Translate this page into:
Benzohydrazide as a good precursor for the synthesis of novel bioactive and anti-oxidant 2-phenyl-1,3,4-oxadiazol-aminoacid derivatives: Structural determination, biological and anti-oxidant activity
⁎Corresponding author. brkhch28@live.fr (Khaled Briki),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The synthesis and biological assessment of 2,5-disubstituted-1,3,4-oxadiazole derivatives from benzo hydrazide and amino acids as novel potential antioxidant and antibacterial agents have been reported. The structures of the new compounds were characterized by physicochemical properties and spectral methods. The synthesized compounds were screened for their in vitro antibacterial activity against three Gram-positive bacterial strains, namely Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 14579, Listeria innocua ATCC 33090, and two Gram-negative bacterial strains, namely Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, and antifungal activity against Candida albicans ATCC 10231 in comparison with Amoxicillin, Tetracycline, Gentamicin and Oxacillin as standards. Most of the compounds have excellent efficacy, and some of them, such as 5i, 5g, 5d, 5c, and 5j can inhibit their activity better or very close to that of Amoxicillin, Tetracycline, Gentamicin, Oxacillin used as standards. Compounds 5b and 5i provided good results against L. innocua with inhibition values of IZ = 14 mm and IZ = 22 mm, respectively, while the rest of the compounds and antibiotics were unable to inhibit it. Compounds 5c, 5d, 5g and 5j showed excellent activity against C. albicans with values between 31 mm and 34 mm. These results were better than all the standards used. The MIC value (25 µg/ml) for derivatives 5(c-e), 5g and 5(i-j) against B. cereus represent the best activity of the tested compounds. All the target compounds were also screened for radical scavenging antioxidant activities by DPPH, FRAP, and TAC assays and found to be excellent antioxidant agents. According to the results, it was observed that most derivatives synthesized showed excellent activity with a concentration of 250 µg/ml as an antioxidant agent (76 % < RSA < 95.5 %) which gave an inhibition percentage that was better or very close to that of ascorbic acid and better than BHT.
Keywords
Synthesis
Benzohydrazide
1,3,4-oxadiazole
Amino acids
Antibacterial and antifungal activity
Antioxidant
1 Introduction
One of the groups of heterocyclic compounds with a wide spectrum of biological activity is oxadiazoles (Kawale et al., 2023; Berillo and Dyusebaeva, 2022; Di Franco et al., 2021; Parrino et al., 2021; Queiroz et al., 2020; Mustafa, 2018). 1,3,4-oxadiazole has applications in diverse areas of medicine, including antibacterial antibacterial (Wang et al., 2023; AL-Sharabi et al., 2023; Abbassi et al., 2021; Briki et al., 2020; Yang et al., 2021; Othman et al., 2019; Desai and Dodiya, 2014), antifungal (Glomb and Świątek, 2021; Wen et al., 2023; Patil et al., 2021; Capoci et al., 2019), antioxidant (Mahi et al., 2023; Bhandari et al., 2023; Shridhar et al., 2016), anticonvulsant (Fakhrioliaei et al., 2023; Nazar et al., 2020), antidepressant (Singh et al., 2020), antiviral (Chaudhary et al., 2023; Hassan et al., 2010; Li et al., 2011), antidiabetic (Qazi et al., 2023; Radia et al., 2021; Wang et al., 2022; Kavitha et al., 2017), antiinflammatory (Dewangan et al., 2018; Chawla et al., 2018; Rahul et al., 2023), anticancer (Chen et al., 2024; Carbone et al., 2023; Afzal et al., 2023; Nayak et al., 2021; Vaidya et al., 2021; Morsy et al., 2017; Gudipati et al., 2011), Antiseizure (Rasool et al., 2023) and anti-Alzheimer's (Naseem et al., 2023).
Several examples containing the 1,3,4-oxadiazole group used in clinical medicine (Fig. 1) such as raltegravir 1 for the treatment of HIV infection (Chaudhary and Upadhyay, 2023), tiodazosin 2 for antihypertensive agents (Desai et al., 2022), zibotentan 3 as an investigational anticancer drug candidate (Bajaj et al., 2021), nesapidil is a vasodilator, that is used as an antiarrythmic and antihypertensive therapy (Paruch et al., 2020) and furamizole 4 for antimicrobial (Siwach and Verma, 2020).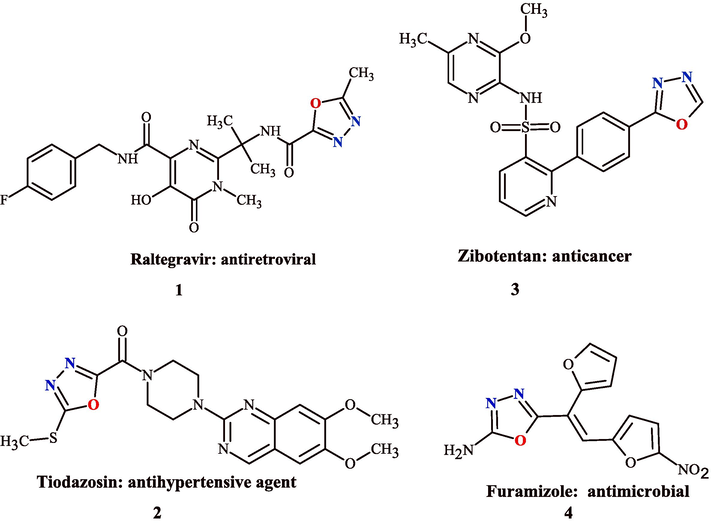
The chemical structures of drugs used in medical practice containing 1,3,4-oxadiazole.
Another sub-class of 2,5-disubstituted-1,3,4-oxadiazole derivatives represented by the general formula (Fig. 2):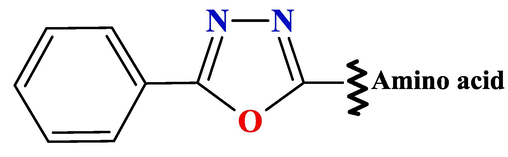
The general chemical structure of the sub-class of 2,5-disubstituted-1,3,4-oxadiazole derivatives.
Some of these compounds were capable of suppressing and/or inhibiting the programmed cell death 1 (PD-1) signally pathway (Aksenov et al., 2022; Brotschi,et al., 2020; Sasikumar et al., 2018). Our interest is to synthesize some derivatives of the 2,5(R)-disubstituted-1,3,4-oxadiazole in different way other than what was reported.
There are several strategies for the synthesis of 2,5- disubstituted -1,3,4-oxadiazole derivatives by treating hydrazide derivatives with carboxylic acid in one or two steps. For one step synthesis, 1,3,4-oxadiazole can be prepared by reacting hydrazide derivatives with: ketones in the presence of I2 and K2CO3 (Chen et al., 2024; Gao et al., 2015), ClF2COONa with K3PO4 (Yan et al., 2012), nucleophilic addition of carbon disulfide (CS2) in the presence of a basic KOH (Wang et al., 2024; Rana et al., 2023), 1,10-carbonyldiimidazole (CDI) (Savariz et al., 2014), CO2 with paraformaldehyde in the presence NaI and t-BuOK (Yang et al., 2016), derivatives of carboxylic acid with polyphosphoric acid (PPA) (Luczynski and Kudelko, 2022), 1,1′-carbonyldiimidazole (CDI) with triphenylphosphine (Ph3P) and tetrabroomethane (CBr4) (Rajapakse et al., 2006), hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) with N,N-diisopropylethylamine (DIPEA) (Li and Dickson, 2009), sulfuryl chloride (SO2Cl2) (De Oliveira et al., 2012; Mihailović et al., 2017) or phosphorus oxychloride (POCl3) as the cyclization agent (Dong et al., 2022; Salama, 2020; Faraz et al., 2017; Amarouche et al., 2022). In this study, we used the POCl3 method by adding a hydrazide derived from benzoic acid with a series of aminoacids.
As for the preparation of 1,3,4-oxadiazole in two steps, the reactant isothiocyanate is used to obtain thiosomicarbazide and then one of the following catalysts is added: mercuric acetate (Hg(AcO)2) (Liu et al., 2014), iodobenzene diacetate (PhI(AcO)2) (Hassan and Rauf, 2016), N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC. HCl) (Naaz et al., 2020), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) (Hamdy et al., 2017; He et al., 2009), N,N-diisopropylethylamine (DIEA) (Maghari et al., 2013), potassium bisulfate (KHSO4) (Long et al., 2021).
In the present paper, we have explored the synthesis of some 2-phenyl- 1,3,4-oxadiazole-5(R)-amino acid derivatives and testing their antioxidant and antimicrobial activity. All the synthesized compounds were primarily detected by careful TLC, melting point, and characterized by spectroscopic methods (UV–Visible, IR, 1HNMR, 13CNMR).
