Translate this page into:
Assessment of antimicrobial and enzymes inhibition effects of Allium kastambulense with in silico studies: Analysis of its phenolic compounds and flavonoid contents
⁎Corresponding authors. dkisa@bartin.edu.tr (Dursun Kısa), ptaslimi@bartin.edu.tr (Parham Taslimi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Type 2 diabetes and obesity-related metabolic diseases have been treated with traditional medicinal plants for centuries. In this study, the effects of Allium kastambulense plant extracts on different enzyme activities were investigated, and the results were added as graphics and tables after calculating. This study aimed to identify and quantify the phenolic composition of Allium kastambulense Bosse and determine the anti-lipase, anti-urease, anti-melanogenesis, antidiabetic, anti-alzheimer, and antibacterial properties. IC50 results for all enzymes were obtained between 0.55 and 138 µg/mL, and this plant inhibited HMG_CoA R and tyrosinase enzymes more with IC50 values of 0.55 and 59.17 µg/mL, respectively. The interactions of active compounds showing activity against different enzymes were examined with molecular docking studies. The most active compound 3, (rosmarinic acid) has −10.90 kcal/mol binding energy value against HMG_CoA R, and also the potential structure compound 2, (+catechin), which has activity against α-amylase, α-glycosidase, and lipase enzymes, was –8.30, −8.40 and −9.70 kcal/mol, respectively. Finally, antimicrobial effects, total phenolic, and flavonoid content, determined with its higher total phenolic (22.63 mg GAE/g extract) and flavonoid (6.41 mgQE/g extract) contents and main chemical compounds of this plant were gentisic acid, (+) catechin, and rosmarinic acid, respectively.
Keywords
Allium kastambulense
Phenolic compound
Antimicrobial
Enzyme inhibition
Molecular docking
1 Introduction
In biochemistry, important plant foods in the traditional diet like onion, herbs, and beans have high phenolic phytochemicals. Also, between significant plant foods, onions, Allium species, and garlic have long been utilized for a large range of aims, including nutrition, medicine, condiment, foodstuff, flavoring, and the therapy of widespread ailments as folk medicine (Zhu et al., 2016). Allium kastambulense (Amaryllidaceae) is an endemic wild plant that spreads between the provincial borders of Kastamonu and Bartın in the northern Anatolia region (Turkey) (Yaman et al., 2020). Studies on A. kastambulense are extremely limited; apart from the taxonomic study on identifying the plant, any previous research could not be reached. Allium is a heterogeneous taxon and has received attention for their biological activities. The genus includes many representatives, widely used as a food constituent or as a remedy for curing and preventing various disorders since ancient times (Maccelli et al., 2020; Bastaki et al., 2021; Kyung, 2012). Studies have been conducted discussing the role of Alliums in the prevention of chronic human ailments such as cardioprotective effect (Rocchetti et al., 2022; Zeng et al., 2017).
Angiotensin-converting enzyme (ACE) is an enzyme that converts angiotensin I (Ang I) peptide to angiotensin II (Ang II) peptide with vasoconstrictor properties, and the relationship of ACE with hypertension (HT) through the renin-angiotensin-aldosterone system is known (Rocchetti et al., 2022). Angiotensin-converting enzyme (ACE) is an essential component of RAS and mediates many systemic and local effects in the cardiovascular system. ACE is produced in the endothelium of somatic tissues as a transmembrane protein inhibited by ACE inhibitors (Verdecchia et al., 2010). ACE in tissues such as kidney, testis, brain, especially lung; It is found in significant proportions in macrophages and blood vessels and physiological fluids such as semen and plasma (Jia et al., 2009). Cholinesterase enzymes are found in many tissue cells, plasma, and body fluids. These enzymes are divided into two as butyrylcholinesterase (BChE) and acetylcholinesterase (AChE) according to their inhibitor sensitivity and substrate specificity (Öztaşkın et al., 2017). HMG CoA reductase is the enzyme that controls cholesterol synthesis. Also, it is an endoplasmic reticulum inner membrane protein, and the enzyme's catalytic domain extends into the cytosol (Schulz et al., 2004).
Both collagenase and elastase are essential enzymes in the breakdown of connective tissue in arthritis. The collagenase enzyme is particularly destructive in cartilage destruction. Recently, elastase can also degrade collagen. Collagenases are active or latent in many tissues, including synovial fluid and tissue (Eckhard et al., 2013). The 'dendritic' cells, especially in the synovium, are a rich source of latent collagenase. Indeed, elastase is found in neutrophil granulocytes, and this enzyme helps destroy articular cartilage in rheumatoid arthritis (Nema et al., 2013). The rate of joint destruction is usually rapid in severe inflammatory diseases such as untreated gout or active rheumatoid arthritis and slower in degenerative joint diseases such as osteoarthrosis (Chiocchio et al., 2018). Urease is a metalloenzyme containing nickel in its structure. This enzyme catalyzes the hydrolysis of urea to form carbon dioxide and ammonia (Nastri et al., 2000). Tyrosinase is also known as polyphenol oxidase, monophenol oxidase, phenolase, or catecholase. The name tyrosinase was given because of the enzyme's specificity as a substrate for tyrosine (monohydroxyphenylalanine) and dihydroxyphenylalanine (Chen et al., 2017). α-Amylase and α-glycosidase enzymes are involved in the digestion of starch taken with diabetes. Indeed, the postprandial blood sugar level can be regulated with the inhibition of these enzymes (Yao et al., 2013).
Furthermore, molecular docking is performed to model the interaction between a ligand and a target based on the spatial pose and energy of the compound compared to each other. It could be used in prediction studies in the phenolic compounds of Allium kastambulense - enzymes (ACE, AChE, BChE, HMG_CoA R, α-amylase, collagenase, α-glycosidase, lipase, tyrosinase, and urease) interaction and give biochemical activity insights between the phenolic compounds of A. kastambulense with aforementioned targets.
The phytochemical content of plants varies according to the environment in which they are grown, climatic characteristics (locality, temperature), and altitude. In this study, the phytochemical content, enzyme inhibition, antibacterial and antifungal effects of Allium kastambulense, an endemic plant in Turkey, were investigated for the first time to reveal its medicinal importance.
2 Materials and methods
2.1 Plant material and extraction procedure
Plant samples (Allium kastambulense Kollmann) were collected during the flowering period in 2020 from Kurucaşile-Bartın province of Turkey and were allowed to air dry at room temperature. The plant was identified by the taxonomist Prof. Dr. Zafer KAYA, and voucher specimens (BofHerb_176) have been deposited in the herbarium of the Faculty of Forestry (Bartin University, Turkey).
The aerial parts of the air-dried plant material, including the flowers, were powdered in a mechanic grinder, and 4 g of powder was extracted in 20 mL of the solvents (methanol-chloroform, 4:1) at room temperature. The mixture was sonicated at 37 °C for 30 min and centrifuged at 5000 × g for 10 min at ambient temperature. The supernatant was then filtered (0.22 µm), and the solvent was evaporated at an ambient temperature lower than 50 °C. The crude extract was stored at −20 °C for further analysis.
2.2 Determination of the total phenolic content
Quantification of the total phenolic compounds (TPC) in the extracts of A. kastambulense was determined by the Folin-Ciocalteu reagent (Singleton et al., 1965). Briefly, 100 µL of dilutions sample solution was mixed with 100 µL of folin-ciocalteu’s reactive and 1.5 mL of dH2O. After the 3 min. of incubation at 25 °C, 300 µL of 2 % aqueous NaHCO3 was added to the reaction medium, and the mixture was allowed to incubate for two hours at 25 °C. The absorbance of samples was recorded at 765 nm. TFC quantity was calculated as mg of gallic acid equivalents (GAEs) per gram of plant extract.
