Translate this page into:
Assessment on in vitro medicinal properties and chemical composition analysis of Solanum virginianum dried fruits
⁎Corresponding authors. saraskri1591@gmail.com (K. Saraswathi), armankhan0301@gmail.com (Ameer Khusro), umar.sahibzada@gmail.com (Muhammad Umar Khayam Sahibzada)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The study was aimed to screen the presence of phytoconstituents and determine distinct in vitro medicinal traits of aqueous and ethanolic extracts of Solanum virginianum dried fruits. Aqueous and ethanolic extract showed total phenolic content of 207.5 ± 0.16 and 268.4 ± 0.42 GAE/mg, respectively. Likewise, total flavonoid content of 50.12 ± 0.39 and 192.88 ± 0.27 QE/mg was estimated for the aqueous and ethanolic extract, respectively. In vitro antibacterial, antioxidant, α-amylase inhibition, anti-inflammatory, and anticancer attributes of extracts were assessed using standard protocols. The antibacterial traits of both the extracts were assessed against certain pathogenic bacteria which exhibited maximum zone of inhibition of 22.3 ± 0.6 mm against Staphylococcus aureus. Antioxidant tests showed not only significant scavenging of DPPH, superoxide, hydroxyl, and ABTS●+ radicals but also estimated ferric reducing power and phosphomolybdenum reduction activities of extracts in a concentration dependent manner. The aqueous extract (54.12 ± 0.44–86.80 ± 0.27%) depicted higher rate of α-amylase inhibition than ethanolic extract (23.07 ± 0.47–81.61 ± 0.43%) at distinct concentrations. Similarly, the aqueous extract protected the haemolysis (46.19 ± 0.14–66.21 ± 0.17%) effectively as compared to the ethanolic extract (12.67 ± 0.19–38.03 ± 0.41%). The aqueous and ethanolic extract showed cytotoxicity against HepG2 cell lines in the range of 32.23 ± 0.34–54.82 ± 0.26% and 49.25 ± 0.38–73.2 ± 0.3%, respectively. Additionally, the GC–MS analysis confirmed the availability of total 15 predominant bioactive constituents in both extracts. Findings of this context indicated pronounced applications of S. virginianum fruits as future therapeutic.
Keywords
Fruit extracts
GC–MS
Medicinal properties
Solanum virginianum
1 Introduction
Medicinal plants are prominent hub of several bioactive phytoconstituents. These phytochemicals from natural resources have gained immense prominence in recent years, contributing to the discovery of new bioactive compounds and perhaps the development of new drugs (Tekuri et al., 2019). The application of herbal drugs as therapeutics has been a tremendous area of research since ancient periods due to its safer consumption with low adverse effects (Barbabosa-Pliego et al., 2020; Prasathkumar et al., 2021). Currently, 80% of world population in developing countries is dependent on herbal medicines because of several pharmacological characteristics (Sen and Chakraborty, 2016). Despite the numerous reports on plants of different families as therapeutic agents, certain medicinal plants still require further pharmaceutical investigations towards drug discovery process.
Solanaceae family contains 98 genera and more than 3000 species of plants globally (Pereira et al., 2019). Plants of this family are well known for ethnomedicinal and dietary uses (Pinela et al., 2019; Pereira et al., 2021a). Solanum virginianum (Solanaceae), commonly called as ‘Kantakari’, is an itchy perennial herb. This herb is also called as ‘Indian night shade’ or ‘yellow berried night shade’ plant. Different parts of this plant are a rich source of varied phytochemicals viz. phenols, saponins, terpenoids, alkaloids, flavonoids, glycosides, and amino acids (Amir and Kumar, 2004). In Ayurvedic system of medicine, this whole plant is used as antimicrobial, antitumor, antipyretic, anti-inflammatory, anti-allergy, anti-fertility, hypoglycaemic, antioxidant, and anti-histamine agents. In addition, the plant is used for treating asthma, skin infection, headache, hair fall, and cough too (Borgato et al., 2007).
Fruits of Solanum spp. are known to exhibit several biological traits, including anthelmintic, wound healing, antipyretic, laxative, antiasthmatic, antibacterial, antioxidant, larvicidal, anti-inflammatory, anti-diabetic, hepatoprotective, anti-urolithiatic, anti-fertility, and aphrodisiac activities (Nithya et al., 2018; Tekuri et al., 2019; Pereira et al., 2020; Pereira et al., 2021b). In fact, fruits of this Solanum spp. contain plethora of effective bioactive phytocomponents such as solanosine, solasodine, carpesterol, campesterol, daucosterol, caffeic acid, coumarins, triterpinoids, solanocarpine, solamorgine, solanocarpidine, lupeol, and diosgenine which are considered potent therapeutic agents (Jayakumar and Murugan, 2016; Tekuri et al., 2019; Moyo et al., 2020).
In view of the multi-therapeutic reports mentioned above, it is clear that different species of Solanum have been extensively studied for distinct pharmacological properties and have been suggested possible utilization of its biologically active components to design effective drugs against various health problems (Tekuri et al., 2019). Surprisingly, certain medicinal aspects of S. virginianum are undetermined or less studied so far. Therefore, this study was aimed not only to assess different in vitro biological activities of solvent extracts of S. virginianum fruits but also analyze the availability of different compounds in the extracts using Gas Chromatography-Mass Spectrometry (GC–MS).
2 Materials and methods
2.1 Chemicals and reagents
Chemicals and reagents used in this investigation were of analytical grade and procured from Merck India Private Limited.
2.2 Plant sample collection and extract preparation
Fresh fruits of S. virginianum were collected from herbal market, Chennai, Tamil Nadu, India in the month of March and were identified by Dr. G. Jeya Jothi, Taxonomist, Department of Plant Biology and Biotechnology, Loyola College, Chennai, India. The shed dried (at 35 °C) fruits of S. virginianum (Fig. 1) were semi-coarsely macerated, mixed with absolute ethanol (99.9%) in the ratio of 1:10 for 3 days, filtered, and condensed at 50 °C for obtaining ethanolic extract. The aqueous extract was prepared by soaking the macerated dried fruits of S. virginianum in sterilized distilled water (1:10) and heating for 15 min. The filtered content was then condensed at 50 °C for getting pale yellow extract (Harborne, 1998).
