 μM. Under 100 μM concentration, clitorin and deacetyltanghinin have lethal toxicity. As for manghaslin, it showed non-toxicity at both 50 and 100 μM concentrations and considerably reduced the melanin content in zebrafish to 72.57 ± 3.76% and 44.91 ± 2.40%, respectively (Fig. 6, Table 4).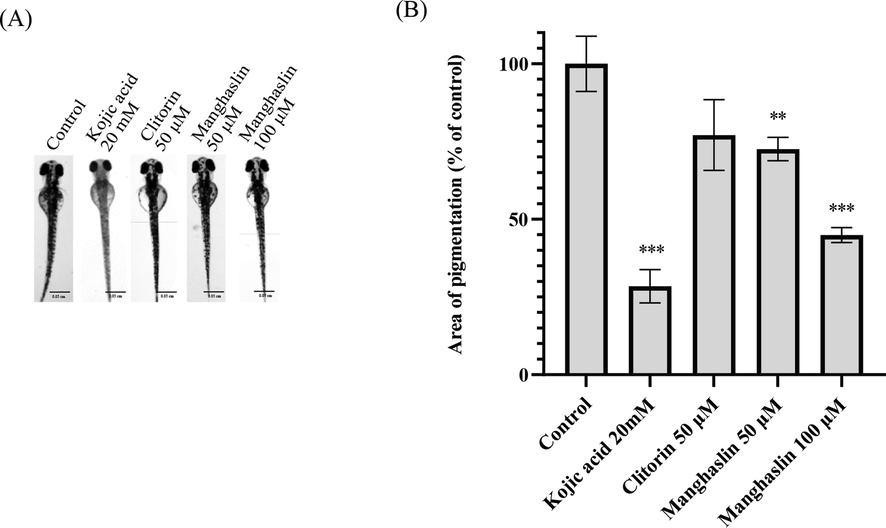
The whitening effect of clitorin and manghaslin. (A) Scale of zebrafish photo = 0.05 cm. (B)The area of pigmentation of zebrafish. Results were expressed as % of control and mean ± SEM, N = 8, * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
Compounds
Concentration
Mortality rate (%)
Non-hatching rate (%)
Malformation rate (%)
Area of pigmentation (%)
Mean ± SEM1
N = 8
N = 8
Clitorin
100 μM
100
0
0
NA
50 μM
0
0
0
77.05 ± 11.37
Manghaslin
100 μM
0
0
0
44.91 ± 2.40***
50 μM
0
0
0
72.57 ± 3.76**
4 Discussion
For large scale screening of skin-whitening products using zebrafish platform, image quantification of melanin content is an efficient method for tracking the effective candidates in spite of its approximation of assessing melanin (Choi et al., 2007, Lajis, 2018). The zebrafish generating melanin pigments on its surface allows direct observation of the pigmentation process under the microscope. By imaging 200 zebrafishes in various groups and arbitrarily scoring for the percentage of pigmentation reduction on body, Choi et al. evaluated the melanin inhibitory effects of four small molecules and found the results were consistent with those obtained from the widely used spectrophotometric method and tyrosinase activity assays (Choi et al., 2007). In our study, we used ImageJ to rapidly quantify the melanin pigmentation area for assessment of melanin inhibitory effect. While this method of observation is simple and convenient, it efficiently screens and identifies candidates with melanin inhibitory effects. In the presence of a large sample, image quantification appears to be sufficiently effective in discerning melanin pigmentation, which corroborates Choi's study. Other than image, there are also other methods to quantify melanin content. For example, Galval et al. reported Ramen spectroscopy using a confocal microscope could quantify melanin in feathers and hairs (Galván et al., 2013). A single Ramen spectrum could be obtained at each detection point, and the melanin content would then be estimated according to the characteristic bands of the spectrum after fitting to a Gaussian function. In addition, Fernandes et al. assessed the content of melanin in cells through fluorescence spectroscopy (Fernandes et al., 2016) based on the property that pigments acquire fluorescence after oxidation with hydrogen peroxide.
