Translate this page into:
Bioactive principles in exudate gel from the leaf of Aloe fleurentiniorum, traditionally used as folkloric medicine by local people of Aridah and Fayfa mountains, Saudi Arabia
⁎Corresponding author. drsmsivakumar@gmail.com (Sivakumar Sivagurunathan Moni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aloe fleurentiniorum is a desert plant that is occasionally used to treat wounds by local people of Aridah and Fayfa mountains in Saudi Arabia. However, medicinal value of the plant has not been scientifically established. The purpose of this study was to determine the bioactive phytocomponents contained in the exudate gel (EG) from the leaves of Aloe fleurentiniorum using GC–MS and FT-IR studies, as well as antibacterial assays. Medicinally important bioactive compounds were identified using GC–MS analysis. The bioactive compounds are “pregn-5-ene-3α,20-diol”, “16α-methyl-pregnane-11,20-dione”, “3-hydroxy-(3α,5α)-, ursodeoxycholic acid”, “1-heptatriacotanol”, “allopregnane-7α,11α-diol-3,20-dione”, “D-arabino-hexopyranoside”, “2-octadecenoic acid methyl ester”, “D-ribo-hexose,2,6-dideoxy-3-O-methyl glucosamine”, “N-acetyl-N-benzoyl-alpha-D-glucopyranoside”, “nonyl 1-thio pregnenolone”, “5-cholestene-3-ol, 24-methyl-cholestanol”, and “D-allose hexadecanoic acid methyl ester”. Furthermore, specific groups and their respective chemical compounds were identified via FT-IR spectroscopy studies. The FT-IR spectroscopy of EG showed various functional groups at 3354, 2945, 2832, 2523, 2046, 1707, 1451, 1108, 1031, 880, 737 and 610 cm−1. The FT-IR peaks revealed the likely presence of various compounds such as glycosides, flavonoids, steroids, saponins, flavonoids, amino sugars, cutin and isothiocyanate. Moreover, EG produced a wide range of antibacterial effects on some screened human pathogenic bacteria.
Keywords
Desert plant
Aloe fleurentiniorum
Bioactive constituents
Anti-bacterial activity
1 Introduction
Wounds are caused by injuries to the skin that interrupt the other soft tissues in and around them. Wound healing is a complex process of tissue repair and rejuvenation in response to injury (Sivakumar et al., 2018; Qing 2017). Topical antimicrobial drugs have been used successfully in the management of infected wounds. Resistance to antimicrobial drugs in wound management may result in therapeutic failure and complications. There is a rising need for development of novel wider-spectrum and topical antimicrobial agents. However, there is no assurance that newer topical applications will not develop resistance to microorganisms. The World Health Organization (WHO) recognises that herbal medicines and phytonutrients are becoming increasingly popular around the world (WHO, 2004). Indeed, approximately 25% of medications administered globally are derived from plants (Rates, 2001). As a result, numerous efforts devoted to research over the last four decades have concentrated on scientific validation of the efficacy of plants/herbs as medicinal agents for treating a variety of illnesses.
Aloe fleurentiniorum is a succulent with a great ecological significance in plant diversity of Saudi Arabia, is widely distributed in the rocky region (Fig. 1). Aloe fleurentiniorum is a member of the Asphodelaceae family and one of the most unique plant in the southern region of Saudi Arabia. Exudate gel (EG) produced from the leaves of Aloe fleurentiniorum has been traditionally used in Saudi Arabia to aid in wound healing. However, neither the wound-healing nor antibacterial capabilities of this plant have been scientifically proven. As a result, the current study sought to investigate the presence of bioactive principles and anti-bacterial activities of EG.
The collecting spot, growth of plant Aloe fleurentiniorum in the hilly region with shrubs on the rocks of Rijal Alma, Saudi Arabia.
2 Materials and methods
2.1 Materials
Bacteriological media was purchased from Scharlau, Spain. All the materials used in this study were supplied by Ejadah Medical Supplies Est, Riyadh, Saudi Arabia.