2.3 Determination of the total flavonoid contents
The total flavonoid content (TFC) was done by the AlCI3 (SigmaAldrich, Germany) colorimetric method with slight modifications (Pękal and Pyrzynska, 2014). The reaction components in the mixture were as follows: 20 µL of extract, 20 µL of AlCI3, and 20 µL of CH3COONa; and the final volume was accomplished to 1 mL with ethanol. After 30 min incubation at room conditions, the absorbance was read at 425 nm using a spectrophotometer (Cary 60, Agilent). TFC was expressed as mg of quercetin equivalents (QE) per gram of extract.
2.4 Profile of individual phenolic compounds
Quantitative analysis of the phenolic compounds in the extract was analyzed using the HPLC (High Performance Liquid Chromatography) system (Shimadzu Scientific Instruments, Japan) coupled with an LC 20AT pump and SPD-M20A model. GL Sciences InertSustain C18 Analytical Column (4.6 mm × 250 mm, 5 µm particle size) was used, and its temperature was kept to 25 °C. The samples were transferred to vials for the automatic injection of 20 µL to the equipment. The mobile phase was formed solvent A (methanol) and solvent B–acidified water (2% acetic acid, v/v), and the elution gradient was applied to the reverse phase column. The flow rate used for the gradient elution was 1 mL min−1. Then, each phenolic compound was identified and quantified after comparing retention times, UV–vis spectra, and chromatographic profile with commercial standard compounds. The results were expressed as mg/g dry extract.
2.5 Enzymes inhibition studies
2.5.1 Angiotensin-converting enzyme (ACE) inhibition assay
The ACE inhibitory activity was performed in 50 mM Tris-HCI buffer with 0.3 M NaCl at pH 7.5 according to a previously described method (Hou et al., 2003). Briefly, the total volume of the final mixture was 150 μL, which contains 10 µL of enzyme solution, 10 µL of test extracts, and 150 µL of the synthetic substrate N-[3-(2–furyl)acryloyl]-Phe-Gly-Gly (FAPGG, 0.88 mM) solution (prepared in the buffer). For the positive control group, captopril was used, and after adding the substrate, absorbance was recorded at 340 nm for 5 min at 37 °C using a microplate reader (MultiskanGO Microplate Reader, Thermo Scientific)
2.5.2 AChE/BChE inhibition assay
The cholinesterase (ChE) inhibitory activity of the extracts was referred to the spectrophotometric method designed by Ellman (Ellman et al., 1961). The reaction mixture of well microplate comprises 130 µL of Tris-HCI (pH 8.0, 100 mM), 10 µL of AChE/BChE enzymes solution, sample solution (10 µL) and DTNB (5,5′-dithio-bis(2-nitro-benzoic). After the mixture was incubated at 25 °C for 10 min, the reaction was initiated by adding AChI/BChI (acetylthiocholine iodide/or butyrylcholine iodide). The activity of both enzymes was determined by reading the increasing absorbance at 412 nm for 5 min at 25 °C using the microplate reader.
2.5.3 α‑Glycosidase inhibition studies
α-Glucosidase inhibition activity was evaluated using p-nitrophenyl α-D-glucopyranoside (p-NPG) as substrate (Tao et al., 2013). 120 µL of phosphate buffer (100 mM, pH 6.8), 10 µL of extract solution, and 20 µL of α-glycosidase enzyme solution were mixed to incubate at 37 °C for 10 min. Then, p-NPG (50 µL, 2 mM) was added to the reaction medium, and the absorbance value of p-NP released was recorded at 405 nm.
2.5.4 α‑Amylase inhibition studies
The α-amylase inhibition activity was determined to the Caraway iodine/potassium iodide (IKI) method with slight modifications (Yang et al., 2012). For use as a substrate, 1 g of starch was dissolved in 100 mL of phosphate buffer (20 mM, pH 6.9 with 6 mM NaCI) by heating for 30 min for the substrate solution. In brief, 30 µL of KH2PO4, 20 µL of the test solution, and 30 µL of α-amylase solution were mixed, and they were preincubated at 37 °C for 10 min. Then, 50 µL of the substrate was added to each well, and the reaction mixture was incubated at 37 °C for 10 min. After the incubation, the reaction was stopped with 25 µL of 10% HCl. This was followed by the addition of about 100 μL of IKI solution. The absorbance of the was spectrophotometrically measured at 630 nm.
2.5.5 Pancreatic lipase inhibition studies
The lipase inhibition activity assay was performed colorimetrically using p-nitrophenylpalmitate (p-NPP) as a substrate described method (El-Korany et al., 2020). The reaction mixture occurs 140 µL of Tris-HCI (pH 8.2, 100 mM), 20 µL of pancreatic lipase, and 20 µL of sample extract and the mixture was incubated at 37 °C for 10 min. Then, 20 µL of p-NPP (10 mM) dissolved in acetonitrile and isopropanol (1:1) was added to the mixture, and enzymatic reaction was carried out at 37 °C for 30 min using a shaking incubator. The absorbance change of p-NP liberated was read at 410 nm by a spectrophotometer (MultiskanGO Microplate Reader, Thermo Scientific).
2.5.6 Tyrosinase inhibition assay
The tyrosinase inhibitory activity of plant extracts was evaluated using L-DOPA as a substrate according to the method previously described by (Masuda et al., 2005). In each well microplates, KH2PO4 (pH 6.8, 100 mM, 150 µL), 10 μL of mushroom tyrosinase solution, and 20 μL of plant extracts were mixed and incubated at 37 °C for 10 min. Kojic acid was used as a positive control. After the standing, L-DOPA (2.5 mM, 20 µL) was added to the reaction environment, and the absorbance of the dopachrome product was measured at 475 nm.
2.5.7 Urease inhibition assay
The urease inhibition study was carried out using indophenols method to quantify ammonia (Ikram et al., 2017). In brief, 50 μL of KH2PO4 buffer (100 mM, pH 8.2), which contains 100 mM urea µL, 25 μL of enzyme solution (jack bean urease), and 20 μL of test compound were incubated in a 96 well plate at 37 °C for 15 min. Then, the ammonia liberated was allowed to complex with 45 µL of a phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside - Na2[Fe(CN)5NO] · 2H2O), and 70 μL of an alkali reagent (0.5% w/v NaOH and 0.1% NaOCl). After the 20 min incubation, the absorbance of the color change in each well was measured at 630 nm using a spectrophotometer (MultiskanGO Microplate Reader, Thermo Scientific).
2.5.8 HMG-CoA reductase (HMGR) inhibition studies
The HMG-CoA reductase inhibitory assay was determined based on the spectrophotometric measurement of the decrease in absorbance at 340 nm due to oxidation of NADPH by HMGR in the presence of substrate HMG-CoA (Holdgate et al., 2003). The HMG-CoA reductase screening kit was purchased from BioVision Inc. (Milpitas, CA, USA). Briefly, HMG-CoA reductase enzyme, plant extract, HMG-CoA substrate, NADPH, and assay buffer were added to the wells in a 96-well microplate. The reaction was immediately measured at 340 nm in the microplate reader for 10 min at 37 °C.
2.5.9 Collagenase inhibition assay
The assay was performed based on the spectrophotometric method according to the previous method (Thring et al., 2009) with some modifications. Collagenase from Clostridium histolyticum (ChC – EC.3.4.23.3) was dissolved in 50 mM tricine buffer (pH 7.5 with 10 mM CaCI2 and 400 mM NaCI), and synthetic substrate N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was used as a substrate. The reaction mixture comprises 110 μL of tricine, 20 μL of ChC and 20 μL of the sample solution and the mixture was incubated at 25 °C for 15 min. Then, the FALGPA (0.8 mM, 50 μL) was added to each well to start the reaction, and the decrease in absorbance was recorded at 340 nm for 5 min using the microplate reader. EGCG (epigallocatechin gallate) was used as a positive control.
2.6 Antimicrobial studies
Antibacterial and antifungal studies of Allium kastambulense were carried out by performing the mic test. Briefly, the antibacterial and antifungal activity of A. kastambulense was determined against fungal strains (C. albicans and C. utilis) gram-positive bacterial strains (S. aureus and E. faecalis) and gram-negative bacterial strains (K. pneumonia, and E. coli) and from frozen stocks for 24 h at 37 °C. New cultures were then prepared up to 0.5 McFarland Units at 37 °C.