Prior to performing the screening of melanin reducing natural extracts, 8 compounds -which were either commercially available as cosmetic whitening ingredients or patented with skin improving functions- were tested in this study to verify the effectiveness and feasibility of zebrafish as a model for measuring melanin inhibitory effect. Under non-cytotoxic concentrations, we observed that azelaic acid, transamin, kojic acid, and PTU exhibited obvious skin-whitening effect and had a dose-dependent effect on melanogenesis of zebrafish, all of which were in congruence with the literature (Cabanes et al., 1994, Ryazanova et al., 2012, Couteau and Coiffard, 2016, Cho et al., 2017). Verbascoside, also found in sample #42, and sodium ascorbate significantly reduced the melanin content at 500 μM and 20 mM respectively. On the other hand, salicylic acid did not show the melanin reduction, which can be attributed to its exfoliation function (Kligman, 2001). Since zebrafish lacks outworn stratum corneum cells on the outermost skin surface, this mechanism is irrelevant to melanogenic effect on the zebrafish (Li and Uitto, 2014). The other compound that failed to decrease the melanin content was mangiferin. Mangiferin possessses various bioactivities, including: antioxidative, antimicrobial, antidiabetic, anti-allergic, anti-cancerous, hypocholesterolemic, and immunomodulatory activities (Imran et al., 2017). However, there is limited evidence on its anti-melanogenic activity. Although the tyrosinase inhibitory rate of 3% mangiferin was reported to be 25.1% in a Japanese patent (Shigenao et al., 2015), the anti-melanogenic effect of mangiferin was not observed on B16 cells in previous literature (Homma et al., 2020). This is contradictory to the skin-whitening claims made by the patent and can be attributed to the limitations of enzyme assay.
We next designed a two-dosage screening strategy to search for safe and effective whitening compounds from natural sources. To evaluate the safety, cytotoxicity and whitening effect of natural extracts, 100 μg/ml -a concentration commonly used for testing of natural extracts- was deemed the lower concentration. A key assumption made was that if a compound retains its effectiveness at high concentration without being toxic, it is likely safe and suitable for product development. Consequently, a concentration of around 10 mg/ml was used for higher concentration testing. Through the dosage cut-off selections, a total of 13 natural extracts were identified with reducing melanogenesis under indicated concentration of extract treatment. The corresponding studies on the identified natural sources are discussed.
The tyrosinase inhibition activity of Wolfiporia cocos (Schw.) Ryv. & Gilbn extracts (the same species as #7) had been reported. Its whitening effect is considerably better than 19 medicinal herbs, including Paeoniae radix rubra, Rhodiola rosea, Ampelopsis japonica, Scutellaria baicalensis Georgi, and Panax ginseng (Wang et al., 2017). This is consistent with what was observed in our study, where the melanin inhibitory effect of W. cocos was noticeably better than the other natural extracts. Moreover, the literature further suggested W. cocos ethanol extracts inhibit melanin production through regulating tyrosinase-related proteins TRP-1 and TRP-2 (Park et al., 2014), and the poricoic acid A extracted from W. cocos can effectively inhibit tyrosinase (Hu et al., 2017).
From the extract of Pinellia ternata (Thunb.) Breit. (#10), 11 compounds possessing whitening effects have been isolated, these compounds are: [6]-gingerol, 1,3-diolein, 1,3-dilinolein, cyclolignanyingoside A, isolariciresinol, isolariciresinol-9-O-β-D-glucopyranoside, medioresinol, pinoresinol-4′-O-β-D-glucopyranoside, dehydrodiconiferyl, alcohol-9-O-β-D-glucopyranoside, and dehydrodiconiferyl alcohol-9′-O-β-D-glucopyranoside (Wu et al., 2015, An et al., 2016, Kang et al., 2017). [6]-gingerol could reduce the melanin content of B16 melanoma cells (Huang et al., 2011, An et al., 2016). Both 1,3-diolein and 1,3-dilinolein have been patented for the prevention and treatment of skin melanosis in South Korea (Hwang and Ku, 2011). 7 other compounds were reported to be tyrosinase inhibitors, with isolariciresinol and medioresinol showing the highest inhibitory activities (Wu et al., 2015). Additionally, the whitening mechanism of pinoresinol-4′-O-β-D-glucopyranoside was investigated as well (Akihisa et al., 2013).
