Translate this page into:
Bioactivity and molecular docking of synthesized macromolecular ligand and its complex
⁎Corresponding author at: College of clothing and textiles, Qingdao University, 266071, China. maryam1988@126.com (Maryam Fatima)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The development of drug resistant strains of the pathogenic fungi despite the availability of large number of drugs demands the development of new and more potential drug molecules. Dendrimer based drug molecules are comparatively less researched upon and a recent advancement in this field. The present study is concerned with the preparation of macromolecular ligand and its complex using ethylenediamine and methylmethacrylate as starting material. The reaction goes via Michael addition reaction and synthesized dendritic units were used as ligands to obtain metal-ligand complexes. Obtained ligand and its complex were characterized in terms of different techniques such as FTIR, 1H NMR, ESI-MS, UV–Vis and Elemental analysis. Further, the ligand and its complex were used to determine antifungal activity against C. albicans ATCC 90028 by MICs and Disk diffusion assay. Comet assay and Molecular docking techniques were used to show toxicity effects and ligand–DNA interactions respectively. Ligand and its complex were obtained in very good yield with square planar geometry and having good antifungal potential against C. albicans. It was also found from Molecular docking and Comet assay, that the Copper complex interacts strongly with DNA and shows less toxicity than ligand. The compounds can serve as promising leads for the development of new antifungal agents.
Keywords
Michael addition
MICs
Disk diffusion assay
Comet assay
Docking
Antifungal
1 Introduction
Consumers worldwide are looking for those materials which are hygienic, non-toxic, biodegradable and environment friendly. However, the major hindrance which comes underway in developing such materials is pathogens. Recent research has shown that materials such as textiles, food packaging products, medical products and food storage products are deteriorated by pathogens. This is main reason for spreading the infection all over the world due to which majority of people are dying from last several years (Munoz-Bonilla and Fernández-García, 2012). Among all infections, the most spreading is mycotic infection caused by Candida and Aspergillus species. Out of all Candida only Candida albicans is main challenge because it causes many infectious diseases (Bhatti et al., 2006). Lot of effective and safe antifungal antibiotics which can fight against these diseases is available in the market but they are very limited in number. Also, they are not able to reduce the mortality rate of these funguses because of their resistance against antifungals (Casalinuovo et al., 2004). Enhancing health awareness has motivated researchers to synthesize new antifungals with high efficacy, high selectivity and good potential to fight against these infectious diseases.
Dendrimers are one such class of bioactive compounds which have been recently discovered. These compounds are soluble in water and therefore easily encourage drugs to move across biomembranes (Chauhan et al., 2004; Devarakonda et al., 2005; Kolhe et al., 2003). The topology of the dendrimers is based on tree. Dendrimers are highly branched molecules, monodisperse in nature, intermediate molecular mass and also have properties of host-guest. The size, branching, surface functionalities can be easily controlled in dendrimers and can be used to increase the solubility of those drugs which are very less soluble (Milhem et al., 2000). Basic PAMAM dendrimers has many applications, for example they are used as additives in many routes of administration (intravenous, oral, trans-dermal or ocular) (Esfand and Tomalia, 2001; Gardiner et al., 2008). There are many advantages of dendrimers over the linear polymers such as density and reactivity of functional groups present at surface are very high due to which dendrimers are bioactive molecules. The surface can be modified according to the need, the size of dendrimers can be easily controlled for many biomedical uses, and penetration power of dendrimers is very high through cell membranes. Many dendrimers are reported to posses’ anticancer, anti-inflammatory and antimicrobial activities. Dendrimer are also used as drug carriers and acts as active part for therapy i.e. used for combinational therapy (Esfand and Tomalia, 2001; Tulu et al., 2009).
With this aim, we report herein the synthesis and biological evaluation of macromolecular ligand (dendrimer) and its complex. Many physicochemical and spectroscopic techniques were used to characterize synthesized ligand and metal complexes. Finally, the ligand and its complex were investigated for their antifungal activities against Candida albicans ATCC 90028. Further, Molecular docking and Comet assay was done in order to show interaction with DNA and toxicity.
2 Experimental
2.1 Materials and methods
Ethylenediamine and methylmethacrylate were obtained from S.D. Fine Chem., Mumbai. And metal salt (copper chloride dihydrate) was purchased from E. Merck, Germany. All the chemicals required no further purification and were of AR grades. For Antifungal study, stock culture of microorganism was preserved in nutrient agar slant at 4 °C. REMI CIS 24 BL shaker was used to grow Candida Albicans in YPD media at 200 rpm and 37 °C. For YPD media glucose, peptone and yeast extract were obtained from Hi Media, India.