2.7 Molecular docking study
Molecular docking analysis is widely applied to predict protein–ligand interaction energies (Bal et al., 2021; Albayrak et al., 2021). In this part, to examine the enzyme inhibition activity data of the phenolic compounds [gentisic acid (1), catechin (2), and rosmarinic acid (3)] of Allium kastambulense, molecular docking based calculations were implemented on ACE, AChE, BChE, HMG_CoA R, α-amylase, collagenase, α-glycosidase, lipase, tyrosinase, and urease by using Autodock vina v.1.2.0 (Eberhardt et al., 2021; Trott and Olson, 2010). The phenolic compounds of Allium kastambulense were drawn, afterwards, geometry optimization and energy minimization were done to utilize DFT/B3LYP/6-31G* basis set in Gaussian09 (G09) (Frisch et al., 2009) software. The AutoDockTools 1.5.7 package (Sanner, 1999; Morris et al., 2009) was used to generate input files before docking. The target models were uploaded from the PDB databank. Discovery Studio (DS) 3.5 (Accelrys Software Inc, 2013) was also applied to prepare the targets for docking processes. The grid of the possible complexes was defined with literature and via subprotocol of DS 3.5. The grid box of size 40 × 40 × 40 representing X, Y, and Z coordinates, with a grid point spacing of 0.375 Å. The value of exhaustiveness was set to 16. In the meantime, the default parameters were applied for Autodock Vina if it was not mentioned. The top docked complex for each target as evaluated by the Vina binding energy was selected. The acquired data were analyzed, and the models remarked greatest ligand-enzyme bindings were selected and gathered for binding energy predictions. When the respective compounds’ docking results were analyzed, it was concluded that the phenolic compounds of Allium kastambulense high interacted with HMG_CoA R, α-amylase, α-glycosidase, and lipase as targets and did not enough interaction with other enzyme structures as given in supplementary part of the study.
2.8 Data analysis
Descriptive analysis and IC50 values for enzyme inhibition antibacterial and antifungal properties were performed using the GraphPad (Prism 9) program.
3 Results and discussion
3.1 The enzyme inhibition results of Allium kastambulense plant enzyme inhibition (IC50 values) against ACE, AChE, BChE, HMG_CoA R, α-amylase, collagenase, α-glycosidase, lipase, tyrosinase, and urease
Inhibitors obtained from plants; are classified under two headings as phenols/polyphenols and aldehydes/derivatives. Plant phenols/polyphenols are chemical compounds commonly found in nature that give their color to many flowers and fruits. While they are usually in the form of complexes found in the root, bark, and leaves of some plants, they can sometimes be found as simple compounds found in fresh fruits, vegetables, and tea. In the studies, it is emphasized that all flavonoids inhibit the enzyme due to their ability to chelate with the metal in the active site of the enzymes (Pandey and Rizvi, 2009).
By inhibiting metabolic enzymes, synthetic ACE inhibitor compounds have long been used as antihypertensive factors. However, the unwanted side effects of these drugs are taking their toll in the form of side effects with high rate. Indeed, bioactive food components like peptides, flavonoids, flavanols, anthocyanins, alkaloids, polyphenols, phenolic acids, tannins, polysaccharides, resveratrol, and sterol have been identified as green ACE inhibitors (Pouwels et al., 2014; Dial et al., 2014). Studies on ACE inhibitors to treat high blood pressure increase day by day. For this enzyme, IC50 value was obtained 60.46 with r2: 0.977, which is slightly weaker than the value of the captopril (21.36, r2: 0.988), but it is similar it is at the level of µg/mL.
Alzheimer's disease (AD) is a neurodegenerative disorder with symptoms like impaired cognitive abilities, decreased memory, and personality changes. It is characterized by disruption of cholinergic neurotransmission and expression of a high percentage of amyloid β peptide in the brain (Taslimi and Gulçin, 2018). In AD, while AChE levels decrease in the brain, there is an increase in BChE levels, and a decrease in AChE levels is observed due to high cholinesterase activity. Cholinesterase inhibitors are used to treat this disease so that cognitive and behavioral disorders can be treated to a certain extent by bringing acetylcholine concentration to normal levels. High cholesterol levels in the plasma can trigger the formation of amyloid β peptides in the brain. One of the new research topics is that statins used for cholesterol-lowering may also protect from AD by lowering the cholesterol level in the brain and inhibiting the increased BChE activity (Ökten et al., 2019). For these enzymes (AChE and BChE), IC50 values were obtained 88.49 with r2:1.94 and 138.40 with r2: 0.989, respectively, and these values are slightly weaker than the value of the tacrine (23.36 and 24.84), but they are similar as it is at the level of µg/mL.
HMG-CoA reductase inhibitors and statins are thought to have inhibitory properties on cholinesterases. It is known that statins and similar lipid-reducing agents not only reduce cholesterol levels but also have protective effects against Alzheimer's disease. It is also the subject of new research that they can be protective-preventive agents for diseases like lipid metabolism, cardiovascular diseases, and stroke (Saeedi Saravi et al., 2017). For this enzyme, IC50 value was obtained 0.55 with r2: 0.983, which is better than the value of the atorvastatin (8.78, r2: 0.997), but it is similar as it is at the level of µg/mL.
Inhibition of collagenase enzymes has been one of the primary goals in the cosmetic industry for research persons to find novel antiwrinkle and skin-lightening molecules, especially with rich polyphenol contents, and prove their effectiveness (Wittenauer et al., 2015). For this enzyme, IC50 value was obtained 81.74 with r2: 0.978, and this value is slightly weaker than the value of the ECGG (epigallocatechin gallate) (2.52, r2: 0.998), but it is similar as it is at the level of µg/mL.
Tyrosinase inhibitors are also widely used to treat neurodegeneration (Wang et al., 2018). For this enzyme, IC50 value was obtained 59.17 with r2: 0.979, and this value is slightly weaker than the value of the kojic acid (3.49, r2: 0.999), but it is similar as it is at the level of µg/mL.
Indeed, studies on urease enzyme inhibitor compounds are very significant to advance drugs to be utilized in the therapy of some diseases caused by pathogens containing urease enzyme in animals and humans to repair these minus impacts on the environment (Mira et al., 2017). For this enzyme, IC50 value was obtained 114.4 with r2: 0.993, which is slightly weaker than the value of the thiourea (20.36, r2: 0.989), but it is similar as it is at the level of µg/mL.
Inhibition of alpha-glucosidase in the small intestine that converts complex carbohydrates into absorbable forms is an essential approach in the therapy of Type 2 diabetes, which is characterized by high postprandial blood glucose levels (Gulçin et al., 2018). For this enzyme, IC50 value was obtained 78.01 with r2: 0.987, which is better than the value of the acarbose (174.30, r2: 0.993), but it is similar as it is at the level of µg/mL.