Anoectochilus formosanus Hayata (#29) isolated compounds isorhamnetin-3-O-glucoside (Wang et al., 2002) and gastrodin (Du et al., 2000) were reported to inhibit the enzyme activity of mushroom tyrosinase (Karioti et al., 2007, Pei et al., 2013). In addition, gastrodin can significantly reduce the melanin production in B16 cells in a dose–response manner (Chen et al., 2013).
As for #42, extracts of Urena lobate L. decreased the activity of tyrosinase by 6.9% (Chen et al., 2017). Ergosterol peroxide (Shrestha et al., 2016) and mangiferin (Ghosh, 2004) were isolated from the plant. Ergosterol peroxide could inhibit melanogenesis in B16 melanoma cells (Xu et al., 2010, Hong et al., 2013). Several patent documents disclosed the use of compositions containing mangiferin or its derivatives for cosmetic applications with its antioxidant and anti-tyrosinase activities (Telang et al., 2013).
Plantago asiatica L. (#76) contains verbascoside and plantamajoside (Miyase et al., 1991). Verbascoside is a tyrosinase inhibitor (Muñoz et al., 2013); and plantamajoside was patented with skin improvement effect (Yoon et al., 2015).
Celtis sinensis Pers (#60) and Duchesnea indica (Andr.) Focke (#69) can significantly promote the content of melanin. There is no literature report on the affected ingredients, but the ethyl acetate part of Duchesnea indica (Andr.) Focke has a positive effect on tyrosinase little inhibitory activity (Ra et al., 2018), which is different from our results.
Paederia foetida L. (#77) consists of asperuloside (Chanda et al., 2017), which can reduce the melanin content in B16 cells(Akihisa et al., 2012).
Cerbera manghas L. (#81) consists of loganin (Yamauchi et al., 1990), which inhibits melanogenesis in B16 cells by suppressing the expression of tyrosinase (Jung et al., 2019).
Hexagonia apiaria Pers. (#82) consists of compounds such as ergosterol peroxide and ursolic acid (Thang et al., 2015). However, ursolic acid was found to be toxic to the B16 cells in spite of its ability to reduce melanin content (Song et al., 2013). We observed the outcome likewise.
From the extract of the heartwood of Pinus morrisonicola Hayata (#96), genkwanin, chrysin, galangin had been isolated (Fang et al., 1987). Genkwanin and chrysin were patented for their effects in whitening (Sang-jin et al., 2005, Moo-hyun et al., 2013). Galangin is a competitive inhibitor of tyrosinase which can reduce melanin production in B16 melanoma cells (Lu et al., 2007).
C. manghas L. (#81) is a toxic plant found in coastal tropical areas. By using the B16-F10 cell model, we assessed its whitening effect and impact on cell viability within the concentration range of 25 to 100 μg/mL. In addition, we also successfully purified two antioxidants, clitorin and manghaslin, which are flavonoids (Ma et al., 2018) with cell line and tissue specificity (Sak, 2014) and a highly cytotoxic deacetylinosine (Sarot et al., 2004). β-sitosterol, β-amyrin acetate, and α-amyrin acetate were also found from this plant.
Besides the use of in vitro tyrosinase inhibition assay and the B16 murine melanoma model for screening molecules, in vivo animal models sharing human homology are also crucial for pre-clinical studies of compound candidates with anti-tyrosinase and anti-melanogenesis properties. In this study, we used zebrafish as an appropriate animal model to establish a high-throughput platform for evaluating the skin-whitening property of natural sources. Manghaslin demonstrated promising anti-melanogenesis properties with no cytotoxicity in our preliminary assessment. Further investigation would be required to validate the findings and confirm its skin-whitening benefit as a commercial product.