For elemental Analysis (C,H,N) Vario EL elemental Analyzer was used. UV–Vis. spectrophotometer used to record electronic spectra was Perkin Elmer Lambda-40 double-beam UV–Visible spectrophotometer, United Kingdom. IR spectra of the compounds were obtained using KBr pellets (4000–400 cm−1) on Perkin Elmer 1750 FTIR spectrophotometer (CT 06859 USA), USA. Bruker (DRX-400) spectrometer (USA) was used to record the 1HNMR spectra of compounds. To record Mass Spectra Model-Q-TOF Micromass ESI (Cambridge, UK) source was used. Melting points of the compounds were recorded on Veego Instrument (REC-2203882), USA.
2.2 Synthesis of ligand (L)
2.2.1 Synthesis of compound (I)
Ethylenediamine (0.02 mol) and methylmethacrylate (0.02 mol) were taken in a round bottom flask and were dissolved in minimum amount of solvent. The solution was stirred for 24 h at room temperature. The resultant liquid was slightly viscous and creamish in color.
2.2.2 Synthesis of compound (II)
The synthesized compound (I) (0.02 mol) was placed in a round bottom flask in an ice bath. To this solution glycerol (0.02 mol) was added and stirred. To the above solution concentrated H2SO4 (1 mL) was added drop wise with continuous stirring. The solution was stirred continuously in an ice bath for 4–5 h. The liquid (II) obtained was highly viscous and yellow in color.
2.2.3 Synthesis of final ligand (III)
The synthesized compound (II) was placed in a round bottom flask and kept at 0°. To the round bottom flask 1 mL H2SO4 was added and stirred for 4 h in an ice bath. The liquid formed was pale yellow in color.
Yield: 96%, mol. wt. 678.86 g mol−1, golden yellow oily liquid, anal. calc. for C26H56N10O4(%): C, 53.08; H, 9.21; N, 16.51. found: C, 50.16; H, 8.98; N, 15.98; I.R. (KBr pellets, cm−1): 3270.09 (—NH2, str.), 1458.91 (C—O, ester), 1558.63 (N—H, bend), 1636.41 (C⚌O), 1043.54 (C—O, ether); 1H NMR (D2O, ppm): 3.180 (CH2,t), 3.453 (CH2,t), 3.492 (CH2,d), 3.387 (CH,m), 0.901 (CH3,d), 3.609 (CH2,d), 4.667 (CH,m); ESI-MS (m/z): 441.3 [M-C8H28N8+H+], 220.2 [M-C20H52N8O4+H+], 104.0 [M-C28H52N8O8-4H+]
2.3 Synthesis of metal complex
A methanolic solution of Ligand (1 mmol) was added to a methanolic solution of CuCl2·2H2O (2 mmol) with constant stirring at 40–50 °C for 5–6 h. The precipitated complex was filtered and washed with methanol and dried under vacuum.
Yield: 62%, mol. wt. 947.76 g mol−1, green powder, anal. calc. for C26H56N10O4(%): C, 38.02; H, 6.59; N, 11.82. found: C, 36.99; H, 6.12; N, 11.02; I.R. (KBr pellets, cm−1): 3155.91 (NH2, str.), 1722.88 (C⚌O), 1403.03 (C—O, ester), 1129 (C—O, ether).
2.4 Solution stability
The stability of the complex was estimated at physiological pH by monitoring their UV–Vis spectra. In aqueous solution of PBS, 10−4 M solution of the complex was prepared at pH 7.4. To calculate the hydrolysis profile of complex, the electronic spectra was then recorded over 24 h at room temperature (wani et al., 2014).
2.5 Biological screening
2.5.1 Minimal inhibitory concentration (MIC)
The lowest concentration of the test compound at which there is inhibition in growth of microorganism is known as minimal inhibitory concentration (MIC). According to the CLSI guidelines (Wayne, 2008) for fungi, broth micro dilution method was used to calculate MIC in vitro in liquid medium. Positive control (drug free control) used was Fluconazole.