The study on inhibition of AChE, BChE, and tyrosinase reported that various extracts of Allium stylosum O. Schwarz exhibited inhibitory properties against studied enzymes. They declared that inhibition potency changed to plant tissues (bulb, leaves, and flower), and IC50 values were between 8.95 ± 1.24 μg/mL and 256.15 ± 2.49 µg/mL (Emir and Emir, 2021). In another study on Allium, methanol extracts of Allium tuncelianum demonstrated effective inhibition ability against AChE and α-glycosidase with IC50 values 11.25 μg/mL and 9.85 μg/mL, respectively (Takim et al., 2021). Details of enzyme inhibition results are given in Fig. 1 and Table 1.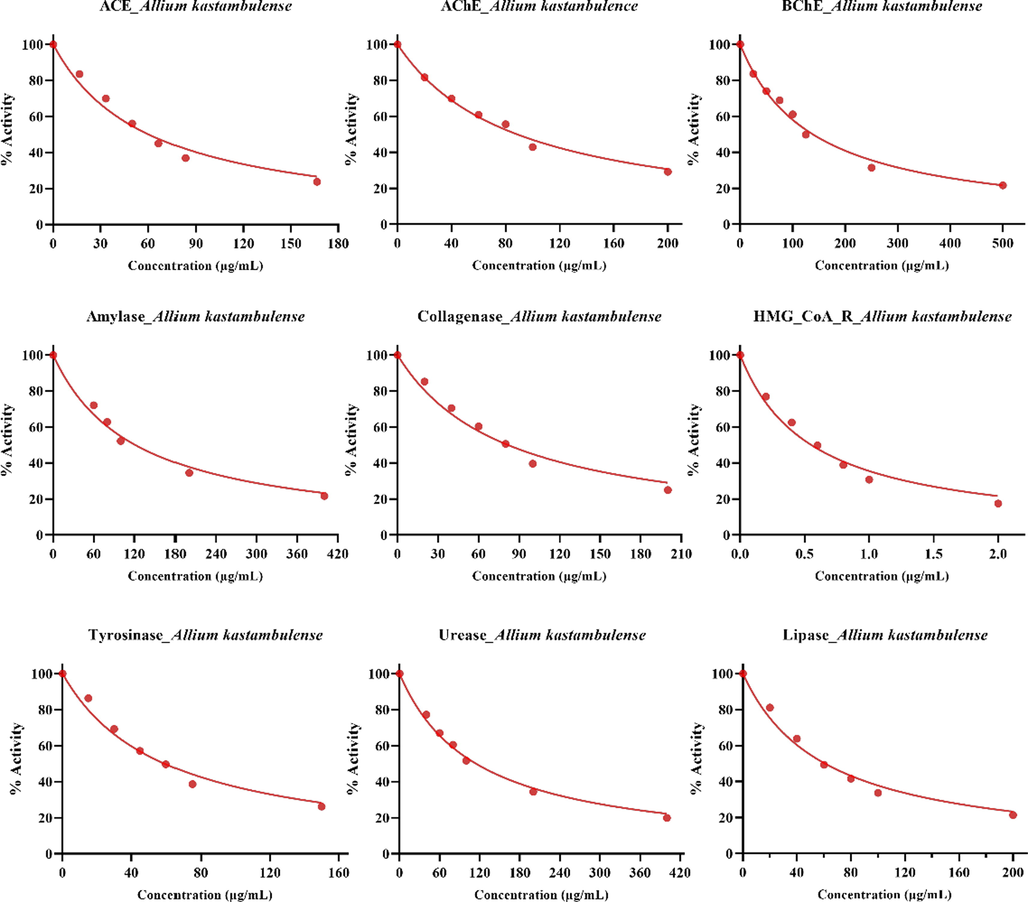
IC50 graphs of enzyme inhibitory results of the A. kastambulense extracts.
Enzymes
Allium kastambulense (µg/mL)
Standards (µg/mL)
IC50
r2
IC50
r2
ACE
60.46 ± 1.78
0.977
21.36 ± 1.33
0.988
AChE
88.49 ± 1.94
0.991
23.36 ± 1.36
0.985
BChE
138.4 ± 2.14
0.989
24.84 ± 1.39
0.992
HMG_CoA R
0.551 ± 0.00
0.983
8.78 ± 0.94
0.997
α-Amylase
121.5 ± 2.08
0.986
131.2 ± 2.11
0.995
Collagenase
81.74 ± 1.91
0.978
2.52 ± 0.40
0.998
α-Glycosidase
78.00 ± 1.89
0.987
174.3 ± 2.24
0.993
Lipase
60.76 ± 1.78
0.984
385.5 ± 2.58
0.991
Tyrosinase
59.17 ± 1.77
0.979
3.49 ± 0.54
0.999
Urease
114.4 ± 2.01
0.993
20.36 ± 1.30
0.989
3.2 Main chemical compounds of methanolic extracts of A. kastambulense
The quantitative analysis of the phenolic compounds in A. kastambulense was performed by HPLC equipment, and the main chemical compounds are shown in Table 2 below. Gentisic acid, (+) catechin, and rosmarinic acid were determined as the most abundant metabolites among 11 metabolites identified in Allium kastambulense plant extract. Their values were calculated as 272.33, 173.00, and 120,06 µg g−1, respectively. Previous study results are in agreement with our current results. In plant species (Pancratium maritimum L., Sternbergia colchiciflora W. K., Galanthus nivalis L., G. elwesii Hook., and Leucojum aestivum L.) belonging to the same family as Allium kastambulense plant, seven phenolic substances (protocateochuic) hydroxybenzoic, vanillic, caffeic, syringic, p-coumaric, and ferulic acid) were determined in 90% ethanolic extraction (Benedec et al., 2018). Phenolic compounds were identified by the HPLC-MS method in the aerial parts (Galanthus nivalis L., Narcissus pseudonarcissus L., N. poeticus L., and Leucojum vernum L.) of species belonging to the Amaryllidaceae family grown in Romania. Chlorogenic (1425.56 ± 10.63–1925.69 ± 6.31 μg/g) and p-coumaric acid (46.54 ± 0.45–270.51 ± 0.42) were stated as the main compounds in all species (Nikolova and Gevrenova, 2005). In the previous study on Allium species, the research group reported that extracts of Allium paniculatum L. subsp. paniculatum L. and Allium paniculatum L. subsp. villosulum (Hal.) contained 29 phenolic compounds in both plants’ bulb, flower, and stem by LC–ESI–MS/MS (Emir et al., 2021).
Phenolic Compounds
Retention time (min)
Calibration equation values
Linear regression (r2)
Amount (µg/g)
(+) Catechin
10.401
y=(1.25217e-005)X+(2.26219)
0,9992410
173,0043
Chlorogenic Acid
11.165
y=(1.25217e-005)X+(2.26219)
0,9992410
115,7491
Gentisic Acid
12.397
y=(1.25217e-005)X+(2.26219)
0,9992410
272,3345
Vanilic Acid
13.391
y=(1.25217e-005)X+(2.26219)
0,9992410
42,67709
Chicoric Acid
14.973
y=(1.25217e-005)X+(2.26219)
0,9992410
93,01739
p-Coumaric Acid
17.276
y=(1.25217e-005)X+(2.26219)
0,9992410
23,31379
Hydroxybenzoic Benzoic Acid
25.039
y=(1.25217e-005)X+(2.26219)
0,9992410
17,45356
Rosmarinic Acid
28.201
y=(1.25217e-005)X+(2.26219)
0,9992410
120,0628
Quercetin
41.888
y=(1.25217e-005)X+(2.26219)
0,9992410
63,04819
Eugenol
46.379
y=(1.25217e-005)X+(2.26219)
0,9992410
12,76878
Apigenin
47.023
y=(1.25217e-005)X+(2.26219)
0,9992410
9,97071
3.3 Total phenolic compounds (TPC) and total flavonoid contents (TFC) results
In the present study, TPC and TFC results of methane extract of Allium kastambulense were determined and represented in Table 3. It was found that the plant contains total phenolics and total flavonoids at 22.63 mg GAE/g extract and 6.41 mg QE/g extract, respectively. These results confirmed that polar extracts include much more phenolics in their structures compared to non-polar extracts such as hexane. In studies conducted with different species of Allium plant, different results were obtained according to the part of the plant used, type of the solvent, and extraction procedure. For instance, Burlando et al. reported that the TPC of the aerial parts of Hedysarum coronarium, extracted with MTBE/ethyl acetate/acetone obtaining two extracts (PSE) and (VSE) were 62 and 38 mg GAE/g extract, respectively (Burlando, 2017). In another study, TPC of hexane, methanol, and water extracts from Hedysarum aucheri was reported to be 5.17, 56.9, and 15.7 GAE/g DW, respectively (Uyar, 2017).