(a) Fresh and (b) Dried fruits of S. virginianum.
2.3 Qualitative phytochemical analysis
Aqueous and ethanolic extracts of S. virginianum dried fruits were subjected to determine the presence of different classes of phytoconstituents (alkaloids, flavonoids, terpenoids, steroids, phenols, tannins, glycosides, saponins, quinones, and carbohydrates) using standard methodologies (Harborne, 1998).
2.4 Determination of total phenols and flavonoids content
Total phenolic content of the aqueous and ethanolic extracts of S. virginianum dried fruits were estimated by Folin-Ciocalteau reagent method (Gutiérrez-Morales et al., 2017) and was calculated as gallic acid equivalent per milligrams of extract (GAE/mg of extract). Total flavonoid contents of aqueous and ethanolic extracts of S. virginianum fruits were determined by aluminium chloride reagent test by calculating quercetin equivalent/mg (QE/mg) of extract (Kefayati et al., 2017).
2.5 Antibacterial assessment
Antibacterial properties of the aqueous and ethanolic extracts of S. virginianum fruits were determined against Gram positive (Staphylococcus aureus, Micrococcus luteus, and Bacillus subtilis) and Gram negative (Proteus vulgaris, Escherichia coli, and Shigella flexneri) bacteria as per the method of Salem et al. (2018). The overnight grown indicator bacteria were evenly swabbed on freshly prepared Mueller Hinton agar medium plates and wells (5 mm in diameter) were created in each plate using sterile cork-borer. The aqueous and ethanolic extracts (100 µL) were loaded into respective wells. Tetracycline (30 µg) was used as standard. After 24 h, the inhibitory zone for each concentration of aqueous extract, ethanolic extract, and standard was calculated by measuring the zone diameter (in mm).
2.6 In vitro antioxidant activities-
2.6.1 1, 1- diphenyl 2-picrylhydrazyl (DPPH) degradation
Antioxidant activity of each extract (20–120 μg/mL) was measured by following the method of Khalaf et al. (2008). One mL of 0.1 mM DPPH solution prepared in methanol was mixed with 1 mL of various concentrations (20–120 μg/mL) of aqueous and ethanolic extracts of S. virginianum fruits. The mixture was then incubated at room temperature for 30 min in dark. The decrease in absorbance was measured at 517 nm using UV–Vis spectrophotometer. Ascorbic acid was used as standard and the DPPH radical scavenging (%) was quantified as: DPPH scavenging (%) = [(Absorbancecontrol– Absorbancesample)/Absorbancecontrol]× 100
2.6.2 Superoxide radical (O2–) scavenging
Superoxide radical (O2–) scavenging activities of different concentrations of aqueous and ethanolic extracts (20–120 μg/mL) were estimated by following the methodology of Bagul et al. (2003). Extracts were mixed with 1 mL of 50 mM phosphate buffer (pH 7.6), 200 µL of 1.5 mM riboflavin, 200 µL of 12 mM EDTA, and 100 µL of 50 mM NBT solutions. The reaction was started by incubating the reaction mixture for 15 min. After incubation, the absorbance was read at 590 nm using UV–Vis spectrophotometer. Ascorbic acid was used as standard. The superoxide (O2–) scavenging (%) was quantified as:
2.6.3 Hydroxyl radical scavenging
Hydroxyl radical scavenging activities of aqueous and ethanolic extracts were measured by the salicylic acid method (Smirnoff and Cumbes, 1989). One mL of aqueous and ethanolic extracts at varied concentrations (20–120 μg/mL) was mixed with 500 µL of 9 mM salicylic acid, 500 µL of 9 mM ferrous sulphate, and 50 µL of 9 mM H2O2 solution. The reaction mixture was incubated for 60 min at 37 °C. After incubation, the absorbance of the mixtures was measured at 510 nm using UV–Vis spectrophotometer. Ascorbic acid was used as standard reference and hydroxyl radical scavenging (%) was estimated as: Hydroxyl radical scavenging (%) = [(Absorbancecontrol– Absorbancesample)/Absorbancecontrol]× 100
2.6.4 ABTS●+ radical cation degradation
Antioxidant capacities of aqueous and ethanolic extracts were calculated by degrading ABTS●+ radical (Arnao et al., 2001). Initially, ABTS●+ was obtained by reacting 7 mM ABTS solution in 5 mM phosphate-buffered saline (pH 7.4) with 2.45 mM potassium persulfate, and the mixture was left to stand in dark at room temperature for 12–16 h before use. The ABTS solution (stable for 2 days) was diluted with 5 mM phosphate-buffered saline (pH 7.4) until the absorbance of 0.70 ± 0.02 at 734 nm was obtained. To the various concentrations (2–12 µg/mL) of aqueous and ethanolic extract, 500 µL of diluted ABTS●+ solution was added. After 10 min of incubation, the absorbance was measured at 734 nm using UV–Vis spectrophotometer. The ABTS●+ degradation (%) was determined as:
2.6.5 Ferric (Fe3+) reduction power
The reduction power of aqueous and ethanol extracts was determined by Fe3+ reduction method (Oyaizu, 1986). One mL of aqueous and ethanolic extract at different concentrations (20–120 µg/mL) was mixed with 1 mL of phosphate buffer (0.2 M, pH 6.6) and 1 mL of potassium ferricyanide solution (1 % w/v). The mixtures were then incubated at 50 °C in water bath for 30 min. Trichloroacetic acid (0.5 mL; 10 % w/v) was further added to each reaction mixture, followed by the addition of 0.1 mL of freshly prepared ferric chloride (0.1% w/v) solution. The absorbance was read at 700 nm using UV–Vis spectrophotometer. Ascorbic acid was used as standard and Fe3+ reductions were quantified as:
2.6.6 Phosphomolybdenum reductions
The antioxidative capacity of each extract was assessed by Mo6+ reduction method (Prieto et al., 1999). The extracts at different concentrations (20–120 μg/mL) were mixed with 1 mL of reagent solution containing ammonium molybdate (4 mM), sodium phosphate (28 mM), and sulphuric acid (600 mM). The reaction mixture was incubated in water bath at 95 °C for 90 min. The absorbance of the coloured complex was measured at 695 nm using UV–Vis spectrophotometer. Ascorbic acid was used as standard and Mo6+ reductions (%) were calculated as:
2.7 In vitro anti-diabetic property
Alpha (α)-amylase inhibitory trait was carried out by starch-iodine assay (Hossan et al., 2009). The sample mixture was composed of various concentrations (10–120 µg/mL) of S. virginianum fruit extracts and 10 µL of α-amylase enzyme (1% w/v), prepared in 0.02 M sodium phosphate buffer (pH 6.9 constituting 6 mM NaCl). The sample mixture was kept at 37 °C for 10 min, followed by the addition of starch (1% w/v; 500 µL) in each reaction and incubation at 37 °C for 1 h. One hundred microlitres of 1 N HCl was added into the solution to stop the reaction, and then 200 μL of iodine reagent (5 mM iodine and 5 mM potassium iodide) was mixed. The optical density was recorded at 565 nm after observing the change in the colour of the solution. The control reaction representing 100% enzyme activity did not contain any of the extract. Acarbose was used as the standard. The α-amylase inhibitory trait (%) was determined as:
2.8 In vitro anti-inflammatory property-
2.8.1 Membrane stabilization- Red blood cells (RBCs) suspension preparation
Blood sample collected from human volunteers in sterile tubes was centrifuged at 3500 rpm for 10 min and then washed with saline. Blood volume was checked and reconstituted as 10% (v/v) suspension using saline.