2.5.2 Disk diffusion assays
The method given by Ahmad et al. (2010) was performed for disk diffusion assay. In liquid media the strains were inoculated and left to grow overnight at 37 °C. Once the cells were grown, these cells were then washed three times with double distilled water. Then, in molten agar media cells of half strength, 1x105 cells were inoculated at 42 °C. All the cells then poured in petridishes of diameter 100 mm. On solidifying the upper layer, sterile paper discs (4 mm) soaks with test compounds. And then placed these discs on agar surface and incubate it for 48 h at 37 °C. After the incubation, calculate the size and pattern of growth zone around discs.
2.5.3 Comet assay
Comet assay (single cell gel electrophoresis) Comet assay was performed under alkaline conditions according to the procedure of Singh et al. (1988) (Singh et al., 1988), with slight modifications. Fully frosted microscopic slides pre- coated with 1.0 percent normal melting agarose were used at about 50 1C (dissolved in Ca2+ and Mg2+ free PBS of pH 7.5). Around 10,000 cells were mixed with 90 mL of 1.0 percent low melting agarose to form a cell suspension and pipette over the first layer and covered immediately by a coverslip. The slides were placed on a flat tray and kept on ice for 10 min to solidify the agarose. The coverslips were removed and a third layer of 0.5 percent low melting agarose (80 mL) was pipetted. Cover slips were placed over it and it was allowed to solidify on ice for 5 min. The coverslips were removed and the slides were immersed in cold lyses solution containing 2.5 M NaCl, 100 mM EDTA and10 mM Tris, pH 10, 1% Triton X-100 was added before use for a minimum of 1 h at 41C. After lyses, DNA was allowed to unwind for 30 min in alkaline electrophoretic solution consisting of 300 mM NaOH and 1 mM EDTA, pH > 13. Electrophoresis was performed at 4 1C in field strength of 0.7 V/cm and 300 mA current. The slides were then neutralized with cold 0.4 M Tris, pH 7.5, stained with 75 mL ethidium bromide (20 mg/mL) and covered with a coverslip. They were then placed in a humidified chamber to prevent drying of the gel and analyzed the same day. Slides were scored using an image analysis system (Komet5.5; Kinetic Imaging, Liverpool, UK) attached to an Olympus (CX41) fluorescent microscope (Olympus Optical Co., Tokyo, Japan) and a COHU 4910-integrated CC camera (equipped with 510–560 nm excitation and 590 nm barrier filters) (COHU, San Diego, USA). Images from 50cells (25from each replicate slide) were analyzed. Tail length (migration of DNA from the nucleus in micrometer used as an index of DNA breakage) was chosen as the parameter taken to assess lymphocytes DNA damage.
2.5.4 Docking
The molecular docking of Cu-complex with 1.BNA protein was done by PyRx software. Initially the crystal structure of protein B-DNA dodecamer d(CGCAAATTTCGC)2 (PDB ID:1BNA) was downloaded by PDB website. Then the structure of Cu-complex was made in the Marvin Sketch software and was saved as PDB file. Finally, both protein and Cu-complex was docked in PyRx and results were analyzed. Moreover, to understand the interaction clearer and visualize docked pose, all resulted structures were analyzed in PyMol (Delano, 2002) and Marvin Sketch software.
3 Result and discussion
The proposed structure of the ligand and its metal complexes were supported by spectroscopic data. The compounds were hygroscopic, soluble in water, ethanol and methanol and insoluble in other common organic solvents. All the compounds were characterized by CHNS analysis and UV–Vis, FT-IR, 1H NMR and ES-MS spectroscopic techniques. The Michael addition of ethylenediamine to methylmethacrylate led to the formation of ligand (L). The Synthesized ligand coordinated with Cu(II) metal ion (Fig. 1).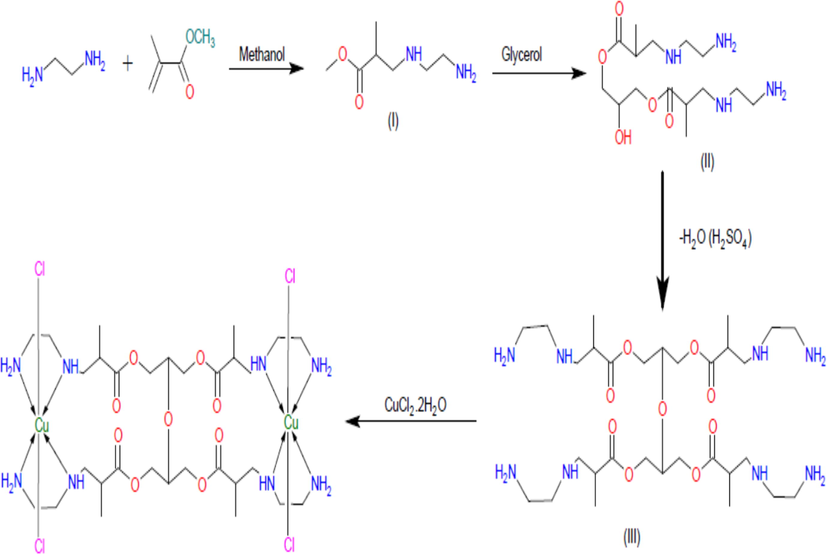
Schematic representation of the synthesis of dendrimeric ligand (L) and its copper(II) complex (proposed structures of L and CuL).