Extracts
TPC (mg/g DW)
TFC (mg/g DW)
Allium kastambulense
22.63 ± 0.50
6.41 ± 0.26
3.4 Antibacterial and antifungal
Antibacterial and antifungal activities of the Allium kastambulense tested are shown in Fig. 2 and Table 4. MIC values ranged from 246.4 and 305.7 μg/mL for fungal strains (C. albicans and C. utilis) 441.1 and 613.2 μg/mL for gram-positive bacterial strains (S. aureus and E. faecalis) and 53.20 and 577 μg/mL for gram-negative bacterial strains (K. pneumonia, and E. coli).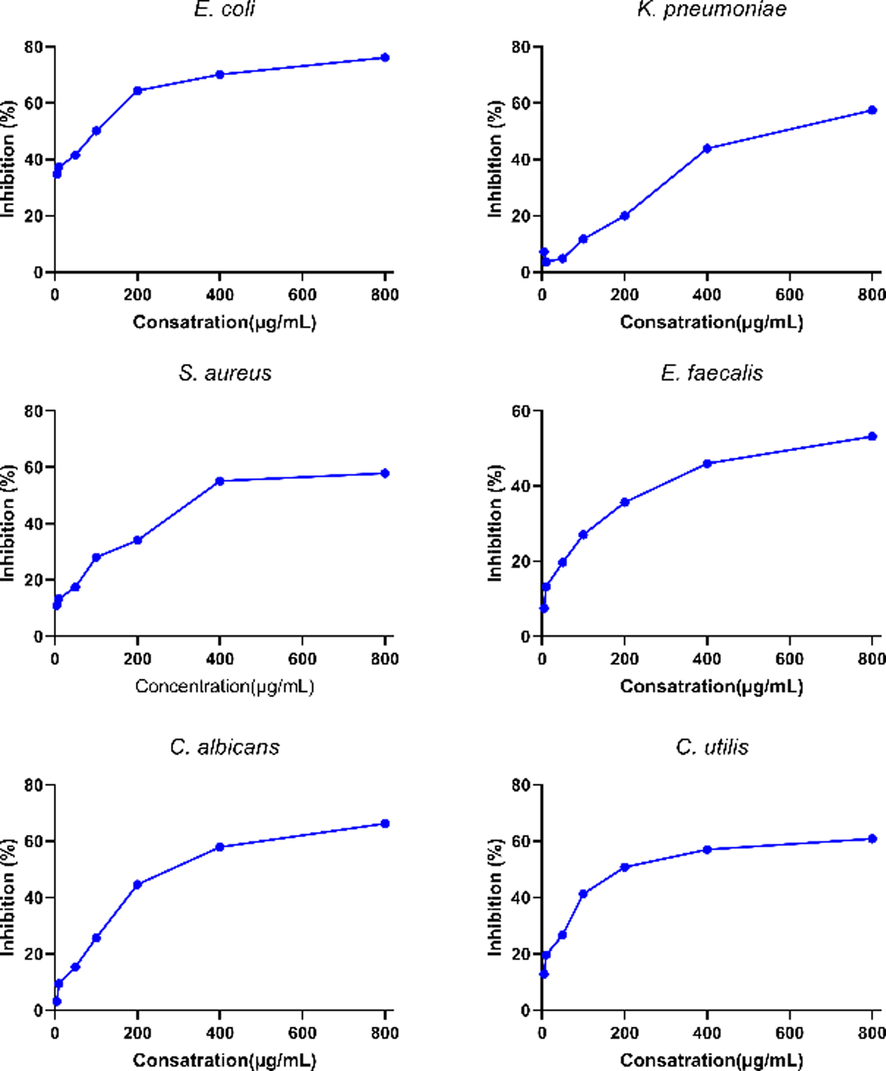
Antibacterial and antifungal activities of the A. kastambulense.
Microorganisms
MIC Values of A. kastambulense(µg/mL)
E. coli
53.20 ± 1.726
K. pneumoniae
577 ± 2.761
S. aureus
441.1 ± 2645
E. faecalis
613.2 ± 2.788
C. albicans
305.7 ± 2.485
C. utilis
246.4 ± 2.392
Antimicrobial results may differ due to different solvents used for herbal extraction, plant parts, method, microorganisms in the environment, the region where the plants are grown, and the harvesting times of the plants. When the literature is examined, it is seen that many biological studies of different Allium species have been done (Ivanova, 2009; Kyung, 2012; Leontiev, 2018; Zarei Mahmoudabadi and Gharib Nasery, 2009). Similar to the antimicrobial studies of Allium species in the literature (Athaillah and Lestari, 2020; Batiha and Saber, 2020; Bernaldez et al., 2021; Chand, 2021; Rama Narsimha Reddy and Srividya Lonkala; Saxena et al., 2018); Allium kastambulense showed antibacterial properties against pathogenic bacteria in our study. The ratios of phytochemical components such as saponins, anthraquinones, tannins, flobatannins, alkaloids, and flavonoids in the plant extract directly affect the antimicrobial properties of the plant. For further studies, in vivo, and in vitro studies should be continued and increased for Allium species.
3.5 Molecular docking result
Although there are in silico studies with target enzymes that are widely discussed in the literature, our aim in this study was to explain the enzyme inhibition analyzes of the extracted phenolic compounds were clarified and evaluated with the help of computational methods. The four targets with the best interaction values and binding energies as a result of the interactions of each target with the three phenolic compounds in the largest amount are HMG_CoA R, α-amylase, α-glycosidase, and lipase, respectively. Compound 3 shows the best result with the first target enzyme in the docking study. Then compound 2 and compound 1 are the structure. In fact, these compounds show a better binding tendency of the enzyme than the control compound, as given in Table 5. Detailed interaction data of the complexes obtained are in the supplementary file.