2.2 Study area, plant collection, and identification
Rijal Almaa was the study area, which is located in Asir province in southern Saudi Arabia, approximately 45 km west of Abha with coordinates N 2015840.65 E 211634.87. It is a mountainous region the Al-Souda Centre borders it on the east, Muhayil on the north, Al-Darb on the south, and Jizan on the west. Aloe fleurentiniorum was harvested in November 2019 from the Rijal Almaa hills (Fig. 1). The part of the plant used for this study was the leaves. The biological specimens transported to the laboratory in paper-wrapped perforated polyethylene bags. The leaves sample was thoroughly washed with Millipore water, to remove impurities using Millipore Milli-Q, Integral 10 Water Purification System, USA, followed by rinsing in distilled water to remove any residual impurities. The plant was identified by a plant taxonomist, and a voucher specimen (reference number = JAZUH 1621) was deposited in Jazan University herbarium.
2.3 Exudate collection
The exudate from Aloe fleurentiniorum (Fig. 2) was collected by performing a sterile leaf incision and keeping the leaf in a slanting position. The gel collected was designated as EG. The sample was collected in a sterile 50 mL glass reagent bottle and refrigerated at 2–8 °C.
The EG is extracted from the leaf of Aloe fleurentiniorum using a sterile blade and then dropped into a glass reagent bottle. By visual appearance, the EG was viscous, pale yellow in color, and homogeneous in texture.
2.4 Physical characterization of EG
Organoleptic qualities were determined by the physical appearance, color, homogeneity, and texture of EG. Visual inspection was used to determine the homogeneity of EG in a clean glass beaker and any aggregates observed were noted. A Brookfield digital viscometer (Model LVDV-E, USA) was used to determine the rheological characteristics of the EG sample. Briefly, 25 mL of EG was placed in a clean glass beaker, kept in a sample holder and left to settle for 5 min before being analyzed. The viscosity was then determined by S63 spindle at a rotating speed of 30 revolutions per minute at room temperature (Makeen et al. (2020).
2.5 Gas chromatography–mass spectrometry (GC–MS) analysis
Thermo Scientific GCMS with AS 3000 autosampler, trace ultra-GC, and ISQ detector was used for the GC–MS analysis. In a volume ratio of 1:10, the sample was diluted in methanol. The sample components were separated using a Thermo Scientific TR 5MS with dimensions of 30 m × 0.25 mm (internal diameter) × 0.25 m (film thickness) and a flow rate of 1.2 mL/min of helium carrier gas (constant flow mode). The mass spectrophotometer was operated and spectral analysis was performed using Xcalibur software. Interpretation of mass spectrum was performed using National Institute Standard and Technology (NIST) and Mainlib inbuilt software libraries.
2.6 Fourier transform infrared spectroscopy (FT-IR) studies
FT-IR analysis was used to identify the functional groups in EG. The functional groups of the samples were analyzed by using Nicolet iS10 FT-IR spectrophotometer, Thermo Fisher Scientific Inc., USA. KBR pellet technique was followed and the spectra of the pellet sample were obtained at 400–4000 cm−1 with a resolution of 4 cm−1.
2.7 Determination of anti-bacterial activity of EG
The anti-bacterial activity of EG was determined on Gram positive bacteria Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, and Gram-negative bacteria Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa. A 24-h culture of each bacterial species was prepared and standardized. The colony-forming unit per milliliter (CFU/mL) was used to determine cell viability. The minimal inhibitory concentration was determined using the broth dilution method. EG concentrations of 50, 40, 30, 20, 10, 5, 2.5, and 1.25 % (v/v) were generated by serial dilution with Millipore water. The minimal inhibitory concentration (MIC) was established based on the visible growth of bacteria in the broth. Susceptibility testing was performed using the previously reported method (Makeen et al., 2020). The bacterial cultures were sub-cultured from the stock culture and then tested for anti-bacterial activity after a 24-hour incubation period at 37 ° C. The anti-bacterial assay was performed by using Muller Hinton (MH) agar plates. The spread plate technique was used to inoculate bacterial species. Individual cultures were streaked over MH agar plates with a sterile cotton swab dipped in individual homogeneous (CFU/mL) cultures and the Petri dish was rotated to evenly disseminate the culture. The inoculation method was performed twice for better culture growth. The plates were allowed to dry for about 10 min before administering EG samples and standard ciprofloxacin in order to diffuse the culture to MH agar media. A regular sterile stainless-steel borer was used to punch 10-mm-diameter holes in the inoculated MH agar plates. The samples and standard were put in separate wells on separate plates. Based on MIC studies, 20% v/v concentrations of EG and standard ciprofloxacin was used at a concentration of 50 µg/mL were placed in their respective wells (Cappuccino and Sherman, 2014), and the plates were incubated for 24 h in a bacteriological incubator (EN 400, Nuve, Turkey) at 37° C. After a 24-h incubation at 37 °C, the anti-bacterial spectrum of EG was determined in terms of zones of inhibition on the plates. The anti-bacterial potential is directly proportional to the diameter of the zone of inhibition.