2.8.2 Heat-induced haemolytic activity
The mixture (2 mL) contained 1 mL of varied doses (20–120 µg/mL) of extracts and 1 mL of 10% RBC’s suspension. Control test tube contained only saline. The test tubes were incubated at 60 °C for 30 min and then cooled at room temperature. The mixture was centrifuged at 3500 rpm for 15 min and absorbance of the supernatant was recorded at 560 nm using Diclofenac as standard (James et al., 2009). The inhibition of haemolytic activity (%) was estimated as:
2.9 In vitro anti-proliferative activity
Human liver cancer cell line (HepG2) cell lines obtained from NCCS (National Centre for Cell Science, Pune), was cultured in Rosewell Park Memorial Institute medium (RPMI) supplemented with 10% fetal bovine serum and antibiotics. The anti-proliferative activity of varied concentrations (200–400 µg/mL) of aqueous and ethanolic extracts was determined by MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)] assay (Mosmann, 1983).
2.10 GC–MS analysis
GC–MS chromatogram of each extract was obtained by PerkinElmer Turbo Mass Spectrophotometer (Norwalk, CTO6859, and USA) using Helium as a carrier gas with a flow rate of 0.5 mL/min. The extract (1 µL) was injected and temperature of the inlet was set at 250 °C. The temperature programme of the oven was set initially at 110 °C for 4 min, and then increased up to 280 °C with total run time of 90 min. The components in each extract were identified based on retention time by comparing with the mass spectrum of the known compounds (Esther Lydia et al., 2019).
2.11 Statistical analysis
All the experiments were done in triplicates and values were expressed as mean ± standard deviation (SD). The statistical analysis was carried out by one-way ANOVA using SPSS version 12.0 (SPSS, Inc., Chicago, IL) and P < 0.05 was considered significant.
3 Results
3.1 Phytochemical analyses (qualitative and quantitative)
S. virginianum fruit extracts (aqueous and ethanolic) revealed the availability of major phytochemicals such as alkaloid, flavonoids, terpenoid, steroids, phenolics, glycosides, saponin, and carbohydrate. On the other hand, quinines were absent in both the aqueous and ethanolic extracts (Table 1A). The total phenolic content of 207.5 ± 0.16 and 268.4 ± 0.42 GAE/mg of extract was estimated for the aqueous and ethanolic extract, respectively. Likewise, the total flavonoid content of 50.12 ± 0.39 and 192.88 ± 0.27 QE/mg of extract was estimated for the aqueous and ethanolic extract, respectively (Table 1B). Total phenolic and flavonoid contents of both the extracts varied significantly (P < 0.05). (+)-Positive; (-)-Negative Values represent mean ± SD. abValues with different superscript letters are significantly (P < 0.05) different.
S. No
Phytochemicals
Aqueous extract
Ethanolic extract
1
Alkaloids
Hager's test
+
+
Mayer's test
+
+
2
Flavonoids-Sodium hydroxide test
+
+
3
Terpenoids-Liebermann burchard test
+
+
4
Steroids-Acetic anhydride test
+
+
5
Phenols-Ferric chloride test
+
+
6
Tannins-Lead acetate test
+
+
7
Glycosides-Legal test
+
+
8
Saponins-Foam test
+
+
9
Quinones-Sulphuric acid test
–
–
10
Carbohydrates-Barfoed test
+
+
Phytochemicals
Extracts
Aqueous
Ethanolic
Phenols
207.5 ± 0.16b mg GAE/g
268.4 ± 0.42a mg GAE/g
Flavonoids
50.12 ± 0.39b mg QE/g
192.88 ± 0.27a mg QE/g
3.2 Antibacterial activity
The antibacterial activities of the aqueous extract of S. virginianum fruits are illustrated in Table 2A. The aqueous extract exhibited potentially significant (P < 0.05) growth inhibitory activities against M. luteus and B. subtilis with maximal zone of inhibition of 15.6 ± 0.3 mm at 500 µg/mL of concentration. The extract depicted comparatively reduced activities against P. vulgaris, E. coli, and S. flexneri at varied concentration. The extract depicted no inhibition against S. aureus at all concentrations. Tetracycline revealed remarkable growth inhibitory activities against all the bacterial pathogens as compared to the aqueous extract. Values represent mean ± SD; mm-millimetres; NA-No activity abcValues with different superscript letters are significantly (P < 0.05) different.