2 Experimental
2.1 Material and methods
2.1.1 Chemistry
The reagent and solvents used were obtained from commercial sources. Analytical thin layer chromatography was carried out on TLC plates of 3 × 15 cm coated with silica gel G for reaction monitoring and for the determination of retardation factor. Spots of TLC were located by the iodine chamber. The final products were purified by recrystallization. Melting points of newly synthesized derivatives were determined on a digital melting point apparatus (BÜCHI 540). The λmax was calculated by using UV–Visible 1800 Shimadzu spectrophotometer. The IR spectra were recorded on a FTIR-Shimadzu spectrometer (University of Laghouat, Amar Telidji, Algeria), ν units of cm−1. The 1H and 13C NMR spectra recorded on a Bruker AC 400 MHz spectrometer in DMSO‑d6 and referenced to TMS (University of AinShamss, Department of Pharmacy, Egypt). Chemical shift values are expressed in ppm and are abbreviated as follows: s (singlet), d (doublet), t (triplet), q (quadruplet), m (multiplet), o (ortho), m (meta), p (para) and ph ((linking phenyl group). Numbering 1,2,..etc for oxadiazole ring, while 1′,2′, …etc for amino acid moiety (see scheme 3).
2.1.1.1 Synthesis of compounds 2–3 and 5(a-j)
Methyl benzoate (2):
To a mixture of benzoic acid 1 (10 g, 0,081 mol) in methanol (80 ml), H2SO4 (2,5 ml) was added dropwise with stirring. The mixture refluxed on a water bath at 80 °C for 3 h. TLC eluted with ethanol/Chloroform (1/1) showed Rf = 0,62 for trace of starting acid 1 and Rf = 0,77 for methyl benzoate 2. After cooling the solution to room temperature, the solution was neutralized with 5 % aqueous NaHCO3 (110 ml) until pH 7. The solution was washed and extracted again with chloroform several times. The organic layers were dried over anhydrous MgSO4 and filtered. The filtrate evaporated to dryness to give product 2 with a yield of 81 %.
Benzohydrazide (3):
Methyl benzoate 2 (11 g, 0.080 mol), ethanol (83 ml) and hydrazine hydrate (64 %, 40 ml) were mixed and heated under reflux at 80 °C for 2 h. TLC eluted with ethyl acetate/hexane 1:1 showed Rf = 0.15 for hydrazide 3 and Rf = 0.73 for trace of starting ester 2. Aqueous ethanol evaporated under reduced pressure and a product was recrystallized from ethanol to give benzohydrazide 3 with a yield of 84 %.
2.1.1.2 General procedures for the synthesis of 2,5-disubstitued-1,3,4-oxadiazole 5(a-j)
The mixture of benzohydrazide (3, 0.01 mol) and the amino acid [4(a-j), 0.01 mol] were dissolved in 7 ml of phosphorus oxychloride POCl3 and allowed to stand at room temperature for 30 min. The mixture was refluxed for 4–7 h at 80 °C, and then concentrated by evaporation in a rotary evaporator under reduced pressure. The rest of the mixture was slowly poured onto crushed ice and kept overnight. The solid was separated and washed by warm water and the filtrate was basified with saturated sodium hydroxide. The precipitate was filtered and recrystallized from ethanol several times to give the white solid compounds 5(a-j).
1-(5-Phenyl-1,3,4-oxadiazol-2-yl)methanamine (46182–58-5) 5a
TLC (ethanol/chloroform 1:4): Rf (5a) = 0.43; Yield 58 % (0.99 g); m.p: 200 °C; UV (H2O), λ (nm): 3.108 (226, π - π*), 0.435 (269, n - π*); IR (νmax, cm−1): 3248.1, 3001.2 (NH2, NH), 2900.9 (CHarom), 1604.7 (C = N), 1489.0 (C = C), 1165 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.14 (s, 3H, +NH3-1′), 7.57–7.31 (m, 5H, -ph), 3.88–3.81 (m, 2H, H1′). 13CNMR (400 MHz, DMSO‑d6) δ: 169.2 (C5), 166.0 (C2), 132.5 (p-ph), 127.5 (m-ph), 122.0 (o-ph), 117.0 (C1-ph), 16.4 (C1′).
(1R)-3-Methyl-1-(5-phenyl-1,3,4-oxadiazol-2-yl)butan-1-amine 5b
TLC (ethanol/chloroform 1:4): Rf (5b) = 0.41; Yield 68 % (1,57 g); m.p: 206 °C; UV (H2O), λ (nm): 1.00 (229, π - π*), 0.34 (257, n - π*); IR (νmax, cm−1): 3248.1, 3001.2 (NH2, NH), 2900.9 (CHarom), 1604 (C⚌N), 1489 (C⚌C), 1165 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.48–8.45 (s, 3H, +NH3-1′), 7.90–7.30 (m, 5H, -ph), 3.86 (m, 1H, H1′), 1.95 (m, 2H, H2′), 1.6 (m, 1H, H3′), 1.15 (m, 6H, H4′, H5′); 13CNMR (400 MHz, DMSO‑d6) δ: 165.9 (C5), 157.6 (C2), 134.9 (p-ph), 130.2 (m-ph), 120.10 (o-ph), 117.4 (C1-ph), 62.6 (C1′), 16. 5 (C2′-C5′).
(2R)-2-Amino-2-(5-phenyl-1,3,4-oxadiazol-2-yl)ethanethiol 5c
TLC (ethanol/chloroform 1:1): Rf (5c) = 0.51; Yield 72 % (1.59 g); m.p: 202 °C; UV (H2O), λ (nm): 3.035 (229, π - π*), 0.412 (262, n - π*); IR (νmax, cm−1): 3147.8 (NH), 2985.8 (CHarom), 2893.2 (CH2) 2630.9 (SH), 1635.6 (C⚌N), 1489 (C⚌C), 1203.5 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.74 (m, 3H, +NH3-1′), 7.82–7.31 (m, 5H, -ph), 4.15 (m, 1H, H1′), 3.31 (m, 2H, H2′), 2.51 (s, 1H, SH); 13CNMR (400 MHz, DMSO‑d6) δ: 169.4 (C5), 133.31 (C2), 131.0 (p-ph), 130.0 (m-ph), 129.5 (o-ph), 62.3 (C1′), 16.5 (C2′).
(1R)-3-(Methylsulfanyl)-1-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-amine 5d
TLC (ethanol/chloroform 1:1): Rf (5d) = 0.48; Yield 55 % (1,36 g); m.p: 135 °C; UV (H2O), λ (nm): 1.031 (231, π - π*), 0.185 (262, n - π*); IR (νmax, cm−1): 3248.13, 3140.1 (NH), 2893.22 (CHarom), 2831.5, 2708.0 (CH2, CH3); 1481.3 (C⚌N), 149.61 (C⚌C), 1249.8 (S-CH3); 1087.6 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.69 (m, 3H, +NH3-1′), 7.93 (t, 1H, J = 0.9 Hz, p-ph), 7.59, (t, 2H, J = 2.5 Hz, m-ph), 7.49 (d, 2H, J = 7.5 Hz, o-ph), 3.87 (m, 2H, H1′), 3.41 (m, 2H, H2′), 2.56 (m, 2H, H3′), 2.05 (s, 3H, H4′); 13CNMR (400 MHz, DMSO‑d6) δ: 166.6 (C5), 159.2 (C2), 135.0 (p-ph), 129.5 (m-ph), 119.8 (o-ph), 117.6 (C1-ph), 62.6 (C1′), 62.5 (C2′), 61.8 (C3′), 16.5 (C4′).
1-[(4R)-4-Amino-4-(5-phenyl-1,3,4-oxadiazol-2-yl)butyl]guanidine 5e
TLC (ethanol/chloroform 1:1): Rf (5e) = 0.42; Yield 63 % (1,72 g); m.p: 187 °C; UV (H2O), λ (nm): 3.435 (228, π - π*), 0.441 (262, n - π*); IR (νmax, cm−1): 3332.9 (NH), 3171 (CHarom), 2893 (CH2); 1651 (C⚌N), 1481 (C⚌C), 1211 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.72 (s, 2H, C5′=+NH2), 8.55 (s, 2H, H2N+-C5′), 8.54 (s, 3H, C5-+NH3), 7.96 (t, 1H, J = 1.5 Hz, p-ph), 7.60 (t, 2H, J = 2 Hz, m-ph), 7.50 (d, 2H, J = 7.5 Hz, o-ph), 4.18 (m, H1′), 3.86 (m, H2′), 3.13 (m, H3′), 1.83 (m, 1H, C5′=NH), 1.63 (m, 2H, C5′–NH2), 1.54 (m, 2H, C1′–NH2); 13CNMR (400 MHz, DMSO‑d6) δ: 165.3 (C5), 158.6 (C2), 134.5 (p-ph), 129.6 (C5′), 129.5 (m-ph), 119.5 (o-ph), 117.5 (C1-ph), 61.6 (C1′), 61.5 (C4′), 16.5 (C2′, C3′).