3 Results and discussion
Natural products are a distinctive source of bioactive compounds with a wide molecular diversity and potentially therapeutic effects. Traditionally, plants have been utilized to cure a range of disorders due to their high concentration of biologically active molecules having therapeutic qualities (Zamawe et al. 2018; Roumita et al. 2018). Many plants are used as folkloric medicinal agents in Saudi Arabia, especially in the southern parts of the country (Aati et al. 2019; Nasser 2017). The EG from Aloe fleurentiniorum is used in folkloric medicine by the people of Asir province for treating wounds. Therefore, the current study was conducted to investigate the antibacterial spectrum of the EG with respect to some Gram-positive and Gram-negative pathogenic bacteria in humans. At room temperature, the EG was pale yellow, highly viscous, and homogeneous, with a consistent consistency and smooth texture (Table 1). Results from GC–MS analysis of EG revealed the presence of several bioactive compounds, as shown in the chromatogram in Fig. 3. The presence of various bioactive constituents is presented in Table 2 and their structure is represented in Fig. 4. EG contained steroids, saponins, proteins, carbohydrates and tannins.
Concentration (% v/v)
Physical parameters
Physical appearance
Texture
Homogeneity
Viscosity Centipoise (cP)
50
Opaque
Smooth
Yes
425
40
Opaque
Smooth
Yes
378
30
Opaque
Smooth
Yes
272
20
Transparent
Smooth
Yes
167
10
Transparent
Smooth
Yes
86
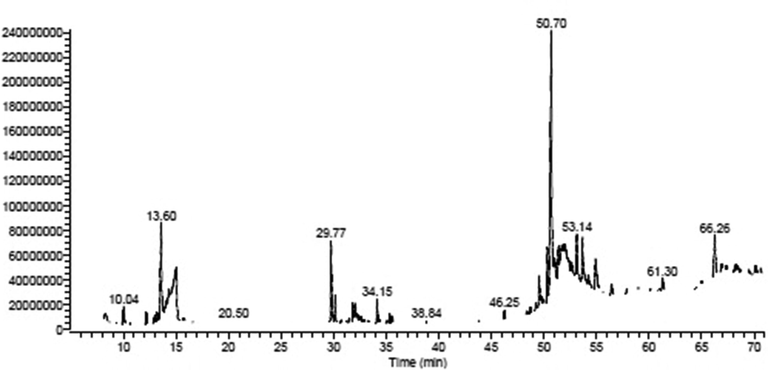
GC–MS chromatogram of EG from the leaf of Aloe fleurentiniorum, displaying several peaks.