3.1 Spectral deconvolution
3.1.1 Solution stability
For the therapeutically active drugs aquation process is very important for their action. To study the solution stability of the complexes generally UV–Vis spectroscopy is used at physiological pH.
The UV–Vis spectra of the copper complex (CuL) in PBS show no shifts in the bands even after 24 h and also no precipitation was observed. But the intensity of the peak was decrease after 24 h, that because water (used as solvent) is polar solvent and decreases the intensity. The compound is stable in its solution state because no hypsochromic shift or bathochromic shift was observed in the spectra after 24 h. These observations indicate that the copper complex (CuL) is stable in its solution state (Singh et al., 2015).
3.1.2 IR spectra
The FT-IR spectra confirmed the structure of ligand (L) and its copper complex. KBr pellets were used to record the FT-IR spectra. The characteristic peaks of ligand (L) were observed at 3270.09 cm−1, 1458.91 cm−1, 1558.63 cm−1, 1636.41 cm−1 and 1043.54 cm−1 in its FT-IR spectra which corresponds to the vibrations of NH2, C—O (ester), N—H (bend), C⚌O and C—O (ether) groups, respectively. Peaks observed for copper complex (CuL) in the spectrum were at 3155.91 cm−1, 1722.88 cm−1, 1403.03 cm−1 and 1129 cm−1 assigned to groups NH2, C⚌O, C—O (ester) and C—O (ether), respectively. In the copper complex (CuL) similar peaks were observed but with slight shifting in the frequencies (Fig. S1a and S1b, supplementary material). This indicates that the metal (i.e. copper metal) coordinates with the ligand.
3.1.3 NMR spectra
The 1HNMR spectrum of the synthesized ligand was recorded using D2O as solvent and TMS as internal standard (Fig. S2, supplementary material). The protons of NH and NH2 are highly acidic in nature due to which there is rapid exchange reaction with deuterium protons in solvent is possible and no signals of these two protons were observed in 1HNMR spectra. In Fig. S3, supplementary materials, the protons of the ligand were showed as A, B, C, D, E, F and G.
The signals observed at 3.180 ppm (triplet) and 3.453 ppm (triplet) were due to protons A and B, respectively. The doublet observed at 3.492 ppm, 0.901 ppm and 3.609 ppm, were due to C, E and F protons, respectively. At 3.387 ppm and 4.667 ppm, multiplet is observed due to protons D and G, respectively. Downfield shifts were observed in copper complex (CuL) (Fig. S4, supplementary material) because of the coordinating effect (Chaudhary and Singh, 2004; Kemp, 1975).
3.1.4 Electronic spectra
Absorption spectrum was scanned to determine the nature and types of electronic transitions in metal complex (i.e. CuL). The spectrum was scanned in the range of 200–800 nm using double distilled water as solvent. The high energy transitions were observed for the copper complex (CuL) at 215, 255 and 337 nm corresponding to n → ϭ*, π → π*, n → π* transitions, respectively. The transitions n → π*, π → π* and n → ϭ* might be assigned to the amide carbonyl, C⚌O (unsaturation) group and to heteroatoms present, respectively. Besides, it exhibited a broad low energy transition centered at 528.12 nm corresponding to 2A1g → 2Eg and this broad peak at 528.12 nm (2A1g → 2Eg transition) indicates the possible geometry of the complex to be square planar (Naik et al., 2009; Singh et al., 1975; Naz and Iqbal, 2009).