S. No
Bacteria
Zone of inhibition (mm)
250 µg/mL
375 µg/mL
500 µg/mL
Tetracycline (30 µg)
1
S. aureus
NA
NA
NA
12.3 ± 0.6
2
M. luteus
14.3 ± 0.6b
15.3 ± 0.6a
15.6 ± 0.3a
28.3 ± 0.6
3
B. subtilis
14.6 ± 0.3b
14.3 ± 0.6b
15.6 ± 0.3a
20.6 ± 0.3
4
P. vulgaris
8.6 ± 0.3c
9.6 ± 0.3b
11.3 ± 0.6a
15.3 ± 0.6
5
E. coli
12.3 ± 0.6c
14.3 ± 0.6b
14.3 ± 0.6a
21.6 ± 0.3
6
S. flexneri
8.3 ± 0.6b
8.6 ± 0.3b
9.6 ± 0.3a
28.6 ± 0.3
Table 2B shows the antibacterial activities of different concentrations of ethanolic extract of S. virginianum fruits against varied pathogens which revealed maximum zone of inhibition of 22.3 ± 0.6 mm against S. aureus, followed by E. coli (18.3 ± 0.6 mm), B. subtilis (17.6 ± 0.3 mm), S. flexneri (17.6 ± 0.3 mm), P. vulgaris (16.6 ± 0.3 mm), and M. luteus (9.6 ± 0.3 mm). The antibacterial activity of ethanolic extract varied significantly (P < 0.05) at higher concentration. Tetracycline showed higher activities than ethanolic extract against all the pathogens, S. flexneri being the most susceptible (31.6 ± 0.3 mm). Values represent mean ± SD; mm-millimetres abValues with different superscript letters are significantly (P < 0.05) different.
S. No
Bacteria
Zone of inhibition (mm)
250 µg/mL
375 µg/mL
500 µg/mL
Tetracycline (30 µg)
1
S. aureus
21.6 ± 0.3b
21.3 ± 0.6b
22.3 ± 0.6a
22.6 ± 0.3
2
M. luteus
8.6 ± 0.3b
9.3 ± 0.6a
9.6 ± 0.3a
28.3 ± 0.6
3
B. subtilis
16.3 ± 0.6b
17.3 ± 0.6a
17.6 ± 0.3a
24.3 ± 0.6
4
P. vulgaris
15.3 ± 0.6b
16.6 ± 0.3a
16.6 ± 0.3a
28.6 ± 0.3
5
E. coli
16.6 ± 0.3b
18.3 ± 0.6a
18.3 ± 0.6a
18.6 ± 0.3
6
S. flexneri
16.6 ± 0.3b
17.3 ± 0.6a
17.6 ± 0.3a
31.6 ± 0.3
3.3 In vitro antioxidant activities
DPPH degradation characteristics of S. virginianum fruits extracts are described in Fig. 2a. The ethanolic extract exhibited significantly (P < 0.05) higher DPPH scavenging traits (38.43 ± 0.22–92.52 ± 0.48%) than the aqueous extract (17.44 ± 0.20–85.95 ± 0.35%) in a dose dependent manner (20–120 µg/mL). Fig. 2b illustrates the superoxide (O2.–) radical degrading activities of both extracts. Results showed significantly (P < 0.05) good ability of aqueous extract to scavenge Superoxide (O2.–) radical (8.03 ± 0.11–62.20 ± 0.45%) with respect to the ethanolic extract (2.69 ± 0.13–56.22 ± 0.41%) in a concentration dependent manner (20–120 µg/mL). Hydroxyl radical scavenging traits of S. virginianum fruits extracts are shown in Fig. 2c which revealed significantly (P < 0.05) higher inhibition attribute of ethanolic extract (30.4 ± 0.29–62.10 ± 0.41%) than that of the aqueous extract (17.70 ± 0.23–57.10 ± 0.42%) at varied concentration (20–120 µg/mL). Fig. 2d illustrates ABTS●+ radical cation scavenging characteristics of the aqueous and ethanolic extract. The ethanolic extract demonstrated significantly (P < 0.05) pronounced scavenging activities (62.68 ± 0.18–93.11 ± 0.46%) as compared to the aqueous extract (50.72 ± 0.42–93.84 ± 0.15%) at diverse concentrations (2–12 µg/mL). Fig. 2e shows Fe3+ reduction abilities of S. virginianum fruits extracts. Results revealed significantly (P < 0.05) higher reduction activities of the aqueous extract (48.19 ± 0.43–74.12 ± 0.11%) than that of the ethanolic extract (9.41 ± 0.45–52.17 ± 0.47%) in a