Manghaslin is an anti-inflammatory agent and an acetylcholine esterase (AchE) inhibitor with an IC50 of 94.92 (Olennikov et al., 2017, Kırmızıbekmez et al., 2019). It belongs to a class of organic compounds recognized as flavonoid-3-o-glycosides. Flavonoids, including several major polyphenolic groups of compounds, are commonly found in various vegetables and medicinal herbs (Testai, 2015). These compounds have gained much attention because of their extensive biological effects such as anti-oxidative, free radical scavenging, anti-tumor, anti-inflammation, and anti-viral activities (Kumar and Pandey, 2013). Some flavonoids, such as hesperidin, baicalein, quercetin, kaempferol, epigallocatechin-3-gallate (EGCG), and luteolin, have been studied for their effects on melanogenesis, as well as their regulatory mechanisms on melanin synthesis in the B16 mouse melanoma cell lines, while enormous gene loci have corresponding orthologues with human genes. In terms of mechanism of action, hesperidin and catechins, including EGCG, inhibit MITF (Kim et al., 2004, Lee D.-Y et al., 2015), whereas EGCG also inhibits tyrosinase production in addition to the two targets stated prior (Kim et al., 2004, Sato and Toriyama, 2009). Baicalein, on the other hand, inhibits MITF via an alternative pathway (Li et al., 2010). Kaempferol and quercetin directly target tyrosinase to down-regulate its effect on melanogenesis (Nagata et al., 2004, Rho et al., 2011). Apigenin targets TYRP-2 and TYRP-1 for melanogenic inhibition (Ye et al., 2010). Interestingly, luteolin activates tyrosinase production but inhibits melanin synthesis by increasing the mRNA level of ASIP (Agouti-signaling protein), which is a negative regulator of a-MSH-mediated cAMP-PKA signaling (Suzuki et al., 1997). It is known that different side group modifications on the the common three rings of flavonoids express distinct biological functions (Karak, 2019). While manghaslin belongs to the group of flavonoids that are widely applied as a nutraceutical, little literature has been published about its biological functions, especially its whitening effect. Our study is the first in the field to demonstrate the anti-melanogenesis property of manghaslin isolated from C. manghas. The anti-melanogenesis property of manghaslin suggests its potential to serve as a skin-whitening agent and treatment for melanoma. In view of the chemical structure, the only difference between clitorin and manghaslin is the hydroxyl group in positions 3′ of manghaslin, which might relate to the cytotoxicity and antioxidant activity. In addition, magnhaslin, luteolin, and quercetin share the common structure with different modifications in position 3 as well. Future studies should focus on elucidating the regulatory mechanism of manghaslin on melanin production and exploring its possible functions in scavenging the reactive oxygen species (ROS), regulating the cell cycle, and modulating the immune system.
5 Conclusions
Preliminary experimental results showed that while most natural extracts have limited ability to reduce melanin production at a low concentration, there were both greater effectiveness and cytotoxicity at a higher concentration. By quantifying the melanin containing area and its color intensity on a zebrafish larva using a dynamic image capture lens and analysis software, the platform was able to rapidly screen 123 kinds of natural extracts in Taiwan, and efficiently assess their skin-whitening effect to predict their potential in the cosmetic market. Moreover, the whitening effect of C. manghas extract was verified by both zebrafish and B16 cell models, and a compound manghaslin isolated from the extract was identified to possess the effect of melanin reduction. In summary, we discovered an unpublished yet promising compound in C. manghas L. with skin-whitening effect.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the Ministry of Science and Technology of the Republic of China (Taiwan) [grant number MOST 107–2320-B-038–019 -MY3].
CRediT authorship contribution statement
Sui-Wen Hsiao: Validation, Formal analysis, Data curation, Visualization. I-Chih Kuo: Writing – original draft. Li-Ling Syu: Validation, Investigation, Writing – original draft. Tzong-Huei Lee: Resources, Writing – review & editing. Chia-Hsiung Cheng: Methodology, Supervision, Project administration. Hui-Ching Mei: Conceptualization, Writing – review & editing. Ching-Kuo Lee: Conceptualization, Resources, Supervision, Project administration, Funding acquisition.