ACE
Binding Energy (kcal/mol)
1
−1.70
2
−4.40
3
−4.10
*Captopril
−5.60
AChE
Binding Energy (kcal/mol)
1
−4.80
2
−6.60
3
−6.30
*Tacrine(TAC)
−7.37
BChE
Binding Energy (kcal/mol)
1
−2.20
2
−5.40
3
−5.00
*Tacrine(TAC)
−6.81
HMG_CoA R
Binding Energy (kcal/mol)
1
−6.90
2
−10.10
3
−10.20
*Atorvastatin
−6.80
α-Amylase
Binding Energy (kcal/mol)
1
−6.40
2
−8.30
3
−7.20
*Acarbose (ACR)
−5.37
Collagenase
Binding Energy (kcal/mol)
1
−3.30
2
−5.00
3
−5.60
*EGCG
−9.10
α-Glycosidase
Binding Energy (kcal/mol)
1
−6.60
2
−8.40
3
−8.20
*Acarbose (ACR)
−7.53
Lipase
Binding Energy (kcal/mol)
1
−6.00
2
−9.70
3
−8.50
*Orlistat
−7.20
Tyrosinase
Binding Energy (kcal/mol)
1
−3.60
2
−6.80
3
−7.20
*Kojic acid
−4.60
Urease
Binding Energy (kcal/mol)
1
−1.50
2
−2.50
3
−0.50
*Thiourea
−3.90
Firstly, the control compound of HMG_CoAR, atorvastatin, formed hydrogen bond with Ile746, Ser745, Ala751, Ser775, and Ser745 residue, halogen bond with Val772, and hydrophobic interaction with Ala754 and Leu853 (Fig. S2). The compound 3 has hydrogen bond (Arg568, Asn755, Ser852, and Glu559) and hydrophobic interaction (Cys561 and Leu853) with the target enzyme. Second, compound 2 made electrostatic interaction with the Arg568 residue at the target's binding site, as well as hydrogen bonds and hydrophobic interactions. Compound 1 showed a H-bond only with Leu862 and hydrophobic interaction with Ala856. These interactions of the aforementioned compounds and their comparison with the control compound are summarized in Fig. 3 and Table S1.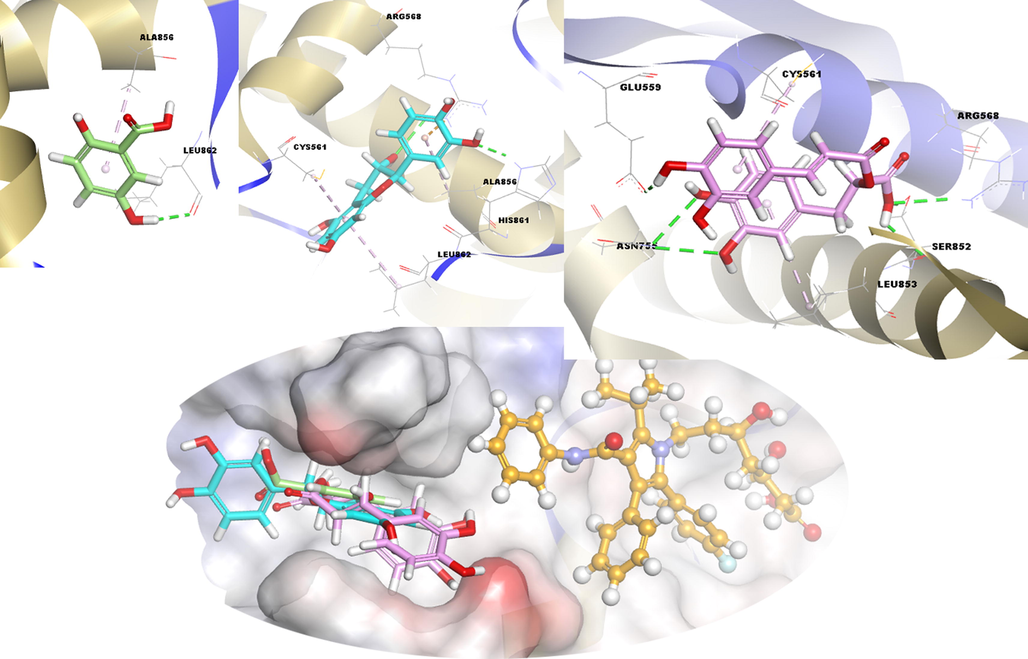
3D docking interactions and superimposed form of gentisic acid (1, light green color, stick form), (+) catechin (2, light blue color, stick form), rosmarinic acid (3, light pink color, stick form), and atorvastatin (orange, ball and stick form) as positive compound with HMG_CoA R, respectively.
As a result of the process of the same compounds with the second enzyme α-amylase, compound 2 exhibits better interaction and binding tendency. Then, compounds 3 and 1 follow. The control compound, acarbose (ACR, Table S3), formed hydrogen bond and hydrophobic interaction as a result of docking interaction with α-amylase enzyme. Based on this situation, compound 2 of the investigated compounds exhibited stronger non-bonding interactions with the enzyme. Especially the electrostatic interactions with Asp197 and Asp300 made the structure more effective than the others. Compound 3 exhibited hydrogen bonding with Glu233 and hydrophobic interaction with Tyr62. Although compound 1 forms hydrogen and hydrophobic interactions with the target enzyme, an undesirable situation has been revealed in a region other than the binding site of the related target. These situations are presented in detail in Fig. 4 and Table S1.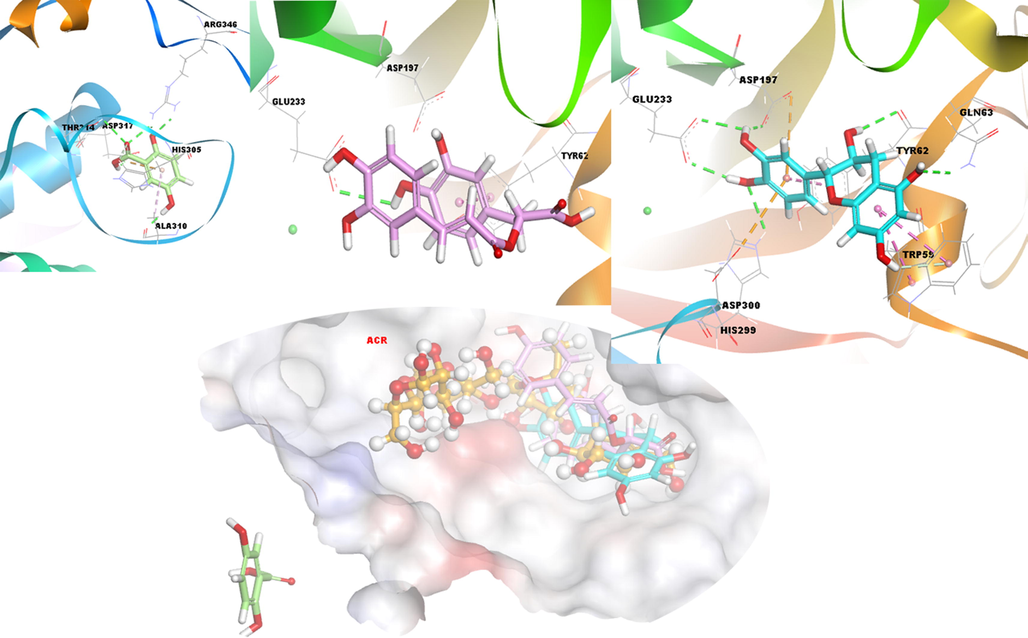
3D docking interactions and superimposed form of gentisic acid (1, light green color, stick form), rosmarinic acid (3, light pink color, stick form), (+) catechin (2, light blue color, stick form) and acarbose (ACR) (orange, ball and stick form) as positive compound with α-amylase, respectively.
Compound 2 shows the best results both numerically and visually in possible complexes with the α-glucosidase enzyme given in Table 5 and Fig. 5. When we consider the docking result in detail, compound 2 occurred hydrogen bond with Tyr313, Asp408, and Glu276, electrostatic interaction with Asp349 and Asp408 residue, and also pi-pi-T-shaped and pi-alkyl interactions with Tyr71 and Arg312 amino acid in the binding site of the target, respectively. Then, compound 3 formed hydrogen bonds with Arg312, Arg439, Asp349, Glu276, and Asp349, electrostatic interaction with Arg439, and hydrophobic interaction with Arg439 and Arg312. Finally, the condition that compound 1 exhibits in the same amylase is also observed in Fig. 5 and Table S1 for this target enzyme. In the meantime, comparing the interactions of the positive compound acarbose shown in Fig. S4, the effectiveness of compounds 2 and 3 with the target is seen.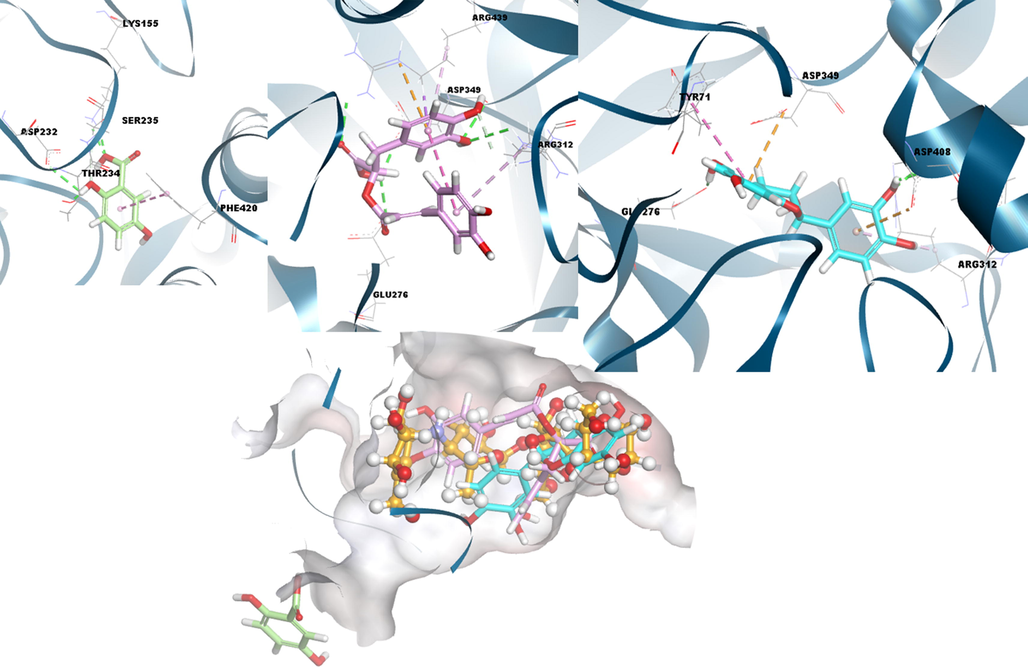
3D docking interactions and superimposed form of gentisic acid (1, light green color, stick form), rosmarinic acid (3, light pink color, stick form), (+) catechin (2, light blue color, stick form) and acarbose (ACR) (orange, ball and stick form) as positive compound with α-glycosidase, respectively.