concentration dependent manner (20–120 µg/mL). Phosphomolybdenum reduction activities of the aqueous and ethanolic extract of S. virginianum fruits are illustrated in Fig. 2f. The ethanolic extract showed significantly (P < 0.05) pronounced rate of Mo6+ reduction activities (68.18 ± 0.16–88.96 ± 0.21%) as compared to the aqueous extract (43.07 ± 0.39–88.14 ± 0.44%) at varied doses. On the other hand, ascorbic acid (standard) revealed significantly (P < 0.05) higher antioxidant activities than aqueous and ethanolic extracts.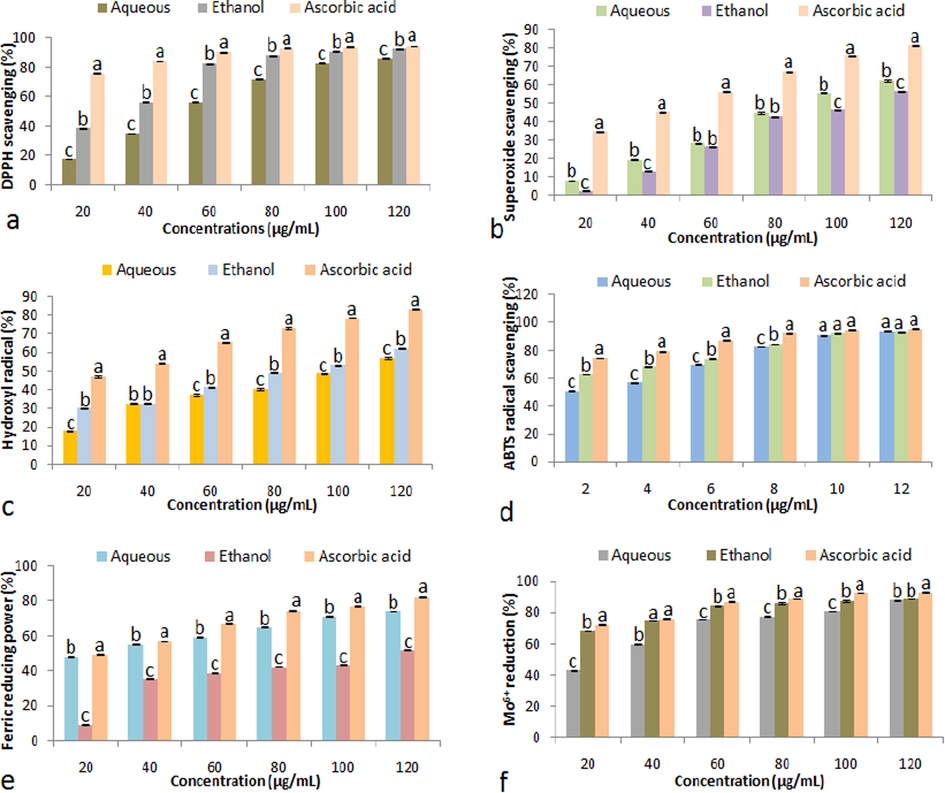
(a) DPPH radical scavenging, (b) Superoxide radical scavenging, (c) Hydroxyl radical scavenging, (d) ABTS●+ radical cation scavenging, (e) Ferric reduction activity, and (f) Mo6+ reduction activities of fruit extracts (aqueous and ethanolic) and ascorbic acid. Data represent mean ± SD. abcValues with different superscript letters are significantly (P < 0.05) different.
3.4 In vitro anti-diabetic traits
α-amylase inhibitory traits of aqueous and ethanolic extract of S. virginianum fruits at distinct concentrations (20–120 µg/mL) are shown in Fig. 3. The aqueous extract (54.12 ± 0.44–86.80 ± 0.27%) depicted significantly (P < 0.05) higher rate of α-amylase inhibition than the ethanolic extract (23.07 ± 0.47–81.61 ± 0.43%) in a concentration dependent manner. Acarbose revealed significantly (P < 0.05) higher inhibition of α-amylase (58.36 ± 0.30–88.24 ± 0.16%) than aqueous and ethanolic extracts at all concentrations.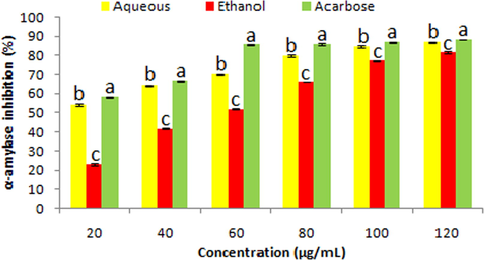
α-amylase inhibition traits of fruit extracts (aqueous and ethanolic) and acarbose. Data represent mean ± SD. abcValues with different superscript letters are significantly (P < 0.05) different.
3.5 In vitro anti-inflammatory property
Both extracts showed effectiveness towards the inhibition of heat induced haemolysis at varied concentrations. However, results exhibited that the aqueous extract at all concentrations (20–100 µg/mL) protected the haemolysis (46.19 ± 0.14–66.21 ± 0.17%) significantly (P < 0.05) higher than the ethanolic extract (12.67 ± 0.19–38.03 ± 0.41%). Diclofenac showed maximum (P < 0.05) inhibition of haemolysis (48.26 ± 0.11–70.39 ± 0.28%) as compared to the extracts (Fig. 4).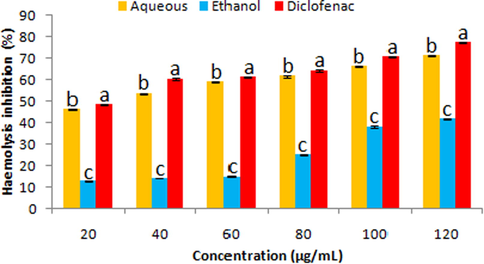
Haemolysis inhibition traits of fruit extracts (aqueous and ethanolic) and diclofenac. Data represent mean ± SD. abcValues with different superscript letters are significantly (P < 0.05) different.
3.6 In vitro anticancer activity
The extracts exhibited significant (P < 0.05) rate of cytoxicity against HepG2 cell line at varied concentrations. The aqueous extract demonstrated anticancer activity in the range of 32.23 ± 0.34–54.82 ± 0.26% along with shrinkage and rounding of cell lines (Fig. 5). On the other hand, the ethanolic extract revealed higher cytotoxicity (49.25 ± 0.38–73.2 ± 0.3%) than the aqueous extract with significant morphological changes in the cell line (Fig. 6).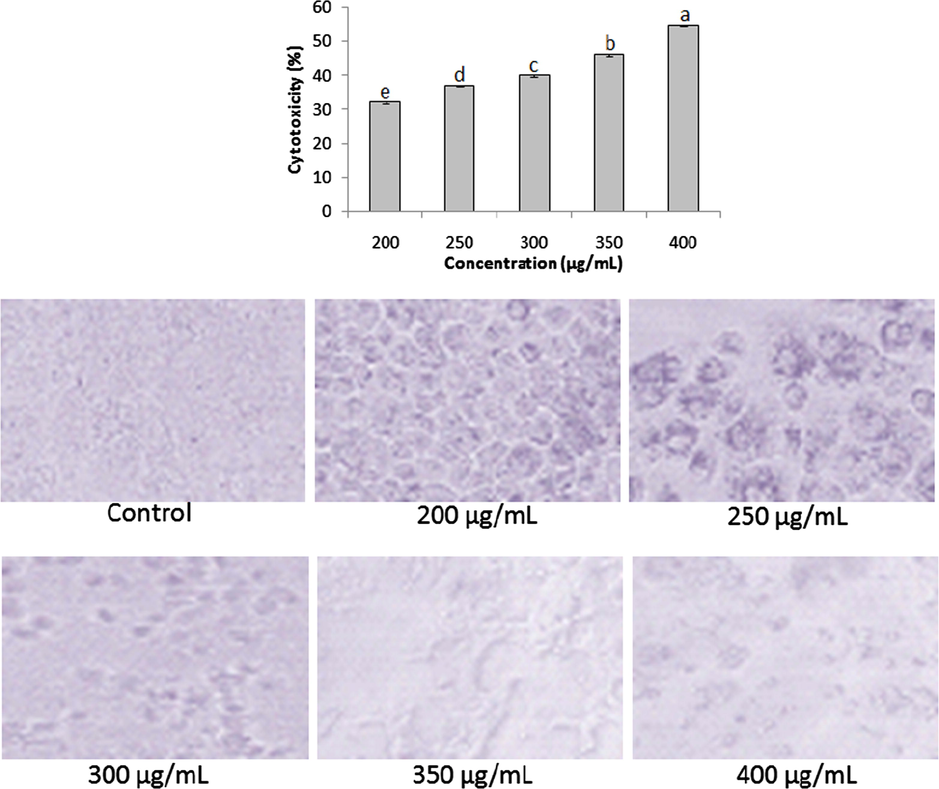
Anticancer activities of aqueous extract at varied concentrations (200–400 µg/mL). Data represent mean ± SD. abcdeValues with different superscript letters are significantly (P < 0.05) different.