Acknowledgements
We are grateful to Dr. Shwu-Huey Wang for the NMR data acquisition by the TMU Core Facility. Chien-Min Chen and Hsiu-Wen Huang for providing research materials by Taiwan Endemic Species Research Institute.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Melanogenesis-inhibitory saccharide fatty acid esters and other constituents of the fruits of Morinda citrifolia (Noni) Chem. Biodivers.. 2012;9:1172-1187.
- [Google Scholar]
- Melanogenesis-inhibitory activity of aromatic glycosides from the stem bark of Acer buergerianum. Chem. Biodivers.. 2013;10:167-176.
- [Google Scholar]
- Utilization of [6]-gingerol as an origin discriminant marker influencing melanin inhibitory activity relative to its content in Pinellia ternata. J. Appl. Biol. Chem.. 2016;59:323-330.
- [Google Scholar]
- Isolation of β-amyrin acetate from the leaf of Ficus sycomorous L. UMYU Journal of Pure and Industrial Chemical Research.. 2022;2:64-72.
- [Google Scholar]
- Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol.. 1994;46:982-985.
- [Google Scholar]
- Screening of marine algae for potential tyrosinase inhibitor: those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol.. 2011;38:354-363.
- [Google Scholar]
- Targeted metabolite analysis of Iridoids of paederia foetida: A popular tribal edible plant of northeast. India. Asian J. Chem.. 2017;29:1713-1717.
- [Google Scholar]
- Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Chaturvedula and Prakash, International Current Pharmaceutical Journal.. 2012;1:239-242.
- [Google Scholar]
- Chemical composition and antioxidant, bactericidal, and matrix metalloproteinase inhibition activity of food-related plant. LWT.. 2017;82:411-419.
- [Google Scholar]
- Effects of gastrodin on formation of B16 melanoma cell melanogenesis. J. Anhui Agric. Sci.. 2013;41:5280-5282.
- [Google Scholar]
- Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep.. 2015;5:1-8.
- [Google Scholar]
- Melanogenesis inhibitors from the rhizoma of Ligusticum sinense in B16–F10 melanoma cells in vitro and zebrafish in vivo. Int. J. Mol. Sci.. 2018;19:3994.
- [Google Scholar]
- Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci.. 2017;88:96-102.
- [Google Scholar]
- Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res.. 2007;20:120-127.
- [Google Scholar]
- Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics. 2016;3:27.
- [Google Scholar]
- Glycosidic constituents from in vitro Anoectochilus formosanus. Chem. Pharm. Bull.. 2000;48:1803-1804.
- [Google Scholar]
- Fluorescent quantification of melanin. Pigment Cell Melanoma Res.. 2016;29:707-712.
- [Google Scholar]
- Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment Cell Melanoma Res.. 2013;26:917-923.
- [Google Scholar]
- Ghosh, K., 2004. A furocoumarin, Imperatorin isolated from Urena lobata L.(Malvaceae). Molbank. 2004, M382.
- Examining the impact of skin lighteners in vitro. Oxid. Med. Cell. Longev.. 2013;2013
- [Google Scholar]
- Chemical constituents of Stenotaenia macrocarpa Freyn & Sint. (Apiaceae) Biochem. Syst. Ecol.. 2021;96:104272
- [Google Scholar]
- Anti-melanogenic activity of salacinol by inhibition of tyrosinase oligosaccharide processing. J. Biochem.. 2020;167:503-511.
- [Google Scholar]
- Inhibition of melanin production and tyrosinase expression of ergosterol derivatives from Phellinus pini. Nat. Prod. Sci.. 2013;19:258-262.
- [Google Scholar]
- Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. PNAS. 2006;103:9081-9085.
- [Google Scholar]
- Extract of Ganoderma formosanum mycelium as a highly potent tyrosinase inhibitor. Sci. Rep.. 2016;6:1-9.
- [Google Scholar]
- Tyrosinase inhibitory activity of total triterpenes and poricoic acid A isolated from Poria cocos. Chin. Herb. Med.. 2017;9:321-327.