Sample ID
Bioactive compound
Molecular formula
Retention time (Minutes)
Molecular weight
1
Pregn-5-ene-3α,20-diol, 16α-methyl-
C22H36O2
66.26
332
2
Pregnane-11,20-dione, 3-hydroxy-, (3α,5α)-
C21H32O3
53.14
332
3
Ursodeoxycholic acid
C24H40O4
50.70
392
4
1-Heptatriacotanol
C37H76O
46.25
536
5
Allopregnane-7α,11α-diol-3,20-dione
C21H32O4
38.84
348
6
D-arabino- hexopyranoside
C15H28O8
34.15
336
7
2-octadecenoic acid, methyl ester
C19H36O2
29.97
296
8
D-ribo-hexose,2,6-dideoxy-3-o-methyl
C7H14O4
13.60
162
9
Glucosamine, N-acetyl-N-benzoyl
C15H19NO7
10.04
325
10
α-d-Glucopyranoside, nonyl 1-thio
C15H30O5S
50.33
710
11
Pregnenolone
C21H32O2
60.19
316
12
5-Cholestene-3-ol, 24-methyl-
C28H48O
60.35
400
13
Cholestanol
C27H48O
60.64
388
14
D-allose
C6H12O6
23.66
180
15
Hexadecanoic acid, methyl ester
C17H34O2
31.47
270
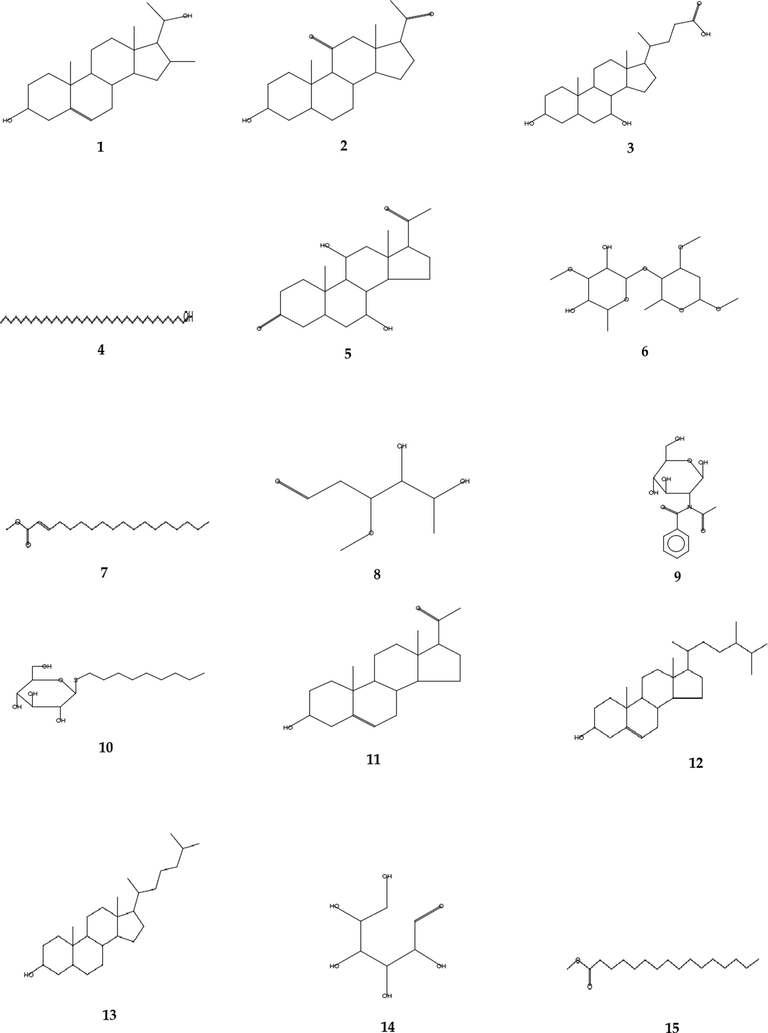
The structure of bioactive compounds of EG from the leaf of Aloe fleurentiniorum, determined through GC–MS analysis. (1) Pregn-5-ene-3α,20-diol, 16α-methyl-; (2) Pregnane-11,20-dione, 3-hydroxy-, (3α,5α)-; (3) Ursodeoxycholic acid; (4) 1-Heptatriacotanol; (5) D-arabino- hexopyranoside; (6) 2-octadecenoic acid, methyl ester; (7) 2-octadecenoic acid, methyl ester; (8) D-ribo-hexose,2,6-dideoxy-3-o-methyl; (9) Glucosamine, N-acetyl-N-benzoyl; (10) α-d-Glucopyranoside, nonyl 1-thio; (11) Pregnenolone; (12) 5-Cholestene-3-ol, 24-methyl-; (13) Cholestanol; (14) D-allose; (15) Hexadecanoic acid, methyl ester.