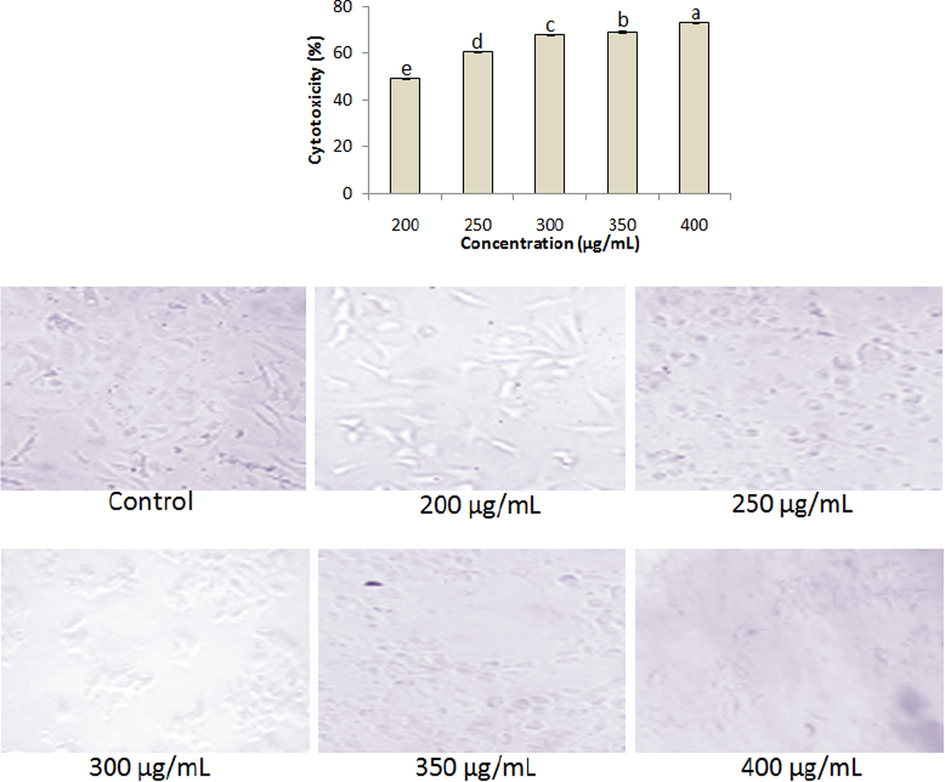
Anticancer activities of ethanolic extract at varied concentrations (200–400 µg/mL). Data represent mean ± SD. abcdeValues with different superscript letters are significantly (P < 0.05) different.
3.7 GC–MS analysis
GC–MS analysis of the aqueous extract of S. virginianum fruits is shown in Table 3 and Fig. 7. Methyl tetradecanoate, 1-octadecene, 9-methyl-10,12-hexadecadien-1-ol acetate, 9-hexadecenoic acid, methyl ester, (Z)-, 2-hexadecenoic acid,2,3-dimethyl-, methyl ester, (E)-, 9-eicosene, (E)-, methyl eicosa- 5,8,11,14,17-pentaenoate, 1-tricosene, and 3-eicosene, (E)- were reported prime components in the aqueous extract. On the other hand, E-2-octenyl tiglate, methyl tetradecanoate, flavone, 9-hexadecenoic acid, hexadecenoic acid, methyl ester, and 9-octadecenoic acid (Z)-, methyl ester were predominantly present in the ethanolic extract (Table 4 and Fig. 8).