3.1.5 Mass spectra
To check the fragmentation of the ligand ESI-MS recorded in positive ion mode shown in (Fig. S5, supplementary material). The structure of the ligand was well supported by mass spectra. Due to the branching structure of the compound, the molecular ion peaks were not observed but other structurally characteristic peaks were observed. The fragment peaks showed in the mass spectra of ligand (L) at m/z values of 441.3, 220.2 and 104.0 corresponds to the fragments [M-C8H28N8+H+], [M-C20H52N8O4+H+] and [M-C28H52N8O8-4H+] respectively.
3.1.6 Biological evaluation
3.1.6.1 Antifungal activity
The synthesized ligand (L) and its copper complex (CuL) were evaluated for the in vitro antifungal activity. The experiment has been repeated three times under similar conditions. Double distilled water was used as a negative control and Fluconazole as positive standard for antifungal activity. The results obtained have been presented in the Tables 1 and 2. It can be interpreted from the data presented in Table 1, that complex (CuL) exhibited pronounced zones of inhibition against Candida Albicans where zone of inhibition was found to be 12 mm, 15 mm and 20 mm almost in proximity to reference drug Fluconazole. Complex displayed nearly same zones of inhibitions almost against fungal strain which was further evident from their respective MIC’s values for the same fungal strain (Fig. 2). The MIC value of copper complex for fungal strain was recorded to be 0.16 µg/mL. Similarly ligand (L) exhibited moderate profile of activity against fungal strain used in the study and was found to be less active against C. albicans in comparison to copper complex. Among the synthesized compounds, complex CuL displayed promising MIC value of 0.16 µg/mL against Candida Albicans in to reference drug Fluconazole (0.004 µg/mL). From the data given in Tables 1 and 2, it is quite obvious that the compounds which are exhibiting efficacious zone of inhibition are having lower MIC’s and are therefore active antifungal agents at the fixed concentration against different fungal strains. Earlier it was reported that the dendrimers increase the antifungal activity of antifungal drug but doesn’t shows antifungal activity when used alone (Khairnar et al., 2010). But in present study not only dendrimer (viz. ligand) shows antifungal activity but also M-L complexes possess excellent antifungal activity as compared to other metal complexes with Schiff’s base as reported earlier (Sheikh et al., 2013; LV et al., 2006). This result is significant as the conventional drug Fluconazole is fungi static and hence leads to antifungal drug resistance. The results revealed that the metal complex is more active than their respective ligand (L). The dispersion of the complexes into lipid membrane increases and that is why they are more biological active. The chelation theory is considered for the increase in the toxicity metal complexes. The chelation of metals there is a partial sharing of the positive charge of metal with donor groups and also the π electron delocalized over the chelate ring so it decreases the polarity of the metal ions used. The lipophilic character of metal ion improved because of chelation and these results the succeeding permeation of metal ions through semi permeable lipid layers of cell membrane (Yang et al., 1997). The presence of lipophilic and polar substituent such as C⚌N, O—H and NH2 are expected to enhance the fungal toxicity. So, the rate of entry of molecules inside the cell was control by lipophilicity which is then customized by metal coordination and that is why metal complexes are more active than ligand itself (Farrell, 2007). In view of the above results, the activity of the ligand and its complexes was found to be in the order: CuL ˃ L.
Test agents
MIC (µg ml−1) C. albicans ATCC 90028
L (ligand)
1
CuL (copper complex)
0.16
Fluconazole (standard drug)
0.004
Test concentration (mg ml−1)
Zone of Inhibition (mm)
L (ligand)
CuL (copper complex)
Fluconazole
0.004
*
*
25
0.2
8
8
*
0.5
14
12
*
1.0
18
15
*
1.5
*
20
*
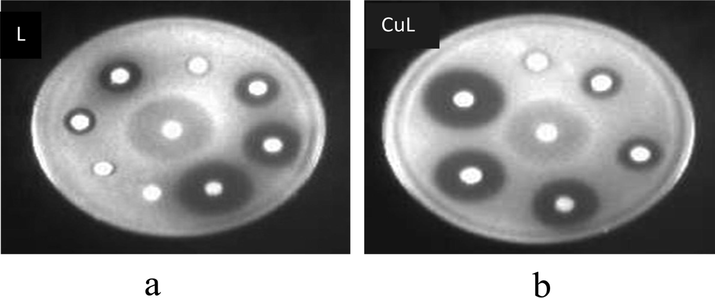
Disk diffusion assay of C. albicans ATCC 90028 showing zones of inhibition in the presence of (a) Ligand (L) (b) Copper complex (CuL). The concentration of the test compounds ranged from 0.1 to 1.0 mg per disk. Fluconazole (FLU) (4 µg) was used as a positive control.