The steroidal derivatives pregn-5-ene-3α,20-diol and 16α-methyl- were identified in EG with maximum retention time, followed by pregnane-11,20-dione, 3-hydroxy-, (3α,5α)-. In our earlier study, pregnane-11,20-dione-3-hydroxy-, (3α,5α) – was identified as one of the constituents of flowers from Caralluma retrospiciens (Saad et al., 2020). In the present study, the peak at 50.70 min showed the presence of ursodeoxycholic acid (UDCA) which produced the highest peak. Earlier research indicated that the ethanolic extract of Caralluma umbellata contains UDCA, which possessed antimicrobial properties (Evanjalin and Mohan, 2018). UDCA have been reported as potent antioxidant properties that shows an effective hepatoprotective agent against liver dysfunction caused by Co-amoxyclav (El-Sherbiny et al., 2009).
1-heptatriacotanol was detected in EG at retention time of 46.25 min. Lou Junwei et al. (2018) reported that 1-heptatriacotanol was one of the major constituents of Pterocarpus cambodianus, and also indicated that it exerted anti-hypercholesterolemic effects. An earlier study reported that 1-heptatriacotanol produced antioxidant, anticancer, anti-inflammatory and sex-hormonal properties (Mohammed et al., 2016). In our earlier studies, 1-heptatriacotanol was identified in the exudate of Caralluma retrospiciens (Makeen et al., 2020). Studies by Dey et al. (2017) and Olufunmiso et al. (2018) have demonstrated the presence of 1-heptatriacotanol in Aloe aspera, and in ethanolic extract of Trilepisium madagascariense, as well as its anti-bacterial properties. 2-Octadecenoic acid, methyl ester (methyl octadecenoate) was identified in the EG of Aloe fleurentiniorum. Similarly, 2-octadecenoic acid methyl ester has been identified in the exudate of Caralluma retrospiciens (Makeen et al., 2020). Allopregnane-7α,11α-diol-3,20-dione; D-arabino-hexopyranoside; D-ribo-hexose, 2,6-dideoxy-3-O-methyl; Glucosamine N-acetyl-N-benzoyl-; α-d-glucopyranoside, nonyl 1-thio; Pregnenolone; 5-Cholestene-3-ol; 24-methyl-; Cholestanol; D-allose were identified as constituents of EG from Aloe fleurentiniorum.
These compounds have also been reported as constituents of exudate of Caralluma retrospiciens (Makeen et al., 2020). 5-cholestene-3-ol has antibacterial, anticancer, antiarthritic, anti-inflammatory, anti-asthmatic, and hepatoprotective characteristics, according to a previous study (Al-Rubaye et al., 2017; Sindhu and Neelamegam, 2015). D-allose is a rare monosaccharide which is resistant to Xanthomonas oryzae pv (Kano et al., 2013; Akihito et al., 2009). Interestingly, D-allose has been shown to have anti-tumor activity against human non-small cell lung cancer in vitro and to inhibit tumor progression in vivo (Kanaji et al., 2018). In this study, the saturated fatty acid hexadecanoic acid methyl ester (palmitic acid methyl ester) was identified in the EG of Aloe fleurentiniorum. In an earlier study, palmitic acid has been identified as one of the constituents of the flower of Caralluma retrospiciens (Saad et al., 2020).