S. No
Compound name
Compound structure
RT
Molecular weight (g/mol)
1
Methyl tetradecanoate
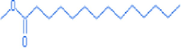
15.08
242.51
2
1-octadecene
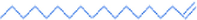
15.77
252.57
3
9-methyl-10,12-hexadecadien-1-ol acetate
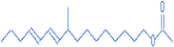
16.6
294
4
9-hexadecenoic acid, methyl ester, (Z)-
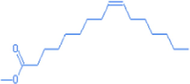
17.05
268.55
5
2-hexadecenoic acid,2,3-dimethyl-, methyl ester, (E)-
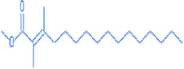
19
296.63
6
9-eicosene, (E)-

19.82
280.63
7
Methyl eicosa- 5,8,11,14,17-pentaenoate

20.57
316.60
8
1-tricosene

21.62
322
9
3-eicosene, (E)-

17.87
280.63
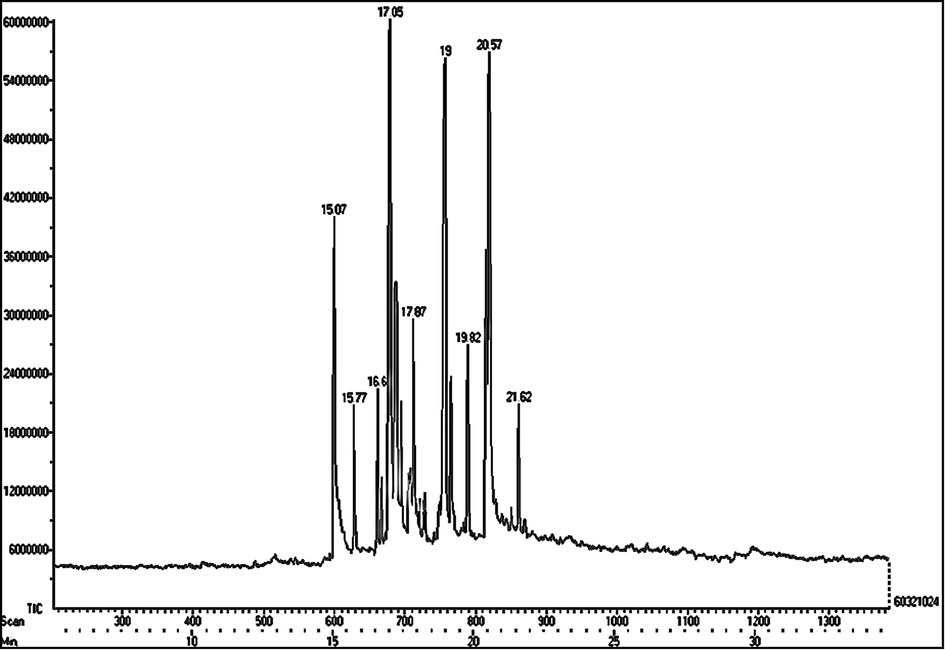
GC–MS chromatogram of aqueous extract of S. virginianum fruits.
S. No
Compound names
Compound structure
RT
Molecular weight (g/mol)
1
E-2-octenyl tiglate
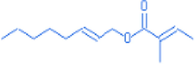
14.02
209.89
2
Methyl tetradecanoate
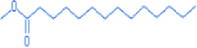
15.2
242.41
3
Flavone
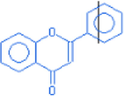
16.1
222
4
9-hexadecenoic acid
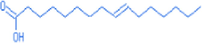
17.12
254
5
Hexadecenoic acid, methyl ester
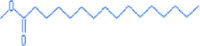
17.32
270.48
6
9-octadecenoic acid (Z)-, methyl ester
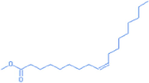
19.07
296.58
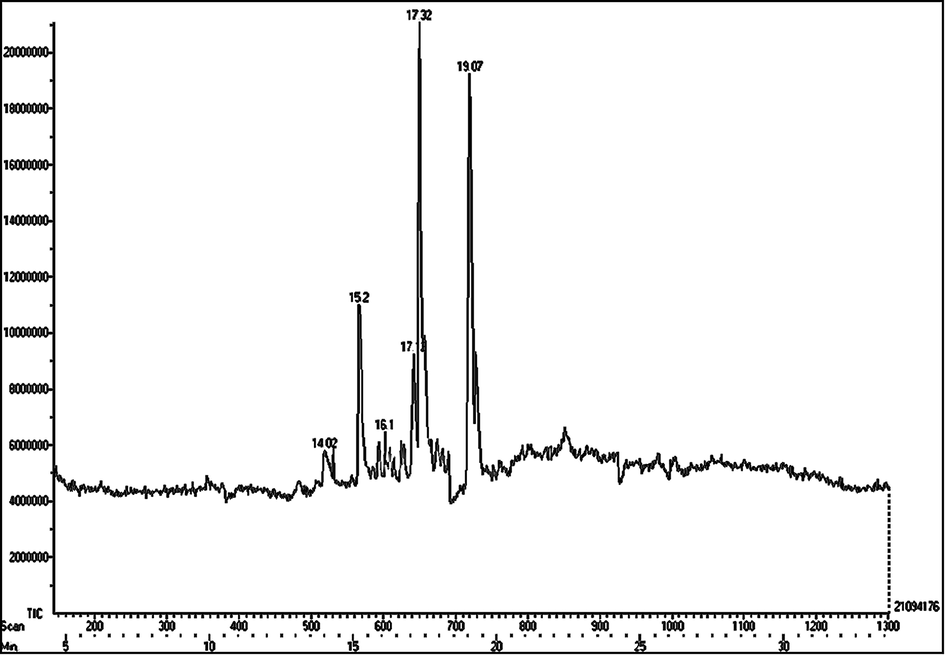
GC–MS chromatogram of ethanolic extract of S. virginianum fruits.
4 Discussion
Over the past few decades, the utilization of plants as medicinal practices has escalated throughout the world (Barbabosa-Pliego et al., 2020). India is known to practice herbs-derived traditional medicines since ancient periods. The therapeutic characteristics of medicinal plants are mainly because of the presence of diversified secondary metabolites such as alkaloids, polyphenols, steroids, flavanoids, terpenoids, and coumarins (Eftekhari et al., 2021). In this context, both extracts of S. virginianum fruits depicted the availability of prime phytoconstituents such as alkaloids, flavonoids, terpenoids, steroids, phenolics, glycosides, saponins, and carbohydrates. In addition, the aqueous and ethanolic extracts showed total phenolic and total flavonoid contents in extensive amount. Phenolics, alkaloids, steroids, flavanoids, sapogenins, triterpenoids, coumarins, and fatty acids (palmitic acid, oleic acid, and linoleic acid) were reported as important phytochemicals in Solanum spp. (Tekuri et al., 2019).