3.1.6.2 Comet assay
To examine that the synthesized complexes were interact with DNA strands or damage it we carried an experiment called as Comet assay. Comet assay is a genotoxicity experiment. This method is basically used to examine DNA fragmentations in human cells and then detect its genotoxic effect as well as anti-genotoxic activity i.e. protective against the breakage of chromosomes i.e. clastogenic challenge. We get the spherical shape it there is no damage occurs in the cell whereas if the cell got fragments do the nucleotides migrates and form a tail known as comet. Level of DNA damage was depending on tail length. If there is high level of DNA damage then the tail length is longer. In this experiment, three complexes were treated with cells and formed comets. But there is no comet observed in case of control i.e. untreated cells. The damage in nucleotide in the tail was calculated using fluorescence percentage. In present experiment it was observed that copper complex slightly damage nucleotide whereas damage by ligand is more. So, it can be concluded that the ligand is more toxic if we compare it with its copper complex (Fig. 3).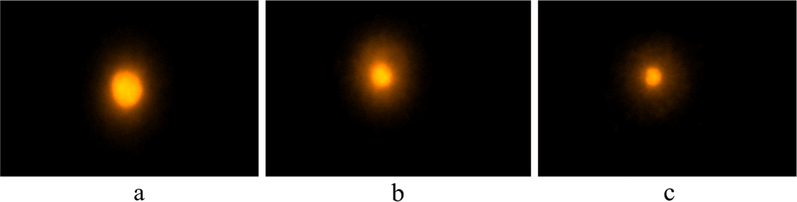
Comet Assay-Single cell gel electrophores is of human peripheral Lymphocytes. Complex induced double-strand DNA breaks in lymphocytes cells. Cells were untreated (a) or treated with 20 μM complex. (a) Test sample with tail length 3.43 ± 0.05 µm, (b) Ligand with tail length 10.42 ± 0.047 µm and (c) Copper Complex with Tail length 7.23 ± 0.039 µm.
3.1.6.3 Docking
To predict the strength of binding of ligand with receptor binding sites by changing some its position we used a computational method known as Docking. To recognize exact sequence, the molecules were designed accordingly. To understand the nucleic acid molecular detection and also to develop new chemotherapeutic drugs, the use nucleic acid is very important.
The DNA duplex we used are of sequence d(CGCGAATTCGCG)2 dodecamer (PDB 1D:1BNA) and check its binding with the copper complex. All the results are shown in Table 3 and Figs. 4–6. Fig. 4 shows the strong binding of Copper complex with protein 1BNA. The Cu-complex shows that there are 8 binding modes by which it is interacting with 1BNA protein (Table 3). All the binding modes have negative binding energies which means that the bonding is strong between Cu-complex and 1BNA protein. If the binding energy is more negative then there is strong binding of DNA and molecules. So, we select the highest negative binding energy mode for visualization in Pymol software (Fig. 6). The results show acceptable bonding of copper complex with 1BNA protein at two sites, one is at Adenine(C6) part of the A-chain and another one is Guanine(C4) part of the same chain. Further, the polar interactions have been seen among heteroatoms in both A and B chains.
Ligand
Binding affinity(kcal/mol)
Mode
RMSD lower bound
RMSD upper bound
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−6.8
0
0.0
0.0
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−6.6
1
1.064
1.448
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−6.4
2
2.443
3.757
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−6.2
3
31.562
2.368
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−5.9
5
8.929
13.462
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−5.7
6
6.372
9.784
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−6.1
4
2.965
8.937
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−5.5
7
2.529
8.742
A_BNA1.Ligand_uff_Cu-complex = 8028.35
−5.5
8
4.024
7.852
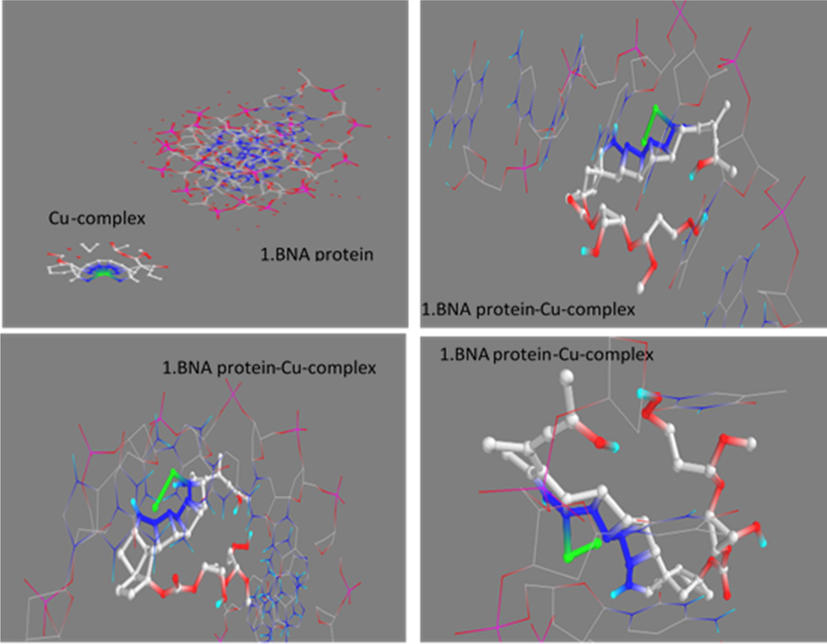
Docked pose of copper complex with DNA at different modes.