FT-IR spectroscopy revealed multiple peaks for EG in several fingerprint regions (Fig. 5). The existence of functional groups and their respective compounds was verified through spectral analysis of EG, which revealed distinct peaks (Table 3). The FT-IR spectrum analysis of EG revealed a bell-shaped curve at 3354 cm−1, indicating the presence of phenolic hydroxyl groups with stretching vibrations and corresponding compounds, as depicted in Table 3. Similarly, peaks at 2945, 2832, 2523, 2046, 1707, 1451, 1108, 1031, 880, 737 and 610 cm−1 representing asymmetric = CH2 stretching vibration, symmetrical = CH2 stretching vibration, –OH stretching vibration, -NCS group (isothiocyanate), =C = O str/C = C (olefinic) stretching vibration, =CH2 (alkane) bending/O-H bending vibration, C-O-C (ether or ester) stretching vibration, S = O (sulfur-oxy compounds) stretching vibration, =C-H bending vibration, methylene -(CH2)n, and alkyne = C-H bending vibration/–OH respectively. The MIC varied depending on the microorganism examined. The results showed that the inhibitory concentration was strongly impacted by the viscosity grade of EG. Str = Stretching; S = Strong; M = Medium; W = Weak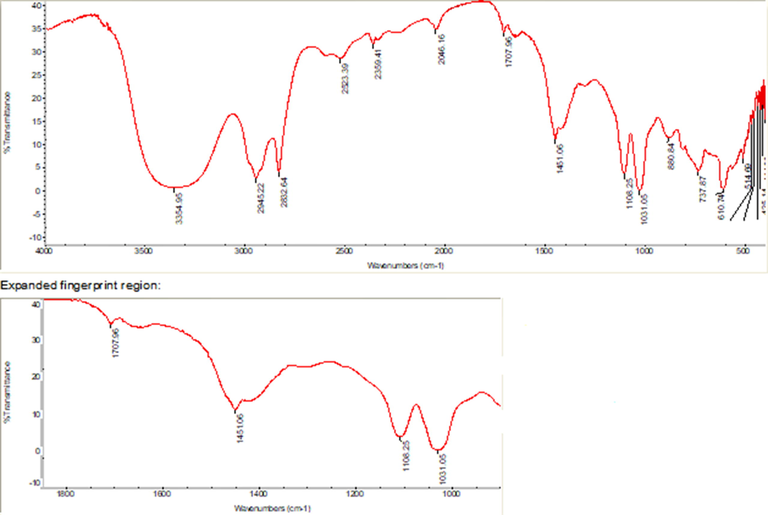
FT-IR Spectral characterization of EG from the leaf of Aloe fleurentiniorum.
Wave number (cm−1)
Intensity Estimation
Functional group/assignment
Possible Phytocompounds
3354
S
O-H str. (Phenolic hydroxyl)
Glycosides, Flavonoids
2945
S
CH2 str. (Asymmetrical)
Aliphatic compounds
Steroids, Saponins, flavonoids
2832
S
CH2 str. (Symmetrical)
Aliphatic compounds
Steroids (Cholestanol, Pregnenolone, Pregn-5-ene-3á,20-diol, Alfaxalone), Saponins, flavonoids
2523
W
O-H str (Hydroxyl group)
Glycosides, Flavonoids, Amino sugar Glucosamine, Steroids (Cholestanol, Pregnenolone, Pregn-5-ene-3á,20-diol, Alfaxalone), D-ribo-hexose
2046
W
-NCS (Isothiocyanate)
Isothiocyanate
1707
M
C = O str
C = C str (Olefinic)Steroids (Cholestanol,Pregnenolone, Pregn-5-ene-3á,20-diol, Alfaxalone), Flavonoids, 2-Octadecenoic acid, methyl ester, D-ribo-hexose
1451
S
CH2 bend (Alkane)
O-H bendingAliphatic compounds,
Steroids, Saponins, flavonoids,
2-octadecenoic acid, methyl ester, Steroids (Cholestanol, Pregnenolone, Pregn-5-ene-3á,20-diol),
Glycosides (D-arabino-hexopyranoside), 5-Cholestene-3-ol
1108
S
C-O-C Str (Ether, Ester)
Glycosides (D-arabino-hexopyranoside), Amino sugar Glucosamine
1031
S
S = O str. (Sulfur-oxy compounds)
Cutin
880
W
Out of plane C-H bending
Glucose/Glactose, Tannins
737
M
Methylene -(CH2) n- rocking
Steroids (Cholestanol, Pregnenolone, Pregn-5-ene-3á,20-diol, Alfaxalone),
2-octadecenoic acid, methyl ester,
Glycosides (D-arabino-hexopyranoside)
610
M
Alkyne C-H bend
Alcohol, OH out-of-plane bendGlycosides (D-arabino-hexopyranoside)