- [Google Scholar]
- Inhibitory effect of [6]-gingerol on melanogenesis in B16F10 melanoma cells and a possible mechanism of action. Biosci. Biotech. Bioch.. 2011;75:1067-1072.
- [Google Scholar]
- Melanosome distribution in keratinocytes in different skin types: melanosome clusters are not degradative organelles. J, Invest. Dermatol.. 2018;138:647-656.
- [Google Scholar]
- Hwang, J. S. and J. H. Ku, 2011. A composition for improving, preventing or treating skin hyper-pigmented diseases and for whitening skin containing 1,3-diolein or 1,3-dilinoleoyl-rac-glycerol. Korea.
- Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis.. 2017;16:1-17.
- [Google Scholar]
- Loganin inhibits α-MSH and IBMX-induced melanogenesis by suppressing the expression of tyrosinase in B16F10 melanoma cells. Life Sci.. 2019;29:1200-1207.
- [Google Scholar]
- Phytochemical study of the low polar constituents of Pinellia ternata. Chem. Nat. Compd.. 2017;53:1152.
- [Google Scholar]
- Plants and natural products for the treatment of skin hyperpigmentation–a review. Planta Med.. 2018;84:988-1006.
- [Google Scholar]
- Biological activities of flavonoids: an overview. Int. J. Pharm. Sci. Res.. 2019;10:1567-1574.
- [Google Scholar]
- Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg. Med. Chem.. 2007;15:2708-2714.
- [Google Scholar]
- Melanogenesis inhibition of β-lapachone, a natural product from Tabebuia avellanedae, with effective in vivo lightening potency. Arch. Dermatol. Res.. 2015;307:229-238.
- [Google Scholar]
- (−)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesisvia decreased MITF production. Arch. Pharm. Res.. 2004;27:334-339.
- [Google Scholar]
- Phenolic compounds from the aerial parts of Clematis viticella L. and their in vitro anti-inflammatory activities. Nat. Prod. Res.. 2019;33:2541-2544.
- [Google Scholar]
- Technologies for cutaneous exfoliation using salicylic acid. Dermatol. Ther.. 2001;14:225-227.
- [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;2013
- [Google Scholar]
- A zebrafish embryo as an animal model for the treatment of hyperpigmentation in cosmetic dermatology medicine. Medicina. 2018;54:35.
- [Google Scholar]
- Antimelanogenic effects of picrionoside a isolated from the leaves of Korean ginseng. Biol. Pharm. Bull.. 2015;38:1663-1667.
- [Google Scholar]
- Composition for skin cell regeneration, anti-wrinkle, anti-imflamation, and skin whitening. Korea; 2014.
- Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci.. 2015;16:18384-18395.
- [Google Scholar]
- Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med.. 2010;25:923-927.
- [Google Scholar]
- Zebrafish as a model system to study skin biology and pathology. J, Invest. Dermatol.. 2014;134:e21
- [Google Scholar]
- Zebrafish on a chip: a novel platform for real-time monitoring of drug-induced developmental toxicity. PLoS One. 2014;9:e94792
- [Google Scholar]
- Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J. Enzyme Inhib. Med. Chem.. 2007;22:433-438.
- [Google Scholar]
- A powerful on line ABTS+–CE-DAD method to screen and quantify major antioxidants for quality control of Shuxuening Injection. Sci. Rep.. 2018;8:1-10.
- [Google Scholar]
- Phenylethanoid glycosides from Plantago asiatica. Phytochemistry. 1991;30:2015-2018.
- [Google Scholar]
- Composition for antioxidant, anti-imflamation, and skin whitening. Korea; 2013.
- Tyrosinase inhibitors from Calceolaria integrifolia s.l.: Calceolaria talcana aerial parts. J. Agric. Food Chem.. 2013;61:4336-4343.
- [Google Scholar]
- Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res.. 2004;17:66-73.
- [Google Scholar]
- Isolation and Characterization of α-Amyrin from Stem Bark of Ficus exasperata (Vahl) Biotechnology Journal International.. 2016;16:1-7.
- [Google Scholar]
- The melanocyte and the epidermal melanin unit: an expanded concept. Dermatol. Clin.. 2007;25:271-281.