Finally, as in α-amylase and α-glycosidase compound 2 and compound 3 and 1, respectively, show a high binding affinity between the complex structures they form with the lipase enzyme. As a result of the detailed analyses in Table S1 presented in the Supplementary files, the electrostatic interaction with His264 increased its binding affinity to the target enzyme, unlike other phenolic compounds and the control compound, orlistat. This expression is also beautifully illustrated in Fig. 6. When the orientations of the positive control structure given in Fig. S5 in the interaction and active site of the target are evaluated with the compounds we have discussed, the efficacy of compounds 2 and 3 is immediately striking.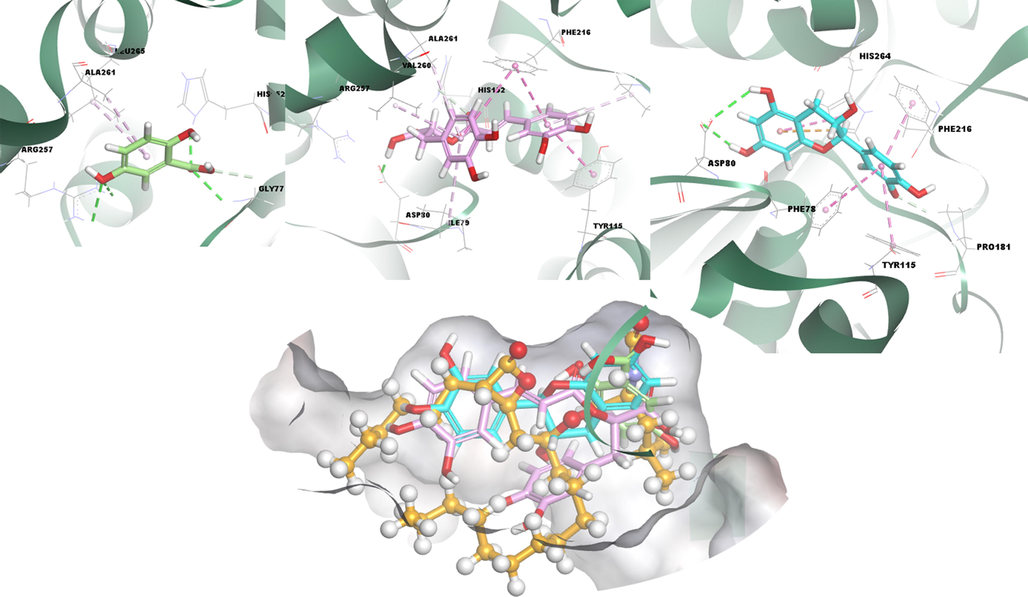
3D docking interactions and superimposed form of gentisic acid (1, light green color, stick form), rosmarinic acid (3, light pink color, stick form), (+) catechin (2, light blue color, stick form) and orlistat (orange, ball and stick form) as positive compound with lipase, respectively.
Molecular docking calculations were exerted to predict the biological activities of the first three compounds with the highest amount among the compounds extracted from A. kastambulense against different target structures and to understand the interaction mechanisms. As a result of the calculations and the findings obtained, it was observed that compound 2 and compound 3 exhibited better activity against target proteins than compound 1. In addition, hydrogen bonding, hydrophobic interaction as well as electrostatic interactions have a large share in the interactions of the related structures with the HMG_CoA R target, compared to other α-amylase, α-glycosidase, lipase enzymes that exhibit high binding tendencies. Besides the interactions, when the compounds are considered topologically, it is seen in Figs. 3-6 that the surface area of the related phenolic compounds and their conformation in the interaction region are important properties on enzyme inhibitions.
4 Conclusion
When we examined all enzyme results in this study, the results of the four enzymes were better than the standard. We had good results for the HMG_CoA R, α-amylase, α-glycosidase, and lipase enzymes, and these IC50 results are in order: 0.55, 121.5, 78.00, and 60.76 µg/mL. The plant also inhibited other enzymes, and all are acceptable. Angiotensin-converting enzyme (ACE) inhibition is considered a useful therapeutic approach in the treatment of hypertension. Therefore, the development of drugs that inhibit ACE to control high blood pressure has gained great importance. Many studies have been conducted on the synthesis of ACE inhibitors such as captopril, enalapril, alasepril, and lisinopril, which are still used to treat essential hypertension and heart failure in humans. As a result of the evaluation of the interactions on ten different targets for the three compounds, which were extracted in the highest amount, with the Molecular docking study, which is one of the in silico techniques, the electrostatic interactions have great contributions to the hydrogen bonding and hydrophobic interactions as well as the high binding affinity with HMG_CoA R, α-amylase, α-glycosidase and lipase. In addition, the surface area of the investigated compounds and their orientation in the active site of the target enzyme were evaluated by comparing them with the control molecule of each target. Natural products are widely used for therapeutic purposes in alternative medicine. Gentisic acid, (+) catechin, and rosmarinic acid were determined to be the most abundant metabolites in Allium kastambulense. In this study, antibacterial and antifungal potency of A. kastambulense plant against four bacterial species and two fungal species were investigated. As a result of the work, it has been shown that the methanol extract of A. kastambulense plant shows antifungal and antibacterial properties in a dose-dependent manner.
Acknowledgments
This study was financially supported by the TÜBİTAK (The Scientific and Technological Research Council of Turkey) (Grand ID: 120Z005). The authors thank Esin Akı Yalcin and the research group for technical assistance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Accelrys Software Inc. Discovery Studio Modeling Environment, Release 3.5 Accelrys Software Inc, San Diego, 2013.
- Benzimidazolium salts bearing 2-methyl-1,4-benzodioxane group: synthesis, characterization, computational studies, in vitro antioxidant and antimicrobial activity. Biointerface Res. Appl. Chem.. 2021;11(5):13333-13346.
- [Google Scholar]
- Antibacterial Activity Test of Ethanol Extract from Dried Simplicia Garlic (Allium Sativum L.) Against Bacillus Cereus Bacteria. J. Pharm. Sci. 2020
- [Google Scholar]
- Silver N-heterocyclic carbene complexes bearing fluorinated benzyl group: Synthesis, characterization, crystal structure, computational studies, and inhibitory properties against some metabolic enzymes. Appl. Organomet. Chem.. 2021;35(9):e6312
- [Google Scholar]
- Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem.. 2021;476:4301-4321.
- [CrossRef] [Google Scholar]
- Chemical Constituents and Pharmacological Activities of Garlic (Allium Sativum L.): A Review. Nutrients 2020
- [Google Scholar]
- Sources for developing new medicinal products: Biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement Altern. Med.. 2018;18:1-12.
- [CrossRef] [Google Scholar]
- Antibacterial Activity of Soap Formulated from Garlic (Allium Sativum L.) Extract. J. Adv. Microbiol. 2021
- [Google Scholar]
- The bioactivity of Hedysarum coronarium extracts on skin enzymes and cells correlates with phenolic content. Pharm. Biol.. 2017;55(1):1984-1991.