The MIC of EG was 20% (v/v) for each microorganism examined (Table 4). In a prior investigation, it was determined that Caralluma retrospiciens had a 50% (v/v) MIC (Makeen et al., 2020). Interestingly, the MIC of EG from Aloe fleurentiniorum was better than that of EG from Caralluma retrospiciens. In the present study, there was a broad spectrum of antibacterial activity against both Gram-positive and Gram-negative bacteria that were examined and the spectrum of activity were more or less similar amongst the screened bacterial organisms (Table 5). However, the anti-bacterial effect produced by EG was lower than that of the standard drug ciprofloxacin (50 µg/mL). (+) Presence of bacterial growth; (-) No bacterial growth # Each value is the mean of 6 batches with standard deviation. *** Extremely significant at p < 0.001, the effect is lesser than standard ciprofloxacin; ** Significant at p < 0.01, the effect is lesser than standard ciprofloxacin; * Significant at p < 0.05, the effect is lesser than standard ciprofloxacin
Bacterial organisms
Concentration of 24-h culture CFU /mL
Minimum inhibitory concentration of Exudate gel (EG) (% v/v)
50
40
30
20
10
Bacillus subtilis
4 × 10-5
–
–
–
–
+
Staphylococcus aureus
3 × 10-5
–
–
–
–
+
Streptococcus pyogenes
3 × 10-3
–
–
–
–
+
Escherichia coli
4 × 10-4
–
–
–
–
+
Pseudomonas aeruginosa
3 × 10-5
–
–
–
–
+
Klebsiella pneumoniae
2 × 10-5
–
–
–
–
+
Organisms
Zone of inhibition (mm) of EG analyte
Concentration CFU/mL
EG (20%)
Ciprofloxacin (50 µg/mL)
Bacillus subtilis
4 × 10-5
20.16 ± 0.75***
26 ± 1.4
Staphylococcus aureus
3 × 10-5
22.16 ± 1.16**
26.5 ± 1
Streptococcus pyogenes
3 × 10-3
21.83 ± 1.5*
24.8 ± 1.7
Escherichia coli
4 × 10-4
21 ± 1.4**
26 ± 1.5
Pseudomonas aeruginosa
3 × 10-5
20.5 ± 1.9*
23.3 ± 1.6
Klebsiella pneumonia
2 × 10-5
20.6 ± 2.1*
23.5 ± 1.9
4 Conclusion
The current study presents scientific data in support of Aloe fleurentiniorum leaf exudate gel's therapeutic capabilities. The findings demonstrated a wide range of bioactive chemicals with anti-bacterial action against Gram-positive and Gram-negative bacteria. The study suggesting that potent antibacterial agent can be isolated for the beneficial of human health care and welfare.
Acknowledgments
The authors are thankful to the Pharmacy Practice Research Unit (PPRU), College of Pharmacy, Jazan University, for allotting the project. The project reference number was graduation research project, PPRU-14-02. The authors also thank Dr. Remesh Moochikkal, herbarium curator, for identifying the seaweed, and for maintaining the samples in the herbarium of Jazan University.
Author’s contributions
Sivakumar Sivagurunathan Moni was involved in conceptualization and methodology. Muhammad H. Sultan analysed the result. Hafiz A. Makeen was in charge of resources, while Osama A. Madkhali did statistical analysis. Mohammed Ali Bakkari drafted the manuscript, while Saad S. Alqahtani and Saeed Alshahrani reviewed the manuscript. M. Intakhab Alam, Mohamed Eltaib Elmobark, Zia ur Rehman and Md Shamsher Alam were involved in methodology. The plant samples were collected and processed by Abdulrahman Yahya Faqihi, Hassan Mansour Mogaidi, Hussein Khobrani, Issa Albana, Ahmed Motab Sharahili, and Santhosh Joseph Menachery.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J Ethnobiol Ethnomed.. 2019;15:1-9.