(1R)-1-(5-Phenyl-1,3,4-oxadiazol-2-yl)pentane-1,5-diamine 5f
TLC (ethanol/chloroform 1:4): Rf (5f) = 0.37; Yield 73 % (1,79 g); m.p: 204 °C; UV (H2O), λ (nm): 2.67 (224, π - π*), 0.25 (273, n - π*); IR (νmax, cm−1): 3132.4 (NH), 3093.8 (CHarom), 2978.0 (CH2); 1620.2 (C⚌N), 1481.3 (C⚌C), 1219.0 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 14.93 (m, 3H, C1′-+NH3), 14.65 (m, 3H, C5′-+NH3), 7.51 (m, 5H, -ph), 4.36 (m, 1H, H1′), 3.94–3.84 (m, 2H, H5′), 3.35–3.25 (m, 6H, H2′-H4′), 1,20 (m, 2H, C5′–NH2); 13CNMR (400 MHz, DMSO‑d6) δ: 170.0 (C5),169.7 (C2), 154.4 (p-ph), 134.0 (m-ph), 128(o-ph), 127.4 (C1-ph), 51.6 (C1′, C4′), 25.6 (C2′), 25.0 (C3′).
(3R)-3-Amino-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propanamide (1518828-37-9) 5g
TLC (ethanol/chloroform 1:4): Rf (5g) = 0.46; Yield 55 % (1.27 g); m.p: 184 °C; UV (H2O), λ (nm): 3.556 (227, π - π*), 0.608 (273, n - π*); IR (νmax, cm−1): 3439.4 (NH2), 3038.3 (CHarom), 1618.9 (C⚌O), 1482.0 (C⚌N), 1402 (C⚌C), 1092.4 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 10.94 (s, 3H, C1′-+NH3), 7.70–7.20 (m, 5H, -ph), 4.1 (m, H1′), 2.90 (d, H2′); 13CNMR (400 MHz, DMSO‑d6) δ: 171.5 (C3′), 170.1 (C5), 165 (C2), 151.0 (p-ph), 129.5 (m-ph), 129.0 (o-ph), 119.0 (C1-ph), 116.0 (C2′), 48.3 (C1′).
(1R)-2-(1H-indol-3-yl)-1-(5-phenyl-1,3,4-oxadiazol-2-yl)ethanamine 5h
TLC (ethanol/chloroform 1:4): Rf (5h) = 0.43; Yield 53 % (1,61 g); m.p: 198 °C; UV (H2O), λ (nm): 3.624 (226, π - π*), 2.446 (276, n - π*); IR (νmax, cm−1): 3425.9 (NH2), 3288.0 (NH), 2982.3 (CHarom), 1578.5 (C = N), 1436.7 (C⚌C), 1206.2 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 11.90 (s, 2H, +NH2), 11.17 (s, 3H, C1′-+NH3), 8.76 (s, =N+H), 7.97 (m, 1H, C4′), 7.60 (t, 1H, J = 1.5 Hz, p-ph), 7.53 (t, 2H, J = 1.5 Hz, m-ph), 7.36 (d, 2H, J = 4 Hz, o-ph), 7.25 (d, 2H, J = 1.5 Hz, H8′), 7.07 (t, 1H, J = 3.5 Hz, H10′), 7.05 (t, 1H, J = 4 Hz, H9′), 7.00 (t, 1H, J = 2.5 Hz, H11′), 6.81 (m, 1H, H7′), 4.13–3.89 (m, 3H, H1′, H2′); 13CNMR (400 MHz, DMSO‑d6) δ: 171.11 (C5), 169.69 (C2), 138.0 (C4′), 136.6 (C1-ph), 136.0 (C8′), 130.0 (C10′), 129.7 (C7′), 129.2 (C11′), 127.5 (C6′), 127.4 (C9′), 127.4 (p-ph), 125.4 (m-ph), 119.1 (o-ph), 62.5 (C1′), 118.7 (C11′), 111.7 (C3′), 33.1 (C2′).
(1R)-2-(1H-imidazol-4-yl)-1-(5-phenyl-1,3,4-oxadiazol-2-yl)ethanamine 5i
TLC (ethanol/chloroform 1:4): Rf (5i) = 0.46; Yield 78 % (1.98 g); m.p: 180 °C; UV (H2O), λ (nm): 3.613 (227, π - π*), 0.425 (263, n - π*); IR (νmax, cm−1): 3424.9 (NH2), 2930.3 (CHarom), 1620.8 (C⚌N), 1422.2 (C⚌C), 1143.3 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 14.67 (s, 3H, -+NH3), 9.05 (s, 2H, -+NH2), 8.73 (s, 1H, -+NH), 8.20–7.32 (m, 5H + 2H, ph & diazole), 4.30 (m, H1′), 3.30 (m, H2′); 13CNMR (400 MHz, DMSO‑d6) δ: 169.6 (C5), 158.0 (C2), 148.0 (C6′), 134.0 (C4′), 133.0 (p-ph), 129.7 (m-ph), 129.1 (o-ph), 116.4 (C1-ph), 62.0 (C1′), 51.6 (C2′).
2-phenyl-5-[(2R)-(pyrrolidin-2-yl)]-1,3,4-oxadiazole (1201915–88-9) 5j
TLC (ethanol/chloroform 1:4): Rf (5j) = 0.33; Yield 63 % (1,35 g); m.p: 188 °C; UV (H2O), λ (nm): 3.393 (229, π - π*), 0.618 (264, n - π*); IR (νmax, cm−1): 3248.1 (NH), 2893.2 (CHarom), 1573.91 (C⚌N), 1481.3 (C⚌C), 1087.8 (C-O-C); 1HNMR (400 MHz, DMSO‑d6) δ: 8.61 (s, 2H, -+NH2), 7.57–7.32 (m, 5H, -ph), 3.85 (m, H1′), 3.81 (m, 2H, H3′), 1.92 (m, 4H, H4′, H5′); 13CNMR (400 MHz, DMSO‑d6) δ: 172.3 (C5), 165.7 (C2), 157.5 (o-ph), 135.0 (m-ph), 157.5 (o-ph), 119.8 (C1-ph), 61.9 (C1′), 59.7 (C3′), 45.8 (C5′), 28.3 (C4′).
2.1.2 Biological activity
2.1.2.1 Antibacterial activity
Microbial strains
The antibacterial activity of the different compounds 2, 3 and 5(a-j) was assessed using three Gram-positive bacteria, viz. Bacillus cereus ATCC 14579, Staphylococcus aureus ATCC 25923, Listeria innocua ATCC 33090, and two Gram-negative bacteria, viz. Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853. The antifungal activity of the different compounds was also assessed using Candida albicans ATCC 10231. The inhibition zones were compared with the controls AX 25: Amoxicillin, TE 30: Tetracycline, CN 120: Gentamicin OX 1: Oxacillin. All bacterial strains (KWIK-STIK format) were purchased from Micro Biologics, MN, USA.
Disc diffusion method
The antibacterial and antifungal activities of different compounds were evaluated by determining the zone of inhibition using the disc diffusion method, as reported by the Clinical and Laboratory Standards Institute CLSI (CLSI, 2012a; CLSI, 2004). Microbial suspensions, prepared from young cultures (18–24 h), were standardized at 0.5 Mc Farland using a densitometer and spread over Mueller Hinton (MH) agar medium. Sterile paper discs (6 mm in diameter, Whatman no.1), impregnated with 10 µL of compound diluted in water to a concentration of 10 mg/ml, and were placed on the surface of the inoculated agars. After incubation at 37 °C for 24 h, the antimicrobial activities were assessed by measuring the diameter of the inhibition zones against the tested microorganism.
Minimal inhibitory concentration
The minimal inhibitory concentration (MIC) of compounds that showed good antibacterial activity was determined using the method described by CLSI (CLSI, 2012b). MH plates containing different concentrations (100, 50, 25, 12.5, 6.25 and 3.12 µg/ml) of compounds were prepared and spread on the surface with standardized bacterial suspension (0.5 McFarland) in order to visualize no bacterial growth after incubation at 37 °C for 24 h in the case of bacteria and 48 h in the case of fungi. Control plates prepared under the same procedure without the bacterial inoculums were assessed simultaneously. The MIC corresponded to the lowest concentration preventing visible growth.
2.1.2.2 Antioxidant activity
All used chemicals, reagents, solvents, and reference standards are of the purest analytical quality available on the market. 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), butylated hydroxytoluene (BHT), ascorbic acid (AA), dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4), potassium ferricyanide (K3Fe(CN)6), trichloroacetic acid (TCA), iron (III) chloride (FeCl3), sodium phosphate (Na3PO4), ammonium molybdate ((NH4)6Mo7O24), and sulfuric acid were the materials obtained from Sigma-Aldrich. Mueller Hinton (MH) agar medium was obtained from Merck.