- [Google Scholar]
- “Antıbacterıal Effect of Garlıc (Allıum Satıvum) And Gınger (Zıngıber Offıcınale) Agaınst Staphylococcus Aureus, Salmonella Typhı, Escherıchıa Colı And Bacıllus Cereus. J. Microbiol., Biotechnol. Food Sci. 2021
- [Google Scholar]
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and L-ascorbic acid on tyrosinase inhibition. Z Naturforsch C. 2017;72:119-121.
- [Google Scholar]
- Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Products. 2018;122:498-505.
- [Google Scholar]
- Antihypertensive agents acting on the renin-angiotensin system and the risk of sepsis. Br. J. Clin. Pharmacol.. 2014;78:1151-1158.
- [Google Scholar]
- AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021
- [Google Scholar]
- Structural basis for activity regulation and substrate preference of clostridial collagenases G, H, and T. J. Biol. Chem.. 2013;288:20184-20194.
- [Google Scholar]
- El-Korany, S.M., Helmy, O.M., El-Halawany, A.M., Ragab, Y.E., Zedan, H.H., 2020. Kojic acid repurposing as a pancreatic lipase inhibitor and the optimization of its production from a local Aspergillus oryzae soil isolate. BMC Biotechnol. 20(1):52. Published 2020 Oct 2. doi:10.1186/s12896-020-00644-9
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [Google Scholar]
- Chemical and biological comparison of different parts of two Allium species: Allium paniculatum L. subsp. villosulum (Hal.) Stearn and Allium paniculatum L. subsp. paniculatum L. Chem. Pap.. 2021;75:411-419.
- [CrossRef] [Google Scholar]
- Phytochemical analyses with LC-MS/MS and in vitro enzyme inhibitory activities of an endemic species “Allium stylosum O. Schwarz” (Amaryllidaceae) S. Afr. J. Bot.. 2021;136:70-75.
- [CrossRef] [Google Scholar]
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., et. al., 2009. Gaussian 09, Revision E.01. Gaussian, Inc., Wallingford CT.
- Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol.. 2018;119:741-746.
- [Google Scholar]
- Molecular mechanism for inhibition of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase by rosuvastatin. Biochem. Soc. Trans.. 2003;31(3):528-531. PMID: 12773150
- [CrossRef] [Google Scholar]
- Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. J. Agric. Food Chem.. 2003;51:1706-1709.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one. Open Chem.. 2017;15:308-319.
- [CrossRef] [Google Scholar]
- Chemical Composition and Antimicrobial Activity of Wild Garlic Allium Ursinum of Bulgarian Origin. Nat. Prod. Commun. 2009
- [Google Scholar]
- Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol.. 2009;297:L84-L96.
- [Google Scholar]
- Antimicrobial properties of allium species. Curr. Opin. Biotechnol.. 2012;23:142-147.
- [Google Scholar]
- A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018
- [Google Scholar]
- Metabolic profiling of different wild and cultivated Allium species based on high-resolution mass spectrometry, highperformance liquid chromatography-photodiode array detector, and color analysis. J. Mass Spectrom.. 2020;2020(55):e4525
- [CrossRef] [Google Scholar]
- Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem.. 2005;69:197-201.
- [CrossRef] [Google Scholar]
- Otto Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric. Ecosys. Environ.. 2017;248:105-112.
- [Google Scholar]
- Auto dock4 and auto dock tools 4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30:2785-2791.
- [Google Scholar]
- Nastri, A., Toderi, G., Bernati, E., Govi, G., 2000. Ammonia volatilization and yield response from urea applied to wheat with urease (NBPT) and nitrification (DCD) inhibitors. Agrochim, 44 (5–6), pp. 231-239.
- Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica. Pharm. Biol.. 2013;51:1182-1187.
- [Google Scholar]
- Determination of phenolic acids in Amaryllidaceae species by high performance liquid chromatography. Pharm. Biol.. 2005;43:289-291.
- [Google Scholar]
- Synthesis, characterization, crystal structures, theoretical calculations and biological evaluations of novel substituted tacrine derivatives as cholinesterase and carbonic anhydrase enzymes inhibitors. J. Mol. Struct.. 2019;2019(1175):906-915.
- [Google Scholar]
- Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg. Chem.. 2017;74:104-114.
- [Google Scholar]
- Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev.. 2009;2:270-278.
- [Google Scholar]
- Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods. 2014;7:1776-1782.
- [CrossRef] [Google Scholar]
- Rama Narsimha Reddy, A., Srividya Lonkala, M., 2019. Antibacterial Activity of Carica Papaya Leaves and Allium Sativum Cloves Alone and in Combination against Multiple Strains. Pharmacognosy J.
- Rocchetti, G., Zhang, L., Bocchi, S., Giuberti, G., Ak, G., Elbasan, F., Yıldıztugay, E., Ceylan, R., Picot-Allain, M.C.N., mahomoodally, M.F., Lucini, L., Zengin, G., 2022. The functional potential of nine Allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chemistry 368, 130782. ttps://doi.org/10.1016/j.foodchem.2021.130782.
- The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab. Brain Dis.. 2017;32(4):949-965.
- [Google Scholar]
- Phython: a programming language for software integration and development. J. Mol. Graph. Model. 1999;17:57-61.
- [Google Scholar]
- Antıbacterıal Actıvıty Of Aqueous Extract of Garlıc (Allıum Satıvum) On Standard Straıns. J. Evol. Med. Dental Sci. 2018
- [Google Scholar]
- HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J. Neurochem.. 2004;89(1):24-32.
- [Google Scholar]
- Singleton, Vernon. L., Rosi, J.A., 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Oenol. Vitic. 16:144–158
- Anticancer, anticholinesterase and antidiabetic activities of tunceli garlic (Allium tuncelianum): determining its phytochemical content. J. Food Meas. Charact.. 2021;15:3323-3335.
- [CrossRef] [Google Scholar]
- Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr.. 2013;27:148-155.
- [CrossRef] [Google Scholar]
- Taslimi, P., Gulçin, İ., 2018. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem., 2018, 42(3), e12516.
- Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern. Med.. 2009;9:1-11.
- [CrossRef] [Google Scholar]
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2010;31(2):455-461.
- [Google Scholar]
- Total phenolic, flavonoid, fatty acid contents and cytotoxic, antioxidant, and antimicrobial activities of Hedysarum aucheri. J. Pharm. Res. Int. 2017:1-13.
- [Google Scholar]
- Angiotensin converting enzyme inhibitors and angiotensin receptor blockers in the treatment of hypertension: should they be used together. Curr. Vasc. Pharmacol.. 2010;8:742-746.
- [Google Scholar]
- Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules. 2018;23:106.
- [Google Scholar]
- Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179-187.
- [Google Scholar]
- Yaman, B., kaya, Z., Tunçkol, B., Özel, H.B., 2020. The endemic plants in Bartın (Turkey), and their conservation status. Biologia Nyssana, 11(1) 23-29. DOI: 10.5281/zenodo.4060285.
- Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia. 2012;83:1169-1175.
- [CrossRef] [Google Scholar]
- Yao, X.C., Zhu, L., Chen, Y.X., Tian, J., Wang, Y.W., 2013. In vivo and in vitro antioxidant activity and α-glucosidase, α-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chemistry, 139 (1–4), pp. 59-66
- Anti Fungal Activity of Shallot, Allium Ascalonicum Linn. (Liliaceae), in Vitro. J. Med. Plants Res. 2009
- [Google Scholar]
- Zeng, Y., Li, Y., Yang, J., Pu, X., Du, J., Yang, X., Yang, T., Yang, S., 2017. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Hindawi Evidence-Based Complementary and Alternative Medicine. Article ID 9402849. https://doi.org/10.1155/2017/9402849.ç
- Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innovative Food Sci. Emerg. Technol.. 2016;3:1-9.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103810.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