- [CrossRef] [Google Scholar]
- A Rare Sugar, d-Allose, Confers Resistance to Rice Bacterial Blight with Upregulation of Defense-Related Genes in Oryza sativa. Genetics and Resistance. 2009;100(1):85-90.
- [CrossRef] [Google Scholar]
- Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int. J. Pharmacogn. Phytochem. Res.. 2017;9:537-552.
- [Google Scholar]
- Microbiology - A Laboratory Manual. USA: Pearson Education Inc; 2014.
- Variation in phytochemical composition reveals distinct divergence of Aloe vera (L.) Burm.f. from other Aloe Species: Rationale behind selective preference of Aloe vera in nutritional and therapeutic Use. J. Evid. Based Complementary Altern. Med.. 2017;22(4):624-631.
- [CrossRef] [Google Scholar]
- Determination of bioactive components of Caralluma umbellata haw. (apocynaceae) by gas chromatography and mass spectroscopy analysis. Asian J. Pharm. Clin. Res. 2018;11(5):194-199.
- [Google Scholar]
- Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by amoxicillin-clavulanic acid in rats. Ann. Hepatol.. 2009;8(2):34-140.
- [CrossRef] [Google Scholar]
- Additive antitumour effect of D-allose in combination with cisplatin in non-small cell lung cancer cells“. Oncology Reports. 2018;39(3):1292-1298.
- [CrossRef] [Google Scholar]
- The rare sugar D-allose acts as a triggering molecule of rice defence via ROS generation. J. Exp. Bot.. 2013;64(16):4939-4951.
- [CrossRef] [Google Scholar]
- Molecules and functions of rosewood: Pterocarpus cambodianus. Arab. J. Chem.. 2018;11(6):763-770.
- [CrossRef] [Google Scholar]
- Documentation of bioactive principles of the exudate gel (EG) from the stem of Caralluma retrospiciens (Ehrenb) and in vitro anti-bacterial activity – Part A. Arab. J. Chem.. 2020;13(8):6672-6681.
- [CrossRef] [Google Scholar]
- Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. J. Pharmacognosy Phytother.. 2016;8(2):8-24.
- [CrossRef] [Google Scholar]
- Ethnopharmacological survey of medicinal plants in Albaha Region. Saudi Arabia. Pharmacognosy Res.. 2017;9(4):401.
- [CrossRef] [Google Scholar]
- Bioactive compounds and in vitro antimicrobial activities of ethanol stem bark extract of Trilepisium madagascariense DC. Int. J. Pharmacol.. 2018;14:901-912.
- [CrossRef] [Google Scholar]
- The molecular biology in wound healing & nonhealing wound. Chin J Traumatol. 2017;20(4):189-193.
- [CrossRef] [Google Scholar]
- Antibiotic-potentiating activity, phytochemical profile, and cytotoxicity of Acalypha integrifolia Willd. (Euphorbiaceae) J. Herb Med.. 2018;11:53-59.
- [CrossRef] [Google Scholar]
- Documentation of bioactive principles of the flower from Caralluma retrospiciens (Ehrenb) and in vitro anti-bacterial activity – Part B. Arab. J. Chem.. 2020;13(10):7370-7377.
- [CrossRef] [Google Scholar]
- Evaluation of phytochemical profile in the ethanol extracts of Malachra capitate (L) L by GC-MS analysis. World Journal of Pharmaceutical Research. 2015;4(8):1342-1353.
- [Google Scholar]
- Therapeutic potential of oleic acid nanovesicles prepared from petroleum ether extract of Sargassum binderi in streptozotocin–induced diabetic wound in Wistar rats. Trop. J. Pharm. Res.. 2018;17(11):2123-2128.
- [CrossRef] [Google Scholar]
- WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva, Switzerland: World Health Organization; 2004. https://apps.who.int/iris/handle/10665/43034
- Effectiveness and safety of herbal medicines for induction of labour: a systematic review and meta-analysis. BMJ Open.. 2018;8(10):e022499.
- [CrossRef] [Google Scholar]