Free radical scavenging activity
Blois was used to evaluate the DPPH radical scavenging activity (Blois, 1958). Solutions of compounds 2, 3 and 5(a–j) in different concentrations (8, 16, 32, 62.5, 125, and 250 μg/ml) were prepared in water. 1000 µL of DPPH methanolic solution (0.2 mM) was mixed with 500 µL of samples at different concentrations in water. The mixture was well shaken and then allowed to settle at room temperature for 30 min. The absorbance was then measured in a UV–Vis spectrophotometer at 517 nm in comparison to the control. Using the following formula, the proportion of radical-scavenging capacity was determined: Radical-scavenging activity (%) = [(1 – A sample) / A control] x 100, where A control is the absorbance of the control reaction (containing all reagents except the test sample), and A sample is the absorbance of the samples/references. The concentration providing 50 % inhibition (IC 50) was calculated from the graph of RSA percentage against compound concentration. RSA of AA and BHT were also estimated for reference.
Ferric-reducing antioxidant power (FRAP)
The ferric-reducing antioxidant power (FRAP) was determined using the Oyaizu method (Oyaizu, 1986). Solutions of compounds 2, 3 and 5(a–j) in different concentrations (8, 16, 32, 62.5, 125, and 250 μg/ml) were prepared in water. Samples at varying concentrations were combined with 500 μL of 1 % potassium ferricyanide [K3Fe(CN)6] and 1000 μL phosphate buffer (2 mM, pH 6.6). For 20 min, the mixture was incubated at 50 °C. After adding 1000 μL of 10 % trichloroacetic acid (TCA), the mixture was centrifuged for 10 min at 3000 rpm. After filtering the supernatant, 500 μL of it was combined with 1.5 ml of distilled water and 500 μL of 0.1 % FeCl3. After giving the mixture a good shake, it was left to sit at room temperature for 30 min. In a UV–Vis spectrophotometer, the absorbance was measured at 700 nm against the control and compared to the references (AA and BHT).
Total antioxidant capacity (TAC) by phosphomolybdenum method
Total antioxidant activity (TAC) was measured as described by Prieto (Prieto et al., 1999). Solutions of compounds 2, 3 and 5(a–j) in different concentrations (8, 16, 32, 62.5, 125, and 250 μg/ml) were prepared in water. 1000 µL of molybdate reagent containing 28 mM sodium phosphate, 4 mM ammonium molybdate and 6 mM sulphuric acid was added to100 μL of different concentrations of the samples. The tubes were incubated at 95 °C for 90 min and then the mixture was cooled at room temperature. The absorbance was recorded at 695 nm against the control using a UV–Vis spectrophotometer.
Statistical analyses
The averages and standard deviations were obtained in triplicate. One-way analysis of variance (ANOVA) followed by Tukey’s multiple range tests was performed to establish the significant differences at p-value < 0.05 using the Statistical Package for Social Science (SPSS 20.0 for windows, SPSS Inc., Chicago, IL, USA).
3 Results and discussion
3.1 Chemistry
The final heterocyclic products 2,5-disubstituted-1,3,4-oxadiazole 5(a-j) have been synthesized from benzohydrazide 3 and amino acids 4(a-j) as summarized in Scheme 1.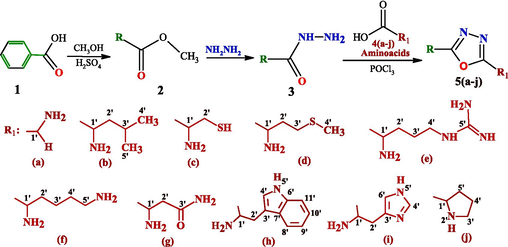
Synthesis of different 2,5-disubstituted-1,3,4-oxadiazole derivatives.
Methyl benzoate 2 was prepared by esterification of benzoic acid 1 in the presence of H2SO4 in methanol. Hydrazide 3 was obtained by the reaction of esters 2 with hydrazine hydrate in ethanol. The hydrazide 3 was utilized as starting material with ten amino acids for the synthesis of 2,5-disubstituted-1,3,4-oxadiazole 5(a-j) by using POCl3 as the cyclization agent. The amino acids were selected: glycine 4a, l-leucine 4b, l-cysteine 4c, l-methionine 4d, l-arginine 4e, l-lysine 4f, l-asparagine 4g, l-tryptophan 4h, l-histidine 4i and l-proline 4j.
The proposed reaction mechanism is shown in Scheme 2.
Proposed mechanism for synthesis of 2,5-disubstituted-1,3,4-oxadiazole derivatives.
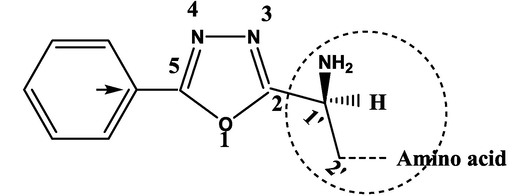
Numbering of 5-Phenyl-1,3,4-oxadiazol-2-yl moiety-amino acid.
3.2 Structural determination by spectroscopic methods
The determination of the ten synthetic compounds 5(a-j) were based upon the characteristic functional groups as following:
The methyl benzoate (2) was obtained and detected by the IR spectrum, which showed two bands at 1722 and 1278 cm−1 for the C⚌O and C-O-C respectively of group ester. The ester 2 refluxed with hydrazine hydrate 64 % to produce hydrazide 3 in yield 84 %. IR spectrum showed bands at 3327.89–3009.3 cm−1 for NH2 and 1659.44 cm−1 for NC⚌O stretching. The yields of the compounds 2,5-disbstituted-1,3,4-oxadiazole 5(a-j) ranged from 53 to 78 %, and they were all produced as white solids. The molecules have a melting point that ranges from 135 to 206 °C. In the UV spectrum, all compounds have an absorption maximum ((λmax, abs) which indicates the presence of electronic transitions of the type n → π* and π → π*. The maximum wavelengths appeared very close for the 1,3,4-oxadiazole derivatives 5(a-j), this is between 224 and 231 nm (Costaet al., 2016; Oliveira et al., 2023). IR spectrum showed bands for NH2 at 3267 cm−1, and CO-N stretching’s at 1586 cm−1. The characteristic C⚌N band (1651–1512 cm−1) of medium intensity and a medium-strong band at 1242,16–1163 cm−1 were identified in each IR spectra, the latter could be attributed to the C–O–C vibration or heteroatom ring deformation of the oxadiazole ring.
The characteristic C⚌N band (1651–1481 cm−1) of medium intensity and a medium-strong band at 1219–1087 cm−1 were identified in each IR spectra, the latter could be attributed to the C–O–C vibration or heteroatom ring deformation of the oxadiazole ring. This difference is due to the type of radical of the amino acid attached at position 5 in the oxadiazole ring.
The 5-phenyl-1,3,4-oxadiazol-2-yl moiety is the common fragment which is repeated in all the ten synthetic compounds 5(a-j) is designated as in the following general structure:
The NMR of 5-Phenyl-1,3,4-oxadiazol-2-yl moiety is as following:
1H NMR showed the characteristic signals in ppm: Phenyl group: 2-(o-H) between 9 → 7; 1-(p-H) between 8.5 → 7.5; 2-(m-H) between 7.5 → 7. Oxadiazole: Non. Amino acid moiety: H1′, 4.5 → 3.5. For rest of amino acid, see experimental part.
13C NMR showed the characteristic signals in ppm: Phenyl locations: 1-ph, between 135 → 125; para-C, between 130 → 129; ortho-C, between 130 → 116; meta-C, between 117 and 105. Oxadiazole: C5, 171 → 165; C2, 165 → 130. Amino acid moiety locations, C1′, between 62.5 → 61.6, and so on (See experimental part).