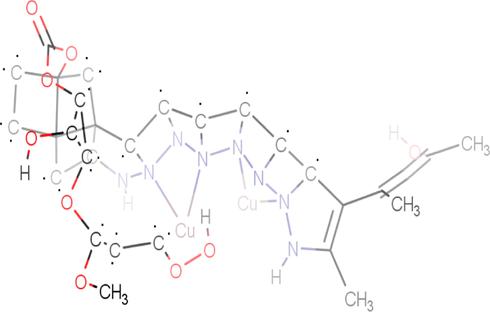
Chemical structure of protein-Cu-complex obtained in Marvin Sketch.
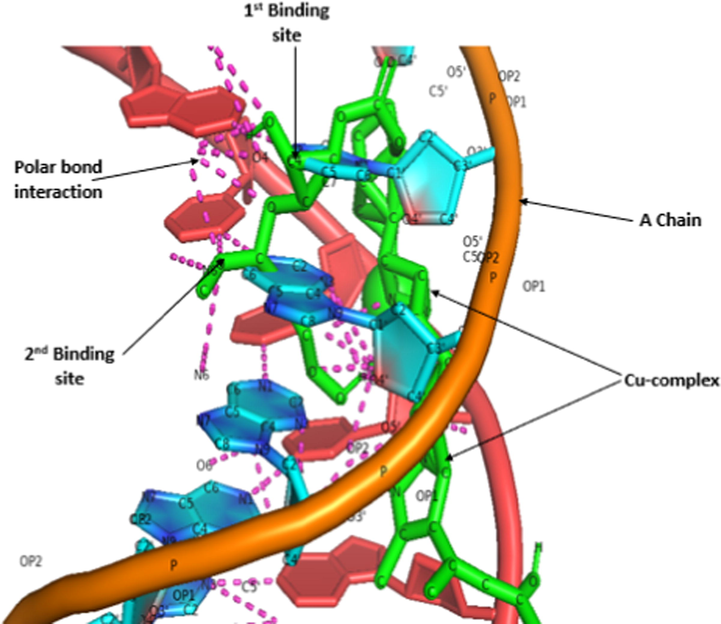
Interaction between protein chains and Cu-complex.
4 Conclusion
In present work, we described synthesis of macromolecular ligand (dendrimer) and its copper complex. The dendrimers are gaining popularity now a day because of their potential use in biomedical applications. A large number of researches are being carried out at various research facilities for the development of biomedical important dendrimers and dendrimer metal complexes. In view of cost, time factor for synthesis and biomedical application we have succeeded in synthesizing a dendritic macromolecule and its complex. It was demonstrated that both ligand and its complex were obtained in good yields and Copper (II) complex have square planer geometry. Both the ligand and its complex displayed significant antifungal activities against Candida albicans (ATCC 90028). The MIC values of compounds indicates that each test compound is effective as antifungal. Solid media growth was appreciably inhibited by metal complexes and we see that halo was completely clear. These results indicated that the synthesized compounds have probable fungicidal activity. Comet assay results showed that Copper Complex is less toxic as compared to ligand, predicted by measuring the length of the tail produced. From Molecular docking it is evident that Copper complex interacts very strongly.
Acknowledgement
The authors are thankful to sophisticated analytical instrumentation facility, Punjab University Chandigarh, India, for providing ESI-MS. Jamia Hamdard University, new delhi, India, for providing facility of NMR and UGC New Delhi, India, for providing UGC-BSR meritorious research fellowship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathog.. 2010;48:35-41.