The over-exploitation of antibiotics to treat several diseases is a huge concern for the humankind, particularly in view of the development of drug-resistance microbes (Ameer et al., 2020; Khusro et al., 2021). Plant extracts are considered potential alternatives to the existing antibiotics which are known to inhibit the growth of several pathogens, thereby treating different microbial infections (Pedraza-Hernández et al., 2021). In this context, the aqueous and ethanolic extracts of S. virginianum fruits revealed promising antibacterial characteristics against certain pathogens. Similar observations were reported by Sheeba (2010), Abbas et al. (2014), and Nithya et al. (2018) too who demonstrated significant antibacterial activities of different extracts of Solanum spp. against distinct gram positive and gram negative bacterial pathogens.
Free radicals are one of the prime causative agents to convert non-cancerous cells into cancerous cells by reacting with biological macromolecules (Liu et al., 2007; Aarti and Khusro, 2019). Medicinal plants are known to mitigate oxidative damages in DNA caused due to free radicals. In fact, anti-oxidative agents of potential herbs cause scavenging of free radicals and control metabolic activities in human body (Preetam Raj et al., 2016). In this investigation, the aqueous and ethanolic extract of S. virginianum fruits exhibited their potentialities to scavenge different reactive oxygen species in a dose dependent manner. Our reports favoured the findings of Muruhan et al. (2013), Kumar and Pandey (2014), and Nithya et al. (2018) who assessed remarkable antioxidative traits of Solanum spp. extracts. Antioxidant activities of Solanum spp. are suggested due to the presence of phenolic and flavonoid components in high amount (Poongothai et al., 2014).
The discovery of new anti-diabetic agents from non-toxic sources is of immense demand at present due to the high prevalence of diabetes globally. Plethora of plants has been investigated in the past to identify potential anti-diabetic agents (Salehi et al., 2019). However, identifying ideal anti-diabetic agents from un/less natural resources is an ongoing process. Considering this, an attempt was undertaken in this context to determine in vitro anti-diabetic characteristics of S. virginianum fruits which revealed significant abilities of its aqueous and ethanolic extract in inhibiting α-amylase. Sridevi et al. (2007) and Poongothai et al. (2014) depicted anti-diabetic properties of Solanum surattense leaves extract. In a different study, Gupta et al. (2011) confirmed the protective role of S. surattense extracts in preventing diabetes induced oxidative damage.
Inflammation is a result of infection, injury, or trauma. In view of the adverse effects of exiting steroidal and non-steroidal drugs, there is an urgency to search new anti-inflammatory agent from natural resources. S. surattense fruits are already being utilized as traditional medicine for reducing inflammation (Tekuri et al., 2019). In this investigation, the aqueous extract of S. virginianum fruits at all concentrations protected the haemolysis effectively as compared to the ethanolic extract. Ramanarayana Reddy et al. (2014) determined in vivo anti-inflammatory characteristic of S. surattense ethanolic extract. In contrary, Anwikar and Bhitre (2010) demonstrated synergistic anti-inflammatory role of S. xanthocarpum and Cassia fistula with higher rate of inhibition as compared to the positive control (diclofenac sodium).
The discovery of potential anticancer agents from plethora of plants has emphasized the researchers to continue searching new anticancer agents from natural resources. Previous studies demonstrated anticancer efficacy of different solvent extracts of S. surattense against various cell lines (Kumar and Pandey, 2014; Burger et al., 2018). Furthermore, findings suggested that the anticancer efficiency of extracts might be due to the presence of different groups of flavonoids which inhibited cancer cells proliferation. In the line of previous reports, in this investigation, the aqueous and ethanolic extracts of S. virginianum fruits revealed remarkable cytotoxicity against HepG2 cell line which widened its pharmacological applications as ideal anticancer agent. Cham (2017) and Sethi et al. (2018) demonstrated anticancer activities of solasodine and diosgenin of Solanum spp. against Hep2B and HCT 116 cell line, respectively.
The analytical assays of S. virginianum fruits extracts (aqueous and ethanolic) showed the presence of major bioactive components viz. methyl tetradecanoate, 1-octadecene, 9-methyl-10,12-hexadecadien-1-ol acetate, 9-hexadecenoic acid, methyl ester, (Z)-, 2-hexadecenoic acid,2,3-dimethyl-, methyl ester, (E)-, 9-eicosene, (E)-, methyl eicosa- 5,8,11,14,17-pentaenoate, 1-tricosene, and 3-eicosene, (E)-, E-2-octenyl tiglate, methyl tetradecanoate, flavone, 9-hexadecenoic acid, hexadecenoic acid, methyl ester, and 9-octadecenoic acid (Z)-, methyl ester. The promising antibacterial, antioxidant, anti-diabetic, anticancer, and anti-inflammatory attributes of S. virginianum fruits’ extracts might be due to these bioactive compounds. Previous studies also reported antimicrobial, antioxidant, anti-inflammatory, and cancer preventive roles of different phytocomponents, particularly 9-octadecenoic acid (Z)-, methyl ester, 9-hexadecenoic acid, methyl tetradecanoate, 1-tricosene, and 3-eicosene present in disparate plant species (Singh et al., 2008; Krishnamoorthy and Subramaniam, 2014; Abubakar and Majinda, 2016; Choudhary et al., 2019; Kim et al., 2020).
5 Conclusions
In conclusion, S. virginianum fruits extracts revealed the presence of major phytochemicals. The ethanolic extract of fruits depicted higher antibacterial activities than the aqueous extract against certain bacterial pathogens. The promising rate of in vitro antioxidant and α-amylase inhibition properties were estimated for both the extracts. However, the aqueous extract at all concentrations protected the haemolysis effectively as compared to the ethanolic extract. The aqueous and ethanolic extract revealed remarkable cytotoxicity against HepG2 cell line with significant morphological variations. The GC–MS analysis confirmed a total of 15 predominant components in fruit extracts. Further in vivo studies are required to confirm its future therapeutic applications in humans.
Acknowledgement
Authors are grateful to IIT (SAIF, Madras) and ARMATS Biotek Training and Research Institute for providing essentials related to this research. Authors like to thank Taif University, Taif, Saudi Arabia for their support (Taif University Researchers Supporting Project number: TURSP-2020/80, Taif University, Taif, Saudi Arabia).