- [Google Scholar]
- Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int. J. Mol. Sci.. 2017;18:1685.
- [Google Scholar]
- Whitening effect of Poria cocas ethanol extract by inhibition of melanin synthesis. Life Sci.. 2014;24:485-490.
- [Google Scholar]
- Inhibition of tyrosinase by gastrodin: An integrated kinetic-computational simulation analysis. Process Biochem.. 2013;48:162-168.
- [Google Scholar]
- Antioxidant and Biological Activity of Duchesnea Indica (Andr.) Focke Extracts. Res. J. Pharm., Biol. Chem. Sci.. 2018;9:87-95.
- [Google Scholar]
- ‘Fish matters’: the relevance of fish skin biology to investigative dermatology. Exp. Dermatol.. 2010;19:313-324.
- [Google Scholar]
- Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules. 2011;16:3338-3344.
- [Google Scholar]
- The phenylthiourea is a competitive inhibitor of the enzymatic oxidation of DOPA by phenoloxidase. J. Enzyme Inhib. Med. Chem.. 2012;27:78-83.
- [Google Scholar]
- A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res.. 2015;307:239-247.
- [Google Scholar]
- Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev.. 2014;8:122.
- [Google Scholar]
- C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same. Korea; 2005.
- New Cytotoxic Cardenolide Glycoside from the Seeds of Cerbera manghas. Chem. Pharm. Bull.. 2004;52:1023-1025.
- [Google Scholar]
- Shigenao, O., T. Junji and M. Hiromichi, 2015. Tyrosinase inhibitor. Japan.
- Phytochemical constituents of the Urena lobata Fruit. Chem. Nat. Compd.. 2016;52:178-180.
- [Google Scholar]
- Triterpenoids from Fragaria ananassa calyx and their inhibitory effects on melanogenesis in B16–F10 mouse melanoma cells. Nat. Prod. Res.. 2013;27:2219-2223.
- [Google Scholar]
- Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to α-melanotropin. J, Invest. Dermatol.. 1997;108:838-842.
- [Google Scholar]
- Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin. Ther. Pat.. 2013;23:1561-1580.
- [Google Scholar]
- Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci.. 2015;135:68-76.
- [Google Scholar]
- Chemical constituents from the fruiting bodies of Hexagonia apiaria and their anti-inflammatory activity. J. Nat. Prod.. 2015;78:2552-2558.
- [Google Scholar]
- Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B. 2014;4:141-145.
- [Google Scholar]
- Profiling and characterization antioxidant activities in Anoectochilus formosanus Hayata. J. Agric. Food Chem.. 2002;50:1859-1865.
- [Google Scholar]
- A comparative study on whitening and antioxidant activities from 19 kinds of Chinese herbs. J. Anhui Univ.. 2017;41:86-94.
- [Google Scholar]
- Wang, F.-F., 2014. Skin-whitening hand cream. China.
- Tyrosol and its analogues inhibit alpha-melanocyte-stimulating hormone induced melanogenesis. Int. J. Mol. Sci.. 2013;14:23420-23440.
- [Google Scholar]
- A new cyclolignan glycoside from the tubers of Pinellia ternata. J. Asian Nat. Prod. Res.. 2015;17:1097-1103.
- [Google Scholar]
- Secondary metabolites of Volvariella bombycina and their inhibitory effects on melanogenesis. J. Microbiol. Biotechnol.. 2010;20:78-81.
- [Google Scholar]
- 10-O-benzoyltheveside and 10-dehydrogeniposide from the leaves of Cerbera manghas. Phytochemistry. 1990;29:2327-2328.
- [Google Scholar]
- Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18:32-35.
- [Google Scholar]
- Yoon, S.-J., J. Moo-Hyun and P. Seon-Gyu, 2015. Composition for skin cell regeneration, anti-wrinkle, antioxidant, anti-inflammation and skin whitening comprising Plantamajoside. Korea.
- Flavonoids in the leaves of Trillium undulatum Willdenow. Int. J. Plant Res.. 1997;110:379-381.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105035.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