- [Google Scholar]
- Review of epidemiology, diagnosis and treatment of invasive mould infections in allogeneic hematopoitic stem cell transplant recipients. Mycopathologia. 2006;162(1):1-15.
- [Google Scholar]
- Fluconazole resistance in Candida Albicans: a review of mechanism. Eur. Rev. Med. Pharmacol. Sci.. 2004;8(2):69-77.
- [Google Scholar]
- Synthetic, spectroscopic and tocicological aspects of novel eighteen to twenty two membered tertraaza macrocycles and their bivalent manganese complexes. Ind. J. Chem.. 2004;43:2529-2535.
- [Google Scholar]
- Solubility enhancement of indomethacin using dendrimer complex and biodisposition profile of these complexes in arthritic rats. J. Drug Target.. 2004;12:575-583.
- [Google Scholar]
- DeLano Scientific. CA, USA: San Carlos; 2002.
- Comparison of the aqueous stabilization of pratically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int. J. Pharm.. 2005;304:193-209.
- [Google Scholar]
- Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today.. 2001;6:427-436.
- [Google Scholar]
- Recent developments in the chemistry of 1,3,2-diazaborolines-(2,3-dihydro-1H-1,3,2-diazaboroles) Coord. ChemRev.. 2007;232(1–2):1-31.
- [Google Scholar]
- PAMAM dendrimers for the delivery of the antibacterial triclosan. J. Enzyme Inhib. Med. Chem.. 2008;23:623-628.
- [Google Scholar]
- Organic Spectroscopy. London, UK: Macmillan Press Ltd.; 1975.
- Dendrimers: Potential tool for enhancement of antifungal activity. Int. J. PharmTech Res.. 2010;2(1):736-739.
- [Google Scholar]
- Drug complexation, invitro release and cellular entry of dednriemrs and hyperbranched molecules. Int. J. Pharm.. 2003;259:143-160.
- [Google Scholar]
- Synthesis, structure and biological activity of cobalt (II) and copper (II) complexes of valine-derived Schiff bases. J. Inorg. Biochem.. 2006;100:1888-1896.
- [Google Scholar]
- Polyamidoamine starbust dendrimers as solubility enhancers. Int. J. Pharm.. 2000;197(1–2):239-241.
- [Google Scholar]
- Polymeric materials with antimicrobial activity. Prog. Polym. Sci.. 2012;37:281-339.
- [Google Scholar]
- Bimetallic complexes of a potentially pentadentate, acyclic, symmetrical compartmental Schiff base ligand that provides suitable topology for an exogenous bridge. Transit. Met. Chem.. 2009;27:333-336.
- [Google Scholar]
- Synthesis, spectroscopic and biological studies of transition metal complexes of novel schiff bases derived from amoxicillin and sugars. J. Chem. Soc. Pak.. 2009;31:440-446.
- [Google Scholar]
- Synthesis and in vitro biology of co(II), Ni(II), Cu(II) and Zn(II) complexes of functionalized beta-diketone bearing energy buried potential antibacterialand antiviral o, o pharmacophore sites. J. Saudi Chem. Soc.. 2013;17:269-276.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res.. 1988;175:184-191.
- [Google Scholar]
- Synthesis and structural studies of some cobalt(II) cadmium complexes with Lewis bases. J. Inorg. Nucl. Chem.. 1975;37:679-683.
- [Google Scholar]
- Synthesis, characterization and antimicrobial activity of water soluble dendritic macromolecules. Eur. J. Med. Chem.. 2009;44:1093-1099.
- [Google Scholar]
- Design and synthesis of Co(II) and Cu(II) complexes of a dendrimeric chelate: promising anticandidal potential of chelotherapeutic agents. J. Coord. Chem.. 2015;68(12):2096-2106.
- [Google Scholar]
- Copper(II), nickel(II), and ruthenium(III) complexes of an oxopyrrolidine-based heterocyclic ligand as anticancer agents. J. coord. Chem.. 2014;12:2110-2130.
- [Google Scholar]
- Wayne, P.A., 2008. Clinical and laboratory standards institute, third ed., 28, M27-A3, USA.
- Study of the interaction between novel ruthenium(II)–polypyridyl complexes and calf thymus DNA. J. Inorg. Biochem.. 1997;66:141-144.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.09.009.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