3.3 Biological activity
3.3.1 Antibacterial activity of synthetic compounds 2, 3 and 5(a-j) at concentration 10 and 0.1 mg/ml
All synthetic compounds 2, 3 and 5(a-j) were assessed in vitro using the paper-disk diffusion method for their antibacterial activity against Gram-negative bacteria (E. coli ATCC 25922, P. aeruginosa ATCC 27853), Gram-positive bacteria (S. aureus ATCC 25923, B. cereus ATCC 14579, L. innocua ATCC 33090), and was also assessed for the antifungal activity using C. albicans ATCC 10231 (Table 1 and Fig. 3). The disk diffusion results were compared against a panel of control antibiotics belonging to three different classes (penicillins, tetracycline and aminoglycosides). Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 14579, Listeria innocua ATCC 33090, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 10231; IZ: Inhibition Zone in mm; T1: AX 25 Amoxicillin, T2: TE 30 Tetracycline, T3: CN 120 Gentamicin, T4: OX 1 Oxacillin
Inhibition Zone IZ in mm
Comp
Gram-positive bacteria
Gram- negative bacteria
Fungi
S. aureus
B. cereus
L. innocua
E. Coli
P. aeruginosa
C. albicans
C1
C2
C1
C2
C1
C2
C1
C2
C1
C2
C1
C2
2
−
−
−
−
−
−
13
−
−
−
−
−
3
−
−
25
8
−
−
11
−
−
−
10
−
5a
14
−
29
12
−
−
23
9
−
−
−
−
5b
21
8
31
8
14
8
25
9
−
−
−
−
5c
25
8
33
15
−
−
28
9
30
8
34
8
5d
23
8
34
15
−
−
30
9
32
10
33
8
5e
21
8
32
15
−
−
27
9
−
−
−
−
5f
−
−
20
7
−
−
−
−
−
−
−
−
5g
22
9
33
15
−
−
19
9
32
11
31
10
5h
−
−
24
7
−
−
−
−
−
−
−
−
5i
24
9
34
15
22
8
23
9
−
−
−
−
5j
25
8
32
16
−
−
25
9
33
11
34
8
T1
16
24
−
12
25
−
T2
08
30
−
11
20
19
T3
29
27
−
31
26
29
T4
18
21
−
09
−
−
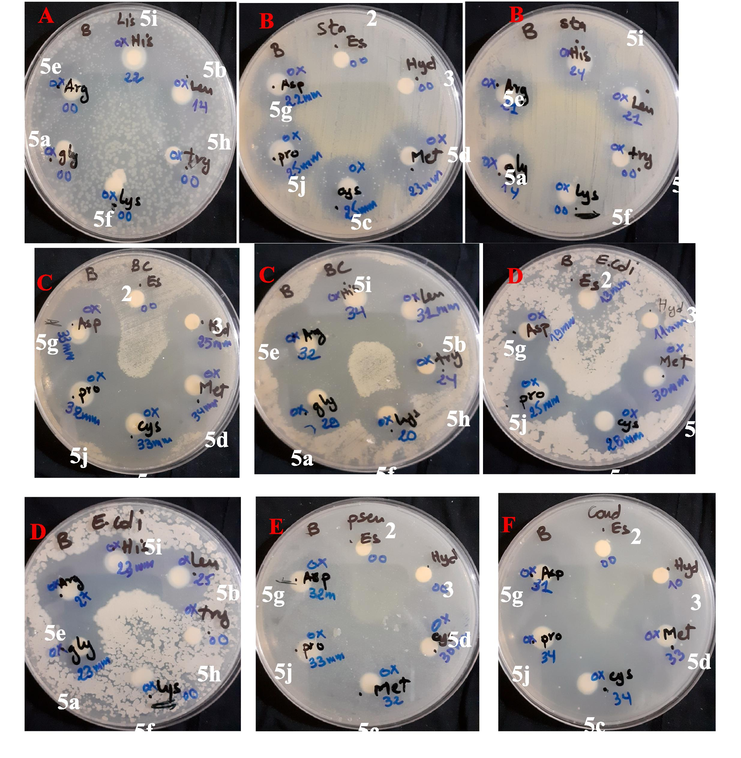
(A-E): antibacterial and antifungal activities of synthesized compounds against tested microorganisms, (A) L. innocua ATCC 33090, (B) S. aureus ATCC 25923, (C) B. cereus ATCC 14579, (D) E. coli ATCC 25922, (E) P. aeruginosa ATCC 27853 and (F) C. albicans ATCC 10231 at 10 mg/ml.
In primary screening 10 mg/ml concentrations were used, and then we used concentrations of 0.1 mg/ml (Table 1). The only compound found to be active in this primary screening was further tested in a second set of dilution 100 μg.ml−1 against all microorganisms, the results are presented in Table 2. The disk diffusion results were compared against a panel of control antibiotics belonging to three different classes (penicillins, tetracycline and aminoglycosides). The results of the antimicrobial activity (Table 1, Fig. 3 and supplementary file) demonstrated variation in the potency against the different microbial strains for each compound tested. Most of the compounds have excellent efficacy, and some of them, such as 5i, 5g, 5d, 5c, and 5j can inhibit their activity better or very close to that of the antibiotics used as standards (T1-4). Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 14579, Listeria innocua ATCC 33090, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 10231.
No
CompGram-positive bacteria
Gram- negative bacteria
Fungi
S. aureus
B. cereus
L. innocua
E. Coli
P. aeruginosa
C. albicans
3
−
100
−
−
−
−
5a
−
50
−
100
−
−
5b
100
100
100
100
−
−
5c
100
25
−
100
100
100
5d
100
25
−
100
50
100
5e
100
25
−
100
−
−
5f
−
100
−
−
−
−
5g
100
25
−
100
50
50
5h
−
100
−
−
−
−
5i
100
25
100
100
−
−
5j
100
25
−
100
50
100
In general, most prepared 2-phenyl-1,3,4-oxadiazol-aminoacid derivatives have shown to be susceptible to excellent potency against Gram-positive (14 mm IZ 34 mm), Gram-negative bacteria (11 mm IZ 33 mm), and Fungi (31 mm IZ 34 mm). The results were better or comparable to those of the reference antibiotics Amoxicillin, Tetracycline, Gentamicin and Oxacillin.
At concentration C1 = 10 mg/ml (Table 1), only two compounds 5b (IZ = 14 mm) and 5i (IZ = 22 mm) presented good results against L. innocua (Table 1 and Fig. 3A), as the rest of the compounds and antibiotics were unable to inhibit it. They also presented a good to excellent inhibition zone (21 mm IZ 34 mm) against S. aureus, B. cereus, E. coli (Table 1 and Fig. 3(B, C, D). Compounds 5(b-e), 5g, and 5(i-j) revealed superior antibacterial activity against S. aureus (14 mm IZ 25 mm) compared to all the used control antibiotics expect for Gentamycin (IZ = 34 mm). Where they showed comparable inhibition zone diameter. In addition, the same compounds revealed superior activity against B. cereus compared to all the tested control antibiotics. Compounds 5c, 5d containing the sulfur radical, 5g containing the amide radical and 5j containing the pyrrolidine radical showed good to very excellent activity (19 mm IZ 34 mm) against all microorganisms except L. innocua. All compounds except ester 2 provided excellent activity against B. cereus (24 mm IZ 34 mm). As for P. aeruginosa and fungal strain C. albicans, the compounds 5c, 5d, 5g and 5j have an inhibition zone of 30 mm IZ 34 mm (Table 1 and Fig 0.3(E, F)) and gave better activity against results than all the standards used.
At concentration C2 = 0.1 mg/ml (Table 1), most of the active compounds presented an inhibition zone ranging between 8 mm and 11 mm for each tested microorganism except for B. cereus which was affected by compounds 5(c-e), 5g and 5i with an inhibition zone of 15 mm, while the compound 5j provided the best inhibition against B. cereus with an inhibition zone of 16 mm. 1,3,4-oxadiazole-aminoacid then subjected to minimum inhibition concentration (MIC) testing. Results were summarized in Table 2 and supplementary file. The MIC values for the tested compounds against S. aureus, L. innocua, E. coli, and C. albicans were 100 μg/mL, which is four-fold higher than the MIC (25 µg/ml) of the other derivatives 5(c-e), 5g and 5(i-j) against B. cereus representing the best activity of the tested compounds.
Also, the MIC of the compounds 5d, 5g and 5j was estimated at 50 µg/ml against P. aeruginosa. These results indicated that the compounds have effective activity against all tested microorganisms and indicated also that the improved potency of the prepared compounds could be attributed to the type of amino acid moiety attached to the 1,3,4-oxadiazole.
3.3.2 Antioxidant activity
Phenyl-oxadiazole derivatives are known for their antioxidant activity due to their potent free radical scavenging activity that could be tolerated through the extended conjugating system composed of the phenyl group and the oxadiazole ring. The following references demonstrate this activity for oxadiazole derivatives (Mihailović et al., 2017; Rana et al., 2023).
The antioxidant activity of synthesized 2-phenyl-1,3,4-oxadiazole derivatives 5(a-j), ester 2 and hydrazide 3 are shown in Table 3. RSA: Radical Scavenging Ability; RPA: Reducing Power Activity; TAC: Total antioxidant capacity. Values are expressed as means ± SD from triplicate. a, b, c: Different letters in the same column indicate significant differences (p < 0.05).
DPPH
FRAP
TAC
Com
RSA % at 250 µg /ml
IC 50 value (µg)
RPA % at 250 µg /ml
IC 50 value (µg)
TAC % at
250 µg /ml
IC 50 value (µg)
2
36,15 ± 0,64 h
> 250 a
59,71 ± 0,77 e
13,71 ± 0,19 h,i
90,60 ± 0,16 a,b
2,56 ± 0,31f
3
92,79 ± 0,41 d,e
15,97 ± 0,35 k
89,49 ± 1,91b
23,08 ± 0,30 g
91,20 ± 0,02 a,b
4,00 ± 0,31f
5a
95,01 ± 0,31b,c
29,30 ± 0,35 h
98,25 ± 4,03 a
35,87 ± 0,40c
98,32 ± 0,31 a
20,76 ± 0,34 a
5b
80,74 ± 0,26 g
54,20±,031 d
80,02 ± 0,70c
39,44 ± 0,29b
96,41 ± 3,35 a
12,76 ± 0,35b,c,d
5c
93,71 ± 0,02c,d
31,96±,035 g
98,24 ± 4,78 a
38,54 ± 0,36b
94,25 ± 3,65 a,b
13,64 ± 0,35b
5d
80,66 ± 0,28 g
25.76 ± 0,31 i
99,21 ± 4,23 a
34,16 ± 0,36c,d
93,89 ± 3,47 a,b
7,90 ± 0,36 e
5e
93,94 ± 0,13c,d
16,03 ± 0,36 k
97,62 ± 3,75 a
29,71 ± 0,33 e
93,40 ± 3,47 a,b
11,45 ± 0,36c,d
5f
33,62 ± 0,11 i
> 250 a
76,12 ± 1,48c
15,22 ± 0,30 h
98,43 ± 0,14 a
6,94 ± 0,33 e
5 g
91,48 ± 0,73 e,f
73,22 ± 0,29c
97,46 ± 2,31 a
11,52 ± 0,31 j
92,41 ± 1,56 a,b
12,89 ± 0,33b,c,d
5 h
80,43 ± 0,26 g
132.59 ± 0,29b
88,08 ± 2,77b
25,00 ± 0,29f
88,19 ± 0,20b
6,05 ± 0,31 e
5i
91,02 ± 0,35f
50,03 ± 0,11 e
97,93 ± 2,18 a
12,41 ± 0,36 i,j
88,07 ± 0,31b
11,11 ± 0,36 d
5j
95,47 ± 0,09b
55,71 ± 0,37 d
98,57 ± 4,31 a
33,89 ± 0,38 d
90,63 ± 2,91 a,b
13,17 ± 0,36b,c
BHT
93,02 ± 0,26 g
21,44 ± 0,35 j
60,66 ± 1,21 e
54,34 ± 0,24 a
94,59 ± 1,10 a,b
3,52 ± 0,31f
AA
97,70 ± 0,32 a
38,33 ± 0,36f
71,49 ± 0,71 d
10,71 ± 0,26 j
91,69 ± 0,03 a,b
6,19 ± 0,34 e
3.3.2.1 Free radical scavenging activity
The antioxidant ability of synthesized 2-phenyl-1,3,4-oxadiazole derivatives 5(a-j), ester 2 and hydrazide 3 were measured by DPPH assay using 2,6-di-tert-butyl-4-methylphenol (butylated hydroxytoluene, BHT) and Ascorbic Acid (AA) as standards. Table 3 displays the percentage of the produced compounds' radical scavenging capacity (RSA) at doses (250 µg/ml, IC50). The proportion of RSA and the concentration of the compounds were shown to be positively correlated, with the percentage of RSA rising as the concentration of the compounds increased.
The results obtained for the anti-radical strength of compounds 2, 3, and 5(a-j) and of BHT and AA determined by DPPH (Table 3), showed radical activity in the trapping by electron transfer or their ability to donate hydrogen. All derivatives synthesized except 2 and 5f showed excellent activity with a concentration of 250 µg/ml as an antioxidant agent (80 % < RSA < 95.5 %), in particular 5a, 5c, 5e and 5j which gave an inhibition percentage very close to AA (RSA = 97.70 %) and better than BHT (RSA = 93.02 %). The highest inhibition percentages are 95.47 % (5j), 95.01 % (5a), 93.94 % (5e), 93.71 % (5c), 92.79 % (3), 91.48 % (5g), 91.02 % (5i), 80.74 % (5b), 80.66 % (5d), 80.43 % (5h), 36.15 (2), and 33.62 % (5f). Because 1,3,4-oxadiazole synthesized contains donor groups such as amine: amine (NH2 found in all compounds); pyrrolidin (in 5j); guanidine (in 5e); sulfanyl (in 5c and 5d); amide [ in 5g]; 1H-imidazol (in 5i) and 1H-indol (in 5h).
By graphing the computed inhibition percentages against various concentrations of the fractions utilized, the concentration of the produced compounds required to scavenge 50 % of the free radicals is determined graphically. The lower the value of the IC50, the more effective the compounds prepared are in eliminating DPPH* free radicals which means more antioxidant activity. The results showed that compounds 3, 5e, 5d, 5a and 5c are more effective as antioxidants based on the ability to scavenge DPPH free radicals: IC50(3) = 15.97 µg/ml < IC50(5e) = 16.03 µg/ml < IC50(BHT) = 21.44 µg/ml < IC50(5d) = 25.76 µg/ml < IC50(5a) = 29.30 µg/ml < IC50(5c) = 31.95 µg/ml < IC50(AA) = 38.33 µg/ml < IC50(5i) = 50.03 µg/ml < IC50(5b) = 54.20 µg/ml < IC50(5j) = 55.71 µg/ml < IC50(5g) = 73.22 µg/ml < IC50(5 h) = 132.95 µg/ml < IC50(2 and 5f).
3.3.2.2 Ferric-reducing antioxidant power (FRAP)
FRAP was used to assess the reducing power activity (RPA) of the synthesized 1,3,4-oxadiazoles derivatives 5(a-j), 2 and 3. The assay measures a compound's capacity to convert ferric ions (Fe3+) to ferrous ions (Fe2+) at a low pH. These ions then react with 2,4,6-trypyridyl-s-triazine (TPTZ) to generate a complex, vivid blue substance (Christodoulouet al., 2022; Munteanu and Apetrei, 2021; Benzie and Strain, 1996).
All synthesized compounds were shown to be good to extremely effective in reducing iron(III) to iron(II) ions in the FRAP test (Table 3). At a concentration of 250 µg/ml, all derivatives synthesized except 2 showed excellent activity as an antioxidant agent (RSA > 76 %) better than AA (71.49 %) and BHT (60.66 %), in particular 5(a, c-e, g, i, j) which gave a higher inhibition percentage (>97 %) because they contain sulfur, amide and amino groups. From the IC50 values of the FRAP test, It can be concluded that the effect of antioxidants followed the following order: AA > 5g > 5i > 2 > 5f > 3 > 5h > 5e > 5j > 5d > 5a > 5c > 5b > BHT. According to the findings, the amine group improves antioxidant capacity.
3.3.2.3 Total antioxidant capacity (TAC)
The total antioxidant capacity of synthesized 1,3,4-oxadiazoles derivatives 5(a-j), 2 and 3 were assessed against BHT and AA by phosphomolybdenum assay which is a quantitative spectroscopic method based on the reduction of Mo (VI) to Mo (V) and subsequent formation of a green phosphate Mo (V) complex at low pH (Alam et al., 2013).
The TAC of the fractions was measured spectrophotometrically by the synthesized compounds with maximum absorption at 695 nm. From results (Table 3), at 250 µg/ml, all synthesized compounds were shown to be excellently effective in reducing Mo (VI) to Mo (V) ions in the TAC test (>88 %).
The 1,3,4-oxadiazole compounds 5(a, b, f) with aliphatic chain and amino groups have the best antioxidant activity (>96 %) than BHT (94.59 %), followed by other compounds that have better or close activity than AA (91.69 %). From the IC50 values, the following compounds 2, 3, 5 h, 5f and 5d showed excellent antioxidant activity with values of 2.56, 4.00, 6.05, 6.94 and 7.90 µg/ml respectively, compared to AA and BHT, while other compounds except 5a (20.76 %) presented good results: 11 µg/ml < IC50 < 14 µg/ml.
4 Conclusion
The ten 2,5-disubstituted-1,3,4-oxadiazolyl derivatives 5(a-j) were successfully synthesized and fully characterized by UV–Vis IR, 1H NMR and 13C NMR.
The most derivatives synthesized showed excellent antioxidant activity with a concentration of 250 µg/ml as an antioxidant agent (76 % < RSA < 95.5 %), in particular 5a, 5c, 5e and 5j which gave an inhibition percentage very close to AA. The results of IC50 values showed that compounds 3, 5e, 5d, 5a and 5c are more effective as antioxidants due to their ability to scavenge DPPH free radicals, and for the FRAP test, it can be concluded that the effect of antioxidants followed the following order: AA > 5 g > 5i > 5f > 3 > 5 h > 5e > 5j > 5d > 5a > 5c > 5b > BHT. TAC test showed that the following compounds 5 h, 5f and 5d have excellent antioxidant activity with IC50 values of 6.05, 6.94 and 7.90 µg/ml respectively compared to AA and BHT.
The 1,3,4-oxadiazole derivatives 5c, 5d, 5g, 5i and 5j showed excellent antibacterial and antifungal activity better or very close to that of the antibiotics used as standards. Compounds 5b and 5i provided good results against L. innocua with inhibition values of IZ = 14 mm and IZ = 22 mm, respectively, while the rest of the compounds and antibiotics were unable to inhibit it. Compounds 5c, 5d, 5 g and 5j showed excellent activity against C. albicans with values between 31 mm and 34 mm. These results were better than all the standards used. The MIC value (25 µg/ml) for derivatives 5(c-e), 5 g and 5(i-j) against B. cereus represent the best activity of the tested compounds. In light of the results obtained in this study, the potent antioxidant and antimicrobial activities of the synthesized compounds could provide a promising application in the field of food, pharmaceutical, and cosmetics industry.
CRediT authorship contribution statement
Khaled Briki: Conceptualization, Data curation, Methodology, Writing – original draft. Talal Lahreche: Conceptualization, Formal analysis, Methodology, Writing – original draft. Mouna Souad Abbassi: Conceptualization, Formal analysis, Methodology, Writing – original draft. Mokhtar Boualem Lahrech: Conceptualization, Supervision, Validation, Writing – review & editing. Adil Ali Othman: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Ahmed M. Elissawy: Formal analysis, Validation, Visualization, Writing – review & editing. Abdel Nasser B. Singab: Conceptualization, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of diazole-thiols derivatives from L-cysteine: characterization, complex formation with Ni (II), Cu (II) and evaluation of their antibacterial activity. J. Saudi Chem. Soc.. 2021;25(5):101230
- [Google Scholar]
- Synthesis and anticancer evaluation of 4-Chloro-2-((5-aryl-1, 3, 4-oxadiazol-2-yl) amino) phenol analogues: an insight into experimental and theoretical studies. Molecules. 2023;28(16):6086.
- [Google Scholar]
- Electrophilically activated nitroalkanes in the synthesis of substituted 1, 3, 4-oxadiazoles from amino acid derivatives. Chem. Heterocycl. Compd.. 2022;58(1):32-36.
- [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.. 2013;21(2):143-152.
- [Google Scholar]
- Synthesis, antimicrobial activity, electrochemical studies and molecular modeling studies of novel 1, 3, 4-oxadiazole derivatives. J. Mol. Struct.. 2023;1289:135775
- [Google Scholar]
- Synthesis of some 2-substituted pyrrolidine alkaloid analogues: N-benzyl-2-(5-substituted 1, 3, 4-oxadiazolyl) pyrrolidine derivatives and pharmacological screening. J. Saudi Chem. Soc.. 2022;26(3):101448
- [Google Scholar]
- Design, synthesis, modelling studies and biological evaluation of 1, 3, 4-oxadiazole derivatives as potent anticancer agents targeting thymidine phosphorylase enzyme. Bioorg. Chem.. 2021;111:104873
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Synthesis of hydrazides of heterocyclic amines and their antimicrobial and spasmolytic activity. Saudi Pharm. J.. 2022;30(7):1036-1043.
- [Google Scholar]
- Design, synthesis, molecular docking and antioxidant evaluation of Benzimidazole-1, 3, 4 oxadiazole derivatives. J. Mol. Struct.. 2023;1276:134747
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-1200.
- [Google Scholar]
- Synthesis, characterization and biological activity of Hg (II), Fe (III) complexes with 1, 3, 4-oxadiazole, 1, 2, 4-triazole derivatives from L-methionine. J. Saudi Chem. Soc.. 2020;24(12):1051-1059.
- [Google Scholar]
- From oxadiazole to triazole analogues: optimization toward a dual orexin receptor antagonist with improved in vivo efficacy in dogs. ChemMedChem. 2020;15(5):430-448.
- [Google Scholar]
- Two new 1, 3, 4-oxadiazoles with effective antifungal activity against Candida albicans. Front. Microbiol.. 2019;10:2130.
- [Google Scholar]
- 1, 3, 4-oxadiazole and 1, 3, 4-thiadiazole nortopsentin derivatives against pancreatic ductal adenocarcinoma: synthesis, cytotoxic activity, and inhibition of CDK1. Mar. Drugs. 2023;21(7):412.
- [Google Scholar]
- Recent advancement in synthesis and bioactivities of 1, 3, 4- oxadiazole. Curr. Org. Synth.. 2023;20(6):663-677.
- [Google Scholar]
- Exploring 1, 3, 4-oxadiazole scaffold for anti-inflammatory and analgesic activities: a review of literature from 2005–2016. Mini Rev. Med. Chem.. 2018;18(3):216-233.
- [Google Scholar]
- Synthesis, biological evaluation and network pharmacology based studies of 1, 3, 4-oxadiazole bearing azaphenols as anticancer agents. Arab. J. Chem.. 2024;17(1):105386
- [Google Scholar]
- Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants. 2022;11(11):2213.
- [Google Scholar]
- CLSI, 2004. Method for antifungal disk diffusion susceptibility testing of yeasts, approved guideline. CLSI document M44-A. CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA.
- CLSI, 2012b. Performance standards for antimicrobial disk susceptibility tests, approved standard, 7th ed., CLSI document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- CLSI, 2012a. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard, 9th ed., CLSI document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- Synthetic approaches and pharmacological activity of 1, 3, 4-oxadiazoles: a review of the literature from 2000–2012. Molecules. 2012;17(9):10192-10231.
- [Google Scholar]
- Synthesis, characterization and in vitro antimicrobial screening of quinoline nucleus containing 1, 3, 4-oxadiazole and 2-azetidinone derivatives. J. Saudi Chem. Soc.. 2014;18(5):425-431.
- [Google Scholar]
- Oxadiazole: a highly versatile scaffold in drug discovery. Arch. Pharm.. 2022;355(9):2200123.
- [Google Scholar]
- Synthesis and molecular docking study of novel hybrids of 1, 3, 4-oxadiazoles and quinoxaline as a potential analgesic and anti-inflammatory agents. J. Heterocycl. Chem.. 2018;55(12):2901-2910.
- [Google Scholar]
- CHK1 inhibitor sensitizes resistant colorectal cancer stem cells to nortopsentin. Iscience. 2021;24(6):102664
- [Google Scholar]
- Optimized POCl3-assisted synthesis of 2-amino-1, 3, 4-thiadiazole/1, 3, 4-oxadiazole derivatives as anti-influenza agents. Arab. J. Chem.. 2022;15(4):103712
- [Google Scholar]
- Hybridization of the effective pharmacophores for treatment of epilepsy: design, synthesis, in vivo anticonvulsant activity, and in silico studies of phenoxyphenyl-1, 3, 4-oxadiazole-thio-N-phenylacetamid hybrids. BMC Chem.. 2023;17(1):80.
- [Google Scholar]
- Synthetic trends followed for the development of 1, 2, 3-triazole derivatives. Int. J. Drug Dev.. 2017;9:22-25.
- [Google Scholar]
- Direct annulation of hydrazides to 1, 3, 4-oxadiazoles via oxidative C (CO)–C (methyl) bond cleavage of methyl ketones. Org. Lett.. 2015;17(12):2960-2963.
- [Google Scholar]
- Antimicrobial activity of 1, 3, 4-oxadiazole derivatives. Int. J. Mol. Sci.. 2021;22(13):6979.
- [Google Scholar]
- Synthesis, characterization and anticancer activity of certain 3-{4-(5-mercapto-1, 3, 4-oxadiazole-2-yl) phenylimino} indolin-2-one derivatives. Saudi Pharm. J.. 2011;19(3):153-158.
- [Google Scholar]
- Synthesis and evaluation of 5-(1H-indol-3-yl)-N-aryl-1, 3, 4-oxadiazol-2-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett.. 2017;27(4):1037-1040.
- [Google Scholar]
- Synthesis, antimicrobial and antiviral testing of some new 1-adamantyl analogues. Saudi Pharm. J.. 2010;18(3):123-128.
- [Google Scholar]
- Synthesis and multi-spectroscopic DNA binding study of 1, 3, 4-oxadiazole and 1, 3, 4-thiadiazole derivatives of fatty acid. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2016;153:510-516.
- [Google Scholar]
- Synthesis, characterization, and optical properties of novel 2, 5-bis [4-(2-(-arylvinyl) phenyl]-1, 3, 4-oxadiazoles. Turk. J. Chem.. 2009;33(3):393-397.
- [Google Scholar]
- Synthesis, characterization and biological evaluation of novel 2, 5 substituted-1, 3, 4 oxadiazole derivatives. Saudi Pharm. J.. 2017;25(3):337-345.
- [Google Scholar]
- A mini review on design, synthesis and biological activities of oxadiazole derivatives. Lat. Am. J. Pharm.. 2023;42(3):612-621.
- [Google Scholar]
- A mild, one-pot preparation of 1, 3, 4-oxadiazoles. Tetrahedron Lett.. 2009;50(47):6435-6439.
- [Google Scholar]
- 1, 3, 4-oxadiazole: a privileged structure in antiviral agents. Mini Rev. Med. Chem.. 2011;11(13):1130-1142.
- [Google Scholar]
- Synthesis and biological evaluation of novel 2-(substituted isoxazol-4-yl)-5-arylamino-1, 3, 4-oxadiazoles. Res. Chem. Intermed.. 2014;40(4):1575-1581.
- [Google Scholar]
- A KHSO4 mediated facile synthesis of 2-amino-1, 3, 4-oxadiazole derivatives. Tetrahedron. 2021;96:132382
- [Google Scholar]
- Synthesis and biological activity of 1, 3, 4-oxadiazoles used in medicine and agriculture. Appl. Sci.. 2022;12(8):3756.
- [Google Scholar]
- A new and efficient synthesis of 1, 3, 4-oxadiazole derivatives using TBTU. Tetrahedron. 2013;69(8):2075-2080.
- [Google Scholar]
- Synthesis, pharmacological and molecular docking investigations of 1, 3, 4-oxadiazole-5-thionyl derivatives of extracted cis-clerodane diterpenoid from Cistus monspeliensis. Med. Chem. Res.. 2023;32(1):128-143.
- [Google Scholar]
- Synthesis and antioxidant activity of 1, 3, 4-oxadiazoles and their diacylhydrazine precursors derived from phenolic acids. RSC Adv.. 2017;7(14):8550-8560.
- [Google Scholar]
- Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J.. 2017;25(6):873-883.
- [Google Scholar]
- Analytical methods used in determining antioxidant activity: a review. Int. J. Mol. Sci.. 2021;22(7):3380.
- [Google Scholar]
- Synthesis, characterization and antibacterial activity of novel heterocycle, coumacine, and two of its derivatives. Saudi Pharm. J.. 2018;26(6):870-875.
- [Google Scholar]
- Design and synthesis of newer 1, 3, 4-oxadiazole and 1, 2, 4-triazole based Topsentin analogues as anti-proliferative agent targeting tubulin. Bioorg. Chem.. 2020;95:103519
- [Google Scholar]
- Therapeutic potential of 1, 3, 4-oxadiazoles as potential lead compounds for the treatment of Alzheimer's disease. RSC Adv.. 2023;13(26):17526-17535.
- [Google Scholar]
- 1, 3, 4-Oxadiazole-containing hybrids as potential anticancer agents: recent developments, mechanism of action and structure-activity relationships. J. Saudi Chem. Soc.. 2021;25(8):101284
- [Google Scholar]
- Recent progress of 1, 3, 4-oxadiazoles as anticonvulsants: future horizons. Arch. Pharm.. 2020;353(7):1900342.
- [Google Scholar]
- Non-symmetrical 1, 3, 4-oxadiazole derivatives: synthesis, characterization, and computational study of their optical properties. Chem. Phys. Impact.. 2023;6:100162
- [Google Scholar]
- 1, 3, 4-oxadiazole, 1, 3, 4-thiadiazole and 1, 2, 4-triazole derivatives as potential antibacterial agents. Arab. J. Chem.. 2019;12(7):1660-1675.
- [Google Scholar]
- Distribution of ascorbic acid analogs and associated glucosamine fractionated by organ ic solvent and thin-layer chromatography. Nippon Shokuin Kogyo Gakkaishi.. 1986;35:771-775.
- [Google Scholar]
- 1, 2, 4-oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem.. 2021;209:112892
- [Google Scholar]
- Antimicrobial and antiprotozoal activity of 3-acetyl-2, 5-disubstituted-1, 3, 4-oxadiazolines: a review. Med. Chem. Res.. 2020;29:1-16.
- [Google Scholar]
- Synthesis of 1,3,4-oxadiazole derivatives with its antifungal activity evaluation. Asian J. Pharm. Technol.. 2021;11(2):146-148.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269(2):337-341.
- [Google Scholar]
- Qazi, A.I., Ahmad, B., Sahibzada, M.U.K., Anwar, F., Khusro, A., Alhumaydhi, F.A., Abdel-rahman Mohamed, A., Mostafa-Hedeab, G., Emran, T.B., 2023. Evaluation of Antidiabetic Activity of Oxadiazole Derivative in Rats. Evidence-Based Complementary and Alternative Medicine. 2023.
- Experimental and theoretical study on structure-tautomerism among edaravone, isoxazolone, and their heterocycles derivatives as antioxidants. Saudi Pharm. J.. 2020;28(7):819-827.
- [Google Scholar]
- Design and synthesis of novel 1, 3, 4-oxadiazole based azaspirocycles catalyzed by NaI under mild condition and evaluated their antidiabetic and antibacterial activities. J. Heterocycl. Chem.. 2021;58(2):612-621.
- [Google Scholar]
- The biological potential and synthetic diversity of 1, 3, 4-oxadiazole multiplexed with various heterocyclic compounds. J. Turkish Chem. Soc. Sect. A: Chem.. 2023;10(2):267-276.
- [Google Scholar]
- A mild and efficient one pot synthesis of 1, 3, 4-oxadiazoles from carboxylic acids and acyl hydrazides. Tetrahedron Lett.. 2006;47(28):4827-4830.
- [Google Scholar]
- Synthesis, computational studies, antioxidant and anti-inflammatory bio-evaluation of 2, 5-Disubstituted-1, 3, 4-oxadiazole derivatives. Pharmaceuticals. 2023;16(7):1045.
- [Google Scholar]
- A facile synthesis of 1, 3, 4-oxadiazole-based carbamothioate molecules: antiseizure potential, EEG evaluation and in-silico docking studies. Arab. J. Chem.. 2023;16(4):104610
- [Google Scholar]
- Synthesis of new 2-amino-1, 3, 4-oxadiazole derivatives with anti-salmonella typhiactivity evaluation. BMC Chemistry.. 2020;14(1):1-8.
- [Google Scholar]
- Sasikumar, P.G.N., Ramachandra, M., Prasad, A. Naremaddepalli, S.S.S., Aurigene Discovery Technologies Ltd., 2018. 3-substituted-1, 2, 4-oxadiazole and thiadiazole compounds as immunomodulators. U.S. Patent Application 15/556, 805.
- Synthesis and antitumor activity of novel 1-substituted phenyl 3-(2-oxo-1, 3, 4-oxadiazol-5-yl) β-carbolines and their Mannich bases. Bioorg. Med. Chem.. 2014;22(24):6867-6875.
- [Google Scholar]
- Synthesis and biological activities of Bis alkyl 1, 3, 4-oxadiazole incorporated azo dye derivatives. Arab. J. Chem.. 2016;9:S1643-S1648.
- [Google Scholar]
- Synthesis and pharmacological evaluation of 3-[5-(aryl-[1, 3, 4] oxadiazole-2-yl]-piperidine derivatives as anticonvulsant and antidepressant agents. Arab. J. Chem.. 2020;13(5):5299-5311.
- [Google Scholar]
- Therapeutic potential of oxadiazole or furadiazole containing compounds. BMC Chem.. 2020;14:1-40.
- [Google Scholar]
- 1, 3, 4-oxadiazole and its derivatives: a review on recent progress in anticancer activities. Chem. Biol. Drug Des.. 2021;97(3):572-591.
- [Google Scholar]
- Research progress on the synthesis and pharmacology of 1, 3, 4-oxadiazole and 1, 2, 4-oxadiazole derivatives: a mini review. J. Enzyme Inhib. Med. Chem.. 2022;37(1):2304-2319.
- [Google Scholar]
- Novel quinazolin-4 (3H)-one bionic-alkaloids bearing an 1, 3, 4-oxadiazole fragment as potential fungicides inhibiting Botrytis cinerea: design, synthesis and bioactive-guided structural optimization. Arab. J. Chem.. 2024;17(1):105455
- [Google Scholar]
- Novel 1, 3, 4-oxadiazole thioether and sulfone derivatives bearing a flexible N-heterocyclic moiety: synthesis, characterization, and anti-microorganism activity. Arab. J. Chem.. 2023;16(2):104479
- [Google Scholar]
- Synthesis, antifungal activity, and molecular simulation study of L-carvone-derived 1, 3, 4-oxadiazole-thioether compounds. Chem. Biodivers.. 2023;20(7):e202300794.
- [Google Scholar]
- Propylene oxide assisted one-pot, tandem synthesis of substituted-1, 3, 4-oxadiazole-2 (3H)-ones in water. Tetrahedron. 2012;68(38):7978-7983.
- [Google Scholar]
- A novel electrochemical conversion of CO2 with aryl hydrazines and paraformaldehyde into 1, 3, 4-oxadiazol-2 (3H)-one derivatives in one step. Electrochem. Commun.. 2016;72:109-112.
- [Google Scholar]
- Design, synthesis and antibacterial studies of 1, 3, 4-oxadiazole-fluoroquinolone hybrids and their molecular docking studies. ChemistrySelect. 2021;6(46):13209-13214.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105767.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







